Abstract
The p24 protein, one of the three proteins implicated in local movement of potato virus X (PVX), was expressed in transgenic tobacco plants (Nicotiana tabacum Xanthi D8 NN). Plants with the highest level of p24 accumulation exhibited a stunted and slightly chlorotic phenotype. These transgenic plants facilitate the cell-to-cell movement of a mutant of PVX that contained a frameshift mutation in p24. Upon inoculation with tobacco mosaic virus (TMV), the size of necrotic local lesions was significantly smaller in p24+ plants than in nontransgenic, control plants. Systemic resistance to tobamoviruses was also evidenced after inoculation of p24+ plants with Ob, a virus that evades the hypersensitive response provided by the N gene. In the latter case, no systemic symptoms were observed, and virus accumulation remained low or undetectable by Western immunoblot analysis and back-inoculation assays. In contrast, no differences were observed in virus accumulation after inoculation with PVX, although more severe symptoms were evident on p24-expressing plants than on control plants. Similarly, infection assays conducted with potato virus Y showed no differences between control and transgenic plants. On the other hand, a considerable delay in virus accumulation and symptom development was observed when transgenic tobacco plants containing the movement protein (MP) of TMV were inoculated with PVX. Finally, a movement defective mutant of TMV was inoculated on p24+ plants or in mixed infections with PVX on nontransgenic plants. Both types of assays failed to produce TMV infections, implying that TMV MP is not interchangeable with the PVX MPs.
Cell-to-cell movement of plant viruses occurs through plasmodesmal channels in a process mediated by virus-encoded proteins that are dispensable for replication and virion assembly (14). A considerable amount of evidence related to the interactions of movement proteins (MPs) with various viral, cytoplasmic, and plasmodesmal components has been reported in the last few years, but a full understanding of cell-to-cell movement of virus infection is still lacking (30).
In many plant virus groups, cell-to-cell movement is mediated by a single MP. The first of these proteins to be identified was the 30-kDa protein (p30) of tobacco mosaic virus (TMV), which was shown to affect viral host range to be essential for virus movement (15, 18), to be localized to plasmodesmata (16, 17, 34, 42, 50), and to alter size exclusion limits of plasmodesmata (51, 54). In addition, it was shown that p30 is phosphorylated in infected protoplasts (53) and in vitro (8). Also, in vitro assays demonstrated that the TMV MP has a nonspecific nucleic acid-binding activity which may be involved in unfolding the single-stranded genomic RNA in a manner compatible with transport through plasmodesmata (7). By using deletion analysis, the putative phosphorylation site, the activity-increasing size exclusion limit, and the RNA-binding activity have been tentatively mapped to specific domains of the TMV MP (6, 7, 51). More recently, immunofluorescence assays showed an association between p30 and cytoskeletal proteins (23, 32).
Potato virus X (PVX) contains a positive-sense genomic RNA comprising five open reading frames (ORFs) numbered 1 to 5 in the 5′-to-3′ direction. They encode polypeptides of 166 kDa (viral replicase), 24 kDa (p24), 12 kDa (p12), 8 kDa (p8), and 25 kDa (viral coat protein [CP]) (25, 40). In contrast to TMV, cell-to-cell movement of PVX is mediated by the proteins encoded by ORF2 to -4 (the triple gene block). Participation of the triple-gene-block polypeptides in viral transport was implicated from experiments performed with white clover mosaic potexvirus (WCIMV), in which viral spread was prevented by mutations affecting each of these genes (4).
In all potexviruses described so far, proteins homologous to p24 contain the amino acid motif G/AX2GXGKS/T, which is also present in several nucleoside triphosphatases (NTPases) and helicases (22); furthermore, the homologous protein of foxtail mosaic potexvirus (FMV) exhibits GTPase activity (44). The potential involvement of the NPTase/helicase motif in viral transport was suggested in WCIMV by site-directed mutagenesis studies in which the sequence GKS was changed to AAA and completely inhibited cell-to-cell movement (4). In addition, it was shown that FMV p26 binds nonspecifically to RNA, suggesting that this protein is also involved in unfolding of viral RNA (44).
Subcellular localization studies using gold-conjugated antibodies revealed that both PVX p24 and its FMV homolog are associated with cytoplasmic components in infected tissues (11, 44). In contrast, PVX p12 and PVX p8 contain amino acids sequences resembling membrane-spanning domains (35, 45) and remain associated with membranes in cell extracts (37). Similar arrays of three overlapping genes are present in the carla-, furo-, and hordeivirus genomes (20, 36). In each of these virus groups, at least one of the triple-gene-block polypeptides has been shown to be involved in viral movement (21, 43).
Despite the low similarity in amino acid sequences between the MPs of different viral groups (38), the local movement of totally unrelated viruses can be frequently complemented in mixed infections (3). For example, PVX can complement the tomato mosaic tobamovirus (ToMV) mutant Ls1, which is unable to move at nonpermissive temperatures (48). In addition, TMV movement can be complemented by PVX in tomato plants that carry Tm2, a gene that restricts the spread of TMV (47). From these experiments, it was concluded that the PVX transport system includes one or more functions that complement or facilitate TMV movement. However, no sequence similarities were found between the MPs of TMV and PVX proteins encoded in the triple gene block (38).
Complementation between the movement systems of TMV and PVX suggested to us that a nonspecific type of resistance to these viruses may be induced by expressing their MPs in transgenic plants, in a way similar to that described for other plant viruses (5, 9, 29, 31). In this paper, we report results showing that transgenic tobacco plants expressing the PVX p24 are resistant to infection by two different members of the tobamovirus group and, reciprocally, that transgenic tobacco plants expressing the TMV MP are resistant to PVX. However, the transgenic plants did not complement movement-defective mutants of the heterologous virus to spread either locally or systemically.
MATERIALS AND METHODS
Plant lines and virus isolates.
Nicotiana tabacum cv. Xanthi D8 NN was obtained from Y. Chupeau (INRA, Versailles, France) and used in all transformation assays. Transgenic N. tabacum cv. Xanthi nn line 277 (p30+) (12) and Xanthi NN line 2005 (p30+) (13) express genes encoding the TMV MP and complement a mutant of TMV that lacks a functional MP (24). The N gene confers hypersensitive resistance to TMV, producing necrotic local lesions upon infection with this virus. R2 progeny of transgenic plant lines were used in this study. N. tabacum cv. Xanthi SX nn and N. tabacum cv. Xanthi NN were used as nontransgenic controls.
PVX (strain CP) and potato virus Y (PVY) (strain O) isolates were obtained from the International Potato Center (Lima, Peru) and propagated in N. tabacum cv. White Burley. PVX was purified as described by Orman et al. (40). PVX inoculations were performed with 1 to 10 μg of purified virions per ml. The PVY inoculum was freshly prepared from systemically infected N. tabacum at 15 to 20 days postinfection (dpi). Source plant leaves were ground in liquid nitrogen, and extracts were diluted 1:100 in 20 mM sodium phosphate (pH 7.0). Two leaves per plant were inoculated with 50 μl of inoculum per leaf.
TMV U1 (common) strain was isolated from leaves of infected plants at 7 to 10 dpi. One to 2 g of leaf tissue was ground in 2 ml of 0.5 M phosphate buffer (pH 7.0)–14.3 mM β-mercaptoethanol. Two volumes of water-saturated chloroform-butanol (50:50) was added and mixed. Samples were centrifuged for 15 min at 10,000 × g. The aqueous phase was transferred to microcentrifuge tubes, and virus particles were precipitated in 4% polyethylene glycol 8000 for 10 min on ice. Virus was collected by centrifugation for 10 min at 10,000 × g. Pellets were resuspended in 10 mM phosphate buffer (pH 7.0) and clarified by centrifugation. Virus was further precipitated in 4% polyethylene glycol–1% NaCl and collected by centrifugation. Progeny of a cloned cDNA derived from the Ob tobamovirus (10, 41, 49) was serially passaged twice on leaves of the local lesion indicator host Chenopodium amaranticolor (49) before inoculation to tobacco plants. Systemically infected leaves were harvested, and the virus was purified as described by Padgett and Beachy (41). Virus concentrations were estimated by absorbance at 260 nm.
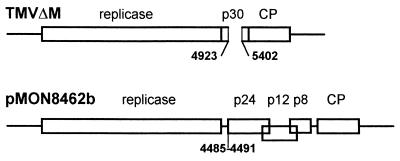
Plasmid pMON8453, containing a full-length cDNA copy of PVX, has been previously described (27). A modified version of pMON8453 was created to prevent translation of PVX ORF2 by deletion of nucleotides 4485 to 4491 and subsequent insertion of an EcoRV site at this region. The resulting clone, pMON8462b (Fig. 1), lacks the AUG initiation codon for ORF2. There are no other inframe methionine codons in the ORF2 sequence, and there are no ORFs of significant length in any reading frame until the AUG for ORF3 is reached. Plasmids pMON8453 and pMON8462b were linearized with SpeI, blunted with T4 DNA polymerase plus dNTPs, and used for the production of infectious transcripts. Transcription by bacteriophage T7 RNA polymerase (Promega) was performed as described by Nielsen and Shapiro (39) except that the concentrations of ATP, CTP, and UTP were increased to 1 mM each and bovine serum albumin was added to a final concentration of 100 μg/ml. One volume of 20 mM sodium phosphate was added, and 1 μg of infectious RNA was inoculated on each plant. TMV clone TMVΔM (kindly provided by C. Holt) carries a deletion in the p30 gene encompassing nucleotides 4923 to 5402 (Fig. 1). Infectious transcripts were propagated in plant line 277, a transgenic plant line that expresses the TMV MP gene (12), and virions were purified by the method described above for TMV.
FIG. 1.
Schematic diagram of viral constructs used in this study. Straight lines indicate untranslated regions. Rectangles enclose ORFs. Mutant TMVΔM contained a deletion which is indicated by a gap in the rectangle. Mutant pMON8462b contained a mutation in the start codon which is indicated by a line. Numbers indicate nucleotide positions.
DNA cloning. (i) pORF2.
A cDNA fragment encoding the PVX ORF2 comprising nucleotides 4484 to 5170 was obtained by PCR using clone 5X41 (40) as a template and oligonucleotides 5′ GACTGGATCCAGATGGATATTCTCATC 3′ and 5′ GATTGCCCGGGCGGTCAGTC 3′ as mutagenic primers. The underlined bases denote mismatches to introduce BamHI and SmaI sites at the 5′ and 3′ ends of the oligonucleotides, respectively. The amplification program was 20 cycles of 1 min at 94°C, 1 min at 50°C, and 1 min at 72°C, using a model IHB2024 Hybaid thermocycler. Reaction buffer and Taq polymerase were purchased from Promega. Following amplification, the PCR fragment was digested with BamHI and SmaI and subcloned in the pGEM-4Z vector (Promega) previously digested with BamHI and HincII, creating plasmid pORF2.
(ii) pMAL-p24.
pORF2 DNA was first digested with BamHI, blunted with Klenow polymerase and dNTPs, and redigested with PstI. The resulting DNA fragment, corresponding to the PVX ORF2, was purified following agarose gel electrophoresis (GeneClean; Bio 101) and ligated to the expression vector pMAL-c (New England Biolabs) to create plasmid pMAL-p24.
(iii) pBl-p24.
The HindIII-BamHI fragment from plasmid pORF2 was inserted into the binary expression vector pBl121 (26) that was previously treated with SstI, blunted with bacteriophage T4 DNA polymerase, and then digested with BamHI in order to excise the β-glucuronidase gene and generate compatible cloning sites. The resulting plasmid was named pBl-p24.
All plasmid constructs were confirmed by restriction analysis, and DNA sequencing was performed with a commercial kit (Sequenase 2.0 DNA sequencing kit; U.S. Biochemical) as instructed by the manufacturer.
Purification of MBP-p24 fusion protein.
PVX p24 was expressed from pMAL-p24 as a fusion protein with maltose-binding protein (MBP) and purified by affinity chromatography. Briefly, a 16-h culture of Escherichia coli XL1−Blue cells transformed with pMAL-p24 was diluted to 1:100 in 1 liter of LB medium containing 100 μg of ampicillin per ml and grown at 37°C to an optical density at 600 nm of 0.5. Isopropylthiogalactopyranoside was added to a final concentration of 0.3 mM, and cells were incubated for an additional 2 h to induce expression of the MBP-p24 fusion protein. Induced cells were harvested by centrifugation and lysed by sonication in 25 ml of 10 mM phosphate–30 mM NaCl–0.25% Tween 20–10 mM β-mercaptoethanol–10 mM EDTA–10 mM EGTA. For purification, a 1:5 dilution of the crude extract was loaded into an MBP affinity chromatography column of amylose resin (New England Biolabs). The column was washed twice with 20 mM Tris-HCl (pH 7.4)–0.2 M NaCl to eliminate nonspecific ligands, and proteins were eluted with the same buffer supplemented with 10 mM β-mercaptoethanol and 10 mM maltose. Eluted protein was separated in a 6% polyacrylamide gel by electrophoresis and show to be a single band of 66 kDa.
Immunization of rabbits.
One milligram of purified MBP-p24 was mixed with 1 ml of Freund’s adjuvant (complete for the initial intradermal injections; incomplete for subsequent intramuscular injections) and injected into two rabbits that had been previously bled to collect preimmune serum. Inoculations were repeated 4 and 6 weeks later. Antisera were collected 10 days after the last injection, and serial dilutions were tested in dot blot assays for titer determination. Antiserum used in this work was used at working dilutions of 1:1,000 and 1:2,000.
Plant transformation.
Tobacco leaf disks from N. tabacum cv. Xanthi D8 NN plants were transformed as described by An et al. (1). To ensure the establishment of independent transgenic plants, only one shoot per explant was selected.
Analysis of transgenic plants.
Kanamycin-resistant R0 plants were self-pollinated, and their seeds (R1 generation) were germinated under growth chamber conditions. p24 accumulation was analyzed by enhanced chemiluminescence (ECL) Western blot assays (ECL kit; Amersham) in 5 to 30 R1 plants of each of the 11 R0 lines obtained. R2 plants were obtained by self-pollination of R1 plants. Expression of the transgene was detected in all R2 seedlings obtained from lines p24-C, p24-D, p24-E, p24-H, and p24-I. The amount of p24 was estimated from Western blots autoradiographs scanned with the NIH Image 1.60 software. A maximum value of 1 was set for the highest-expressing plant, and values of 1 to 0.7, 0.7 to 0.3, and less than 0.3 were ranked as corresponding to high, intermediate, and low expressors, respectively.
Infection assays.
Eight to twelve plants per line were assayed in each infection test, and all experiments were repeated at least twice. Plants were grown in a growth room under artificial light (14 h/day) at 25°C. After 6 to 8 weeks, plants were mechanically inoculated with purified virus particles, sap extracts, or viral RNA, using carborundum (330 grit; Fisher Scientific) as an abrasive. Plants were rinsed with water immediately after inoculation and placed in growth chambers. TMV local lesions were counted and measured with a micrometer.
Tobacco protoplasts.
Tobacco protoplasts were generated from leaf tissue as follows. Leaf tissue was surface sterilized and washed with water, and 1 g of tissue was placed in a petri plate and digested overnight with enzyme solution (0.6 M mannitol, 0.1% 2-(N-morpholino)ethanesulfonic acid [MES], 0.015 g of cellulase per ml, 0.002 g of macerase per ml). Protoplasts were then filtered through a 300-μm-pore-size sieve and transferred to 15-ml polypropylene tubes. A cushion of 0.6 M sucrose was added to each tube, and protoplasts were spun at 1,200 × g at room temperature during 4 min in a swinging-bucket rotor. The upper phase was transferred to a new 15-ml tube, and protoplasts were pelleted as described above. Protoplasts were resuspended in 0.6 M mannitol–0.1% MES and spun again. This procedure was repeated twice. Samples of 2 million protoplasts were inoculated by electroporation with in vitro transcripts as described by Watanabe et al. (52) and cultured in 35-mm-diameter dishes at 23°C. For Western blot analysis, protoplasts (4 × 105 ml−1) were harvested at 48 h by centrifugation followed by lysis in Laemmli buffer (28). Samples containing the equivalent of 105 protoplasts were separated in 12.5% polyacrylamide gels containing sodium dodecyl sulfate (SDS). The proteins were electroblotted onto nitrocellulose and subjected to Western blot analysis using antibodies raised against PVX CP or TMV CP, followed by development with an ECL immunodetection kit (Amersham). The amount of PVX CP was estimated from autoradiographs scanned with the NIH Image 1.60 software.
Western blotting and ELISA.
Tobacco leaves were ground in liquid nitrogen and homogenized in buffer A (100 mM Tris-HCl [pH 6.8], 10 mM EDTA, 1% SDS), boiled for 5 min, and centrifuged at 10,000 × g for 15 min at 4°C. Supernatants were transferred to new tubes, and 4 volumes of cold acetone were added and thoroughly mixed. After incubation for 2 min on ice and centrifugation for 5 min at 10,000 × g, 4°C, the supernatant was discarded and pellets were held on ice. The pellets were resuspended in buffer A and clarified by centrifugation, and protein content of the supernatant was determined by the bicinchoninic acid system (Pierce) (46). Aliquots containing equivalent amounts (30 to 40 μg) of soluble protein were mixed with loading buffer, boiled for 3 min, subjected to electrophoresis in 10 to 12% polyacrylamide gels containing 0.1% SDS (28), and then electrotransferred to nylon membranes (Immobilon-P). PVX p24 was detected by using the antibody raised against the MBP-p24 fusion protein. TMV and Ob CPs were detected by using an antibody raised against TMV U1. Specifically bound antibodies were visualized by using an ECL immunodetection kit (Amersham). Enzyme-linked immunosorbent assays (ELISA) to detect PVX and PVY were carried out with anti-CP antibodies (PVX and PVY detection kits; Boehringer) as instructed by the manufacturer.
RESULTS
Evaluation of anti-p24 antibody.
Polyclonal antibodies to p24 were obtained by expressing the protein in E. coli cells as a fusion to the C terminus of MBP. The MBP-p24 fusion protein was purified by affinity chromatography and used as antigen to obtain polyclonal antibodies in rabbits. When leaf extracts from healthy and PVX-infected tobacco plants were tested in Western blot assays, the anti-MPB-p24 antibodies reacted specifically with a major polypeptide of 24 kDa that was present only in infected leaves (data not shown). As previously reported (11), bands of higher molecular weight were not detected in infected tissues, suggesting that p24 is not posttranslationally processed or derived from a precursor polypeptide.
Generation of p24 transgenic tobacco plants.
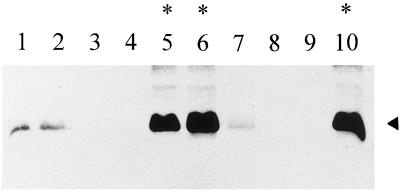
Tobacco leaf explants were transformed with a chimeric gene comprising the cauliflower mosaic virus 35S promoter, the coding sequence for p24, and the nos 3′ end; 11 R0 plants were self-pollinated to establish lines p24-A to -K. Seedlings from the R1 generation were analyzed for p24 accumulation by Western blot assays using anti-MBP-p24 antibodies. In five independent lines, p24-C, -D, -E, -H, and -I, segregation of p24 was approximately 3:1 (presence:absence), suggesting that a single copy of the transgene was expressed (Fig. 2). Seedlings that accumulated the highest levels of p24 exhibited a distinctive phenotype consisting of stunted plants with short internodes, smaller leaves, and slightly chlorotic appearance (Fig. 3A). Plants in the R2 generation obtained from these high-level expressors also showed a correlation between the stunted phenotype and levels of p24, suggesting that expression of this protein above a certain threshold has a pleiotropic effect on normal plant development. R2 lines p24-C and p24-D, with high levels of p24 and altered phenotype, and lines p24-E, p24-H and p24-I, with intermediate or low levels of p24 and normal phenotype, were selected for subsequent experiments (Table 1).
FIG. 2.
Western blot analysis of proteins extracted from p24 transgenic plants. Membranes with blotted proteins (40 μg/per lane) were reacted with anti-p24 serum. Specific binding of the antibody was detected by chemiluminescence. The position of p24 is indicated by the arrowhead. Five p24 transgenic plants of the R1 generation from p24-C (lanes 1 to 5) and p24-D (lanes 6 to 10) lines were analyzed. Plants showing a stunted and chlorotic phenotype are denoted by asterisks.
FIG. 3.
(A) Phenotype of Xanthi D8 NN (right) and p24-C (left) plants, shown 8 weeks after germination. (B) Detached systemic leaves from a p24-C plant (left) infected with pMON8462b (p24−) and from a Xanthi D8 NN plant (right) infected with pMON8453 (p24+). Leaves shown are from plants at 18 dpi. (C and D) Detached inoculated leaves of p24-C (C) and Xanthi D8 NN (D) plants inoculated with TMV-U1 (50 ng/ml) and photographed at 12 dpi. (E) p24-C plant (left) and Xanthi D8 NN plant (right) inoculated with Ob (0.2 mg/ml) and photographed at 15 dpi. (F) Detached systemic leaf of a p24-C plant inoculated with PVX (5 μg/ml) and shown at 15 dpi.
TABLE 1.
Transgenic plant lines used in this study
| Plant line | Transgene | Relative expression level |
|---|---|---|
| p24-C | PVX p24 | Higha |
| p24-D | PVX p24 | Higha |
| p24-E | PVX p24 | Intermediatea |
| p24-H | PVX p24 | Intermediatea |
| p24-I | PVX p24 | Lowa |
| 2005 | TMV p30 | Highb |
| 277 | TMV p30 | Highb |
Determined from Western blots as described in Materials and Methods.
Determined as described in reference 2.
Evaluation of p24 activity in the transgenic plants.
To evaluate the activity of p24 in transgenic plants, complementation assays were performed with PVX infectious transcripts carrying a frameshift mutation in ORF2. RNA transcripts from clones pMON8453 (p24+) and pMON8462b (p24−) (Fig. 1) were inoculated onto nontransformed line Xanthi D8 NN and onto transgenic lines p24-C and p24-E. Plants were monitored by ELISA for appearance of systemic symptoms and for CP accumulation in the inoculated and uninoculated leaves. While Xanthi D8 NN plants inoculated with transcripts of pMON8453 (p24+) were fully infected at 16 dpi, no symptoms of infection or PVX accumulation were detected in this line after inoculation with transcripts from pMON8462b (p24−) (data not shown). In contrast, there was development of systemic symptoms when transcripts of pMON8462b (p24−) were inoculated onto lines p24-C (Fig. 3B) and p24-E (data not shown), indicating that the transgenic protein is able to support viral transport when provided in trans. Disease symptoms observed in these complementation assays were similar to those induced by PVX on nontransgenic plants, except that the chlorotic appearance of p24+ plants was instead a dark green color in the center of the infection rings, which were sometimes masked by the chlorotic background of the transgenic plants (Fig. 3B). To assess viral replication of wild-type and mutant virus, transcripts from pMON8453 (p24+) and pMON8462b (p24−) were electroporated into tobacco protoplasts. As estimated from PVX CP accumulation measured in Western blot immunoassays (see Materials and Methods), p24-defective and nondefective transcripts replicated to similar extents in these protoplasts (data not shown). As an additional control, back-inoculation experiments in which nontransgenic plants were inoculated with sap from p24-C plants previously infected with transcripts from pMON8462b (p24−) failed to induce symptoms or accumulate virus, confirming that infection of p24-C plants was not due to a recovery of wild-type virus.
Resistance studies on p24 transgenic plants.
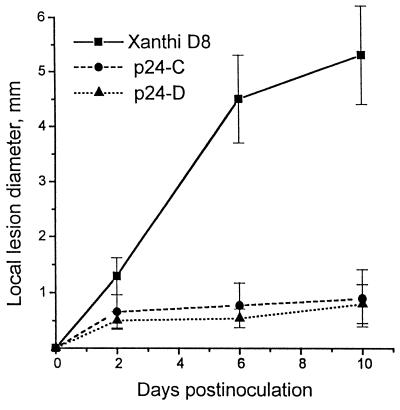
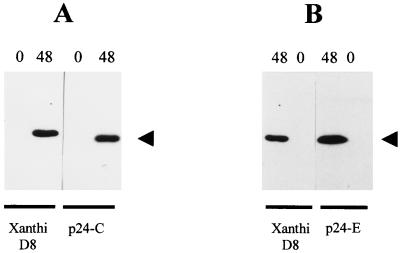
The complementation between PVX and ToMV Ls1 reported by Taliansky et al. (48) prompted us to examine the effect of TMV inoculation on p24 transgenic plants. Plants from lines p24-C, p24-D, and Xanthi D8 NN were challenged with TMV, and the progress of infection was monitored by scoring the size and number of necrotic local lesions. At 10 dpi, when growth of local lesions was arrested, the average diameter of lesions was much smaller in p24 transgenic plants (0.7 to 0.9 mm) than in control plants (5.4 mm) (Fig. 3C, 3D, and 4). Inoculation with TMV RNA produced the same results (data not shown). A separate experiment including high-, intermediate-, and low-expression plants was carried out. While the average lesion size of intermediate expressors (1.10 mm) was slightly higher than those of high expressors (0.89 and 0.91 mm), values corresponding to low expressors (3.90 mm) did not significantly differ from those found in control plants (Table 2). Remarkably, the total number of local lesions did not significantly differ between p24 transgenic and control lines (Fig. 3C and D). To assess whether TMV replication or movement was affected on p24+ plants, protoplasts from lines p24-C and p24-E were inoculated with TMV infectious transcripts. As shown in Fig. 5, viral accumulation reached normal levels on p24 protoplasts, indicating that viral spread was impaired in p24+ plants.
FIG. 4.
Time course of local lesion development on inoculated leaves of p24 and nontransgenic Xanthi D8 NN plants. Ten plants per line were inoculated with TMV U1 (50 ng/ml). The values represent the mean measurements of 100 individual local lesions from different plants of the same line. Standard deviations are represented by vertical bars.
TABLE 2.
Lesion sizes induced on different p24+ plants inoculated with TMV
| Plant linea | Relative expression levelb | Mean lesion diam (mm) |
|---|---|---|
| p24-C (R2) | High | 0.91 (0.45c) |
| p24-D (R2) | High | 0.89 (0.31) |
| p24-E (R2) | Intermediate | 1.10 (0.48) |
| p24-I (R1) | Low | 3.90 (0.69) |
| Xanthi NN | 3.81 (0.76) |
Plant generation is indicated in parentheses. Four plants from each line were inoculated with purified TMV virions (50 ng/μl). Thirty lesions per plant were measured at 6 dpi.
Determined from Western blots as described in Materials and Methods.
Standard deviation.
FIG. 5.
Western blot analysis of proteins extracted from tobacco protoplasts. Membranes with blotted proteins were reacted with anti-TMV CP serum. Specific binding of the antibody was detected by chemiluminescence. The position of TMV CP is indicated by an arrowhead. Times (hours postinoculation) are indicated at the top of each panel, and plant lines are indicated at the bottom. Shown are results of two independent experiments using protoplasts from p24-C (A) and p24-E (B) plant lines.
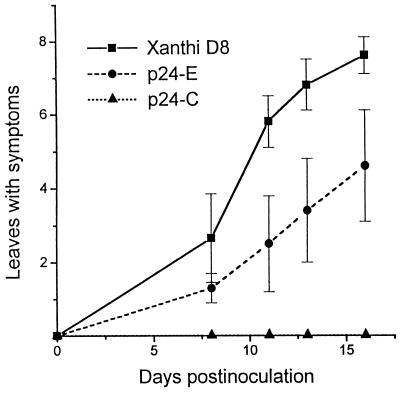
To determine if p24+ plants were also resistant to systemic infection by another tobamovirus, studies were carried out with Ob, a member of the tobamovirus group which is able to overcome N-gene-mediated resistance (41). Thus, plants from lines p24-C, p24-E, and Xanthi D8 NN were inoculated with Ob and examined for development of systemic symptoms and virus accumulation. By 15 dpi, none of the p24-C plants showed symptoms (Fig. 3E and 6), while p24-E plants showed a considerable delay in the appearance of symptoms (Fig. 6). By 30 dpi, plants from line p24-E showed the same degree of symptom severity as nontransgenic plants, but p24-C plants remained symptomless (data not shown). To ascertain that the absence of symptoms reflected a reduction of virus accumulation, the concentration of Ob was estimated by Western blot analysis and back-inoculation assays on the local lesion host C. amaranticolor. No Ob CP was detected in uninoculated leaves from p24-C plants at 20 dpi. By contrast, nontransgenic plants were fully infected at this time. Likewise, no local lesions developed on any of the inoculated leaves of C. amaranticolor plants back-inoculated with undiluted sap from uninoculated leaves of each Ob-inoculated p24-C plant.
FIG. 6.
Development of Ob infection on p24 and Xanthi D8 NN plants, indicated by number of leaves showing disease symptoms as a function of days after inoculation. Twelve plants per line were inoculated with Ob (0.2 μg/ml). Values represent the average number of leaves showing symptoms, and standard deviations are represented by vertical bars. The experiment was repeated twice.
To characterize the response to infection by members of other viral families, R2 seedlings of p24+ plants were challenged with PVY and PVX. Lines p24-C, p24-H, and Xanthi D8 NN were inoculated with PVX, and presence of virus in uninoculated and inoculated leaves was monitored by ELISA at different times postinfection. No differences were observed in the amount of virus or rate of symptom development between control and transgenic plants (data not shown). However, systemically infected leaves of p24+ plants displayed more severe symptoms than nontransgenic plants; symptoms included chlorotic rings limited by a thin necrotic border (Fig. 3F). Similarly, plants from lines p24-C and Xanthi D8 NN were inoculated with PVY. Virus accumulation in the two plant groups followed similar temporal patterns, and no differences were found in levels of virus accumulation (data not shown).
Resistance studies on p30 transgenic plants.
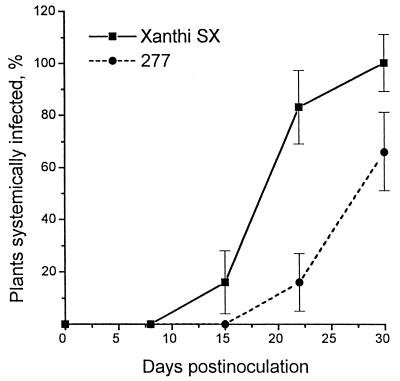
To determine whether TMV p30 reduced infection by PVX, plants from lines 277 (p30+) (Table 1) and Xanthi SX nn were inoculated with PVX, and the development of infection was monitored in inoculated and uninoculated leaves. Although levels of virus accumulation in the inoculated leaves were similar in 277 (p30+) and control plants, there was a significant delay in the development of symptoms and virus accumulation in the uninoculated leaves of 277 (p30+) plants (Fig. 7).
FIG. 7.
Plants infected with PVX as a function of time after inoculation. Ten plants per line were inoculated with PVX (1 μg/ml), and uninoculated leaves were analyzed by ELISA at various dpi. Vertical bars represent standard deviations. The experiment was repeated twice, and values represent the mean of both experiments.
Complementation assays.
The fact the p24+ and p30+ plants showed protection to TMV and PVX, respectively, suggested that the two viral transport systems would share functions reciprocally interfering with each other. Based on this proposal, we performed a series of preliminary experiments to explore whether PVX and TMV MPs can be interchanged. Thus, seedlings from lines 2005 (p30+), 277 (p30+), p24-C (Table 1), and Xanthi D8 NN were inoculated with in vitro transcripts of pMON8462b (p24−), and PVX infection was monitored by ELISA at different dpi. While p24+ plants were fully infected at 15 dpi, no symptoms or PVX accumulation were detected in Xanthi D8 NN, 2005, or 277 plants up to 35 dpi (data not shown).
To determine if PVX p24 can complement a TMV p30 deletion mutant, plants from lines p24-C, p24-E, 2005, and Xanthi D8 NN were inoculated with TMVΔM (Fig. 1). As expected (24), confluent local lesions developed on the inoculated leaves of 2005 plants by 3 dpi. In contrast, no lesions appeared on the other lines, indicating that p24 does not provide the functions for local spread of the TMV mutant (data not shown). In addition, we performed a series of coinfection assays to determine if other PVX MPs were able to complement TMVΔM. Thus, Xanthi D8 NN plants were coinoculated with various concentrations of TMVΔM (0.01, 0.1, 1, and 10 mg/ml) and PVX (0.1 and 1 mg/ml). Though these assays were repeated several times, no lesions induced by TMVΔM were observed on inoculated leaves, and only replication of PVX could be detected (data not shown).
DISCUSSION
Tobacco plants expressing high levels of PVX p24 exhibit a stunted phenotype, including shorter internodes, smaller leaf sizes, and a slightly chlorotic condition. Since these plants become systemically infected after inoculation with a p24-defective mutant of PVX, it was concluded that the transgenic protein is functional and able to act in trans. In addition, p24+ plants showed more severe symptoms than did nontransgenic plants upon infection with PVX, including peculiar necrotic rings surrounding nonchlorotic tissue. These observations suggest that, in addition to its role in virus movement, p24 may be involved in symptom development.
Upon challenge with TMV, p24+ plants exhibited a considerable level of resistance which was perceived as a reduction in the area of virus-induced lesions. The fact that TMV replicated normally in p24+ protoplasts, together with the development of approximately equivalent numbers of local lesions in p24+ and nontransgenic plants, indicates that neither the establishment of TMV infection nor replication is affected by p24 and that p24 acts by interfering with TMV movement. This interpretation is also supported by the results obtained with Ob, in which those plants showing the highest levels of p24 expression did not develop systemic infection. In contrast, no differences with control plants were observed when p24+ plants were challenged with either PVY or PVX, implying that the resistance-inducing mechanism is specific to certain viral groups. Remarkably, a considerable delay in systemic infection was observed when p30+ plants were inoculated with PVX, though no differences could be detected in inoculated leaves. It was previously shown that transgenic plants containing a dysfunctional p30 developed resistance to heterologous viruses in systemic rather than in inoculated leaves (9). Similarly, resistance to PVX could reflect a differential activity of p30 inhibiting long-distance transport but not cell-to-cell spread. A summary of the infection assays performed in this work is shown in Table 3.
TABLE 3.
Summary of infections of transgenic and nontransgenic plants
| Virus | Infection profilea of indicated tobacco plants
|
||
|---|---|---|---|
| Nontransgenic | p24 transgenic | p30 transgenic | |
| PVX | + | + | D |
| pMON8462b (PVX p24−) | − | + | − |
| TMV | + | R | + |
| TMVΔM | − | − | + |
| PVY | + | + | ND |
| Ob | + | R + D | + |
+, infection; −, no infection; R, resistance; D, delayed infection; ND, not determined. Viral infection was established on the basis of symptom development and immunological assays.
No TMV infection was detected when p24+ plants were inoculated with TMVΔM or when this mutant was coinoculated with PVX on nontransgenic plants, indicating that neither p24 nor other PVX MPs can complement p30 under these conditions. This result does not necessarily conflict with the movement complementation previously reported for PVX and ToMV Ls1 (48). This interaction could be explained if Ls1 p30 retains some but not all of its functional properties at nonpermissive temperatures, while TMVΔM carries an extensive deletion. On the other hand, at least one of the p24 functions is not exerted by p30, since p30-expressing plants do not support infection by a p24-defective PVX mutant. Taken together, these experiments show that the TMV and PVX transport systems are not completely interchangeable. However, our experiments cannot exclude the possibility that some functional determinants are shared by the two proteins and that the two viruses can act cooperatively in certain cases.
Two general models can be postulated to explain virus resistances in p24- and p30-expressing plants. In the first model, p24 and p30 contain multiple functional domains: those performing equivalent activities and those specific to each virus protein. This combination of shared and nonshared domains would cause the proteins to appear to be partially functional for the heterologous virus and, consequently, dominant negative effectors of the nonshared function(s). Evidence for the presence of shared functions includes the reported complementation between PVX and the ToMV Ls1 mutant (48) and the RNA-binding activity demonstrated in both proteins (7, 44). Hence, the resistance observed in p24+ and p30+ transgenic plants could be explained as a competition for a limiting cellular factor which is required for both systems. This model is supported both by the correlation between the p24 expression level and the degree of protection against TMV and Ob and by the susceptibility of p24- and p30-expressing plants to the homologous viruses (reference 9 and this work).
Alternatively, the resistance found in p24+ and p30+ plants could be explained by a mechanism inducing a constitutive defense response that restricts virus spread. A predicted consequence of this model is that such response should be nonspecific and likely to act against a broad range of viruses, including potex-, poty-, and tobamoviruses. The fact that p24+ plants are susceptible to PVX and PVY, and that p30+ plants are susceptible to TMV and other viruses, argues against this type of mechanism.
Genetically engineered protection, derived from viral and nonviral genes, has been widely explored as an alternative approach to plant virus control. CP-mediated resistance proved to be the most successful approach to accomplish this goal, but it is usually limited to closely related viruses (19). On the other hand, a broader antiviral resistance has been obtained by expression of viral MPs in transgenic plants, either in native or mutated versions. Thus, tobacco plants expressing a mutated form of the TMV p30 were found to be resistant to infection by several tobamo-, tobra-, nepo-, alfamo-, caulimo-, and cucumoviruses (9, 29). Likewise, transgenic plants expressing a mutated version of WCIMV p13 protein were shown to be resistant to this and other potexviruses and to potato carlavirus S (5). In addition, resistance to TMV was induced by expression of the brome mosaic virus 32-kDa MP in tobacco plants. Since tobacco is not a host for brome mosaic virus (31), this finding suggests that expression of MPs that are nonfunctional in a particular host could interfere with viruses adapted to replicate in it. Our results demonstrate that the nonmodified PVX and TMV MPs can confer protection in a rather specific manner which is not effective in the case of the homologous virus. To be applied under agricultural conditions, this strategy must first be adapted to suppress potential problems associated with MP expression and undesirable effects observed on plant phenotype and to develop MP mutants that are incapable of normal function (5, 29).
ACKNOWLEDGMENTS
We are grateful to Sally Leitner for growth and maintenance of plant material and to Curtis Holt for providing the TMVΔM virus.
X.A., G.C., S.C., and A.M. were supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina; X.A. was also supported by a UNESCO fellowship. Other support was provided by NSF grant MCB 9209530 to R.N.B.
REFERENCES
- 1.An G, Ebert P R, Mitra A, Ha S M. In: Plant molecular biology manual, section A3. Gelvin S B, Schilperoort R A, editors. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1988. pp. 1–19. [Google Scholar]
- 2.Arce Johnson, P., U. Reimann-Philipp, H. Padgett, R. Rivera-Bustamante, and R. N. Beachy. Requirement of the movement protein for long distance spread of tobacco mosaic virus in grafted plants. Mol. Plant-Microbe Interact., in press.
- 3.Atabekov J G, Taliansky M E. Expression of a plant virus-coded transport function by different viral genomes. Adv Virus Res. 1990;38:201–248. doi: 10.1016/s0065-3527(08)60863-5. [DOI] [PubMed] [Google Scholar]
- 4.Beck D L, Guilford P J, Voot D M, Andersen M T, Forster R L S. Triple gene block proteins of white clover mosaic potexvirus are required for transport. Virology. 1991;183:695–702. doi: 10.1016/0042-6822(91)90998-q. [DOI] [PubMed] [Google Scholar]
- 5.Beck D L, Van Dolleweerd C J, Lough T J, Balmori E, Voot D M, Andersen M T, O’Brien I E W, Forster R L S. Disruption of virus movement confers broad-spectrum resistance against systemic infection by plant viruses with a triple gene block. Proc Natl Acad Sci USA. 1994;91:10310–10314. doi: 10.1073/pnas.91.22.10310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berna A, Gafny R, Wolf S, Lucas W J, Holt C A, Beachy R N. The TMV movement protein: role of the C-terminal 73 amino acids in subcellular localization and function. Virology. 1991;182:682–689. doi: 10.1016/0042-6822(91)90609-f. [DOI] [PubMed] [Google Scholar]
- 7.Citovsky V, Wong M L, Shaw A A L, Venkataram Prasad B V, Zambryski P. Visualization and characterization of tobacco mosaic virus movement protein binding to single-stranded nucleic acids. Plant Cell. 1992;4:397–411. doi: 10.1105/tpc.4.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Citovsky V, McLean B G, Zupan J R, Zambryski P. Phosphorylation of tobacco mosaic virus cell-to-cell movement protein by a developmentally regulated plant cell wall-associated protein kinase. Genes Dev. 1993;7:904–910. doi: 10.1101/gad.7.5.904. [DOI] [PubMed] [Google Scholar]
- 9.Cooper B, Lapidot M, Heick J A, Dodds J A, Beachy R N. A defective movement protein of TMV in transgenic plants confers resistance to multiple viruses whereas the functional analog increases susceptibility. Virology. 1995;206:307–313. doi: 10.1016/s0042-6822(95)80046-8. [DOI] [PubMed] [Google Scholar]
- 10.Csillery G, Tobias I, Rusko J. A new pepper strain of tomato mosaic virus. Acta Phytopathol Acad Sci Hung. 1983;18:195–200. [Google Scholar]
- 11.Davies C, Graham H, Baulcombe D C. Sub-cellular localization of the 25-kDa protein encoded in the triple gene block of potato virus X. Virology. 1993;197:166–175. doi: 10.1006/viro.1993.1577. [DOI] [PubMed] [Google Scholar]
- 12.Deom C M, Oliver M J, Beachy R N. The 30-kilodalton gene product of tobacco mosaic virus potentiates virus movement. Science. 1987;237:384–389. doi: 10.1126/science.237.4813.389. [DOI] [PubMed] [Google Scholar]
- 13.Deom C M, Wolf S, Holt C A, Lucas W J, Beachy R N. Altered function of the tobacco mosaic virus movement protein in a hypersensitive host. Virology. 1991;180:251–256. doi: 10.1016/0042-6822(91)90029-b. [DOI] [PubMed] [Google Scholar]
- 14.Deom C M, Lapidot M, Beachy R N. Plant virus movement proteins. Cell. 1992;69:221–224. doi: 10.1016/0092-8674(92)90403-y. [DOI] [PubMed] [Google Scholar]
- 15.Deom C M, Xian Z H, Beachy R N, Weissinger A K. Influence of heterologous tobamovirus movement protein and chimeric-movement protein genes on cell-to-cell and long-distance movement. Virology. 1994;205:198–209. doi: 10.1006/viro.1994.1635. [DOI] [PubMed] [Google Scholar]
- 16.Ding B, Haudenshield J S, Hull R J, Wolf S, Beachy R N, Lucas W J. Secondary plasmodesmata are specific sites of localization of the tobacco mosaic virus movement protein in transgenic tobacco plants. Plant Cell. 1992;4:915–928. doi: 10.1105/tpc.4.8.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epel B L, Padgett H S, Heinlein M, Beachy R N. Plant virus movement protein dynamics probed with a GFP-protein fusion. Gene. 1996;173:75–79. doi: 10.1016/0378-1119(95)00678-8. [DOI] [PubMed] [Google Scholar]
- 18.Fenczik C A, Padgett H S, Holt C A, Casper S J, Beachy R N. Mutational analysis of the movement protein of odontoglossum ringspot virus to identify a host-range determinant. Mol Plant-Microbe Interact. 1995;8:666–673. doi: 10.1094/mpmi-8-0666. [DOI] [PubMed] [Google Scholar]
- 19.Fitchen J H, Beachy R N. Genetically engineered protection against viruses in transgenic plants. Annu Rev Microbiol. 1993;47:739–763. doi: 10.1146/annurev.mi.47.100193.003515. [DOI] [PubMed] [Google Scholar]
- 20.Forster R L S, Bevan M W, Harbison S, Gardner R C. The complete sequence of the potexvirus white clover mosaic virus. Nucleic Acids Res. 1988;16:291–304. doi: 10.1093/nar/16.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilmer D, Bouzoubaa S, Hehn A, Guilley H, Richards K, Jonard G. Efficient cell-to-cell movement of beet necrotic yellow vein virus requires 3′ proximal genes located on RNA2. Virology. 1992;189:40–47. doi: 10.1016/0042-6822(92)90679-j. [DOI] [PubMed] [Google Scholar]
- 22.Gorbalenya A E, Koonin E V, Donchenko A P, Blinov V M. A novel superfamily of nucleoside triphosphate-binding motif containing proteins which are probably involved in duplex unwinding in DNA and RNA replication and recombination. FEBS Lett. 1988;235:16–24. doi: 10.1016/0014-5793(88)81226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinlen M, Epel B L, Padgett H S, Beachy R N. Interaction of tobamovirus movement proteins with the plant cytoskeleton. Science. 1995;270:1983–1985. doi: 10.1126/science.270.5244.1983. [DOI] [PubMed] [Google Scholar]
- 24.Holt C A, Beachy R N. In vivo complementation of infectious transcripts from mutant tobacco mosaic virus cDNAs in transgenic plants. Virology. 1991;181:109–117. doi: 10.1016/0042-6822(91)90475-q. [DOI] [PubMed] [Google Scholar]
- 25.Huisman M J, Linthorst H J M, Bol J F, Cornelissen B J C. The complete nucleotide sequence of potato virus X and its homologies at the amino acid level with various plus-stranded RNA viruses. J Gen Virol. 1988;69:1789–1798. doi: 10.1099/0022-1317-69-8-1789. [DOI] [PubMed] [Google Scholar]
- 26.Jefferson R A, Kavanagh T A, Bevan M W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim K H, Hemenway C. The 5′ nontranslated region of potato virus X affects both genomic and subgenomic RNA synthesis. J Virol. 1996;70:5533–5540. doi: 10.1128/jvi.70.8.5533-5540.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Lapidot M, Gafny R, Ding B, Wolf S, Lucas W J, Beachy R N. A dysfunctional movement protein of tobacco mosaic virus that partially modifies the plasmodesmata and limits virus spread in transgenic plants. Plant J. 1993;4:959–970. [Google Scholar]
- 30.Lucas W J, Gilbertson R L. Plasmodesmata in relation to viral movement within leaf tissues. Annu Rev Phytopathol. 1994;32:387–411. [Google Scholar]
- 31.Malyshenko S I, Kondakova O A, Nazarova J V, Kaplan I B, Taliansky M E, Atabekov J G. Reduction of tobacco mosaic virus accumulation in transgenic plants producing non-functional viral transport proteins. J Gen Virol. 1993;74:1149–1156. doi: 10.1099/0022-1317-74-6-1149. [DOI] [PubMed] [Google Scholar]
- 32.McLean B G, Zupan J, Zambryski P C. Tobacco mosaic virus movement protein associates with the cytoskeleton in tobacco cells. Plant Cell. 1995;7:2101–2114. doi: 10.1105/tpc.7.12.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meshi T, Watanabe Y, Saito T, Sugimoto A, Maeda T, Okada Y. Function of the 30 kD protein of tobacco mosaic virus: Involvement in cell-to-cell movement and dispensability for replication. EMBO J. 1987;6:2557–2563. doi: 10.1002/j.1460-2075.1987.tb02544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore P J, Fenczik C A, Deom C M, Beachy R N. Developmental changes in plasmodesmata in transgenic tobacco expressing the movement protein of tobacco mosaic virus. Protoplasma. 1992;170:115–127. [Google Scholar]
- 35.Morozov S Y, Lukasheva L I, Chernov B K, Skryabin K G, Atabekov J G. Nucleotide sequence of the open reading frames adjacent to the coat protein cistron in potato virus X genome. FEBS Lett. 1987;213:438–442. [Google Scholar]
- 36.Morozov S Y, Dolja V V, Atabekov J G. Probable reassortment of genomic elements among elongated RNA-containing plant viruses. J Mol Evol. 1989;29:52–62. doi: 10.1007/BF02106181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morozov S Y, Miroshnichenko N A, Zelenina D A, Fedorkin O N, Solovijev A G, Lukasheva L I, Atabekov J G. Expression of RNA transcripts of potato virus X full-length and subgenomic cDNAs. Biochimie. 1990;72:677–684. doi: 10.1016/0300-9084(90)90051-h. [DOI] [PubMed] [Google Scholar]
- 38.Mushegian A R, Koonin E V. Cell-to-cell movement of plant viruses. Insights from amino acid sequence comparisons of movement proteins and from analogies with cellular transport systems. Arch Virol. 1993;133:239–257. doi: 10.1007/BF01313766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nielsen D A, Shapiro D J. Preparation of capped RNA transcripts using T7 RNA polymerase. Nucleic Acids Res. 1986;14:5936. doi: 10.1093/nar/14.14.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orman B E, Celnik R M, Mandel A M, Torres H N, Mentaberry A N. Complete cDNA sequence of a South American isolate of potato virus X. Virus Res. 1990;16:293–306. doi: 10.1016/0168-1702(90)90054-f. [DOI] [PubMed] [Google Scholar]
- 41.Padgett H S, Beachy R N. Analysis of a tobacco mosaic virus strain capable of overcoming N gene-mediated resistance. Plant Cell. 1993;5:577–586. doi: 10.1105/tpc.5.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Padgett, H. S., B. L. Epel, M. H. Heinlein, Y. Watanabe, and R. N. Beachy. Distribution of tobamovirus movement protein in infected cells and implications for cell-to-cell spread of infection. Plant J., in press. [DOI] [PubMed]
- 43.Petty I T D, French R, Jones R W, Jackson A O. Identification of barley stripe mosaic virus genes involved in viral RNA replication and systemic movement. EMBO J. 1990;9:3453–3457. doi: 10.1002/j.1460-2075.1990.tb07553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rouleau M, Smith R J, Bancroft J B, Mackie G A. Purification, properties, and subcellular localization of foxtail mosaic potexvirus 26-kDa protein. Virology. 1994;204:254–265. doi: 10.1006/viro.1994.1530. [DOI] [PubMed] [Google Scholar]
- 45.Rupasov V V, Morozov S Y, Kanyuka K V, Zhuria V Y, Lukasheva L I, Zavriev S K. Nucleotide sequence and structural organization of 3′-terminal region of genomic RNA of potato virus M. Mol Biol. 1990;24:448–459. [PubMed] [Google Scholar]
- 46.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson V J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 47.Taliansky M E, Malyshenko S I, Pshennikova E S, Atabekov J G. Plant virus-specific transport function. A factor controlling virus host range. Virology. 1982;122:327–331. doi: 10.1016/0042-6822(82)90232-x. [DOI] [PubMed] [Google Scholar]
- 48.Taliansky M E, Malyshenko S I, Pshennikova E S, Kaplan I B, Ulanova E F, Atabekov J G. Plant virus-specific transport function. Virus genetic control required for systemic spread. Virology. 1982;122:318–326. doi: 10.1016/0042-6822(82)90231-8. [DOI] [PubMed] [Google Scholar]
- 49.Tobias I, Rast A T B, Maat D Z. Tobamoviruses of pepper, eggplant, and tobacco: comparative host reactions and serological relationships. Neth J Plant Pathol. 1982;88:257–268. [Google Scholar]
- 50.Tomenius K, Clapham D, Meshi T. Localization by immunogold-cytochemistry of the virus-coded 30K protein in plasmodesmata of leaves infected with tobacco mosaic virus. Virology. 1987;160:363–371. doi: 10.1016/0042-6822(87)90007-9. [DOI] [PubMed] [Google Scholar]
- 51.Waigmann E, Lucas W J, Citovsky V, Zambryski P. Direct functional assay for tobacco mosaic virus cell-to-cell movement protein and identification of a domain involved in increasing plasmodesmal permeability. Proc Natl Acad Sci USA. 1994;91:1433–1437. doi: 10.1073/pnas.91.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watanabe Y, Meshi T, Okada T. Infection of tobacco protoplasts with in vitro transcribed tobacco mosaic virus RNA. FEBS Lett. 1987;219:65–69. [Google Scholar]
- 53.Watanabe Y, Ogawa T, Okada Y. In vivo phosphorylation of the 30-kDa protein of tobacco mosaic virus. FEBS Lett. 1992;313:181–184. doi: 10.1016/0014-5793(92)81440-w. [DOI] [PubMed] [Google Scholar]
- 54.Wolf S, Deom C M, Beachy R N, Lucas W J. Movement protein of tobacco mosaic virus modifies plasmodesmatal size exclusion limit. Science. 1989;246:377–379. doi: 10.1126/science.246.4928.377. [DOI] [PubMed] [Google Scholar]