Summary
Using advanced cyclic testing techniques improves accuracy in estimating capacity fade and incorporates real-world scenarios in battery cycle aging assessment. Here, we present a protocol for conducting cyclic tests in lithium-ion batteries to estimate capacity fade. We describe steps for implementing strategies for accounting for variations in rest periods, charge-discharge rates, and temperatures. We also detail procedures for validating tests experimentally within a climate-controlled chamber and for developing an empirical model to estimate capacity fading under various testing objectives.
For complete details on the use and execution of this protocol, please refer to Mulpuri et al.1
Subject areas: Energy, Chemistry, Material sciences
Graphical abstract

Highlights
-
•
Development of comprehensive protocols for advanced cyclic testing in Li-ion batteries
-
•
Procedures for creating an environmental cell testing chamber and controller
-
•
Empirical modeling techniques for accurate battery capacity fade estimation
-
•
Advanced cell testing in a high-performance computing simulation environment
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Using advanced cyclic testing techniques improves accuracy in estimating capacity fade and incorporates real-world scenarios in battery cycle aging assessment. Here, we present a protocol for conducting cyclic tests in lithium-ion batteries to estimate capacity fade. We describe steps for implementing strategies for accounting for variations in rest periods, charge-discharge rates, and temperatures. We also detail procedures for validating tests experimentally within a climate-controlled chamber and for developing an empirical model to estimate capacity fading under various testing objectives.
Before you begin
This protocol implements advanced cyclic testing methods presented in Mulpuri et al.1 To perform these tests, battery modeling software and an environmentally controlled test chamber are needed in which the battery can be subjected to user-defined protocols by utilizing the cell models and corresponding cell parameters. The battery modeling software is used to perform the tests in a simulation environment using an open-source Python Battery Mathematical Modelling (PyBaMM),2 and the environmentally controlled test chamber is used for validating the results. This open-source battery modeling software package allows continuum model simulation using different types of models such as Doyle-Fuller Newman (DFN), Single Particle Model (SPM), and SPM with electrolyte effects (SPMe), on which our work is based on SPMe. SPMe uses asymptotic and numerical analysis to solve the model equations. Any other software supporting battery modeling is also promoted; however, this work uses PyBaMM because of its versatility and usability in different operating systems (Windows, MacOS, and Linux/GNU operating systems) and its availability as open source. The validation of the simulation results is necessary; hence, an environmentally controlled test chamber is used to perform manufacturer-specified cyclic tests and proposed advanced cyclic tests. The abbreviations and notations used in the present article are defined in Table 1.
Table 1.
Abbreviations and notations
| Abbreviation/ Notation | Definition |
|---|---|
| DFN | Doyle-Fuller-Newman |
| SPM | Single Particle Model |
| SPMe | SPM with electrolyte effects |
| SEI | Solid Electrolyte Interface |
| LLI | Loss of Lithium Inventory |
| Q | Total charge throughput (Ah) |
| T | Total duration of the cycle (hr) |
| tamb | Cell ambient temperature |
| tnom | Nominal temperature |
| NTC | Negative Temperature Coefficient |
| SPI | Serial Peripheral Interface |
| SMPS | Switch Mode Power Supply |
| RAM | Random Access Memory |
| HPC | High Power Computing |
Development of environmentally controlled cell testing chamber
Timing: 12 weeks
An environmentally controlled cell testing chamber is developed in-house within the E-Mobility Lab (EML), Indian Institute of Technology (IIT) Guwahati, Assam, India, for validating the simulation results. An essential design criterion involves ensuring that the cell test chamber maintains an error accuracy within ‒1°C to +1°C of the target temperature. This precision is crucial because even slight fluctuations in temperature can significantly impact the accuracy of battery cell test results. Given the variable temperature conditions in the testing lab, exerting control over temperature for precise performance evaluations is imperative. Furthermore, the temperature control system must consistently uphold the desired levels within our specified test temperature range (‒20°C to 80°C) throughout the length of the test. Hence, robust temperature insulation is also needed to prevent thermal leakage. The developed environmentally controlled test chamber setup is shown in Figure 1. The detailed steps to develop various constituents of the testing chamber are described as follows.
-
1.Acrylic Chamber Development.
-
a.Construct the Cell Test Chamber using Acrylic, with dimensions of 65(l) × 60(b) × 45(h) cm, and a thickness of 10 mm, providing an internal volume of around 175 L for enhanced visibility during testing.
-
b.Design a six-channel adjustable cell holder to effectively accommodate various lithium-ion cell geometries.
-
c.Select materials for the chamber interior capable of withstanding temperatures ranging from ‒20°C to 80°C.
-
a.
Note: The chamber is front-loading with a hinged lid, facilitating convenient product testing applications.
Note:Table 2 shows the thermal specifications of the components/material used inside the chamber.
-
2.Three-layered Insulation Housing.
-
a.Encase the acrylic chamber within a three-layered insulation housing to prevent temperature leakage.
-
b.Construct the insulation housing with a glasswool layer sandwiched between two layers of plywood, ensuring robust insulation with dimensions of 81(l) × 75(b) × 75(h) and a thickness of 19 mm.
-
c.Apply double-layered adhesive gaskets at the openings and hinges of both the acrylic and insulation units to prevent minor leakages.
-
a.
-
3.Heating Unit.
-
a.Install eight cartridge heaters within the chamber to achieve temperatures of 80°C.
-
b.Connect all eight heaters in series, each operating on a 12 V DC supply with a power rating of 40 W.
-
c.Optimize their placement at various locations inside the chamber for uniform heating.
-
a.
-
4.Refrigeration Unit.
-
a.Integrate a generic refrigeration unit based on fundamental thermodynamic principles within the chamber.
-
b.Circulate refrigerant R14a through key components—evaporator, capillary valve, condenser, and compressor—to extract heat within the chamber, releasing it via the condenser.
-
c.Place a DC cooling fan behind the evaporator within the chamber to ensure effective cooling distribution.
-
a.
Note: Refrigerant will be a high-pressure vapor at the compressor outlet, high-pressure liquid at the condenser outlet, low-pressure liquid at the capillary valve outlet and low-pressure vapor at the evaporator outlet.
Note: The time taken to reach the desired temperature varies depending on whether it is a higher or lower temperature. Elevating from an ambient room temperature of approximately 25°C to a higher desired temperature of 50°C takes less than 30 min. Conversely, reducing the temperature from 25°C ambient to a lower desired temperature of 0°C takes a maximum of around 90 min.
Figure 1.
Environmentally controlled test chamber setup integrated with heating and refrigeration units
This setup features a battery holder and sensor circuitry for monitoring battery and ambient temperatures, voltage, and current. The cell testing chamber is enclosed in a triple-layered plywood-glass wool-plywood structure. The chamber, as well as battery parameters, is controlled by a test chamber controller for regulating current, voltage, temperature, and data acquisition, which are linked to the local desktop for visualization and analysis.
Table 2.
Thermal specifications of materials and components used in the testing chamber
| Component | Purpose | Minimum (°C) | Maximum (°C) |
|---|---|---|---|
| Acrylic Thermo Plastic | Cell Testing Chamber | ‒40 | 80 |
| High Density Poly-Ethylene (HDPE) | Ports and Outlets | ‒45 | 120 |
| PolyTetraFluoroEthylene (PTFE) | Wiring | ‒240 | 260 |
| Aluminum | Battery Holders and Terminals | ‒45 | 600 |
| Buna-O-Ring | Sealing and Adhesives | ‒40 | 120 |
Data measurement unit
Timing: 1 week
-
5.Cell Voltage Measurement.
-
a.Measure the terminal voltage across each cell in each channel using a 4-wire measurement setup, utilizing 4 leads to separate mains current and voltage sensing.
-
b.Achieve precise voltage measurement by separating current and voltage sensing wires, eliminating the flow of battery current through voltage sensing wires.
-
a.
Note:Figure 2 illustrates the 4-wire measurement setup, where the main current passes through one pair (mains pair), and the other pair is used for sensing the voltage (sense pair).
-
6.Cell Current Measurement.
-
a.Measure cell current using the ACS712 current sensor module, employing 6 modules for each channel capable of sensing up to 30 A current flow.
-
b.Power each module with a supply voltage of around 4.5 V–5.5 V DC, with a sensitivity of 100 mV/A.
-
c.Read the current signal via any microcontroller or Arduino after proper calibration.
-
a.
-
7.Chamber Humidity and Temperature Measurement.
-
a.Utilize the DHT22 digital sensor to measure chamber humidity, requiring a supply voltage of around 3.5 V–5.5 V.
-
b.Measure chamber temperature using the DS18B20 waterproof temperature probe, powered with a supply voltage between 3.0 V and 5.5 V.
-
a.
Note: DHT22 digital sensor has an accuracy of ±1%, and DS18B20 waterproof temperature probe has a range of ‒55°C–125°C and an accuracy of ±0.5°C (from ‒10°C to 85°C).
-
8.Cell Temperature Measurement.
-
a.Measure the surface temperature of each cell using a 100 kΩ NTC Thermistor temperature sensor.
-
b.Employ a total of 6 such thermistor cables for each cell in 6 channels.
-
a.
Note: 100 kΩ NTC Thermistor temperature sensor has a temperature range of ‒40°C–270°C and a maximum power rating of up to 45 mW.
CRITICAL: The precision in measurement is of significant importance and must be ensured throughout. The measuring instruments and sensors need to be calibrated against standard scales. In this work, each sensor within the cell testing chamber and the charge-discharge controller is meticulously calibrated against known values before integration into the chamber and controller. This calibration resulted in individual offset values in measurement, which allowed us to calibrate the sensors and instruments precisely using the respective offset values, hence improving the accuracy of measurements.
Figure 2.
4-wire voltage measurement
(A) Schematic representation, (B) Experimental setup.
Designing chamber charge-discharge controller
Timing: 4 weeks
-
9.Cell Charging Unit.
-
a.Charge each cell individually using a step-down DC-DC buck converter module of 300 W, 20 A ratings for each channel.
-
a.
Note: The input supply voltage to each DC-DC buck converter module can be between 6 V and 40 V with a corresponding output voltage of 1.25 V–36 V.
Note: The maximum charging current in this protocol is limited to 5 A, but the charger modules are tested at a rated current of 20 A for safety, reliability, and scalability reasons.
-
10.Cell Discharging Unit.
-
a.Design a constant current regulated programmable electronic load to discharge each cell.
-
a.
Note: Energy dissipation is carried out through MOSFET operating in linear regions within the electronic load.
Note: Through a closed-loop system, an operational amplifier controls the gate terminal via a feedback signal from the voltage across the current sensing resistor.
-
11.Microcontroller Unit.
-
a.Sense critical data, including voltage, current, and temperature of each cell across all channels, chamber temperature, and humidity using the central microcontroller unit Arduino 2560 ATMEGA pro. Any other available microcontroller unit can also be used if the measurement and control requirements as defined in this protocol are met.
-
b.Measure and store data in local storage servers using the serial peripheral interface (SPI) bus.
-
a.
Note: This microcontroller also controls and supervises the chamber temperature and humidity. The controller operates on a 16 MHz clock speed with a flash memory of 256 KB.
Note: An input voltage between 6 V and 9 V is recommended, with a limit of 12 V to operate the microcontroller. However, the input value of voltage will change with the change in the type of microcontroller being used.
-
12.Chamber Controller Enclosure.
-
a.Develop a robust enclosure box to ensure safe, efficient, and reliable operation of the testing chamber, housing the entire charge-discharge unit, microcontroller unit, and Switch Mode Power Supply (SMPS) with ventilation and cooling features.
-
a.
Note: SMPS provides the voltage input required to operate the sensor circuitry, microcontroller, and relay modules across the test bed.
Installing PyBaMM package
-
13.Python Version Requirements.
-
a.Prior to using PyBaMM, install Python version >3.8.
-
b.Create a virtual environment before installing PyBaMM to prevent alterations in existing Python files.
-
a.
Note: PyBaMM is compatible with minimum hardware requirements, including a 64-bit Central Processing Unit (CPU) with Intel/AMD architecture, 4 GB RAM, and 5 GB of free disk space. The supported operating systems encompass Windows, MacOS, and Linux/GNU.
-
14.Install PyBaMM.
-
a.On Windows and GNU/Linux, install PyBaMM using “pip install pybamm” command.
-
b.On MacOS, install the “sundials” library before installing PyBaMM using “brew install sundials” command, and then install PyBamm using “pip install pybamm” command.
-
a.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| PyBaMM | PyBaMM | www.pybamm.org |
| Origin Pro 2023 | OriginLab | www.originlab.com |
| Other | ||
| Param Ishan – high-performance computing platform | IIT Guwahati, India | www.iitg.ac.in/param-ishan |
| Climatic cell test chamber | E-Mobility Lab, IIT Guwahati, India | N/A |
| Acrylic sheet | M/S Chinkoi Udyog | HSN:39203090 |
| Plywood | M/S Century Udyog | HSN:44123190 |
| Glasswool | M/S The Dream Décor | HSN:70199010 |
| Self-adhesive gasket | M/S Bigtime Enterprises | HSN:40082990 |
| Aluminum foil self-adhesive | M/S Bhabha | HSN:3919 |
| Fire extinguisher | Alertfire | HSN:8424 |
| Silicone sealant | M/S Ambey Traders | HSN:3917 |
| Battery charge controller | E-Mobility Lab, IIT Guwahati, India | N/A |
| Switch mode power supply (SMPS) | M/S Rasila Pragwat | HSN:8504 |
| 8-Channel relay module | Electronics Comp | HSN:85177010 |
| 8-Channel logic level converter | Electronics Comp | TXS0108E |
| Micro SD card module – breakout board | Electronics Comp | HSN:85381010 |
| 4-Channel ADC gain amplifier module | Electronics Comp | EC3214 |
| PVC-coated 30AWG wire | ROBU.in | SKU:305193 |
| PCB design and fabrication | M/S Circuit Systems Ltd. | www.pcbpower.com |
| LG-M50 INR21700 cells | Genuine Power | www.genuinepower.co.in |
| Electrical power sources and local computers | E-Mobility Lab, IIT Guwahati, India | www.iitg.ac.in/e_mobility/ |
| Chamber heating unit – ceramic cartridge heater | ROBU.in | SKU: 12067 |
| Chamber cooling unit – evaporator (aluminum metal) | M/S OK A1 Cooling | NA |
| Chamber cooling unit – compressor | Whirlpool | ASD43KL |
| Chamber cooling unit – capillary tube (copper) | M/S OK A1 Cooling | N/A |
| Chamber cooling unit – condenser (mild steel) | M/S OK A1 Cooling | N/A |
| Main microcontroller – Mega2560 Pro ATMEGA | ROBU.in | SKU:699585 |
| Current sensor module | Allegro MicroSystems | ACS712 |
| Chamber humidity sensor | Aosong Electronics | DHT22 |
| Chamber temperature sensor | Dallas Semiconductor | DS18B20 |
| NTC 100K thermistor | ROBU.in | SKU: 84428 |
Step-by-step method details
As the cyclic testing procedures are carried out on all kinds of batteries, the implementation of the presented protocols is feasible on any lithium-ion cell chemistry and is applicable to diverse lithium-ion cell geometries, including cylindrical, pouch, coin, and prismatic cells. However, specific current and voltage thresholds and amplitudes may vary based on manufacturer-specified rated values, which must be considered while performing the test. An LGM50 21700 cylindrical cell is chosen in this work, and the step-by-step methods are presented below. The selected cell had a nominal voltage of 3.6 V and a nominal capacity of 5 Ah. Table 3 details the other key cell specifications and parameters provided by the manufacturer.3
Table 3.
Parameters and specifications for LGM50 21700 cylindrical cell provided by the manufacturer
| Macroscale geometry | ||
| Negative current collector thickness | 1.20E-05 | [m] |
| Negative electrode thickness | 8.52E-05 | [m] |
| Separator thickness | 1.20E-05 | [m] |
| Positive electrode thickness | 7.56E-05 | [m] |
| Positive current collector thickness | 1.60E-05 | [m] |
| Electrode height | 6.50E-02 | [m] |
| Electrode width | 1.58 | [m] |
| Cell cooling surface area | ||
| Cell volume | 5.31E-03 | [m2] |
| Current collector properties | 2.42E-05 | [m3] |
| Negative current collector conductivity | 58411000 | [S.m-1] |
| Positive current collector conductivity | 36914000 | [S.m-1] |
| Density | ||
| Negative current collector density | 8960 | [kg.m-3] |
| Positive current collector density | 2700 | [kg.m-3] |
| Specific heat capacity | ||
| Negative current collector-specific heat capacity | 385 | [J.kg-1.K-1] |
| Positive current collector specific heat capacity | 897 | [J.kg-1.K-1] |
| Thermal conductivity | ||
| Negative current collector thermal conductivity | 401 | [W.m-1.K-1] |
| Positive current collector thermal conductivity | 237 | [W.m-1.K-1] |
Cycle life experimentation
Timing: 1.5 week
-
1.Set the cell testing chamber to 25°C before initiating the cycle life experimentation.
-
a.Maintain this temperature throughout the duration of the experiment.
-
a.
-
2.
Soak the cell at the test temperature of 25°C for at least 2 h to ensure uniform temperature distribution.
Note:Figure 3 illustrates the manufacturer-specified cycle that needs to be implemented in the cell testing chamber. It outlines the specific parameters and conditions under which the LGM50 cell is tested within the chamber.
Note: Additionally, Figure 4 presents a comprehensive flowchart that depicts the sequence of steps and methodologies employed in the cycle life experimentation.
-
3.Conduct a manufacturer-specified cycle life experiment for 50 cycles within the cell testing chamber on the chosen LGM50 cell at an ambient temperature of 25°C.
-
a.Begin the discharging phase as depicted in Figure 3, discharging the cell at a constant current of 2.4 A (0.5C) until the voltage reaches 2.85 V (T1), followed by a 20-min rest period (T2).
-
b.Initiate the charging process by applying a constant current rate of 0.3C (1.44 A) until the cell voltage reaches 4.1 V (T3).
-
c.Maintain constant voltage charging until the current reaches 240 mA (C/100) (T4).
-
d.Allow the cell to rest for 10 min (T5).
-
a.
-
4.
Repeat this cycle for 50 cycles while ensuring the ambient temperature inside the chamber remains at 25°C.
Note: By performing this cycle life experiment, charging characteristics, discharge behavior, and life cycle performance can be assessed.
Note:Figure 5 shows the preliminary result of terminal voltage to provide an overview of the expected outcome following the implementation of the described protocol.
Note: For a detailed discussion of the complete set of results, consult Mulpuri et al.1
Note: According to the International Electrotechnical Commission (IEC) 62620 standard,4 to attain a uniform temperature across the cell, it needs to be stored at the desired temperature (± 5°C) for not less than 1 hour and not more than 4 hours.
CRITICAL: The timing mentioned for the cycle life experimentation method is based on the charge-discharge rates of the protocol and the ratings of the chosen cell.
Figure 3.
Manufacturer-specified test cycle implemented for cycle life experimentation within the cell testing chamber
Figure 4.
Flowchart for implementing cycle life experimentation within the cell testing chamber
Figure 5.
Terminal voltage profile of LGM50-21700 cell obtained from cycle life experimentation in cell testing chamber
Proposed advanced test cycle experimentation
Timing: 2–3 days
-
5.Set the cell testing chamber to 25°C before initiating the cycle life experimentation.
-
a.Maintain this temperature throughout the duration of the experiment.
-
a.
-
6.
Soak the cell at the test temperature of 25°C for a minimum of 2 h to ensure uniform temperature distribution.
Note:Figure 6 illustrates the chosen proposed test cycle that needs to be implemented in the cell testing chamber. It outlines the specific parameters and conditions under which the LGM50 cell is tested within the chamber.
Note: Additionally, Figure 7 presents a comprehensive flowchart that depicts the sequence of steps and methodologies employed in the implementation of the proposed test cycle experimentation within the cell testing chamber.
-
7.Implement the test cycle chosen from the proposed 1000 advanced cyclic tests1 and replicate it 15 times in the cell testing chamber.
-
a.Initiate the chosen cycle with a discharge duration of 30 min and a discharge C-rate of 2.5 A (0.5C), followed by a 1-h rest period.
-
b.Charge the cell at a constant current rate of 1.5 A (0.3C) until the cell voltage reaches 4.1 V.
-
c.Maintain the cell at a constant voltage of 4.1 V until the current reaches 0.25 A (0.05C), followed by a 1-h rest period.
-
a.
-
8.
Repeat this cycle 15 times while ensuring the ambient temperature inside the chamber remains at 25°C.
Note: The implementation of this protocol served to validate the climatic test chamber and PyBaMM simulation environment for the proposed advanced cyclic tests, with the primary objective being the acquisition of terminal voltage profiles across the cell.
Note:Figure 8 depicts the terminal voltage profile obtained after implementing the described protocol in the climatic test chamber. This profile is essential for subsequent calibration, with simulation results described in further sections.
Note: For a more comprehensive analysis and discussion of the advanced cyclic test results, readers are directed to refer to Mulpuri et al.1
Figure 6.
Proposed test cycle implemented for experimentation within the cell testing chamber
Figure 7.
Flowchart for implementing proposed test cycle experimentation within the cell testing chamber
Figure 8.
Terminal voltage profile of LGM50-21700 cell obtained from implementing proposed test cycle experimentation in cell testing chamber
Advanced cyclic tests simulations @ 25°C
Timing: 1 week
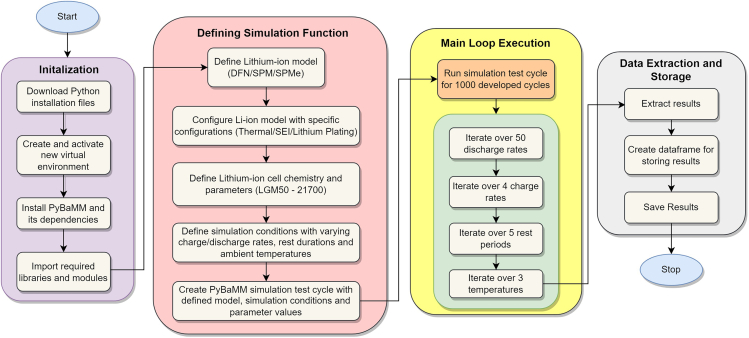
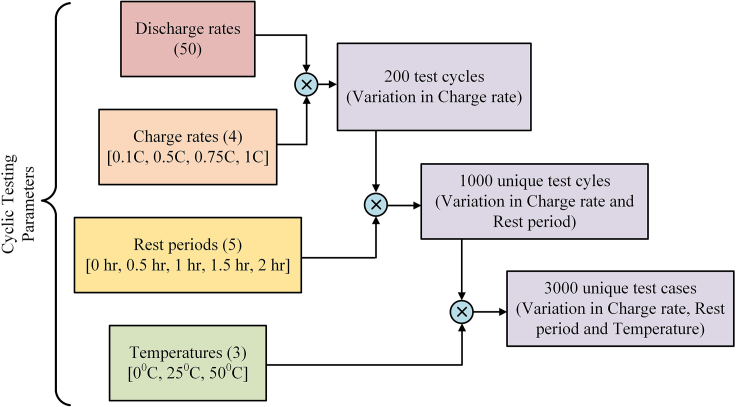
The designed set of simulation-based experiments is performed in the simulation environment PyBaMM. A detailed flowchart depicting the sequence of steps from setting up PyBaMM to performing simulations and storing the results is shown in Figure 9. The developed 1000 test cycles1 are obtained by varying discharge capacities, durations, charge rates, and rest periods as shown in Figure 10.
-
9.
Choose five different durations ranging from 0.5 to 1.5-h periods in steps of 0.25 h as an initial step.
-
10.
Select ten discharge capacities from 1 to 4.375 Ah. Utilize these in combination with the chosen discharge durations to adapt a two-factorial, multi-level design criterion problem5 to design 50 discharge rates, as outlined in Table 4.
-
11.
Study each discharge rate with four different rest periods of 0.5 h, 1 h, 1.5 h, and 2 h, along with a case without any rest period.
-
12.
Include charge periods to complete a cycle. Investigate each discharge rate from the 50 designed rates with four charge rates of 0.1C, 0.5C, 0.75C, and 1C.
-
13.
Combine 50 discharge rates at five rest periods and four charge rates to form 1000 unique test cycles.
-
14.
Simulate all 1000 designed cycles at a constant ambient temperature of 25°C.
Note: In subsequent steps, these 1000 cycles are simulated at constant ambient temperatures of 0°C and 50°C.
Note: Refer to Figure 11 for an illustration of the design methodology behind developing the 1000 test cycles.
CRITICAL: For faster computations, it is recommended to implement simulations in a High-Performance Computing (HPC) environment to perform complex simulations at high speeds. In this protocol, we utilized the Param-Ishan supercomputing facility at the Indian Institute of Technology Guwahati for the described simulations.
Figure 9.
Flowchart of simulation process in PyBaMM from initialization to data storage in implementing the advanced cyclic tests at specified conditions
Figure 10.
Advanced cyclic testing protocols developed depicting the variations
(A) discharge capacity, (B) discharge duration, (C) charge rate, and (D) rest period. The test cycles depict different phases viz. Discharge period (D), Rest Period after discharging (Rd), Charge Period (C), Rest Period after charging (Rc). The notations (iD1, iD2...iDn) and (D1, D2...Dn) denote varied discharge capacities and durations, respectively. The notations (iC1, iC2...iCn) and (C1, C2...Cn) denotes varied charge rates and durations, respectively. The notations (Rd1, Rd2...Rdn) and (Rc1, Rc2...Rcn) denotes varied rest periods after discharge and charge periods, respectively.
Table 4.
Fifty designed discharge rates correspond to respective discharge durations (hr) and discharge capacities (Ah)
| C-rate | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Discharge duration (hr) | Discharge capacity (Ah) |
|||||||||
| 1 | 1.25 | 1.5 | 1.875 | 2 | 2.5 | 3 | 3.75 | 4 | 4.375 | |
| 0.5 | 0.40 | 0.50 | 0.60 | 0.75 | 0.80 | 1.00 | 1.20 | 1.50 | 1.60 | 1.75 |
| 0.75 | 0.27 | 0.33 | 0.40 | 0.50 | 0.53 | 0.67 | 0.80 | 1.00 | 1.07 | 1.17 |
| 1 | 0.20 | 0.25 | 0.30 | 0.38 | 0.40 | 0.50 | 0.60 | 0.75 | 0.80 | 0.88 |
| 1.25 | 0.16 | 0.20 | 0.24 | 0.30 | 0.32 | 0.40 | 0.48 | 0.60 | 0.64 | 0.70 |
| 1.5 | 0.13 | 0.17 | 0.20 | 0.25 | 0.27 | 0.33 | 0.40 | 0.50 | 0.53 | 0.58 |
Figure 11.
Design methodology behind the development of 3000 unique test cases for advanced cyclic test simulations
Advanced cyclic tests simulations @ 0°C
Timing: 1 week
-
15.
Repeat the 1000 unique cycles developed in the “advanced cyclic tests simulations @ 25°C” step for a constant ambient temperature of 0°C.
Advanced cyclic test simulations @ 50°C
Timing: 1 week
-
16.
Repeat the 1000 unique cycles developed in the “advanced cyclic tests simulations @ 25°C” step for a constant ambient temperature of 50°C.
Note: It is important to highlight that the simulations at each temperature are executed in the High-Performance Computing (HPC) facility at the Indian Institute of Technology Guwahati. In this configuration, all 1000 cycles for a particular temperature are simulated simultaneously, leveraging parallel computing to enable multiprocessing within each temperature simulation. As a result, the individual execution time for each temperature set is one week.
However, it is worth noting that if additional HPC resources become available, the code can be further optimized to conduct simulations for all three temperatures in parallel. With this, the total simulation time would be significantly reduced, potentially completing the 3000 simulations in less than the specified 3 weeks.
Note: The HPC facility comprises 126 modes, each equipped with two Intel Xeon E5-2680 v3 processors (12 cores, 2.5 GHz) and 64 GB of RAM. For implementing the advanced test cycle simulations, all 3000 simulations were performed across three temperature conditions (0°C, 25°C, 50°C), with each category involving 1000 simulations.
For each simulation batch, 45 cores were allocated by the HPC facility, allowing for 45 simulations, as each core handles one simulation. Hence, a total of 23 batches (∼1000/45) were made to complete 1000 simulations per temperature condition. Each batch, running 45 simulations, took an average of 7 h to complete. Consequently, completing all batches for one temperature condition required approximately 161 h, equalling around 6.7 days.
In terms of memory usage, each node’s 64 GB RAM is shared among its 24 cores, leading to an allocation of approximately 2.67 GB RAM per core for each simulation.
Empirical modeling for capacity fade estimation
Timing: 3 days
-
17.
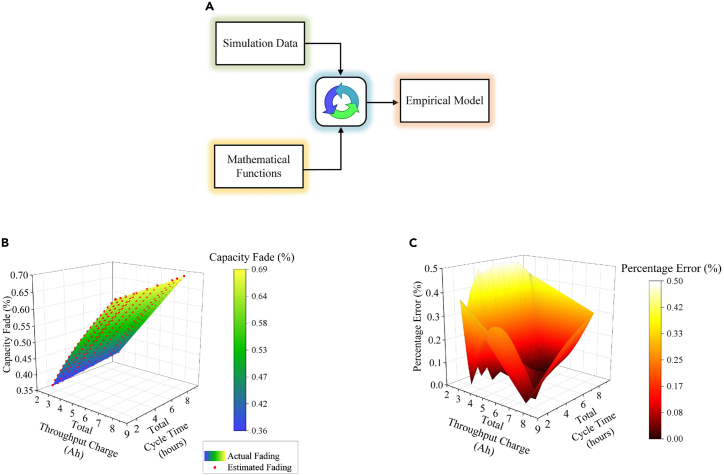
Fit the simulation results to mathematical functions to develop the empirical model (Figure 12A) using a non-linear least square method based curve fitting technique.
-
18.
Model the critical capacity fading phenomenon, including SEI layer growth and Lithium plating, individually to form the empirical model.
-
19.
Identify the parameters influencing the critical degradation phenomenon.
Note: The parameters on which the critical degradation phenomenon depends are the total charge throughput (Q), the total duration of the cycle (T), and the cell ambient temperature (tamb).
-
20.
Develop two non-linear equations corresponding to SEI layer growth and lithium plating phenomena, with the three parameters as functions, to model capacity fading.
Note:Equations 1 and 2 models the capacity fades due to SEI layer growth and the lithium plating phenomenon.
| (Equation 1) |
| (Equation 2) |
| (Equation 3) |
-
21.
Select terms in the equations based on observed behavior of the degradation phenomena from the results.
-
22.
Capture the predominantly time-dependent nature of SEI layer growth by including the total cycle duration (T) (Refer Equation 1).
Note: SEI layer growth occurs without energy exchange (Q = 0). Hence, the independence in terms of Q and T can be seen in Equations 1, unlike in Equation 2
-
23.
Introduce a dependent term involving Q and T in Equation 2 to model lithium plating, which occurs with energy exchange.
-
24.
Include an exponential term () to capture the temperature effect, considering both phenomena are temperature dependent.
-
25.
Determine nine coefficients (a, b, c, d, e, f, g, h, i) within both equations through curve-fitting techniques.
-
26.
Subject the developed equations to curve fitting using obtained results to evaluate optimal coefficients for any values of Q, T, ambient temperature (tamb), and nominal temperature (tnom).
Note: The obtained coefficients of the empirical model are presented in Table 5.
Note: The capacity fading can be mathematically determined from the obtained coefficients for any discharge duration, discharge capacity, charge-discharge rate, and ambient temperature.
-
27.
Validate the model’s accuracy and reliability by estimating capacity fading at a fixed ambient temperature of 25°C.
-
28.
Compare the estimated capacity fade from the empirical model with actual data obtained from simulating 1000 advanced cyclic tests at 25°C ambient temperature.
Note:Figure 12B shows a comparison between the estimated capacity fade from the empirical model and the actual data obtained from simulating 1000 advanced cyclic tests at 25°C ambient temperature.
-
29.
Conduct error analysis to assess the precision of the empirical model by comparing its outcomes with actual simulation data.
Note:Figure 12C shows the error plot highlighting the model’s accuracy, with the maximum error recorded being less than 0.5%.
Note: This low error margin and the model’s robustness are paramount to ensure that the capacity fading estimations closely align with real-world battery degradation.
Figure 12.
Empirical model development and accuracy validation
(A) Model development methodology, (B) Actual vs. estimated fading at 25°C ambient temperature, (C) Error plot (%) between actual vs. estimated fading at 25°C ambient temperature.
Table 5.
Coefficients of the empirical model for capacity fading estimation
| Ambient temperature (0K) | a | b | c | d | e | f | g | h | i |
|---|---|---|---|---|---|---|---|---|---|
| 273.15 | 0.15 | 0.54 | 0.01 | 0.19 | 1.05 | 0.04 | −0.13 | 0.13 | 1.05 |
| 298.15 | 0.15 | 0.54 | 0.01 | 0.21 | 1.05 | 0.04 | −0.13 | 0.13 | 1.05 |
| 323.15 | 0.15 | 0.54 | 0.01 | 0.23 | 1.04 | 0.05 | −0.13 | 0.13 | 1.05 |
The coefficients are derived from fitting the capacity fading data obtained after performing the advanced cyclic testing simulations.
Expected outcomes
The proposed advanced cyclic tests can be implemented following the protocols detailed above. By performing these tests, the impact of critical parameters such as discharge duration, discharge capacity, charge-discharge rate, and ambient temperature on the capacity fade, and the degradation phenomenon can be analyzed in detail. In addition, the critical evaluation to further understand the influence of SEI layer growth and lithium plating on the overall capacity fading and their contribution toward the overall fade can be determined at different operating conditions. Figure 13 offers supporting visualizations of the expected outcomes, illustrating the extent of total capacity fade under different conditions of temperature (Figures 13A and 13B, 13C), discharge rate (Figure 13D), charge rates (Figure 13E), and rest periods (Figure 13F). For a more detailed discussion and comprehensive insights into the results of these simulations, readers are encouraged to refer Mulpuri et al.1
Figure 13.
Extended results depicting the outcomes of advanced cyclic testing protocols simulations
(A) Capacity fade due to SEI layer growth under different ambient temperatures, (B) Capacity fade due to lithium plating under different ambient temperatures, (C) Total capacity fade under different ambient temperatures; Total LLI (%) vs. cycle number (D) under different discharge rates, (E) under different charge rates, and (F) under different rest periods.
The amalgamation of the experiments and simulation leading to simulation-based experiments' can be used to develop a robust empirical model for estimating accurate capacity fade under specific testing objectives. Further, performing the testing in a customized environmentally controlled cell testing chamber enables incorporating a critical parameter- a temperature that hugely impacts the estimated results, thereby enhancing the work’s reliability.
Limitations
For the chosen LGM50 cell, the advanced cyclic tests are conducted for 500 cycles. The validity of the proposed empirical model is not explored beyond 500 cycles. Moreover, the coefficients estimated for the empirical relations are limited to the chosen LGM50 cell and its chemistry. The coefficient will vary with different cells and their respective chemistries. However, the nature of the empirical relations remains the same as the equations are modeled considering the interdependency of various critical parameters on cell degradation.
Troubleshooting
Potential problems related to battery testing
In any battery study, testing the batteries becomes a crucial component. While testing the batteries under controlled environments, mitigating risks and failures is essential. Common failures associated with testing lithium-ion batteries include undercharging, overcharging, and overheating.
Problem 1
Release of flammable gases occurs during high-temperature testing due to thermal runaway issues (before you begin: step 1).
Potential solution
Develop the cell testing chamber using high temperature-tolerant materials and components to mitigate the risk of thermal runaway. Choose materials with temperature tolerance limits exceeding the test temperature limits, such as acrylic thermoplastic with a tolerance range of ‒40°C to 80°C, making it suitable for chamber development. Additionally, incorporate specific gas sensors connected to the controller into the test chamber for safety notifications.
Problem 2
The chamber is at risk of exploding due to high-pressure conditions during thermal runaway (before you begin: step 1).
Potential solution
Install a pressure relief port within the chamber to accommodate pressure contractions and expansions caused by the release of flammable gases, effectively addressing the issue.
Problem 3
The wiring inside the chamber poses a risk of short circuit (before you begin: step 1).
Potential solution
Ensure proper insulation of electrical equipment, including wiring, sensors, and battery terminals, with materials capable of withstanding test temperatures. Utilize polytetrafluoroethylene (PTFE) polymer insulation to safeguard against short circuits. PTFE insulation offers excellent thermal and electrical properties, making it suitable for operation in harsh environments.
Problem 4
Leakage issues from the chamber can destabilize temperature and humidity due to improper sealing (before you begin: step 2).
Potential solution
Implement a three-layered insulation housing around the acrylic chamber to ensure robust thermal insulation. This housing consists of plywood-glass wool-plywood construction, effectively preventing leakages. Seal the chamber and insulation housing openings with adhesive tapes to eliminate any potential leakages. Additionally, coat the outer layer of plywood with heat-reflective paint to mitigate the influence of ambient heat, ensuring the chamber remains completely adiabatic and stable in temperature.
Potential problems related to implementing advanced cyclic testing protocols in the simulation environment
Problem 5
Running 1000 advanced test cycles, particularly under diverse operating conditions and configurations, demands substantial computational power, leading to inadequate computational resources (step 14).
Potential solution
Optimize the simulation code for parallel computing to effectively utilize High-Performance Computing (HPC) resources. Implement batch processing and job scheduling techniques to manage queues efficiently and overcome resource limitations or queuing delays.
Problem 6
Errors in the simulation environment configuration, such as incorrect settings or parameters in the PyBaMM environment, can result in inaccurate simulation results (step 14).
Potential solution
Double-check all simulation conditions, libraries, cell models, and model parameters before initiating simulations. Conduct pilot runs with a small subset of cycles to verify the correct configuration of the simulation environment and prevent errors.
Potential problems related to empirical model development, implementation, and analysis
Problem 7
Curve fitting encounters convergence issues, particularly with the non-linear least squares method, when initial parameter guesses deviate significantly from optimal values. Sensitivity to initial conditions or simulation parameters may result in improper prediction of optimal coefficients (step 17).
Potential solution
Experiment with various initial parameter values, leveraging basic system understanding to provide reasonable initial estimates. Conduct a sensitivity analysis to identify critical parameters affecting model stability, facilitating better convergence in curve fitting.
Problem 8
The empirical model’s applicability may be limited if calibration is conducted for only specific discharge rates, charge rates, rest periods, and ambient temperatures (step 29).
Potential solution
Enhance the model’s versatility by expanding the range of operating conditions during curve fitting. Regularly validate the model against additional simulation data or experimental results to broaden its applicability and ensure accuracy across varied operating conditions.
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Bikash Sah (bikash.sah@h-brs.de).
Technical contact
Sai Krishna Mulpuri (m.sai@iitg.ac.in).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
The data reported in this paper will be shared by the lead contact upon request.
-
•
All original code has been deposited at GitHub (https://github.com/mlnsai/Advanced-Cyclic-Tests_Protocol) and Zenodo (https://doi.org/10.5281/zenodo.10620554), and is publicly available as of the date of publication.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
This work was supported by the Ministry of Education (MoE), Government of India through the Prime Minister’s Research Fellowship (PMRF) Scheme and E-Mobility Lab, Indian Institute of Technology, Guwahati, Assam, India.
Author contributions
S.K.M., B.S., and P.K. performed the conceptualization of the research. S.K.M. performed data curation, formal analysis, investigation, methodology, resources, software, visualization, writing – original draft, review, and editing. B.S. supported throughout the formal analysis, methodology, investigation, software, and visualization processes. B.S. and P.K. performed supervision, validation, and writing – review and editing.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Sai Krishna Mulpuri, Email: m.sai@iitg.ac.in.
Bikash Sah, Email: bikash.sah@h-brs.de.
References
- 1.Mulpuri S.K., Sah B., Kumar P. Unraveling capacity fading in lithium-ion batteries using advanced cyclic tests: A real-world approach. iScience. 2023;26 doi: 10.1016/j.isci.2023.107770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sulzer V., Marquis S.G., Timms R., Robinson M., Chapman S.J. Python battery mathematical modelling (pybamm) J. Open Res. Softw. 2021;9:14. doi: 10.5334/jors.309. [DOI] [Google Scholar]
- 3.Chen C.H., Brosa Planella F., O’Regan K., Gastol D., Widanage W.D., Kendrick E. Development of experimental techniques for parameterization of multi-scale lithium-ion battery models. J. Electrochem. Soc. 2020;167 doi: 10.1149/1945-7111%2Fab9050. [DOI] [Google Scholar]
- 4.International Electrotechnical Commission "IEC 62620:2014+AMD1:2023 CSV, Secondary cells and batteries containing alkaline or other non-acid electrolytes - Secondary lithium cells and batteries for use in industrial applications." International Standard, 1.1 Edition. 2023. https://webstore.iec.ch/publication/85493
- 5.Krzywinski M., Altman N. Two-factor designs. Nat. Methods. 2014;11:1187–1188. doi: 10.1038/nmeth.3180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
-
•
The data reported in this paper will be shared by the lead contact upon request.
-
•
All original code has been deposited at GitHub (https://github.com/mlnsai/Advanced-Cyclic-Tests_Protocol) and Zenodo (https://doi.org/10.5281/zenodo.10620554), and is publicly available as of the date of publication.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.