Abstract
Despite its high prevalence among dementias, Lewy body dementia (LBD) remains poorly understood with a limited, albeit growing, evidence base. The public‐health burden that LBD imposes is worsened by overlapping pathologies, which contribute to misdiagnosis, and lack of treatments. For this report, we gathered and analyzed public‐domain information on advocacy, funding, research outputs, and the therapeutic pipeline to identify gaps in each of these key elements. To further understand the current gaps, we also conducted interviews with leading experts in regulatory/governmental agencies, LBD advocacy, academic research, and biopharmaceutical research, as well as with funding sources. We identified wide gaps across the entire landscape, the most critical being in research. Many of the experts participated in a workshop to discuss the prioritization of research areas with a view to accelerating therapeutic development and improving patient care. This white paper outlines the opportunities for bridging the major LBD gaps and creates the framework for collaboration in that endeavor.
Highlights
A group representing academia, government, industry, and consulting expertise was convened to discuss current progress in Dementia with Lewy Body care and research.
Consideration of expert opinion,natural language processing of the literature as well as publicly available data bases, and Delphi inspired discussion led to a proposed consensus document of priorities for the field.
Keywords: funding sources, Lewy body dementia, natural language processing, research trends
1. INTRODUCTION
Lewy body dementia (LBD) comprises the clinical syndromes of dementia with Lewy bodies (DLB) and Parkinson's disease dementia (PDD), with the application of either of the two syndromic terms based on the timing of the onset of cognitive decline as it relates to the timing of the onset of parkinsonism. 1 , 2 LBD affects approximately 1.4 million individuals in the United States alone. 3 Early symptoms may include cognitive changes, parasomnia, fluctuating attention and alertness, autonomic dysregulation, neuropsychiatric features (e.g., depression and visual hallucinations), and changes in movement. 4 , 5 , 6 Unfortunately, many of these symptoms are not unique to LBD, and this diagnosis may go unrecognized. Furthermore, Alzheimer's disease (AD) co‐pathology occurs in about half of LBD cases at autopsy, 7 which is associated with a later or more subtle emergence of core LBD features. 8 As a result, missed or delayed diagnoses of LBD are common, 9 and patients often require numerous assessments before an accurate diagnosis is made. 10
On average, a diagnosis of DLB occurs 18 months after the onset of symptoms. Indeed, results from the DIAMOND Lewy Study suggest that diagnosis of LBD takes twice as long as AD from referral. 11 According to current criteria for probable DLB, the patient should exhibit dementia and at least two of four core clinical features, or one core feature and one indicative feature comprised of polysomnography evidence of rapid eye movement (REM) sleep behavior disorder, functional imaging of reduced dopamine transporter uptake, or cardiac imaging of postganglionic sympathetic innervation. 1 No fully definitive laboratory tests exist, though recent alpha‐synuclein seeding amplification assays (e.g., real‐time quaking‐induced conversion [RT‐QuIC]) now seem to offer a highly reliable binary yes/no determination of the presence of Lewy body pathology in the brain, in living patients, but does not correspond to symptom severity, and is not yet currently in clinical practice. 12 Furthermore, diagnosis at early or prodromal stages remains difficult given the subtlety of symptoms. To add to the burden, there are no Food and Drug Administration (FDA)‐approved treatments. As a result of misdiagnosis/late diagnosis and the lack of validated, widely available biomarkers outside of the research setting and approved treatments, there is insufficient public awareness, limited patient advocacy, and hence less funding for basic research. So, not surprisingly, very few therapeutic options are available, and there is comparatively little in the Research and Development pipeline. 13 , 14
To gain an understanding of these issues, the Boston Consulting Group (BCG) set up a Lewy Body Dementia Project Team to map the LBD landscape. The work included interviews with thought leaders active in all aspects (researchers, clinicians, pharmaceutical representatives, funding agents, and patient advocates), literature and database analyses, and desk research – all leading to a five‐step framework that identified gaps and potential opportunities. The five areas analyzed were:
Advocacy and awareness
Funding
Research
Pharmaceutical and biotechnology industry activity
Patient impact
The Project Team organized a workshop with LBD and other dementia experts to discuss ways of addressing the critical gaps and to define goals. The workshop highlighted the need for collaboration across advocacy groups, research teams, clinicians, biopharmaceutical companies, and regulatory agencies.
The Project Team's overall finding was that serious gaps exist across the board, creating a vicious cycle that thwarts progress. The gaps in research turn out to be particularly important: resolving them will help to overcome the gaps in all the other stages. One consensus conclusion was that research, such as identifying LBD biomarkers, devising novel diagnostics for early DLB, and elucidating DLB's natural history, would enhance clinical research, facilitate more effective therapeutic trials, likely accelerate development of treatments, and ultimately lead to improved patient care, awareness, and advocacy.
2. METHODS
Preparation for the workshop included interviews, desk research, and natural‐language‐processing analyses of publications and grants. The interviews were conducted with 35 leaders in academia, industry, regulatory/government agencies, and patient advocacy. For the other research, the Project Team searched public sources and databases (e.g., scientific publications foundation websites, clinicaltrials.gov, National Institutes of Health (NIH) RePORTER) to identify major initiatives for LBD and related dementias, to assess funding levels, and to characterize the therapeutic and diagnostic landscape.
To assess the current therapeutic pipeline, the Project Team leveraged search methods described in Lee et al., 15 : the search term “Dementia with Lewy Bodies” was searched on clinicaltrials.gov. Behavioral interventions, cognitive therapies, and diagnostic, biomarker, and imaging studies were excluded, as were trials that did not specify DLB or DLB and PDD as the type of dementia being treated. When not available on clinicaltrials.gov, company websites, news articles, FDA labels, and literature searches were used to identify the mechanism of action, sponsor, and historical trials and approvals. By consulting earlier publications, we were able to compare LBD's research and development pipeline with those for Parkinson's disease (PD) and AD.
To focus on areas related to the identified gaps or proposed solutions, we conducted natural‐language processing both on NIH‐funding public documents and on the current scientific literature (Figures S1‐S2). For analysis of NIH grants, we examined 2200 titles and abstracts available through NIH RePORTER based on the following search criteria:
(NIH Spending Category = (Lewy Body Dementia) OR (NIH Spending Category = ((Alzheimer's Disease including Alzheimer's Disease Related Dementias (AD/ADRD)) OR (Alzheimer's Disease Related Dementias (ADRD) OR (Dementia)) AND Project Title/Abstract = (“Lewy Body” OR “Lewy Bodies”)) OR Project Title = ((“Lewy Body” OR “Lewy Bodies”) AND Dementia) OR Project Abstract = ((“Lewy Body” OR “Lewy Bodies”) AND Dementia)) AND Fiscal Year = (All).
For scientific literature published since 2016, we consulted 2600 articles on LBD. The Web of Science database was used with the following search:
(Title = ((Lewy NEAR/2 (body OR bodies)) NEAR/2 dementia*) OR Topic = ((Lewy NEAR/2 (body OR bodies)) NEAR/2 dementia*)) AND Publication Year > = (2016).
Natural‐language processing and cluster analysis helped in identifying key topics. We used the Quid tool from NetbaseQuid to process the grant and scientific literature data, respectively. The abstracts and titles of the articles/grants were analyzed algorithmically to identify shared terminology and phrases. The articles/grants were then connected according to the semantic similarity of their descriptions and titles and clusters created using the Louvain algorithm. The dynamic visual representation of the outcome enabled the expert team to sense‐check and manually curate the outcome and titles of the clusters to ensure like articles/grants were clustered together.
The workshop took place on January 26, 2023, with about two dozen participants. The first focal discussion reviewed and analyzed the scientific status quo, and addressed three specific questions:
Is the LBD research community focused on topics with highest potential to move the needle for LBD?
Would adjusting the focus lead to faster progress?
Where are opportunities for new ideas, approaches, and collaborations?
The second half of the workshop concentrated on the gaps in diagnostics and aimed to develop a consensus on areas of highest opportunity. Three diagnostic‐focused questions guided the session:
What are the leading approaches to bridging current gaps, and how might they be prioritized?
What are the risks and mitigations?
What is the timeline?
The participants developed a list of potential approaches to address gaps in diagnostics and biomarkers, building on a set of starting ideas suggested by the project team. Then, participants used virtual polling to rank (1) the potential impact, and (2) the likelihood of success in 2–4 years for each approach. This was followed by an in‐depth comparison and discussion. The same poll was performed twice, with discussion between, to support consensus building – a process known as the Delphi method.
3. THE CURRENT AND FUTURE STATE OF LEWY BODY DEMENTIA
3.1. Current outlook of the LBD field: Assessing the major gaps
Despite comparable prevalence to PD without dementia, LBD remains under the radar of the public, federal agencies, and many medical professionals, patients, and families. Resources available for patient care and awareness are varied, ranging from LBD‐focused approaches to more general approaches to dementia. Initiatives for patient care and advocacy are sponsored through the LBD Association (LBDA – initiatives include LBD Support Groups), LBD Research Centers of Excellence), National Institute of Aging (NIA), National Institute of Neurological Disorders and Stroke (NINDS – initiatives include Alzheimer's Disease Education and Referral centers, i.e., ADEAR), and the Administration for Community Living (ACL – initiatives include the Alzheimer's and Dementia Program Center). Public awareness of LBD over the past decade has increased, largely thanks to documentaries (e.g., SPARK, Robin's Wish, LEWY) and campaigns (e.g., #FutureofPD, “Let's talk about dementia”), with first‐hand accounts by patients and caregivers identified as the most powerful tool to expand public awareness. Despite this increased awareness overall, low diagnostic rates and limited awareness hamper substantial advocacy. A further impediment to public understanding and advocacy is the overlap that LBD has with other conditions, notably AD (dementia) and PD (movement disorders).
The increase of early, accurate diagnosis that drives public awareness is crucial to move research forward, identify novel diagnostic and therapeutic targets, and increase funding and interest in the biopharma industry. LBD research has minimal foundational support compared to PD or AD, and fewer government grants. Overall, LBD‐focused research has been receiving less than one‐eighth of the funding per patient that AD and PD receive (Figure S3). The main funder of LBD research in the United States (US) has been the NIH, with most funding supported by the NIA and NINDS. It should be noted that our analysis focused on the US funding through the NIH, there remains a limitation given the reasonably large corpus of research outside of United States (and not funded by NIH).
The main areas receiving LBD funding (nearly half of NIH‐funded projects) have been the molecular mechanisms underlying the disease (Figure 1). A quarter of all funded projects focus on regulation of alpha‐synuclein (the protein responsible for the accumulation of Lewy bodies in the brain) or more broadly on protein degradation/aggregation pathways. Such concentration suggests that other research and development gaps may be relatively underfunded – neuroinflammation/immune‐mediated mechanisms, for example. There are several areas in which LBD funding did increase rapidly during our analysis period of 2016–2021 – for instance, cerebrospinal fluid (CSF) biomarker analysis; RT‐QuIC‐based detection; and caregiving, rehabilitation, and telehealth in dementia. Such expansion could help to improve diagnostic and therapeutic strategies and ultimately improve the patient experience.
FIGURE 1.
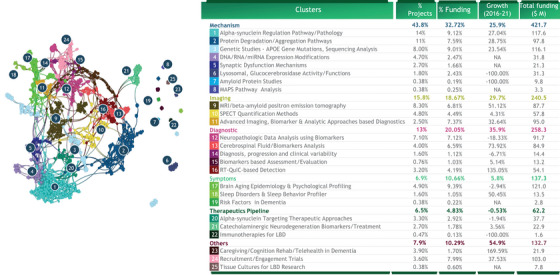
Cumulative funding from NIH and foundations. The semantic network of ∼1k projects funded by NIH in the field of “Lewy Body Dementia.” The proximity of clusters indicates technology affinity/overlapping. ∼30 projects could not be clustered. Funding amounts are based on 2.2k projects related to ”Lewy Body Dementia" funded by NIH Growth measured as CATR. Dot size is associated with the amount of funding associated with each grant. Source: NIH, BCG analysis BCG Center for Growth & Innovation Analytics. BCG, Boston Consulting Group; NIH, National Institutes of Health
The Project Team interviews investigated other gaps and areas of opportunity in the research step of the framework. Regarding human capital and the LBD workforce, several of the interviewees reported a relatively small, close‐knit community of senior researchers in the field of LBD, with the researchers themselves anxious about the lack of support for new early‐career researchers to enter the field. This gap could readily be addressed via funding, collaboration, and data‐sharing, thereby attracting new recruits who would inject new perspectives and enrich the workforce infrastructure in LBD.
To investigate knowledge capital, the Project Team examined LBD‐, AD‐, and PD‐related reports published between 2011 and 2020. LBD can claim only a very small fraction of publications compared to AD and PD, and the annual output grew only 7.7% during the decade. 16 To identify recent (since 2016) trends in those LBD publications, we used natural language processing to characterize articles published in 2016–2021 (Figure 2). Once again, alpha‐synuclein dominated. Topics that registered high growth were RT‐QuIC‐based detection, prodromal diagnosis, cholinergic and acetylcholine evaluation, caregiving and cognition rehab, and assessment toolkits. Despite the increase in publications in these areas, diagnostics and quantitative biomarkers are likely to need more attention and progress, to accelerate therapeutic development. See below for further details.
FIGURE 2.

Analysis of LBD publication landscape. The semantic network of ∼2.6k articles in the field of “Lewy Body Dementia” published since 2016. The proximity of clusters indicates technological affinity/overlapping. ∼40 articles could not be clustered and hence not shown in the above analysis. Source: Web of Science, BCG analysis BCG Center for Growth & Innovation Analytics. BCG, Boston Consulting Group; LBD, Lewy body dementia
The Project Team also compared the topics of focus in scientific publications to the funding landscape described above by examining the share of knowledge capital, the growth rate, and the total funding levels (Figure 3). Several trends emerged. As discussed, alpha‐synuclein holds a major share of both funding and knowledge capital, but slower growth suggesting diversification of research areas. There were above average levels of funding growth for several diagnostics related topics, including RT‐QuIC based detection that so far has been the focus of very few publications, and CSF biomarker analyses that already hold a large share of the publication landscape. Drug discovery topics in LBD were underrepresented in publications and funding, with very little growth in this area. However, it should be acknowledged that therapeutic advances in modifying disease progression in AD and PD could have major implications for the treatment of LBD. The molecular pathology in LBD and Parkinson's is similar, and many individuals with LBD have concomitant amyloid brain pathology, albeit qualitatively different to that found in AD.
FIGURE 3.
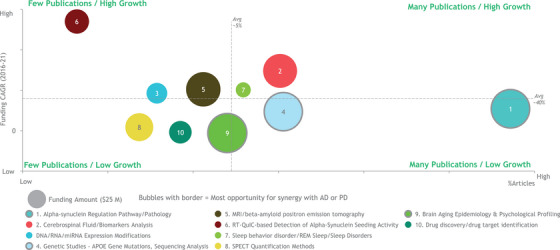
Growth of grant funding and articles in the top 10 areas of LBD research. Lysosomal Glucocerebrosidase activity/functions excluded from plot due to too low n to calculate CAGR; Caregiving/cognition rehab excluded due to out of scope of workshop focus. Growth measured by CAGR. CAGR values for ‘3′ calculated using 2017–2021 data. Projects under “Alpha‐synuclein Targeting Therapeutic Approaches” categorized as “Drug discovery/drug target identification.” Source: BCG analysis and Lee et al.7. BCG, Boston Consulting Group; CAGR, compound annual growth rate; LBD, Lewy body dementia
Despite the prevalence of LBD, it remains far behind AD and PD in its share of the therapeutic pipeline in the pharmaceutical and biotechnology industries (Figure 4). 17 , 18 , 19 , 20 Most therapies are repurposed from AD and PD rather than being specifically designed for LBD. These industries currently lack screening biomarkers, the endpoints, measurements, and patient access that would enable them to make serious progress toward LBD therapeutics. During interviews, industry leaders noted limitations in validated clinical and biomarker instruments, a shortage of collaborative infrastructure, and insufficient understanding of LBD's natural history through research cohorts. The patient experience is exacerbated by difficulties in clarity of diagnosis and by underuse of diagnostic criteria: many patients have a prolonged series of interactions with their doctors and specialists yet remain misdiagnosed and without specific or effective therapy (Figure S4).
FIGURE 4.
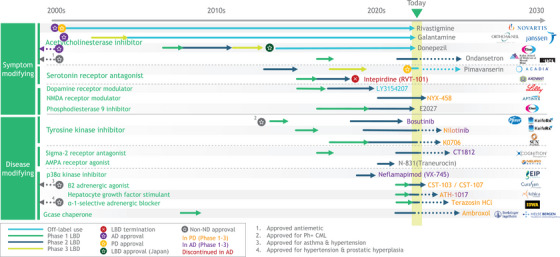
LBD, AD, and PD therapeutic pipeline. Source: www.clinicaltrials.gov. AD, Alzheimer's disease; LBD, Lewy body dementia; PD, Parkinson's disease
3.2. Looking ahead: Next steps identified to move the needle on LBD
3.2.1. Scientific landscape – Next steps to enhance the therapeutic pipeline
The first step is to establish consistent, reliable diagnosis of DLB in clinical and research settings, and its differentiation from, and overlap with, AD and PD. 21 Consider one primary difference in DLB diagnosis – the presence of a specific set of neuropsychiatric symptoms. Constellations of DLB symptoms can be used to inform the diagnosis, but the scales used to delineate the presence of symptoms and measure their severity are not optimized for DLB. For example, the routinely used Neuropsychiatric Inventory (NPI) is tuned to detect and measure the severity of a wide variety of hallucinations but not specifically visual hallucinations, a core symptom in DLB 22 – hence the need for better clinical tools to diagnose psychiatric symptoms in DLB. To make matters worse, there is little commonality across examinations used by clinicians – hence the need for standardized neuropsychiatric tools and metrics capable of dealing with DLB's complexities.
A step toward this is the incorporation of the Lewy Body Module in the Uniform Data Set of the NIA's Alzheimer's Centers. However, the total number of individual participants who are followed longitudinally is relatively small, and frequently orthogonal data – including imaging and biomarkers – are not uniformly collected. This sort of large scale, longitudinal research data collected in a uniform way is critical to a better understanding of the drivers of disease progression and patterns of co‐morbidity and mixed pathology among AD, PD, and DLB. Furthermore, a well characterized cohort is an important basis for improved diagnostic and therapeutic strategies.
What are the prospects, then, for reliable LBD biomarkers? Some relatively recent biomarkers look promising, including RT‐QuIC and similar alpha‐synuclein aggregation assays, using CSF or, increasingly, skin biopsies, and a recent study suggesting the possibility of serum. 23 Molecular biomarkers such as these are anticipated to accelerate studies of prodromal DLB 24 including mild cognitive impairment with Lewy bodies; as well as REM sleep behavioral disorder, a common precursor to DLB. Such biomarkers may ultimately prove useful in identifying presymptomatic disease as well, just as amyloid PET imaging has done in AD. These biomarkers currently provide a binary yes/no indication of the presence of alpha‐synuclein, but they do not indicate the location, amount, or distribution of the alpha‐synuclein deposits. Utilizing a suite of imaging modalities (e.g., functional and structural) including functional MRIs and dopamine transporter (DAT) scans, could provide both diagnostic information and potential insight into disease progression. Indeed, while more and better refined biomarkers that directly reflect alpha‐synuclein pathology are clearly needed, DAT uptake imaging is a validated indirect biomarker. Low DAT uptake in the basal ganglia demonstrated by SPECT or PET imaging can be used to track disease progression. 25 , 26 , 27 , 28 DAT imaging has been validated and is FDA‐ and European Union (EU)‐approved for suspected DLB, although it is less sensitive early in the course (e.g., during mild cognitive impairment). Hence, there is a need for quantitative and imaging biomarkers, to elucidate the progression of the disease and to inform therapeutic research. Early biomarkers will enable improved clinical trials, identifying new therapeutics, and afford the opportunity for clinicians to treat before the development of symptoms.
While identification of biomarkers is critical to diagnosis of DLB, a proper understanding of the overlapping pathophysiological mechanisms of DLB, AD, and PD and other neurodegenerative processes contributing to cognitive decline is imperative to pinpoint the best biomarkers. The resulting insights would turn the complexity of mixed pathologies into an opportunity. Notably, these mixed pathologies occur more commonly in women. 29 Ignoring these pathological features in research studies may result in health inequities. We are aware of several expert groups currently discussing the framework of PD and a‐synucleinopathies. These “cross‐disease” mechanisms include defects in proteostasis and the aggregation of proteins. Similar gains in understanding cross‐disease mechanism likely apply to research in inflammatory systems – the role of innate and adaptive immunity, 30 isoform‐specific actions of apolipoprotein E, complement, autophagy, lysosomal function, and so on.
Additional examples of overlap will likely emerge if a focused effort, similar to the efforts supported by the Accelerating Medicines Partnership for Parkinson's Disease (AMP‐PD) and Michael J. Fox Foundation (MJFF), were able to identify the right targets and useful biomarkers. The AMP‐AD data sets include LBD (see https:amp‐pd.org) and can be used to mine “omics” approaches (e.g., transcriptomics, proteomics, metabolomics, genomics), utilizing large datasets, and then, with the correct statistics, identify novel targets. Investigations of the underlying neural systems biology in disease and other genetic risk factors leveraging these “omics” approaches are currently behind comparable efforts in other diseases. Other newer technologies may be useful to encourage studies related to DLB: for example, by using spatial transcriptomics in brain cryosections, researchers might finally be able to reveal the amount, location, and distribution of changes associated with Lewy bodies at various stages of the disease.
Currently, large datasets tend to be fragmented across multiple repositories, making it difficult for scientists to reanalyze or analyze a combined or complete dataset. So, another useful early‐stage step would be to consolidate data for the DLB research and development community – establishing a centralized repository of single‐cell data, for example– in the way that Synapse has done for AMP‐AD and the Terra platform for AMP‐PD scientists. Furthermore, development of a standardized biological staging system for DLB and PDD (as recently outlined by MJFF) would likely push advancements in the therapeutic pipeline.
3.2.2. Future research agenda – Moving forward through improved diagnostic strategies
Our preworkshop analyses and early discussions revealed serious gaps in diagnostic tools. The top two were: diagnostics for clinical use and biomarkers for tracking clinical‐trial effectiveness. The Project Team felt that these two targets should be pursued in tandem, in the hope of enhancing both patient care and clinical trials. A poll among the workshop participants then highlighted the potentially most impactful solutions to explore in the next 2 to 4 years (Figure 5): a broad roll‐out of seed aggregation assays (such as protein misfolding cyclic amplification (PMCA) and RT‐QuIC); adaptation of seeding aggregation assays for alternative biosamples; and a set of patient‐cohort biomarker studies to connect natural history and molecular markers.
FIGURE 5.
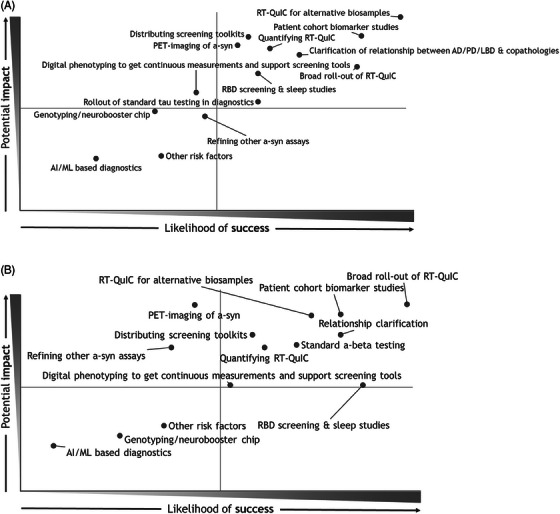
The potential impact and likelihood of success for identified LBD priorities. The outcome of the first (A) and second (B) votes for the prioritization of key approaches and likelihood of success by the Delphi method. LBD, Lewy body dementia
Regarding the roll‐out: As mentioned, seeding assays like RT‐QuIC for diagnosis of neuronal alpha‐synucleinopathies have recently become more available, but even in academic medical centers is still uncommon. Further hindering expansion for the use of seeding aggregation assays, primary care or nonspecialist MDs are unlikely to order synuclein tests appropriately without considerable education. Hence, the need for a broader roll‐out and education over the next several years.
With respect to the adaptation of seeding assays for alternative biosamples: The current RT‐QuIC situation has further drawbacks – at present, the procedure involves obtaining CSF as part of the diagnostic evaluation. However, there is a very recent report of success analyzing serum 23 and skin biopsy assays being integrated into LBD research studies, 31 and we are optimistic about these sorts of assays becoming more widely available. The hope is that, in the next 5 years, assays will be developed that are quicker, blood‐based, and quantitative – whether upgraded seeding assays or other ultrasensitive, protein‐conformation‐sensitive assays, such as soluble oligomer binding assay (SOBA). 30 Development of alternative biosample seeding assays would in turn lead to better opportunities for trial recruitment.
Regarding patient‐cohort biomarker studies: During patient‐cohort studies, blood and CSF samples are routinely collected, but not all the samples are used for specific endpoints of these studies – instead, many of these biological samples are banked for future use. In the case of studies with carefully phenotyped DLB patients, any banked samples (and any whole genome sequencing) could and should be made available to other researchers in the field. One such study currently under way is that of the NINDS‐funded DLB consortium, which is deeply phenotyping a small set of CSF and blood samples from DLB patients. Biobanking is enormously beneficial in helping to define disease progression and in creating research and development momentum. The biorepositories provide samples that enable biomarker discovery, help under‐resourced investigators by providing preliminary evidence, flag potential collaborations, and generally reinvigorate the field. As such, they need appropriate funding and proper publicizing throughout the dementia community.
The team also felt that engaging strategies for digital phenotyping of movement, gait, and sleep provide potentially promising data that could inform clinical decision making and clinical trial design. Wearable devices would provide continuous measurements and support screening tools. It is much simpler to measure and assess motor symptoms in this way than to measure and assess cognitive and behavioral symptoms. Ideally, wearable digital tools could be developed to measure more advanced symptoms too. Such tools could provide continuous measurements and assessments – patients could reduce their visits to the clinic, and yet provide far more data than they currently can. Unfortunately, for both kinds of symptoms, little consensus exists yet on their relevance to LBD diagnosis and treatment. Hence, the need for further research and consensus, to refine digital phenotyping methods and improve the metrics. Similarly, development of LBD screening toolkits and rapid dissemination of these toolkits (such as the DIAMOND‐Lewy toolkit in the United Kingdom 32 ) could provide a rich source of information and enable more rapid diagnosis of LBD.
Another important limitation is the lack of diversity in these cohorts. Most findings stem from studies including people identifying as non‐Hispanic White with higher levels of education and more access to resources, the generalizability and applicability of these findings remains questionable. For instance, significant sex and gender differences for the prevalence and impact of underlying Lewy body pathology, 29 and potentially lower diagnostic accuracy in people from minoritized ethnoracial groups 33 need to be better understood. As efforts focus on harmonizing data from cohorts in different centers and countries, 16 outreach to and inclusion of underrepresented communities also needs to be emphasized to support diagnostic and therapeutic advances in DLB.
4. CONCLUSIONS
DLB is caught in a vicious cycle. In the absence of accurate early diagnostics, far too many patients tend to remain undiagnosed or misdiagnosed for an unduly long time, which prevents awareness of the disease and its advocacy. This in turn results in less cultivation of funding sources. Limited funding constrains the amount, scale, and novelty of research as well as posing difficulties in attracting early‐career researchers. Without validated diagnostics and biomarkers (coming from basic research), biopharma is severely constrained in sourcing patients, measuring clinical outcomes, and generating specific therapeutic interventions. Subsequently, biopharma cannot leverage its considerable resources to drive public awareness and clinician education.
All the gaps in the DLB landscape will ultimately need to be addressed, but one in particular needs urgent attention – the gap in research. Three priority areas were endorsed by the workshop. The top priority is to establish reliable, consistent diagnosis of DLB in clinical and research settings. That will require improved clinical tools, especially those for assessing the complex psychiatric symptoms of DLB, and it will also require the standardization of diagnostic metrics, so that clinicians can at last take a common approach to the condition. Tool kits need to be developed and disseminated, keeping in mind the need to create or adapt tools in different languages and that are sensitive to cultural and socioeconomic status issues. This is not simple to do, but one of the cornerstones of moving the needle for DLB.
To optimize diagnostics (and eventually therapeutic strategies too), researchers need to gain a better understanding of the disease's natural progression and its patterns of co‐morbidity with AD and PD. For that purpose, efforts should be made to increase the number of patients followed longitudinally (currently too small) and to collect longitudinal biomarker and imaging data. Those actions would also provide insights into the full spectrum of proteinopathy to treat the heterogeneity of the disease and reduce the current emphasis on differentiating DLB from PD and AD – since about 80% of DLB patients have mixed pathologies. Furthermore, these studies should focus on early and unique symptoms of DLB, such as REM sleep behavior disorder which presents in over 80% of those with DLB and typically precedes cognitive and neuropsychiatric symptoms 34
The other main priority is to optimize biomarkers – accelerate the development of quantitative biomarkers, and ultimately make biomarkers cheaper and more accessible. That will require enhancements to seeding assays and adaptations for alternative biospecimens, particularly blood. DLB research should seek greater collaboration with PD and AD research in areas of overlap – for instance, on the cell biology of alpha‐synuclein. Such collaboration would not only make more efficient use of resources but also would help to break down the silos in research on these diseases – diseases that are really on a spectrum.
The group therefore noted several low‐hanging‐fruit opportunities: coordination, harmonization, and consensus‐building around standardized assessments and diagnostic‐tool development; consistent metrics and outcome measures; consolidation of datasets; standardized phenotyping; availability of biological samples; and greater data‐sharing. Convening small work groups to discuss and pursue these opportunities would provide a valuable foundation for all future research.
In the future, it may also be worthwhile to directly address workforce/talent development and to revisit the analysis of gaps in the current research portfolio, consequent to progress made over the next 2–4 years. For both, increased funding remains imperative. The most powerful funding lever is awareness, and that is best generated by robust patient/caregiver stories. Only through enhanced diagnostics will those stories reach a critical mass.
Overall, the field of LBD may well be at a positive inflection point. Serious improvements could be imminent in diagnostics, biomarkers, and natural‐history studies, and will eventually lead to novel therapeutics and a transformative patient experience.
CONFLICT OF INTEREST STATEMENT
The opinions expressed in this article are the author's own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government. At the time of the research, K.A., W.B., H.K., M.K., S.O.M., V.P., M.S.R., J.C.S., and L.W. were employees of Boston Consulting Group which funded the research for this article through BCG's health care practice. B.F.B. is an investigator for clinical trials sponsored by Biogen, Alector, EIP Pharma, Cognition Therapeutics, and Transposon; a Scientific Advisory Board member for the Tau Consortium, AFTD, LBDA, and GE HealthCare; and serves as a member of the Data Safety Monitoring Board for a clinical trial on mesenchymal stem cells in MSA. J.H.C. is a former employee of Novartis, current employee and stockholder of Latus Bio, Inc., and serves as a member of the Comprehensive Center for X‐Linked Parkinsons Dementia (CCXD) Scientific Advisory Board. M.D. and M.C.I. are employees of Eisai, Inc., which has drug development programs in neurodegenerative disease. D.G. is a consultant for GE Healthcare. J.E.G. provides consultations to Biogen, BMS, Eisai, Eli Lilly, GE Healthcare, and Lundbeck, serves on the advisory board for Passage Bio, is the Chief Scientific Officer and stockholder at Cognivue which develops cognitive assessment tools. S.G. consults for EIP Pharma and serves on the advisory boards of Hillhurst Biopharmaceuticals, EIP Pharma, and the LBDA RCOE. K.K. consults for Biogen and serves on a Data Safety Monitoring Board at Takeda. A.K., holds stock in Sage Therapeutics and Delix Therapeutics. J.B.L. serves as an advisor for LBDA and the Cleveland Alzheimer's Association Chapter Board. J.T.O. consults for Biogen and Roche, serves on advisory boards for TauRx, NovoNordisk, Lilly, and the Lewy Body Society, as well as chairs the Research Strategy Council for the Alzheimer's Society. S.W.S. serves on the Scientific Advisory Council of the Lewy Body Dementia Association; receives research support from Cerevel Therapeutics; and serves on the scientific advisory council for the LBDA and Multiple System Atrophy Coalition. R.S. consults for AbbVie, AC Immune, Acumen, Alector, Alnylam, Bristol Myers Squibb, Genentech, Janssen, JOMDD, Nervgen, Neuraly, Neurocentria, Oligomerix, Prothena, Renew, Roche, Shionogi, Vigil Neuroscience, Ionis, and Vaxxinity. A.T. is an employee of the Lewy Body Dementia Association. J.P.T. consults for EIP Pharma, Kyowa‐Kirin, and Sosei‐Heptares; and serves on the scientific advisory committee for the LBDA and the Lewy Body Society. R.A.W. provided freelance medical writing services for this manuscript. The following authors have no additional disclosures (funding support related to this manuscript is listed above): E.B., L.B., T.J.F., B.H., I.M., P.J.M., T.M., and S.R. Author disclosures are available in the S2.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
We thank Eliezer Masliah and Walter Koroshetz for their thoughtful discussions and editing of the manuscript. E.B. was supported by National Institute on Aging (NIA) grant K99AG073453 and the Lewy Body Dementia Association. B.F.B was supported by NIH grants P30AG062677, U01NS100620, U19AG071754; Lewy Body Dementia Association; Mayo Clinic Dorothy and Harry T. Mangurian Jr. Lewy Body Dementia Program; Little Family Foundation; Ted Turner and Family LBD Functional Genomics ConProgram; American Brain Foundation. T.J.F was supported by NIA grants U19AG071754, U01NS100620, and P30AG062677. K.K. was supported by the NIH grant U01NS100620. J.B.L was supported by NIH grants U01NS100610, U01AG0733, P30AG072959, the Lewy Body Dementia Association, Douglas Herthel DVM Memorial Fund, and GE Healthcare. S.W.S. was supported by the intramural research program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health (program #: ZIANS003154). J.P.T. was supported by the National Institute for Health Research for the Newcastle (NIHR) Biomedical Research Centre.
Agarwal K, Backler W, Bayram E, et al. Lewy body dementia: Overcoming barriers and identifying solutions. Alzheimer's Dement. 2024;20:2298–2308. 10.1002/alz.13674
REFERENCES
- 1. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology. 2017;89:88‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lippa CF, Duda JE, Grossman M, et al. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007;68:812‐819. [DOI] [PubMed] [Google Scholar]
- 3. Vann Jones SA, O'Brien JT. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol Med. 2014;44:673‐683. [DOI] [PubMed] [Google Scholar]
- 4. Goldman JG, Forsberg LK, Boeve BF, et al. Challenges and opportunities for improving the landscape for Lewy body dementia clinical trials. Alzheimers Res Ther. 2020;12:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jellinger KA. Depression in dementia with Lewy bodies: a critical update. J Neural Transm. 2023;130(10):1207‐1218 [DOI] [PubMed] [Google Scholar]
- 6. Mehraram R, Peraza LR, Murphy NRE, et al. Functional and structural brain network correlates of visual hallucinations in Lewy body dementia. Brain. 2022;145:2190‐2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Irwin DJ, Grossman M, Weintraub D, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol. 2017;16:55‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferman TJ, Aoki N, Boeve BF, et al. Subtypes of dementia with Lewy bodies are associated with α‐synuclein and tau distribution. Neurology. 2020;95:e155‐e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galvin JE, Duda JE, Kaufer DI, Lippa CF, Taylor A, Zarit SH. Lewy body dementia: the caregiver experience of clinical care. Parkinsonism Relat Disord. 2010;16:388‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Surendranathan A, Kane JPM, Bentley A, et al. Clinical diagnosis of Lewy body dementia. BJPsych Open. 2020;6:e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Brien JT, Taylor JP, Thomas A, et al. Improvingthe diagnosis and management of Lewy body dementia: the DIAMOND‐Lewy researchprogramme including pilot cluster RCT. Southampton (UK): NIHR Journals Library. 2021. Programme Grants for Applied Research, No. 9.7. https://www.ncbi.nlm.nih.gov/books/NBK572416/ [PubMed] [Google Scholar]
- 12. Rossi M, Candelise N, Baiardi S, et al. Ultrasensitive RT‐QuIC assay with high sensitivity and specificity for Lewy body‐associated synucleinopathies. Acta Neuropathol. 2020;140:49‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taylor JP, McKeith IG, Burn DJ, et al. New evidence on the management of Lewy body dementia. Lancet Neurol. 2020;19:157‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abdelnour C, Gonzalez MC, Gibson LL, et al. Dementia with Lewy bodies drug therapies in clinical trials: systematic review up to 2022. Neurol Ther. 2023;12:727‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee G, Cummings J, Decourt B, Leverenz JB, Sabbagh MN. Clinical drug development for dementia with Lewy bodies: past and present. Expert Opin Investig Drugs. 2019;28:951‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. D'Antonio F, Kane JPM, Ibañez A, et al. Dementia with Lewy bodies research consortia: a global perspective from the ISTAART Lewy Body Dementias Professional Interest Area working group. Alzheimers Dement. 2021;13:e12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. MacDonald S, Shah AS, Tousi B. Current therapies and drug development pipeline in Lewy Body Dementia: an update. Drugs Aging. 2022;39:505‐522. [DOI] [PubMed] [Google Scholar]
- 18. McFarthing K, Rafaloff G, Baptista M, et al. Parkinson's Disease drug therapies in the Clinical Trial Pipeline: 2022 Update. J Parkinsons Dis. 2022;12:1073‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cummings J, Lee G, Nahed P, et al. Alzheimer's disease drug development pipeline: 2022. Alzheimers Dement. 2022;8:e12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Armstrong MJ. Advances in dementia with Lewy bodies. Ther Adv Neurol Disord. 2021:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Toledo JB, Abdelnour C, Weil RS, et al. Dementia with Lewy bodies: impact of co‐pathologies and implications for clinical trial design. Alzheimers Dement. 2023;19:318‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rodriguez‐Porcel F, Wyman‐Chick KA, Abdelnour Ruiz C, et al. Clinical outcome measures in dementia with Lewy bodies trials: critique and recommendations. Transl Neurodegener. 2022;11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Okuzumi A, Hatano T, Matsumoto G, et al. Propagative α‐synuclein seeds as serum biomarkers for synucleinopathies. Nat Med. 2023;29:1448‐1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McKeith IG, Ferman TJ, Thomas AJ, et al. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology. 2020;94:743‐755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McKeith I, O'Brien J, Walker Z, et al. Sensitivity and specificity of dopamine transporter imaging with 123I‐FP‐CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol. 2007;6:305‐313. [DOI] [PubMed] [Google Scholar]
- 26. Thomas AJ, Donaghy P, Roberts G, et al. Diagnostic accuracy of dopaminergic imaging in prodromal dementia with Lewy bodies. Psychol Med. 2019;49:396‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jreige M, Kurian GK, Perriraz J, et al. The diagnostic performance of functional dopaminergic scintigraphic imaging in the diagnosis of dementia with Lewy bodies: an updated systematic review. Eur. J. Nucl. 2023;50:1988‐2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Durcan R, Roberts G, Hamilton CA, et al. Serial Nigrostriatal Dopaminergic Imaging in mild cognitive impairment with Lewy bodies, Alzheimer disease, and age‐matched controls. Neurology. 2023;101:e1196‐e1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chiu SY, Wyman‐Chick KA, Ferman TJ, et al. Sex differences in dementia with Lewy bodies: focused review of available evidence and future directions. Parkinsonism Relat Disord. 2023;107:105285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shea D, Colasurdo E, Smith A, et al. SOBA: development and testing of a soluble oligomer binding assay for detection of amyloidogenic toxic oligomers. Proc Natl Acad Sci U S A. 2022;119:e2213157119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gibbons CH, Freeman R, Bellaire B, Adler CH, Moore D, Levine T. Synuclein‐One study: skin biopsy detection of phosphorylated α‐synuclein for diagnosis of synucleinopathies. Biomark Med. 2022;16:499‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thomas AJ, Taylor JP, McKeith I, et al. Development of assessment toolkits for improving the diagnosis of the Lewy body dementias: feasibility study within the DIAMOND Lewy study. Int J Geriatr Psychiatry. 2017;32:1280‐1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bayram E, Holden SK, Fullard M, Armstrong MJ. Race and Ethnicity in Lewy Body Dementia: a Narrative Review. J Alzheimers Dis. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pao WC, Boeve BF, Ferman TJ, et al. Polysomnographic findings in dementia with Lewy bodies. Neurologist. 2013;19:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


