Abstract
Background
The link between excess adiposity and carcinogenesis has been well established for multiple malignancies, and cancer is one of the main contributors to obesity-related mortality. The potential role of different weight-loss interventions on cancer risk modification has been assessed, however, its clinical implications remain to be determined. In this clinical review, we present the data assessing the effect of weight loss interventions on cancer risk.
Methods
In this clinical review, we conducted a comprehensive search of relevant literature using MEDLINE, Embase, Web of Science, and Google Scholar databases for relevant studies from inception to January 20, 2024. In this clinical review, we present systematic reviews and meta-analysis, randomized clinical trials, and prospective and retrospective observational studies that address the effect of different treatment modalities for obesity in cancer risk. In addition, we incorporate the opinions from experts in the field of obesity medicine and oncology regarding the potential of weight loss as a preventative intervention for cancer.
Results
Intentional weight loss achieved through different modalities has been associated with a reduced cancer incidence. To date, the effect of weight loss on the postmenopausal women population has been more widely studied, with multiple reports indicating a protective effect of weight loss on hormone-dependent malignancies. The effect of bariatric interventions as a protective intervention for cancer has been studied extensively, showing a significant reduction in cancer incidence and mortality, however, data for the effect of bariatric surgery on certain specific types of cancer is conflicting or limited.
Conclusion
Medical nutrition therapy, exercise, antiobesity medication, and bariatric interventions, might lead to a reduction in cancer risk through weight loss-dependent and independent factors. Further evidence is needed to better determine which population might benefit the most, and the amount of weight loss required to provide a clinically significant preventative effect.
Keywords: Bariatric surgery, Cancer, Obesity, Prevention, Weight loss
Abbreviations
- BMI
Body Mass Index
- IARC
International Agency for Research on Cancer
- WHI
Women's Health Initiative
- HR
Hazard Ratio
- 95% CI
95% Confidence Interval
- ILI
Intensive Lifestyle Intervention
- AOM
Antiobesity Medication
- RR
Risk Ratio
- IL
Interleukin
- hsCRP
High-sensitivity C-reactive protein
- FDA
U.S. Food and Drug Administration
- MTC
Medullary Thyroid Cancer
- GIP
Glucose-dependent insulinotropic polypeptide
- GLP-1
Glucagon-like Peptide-1
- SOS
Swedish Obese Subject (SOS) study
- RYGB
Roux-en-Y Gastric Bypass
- SG
Sleeve Gastrectomy
- ccRCC
Clear Cell Renal Cell Carcinoma
- MC4R
Melanocortin-4 Receptor
- MEN-2
Multiple Endocrine Neoplasia Type 2 Syndrome
1. Introduction
The burden of obesity and its associated comorbidities has been steadily increasing to reach epidemic proportions. It has been estimated that by 2030, around 20% of the world's adult population will have obesity, with a more rapid and steep increase in prevalence in developing regions [1]. Remarkably, in the United States, the prevalence of obesity is expected to increase from 42% (2017 prevalence) to 50% by 2030 [2]. A study reported that the global disability-adjusted life years attributable to high body mass index (BMI) have nearly doubled from 1990 to 2017 in women (33.1 million to 70.7 million) and men (31.9 million to 77.0 million), mostly related to an increase in cardiovascular disease, diabetes, kidney disease, and cancer [3].
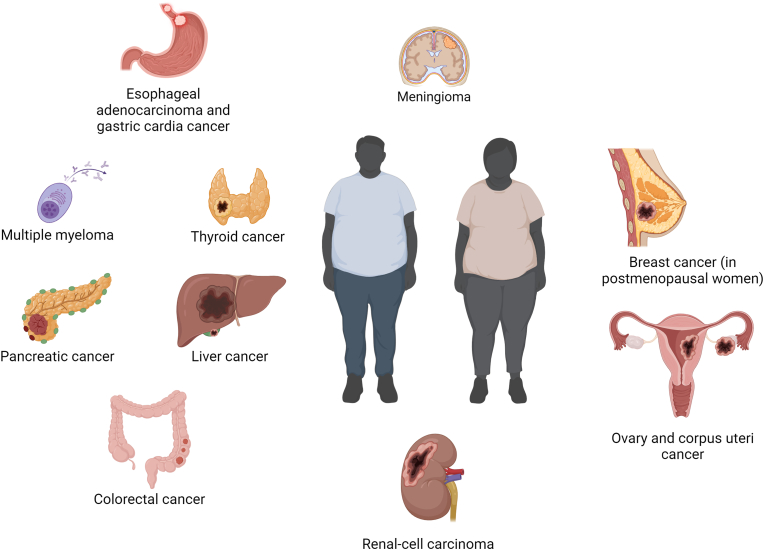
Notably, a substantial proportion of malignancies can be attributed to a high BMI [4]. For instance, in 2014, out of all new cancer diagnoses in the United States, about 40% were obesity-associated malignancies, disproportionately affecting women and people aged >50 years [5]. According to the International Agency for Research on Cancer (IARC), there is sufficient evidence to indicate a relation between excess adiposity and cancer in the following anatomical sites: esophageal, gastric cardia, colorectal, liver, gallbladder, pancreas, breast (in post-menopausal patients), endometrium, ovary, kidney, meningioma, thyroid, and the bone marrow (multiple myeloma) (Fig. 1) [6]. Additionally, there is data indicating a probable relationship for advanced prostate cancer [7], and limited evidence for male breast cancer [8] and diffuse large B-cell lymphoma [9].
Fig. 1.
Anatomical sites with strong evidence for association with excess adiposity as established by the International Agency for Research on Cancer.
With the rate of obesity increasing at an alarming pace [10], multiple treatment modalities have been developed, including lifestyle interventions, antiobesity medications, and bariatric procedures, with the ultimate goal of achieving sufficient weight loss to decrease the risk of developing adiposity-associated diseases, or improve their outcomes if present [11]. As some cancers have a strong association with excess adiposity, promoting evidence-based and individualized treatments for overweight and obesity could therefore contribute to their prevention. In this review, we present data on the protective effect of treatment modalities for overweight and obesity on the risk of developing cancer.
2. Methods
In this clinical review, we explore the role of treatment modalities for overweight and obesity as a potential preventative intervention for certain types of cancer. We searched the MEDLINE, Embase, Web of Science, and Google Scholar databases for relevant studies from inception to January 20, 2024, in English language. We present publications (e.g., systematic reviews and meta-analysis, randomized clinical trials, and prospective and retrospective observational studies) that focus on the effect of treatment modalities for overweight and obesity in cancer prevention. We included the following treatment modalities: lifestyle interventions (medical nutrition therapy and exercise), antiobesity medications, and bariatric procedures. We also present an expert opinion regarding weight-centric prevention of cancer.
3. Results
3.1. Effects of excess adiposity in carcinogenesis
Multiple mechanisms have been proposed to explain the relationship between excess adiposity and cancer. The contribution of each mechanism to carcinogenesis varies by the anatomic site involved and by the presence of specific defining characteristics in a population [12]. The interplay of different mechanisms leading to malignancy in patients with excess adiposity is being actively studied. In Table 1, we summarize the currently proposed pathogenic and molecular mechanisms underlying an increased malignancy risk by anatomic site for obesity-associated cancers, as established by the IARC [6].
Table 1.
Mechanisms of carcinogenesis for obesity-associated cancers.
| Cancer site | Adiposity-associated pathogenic mechanisms | Proposed adiposity-associated molecular mechanisms for carcinogenesis |
|---|---|---|
| Esophageal adenocarcinoma [[124], [125], [126], [127]] |
Abdominal adiposity predisposes to gastroesophageal reflux disease, increasing the risk of Barrett's esophagus and esophageal adenocarcinoma. Additional contributors: chronic inflammation and hyperinsulinemia. | Increased expression of leptin receptors in patients with obesity and esophageal adenocarcinoma could stimulate proliferation and inhibit apoptosis in esophageal adenocarcinoma cells, promoting progression of the disease. |
| Hyperinsulinemia in vivo leads to IGF-1 receptor upregulation and promotion of esophageal carcinogenesis through cell growth and proliferation. | ||
| Gastric cardia [[128], [129], [130], [131], [132]] |
Chronic inflammation and hyperinsulinemia. | Malignant gastric cells have higher expression of IGF-1, which could promote cell proliferation. |
| There is correlation between leptin levels and leptin tissue expression and clinicopathological variables in gastric cancer, suggesting its carcinogenic role. | ||
| IL-mediated chronic inflammation could contribute to cell proliferation and invasion. | ||
| Colorectal [[133], [134], [135], [136], [137]] |
Chronic inflammation and hyperinsulinemia. | Chronic inflammation related to visceral adiposity may participate tumorigenesis and immune escape, leading to cancer development and progression. |
| Leptin and other adipokines potentially induce growth of neoplastic colorectal cells. | ||
| Insulin and IGF-1 signaling favors mitogenic and proangiogenic signals in colorectal. | ||
| Liver [[138], [139], [140], [141]] |
Chronic inflammation, hyperinsulinemia, and alterations in the gut microbiome. | Adiposity induced proinflammatory cytokines, such as TNFα and IL-6 might contribute to liver tumorigenesis. |
| Obesity-associated alterations in gut microbiome metabolites might contribute to DNA damage and activation of a senescence-associated secretory phenotype in hepatic stellate cells, essential for liver tumorigenesis. | ||
| Insulin and IGF-1 signaling in the liver might contribute to liver tumorigenesis. | ||
| Gallbladder [142,143] |
Chronic inflammation from gallstones, which patients with obesity are at risk of. | Not widely studied. |
| Pancreas [[144], [145], [146], [147], [148], [149]] |
Chronic inflammation and hyperinsulinemia. | Adipokines and other adiposity-associated inflammatory mediators activate oncogenic downstream pathways. |
| Insulin and IGF-1 stimulate pancreatic duct acinar cell proliferation through mTOR signaling. | ||
| Breast (post-menopausal), Endometrium and Ovary [16,[150], [151], [152], [153], [154], [155], [156], [157], [158], [159], [160]] |
Hyperestrogenism, chronic inflammation, oxidative stress, and hyperinsulinemia. | Excess adiposity and its associated inflammatory environment increase adipose-tissue aromatase expression and activity, leading to androgen conversion to estrogen. |
| Estrogen and insulin/IGF-1 are major synergistic mitogens for epithelial cells, inducing cell cycle progression. | ||
| Excess adiposity promotes oxidative stress, which denatures cell structures leading to genetic instability and tumorigenesis. | ||
| Leptin stimulates breast, endometrial, and ovarian cancer cell growth and impairs apoptosis through activation of multiple signaling pathways. In addition, leptin increases the expression of aromatase, further contributing to hyperestrogenism. | ||
| Clear Cell Renal cell carcinoma (ccRCC) [[161], [162], [163], [164]] |
Microenvironment alterations, metabolic reprogramming, and chronic inflammation. | Genes associated with an increased risk of ccRCC are associated with metabolic stress pathways. |
| Lipidomic signatures of ccRCC contributes to cell proliferation. | ||
| Expression of different adipokines has been suggested to modify the risk for ccRCC. | ||
| Meningioma [[165], [166], [167], [168], [169]] |
Hyperestrogenism. | Excess adiposity and its associated inflammatory environment increase adipose-tissue aromatase expression and activity, leading to androgen conversion to estrogen. Most meningiomas express progesterone, estrogen, or androgen receptors, and estrogen is a potent enhancer of meningioma cell proliferation in vitro. |
| Thyroid [[170], [171], [172], [173], [174], [175], [176], [177]] |
Chronic inflammation. Possibly hyperinsulinemia and hyperestrogenism, although their roles are less well defined. | Increased adipokines and oxidative stress promote malignancy. |
| In vitro, insulin promotes thyroid cell proliferation and migration, and insulin resistance correlates with thyroid nodule vascularity. | ||
| There is increased estrogen α-receptor expression in thyroid cancer cells, especially in post-menopausal women. Its role is unclear. | ||
| Multiple myeloma [[178], [179], [180], [181], [182]] |
Chronic inflammation. Possibly hyperinsulinemia although its role is less well defined. | Increase in bone marrow adipose tissue leads to increased circulating adipokines. |
| In vivo and in vitro studies show increased adipocyte-secreted cytokine angiotensin II promotes tumorigenesis. | ||
| Insulin is a potent growth factor for multiple myeloma cells in vitro. |
The adipose tissue is a highly active endocrine organ responsible for secreting multiple adipokines that regulate essential processes such as energy homeostasis and inflammation [13]. For instance, adiponectin is an insulin-sensitizing and anti-inflammatory adipokine [14]. Hypoadiponectinemia is present among patients with obesity and has been associated with an increased risk of malignancy, due to alterations in receptor-mediated signaling pathways, insulin sensitivity, inflammatory response, and angiogenesis [15]. In contrast, high leptin and visfatin levels, two other adipokines, have also been implicated in tumorigenesis [16]. Similarly, alterations in adipose tissue maturation might contribute to low-grade chronic inflammation, resulting in the release of multiple proinflammatory cytokines and angiogenic factors, that could contribute to carcinogenesis in multiple anatomic sites [17]. Although data is inconclusive, alterations in gut microbiota in patients with obesity might further promote chronic inflammation and the risk for developing cancer [18,19]. Moreover, adipose tissue within the tumor microenvironment might contribute to carcinogenesis by secretion of signaling molecules and by providing an energy reservoir for proliferating cells [20,21].
Notably, adiposity associated diseases can also increase the risk of malignancy. For instance, abdominal obesity predisposes to gastroesophageal reflux disorder and Barrett's esophagus due to increased intra-abdominal pressure [22]. However, in the case of gastroesophageal reflux, as it does not fully explain the increased risk of esophageal adenocarcinoma in patients with obesity, it has been hypothesized that adipose tissue-mediated inflammation might contribute to dysplastic and neoplastic progression [23,24].
To conclude this section, while we present data on the risk of cancer in patients with increased adiposity, it is important to note that some observational studies have reported that patients with higher BMI might have a decreased risk for certain types of cancer, including breast cancer in premenopausal patients [25,26]. This controversial epidemiological phenomenon has been named the ‘obesity paradox’ [27]. The mechanism underlying this protective effect has not been completely elucidated. Finally, the effect of intentional weight loss on certain biomarkers associated to cancer have shown to be small or inconsistent, and as a result, merit further investigation [28].
3.2. Role of intentional weight loss in cancer prevention
Considering the strong association between excess adiposity and certain types of cancer, multiple studies have assesed the effect of intentional weight loss in cancer risk. One of the most studied populations is postmenopausal women [[29], [30], [31], [32]]. For instance, the observational study branch of the Women's Health Initiative (WHI), which included 93,676 postmenopausal women across the US, has provided valuable information [33,34]. In this study, postmenopausal women who intentionally achieved at least a 5% reduction in total body weight over a 3-year follow-up had a significantly lower risk of obesity-associated cancers as compared to women with stable weight (HR: 0.88, 95%CI: 0.80–0.96, P < 0.05) [35]. A stratified analysis by BMI showed that this effect was only present among women with obesity [35]. This protective effect was exemplified with endometrial cancer. Postmenopausal women that achieved a weight loss of at least 5% had a significantly lower risk of endometrial cancer, with a stronger association among women that had obesity at baseline [36]. In addition, women that gained at least 10 pounds of total body weight over a 3-year period had a significantly higher risk of endometrial cancer [36]. In accordance with the WHI data, results from the Iowa Women's Health Study showed that postmenopausal women that intentionally lost at least 20 pounds during adulthood had a 18% reduced incidence of obesity-associated cancers [37]. Moreover, a pooled analysis including ten prospective cohorts from multiple countries showed that sustained weight loss led to a decreased risk of breast cancer in women aged over 50 years not taking menopausal hormonal therapy [38]. These findings suggest that weight loss could be a preventative intervention for obesity-associated cancer in postmenopausal women, yet, the effects of intentional weight loss in other populations have not been completely elucidated.
Outside of studies in postmenopausal women, the Look AHEAD trial gave some insight in the effect of weight loss in patients with type 2 diabetes. In this trial, individuals with overweight or obesity and type 2 diabetes mellitus were randomized to either conventional diabetes education or an intensive lifestyle intervention (ILI) to promote weight loss [39]. Even though this trial was initially conceived to assess for improvement in cardiovascular outcomes, a subsequent analysis was done to evaluate the effect of the ILI in the risk of cancer. Compared to patients receiving conventional diabetes education, patients that were randomized to the ILI had a greater weight loss at 1 year (8.6% vs. 0.7%), and had a lower incidence of obesity-associated cancers (6.1 vs. 7.3 cases per 1000 person-years; HR: 0.84, 95% CI: 0.58–1.04) [40]. It is important to note that the difference in cancer risk was not statistically significant for the duration of the follow-up (median: 11 years), likely due to inadequate statistical power or misclassification of cancer location. Notably, sex did not affect the effect of the ILI on cancer risk (p = 0.68) [40].
Weight cycling (i.e., repeated cycles of intentional weight loss followed by weight regain) is common among people with overweight or obesity, with a study reporting that 42% of men and 57% of women had experienced at least one episode of weight cycling throughout their adult lives [41]. Contrary to weight loss maintenance, some studies have suggested that weight cycling could contribute to an increased risk of cancer by promoting an inflammatory state and altering immune surveillance [[42], [43], [44]]. However, results from a large prospective cohort study showed that after adjusting for BMI and other covariates, weight cycling was not associated with an increased risk of cancer in men (HR:0.96, 95% CI: 0.83–1.11) or women (HR:0.96, 95% CI: 0.86–1.08), regardless of a history of obesity [45]. Importantly, this study reported that weight cycling was not protective against cancer [45]. Prevention of weight regain is essential after the implementation of any weight loss intervention, and long-term management might be needed to prevent further episodes of weight cycling in some patients.
3.3. Effect of weight loss interventions on cancer risk
Given the effect of intentional weight loss in relation to cancer risk reduction, we will now summarize the evidence of different weight loss interventions on cancer risk, including lifestyle interventions (i.e., medical nutrition therapy and exercise), antiobesity medications (AOMs), and bariatric surgery.
3.3.1. Lifestyle interventions
3.3.1.1. Medical nutrition therapy
A meta-analysis including 8 randomized clinical trials suggests that diet-induced weight loss leads to a modest, but non statistically significant decreased risk of cancer mortality (pooled RR: 0.58, 95% CI: 0.30–1.11) [46]. In the same study, nineteen trials had very low quality evidence for patients developing new cancer, with only 103 events reported among 6330 participants, highlighting the low number of events in these trials. Besides this, due the limited follow-up duration for these trials and the significant heterogeneity in weight loss outcomes, further large-scale studies with a longer follow-up duration are required to better elucidate the effects of diet-induced weight loss in the reduction of cancer risk.
Although the data assessing the effect of weight loss from dietary interventions in cancer risk is not conclusive, previous interventional studies have demonstrated that weight loss achieved through diet leads to a decrease in multiple biomarkers associated with cancer [47]. For instance, diet-induced weight loss in a cohort of 10 premenopausal women with obesity led to significant reduction in serum tumor necrosis factor-α and interleukin (IL)-8 concentrations, while also downregulating the expression of pro-inflammatory cytokines and gene pathways associated with colorectal cancer in rectosigmoid biopsies [48]. Similarly, in a trial including 20 participants with obesity, an 8-week calory-restricted liquid diet leading to substantial weight loss (13.6%) was associated with a significant decrease in colonic tissue expression of Ki-67, an important cell proliferation marker [49]. In accordance with these data, a trial in postmenopausal women showed that a dietary intervention led to a reduction in body weight and favorable hormonal changes that could reduce the risk of breast cancer, including a decrease in total and free serum testosterone levels, and an increase in sex hormone binding globulin and IGF binding protein 1 and 2 [50].
There is data suggesting that dietary macronutrient composition could affect cancer risk [51]. For instance, previous studies have reported that increased consumption of red meat and processed meat have a strong association with colorectal cancer [52,53]. The Mediterranean diet, a dietary pattern consisting of high amounts of fruits, vegetables, whole grains, cereals, nuts, legumes, fish, and extra virgin olive oil, and reduced consumption of red meats, has been associated with a decreased risk of cancer in some observational and prospective studies [54,55]. A meta-analysis including 56 observational studies showed that high-adherence to a Mediterranean diet led to a reduction in overall cancer mortality and a decrease in the incidence of colorectal, breast, gastric, prostate, liver and head and neck cancer [56]. The effect of other dietary patterns has not been as widely studied.
The clinical implications of these findings remain to be determined. Currently, a caloric deficit of 500–750 kcal/d, usually achieved with a daily calory intake of 1200–1500 kcal for women and 1500–1800 kcal for men, is recommended to promote weight loss in patients with overweight or obesity [57,58]. With this information in mind, a comprehensive dietary approach incorporating caloric restriction to promote weight loss while preserving a healthy diet composition pattern can become a valuable cancer preventative strategy for people with overweight or obesity.
3.3.1.2. Physical activity
Previous reports have suggested that the addition of exercise training to hypocaloric diet leads to a more significant reduction in inflammatory biomarkers associated to malignancy in postmenopausal women, including C-reactive protein and IL-6 [59]. A clinical trial randomized 439 postmenopausal women with overweight or obesity to different lifestyle interventions: a) caloric restriction diet, b) aerobic exercise, c) combined diet + exercise or d) control [60]. After 1 year, the diet + exercise, diet, and exercise group lost 10.8%, 8.5% and 2.4% of their body weight, respectively. When compared to controls, the diet, and the diet + exercise group, had a significant decrease in most studied inflammatory biomarkers, however, this effect was not present for the exercise group [60]. In addition, only patients that lost ≥5% of their weight in the diet and diet + exercise group had a significant reduction in high-sensitivity C reactive protein (hs-CRP) [60]. In a similar study, 243 postmenopausal women with overweight or obesity were randomized to either a 16-week intervention consisting of either diet only (caloric restriction of 3500 kcal/week with habitual physical activity level), diet + exercise (caloric deficit of 1750 kcal/week with an intensive physical activity regimen consisting of a 4 h/week combined endurance and strenght exercise program), or no intervention (i.e., habitual diet and physical activity level) [61]. The diet and the diet + exercise groups lost 6.1% and 6.9% of their body weight, respectively. When compared to controls, leptin levels significantly decreased for both groups. In contrast, the hsCRP levels decreased for both groups, but results were only significant for the diet + exercise group [61]. Moreover, data from prospective and interventional studies suggest that increased physical activity levels are associated with decreased serum estrogen and testosterone levels in postmenopausal women which can consequnetly decrease estrogen-associated cancers in this population [[62], [63], [64], [65], [66]].
Currently, comprehensive lifestyle intervention programs usually recommend increasing aerobic physical activity for ≥150 min/wk, however, levels closer to 225–420 min/wk might be needed to lose 5–7.5 kg, and 200–300 min/wk might be needed to avoid weight regain in the long-term [67]. Although there is no conclusive evidence showing the direct effect of exercise in cancer risk reduction, these data suggest that the addition of exercise to medical nutrition therapy as part of a comprehensive weight loss intervention, can result in a more favorable reduction in inflammatory and hormonal biomarkers associated with the development of obesity-associated cancers.
Medical nutrition therapy and exercise continue to be the backbone of comprehensive weight management programs, however, substantial and durable weight loss might be difficult to achieve in a significant proportion of patients with this methods alone. For instance, about 60% of patients do not achieve a total body weight loss percentage of at least 10% with currently recommended caloric deficits [68]. Hence, multiple patients with obesity require pharmacological and/or surgical interventions to achieve clinically significant weight loss.
3.3.2. Antiobesity medications
AOM are an effective treatment for overweight and obesity. AOMs not only induce weight loss, but if used correctly, i.e., long-term, can also promote weight loss maintenance. The use of AOMs has increased in the US over the past few years, however, access to these medications remains limited, mostly due to inadequate insurance coverage and high costs for patients [[69], [70], [71], [72], [73]]. At the time of this review, the Food and Drug Administration (FDA) has approved the use of seven different agents for chronic overweight and obesity treatment. Even though the effect of AOMs in the risk of developing cancer has not been directly examined, the efficacy of AOMs to promote weight loss as part of a comprehensive weight loss intervention has been well established [74,75]. As with other weight loss interventions, there is significant heterogeneity in weight loss outcomes among different AOMs and even within the same AOM [76]. Newer glucagon-like peptide-1 (GLP-1) receptor agonists (i.e., semaglutide) and glucose-dependent insulinotropic polypeptide (GIP) co-agonists (i.e., tirzepatide) have showed significantly superior weight loss outcomes in comparison to other antiobesity medications [77,78]. In Table 2, we show the reported weight loss outcomes and evidence on potential interactions with cancer risk of FDA-approved AOMs for chronic weight management.
Table 2.
Antiobesity medications approved by the Food and Drug Administration for chronic weight management.
| Antiobesity medication | Mechanism of action | Reported total body weight loss percentage | Potential interactions with cancer risk |
|---|---|---|---|
| Orlistat [[183], [184], [185], [186], [187], [188], [189], [190]] |
Lipase inhibitor. | 10.2% | In vitro study suggested that orlistat induced caspase-dependent apoptosis and protective autophagy in ovarian cancer cells. |
| In vitro and in vivo studies suggest that orlistat led to decreased colonic inflammation and cancer cell proliferation. | |||
| Orlistat is a fatty acid synthase inhibitor, potentially having antiproliferative effects. | |||
| Phentermine –Topiramate [191,192] |
Appetite suppressant. | 6.7%–14.4% | An in vitro study suggested that topiramate inhibited proliferation and promoted apoptosis in ovarian cancer cells. |
| Naltrexone – bupropion [[193], [194], [195], [196]] |
Appetite suppressant. | 6.7%–11.5% | In vitro and in vivo studies have suggested that low-dose naltrexone might promote apoptosis and inhibit proliferation of colorectal cancer cells and cervical cancer cells. |
| Setmelanotidea [197] |
Melanocortin 4 receptor (MC4R) agonist. | 12.5%–25.6% | Half of patients treated with setmelanotide develop skin hyperpigmentation disorders. Melanoma has been proposed as a potential risk and is actively being monitored. Subjects with a history or close family history of melanoma were excluded from clinical trials. To date, no evidence exists of an increased risk of melanoma in patients treated with setmelanotide. |
| Liraglutide [79,198,199] |
Glucagon-like peptide 1 (GLP-1) receptor agonist. | 6.2%–8.0% | Concerns have been raised about the possibility of increased risk of medullary thyroid cancer (MTC) and pancreatic cancer with the use of GLP-1 receptor agonists. |
| Semaglutide [77,88,89,200] |
9.6%–14.9% | While data from animal models have suggested a possible connection between the use of GLP-1 receptor agonists and MTC, the risk of MTC in humans with the use of these medications has not been established clinically. Currently, GLP-1 receptor agonists are contraindicated in patients with a personal or family history of MTC or multiple endocrine neoplasia type 2 syndrome (MEN2). | |
| Tirzepatide [78,201] |
GLP-1 and Glucose-dependent insulinotropic polypeptide (GIP) dual agonist. | 14.7%–20.9% | Evidence suggests that the risk of pancreatic and thyroid cancer, and overall neoplasias are not increased in patients using GLP-1 receptor agonists. |
Setmelanotide has been approved for chronic weight loss management in patients with select genetic variants in the leptin-melanocortin pathway.
AOMs are a safe intervention, with most reported adverse events being mild or moderate, usually self-resolving, and generally not leading to medication discontinuation [76]. Out of the currently FDA-approved AOMs, concerns have been raised about the risk of medullary thyroid cancer (MTC) with GLP-1 receptor agonists, liraglutide, semaglutide and tirzepatide. Reports from studies with rodents suggested that GLP-1 receptor agonists led to thyroid C-cell proliferation, with an increased risk of malignancy [79]. This resulted in the FDA issuing a boxed warning for all GLP-1 receptor agonist, and a contraindication for patients with a personal or family history of MTC or Multiple Endocrine Neoplasia syndrome type 2, a known genetic syndrome associated with MTC. Some reports have suggested a disproportionately higher proportion of thyroid malignancies in patients receiving GLP-1 receptor agonists [[80], [81], [82]], however, a meta-analysis including data from 45 trials of different GLP-1 receptor agonists concluded that there was no significant effect on thyroid cancer risk (RR: 1.30, 95% CI: 0.86–1.97) [83]. In addition, a study that randomized 9340 patients with type 2 diabetes to either liraglutide or placebo reported that liraglutide did not significantly increase calcitonin levels at 36 months, with no episodes of C-cell hyperplasia or MTC in patients treated with liraglutide [84]. Similarly, a concern for an increased risk of pancreatic cancer with the use of GLP-1 receptor agonists has been raised, as some studies in animal models showed that incretin-based therapies led to pancreatic acinar and duct cell proliferation [85,86]. Previous meta-analyses found that there is no increased risk of pancreatic cancer in patients taking GLP-1 receptor agonists [87,88]. Moreover, a recent meta-analysis including data from 37 randomized clinical trials and 19 real world-studies with 46,719 patients showed that compared to placebo, semaglutide was not associated with an increased risk of pancreatic cancer (OR: 0.25, 95% CI: 0.03–2.24, p = 0.21), thyroid cancer (OR: 2.04, 95% CI: 0.33–12.61, p = 0.82) or all neoplasias (OR: 0.95, 95% CI: 0.44–1.89) [89].
The selection of an antiobesity medication as an added tool for obesity treatment remains a clinical challenge. A recent report by the Institute for Clinical and Economic Review suggested that semaglutide was the most effective AOM, however, in terms of cost-effectiveness phentermine-topiramate was a superior alternative [90]. In this context, an individualized shared-decision making approach taking into consideration comorbidities, safety profile, insurance coverage and patient preferences might be required to optimize weight loss response [91].
3.3.3. Bariatric surgery
3.3.3.1. Overall effect of bariatric surgery in cancer risk
Bariatric surgery remains the most effective intervention for obesity, often leading to substantial and durable weight loss and comorbidity resolution [92]. Previous studies have shown a decreased incidence and mortality rate associated with cancer in people with obesity undergoing bariatric surgery [[93], [94], [95], [96]]. A meta-analysis including 32 studies showed that bariatric surgery was associated with a significant reduction in the overall incidence of cancer (RR: 0.62, 95% CI: 0.46–0.84, P = 0.002) and cancer-related mortality (RR: 0.51, 95% CI: 0.42–0.62, p < 0.001), however it should be noted that almost two-thirds of the studies included for this review had a serious or critical risk of bias, and there was significant heterogeneity among included studies [97]. A prospective intervention trial in Sweden, the Swedish Obese Subject (SOS) study, followed 4047 participants with obesity, out of which 2010 underwent bariatric surgery, and 2037 served as matched controls [98]. During the follow-up period, 286 new cases of cancer occurred, 117 and 169 in the surgery and the control group, respectively. Patients that underwent bariatric surgery had a lower risk of incident cancer when compared to the control group (HR: 0.67, 95% CI: 0.53–0.85, p = 0.0009). A differential effect was observed depending on sex. While authors reported a significant decrease in risk of incident cancer in women (HR: 0.58, 95% CI: 0.44–0.77, p = 0.0001), this was not the case for men (HR: 0.97, 95% CI: 0.61–1.52, p = 0.90) [98]. Similarly, a meta-analysis including eight population-based studies reported that bariatric surgery led to a lower risk of developing obesity-associated and overall cancer incidence, however, after stratifying by sex, the effect of bariatric surgery was less prominent in men [99]. This diverging effects according to sex could be the result of the strong protective effect of weight loss on hormone-dependent breast and endometrial cancer, which represent a significant proportion of cancers in postmenopausal women [95]. It is important to note that, Schauer et al. demonstrated that weight loss at one year was an independent predictor of cancer risk reduction, while bariatric surgery by itself was not; with an estimated 14% decrease in cancer risk for every 10% of body weight loss [100]. These results suggest that the reduction of cancer risk in patients undergoing bariatric surgery is mostly weight-dependent, but potential weight-independent metabolic benefits of bariatric surgery in cancer risk remain unclear [101].
3.3.3.2. Effect of bariatric surgery on cancer by anatomic site
The protective effect of bariatric surgery for hormone-related malignancies has been more widely studied [[102], [103], [104], [105]]. A meta-analysis including seven prospective and retrospective cohort studies showed that bariatric surgery led to a 59% reduction in the aggregate risk of breast, ovarian, and endometrial cancer (RR: 0.41, 95% CI: 0.31,0.56, p < 0.05) [102]. Moreover, Feigelson et al. showed that bariatric surgery led to a reduction in premenopausal and postmenopausal breast cancer cases, with a more prominent effect for estrogen-receptor negative breast cancer in premenopausal women, and estrogen-receptor positive breast cancer for postmenopausal women [106]. These protective effects could be attributed to weight-loss induced favorable alterations in sex hormone parameters [50,107].
Similarly, in a recent study, Clapp et al. assessed the effect of bariatric surgery on non-hormone related cancer (i.e., excluding breast, endometrium, ovary, prostate, testis, thyroid, and osteosarcoma) [94]. This meta-analysis included 15 retrospective studies, and showed that bariatric surgery led to a decrease incidence of non-hormone related cancer when compared to the non-surgical group (OR: 0.65, 95% CI: 0.53,0.80, p < 0.002) [94]. Wilson et al. reported that bariatric surgery led to a reduction in the incidence of hepatocellular, colorectal, pancreatic and gallbladder cancer, and female-specific hormone dependent cancers (i.e., breast, endometrial and ovarian cancer), without evidence for a protective effect on the incidence of esophageal, gastric, thyroid, kidney and prostate cancer or multiple myeloma [97].
The effect of bariatric surgery in the risk of developing colorectal cancer has been a subject of controversy, as data from observational studies has shown discordant results [[108], [109], [110], [111], [112], [113], [114], [115], [116]]. A population-based cohort study indicated that Roux-en-Y gastric bypass (RYGB), but not banding or sleeve gastrectomy, led to an increased risk of colorectal cancer (OR: 2.63, 95% CI: 1.17–5.95, P < 0.05) [111]. It has been hypothesized that bariatric surgery-induced alterations in microbiome and increased bile salt exposure might lead to local mucosal changes that favor carcinogenesis in the colon [[117], [118], [119]]. However, a recent meta-analysis including 13 retrospective studies, and a population of over 3 million, indicated a decreased risk of developing colorectal cancer in patients undergoing bariatric surgery, with a pooled RR of 0.63 (95% CI: 0.50–0.79, P < 0.05) [120]. The effect of bariatric surgery on colorectal cancer remains unclear, and additional studies with longer follow-up periods are required to assess this. Overall, there is evidence favoring the protective effect of bariatric surgery in the risk of liver, breast, endometrium, ovary, and pancreas cancer, however, it is worth mentioning that most of the studies assessing the risk for future cancer after bariatric surgery have been retrospective in nature, with follow-up time often being insufficient. The significant heterogeneity and high risk of bias for most of these studies limit the conclusions that can be drawn, and further studies are needed to better assess the potential protective effect of bariatric surgery in other types of cancer [97].
3.3.3.3. Differential effect among bariatric interventions
Currently, the most frequently performed bariatric surgeries in the United States are the sleeve gastrectomy (SG) and the RYGB [121]. Significant heterogeneity has been reported in weight loss outcomes of these two interventions. The RYGB results in greater weight loss compared to the SG, but carries a higher burden of complications [122]. The differential effect of different types of bariatric surgeries on cancer risk has been previously examined.
Within the SOS study cohort, a significantly lower risk of incident cancer was present in women undergoing gastric banding (HR: 0.54, 95% CI: 0.31–0.93, p = 0.026) and vertical banded gastroplasty (HR: 0.60, 95% CI: 0.44–0.82, p = 0.0012), but not in women undergoing RYGB or men undergoing any of these surgical interventions [98]. In contrast, other studies have reported that within bariatric surgeries, RYGB led to a greater reduction in obesity-related cancers than the sleeve gastrectomy and gastric banding [94,111].
More recently, multiple endoscopic bariatric and metabolic therapies have been proposed as non-invasive and efficacious interventions for weight management, with options including the intragastric balloon and the endoscopic sleeve gastroplasty [123]. The effect of these endoscopic interventions on cancer risk has not been assessed directly.
4. Expert opinion: weight-centric approach to cancer prevention
It is now well established that overweight and obesity in adulthood is a risk factor for many cancers [6]. This likely involves increased inflammation from visceral fat, increased insulin levels, insulin-like growth factors, and increased sex hormones. It is astounding that more than 40% of women and 35% of men in the United States live with obesity, with projections of its prevalence continuing to rise. The rates vary among different racial and ethnic groups, with a higher incidence of obesity seen in Non-Hispanic Blacks, American Indian/Pacific Islanders, and Hispanics. Concerningly, rates of obesity in children are also increasing across the world. Despite the knowledge that obesity increases the risk of cancer, cardiovascular disease, and diabetes/metabolic disorders, rates continue to rise. This is likely due to the wide availability and ease of eating ultra-processed and packaged foods, the lack of availability of fresh whole foods as seen in food deserts, other behavioral patterns, and the inability to sustain behavior and lifestyle changes. Losing weight remains a significant challenge for many patients. While medications or bariatric procedures are promising for some patients, there is also a growing movement and research to support incorporating health coaches to help patients with behavior change, which includes changing diet quality and patterns, increasing exercise, reducing stress, and improving sleep quality. Lifestyle changes are essential to continue, even if patients have bariatric surgery or are on medications, to help preserve lean mass and maintain weight. Given the morbidity and mortality associated with obesity, healthcare providers need to recognize overweight/obesity in our patients and refer them for appropriate counseling and programs to assist with weight loss, which will decrease the worldwide morbidity and mortality of cancer. Governments worldwide must ensure public education, the availability of higher quality food and provide coverage for weight loss programs, medications, and procedures that will ultimately be lifesaving.
5. Conclusions
Excess adiposity is a well-defined risk factor for multiple malignancies, and there is growing evidence that weight loss achieved through different modalities, including lifestyle interventions, AOMs, and bariatric procedures, can decrease the risk of developing cancer. However, further prospective studies are needed to provide more robust evidence supporting weight loss strategies as a preventative intervention for cancer. Similarly, studies are needed to better characterize the amount of weight loss required to have the greatest benefit from these interventions, and whether specific populations should be targeted. Population-based interventions to promote a healthy weight should also be evaluated to determine if there is a potential role for weight loss as a primary prevention strategy for cancer.
-
•
Obesity increases cancer risk in different anatomical locations through multiple mechanisms.
-
•
Weight-loss interventions can decrease cancer risk in patients with excess adiposity, with the most evidence available for post-menopausal women.
-
•
There is some conflicting data in terms of cancer protective effect after bariatric surgery for certain anatomical locations.
Source of funding
Beyond payment to the research staff by Mayo Clinic, this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of artificial intelligence (AI) and AI-assisted technologies
During the preparation of this work the authors did not use AI or AI-assisted technologies.
Declaration of competing interest
Dr. Acosta is a stockholder in Gila Therapeutics, Phenomix Sciences; he served as a consultant for Rhythm Pharmaceuticals, General Mills, and Amgen Pharmaceuticals. Dr. D'Andre is supported by Minnesota Clinical Trials Network (MNCCTN) and Mayo Clinic internal grants to support unrelated research. Dr. Hurtado is supported by the NIH (K12AR084222). No disclosures on behalf of the rest of the authors.
Acknowledgements
No acknowledgements.
References
- 1.Kelly T., Yang W., Chen C.S., Reynolds K., He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes. 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 2.Ward Z.J., Bleich S.N., Cradock A.L., Barrett J.L., Giles C.M., Flax C., et al. Projected U.S. State-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440–2450. doi: 10.1056/NEJMsa1909301. [DOI] [PubMed] [Google Scholar]
- 3.Dai H., Alsalhe T.A., Chalghaf N., Riccò M., Bragazzi N.L., Wu J. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: an analysis of the Global Burden of Disease Study. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold M., Pandeya N., Byrnes G., Renehan P.A.G., Stevens G.A., Ezzati P.M., et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16:36–46. doi: 10.1016/S1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steele C.B., Thomas C.C., Henley S.J., Massetti G.M., Galuska D.A., Agurs-Collins T., et al. 2017. Vital signs: trends in incidence of cancers associated with overweight and obesity — United States, 2005–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lauby-Secretan B., Scoccianti C., Loomis D., Grosse Y., Bianchini F., Straif K. Body fatness and cancer — viewpoint of the IARC working group. N Engl J Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Cancer Research Fund . Continuous Update Project Expert Report; 2018. Diet, nutrition, physical activity and cancer: a global perspective. [Google Scholar]
- 8.Brinton L.A., Cook M.B., McCormack V., Johnson K.C., Olsson H., Casagrande J.T., et al. Anthropometric and hormonal risk factors for male breast cancer: male breast cancer pooling project results. J Natl Cancer Inst. 2014;106:djt465. doi: 10.1093/jnci/djt465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel A.V., Diver W.R., Teras L.R., Birmann B.M., Gapstur S.M. Body mass index, height and risk of lymphoid neoplasms in a large United States cohort. Leuk Lymphoma. 2013;54:1221–1227. doi: 10.3109/10428194.2012.742523. [DOI] [PubMed] [Google Scholar]
- 10.Boutari C., Mantzoros C.S. A 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism. 2022;133 doi: 10.1016/j.metabol.2022.155217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurtado A.M., Acosta A. Precision medicine and obesity. Gastroenterol Clin N Am. 2021;50:127–139. doi: 10.1016/j.gtc.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avgerinos K.I., Spyrou N., Mantzoros C.S., Dalamaga M. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–135. doi: 10.1016/j.metabol.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Kershaw E.E., Flier J.S. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi T., Kadowaki T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes. 2008;32:S13–S18. doi: 10.1038/ijo.2008.233. [DOI] [PubMed] [Google Scholar]
- 15.Dalamaga M., Diakopoulos K.N., Mantzoros C.S. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. 2012;33:547–594. doi: 10.1210/er.2011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sánchez-Jiménez F., Pérez-Pérez A., de la Cruz-Merino L., Sánchez-Margalet V. Obesity and breast cancer: role of leptin. Front Oncol. 2019;9:596. doi: 10.3389/fonc.2019.00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iyengar N.M., Gucalp A., Dannenberg A.J., Hudis C.A. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. 2016;34:4270–4276. doi: 10.1200/JCO.2016.67.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marzullo P., Bettini S., Menafra D., Aprano S., Muscogiuri G., Barrea L., et al. Spot-light on microbiota in obesity and cancer. Int J Obes. 2021;45:2291–2299. doi: 10.1038/s41366-021-00866-7. [DOI] [PubMed] [Google Scholar]
- 19.Cani P.D., Jordan B.F. Gut microbiota-mediated inflammation in obesity: a link with gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 2018;15:671–682. doi: 10.1038/s41575-018-0025-6. [DOI] [PubMed] [Google Scholar]
- 20.Nieman K.M., Kenny H.A., Penicka C.V., Ladanyi A., Buell-Gutbrod R., Zillhardt M.R., et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nieman K.M., Romero I.L., Van Houten B., Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831:1533–1541. doi: 10.1016/j.bbalip.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhan J., Yuan M., Zhao Y., Zhang X., Qiao T., Ji T., et al. Abdominal obesity increases the risk of reflux esophagitis: a systematic review and meta-analysis. Scand J Gastroenterol. 2022;57:131–142. doi: 10.1080/00365521.2021.1994643. [DOI] [PubMed] [Google Scholar]
- 23.Singh S., Sharma A.N., Murad M.H., Buttar N.S., El-Serag H.B., Katzka D.A., et al. Central adiposity is associated with increased risk of esophageal inflammation, metaplasia, and adenocarcinoma: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11 doi: 10.1016/j.cgh.2013.05.009. 1399-412.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelsen E.M., Kirihara Y., Takahashi N., Shi Q., Lewis J.T., Namasivayam V., et al. Distribution of body fat and its influence on esophageal inflammation and dysplasia in patients with Barrett's esophagus. Clin Gastroenterol Hepatol. 2012;10:728–734. doi: 10.1016/j.cgh.2012.03.007. quiz e61-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki R., Orsini N., Saji S., Key T.J., Wolk A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status-A meta-analysis. Int J Cancer. 2009;124:698–712. doi: 10.1002/ijc.23943. [DOI] [PubMed] [Google Scholar]
- 26.Baer H.J., Tworoger S.S., Hankinson S.E., Willett W.C. Body fatness at young ages and risk of breast cancer throughout life. Am J Epidemiol. 2010;171:1183–1194. doi: 10.1093/aje/kwq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trestini I., Carbognin L., Bonaiuto C., Tortora G., Bria E. The obesity paradox in cancer: clinical insights and perspectives. Eat Weight Disord. 2018;23:185–193. doi: 10.1007/s40519-018-0489-y. [DOI] [PubMed] [Google Scholar]
- 28.Byers T., Sedjo R.L. Does intentional weight loss reduce cancer risk? Diabetes Obes Metabol. 2011;13:1063–1072. doi: 10.1111/j.1463-1326.2011.01464.x. [DOI] [PubMed] [Google Scholar]
- 29.Teras L.R., Goodman M., Patel A.V., Diver W.R., Flanders W.D., Feigelson H.S. Weight loss and postmenopausal breast cancer in a prospective cohort of overweight and obese US women. Cancer Causes Control. 2011;22:573–579. doi: 10.1007/s10552-011-9730-y. [DOI] [PubMed] [Google Scholar]
- 30.Eliassen A.H., Colditz G.A., Rosner B., Willett W.C., Hankinson S.E. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296:193–201. doi: 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- 31.Moy F.M., Greenwood D.C., Cade J.E. Associations of clothing size, adiposity and weight change with risk of postmenopausal breast cancer in the UK Women's Cohort Study (UKWCS) BMJ Open. 2018;8 doi: 10.1136/bmjopen-2018-022599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teras L.R., Patel A.V., Wang M., Yaun S.-S., Anderson K., Brathwaite R., et al. Sustained weight loss and risk of breast cancer in women 50 Years and older: a pooled analysis of prospective data. JNCI: Journal of the National Cancer Institute. 2019;112:929–937. doi: 10.1093/jnci/djz226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hays J., Hunt J.R., Hubbell F.A., Anderson G.L., Limacher M., Allen C., et al. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 34.Luo J., Hendryx M., Chlebowski R.T. Intentional weight loss and cancer risk. Oncotarget. 2017;8:81719–81720. doi: 10.18632/oncotarget.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo J., Hendryx M., Manson J.E., Figueiredo J.C., LeBlanc E.S., Barrington W., et al. Intentional weight loss and obesity-related cancer risk. JNCI Cancer Spectr. 2019;3:pkz054. doi: 10.1093/jncics/pkz054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo J., Chlebowski R.T., Hendryx M., Rohan T., Wactawski-Wende J., Thomson C.A., et al. Intentional weight loss and endometrial cancer risk. J Clin Oncol. 2017;35:1189–1193. doi: 10.1200/JCO.2016.70.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parker E.D., Folsom A.R. Intentional weight loss and incidence of obesity-related cancers: the Iowa Women's Health Study. Int J Obes Relat Metab Disord. 2003;27:1447–1452. doi: 10.1038/sj.ijo.0802437. [DOI] [PubMed] [Google Scholar]
- 38.Teras L.R., Patel A.V., Wang M., Yaun S.S., Anderson K., Brathwaite R., et al. Sustained weight loss and risk of breast cancer in women 50 Years and older: a pooled analysis of prospective data. J Natl Cancer Inst. 2020;112:929–937. doi: 10.1093/jnci/djz226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wing R.R., Bolin P., Brancati F.L., Bray G.A., Clark J.M., Coday M., et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeh H.C., Bantle J.P., Cassidy-Begay M., Blackburn G., Bray G.A., Byers T., et al. Intensive weight loss intervention and cancer risk in adults with type 2 diabetes: analysis of the Look AHEAD randomized clinical trial. Obesity. 2020;28:1678–1686. doi: 10.1002/oby.22936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevens V.L., Jacobs E.J., Sun J., Patel A.V., McCullough M.L., Teras L.R., et al. Weight cycling and mortality in a large prospective US study. Am J Epidemiol. 2012;175:785–792. doi: 10.1093/aje/kwr378. [DOI] [PubMed] [Google Scholar]
- 42.Anderson E.K., Gutierrez D.A., Kennedy A., Hasty A.H. Weight cycling increases T-cell accumulation in adipose tissue and impairs systemic glucose tolerance. Diabetes. 2013;62:3180–3188. doi: 10.2337/db12-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbosa-da-Silva S., Fraulob-Aquino J.C., Lopes J.R., Mandarim-de-Lacerda C.A., Aguila M.B. Weight cycling enhances adipose tissue inflammatory responses in male mice. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shade E.D., Ulrich C.M., Wener M.H., Wood B., Yasui Y., Lacroix K., et al. Frequent intentional weight loss is associated with lower natural killer cell cytotoxicity in postmenopausal women: possible long-term immune effects. J Am Diet Assoc. 2004;104:903–912. doi: 10.1016/j.jada.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 45.Stevens V.L., Jacobs E.J., Patel A.V., Sun J., McCullough M.L., Campbell P.T., et al. Weight cycling and cancer incidence in a large prospective US cohort. Am J Epidemiol. 2015;182:394–404. doi: 10.1093/aje/kwv073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma C., Avenell A., Bolland M., Hudson J., Stewart F., Robertson C., et al. Effects of weight loss interventions for adults who are obese on mortality, cardiovascular disease, and cancer: systematic review and meta-analysis. BMJ. 2017:j4849. doi: 10.1136/bmj.j4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X., Rhoades J., Caan B.J., Cohn D.E., Salani R., Noria S., et al. Intentional weight loss, weight cycling, and endometrial cancer risk: a systematic review and meta-analysis. Int J Gynecol Cancer. 2019;29:1361–1371. doi: 10.1136/ijgc-2019-000728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pendyala S., Neff L.M., Suárez-Fariñas M., Holt P.R. Diet-induced weight loss reduces colorectal inflammation: implications for colorectal carcinogenesis. Am J Clin Nutr. 2011;93:234–242. doi: 10.3945/ajcn.110.002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beeken R.J., Croker H., Heinrich M., Obichere A., Finer N., Murphy N., et al. The impact of diet-induced weight loss on biomarkers for colorectal cancer: an exploratory study (INTERCEPT) Obesity. 2017;25(Suppl 2):S95–s101. doi: 10.1002/oby.21984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaaks R., Bellati C., Venturelli E., Rinaldi S., Secreto G., Biessy C., et al. Effects of dietary intervention on IGF-I and IGF-binding proteins, and related alterations in sex steroid metabolism: the Diet and Androgens (DIANA) Randomised Trial. Eur J Clin Nutr. 2003;57:1079–1088. doi: 10.1038/sj.ejcn.1601647. [DOI] [PubMed] [Google Scholar]
- 51.Papadimitriou N., Markozannes G., Kanellopoulou A., Critselis E., Alhardan S., Karafousia V., et al. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat Commun. 2021;12:4579. doi: 10.1038/s41467-021-24861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Händel M.N., Rohde J.F., Jacobsen R., Nielsen S.M., Christensen R., Alexander D.D., et al. Processed meat intake and incidence of colorectal cancer: a systematic review and meta-analysis of prospective observational studies. Eur J Clin Nutr. 2020;74:1132–1148. doi: 10.1038/s41430-020-0576-9. [DOI] [PubMed] [Google Scholar]
- 53.Yiannakou I., Barber L.E., Li S., Adams-Campbell L.L., Palmer J.R., Rosenberg L., et al. A prospective analysis of red and processed meat intake in relation to colorectal cancer in the black women's health study. J Nutr. 2022;152:1254–1262. doi: 10.1093/jn/nxab419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guasch-Ferré M., Bulló M., Martínez-González M., Ros E., Corella D., Estruch R., et al. Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Med. 2013;11:164. doi: 10.1186/1741-7015-11-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwingshackl L., Hoffmann G. Does a mediterranean-type diet reduce cancer risk? Curr Nutr Rep. 2016;5:9–17. doi: 10.1007/s13668-015-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schwingshackl L., Schwedhelm C., Galbete C., Hoffmann G. Adherence to mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients. 2017;9 doi: 10.3390/nu9101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jensen M.D., Ryan D.H., Apovian C.M., Ard J.D., Comuzzie A.G., Donato K.A., et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. Circulation. 2014;129:S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Acosta A., Streett S., Kroh M.D., Cheskin L.J., Saunders K.H., Kurian M., et al. White paper AGA: POWER - practice guide on obesity and weight management, education, and resources. Clin Gastroenterol Hepatol. 2017;15 doi: 10.1016/j.cgh.2016.10.023. 631-49.e10. [DOI] [PubMed] [Google Scholar]
- 59.You T., Berman D.M., Ryan A.S., Nicklas B.J. Effects of hypocaloric diet and exercise training on inflammation and adipocyte lipolysis in obese postmenopausal women. J Clin Endocrinol Metab. 2004;89:1739–1746. doi: 10.1210/jc.2003-031310. [DOI] [PubMed] [Google Scholar]
- 60.Imayama I., Ulrich C.M., Alfano C.M., Wang C., Xiao L., Wener M.H., et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: a randomized controlled trial. Cancer Res. 2012;72:2314–2326. doi: 10.1158/0008-5472.CAN-11-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Gemert W.A., May A.M., Schuit A.J., Oosterhof B.Y., Peeters P.H., Monninkhof E.M. Effect of weight loss with or without exercise on inflammatory markers and adipokines in postmenopausal women: the SHAPE-2 trial, A randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2016;25:799–806. doi: 10.1158/1055-9965.EPI-15-1065. [DOI] [PubMed] [Google Scholar]
- 62.Chan M.F., Dowsett M., Folkerd E., Bingham S., Wareham N., Luben R., et al. Usual physical activity and endogenous sex hormones in postmenopausal women: the European prospective investigation into cancer-norfolk population study. Cancer Epidemiol Biomarkers Prev. 2007;16:900–905. doi: 10.1158/1055-9965.EPI-06-0745. [DOI] [PubMed] [Google Scholar]
- 63.Oh H., Arem H., Matthews C.E., Wentzensen N., Reding K.W., Brinton L.A., et al. Sitting, physical activity, and serum oestrogen metabolism in postmenopausal women: the Women's Health Initiative Observational Study. Br J Cancer. 2017;117:1070–1078. doi: 10.1038/bjc.2017.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McTiernan A., Tworoger S.S., Ulrich C.M., Yasui Y., Irwin M.L., Rajan K.B., et al. Effect of exercise on serum estrogens in postmenopausal women: a 12-month randomized clinical trial. Cancer Res. 2004;64:2923–2928. doi: 10.1158/0008-5472.can-03-3393. [DOI] [PubMed] [Google Scholar]
- 65.Dallal C.M., Brinton L.A., Matthews C.E., Pfeiffer R.M., Hartman T.J., Lissowska J., et al. Association of active and sedentary behaviors with postmenopausal estrogen metabolism. Med Sci Sports Exerc. 2016;48:439–448. doi: 10.1249/MSS.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wiggs A.G., Chandler J.K., Aktas A., Sumner S.J., Stewart D.A. The effects of diet and exercise on endogenous estrogens and subsequent breast cancer risk in postmenopausal women. Front Endocrinol. 2021;12 doi: 10.3389/fendo.2021.732255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Donnelly J.E., Blair S.N., Jakicic J.M., Manore M.M., Rankin J.W., Smith B.K. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 68.Liu D., Huang Y., Huang C., Yang S., Wei X., Zhang P., et al. Calorie restriction with or without time-restricted eating in weight loss. N Engl J Med. 2022;386:1495–1504. doi: 10.1056/NEJMoa2114833. [DOI] [PubMed] [Google Scholar]
- 69.Suissa K., Schneeweiss S., Kim D.W., Patorno E. Prescribing trends and clinical characteristics of patients starting antiobesity drugs in the United States. Diabetes Obes Metabol. 2021;23:1542–1551. doi: 10.1111/dom.14367. [DOI] [PubMed] [Google Scholar]
- 70.Gomez G., Stanford F.C. US health policy and prescription drug coverage of FDA-approved medications for the treatment of obesity. Int J Obes. 2018;42:495–500. doi: 10.1038/ijo.2017.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gasoyan H., Sarwer D.B. Addressing insurance-related barriers to novel antiobesity medications: lessons to be learned from bariatric surgery. Obesity. 2022;30:2338–2339. doi: 10.1002/oby.23556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baum C., Andino K., Wittbrodt E., Stewart S., Szymanski K., Turpin R. The challenges and opportunities associated with reimbursement for obesity pharmacotherapy in the USA. Pharmacoeconomics. 2015;33:643–653. doi: 10.1007/s40273-015-0264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Levi J., Wang J., Venter F., Hill A. Estimated minimum prices and lowest available national prices for antiobesity medications: improving affordability and access to treatment. Obesity. 2023 doi: 10.1002/oby.23725. [DOI] [PubMed] [Google Scholar]
- 74.Calderon G., Gonzalez-Izundegui D., Shan K.L., Garcia-Valencia O.A., Cifuentes L., Campos A., et al. Effectiveness of anti-obesity medications approved for long-term use in a multidisciplinary weight management program: a multi-center clinical experience. Int J Obes. 2022;46:555–563. doi: 10.1038/s41366-021-01019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khera R., Murad M.H., Chandar A.K., Dulai P.S., Wang Z., Prokop L.J., et al. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA. 2016;315:2424–2434. doi: 10.1001/jama.2016.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahmad N.N., Robinson S., Kennedy-Martin T., Poon J.L., Kan H. Clinical outcomes associated with anti-obesity medications in real-world practice: a systematic literature review. Obes Rev. 2021;22 doi: 10.1111/obr.13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilding J.P.H., Batterham R.L., Calanna S., Davies M., Van Gaal L.F., Lingvay I., et al. Once-Weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 78.Jastreboff A.M., Aronne L.J., Ahmad N.N., Wharton S., Connery L., Alves B., et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205–216. doi: 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
- 79.Bjerre Knudsen L., Madsen L.W., Andersen S., Almholt K., de Boer A.S., Drucker D.J., et al. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151:1473–1486. doi: 10.1210/en.2009-1272. [DOI] [PubMed] [Google Scholar]
- 80.Bezin J., Gouverneur A., Pénichon M., Mathieu C., Garrel R., Hillaire-Buys D., et al. GLP-1 receptor agonists and the risk of thyroid cancer. Diabetes Care. 2023;46:384–390. doi: 10.2337/dc22-1148. [DOI] [PubMed] [Google Scholar]
- 81.Mali G., Ahuja V., Dubey K. Glucagon-like peptide-1 analogues and thyroid cancer: an analysis of cases reported in the European pharmacovigilance database. J Clin Pharm Therapeut. 2021;46:99–105. doi: 10.1111/jcpt.13259. [DOI] [PubMed] [Google Scholar]
- 82.Elashoff M., Matveyenko A.V., Gier B., Elashoff R., Butler P.C. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141:150–156. doi: 10.1053/j.gastro.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hu W., Song R., Cheng R., Liu C., Guo R., Tang W., et al. Use of GLP-1 receptor agonists and occurrence of thyroid disorders: a meta-analysis of randomized controlled trials. Front Endocrinol. 2022;13 doi: 10.3389/fendo.2022.927859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hegedüs L., Sherman S.I., Tuttle R.M., von Scholten B.J., Rasmussen S., Karsbøl J.D., et al. No evidence of increase in calcitonin concentrations or development of C-cell malignancy in response to liraglutide for up to 5 Years in the LEADER trial. Diabetes Care. 2018;41:620–622. doi: 10.2337/dc17-1956. [DOI] [PubMed] [Google Scholar]
- 85.Perfetti R., Merkel P. Glucagon-like peptide-1: a major regulator of pancreatic beta-cell function. Eur J Endocrinol. 2000;143:717–725. doi: 10.1530/eje.0.1430717. [DOI] [PubMed] [Google Scholar]
- 86.Gier B., Matveyenko A.V., Kirakossian D., Dawson D., Dry S.M., Butler P.C. Chronic GLP-1 receptor activation by exendin-4 induces expansion of pancreatic duct glands in rats and accelerates formation of dysplastic lesions and chronic pancreatitis in the KrasG12D mouse model. Diabetes. 2012;61:1250–1262. doi: 10.2337/db11-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pinto L.C., Falcetta M.R., Rados D.V., Leitão C.B., Gross J.L. Glucagon-like peptide-1 receptor agonists and pancreatic cancer: a meta-analysis with trial sequential analysis. Sci Rep. 2019;9:2375. doi: 10.1038/s41598-019-38956-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cao C., Yang S., Zhou Z. GLP-1 receptor agonists and pancreatic safety concerns in type 2 diabetic patients: data from cardiovascular outcome trials. Endocrine. 2020;68:518–525. doi: 10.1007/s12020-020-02223-6. [DOI] [PubMed] [Google Scholar]
- 89.Nagendra L., Bg H., Sharma M., Dutta D. Semaglutide and cancer: a systematic review and meta-analysis. Diabetes Metabol Syndr: Clin Res Rev. 2023;17 doi: 10.1016/j.dsx.2023.102834. [DOI] [PubMed] [Google Scholar]
- 90.Atlas S.J.K.K., Beinfeld M., Lancaster V., Nhan E., Lien P.W., Shah K., Touchette D.R., Moradi A., Rind D.M., Pearson S.D., Beaudoin F. Institute for Clinical and Economic Review; 2022. Medications for obesity management: effectiveness and value; evidence report. [Google Scholar]
- 91.Cifuentes L., Hurtado A.M., Eckel-Passow J., Acosta A. Precision medicine for obesity. Dig Dis Interv. 2021;5:239–248. doi: 10.1055/s-0041-1729945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O'Brien P.E., Hindle A., Brennan L., Skinner S., Burton P., Smith A., et al. Long-term outcomes after bariatric surgery: a systematic review and meta-analysis of weight loss at 10 or more years for all bariatric procedures and a single-centre review of 20-year outcomes after adjustable gastric banding. Obes Surg. 2019;29:3–14. doi: 10.1007/s11695-018-3525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang K., Luo Y., Dai H., Deng Z. Effects of bariatric surgery on cancer risk: evidence from meta-analysis. Obes Surg. 2020;30:1265–1272. doi: 10.1007/s11695-019-04368-4. [DOI] [PubMed] [Google Scholar]
- 94.Clapp B., Portela R., Sharma I., Nakanishi H., Marrero K., Schauer P., et al. Risk of non-hormonal cancer after bariatric surgery: meta-analysis of retrospective observational studies. Br J Surg. 2022;110:24–33. doi: 10.1093/bjs/znac343. [DOI] [PubMed] [Google Scholar]
- 95.Schauer D.P., Feigelson H.S., Koebnick C., Caan B., Weinmann S., Leonard A.C., et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann Surg. 2019;269:95–101. doi: 10.1097/SLA.0000000000002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Adams T.D., Gress R.E., Smith S.C., Halverson R.C., Simper S.C., Rosamond W.D., et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 97.Wilson R.B., Lathigara D., Kaushal D. Systematic review and meta-analysis of the impact of bariatric surgery on future cancer risk. Int J Mol Sci. 2023:24. doi: 10.3390/ijms24076192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sjostrom L., Gummesson A., Sjostrom C.D., Narbro K., Peltonen M., Wedel H., et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10:653–662. doi: 10.1016/S1470-2045(09)70159-7. [DOI] [PubMed] [Google Scholar]
- 99.Wiggins T., Antonowicz S.S., Markar S.R. Cancer risk following bariatric surgery-systematic review and meta-analysis of national population-based cohort studies. Obes Surg. 2019;29:1031–1039. doi: 10.1007/s11695-018-3501-8. [DOI] [PubMed] [Google Scholar]
- 100.Schauer D.P., Feigelson H.S., Koebnick C., Caan B., Weinmann S., Leonard A.C., et al. Association between weight loss and the risk of cancer after bariatric surgery. Obesity. 2017;25(Suppl 2) doi: 10.1002/oby.22002. S52-s7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fouladi F., Carroll I.M., Sharpton T.J., Bulik-Sullivan E., Heinberg L., Steffen K.J., et al. A microbial signature following bariatric surgery is robustly consistent across multiple cohorts. Gut Microb. 2021;13 doi: 10.1080/19490976.2021.1930872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ishihara B.P., Farah D., Fonseca M.C.M., Nazario A. The risk of developing breast, ovarian, and endometrial cancer in obese women submitted to bariatric surgery: a meta-analysis. Surg Obes Relat Dis. 2020;16:1596–1602. doi: 10.1016/j.soard.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 103.Hassinger T.E., Mehaffey J.H., Hawkins R.B., Schirmer B.D., Hallowell P.T., Schroen A.T., et al. Overall and estrogen receptor-positive breast cancer incidences are decreased following bariatric surgery. Obes Surg. 2019;29:776–781. doi: 10.1007/s11695-018-3598-9. [DOI] [PubMed] [Google Scholar]
- 104.Doumouras A.G., Lovrics O., Paterson J.M., Sutradhar R., Paszat L., Sivapathasundaram B., et al. Bariatric surgery and breast cancer incidence: a population-based, matched cohort study. Obes Surg. 2022;32:1261–1269. doi: 10.1007/s11695-022-05946-9. [DOI] [PubMed] [Google Scholar]
- 105.Anveden Å., Taube M., Peltonen M., Jacobson P., Andersson-Assarsson J.C., Sjöholm K., et al. Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects Study. Gynecol Oncol. 2017;145:224–229. doi: 10.1016/j.ygyno.2017.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Feigelson H.S., Caan B., Weinmann S., Leonard A.C., Powers J.D., Yenumula P.R., et al. Bariatric surgery is associated with reduced risk of breast cancer in both premenopausal and postmenopausal women. Ann Surg. 2020;272:1053–1059. doi: 10.1097/SLA.0000000000003331. [DOI] [PubMed] [Google Scholar]
- 107.Ernst B., Wilms B., Thurnheer M., Schultes B. Reduced circulating androgen levels after gastric bypass surgery in severely obese women. Obes Surg. 2013;23:602–607. doi: 10.1007/s11695-012-0823-9. [DOI] [PubMed] [Google Scholar]
- 108.Derogar M., Hull M.A., Kant P., Östlund M., Lu Y., Lagergren J. Increased risk of colorectal cancer after obesity surgery. Ann Surg. 2013;258:983–988. doi: 10.1097/SLA.0b013e318288463a. [DOI] [PubMed] [Google Scholar]
- 109.Aravani A., Downing A., Thomas J.D., Lagergren J., Morris E.J.A., Hull M.A. Obesity surgery and risk of colorectal and other obesity-related cancers: an English population-based cohort study. Cancer Epidemiol. 2018;53:99–104. doi: 10.1016/j.canep.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tao W., Artama M., von Euler-Chelpin M., Hull M., Ljung R., Lynge E., et al. Colon and rectal cancer risk after bariatric surgery in a multicountry Nordic cohort study. Int J Cancer. 2020;147:728–735. doi: 10.1002/ijc.32770. [DOI] [PubMed] [Google Scholar]
- 111.Mackenzie H., Markar S.R., Askari A., Faiz O., Hull M., Purkayastha S., et al. Obesity surgery and risk of cancer. Br J Surg. 2018;105:1650–1657. doi: 10.1002/bjs.10914. [DOI] [PubMed] [Google Scholar]
- 112.Khalid S.I., Maasarani S., Wiegmann J., Wiegmann A.L., Becerra A.Z., Omotosho P., et al. Association of bariatric surgery and risk of cancer in patients with morbid obesity. Ann Surg. 2022;275:1–6. doi: 10.1097/SLA.0000000000005035. [DOI] [PubMed] [Google Scholar]
- 113.Tsui S.T., Yang J., Zhang X., Docimo S., Jr., Spaniolas K., Talamini M.A., et al. Development of cancer after bariatric surgery. Surg Obes Relat Dis. 2020;16:1586–1595. doi: 10.1016/j.soard.2020.06.026. [DOI] [PubMed] [Google Scholar]
- 114.Bailly L., Fabre R., Pradier C., Iannelli A. Colorectal cancer risk following bariatric surgery in a nationwide study of French individuals with obesity. JAMA Surg. 2020;155:395–402. doi: 10.1001/jamasurg.2020.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Taube M., Peltonen M., Sjöholm K., Palmqvist R., Andersson-Assarsson J.C., Jacobson P., et al. Long-term incidence of colorectal cancer after bariatric surgery or usual care in the Swedish Obese Subjects study. PLoS One. 2021;16 doi: 10.1371/journal.pone.0248550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Christou N.V., Lieberman M., Sampalis F., Sampalis J.S. Bariatric surgery reduces cancer risk in morbidly obese patients. Surg Obes Relat Dis. 2008;4:691–695. doi: 10.1016/j.soard.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 117.Li J.V., Ashrafian H., Bueter M., Kinross J., Sands C., le Roux C.W., et al. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut. 2011;60:1214–1223. doi: 10.1136/gut.2010.234708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang H., Dibaise J.K., Zuccolo A., Kudrna D., Braidotti M., Yu Y., et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2009;106:2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kant P., Hull M.A. Excess body weight and obesity--the link with gastrointestinal and hepatobiliary cancer. Nat Rev Gastroenterol Hepatol. 2011;8:224–238. doi: 10.1038/nrgastro.2011.23. [DOI] [PubMed] [Google Scholar]
- 120.Janik M.R., Clapp B., Sroczyński P., Ghanem O. The effect of bariatric surgery on reducing the risk of colorectal cancer: a meta-analysis of 3,233,044 patients. Surg Obes Relat Dis. 2022 doi: 10.1016/j.soard.2022.10.003. [DOI] [PubMed] [Google Scholar]
- 121.Khorgami Z., Shoar S., Andalib A., Aminian A., Brethauer S.A., Schauer P.R. Trends in utilization of bariatric surgery, 2010-2014: sleeve gastrectomy dominates. Surg Obes Relat Dis. 2017;13:774–778. doi: 10.1016/j.soard.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 122.Chang S.-H., Stoll C.R.T., Song J., Varela J.E., Eagon C.J., Colditz G.A. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surgery. 2014;149:275–287. doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tawadros A., Makar M., Kahaleh M., Sarkar A. Overview of bariatric and metabolic endoscopy interventions. Therapeutic Advances in Gastrointestinal Endoscopy. 2020;13 doi: 10.1177/2631774520935239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen Q., Zhuang H., Liu Y. The association between obesity factor and esophageal caner. J Gastrointest Oncol. 2012;3:226–231. doi: 10.3978/j.issn.2078-6891.2012.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ogunwobi O.O., Beales I.L. Leptin stimulates the proliferation of human oesophageal adenocarcinoma cells via HB-EGF and Tgfalpha mediated transactivation of the epidermal growth factor receptor. Br J Biomed Sci. 2008;65:121–127. doi: 10.1080/09674845.2008.11732814. [DOI] [PubMed] [Google Scholar]
- 126.Howard J.M., Beddy P., Ennis D., Keogan M., Pidgeon G.P., Reynolds J.V. Associations between leptin and adiponectin receptor upregulation, visceral obesity and tumour stage in oesophageal and junctional adenocarcinoma. Br J Surg. 2010;97:1020–1027. doi: 10.1002/bjs.7072. [DOI] [PubMed] [Google Scholar]
- 127.Arcidiacono D., Dedja A., Giacometti C., Fassan M., Nucci D., Francia S., et al. Hyperinsulinemia promotes esophageal cancer development in a surgically-induced duodeno-esophageal reflux murine model. Int J Mol Sci. 2018;19:1198. doi: 10.3390/ijms19041198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Alemán J.O., Eusebi L.H., Ricciardiello L., Patidar K., Sanyal A.J., Holt P.R. Mechanisms of obesity-induced gastrointestinal neoplasia. Gastroenterology. 2014;146:357–373. doi: 10.1053/j.gastro.2013.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang H.-B., Zhou C.-J., Song S-z, Chen P., Xu W-h, Liu B., et al. Evaluation of Nrf2 and IGF-1 expression in benign, premalignant and malignant gastric lesions. Pathol Res Pract. 2011;207:169–173. doi: 10.1016/j.prp.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 130.Matsubara J., Yamada Y., Nakajima T.E., Kato K., Hamaguchi T., Shirao K., et al. Clinical significance of insulin-like growth factor type 1 receptor and epidermal growth factor receptor in patients with advanced gastric cancer. Oncology. 2008;74:76–83. doi: 10.1159/000139127. [DOI] [PubMed] [Google Scholar]
- 131.Zhao X., Huang K., Zhu Z., Chen S., Hu R. Correlation between expression of leptin and clinicopathological features and prognosis in patients with gastric cancer. J Gastroenterol Hepatol. 2007;22:1317–1321. doi: 10.1111/j.1440-1746.2007.04941.x. [DOI] [PubMed] [Google Scholar]
- 132.Kai H., Kitadai Y., Kodama M., Cho S., Kuroda T., Ito M., et al. Involvement of proinflammatory cytokines IL-1β and IL-6 in progression of human gastric carcinoma. Anticancer Res. 2005;25:709–713. [PubMed] [Google Scholar]
- 133.Ahechu P., Zozaya G., Martí P., Hernández-Lizoáin J.L., Baixauli J., Unamuno X., et al. NLRP3 inflammasome: a possible link between obesity-associated low-grade chronic inflammation and colorectal cancer development. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.02918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Martinez-Useros J., Garcia-Foncillas J. Obesity and colorectal cancer: molecular features of adipose tissue. J Transl Med. 2016;14:21. doi: 10.1186/s12967-016-0772-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shah M.S., Fogelman D.R., Raghav K.P., Heymach J.V., Tran H.T., Jiang Z.Q., et al. Joint prognostic effect of obesity and chronic systemic inflammation in patients with metastatic colorectal cancer. Cancer. 2015;121:2968–2975. doi: 10.1002/cncr.29440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bartucci M., Svensson S., Ricci-Vitiani L., Dattilo R., Biffoni M., Signore M., et al. Obesity hormone leptin induces growth and interferes with the cytotoxic effects of 5-fluorouracil in colorectal tumor stem cells. Endocr Relat Cancer. 2010;17:823–833. doi: 10.1677/ERC-10-0083. [DOI] [PubMed] [Google Scholar]
- 137.Vigneri P.G., Tirrò E., Pennisi M.S., Massimino M., Stella S., Romano C., et al. The insulin/IGF system in colorectal cancer development and resistance to therapy. Front Oncol. 2015;5:230. doi: 10.3389/fonc.2015.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Park E.J., Lee J.H., Yu G.Y., He G., Ali S.R., Holzer R.G., et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yoshimoto S., Loo T.M., Atarashi K., Kanda H., Sato S., Oyadomari S., et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 140.Loo T.M., Kamachi F., Watanabe Y., Yoshimoto S., Kanda H., Arai Y., et al. Gut microbiota promotes obesity-associated liver cancer through PGE(2)-mediated suppression of antitumor immunity. Cancer Discov. 2017;7:522–538. doi: 10.1158/2159-8290.CD-16-0932. [DOI] [PubMed] [Google Scholar]
- 141.Sakurai Y., Kubota N., Takamoto I., Obata A., Iwamoto M., Hayashi T., et al. Role of insulin receptor substrates in the progression of hepatocellular carcinoma. Sci Rep. 2017;7:5387. doi: 10.1038/s41598-017-03299-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li L., Gan Y., Li W., Wu C., Lu Z. Overweight, obesity and the risk of gallbladder and extrahepatic bile duct cancers: a meta-analysis of observational studies. Obesity. 2016;24:1786–1802. doi: 10.1002/oby.21505. [DOI] [PubMed] [Google Scholar]
- 143.Sharma A., Sharma K.L., Gupta A., Yadav A., Kumar A. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: recent update. World J Gastroenterol. 2017;23:3978. doi: 10.3748/wjg.v23.i22.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Eibl G., Rozengurt E. Obesity and pancreatic cancer: insight into mechanisms. Cancers. 2021;13:5067. doi: 10.3390/cancers13205067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Logsdon C.D., Lu W. The significance of ras activity in pancreatic cancer initiation. Int J Biol Sci. 2016;12:338–346. doi: 10.7150/ijbs.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chang H.H., Moro A., Chou C.E.N., Dawson D.W., French S., Schmidt A.I., et al. Metformin decreases the incidence of pancreatic ductal adenocarcinoma promoted by diet-induced obesity in the conditional KrasG12D mouse model. Sci Rep. 2018;8:5899. doi: 10.1038/s41598-018-24337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Philip B., Roland C.L., Daniluk J., Liu Y., Chatterjee D., Gomez S.B., et al. A high-fat diet activates oncogenic Kras and COX2 to induce development of pancreatic ductal adenocarcinoma in mice. Gastroenterology. 2013;145:1449–1458. doi: 10.1053/j.gastro.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hao F., Xu Q., Zhao Y., Stevens J.V., Young S.H., Sinnett-Smith J., et al. Insulin receptor and GPCR crosstalk stimulates YAP via PI3K and PKD in pancreatic cancer cells. Mol Cancer Res. 2017;15:929–941. doi: 10.1158/1541-7786.MCR-17-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rozengurt E., Sinnett-Smith J., Kisfalvi K. Crosstalk between insulin/insulin-like growth factor-1 receptors and G protein-coupled receptor signaling systems: a novel target for the antidiabetic drug metformin in pancreatic cancer: fig. 1. Clin Cancer Res. 2010;16:2505–2511. doi: 10.1158/1078-0432.CCR-09-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen C., Chang Y.C., Lan M.S., Breslin M. Leptin stimulates ovarian cancer cell growth and inhibits apoptosis by increasing cyclin D1 and Mcl-1 expression via the activation of the MEK/ERK1/2 and PI3K/Akt signaling pathways. Int J Oncol. 2013;42:1113–1119. doi: 10.3892/ijo.2013.1789. [DOI] [PubMed] [Google Scholar]
- 151.Risch H.A. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. J Natl Cancer Inst. 1998;90:1774–1786. doi: 10.1093/jnci/90.23.1774. [DOI] [PubMed] [Google Scholar]
- 152.Syed V., Ulinski G., Mok S.C., Yiu G.K., Ho S.M. Expression of gonadotropin receptor and growth responses to key reproductive hormones in normal and malignant human ovarian surface epithelial cells. Cancer Res. 2001;61:6768–6776. [PubMed] [Google Scholar]
- 153.Mungenast F., Thalhammer T. Estrogen biosynthesis and action in ovarian cancer. Front Endocrinol. 2014;5:192. doi: 10.3389/fendo.2014.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Simone V., D'Avenia M., Argentiero A., Felici C., Rizzo F.M., De Pergola G., et al. Obesity and breast cancer: molecular interconnections and potential clinical applications. Oncol. 2016;21:404–417. doi: 10.1634/theoncologist.2015-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Morris P.G., Hudis C.A., Giri D., Morrow M., Falcone D.J., Zhou X.K., et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res. 2011;4:1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mawson A., Lai A., Carroll J.S., Sergio C.M., Mitchell C.J., Sarcevic B. Estrogen and insulin/IGF-1 cooperatively stimulate cell cycle progression in MCF-7 breast cancer cells through differential regulation of c-Myc and cyclin D1. Mol Cell Endocrinol. 2005;229:161–173. doi: 10.1016/j.mce.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 157.Słabuszewska-Jóźwiak A., Lukaszuk A., Janicka-Kośnik M., Wdowiak A., Jakiel G. Role of leptin and adiponectin in endometrial cancer. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23105307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Macciò A., Madeddu C., Gramignano G., Mulas C., Floris C., Massa D., et al. Correlation of body mass index and leptin with tumor size and stage of disease in hormone-dependent postmenopausal breast cancer: preliminary results and therapeutic implications. J Mol Med (Berl) 2010;88:677–686. doi: 10.1007/s00109-010-0611-8. [DOI] [PubMed] [Google Scholar]
- 159.Seibold P., Hein R., Schmezer P., Hall P., Liu J., Dahmen N., et al. Polymorphisms in oxidative stress-related genes and postmenopausal breast cancer risk. Int J Cancer. 2011;129:1467–1476. doi: 10.1002/ijc.25761. [DOI] [PubMed] [Google Scholar]
- 160.Wenyan T., Fei T., Jinping G., Chao G., Guoyan L., Yanfang Z., et al. Estrogen and insulin synergistically promote endometrial cancer progression via crosstalk between their receptor signaling pathways. Cancer Biology & Medicine. 2019;16:55. doi: 10.20892/j.issn.2095-3941.2018.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Linehan W.M., Srinivasan R., Schmidt L.S. The genetic basis of kidney cancer: a metabolic disease. Nat Rev Urol. 2010;7:277–285. doi: 10.1038/nrurol.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang H.P., Zou J., Xu Z.Q., Ruan J., Yang S.D., Yin Y., et al. Association of leptin, visfatin, apelin, resistin and adiponectin with clear cell renal cell carcinoma. Oncol Lett. 2017;13:463–468. doi: 10.3892/ol.2016.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Qi X., Li Q., Che X., Wang Q., Wu G. The uniqueness of clear cell renal cell carcinoma: summary of the process and abnormality of glucose metabolism and lipid metabolism in ccRCC. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.727778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lucarelli G., Loizzo D., Franzin R., Battaglia S., Ferro M., Cantiello F., et al. Metabolomic insights into pathophysiological mechanisms and biomarker discovery in clear cell renal cell carcinoma. Expert Rev Mol Diagn. 2019;19:397–407. doi: 10.1080/14737159.2019.1607729. [DOI] [PubMed] [Google Scholar]
- 165.Walsh K.M., Zhang C., Calvocoressi L., Hansen H.M., Berchuck A., Schildkraut J.M., et al. Pleiotropic MLLT10 variation confers risk of meningioma and estrogen-mediated cancers. Neurooncol Adv. 2022;4:vdac044. doi: 10.1093/noajnl/vdac044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Custer B., Longstreth W.T., Jr., Phillips L.E., Koepsell T.D., Van Belle G. Hormonal exposures and the risk of intracranial meningioma in women: a population-based case-control study. BMC Cancer. 2006;6:152. doi: 10.1186/1471-2407-6-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dresser L., Yuen C.A., Wilmington A., Walker M., Vogel T.J., Merrell R.T., et al. Estrogen hormone replacement therapy in incidental intracranial meningioma: a growth-rate analysis. Sci Rep. 2020;10 doi: 10.1038/s41598-020-74344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jay J.R., MacLaughlin D.T., Riley K.R., Martuza R.L. Modulation of meningioma cell growth by sex steroid hormones in vitro. J Neurosurg. 1985;62:757–762. doi: 10.3171/jns.1985.62.5.0757. [DOI] [PubMed] [Google Scholar]
- 169.Leães C.G., Meurer R.T., Coutinho L.B., Ferreira N.P., Pereira-Lima J.F., da Costa Oliveira M. Immunohistochemical expression of aromatase and estrogen, androgen and progesterone receptors in normal and neoplastic human meningeal cells. Neuropathology. 2010;30:44–49. doi: 10.1111/j.1440-1789.2009.01047.x. [DOI] [PubMed] [Google Scholar]
- 170.Zhao J., Wen J., Wang S., Yao J., Liao L., Dong J. Association between adipokines and thyroid carcinoma: a meta-analysis of case-control studies. BMC Cancer. 2020;20 doi: 10.1186/s12885-020-07299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Young O., Crotty T., O'Connell R., O'Sullivan J., Curran A.J. Levels of oxidative damage and lipid peroxidation in thyroid neoplasia. Head Neck. 2010;32:750–756. doi: 10.1002/hed.21247. [DOI] [PubMed] [Google Scholar]
- 172.Rubio G.A., Catanuto P., Glassberg M.K., Lew J.I., Elliot S.J. Estrogen receptor subtype expression and regulation is altered in papillary thyroid cancer after menopause. Surgery. 2018;163:143–149. doi: 10.1016/j.surg.2017.04.031. [DOI] [PubMed] [Google Scholar]
- 173.Liu J., Xu T., Ma L., Chang W. Signal pathway of estrogen and estrogen receptor in the development of thyroid cancer. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.593479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang K., Yang Y., Wu Y., Chen J., Zhang D., Mao X., et al. The association between insulin resistance and vascularization of thyroid nodules. The Journal of Clinical Endocrinology & Metabolism. 2015;100:184–192. doi: 10.1210/jc.2014-2723. [DOI] [PubMed] [Google Scholar]
- 175.Xu N., Liu H., Wang Y., Xue Y. Relationship between insulin resistance and thyroid cancer in Chinese euthyroid subjects without conditions affecting insulin resistance. BMC Endocr Disord. 2022;22:58. doi: 10.1186/s12902-022-00943-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kim W.G., Cheng S.Y. Mechanisms linking obesity and thyroid cancer development and progression in mouse models. Horm Cancer. 2018;9:108–116. doi: 10.1007/s12672-017-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang X., Sheng X., Miao T., Yao K., Yao D. Effect of insulin on thyroid cell proliferation, tumor cell migration, and potentially related mechanisms. Endocr Res. 2019;44:55–70. doi: 10.1080/07435800.2018.1522641. [DOI] [PubMed] [Google Scholar]
- 178.Li Z., Liu H., He J., Wang Z., Yin Z., You G., et al. Acetyl-CoA synthetase 2: a critical linkage in obesity-induced tumorigenesis in myeloma. Cell Metabol. 2021;33:78–93.e7. doi: 10.1016/j.cmet.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fowler J.A., Lwin S.T., Drake M.T., Edwards J.R., Kyle R.A., Mundy G.R., et al. Host-derived adiponectin is tumor-suppressive and a novel therapeutic target for multiple myeloma and the associated bone disease. Blood. 2011;118:5872–5882. doi: 10.1182/blood-2011-01-330407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fairfield H., Dudakovic A., Khatib C.M., Farrell M., Costa S., Falank C., et al. Myeloma-modified adipocytes exhibit metabolic dysfunction and a senescence-associated secretory phenotype. Cancer Res. 2021;81:634–647. doi: 10.1158/0008-5472.CAN-20-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sprynski A.C., Hose D., Kassambara A., Vincent L., Jourdan M., Rossi J.F., et al. Insulin is a potent myeloma cell growth factor through insulin/IGF-1 hybrid receptor activation. Leukemia. 2010;24:1940–1950. doi: 10.1038/leu.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hosgood H.D., Gunter M.J., Murphy N., Rohan T.E., Strickler H.D. The relation of obesity-related hormonal and cytokine levels with multiple myeloma and non-hodgkin lymphoma. Front Oncol. 2018;8 doi: 10.3389/fonc.2018.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sjöström L., Rissanen A., Andersen T., Boldrin M., Golay A., Koppeschaar H.P., et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352:167–172. doi: 10.1016/s0140-6736(97)11509-4. [DOI] [PubMed] [Google Scholar]
- 184.Peng H., Wang Q., Qi X., Wang X., Zhao X. Orlistat induces apoptosis and protective autophagy in ovarian cancer cells: involvement of Akt-mTOR-mediated signaling pathway. Arch Gynecol Obstet. 2018;298:597–605. doi: 10.1007/s00404-018-4841-2. [DOI] [PubMed] [Google Scholar]
- 185.Czumaj A., Zabielska J., Pakiet A., Mika A., Rostkowska O., Makarewicz W., et al. In vivo effectiveness of orlistat in the suppression of human colorectal cancer cell proliferation. Anticancer Res. 2019;39:3815–3822. doi: 10.21873/anticanres.13531. [DOI] [PubMed] [Google Scholar]
- 186.Sokolowska E., Presler M., Goyke E., Milczarek R., Swierczynski J., Sledzinski T. Orlistat reduces proliferation and enhances apoptosis in human pancreatic cancer cells (PANC-1) Anticancer Res. 2017;37:6321–6327. doi: 10.21873/anticanres.12083. [DOI] [PubMed] [Google Scholar]
- 187.Schcolnik-Cabrera A., Chávez-Blanco A., Domínguez-Gómez G., Taja-Chayeb L., Morales-Barcenas R., Trejo-Becerril C., et al. Orlistat as a FASN inhibitor and multitargeted agent for cancer therapy. Expet Opin Invest Drugs. 2018;27:475–489. doi: 10.1080/13543784.2018.1471132. [DOI] [PubMed] [Google Scholar]
- 188.Jin B.R., Kim H.J., Sim S.A., Lee M., An H.J. Anti-obesity drug orlistat alleviates western-diet-driven colitis-associated colon cancer via inhibition of STAT3 and NF-κB-Mediated signaling. Cells. 2021;10 doi: 10.3390/cells10082060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jovankić J.V., Nikodijević D.D., Milutinović M.G., Nikezić A.G., Kojić V.V., Cvetković A.M., et al. Potential of Orlistat to induce apoptotic and antiangiogenic effects as well as inhibition of fatty acid synthesis in breast cancer cells. Eur J Pharmacol. 2023;939 doi: 10.1016/j.ejphar.2022.175456. [DOI] [PubMed] [Google Scholar]
- 190.Huang H.Q., Tang J., Zhou S.T., Yi T., Peng H.L., Shen G.B., et al. Orlistat, a novel potent antitumor agent for ovarian cancer: proteomic analysis of ovarian cancer cells treated with Orlistat. Int J Oncol. 2012;41:523–532. doi: 10.3892/ijo.2012.1465. [DOI] [PubMed] [Google Scholar]
- 191.Allison D.B., Gadde K.M., Garvey W.T., Peterson C.A., Schwiers M.L., Najarian T., et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP) Obesity. 2012;20:330–342. doi: 10.1038/oby.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xu G., Fang Z., Clark L.H., Sun W., Yin Y., Zhang R., et al. Topiramate exhibits anti-tumorigenic and metastatic effects in ovarian cancer cells. Am J Transl Res. 2018;10:1663–1676. [PMC free article] [PubMed] [Google Scholar]
- 193.Greenway F.L., Fujioka K., Plodkowski R.A., Mudaliar S., Guttadauria M., Erickson J., et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595–605. doi: 10.1016/S0140-6736(10)60888-4. [DOI] [PubMed] [Google Scholar]
- 194.Wadden T.A., Foreyt J.P., Foster G.D., Hill J.O., Klein S., O'Neil P.M., et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity. 2011;19:110–120. doi: 10.1038/oby.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Liu N., Yan L., Shan F., Wang X., Qu N., Handley M.K., et al. Low-dose naltrexone plays antineoplastic role in cervical cancer progression through suppressing PI3K/AKT/mTOR pathway. Transl Oncol. 2021;14 doi: 10.1016/j.tranon.2021.101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ma M., Wang X., Liu N., Shan F., Feng Y. Low-dose naltrexone inhibits colorectal cancer progression and promotes apoptosis by increasing M1-type macrophages and activating the Bax/Bcl-2/caspase-3/PARP pathway. Int Immunopharm. 2020;83 doi: 10.1016/j.intimp.2020.106388. [DOI] [PubMed] [Google Scholar]
- 197.Clément K., van den Akker E., Argente J., Bahm A., Chung W.K., Connors H., et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020;8:960–970. doi: 10.1016/S2213-8587(20)30364-8. [DOI] [PubMed] [Google Scholar]
- 198.Pi-Sunyer X., Astrup A., Fujioka K., Greenway F., Halpern A., Krempf M., et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 199.Wadden T.A., Hollander P., Klein S., Niswender K., Woo V., Hale P.M., et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes. 2013;37:1443–1451. doi: 10.1038/ijo.2013.120. [DOI] [PubMed] [Google Scholar]
- 200.Davies M., Færch L., Jeppesen O.K., Pakseresht A., Pedersen S.D., Perreault L., et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397:971–984. doi: 10.1016/S0140-6736(21)00213-0. [DOI] [PubMed] [Google Scholar]
- 201.Garvey W.T., Frias J.P., Jastreboff A.M., le Roux C.W., Sattar N., Aizenberg D., et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2023;402:613–626. doi: 10.1016/S0140-6736(23)01200-X. [DOI] [PubMed] [Google Scholar]