Abstract
Background
Exercise is recommended for patients with chronic heart failure (CHF) and its intensity is usually set as a percentage of the maximal work rate (MWR) during cardiopulmonary exercise testing (CPX) or a symptom-limited incremental test (SLIT). As these tests are not always available in cardiac rehabilitation due to logistic/cost constraints, we aimed to develop a predictive model to estimate MWR at CPX (estMWR@CPX) in CHF patients using anthropometric and clinical measures and the 6-min walk test (6 MWT), the most widely used exercise field test.
Methods
This is a multicentre cross-sectional retrospective study in a cardiac rehabilitation setting. Six hundred patients with HF in New York Heart Association (NYHA) functional class I-III underwent both CPX and 6 MWT and, through multivariable linear regression analysis, we defined several predictive models to define estMWR@CPX.
Results
The best model included 6 MWT, sex, age, weight, NYHA class, left ventricular ejection fraction (LVEF), smoking status and chronic obstructive pulmonary disease COPD (adjusted R2 = 0.55; 95% LoA −39 to 33 W). When LVEF was excluded as a predictor, the resulting model performed only slightly worse (adjusted R2 = 0.54; 95% LoA −42 to 34 W). Only in 34% of cases was the percentage difference between estMWR@CPX and real MWR@CPX <10% in absolute value. EstMWR@CPX tended to overestimate low values and underestimate high values of true MWR@CPX.
Conclusions
Our results showed a lack of accuracy in the predictive model evaluated; therefore, for an accurate prescription of cycle-ergometer exercise training, it is necessary to assess MWR by CPX or SLIT.
Keywords: Cardiopulmonary exercise testing, 6-Min walking test, Chronic heart failure
Highlights
-
•
In patients with CHF, we assessed equations that predicted the work rate at CPX from 6 MWT
-
•
The equations found were not accurate.
-
•
In patients with CHF, CPX should be performed before cardiac rehabilitation.
List of abbreviations
- 6 MWT
6-min walk test
- BMI
body mass index
- CHF
chronic heart failure
- COPD
Chronic Obstructive Pulmonary Disease
- CPX
cardiopulmonary exercise testing
- estMWR@CPX
estimate MWR at CPX
- ICS =
Istituti Clinici Scientifici
- LoA
Limits of agreement
- LVEF
left ventricular ejection fraction
- MWR
maximal work rate
- MWR@CPX
MWR at CPX
- NHYA
New York Heart Association
- RER
respiratory exchange ratio
- RPE
Rating of Perceived Exertion
- SLIT
symptom-limited incremental test
- VE
ventilation
- VCO2
carbon dioxide production
- VO2
oxygen uptake
- W
Watt
1. Introduction
A large body of evidence supports the role of exercise training as a tool for improving functional capacity and quality of life and reducing hospitalization in patients with chronic heart failure (CHF) [1]. Because of these proven benefits, exercise rehabilitation is recommended as a class 1 intervention in the European Society of Cardiology guidelines for the diagnosis and treatment of acute and chronic heart failure [2,3]. Currently, the exercise intensity is usually recommended as a percentage of the patient's maximal aerobic capacity [usually assessed by maximal oxygen uptake (VO2 max), maximal work rate (MWR)] [4].
The gold-standard assessment of maximal aerobic capacity and training intensity is the cardiopulmonary exercise testing (CPX), typically performed on a treadmill or cycle ergometer, with incremental increases in exercise workload up to the maximal effort, usually limited by symptoms or fatigue [5]. However, CPX is not widely used in the cardiorespiratory rehabilitation setting due to the cost of equipment and the need for specialized staff to perform and interpret the CPX [[6], [7], [8]]. As a result, exercise capacity is often measured using field-based walking tests [[6], [7], [8]] which are easy to perform and interpret and inexpensive. The 6-min walk test (6 MWT), which measures the distance walked by the patient in 6 min at a self-selected pace, is currently the most widely used exercise field test [9].
Several studies in COPD patients have investigated the relationship between 6 MWT and the MWR at CPX (MWR@CPX) [[10], [11], [12], [13]]. These have led to the development of some regression equations to estimate MWR@CPX from the distance walked and other anthropometric parameters in order to set the correct workload during cycle-ergometer exercise training [13]. In CHF patients, the distance walked during the 6 MWT was found significantly related to peak VO2 at CPX (r = 0.63) [14] and some studies have estimated peak VO2 from 6 MWT for prognostic and clinical purposes [[15], [16], [17]]. However, to our knowledge, no studies have directly estimated the MWR@CPX for exercise prescription purposes. Considering the above studies in patients with COPD, we hypothesized that if a significant relationship between 6 MWT and MWR was also found in patients with CHF, this would facilitate the prescription of cycle ergometer training in a rehabilitation setting.
Therefore, the aim of this cross-sectional study was to develop a predictive model to estimate the MWR@CPX (estMWR@CPX) in a large cohort of CHF patients using the distance walked at 6 MWT and a set of anthropometric and clinical measures as covariates.
2. Materials and methods
In this multicentre cross-sectional study, we retrospectively analyzed data from patients with CHF who underwent both CPX and 6 MWT at the Istituti Clinici Scientifici (ICS) Maugeri of Lumezzane, Montescano, and Veruno (Italy) and at the Istituto Cardiologico Monzino (Milano, Italy) between October 2008 and November 2020.
2.1. Patients
We selected CHF patients aged 40–80 years in NYHA class I-III who had undergone 6 MWT and CPX, for the assessment of functional capacity, with less than a 1-week interval between the two tests. The CPX was performed in the absence of established absolute and relative contraindications [18]. Thus, patients were clinically stable on optimal medical therapy with standard HF medications. Exclusion criteria were the use of long‐term oxygen therapy, previous heart transplantation or left ventricular assist device, severe aortic stenosis, neuromuscular or orthopaedic co‐morbidities, and concomitant moderate or more severe chronic obstructive pulmonary disease. The presence of a permanent pacemaker, implantable cardioverter defibrillator, or cardiac resynchronization therapy was not exclusion criteria.
2.2. Outcome measure
The CPX was performed and interpreted according to current international guidelines [19,20] using a stationary ergo spirometer (Quark PFT, COSMED, Rome, Italy) with an electronically braked cycle ergometer (Ergoselect 200, Ergoline GmbH, Bitz, Germany). The load increase protocol was set at 10 W/min and patients pedalled at 60 rpm. In the absence of clinical events (including ECG abnormalities, attainment of maximal HR, hypertensive crisis, hemodynamic instability, and significant desaturation with SpO2 <85%), CPX was stopped when patients reported reaching maximal effort due to muscle fatigue or dyspnea. The MWR achieved (Wmax) was the parameter we used as the dependent variable in our estimation equations.
From the breath‐by‐breath analysis of expiratory gases and ventilation (VE), the respiratory exchange ratio (RER) was measured as VCO2/VO2, and the exercise performance achieved was defined as maximal or near maximal, based on a cut‐off value of 1.05 [[20], [21], [22]].
The 6 MWT was performed, according to the ATS/ERS guidelines [23,24], along a flat corridor of at least 20 m. Each patient received standardized pre-test instructions and verbal encouragement at every minute during the test. Two tests were performed: the first was for familiarization and was not included in the analysis.
A standard 2D-echocardiography was performed in all patients as recommended by the American Society of Echocardiography [25].
The following patient data were also taken into account: demographic (age, sex), anthropometric [weight, height, body mass index (BMI)], and clinical data (hemoglobin, ejection fraction, and presence of any comorbidities and risk factors).
2.3. Statistical analysis
Descriptive statistics are reported as mean ± SD or N (percentage frequency) for continuous and categorical variables, respectively. The association between pairs of variables was assessed by Pearson's correlation coefficient or Kendall's rank correlation coefficient, as appropriate.
Simple and multivariable linear regression analyses were performed to develop predictive models for estimating Wmax at CPET (response variable) using the distance walked at 6 MWT, demographic, anthropometric, and clinical variables as predictors. Specifically, a first very simple model including only the 6 MWT was developed, then a multivariable model including sex, age, and weight in addition to the 6 MWT was developed. Finally, a backward stepwise regression analysis with elimination at the 0.05 significance level was performed including all available candidate predictors to obtain the best possible model.
The agreement between the predicted and the true values of Wmax was assessed using Bland-Altman plots, with computation of the nonparametric 95% limits of agreement (95%LoA, the 2.5-th and 97.5-th percentiles of the difference between paired measurements), which provide an estimate of the interval within which 95% of the differences are expected to lie.
All statistical tests were two-tailed and statistical significance was set at p < 0.05. All analyses were carried out using MATLAB, release 2014a (The MathWorks, Natick, MA, U.S.A.) and the SAS/STAT statistical package, release 9.2 (SAS Institute Inc., Cary, NC, U.S.A.).
3. Results
Of the 728 HF patients screened, 600 (mean age 63 ± 10 years, mean left ventricular ejection fraction 34 ± 10%, NYHA class I-III) met the inclusion/exclusion criteria and were included in this analysis.
Of these, 533 were men (89%), 43% had hypertension, 27.5% had diabetes and 10% had COPD as a comorbidity. Regarding medications, 94% were taking β-blockers, 92% were taking diuretics and 58% were taking statins (Table 1SM in the supplementary material).
The association (Pearson's r) between true MWR@CPX and relevant variables is shown in Table 1. MWR@CPX was significantly associated with 6 MWT, 6MWTwork (obtained as the distance walked at 6 MWT * weight) [26] and anthropometric and clinical data. Fig. 1SM in the supplementary material shows a scatterplot of the relationship between true MWR@CPX and 6 MWT, together with the linear regression line (equation: estMWR@CPX=4.43 + 0.191*6 MWT, adjusted R2 = 0.28).
We then built a simple model including, in addition to 6 MWT, sex, age and weight: the equation obtained was estMWR@CPX = −18.77 + 0.16*6 MWT +9.1*sex (1 Male, 0 Female) - 0.25*Age + 0.55*Weight (Fig. 1). All variables included in the model were significant (i.e. independent predictors of MWR@CPX). The adjusted coefficient of determination (R2) was 0.38. The 95% LoA ranged from −56 to 37 W.
We then performed a backward stepwise regression analysis including all available variables: 6 MWT, age, weight, sex, NYHA class, left ventricular ejection fraction (LVEF), smoking status, and COPD were retained at the 0.05 significance level. The resulting model equation was: estMWR@CPX = 8.83 + 0.14*6 MWT +12.74*sex (1 Male, 0 Female) - 0.32*Age + 0.51*Weight - 19.97*NYHA2 (1 if NYHA class = 2, 0 otherwise) - 31.43*NYHA3 (1 if NYHA class = 3, 0 otherwise) + 0.33*LVEF - 5.58*Smoke (0 Non-smoker, 1 Ex-smoker, 2 Smoker) - 6.55*COPD (0 no COPD, 1 COPD). The adjusted R2 was 0.55 and the 95% LoA ranged from −39 to 33 W.
Because LVEF may not be readily available in all clinical settings, we also run the backward stepwise regression analysis without this variable (Fig. 1). The resulting equation was: MWR@CPX = 17.57 + 0.14*6 MWT +11.50*sex (1 Male, 0 Female) - 0.30*Age + 0.53*Weight - 21.01*NYHA2 (1 if NYHA class = 2, 0 otherwise) - 33.43*NYHA3 (1 if NYHA class = 3, 0 otherwise) - 5.95*Smoke (0 Non-smoker, 1 Ex-smoker, 2 Smoker) - 6.17*COPD (0 no COPD, 1 COPD). The adjusted R2 was 0.54 with 95% LoA ranging from −42 to 34 W.
Fig. 1.
Results for model: estMWR@CPX = 17.57 + 0.14*6 MWT +11.50*sex - 0.30*age +0.53*weight - 21.01*NYHA2 - 33.43*NYHA3 - 5.95*smoking - 6.17*COPD.
Legend: Top panel: Bland-Altman plot (difference between the mean values of estimated and true MWR@CPX). Bottom left panel: graphical representation of the effect of the considered predictors on estimated MWR@CPX. Diamonds represent the expected average change in estMWR@CPX moving from one extreme value to the other of each variable in the model, adjusting for all other variables. The lines around the circles represent the 95% confidence interval: crossing the zero effect line indicates a lack of significance. Bottom right panel: plot of the residuals as a function of predicted values. Sex: 1 male, 0 female; NYHA2: 1 if NYHA class = 2, 0 otherwise; NYHA3: 1 if NYHA class = 3, 0 otherwise; smoking: 0 non-smoker, 1 ex-smoker, 2 smoker; COPD: 0 no COPD, 1 COPD.
Focusing on the Bland-Altman plots, it can be seen that for all models developed, the predicted values of MWR@CPX tended to overestimate low values of true MWR@CPX (positive error) and underestimate high values of MWR@CPX (negative error).
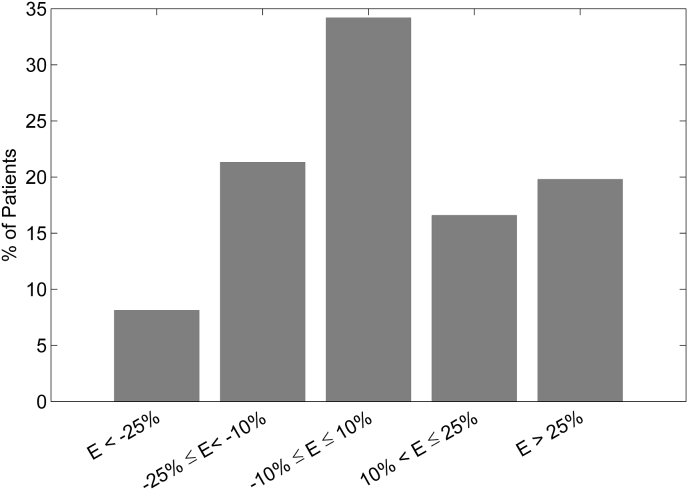
The error (estMWR@CPX – true MWR@CPX) obtained with the last model described (i.e. the one without LVEF), was, in absolute value, less than 5 Watts in 21.7% of patients, between 5 and 10 Watts in 21.6% of patients and greater than 10 Watts in 56.7% of patients. The histogram showing the distribution of patients according to the percentage error (100*(estMWR@CPX – true MWR@CPX)/true MWR@CPX) is shown in Fig. 3. The absolute percentage error was smaller than 10% in 34.2% of patients, between 10% and 25% in 37.9% of patients and greater than 25% in the remaining 27.9% of patients.
Fig. 2.
Histogram showing the distribution of patients according to the percentage difference (E) between estimated and true MWR@CPX.
For the sake of comparison with previous findings reported in COPD patients, we also tested 6MWTwork as a predictor instead of the distance walked at 6 MWT, but no improvement in the predictive ability was observed.
4. Discussion
Our results show that the models we developed to estimate MWR@CPX in CHF patients using the 6 MWT and a set of anthropometric and clinical measures were not sufficiently accurate. In only 34% of our CHF patients was the difference between estimated and actual MWR@CPX less than 10%. Using the estMWR@CPX regression equations would lead to an overestimation or underestimation of the true MWR@CPX. This means that if we applied the regression calculated with the present data set, most HF patients would not start cycle-ergometer training with an optimal workload. Therefore, in our opinion, in HF patients it is mandatory to assess the MWR directly by CPX or a symptom-limited incremental test (SLIT) to accurately define a tailored cycle-ergometer training program.
In addition, the performance of our best model (R2 = 0.55) was worse than reported in COPD patients, both in studies with small cohorts (R2 = 0.67 and R2 = 0.80) [11,13] and in a study with a very large cohort (N = 2096, R2 = 0.67) [27]. Of note, despite the better coefficient of determination reported in COPD patients, Sillen et al. [27] also concluded that this estimation method was not accurate enough to be used in practice.
A possible explanation for the low accuracy of the 6 MWT in estimating MWR@CPX is that the CPX and 6 MWT measure different aspects of exercise tolerance. From a physiological point of view, this could be explained by the principle of exercise specificity [28]: the muscles involved, the movement and the type of tasks required are different between the two tests. In addition, the 6 MWT is a constant exercise test, whereas the CPX usually uses a ramp exercise protocol, and gas exchange and ventilatory kinetics are therefore different [29]. In fact, in patients with severe CHF, peak aerobic capacity is higher on the 6 MWT than on the CPX in almost half of the patients, as is ventilation and heart rate [30]. As the 6 MWT has a constant VO2 for at least 5 min of exercise, these data clearly show that the 6 MWT elicits a greater effort than the CPX in patients with severe HF and therefore the data between the two are not easily comparable.
Therefore, our results suggest that the 6 MWT cannot be used to estimate training work rate when this is calculated as a percentage of peak exercise capacity. Accordingly, a pre-rehabilitation CPX is necessary especially when the focus is on high-intensity cycle-ergometer training. In this regard, the American Heart Association [31], the American Association of Cardiovascular and Pulmonary Rehabilitation [32], the Canadian Association of Cardiac Rehabilitation [33], and the European Association of Preventive Cardiology [[34], [35], [36], [37]] all recommend an ECG-monitored exercise testing as a pre-rehabilitation assessment. Moreover, in order to facilitate interventions on lifestyle and during phase II/phase III cardiac rehabilitation programs, the Italian Alliance for Cardiovascular Rehabilitation and Prevention (ITACARE-P) has recently developed a CPX reporting form specifically oriented to exercise prescription [38].
Despite the recommendations of these leading cardiovascular societies, in Australasia and the UK a lower standard is used to determine functional capacity, based on less technical exercise testing in the form of either the 6 MWT or a shuttle walk test [38]. However, the authors acknowledge that an ECG-monitored exercise stress test should be performed in high-risk patients (those with decompensated HF, uncontrolled arrhythmias, or experiencing angina at rest or on minimal exercise) and in those who wish to participate in a high-intensity exercise program (>75% of maximal heart rate) [39].
Thus, we confirm that it is important to perform a CPX or, at least, a functional sign/symptom-limited exercise test before starting a rehabilitation program using the cycle ergometer for training [3]. Of note, programs based on walking as the main exercise modality are also beneficial for patients with CHF [40] and, for (brisk) walking programs, the use of an incremental shuttle walk test [41] or a 6 MWT has been suggested.
If logistic constraints (unavailability of equipment or skilled personnel) or the setting (e.g. home maintenance programs after cardiac rehabilitation) prevent the assessment of MWR with CPX or an ECG-monitored exercise test, the optimal training intensity should be defined according to current guidelines. Further studies are needed to investigate whether the use of some equations to estimate maximal work rate using the distance walked on the 6 MWT (even if not precise), in combination with the rating of perceived exertion (RPE) and close monitoring could be useful to safely set the correct workload target in these contexts. Further studies will also evaluate whether the assessment of oxygen consumption on the 6 MWT can help to improve the estimation of target exercise load.
4.1. Study limitation
The main limitation of this study is its retrospective nature: however, we are confident in the accuracy of the data collected as our laboratories are equipped with advanced technological devices and highly qualified experts in exercise evaluation.
5. Clinical implication and conclusions
The use of the 6 MWT to prescribe the intensity of exercise to be performed during cycle ergometer training in patients with HF is becoming increasingly common. This is because, compared with cardiopulmonary exercise testing, the 6 MWT is easier to perform, less time-consuming and does not require specialized personnel and equipment to perform and interpret the test. In this study, we investigated the feasibility of building a model that provides an equation to predict the watts in the CPT using the meters walked in the 6 MWT, using anthropometric, demographic and clinical parameters as adjusting factors.
Unfortunately, the results were negative, as the estimation of MWR@CPX using the 6 MWT in patients with HF lacks accuracy, even when conventional clinical variables are added to the model.
The study highlights the importance of the cardiopulmonary testing (at least one exercise test) when prescribing exercise to patients with HF and suggests caution against using the 6 MWT to prescribe exercise training intensity.
Funding sources
This study was supported by the “Ricerca Corrente” funding scheme of the Ministry of Health, Italy.
CRediT authorship contribution statement
Giancarlo Piaggi: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. Mara Paneroni: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. Roberto Maestri: Formal analysis, Visualization, Writing – review & editing. Elisabetta Salvioni: Data curation, Investigation, Writing – review & editing. Ugo Corrà: Data curation, Investigation, Writing – review & editing. Angelo Caporotondi: Data curation, Investigation, Writing – review & editing. Simonetta Scalvini: Data curation, Supervision, Writing – review & editing. Piergiuseppe Agostoni: Data curation, Supervision, Writing – review & editing. Maria Teresa La Rovere: Conceptualization, Data curation, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
All Authors declare no conflict of interest relevant to the research, analysis, or interpretation presented in the manuscript.
Acknowledgements
The authors thank Laura Comini and Adriana Olivares for their technical support.
Handling Editor: D Levy
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcrp.2024.200247.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Taylor R.S., Long L., Mordi I.R., et al. Exercise-based rehabilitation for heart failure: Cochrane systematic review, Meta-analysis, and Trial Sequential analysis. JACC Heart Fail. 2019;7:691–705. doi: 10.1016/j.jchf.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 2.Authors/Task Force Members. McDonagh T.A., Metra M., Adamo M., et al. ESC Scientific Document Group 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022;24:4–131. doi: 10.1002/ejhf.2333. [DOI] [PubMed] [Google Scholar]
- 3.Mezzani A., Hamm L.F., Jones A.M., et al. European association for cardiovascular prevention and rehabilitation; American association of cardiovascular and pulmonary rehabilitation; Canadian association of cardiac rehabilitation aerobic exercise intensity assessment and prescription in cardiac rehabilitation: a joint position statement of the European association for cardiovascular prevention and rehabilitation, the American association of cardiovascular and pulmonary rehabilitation and the Canadian association of cardiac rehabilitation. Eur J Prev Cardiol. 2013;20:442–467. doi: 10.1177/2047487312460484. [DOI] [PubMed] [Google Scholar]
- 4.Shoemaker M.J., Dias K.J., Lefebvre K.M., Heick J.D., Collins S.M. Physical therapist clinical practice guideline for the Management of Individuals with heart failure. Phys. Ther. 2020;100:14–43. doi: 10.1093/ptj/pzz127. [DOI] [PubMed] [Google Scholar]
- 5.Task Force of the Italian Working Group on Cardiac Rehabilitation and Prevention (Gruppo Italiano di Cardiologia Riabilitativa e Prevenzione, GICR) Working Group on Cardiac Rehabilitation and Exercise Physiology of the European Society of Cardiology Statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction: recommendations for performance and interpretation Part III: interpretation of cardiopulmonary exercise testing in chronic heart failure and future applications. Eur. J. Cardiovasc. Prev. Rehabil. 2006;13:485–494. doi: 10.1097/01.hjr.0000201518.43837.bc. [DOI] [PubMed] [Google Scholar]
- 6.Griffo R., Tramarin R., Volterrani M., et al. Società Italiana Cardiologia Ospedalità Accreditata. The Italian survey on cardiac rehabilitation - 2013 (ISYDE.13-Directory): national availability and organization of cardiac rehabilitation facilities. G. Ital. Cardiol. 2016;17:217–224. doi: 10.1714/2190.23666. [DOI] [PubMed] [Google Scholar]
- 7.Yohannes A.M., Connolly M.J. Pulmonary rehabilitation programmes in the UK: a national representative survey. Clin. Rehabil. 2004;18:444–449. doi: 10.1191/0269215504cr736oa. [DOI] [PubMed] [Google Scholar]
- 8.Brooks D., Sottana R., Bell B., Hanna M., Laframboise L., Selvanayagarajah S. Characterization of pulmonary rehabilitation programs in Canada in 2005. Can Respir J. 2007;14:87–92. doi: 10.1155/2007/951498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellet R.N., Adams L., Morris N.R. The 6-minute walk test in outpatient cardiac rehabilitation: validity, reliability and responsiveness--a systematic review. Physiotherapy. 2012;98:277–286. doi: 10.1016/j.physio.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Cavalheri V., Hernandes N.A., Camillo C.A., Probst V.S., Ramos D., Pitta F. Estimation of maximal work rate based on the 6-minute walk test and fat-free mass in chronic obstructive pulmonary disease. Arch. Phys. Med. Rehabil. 2010;91:1626–1628. doi: 10.1016/j.apmr.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Hill K., Jenkins S.C., Cecins N., Philippe D.L., Hillman D.R., Eastwood P.R. Estimating maximum work rate during incremental cycle ergometry testing from six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch. Phys. Med. Rehabil. 2008;89:1782–1787. doi: 10.1016/j.apmr.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 12.Kozu R., Jenkins S., Senjyu H., Mukae H., Sakamoto N., Kohno S. Peak power estimated from 6-minute walk distance in Asian patients with idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease. Respirology. 2010;15:706–713. doi: 10.1111/j.1440-1843.2010.01744.x. [DOI] [PubMed] [Google Scholar]
- 13.Luxton N., Alison J.A., Wu J., Mackey M.G. Relationship between field walking tests and incremental cycle ergometry in COPD. Respirology. 2008;13:856–862. doi: 10.1111/j.1440-1843.2008.01355.x. [DOI] [PubMed] [Google Scholar]
- 14.Faggiano P., D'Aloia A., Gualeni A., Lavatelli A., Giordano A. Assessment of oxygen uptake during the 6-minute walking test in patients with heart failure: preliminary experience with a portable device. Am. Heart J. 1997;134(2 Pt 1):203–206. doi: 10.1016/s0002-8703(97)70125-x. [DOI] [PubMed] [Google Scholar]
- 15.Ross R.M., Murthy J.N., Wollak I.D., Jackson A.S. The six-minute walk test accurately estimates mean peak oxygen uptake. BMC Pulm. Med. 2010;10:31. doi: 10.1186/1471-2466-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingle L., Goode K., Rigby A.S., Cleland J.G., Clark A.L. Predicting peak oxygen uptake from 6-min walk test performance in male patients with left ventricular systolic dysfunction. Eur. J. Heart Fail. 2006;8:198–202. doi: 10.1016/j.ejheart.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Cahalin L.P., Mathier M.A., Semigran M.J., Dec G.W., DiSalvo T.G. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest. 1996;110:325–332. doi: 10.1378/chest.110.2.325. [DOI] [PubMed] [Google Scholar]
- 18.Albouaini K., Egred M., Alahmar A., Wright D.J. Cardiopulmonary exercise testing and its application. Postgrad Med J. 2007;83:675–682. doi: 10.1136/hrt.2007.121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers J., Bellin D. Ramp exercise protocols for clinical and cardiopulmonary exercise testing. Sports Med. 2000;30:23–29. doi: 10.2165/00007256-200030010-00003. [DOI] [PubMed] [Google Scholar]
- 20.ATS/ACCP Statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 21.Agostoni P., Dumitrescu D. How to perform and report a cardiopulmonary exercise test in patients with chronic heart failure. Int. J. Cardiol. 2019;288:107–111. doi: 10.1016/j.ijcard.2019.04.053. [DOI] [PubMed] [Google Scholar]
- 22.Wasserman K., Hansen J.E., Sue D.Y., Stringer W.W., Whipp B.J. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications. Lippincott Williams & Wilkins; 2005. Clinical exercise testing; pp. 137–138. [Google Scholar]
- 23.The American Thoracic Society ATS statement: guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton D.M., Haennel R.G. Validity and reliability of the 6-minute walk test in a cardiac rehabilitation population. J Cardiopulm Rehabil. 2000;20:156–164. doi: 10.1097/00008483-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Evangelista A., Flachskampf F., Lancellotti P., et al. European Association of Echocardiography. European Association of Echocardiography recommendations for standardization of performance, digital storage and reporting of echocardiographic studies. Eur. J. Echocardiogr. 2008;9:438–448. doi: 10.1093/ejechocard/jen174. [DOI] [PubMed] [Google Scholar]
- 26.Carter R., Holiday D.B., Nwasuruba C., Stocks J., Grothues C., Tiep B. 6-minute walk work for assessment of functional capacity in patients with COPD. Chest. 2003;123:1408–1415. doi: 10.1378/chest.123.5.1408. [DOI] [PubMed] [Google Scholar]
- 27.Sillen M.J., Vercoulen J.H., van 't Hul A., et al. Inaccuracy of estimating peak work rate from six-minute walk distance in patients with COPD. COPD. 2012;9:281–288. doi: 10.3109/15412555.2012.655866. [DOI] [PubMed] [Google Scholar]
- 28.Ketelhut S., Ketelhut R.G. Type of exercise training and training methods. Adv. Exp. Med. Biol. 2020;1228:25–43. doi: 10.1007/978-981-15-1792-1_2. [DOI] [PubMed] [Google Scholar]
- 29.Troosters T., Vilaro J., Rabinovich R., et al. Physiological responses to the 6-min walk test in patients with chronic obstructive pulmonary disease. Eur. Respir. J. 2002;20:564–569. doi: 10.1183/09031936.02.02092001. [DOI] [PubMed] [Google Scholar]
- 30.Mapelli M., Salvioni E., Paneroni M., et al. Brisk walking can be a maximal effort in heart failure patients: a comparison of cardiopulmonary exercise and 6-min walking test cardiorespiratory data. ESC Heart Fail. 2022;9:812–821. doi: 10.1002/ehf2.13781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balady G.J., Williams M.A., Ades P.A., et al. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American heart association exercise, cardiac rehabilitation, and prevention Committee, the council on clinical Cardiology; the councils on cardiovascular nursing, epidemiology and prevention, and nutrition, physical activity, and metabolism; and the American association of cardiovascular and pulmonary rehabilitation. Circulation. 2007;115:2675–2682. doi: 10.1161/CIRCULATIONAHA.106.180945. [DOI] [PubMed] [Google Scholar]
- 32.American Association of Cardiovascular and Pulmonary Rehabilitation . fifth ed. Human Kinetics; Champaign, IL: 2013. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. [Google Scholar]
- 33.Stone J.A., Arthur H.M., Suskin N., et al. third ed. Canadian Association of Cardiac Rehabilitation; Winnipeg, Canada: 2009. Canadian Guidelines for Cardiac Rehabilitation and Cardiovascular Disease Prevention: Translating Knowledge into Action. [Google Scholar]
- 34.Piepoli M.F., Corrà U., Benzer W., et al. Secondary prevention through cardiac rehabilitation: from knowledge to implementation. A position paper from the cardiac rehabilitation section of the European association of cardiovascular prevention and rehabilitation. Eur. J. Cardiovasc. Prev. Rehabil. 2010;17:1–17. doi: 10.1097/HJR.0b013e3283313592. [DOI] [PubMed] [Google Scholar]
- 35.Pavy B., Iliou M.C., Verges-Patois B., et al. French Society of Cardiology guidelines for cardiac rehabilitation in adults. Arch Cardiovasc Dis. 2012;105:309–328. doi: 10.1016/j.acvd.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Bjarnason-Wehrens B., Mayer-Berger W., Meister E.R., et al. Recommendations for resistance exercise in cardiac rehabilitation. Recommendations of the German federation for cardiovascular prevention and rehabilitation. Eur. J. Cardiovasc. Prev. Rehabil. 2004;11:352–361. doi: 10.1097/01.hjr.0000137692.36013.27. [DOI] [PubMed] [Google Scholar]
- 37.Royal Dutch Society for Physical Therapy KNGF clinical practice guideline for physical therapy in patients undergoing cardiac rehabilitation. Dutch J Phys Ther. 2011;121:1–47. [Google Scholar]
- 38.Ruzzolini M., Ambrosetti M. Cardiopulmonary exercise testing in cardiac rehabilitation: from the reporting form to structured exercise prescription. A proposal from the Italian alliance for cardiovascular rehabilitation and prevention (Itacare-P) Int J Cardiol Cardiovasc Risk Prev. 2023;18 doi: 10.1016/j.ijcrp.2023.200191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price K.J., Gordon B.A., Bird S.R., Benson A.C. A review of guidelines for cardiac rehabilitation exercise programmes: is there an international consensus? Eur J Prev Cardiol. 2016:231715–231733. doi: 10.1177/2047487316657669. [DOI] [PubMed] [Google Scholar]
- 40.Piotrowicz E., Zieliński T., Bodalski R., et al. Home-based telemonitored Nordic walking training is well accepted, safe, effective and has high adherence among heart failure patients, including those with cardiovascular implantable electronic devices: a randomised controlled study. Eur J Prev Cardiol. 2015;22:1368–1377. doi: 10.1177/2047487314551537. [DOI] [PubMed] [Google Scholar]
- 41.Morales F.J., Martínez A., Méndez M., et al. A shuttle walk test for assessment of functional capacity in chronic heart failure. Am. Heart J. 1999;138:291–298. doi: 10.1016/s0002-8703(99)70114-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.