Abstract
In children, psychotic-like experiences (PLEs) are related to risk of psychosis, schizophrenia, and other mental disorders. Maladaptive cognitive functioning, influenced by genetic and environmental factors, is hypothesized to mediate the relationship between these factors and childhood PLEs. Using large-scale longitudinal data, we tested the relationships of genetic and environmental factors (such as familial and neighborhood environment) with cognitive intelligence and their relationships with current and future PLEs in children. We leveraged large-scale multimodal data of 6,602 children from the Adolescent Brain and Cognitive Development Study. Linear mixed model and a novel structural equation modeling (SEM) method that allows estimation of both components and factors were used to estimate the joint effects of cognitive phenotypes polygenic scores (PGSs), familial and neighborhood socioeconomic status (SES), and supportive environment on NIH Toolbox cognitive intelligence and PLEs. We adjusted for ethnicity (genetically defined), schizophrenia PGS, and additionally unobserved confounders (using computational confound modeling). Our findings indicate that lower cognitive intelligence and higher PLEs are significantly associated with lower PGSs for cognitive phenotypes, lower familial SES, lower neighborhood SES, and less supportive environments. Specifically, cognitive intelligence mediates the effects of these factors on PLEs, with supportive parenting and positive school environments showing the strongest impact on reducing PLEs. This study underscores the influence of genetic and environmental factors on PLEs through their effects on cognitive intelligence. Our findings have policy implications in that improving school and family environments and promoting local economic development may enhance cognitive and mental health in children.
Research organism: Human
eLife digest
Childhood is a critical period for brain development. Difficult experiences during this developmental phase may contribute to reduced intelligence and poorer mental health later in life. Genetics and environmental factors also play roles. For example, having family support or a higher family income has been linked to better brain health outcomes for children.
Delusions or hallucinations, or other psychotic-like experiences during childhood, are linked with poor mental health later in life. Children who experience psychotic-like episodes between the ages of nine and eleven have a higher risk of developing schizophrenia or related conditions. Environmental circumstances during childhood also appear to play a crucial role in shaping the risk of schizophrenia or related conditions.
Park, Lee et al. show that positive parenting and supportive school and neighborhood environments boost child intelligence and mental health. In the experiments, Park, Lee et al. analyzed data on 6,602 children to determine how genetics and environmental factors shaped their intelligence and mental health. The models show that children with higher intelligence have a lower risk of psychosis. Both genetics and supportive environments contribute to higher intelligence.
Complex interactions between biology and social factors shape children’s intelligence and mental health. Beneficial genetics and coming from a family with more financial resources are helpful. Yet, social environments, such as having parents who use positive child-rearing practices, or having supportive schools or neighborhoods, have protective effects that can offset other disadvantages. Policies that help parents, encourage supportive school environments, and strengthen neighborhoods may boost children’s intelligence and mental health later in life.
Introduction
Childhood is the critical developmental period in human life. Cognitive intelligence and mental health in this period significantly impact key life outcomes at later ages, including academic performance, economic productivity, physical health, intelligence, and psychopathology (Shonkoff, 2012; Walker et al., 2022). Literature shows the significant impact of social adversities on cognitive ability and mental health in early childhood. Lower family socioeconomic status (SES), particularly household income, is linked to lower neurocognitive ability and higher risk of psychopathology in childhood (Hair et al., 2015; Noble et al., 2015; Peverill et al., 2021; Tomasi and Volkow, 2021; Weissman et al., 2018).
Additional to family SES, the importance of neighborhood social environment on children’s neurocognitive ability has been also emphasized (Gard et al., 2021; Tooley et al., 2020). Adverse neighborhood environment, such as the percent of families below poverty line, low education levels, and exposure to violence, is associated with lower cognitive performance (CP) and a greater risk for psychosis in children (Butler et al., 2018; Karcher et al., 2021; Rakesh et al., 2021; Taylor et al., 2020). Conversely, as protective factors against familial and neighborhood socioeconomic challenges, supportive parenting (Brody et al., 2017; Brody et al., 2019; Holmes et al., 2018; Luby et al., 2012; Luby et al., 2016) and positive school environment (Gard et al., 2021; Piccolo et al., 2019; Rakesh et al., 2021) have been highlighted to improve child cognition and mental health.
Psychotic-like experiences (PLEs), which are prevalent in childhood, indicate the risk of psychosis (van der Steen et al., 2019; van Os and Reininghaus, 2016). Although they are not a direct precursor of schizophrenia, children reporting PLEs in ages of 9–11 years are at higher risk of psychotic disorders in adulthood (Kelleher and Cannon, 2011; Poulton et al., 2000). PLEs also point toward the potential for other psychopathologies including mood, anxiety, and substance disorders (van der Steen et al., 2019), are linked to deficits in cognitive intelligence (Cannon et al., 2002; Kelleher and Cannon, 2011) and show a stronger association with environmental risk factors during childhood than other internalizing/externalizing symptoms (Karcher et al., 2021).
Maladaptive cognitive intelligence may act as a mediator for the effects of genetic and environmental risks on the manifestation of psychotic symptoms (Cannon et al., 2000; Keefe et al., 2006; Reichenberg et al., 2005). Abnormal neurodevelopment, influenced by genetic factors, combined with disrupted cognitive processes resulting from socioenvironmental adversity, may eventually give rise to the positive symptoms of schizophrenia, relevant to PLEs (Garety et al., 2001; Howes and Murray, 2014). Family studies show a decline in cognitive intelligence preceding psychotic symptoms is related to genetic risk (Cosway et al., 2000; Curtis et al., 2001). In more recent studies, these associations have led to the model positing that cognitive intelligence mediates the genetic risk for psychopathology and PLEs (Karcher et al., 2022; Pat et al., 2022).
To minimize potential bias in the estimates of environmental effects, it is crucial to adjust for genetic confounding (Sariaslan et al., 2016), given the substantial genetic influence on intelligence (Bouchard and McGue, 1981; Deary et al., 2006; Plomin and Spinath, 2004) and PLEs (Bentall and Fernyhough, 2008; Maxwell et al., 2023). Recent advances in genetics have led to the development of the polygenic score (PGS) approach: a computational method to estimate the genetic loading for a complex trait using statistical associations of each single-nucleotide polymorphism (SNP) identified by genome-wide association studies (GWAS) (Cho et al., 2022). Particularly, PGS for two related but distinct phenotypes—CP and educational attainment (EA)—holds significant importance. As the two most frequently used proxies of cognitive intelligence in genetic studies, PGSs for CP and EA are positively correlated with intelligence, EA, income, self-rated health, and height (Judd et al., 2020; Lee et al., 2018; Okbay et al., 2022; Selzam et al., 2019). Furthermore, the PGS for EA is associated with a wide range of biological and social outcomes, including brain morphometry (Judd et al., 2020; Karcher et al., 2022), psychopathologies such as PLEs, autism, depression, Alzheimer’s disease, neuroticism (Karcher et al., 2022; Okbay et al., 2022), cognitive decline (Joo et al., 2022; Karcher et al., 2022; Ritchie et al., 2020), body mass index (BMI), time spent watching television, geographic residence (Abdellaoui et al., 2022), and wealth inequality (Barth et al., 2020). Similar to the terms used in prior research, we will collectively refer to these two PGSs of focus as ‘cognitive phenotypes PGSs’ throughout this paper (Joo et al., 2022; Okbay et al., 2022; Selzam et al., 2019). An important gap in the literature is the lack of integrated assessment of the effects of genetic and environmental factors at multiple levels (e.g., familial vs neighborhood) to dissect the genetic and environmental effects underlying abnormal cognitive intelligence and the PLEs. Addressing this with large multimodal data will allow for a more complete understanding of the factors related to the development of PLEs.
In this study, we systematically explore the longitudinal trajectories of genetic and environmental influences on PLEs, mediated through cognitive intelligence. Toward this goal, we firstly assess the associations of cognitive phenotype PGSs, family and neighborhood SES, and supportive environment with children’s cognitive intelligence and longitudinally measured PLEs. To maintain robustness of our assessment, we employed statistical and computational approaches to carefully consider potential confounding. We then test the mediating effect of cognitive intelligence on the relationship between genetic and environmental factors and PLEs. Our investigation traces these effects from the baseline and through the 1- and 2-year follow-ups, providing a nuanced understanding on the role of cognitive phenotype PGSs, family SES, neighborhood SES, and positive family and school environments in shaping PLEs in children aged 9–10 years.
Materials and methods
Study participants
We used the multimodal genetic and environmental data of 11,878 preadolescent children aged 9–10 years old collected from 21 research sites of the Adolescent Brain Cognitive Development (ABCD) Study, one of the largest longitudinal studies for children’s neurodevelopment in the United States. We analyzed the baseline, first year, and second year follow-up datasets included in ABCD Release 4.0, downloaded on January 25, 2022. After k-nearest neighbor imputation of missing values of covariates (categorical variables: sex, genetic ancestry, marital status of the caregiver, ABCD research sites; continuous variables: age, BMI, family history of psychiatric disorders; 4.67% of total observations imputed) using the R package VIM (Kowarik and Templ, 2016), we removed participants with missing data on study variables (missing genotype: N = 3260; follow-up observations: N = 1180; neighborhood information: N = 694; cognitive intelligence tests: N = 126; PLEs: N = 5; positive environment: N = 11). The final samples included 6602 multiethnic children, which comprised 890 of African ancestry (13.48%), 91 of East Asian ancestry (1.38%), 5211 of European ancestry (78.93%), 229 of Native American ancestry (3.47%), and 181 not specified (2.74%).
Data
NIH toolbox CP
Children’s neurocognitive abilities were assessed using the NIH Toolbox Cognitive Battery, which has seven cognitive instruments for examining executive function, episodic memory, language abilities, processing speed, working memory, and attention (Thompson et al., 2019). We utilized baseline observations of uncorrected composite scores of fluid intelligence (Dimensional Change Card Sort Task, Flanker Test, Picture Sequence Memory Test, and List Sorting Working Memory Test), crystallized intelligence (Picture Vocabulary Task and Oral Reading Recognition Test), and total intelligence (all seven instruments) provided in the ABCD Study dataset.
Psychotic-like experiences
Baseline and 1- and 2-year follow-up of PLEs were measured using the children’s responses to the Prodromal Questionnaire-Brief Child Version. In line with previous research (Karcher et al., 2018; Karcher et al., 2020; Karcher et al., 2021), we computed Total Score and Distress Score, each indicating the number of psychotic symptoms and levels of total distress. Considering self- and parent-reports of psychopathology may differ (Achenbach, 2006), we additionally used parent-rated PLEs derived from four items of the Child Behavior Checklist according to previous studies (Karcher et al., 2018; Karcher et al., 2020; Karcher et al., 2021). Self- and parent-reported PLEs had significant positive correlation (Pearson’s correlation of baseline year: r = 0.095–0.0989, p < 0.0001; 1-year follow-up: r = 0.1322–0.1327, p < 0.0001; 2-year follow-up: r = 0.1569–0.1632, p < 0.0001).
Polygenic scores
To investigate the aggregated effect of genetic components, we estimated PGS of two representative cognitive phenotypes for each participant: EA and CP (Cho et al., 2022). We used the summary statistics released from a GWAS (Lee et al., 2018) of European-descent individuals for EA (n = 1,131,881) and CP (n = 257,841). EA was measured as the years of schooling; CP, measured as the respondent’s score on cognitive ability assessments of general cognitive function and verbal–numerical reasoning, was assessed in participants from the COGENT consortium and the UK Biobank. To construct PGS of schizophrenia for sensitivity analyses, we used the summary statistics from the multiple GWAS of European sample (n = 65,967; Ruderfer et al., 2018) and East Asian sample (n = 58,140; Lam et al., 2019). We applied PRS-CSx, a high-dimensional Bayesian regression framework that improves cross-population prediction via continuous shrinkage prior to SNP effect sizes (Ge et al., 2019) (for details, see Appendix 1). The two PGSs for cognitive phenotypes had a positive significant correlation (Pearson’s correlation: r = 0.4331, p < 0.0001).
Family-, neighborhood-, and school-level environment
We assessed children’s family-level SES with family income, parental education, and family’s financial adversity based on parent self-reporting (Karcher et al., 2020; Taylor et al., 2020; Tomasi and Volkow, 2021). Higher family income and parental education and lower family’s financial adversity denote a higher family SES.
Neighborhood-level SES was assessed using the Area Deprivation Index (ADI), the percentage of individuals below −125% of the poverty level (henceforth ‘poverty’), and years of residence, which were associated with PLEs in prior research (Karcher et al., 2021). Higher values of ADI and poverty and fewer years of residence indicate a lower neighborhood SES.
Based on existing literature (Karcher et al., 2021; Rakesh et al., 2021), we measured the level of positive parenting behavior and positive school environment to assess the effect of positive family and school environment on each individual.
Statistical modeling
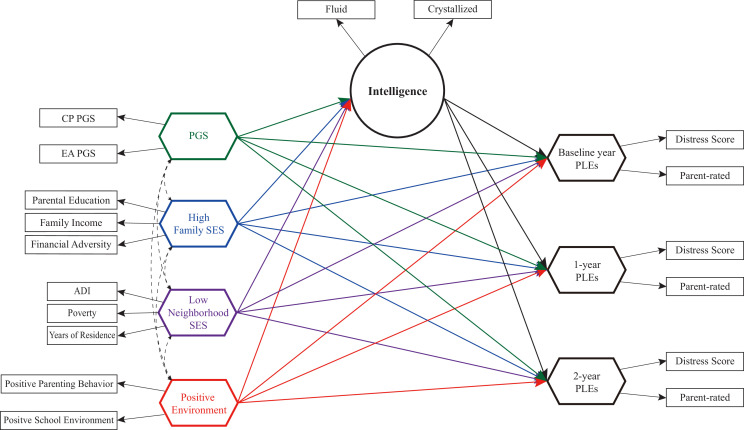
In this study, we employ linear mixed models and a novel structural equation modeling (SEM) method to examine the longitudinal trajectories of genetic and environmental influences on PLEs mediated by cognitive intelligence. We specifically investigate the mediating role of cognitive intelligence within the impacts of cognitive phenotype PGSs, high family SES, low neighborhood SES, and positive family and school environments on PLEs. These influences are examined across three periods of PLEs observations: baseline, 1-year follow-up, and 2-year follow-up (Figure 1).
Figure 1. Study diagram for longitudinal trajectories of genetic and environmental influences on psychotic-like experiences (PLEs) through cognitive intelligence.
This study examines the mediating role of cognitive intelligence within the effects of cognitive phenotype polygenic scores (PGSs), high family socioeconomic status (SES), low neighborhood SES, and positive family and school environments on PLEs observed at baseline, 1-year follow-up, and 2-year follow-up in children aged 9–10 years. Both direct and indirect effects, as well as total effects, were evaluated for statistical significance.
Linear mixed models
Using linear mixed models to minimize potential population stratification from different household environments and geographical locations (Cho et al., 2022), we analyzed the genetic and environmental effects on cognitive intelligence and PLEs. Key variables of interest were PGS, family SES, neighborhood SES, and positive environment. To avoid multicollinearity, CP and EA PGSs were used separately in two other models. As a random factor, the variable indicating ABCD research sites was used. Variables within each model had no signs of multicollinearity (Fox and Monette, 1992) (generalized variance inflation factor <2.0 for every variable in all models). The child’s sex, age, genetic ancestry, BMI, marital status of the caregiver, and family history of psychiatric disorders were included as covariates in the model (Karcher et al., 2021). All continuous variables were standardized (z-scaled) beforehand to get standardized estimates, and the analyses were conducted with the lme4 package in R version 4.1.2. Throughout this paper, threshold for statistical significance was set at p < 0.05, with correction for multiple comparisons using the false discovery rate. 95% bootstrapped confidence intervals were obtained with 5000 iterations.
Path modeling
To test the plausibility of whether cognitive intelligence may mediate the association between genetic and environmental factors and PLEs, we used an up-to-date SEM method, integrated generalized structured component analysis (IGSCA) (Hwang et al., 2021b). This approach is suited to our study using the multimodal genetic and environmental variables in that it estimates models with both factors and components as statistical proxies for the constructs.
Standard SEM using latent factors (i.e., indirectly measured indicators that explain the covariance among observed variables) to represent indicators such as PGS or family SES relies on the assumption that observed variables within each construct share a common underlying factor. If this assumption is violated, standard SEM cannot effectively control for estimation biases. The IGSCA method addresses this limitation by allowing for the use of composite indicators (i.e., components)—defined as a weighted sum of observed variables—as constructs in the model, more effectively controlling bias in estimation compared to the standard SEM. During estimation, the IGSCA determines weights of each observed variable in such a way as to maximize the variances of all endogenous indicators and components.
We assessed path-analytic relationships among the six key constructs: cognitive phenotypes PGSs, family SES, neighborhood SES, positive family and school environment, general intelligence, and PLEs. Considering that the observed variables of the PGSs, family SES, neighborhood SES, positive family and school environment, and PLEs are evaluated as a composite index by prior research, the IGSCA method can mitigate bias more effectively by representing these constructs as components. Notably, investigations carried out by Judd et al., 2020 and Martin et al., 2015 utilized composite indicators to examine the genetic influence on EA and Attention-Deficit/Hyperactivity Disorder. Moreover, socioenvironmental influences are often treated as composite indicators as highlighted in Judd et al., 2020. When considering the psychosis continuum, studies like that of van Os et al., 2009 postulate that psychotic disorders are likely underpinned by a multiplicity of background factors rather than a single common factor. This perspective is substantiated by a multitude of prior research that deploys composite indices for the measurement of psychotic symptoms. For these reasons, we statistically represented these constructs as the weighted sums of their observed variables or components (Cho et al., 2022). On the other hand, we represented general intelligence as a common factor that determines the underlying covariance pattern of fluid and crystallized intelligence, based on the classical g theory of intelligence (Jensen, 1998; Spearman, 1904).
The IGSCA model included the same covariates used in the linear mixed model as well as the ABCD research site as an additional covariate. We applied GSCA Pro 1.1 (Hwang et al., 2021a) to fit the IGSCA model to the data and checked the model’s goodness-of-fit index (GFI) (Jöreskog and Sörbom, 1986), standardized root mean square residual (SRMR), and total variance of all indicators and components explained (FIT) to assess its overall goodness-of-fit. Ranging from 0 to 1, a larger FIT value indicates more variance of all variables is explained by the specified model (e.g., FIT = 0.50 denotes that the model explains 50% of the total variance of all variables) (Hwang et al., 2021a). The rules-of-thumb cutoff criteria in IGSCA is GFI ≥0.93 and SRMR ≤0.08 for an acceptable fit (Cho et al., 2020). Finally, we conducted conditional process analyses to investigate further the indirect and total effects of the constructs in the model. As a trade-off for obtaining robust nonparametric estimates without distributional assumptions for normality, the IGSCA method does not return exact p values (Hwang et al., 2021b). As a reasonable alternative, we obtained 95% confidence intervals based on 5000 bootstrap samples to test the statistical significance of parameter estimates.
Sensitivity analyses
To ensure robustness of the main analyses results, we conducted multiple sensitivity analyses. As the European-descent-based GWAS was used for constructing PGS, we reran the main analyses using participants of European ancestry (n = 5211) to adjust for ethnic confounding. Next, we tested effects of gene × environment interactions on cognitive intelligence and PLEs, respectively. We also tested the effects of cognitive phenotypes PGS adjusting for schizophrenia PGS, given the association of schizophrenia PGS and cognitive deficit in psychosis patients (Shafee et al., 2018) and individuals at-risk of psychosis (He et al., 2021). Lastly, we adjusted for unobserved confounding bias in the linear mixed model, using a recently developed framework for causal inference based on null treatments approach (Miao et al., 2023). Designed to discern causal effects from multiple treatment variables within non-randomized, observational data, the null treatments approach hinges on the assumption that no fewer than half of the confounded treatments exert no causal influence on the outcome. It circumvents the need for prior knowledge regarding which treatments are null and eliminates the necessity for independence among treatments. Given our model’s inclusion of numerous treatment variables with shared variances due to the presence of unobserved confounders (Abdellaoui et al., 2022; Okbay et al., 2022)—including cognitive phenotypes PGS, family and neighborhood SES, positive family and school environments— we opted to employ this method.
Results
Demographics
Table 1 presents the demographic characteristics of the final samples. For multiethnic subjects (main analyses, n = 6602), 47.15% were female, and the parents of 70.21% were married. In European ancestry samples (sensitivity analyses, n = 5211), 46.71% were female, and the parents of 77.47% were married. Children of European ancestry showed significantly different marital status (p < 0.0001), lower BMI (p < 0.0001), and family history of psychiatric disorders (p < 0.0001) compared to children of other genetic ancestries. Our linear mixed model and IGSCA analyses were adjusted using sex, age, marital status, BMI, family history of psychiatric disorders, and ABCD research sites as covariates.
Table 1. Demographic characteristics of the study participants.
Of the initial 11,878 ABCD samples, we obtained data for the variables of interest for 6602 multiethnic children. For multiethnic subjects (main analyses, n = 6602), 47.15% were female, and the parents of 70.21% were married. In European ancestry samples (sensitivity analyses, n = 5211), 46.71% were female, and the parents of 77.47% were married. Children of European ancestry had significantly different marital status (p < 0.0001), lower body mass index (BMI; p < 0.0001), and higher family history of psychiatric disorders (p < 0.0001) than children of multiethnic ancestries. There were no significant differences in other characteristics between the two ancestry groups. The 6602 multiethnic participants consisted of 890 African-ancestry (13.48%), 229 Native American ancestry (3.47%), 91 East Asian ancestry (1.38%), 181 not specified (2.74%), and 5211 European ancestry (78.93%) children. Differences between genetic ancestry groups were calculated using χ2 tests for categorical variables and t-tests for continuous variables.
| Demographic characteristics | European ancestry (n = 5211) | Multiethnic (n = 6602) | Test statistics | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Ratio (%) | Mean (SD) | N | Ratio (%) | Mean (SD) | t(df)/χ2(df) | p value | ||
| Sex | Male | 2777 | 53.29 | 3489 | 52.85 | −0.4795 (11811) | 0.6316 | ||
| Female | 2434 | 46.71 | 3113 | 47.15 | |||||
| Marital status of the caregiver | Married | 4037 | 77.47 | 4635 | 70.21 | −10.2326 (11811) | <0.0001 | ||
| Widowed | 38 | 0.73 | 50 | 0.76 | |||||
| Divorced | 485 | 9.31 | 610 | 9.24 | |||||
| Separated | 155 | 2.97 | 232 | 3.51 | |||||
| Never married | 275 | 5.28 | 718 | 10.88 | |||||
| Living with partner | 221 | 4.24 | 357 | 5.41 | |||||
|
Age (rounded to chronological month) |
5211 | 118.99 (7.46) | 6602 | 118.94 (7.41) | 0.3652 (11811) | 0.715 | |||
| BMI | 18.29 (3.67) | 18.72 (4.12) | −5.8889 (11811) | <0.0001 | |||||
|
Family history of psychiatric disorders (proportion of first-degree relatives who experienced mental illness) |
0.10 (0.11) | 0.09 (0.11) | 4.4296 (11811) | <0.0001 | |||||
| Genetic ancestry | African | - | 890 | 13.48 | |||||
| Native American | - | 229 | 3.47 | ||||||
| East Asian | - | 91 | 1.38 | ||||||
| European | 5211 | 100 | 5211 | 78.93 | |||||
| Not specified | - | 181 | 2.74 | ||||||
Genetic influence on cognitive phenotypes correlates positively with intelligence and negatively with PLEs
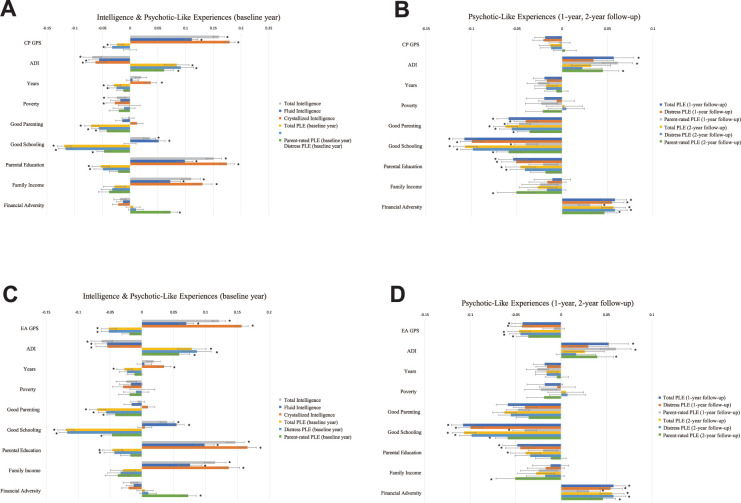
As shown in Figure 2, higher PGSs of cognitive capacity phenotypes correlated significantly with higher intelligence (CP PGS: β = 0.1113–0.1793; EA PGS: β = 0.0699–0.1567). While CP PGS was associated only with lower baseline year Distress Score PLEs (β = −0.0323), EA PGS was associated with lower baseline year and follow-up PLEs of all measures (baseline: β = −0.0518 to −0.0519; 1 year: β = −0.0423 to −0.043; 2 years: β = −0.036 to −0.0463). No significant correlations were found between CP PGS and follow-up PLEs (p > 0.05). The effects of EA PGS were larger on baseline year PLEs than follow-up PLEs. The effect sizes of EA PGS on children’s PLEs were larger than those of CP PGS (EA PGS: β = −0.036 to −0.0519; CP PGS: β = −0.0323) (Supplementary file 1).
Figure 2. Linear models testing genetic, socioeconomic, and environmental factors associated with cognitive intelligence and PLEs.
Standardized coefficients of a linear mixed model with CP PGS (A, B) and EA PGS (C, D). The analyses included 6602 samples of multiethnicity. Cognitive intelligence and PLEs correlated with the PGSs, residential disadvantage, positive environment, and family SES in the opposite directions. Error bars indicate 95% bootstrapped confidence intervals with 5000 iterations. CP and EA denote cognitive performance and education attainment, respectively; PGS, polygenic scores; SES, socioeconomic status; PLEs, psychotic-like experiences; ADI, Area Deprivation Index; Poverty, percentage of individuals below −125% of the poverty level; Years, years of residence (*p-FDR <0.05).
Family and neighborhood SES correlates positively with intelligence and negatively with PLEs
Parental education associated positively with all types of intelligence (β = 0.0699 to 0.1745) and negatively with baseline year Total and Distress Score PLEs (β = −0.0528 to −0.043), 1-year follow-up PLEs (β = −0.0538 to −0.0449), and 2-year follow-up PLEs (β = −0.0459 to −0.0389). Family income correlated positively with intelligence (β = 0.0723 to 0.1365) and negatively with 2-year follow-up parent-rated PLEs (β = −0.0503 to −0.0502). Family’s financial disadvantage correlated negatively with baseline year parent-rated PLEs (β = 0.0726–0.0728), 1-year follow-up PLEs of all types (β = 0.0307–0.0577), and 2-year follow-up PLEs of all types (β = 0.0461–0.0581).
The ADI correlated significantly negatively with all types of intelligence (β = −0.0684 to −0.054). Additionally, a higher ADI correlated significantly with higher baseline year PLEs (β = 0.0587–0.0914), 1-year follow-up PLEs (β = 0.0523–0.0613), and 2-year follow-up PLEs (β = 0.0397–0.0449).
We found no significant associations of poverty with any of the target variables (p > 0.05). Years of residence correlated significantly with crystallized intelligence (β = 0.035 to 0.0372) and baseline year Total Score PLEs (β = −0.029 to −0.0273) (Supplementary file 1).
Positive family and school environments correlate positively with intelligence and negatively with the influence of PLEs
Positive parenting behaviors showed significant negative correlations with baseline year PLEs (β = −0.0702 to −0.0419), 1-year follow-up PLEs (β = −0.0588 to −0.0397), and 2-year follow-up PLEs (β = −0.0623 to −0.0356) (Figure 2). Positive school environment was associated positively with total intelligence (β = 0.0353 to 0.0397) and fluid intelligence (β = 0.0514 to 0.0545) and negatively with all three measures of baseline year PLEs (β = −0.1193 to −0.0468), 1-year follow-up PLEs (β = −0.1078 to −0.04), and 2-year follow-up PLEs (β = −0.1068 to −0.0586) (Supplementary file 1).
SEM-IGSCA
The IGSCA model showed that intelligence mediated the effects of genes and environments on the risk for psychosis (PLEs) (Figure 3 and Table 2). Estimated factor loadings of latent factor variable and weights of component variables are presented in Supplementary file 1. Correlation matrices between component/factor variables are presented in Appendix 3—figure 1. The model showed a good model fit with a GFI of 0.9735, SRMR of 0.0359, and FIT value of 0.4912 (Cho et al., 2020). Intelligence was under significant direct influences of the cognitive phenotypes PGS (β = 0.2427), family SES (β = 0.2932), neighborhood SES (β = −0.1121), and positive environment (β = 0.0268). Family SES and positive environment had significant negative direct effects on PLEs of all years. Cognitive phenotypes PGS and neighborhood SES showed no significant direct effects on any of the PLEs (p > 0.05). Intelligence significantly mediated the effects of the PGS, family and neighborhood SES, and positive environment on PLEs of all years: the PGS (baseline year: β = −0.035; 1 year: β = −0.0355; 2 years: β = −0.0274), family SES (baseline year: β = −0.0423; 1 year: β = −0.0429; 2 years: β = −0.0331), neighborhood SES (baseline year: β = 0.0162; 1 year: β = 0.0164; 2 years: β = 0.0126), and positive environment (baseline year: β = −0.0039; 1 year: β = −0.0039; 2 years: β = −0.003).
Figure 3. Direct/indirect effects of gene–environment factors to cognitive and PLEs outcomes.
(A) Direct pathways from PGS, high family SES, low neighborhood SES, and positive environment to cognitive intelligence and PLEs. Standardized path coefficients are indicated on each path as direct effect estimates (significance level *p < 0.05). (B) Indirect pathways to PLEs via intelligence were significant for polygenic scores, high family SES, low neighborhood SES, and positive environment, indicating the significant mediating role of intelligence. (C) Relative effect sizes of direct and indirect pathways within the total effects on PLEs. The standardized effect sizes of direct pathways are colored within each bar. In (A–B), child sex, genetic ancestry, body mass index (BMI), marital status, family history of psychiatric disorders, and ABCD research sites were included as covariates. CP PGS and EA PGS denote polygenic scores of cognitive performance and education attainment, respectively; SES, socioeconomic status; PLEs, psychotic-like experiences; Crystallized and Fluid, crystallized and fluid intelligence; ADI, Area Deprivation Index; Poverty, percentage of individuals below −125% of the poverty level; Years, years of residence. Note: * indicates a statistically significant parameter estimate at α = 0.05.
Table 2. Integrated generalized structured component analysis (IGSCA) of multiethnic samples.
Sex, age, genetic ancestry, body mass index (BMI), parental education, marital status of the caregiver, household income, and family’s financial adversity based on parents’ self-report, family history of psychiatric disorders, and ABCD research sites were included as covariates. Family socioeconomic status was included to confirm that the aassociations of polygenic score (PGS), neighborhood disadvantage, and positive environment are meaningful. SE and CI represent standard error and confidence intervals, respectively. Significant effects are marked with a star (*).
| Analysis of total/direct/indirect effects | ||||||
|---|---|---|---|---|---|---|
| Effect type | Paths | Estimate | SE | 95% CI | Significance | |
| Effects from PGS to intelligence (baseline year) | ||||||
| Direct effect | PGS → intelligence | 0.242736 | 0.01277 | 0.218202 | 0.267954 | * |
| Effects from high family SES to intelligence (baseline year) | ||||||
| Direct effect | High family SES → intelligence | 0.293171 | 0.016737 | 0.260337 | 0.326413 | * |
| Effects from low neighborhood SES to intelligence (baseline year) | ||||||
| Direct effect | Low neighborhood SES → intelligence | −0.1121 | 0.016768 | −0.14568 | −0.08118 | * |
| Effects from positive environment to intelligence (baseline year) | ||||||
| Direct effect | Positive environment → intelligence | 0.026793 | 0.012552 | 0.003984 | 0.052633 | * |
| Effects from intelligence to psychotic-like experiences (all years) | ||||||
| Direct effect | Intelligence → psychotic-like experiences (baseline year) | −0.14421 | 0.027683 | −0.20344 | −0.09516 | * |
| Direct effect | Intelligence → psychotic-like experiences (1-year follow-up) | −0.14638 | 0.027507 | −0.20834 | −0.09983 | * |
| Direct effect | Intelligence → psychotic-like experiences (2-year follow-up) | −0.11276 | 0.028708 | −0.17428 | −0.063 | * |
| Effects from PGS to psychotic-like experiences (baseline year) | ||||||
| Total effect | PGS → psychotic-like experiences | −0.05017 | 0.011354 | −0.07292 | −0.02853 | * |
| Indirect effect | PGS → intelligence → psychotic-like experiences | −0.035 | 0.007126 | −0.0508 | −0.02273 | * |
| Direct effect | PGS → psychotic-like experiences | −0.01516 | 0.01347 | −0.04085 | 0.012389 | |
| 7 Effects from high family SES to psychotic-like experiences (baseline year) | ||||||
| Total effect | High family SES → psychotic-like experiences | −0.12126 | 0.019087 | −0.15851 | −0.08313 | * |
| Indirect effect | High family SES → intelligence → psychotic-like experiences | −0.04228 | 0.008652 | −0.06139 | −0.02707 | * |
| Direct effect | High family SES → psychotic-like experiences | −0.07898 | 0.020747 | −0.11856 | −0.03698 | * |
| Effects from low neighborhood SES to psychotic-like experiences (baseline year) | ||||||
| Total effect | Low neighborhood SES → psychotic-like experiences | 0.050374 | 0.018277 | 0.013545 | 0.085934 | * |
| Indirect effect | Low neighborhood SES → intelligence → psychotic-like experiences | 0.016166 | 0.003944 | 0.009843 | 0.025298 | * |
| Direct effect | Low neighborhood SES → psychotic-like experiences | 0.034209 | 0.0184 | −0.00268 | 0.069813 | |
| Effects from positive environment to psychotic-like experiences (baseline year) | ||||||
| Total effect | Positive environment → psychotic-like experiences | −0.15256 | 0.013871 | −0.17965 | −0.1252 | * |
| Indirect effect | Positive environment → intelligence → psychotic-like experiences | −0.00386 | 0.002065 | −0.00859 | −0.00058 | * |
| Direct effect | Positive environment → psychotic-like experiences | −0.14869 | 0.014025 | −0.17573 | −0.12073 | * |
| Effects from PGS to psychotic-like experiences (1-year follow-up) | ||||||
| Total effect | PGS → psychotic-like experiences | −0.035895 | 0.011646 | −0.058499 | −0.013458 | * |
| Indirect effect | PGS → intelligence → psychotic-like experiences | −0.03553 | 0.007062 | −0.05176 | −0.02376 | * |
| Direct effect | PGS → psychotic-like experiences | −0.00036 | 0.013579 | −0.02566 | 0.028107 | |
| Effects from high family SES to psychotic-like experiences (1-year follow-up) | ||||||
| Total effect | High family SES → psychotic-like experiences | −0.11184 | 0.018291 | −0.1478 | −0.07584 | * |
| Indirect effect | High family SES → intelligence → psychotic-like experiences | −0.04291 | 0.008569 | −0.06242 | −0.0288 | * |
| Direct effect | High family SES → psychotic-like experiences | −0.06892 | 0.019586 | −0.10522 | −0.02866 | * |
| Effects from low neighborhood SES to psychotic-like experiences (1-year follow-up) | ||||||
| Total effect | Low neighborhood SES → psychotic-like experiences | 0.032947 | 0.018055 | −0.00264 | 0.068773 | |
| Indirect effect | Low neighborhood SES → intelligence → psychotic-like experiences | 0.016409 | 0.004003 | 0.010133 | 0.025893 | * |
| Direct effect | Low neighborhood SES → psychotic-like experiences | 0.016538 | 0.018503 | −0.02066 | 0.051855 | |
| Effects from positive environment to psychotic-like experiences (1-year follow-up) | ||||||
| Total effect | Positive environment → psychotic-like experiences | −0.13149 | 0.013154 | −0.15756 | −0.10589 | * |
| Indirect effect | Positive environment → intelligence → psychotic-like experiences | −0.00392 | 0.00208 | −0.0087 | −0.00059 | * |
| Direct effect | Positive environment → psychotic-like experiences | −0.12757 | 0.013237 | −0.15343 | −0.10137 | * |
| Effects from PGS to psychotic-like experiences (2-year follow-up) | ||||||
| Total effect | PGS → psychotic-like experiences | −0.03643 | 0.012196 | −0.06027 | −0.01272 | * |
| Indirect effect | PGS → intelligence → psychotic-like experiences | −0.02737 | 0.007142 | −0.04307 | −0.01508 | * |
| Direct effect | PGS → psychotic-like experiences | −0.00906 | 0.014737 | −0.03696 | 0.021152 | |
| Effects from high family SES to psychotic-like experiences (2-year follow-up) | ||||||
| Total effect | High family SES → psychotic-like experiences | −0.11632 | 0.018067 | −0.15258 | −0.08174 | * |
| Indirect effect | High family SES → intelligence → psychotic-like experiences | −0.03306 | 0.008796 | −0.05228 | −0.01807 | * |
| Direct effect | High family SES → psychotic-like experiences | −0.08326 | 0.019462 | −0.12066 | −0.04392 | * |
| Effects from low neighborhood SES to psychotic-like experiences (2-year follow-up) | ||||||
| Total effect | Low neighborhood SES → psychotic-like experiences | 0.01921 | 0.018684 | −0.01767 | 0.055261 | |
| Indirect effect | Low neighborhood SES → intelligence → psychotic-like experiences | 0.012641 | 0.003814 | 0.006533 | 0.0215 | * |
| Direct effect | Low neighborhood SES → psychotic-like experiences | 0.006569 | 0.019176 | −0.03173 | 0.042823 | |
| Effects from positive environment to psychotic-like experiences (2-year follow-up) | ||||||
| Total effect | Positive environment → psychotic-like experiences | −0.13627 | 0.013881 | −0.1635 | −0.10926 | * |
| Indirect effect | Positive environment → intelligence → psychotic-like experiences | −0.00302 | 0.001703 | −0.0069 | −0.00043 | * |
| Direct effect | Positive environment → psychotic-like experiences | −0.13325 | 0.014009 | −0.16069 | −0.10565 | * |
For all observed years, positive environment had largest total effects on PLEs (baseline year: β = −0.152; 1 year: β = −0.1316; 2 years: β = −0.1364), followed by family SES (baseline year: β = −0.1216; 1 year: β = −0.1119; 2 years: β = −0.1164), neighborhood SES (baseline year: β = 0.0504; 1 year: β = 0.0329; 2 years: β = 0.0192), and PGS (baseline year: β = −0.0498; 1 year: β = −0.036; 2 years: β = −0.0365). The total effects of each indicator on PLEs were significant except for those of neighborhood SES (p > 0.05).
Sensitivity analyses
As sensitivity analyses, we reran our main analyses with adjustment for ethnic confounding, schizophrenia PGS, and unobserved confounding, respectively. Results of linear mixed model and IGSCA analyses were consistent (Supplementary file 1). See Appendix 2 for detailed results.
Discussion
This study investigated the relationships of the genetic and environmental influences on the development of intelligence and the PLEs in youth, leveraging genetic data from the large epidemiological samples and a multi-level environmental (socioeconomic) data. Our results support the model that genetic factors (PGS for cognitive phenotypes), socioeconomic conditions, and family and school environments may influence cognitive intelligence in children, and this impact may lead to the individual variability of the current and future PLEs in children. Our analysis with integrated data shows the contributions of genetic and environmental factors, respectively, to cognitive and mental wellness in children, and further provides policy implications to improve them.
Our SEM shows that cognitive intelligence mediates the environmental and genetic influence on the current and future PLEs. The environmental factors (family SES, neighborhood SES, and positive parenting and schooling) and PGS of cognitive phenotypes exhibit significant indirect effects via cognitive intelligence on PLEs. Prior research identifying the mediation of cognitive intelligence focused on either genetic (Karcher et al., 2022) or environmental factors (Lewis et al., 2020) alone. Studies with older clinical samples have shown that cognitive deficit may be a precursor for the onset of psychotic disorders (Eastvold et al., 2007; Fett et al., 2020; Vorstman et al., 2015). Our study advances this by demonstrating the integrated effects of genetic and environmental factors on PLEs through the cognitive intelligence in 9- to 11-year-old children. Such comprehensive analysis contributes to assessing the relative importance of various factors influencing children’s cognition and mental health, and it can aid future studies designed for identifying health policy implications. Considering the directions and magnitudes of the effects, though the effects of PGS remain significant, aggregated effects of environmental factors account for much greater degrees on PLEs.
Our results of cognitive intelligence mediating the genetic and environmental effects on PLEs may be related to several potential mechanisms. Children raised in higher family SES may have sufficient nutrition and cognitive stimulants, whereas children living in deprived neighborhoods may be exposed to higher rates of crime, air pollution, and substance abuse (Lewis et al., 2020; Marshall et al., 2020; Taylor et al., 2020; Tomasi and Volkow, 2021). Environmental enrichment may be associated with longer periods of neural plasticity (e.g., myelination, maturation of brain circuitry), leading to higher cognitive ability and lower risk of mental disorders like PLEs (Tooley et al., 2021). This may be further linked to the cognitive reserve theory. The theory suggests that genetic influence for cognitive phenotypes and environmental enrichment promotes more efficient, flexible brain networks, which may lead to greater resilience against psychopathology (Stern, 2009). Indeed, prior clinical studies show the linkage between cognitive reserve and psychosis (Amoretti et al., 2018; Leeson et al., 2011).
Our results indicate that genetic influences on cognitive phenotypes are significantly linked to PLEs. PGSs for CP and EA were strongly correlated with PLEs (baseline year, 1-year follow-up, and 2-year follow-up). These associations were robust after adjustment for schizophrenia PGS, ethnic confounding and unobserved confounders. Cognitive phenotypes PGS generally show higher predictive performance than PGS of any other traits (Lee et al., 2018; Okbay et al., 2022; Plomin and von Stumm, 2018). Genetic variants associated with CP and EA are related to complex traits across the life span, including neuroticism, depressive symptoms, smoking in adulthood, cognitive decline at a later age (Joo et al., 2022), risk for Alzheimer’s disease (Lee et al., 2018; Okbay et al., 2022), brain volume, area, and thickness, as well as psychotic disorders (Karcher et al., 2022). Prior gene expression studies suggest that polygenic signals for schizophrenia, bipolar disorder, and EA are significantly enriched in the central nervous system, particularly the cerebellum (Finucane et al., 2018). Our findings emphasize the importance of cognitive phenotypes PGS as a biomarker which not only implicates cognitive traits but also exhibits genetic overlap with the PLEs.
The differing magnitudes of the PGS impact between EA and CP warrant attention. The effects of the EA PGS on the PLEs of all years were 160.68–371.67% larger than those of CP PGS. This discrepancy may result from that the larger sample size of EA GWAS than that of CP GWAS. Alternatively, the discrepancies in effect sizes may suggest different genetic compositions between EA and CP. Recent literature documents that more than half of the polygenic signal for EA is related to noncognitive and social skills required for successful EA (Demange et al., 2021), whereas CP may rather be linked to cognitive skills. This observation also supports the well-established relationships of the EA PGS with socioeconomic and life-course outcomes (e.g., social mobility Belsky et al., 2018), voter turnout (Aarøe et al., 2021), BMI, income, time spent watching television, geographic residence (Abdellaoui et al., 2022), and wealth inequality (Barth et al., 2020), which may be influenced by unobserved environmental factors (Young et al., 2019). In our analysis, the utilization of two PGSs a more comprehensive evaluation, contributing to an estimation of the genetic and environmental factors that attempted to minimize confounding bias.
Furthermore, the significant effects of cognitive phenotypes PGS on cognitive intelligence (β = 0.0699–0.1793) remained robust and similar in magnitude after adjusting for genetic ancestry (β = 0.0754–0.1866) and other (unobserved) confounding (β = 0.0546–0.1776). As we controlled for family-, neighborhood-, and school-level environmental factors and unobserved confounders, our results may be interpreted as significant genetic influences on individual’s cognitive intelligence. This interpretation is supported by a recent study (Isungset et al., 2022): Despite of the socioeconomic differences in Norway (a typical social democratic welfare state) and the United States (a typical liberal welfare state), the magnitudes of genetic influence on cognitive intelligence were similar (Norway: β = 0.18; United States: β = 0.17). Cognitive phenotypes PGS is an important genetic factor across the nations and societies. Therefore, analyses omitting the genetic influence may be subject to overestimation of the socioeconomic impact (Plomin and von Stumm, 2018; Sariaslan et al., 2016).
This study shows that a high SES and positive environment, particularly positive parenting behavior and school environment, is associated with higher intelligence and a lower risk for PLEs in children. While prior research has emphasized the dominant role of family SES (e.g., family income) (Tomasi and Volkow, 2021), our SEM analyses (IGSCA) showed that positive environmental factors such as supportive parenting and schooling have a greater impact on children’s PLEs. Specifically, the effect sizes were the highest in supportive family and school environment, followed by family and neighborhood SES. Even after adjusting for genetic ancestry and unobserved confounders, the strong associations of positive parenting and schooling with higher intelligence and fewer PLEs remained significant. These findings suggest that interventions that target positive family and school environments may be particularly effective. Recent research supports this notion, showing that interventions that promote supportive parenting and inclusive school environments can improve neurocognitive ability, academic performance, and decrease risk behaviors such as drinking and emotional eating (Brody et al., 2017; Brody et al., 2019; Holmes et al., 2018).
Moreover, our results showed that positive parenting and schooling in baseline year were associated not only with baseline year PLEs but also with PLEs 1–2 years later. This is in line with prior research showing that intervention focused on parenting behavior and school environment have long-lasting positive effects that extend into adulthood and even across generations (Cunha and Heckman, 2007; Hill et al., 2020).
While policy implications in observational studies like ours might be limited, our findings show the importance of comprehensive approaches considering the entire ecosystem of children’s lives—including residential, family, and school environment—for future research aimed to enhance children’s intelligence and mental health. When we combine the total effect sizes of neighborhood and family SES, as well as positive school environment and parenting behavior , they considerably surpass the total effect sizes of cognitive phenotypes PGSs . It has been suggested that a holistic and quantitative approach that takes into account the comprehensive ecosystem of family, school, and residential environments may ensure policy effects and efficient use of resources (Cree et al., 2018; Garner et al., 2021; Shonkoff, 2012). For example, the Health Impact in 5 Years Initiative of the US Centers for Disease Control and Prevention (CDC, 2018) includes 14 evidence-based interventions, such as providing school-based prevention programs, public transportation, home improvement loans, and earned income tax credits, to tackle the social determinants of public health. Our study strengthens the idea that an interdisciplinary science-driven, coordinated approach to intervening in the select environmental factors may allow practical improvements in child development, particularly in those who are at a disadvantage.
Our study has some limitations. First, due to data availability constraints in the ABCD Study, we only utilized baseline observations for NIH Toolbox cognitive intelligence, and we could not test whether PLEs might be a mediator of intelligence. Second, the generalizability of our findings may be limited since most of the participants included in our analysis are from European ancestry. Although the ABCD Study aimed to achieve its representativeness by recruiting from an array of school systems located around each of the 21 research sites, chosen for their diversity in geography, demographics, and SES, it is not fully representative of the US population (Compton et al., 2019). Third, the duration of the follow-up period utilized in this study is relatively short (1- and 2-year follow-up), which may limit the interpretability of our findings for understanding cognitive and psychiatric development during later childhood. Future research could potentially benefit from employing longer follow-up periods, as more follow-up observations are being collected in the ABCD Study. Fourth, while we used a wide range of statistical methods to adjust for confounding bias from observed and unobserved variables (e.g., genetic ancestry), we did not account for other types of potential bias such as sample selection bias. Fifth, despite a number of causal inference methods used in this study, the ABCD Study is a non-randomized dataset. Given the observational nature of the ABCD Study, interpreting our results as actual causality requires more caution. Finally, we did not include all important environmental variables, such as air pollution (Marshall et al., 2020) and social capital (Krabbendam and van Os, 2005), which are not collected in the ABCD Study.
In conclusion, our study provides potential pathways of genetic factors of cognitive phenotypes and environmental factors of family, school, and neighborhood to cognitive and mental wellness in children. Our findings underscore the importance of a comprehensive approach that considers both biological and socioeconomic features in promoting young children’s cognitive ability and mental health. Given the importance of child development, it requires joint efforts from multiple disciplines.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1C1C1006503, 2021K1A3A1A2103751212, 2021M3E5D2A01022515, RS-2023-00266787, RS-2023-00265406, 021R1I1A1A01054995, and RS-2023-00250759), by Creative-Pioneering Researchers Program through Seoul National University (No. 200-20230058), and by Institute of Information & communications Technology Planning & Evaluation (IITP) grant funded by the Korea government (MSIT) [No. 2021-0-01343, Artificial Intelligence Graduate School Program (Seoul National University)], and the New Faculty Startup Fund from Sungkyunkwan University (S-2023-1896-000-01).
Appendix 1
Genotype data
The ABCD Study collected saliva samples of study participants at the baseline visit and shipped the samples from the collection site to the Rutgers University Cell and DNA Repository. Genomic DNA was extracted and genotyped using the Affymetrix NIDA Smokescreen array (733,293 SNPs). We removed any inferior SNPs with (1) genotype call rate <95%, (2) sample call rate <95%, and (3) rare variants with minor allele frequency (MAF) <0.01. Variant imputation was performed with the Michigan Imputation Server (Das et al., 2016) using the 1000 Genome phase 3 version 5 multiethnic Grch37/hg19 reference panel with Eagle ver2.4 phased output (Loh et al., 2016). For the imputed 12,046,090 SNPs, we only retained data from any individuals with <5% missing genotypes; without extreme heterozygosity (F coefficient <3 standard deviations from the population mean); and SNPs with >0.4 imputation quality INFO score, <5% missingness rate, >0.01 MAF and Hardy–Weinberg equilibrium (p > 10−6). The genetic ancestry of each participant was determined with the fastSTRUCTURE algorithm (Raj et al., 2014), available from ABCD Release 4.0. Considering the diverse ethnic and racial backgrounds of the study participants, we estimated both kinship coefficients (K.C.s) and ancestrally informative principal components (P.C.s) to additionally control familial relatedness and ancestry admixture using PC-Air (Conomos et al., 2015) and PC-Relate (Conomos et al., 2016). We selected unrelated participants that were inferred to be more distant than fourth-degree relatives (K.C. >0.022) and removed any outliers that fell significantly outside (>6 S.D. limits) the center in P.C. space. In the rest of this paper, we used final genotype data (11,301,999 variants) of 10,199 unrelated multiethnic samples, including 7893 European-ancestry participants, after Q.C.
Polygenic scores
Hyperparameters of PGSs were optimized in the held-out validation set of 1579 unrelated participants, consisting of 88 of African ancestry (5.57%), 25 of East Asian ancestry (1.58%), 1365 of European ancestry (86.45%), 88 of Native American ancestry (2.91%), and 55 not specified (3.48%). The validation set was created during the quality control process of the study genotype data when we selected unrelated study participants of the original ABCD samples with pairwise kinship coefficients of less than 0.022 among them. To select the optimal hyperparameter, we fitted linear regression models for intelligence composite scores within the validation set and evaluated the model performance in terms of the highest R2 and effect size (beta). The models were adjusted for age, sex, and the first 10 principal components of genotype data. For the PGS of multiethnic participants, genetic ancestry determined by ADMIXTURE (Alexander et al., 2009) was additionally included as a covariate. The validation samples were only used for hyperparameter tuning for PGS optimization and were excluded from any further analyses. The PGSs were residualized against the first 10 ancestrally informative P.C.s to adjust for population stratification.
Parent-rated psychotic-like experiences
As self- and parent-reports of psychopathology often differ, parent-rated PLEs derived from four items of the Child Behavior Checklist:
‘Hears sounds or voices that other people think aren’t there.’
‘Sees things that other people think aren’t there.’
‘Does things that other people think are strange.’
‘Has thoughts that other people would think are strange.’
Each question was scored from 0 = not true, 1 = somewhat or sometimes true, and 2 = very true or often true.
Family and neighborhood SES
We assessed children’s family SES based on family income, parental education, and family’s financial adversity. All three variables were based on self-reported responses of children’s primary caregiver. For family income, the caregivers were asked ‘What is your TOTAL COMBINED FAMILY INCOME for the past 12 months? This should include income (before taxes and deductions) from all sources, wages, rent from properties, social security, disability and/or veteran’s benefits, unemployment benefits, workman’s compensation, help from relative (include child payments and alimony), and so on. If Separated/Divorced, please average the two household incomes.’ Answer choices are shown below:
Less than $5000
$5000 through $11,999
$12,000 through $15,999
$16,000 through $24,999
$25,000 through $34,999
$35,000 through $49,999
$50,000 through $74,999
$75,000 through $99,999
$100,000 through $199,999
$200,000 and greater
Parental education was measured as the highest grade or level of school completed or highest degree received by the primary caregiver. (‘What is the highest grade or level of school you have completed or the highest degree you have received?’). Answer choices were:
Never attended/Kindergarten only
1st grade
2nd grade
3rd grade
4th grade
5th grade
6th grade
7th grade
8th grade
9th grade
10th grade
11th grade
12th grade
High school graduate
GED or equivalent diploma
Some college
Associate degree: Occupational
Associate degree: Academic Program
Bachelor’s degree (ex. BA)
Master’s degree (ex. MA)
Professional School degree (ex. MD)
Doctoral degree (ex. PhD)
Family’s financial adversity is measured with Parent-Reported Financial Adversity Questionnaire, reflecting family’s financial ability to pay for basic life expenses (Diemer et al., 2013). The questionnaire asked whether the child’s caregiver and family experienced any of the following difficulties within the past 12 months:
‘Needed food but couldn’t afford to buy it or couldn’t afford to go out to get it?’
‘Were without telephone service because you could not afford it?’
‘Didn’t pay the full amount of the rent or mortgage because you could not afford it?’
‘Were evicted from your home for not paying the rent or mortgage?’
‘Had services turned off by the gas or electric company, or the oil company wouldn’t deliver oil because payments were not made?’
‘Had someone who needed to see a doctor or go to the hospital but didn’t go because you could not afford it?’
‘Had someone who needed a dentist but couldn’t go because you could not afford it?’
Each of the seven items was scored with 0=no or 1=yes.
Neighborhood SES was measured by Residential History Derived Scores based on the census tracts of each respondent’s primary address. The national percentile score of ADI (Karcher et al., 2021; Rakesh et al., 2021) was calculated from the 2011–2015 American Community Survey 5-year summary. Components used for deriving ADI includes:
Percentage of population aged ≥25 years with <9 years of education
Percentage of population aged ≥25 years with at least a high school diploma
Percentage of employed persons aged ≥16 years in white collar occupations
Median family income
Income disparity defined by Singh as the log of 100x ratio of the number of households with <10,000 annual income to the number of households with >50,000 annual income.
Median home value
Median gross rent
Median monthly mortgage
Percentage of owner
Percentage of occupied housing units with >1 person per room (crowding)
Percentage of civilian labor force population aged ≥16 years unemployed (unemployment rate)
Percentage of families below the poverty level
Percentage of population below 138% of the poverty threshold
Percentage of single
Percentage of occupied housing units without a motor vehicle
Percentage of occupied housing units without a telephone
Percentage of occupied housing units without complete plumbing (log)
Positive family and school environment
Positive parenting behavior was assessed using the ABCD Children’s Report of Parental Behavioral Inventory. We used the average values of mean summary scores of five questionnaire items about first and second caregivers. Single values were used for respondents lacking a second caregiver. Positive school environment was assessed as the sum of children’s responses to 12 items in the ABCD School Risk and Protective Factors Survey.
Prior work used response items about the first caregiver for positive parenting behavior and the first six items about the school environment for positive school environment (Rakesh et al., 2021). However, to obtain a comprehensive and accurate assessment, we also included items about the second caregiver and six additional questions about the school environment.
Children were asked to choose between 1 = Not like him/her, 2 = Somewhat like him/her, or 3 = A lot like him/her about the five questions regarding the first and second caregivers:
Smiles at me very often.
Is able to make me feel better when I am upset.
Believes in showing his/her love for me.
Is easy to talk to.
Makes me feel better after talking over my worries with him/her
The following questions were asked to assess positive school environment:
In my school, students have lots of chances to help decide things like class activities and rules.
I get along with my teachers.
My teacher(s) notices when I am doing a good job and lets me know about it.
There are lots of chances for students in my school to get involved in sports, clubs, or other school activities outside of class.
I feel safe at my school.
The school lets my parents know when I have done something well.
I like school because I do well in class.
I feel I’m just as smart as other kids my age.
There are lots of chances to be part of class discussions or activities.
In general, I like school a lot.
Usually, school bores me.
Getting good grades is not so important to me.
Each of the 12 items was scored with 1 = NO!, 2 = no, 3 = yes, 4 = YES!. Item 11 and 12 were reverse coded when obtaining summary scores.
Appendix 2
Results of linear mixed models with European samples
As the European-descent-based GWAS was used for constructing PGS, we reran the main analyses using participants of European ancestry (n = 5211) to adjust for ethnic confounding (females 46.71%, mean age 118.99 [SD 7.46]).
The results of linear mixed models were similar to those of main analyses (Supplementary file 1). Higher intelligence was significantly associated with higher CP and EA PGS (βs > 0.0754, p < 0.0001), lower ADI (βs < −0.0503, p = 0.0253), more years of residence (βs > 0.0268, p = 0.0311), positive school environment (βs > 0.0365, p = 0.004), higher parental education (βs > 0.1067, p < 0.0001), more family income (βs > 0.0561, p = 0.0166), and less family’s financial disadvantages (βs < −0.0427, p = 0.011). No significant association of poverty and supportive parenting behavior with intelligence was found (p > 0.05).
More Total and Distress Score PLEs were significantly associated with lower CP and EA PGS (baseline: βs < −0.0316, p = 0.0212; 1 year: βs < −0.0422, p = 0.0016; 2 years: βs < −0.0489, p = 0.0002), higher ADI (baseline: βs > 0.0554, p = 0.0421), less supportive parenting (baseline: βs < −0.0722, p < 0.0001; 1 year: βs < −0.0473, p = 0.0013; 2 years: βs < −0.0693, p < 0.0001), less positive school environment (baseline: βs < −0.1076, p < 0.0001; 1 year: βs < −0.0896, p = 0.0013; 2 years: βs < −0.0847, p < 0.0001), lower parental education (baseline: βs < −0.0632, p = 0.0011; 1 year: βs < −0.0567, p = 0.004; 2 years: βs < −0.0463, P=0.0198), more family’s financial disadvantage (1 year: βs > 0.0605, p = 0.005; 2 years: βs > 0.0646, p < 0.0001). No significant association of years of residence, poverty, and family income with Total and Distress Score PLEs was found (p > 0.05). Parent-rated PLEs was positively associated with ADI (baseline: β = 0.0486, p = 0.0378; 1 year: βs > 0.0527, p = 0.0225) and negatively with positive school environment (baseline: βs < −0.0522, p = 0.0018; 2 years: βs < −0.0601, p = 0.0009) and family’s financial disadvantage (2 years: βs > 0.0501, p = 0.0216).
Results of IGSCA with European samples
The results of IGSCA in European ancestry samples were similar to those in multiethnic participants (Supplementary file 1). It showed a good model fit with a GFI of 0.9695, SRMR of 0.0397, and FIT value of 0.4854.
Intelligence was under a significant direct influence of the cognitive phenotypes PGS (β = 0.2987 [95% CI = 0.2673 to 0.3281]), neighborhood SES (β = −0.0931 [95% CI = −0.1303 to −0.0586]), family SES (β = 0.3034 [95% CI = 0.2683 to 0.3413]), and positive environment (β = 0.0396 [95% CI = 0.0104 to 0.0698]). Neighborhood SES (β = 0.0411 [95% CI = 0.0037 to 0.0784]), family SES (β = −0.0531 [95% CI = −0.0956 to −0.0101]), and positive environment (β = −0.1473 [95% CI = −0.1779 to −0.1163]) showed significant direct influence on baseline year PLEs, but cognitive phenotypes PGS did not. Constructs that had significant direct effects on PLEs of 1- and 2-year follow-up were family SES (βs < −0.0850) and positive environment (βs < −0.1222).
Intelligence had a significant mediating effect on the pathways of the PGS (β = −0.0555 [95% CI = −0.0754 to −0.0392]), family SES (β = −0.0563 [95% CI = −0.07749 to −0.0392]), neighborhood SES (β = 0.0173 [95% CI = 0.0098 to 0.0271]), and positive environment (β = −0.0074 [95% CI = −0.0142 to −0.0019]) to baseline year PLEs. For PLEs of 1- and 2-year follow-up, the mediation effects of intelligence were also significant with all four constructs: PGS (1 year: β = −0.0397 [95% CI = −0.0586 to −0.0235]; 2 years: β = −0.0204 [95% CI = −0.0389 to −0.0036]), family SES (1 year: β = −0.0404 [95% CI = −0.0601 to −0.0238]; 2 years: β = −0.0207 [95% CI = −0.0399 to −0.0036]), neighborhood SES (1 year: β = 0.0124 [95% CI = 0.0064 to 0.0208]; 2 years: β = 0.0064 [95% CI = 0.0011 to 0.0132]), and positive environment (1 year: β = −0.0053 [95% CI = −0.0108 to −0.0012]; 2 years: β = −0.0027 [95% CI = −0.0067 to −0.0002]). Positive environment had the largest total effects on PLEs among the constructs (baseline year: β = −0.1547; 1 year: β = −0.1274; 2 years: β = −0.1386).
Results of linear mixed models adjusted for schizophrenia PGS with multiethnic samples
We also assessed whether the effects of cognitive phenotypes PGS in the linear mixed model are significant including schizophrenia PGS. The results showed that inclusion of schizophrenia PGS in the models did not change much of the main results (Supplementary file 1). Schizophrenia PGS was negatively associated with total and fluid intelligence in EA PGS model (Total intelligence β = −0.0293, 95% CI = −0.0488 to −0.01, p = 0.0048; Fluid intelligence β = −0.0317, 95% CI = −0.0522 to −0.0097, p = 0.0062). No significant association was found between schizophrenia PGS and PLEs of all three time points (p > 0.05).
Results of linear mixed models adjusted for unobserved confounders with multiethnic samples
Unobserved confounding variables may bias linear regression estimates. In particular, linear estimates may be subject to collider bias when those unobserved confounders and genetic factors are jointly associated with environmental factors and target traits (Akimova et al., 2021). Thus, bias from unobserved confounding variables were adjusted using the null treatments approach (Miao et al., 2023). This approach can identify the causal effects of multiple treatment variables in presence of unobserved confounders. Assuming that fewer than half of the treatments have causal effects on the outcome, it first estimates the joint distribution of treatments–confounders to obtain asymptotic bias using a factor model. Second, it estimates the relationship between treatments, confounders, and outcome using standard density estimation methods (e.g., least squares regression). Finally, by eliminating the asymptotic bias from the estimated treatments–confounders–outcome relationship, it can adjust for unobserved confounding even without specifying which treatments are null.
The significance of effects of PGSs was mostly preserved after adjusting for unobserved confounders (Supplementary file 1). Higher cognitive phenotypes PGSs correlated significantly with higher intelligence (CP PGS: βs > 0.1111, p < 0.0001; EA PGS: βs > 0.0546, p = 0.0002). CP PGS was associated with lower baseline year Distress Score PLEs (β = −0.0348, p = 0.021) and EA PGS was associated with lower Total and Distress Score PLEs of baseline year and follow-up years (baseline: βs < −0.0534, p = 0.0002; 1 year: β = −0.0375, p = 0.0149; 2 years: βs < −0.0391, p = 0.017). We found no significant associations of neighborhood SES variables with any of the target variables (p > 0.05).
While positive parenting behaviors did not have significant association with intelligence, positive school environment was significantly associated with higher total and fluid intelligence (βs > 0.0328, p = 0.0242). Positive parenting behaviors had significant negative correlations with all types of PLEs of baseline year and follow-up years (baseline: βs <−0.0412, p = 0.036; 1 year: βs < −0.0435, p = 0.0316; 2 years: βs <−0.0558, p = 0.0017). Positive school environment was also negatively associated with all types of PLEs of baseline year and follow-up years (baseline: βs < −0.0464, p = 0.036; 1 year: βs < −0.0952, p < 0.0001; 2 years: βs < −0.0583, p = 0.0095).
Parental education and family income were not significantly associated with all types of intelligence and PLEs of all three time points. Family’s financial disadvantage did not show significant association with intelligence and baseline year PLEs, but it had positive associations with 1-year follow-up Total Score (β = 0.0604, p = 0.0113) and Distress Score PLEs (β = 0.0532, p = 0.0197) and 2-year follow-up Total Score (β > 0.0544, p = 0.0284) and Distress Score PLEs (β = 0.0584, p = 0.0224).
Appendix 3
Appendix 3—figure 1. Component correlation matrix of integrated generalized structured component analysis (IGSCA).
(A) Correlation between all component/factor variables of the IGSCA model. (B) Correlation between all observed variables used to construct the relevant component/factor variables in the IGSCA model. CP PGS and EA PGS denote polygenic scores of cognitive performance and education attainment, respectively; PLEs, psychotic-like experiences; Crystallized and Fluid, crystallized and fluid intelligence; ADI, Area Deprivation Index; Poverty, percentage of individuals below −125% of the poverty level; Years, years of residence.
Funding Statement
The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication.
Contributor Information
Yoonjung Yoonie Joo, Email: yoonjungjoo@skku.edu.
Jiook Cha, Email: connectome@snu.ac.kr.
Charlotte Cecil, Erasmus MC, Netherlands.
Ma-Li Wong, State University of New York Upstate Medical University, United States.
Funding Information
This paper was supported by the following grants:
National Research Foundation of Korea 2021R1C1C1006503 to Jiook Cha.
National Research Foundation of Korea 2021K1A3A1A2103751212 to Jiook Cha.
National Research Foundation of Korea 2021M3E5D2A01022515 to Jiook Cha.
National Research Foundation of Korea RS-2023-00266787 to Jiook Cha.
National Research Foundation of Korea RS-2023-00265406 to Jiook Cha.
Seoul National University Creative-Pioneering Researchers Program through Seoul National University 200-20230058 to Jiook Cha.
Seoul National University Semi-Supervised Learning Research Grant by SAMSUNG A0426-20220118 to Jiook Cha.
Institute for Information and Communications Technology Planning and Evaluation NO.2021-0-01343 to Jiook Cha.
Institute for Information and Communications Technology Planning and Evaluation Artificial Intelligence Graduate School Program to Jiook Cha.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Validation, Investigation, Methodology, Writing - original draft, Project administration, Writing – review and editing.
Investigation, Writing - original draft, Writing – review and editing.
Methodology.
Investigation, Methodology.
Investigation.
Investigation.
Supervision, Validation, Investigation, Writing – review and editing.
Conceptualization, Supervision, Funding acquisition, Writing – review and editing.
Additional files
Data availability
All codes used in this study can be found at https://github.com/Transconnectome/Intell_PLE_Pathway (copy archived at Park, 2024). The original ABCD Study dataset is freely accessible to all qualified researchers upon submission of an access request through the National Institutes of Mental Health Data Archive (nda.nih.gov). Each of the participating centers secured comprehensive written informed consent from parents and assent from all children involved. The research protocols received approval from the University of California, San Diego's Institutional Review Board (IRB) under approval number 160091, as well as from the IRBs of the 21 data collection sites involved (Auchter et al., 2018). Due to ABCD Study's policy in data sharing, we provide a synthetic dataset instead of real observations. This synthetic dataset was generated with conditional GAN (Xu et al., 2019) to imitate the data structure of our final study samples. After automatic hyperparameter optimization with Optuna (Akiba et al., 2019), the synthetic dataset showed overall quality score of 84.15%. Note that analyses results from the synthetic data may not be 100% identical to the results presented in this paper, due to the differences between synthetic vs original dataset.
References
- Aarøe L, Appadurai V, Hansen KM, Schork AJ, Werge T, Mors O, Børglum AD, Hougaard DM, Nordentoft M, Mortensen PB, Thompson WK, Buil A, Agerbo E, Petersen MB. Genetic predictors of educational attainment and intelligence test performance predict voter turnout. Nature Human Behaviour. 2021;5:281–291. doi: 10.1038/s41562-020-00952-2. [DOI] [PubMed] [Google Scholar]
- Abdellaoui A, Dolan CV, Verweij KJH, Nivard MG. Gene–environment correlations across geographic regions affect genome-wide association studies. Nature Genetics. 2022;54:1345–1354. doi: 10.1038/s41588-022-01158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach TM. As others see us. Current Directions in Psychological Science. 2006;15:94–98. doi: 10.1111/j.0963-7214.2006.00414.x. [DOI] [Google Scholar]
- Akiba T, Sano S, Yanase T, Ohta T, Koyama M. Optuna: a next-generation hyperparameter optimization framework.KDD. 19 Proceedings of the 25th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining; Anchorage. 2019. [DOI] [Google Scholar]
- Akimova ET, Breen R, Brazel DM, Mills MC. Gene-environment dependencies lead to collider bias in models with polygenic scores. Scientific Reports. 2021;11:9457. doi: 10.1038/s41598-021-89020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Research. 2009;19:1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoretti S, Cabrera B, Torrent C, Mezquida G, Lobo A, González-Pinto A, Parellada M, Corripio I, Vieta E, de la Serna E, Butjosa A, Contreras F, Sarró S, Penadés R, Sánchez-Torres AM, Cuesta M, Bernardo M, PEPsGroup Cognitive reserve as an outcome predictor: first-episode affective versus non-affective psychosis. Acta Psychiatrica Scandinavica. 2018;138:441–455. doi: 10.1111/acps.12949. [DOI] [PubMed] [Google Scholar]
- Auchter AM, Hernandez Mejia M, Heyser CJ, Shilling PD, Jernigan TL, Brown SA, Tapert SF, Dowling GJ. A description of the ABCD organizational structure and communication framework. Developmental Cognitive Neuroscience. 2018;32:8–15. doi: 10.1016/j.dcn.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth D, Papageorge NW, Thom K. Genetic endowments and wealth inequality. The Journal of Political Economy. 2020;128:1474–1522. doi: 10.1086/705415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Domingue BW, Wedow R, Arseneault L, Boardman JD, Caspi A, Conley D, Fletcher JM, Freese J, Herd P, Moffitt TE, Poulton R, Sicinski K, Wertz J, Harris KM. Genetic analysis of social-class mobility in five longitudinal studies. PNAS. 2018;115:E7275–E7284. doi: 10.1073/pnas.1801238115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentall RP, Fernyhough C. Social predictors of psychotic experiences: specificity and psychological mechanisms. Schizophrenia Bulletin. 2008;34:1012–1020. doi: 10.1093/schbul/sbn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard TJ, McGue M. Familial studies of intelligence: a review. Science. 1981;212:1055–1059. doi: 10.1126/science.7195071. [DOI] [PubMed] [Google Scholar]
- Brody GH, Gray JC, Yu T, Barton AW, Beach SRH, Galván A, MacKillop J, Windle M, Chen E, Miller GE, Sweet LH. Protective prevention effects on the association of poverty with brain development. JAMA Pediatrics. 2017;171:46–52. doi: 10.1001/jamapediatrics.2016.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Yu T, Nusslock R, Barton AW, Miller GE, Chen E, Holmes C, McCormick M, Sweet LH. The protective effects of supportive parenting on the relationship between adolescent poverty and resting-state functional brain connectivity during adulthood. Psychological Science. 2019;30:1040–1049. doi: 10.1177/0956797619847989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler O, Yang X-F, Laube C, Kühn S, Immordino-Yang MH. Community violence exposure correlates with smaller gray matter volume and lower IQ in urban adolescents. Human Brain Mapping. 2018;39:2088–2097. doi: 10.1002/hbm.23988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Bearden CE, Hollister JM, Rosso IM, Sanchez LE, Hadley T. Childhood cognitive functioning in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophrenia Bulletin. 2000;26:379–393. doi: 10.1093/oxfordjournals.schbul.a033460. [DOI] [PubMed] [Google Scholar]
- Cannon M, Caspi A, Moffitt TE, Harrington H, Taylor A, Murray RM, Poulton R. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Archives of General Psychiatry. 2002;59:449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- CDC Health Impact in 5 years. 2018. [October 23, 2021]. https://www.cdc.gov/policy/hst/hi5/index.html
- Cho G, Hwang H, Sarstedt M, Ringle CM. Cutoff criteria for overall model fit indexes in generalized structured component analysis. Journal of Marketing Analytics. 2020;8:189–202. doi: 10.1057/s41270-020-00089-1. [DOI] [Google Scholar]
- Cho G, Sarstedt M, Hwang H. A comparative evaluation of factor- and component-based structural equation modelling approaches under (in)correct construct representations. The British Journal of Mathematical and Statistical Psychology. 2022;75:220–251. doi: 10.1111/bmsp.12255. [DOI] [PubMed] [Google Scholar]
- Compton WM, Dowling GJ, Garavan H. Ensuring the best use of data: the adolescent brain cognitive development study. JAMA Pediatrics. 2019;173:809–810. doi: 10.1001/jamapediatrics.2019.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conomos MP, Miller MB, Thornton TA. Robust inference of population structure for ancestry prediction and correction of stratification in the presence of relatedness. Genetic Epidemiology. 2015;39:276–293. doi: 10.1002/gepi.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conomos MP, Reiner AP, Weir BS, Thornton TA. Model-free estimation of recent genetic relatedness. American Journal of Human Genetics. 2016;98:127–148. doi: 10.1016/j.ajhg.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosway R, Byrne M, Clafferty R, Hodges A, Grant E, Abukmeil SS, Lawrie SM, Miller P, Johnstone EC. Neuropsychological change in young people at high risk for schizophrenia: results from the first two neuropsychological assessments of the Edinburgh High Risk Study. Psychological Medicine. 2000;30:1111–1121. doi: 10.1017/s0033291799002585. [DOI] [PubMed] [Google Scholar]
- Cree RA, Bitsko RH, Robinson LR, Holbrook JR, Danielson ML, Smith C, Kaminski JW, Kenney MK, Peacock G. Health care, family, and community factors associated with mental, behavioral, and developmental disorders and poverty among children aged 2-8 years - United States, 2016. MMWR. Morbidity and Mortality Weekly Report. 2018;67:1377–1383. doi: 10.15585/mmwr.mm6750a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha F, Heckman J. The technology of skill formation. American Economic Review. 2007;97:31–47. doi: 10.1257/aer.97.2.31. [DOI] [Google Scholar]
- Curtis CE, Calkins ME, Grove WM, Feil KJ, Iacono WG. Saccadic disinhibition in patients with acute and remitted schizophrenia and their first-degree biological relatives. The American Journal of Psychiatry. 2001;158:100–106. doi: 10.1176/appi.ajp.158.1.100. [DOI] [PubMed] [Google Scholar]
- Das S, Forer L, Schönherr S, Sidore C, Locke AE, Kwong A, Vrieze SI, Chew EY, Levy S, McGue M, Schlessinger D, Stambolian D, Loh P-R, Iacono WG, Swaroop A, Scott LJ, Cucca F, Kronenberg F, Boehnke M, Abecasis GR, Fuchsberger C. Next-generation genotype imputation service and methods. Nature Genetics. 2016;48:1284–1287. doi: 10.1038/ng.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Spinath FM, Bates TC. Genetics of intelligence. European Journal of Human Genetics. 2006;14:690–700. doi: 10.1038/sj.ejhg.5201588. [DOI] [PubMed] [Google Scholar]
- Demange PA, Malanchini M, Mallard TT, Biroli P, Cox SR, Grotzinger AD, Tucker-Drob EM, Abdellaoui A, Arseneault L, van Bergen E, Boomsma DI, Caspi A, Corcoran DL, Domingue BW, Harris KM, Ip HF, Mitchell C, Moffitt TE, Poulton R, Prinz JA, Sugden K, Wertz J, Williams BS, de Zeeuw EL, Belsky DW, Harden KP, Nivard MG. Investigating the genetic architecture of noncognitive skills using GWAS-by-subtraction. Nature Genetics. 2021;53:35–44. doi: 10.1038/s41588-020-00754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diemer MA, Mistry RS, Wadsworth ME, López I, Reimers F. Best Practices in conceptualizing and measuring social class in psychological research. Analyses of Social Issues and Public Policy. 2013;13:77–113. doi: 10.1111/asap.12001. [DOI] [Google Scholar]
- Eastvold AD, Heaton RK, Cadenhead KS. Neurocognitive deficits in the (putative) prodrome and first episode of psychosis. Schizophrenia Research. 2007;93:266–277. doi: 10.1016/j.schres.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett AKJ, Velthorst E, Reichenberg A, Ruggero CJ, Callahan JL, Fochtmann LJ, Carlson GA, Perlman G, Bromet EJ, Kotov R. Long-term changes in cognitive functioning in individuals with psychotic disorders. JAMA Psychiatry. 2020;77:387. doi: 10.1001/jamapsychiatry.2019.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane HK, Reshef YA, Anttila V, Slowikowski K, Gusev A, Byrnes A, Gazal S, Loh P-R, Lareau C, Shoresh N, Genovese G, Saunders A, Macosko E, Pollack S, Brainstorm Consortium. Perry JRB, Buenrostro JD, Bernstein BE, Raychaudhuri S, McCarroll S, Neale BM, Price AL. Heritability enrichment of specifically expressed genes identifies disease-relevant tissues and cell types. Nature Genetics. 2018;50:621–629. doi: 10.1038/s41588-018-0081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Monette G. Generalized collinearity diagnostics. Journal of the American Statistical Association. 1992;87:178–183. doi: 10.1080/01621459.1992.10475190. [DOI] [Google Scholar]
- Gard AM, Maxwell AM, Shaw DS, Mitchell C, Brooks-Gunn J, McLanahan SS, Forbes EE, Monk CS, Hyde LW. Beyond family-level adversities: exploring the developmental timing of neighborhood disadvantage effects on the brain. Developmental Science. 2021;24:e12985. doi: 10.1111/desc.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garety PA, Kuipers E, Fowler D, Freeman D, Bebbington PE. A cognitive model of the positive symptoms of psychosis. Psychological Medicine. 2001;31:189–195. doi: 10.1017/s0033291701003312. [DOI] [PubMed] [Google Scholar]
- Garner A, Yogman M, COMMITTEE ON PSYCHOSOCIAL ASPECTS OF CHILD AND FAMILY HEALTH, SECTION ON DEVELOPMENTAL AND BEHAVIORAL PEDIATRICS, COUNCIL ON EARLY CHILDHOOD Preventing childhood toxic stress: partnering with families and communities to promote relational health. Pediatrics. 2021;148:e2021052582. doi: 10.1542/peds.2021-052582. [DOI] [PubMed] [Google Scholar]
- Ge T, Chen CY, Ni Y, Feng YCA, Smoller JW. Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nature Communications. 2019;10:1776. doi: 10.1038/s41467-019-09718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair NL, Hanson JL, Wolfe BL, Pollak SD. Association of child poverty, brain development, and academic achievement. JAMA Pediatrics. 2015;169:822–829. doi: 10.1001/jamapediatrics.2015.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Jantac Mam-Lam-Fook C, Chaignaud J, Danset-Alexandre C, Iftimovici A, Gradels Hauguel J, Houle G, Liao C, ICAAR study group. Dion PA, Rouleau GA, Kebir O, Krebs M-O, Chaumette B. Influence of polygenic risk scores for schizophrenia and resilience on the cognition of individuals at-risk for psychosis. Translational Psychiatry. 2021;11:518. doi: 10.1038/s41398-021-01624-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KG, Bailey JA, Steeger CM, Hawkins JD, Catalano RF, Kosterman R, Epstein M, Abbott RD. Outcomes of childhood preventive intervention across 2 generations. JAMA Pediatrics. 2020;174:764. doi: 10.1001/jamapediatrics.2020.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CJ, Barton AW, MacKillop J, Galván A, Owens MM, McCormick MJ, Yu T, Beach SRH, Brody GH, Sweet LH. Parenting and salience network connectivity among African Americans: a protective pathway for health-risk behaviors. Biological Psychiatry. 2018;84:365–371. doi: 10.1016/j.biopsych.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. The Lancet. 2014;383:1677–1687. doi: 10.1016/S0140-6736(13)62036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang H, Cho G, Choo H. GSCA Pro—free stand-alone software for structural equation modeling. Structural Equation Modeling: A Multidisciplinary Journal. 2021a;01:RG.2.2.28162.61127/1. doi: 10.13140/RG.2.2.28162.61127/1. [DOI] [Google Scholar]
- Hwang H, Cho G, Jung K, Falk CF, Flake JK, Jin MJ, Lee SH. An approach to structural equation modeling with both factors and components: Integrated generalized structured component analysis. Psychological Methods. 2021b;26:273–294. doi: 10.1037/met0000336. [DOI] [PubMed] [Google Scholar]
- Isungset MA, Conley D, Zachrisson HD, Ystrom E, Havdahl A, Njølstad PR, Lyngstad TH. Social and genetic associations with educational performance in a Scandinavian welfare state. PNAS. 2022;119:25. doi: 10.1073/pnas.2201869119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AR. The g Factor: The Science of Mental Ability. Praeger Publishers/Greenwood Publishing Group; 1998. [Google Scholar]
- Joo YY, Cha J, Freese J, Hayes MG. Cognitive capacity genome-wide polygenic scores identify individuals with slower cognitive decline in aging. Genes. 2022;13:1320. doi: 10.3390/genes13081320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöreskog KG, Sörbom D. PRELIS: A Program for Multivariate Data Screening and Data Summarization. Mooresville: Scientific Software; 1986. [Google Scholar]
- Judd N, Sauce B, Wiedenhoeft J, Tromp J, Chaarani B, Schliep A, van Noort B, Penttilä J, Grimmer Y, Insensee C, Becker A, Banaschewski T, Bokde ALW, Quinlan EB, Desrivières S, Flor H, Grigis A, Gowland P, Heinz A, Ittermann B, Martinot J-L, Paillère Martinot M-L, Artiges E, Nees F, Papadopoulos Orfanos D, Paus T, Poustka L, Hohmann S, Millenet S, Fröhner JH, Smolka MN, Walter H, Whelan R, Schumann G, Garavan H, Klingberg T. Cognitive and brain development is independently influenced by socioeconomic status and polygenic scores for educational attainment. PNAS. 2020;117:12411–12418. doi: 10.1073/pnas.2001228117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcher NR, Barch DM, Avenevoli S, Savill M, Huber RS, Simon TJ, Leckliter IN, Sher KJ, Loewy RL. Assessment of the prodromal questionnaire-brief child version for measurement of self-reported psychoticlike experiences in childhood. JAMA Psychiatry. 2018;75:853–861. doi: 10.1001/jamapsychiatry.2018.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcher NR, Perino MT, Barch DM. An item response theory analysis of the prodromal questionnaire-brief child version: developing a screening form that informs understanding of self-reported psychotic-like experiences in childhood. Journal of Abnormal Psychology. 2020;129:293–304. doi: 10.1037/abn0000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcher NR, Schiffman J, Barch DM. Environmental risk factors and psychotic-like experiences in children aged 9–10. Journal of the American Academy of Child & Adolescent Psychiatry. 2021;60:490–500. doi: 10.1016/j.jaac.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcher NR, Paul SE, Johnson EC, Hatoum AS, Baranger DAA, Agrawal A, Thompson WK, Barch DM, Bogdan R. Psychotic-like experiences and polygenic liability in the adolescent brain cognitive development study. Biological Psychiatry. 2022;7:45–55. doi: 10.1016/j.bpsc.2021.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RSE, Perkins DO, Gu H, Zipursky RB, Christensen BK, Lieberman JA. A longitudinal study of neurocognitive function in individuals at-risk for psychosis. Schizophrenia Research. 2006;88:26–35. doi: 10.1016/j.schres.2006.06.041. [DOI] [PubMed] [Google Scholar]
- Kelleher I, Cannon M. Psychotic-like experiences in the general population: characterizing a high-risk group for psychosis. Psychological Medicine. 2011;41:1–6. doi: 10.1017/S0033291710001005. [DOI] [PubMed] [Google Scholar]
- Kowarik A, Templ M. Imputation with the R Package VIM. Journal of Statistical Software. 2016;74:1–16. doi: 10.18637/jss.v074.i07. [DOI] [Google Scholar]
- Krabbendam L, van Os J. Schizophrenia and urbanicity: a major environmental influence--conditional on genetic risk. Schizophrenia Bulletin. 2005;31:795–799. doi: 10.1093/schbul/sbi060. [DOI] [PubMed] [Google Scholar]
- Lam M, Chen C-Y, Li Z, Martin AR, Bryois J, Ma X, Gaspar H, Ikeda M, Benyamin B, Brown BC, Liu R, Zhou W, Guan L, Kamatani Y, Kim S-W, Kubo M, Kusumawardhani AAAA, Liu C-M, Ma H, Periyasamy S, Takahashi A, Xu Z, Yu H, Zhu F, Schizophrenia Working Group of the Psychiatric Genomics Consortium. Indonesia Schizophrenia Consortium. Genetic REsearch on schizophreniA neTwork-China and the Netherlands (GREAT-CN) Chen WJ, Faraone S, Glatt SJ, He L, Hyman SE, Hwu H-G, McCarroll SA, Neale BM, Sklar P, Wildenauer DB, Yu X, Zhang D, Mowry BJ, Lee J, Holmans P, Xu S, Sullivan PF, Ripke S, O’Donovan MC, Daly MJ, Qin S, Sham P, Iwata N, Hong KS, Schwab SG, Yue W, Tsuang M, Liu J, Ma X, Kahn RS, Shi Y, Huang H. Comparative genetic architectures of schizophrenia in East Asian and European populations. Nature Genetics. 2019;51:1670–1678. doi: 10.1038/s41588-019-0512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, Nguyen-Viet TA, Bowers P, Sidorenko J, Karlsson Linnér R, Fontana MA, Kundu T, Lee C, Li H, Li R, Royer R, Timshel PN, Walters RK, Willoughby EA, Yengo L, 23andMe Research Team. COGENT (Cognitive Genomics Consortium) Social Science Genetic Association Consortium. Alver M, Bao Y, Clark DW, Day FR, Furlotte NA, Joshi PK, Kemper KE, Kleinman A, Langenberg C, Mägi R, Trampush JW, Verma SS, Wu Y, Lam M, Zhao JH, Zheng Z, Boardman JD, Campbell H, Freese J, Harris KM, Hayward C, Herd P, Kumari M, Lencz T, Luan J, Malhotra AK, Metspalu A, Milani L, Ong KK, Perry JRB, Porteous DJ, Ritchie MD, Smart MC, Smith BH, Tung JY, Wareham NJ, Wilson JF, Beauchamp JP, Conley DC, Esko T, Lehrer SF, Magnusson PKE, Oskarsson S, Pers TH, Robinson MR, Thom K, Watson C, Chabris CF, Meyer MN, Laibson DI, Yang J, Johannesson M, Koellinger PD, Turley P, Visscher PM, Benjamin DJ, Cesarini D. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nature Genetics. 2018;50:1112–1121. doi: 10.1038/s41588-018-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeson VC, Sharma P, Harrison M, Ron MA, Barnes TRE, Joyce EM. IQ trajectory, cognitive reserve, and clinical outcome following A first episode of psychosis: A 3-year longitudinal study. Schizophrenia Bulletin. 2011;37:768–777. doi: 10.1093/schbul/sbp143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G, Dykxhoorn J, Karlsson H, Khandaker GM, Lewis G, Dalman C, Kirkbride JB. Assessment of the role of IQ in associations between population density and deprivation and nonaffective psychosis. JAMA Psychiatry. 2020;77:729–736. doi: 10.1001/jamapsychiatry.2020.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh P-R, Danecek P, Palamara PF, Fuchsberger C, A Reshef Y, K Finucane H, Schoenherr S, Forer L, McCarthy S, Abecasis GR, Durbin R, L Price A. Reference-based phasing using the Haplotype Reference Consortium panel. Nature Genetics. 2016;48:1443–1448. doi: 10.1038/ng.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Barch DM, Belden A, Gaffrey MS, Tillman R, Babb C, Nishino T, Suzuki H, Botteron KN. Maternal support in early childhood predicts larger hippocampal volumes at school age. PNAS. 2012;109:2854–2859. doi: 10.1073/pnas.1118003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Belden A, Harms MP, Tillman R, Barch DM. Preschool is a sensitive period for the influence of maternal support on the trajectory of hippocampal development. PNAS. 2016;113:5742–5747. doi: 10.1073/pnas.1601443113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall AT, Betts S, Kan EC, McConnell R, Lanphear BP, Sowell ER. Association of lead-exposure risk and family income with childhood brain outcomes. Nature Medicine. 2020;26:91–97. doi: 10.1038/s41591-019-0713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Hamshere ML, Stergiakouli E, O’Donovan MC, Thapar A. Neurocognitive abilities in the general population and composite genetic risk scores for attention-deficit hyperactivity disorder. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2015;56:648–656. doi: 10.1111/jcpp.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell J, Ronald A, Cardno AG, Breen G, Rimfeld K, Vassos E. Genetic and geographical associations with six dimensions of psychotic experiences in adolesence. Schizophrenia Bulletin. 2023;49:319–328. doi: 10.1093/schbul/sbac149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao W, Hu W, Ogburn EL, Zhou XH. Identifying effects of multiple treatments in the presence of unmeasured confounding. Journal of the American Statistical Association. 2023;118:1953–1967. doi: 10.1080/01621459.2021.2023551. [DOI] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, Akshoomoff N, Amaral DG, Bloss CS, Libiger O, Schork NJ, Murray SS, Casey BJ, Chang L, Ernst TM, Frazier JA, Gruen JR, Kennedy DN, Van Zijl P, Mostofsky S, Kaufmann WE, Kenet T, Dale AM, Jernigan TL, Sowell ER. Family income, parental education and brain structure in children and adolescents. Nature Neuroscience. 2015;18:773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okbay A, Wu Y, Wang N, Jayashankar H, Bennett M, Nehzati SM, Sidorenko J, Kweon H, Goldman G, Gjorgjieva T, Jiang Y, Hicks B, Tian C, Hinds DA, Ahlskog R, Magnusson PKE, Oskarsson S, Hayward C, Campbell A, Porteous DJ, Freese J, Herd P, 23andMe Research Team. Social Science Genetic Association Consortium. Watson C, Jala J, Conley D, Koellinger PD, Johannesson M, Laibson D, Meyer MN, Lee JJ, Kong A, Yengo L, Cesarini D, Turley P, Visscher PM, Beauchamp JP, Benjamin DJ, Young AI. Polygenic prediction of educational attainment within and between families from genome-wide association analyses in 3 million individuals. Nature Genetics. 2022;54:437–449. doi: 10.1038/s41588-022-01016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. Intell_Ple_Pathway. swh:1:rev:c2e8f273b79833a0ce01380ed47897fd831e7b88Software Heritage. 2024 https://archive.softwareheritage.org/swh:1:dir:ae6409796aa88f926645275070b82b0cb95aa3e7;origin=https://github.com/Transconnectome/Intell_PLE_Pathway;visit=swh:1:snp:4af519639403a1d5bf3b27528678311d7caa58af;anchor=swh:1:rev:c2e8f273b79833a0ce01380ed47897fd831e7b88
- Pat N, Riglin L, Anney R, Wang Y, Barch DM, Thapar A, Stringaris A. Motivation and Cognitive abilities as mediators between polygenic scores and psychopathology in children. Journal of the American Academy of Child & Adolescent Psychiatry. 2022;61:782–795. doi: 10.1016/j.jaac.2021.08.019. [DOI] [PubMed] [Google Scholar]
- Peverill M, Dirks MA, Narvaja T, Herts KL, Comer JS, McLaughlin KA. Socioeconomic status and child psychopathology in the United States: a meta-analysis of population-based studies. Clinical Psychology Review. 2021;83:101933. doi: 10.1016/j.cpr.2020.101933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo LR, Merz EC, Noble KG, Pediatric Imaging, Neurocognition, and Genetics Study School climate is associated with cortical thickness and executive function in children and adolescents. Developmental Science. 2019;22:e12719. doi: 10.1111/desc.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, Spinath FM. Intelligence: genetics, genes, and genomics. Journal of Personality and Social Psychology. 2004;86:112–129. doi: 10.1037/0022-3514.86.1.112. [DOI] [PubMed] [Google Scholar]
- Plomin R, von Stumm S. The new genetics of intelligence. Nature Reviews. Genetics. 2018;19:148–159. doi: 10.1038/nrg.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H. Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Archives of General Psychiatry. 2000;57:1053–1058. doi: 10.1001/archpsyc.57.11.1053. [DOI] [PubMed] [Google Scholar]
- Raj A, Stephens M, Pritchard JK. fastSTRUCTURE: variational inference of population structure in large SNP data sets. Genetics. 2014;197:573–589. doi: 10.1534/genetics.114.164350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakesh D, Seguin C, Zalesky A, Cropley V, Whittle S. Associations between neighborhood disadvantage, resting-state functional connectivity, and behavior in the adolescent brain cognitive development study: the moderating role of positive family and school environments. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging. 2021;6:877–886. doi: 10.1016/j.bpsc.2021.03.008. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Weiser M, Rapp MA, Rabinowitz J, Caspi A, Schmeidler J, Knobler HY, Lubin G, Nahon D, Harvey PD, Davidson M. Elaboration on premorbid intellectual performance in schizophrenia. Archives of General Psychiatry. 2005;62:1297. doi: 10.1001/archpsyc.62.12.1297. [DOI] [PubMed] [Google Scholar]
- Ritchie SJ, Hill WD, Marioni RE, Davies G, Hagenaars SP, Harris SE, Cox SR, Taylor AM, Corley J, Pattie A, Redmond P, Starr JM, Deary IJ. Polygenic predictors of age-related decline in cognitive ability. Molecular Psychiatry. 2020;25:2584–2598. doi: 10.1038/s41380-019-0372-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderfer DM, Ripke S, McQuillin A, Boocock J, Stahl EA, Pavlides JMW, Mullins N, Charney AW, Ori APS, Loohuis LMO, Domenici E, Di Florio A, Papiol S, Kalman JL, Trubetskoy V, Adolfsson R, Agartz I, Agerbo E, Akil H, Albani D, Albus M, Alda M, Alexander M, Alliey-Rodriguez N, Als TD, Amin F, Anjorin A, Arranz MJ, Awasthi S, Bacanu SA, Badner JA, Baekvad-Hansen M, Bakker S, Band G, Barchas JD, Barroso I, Bass N, Bauer M, Baune BT, Begemann M, Bellenguez C, Belliveau RA, Bellivier F, Bender S, Bene J, Bergen SE, Berrettini WH, Bevilacqua E, Biernacka JM, Bigdeli TB, Black DW, Blackburn H, Blackwell JM, Blackwood DHR, Pedersen CB, Boehnke M, Boks M, Borglum AD, Bramon E, Breen G, Brown MA, Bruggeman R, Buccola NG, Buckner RL, Budde M, Bulik-Sullivan B, Bumpstead SJ, Bunney W, Burmeister M, Buxbaum JD, Bybjerg-Grauholm J, Byerley W, Cahn W, Cai G, Cairns MJ, Campion D, Cantor RM, Carr VJ, Carrera N, Casas JP, Casas M, Catts SV, Cervantes P, Chambert KD, Chan RCK, Chen EYH, Chen RYL, Cheng W, Cheung EFC, Chong SA, Clarke TK, Cloninger CR, Cohen D, Cohen N, Coleman JRI, Collier DA, Cormican P, Coryell W, Craddock N, Craig DW, Crespo-Facorro B, Crowley JJ, Cruceanu C, Curtis D, Czerski PM, Dale AM, Daly MJ, Dannlowski U, Darvasi A, Davidson M, Davis KL, de Leeuw CA, Degenhardt F, Del Favero J, DeLisi LE, Deloukas P, Demontis D, DePaulo JR, di Forti M, Dikeos D, Dinan T, Djurovic S, Dobbyn AL, Donnelly P, Donohoe G, Drapeau E, Dronov S, Duan J, Dudbridge F, Duncanson A, Edenberg H, Edkins S, Ehrenreich H, Eichhammer P, Elvsashagen T, Eriksson J, Escott-Price V, Esko T, Essioux L, Etain B, Fan CC, Farh KH, Farrell MS, Flickinger M, Foroud TM, Forty L, Frank J, Franke L, Fraser C, Freedman R, Freeman C, Freimer NB, Friedman JI, Fromer M, Frye MA, Fullerton JM, Gade K, Garnham J, Gaspar HA, Gejman PV, Genovese G, Georgieva L, Giambartolomei C, Giannoulatou E, Giegling I, Gill M, Gillman M, Pedersen MG, Giusti-Rodriguez P, Godard S, Goes F, Goldstein JI, Gopal S, Gordon SD, Gordon-Smith K, Gratten J, Gray E, Green EK, Green MJ, Greenwood TA, Grigoroiu-Serbanescu M, Grove J, Guan W, Gurling H, Parra JG, Gwilliam R, de Haan L, Hall J, Hall MH, Hammer C, Hammond N, Hamshere ML, Hansen M, Hansen T, Haroutunian V, Hartmann AM, Hauser J, Hautzinger M, Heilbronner U, Hellenthal G, Henskens FA, Herms S, Hipolito M, Hirschhorn JN, Hoffmann P, Hollegaard MV, Hougaard DM, Huang H, Huckins L, Hultman CM, Hunt SE, Ikeda M, Iwata N, Iyegbe C, Jablensky AV, Jamain S, Jankowski J, Jayakumar A, Joa I, Jones I, Jones LA, Jonsson EG, Julia A, Jureus A, Kahler AK, Kahn RS, Kalaydjieva L, Kandaswamy R, Karachanak-Yankova S, Karjalainen J, Karlsson R, Kavanagh D, Keller MC, Kelly BJ, Kelsoe J, Kennedy JL, Khrunin A, Kim Y, Kirov G, Kittel-Schneider S, Klovins J, Knight J, Knott SV, Knowles JA, Kogevinas M, Konte B, Kravariti E, Kucinskas V, Kucinskiene ZA, Kupka R, Kuzelova-Ptackova H, Landen M, Langford C, Laurent C, Lawrence J, Lawrie S, Lawson WB, Leber M, Leboyer M, Lee PH, Keong JLC, Legge SE, Lencz T, Lerer B, Levinson DF, Levy SE, Lewis CM, Li JZ, Li M, Li QS, Li T, Liang KY, Liddle J, Lieberman J, Limborska S, Lin K, Linszen DH, Lissowska J, Liu C, Liu J, Lonnqvist J, Loughland CM, Lubinski J, Lucae S, Macek M, MacIntyre DJ, Magnusson PKE, Maher BS, Mahon PB, Maier W, Malhotra AK, Mallet J, Malt UF, Markus HS, Marsal S, Martin NG, Mata I, Mathew CG, Mattheisen M, Mattingsdal M, Mayoral F, McCann OT, McCarley RW, McCarroll SA, McCarthy MI, McDonald C, McElroy SL, McGuffin P, McInnis MG, McIntosh AM, McKay JD, McMahon FJ, Medeiros H, Medland SE, Meier S, Meijer CJ, Melegh B, Melle I, Meng F, Mesholam-Gately RI, Metspalu A, Michie PT, Milani L, Milanova V, Mitchell PB, Mokrab Y, Montgomery GW, Moran JL, Morken G, Morris DW, Mors O, Mortensen PB, Mowry BJ, Mühleisen TW, Müller-Myhsok B, Murphy KC, Murray RM, Myers RM, Myin-Germeys I, Neale BM, Nelis M, Nenadic I, Nertney DA, Nestadt G, Nicodemus KK, Nievergelt CM, Nikitina-Zake L, Nimgaonkar V, Nisenbaum L, Nordentoft M, Nordin A, Nöthen MM, Nwulia EA, O’Callaghan E, O’Donovan C, O’Dushlaine C, O’Neill FA, Oedegaard KJ, Oh SY, Olincy A, Olsen L, Oruc L, Van Os J, Owen MJ, Paciga SA, Palmer CNA, Palotie A, Pantelis C, Papadimitriou GN, Parkhomenko E, Pato C, Pato MT, Paunio T, Pearson R, Perkins DO, Perlis RH, Perry A, Pers TH, Petryshen TL, Pfennig A, Picchioni M, Pietilainen O, Pimm J, Pirinen M, Plomin R, Pocklington AJ, Posthuma D, Potash JB, Potter SC, Powell J, Price A, Pulver AE, Purcell SM, Quested D, Ramos-Quiroga JA, Rasmussen HB, Rautanen A, Ravindrarajah R, Regeer EJ, Reichenberg A, Reif A, Reimers MA, Ribases M, Rice JP, Richards AL, Ricketts M, Riley BP, Rivas F, Rivera M, Roffman JL, Rouleau GA, Roussos P, Rujescu D, Salomaa V, Sanchez-Mora C, Sanders AR, Sawcer SJ, Schall U, Schatzberg AF, Scheftner WA, Schofield PR, Schork NJ, Schwab SG, Scolnick EM, Scott LJ, Scott RJ, Seidman LJ, Serretti A, Sham PC, Weickert CS, Shehktman T, Shi J, Shilling PD, Sigurdsson E, Silverman JM, Sim K, Slaney C, Slominsky P, Smeland OB, Smoller JW, So HC, Sobell JL, Soderman E, Hansen CS, Spencer CCA, Spijker AT, St Clair D, Stefansson H, Stefansson K, Steinberg S, Stogmann E, Stordal E, Strange A, Straub RE, Strauss JS, Streit F, Strengman E, Strohmaier J, Stroup TS, Su Z, Subramaniam M, Suvisaari J, Svrakic DM, Szatkiewicz JP, Szelinger S, Tashakkori-Ghanbaria A, Thirumalai S, Thompson RC, Thorgeirsson TE, Toncheva D, Tooney PA, Tosato S, Toulopoulou T, Trembath RC, Treutlein J, Trubetskoy V, Turecki G, Vaaler AE, Vedder H, Vieta E, Vincent J, Visscher PM, Viswanathan AC, Vukcevic D, Waddington J, Waller M, Walsh D, Walshe M, Walters JTR, Wang D, Wang Q, Wang W, Wang Y, Watson SJ, Webb BT, Weickert TW, Weinberger DR, Weisbrod M, Weiser M, Werge T, Weston P, Whittaker P, Widaa S, Wiersma D, Wildenauer DB, Williams NM, Williams S, Witt SH, Wolen AR, Wong EHM, Wood NW, Wormley BK, Wu JQ, Xi S, Xu W, Young AH, Zai CC, Zandi P, Zhang P, Zheng X, Zimprich F, Zollner S, Corvin A, Fanous AH, Cichon S, Rietschel M, Gershon ES, Schulze TG, Cuellar-Barboza AB, Forstner AJ, Holmans PA, Nurnberger JI, Andreassen OA, Lee SH, O’Donovan MC, Sullivan PF, Ophoff RA, Wray NR, Sklar P, Kendler KS. Genomic Dissection of bipolar disorder and schizophrenia, including 28 subphenotypes. Cell. 2018;173:1705–1715. doi: 10.1016/j.cell.2018.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariaslan A, Fazel S, D’Onofrio BM, Långström N, Larsson H, Bergen SE, Kuja-Halkola R, Lichtenstein P. Schizophrenia and subsequent neighborhood deprivation: revisiting the social drift hypothesis using population, twin and molecular genetic data. Translational Psychiatry. 2016;6:e796. doi: 10.1038/tp.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzam S, Ritchie SJ, Pingault J-B, Reynolds CA, O’Reilly PF, Plomin R. Comparing Within- and between-family polygenic score prediction. American Journal of Human Genetics. 2019;105:351–363. doi: 10.1016/j.ajhg.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafee R, Nanda P, Padmanabhan JL, Tandon N, Alliey-Rodriguez N, Kalapurakkel S, Weiner DJ, Gur RE, Keefe RSE, Hill SK, Bishop JR, Clementz BA, Tamminga CA, Gershon ES, Pearlson GD, Keshavan MS, Sweeney JA, McCarroll SA, Robinson EB. Polygenic risk for schizophrenia and measured domains of cognition in individuals with psychosis and controls. Translational Psychiatry. 2018;8:78. doi: 10.1038/s41398-018-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP. Leveraging the biology of adversity to address the roots of disparities in health and development. PNAS. 2012;109:17302–17307. doi: 10.1073/pnas.1121259109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearman C. General Intelligence. Objectively Determined and Measured. The American Journal of Psychology. 1904;15:201–292. doi: 10.2307/1412107. [DOI] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RL, Cooper SR, Jackson JJ, Barch DM. Assessment of neighborhood poverty, cognitive function, and prefrontal and hippocampal volumes in children. JAMA Network Open. 2020;3:e2023774. doi: 10.1001/jamanetworkopen.2020.23774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WK, Barch DM, Bjork JM, Gonzalez R, Nagel BJ, Nixon SJ, Luciana M. The structure of cognition in 9 and 10 year-old children and associations with problem behaviors: Findings from the ABCD study’s baseline neurocognitive battery. Developmental Cognitive Neuroscience. 2019;36:100606. doi: 10.1016/j.dcn.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Associations of family income with cognition and brain structure in USA children: prevention implications. Molecular Psychiatry. 2021;26:6619–6629. doi: 10.1038/s41380-021-01130-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooley UA, Mackey AP, Ciric R, Ruparel K, Moore TM, Gur RC, Gur RE, Satterthwaite TD, Bassett DS. Associations between Neighborhood SES and Functional Brain Netwobetween neighborhrk Development. Cerebral Cortex. 2020;30:1–19. doi: 10.1093/cercor/bhz066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooley UA, Bassett DS, Mackey AP. Environmental influences on the pace of brain development. Nature Reviews. Neuroscience. 2021;22:372–384. doi: 10.1038/s41583-021-00457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Steen Y, Myin-Germeys I, van Nierop M, Ten Have M, de Graaf R, van Dorsselaer S, van Os J, van Winkel R. “False-positive” self-reported psychotic experiences in the general population: an investigation of outcome, predictive factors and clinical relevance. Epidemiology and Psychiatric Sciences. 2019;28:532–543. doi: 10.1017/S2045796018000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for A psychosis proneness-persistence-impairment model of psychotic disorder. Psychological Medicine. 2009;39:179–195. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- van Os J, Reininghaus U. Psychosis as a transdiagnostic and extended phenotype in the general population. World Psychiatry. 2016;15:118–124. doi: 10.1002/wps.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorstman JAS, Breetvelt EJ, Duijff SN, Eliez S, Schneider M, Jalbrzikowski M, Armando M, Vicari S, Shashi V, Hooper SR, Chow EWC, Fung WLA, Butcher NJ, Young DA, McDonald-McGinn DM, Vogels A, van Amelsvoort T, Gothelf D, Weinberger R, Weizman A, Klaassen PWJ, Koops S, Kates WR, Antshel KM, Simon TJ, Ousley OY, Swillen A, Gur RE, Bearden CE, Kahn RS, Bassett AS, International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome Cognitive decline preceding the onset of psychosis in patients with 22q11.2 deletion syndrome. JAMA Psychiatry. 2015;72:377–385. doi: 10.1001/jamapsychiatry.2014.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SP, Chang SM, Wright AS, Pinto R, Heckman JJ, Grantham-McGregor SM. Cognitive, psychosocial, and behaviour gains at age 31 years from the Jamaica early childhood stimulation trial. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2022;63:626–635. doi: 10.1111/jcpp.13499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DG, Conger RD, Robins RW, Hastings PD, Guyer AE. Income change alters default mode network connectivity for adolescents in poverty. Developmental Cognitive Neuroscience. 2018;30:93–99. doi: 10.1016/j.dcn.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Skoularidou M, Cuesta-Infante A, Veeramachaneni K. Modeling tabular data using conditional GAN. Proceedings of the 33rdInternational Conference on Neural Information Processing Systems 33rd Conference on Neural Information Processing Systems (NeurIPS 2019; Vancouver. 2019. [Google Scholar]
- Young AI, Benonisdottir S, Przeworski M, Kong A. Deconstructing the sources of genotype-phenotype associations in humans. Science. 2019;365:1396–1400. doi: 10.1126/science.aax3710. [DOI] [PMC free article] [PubMed] [Google Scholar]