Abstract
Teratogenic and embryotoxic effect of diclofenac sodium (DS) on different developmental stages of the chick-embryos was investigated by examining different parameters such as its mortality rate, hatching, morphological measurements, weighing its internal organs and calculation of different indices. Experiment was divided into four trials with different dose (0.1 mL, 0.2 mL, 0.3 mL in groups A, B, and C, respectively and group D received 0.3 mL saline solution (0.9% NaCl) and group E remained un-injected) administration and observation. Results of first and second trial showed statistically (p<0.01) significant difference in bodyweight, body-length, forelimb and hindlimb length between experimental and control groups. In third trial, diclofenac sodium administration showed a statistically (p<0.01) significant difference in the bodyweight, body-length, forelimb, hindlimb length, liver weight, egg weight (EE ratio) and kidney somatic index (KSI). The beak-size, heart weight, kidney weight, cardiac somatic index (CSI) and hepato somatic index (HSI) were not significant (p>0.05) when compared with the control groups. In trial 4, forelimb, hindlimb length, heart weight, CSI and HSI were statistically (p<0.01) significant. Body-length and liver weight were significant (p<0.05). While bodyweight, beak size, kidney weight and KSI were non-significant (p>0.05). The mortality rate was increased with increase dose of DS and also affected the hatching. DS effect on chick embryos can be applied to humans because the early development of mammals and birds are closely related. So, it was concluded that DS should be used with caution during pregnancy especially during first trimester of pregnancy.
Keywords: Chick embryos, Development, Diclofenac sodium, Mortality, Pregnancy
Graphical Abstract
Highlights
-
•
Teratogenic and embryonic effect of diclofenac sodium on different development stages of chick embryo was investigated.
-
•
Results of first and second trial showed statistically (p<0.01) significant difference in bodyweight, body-length, forelimb and hindlimb length.
-
•
In third trial, DS administration showed a statistically (p<0.01) significant difference in the bodyweight, body-length, forelimb, hindlimb length, liver weight, EE ratio and KSI.
-
•
It was concluded that DS should be used with caution during pregnancy.
1. Introduction
Diclofenac sodium (Dicloran was supplied by SAMI Pharmaceuticals (Pvt) Limited located at Karachi, Sindh, Pakistan) is a nonsteroidal anti-inflammatory drug (NSAID) having anti-inflammatory, analgesic, and antipyretic properties [1], [2], [3]. The mechanism of action of diclofenac sodium is to reduce the activity of cyclooxygenases (COX-1 and COX-2), which can prevent the conversion of arachidonic acid to prostaglandins [4], [5]. Diclofenac works by lowering chemicals in the body that induce pain and inflammation and it is often used by women of reproductive age for many diseases [6]. Diclofenac sodium is commonly used to treat arthritis, gout, dysmenorrhea and other diseases that cause pain and inflammation [7], [8], [9], [10]. Although Diclofenac is an efficient pain reliever, it has side effects in people and animals due to the reduction of prostaglandin production. Diclofenac-mediated nephrotoxicity, hepatotoxicity, and gastrointestinal injuries are the drug's most common adverse effects, which are caused by the drug's stimulation of oxidative damage [11], [12], [13], [14]. The production of reactive metabolites such as 5-hydroxydiclofenac and N,5-dihydroxydiclofenac is thought to be the cause of diclofenac's negative effects. These may result in an excess of oxidants and free radicals in the body, known as reactive oxygen and nitrogen species, causing oxidative stress and hepatocellular damage in rats [15], [16].
Diclofenac sodium is a type of non-steroidal anti-inflammatory drug that usage in obstetrics and gynaecology. NSAID use during pregnancy, on the other hand, has many negative impacts for both the mother and the foetus [1]. Some investigation has found that diclofenac sodium may have teratogenic effects on some organs when used throughout the perinatal period [17], [18]. As birds lay eggs, their embryos produce an allantois, making them more closely related to mammals than reptiles, amphibians, or fish in terms of growth. The developmental mechanisms of birds and mammals are strongly conserved and thus have direct biomedical significance. This allows for a direct assessment of the toxicant's prenatal effects without having to consider maternal metabolism or placental transfer [19]. The goal of the present study was to observe how diclofenac sodium affects the development of chick embryos. It was determined whether it has any potential side effect when used during pregnancy. The nature and extent of these findings were to find out what concentration causes embryo death. Different parameters were examined to evaluate its effect.
2. Materials and method
2.1. Experimental area and maintenance of eggs
The experiment was conducted at Zoology Lab-II of Ghazi University, DG Khan, from September 2020 to April 2021. Total 100 fertilized, non-incubated chicken (Gallus gallus domesticus) eggs were obtained from a Government poultry farm, Bahawalpur, Punjab, Pakistan. The eggs were incubated at a constant temperature of 37.5±2 °C and a humidity level of 65–75% with 45˚ angle of rotation after 2 hours. Eggs were examined to detect embryos by candling. Eggs that do not show embryonic formation were considered unfertilized and added a new one to keep the number of eggs in each group constant.
2.2. Drug used
Diclofenac Sodium (Dicloran injection 75 mg/3 mL injection) was used for the experiment. Dicloran (Diclofenac Sodium) was supplied by SAMI Pharmaceuticals (Pvt) Limited located at Karachi, Sindh, Pakistan. It was obtained commercially from a local market of Dera Ghazi Khan, Punjab, Pakistan.
2.3. Experimental design
Diclofenac sodium was administered within eggs in various doses and the findings were compared to those of control groups of the same age. First, the eggshell was sterilized with 70% ethanol and marked its air space with a permanent marker. With the use of an egg driller, a tiny hole was drilled at the centre of the air sac. Diclofenac sodium was administrated at various doses of 0.1 mL, 0.2 mL, 0.3 mL in groups A, B, and C, respectively. Group D received 0.3 mL saline solution (0.9% NaCl) and Group E remained un-injected. Both served as control. After administration of diclofenac sodium, paraffin wax was used to seal the hole and all the eggs were transferred into an incubator carefully.
The experiment was conducted in 4 trials based on different days of dose administration and observation days. A total of 25 fertilized chicken eggs were used for each trial of the experiment. Eggs were divided into 5 groups, A, B, C, D and E, each group containing 5 eggs. In trials 1 and 2, diclofenac sodium was given at 0 hours’ incubation and opened on the 7th and 14th day of incubation. Embryos were removed from eggs and morphological features viz body length (cm), forelimb length (cm) and hind limb length (cm) of each embryo were measured by ruler/measuring tape. The body weight (gm) of the embryo was taken by using a digital electronic balance (Model no. FA2204). In the third trial, diclofenac sodium was administered on the 14th day and the embryos were examined on the 18th day. Different morphological parameters were measured as described above. Embryos were dissected and their organs were removed from the body. Liver, kidney, and heart weight were weighed (gm) by using a digital electronic balance (Model no. FA2204). Different indices such as The Embryo: Egg weight ratio (EE), HSI, RSI and CSI ratios were calculated by using the following equations [20]:
In trial 4, diclofenac was administered on the 18th day and observed for hatching. Different morphological parameters of chicks were measured. The internal organs were weighed and calculated different indices (except EE ratio) as described above. Different hatching days of chicks were also observed. Based on overall morphology, the embryos of the 7th, 14th, 18th days and chicks of the 21st day were classified into one of three groups: [1] normal development; [2] no development; or [3] under development. Embryos and chicks that showed features of stage 31 (Observation day 7), stage 40 (Observation day 14), stage 44 (Observation day 18) and stage 46 (Observation day 21) according to Hamburger and Hamilton stages were considered normal [21].
Mortality rate in each trial was also observed. Mortality percentage (%) was calculated by using the following formula:
| Mortality Percentage (%) = Dead chicks/embryos/Total number of embryos/chicks × 100 |
2.4. Statistical analysis
All data were analysed by using the SPSS software 20 program. All the data were represented as group Mean ± SE and were compared with the control by using one-way ANOVA followed by Duncan's multiple range test for post hoc analysis. The mortality rate was calculated by mortality percentage (%). p<0.05 was considered statistically significant and highly statistically significant if p<0.01.
3. Results
3.1. Morphological measurements
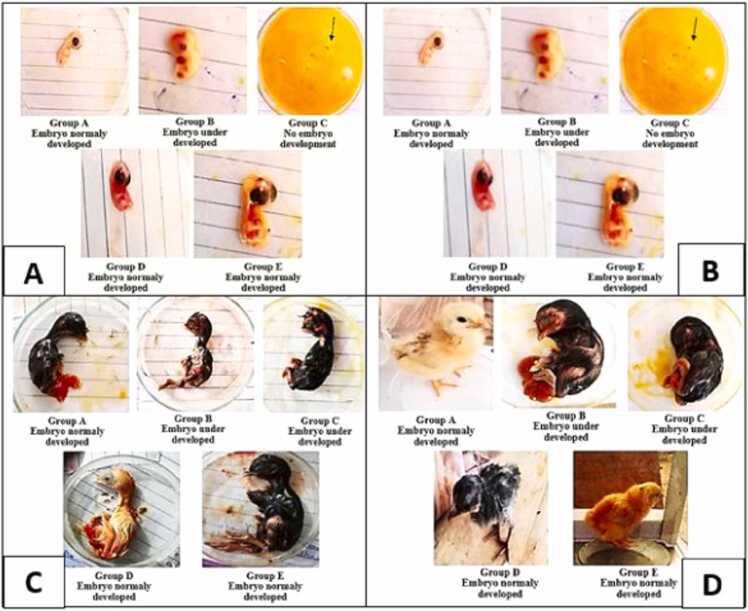
Results of trials 1 and 2 showed there was a highly statistically significant difference (p<0.01) was present between experimental and control groups as shown in Table 1. No development was observed in group C that was given a high dose of diclofenac sodium. Its results revealed that when diclofenac sodium was given at the early stages of development, its high dose retards the development and medium dose also adversely affected the embryos morphologically. The results of trial 3 have been given in Table 2. There was a highly statistically significant difference (p<0.01) was present in body weight, body length, forelimb length and hind limb length as compared to control groups. however, beak size showed no significant difference (p>0.05) [22].
Table 1.
Morphological measurement (Mean±SE) of chick embryos administered different doses of diclofenac sodium at on 0 hours’ incubation.
| Morphological Parameters | Observation days |
Experimental groups |
Control groups |
p-value | |||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | |||
| Body Weight (g) | 7 | 0.46±0.16ab | 0.21±0.11bc | 0.00±0.00c | 0.58±0.04a | 0.62±0.06a | 0.000** |
| 14 | 1.67±0.5b | 0.29±0.09c | 0.00±0.00c | 6.92±0.71a | 5.93±0.32a | 0.000** | |
| Body Length (cm) | 7 | 2.28±0.12ab | 1.80±0.38b | 0.00±0.00c | 2.30±0.06ab | 2.52±0.14a | 0.000** |
| 14 | 3.00±0.45b | 2.00±0.32b | 0.00±0.00c | 5.70±0.41a | 5.80±0.37a | 0.000** | |
| Forelimb length (cm) | 7 | 0.30±0.13ab | 0.10±0.08bc | 0.00±0.00c | 0.38±0.02a | 0.50±0.06a | 0.001** |
| 14 | 1.00±0.32b | 0.20±0.20c | 0.00±0.00c | 1.90±0.15a | 2.00±0.00a | 0.000** | |
| Hind limb length (cm) | 7 | 0.38±0.16bc | 0.24±0.17 cd | 0.00±0.00d | 0.76±0.07a | 0.70±0.06ab | 0.001** |
| 14 | 1.60±0.24c | 0.20±0.20d | 0.00±0.00d | 3.16±0.12a | 2.44±0.27b | 0.000** | |
Values a, b, c, d with different letters in a row differ significantly (** p<0.01)
Table 2.
Morphological measurement (Mean±SE) of chick embryos/chicks administered different doses of diclofenac sodium on 14th and 18th day of incubation.
| Morphological Parameters | Observation days | Experimental groups |
Control groups |
p-value |
|||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | |||
| Body Weight (g) | 18 | 13.26±2.69ab | 5.21±1.70c | 10.52±0.37bc | 20.11±3.76a | 20.24±1.73a | 0.001** |
| 21 | 32.01±2.73 | 29.33±1.17 | 28.92±1.10 | 28.91±2.64 | 29.96±0.94 | 0.758 | |
| Body Length (cm) | 18 | 6.62±0.43bc | 4.84±0.42d | 5.80±0.16 cd | 7.78±0.74ab | 8.18±0.31a | 0.000** |
| 21 | 8.14±0.34bc | 7.90±0.07c | 8.40±0.34bc | 9.28±0.35a | 8.94±0.12ab | 0.011* | |
| Forelimb length (cm) | 18 | 2.06 ±0.16b | 1.60±0.15b | 1.76±0.10b | 2.94±0.24a | 2.82±0.16a | 0.000** |
| 21 | 3.24±0.16b | 3.22±0.07b | 3.28±0.10b | 4.12±0.16a | 4.18±0.14a | 0.000** | |
| Hind limb length (cm) | 18 | 3.06 ±0.38b | 2.48±0.19b | 3.02 ±0.09b | 4.50±0.47a | 4.36±0.19a | 0.000** |
| 21 | 4.36±0.19b | 3.92±0.07b | 4.22±0.32b | 5.70±0.27a | 5.60±0.08a | 0.000** | |
| Beak Size (cm) | 18 | 0.54±0.04 | 0.42±0.07 | 0.50±0.04 | 0.58±0.10 | 0.58±0.02 | 0.394 |
| 21 | 0.70±0.04 | 0.62±0.04 | 0.60±0.03 | 0.68±0.05 | 0.62±0.02 | 0.302 | |
Values a, b, c, d with different letters in a row differ significantly (** p<0.01, * p<0.05)
Table 2 shows the mean and standard error for morphological measurements of 21st day old chicks. Forelimb length and hind limb length were highly statistically significantly different (p<0.01). Body length was significantly different (p>0.05). Body weight and beak size showed a non-significant (p>0.05) difference between experimental and control groups [23].
3.2. Internal organ weight
The result of trial 3 for internal organ weight has been given in Table 3. The difference in kidney, and heart weight between the experimental and control groups was non-significant (p>0.05). Liver weight showed a highly statistically significant difference (p<0.01). As shown in Table 3, the result of trial 4 showed that liver, kidney and heart weight decrease in experimental group B when compared with control groups. Heart weight was statistically significantly different (p<0.01). Liver weight was significant (p<0.05), while kidney weight showed non-significance (p>0.05) with control groups [24].
Table 3.
Internal organ weight (Mean±SE) of chick embryos/chick administered different doses of diclofenac sodium on 14th and 18th day of incubation.
| Internal organ weight | Observation days | Experimental groups |
Control groups |
p-value | |||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | |||
| Liver Weight (g) | 18 | 0.28±0.04b | 0.18±0.09b | 0.24±0.05b | 0.60±0.09a | 0.30±0.04b | 0.002** |
| 21 | 0.72±0.11a | 0.50±0.04b | 0.64±0.03ab | 0.79±0.07a | 0.78±0.06a | 0.031* | |
| Kidney Weight (g) | 18 | 0.05±0.02 | 0.10±0.05 | 0.04±0.01 | 0.07±0.04 | 0.05±0.01 | 0.742 |
| 21 | 0.15±0.05 | 0.10±0.04 | 0.16±0.01 | 0.19±0.02 | 0.20±0.04 | 0.268 | |
| Heart Weight (g) | 18 | 0.25±0.18 | 0.10±0.06 | 0.07±0.01 | 0.17±0.04 | 0.12±0.01 | 0.645 |
| 21 | 0.14±0.01c | 0.12±0.01c | 0.16±0.01bc | 0.21±0.02ab | 0.24±0.04a | 0.002** | |
Values a, b, c with different letters in a row differ significantly (** p<0.01, * p<0.05)
3.3. EE ratio, HSI, KSI and CSI
Table 4 lists the different ratios investigated in trial 3. When compared to control groups, EE and KSI showed high significance (p<0.01), while HSI and CSI were non-significant. These values showed variations in different groups. In trial 4, between the experimental and control groups, HSI and CSI revealed a highly statistically significant difference (p<0.01) [24]. KSI, on the other hand, revealed no significant difference (p>0.05) as given in Table 4.
Table 4.
EE, HIS, KSI, and CSI ratios (Mean±SE) of chick embryos/chick administered different doses of diclofenac sodium on 14th and 18th day of incubation.
| Ratios | Observation days |
Experimental groups |
Control groups |
p-value |
|||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | |||
| EE | 18 | 28.04±4.96b | 11.17±3.95c | 23.50±0.98bc | 41.68±7.33a | 43.34±3.00a | 0.000** |
| - | - | - | - | - | - | - | - |
| HSI | 18 | 2.30±0.40 | 3.09±0.71 | 2.23±0.44 | 3.80±1.18 | 1.76±0.09 | 0.257 |
| 21 | 2.25±0.31ab | 1.72±0.17b | 2.21±0.07ab | 2.76±0.10a | 2.58±0.14a | 0.005** | |
| KSI | 18 | 0.58±0.27b | 1.81±0.50a | 0.42±0.04b | 0.46±0.21b | 0.26±0.04b | 0.004** |
| 21 | 0.47±0.15 | 0.33±0.14 | 0.57±0.04 | 0.66±0.02 | 0.66±0.12 | 0.196 | |
| CSI | 18 | 1.69±1.06 | 1.53±0.60 | 0.66±0.05 | 0.88±0.19 | 0.62±0.03 | 0.526 |
| 21 | 0.46±0.06c | 0.42±0.04c | 0.55±0.06bc | 0.75±0.03ab | 0.82±0.13a | 0.003** | |
Values a, b, c with different letters in a row differ significantly (** p<0.01, * p<0.05)
3.4. Observation of chick embryos/chicks according to Hamburger and Hamilton stages of development
Different doses of diclofenac sodium were given at different stages of development to evaluate whether they were developing normally or not, chick embryos/chicks were compared with the hamburger and Hamilton developmental stages. Embryos that were developing according to the hamburger and Hamilton stage were considered normal. When diclofenac sodium was given at 0 hours’ incubation, it retards development in experimental group C given a high dose as compared with control groups [8]. In group C, no embryo was observed, only blood vessels were present, which showed that the embryo formed but failed to develop as shown by an arrow in Fig. 1 (A, B, C and D). Embryos/chicks of each trial that were either normal, showed no development, or under-developed have been given in Table 5 and Fig. 1.
Fig. 1.
Observation for normal development of chick embryos/chicks according to Hamburger and Hamilton developmental stages in different experimental (A, B, C) and control groups (D, E); A. Chick embryos on 7th incubation day given diclofenac sodium on 0 hr’s incubation; B. Chick embryos on 14th incubation day given diclofenac sodium on 0 hr’s incubation; C. Chick embryos on 18th incubation day given diclofenac sodium on 14th day of incubation; D. Chicks on hatching day given diclofenac sodium on 18th day of incubation.
Table 5.
Observation of chick embryos/chick according to Hamburger and Hamilton stages of development administered different doses of diclofenac sodium.
|
Development |
Observation days |
Hamburger and Hamilton stages |
Experimental model |
|||||
|---|---|---|---|---|---|---|---|---|
|
Experimental groups |
Control groups |
Total |
||||||
| A | B | C | D | E | ||||
| Normal development | 7 | Stage 31 | 3/5 | 1/5 | 0/5 | 5/5 | 5/5 | 14/25 |
| 14 | Stage 40 | 0/5 | 0/5 | 0/5 | 5/5 | 5/5 | 10/25 | |
| 18 | Stage 44 | 4/5 | 2/5 | 3/5 | 4/5 | 5/5 | 18/25 | |
| 21 | Stage 46 | 2/5 | 1/5 | 0/5 | 5/5 | 5/5 | 13/25 | |
| No development | 7 | Stage 31 | 0/5 | 0/5 | 5/5 | 0/5 | 0/5 | 5/25 |
| 14 | Stage 40 | 0/5 | 0/5 | 5/5 | 0/5 | 0/5 | 5/25 | |
| 18 | Stage 44 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/25 | |
| 21 | Stage 46 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/25 | |
| Under-developed | 7 | Stage 31 | 2/5 | 4/5 | 0/5 | 0/5 | 0/5 | 6/25 |
| 14 | Stage 40 | 5/5 | 5/5 | 0/5 | 0/5 | 0/5 | 10/25 | |
| 18 | Stage 44 | 1/5 | 3/5 | 2/5 | 1/5 | 0/5 | 7/25 | |
| 21 | Stage 46 | 3/5 | 4/5 | 5/5 | 0/5 | 0/5 | 12/25 | |
3.5. Mortality percentage (%)
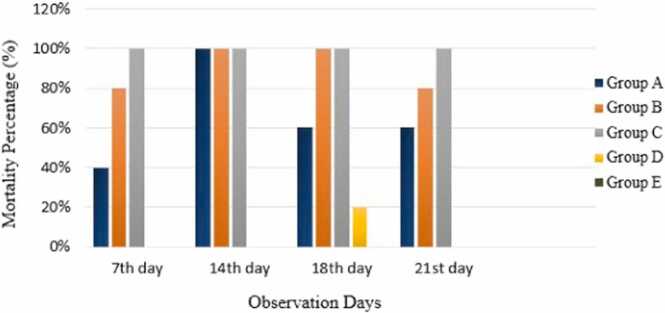
Fig. 2 showed that as the dose of diclofenac sodium was increased, the death rate increased. When diclofenac sodium was administered at 0 hours of incubation the mortality rate was 40%, 80%, and 100% for doses of 0.1 mL, 0.2 mL, and 0.3 mL, respectively on the 7th day of observation, Embryos revealed a 100% mortality rate at all dosages of diclofenac sodium when eggs were opened on the 14th day of incubation. It was observed that giving diclofenac sodium at an early stage increases mortality when the dose was increased [25]. However, after only a few days of drug administration, its low dose also causes the death of all embryos. Control group showed a 0% mortality rate.
Fig. 2.
Hatching of chicks on different days in experimental and control groups; A: 0.1 mL Diclofenac sodium; B: 0.2 mL Diclofenac sodium; C: 0.3 mL Diclofenac sodium; D: 0.3 mL Saline solution; E: Un-Injected.
When diclofenac sodium was given during the development of chick embryos on the 14th day of incubation, it showed 60%, 100%, 100% mortality at 0.1 mL, 0.2 mL and 0.3 mL dose of diclofenac sodium, respectively. Control group D showed 20% while group E showed 0% mortality. There was a gradual increase in mortality rate with an increase in the dose of diclofenac sodium. On the hatching day of chicks, the mortality rate was 60%, 80% and 100% in experimental groups A, B and C, respectively, while control groups D and E showed 0% mortality.
3.6. Day of hatching
Eggs were incubated for 18 days and administered the diclofenac sodium in different concentrations. After treatment, all eggs were again put in an incubator and observed hatching. It was found that chicks in different groups were hatched on different days. The hatching of chicks on different days has been given in Fig. 3. It was concluded that diclofenac sodium has exerted its deleterious effect. When diclofenac sodium was given at the early stages of development or during development, it retards the development and increases the mortality rate. This study provides a suggestion about the use of diclofenac sodium during pregnancy that might cause a teratogenic effect on the human embryo, as the first month of vertebral development in mammals is represented by a chick embryo model. Therefore, it must be used with caution. Further study would be helpful to understand the deleterious effect of diclofenac sodium [26].
Fig. 3.
Mortality percentage (%) of chick embryos/chicks on different observation days administered different doses of diclofenac sodium on different days.
4. Discussion
The current study showed that a high dose of diclofenac sodium had reduced the body weight of 7th, 14th, 18th day old chick embryos and body weight decreased in the 21st day old chicks as compared to control groups. It was in line with the findings of Hussain [23], who reported as high doses had reduced the body weight of broiler chicks, pigeons, quail and mynah. Administration of diclofenac sodium (10 mg/kg) had decreased the percentage of body weight in treated rats as compared to the control group [27]. Praskova [28] had also reported a decrease in body weight after exposure to the high dose of diclofenac sodium. The use of diclofenac daily for 21 days in albino Wistar rats resulted in 18.5% reduction in body weight [29]. In contrast to the present study, it was reported that diclofenac sodium had increased body weight in arthritis-induced rats as compared with the control group [30]. Diclofenac sodium insertion for a long duration had not been associated with reduced body weight [31].
The present study revealed that a high dose of diclofenac sodium decreased the body length in experimental groups as compared with control groups. It was consistent with Ertekin findings that had reported the decreased somite number, crown-rump length in high dose experimental groups compared with a control group [8]. It was not supported by the findings of Praskova that had found no significant difference in the body length of fish when exposed to different concentrations of diclofenac sodium [28]. The current results showed that a high dose of diclofenac sodium decreased the hind limb length as compared to control groups. The same result was reported by Chan when an experiment was conducted on a rat embryo model. And found that Hind limb, flexion and caudal NT were significantly lower when exposed to the high dose of diclofenac sodium, while low dose showed no effect [7].
It was found in present study; diclofenac sodium was administered within eggs on the 14th and 18th day of incubation and observation on the 18th and 21st day showed that a high dose of diclofenac sodium had reduced the kidney weight of the chick as compared to the control group. The present study show correlation with the findings of Hussain who conducted an experiment on broiler chicks, pigeons, quail, and mynah. And found that relative kidney weight was decreased in mynah and quail in the treatment group as compared to the control group. But results were inconsistent with broiler chicks and pigeons as relative kidney weight was increased in the high-dose diclofenac sodium treated group as compared to the control group [23]. It was found that an increase in the relative weight of kidney and liver in pigeon and broiler chicks might be due to swelling and increased organs size due to gross changes that occur during necropsy in birds. Similarly, toxicity due to diclofenac in other birds had been described [32], [33].
5. Conclusion
Present study showed that diclofenac sodium treated groups showed a decrease in liver weight of 18th day old chick embryo and 21st day old chicks as compared with control groups. Experiment was conducted on broiler chicks, pigeons, quail, and mynah. And found that in mynah relative liver weight was decreased in the treatment group. But results were the opposite in broiler chicks, pigeons and quail that showed an increased relative weight of liver at a high dose of diclofenac sodium as compared to control [23]. There was considerable increase (4.3%) in relative liver weight in rats, indicating the negative effects of diclofenac toxicity [29]. The effect of diclofenac sodium on the liver in other mammals and humans has been reported. An increase in the relative liver weight of rats as compared to the control group had also been found [27], [34].
It was evaluated that high dose had reduced the heart weight in diclofenac sodium treated groups as compared to the control group. A similar finding was reported by Gevrek who had found that diclofenac sodium at a dose of 1 mg/kg body weight in rats had decreased the volume of heart ventricle of both females and males in experimental groups than the control group [35]. The current study showed a decrease in the hepato-somatic index of the high dose experimental group as compared with control groups. The same result was found after conducting an experiment on freshwater fish and found reduced hepato-somatic index (HSI) as liver size reduced in them due to the diclofenac sodium (0.2 μg/kg) effect [36]. But the present study was not consistent with Bickley that found no difference in hepato-somatic index between any of the treatment groups after 48 h or 21 d exposure of diclofenac sodium in fish [4], [37].
The present findings demonstrated the diclofenac sodium’s effect on early embryonic development. When given diclofenac sodium on 0 hours of incubation, it retarded the embryo development. The same result was reported by Nielsen who had found that when diclofenac was given at a dose of 40 g/mL inhibited implantation and retarded embryo development. Also, NSAID use during pregnancy is linked to miscarriages [38]. The current study showed the decrease in organ weight of 21st day old chicks (hatched chicks) as compared to control groups It was also found that diclofenac sodium’s usage during delivery had teratogenic effects on other organs [39].
High mortality rate was observed in treatment groups given a high dose of diclofenac sodium as compared to control groups which showed no mortality. Previous study had also showed a high mortality rate in groups treated with a high dose of diclofenac sodium [40]. It was also reported by Ramzan who had found that mortality increases in different treatment groups with increasing the dose of diclofenac used either by intramuscular or oral route [41]. Diclofenac sodium’s toxicity had been reported in vulture populations and death occurred after a few days of exposure [42], [43]. Our study can add the information in SmPC regarding use of diclofenac sodium during the early days of pregnancy. The early stages of development of an embryo in birds and mammal have more similarities such as development of allantois. We can guess the effect of use of diclofenac sodium on embryo of few days without exposing mother. According to Siu et al. [44] diclofenac can accumulate in the foetus fluid and removal of this drug need development of kidney. Nielsen et al. [45] found that when diclofenac was given at a dose of 40 g/mL inhibited implantation and retarded embryo development.
Diclofenac is a “non-steroidal anti-inflammatory drug” (NSAID), acting primarily by inhibiting prostaglandin synthesis through its activity on cyclooxygenase (COX) enzymes, and assigned a pregnancy risk class C designation by the FDA. After oral administration, diclofenac undergoes first-pass metabolism which decreases its systemic bioavailability to 50%, and its elimination half-life is about 2 h; diclofenac crosses the human placenta. The maternal administration of diclofenac results in a rapid accumulation of the drug in the foetus during the first trimester of pregnancy [44]. The use of diclofenac increased the risk of neural tube defects and hypospadias in male neonates [46].
The chick embryo is an excellent model for in vivo investigation of drug toxicity, biocompatibility, biodistribution, and pharmacokinetics because of its simplicity, low cost, strong reproducibility of outcomes, reduced ethical and legal problems, and the mother has little effect on the drug's pharmacokinetics [47], [48], [49]. As birds lay eggs, their embryos produce an allantois, making them more closely related to mammals than reptiles, amphibians, or fish in terms of growth. The developmental mechanisms of birds and mammals are strongly conserved and thus have direct biomedical significance. This allows for a direct assessment of the toxicant's prenatal effects without having to consider maternal metabolism or placental transfer [50].
CRediT authorship contribution statement
Muhammad Arif: Project administration, Methodology, Investigation, Formal analysis, Data curation. Ayesha Iman: Visualization, Validation, Methodology, Investigation, Data curation. Muhammad Khan: Visualization, Software, Methodology, Investigation. Shakeel Ahmad: Visualization, Software, Methodology, Investigation, Data curation. Muhammad Farooq: Writing – review & editing, Visualization, Supervision, Investigation, Data curation, Conceptualization. Khalil Ahmad: Writing – review & editing, Supervision, Investigation, Formal analysis, Data curation, Conceptualization. Sana Khan: Writing – original draft, Software, Methodology, Formal analysis, Conceptualization. Awais Khalid: Formal analysis, Visualization, Writing – review & editing. Muhammad Altaf Nazir: Formal analysis, Investigation, Software, Visualization, Writing – review & editing. Fiaz Mazari: Software, Methodology, Investigation, Data curation. Hafiz Sabir: Software, Methodology, Investigation, Formal analysis, Data curation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors are thankful to the Ghazi University, Dera Ghazi Khan, Pakistan for providing facilities to complete this work.
Handling Editor: Prof. L.H. Lash
Data availability
No data was used for the research described in the article.
References
- 1.Aygün D., Kaplan S., Odaci E., Onger M.E., Altunkaynak M.E. Toxicity of non-steroidal anti-inflammatory drugs: a review of melatonin and diclofenac sodium association. Histol. Histopathol. 2012 doi: 10.14670/HH-27.417. [DOI] [PubMed] [Google Scholar]
- 2.Orabi S.H., Abd Eldaium D., Hassan A., El Sabagh H.S., Abd Eldaim M.A. Allicin modulates diclofenac sodium induced hepatonephro toxicity in rats via reducing oxidative stress and caspase 3 protein expression. Environ. Toxicol. Pharmacol. 2020;74 doi: 10.1016/j.etap.2019.103306. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad K., Nazir M.A., Qureshi A.K., Hussain E., Najam T., Javed M.S., et al. Engineering of Zirconium based metal-organic frameworks (Zr-MOFs) as efficient adsorbents. Mater. Sci. Eng. B. 2020;262 [Google Scholar]
- 4.Scialis R.J., Aleksunes L.M., Csanaky I.L., Klaassen C.D., Manautou J.E. Identification and characterization of efflux transporters that modulate the subtoxic disposition of diclofenac and its metabolites. Drug Metab. Dispos. 2019;47(10):1080–1092. doi: 10.1124/dmd.119.086603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun C., Lin S., Li Z., Liu H., Liu Y., Wang K., et al. iTRAQ-based quantitative proteomic analysis reveals the toxic mechanism of diclofenac sodium on the kidney of broiler chicken. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2021;249 doi: 10.1016/j.cbpc.2021.109129. [DOI] [PubMed] [Google Scholar]
- 6.Kaelin W.G., Jr, Thompson C.B. Clues from cell metabolism. Nature. 2010;465(7298):562–564. doi: 10.1038/465562a. [DOI] [PubMed] [Google Scholar]
- 7.Chan L., Chiu P., Siu S., Lau T. A study of diclofenac-induced teratogenicity during organogenesis using a whole rat embryo culture model. Hum. Reprod. 2001;16(11):2390–2393. doi: 10.1093/humrep/16.11.2390. [DOI] [PubMed] [Google Scholar]
- 8.Ertekin T., Bilir A., Aslan E., Koca B., Turamanlar O., Ertekin A., et al. The effect of diclofenac sodium on neural tube development in the early stage of chick embryos. Folia Morphol. 2019;78(2):307–313. doi: 10.5603/FM.a2018.0080. [DOI] [PubMed] [Google Scholar]
- 9.Parveen S., Naseem H.A., Ahmad K., Shah H.-U.-R., Aziz T., Ashfaq M., et al. Design, synthesis and spectroscopic characterizations of medicinal hydrazide derivatives and metal complexes of malonic ester. Curr. Bioact. Compd. 2023;19(4):31–46. [Google Scholar]
- 10.Rana S., Ahmad K., Muhammad Asif H., Wadood A., Ahmad S., Ashfaq M., et al. Nutritional Assessment among Undergraduate Students of the Islamia University of Bahawalpur. J. Food Nutr. Disord. 2020 [Google Scholar]
- 11.Ahmed A.Y., Gad A.M., El-Raouf O.M.A. Curcumin ameliorates diclofenac sodium-induced nephrotoxicity in male albino rats. J. Biochem. Mol. Toxicol. 2017;31(10) doi: 10.1002/jbt.21951. [DOI] [PubMed] [Google Scholar]
- 12.Nouri A., Izak-Shirian F., Fanaei V., Dastan M., Abolfathi M., Moradi A., et al. Carvacrol exerts nephroprotective effect in rat model of diclofenac-induced renal injury through regulation of oxidative stress and suppression of inflammatory response. Heliyon. 2021;7(11) doi: 10.1016/j.heliyon.2021.e08358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon J.P., Evan Prince S. Aqueous leaves extract of Madhuca longifolia attenuate diclofenac-induced hepatotoxicity: impact on oxidative stress, inflammation, and cytokines. J. Cell. Biochem. 2018;119(7):6125–6135. doi: 10.1002/jcb.26812. [DOI] [PubMed] [Google Scholar]
- 14.Fatima K., Ahmad K., Rajpoot S.R., Tasleem M.W., Asad M., Ali S. Health and Nutritional Status of Certain Lactating Mothers of Bahawalpur, Pakistan.
- 15.Ogbe R.J., Agu S., Luka C.D., Adoga G.I. Comparative study on the effects of Alchornea cordifolia and Cassia spectabilis leaf extracts on diclofenac-induced liver and kidney injuries in rats. Comp. Clin. Pathol. 2020;29:927–935. [Google Scholar]
- 16.Bort R., Ponsoda X., Jover R., Gómez-Lechón M.J., Castell J.V. Diclofenac toxicity to hepatocytes: a role for drug metabolism in cell toxicity. J. Pharmacol. Exp. Ther. 1999;288(1):65–72. [PubMed] [Google Scholar]
- 17.Arslan H., Aktaş A., Elibol E., Esener O.B.B., Türkmen A.P., Yurt K.K., et al. Effects of prenatal diclofenac sodium exposure on newborn testis: a histomorphometric study. Biotech. Histochem. 2016;91(4):277–282. doi: 10.3109/10520295.2016.1151551. [DOI] [PubMed] [Google Scholar]
- 18.Gevrek F., Kara M., RAĞBETLİ M.Ç., ASLAN H. Effects of prenatally exposed diclofenac sodium on rat heart tissue: a stereological and histological study. Turk. J. Med. Sci. 2015;45(3):474–480. doi: 10.3906/sag-1404-173. [DOI] [PubMed] [Google Scholar]
- 19.Smith S.M., Flentke G.R., Garic A. Avian models in teratology and developmental toxicology. Dev. Toxicol. Methods Protoc. 2012:85–103. doi: 10.1007/978-1-61779-867-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh M., Singh U., Ourung B. Evaluation of egg weight and its various measurement attributes in indigenous Aseel breed of chicken. Indian J. Poult. Sci. 2000;35(3):312–314. [Google Scholar]
- 21.Hamburger V., Hamilton H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88(1):49–92. [PubMed] [Google Scholar]
- 22.McBride D.J., Jr, Hahn R.A., Silver F.H. Morphological characterization of tendon development during chick embryogenesis: measurement of birefringence retardation. Int. J. Biol. Macromol. 1985;7(2):71–76. [Google Scholar]
- 23.Hussain I., Khan M.Z., Khan A., Javed I., Saleemi M.K. Toxicological effects of diclofenac in four avian species. Avian Pathol. 2008;37(3):315–321. doi: 10.1080/03079450802056439. [DOI] [PubMed] [Google Scholar]
- 24.Ramzan M., Ashraf M., Hashmi H., Iqbal Z., Anjum A. Evaluation of diclofenac sodium toxicity at different concentrations in relation to time using broiler chicken model. J. Anim. Plant Sci. 2015;25:2. [Google Scholar]
- 25.Lahijani M.S., Tehrani D.M., Varzideh F. Effects of the ELF-MFs on the development of spleens of preincubated chicken embryos. Electromagn. Biol. Med. 2013;32(3):301–314. doi: 10.3109/15368378.2012.712588. [DOI] [PubMed] [Google Scholar]
- 26.Muhammad O., Lama R. Chemical analysis of diclofenac sodium effect on angiogenesis using chorioallantoic membrane assay. Natrosa Lett. 2019;04:108–115. 2022. [Google Scholar]
- 27.Alabi Q.K., Akomolafe R.O., Olukiran O.S., Adeyemi W.J., Nafiu A.O., Adefisayo M.A., et al. The Garcinia kola biflavonoid kolaviron attenuates experimental hepatotoxicity induced by diclofenac. Pathophysiology. 2017;24(4):281–290. doi: 10.1016/j.pathophys.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Shakir S.K., Azizullah A., Murad W., Daud M.K., Nabeela F., Rahman H., et al. Toxic metal pollution in Pakistan and its possible risks to public health. 2016:1-60. [DOI] [PubMed]
- 29.Gupta A., Kumar R., Ganguly R., Singh A.K., Rana H.K., Pandey A.K. Antioxidant, anti-inflammatory and hepatoprotective activities of Terminalia bellirica and its bioactive component ellagic acid against diclofenac induced oxidative stress and hepatotoxicity. Toxicol. Rep. 2021;8:44–52. doi: 10.1016/j.toxrep.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ismail M.F., EL-Maraghy S.A., Sadik N.A. Study of the immunomodulatory and anti-inflammatory effects of evening primrose oil in adjuvant arthritis. Afr. J. Biochem Res. 2008;2:74–80. [Google Scholar]
- 31.Chouhan S., Sharma S. Sub-chronic diclofenac sodium induced alterations of alkaline phosphatase activity in serum and skeletal muscle of mice. Indian J. Exp. Biol. 2011 [PubMed] [Google Scholar]
- 32.Reddy N.P., Anjaneyulu Y., Sivasankari B., Rao K.A. Comparative toxicity studies in birds using nimesulide and diclofenac sodium. Environ. Toxicol. Pharmacol. 2006;22(2):142–147. doi: 10.1016/j.etap.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Swan G.E., Cuthbert R., Quevedo M., Green R.E., Pain D.J., Bartels P., et al. Toxicity of diclofenac to Gyps vultures. Biol. Lett. 2006;2(2):279–282. doi: 10.1098/rsbl.2005.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwaiger J., Ferling H., Mallow U., Wintermayr H., Negele R.D. Toxic effects of the non-steroidal anti-inflammatory drug diclofenac: Part I: histopathological alterations and bioaccumulation in rainbow trout. Aquat. Toxicol. 2004;68(2):141–150. doi: 10.1016/j.aquatox.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Fikret G., Mikail K., Murat Cetin R., Hüseyin A. Effects of prenatally exposed diclofenac sodium on rat heart tissue: a stereological and histological study. Turk. J. Med. Sci. 2015;45(3):474–480. doi: 10.3906/sag-1404-173. [DOI] [PubMed] [Google Scholar]
- 36.Guiloski I.C., Ribas J.L.C., Pereira Ld.S., Neves A.P.P., Silva de Assis H.C. Effects of trophic exposure to dexamethasone and diclofenac in freshwater fish. Ecotoxicol. Environ. Saf. 2015;114:204–211. doi: 10.1016/j.ecoenv.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 37.Peiris J.S., Guan Y., Yuen K. Severe acute respiratory syndrome. Nat. Med. 2004;10(Suppl 12):S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nielsen HS G.L., Larsen H. Risk of adverse birth outcome and miscarriage in pregnant users of non-steroidal anti-inflammatory drugs: population based observational study and case-control study. BMJ. 2001;322(7281):266–270. doi: 10.1136/bmj.322.7281.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arslan H., Aktaş A., Elibol E., Esener O.B.B., Türkmen A.P., Yurt K.K., et al. Effects of prenatal diclofenac sodium exposure on newborn testis: a histomorphometric study. Biotech. Histochem. 2016;91(4):277–282. doi: 10.3109/10520295.2016.1151551. [DOI] [PubMed] [Google Scholar]
- 40.Malovanyy M., Petrushka K., Petrushka I.J.C. Improvement of Adsorption-Ion-Exchange Processes for Waste and Mine Water Purification. Chem. Technol. 2019;(3):372–376. [Google Scholar]
- 41.Ramzan M., Ashraf M., Hashmi H., Iqbal Z., Anjum A. Evaluation of diclofenac sodium toxicity at different concentrations in relation to time using broiler chicken model: Pakistan Agricultural Scientists Forum. 357–365 p.
- 42.Johnson J.A., Gilbert M., Virani M.Z., Asim M., Mindell D.P. Temporal genetic analysis of the critically endangered oriental white-backed vulture in Pakistan. Biol. Conserv. 2008;141(9):2403–2409. [Google Scholar]
- 43.Naidoo V., Swan G.E. Diclofenac toxicity in Gyps vulture is associated with decreased uric acid excretion and not renal portal vasoconstriction. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009;149(3):269–274. doi: 10.1016/j.cbpc.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 44.Siu S.S.N., Yeung J.H.K., Lau T.K. A study on placental transfer of diclofenac in first trimester of human pregnancy. Hum. Reprod. 2000;15(11):2423–2425. doi: 10.1093/humrep/15.11.2423. [DOI] [PubMed] [Google Scholar]
- 45.Risk of adverse birth outcome and miscarriage in pregnant users of non-steroidal anti-inflammatory drugs: population based observational study and case-control study. BMJ. 2001;322(7281):266. [DOI] [PMC free article] [PubMed]
- 46.Zafeiri A., Raja E.A., Mitchell R.T., Hay D.C., Bhattacharya S., Fowler P.A. Maternal over-the-counter analgesics use during pregnancy and adverse perinatal outcomes: cohort study of 151 141 singleton pregnancies. BMJ Open. 2022;12(5) doi: 10.1136/bmjopen-2020-048092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nazem M.N., Amanollahi R., Tavakoli H., Mansouri F. Effect of in Ovo injected methionine on feather follicle formation and its growth in the chicken embryo % Anat. Sci. J. 2015;12(2):83–88. [Google Scholar]
- 48.Vargas A., Zeisser-Labouèbe M., Lange N., Gurny R., Delie F. The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv. Drug Deliv. Rev. 2007;59(11):1162–1176. doi: 10.1016/j.addr.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 49.Haselgrübler R., Stübl F., Essl K., Iken M., Schröder K., Weghuber J. Gluc-HET, a complementary chick embryo model for the characterization of antidiabetic compounds. PLoS ONE. 2017;12(8) doi: 10.1371/journal.pone.0182788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith S.M., Flentke G.R., Garic A. In: Developmental Toxicology: Methods and Protocols. Harris C., Hansen J.M., editors. Humana Press; Totowa, NJ: 2012. Avian models in teratology and developmental toxicology; pp. 85–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.