Abstract
Background
Computed tomography pulmonary angiogram and lung scintigraphy with ventilation/perfusion scan are needed to diagnose pulmonary embolism (PE) in pregnancy. Their associated ionizing radiation doses are considered safe in pregnancy. A standardized patient information tool may improve patient counseling and reduce testing hesitancy.
Objectives
In this context, we sought to address 1) what patients want to know before undergoing these tests and 2) how they want the information to be provided to them.
Methods
We used a qualitative descriptive methodology. We recruited pregnant participants at the McGill University Health Center in Montreal, Canada. Structured interviews explored information needs about PE and diagnostic imaging for PE. The interview transcripts’ themes were analyzed with a hybrid deductive and inductive approach.
Results
Of 21 individuals approached, 20 consented to participate. Four had been previously investigated for PE. Participants requested information about the risks associated with PE and radiation and their effects on maternal and fetal health. They preferred for radiation doses to be presented in comparison with known radiation thresholds for fetal harm. They suggested that a written tool should be developed using an accessible language. Participants also indicated that the tool would be integrated into their decision-making process, emphasizing a lower risk tolerance for their fetus than for themselves.
Conclusion
This single-center group of pregnant patients wished to be informed about the risks of PE and radiation associated with imaging. A written tool could help put information into context and facilitate decision making. These new insights may be used to inform counseling.
Keywords: pregnancy, pulmonary embolism, radiation, ventilation-perfusion scan, patient education, computed tomography angiography
Essentials
-
•
Diagnosing pulmonary embolism during pregnancy may be delayed by concerns for radiation.
-
•
We interviewed pregnant individuals to understand their informational needs about these tests.
-
•
Participants wished to be informed on the risks of pulmonary embolism and radiation exposure.
-
•
A written tool could help put information into context and facilitate decision making.
1. Introduction
Pulmonary embolism (PE) affects 1 to 2 per 1000 pregnancies [1]. Clinical practice guidelines have identified computed tomography pulmonary angiogram (CTPA) and lung scintigraphy with ventilation/perfusion scan (V/Q scan) as the 2 main imaging modalities to diagnose PE during pregnancy [[2], [3], [4], [5]]. While both tests require the use of ionizing radiation, they are considered safe and are well below the radiation threshold known to be associated with fetal toxicity (ie, 50 mGy) [2,6,7]. Importantly, the benefits of accurately diagnosing and promptly treating an acute PE largely outweigh any potential risk associated with the use of CTPA and V/Q scans in pregnancy [2,8].
To counsel patients requiring diagnostic imaging in pregnancy, clinicians must be comfortable describing radiation exposure, outlining the benefit and harm associated with the use of imaging modalities, and answering any additional question or concern about the planned diagnostic tests [9]. However, counseling prior to radiologic testing has been shown to be suboptimal, as physicians discussed radiation risk in a minority of cases [10], with nearly half of the patients having to seek information on their own [11], and a quarter of them not being satisfied with the information provided [12]. Moreover, inadequate knowledge and erroneous perception of the risks associated with radiation exposure during pregnancy have been reported among physicians [13]. In addition, pregnant patients requiring urgent diagnostic testing to rule out PE are often looked after by a wide range of providers with a variable knowledge level of diagnostic testing in pregnancy, potentially leading to miscommunication and diagnostic delay. Accordingly, adequate communication between providers and patients is paramount to alleviate concerns and ensure that clinically indicated urgent testing is performed [14].
A patient information tool specifically designed for pregnant patients requiring urgent imaging to rule out PE may enhance, complement, and standardize counseling by providers. In turn, efficient counseling may reduce diagnostic delays and improve the experience of patients undergoing CTPA or V/Q scans during pregnancy. The purpose of the current study was to assess the patients’ counseling needs for diagnostic testing for PE during pregnancy to inform a future patient information tool. Specifically, we sought to address the following questions: 1) what do patients want to know before undergoing these tests and 2) how do they want the information to be provided to them?
2. Methods
2.1. Study design
We conducted a qualitative descriptive study to assess patients’ counseling needs prior to undergoing diagnostic testing to rule out PE [15]. We have already conducted the needs assessment from the providers’ perspective [16]. This project was part of a larger action project [17], with the overarching goals of codeveloping a patient information tool for pregnant individuals requiring urgent imaging to rule out PE.
2.2. Participant recruitment
Pregnant individuals presenting for routine follow-up visits at the obstetrical clinics of McGill University Health Center were eligible to participate. We planned a hybrid sampling strategy using consecutive convenience sampling as the primary strategy, supplemented by purposive maximum variation sampling, as needed, to recruit pregnant individuals from the obstetrical clinics (antepartum and obstetric medicine clinics) of the McGill University Health Center. For consecutive convenience sampling, we distributed a recruitment handout as part of the registration package of the obstetrical clinics in order to identify patients who would be willing to be approached by the research team for consent procedures. The goal of the research study was disclosed to participants prior to their enrollment as part of the initial recruitment handout. Patients who selected “Yes” to the question “Would you accept to be approached by a member of the research team for the interview?” on the recruitment handout were approached by a member of the research team (S.O.) for consent procedures. For purposive maximum variation sampling, we screened the medical records of patients to identify those with a prior history of venous thromboembolism (VTE) and whether they had known comorbidities (ie, any chronic medical conditions) in case we would need to approach specific patients to ensure representation of all groups in our study. We did not track their specific comorbidities but rather whether they had lived experience of chronic medical conditions. With this approach, we sought to ensure a sample representing patients with a variety of experiences with healthcare services. As we progressed in the interviews, we kept track of the recruitment of participants with prior suspected or confirmed VTE, those with any comorbidity, and participants without any prior comorbidities to ensure inclusion of patients within each group. Ultimately, consecutive sampling alone yielded participants from each category without any additional purposive sampling being required. No financial compensation was offered for participation.
2.3. Reflexivity
Interviews were conducted by one of the research team members (S.O.) who identified as a mixed-race, cisgender, French- and English-speaking woman. At the time of the study, she was a resident physician in General Internal Medicine at McGill University in her final year of training and, she was not part of the participants’ treating team. S.O. had been involved in a prior study assessing the opinions of healthcare providers [16] on the development of this dedicated patient information tool and had prior notions of what should be integrated into the tool. For this study, S.O. performed the coding for the thematic analysis in conjunction with I.M.
I.M. is a physician with specialization in general internal medicine and clinical expertise in obstetrical medicine. Her research program focuses on pregnancy-related severe morbidity, including thromboembolic complications. She frequently assesses and manages pregnant patients with VTE. A.d.P., L.S., and N.-Z.S. are clinician educators or clinician scientists with expertise in qualitative research methodology and educational tool development. They provided insight in terms of protocol development and data analysis in the setting of qualitative research. A.B., A.R., C.S., K.W., M.K., and V.T. are physicians or clinician scientists involved in the diagnosis and management of VTE in pregnant patients. S.L. is a radiologist and is familiar with the imaging protocols used in our institution. S.S.-G. is a registered nurse and an assistant head nurse in the Department of Obstetrics. Their collective expertise advanced the development of the protocol and overall understanding of the data, and they will contribute to the next steps in the larger action research project that this study is a part of.
2.4. Instrument and data collection
Recruited participants were invited to complete an electronic questionnaire using LimeSurvey [18], hosted by McGill University, to assess baseline characteristics of the study population, including age, prior pregnancies, gender identity, and education status. The questionnaire was filled out at the beginning of the encounter with the help of a research team member (S.O.). Based on guidance from the literature on qualitative interview sampling [19,20], we conducted a total of 20 in-person, one-on-one, audio-recorded structured interviews between September 26, 2022, and October 13, 2022. The interview comprised 11 questions regarding any prior experience with imaging during pregnancy, current understanding of the diagnosis of PE and diagnostic testing, perceptions of the information they needed, and how they wished for it to be conveyed. The interview guide was pilot-tested by the first 2 participants, and no modifications were required. The interview guide can be found in the Supplementary Material.
2.5. Statistical analysis
The interview recordings were transcribed verbatim in English or French according to the language spoken by the participant, and transcripts were deidentified prior to analysis. After all interviews were completed, a study investigator (S.O.) performed a thematic analysis for the first 10 transcripts. A hybrid deductive and inductive approach was used to identify concepts and how they interacted with each other and with our research questions [21]. The themes described in the first 10 interviews were cross-verified by another investigator (I.M.). Discrepancies were resolved by returning to the transcript with a discussion until a consensus was reached. One investigator (S.O.) independently completed the thematic analysis for the remaining 10 transcripts. Thematic sufficiency was reached after 16 transcripts. By then, although no additional novel theme could be extracted, we still analyzed all transcripts to both appreciate the recurrence of themes and capture the diversity of their expression. Quotes in French were translated into English for the purpose of this article. Data were analyzed using Quirkos 2.5.3 (computer software, Quirkos Limited, 2023), an online qualitative data analysis software [22]. Data are presented according to the Consolidated Criteria for Reporting Qualitative Research, as shown in the Supplementary Material [23].
3. Results
Between September 26, 2022, and October 13, 2022, we distributed 106 recruitment handouts, and 29 participants agreed to be approached by the study team. A total of 21 participants were approached, 20 of whom consented to participate. The average duration of interviews was 8 minutes (range, 4-17 minutes).
Half of the cohort self-identified as being of White and of North American ethnicity (n = 10, 50%) (Table). Most participants had completed a university degree (n = 8 undergraduate studies and n = 6 postgraduate studies, 70% in total). It was the first, second, and third or more pregnancy for 5 (%), 4 (20%), and 11 (55%) of participants, respectively. Four (20%) participants had prior confirmed or suspected VTE, and 14 (70 %) had medical comorbidities. Eleven (55%) interviews were conducted in English, and 9 (45%) interviews were conducted in French.
Table.
Baseline characteristics of study participants.
| Characteristics | No. of participants (%) |
|---|---|
| Racial and ethnic identity | |
| White, North American | 10 (50) |
| White, European | 2 (10) |
| East Asian | 2 (10) |
| South-East Asian | 1 (5) |
| Black Caribbean | 1 (5) |
| Latin American | 1 (5) |
| Northern African | 1 (5) |
| Mixed heritage | 2 (10) |
| Country of birth | |
| Canada | 13 (65) |
| Other | 7 (35) |
| Gender identity | |
| Woman | 20 (100) |
| Education level | |
| Primary school | 1 (5) |
| Secondary school | 1 (5) |
| College (CEGEP or equivalent) | 4 (20) |
| University undergraduate | 8 (40) |
| University postgraduate | 6 (30) |
| No. of pregnancies | |
| 1 | 5 (25) |
| 2 | 4 (20) |
| 3 or more | 11 (55) |
| VTE experience | |
| Prior confirmed or suspected VTE | 4 (20) |
| No prior confirmed or suspected VTE | 16 (80) |
| Comorbidities | |
| Yes | 14 (70) |
| No | 6 (30) |
CEGEP, Collège d'enseignement général et professionnel; VTE, venous thromboembolism.
The interviews allowed us to identify what information patients wished to receive prior to undergoing urgent diagnostic imaging in the setting of a suspected PE and how this information should be communicated to them. A third question was uncovered throughout the interviews as being a recurrent topic of reflection among interviewees: how would an information tool be integrated into their decision-making process? Below are presented the themes that surfaced from these 3 questions and selected patient quotations illustrating these concepts. Key quotations are shown below, but additional quotations are listed in the Supplementary Material.
3.1. What do patients want to know?
3.1.1. They want to know about PE
While some women had heard about PE before, others had not, and participants referred to PE as “blood clots in the lungs.” Participants expressed wanting to know more about PE in general and reported feeling unfamiliar with this diagnosis and how their risk of PE may be affected by pregnancy. They wished to know more about the symptoms and signs of PE and to be better informed about when to seek medical attention. Even participants with limited prior knowledge of PE perceived this diagnosis as a medical emergency. Participants also stated that they would want to know how PE could impact their pregnancy and their baby’s health, and some expressed concern about the association of PE with fetal death.
I think there should be something that explains why pregnant women might be at risk and what the consequences on the pregnancy would be, I mean beyond it being bad to have blood clots in your lungs. (R017)
3.1.2. They want to know about the imaging test
Participants voiced the need to know about the indication for the test. They wondered about the process of undergoing the test and wished to receive pragmatic explanations about its different stages: they wanted to know if they had to prepare for the test, what products or medications would have to be given for the test, whether there were any sounds or invasive procedures to expect, how long the test would last, and how soon after the test would the results be available.
As I do not know this test, explain that it makes this sound… It will last this long… Information that explains the test itself and the process of the test. (R009, translated from French)
Among participants, there was a sense of comfort from knowing that professional organizations had recommended using these diagnostic tests in pregnancy, and they thought it would be helpful to have that information included in the tool. Participants wanted to know more about the radiation dose when undergoing these tests. However, they indicated that the dose would have to be put into context for information to be meaningful to them (see section 3.1.4, “How do they want the information to be transmitted?”). A subset of participants expressed that they would not want to know the exact radiation dose, which seemed too technical for them; they felt that as long as their physician had recommended the test and had assessed the risk for them and their baby, knowing about the radiation dose was unnecessary.
Obviously if it’s something that Canada recommends, that means they’ve studied it and know that it’s going to be safe. So, it would be reassuring to find out what the recommendations are. (R017)
3.1.3. They want to know about the risks of radiation associated with these tests
All participants wanted to be informed about the test-related risks to their pregnancy and the health of their baby. They expressed concerns about the risks of miscarriage and preterm delivery and the impact on breastfeeding. They also requested specific and concrete information on the test-related risks to fetal health, specifically about the risks of teratogenicity and abnormal neurologic development. In addition to explanations about the immediate impact of ionizing radiation, they inquired about the radiation-related health risks for their child in the intermediate and long term. Of the 20 participants that we interviewed, 3 mentioned being worried about the risks of childhood malignancy.
Could the radiation affect the baby when he is born, or after 5 or 10 years? Sometimes, just the exposure to the radiation will affect the person 10 years, 15 years after. I don’t know if that has been studied, I have no idea. So that’s something I would like to know. (R008)
Participants wanted to know about the risk of radiation on their own general health and impaired fertility for future pregnancies. Many mentioned having questions regarding the long-term effects of radiation on their aging and their risk of chronic illnesses. Specifically, 6 participants asked about their own future risk of malignancy.
Everything that could have an impact if I wished to have other pregnancies. What impact could it have on fertility, on the day-to-day, on menses, on breastfeeding? If it may have an impact on my health as, I don’t know, as chemo, should I expect to lose my hair, that type of impact. (R009, translated from French)
Some participants wished to receive information on techniques used to reduce their exposure to radiation since they felt it would reassure them in this stressful situation. In contrast, others mentioned that they would not necessarily benefit from detailed information about the radiation reduction techniques since it was believed to be too technical but mentioned that they would appreciate generally knowing that such measures had been put in place.
Because if we know, for instance, that even if we are exposed to a lot of radiation, there is always something that can be used in the background to reduce this risk, it allows us to be less stressed and to have less fear related to the exposure. (R007, translated from French)
3.1.4. How do they want the information to be transmitted?
When asked about whether they would benefit from a written information tool, 18 of 20 participants responded that a written information tool would be useful to them. One participant mentioned that she would not need it personally but acknowledged that other women might feel differently. Some participants reported that they would want that information tool to be a physical document that they could bring home and read again later because they could not process all the information provided verbally after a single encounter. In addition, a written information tool was perceived as a starting point that would allow them to discuss their concerns with their physician and their family. Participants also pointed out that a written pamphlet or a visual explanation alone would be insufficient and that they would still expect to receive explanations from their care providers.
Sometimes, when you are just speaking with somebody in person, there are like things you will forget or that get lost in translation. So, like having something that you can refer to back at home would be useful. (R010)
Participants’ preferences varied about how comprehensive the information tool should be. Some expressed wanting very extensive explanations, whereas others preferred a brief summary. Participants also emphasized that they needed a tool that would integrate simple language and avoid medical jargon. Participants suggested that relaying the risk of complications from PE or ionizing radiation in terms of percentages would be clear, concrete, and accessible.
I just want to be told how much risk there is, that they make something that is simplified rather than providing a radiation dose, because that will not help me further. If they want to provide a percentage, let’s say, there is a 4% chance of complications, whatever, that is more interesting to me. It’s clearer. (R004, translated from French)
They also brought up the idea of comparing the radiation associated with CTPA and V/Q scans to known safety thresholds, radiation exposure they received in their daily activities, or other type of more common imaging modalities.
I guess it would be useful information and I think having ways to compare it to other tests that, you know, you go for dental exams how much radiation you get at that point, and it would be good to compare it maybe with other forms of standard radiation we are exposed to, to see it would not be such a great amount. I think it would be a good idea to give other values from other things we are exposed to on a daily basis, throughout our life, to show that the risk is not that high, or the exposure is not that high. (R002)
3.1.5. How is information integrated into their decision-making process?
Most participants wished to receive enough information to be able to weigh the risks and benefits of proceeding with an imaging test. Specifically, they mentioned being more likely to accept the risks associated with ionizing radiation if they could understand the benefits of doing the test or, in other words, the risks of not diagnosing a PE.
For me, it’s important to balance with the benefits of proceeding with the test as well. So, yes, there is radiation, but it’s compensated because of that. (R014, translated from French)
They also reported that their own lack of familiarity with an imaging modality could induce fear and might deter them from consenting to a test. Without sufficient information, some participants reported having previously refused diagnostic imaging tests during pregnancy, including a chest x-ray. Improving their knowledge of a diagnostic test appeared to increase the likelihood of them providing consent to undergo the test.
I have never heard of ventilation/perfusion scan. I would definitely be more apprehensive to have an imaging like that done than a CT because I have a little bit, very minimal, knowledge about it, but I would be more apprehensive to have a ventilation/perfusion scan because I have never heard of them. Is that a new technology? (R002)
Several women also suggested that an information tool could promote a feeling of trust toward the medical team by providing full disclosure and reducing their need to conduct research on the topic. Participants acknowledged that they often did their own research on their medical conditions but sometimes felt overwhelmed by what they found online because they did not know whether the information was reliable. On the contrary, a written tool could reduce the stress related to misinformation.
I think the less you leave to ambiguity, the more you can be reassured. I have a past history of not really trusting [the medical system]. I have had poor experiences in the past, so I will often do my own research at home just to cross-reference. So, if that information is already provided, I don’t have to look it up at home. And then, I’ll feel like it’s really not trying to leave anybody in the dark. (R010)
In general, the tolerance for fetal risk was much lower than the tolerance toward maternal risk, and in their decision making, participants would prioritize minimizing risks to their baby.
I think especially pregnant women are more worried about their babies than themselves. So, they would want to know what the risk to the baby was. And kind of you know a ratio, 1 in 1,000 chances, you know a ratio. It increases your chance this much of growth issues, or developmental issues, or malformations, or whatever. I think if they know their risk is low enough, they might be more willing to accept the imaging studies. (R011)
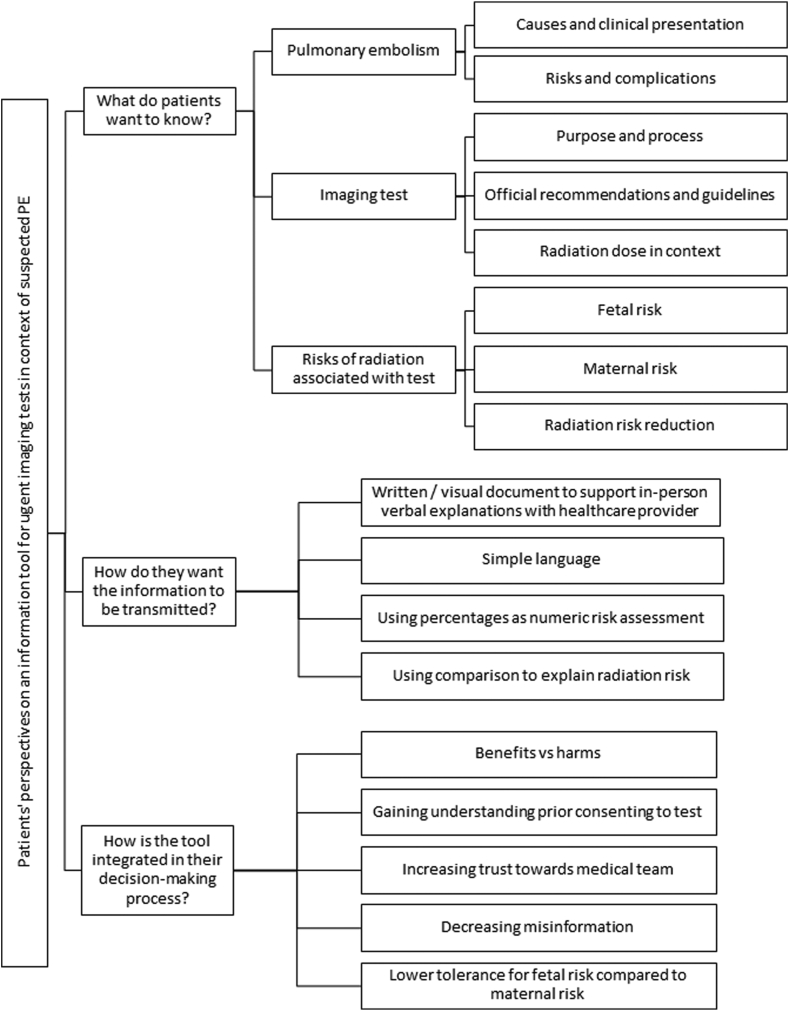
The patients’ perspectives on the content and format of an information tool for urgent imaging testing in the context of suspected PE, as well as their anticipated integration of such tool in their decision-making process, is summarized in the Figure.
Figure.
A schema of patients’ perspectives on the content, format, and role of the information tool in their decision-making process. PE, pulmonary embolism.
4. Discussion
In this study, patient participants expressed their need for an information tool that would explain the risks associated with the diagnosis of PE, the nature of the imaging tests used to diagnose PE, and the risks of undergoing an imaging test that emits radiation, including fetal and maternal risk of malignancy. They deemed a written information tool as useful. The preference for the comprehensiveness of a written tool varied from one individual to another. Participants emphasized the importance of simplified language and perceived radiation dose comparisons with other radiation exposure situations as a way to explain risks in a more accessible manner. They believed that a written information tool could help their decision making by highlighting the risks and benefits of undergoing imaging during pregnancy and promoting trust toward the medical team. While the weight of their preoccupations was shifted toward fetal risk, they still expected to be informed on the potential harms to themselves.
Many participants reported limited knowledge about PE, illustrating the overall low awareness of VTE both in general and high-risk populations, as shown in prior research [[24], [25], [26]]. Indeed, this educational need has now been prioritized by different national awareness campaigns, such as Stop the Clot, Spread the Word [27] or World Thrombosis Day [28]. Patients also reported limited knowledge of CTPA and V/Q scans, echoing the findings of a prior study examining patients’ perceptions of ionizing radiation used for imaging [29,30]. They requested to know more about the necessity and process of diagnostic imaging tests, a finding that had been observed in previous qualitative research with pregnant women undergoing plain radiographs [13]. Participants mentioned that they had limited prior knowledge of radiation and radiation risk reduction techniques, as shown in other research [30,31], and would want to be more informed prior to consenting to an imaging test.
In line with previous studies evaluating radiation risk communication in nonpregnant populations, there was a repeated wish for a numerical risk assessment among the interviewees, ideally including risk comparison with other settings, with the caveat that understanding this type of explanation was more difficult, possibly too technical, for some patients [[31], [32], [33]]. Moreover, patients have previously reported feeling unsatisfied with a statement such as “the risk of radiation is low” [32]. Likewise, several women tended to conduct their own research whenever they felt that the medical team minimized the risk related to an intervention.
As observed in another study on patients’ preferences for radiation risk communication [32], a written pamphlet does not preclude the need for a face-to-face conversation on the risks and benefits of radiation-emitting imaging with the referring physician. Several participants mentioned that they wanted to review the pamphlet with their physician and would be more inclined to accept a test if they trusted that their treating physician had weighed the risks and benefits with their best interests in mind, as seen in prior research in nonpregnant populations [11,13,32,33]. A written information tool was also perceived as useful in relaying information to their partner or family, which could help in their decision-making process, a finding that was also described in research looking at patient education tools for preeclampsia in pregnancy [34].
Considering variations in patients’ preferences, a document created to support patient counseling will need to strike a balance between providing precise, comprehensive education and using accessible terms and language. Previous research examining the quality and readability of educational online resources on preeclampsia identified that most educational materials were above the sixth grade reading level recommended by the US Department of Health and Human Services and the National Institutes of Health [35]. Thus, the understandability of the future tool will need to be evaluated with a diverse group of individuals.
Most participants stated that a written information tool would be useful to guide their decision making. A written format emerged as a possible asset in providing standardized counseling for patients in a focus group among healthcare providers [16]. The sentiment that such a tool should be both complete and accessible to most was shared by healthcare providers [16]. Educational resources on hypertensive disorders of pregnancy revealed conflicting data as to whether written information tools increased patient satisfaction [[36], [37], [38], [39], [40], [41]], as enhanced patient knowledge does not necessarily translate into increased satisfaction or decreased anxiety. As such, the impact of a patient information tool on patient satisfaction and well-being must be further evaluated. In addition, information overload from a counseling tool could be detrimental, particularly among pregnant individuals facing obstetrical complications [34], and this balancing measure must be assessed in future studies. Moreover, counseling in the emergency setting of a suspected PE can be challenging and limited by time. These facts may need to be clarified with patients, as some participants believed the written tool could be used at home to reflect on possible future investigations, which might not be possible in the clinical setting.
The involvement of pregnant patients, including some with prior experiences with diagnostic imaging during pregnancy, is a major strength of our study. Not only did it allow us to identify the content and format of an ideal written tool from a patient’s perspective, but it also revealed insights into how such a tool would be used in their decision-making process. Although prior studies have examined patients’ expectations regarding education about imaging risks and benefits, including risks of ionizing radiation [[10], [11], [12]], this study focused on the pregnant population and diagnostic testing for suspected PE.
Our study has limitations. The majority of participants were highly educated, with 70% having achieved university-level education. In addition, 70% experienced other medical comorbidities, likely reflecting the complexity of care in a tertiary care obstetrics antenatal clinic. Therefore, the type and level of granularity of information requested on radiation risk and PE complications might be a result of their degree of medical literacy, a bias driven by the educated volunteer phenomenon [42]. There could also be some degree of bias since the patients who consented to participate in our study might be looking for more medical information at baseline, with many expressing that they felt reassured by receiving comprehensive explanations from their medical providers and a minority stating that a written tool would raise their anxiety. It is possible that individuals who declined to be approached by our investigator would have felt differently. In this qualitative study, the rationale for tracking whether or not study participants had lived experiences of comorbidities was to develop an understanding of prior experience with the healthcare system rather than to provide a detailed description of the characteristics of our study population. As such, we did not collect any information on specific comorbidities. However, ensuring the representativity of patients with pregnancy-related and nonpregnancy-related conditions will be important in future codevelopment and validation stages of the design process. Finally, our study team included a single radiologist (S.L.). Imaging specialists are usually not involved in deciding whether pretest risk for PE warrants dedicated imaging; they rarely provide direct patient counseling about diagnostic testing for PE in pregnancy, and they typically only participate in the patient’s care path once the decision to pursue testing has already been made. Thus, while their insight was important at this stage, their contribution will be more central in the next phase of our work, where their knowledge of ionizing radiation in pregnancy will be required to codesign the patient information tool.
5. Conclusion
In this single-center study with pregnant patients, highly educated for the most part, we identified specific patient counseling needs prior to undergoing diagnostic imaging to rule out PE in pregnancy. A written tool was deemed useful, especially if it used an accessible language and made comparisons to put information into context. It was perceived as potentially helpful in patients’ own decision making. In the next phase of our research, we will codevelop an educational tool that will integrate patients’ interrogations regarding the fetal and maternal risks of PE and radiation. In turn, we plan to validate this tool with a diverse group of professionals and patients with a broader range of educational backgrounds.
Appendix
Study group participants for the CanVECTOR Network:
Suzie Ouellet, Montreal, Québec, Canada.
Sandrine Hamel, Montreal, Québec, Canada.
Camille Simard, Montreal, Québec, Canada.
Maral Koolian, Montreal, Québec, Canada.
Vicky Tagalakis, Montreal, Québec, Canada.
Isabelle Malhamé, Montreal, Québec, Canada.
Acknowledgments
Funding
S.O. and S.H. were recipients of the Canadian Venous Thromboembolism Research Network studentship award, which provided operational funding for this study. This research also received funding from the McGill University Department of Medicine Scholarly Quality Awards Competition.
Ethics statement
This study received approval from the McGill University Health Center Research and Ethics Board (#2022-8495).
Author contributions
All authors contributed to the development of the study protocol, including the interview questionnaire. A.d.P, L.S., and N.-Z.S. provided special guidance for qualitative research methodology. S.O. and I.M. approached the McGill University Health Center Obstetrics clinic for participant recruitment. S.O. recruited participants, conducted patient interviews, and performed the thematic analysis from interview transcripts. S.O. and I.M. interpreted the results and wrote the manuscript. All authors discussed the results and critically revised the manuscript.
Relationship Disclosure
S.O. and S.H. were recipients of the Canadian Venous Thromboembolism Research Network studentship award. I.M. has received funding from the Canadian Institutes of Health Research for her research program, and she receives salary support from the Fonds de Recherche du Québec en Santé. C.S. has received honoraria from Leo Pharma for leading a previous webinar on venous thromboembolism in pregnancy. L.S. has received prior funding from the Royal College of Physicians and Surgeons of Canada and Karolinska Institute for attending meetings and is on the board of the American College of Physicians, Quebec Chapter. M.K., A.R., S.L., A.B., S.S.-G., V.T., A.d.P., L.S., and N.-Z.S. declare no other conflict of interest.
Footnotes
Handling Editor: Dr Bethany Samuelson Bannow
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2024.102317
Supplementary material
References
- 1.Liu S., Rouleau J., Joseph K.S., Sauve R., Liston R.M., Young D., et al. Epidemiology of pregnancy-associated venous thromboembolism: a population-based study in Canada. J Obstet Gynaecol Can. 2009;31:611–620. doi: 10.1016/S1701-2163(16)34240-2. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen E.T., Hague C., Manos D., Memauri B., Souza C., Taylor J., et al. Canadian Society of Thoracic Radiology/Canadian Association of Radiologists best practice guidance for investigation of acute pulmonary embolism, part 1: acquisition and safety considerations. Can Assoc Radiol J. 2022;73:203–213. doi: 10.1177/08465371211000737. [DOI] [PubMed] [Google Scholar]
- 3.Malhamé I., Tagalakis V., Dayan N. Diagnosing pulmonary embolism in pregnancy: synthesis of current guidelines and new evidence. J Obstet Gynaecol Can. 2020;42:1546–1549. doi: 10.1016/j.jogc.2020.03.025. [DOI] [PubMed] [Google Scholar]
- 4.Leung A.N., Bull T.M., Jaeschke R., Lockwood C.J., Boiselle P.M., Hurwitz L.M., et al. American Thoracic Society documents: an official American Thoracic Society/Society of Thoracic Radiology Clinical Practice Guideline--evaluation of suspected pulmonary embolism in pregnancy. Radiology. 2012;262:635–646. doi: 10.1148/radiol.11114045. [DOI] [PubMed] [Google Scholar]
- 5.Cohen S.L., Feizullayeva C., McCandlish J.A., Sanelli P.C., McGinn T., Brenner B., et al. Comparison of international societal guidelines for the diagnosis of suspected pulmonary embolism during pregnancy. Lancet Haematol. 2020;7:e247–e258. doi: 10.1016/S2352-3026(19)30250-9. [DOI] [PubMed] [Google Scholar]
- 6.Bates S.M., Rajasekhar A., Middeldorp S., McLintock C., Rodger M.A., James A.H., et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2:3317–3359. doi: 10.1182/bloodadvances.2018024802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan W.S., Rey E., Kent N.E., Corbett T., David M., Douglas M.J., et al. VTE in Pregnancy Guideline Working Group; Society of Obstetricians and Gynecologists of Canada. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can. 2014;36:527–553. doi: 10.1016/s1701-2163(15)30569-7. [DOI] [PubMed] [Google Scholar]
- 8.Woo J.K., Chiu R.Y., Thakur Y., Mayo J.R. Risk-benefit analysis of pulmonary CT angiography in patients with suspected pulmonary embolus. AJR Am J Roentgenol. 2012;198:1332–1339. doi: 10.2214/AJR.10.6329. [DOI] [PubMed] [Google Scholar]
- 9.Lowe S. Diagnostic imaging in pregnancy: making informed decisions. Obstet Med. 2019;12:116–122. doi: 10.1177/1753495X19838658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robey T.E., Edwards K., Murphy M.K. Barriers to computed tomography radiation risk communication in the emergency department: a qualitative analysis of patient and physician perspectives. Acad Emerg Med. 2014;21:122–129. doi: 10.1111/acem.12311. [DOI] [PubMed] [Google Scholar]
- 11.Pahade J.K., Trout A.T., Zhang B., Bhambhvani P., Muse V.V., Delaney L.R., et al. What patients want to know about imaging examinations: a multiinstitutional U.S. survey in adult and pediatric teaching hospitals on patient preferences for receiving information before radiologic examinations. Radiology. 2018;287:554–562. doi: 10.1148/radiol.2017170592. [DOI] [PubMed] [Google Scholar]
- 12.Rosenkrantz A.B., Flagg E.R. Survey-based assessment of patients’ understanding of their own imaging examinations. J Am Coll Radiol. 2015;12:549–555. doi: 10.1016/j.jacr.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Ratnapalan S., Bona N., Chandra K., Koren G. Physicians’ perceptions of teratogenic risk associated with radiography and CT during early pregnancy. AJR Am J Roentgenol. 2004;182:1107–1109. doi: 10.2214/ajr.182.5.1821107. [DOI] [PubMed] [Google Scholar]
- 14.Reitan A.F., Sanderud A. What information did pregnant women want related to risks and benefits attending X-ray examinations? J Med Imaging Radiat Sci. 2021;52:79–85. doi: 10.1016/j.jmir.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 2000;23:334–340. doi: 10.1002/1098-240x(200008)23:4<334::aid-nur9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 16.Hamel S., Ouellet S., Simard C., Robert A., Wou K., St-Georges S., et al. Developing a patient information tool for pregnant people requiring diagnostic imaging for pulmonary embolism: a providers’ needs assessment. J Obstet Gynaecol Can. 2022;44:1132–1133. doi: 10.1016/j.jogc.2022.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Baum F., MacDougall C., Smith D. Participatory action research. J Epidemiol Community Health. 2006;60:854–857. doi: 10.1136/jech.2004.028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LimeSurvey GmbH. (n.d.). LimeSurvey: an open source survey tool, http://www.limesurvey.org/; 2022 [accessed September 26, 2022 - October 13, 2022].
- 19.Guest G., Bunce A., Johnson L. How many interviews are enough?: An experiment with data saturation and variability. Field Methods. 2006;18:59–82. [Google Scholar]
- 20.Boddy C.R. Sample size for qualitative research. Qual Mark Res. 2016;19:426–432. [Google Scholar]
- 21.Kiger M.E., Varpio L. Thematic analysis of qualitative data: AMEE Guide No. 131. Med Teach. 2020;42:846–854. doi: 10.1080/0142159X.2020.1755030. [DOI] [PubMed] [Google Scholar]
- 22.Quirkos 2.5.2 Computer Software. 2022. https://www.quirkos.com
- 23.Tong A., Sainsbury P., Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19:349–357. doi: 10.1093/intqhc/mzm042. [DOI] [PubMed] [Google Scholar]
- 24.Wendelboe A.M., McCumber M., Hylek E.M., Buller H., Weitz J.I., Raskob G., et al. Global public awareness of venous thromboembolism. J Thromb Haemost. 2015;13:1365–1371. doi: 10.1111/jth.13031. [DOI] [PubMed] [Google Scholar]
- 25.Le Sage S., McGee M., Emed J.D. Knowledge of venous thromboembolism (VTE) prevention among hospitalized patients. J Vasc Nurs. 2008;26:109–117. doi: 10.1016/j.jvn.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Almodaimegh H., Alfehaid L., Alsuhebany N., Bustami R., Alharbi S., Alkatheri A., et al. Awareness of venous thromboembolism and thromboprophylaxis among hospitalized patients: a cross-sectional study. Thromb J. 2017;15:19. doi: 10.1186/s12959-017-0144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Blood Clot Alliance Stop the clot, spread the word. https://www.stoptheclot.org/spreadtheword/pregnancy/ 2021. [accessed December 4, 2022].
- 28.International Society on Thrombosis and Haemostasis World thrombosis day. Move against thrombosis. https://www.worldthrombosisday.org/ 2023. [accessed December 4, 2022].
- 29.Bastiani L., Paolicchi F., Faggioni L., Martinelli M., Gerasia R., Martini C., et al. Patient perceptions and knowledge of ionizing radiation from medical imaging. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.28561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ludwig R.L., Turner L.W. Effective patient education in medical imaging: public perceptions of radiation exposure risk. J Allied Health. 2002;31:159–164. [PubMed] [Google Scholar]
- 31.Dauer L.T., Thornton R.H., Hay J.L., Balter R., Williamson M.J., Germain J.S. Fears, feelings, and facts: interactively communicating benefits and risks of medical radiation with patients. AJR Am J Roentgenol. 2011;196:756–761. doi: 10.2214/AJR.10.5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies E., Peet D., Taylor M.J., Chung E.M.L. Survey of the publics’ preferences for communication of medical radiation risk. J Radiol Prot. 2022;42 doi: 10.1088/1361-6498/ac4c93. [DOI] [PubMed] [Google Scholar]
- 33.Coakley F.V., Gould R., Yeh B.M., Arenson R.L. CT radiation dose: what can you do right now in your practice? AJR Am J Roentgenol. 2011;196:619–625. doi: 10.2214/AJR.10.5043. [DOI] [PubMed] [Google Scholar]
- 34.Vinogradov R., Smith V.J., Robson S.C., Araujo-Soares V. Informational needs related to aspirin prophylactic therapy amongst pregnant women at risk of preeclampsia – a qualitative study. Pregnancy Hypertens. 2021;25:161–168. doi: 10.1016/j.preghy.2021.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Lange E.M., Shah A.M., Braithwaite B.A., You W.B., Wong C.A., Grobman W.A., et al. Readability, content, and quality of online patient education materials on preeclampsia. Hypertens Pregnancy. 2015;34:383–390. doi: 10.3109/10641955.2015.1053607. [DOI] [PubMed] [Google Scholar]
- 36.Goel K.M., Maxwell R.A., Cooley M.E., Rackett T.M., Yaklic J.L. Quality improvement: patient education for management of hypertension in pregnancy. Hypertens Pregnancy. 2021;40:271–278. doi: 10.1080/10641955.2021.1981371. [DOI] [PubMed] [Google Scholar]
- 37.Parfenova M., Côté A.M., Cumyn A., Pesant M.H., Champagne M., Roy-Lacroix M.È., et al. Impact of an educational pamphlet on knowledge about health risks after hypertensive disorders of pregnancy: a randomized trial. J Obstet Gynaecol Can. 2021;43:182–190. doi: 10.1016/j.jogc.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Gingras-Charland M.E., Côté A.M., Girard P., Grenier A., Pasquier J.C., Sauvé N. Pre-eclampsia educational tool impact on knowledge, anxiety, and satisfaction in pregnant women: a randomized trial. J Obstet Gynaecol Can. 2019;41:960–970. doi: 10.1016/j.jogc.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Walker M.G., Windrim C., Ellul K.N., Kingdom J.C.P. Web-based education for placental complications of pregnancy. J Obstet Gynaecol Can. 2013;35:334–339. doi: 10.1016/S1701-2163(15)30961-0. [DOI] [PubMed] [Google Scholar]
- 40.Van der Merwe J.L., Hall D.R., Harvey J. Does a patient information sheet lead to better understanding of pre-eclampsia? A randomised controlled trial. Pregnancy Hypertens. 2011;1:225–230. doi: 10.1016/j.preghy.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Sauvé N., Powrie R.O., Larson L., Phipps M.G., Weitzen S., Fitzpatrick D., et al. The impact of an educational pamphlet on knowledge and anxiety in women with preeclampsia. Obstet Med. 2008;1:11–17. doi: 10.1258/om.2008.070001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tripepi G., Jager K.J., Dekker F.W., Zoccali C. Selection bias and information bias in clinical research. Nephron Clin Pract. 2010;115:c94–c99. doi: 10.1159/000312871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.