Abstract
Renal fibrosis is the final pathological change in renal disease, and aging is closely related to renal fibrosis. Mitochondrial dysfunction has been reported to play an important role in aging, but the exact mechanism remains unclear. Disulfide-bond A oxidoreductase-like protein (DsbA-L) is mainly located in mitochondria and plays an important role in regulating mitochondrial function and endoplasmic reticulum (ER) stress. However, the role of DsbA-L in renal aging has not been reported. In this study, we showed a reduction in DsbA-L expression, the disruption of mitochondrial function and an increase in fibrosis in the kidneys of 12- and 24-month-old mice compared to young mice. Furthermore, the deterioration of mitochondrial dysfunction and fibrosis were observed in DsbA-L−/− mice with D-gal-induced accelerated aging. Transcriptome analysis revealed a decrease in Flt4 expression and inhibition of the PI3K-AKT signaling pathway in DsbA-L−/− mice compared to control mice. Accelerated renal aging could be alleviated by an AKT agonist (SC79) or a mitochondrial protector (MitoQ) in mice with D-gal-induced aging. In vitro, overexpression of DsbA-L in HK-2 cells restored the expression of Flt4, AKT pathway factors, SP1 and PGC-1α and alleviated mitochondrial damage and cell senescence. These beneficial effects were partially blocked by inhibiting Flt4. Finally, activating the AKT pathway or improving mitochondrial function with chemical reagents could alleviate cell senescence. Our results indicate that the DsbA-L/AKT/PGC-1α signaling pathway could be a therapeutic target for age-related renal fibrosis and is associated with mitochondrial dysfunction.
Keywords: DsbA-L, Flt4, mitochondria, kidney, aging
Introduction
With economic development and improved medical care, human life expectancy has increased significantly over the past few decades. The resulting problem is that population aging has intensified. Aging leads to functional declines in multiple organs and the occurrence of a variety of diseases, including tumors [1], metabolic diseases [2] and cardiovascular diseases [3]. The kidneys are also vulnerable to the effects of aging, and there is a gradual decline in kidney function with age. A decline in age-induced eGFR begins after age 30 in normal individuals and decreases at a rate of approximately 1 ml per year. A study of 610 individuals older than 70 showed that half had an estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73 m2 [4]. These changes make the kidneys less resistant to adverse external stimuli and more vulnerable to damage. Although telomeres [5], inflammation [6] and metabolic disorders [7] have been shown to be related to renal aging, they cannot fully explain the process of renal aging. Therefore, it is still necessary to further understand the molecular mechanism of renal aging and identify a practical target to delay renal aging.
Mitochondria are the energy factories of cells. The kidney is an important organ for material exchange, and kidney cells (especially renal tubule cells) contain abundant mitochondria to provide ATP and maintain kidney function [8]. Mitochondrial dysfunction is also involved in cellular aging, and the mitochondrial free radical theory of aging (MFRTA) has taken center stage in aging theory in the past few decades [9]. During oxidative phosphorylation, mitochondria produce large amounts of reactive oxygen species (ROS), and the increase in ROS can cause oxidative damage to a variety of proteins, lipids and nucleic acids, thus accelerating cell aging [10]. Miao et al. demonstrated that there was mitochondrial dysfunction in aging kidneys, and mitochondrial protectors could effectively delay the aging process in the kidney [11]. This suggests that mitochondrial quality control plays a crucial role in delaying renal aging. However, the underlying mechanism of mitochondrial dysfunction in renal tubule cells has not been elucidated.
Disulfide-bond A oxidoreductase-like protein (DsbA-L) is a mitochondrial protein that was first identified in rat hepatocytes and is involved in the progression of metabolic diseases such as diabetes [12], obesity [13] and diabetic nephropathy [14]. Bai et al. demonstrated that DsbA-L could inhibit the release of mtDNA into the cytoplasm and alleviate cGAS-STING-mediated cellular inflammation in obesity [13]. In addition, liver-specific knockout or overexpression of DsbA-L aggravated or alleviated mitochondrial dysfunction and insulin resistance in high-fat diet-induced mice, respectively [15]. Moreover, previous studies have shown that DsbA-L ameliorates renal injury in DN by maintaining mitochondrial homeostasis [16] and mitochondria-associated ER membrane (MAM) integrity [17]. This evidence suggests that DsbA-L plays an indispensable role in regulating metabolic homeostasis. However, the role of tubular DsbA-L in renal aging and the mechanism are still unknown.
In this study, we detected a decrease in DsbA-L expression in naturally aged mice, D-gal-induced aged mice, and cell models, and these effects were accompanied by abnormal mitochondrial function and increased oxidative stress. These adverse changes were further aggravated in diabetic DsbA-L-deficient mice. Mechanistically, transcriptome analysis showed that DsbA-L upregulated the expression of FMS-related receptor tyrosine kinase 4 (Flt4), which activated SP1/PGC1α through the AKT signaling pathway and ultimately regulated mitochondrial homeostasis. These data indicate that DsbA-L alleviates renal aging by maintaining mitochondrial homeostasis through the Flt4/SP1/PGC1α pathway.
Materials and methods
Animal model
Male C57BL/6 mice (8 weeks of age) were obtained from SLAC Animal and fed until 7, 12, and 24 months of age to obtain a naturally aged mouse model. DsbA-L−/− mice were obtained from Shanghai Bioray Laboratory, Inc., (China) as described in our previous study [14]. The accelerated aging mouse model was established by D-gal treatment (120 mg·kg−1·d−1) for 12 weeks as previously described [18]. The mitochondria-targeted antioxidant mitoquinone (MitoQ) (5 mg/kg, MedChemExpress) was intraperitoneally injected twice weekly after 2 weeks of D-gal injection. SC79 was obtained from MedChemExpress, and 4 mg/kg was administered via intraperitoneal injection twice weekly after 2 weeks of D-gal injection. MitoQ and SC79 were dissolved in dimethyl sulfoxide (DMSO) to form stock solutions, and the final working concentration of DMSO was < 5%. The control group was injected with the same solvent. All mice were housed in a quiet environment at 22–26 °C. The mice were anesthetized by an intraperitoneal injection of 50 mg/kg pentobarbital sodium and killed by exsanguination. Kidney tissue, blood and urine were collected for various biochemical and morphological tests. All animal experiments were performed in the Second Xiangya Hospital of Central South University and were approved by the Committee of Laboratory Animal Care and Use Institutions of Central South University (No. 20220151).
Transcriptome analysis
Transcriptome analysis was performed by Seqhealth Technology Co., Ltd (Wuhan, China). Total RNA was extracted from the kidney cortices of wild-type mice and DsbA-L-knockout mice by using RNAiso Plus (TaKaRa, Japan). The mRNA sequencing library was prepared with 5 μg of total RNA, which was sequenced by a HiSeq X10 system after the concentration and integrity were determined (Illumina, San Diego, CA, USA).
Western blotting
Radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitors and phosphatase inhibitors was used to extract proteins from kidney tissue and HK-2 cells. After the protein concentration was determined by a BCA assay, equivalent amounts of protein were detected by Western blotting as previously described [16]. The following antibodies were used: anti-DsbA-L (1:1000, Abcam, ab92819), anti-p16INK4A (1:1000, Abcam, ab189034), anti-γH2AX (1:1000, Abcam, ab26350), anti-fibronectin (1:1000, Abcam, ab2413), anti-α-SMA (1:2000, Proteintech, 14395-1-AP), anti- collagen 1 (1:1000, Abcam, ab260043), anti-TOMM20 (1:10000, Proteintech, 11802-1-AP), anti-PGC1α (1:5000, Proteintech, 66369-1-Ig), anti-MFN2 (1:5000, Proteintech, 12186-1-AP), anti-DRP1 (1:5000, Proteintech, 12957-1-AP), anti-Fis1 (1:1000, Proteintech, 10956-1-AP), anti-COX IV (1:10000, Proteintech, 11242-1-AP), anti-AKT (1:1000, CST, #9272), and anti-FLT4 (1:1000, Proteintech, 20712-1-AP). The Western blot bands were quantified by ImageJ software. Briefly, there were 4 mice in each group, the experiment was repeated 3 times for each mouse, and the average value of each group was used to determine the ratio relative to the control group.
Immunohistochemical (IHC) staining
After the renal paraffin sections were dewaxed, rehydrated, subjected to antigen repair (0.1 M sodium citrate buffer at pH 6.0, and 90 °C in a water bath for 40 min as the antibody instruction) [19] and blocked (5% BSA), they were incubated with anti-DsbA-L (1:50, GTX82705, Genetex) [16], anti-p16INK4A (1: 200, ab241543, Abcam), anti-fibronectin (1:50, ab2413, Abcam), α-SMA (1:1000, 14395-1-AP, Proteintech), Flt4 (1:200, 20712-1-AP, Proteintech), and CD31 (1:1000, 28083-1-AP, Proteintech) at 4 °C overnight. The sections were then incubated at room temperature with the secondary antibody for 1 h, after which the samples were nucleated, sealed, observed and photographed under a microscope. There were 4 mice in each group, 10 random visual fields of each kidney were observed under the microscope, and the immunohistochemistry results were quantified by IPP software. The average value of each group was used to determine the ratio relative to the control group.
RT and real-time PCR
The TRIzol RNA isolation system (Life Technologies) was used to isolate total RNA according to the manufacturer’s protocol. Real-time PCR was performed using a Platinum SYBR Green qPCR SuperMix-UDG kit (Invitrogen). The relative expression levels of each gene were normalized to β-actin. A DNeasy blood and tissue kit (Qiagen) was used to extract total DNA according to the manufacturer’s protocol. Then, mtDNA was amplified with the COX2 gene primer and normalized to ribosomal protein s18 (RSP18) [11].
Assessment of oxidative stress
DCFH-DA staining
DCFH-DA staining was performed to detect reactive oxygen species levels in cells. Briefly, cells in the different groups were incubated with DCFH-DA (Invitrogen) at room temperature for 10 min according to the instructions and then observed and photographed under a fluorescence microscope. DCFH-DA fluorescence intensity was analyzed by ImageJ software.
Dihydroethidium (DHE) staining
DHE staining was performed to detect reactive oxygen species levels in tissues. Briefly, frozen kidney sections were incubated at room temperature with DHE stain for 10 min and then sealed and photographed under a fluorescence microscope.
IHC staining of 4HNE and 8-OHdG
The expression of 4HNE and 8-OhdG in the kidney was detected by IHC staining as previously described. 4HNE is a marker of intracellular lipid peroxidation, and 8-OHdG is a biomarker of DNA oxidative damage [20].
Transmission electron microscopy and mitochondrial morphology analysis
Fresh kidney tissue was fixed with 2.5% glutaraldehyde and then fixed with 1% osmium tetroxide. The fixed kidney tissue was dehydrated and then embedded in Epon 812. Ultrathin sections were stained with uranyl acetate and lead citrate and observed by transmission electron microscopy. Mitochondrial fragmentation was quantified using ImageJ. Briefly, 6 electron images of renal tubular epithelial cells were randomly selected for each kidney, and mitochondrial morphology was evaluated using ImageJ to determine the mitochondrial aspect ratio (the lengths of major axes/lengths of minor axes). A decrease in the aspect ratio indicates mitochondrial fragmentation, as previously described [16].
Cell culture and treatments
The human proximal tubular epithelial cell line (HK-2 cells) was obtained from ATCC (USA) and cultured in 5% CO2 and 95% air at 37 °C. The DsbA-L overexpression plasmid was transfected into HK-2 cells with Lipofectamine 3000. HK-2 cells were treated with D-gal (Sigma-Aldrich), MitoQ (MedChemExpress) and SC79 (MedChemExpress). After being treated, the cells were prepared and subjected to Western blotting or SA-β-gal staining.
Senescence-associated β-galactosidase (SA-β-gal) staining
Cells or renal tissues were treated, fixed at room temperature for 15 min, incubated at 37 °C overnight with SA-β-gal staining solution (C0602, Beyotime, China) and observed and photographed under a microscope. The positive area of the cells was measured and calculated by Image-Pro Plus as previously described [21, 22].
ATP assay
ATP concentrations were detected by using an ATP Assay Kit (Beyotime Biotechnology, S0026) according to the manufacturer’s instructions [23].
Statistical analysis
Statistical analyses were performed by SPSS 20.0 software. The results are presented as the mean ± SEM and were analyzed by Student’s t test or one-way analysis of variance. When the P value was less than 0.05, there was a significant difference between the two groups.
Results
The expression of DsbA-L in the kidney decreased in a time-dependent manner during aging
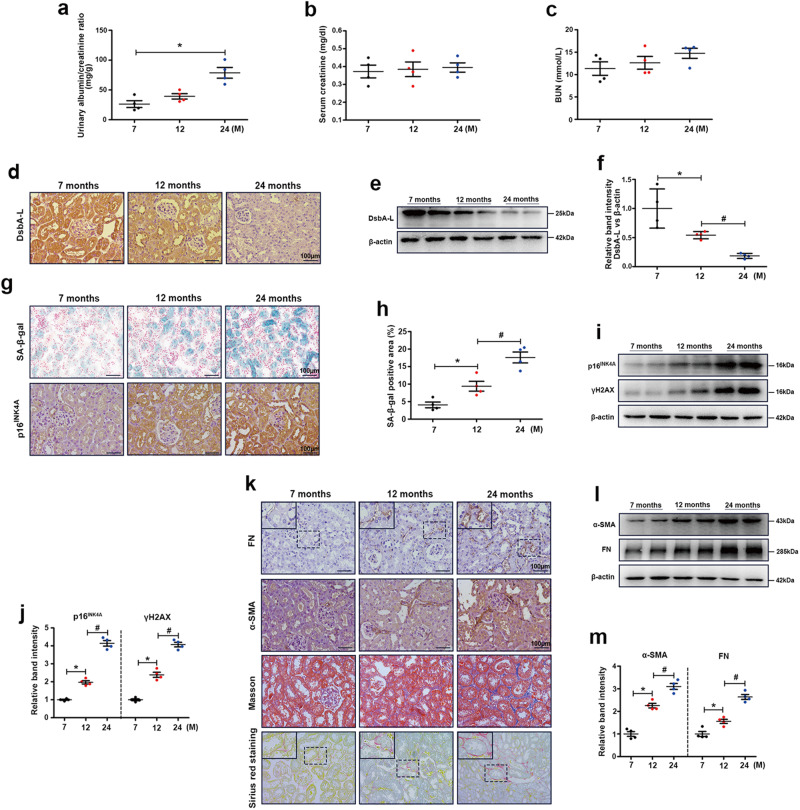
Compared with those of 7-month-old mice, the UACR levels of 24-month-old mice were significantly increased (Fig. 1a), but serum creatinine and BUN levels were not significantly different (Fig. 1b, c). To study the role of kidney DsbA-L in natural aging, we measured DsbA-L expression in the kidneys of 7-, 12-, and 24-month-old mice by immunohistochemistry and found that the expression of DsbA-L decreased gradually with age (Fig. 1d). Similar DsbA-L expression was observed by Western blot analysis (Fig. 1e, f). Furthermore, we examined renal senescence and fibrosis by measuring the activity of aging markers (SA-β-gal (blue area) and p16INK4A). As shown in Fig. 1g, h, SA-β-gal activity and the expression of p16INK4A were increased in the kidney with age. Similar results were observed by Western blot analysis of aging markers (p16INK4A and γH2AX) (Fig. 1i, j). Moreover, Masson and Sirius red staining and IHC staining of FN and α-SMA showed that fibrosis levels were significantly upregulated with age (Fig. 1k). Western blot analysis of FN and α-SMA also showed similar results (Fig. 1l, m).
Fig. 1. DsbA-L expression was downregulated, and cellular senescence was observed in aging kidneys.
The levels of urinary albumin/creatinine ratio (UACR) (a), serum creatinine (b) and BUN (c). IHC staining d and Western blot analysis e, f of DsbA-L expression in the kidneys. g, h SA-β-gal staining and IHC staining of p16INK4A in the kidney in the different groups. i Western blot analysis of p16INK4A and γH2AX in the kidneys. j Representative band intensities of p16INK4A and γH2AX. k IHC staining of FN and α-SMA and Masson and Sirius red staining of the kidney in the different groups. l Western blot analysis of FN and α-SMA in the kidneys. m Representative band intensities of FN and α-SMA. The data are shown as the mean ± SEM. n = 4, *P < 0.05 vs. 7 months. #P < 0.05 vs. 12 months.
Mitochondrial homeostasis was disturbed in a time-dependent manner during aging
Abnormal mitochondrial homeostasis is involved in the progression of aging [24], and DsbA-L is a key protein that maintains mitochondrial homeostasis [13]. Therefore, we examined the changes in mitochondrial homeostasis during renal aging. As shown in Fig. 2a, the level of mtDNA was notably decreased in the kidneys of 24-month-old mice. Moreover, the mRNA levels of the OXPHOS complex III subunit cytochrome b (Cytb), complex V subunit ATP synthase 6 (ATP6), and complex IV subunits cytochrome c oxidase 1 (COX1) and cytochrome c oxidase 2 (COX2) were significantly downregulated in the kidneys of 12-month-old mice and further decreased in 24-month-old mice (Fig. 2b, c). The disturbance in mitochondrial homeostasis may also be involved in the aging process [25, 26], and DsbA-L is involved in the maintenance of mitochondrial homeostasis [13]. Electron microscopy showed that the number of fragmented mitochondria in renal tubule cells increased with the progression of renal aging (Fig. 2d, e). DHE staining showed that the kidneys of 12-month-old mice showed an increase in oxidative stress compared to the kidneys of 7-month-old mice, and oxidative stress was further increased in the 24-month-old kidneys (Fig. 2f, g). Furthermore, we measured the expression of mitochondria-associated proteins (PGC1α, TOMM20, MFN2, Drp1 and Fis1), and the results showed that PGC1α, TOMM20 and MFN2 were significantly decreased, while Drp1 and Fis1 were increased in 12-month-old mice and were further aggravated in 24-month-old mice (Fig. 2h–m). Moreover, double immunofluorescence staining showed that the mitochondrial translocation of DsbA-L (yellow area) was reduced in 24-month-old kidneys (Fig. 2n).
Fig. 2. Disturbed mitochondrial homeostasis was observed in aging renal tissue.
a Mitochondrial DNA (mtDNA) content in the kidney in the different groups. b, c The mRNA expression of mitochondrial-encoded OXPHOS genes (Cytb, ATP6, COX1 and COX2) in the kidney in the different groups. d, e Electron microscopy was used to observe mitochondrial morphology in the kidney in the different groups. f DHE staining was used to detect oxidative stress levels in the kidney in the different groups. g Scatter diagram showing the levels of oxidative stress as detected by DHE staining. h Western blot analysis of PGC1α, TOMM20, MFN2, Drp1 and Fis1 in the kidney in the different groups. i–m Representative band intensities of PGC1α, TOMM20, MFN2, Drp1 and Fis1. n Immunofluorescence double staining of DsbA-L (green) and COX IV (red) in the kidney in the different groups. The data are shown as the mean ± SEM. n = 4, *P < 0.05 vs. 7 months. #P < 0.05 vs. 12 months.
DsbA-L gene deficiency aggravated fibrosis in the kidney in an accelerated aging mouse model
There was no expression of DsbA-L in DsbA-L−/− mice or in DsbA-L−/− mice with accelerated aging (Fig. 3a). SA-β-gal staining (blue area) and IHC staining of p16INK4A showed that aging levels were increased in the kidneys of mice with accelerated aging and DsbA-L−/− mice compared to control mice and were further aggravated in DsbA-L−/− mice with accelerated aging (Fig. 3b, c). Similar results were observed by Western blot analysis of aging markers (p16INK4A and γH2AX) (Fig. 3d, e). Moreover, we examined fibrosis levels in the kidneys by Masson and Sirius staining and IHC staining of FN and α-SMA. The results showed that fibrosis levels were increased in the kidneys of mice with accelerated aging and DsbA-L−/− mice and were aggravated in DsbA-L−/− mice with accelerated aging (Fig. 3f). Western blot analysis of FN, α-SMA and collagen 1 showed a consistent trend (Fig. 3g, h). These results suggest that DsbA-L deficiency exacerbated renal aging and fibrosis levels.
Fig. 3. Aggravated cellular senescence and fibrosis levels in the kidneys of DsbA-L gene-deficient mice.
a Western blot analysis of DsbA-L in the kidney in the different groups. b, c SA-β-gal staining and IHC staining of p16INK4A in the kidney in the different groups. d Western blot analysis of p16INK4A and γH2AX in the kidney in the different groups. e Representative band intensities of p16INK4A and γH2AX. f IHC staining of FN and α-SMA and Masson and Sirius red staining of the kidney in the different groups. g Western blot analysis of FN, α-SMA and collagen 1 in the kidney. h Representative band intensities of FN, α-SMA and collagen 1. The data are shown as the mean ± SEM. n = 4, *P < 0.05 represents DsbA-L−/− vs. control. #P < 0.05 represents D-gal-treated mice vs. control mice. @P < 0.05 represents D-gal-treated DsbA-L−/− mice vs. D-gal-treated mice.
DsbA-L regulated the AKT/SP1/PGC1α signaling pathway via Flt4
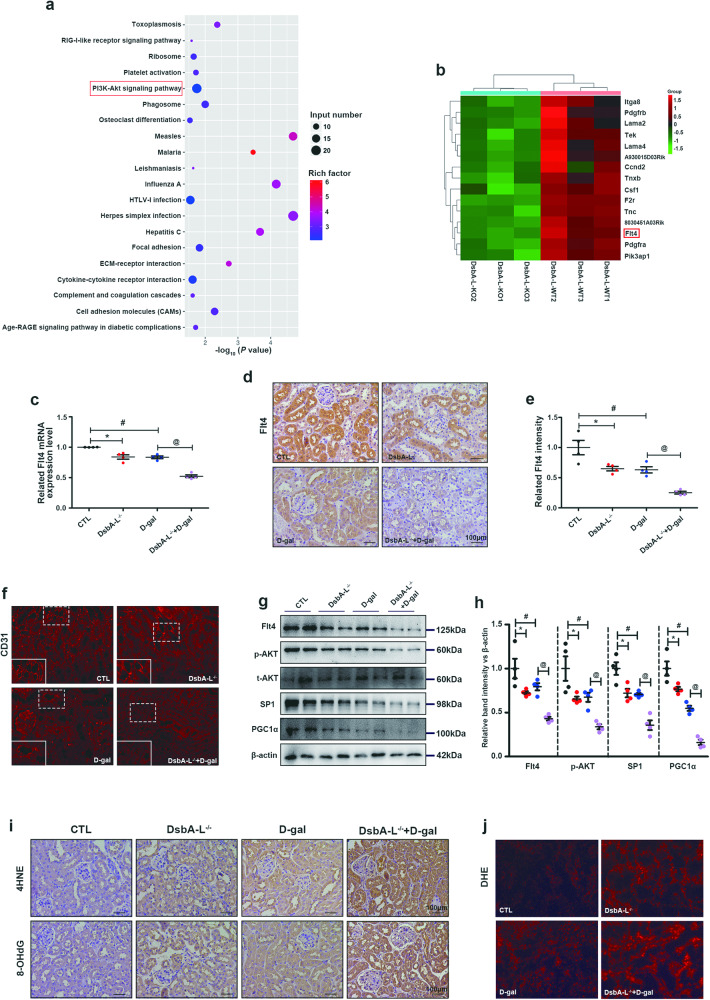
To further explore the role of DsbA-L in renal aging, we compared the renal transcriptomes of DsbA-L−/− mice and wild-type mice. As shown in Fig. 4a, the differentially expressed genes in DsbA-L−/− mice and the control group were mainly concentrated in the AKT signaling pathway. We also found that Flt4 was significantly downregulated in DsbA-L-knockout (Fig. 4b). Flt4, which is also known as vascular endothelial growth factor receptor 3 (VEGFR3), is a member of the VEGFR family [27]. The downregulation of this factor was confirmed by measuring the mRNA expression of Flt4 (Fig. 4c). IHC staining also showed that Flt4 expression was reduced in the kidneys of aging mice compared to those in the control group, and it was further decreased in aging DsbA-L−/− mice (Fig. 4d, e). Moreover, the level of peritubular capillary density was decreased in the kidneys of mice with accelerated aging and DsbA-L−/− mice compared to control mice and was further aggravated in DsbA-L−/− mice with accelerated aging (Fig. 4f). Inhibition of Flt4 expression has been shown to inhibit AKT phosphorylation [28, 29], while activation of Flt4 increases AKT phosphorylation [29]. SP1 is a transcription factor that is commonly expressed in cells, and its expression is regulated by AKT [30–33]. Moreover, SP1 can directly bind to the PGC1α promoter to promote PGC1α (a mitochondrial biogenesis protein) expression [34]. Therefore, we measured the expression of the AKT/SP1/PGC1α signaling pathway in the kidneys in the different groups. Western blotting showed downregulated expression of Flt4, phosphorylated AKT (p-AKT), SP1 and PGC1α in the kidneys of aging mice compared to those in the control group, and these changes were further aggravated in aging DsbA-L−/− mice (Fig. 4g, h). Furthermore, mitochondria are the centers of oxidative stress in cells, and we examined changes in oxidative stress levels in the kidney. IHC staining of 4HNE (an active aldehyde produced by lipid peroxidation that represents the level of lipid peroxidation products) and 8-OHdG (an oxidized nucleoside that represents the level of oxidative damage to DNA) (Fig. 4i) and DHE staining (an intracellular superoxide indicator) (Fig. 4j) showed that oxidative stress was notably increased in the kidneys of aging mice and was further increased in aging DsbA-L−/− mice. These results suggest that DsbA-L may activate the p-AKT/SP1 signaling pathway by regulating the expression of Flt4, thereby promoting the expression of the mitochondrial biogenic protein PGC1α and regulating the level of intracellular oxidative stress.
Fig. 4. Decreased Flt4 and inhibited AKT/SP1/PGC1α signaling were observed in the kidneys of DsbA-L-knockout mice.
a KEGG pathway analysis of the kidneys of DsbA-L−/− mice compared to control mice. b Transcriptomics showed that Flt4 was significantly decreased in the kidneys of DsbA-L−/− mice. c The mRNA level of Flt4. d, e IHC staining of Flt4. f Immunofluorescence analysis of CD31 in the kidney in the different groups. g Western blot analysis of Flt4, p-AKT, total AKT (t-AKT), SP1 and PGC1α in the kidney. h Representative band intensities of Flt4, p-AKT, SP1 and PGC1α. i IHC staining of 4HNE and 8-OHdG in the kidneys. j DHE staining was used to detect oxidative stress levels in the kidney. The data are shown as the mean ± SEM. n = 4, *P < 0.05 represents DsbA-L−/− vs. control. #P < 0.05 represents D-gal-treated mice vs. control. @P < 0.05 represents D-gal -treated DsbA-L−/− mice vs. D-gal-treated mice.
AKT agonists and mitochondrial protectants significantly delayed renal aging in an aging mouse model
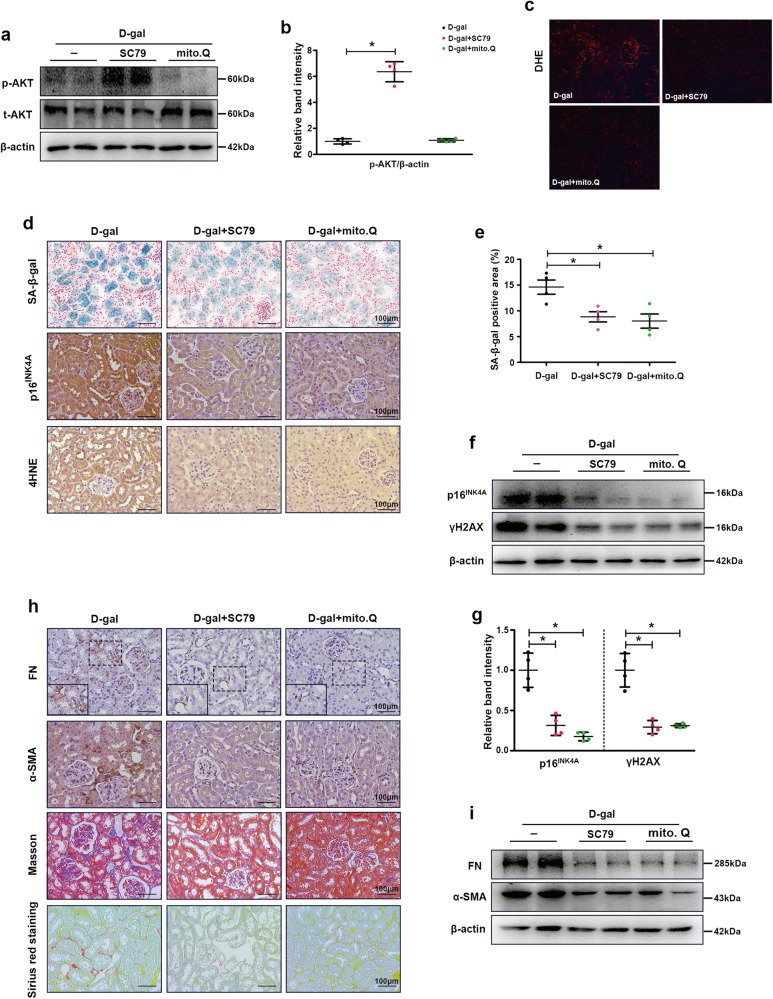
To further explore the role of AKT-mediated mitochondrial homeostasis in renal aging, we treated the aging mouse model with an AKT agonist (SC79) and a mitochondrial protector (MitoQ). Treatment with SC79 significantly activated AKT, while MitoQ seemed to have no effect on AKT phosphorylation (Fig. 5a, b). Furthermore, we examined the effects on intracellular oxidative stress, and the results showed that SC79 and MitoQ significantly reduced oxidative stress levels in the aging mouse model (Fig. 5c). We then examined cellular senescence and oxidative stress in the groups. As shown in Fig. 5d, e, SC79 and MitoQ notably decreased the activity of SA-β-gal (blue area), p16INK4A and 4HNE compared to those in the aging mouse model. Similar results were observed by Western blot analysis of p16INK4A and γH2AX (Fig. 5f, g). Furthermore, renal fibrosis in the different groups was detected by Masson and Sirius red staining, IHC staining and Western blot analysis, and SC79 and MitoQ significantly ameliorated renal fibrosis in aging mice (Fig. 5h, i). These results suggest that activating AKT and enhancing mitochondrial function can delay renal aging and fibrosis.
Fig. 5. AKT agonists (SC79) and mitochondrial protectants (MitoQ) significantly ameliorated cellular senescence and fibrosis levels in the kidneys of aging mice.
a Western blot analysis of p-AKT and t-AKT in the kidney. b Representative band intensity of p-AKT. c DHE staining was used to detect oxidative stress levels in the kidney. d, e SA-β-gal staining and IHC staining of p16INK4A and 4HNE in the kidney. f, g Western blot analysis of p16INK4A and γH2AX in the kidney. h IHC staining of FN and α-SMA and Masson and Sirius red staining in the kidneys. i Western blot analysis of FN and α-SMA in the kidney. The data are shown as the mean ± SEM. n = 4, *P < 0.05 vs. D-gal-treated mice.
Overexpression of DsbA-L ameliorated mitochondrial function and delayed aging by upregulating Flt4 and activating the AKT/SP1/PGC1α axis
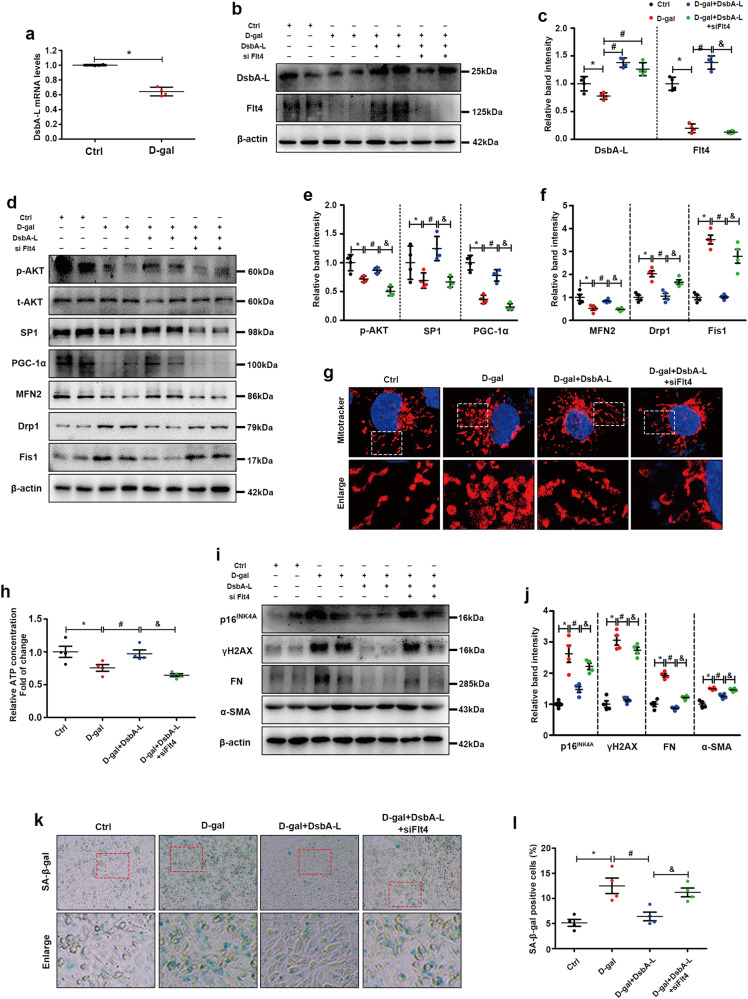
The mRNA expression of DsbA-L was significantly decreased in the D-gal group compared with the control group (Fig. 6a). Western blot analysis showed decreased expression of DsbA-L in HK2 cells exposed to D-gal compared to control cells, and it was upregulated when DsbA-L was overexpressed (Fig. 6b, c). D-gal significantly inhibited Flt4 expression, while DsbA-L overexpression up-regulated Flt4, and the effect of DsbA-L on Flt4 was inhibited by Flt4 siRNA (Fig. 6b, c). Changes in the AKT/SP1/PGC1α axis and mitochondrial proteins were detected by Western blot analysis. As shown in Fig. 6d–f, D-gal treatment downregulated the expression of p-AKT, SP1, PGC1α and MFN2 (mitochondrial fusion protein) and upregulated mitochondrial fission proteins (Drp1 and Fis1), and these changes were ameliorated by the overexpression of DsbA-L. However, these changes were partially inhibited by Flt4 siRNA treatment (Fig. 6d–f). Moreover, mitochondrial morphology was observed by MitoTracker staining. Donut-shaped mitochondria indicate mitochondrial fragmentation [35]. As shown in Fig. 6g, in the control group of HK-2 cells, the mitochondria were filamentous, while the mitochondria became donut-shaped when D-gal was added. Overexpression of DsbA-L could restore filamentous mitochondria, and the protective effect of DsbA-L was partially blocked by Flt4 siRNA (Fig. 6g). Furthermore, we measured intracellular ATP production in the different groups and found that compared with those in the control group, D-gal significantly inhibited intracellular ATP levels, while overexpression of DsbA-L partially restored ATP production, and further inhibition of Flt4 blocked the protective effect of DsbA-L on ATP production (Fig. 6h). We also examined cellular senescence and fibrosis levels. D-gal significantly increased the expression of aging markers (p16INK4A and γH2AX) and fibrosis markers (FN and α-SMA), while overexpression of DsbA-L significantly relieved the adverse effects of D-gal, and the protective effect of DsbA-L was partially blocked by Flt4 siRNA (Fig. 6i, j). Similar changes were observed by SA-β-gal staining (Fig. 6k, l). These results suggest that DsbA-L can ameliorate mitochondrial homeostasis, renal aging and fibrosis by regulating Flt4 and activating the AKT/SP1/PGC1α signaling pathway.
Fig. 6. Overexpression of DsbA-L restored Flt4 expression and ameliorated mitochondrial function, cellular senescence, and fibrosis in D-gal-treated HK-2 cells.
a The mRNA levels of DsbA-L. b, c Western blot analysis of DsbA-L and Flt4 in the different groups. d–f Western blot analysis of p-AKT, t-AKT, SP1, PGC1α, MFN2, Drp1 and Fis1 in the different groups. g MitoTracker staining in the different groups. h ATP concentrations in the different groups. i Western blot analysis of p16INK4A, γH2AX, FN and α-SMA in different groups. j Representative band intensities of p16INK4A, γH2AX, FN and α-SMA in the different groups. k, l SA-β-gal staining in the different groups. The data are shown as the mean ± SEM. n = 4, *P < 0.05 represents D-gal vs. control. #P < 0.05 represents D-gal +DsbA-L vs. D-gal. &P represents D-gal+DsbA-L+siFlt4 vs. D-gal+DsbA-L.
AKT agonists and mitochondrial protectants ameliorated aging in D-gal treated HK-2 cells
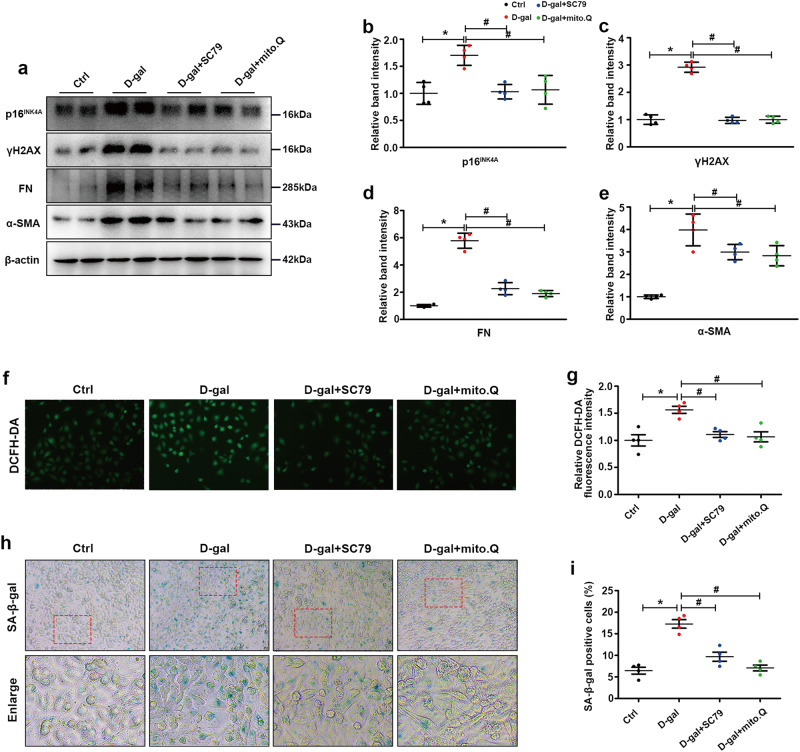
To further verify the protective effect of activating AKT and enhancing mitochondrial function in aging, we treated HK-2 cells exposed to D-gal with SC79 or MitoQ. Western blot analysis showed that SC79 or MitoQ treatment significantly inhibited the expression of p16INK4A, γH2AX, FN and α-SMA in the presence of D-gal (Fig. 7a–e). Similar results were obtained and showed that SC79 or MitoQ notably abrogated the increase in intracellular oxidative stress and cell senescence caused by D-gal, as shown by DCFH-DA staining (Fig. 7f, g) and SA-β-gal staining (Fig. 7h, i), respectively.
Fig. 7. AKT agonists (SC79) and mitochondrial protectants (MitoQ) significantly ameliorated cellular senescence and fibrosis levels in D-gal-treated HK-2 cells.
a Western blot analysis of p16INK4A, γH2AX, FN and α-SMA in the different groups. b–e Representative band intensities of p16INK4A, γH2AX, FN and α-SMA in the different groups. f, g Intracellular oxidative stress levels in the different groups were detected by DCFH-DA staining. h, i SA-β-gal staining in the different groups. The data are shown as the mean ± SEM. n = 4, *P < 0.05 vs. control. #P < 0.05 vs. D-gal.
Discussion
In this study, we demonstrated that the level of renal fibrosis increased during natural aging, and a decrease in DsbA-L expression and abnormal mitochondrial function were observed. Knockout of DsbA-L could accelerate mitochondrial dysfunction and renal aging. Mechanistically, transcriptomics analysis showed that DsbA-L regulated the AKT/SP1/PGC1α signaling pathway by affecting Flt4, thereby maintaining mitochondrial homeostasis and delaying renal aging.
Mitochondria are the energy factories of the cell and produce large amounts of ATP for various cellular activities [36]. The kidney is one of the most vigorous organs with high intracellular metabolism that mediates the excretion of metabolic waste produced by the human body; this process requires a large amount of energy. Renal cells, especially tubular epithelial cells, contain many mitochondria [37]. Mitochondrial dysfunction is involved in the occurrence and development of a variety of kidney diseases, including acute kidney injury (AKI) [38, 39] and DN [23]. Aging has always been a hot topic of research, and delaying aging could reduce the occurrence of various age-related diseases. The role of mitochondria in aging cannot be ignored, and the mitochondrial free radical aging theory (MFRTA) has taken center stage in the aging theory for decades [40]. Multiple functional abnormalities in mitochondria can accelerate aging. Continuous accumulation of dysfunctional mitochondria is an important sign of aging, and abnormal mitophagy can lead to the failure of timely clearance of dysfunctional mitochondria, thus aggravating cell damage [41, 42]. Moreover, there was impaired mitochondrial function, disturbed redox homeostasis and decreased renal function in the kidneys of aged rats compared to control rats [43]. In addition, gene editing or drug-mediated enhancement of mitochondrial function can effectively delay aging [44, 45]. Peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α is a transcriptional coactivator and a major regulator of mitochondrial biogenesis and function [46, 47]. This factor can activate several transcription factors, including nuclear respiration factor (NRF-1 and 2) and mitochondrial transcription factor A (TFAM), thereby increasing the transcription of genes associated with mitochondrial biogenesis and function [48]. Moreover, PGC1α can regulate the occurrence of mitophagy [49]. Kim et al. showed that treatment with fenofibrate could activate PGC1α, thereby improving renal oxidative stress and mitochondrial dysfunction and delaying renal aging in aging mice [50]. Similarly, Wnt/β-catenin regulates mitochondrial function and renal aging through PGC1α [11]. These results reveal the protective role of PGC1α-mediated mitochondrial homeostasis in aging. In this study, we found a decrease in PGC1α expression, mitochondrial dysfunction, and an increase in fibrosis in the kidneys of naturally aged mice and treatment with the mitochondrial protective agent MitoQ could significantly alleviate age-related renal oxidative stress and fibrosis.
DsbA-L is also known as glutathione S-transferase kappa l (GSTK1), and it was originally found to be a mitochondrial matrix protein, but with further study, it was found to be distributed in the endoplasmic reticulum and MAM, as well as mitochondria [13, 17, 51]. Liu et al. found that the N-terminus of DsbA-L contained ER target signals, which could be localized to the ER and interact with the ER chaperone protein Ero1-Lα, thereby alleviating ER stress and upregulating adiponectin expression [51]. In addition, previous studies by us and others have shown that DsbA-L can maintain mitochondrial homeostasis and mitigate disease progression. Adipocyte-specific knockout of DsbA-L impaired mitochondrial function and promoted mtDNA release, thus leading to activation of the cGAS-cGAMP-STING pathway and the inflammatory response, while adipocyte-specific overexpression of DsbA-L protected mice against activation of the cGAS-cGAMP-STING pathway and inflammation induced by a high-fat diet [13]. We also found that knockout of DsbA-L aggravated mitochondrial fragmentation, oxidative stress and kidney damage in diabetic mice [16]. This evidence indicates the importance of DsbA-L in maintaining mitochondrial homeostasis, but unfortunately, the role of DsbA-L in aging has not yet been determined. Here, we found that DsbA-L expression in mouse kidneys decreased with age and was accompanied by mitochondrial dysfunction and increased oxidative stress. Moreover, a more severe aging phenotype, mitochondrial dysfunction, and an increase in oxidative stress were observed in the kidneys of D-gal-treated DsbA-L-knockout mice compared to mice in the D-gal group. In addition, overexpression of DsbA-L in HK-2 cells partially restored the level of mitochondrial dysfunction and fibrosis caused by D-gal.
Transcriptomics was performed to further study the mechanism by which DsbA-L maintains mitochondrial homeostasis. The differentially expressed genes in the kidney in the DsbA-L-knockout group and control group were mainly concentrated in the PI3K/AKT signaling pathway, and the mRNA level of Flt4 in the DsbA-L-knockout group was significantly decreased compared to that in control mice. Flt4, which is also known as vascular endothelial growth factor receptor 3 (VEGFR3), is a member of the VEGFR family [27]. Ito et al. showed that the expression of Flt4 in the lung decreased with age and was further downregulated in LPS-induced lung injury [52]. Similar age-dependent changes in Flt4 expression were observed in iNKT cells [53]. Inhibiting Flt4 expression has been shown to inhibit AKT phosphorylation [28], while activating Flt4 increases AKT phosphorylation [29]. SP1 is a transcription factor that is commonly expressed in cells, and its expression is regulated by AKT [30–32]. SP1 can directly bind to the PGC1α promoter to promote PGC1α expression [34]. PGC1α is a key protein that regulates mitochondrial homeostasis, and abnormal PGC1α expression can lead to mitochondrial dysfunction and accelerate the aging process [54, 55]. In this study, we showed that the AKT/SP1/PGC1α signaling pathway was inhibited, which was accompanied by decreased expression of Flt4 and increased oxidative stress levels in D-gal-treated DsbA-L−/− mice. Moreover, the aging phenotype, renal oxidative stress and fibrosis were effectively improved in aging mice treated with an AKT agonist (SC79) and a mitochondrial protective agent (MitoQ). In addition, overexpression of DsbA-L upregulated the expression of Flt4, thereby activating the AKT/SP1/PGC1α signaling pathway, enhancing mitochondrial function and slowing renal aging and fibrosis. However, although we observed that DsbA-L could promote the expression of Flt4, its specific molecular mechanism remains unclear. Flt4 is mainly synthesized in the ER, and a previous study reported that DsbA-L could be translocated to the ER and interact with the ER-chaperonin protein Ero1-Lα to inhibit endoplasmic reticulum stress and promote protein synthesis [51]. In addition, studies have shown that the elimination of CPT1A downregulates the expression of FLT4 [56]. DsbA-L could effectively activate AMPK [57], which is a key regulator of CPT1A [58–60]. These results indicate that DsbA-L upregulates the expression of Flt4, possibly through ER stress or the AMPK/CPT1A pathway. Although these reports are consistent with our findings, the specific molecular mechanisms need to be further explored. Overall, these data suggest that DsbA-L can enhance mitochondrial function through the Flt4/SP1/PGC1α signaling pathway, thereby alleviating renal aging.
In this study, we demonstrated that the decrease in DsbA-L expression was associated with mitochondrial dysfunction and fibrosis in the kidneys of aging mice. DsbA-L can upregulate the transcription of Flt4, thus activating the AKT/SP1/PGC1α signaling pathway, maintaining mitochondrial homeostasis and slowing renal aging.
Acknowledgements
This work was supported by grants from the Natural Science Foundation of Hunan Province (2021JC0003).
Author contributions
MY and YL performed the experiments and wrote the manuscript. SLL, CBL, NJ, CRL, HZ, YCH, WC, and LL provided technical support for this study and participated in discussions about this study. MY and LS designed this study and edited this manuscript. LS is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All the authors have read and approved the final manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Ming Yang, Yan Liu
References
- 1.Calcinotto A, Kohli J, Zagato E, Pellegrini L, Demaria M, Alimonti A. Cellular senescence: aging, cancer, and injury. Physiol Rev. 2019;99:1047–78. doi: 10.1152/physrev.00020.2018. [DOI] [PubMed] [Google Scholar]
- 2.Amorim JA, Coppotelli G, Rolo AP, Palmeira CM, Ross JM, Sinclair DA. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat Rev Endocrinol. 2022;18:243–58. doi: 10.1038/s41574-021-00626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110:1097–108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaeffner ES, Ebert N, Delanaye P, Frei U, Gaedeke J, Jakob O, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012;157:471–81. doi: 10.7326/0003-4819-157-7-201210020-00003. [DOI] [PubMed] [Google Scholar]
- 5.Ameh OI, Okpechi IG, Dandara C, Kengne AP. Association between telomere length, chronic kidney disease, and renal traits: a systematic review. OMICS. 2017;21:143–55. doi: 10.1089/omi.2016.0180. [DOI] [PubMed] [Google Scholar]
- 6.Cobo G, Lindholm B, Stenvinkel P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol Dial Transpl. 2018;33:iii35–iii40. doi: 10.1093/ndt/gfy175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frassetto LA, Sebastian A, DuBose TD. How metabolic acidosis and kidney disease may accelerate the aging process. Eur J Clin Nutr. 2020;74:27–32. doi: 10.1038/s41430-020-0693-5. [DOI] [PubMed] [Google Scholar]
- 8.Yang M, Li C, Yang S, Xiao Y, Chen W, Gao P, et al. Mitophagy: a novel therapeutic target for treating DN. Curr Med Chem. 2021;28:2717–28. doi: 10.2174/0929867327666201006152656. [DOI] [PubMed] [Google Scholar]
- 9.Son JM, Lee C. Mitochondria: multifaceted regulators of aging. BMB Rep. 2019;52:13–23. doi: 10.5483/BMBRep.2019.52.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liochev SI. Reactive oxygen species and the free radical theory of aging. Free Radic Biol Med. 2013;60:1–4. doi: 10.1016/j.freeradbiomed.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Miao J, Liu J, Niu J, Zhang Y, Shen W, Luo C, et al. Wnt/β-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction. Aging Cell. 2019;18:e13004. doi: 10.1111/acel.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao F, Fang Q, Zhang R, Lu J, Lu H, Wang C, et al. Polymorphism of DsbA-L gene associates with insulin secretion and body fat distribution in Chinese population. Endocr J. 2009;56:487–94. doi: 10.1507/endocrj.K08E-322. [DOI] [PubMed] [Google Scholar]
- 13.Bai J, Cervantes C, Liu J, He S, Zhou H, Zhang B, et al. DsbA-L prevents obesity-induced inflammation and insulin resistance by suppressing the mtDNA release-activated cGAS-cGAMP-STING pathway. Proc Natl Acad Sci USA. 2017;114:12196–201. doi: 10.1073/pnas.1708744114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Han Y, Gao P, Yang M, Xiao L, Xiong X, et al. Disulfide-bond a oxidoreductase-like protein protects against ectopic fat deposition and lipid-related kidney damage in diabetic nephropathy. Kidney Int. 2019;95:880–95. doi: 10.1016/j.kint.2018.10.038. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Bai J, Dong F, Fang H, Zhang Y, Meng W, et al. Hepatic DsbA-L protects mice from diet-induced hepatosteatosis and insulin resistance. FASEB J. 2017;31:2314–26. doi: 10.1096/fj.201600985R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao P, Yang M, Chen X, Xiong S, Liu J, Sun L. DsbA-L deficiency exacerbates mitochondrial dysfunction of tubular cells in diabetic kidney disease. Clin Sci. 2020;134:677–94. doi: 10.1042/CS20200005. [DOI] [PubMed] [Google Scholar]
- 17.Yang M, Zhao L, Gao P, Zhu X, Han Y, Chen X, et al. DsbA-L ameliorates high glucose induced tubular damage through maintaining MAM integrity. EBioMedicine. 2019;43:607–19. doi: 10.1016/j.ebiom.2019.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Tang XQ, Shi Y, Li HM, Meng ZY, Chen H, et al. Tetrahydroberberrubine retards heart aging in mice by promoting PHB2-mediated mitophagy. Acta Pharm Sin. 2023;44:332–44. doi: 10.1038/s41401-022-00956-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi S-R, Shi Y, Taylor CR. Antigen retrieval immunohistochemistry: review and future prospects in research and diagnosis over two decades. J Histochem Cytochem. 2011;59:13–32. doi: 10.1369/jhc.2010.957191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2’ -deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120–39. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 21.Mortuza R, Chen S, Feng B, Sen S, Chakrabarti S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS ONE. 2013;8:e54514. doi: 10.1371/journal.pone.0054514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Gao F, Wei L, Chen L, Qu N, Zeng L, et al. Predict the role of lncRNA in kidney aging based on RNA sequencing. BMC Genomics. 2022;23:254. doi: 10.1186/s12864-022-08479-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Li L, Yang M, Yang J, Zhao C, Han Y, et al. PACS-2 ameliorates tubular injury by facilitating endoplasmic reticulum-mitochondria contact and mitophagy in diabetic nephropathy. Diabetes. 2022;71:1034–50. doi: 10.2337/db21-0983. [DOI] [PubMed] [Google Scholar]
- 24.Akbari M, Kirkwood TBL, Bohr VA. Mitochondria in the signaling pathways that control longevity and health span. Ageing Res Rev. 2019;54:100940. doi: 10.1016/j.arr.2019.100940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu NN, Zhang Y, Ren J. Mitophagy, mitochondrial dynamics, and homeostasis in cardiovascular aging. Oxid Med Cell Longev. 2019;2019:9825061. doi: 10.1155/2019/9825061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leduc-Gaudet JP, Hussain SNA, Barreiro E, Gouspillou G. Mitochondrial dynamics and mitophagy in skeletal muscle health and aging. Int J Mol Sci. 2021;22:8179. [DOI] [PMC free article] [PubMed]
- 27.Monaghan RM, Page DJ, Ostergaard P, Keavney BD. The physiological and pathological functions of VEGFR3 in cardiac and lymphatic development and related diseases. Cardiovasc Res. 2021;117:1877–90. doi: 10.1093/cvr/cvaa291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao L, Zhu Z, Yao C, Huang Y, Zhi E, Chen H, et al. VEGFC/VEGFR3 signaling regulates mouse spermatogonial cell proliferation via the activation of AKT/MAPK and cyclin D1 pathway and mediates the apoptosis by affecting caspase 3/9 and Bcl-2. Cell Cycle. 2018;17:225–39. doi: 10.1080/15384101.2017.1407891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin QY, Zhang YL, Bai J, Liu JQ, Li HH. VEGF-C/VEGFR-3 axis protects against pressure-overload induced cardiac dysfunction through regulation of lymphangiogenesis. Clin Transl Med. 2021;11:e374. doi: 10.1002/ctm2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin HO, An S, Lee HC, Woo SH, Seo SK, Choe TB, et al. Hypoxic condition- and high cell density-induced expression of Redd1 is regulated by activation of hypoxia-inducible factor-1alpha and Sp1 through the phosphatidylinositol 3-kinase/Akt signaling pathway. Cell Signal. 2007;19:1393–403. doi: 10.1016/j.cellsig.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Lin HY, Chen YS, Wang K, Chien HW, Hsieh YH, Yang SF. Fisetin inhibits epidermal growth factor-induced migration of ARPE-19 cells by suppression of AKT activation and Sp1-dependent MMP-9 expression. Mol Vis. 2017;23:900–10. [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Liao R, Qiang Z, Zhang C. Pro-inflammatory cytokine-driven PI3K/Akt/Sp1 signalling and H2S production facilitates the pathogenesis of severe acute pancreatitis. Biosci Rep. 2017;37:BSR20160483. [DOI] [PMC free article] [PubMed]
- 33.Yin P, Zhao C, Li Z, Mei C, Yao W, Liu Y, et al. Sp1 is involved in regulation of cystathionine γ-lyase gene expression and biological function by PI3K/Akt pathway in human hepatocellular carcinoma cell lines. Cell Signal. 2012;24:1229–40. doi: 10.1016/j.cellsig.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Park G, Horie T, Kanayama T, Fukasawa K, Iezaki T, Onishi Y, et al. The transcriptional modulator Ifrd1 controls PGC-1α expression under short-term adrenergic stimulation in brown adipocytes. FEBS J. 2017;284:784–95. doi: 10.1111/febs.14019. [DOI] [PubMed] [Google Scholar]
- 35.Suh J, Kim NK, Shim W, Lee SH, Kim HJ, Moon E, et al. Mitochondrial fragmentation and donut formation enhance mitochondrial secretion to promote osteogenesis. Cell Metab. 2023;35:345-360.e7. [DOI] [PubMed]
- 36.Burke PJ. Mitochondria, bioenergetics and apoptosis in cancer. Trends Cancer. 2017;3:857–70. doi: 10.1016/j.trecan.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quadri MM, Fatima SS, Che RC, Zhang AH. Mitochondria and renal fibrosis. Adv Exp Med Biol. 2019;1165:501–24. doi: 10.1007/978-981-13-8871-2_25. [DOI] [PubMed] [Google Scholar]
- 38.Sun J, Zhang J, Tian J, Virzì GM, Digvijay K, Cueto L, et al. Mitochondria in sepsis-induced AKI. J Am Soc Nephrol. 2019;30:1151–61. doi: 10.1681/ASN.2018111126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Agborbesong E, Li X. The role of mitochondria in acute kidney injury and chronic kidney disease and its therapeutic potential. Int J Mol Sci. 2021;22:11253. [DOI] [PMC free article] [PubMed]
- 40.Sanz A, Fernández-Ayala DJM, Stefanatos RK, Jacobs HT. Mitochondrial ROS production correlates with, but does not directly regulate lifespan in Drosophila. Aging. 2010;2:200–23. doi: 10.18632/aging.100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, et al. Mitophagy and Alzheimer’s disease: cellular and molecular mechanisms. Trends Neurosci. 2017;40:151–66. doi: 10.1016/j.tins.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohammad RS, Lokhandwala MF, Banday AA. Age-related mitochondrial impairment and renal injury is ameliorated by sulforaphane via activation of transcription factor NRF2. Antioxidants. 2022;11:156. [DOI] [PMC free article] [PubMed]
- 44.Rera M, Bahadorani S, Cho J, Koehler CL, Ulgherait M, Hur JH, et al. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 2011;14:623–34. doi: 10.1016/j.cmet.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bharath LP, Agrawal M, McCambridge G, Nicholas DA, Hasturk H, Liu J, et al. Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell Metab. 2020;32:44-55.e6. [DOI] [PMC free article] [PubMed]
- 46.Salazar G, Cullen A, Huang J, Zhao Y, Serino A, Hilenski L, et al. SQSTM1/p62 and PPARGC1A/PGC-1alpha at the interface of autophagy and vascular senescence. Autophagy. 2020;16:1092–110. doi: 10.1080/15548627.2019.1659612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fontecha-Barriuso M, Martin-Sanchez D, Martinez-Moreno JM, Monsalve M, Ramos AM, Sanchez-Niño MD, et al. The role of PGC-1α and mitochondrial biogenesis in kidney diseases. Biomolecules. 2020; 10. [DOI] [PMC free article] [PubMed]
- 48.Chandrasekaran K, Anjaneyulu M, Choi J, Kumar P, Salimian M, Ho C-Y, et al. Role of mitochondria in diabetic peripheral neuropathy: Influencing the NAD+-dependent SIRT1-PGC-1α-TFAM pathway. Int Rev Neurobiol. 2019;145:177–209. doi: 10.1016/bs.irn.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang D, Zhuo Y, Guo Z, He L, Wang X, He Y, et al. SIRT1/PGC-1 pathway activation triggers autophagy/mitophagy and attenuates oxidative damage in intestinal epithelial cells. Biochimie. 2020;170:10–20. doi: 10.1016/j.biochi.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 50.Kim EN, Lim JH, Kim MY, Kim HW, Park CW, Chang YS, et al. PPARα agonist, fenofibrate, ameliorates age-related renal injury. Exp Gerontol. 2016;81:42–50. doi: 10.1016/j.exger.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 51.Liu M, Chen H, Wei L, Hu D, Dong K, Jia W, et al. Endoplasmic reticulum (ER) localization is critical for DsbA-L protein to suppress ER stress and adiponectin down-regulation in adipocytes. J Biol Chem. 2015;290:10143–8. doi: 10.1074/jbc.M115.645416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ito Y, Betsuyaku T, Nagai K, Nasuhara Y, Nishimura M. Expression of pulmonary VEGF family declines with age and is further down-regulated in lipopolysaccharide (LPS)-induced lung injury. Exp Gerontol. 2005;40:315–23. doi: 10.1016/j.exger.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 53.Papadogianni G, Ravens I, Dittrich-Breiholz O, Bernhardt G, Georgiev H. Impact of aging on the phenotype of invariant natural killer T cells in mouse thymus. Front Immunol. 2020;11:575764. doi: 10.3389/fimmu.2020.575764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang Q, Ji A, Li D, Shi L, Gao M, Lv N, et al. Mitochondria damage in ambient particulate matter induced cardiotoxicity: roles of PPAR alpha/PGC-1 alpha signaling. Environ Pollut. 2021;288:117792. doi: 10.1016/j.envpol.2021.117792. [DOI] [PubMed] [Google Scholar]
- 55.He W, Wang P, Chen Q, Li C. Exercise enhances mitochondrial fission and mitophagy to improve myopathy following critical limb ischemia in elderly mice via the PGC1a/FNDC5/irisin pathway. Skelet Muscle. 2020;10:25. doi: 10.1186/s13395-020-00245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiong Y, Liu Z, Zhao X, Ruan S, Zhang X, Wang S, et al. CPT1A regulates breast cancer-associated lymphangiogenesis via VEGF signaling. Biomed Pharmacother. 2018;106:1–7. doi: 10.1016/j.biopha.2018.05.112. [DOI] [PubMed] [Google Scholar]
- 57.Yang M, Luo S, Jiang N, Wang X, Han Y, Zhao H, et al. DsbA-L ameliorates renal injury through the AMPK/NLRP3 inflammasome signaling pathway in diabetic nephropathy. Front Physiol. 2021;12:659751. doi: 10.3389/fphys.2021.659751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yao Q, Li S, Cheng X, Zou Y, Shen Y, Zhang S. Yin Zhi Huang, a traditional Chinese herbal formula, ameliorates diet-induced obesity and hepatic steatosis by activating the AMPK/SREBP-1 and the AMPK/ACC/CPT1A pathways. Ann Transl Med. 2020;8:231. doi: 10.21037/atm.2020.01.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu Z, Zhou W, Yang Y, Wang K, Li F, Dang Y. Quantitative profiling of oxylipin reveals the mechanism of Pien-Tze-Huang on alcoholic liver disease. Evid Based Complement Altern Med. 2021;2021:9931542. doi: 10.1155/2021/9931542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin CW, Peng YJ, Lin YY, Mersmann HJ, Ding ST. LRRK2 regulates CPT1A to promote β-oxidation in HepG2 cells. Molecules. 2020;25:4122. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.