Abstract
Resistance to rituximab B-cell depletion therapy is a clinically pertinent adverse sequela that can have significant implications for the treatment of immune-mediated glomerular diseases. The true incidence of rituximab resistance remains unknown; however, it is an increasingly recognized treatment complication. Resistance typically presents with suboptimal treatment response, rapid B-cell reconstitution, and a relapsing disease course. Although the diverse mechanisms resulting in rituximab resistance are ongoing topics of research, both primary and secondary mechanisms have been identified as key catalysts.
The emergence of human antichimeric antibodies (HACAs) is a major cause of secondary resistance to rituximab therapy and typically appears following repeated drug exposure. Frequently, HACAs develop in the setting of underlying autoimmune disease and contribute to poor B-cell depletion, reduced rituximab therapeutic efficacy, and enhanced drug clearance.
The clinical challenge of rituximab resistance necessitates heightened awareness among clinicians. Screening for HACAs should be considered in individuals with poor clinical response to rituximab, more rapid B-cell reconstitution, and relapsing disease. Detection of HACAs may guide treatment alterations, including addition of further immunosuppressive therapy and transitioning to a humanized B-cell depleting monoclonal antibody.
Index Words: Antidrug antibodies, B-cell depletion therapy, CD20 antigen, glomerular disease, human antichimeric antibodies, rituximab resistance
Autoimmune glomerular diseases are largely driven by autoantibody formation, leading to damage by targeting renal antigens or the formation of antigen-antibody complexes.1 Resultantly, B-cell depletion therapy with rituximab has been increasingly used in the management of various immune-mediated glomerular diseases, both newly diagnosed and relapsing. This agent is a chimeric mouse-human monoclonal antibody (mAb) consisting of a murine variable region directed against the CD20 antigen on B lymphocytes. It induces B-cell depletion through various mechanisms, including Fc receptor γ-mediated antibody-dependent cellular cytotoxicity (ADCC), complement-mediated cell lysis, B-cell growth arrest, and B-cell apoptosis.2
Despite the paucity of data concerning rituximab’s influence on the management of autoimmune disease, resistance to rituximab remains a pertinent adverse clinical sequela. It is defined as a lack of response to rituximab-containing regimens, in particular, disease progression within 6 months of therapy.3 Determining the true incidence of resistance remains challenging. In a recent retrospective, multicenter study from France, rituximab resistance was observed in 12% of participants with antineutrophilic cytoplasmic antibody-associated vasculitis 3 months after initiating rituximab induction therapy.4 Clinical suspicion of rituximab resistance is important in individuals with relapsing disease, suboptimal treatment response, and rapid B-cell reconstitution. It is more commonly observed in autoimmune diseases, such as systemic lupus erythematosus, membranous nephropathy, and rheumatoid arthritis.5
This review focuses on the proposed mechanisms of rituximab resistance, including the emergence of human antichimeric antibodies (HACAs) and treatment strategies to combat the pleiotropic process.
Mechanism of Rituximab Resistance
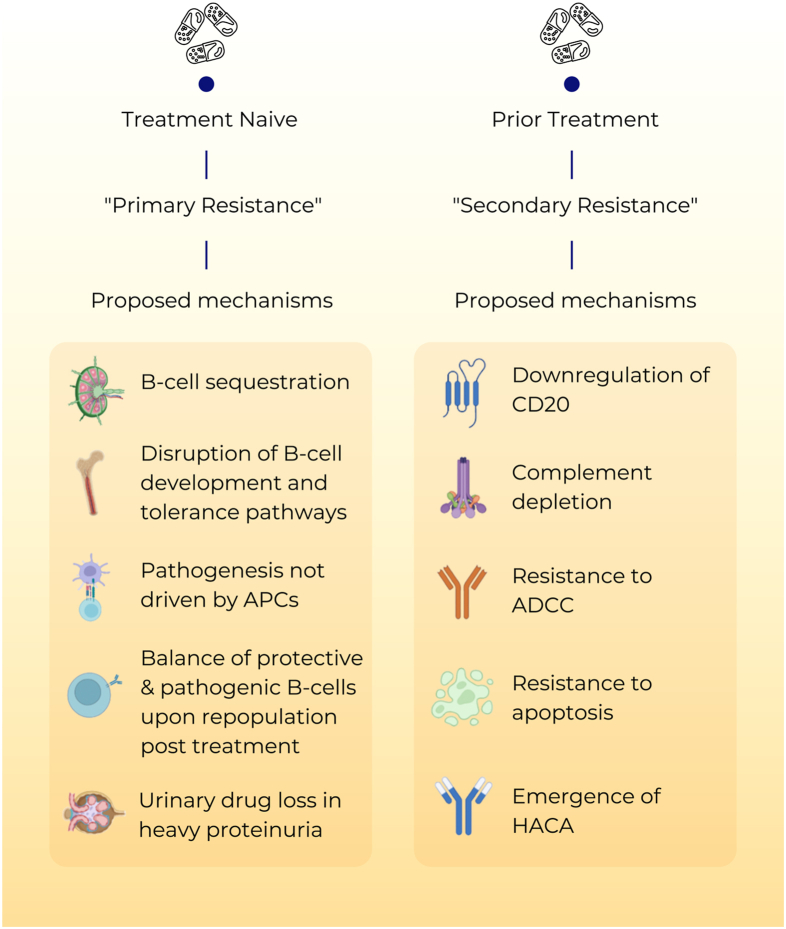
The exploration of the multifaceted mechanisms responsible for rituximab resistance remains a subject of active investigation, with numerous primary and secondary mechanisms proposed as pivotal contributors (Fig 1). Our understanding of the pathophysiologic mechanisms underlying drug resistance has primarily been derived from studies of hematologic malignancies, although there is a notable scarcity of scientific data in the context of managing autoimmune diseases.
Figure 1.
Mechanisms of rituximab resistance. ADCC, antibody-dependent cellular cytotoxicity; APC, antigen-presenting cell; HACA, human antichimeric antibody.
Primary Rituximab Resistance
Primary rituximab resistance has been observed in treatment-naïve individuals. The cause is poorly understood; however, B-cell sequestration, expansion of memory B-cell subsets, and disruption of B-cell development and tolerance pathways may be contributory. The balance of protective and pathogenic B-cell subsets established following B-cell repopulation is also significant.6 In addition, the underlying disease pathophysiology is relevant to primary resistance, particularly when antigen-presenting cells do not play a critical role in pathogenesis. Moreover, the level of CD20 expression at baseline significantly influences the response to treatment. Individuals with autoimmune disease have been found to have a greater frequency of CD19+CD20− B-cell population in peripheral blood preceding rituximab initiation, which can contribute to a suboptimal B-cell depletion.7
Rapid urinary loss of rituximab in heavy proteinuric states is also likely to yield a suboptimal treatment response. This has been illustrated in the Lupus Nephritis Assessment With Rituximab study, where the addition of rituximab to lupus nephritis induction therapy did not influence remission rates.8 Secondary analysis of the Lupus Nephritis Assessment With Rituximab study displayed that heavier proteinuria at the time of rituximab treatment was associated with lower peak rituximab levels, incomplete peripheral B-cell depletion, and lower odds of clinical response to rituximab.9
Secondary Rituximab Resistance
Secondary rituximab resistance occurs with repeated drug exposure. Here, the major effector pathways of rituximab’s mechanism of action are involved.3 Such pathways broadly include the downregulation of CD20, complement depletion, and resistance to complement-dependent cytotoxicity, ADCC, and apoptosis.
The pathophysiologic mechanisms underlying secondary rituximab resistance have largely been examined within the context of hematologic malignancies. Treatment efficacy is known to correlate with CD20 levels, and in rituximab-resistant diseases, these levels are reduced.10 Malignant cell lines resistant to rituximab display an upregulation of complement inhibitory proteins, leading to resistance to complement-dependent cytotoxicity.11 Additionally, the clinical response to rituximab relies heavily on ADCC, and resistance to ADCC is a significant contributor to treatment failure.12 In ADCC, peripheral blood natural killer cells play a pivotal role. Their levels and function can be altered by rituximab, thereby contributing to ADCC resistance in diffuse large B-cell lymphoma.13 Furthermore, cells resistant to rituximab exhibit hyperactivation of intracellular pathways, including p38 mitogen-activated protein kinase, nuclear factor κB, extracellular signal-regulated kinases 1 and 2, and protein kinase B pathways. This hyperactivation promotes cell survival in the setting of B-cell lymphoma and promotes rituximab evasion.14
Finally, the presence of antidrug antibodies is an additional factor contributing to rituximab resistance, particularly in the cases of autoimmune diseases. These antibodies not only target the mAb binding domain but can also modify drug pharmacokinetics by forming immune complexes and enhancing mAb metabolism.
Human Antichimeric Antibodies
The emergence of HACAs to the mouse-derived variable region of rituximab is a recognized consequence of rituximab therapy.15 These antibodies may contribute to increased infusion reactions, poor B-cell depletion, faster B-cell reconstitution, and reduced therapeutic efficacy of rituximab by enhanced drug clearance.
Although the incidence of HACAs to rituximab remains uncertain, these antibodies are more commonly observed in the management of autoimmune diseases compared with hematologic malignancies.5 This could be associated with a highly activated B-lymphocyte status and a tendency of developing antidrug antibodies in the setting of underlying autoimmunity. The formation of HACAs is notably high in systemic lupus erythematosus, where polyclonal B-cell activation is present.16 An incidence of 37.8% has been reported, most commonly in the setting of lupus nephritis, a more active disease and a high Systemic Lupus Erythematosus Disease Activity Index 2000 score. Rituximab HACAs have also been described in the treatment of membranous nephropathy, with reports as high as 23%.17 Here, the HACAs have been drug-neutralizing and associated with faster B-cell reconstitution and higher relapse rates. However, these findings have not been demonstrated in all glomerular diseases because another study found no association between the development of HACAs and relapse in steroid-dependent nephrotic syndrome.18
Enzyme-linked immunoassays can be used for the quantitative assessment of serum rituximab and anti-rituximab antibodies.19 Consideration should be given to screening for HACAs in individuals experiencing rituximab-resistant or relapsing disease, and serial monitoring may be advisable. The detection of HACAs does not necessarily imply treatment failure because drug efficacy is reliant upon the balance between the antibody titers and serum free rituximab levels. Thus, a positive result merely signifies a risk factor for treatment failure and could assist in selecting patients who are likely to be unresponsive to rituximab retreatment.20 It is important to note that immunoassays can solely detect free serum HACAs, and the formation of immune complexes involving HACAs and rituximab may lead to false-negative results. Therefore, serum samples should be obtained immediately before rituximab administration to increase the yield of antibody detection. Unfortunately, however, testing for HACAs and rituximab neutralization assays are not readily accessible, even among resource-rich countries.
Management of Rituximab Resistance
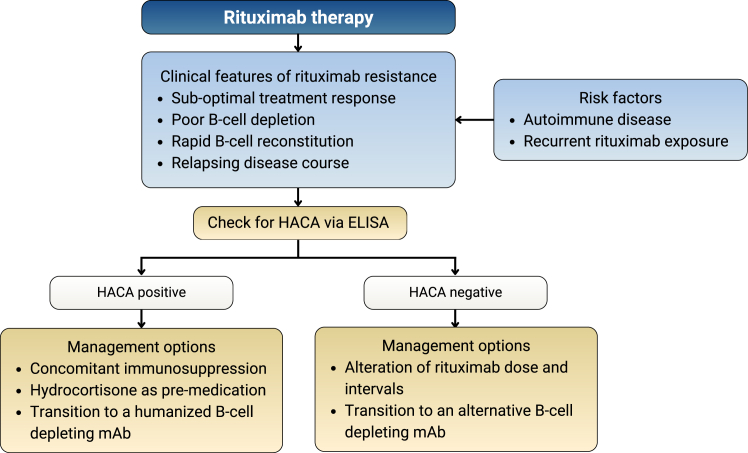
There is little evidence on how to circumvent rituximab resistance; however, a clinical approach to its diagnosis and management can be adopted (Fig 2).
Figure 2.
Clinical approach to diagnosis and management of rituximab resistance. ELISA, enzyme-linked immunoassay; HACA, human antichimeric antibody; mAb, monoclonal antibody.
Numerous strategies may modify rituximab treatment response and considerable interindividual variability is observed. In the absence of HACAs, retreatment with rituximab can be considered as a reasonable initial strategy. Alterations of dose and reduction of treatment intervals may have a role, especially in those with rapid urinary drug loss. However, the optimal strategy remains to be defined. Concomitant therapy with other immunosuppressive agents, such as corticosteroids and antimetabolites, may theoretically diminish the development of HACAs, an observation noted with infliximab mAb treatment.21 Premedication with intravenous hydrocortisone may also be of benefit.22
Most importantly, however, transition to alternative B-cell depleting agents may be required. The rate of immunogenicity and cross-reactivity of rituximab HACAs with other B-cell depleting agents is unknown. Type II anti-CD20 molecules, such as obinutuzumab, are directed against an alternative epitope and cause B-cell depletion primarily by apoptosis rather than complement-dependent cytotoxicity.23 The Fc fragment of the mAb is modified to improve its binding affinity to FcγRIIIa, which in turn dramatically increases ADCC potency compared with rituximab.13 Furthermore, the humanized nature of this mAb negates the risk of HACA development and has proven effective in cases of rituximab refractory membranous nephropathy.24 As such, humanized anti-CD20 mAbs should be considered as alternative treatment options in those with persistent rituximab resistance. Lastly, therapies targeting CD19, such as CD19-targeted chimeric antigen receptor–modified T cells may provide an attractive substitute in some cases of rituximab resistance, particularly in cases demonstrating a greater frequency of CD19+CD20− B cells.25
Conclusion
Increased awareness of the immunogenic consequences of rituximab administration is required among clinicians. Screening for HACAs should be considered in individuals with high-risk autoimmune diseases, such as systemic lupus erythematosus, and in those demonstrating poor clinical response to therapy, more rapid B-cell reconstitution, or relapsing disease. This is particularly significant if future rituximab treatment is to be considered. Detection of HACAs may assist in directing disease treatment options, with consideration of additional immunosuppressive therapy and alternative B-cell depleting agents.
Article Information
GlomCon Editorial Team
A list of the GlomCon Editorial Team can be found at: https://pubs.glomcon.org/editorial-team/.
Authors’ Full Names and Academic Degrees
Tania Salehi, MBBS, Anoushka Krishnan, FRACP, Ayman Al Jurdi, MD, Paolo So, MD, Edgar Lerma, MD, and Nasim Wiegley, MD, on behalf of the GlomCon Editorial Team
Support
There was no monetary or nonmonetary support for the preparation of the manuscript.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received September 14, 2023. Evaluated by 2 external peer reviewers, with direct editorial input from the Editor-in-Chief. Accepted in revised form November 12, 2023.
Footnotes
Complete author and article information provided before references.
References
- 1.Couser W.G., Johnson R.J. The etiology of glomerulonephritis: roles of infection and autoimmunity. Kidney Int. 2014;86(5):905–914. doi: 10.1038/ki.2014.49. [DOI] [PubMed] [Google Scholar]
- 2.Cragg M.S., Walshe C.A., Ivanov A.O., Glennie M.J. The biology of CD20 and its potential as a target for mAb therapy. Curr Dir Autoimmun. 2005;8:140–174. doi: 10.1159/000082102. [DOI] [PubMed] [Google Scholar]
- 3.Rezvani A.R., Maloney D.G. Rituximab resistance. Best Pract Res Clin Haematol. 2011;24(2):203–216. doi: 10.1016/j.beha.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machet T., Quémeneur T., Ledoult E., et al. Rituximab resistance at 3 months of induction therapy in newly diagnosed or relapsing ANCA-associated vasculitis: a French multicentre retrospective study in 116 patients. Joint Bone Spine. 2023;90(5) doi: 10.1016/j.jbspin.2023.105591. [DOI] [PubMed] [Google Scholar]
- 5.Fleischmann R.M. Safety of biologic therapy in rheumatoid arthritis and other autoimmune diseases: focus on rituximab. Semin Arthritis Rheum. 2009;38(4):265–280. doi: 10.1016/j.semarthrit.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Pateinakis P., Pyrpasopoulou A. CD20+ B cell depletion in systemic autoimmune diseases: common mechanism of inhibition or disease-specific effect on humoral immunity? Biomed Res Int. 2014;2014 doi: 10.1155/2014/973609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah K., Klein C., Cambridge G., et al. P167 Overcoming rituximab resistance in autoimmune disease: back to basics. Rheumatology. 2023;62(suppl 2) doi: 10.1093/rheumatology/kead104.208. [DOI] [Google Scholar]
- 8.Rovin B.H., Furie R., Latinis K., et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012;64(4):1215–1226. doi: 10.1002/art.34359. [DOI] [PubMed] [Google Scholar]
- 9.Gomez Mendez L.M., Cascino M.D., Garg J., et al. Peripheral blood B cell depletion after rituximab and complete response in lupus nephritis. Clin J Am Soc Nephrol. 2018;13(10):1502–1509. doi: 10.2215/CJN.01070118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavlasova G., Mraz M. The regulation and function of CD20: an “enigma” of B-cell biology and targeted therapy. Haematologica. 2020;105(6):1494–1506. doi: 10.3324/haematol.2019.243543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czuczman M.S., Olejniczak S., Gowda A., et al. Acquirement of rituximab resistance in lymphoma cell lines is associated with both global CD20 gene and protein down-regulation regulated at the pretranscriptional and posttranscriptional levels. Clin Cancer Res. 2008;14(5):1561–1570. doi: 10.1158/1078-0432.CCR-07-1254. [DOI] [PubMed] [Google Scholar]
- 12.Bonavida B. Postulated mechanisms of resistance of B-cell non-Hodgkin lymphoma to rituximab treatment regimens: strategies to overcome resistance. Semin Oncol. 2014;41(5):667–677. doi: 10.1053/j.seminoncol.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kusowska A., Kubacz M., Krawczyk M., Slusarczyk A., Winiarska M., Bobrowicz M. Molecular aspects of resistance to immunotherapies-advances in understanding and management of diffuse large B-cell lymphoma. Int J Mol Sci. 2022;23(3):1501. doi: 10.3390/ijms23031501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeon M.J., Yu E.S., Choi C.W., Kim D.S. Identification and overcoming rituximab resistance in diffuse large B-cell lymphoma using next-generation sequencing. Korean J Intern Med. 2023;38(6):893–902. doi: 10.3904/kjim.2023.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albert D., Dunham J., Khan S., et al. Variability in the biological response to anti-CD20 B cell depletion in systemic lupus erythaematosus. Ann Rheum Dis. 2008;67(12):1724–1731. doi: 10.1136/ard.2007.083162. [DOI] [PubMed] [Google Scholar]
- 16.Faustini F., Dunn N., Kharlamova N., et al. First exposure to rituximab is associated to high rate of anti-drug antibodies in systemic lupus erythematosus but not in ANCA-associated vasculitis. Arthritis Res Ther. 2021;23(1):211. doi: 10.1186/s13075-021-02589-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyer-Suavet S., Andreani M., Lateb M., et al. Neutralizing anti-rituximab antibodies and relapse in membranous nephropathy treated with rituximab. Front Immunol. 2019;10:3069. doi: 10.3389/fimmu.2019.03069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angeletti A., Bruschi M., Colucci M., et al. Circulating anti-rituximab antibodies do not affect response to rituximab in steroid-dependent nephrotic syndrome. Kidney Int Rep. 2022;7(11):2509–2512. doi: 10.1016/j.ekir.2022.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LISA TRACKER Duo Rituximab 2017. https://www.clsdiagnostics.com/files/ltr005-lisa-tracker-duo-rituximab-jul-2017.pdf
- 20.Teisseyre M., Boyer-Suavet S., Crémoni M., Brglez V., Esnault V., Seitz-Polski B. Analysis and management of rituximab resistance in PLA2R1-associated membranous nephropathy. Kidney Int Rep. 2021;6(4):1183–1188. doi: 10.1016/j.ekir.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maini R.N., Breedveld F.C., Kalden J.R., et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41(9):1552–1563. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 22.Farrell R.J., Alsahli M., Jeen Y.T., Falchuk K.R., Peppercorn M.A., Michetti P. Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn’s disease: a randomized controlled trial. Gastroenterology. 2003;124(4):917–924. doi: 10.1053/gast.2003.50145. [DOI] [PubMed] [Google Scholar]
- 23.Tobinai K., Klein C., Oya N., Fingerle-Rowson G. A review of obinutuzumab (GA101), a novel type II anti-CD20 monoclonal antibody, for the treatment of patients with B-cell malignancies. Adv Ther. 2017;34(2):324–356. doi: 10.1007/s12325-016-0451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klomjit N., Fervenza F.C., Zand L. Successful treatment of patients with refractory PLA2R-associated membranous nephropathy with obinutuzumab: a report of 3 cases. Am J Kidney Dis. 2020;76(6):883–888. doi: 10.1053/j.ajkd.2020.02.444. [DOI] [PubMed] [Google Scholar]
- 25.Mougiakakos D., Krönke G., Völkl S., et al. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N Engl J Med. 2021;385(6):567–569. doi: 10.1056/NEJMc2107725. [DOI] [PubMed] [Google Scholar]