Abstract
The E6 and E7 genes of human papillomaviruses (HPVs) associated with anogenital cancers are largely responsible for the oncogenic activity of these viruses, and regulation of these genes has been intensively studied. Transcription of the E6 and E7 genes is controlled by the viral upstream regulatory region (URR). We have used in vivo footprinting to examine the occupancy by regulatory factors of the HPV type 18 (HPV18) URR enhancer and promoter in the cervical carcinoma cell lines HeLa and C4-II. While corroborating occupancy in vivo of all of the elements previously implicated in the transcriptional control of the HPV18 E6 and E7 genes by in vitro DNase I footprinting, gel retardation assays, and transfection studies, we also detect occupancy in vivo of several enhancer and promoter sequences which have not been previously identified as HPV18 URR regulatory elements. Our data suggest that the HPV18 enhancer and promoter are more densely occupied by DNA-binding proteins than previously thought and raise the possibility that additional, possibly novel factors contribute to transcription of the HPV18 early genes.
Most cervical cancers develop subsequent to infection with human papillomavirus types 16 and 18 (HPV16 and -18), or less commonly other high-risk HPV types, and contain transcriptionally active copies of the viral DNA (56). In all HPV18-associated cervical carcinomas so far examined, and in most but not all HPV16-linked malignancies, viral DNA is integrated into the host genome (5, 11, 45). Viral sequences which encode the E6 and E7 proteins and an 800-bp viral upstream regulatory region (URR) which controls transcription of these viral genes are invariably intact in the integrated viral genome, although the integration event usually interrupts sequences necessary for expression of the E2 protein, the only viral product thought to regulate HPV transcription (13, 42, 44). The E6 and E7 genes encode proteins which interact with and inactivate the tumor suppressors p53 and Rb, respectively (19, 41), and expression of these viral proteins is thought to be crucial to the initiation and progression of cervical tumors (27, 28, 33, 52, 54).
Recognition of the key role of these viral proteins in cervical cancer has stimulated intense efforts to understand how E6/E7 gene expression is regulated in cervical cells. Although posttranscriptional events are clearly important (26), the rate of viral transcription controlled by the URR is a major determinant of E6 and E7 levels. Considerable effort has therefore been made to delineate which HPV16 and HPV18 URR sequences are important for transcriptional regulation of the E6 and E7 oncogenes and to identify the cellular factors which interact with these sequence elements. Attention has largely been focused on the 400 to 500 bp of sequence which are immediately upstream of the E6/E7 transcription start site, since transfection studies have indicated that deleting the remaining sequences of the 800-bp URR diminishes transcription only slightly (20, 47). Transfection studies have further revealed that a region 230 bp long in HPV18 and 400 bp long in HPV16, termed the constitutive enhancer, is critical for efficient transcription, is preferentially active in epithelial cells, and will function with heterologous promoters (10, 16, 46). In both HPV16 and HPV18, DNase I footprinting studies reveal the constitutive enhancer to be densely occupied by cellular regulatory factors, and gel retardation assays suggest that many of these are ubiquitous factors which regulate a wide variety of cellular genes (7, 22, 51). The remaining URR sequences downstream of the enhancer constitute the proximal promoter, which contains binding sites for Sp1 and other cellular factors, e.g., YY1, but which by itself drives only very low levels of viral transcription (21, 47). With a few notable exceptions, many of the same cellular factors appear to interact with both the HPV18 and HPV16 enhancer and promoter; however, the cognate sites for these factors are arranged quite differently in the regulatory regions of the two viruses.
Although invaluable tools in the analysis of regulatory regions, in vitro DNase I footprinting and gel retardation assays utilize naked DNA fragments and cellular extracts and therefore may detect DNA-protein interactions which are not physiologically relevant or may fail to detect all interactions which are occurring in vivo. Complementation of in vitro binding assays with in vivo footprinting studies is therefore becoming increasingly common in promoter analysis. Such studies allow one to detect occupancy of regulatory regions in the unperturbed cell: chromatin structure is intact, the localization and availability of DNA-binding proteins are not perturbed, and the interactions of these proteins with other protein partners are not disrupted by extract preparation (15, 23, 34, 50).
While data from several independent studies agree as to the identity of certain of the factors which recognize sites in the HPV16 and HPV18 URRs, e.g., AP1 and Oct-1 (7, 20, 36, 49), and the functional importance of certain sites, there is less of a consensus regarding other sites, e.g., putative NF1 sites (6, 8). To verify that all sequence elements so far identified by in vitro binding assays and transfection studies in HPV18 are occupied in vivo and to look for additional sites of factor interaction in the HPV18 enhancer and promoter which may have escaped detection, we have performed in vivo footprinting in the cervical carcinoma cell lines HeLa and C4-II, both of which contain integrated, transcriptionally active copies of HPV18. While corroborating occupancy in vivo of most of the elements previously implicated in the transcriptional control of the HPV18 E6 and E7 genes, our results also reveal occupancy in vivo of several enhancer and promoter sequences which have not been previously identified as HPV18 URR regulatory elements. Our data thus suggest that the enhancer and promoter regions of HPV18 are more densely occupied by DNA-binding proteins than previously thought and raise the possibility that additional, possibly novel factors contribute to transcription of the HPV18 early genes.
MATERIALS AND METHODS
Amplification and sequencing of the HPV18 enhancer and promoter.
Genomic DNA (100 ng) from HeLa and C4-II cells was amplified with primers 18E6 (5′-AGGGTCGCCGTGTTGGATCCT-3′; nucleotides [nt] 138 to 118), and primer 1 of bottom-strand footprinting set I (nt 7363 to 7386; sequence listed below). PCR conditions were 40 cycles of 94°C for 1 min, 56°C for 1 min, and 72°C for 1 min. Amplimers (632 bp) were isolated from a 0.9% NuSieve GTG agarose gel (FMC) by melting, phenol extraction, and ethanol precipitation and were sequenced by using an Amplicycle sequencing kit (Perkin-Elmer). Sequencing primers (18E6, primer 1 of bottom-strand footprinting set I, and primer 2 of bottom strand footprinting set III) were end labeled with [γ-33P]ATP.
In vivo footprinting.
Subconfluent HeLa and C4-II cells, growing on 15-cm-diameter dishes in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum, were exposed to dimethyl sulfate (DMS) for 2 min at room temperature as follows: spent medium was removed, DMS was suspended in 10 ml of this medium to 0.2% final concentration by vortexing, and the mixture was added back to the cells. The reaction was stopped by aspiration of the DMS-containing medium and immediate addition of ice-cold phosphate-buffered saline. Cells were scraped from the plates and lysed to isolate nuclei. Genomic DNA was isolated from the lysed nuclei (4), digested with HindIII, cleaved with piperidine, and used for footprinting. Footprinting was also carried out on DNA which was isolated from C4-II and HeLa cells, purified free of protein (naked DNA), cleaved with HindIII, and treated with DMS in vitro (40 μg of naked DNA was exposed to 0.25% DMS for 1 min). Footprinting was carried out on multiple batches of cells and naked DNA independently exposed to DMS to ensure reproducibility of results.
Footprinting analyses were performed by using a ligation-mediated PCR method (32). Footprinting of the top and bottom strands of the HPV18 enhancer and promoter was performed with eight sets of primers, three primers per set. For footprinting of the bottom strand, the primers in set I were primer 1 (5′-GCTTGTTGGGCTATATATTGTCCT-3′), primer 2 (5′-CTGCACACCTTACAGCATCCATTTTATCCTACA-3′), and primer 3 (5′-CTGCACACCTTACAGCATCCATTTTATCCTACAATCCTC-3′). The primers in set II were primer 1 (5′-ATACAGTACGCTGGCACTATTG-3′), primer 2 (5′-GGGCACTGCTCCTACATATTTTGAACCATTG-3′), and primer 3 (5′-GGGCACTGCTCCTACATATTTTGAACCATTGGCG-3′). The primers in set III were primer 1 (5′-CTACATATTTTGAACCATTGGCG-3′), primer 2 (5′-ACCTGGTATTAGTCATTTTCCTGTCCAGG-3′), and primer 3 (5′-ACCTGGTATTAGTCATTTTCCTGTCCAGGTGCG-3′). The primers in set IV were primer 1 (5′-TCCCTATGTAATAAAACTGCTTTTAGG-3′), primer 2 (5′-GCTAATTGCATACTTGGCTTGTACAACTACTTTCAT-3′), and primer 3 (5′-GCTAATTGCATACTTGGCTTGTACAACTACTTTCATGTCC-3′. For footprinting of the top strand, the primers in set I were primer 1 (5′-GCCTAAAAGCAGTTTTATTACATAGGG-3′), primer 2 (5′-GGGAGTGGATATAGTTATGCAAGCAATTGTTGT), and primer 3 (5′-GGGAGTGGATATAGTTATGCAAGCAATTGTTGTAGC-3′). The primers in set II were primer 1 (5′-CTTAGTCATATTATAGTTCATGTTAAGG-3′), primer 2 (5′-GACAGAATGTTGGACATGAAAGTAGTTGTACAA), and primer 3 (5′-GACAGAATGTTGGACATGAAAGTAGTTGTACAAGCC-3′). The primers in set III were primer 1 (5′-GAAAAGTATAGTATGTGCTGCC-3′), primer 2 (5′-CCCAACCTATTTCGGTTGCATAAACTATGTAT-3′), and primer 3 (5′-CCCAACCTATTTCGGTTGCATAAACTATGTAT-3′). The primers in set IV were primer 1 (5′-AAGTGTTCAGTTCCGTGCACA-3′), primer 2 (5′-GTGTTGGATCCTCAAAGCGCGCCA-3′), and primer 3 (5′-CCTCAAAGCGCGCCATGGTATTGTGGTGTG-3′). The positions of the 3′ ends of the eight primers 3 used for footprinting are indicated by arrowheads in Fig. 1.
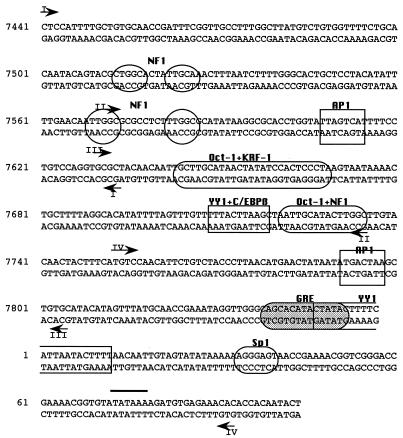
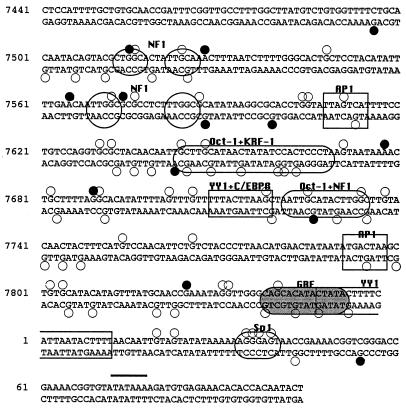
FIG. 1.
Sequence of the HPV18 enhancer (nt 7510 to 7740) and promoter (nt 7741 to 105), showing proposed binding sites for transcription factors, as suggested from previous studies. The arrowheads denote the 3′ ends of primer 3 from each primer set; DMS reactivity patterns on the opposite strand can be visualized starting approximately 30 nt downstream of the 3′ end of primer 3.
Primer 1 was annealed to piperidine-treated DNA and extended with Sequenase, primer 2 was used in conjunction with a linker primer for amplification, and primer 3 was phosphorylated and used to label the amplified fragments. Amplification conditions were 19 cycles of 1 min at 94°C, 2 min of annealing at a temperature which varied with each primer 2, and 3 min at 76°C. To label the fragments, we performed one cycle of 2 min at 94°C, 2 min of annealing at a temperature which varied with each primer 3, and 10 min at 76°C. Labeled fragments were electrophoresed on 6% polyacrylamide–8 M urea sequencing gels, which were dried and exposed to film.
RESULTS
In vivo footprinting of the HPV18 URR enhancer.
C4-II cervical carcinoma cells contain a single integrated and transcriptionally active copy of the HPV18 genome (44), and because all regulatory factor-DNA interactions detected are likely to be relevant to transcription, it was our original intent to use C4-II cells exclusively in this study. However, we obtained much better sensitivity and nearly identical results with HeLa cells, which contain 10 to 50 integrated copies of HPV18 DNA (44), and we therefore show results from both lines interchangeably. We confirmed that the C4-II and HeLa stocks maintained in our laboratory actively transcribe the E6/E7 gene by transcription run-on analysis (data not shown).
Eight sets of primers were used to examine factor occupancy in the enhancer and promoter (Fig. 1). To facilitate the design of these primer sets, as well as the matching of DMS reactivity patterns to sequence, we amplified URR nt 7363 to 138 from each cell line and sequenced these amplimers directly. When compared to the sequence of the prototype HPV18 genome cloned from a cervical biopsy specimen (9 [accession no. X05015]), seven base pair changes were found in the HPV18 DNA integrated in the C4-II cell line and six changes were found in the HPV18 sequences integrated in HeLa cells, with five of these changes common to both cell lines (Table 1). An independent sequencing of the HPV18 DNA cloned from the biopsy specimen (48) and sequencing of HPV18 DNA isolated from HeLa cells by using an enhancer trap strategy (46) revealed very similar sets of changes.
TABLE 1.
Deviations from the depositeda enhancer and promoter sequences in HPV18 sequences in HeLa and C4-II cells
| Position | Nucleotide
|
||
|---|---|---|---|
| GenBank | HeLa | C4-II | |
| 7486 | C | T | C |
| 7529 | C | A | A |
| 7567 | A | C | C |
| 7592 | T | C | C |
| 7670 | A | T | T |
| 7717 | A | A | C |
| 41 | A | A | G |
| 104 | T | C | C |
GenBank accession no. X05015.
To identify sequences in the HPV18 enhancer and promoter to which factors were binding and potentially regulating transcription in vivo, both HeLa and C4-II cells were treated briefly with 0.2% DMS, and the DMS reactivity patterns in DNA isolated from these cells were compared to those of DNA isolated from the same cell lines, purified free of proteins, and then exposed to DMS in vitro. Differences in the susceptibility of guanines to methylation by DMS in DNA exposed in vivo and in vitro are taken to indicate the presence of bound regulatory factors (4). Reactivity to DMS is not perturbed by the association of DNA with histone or nonhistone components of chromatin (30). Adenines are also reactive with DMS, although much less so than guanines (40). We have noticed, however, that adenines which are part of a purine-rich stretch are fairly reactive with DMS. The HPV18 URR enhancer and promoter contain many short A-rich stretches, and these stretches are frequently hyperreactive in DNA from DMS-treated cells, the basis for which is presently unclear. Therefore, in this study, only adenines which are particularly hyperreactive to DMS are considered to mark factor binding sites.
Before a region was designated as a putative site for factor interaction, several criteria had to be met. Changes in methylation sensitivities were not considered significant unless they were consistently seen in multiple footprinting assays which used DNA exposed to DMS in independently performed experiments. Binding of a regulatory factor was generally ascribed only to those regions in which the relative densities of several bands in a set differed between DNA treated in vitro and in vivo. There was often considerable overlap in the sequences which could be visualized with different primer sets, and when this overlap encompassed regions of differences in the reactivity patterns between in vivo- and in vitro-exposed DNA, similar changes in the band patterns had to be seen with both primer sets.
In vivo footprinting of the distal region of the enhancer.
Regulatory proteins which have been proposed to bind to the HPV18 enhancer include the ubiquitous transcription factors NF1 (6, 17), Oct-1 (6, 29), AP1 (14, 49), and YY1 (1), as well as a C/EBP β-YY1 complex (3) and KRF-1 (29), a factor which has not yet been characterized (Fig. 1). We performed in vivo footprinting of the enhancer starting from the distal end. Within the distal portion of the enhancer are two pairs of TTGGC half-sites, one pair at nt 7513 to 7526, where neither half-site is a perfect match to the consensus, and the other at nt 7569 to 7586, where both half-sites are TTGGC. These pairs of half-sites have been proposed to be binding sites for the factor NF1 (17). The sequence TGACTAA, which deviates in only one position from a consensus AP1 site, is found just downstream at nt 7608 to 7614.
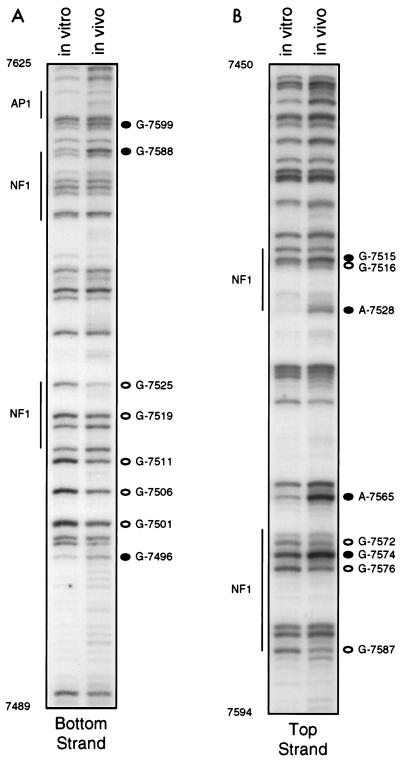
Using bottom primer set I, which allowed us to visualize nt 7490 to 7625, we saw only a few prominent differences in the DMS reactivity patterns of DNA from DMS-treated cells and naked DNA exposed to DMS in vitro (Fig. 2A). However, there were other, less prominent changes in reactivity which were seen with absolute consistency and are likely to signify factor occupancy. Just upstream of the distal pair of sequence-aberrant TTGGC half-sites, G-7496 was hyperreactive to DMS in vivo, while G-7501 and G-7506 were hyporeactive; sequences comprising these guanines have not previously been proposed to constitute a binding site. Within and bordering the TTGGC half-sites, three guanines showed diminished reactivity in vivo: G-7511 and G-7519, which were weakly protected from DMS in vivo, and G-7525, which was strongly protected. These G’s all flank or lie in within sequences corresponding to the distal pair of TTGGC half-sites. Between G-7525 and the end of the region visualized with this primer set, only two other bottom-strand G’s showed clear and reproducible differences in reactivity to DMS. G-7588, just downstream of the proximal pair of TTGGC repeats, was clearly hyperreactive in vivo, while G-7599 showed a less pronounced hyperreactivity. We saw no change in reactivity to DMS of G-7613 in the AP1 site in assays using this set of primers.
FIG. 2.
In vivo footprinting of the distal portion of the HPV18 enhancer (nt 7450 to 7625), using top and bottom primer sets I and DNA from HeLa cells. The left lane in each gel shows the methylation pattern of protein-free DNA exposed to DMS in vitro; the right lane shows the methylation pattern of DNA isolated from DMS-treated cells. Open circles represent G’s or A’s which were hyporeactive to DMS in DNA treated in vivo; closed circles represent G’s or A’s which were hyperreactive to DMS in DNA treated in vivo. Regions corresponding to proposed factor binding sites are denoted by vertical lines.
Footprinting of top-strand sequences (nt 7450 to 7595) of the distal region of the enhancer consistently showed differences in the methylation patterns in sequences comprising both pairs of putative NFI sites (Fig. 2B). Within the proximal pair of TTGGC half-sites, three protected G’s, G-7587, G-7576, and G-7572, and one hypersensitive G, G-7574, were seen in DNA exposed to DMS in vivo, and a marked hypersensitivity at A-7565 was seen just upstream. In the top-strand sequences of the distal pair of imperfect TTGGC half-sites, A-7528 was strikingly hyperreactive to DMS in vivo, G-7515 was also hyperreactive, and G-7516 was hyporeactive. Taken together, our top- and bottom-strand footprinting data clearly suggest that sequences which include the two pairs of TTGGC half-sites are occupied in vivo, although the footprints for the distal and proximal pairs are quite distinct. It is also possible that sequences just upstream of the distal pair of half-sites are occupied by factors, although in this region changes were only seen in bottom-strand sequences.
In vivo footprinting of the central region of the enhancer.
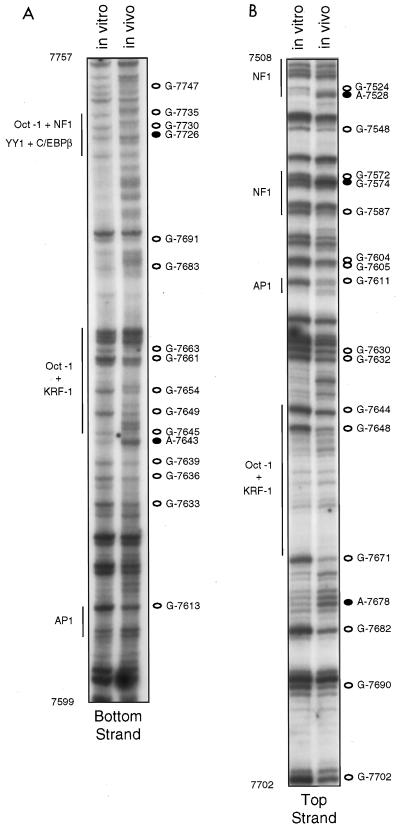
Top-strand primer set II, which was used to footprint the central region of the enhancer, also allowed us to visualize reactivity patterns in the proximal and distal TTGGC repeats, although with less resolution, since these regions were now far from the primers, i.e., near the top of the gel. Nevertheless, this primer set yielded very similar DMS reactivity patterns in top-strand sequences comprising the two pairs of TTGGC repeats (Fig. 3B). With this primer set, top strand reactivity patterns covering sequences between nt 7590 and 7700 could also be visualized, and within these sequences we saw many differences between DNA exposed to DMS in vitro and in vivo (Fig. 3B), suggesting that virtually the entire central portion of the enhancer is occupied by DNA-binding proteins. Clear protection from DMS of G-7611 in the AP1 site was apparent in DNA from DMS-treated cells, and two guanines just upstream (G-7604 and G-7605) were also protected. Just downstream of the AP1 site, we observed protection of G-7630 and G-7632 in a region which does not comprise a known HPV18 enhancer binding site. Sequences between nt 7644 and 7651 match in six of eight positions a consensus Oct-1 site and appear to bind Oct-1 proteins in a nuclear extract (29); immediately downstream and possibly overlapping the Oct-1 site are sequences (nt 7641 to 7675) first identified by DNase I protection studies and subsequently shown to interact with a keratinocyte specific factor, KFR-1 (29). Two G’s within the putative Oct-1 site, G-7644 and G-7648, and G-7671, at the 3′ end of the sequences proposed to interact with KRF-1, were all protected in vivo (Fig. 3B).
FIG. 3.
In vivo footprinting of the central portion of the HPV18 enhancer (nt 7510 to 7760), using top and bottom primer sets II and DNA from C4-II cells. The left lane in each gel shows the methylation pattern of protein-free DNA exposed to DMS in vitro; the right lane shows the methylation pattern of DNA isolated from DMS-treated cells. Open circles represent G’s or A’s which were hyporeactive to DMS in DNA treated in vivo; closed circles represent G’s or A’s which were hyperreactive to DMS in DNA treated in vivo. Regions corresponding to proposed factor binding sites are denoted by vertical lines.
At the proximal end of the central enhancer, additional hyporeactive G’s were seen: G-7682 was clearly protected from DMS in vivo, as were G-7690 and G-7702. Although several A’s upstream of G-7682 were hyperreactive in vivo, A-7678 was especially so. Sequences between nt 7691 and 7700, CACATATTTT, are identical to a site in HPV16 which was previously shown to bind TEF-1 (24), a factor which was initially identified as a simian virus 40 enhancer-binding protein (12). Our data thus show changes in a potential TEF-1 binding site but also indicate occupancy of sequences immediately upstream. In DNase I footprinting assays carried out by Nakshatri et al. (35), nt 7673 to 7704 were protected from DNase I when C33A nuclear extracts were used, although not when extracts of four other cell lines, including HeLa, were used. Two other in vitro footprinting studies failed to detect protection of this region (14, 17).
Footprinting of bottom-strand sequences (nt 7600 to 7760) in the central region of the enhancer with primer set II confirmed that this portion of the enhancer is densely occupied by regulatory factors. Sequences corresponding to the AP1 site were seen at higher resolution with this primer set, and a weak but reproducible protection of G-7613 within this site was seen (Fig. 3A). Downstream of the AP1 site, in the same region where protections of G-7630 and G-7632 were seen with top-strand primer set II, bottom-strand G-7633, G-7636, and G-7639 were weakly protected from DMS in vivo. In bottom-strand sequences comprising the Oct-1 and KRF-1 sites, there were very clear and reproducible differences in the methylation patterns. These include protected G’s at positions 7645, 7649, 7654, 7661, and 7663, as well as a very hypersensitive A at position 7643. Furthermore, in the 75 nt of bottom-strand sequences corresponding to bands in the top third of the gel, numerous and prominent differences in the reactivity patterns between DNA exposed to DMS in vitro and in vivo were apparent (Fig. 3A). G-7691, in the putative TEF-1 site, and G-7683, upstream of this site, were protected. Four adenines between these G’s and three stretches of adenine repeats downstream of G-7693 were clearly hypersensitive to methylation in vivo. Sequences downstream of this A-rich stretch, corresponding to bands near the top of the gel, include a site for a proposed C/EBP β-YY1 complex, termed the switch region (nt 7710 to 7718 [2, 3]) and a putative Oct-1/NF1 binding site (nt 7720 to 7735 [6]). Within these sequences, e.g., at G-7726, G-7730, and G-7735, and in sequences immediately downstream, e.g., G-7747, there were numerous differences in susceptibility to methylation between DNA exposed to DMS in vitro and in vivo (Fig. 3A).
In vivo footprinting of the proximal region of the enhancer.
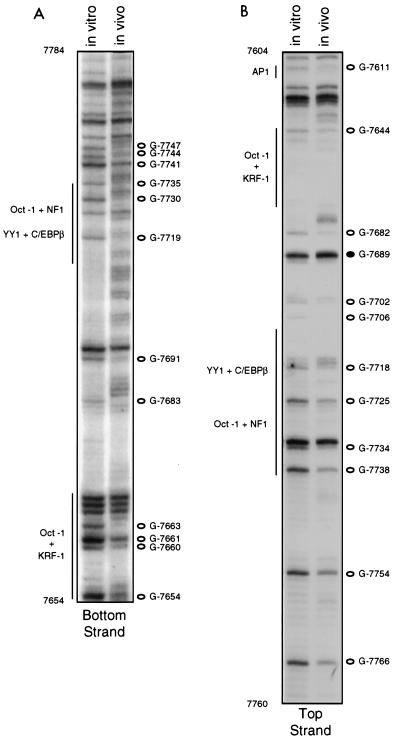
Bands near the top of the gel shown in Fig. 3A were better resolved when footprinting was performed with bottom primer set III. This primer set allowed visualization of DMS reactivity patterns between G-7654 in the KRF-I site and G-7784, 45 nt downstream of the enhancer/promoter boundary. Much of this sequence overlapped with that seen with bottom primer set II, and within the region of overlap, the band patterns for DNA exposed to DMS in vitro and in vivo in assays using these two primer sets were virtually identical (compare Fig. 3A and 4A). The single G in the switch region site, G-7713, was not visible as a band in C4-II cells, for reasons unknown, but was visible and clearly protected from methylation in HeLa cells (data not shown). G-7719, just upstream of the Oct-1 site, showed clear protection in vivo with primer set III, and protections of G-7730, between the Oct-1 and NF1 sites, and G-7735, within the TTGGC half-site, were very obvious as well (Fig. 4A). The marked differences in the bottom-strand methylation patterns in sequences comprising this TTGGC half-site, which borders an Oct-1 site, are in contrast to the minor differences seen for the proximal pair of TTGGC half-sites. One possible explanation for this difference is that binding of Oct-1 to its cognate site enhances occupancy of the adjacent TTGGC site. For an almost identical Oct-1/NF1 motif in HPV16, data obtained from gel shift assays suggested cooperative binding of Oct-1 and NF1 (36). Immediately downstream of the Oct-1/NF1 site, protections of G-7741, G-7744, and G-7747 were also very apparent with this primer set (Fig. 4A).
FIG. 4.
In vivo footprinting of the proximal portion of the HPV18 enhancer (nt 7605 to 7785), using bottom primer set III and DNA from C4-II cells and top primer set III and DNA from HeLa cells. The left lane in each gel shows the methylation pattern of protein-free DNA exposed to DMS in vitro; the right lane shows the methylation pattern of DNA isolated from DMS-treated cells. Open circles represent G’s which were hyporeactive to DMS in DNA treated in vivo; closed circles represent G’s which were hyperreactive to DMS in DNA treated in vivo. Regions corresponding to proposed factor binding sites are denoted by vertical lines.
Using primer set III, we were able to examine top-strand sequences in a region which included the proximal part of the enhancer and the distal part of the promoter. Near the top of the gel, where there was overlap between the sequences visualized with top-strand primer sets II and III, changes in the DMS reactivity patterns were very similar to those seen in Fig. 3 (compare Fig. 3B and 4B). With this primer set, we also detected protection from DMS in vivo of G-7706, just downstream of the putative TEF-1 binding site, as well as protections of G-7718 in the switch region, G-7725 and G-7734 in the Oct-1/NF1 site, and G-7738, G-7754, and G-7766 in the distal end of the promoter (Fig. 4B). The data obtained with top and bottom primer sets III strengthen the notion that enhancer sequences between the AP1 site at 7610 and the enhancer-promoter boundary at nt 7740 are almost fully occupied by factors. Prominent changes in DMS reactivity patterns were also seen in both the top and bottom strands of sequences just downstream of the enhancer-promoter boundary, a region not previously proposed to be a factor binding site.
In vivo footprinting of the promoter.
The HPV18 promoter, which encompasses nt 7738 to 105, contains an AP1 site at its distal end, an Sp1 site at its proximal end, and, between these two sites, a putative glucocorticoid response element (GRE) and a YY1 binding site (Fig. 1). The promoter also contains three binding sites for the viral E2 protein, although neither HeLa nor C4-II cells are thought to express E2, due to disruption of its coding region during integration of the viral DNA. Primer sets III allowed us to examine the distal 30 to 40 bp of the promoter (Fig. 4), and we used primer sets IV to inspect the DMS reactivity patterns of the rest of the promoter.
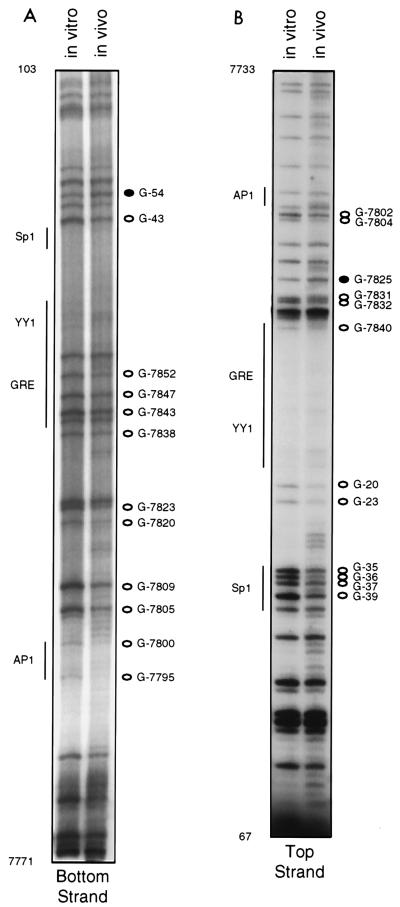
Using bottom primer set IV, we were able to visualize bottom-strand sequences starting at nt 7770 (just upstream of the promoter AP1 site) and extending well into the coding region (Fig. 5A). Because in approximately one-half of the viral copies in HeLa cells, integration has disrupted the URR between the enhancer-promoter junction and the TATA box (42, 46), the bottom strand of the promoter could be footprinted in its entirety only in C4-II cells. Several G’s hyporeactive to DMS in vivo were seen in the lower half of the gel: these included G-7795 and G-7800, within and flanking the AP1 site, G-7805 and G-7809, downstream of the AP1 site, and G-7820 and G-7823, within and flanking the E2 binding site at nt 7822 to 7833 (Fig. 5A). We note with interest that in a previously reported DNase I footprinting study of the HPV18 promoter, protection of two regions, nt 7781 to 7803 and 7809 to 7827, was observed (14). Just downstream of the E2 site are sequences (nt 7839 to 7853) which comprise a proposed GRE and which have been shown to mediate weak hormonal responsiveness (6, 31). Sequences between nt 7841 and 7850 also match the consensus for a TEF-1 binding site, 5′-N(G/A)CAT(T/A)(T/C)(T/C)(T/A)-3′, however (25). Within and flanking these sequences we saw four weakly but consistently protected G’s at positions 7838, 7843, 7847, and 7852 (Fig. 5A). Immediately downstream of these sequences is a stretch of 40 nt, encompassing both the YY1 and Sp1 sites, which is very guanine poor. Bands corresponding to G-8, which lies within the proposed YY1 site, and G-15 were always extremely faint and are barely visible in Fig. 5. However, we have seen protection of G-8 in vivo in several other independently performed footprinting assays of this region. Abutting the 3′ end of the Sp1 site (nt 35 to 40) is a pair of closely spaced E2 binding sites, nt 42 to 53 and 57 to 68, and we consistently observed hyporeactivity in vivo of G-43, just 3′ to the Sp1 site, and hyperreactivity of G-54, which lies between the two E2 sites. Viral sequences encoding the E2 protein are not intact in C4-II cells (42); therefore, if the changes in DMS reactivity seen at this pair of E2 binding sites as well as at nt 7822 to 7833 signify factor binding, such a factor must be of cellular origin.
FIG. 5.
In vivo footprinting of the HPV18 promoter (nt 7730 to 105), using top and bottom primer sets IV and DNA from C4-II cells. The left lane in each gel shows the methylation pattern of protein-free DNA exposed to DMS in vitro; the right lane shows the methylation pattern of DNA isolated from DMS-treated cells. Open circles represent G’s which were hyporeactive to DMS in DNA treated in vivo; closed circles represent G’s which were hyperreactive to DMS in DNA treated in vivo. Regions corresponding to proposed factor binding sites are denoted by vertical lines.
Footprinting performed with top-strand primer set IV allowed us to examine all but the two most proximal top strand G’s in these same E2 sites, and these guanines showed equivalent reactivity to DMS in vivo and in vitro (Fig. 5B). Immediately upstream of the pair of E2 sites, clear protections in vivo of all four G’s (at positions 35, 36, 37, and 39) in a putative Sp1 site were evident. These sequences have been shown by gel retardation assays to bind Sp1 and to be required for HPV18 URR promoter activity (21). G-20 and G-23 were also protected; these G’s are just 5′ to an upstream TATA consensus element which in the HPV18 promoter does not mediate transcription initiation but rather comprises part of the origin of replication in the intact viral genome (38). A cluster of bands whose relative densities consistently differed in the in vitro and in vivo lanes corresponded to nt 7825 to 7840 (Fig. 5B). G-7840, although faint in the lane representing DNA exposed to DMS in vitro, was fainter in the in vivo lane, G-7831 and G-7832 were weakly protected in vivo, and G-7825 was hyperreactive in vivo (Fig. 5B). Considered along with the altered reactivities seen in bottom-strand G’s in this region (Fig. 5A), the altered reactivities of these top-strand G’s further support the notion that a factor or factors constitutively occupy sequences between nt 7830 and 7850. The decreased reactivities of G-7802 and G-7804 seen in vivo (Fig. 5B) may be due to in vivo occupancy of the AP1 site at nt 7792 to 7798; however, changes in reactivities of G’s at positions 7805 and 7809 in the bottom strand were also seen (Fig. 5A), and nt 7807 to 7815 deviate in only one position from a TEF-1 consensus site. We did not detect changes in reactivities of G’s in the top strand of the AP1 site, similar to our failure to detect changes in the bottom strand of the distal AP1 site. Except for G’s in the Sp1 site, most of the guanines in the promoter showed only small differences in reactivity to DMS in vitro and in vivo. Nonetheless, our data raise the possibility that in the promoter also, there are heretofore unrecognized binding sites for regulatory factors.
DISCUSSION
Previous work using in vitro binding assays and/or transfection studies have identified multiple cis-acting elements in the HPV18 URR enhancer and promoter. In vivo footprinting analyses performed in this study confirm that all of these elements are occupied in vivo in both HeLa and C4-II cells. Importantly, these in vivo analyses have also identified several additional candidate factor binding sites; in both HeLa and C4-II cells, much of the proximal 300 bp of the URR exhibits changes in DMS reactivity in vivo suggestive of factor occupancy (Fig. 6).
FIG. 6.
Summary of in vivo footprinting results. Guanines which were hyporeactive to DMS in DNA isolated from DMS-treated cells are indicated by open circles; guanines which were hyperreactive to DMS in DNA isolated from DMS-treated cells are indicated by closed circles. Several adenines which were particularly hyperreactive to DMS are also indicated by closed circles.
One of the candidate sites identified in this study, nt 7675 to 7706, includes 10 bp which are a perfect match to a TEF-1 binding site previously identified in the HPV16 enhancer (24). Importantly, a BLAST search using nt 7675 to 7706 as a query revealed very close matches in 17 other HPV types, including many which are oncogenic for anogenital tissue, e.g., HPV16, -31, -33, -35, and -39. When used in an electrophoretic mobility shift assay, an oligonucleotide corresponding to nt 7675 to 7706 forms a sequence-specific complex with proteins in whole-cell extracts of HeLa, C4-II, and C33A (HPV-negative cervical carcinoma) cells (43). Studies to characterize the factors which interact with this region and to assess its contribution to HPV18 URR-driven transcription are in progress.
Interestingly, changes in DMS reactivity patterns were seen at two other candidate TEF-1 binding sites in the promoter. One of these regions, nt 7639 to 7653, has also been identified as a putative GRE (6, 31). Mutation of this site, while abolishing glucocorticoid responsiveness of a transfected URR reporter construct, also increased its basal expression (6). Such an effect would be consistent with our footprinting data, which suggest basal occupancy of these sequences in vivo. It will be interesting to determine whether in C4-II cells, which exhibit increased HPV18 transcription after treatment with glucocorticoids (53), concomitant changes in DMS reactivity patterns encompassing nt 7639 to 7653 are seen. The other candidate TEF-1 binding site, nt 7807 to 7815, located just downstream of the promoter AP1 site, has not been previously identified as a regulatory element. While we are very interested in the possibility that TEF-1 interacts with multiple sites in the HPV18 URR, the highly degenerate nature of the TEF-1 binding site mandates caution in predicting that a particular nucleotide sequence will bind TEF-1 with high affinity.
We hope to verify that one or more factors can recognize other HPV18 URR sequence stretches in which multiple G’s exhibit altered reactivity to DMS in vivo, strongly suggestive of factor occupancy. Because these sequences were not protected in any of three previously performed DNase footprinting assays, we anticipate that demonstrating binding in vitro may require manipulations of the assay conditions or fractionation of the extracts to enrich for the putative binding protein. Establishing an in vitro system in which factors will recognize the site under investigation is a prerequisite for identifying the factor, especially where the site does not resemble known factor binding sites. Furthermore, the ability to determine which bases are critical for binding allows one to more easily assess the consequences on expression of mutating the candidate cis-acting element.
Enhancer sequences previously shown to bind factors in vitro by DNase I footprinting assays and proposed to be a binding site for a novel keratinocyte specific factor, KFR-1 (29), exhibited one of the most pronounced footprints seen in our analyses. Mutation of this site in the context of the 230-bp HPV18 enhancer fragment diminished enhancer activity by 80% in HeLa cells (26) and in primary human keratinocytes (6). Coupled with these previous results, the observation that the KRF-1 binding site is clearly occupied in vivo underscores the importance of characterizing the factor which recognizes it.
One of the more controversial aspects of HPV18 (and HPV16) transcriptional regulation has been the role of TTGGC sites, which are present in multiple copies and which have been proposed to bind NF1 (although its consensus motif is a partial palindrome TTGGCTN3AGCCAA). Only one of three DNase I footprinting studies detected protections of the distal and proximal pairs of TTGGC half-sites as well as the TTGGC at the enhancer-promoter boundary (17). The second study reported a protection at the proximal pair only (35), and the third study did not report protections at any of the TTGGC motifs (14). Binding of NF1 to oligomers corresponding to either pair of half-sites or to the Oct-1/TTGGC motif in the HPV18 enhancer could be demonstrated only when purified NF1 was used (6), probably reflecting the demonstrated low affinity of NF1 for nonpalindromic sites. Within the context of the intact URR, mutating all three NF1 sites so as to abolish binding of NF1 did not substantially reduce transcription; however, in the context of the intact enhancer (nt 7510 to 7739) or a distal enhancer fragment which extended through the AP1 site (nt 7510 to 7625), deleting the distal 73 bp so as to remove both the distal and proximal pairs of TTGGC half-sites substantially reduced enhancer activity (6). To explain this discrepancy, it was suggested that the deleted sequences contained binding sites for additional factors. Although in vivo footprinting provides no insight into the nature of the factor which occupies the five TTGGC or TTGGC-like sites in the URR enhancer, it does suggest that all of them are occupied in vivo. Equally important, our data do not reveal occupancy of sites other than the TTGGC and AP1 motifs within the region from nt 7510 to 7625.
Our data demonstrating in vivo occupancy of the switch region, sequences which have been shown to be necessary for the YY1 site in the promoter to mediate activation rather than repression of transcription, are in accord with those obtained in a previous study (2). In this earlier study, which focused on the switch region, a single set of top- and bottom-strand primers was used to confirm in vivo occupancy of this site in both HeLa and C4-I cells (derived from the same tumor as C4-II cells), but these primers also allowed visualization of DMS reactivity patterns of flanking sequences as well. Consistent with our results, changes in reactivity to DMS were also seen in sequences just upstream of the switch region (nt 7682 to 7706) as well as in sequences downstream of the putative Oct-1/NF1 site (nt 7741 to 7757). However, our data also suggest occupancy of some sequences not detected in the previous analysis, which may reflect our use of lower DMS concentrations or multiple primer sets which allowed us to view the reactivity patterns at higher resolution.
Data generated from the application of in vivo footprinting to HPV18 transcriptional regulation suggest that the number and variety of trans-acting factors which interact with the enhancer and promoter are even greater than was previously thought. Occupancy of the additional sites identified in our footprinting analyses awaits confirmation by in vitro binding assays and mutagenesis studies. We cannot rule out the possibility that causes other than factor occupancy, e.g., distortions of the helix due to binding of factors to adjacent sites, are responsible for some of the changes in DMS reactivity that we observed. That packaging of DNA into chromatin does not affect the reactivity of guanines to DMS has been thoroughly documented, however: both we and others have footprinted genes which are transcriptionally silent and inaccessible to transcription factors and have seen identical DMS reactivity patterns in vitro and in vivo (18, 37).
While the currently accepted model depicts the URR as occupied in large part by well-characterized and commonly utilized transcription factors, regulatory proteins whose features and target genes are unknown or less well characterized may in fact play a major role in controlling transcription of the HPV18 early genes or in regulating other aspects of the viral life cycle. If further studies confirm this hypothesis, the next challenge will be to identify these proteins and elucidate their roles in HPV18 regulation.
ACKNOWLEDGMENT
This work was supported by the Evelyn Dyba Women’s Health Fellowship award to B.P.
REFERENCES
- 1.Bauknecht T, Angel P, Royer H-D, zur Hausen H. Identification of a negative regulatory domain in the human papillomavirus type 18 promoter: interaction with the transcriptional repressor YY1. EMBO J. 1992;11:4607–4617. doi: 10.1002/j.1460-2075.1992.tb05563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauknecht T, Jundt F, Herr I, Oehler T, Delius H, Shi Y, Angel P, zur Hausen H. A switch region determines the cell-type-specific positive or negative action of YY1 on the activity of the human papillomavirus type 18 promoter. J Virol. 1995;69:1–12. doi: 10.1128/jvi.69.1.1-12.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauknecht T, See R H, Shi Y. A novel C/EBP B-YY1 complex controls the cell-type-specific activity of the human papillomavirus type 18 upstream regulatory region. J Virol. 1996;70:7695–7705. doi: 10.1128/jvi.70.11.7695-7705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker P B, Schutz G. Genomic footprinting. In: Setlow J K, editor. Genetic engineering, principles and methods. Vol. 10. New York, N.Y: Plenum Press; 1988. pp. 1–19. [Google Scholar]
- 5.Berumen J, Unger E R, Casas L, Figueroa P. Amplification of human papillomavirus types 16 and 18 in invasive cervical cancer. Hum Pathol. 1995;26:676–681. doi: 10.1016/0046-8177(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 6.Butz K, Hoppe-Seyler F. Transcriptional control of human papillomavirus (HPV) oncogene expression: composition of the HPV type 18 upstream regulatory region. J Virol. 1993;67:6476–6486. doi: 10.1128/jvi.67.11.6476-6486.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong T, Chan W-K, Bernard H-U. Transcriptional activation of human papillomavirus 16 by nuclear factor 1, AP1, steroid receptors and a possibly novel transcription factor, PVF: a model for the composition of genital papillomavirus enhancers. Nucleic Acids Res. 1990;18:465–470. doi: 10.1093/nar/18.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong T, Apt D, Gloss B, Isa M, Bernard H-U. The enhancer of human papillomavirus type 16: binding sites for the ubiquitous transcription factors Oct-1, NFA, TEF-2, NF1, and AP1 participate in epithelial cell-specific transcription. J Virol. 1991;65:5933–5943. doi: 10.1128/jvi.65.11.5933-5943.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole S T, Danos O. Nucleotide sequence and comparative analysis of the human papillomavirus type 18 genome. J Mol Biol. 1987;193:599–608. doi: 10.1016/0022-2836(87)90343-3. [DOI] [PubMed] [Google Scholar]
- 10.Cripe T M, Haugen T H, Turk J P, Tabatabai F, Schmid III P G, Durst M, Gissman L, Roman A, Turek L B. Transcriptional regulation of the human papillomavirus-16 E6-E7 promoter by a keratinocyte-dependent enhancer, and by viral E2 trans-activator and repressor gene products: implications for cervical carcinogenesis. EMBO J. 1987;6:3745–3753. doi: 10.1002/j.1460-2075.1987.tb02709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cullen A P, Reid R, Campion M, Lorincz A T. Analysis of the physical state of different human papillomavirus DNAs in intraepithelial and invasive cervical neoplasm. J Virol. 1991;65:606–612. doi: 10.1128/jvi.65.2.606-612.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson I, Xiao J H, Rosales R, Staub A, Chambron P. The HeLa cell protein TEF-1 binds specifically and cooperatively to two SV40 enhancer motifs of unrelated sequences. Cell. 1988;54:931–942. doi: 10.1016/0092-8674(88)90108-0. [DOI] [PubMed] [Google Scholar]
- 13.El Awady M K, Kaplan J B, O’Brien S J, Burk R D. Molecular analysis of integrated human papillomavirus 16 sequences in the cervical cancer cell line SiHa. Virology. 1987;159:389–398. doi: 10.1016/0042-6822(87)90478-8. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Carranca A, Thierry F, Yaniv M. Interplay of viral and cellular proteins along the long control region of human papillomavirus type 18. J Virol. 1988;62:4321–4330. doi: 10.1128/jvi.62.11.4321-4330.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrity P A, Chen D, Rothenberg E V, Wold B J. Interleukin-2 transcription is regulated in vivo at the level of coordinated binding of both constitutive and regulated factors. Mol Cell Biol. 1994;14:2159–2169. doi: 10.1128/mcb.14.3.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gloss B, Bernard H U, Seedorf K, Klock G. The upstream regulatory region of the human papilloma virus-16 contains an E2 protein-independent enhancer which is specific for cervical carcinoma cells and regulated by glucocorticoid hormones. EMBO J. 1987;6:3735–3743. doi: 10.1002/j.1460-2075.1987.tb02708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gloss B, Yeo-Gloss M, Meisterernst M, Rogge L, Winnacker E L, Bernard H-U. Clusters of nuclear factor I binding sites identify enhancers of several papillomaviruses but alone are not sufficient for enhancer function. Nucleic Acids Res. 1989;17:3519–3533. doi: 10.1093/nar/17.9.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorzowski J J, Eckerley C A, Halgren R G, Mangurten A B, Phillips B. Methylation-associated transcriptional silencing of the MHC-linked hsp70 genes in mouse cell lines. J Biol Chem. 1995;270:26940–26949. doi: 10.1074/jbc.270.45.26940. [DOI] [PubMed] [Google Scholar]
- 19.Heck D V, Yee C L, Howley P M, Munger K. Efficiency of binding the retinoblastoma protein correlates with the transforming capacity of the E7 oncoproteins of the human papillomaviruses. Proc Natl Acad Sci USA. 1992;89:4442–4446. doi: 10.1073/pnas.89.10.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoppe-Seyler F, Butz K, zur Hausen H. Repression of the human papillomavirus type 18 enhancer by the cellular transcription factor Oct-1. J Virol. 1991;65:5613–5618. doi: 10.1128/jvi.65.10.5613-5618.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoppe-Seyler F, Butz K. Activation of human papillomavirus type 18 E6-E7 oncogene expression by transcription factor SpI. Nucleic Acids Res. 1992;20:6701–6706. doi: 10.1093/nar/20.24.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoppe-Seyler F, Butz K. Cellular control of human papillomavirus oncogene transcription. Mol Carcinog. 1994;10:134–141. doi: 10.1002/mc.2940100304. [DOI] [PubMed] [Google Scholar]
- 23.Hornstra I K, Yang T P. In vivo footprinting and genomic sequencing by ligation-mediated PCR. Anal Biochem. 1993;213:179–193. doi: 10.1006/abio.1993.1407. [DOI] [PubMed] [Google Scholar]
- 24.Ishiji T, Lace M J, Parkkinen S, Anderson R D, Haugen T H, Cripe T P, Xiao J H, Davidson I, Chambron P, Turek L P. Transcriptional enhancer factor (TEF)-1 and its cell-specific co-activator activate human papillomavirus-16 E6 and E7 oncogene transcription in keratinocytes and cervical carcinoma cells. EMBO J. 1992;11:2271–2281. doi: 10.1002/j.1460-2075.1992.tb05286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacquemin P, Hwang J J, Martial J A, Dolle P, Davidson I. A novel family of developmentally regulated mammalian transcription factors containing the TEA/ATTS DNA binding domain. J Biol Chem. 1996;271:21775–21785. doi: 10.1074/jbc.271.36.21775. [DOI] [PubMed] [Google Scholar]
- 26.Jeon S, Lambert P F. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: implications for cervical carcinogenesis. Proc Natl Acad Sci USA. 1995;92:1654–1658. doi: 10.1073/pnas.92.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaur P, McDougall J K, Cone R. Immortalization of primary human epithelial cells by cloned cervical carcinoma DNA containing human papillomavirus type 16 E6/E7 open reading frames. J Gen Virol. 1989;70:1261–1266. doi: 10.1099/0022-1317-70-5-1261. [DOI] [PubMed] [Google Scholar]
- 28.Liu Z, Ghai J, Ostrow R S, Faras A J. The expression levels of the human papillomavirus type 16 E7 correlate with its transforming potential. Virology. 1995;207:260–270. doi: 10.1006/viro.1995.1075. [DOI] [PubMed] [Google Scholar]
- 29.Mack, D. H., and L. A. Laimins. A keratinocyte-specific transcription factor, KRF-1, interacts with AP-1 to activate expression of human papillomavirus type 18 in squamous epithelial cells. Proc. Natl. Acad. Sci. USA 88:9102–9106. [DOI] [PMC free article] [PubMed]
- 30.McGhee J D, Felsenfeld G. Reaction of nucleosome DNA with dimethyl sulfate. Proc Natl Acad Sci USA. 1979;76:2133–2137. doi: 10.1073/pnas.76.5.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medina-Martinez O, Morales-Peza N, Yaniv M, Garcia-Carranca A, Thierry F. A single element mediates glucocorticoid hormone response of HPV18 with no functional interactions with AP1 or hbrm. Virology. 1996;217:392–396. doi: 10.1006/viro.1996.0129. [DOI] [PubMed] [Google Scholar]
- 32.Mueller P R, Wold B. In vivo footprinting of a muscle specific enhancer by ligation mediated PCR. Science. 1989;246:780–785. doi: 10.1126/science.2814500. [DOI] [PubMed] [Google Scholar]
- 33.Munger K, Phelps W C, Bubb V, Howley P M, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63:4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy S P, Gorzowski J J, Sarge K D, Phillips B. Characterization of constitutive HSF2 DNA binding activity in mouse embryonal carcinoma cells. Mol Cell Biol. 1994;14:5309–5317. doi: 10.1128/mcb.14.8.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakshatri H, Pater M M, Pater A. Ubiquitous and cell-type-specific protein interactions with human papillomavirus type 16 and type 18 enhancers. Virology. 1990;178:92–103. doi: 10.1016/0042-6822(90)90382-2. [DOI] [PubMed] [Google Scholar]
- 36.O’Connor M, Bernard H-U. Oct-1 activates the epithelial-specific enhancer of human papillomavirus type 16 via a synergistic interaction with NF1 at a conserved composite regulatory element. Virology. 1995;207:77–88. doi: 10.1006/viro.1995.1053. [DOI] [PubMed] [Google Scholar]
- 37.Pfeifer G P, Tanguay R L, Steigerwald S D, Riggs A D. In vivo footprint and methylation analysis by PCR-aided genomic sequencing: comparison of active and inactive X chromosomal DNA at CpG island and promoter of human PGK-1. Genes Dev. 1990;4:1277–1287. doi: 10.1101/gad.4.8.1277. [DOI] [PubMed] [Google Scholar]
- 38.Remm M, Brain R, Jenkins J R. The E2 binding sites determine the efficiency of replication for the origin of human papillomavirus type 18. Nucleic Acids Res. 1992;20:6015–6021. doi: 10.1093/nar/20.22.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romanczuk H, Villa L L, Schlegel R, Howley P M. The viral transcriptional regulatory region upstream of the E6 and E7 genes is a major determinant of the differential immortalization activities of human papillomavirus types 16 and 18. J Virol. 1991;65:2739–2744. doi: 10.1128/jvi.65.5.2739-2744.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saluz H P, Jost J P. Approaches to characterize protein-DNA interactions in vivo. Crit Rev Eukaryotic Gene Expression. 1993;3:1–29. [PubMed] [Google Scholar]
- 41.Scheffner M, Werness B A, Huibregtse J M, Levine A J, Howley P M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 42.Schneider-Gadicke A, Schwarz E. Different human cervical carcinoma cell lines show similar transcription patterns of human papillomavirus type 18 early genes. EMBO J. 1986;5:2285–2292. doi: 10.1002/j.1460-2075.1986.tb04496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schrock, A., and B. Phillips. Unpublished data.
- 44.Schwarz E, Freese U K, Gissmann L, Mayer W, Roggenbuck B, Stremlau A, zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985;314:111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- 45.Stoler M H, Rhodes C R, Whitbeck A, Wolinsky S M, Chow L T, Broker T R. Human papillomavirus type 16 and 18 gene expression in cervical neoplasias. Hum Pathol. 1992;23:117–128. doi: 10.1016/0046-8177(92)90232-r. [DOI] [PubMed] [Google Scholar]
- 46.Swift F V, Bhat K, Younghusband H B, Hamada H. Characterization of a cell type-specific enhancer found in the human papilloma virus type 18 genome. EMBO J. 1987;6:1339–1344. doi: 10.1002/j.1460-2075.1987.tb02373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thierry F, Garcia-Carranca A, Yaniv M. Elements that control the transcription of genital papillomavirus type 18. Cancer Cells. 1987;5:23–32. [Google Scholar]
- 48.Thierry F, Heard J M, Yaniv M. Characterization of a transcriptional promoter of human papillomavirus 18 and modulation of its expression by simian virus 40 and adenovirus early antigens. J Virol. 1987;61:134–142. doi: 10.1128/jvi.61.1.134-142.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thierry F, Spyrou G, Yaniv M, Howley P. Two AP1 sites binding junB are essential for human papillomavirus type 18 transcription in keratinocytes. J Virol. 1992;66:3740–3748. doi: 10.1128/jvi.66.6.3740-3748.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tommasi S, Pfeifer G P. In vivo structure of the human cdc2 promoter: release of a p130-E2F-4 complex from sequences immediately upstream of the transcription initiation site coincides with induction of cdc2 expression. Mol Cell Biol. 1995;15:6901–6913. doi: 10.1128/mcb.15.12.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turek L P. The structure, function, and regulation of papillomavirus genes in infection and cervical cancer. Adv Virus Res. 1994;44:305–356. doi: 10.1016/s0065-3527(08)60332-2. [DOI] [PubMed] [Google Scholar]
- 52.von Knebel Doeberitz M, Oltersdorf T, Schwartz E, Gissmann L. Correlation of modified human papilloma virus early gene expression with altered growth properties in C4-1 cervical carcinoma cells. Cancer Res. 1988;48:3780–3786. [PubMed] [Google Scholar]
- 53.von Knebel Doeberitz M, Bauknecht T, Bartsch D, zur Hausen H. Influence of chromosomal integration on glucocorticoid-regulated transcription of growth-stimulating papillomavirus genes E6 and E7 in cervical carcinoma cells. Proc Natl Acad Sci USA. 1991;88:1411–1415. doi: 10.1073/pnas.88.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.von Knebel Doeberitz M, Rittmuller C, Aengeneyndt F, Jansen-Durr P, Spitkovsky D. Reversible repression of papillomavirus oncogene expression in cervical carcinoma cells: consequences for the phenotype and E6-p53 and E7-pRB interactions. J Virol. 1994;68:2811–2821. doi: 10.1128/jvi.68.5.2811-2821.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao J H, Davidson I, Matthes H, Garnier J M, Chambon P. Cloning, expression, and transcriptional properties of the human enhancer factor TEF-1. Cell. 1991;65:551–568. doi: 10.1016/0092-8674(91)90088-g. [DOI] [PubMed] [Google Scholar]
- 56.zur Hausen H. Human papillomavirus in the pathogenesis of anogenital cancer. Virology. 1991;184:9–13. doi: 10.1016/0042-6822(91)90816-t. [DOI] [PubMed] [Google Scholar]