Abstract
The O-linked-β-N-acetylglucosamine (O-GlcNAc) glycosylation (O-GlcNAcylation) is a critical post-translational modification that couples the external stimuli to intracellular signal transduction networks. However, the critical protein targets of O-GlcNAcylation in oxidative stress-induced apoptosis remain to be elucidated. Here, we show that treatment with H2O2 inhibited O-GlcNAcylation, impaired cell viability, increased the cleaved caspase 3 and accelerated apoptosis of neuroblastoma N2a cells. The O-GlcNAc transferase (OGT) inhibitor OSMI-1 or the O-GlcNAcase (OGA) inhibitor Thiamet-G enhanced or inhibited H2O2-induced apoptosis, respectively. The total and phosphorylated protein levels, as well as the promoter activities of signal transducer and activator of transcription factor 3 (STAT3) and Forkhead box protein O 1 (FOXO1) were suppressed by OSMI-1. In contrast, overexpressing OGT or treating with Thiamet-G increased the total protein levels of STAT3 and FOXO1. Overexpression of STAT3 or FOXO1 abolished OSMI-1-induced apoptosis. Whereas the anti-apoptotic effect of OGT and Thiamet-G in H2O2-treated cells was abolished by either downregulating the expression or activity of endogenous STAT3 or FOXO1. These results suggest that STAT3 or FOXO1 are the potential targets of O-GlcNAcylation involved in the H2O2-induced apoptosis of N2a cells.
Keywords: O-GlcNAcylation, oxidative stress, apoptosis, STAT3, FOXO1
Introduction
Reactive oxygen species (ROS) are inevitable by-products of cellular energy metabolism and play a critical role in proliferation, differentiation, survival and cellular signal transduction networks. Oxidation and antioxidant reactions are in balance in physiological state. However, the production and removal of ROS will be disrupted and trigger oxidative stress upon the pathological stimulations. Excess ROS causes irreversible cellular damage in DNA, oxidized proteins, glycation products and lipid peroxidation, leading to cytokine necrosis, neuronal degeneration, and cell apoptosis [1]. Numerous studies have discovered that oxidative stress can directly or indirectly induce the pathogenesis of cardiovascular, metabolic and neurodegenerative diseases etc [2–4]. The central nervous system usually exhibits a strong susceptibility to oxidative stress due to its rich mitochondrial and lipid content [5–8], and the neuronal loss is closely related to oxidative stress in a variety of neurodegenerative diseases [9, 10].
O-linked-β-N-acetylglucosamine (O-GlcNAc) glycosylation (O-GlcNAcylation) is a dynamic and indispensable post-translational modification (PTM) in mammals [11, 12], participating in majority of adaptive cellular responses, including transcriptional activation or silencing [13–15], cell cycle regulation [16], nucleoplasmic transport [17], protein-protein interactions [18, 19] and signal transduction [20, 21], etc. Numerous studies have shown that oxidative stress causes changes in the overall level of cellular O-GlcNAc, and indicated O-GlcNAcylation plays an important role in the spatiotemporal regulation of cellular responses to nutrition and stress [22, 23]. In addition, key regulators of redox homeostasis are in turn regulated by O-GlcNAcylation modifications in most cells, tissues and organisms [24, 25].
Signal transducer and activator of transcription 3 (STAT3) is the only embryonic lethal member of the STATs family constitutionally activated in approximately 70% of cancers, such as breast cancer, brain tumor and leukemia, etc [26]. Mutations of STAT3 lead to growth arrest or apoptosis in many cancer cell lines [27]. When stimulated by adverse external factors, STAT3 can either activate the anti-apoptotic genes BCL2, BCL2L1 and MCL, or induce JUNB and IRF1 to promote terminal cell differentiation and growth arrest [28]. Forkhead box transcription factor family (including FOXO1, FOXO3 and FOXO4) is an important regulator of oxidative stress and glucose metabolism which has been shown to mediate the effects through regulation of gene transcription [29, 30]. In terms of the functions of FOXO target genes, they can be classified into three major groups: “stress response and antioxidant defense” [31], “metabolism” [32] and “cell death and proliferation” [33, 34]. In premature glioblastoma cell, activation of the PI3K-AKT pathway increases ROS levels by inhibiting FOXO activity and suppressing transcription of antioxidant and autophagy-related genes (ATG6, ATG7, ATG12, etc.), thereby promoting DNA damage and apoptosis [35, 36]. In addition, oxidative stress signaling in turn disturbs the transcription activity of FOXO by affecting its expression, protein post-translational modifications [37], intracellular localization [38] and protein interactions [39, 40]. Thus, FOXO can also be considered as a sensor of oxidative stress, which delivers the environmental stress signals to induce apoptosis or stress resistance [9, 10].
There is also a complex interaction between STAT3 and FOXO1. It has been shown that FOXO1 disrupts the interaction of STAT3 with target gene promoter complexes either by inhibiting the activation of STAT3 phosphorylation or by directly binding to STAT3 [41, 42]. Conversely, STAT3 also affects FOXO1 transcription, phosphorylation, and nucleoplasmic localization, resulting in proper regulation of cellular processes such as energy metabolism, cell proliferation, and immune activation [43]. Furthermore, FOXO1 and STAT3 binding sites are reported to compete with each other in the function of regulation gene transcription [44]. In addition, STAT3- and FOXO1-dependent signaling pathways also interplay with other pathways such as the vascular endothelial growth factor (VEGF) pathway, the NOTCH pathway and NF-κB pathway to coordinate biological processes [45–47].
Although several key O-GlcNAcylated protein substrates have been identified, the intracellular mechanism of how O-GlcNAcylation signal in neurogenic cell lines mediates the cellular response to harmful stimuli is not fully understood. In this study, we investigated the physiological role of O-GlcNAcylation in oxidative stress-induced apoptosis by pharmacological and genetic manipulation in the N2a cells, and reveal novel transcriptional factors as the targets of O-GlcNAcylation in the N2a cells which mediates the response to oxidative stress and apoptosis.
Materials and methods
Reagents
OSMI-1 (SML1621-2), Thiamet-G (SML0244), Dimethyl sulfoxide (DMSO) (D2650), and Actinomycin D (SBR00013) were purchased from Sigma-Aldrich (St. Louis, Mo, USA). The stock solution of stattic was purchased from Selleck (S7024, Chemicals, Shanghai, China). The stock solution of AS1842856 was purchased from (HY-100596, MedChemExpress, Monmouth Junction, NJ, USA). The stock solution of CHX was purchased from Aladdin (C408230, Chemicals, Shanghai, China). OSMI-1 was dissolved in DMSO at stock concentration of 100 mM. Thiamet-G was dissolved in PBS (C0221A, Beyotime, Shanghai, China) at stock concentration of 5 mM.
Cell culture and transfection
Neuron-2a neuroblastoma (N2a) cells were cultured at 37 °C in a humidified 5% CO2 incubator in MEM+GlutaMAXTM (41090036, Gibco, Grand Island, NY, USA) supplemented with 10% (v/v) fetal bovine serum (WS500T, Ausbian, AUS) and 10,000 µg/mL of penicillin/streptomycin/Glutamax (10378016, Gibco, Grand Island, NY, USA). Cells were grown at 1 × 105 cells / well in 24-well plates (353047, Corning, Midland, MI, USA). Twenty-four hours after incubation, the cells were transfected with plasmids by using LipofectamineTM 2000 (11668019, Thermo Fisher Scientific, MA, USA). Forty-eight hours after transfection, cells were collected for study.
Plasmids
pLEGFP-WT-STAT3 (# 71450), HA-Foxo1 (pCMV5) (#12142) were purchased from Addgene. pEGFP-C1-ncOGT (pCMV5) vector was a gift provided by Dr. Hai-tao Wu (Chinese Academy of Military Medical Sciences). Truncations of mouse Stat3 promoters (signal transducer and activator of transcription 3/NM_213659.3/NP_998824.1) and Foxo1 promoters (forkhead box protein O1/NM_019739.3/NP_062713.2) were amplified via PCR from the N2a cells genomic DNA using specific primers, and were cloned into the psiCHECK™-2 Vector (Promega: No.4345MA10_3A) using ClonExpress® II One Step Cloning Kit (C112-01, Vazyme, Nanjing, China). All plasmids were verified by DNA sequencing (Azenta, Suzhou, China). All the primers used were listed in Supplementary Table S1.
Luciferase reporter assay
A dual fluorescent reporter enzyme system was used to determine the promoter activity. The psiCHECK™-2 vector encodes both Luc and Ruc luminescence. The fluorescence signal of Luc was used to reflect the promoter activity of protein and normalized to the fluorescence signal of Ruc (an internal control for the reporter expression between different samples). N2a cells were seeded in 96-well plates (3917, Costar, Kennebunk, ME, USA). When the cells reached 80% confluence, cells were transfected with mouse Stat3 or Foxo1 promoter reporter plasmid (0.1 μg) and pEGFP-C1-ncOGT plasmid (0.1 μg). After 24 h, the culture medium was replaced by MEM Medium containing OSMI-1 or Thiamet-G for 24 h. Then cells were lysed and luciferase activities were analyzed by using Duo-Lite Luciferase Assay System (DD1205-01, Vazyme, Nanjing, China). The relative fluorescence value was calculated as (Luc-blank)/(Ruc-blank).
RNA interference
The small interfering RNAs (siRNAs) targeting Foxo1 (sc-35383), Stat3 (sc-29494), and siRNA negative control (sc-37007) were purchased from Santa Cruz Biotechnology. N2a cells cultured in 24-well plate were transfected with Foxo1 siRNA, Stat3 siRNA, and siRNA NC using Lipofectamine™ RNAiMAX reagent (Invitrogen, 13778500) in Opti-MEM according to the manufacturer’s instructions. After 48 h transfection, cells were harvested for RT-qPCR and Western blot to evaluate the efficiency of the siRNA interference.
Western blot analysis
Cells were lysed in RIPA Lysis Buffer (P0013B, Beyotime, Shanghai, China) plus protease inhibitor cocktail (539134, Millipore, MA, USA), phosphatase inhibitor cocktail (524628, Millipore), Na3VO4 (450243, Sigma-Aldrich), PMSF (ST506, Beyotime). After a centrifugation at 4 °C at 15,000 × g for 30 min, the supernatant protein was quantified with the BCA protein assay kit (23225, Thermo Fisher Scientific, Waltham, MA, USA) and subsequently applied for Western blot analysis. For subcellular fractions (nuclear and cytoplasmic extracts) were performed using the NE-PER nuclear and cytoplasmic extraction reagents (78833; Thermo Scientific) according to the manufacturer’s recommendations. Protein samples (20 μg) were separated by 10% or 4%-20% SDS polyacrylamide gradient gels (PAGE) (P0052A/P0057A, Beyotime) and transferred to a nitrocellulose membrane (0.45 μM, A29603478, GE Whatman, Maidstone, UK). The membranes were blocked with 5% BSA and incubated with primary antibodies against O-GlcNAc (1:500, MA1-072, Invitrogen, Carlsbad, CA, USA), STAT3 (1:1000, MA1-13042, Invitrogen), P-STAT3 (1:1000, #9134, CST), FOXO1 (1:500, MA5-17078, Invitrogen), P-FOXO1 (1:1000, #9461, CST), OGT (1:1000, O6014, Sigma-Aldrich), MGEA5 (1:2000, 14711-1-AP, Proteintech), Lamin B1 (1:500, sc-374015, SANTA), PCNA (1:1000, #13110, CST), Histone H3 (1:1000, #4499, CST), Cleaved Caspase 3 (1:500, #9664, CST), Caspase 3 (1:1000, #9662, CST), GAPDH (1:2000, sc-365062, SANTA) or Actin (1:2000, A2066, Sigma-Aldrich) overnight at 4 °C, and then incubated with appropriate secondary antibodies at room temperature for 1 h. Blots were imaged on the Odyssey (LI-COR Biosciences, USA) and the grayscale of each band was analyzed by open-source software Image J.
Immunofluorescence staining
N2a cells were seeded onto coverslips and fixed with 4% paraformaldehyde for 20 min at 23°C. After washing three times with PBS, cells were permeabilized with 0.3% Triton X-100 in PBS for 5 min. After incubation with 5% BSA for 1 h at room temperature, the cells were incubated with primary anti-GFP-488 (1:500, A21311, Invitrogen), anti-Myc-Tag (1:500, 2276 S, CST, Boston, USA), anti-HA (1:1000, 2367 S, CST), or anti-Flag (1:1000, F1804, Sigma-Aldrich) antibodies overnight at 4 °C. Then, the cells were rinsed with PBS and secondary antibody (Alexa Fluor 488) (1:300, 111545, Jackson ImmunoResearch, PA, USA) was applied to the cells for 2 h at room temperature followed by 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI) (C1006, Beyotime) staining. For terminal-deoxynucleoitidyl transferase mediated nick end labeling (TUNEL) assay, cells were rinsed using PBS and then fixed with paraformaldehyde. The apoptotic cells were stained by using TUNEL reagents (E-CK-A325, elabscience, Wuhan, China) in accordance with standard method. Fluorescent images were captured by Nikon-A1 confocal laser scanning microscope (Tokyo, Japan) with ×20 air objective. All images were analyzed with open-source software Image J.
Cell viability assay
Cell viability was assessed by the Cell Counting Kit-8 method (CCK-8, 40203ES80, Yeasen, Shanghai, China). In brief, N2a cells were plated onto 24-well culture plates at a density of 1 × 105 per well. The CCK-8 assay solution was added to each well after treatment of N2a cells with H2O2 or vehicles as described above, incubated for another 2 h and then the optical densities were read at a 450-nm wavelength on a microplate reader (Bio-Tek ELX800, Bio-Tek Instrument Inc., America).
Quantitative real-time PCR (RT-qPCR)
N2a Cells were treated with OSMI-1 or DMSO for 24 h, then the culture medium was replaced by MEM Medium containing 5 μg/ml Actinomycin D (SBR00013, Sigma-Aldrich, St. Louis, Mo, USA) for 2, 4 and 8 h, respectively. After 24 h, total RNA was extracted from cells using RNA isolater Total RNA Extraction Reagent (R401-01, Vazyme, Nanjing, China) according to manufacturer’s instructions. 1 μg of total RNA was reverse-transcripted to cDNA using HiScript II 1st Strand cDNA Synthesis Kit (R212-02, Vazyme). qPCR was performed using ChamQ Universal SYBR qPCR Master Mix (Q711-03, Vazyme) and specific primers (Table S1). The expression of each gene was defined from the threshold cycle (CT) and melting temperatures (Tm) were recorded. The relative mRNA content was calculated using the 2−ΔΔCT relative quantification method with GAPDH as an endogenous control. All the primers for FOXO1 and STAT3 used were listed in Supplementary Table S1.
Statistics
Statistical analyses were performed by SPSS (IBM Corporation, Armonk, NY, USA). Student’s t-test or one-way ANOVA was used followed by Tukey’s or Dunnett’s post-hoc test. Two-way ANOVA was used for comparing two independent variables followed by Bonferroni’s post-hoc test, as indicated in the figure legends. Results are shown as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; n.s. not significant.
Results
H2O2 induces apoptosis and decreases O-GlcNAcylation in N2a cells
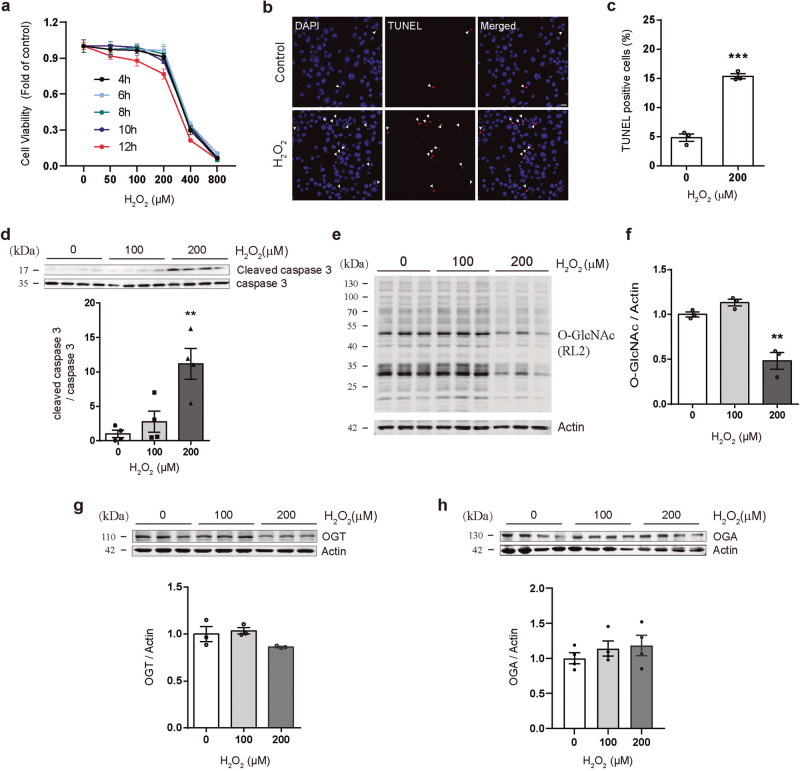
Hydrogen peroxide (H2O2) is a major cell-damaging reactive oxide causing cellular damage that permeates cell membranes and reacts with intracellular iron ions to form highly reactive free radicals. To investigate the oxidative stress effect of H2O2 on neurons, Neuron-2a neuroblastoma cells (N2a) were treated with H2O2 at the different concentrations, and the cell viabilities were determined by the cell counting assay at different time points following H2O2 administration (Fig. 1a). The results showed that 200 μM H2O2 treatment for 12 h significantly inhibited the viability of N2a cells, and the inhibitory effect became more apparent when the dose was increased to 400 μM (Fig. 1a) (100% ± 0.37% in the control group, 96.11% ± 0.79% in the 100 μM group, 76.68% ± 5.11% in the 200 μM group and 5.48% ± 1.62% in the 400 μM group). To determine whether the death of H2O2-treated cells was due to activated apoptosis, we performed the TUNEL assay to detect apoptosis. As expected, 200 μM H2O2 induced cell apoptosis (Fig. 1b, c) (4.83% ± 0.91% in the control group vs 15.36% ± 0.61% in the H2O2 group). In addition, pro-apoptotic cleaved caspase 3 was significantly elevated after stimulation with 200 μM H2O2 (Fig. 1d). Since O-GlcNAcylation functions as a stress sensor for cellular oxidative stress, the protein level of O-GlcNAc 12 h after H2O2 treatment was assessed. Global O-GlcNAcylation level was decreased by 200 μM H2O2 stimulation (Fig. 1e, f), while the protein levels of O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), the bidirectional regulatory enzymes for O-GlcNAcylation, were not significantly changed (Fig. 1g, h). These results indicate that H2O2 treatment induces apoptosis and suppresses the O-GlcNAcylation in N2a Cells.
Fig. 1. H2O2 induces cell apoptosis and decreases O-GlcNAcylation in N2a cells.
a The cell counting assay showed that H2O2 inhibited the viability of N2a cells in a time- and dose-dependent manner. b Representative images of confocal microscopy combined with TUNEL assays (Scale bar = 20 μm). c, d 200 μM H2O2 treatment increased cell apoptosis and cleaved caspase 3 expression. e, f Western blotting (4%–20% SDS-PAGE) revealed that H2O2 treatment induced a reduction in O-GlcNAc levels. g, h Western blotting showed H2O2 treatment had no significant change on OGT and OGA levels. The values are expressed as the means ± SEMs (n = 3 per group). *P < 0.05; **P < 0.01; ***P < 0.001.
Bi-directional regulation of O-GlcNAcylation and H2O2-induced apoptosis by OGT inhibitor OSMI-1 or the OGA inhibitor Thiamet-G
O-GlcNAcylation plays an important role in directing intracellular homeostasis and maintaining multiple signaling mechanisms for cell survival [22]. Intracellular levels of O-GlcNAc are bidirectionally regulated by O-GlcNAc transferase (OGT) and O-GlcNAc hydrolase (OGA) [48]. To investigate the relationship between O-GlcNAcylation and H2O2-induced apoptosis, an inhibitor of OGT OSMI-1 was used to inhibit global O-GlcNAcylation in N2a cells. Treatment of N2a cell with various concentrations of OSMI-1 (0–100 μM) resulted in a dose-dependent decrease in O-GlcNAc levels (Fig. 2a, b). Meanwhile, cell viability analysis showed that OSMI-1 treatment significantly inhibited the viability of N2a cells at a concentration of 40 μM and the inhibitory effect became more apparent when the OSMI-1 dose was increased to 100 μM (Fig. 2c). Instead, treatment of Thiamet-G (TG), an inhibitor of OGA, increased O-GlcNAcylation of N2a cells in a dose-dependent manner while did not affect cell viability (Fig. 2d-f). Considering the potential non-specific effect of high concentrations inhibitors, 60 μM OSMI-1 and 5 μM TG were selected for the following experiments. To determine whether OSMI-1 induced an activated apoptosis, cell apoptosis rate was measured. As shown in Fig. 2g, h, the apoptosis rate of N2a cells was dramatically increased by OSMI-1, while there was no significant change in the TG-treated group (3.21% ± 0.26% in the control group, 10.42% ± 1.18% in the OSMI-1 group, and 2.35% ± 0.63% in the TG group). The apoptosis level was also assessed in cells co-treated OSMI-1 or TG with H2O2. The results showed that OSMI-1 treatment increased H2O2-induced apoptosis in the N2A cells, whereas TG treatment alleviated it (Fig. 2g, h) (4.83% ± 0.64% in the control group, 10.26% ± 0.80% in the DMSO + H2O2 group, 21.07% ± 2.36% in the OSMI-1 + H2O2 group, and 3.33% ± 0.55% in the TG + H2O2 group). These results suggest that the decreased O-GlcNAcylation in N2a cells is closely related with H2O2-induced apoptosis.
Fig. 2. Inhibition of O-GlcNAcylation by OSMI-1 enhances cell apoptosis in N2a cells.
a N2a cells were treated with varying concentrations of OSMI-1 (0–100 μM) for 12 h. Global O-GlcNAcylation was examined via Western blot (10% SDS-PAGE), β-actin was used as an internal control. b OSMI-1 treatment led to a dose-dependent decrease of O-GlcNAc levels (n = 3). c Cell counting assay showed that OSMI-1 inhibited the viability of N2a cells in a dose-dependent manner after incubation for 12 h (n = 4). d N2a cells were treated with variant concentrations of TG (0-10 μM) for 12 h. Global O-GlcNAc were examined via Western blot (10% SDS-PAGE), β-actin was used as an internal control. e TG treatment led to a dose-dependent increase of O-GlcNAc levels (n = 4). f Cell counting assay showed that TG has no significant effect on the viability of N2a cells after incubation for 12 h (n = 5). g, h Representative images and quantification analyses of the TUNEL assays detecting the effect of OSMI-1 (60 μM) and TG (5 μM) on the apoptosis of N2a cells treated with either vehicle or H2O2 (n = 4). Scale bar: 20 μm. DATA are expressed as the means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
The protein expression and promoter activities of STAT3 and FOXO1 are decreased by O-GlcNAcylation inhibition
O-GlcNAcylation responds to various environmental stimuli by regulating cellular physiological processes such as gene transcription and translation, and the disruption of O-GlcNAcylation usually results in aberrant regulation of protein expression [49, 50]. JAK-STAT3 and AKT-FOXO pathways have been reported to play important roles in apoptosis and antioxidant effects under oxidative stress [36, 51–53]. To determine whether H2O2-induced downregulation of O-GlcNAcylation mediates the apoptosis by regulating the expression of STAT3 and FOXO1, the protein levels of STAT3 or FOXO1 were assessed in N2a cells after OSMI-1 administration. Treatment of N2a cells with 60 μM OSMI-1 inhibited STAT3 and FOXO1 expression, whereas treatment of N2a cells with 5–50 μM TG increased STAT3 and FOXO1 expression (Fig. 3). Since the phosphorylation status of STAT3 and FOXO1 is closely related to their transcriptional activity and nucleoplasmic localization, the protein levels of phosphorylated STAT3 and FOXO1 were also determined. The results showed that 60 μM OSMI-1 treatment decreased the phosphorylated STAT3 and FOXO1 in the N2a cells, whereas 5–50 μM TG showed the opposite effect (Fig. 3). We speculate that the altered phosphorylation state of STAT3 and FOXO1 may be partly due to changes of their total protein levels.
Fig. 3. The total and phosphorylated protein level of STAT3 and FOXO1 in N2A cells were bidirectionally regulated by OSMI-1 or TG.
a, b Western blotting showed that OSMI-1 (60 μM) treatment inhibited STAT3 expression and phosphorylation (n = 3). c, d Western blotting showed that OSMI-1 (60 μM) treatment inhibited FOXO1 expression and phosphorylation (n = 3). e, f Western blotting showed that TG (5 and 50 μM) treatment promoted the total and phosphorylated STAT3 protein level (n = 3). g, h Western blotting showed that TG (5 and 50 μM) treatment promoted the total and phosphorylated FOXO1 protein level (n = 3). Data are presented as mean ± SEM (n = 3 per group). *P < 0.05, **P < 0.01, ***P < 0.001.
Transcriptional regulation of the mouse Stat3 and Foxo1 genes was assessed using dual luciferase reporter assays. Various truncated fragments upstream from the transcription start site (TSS) of the Stat3 or Foxo1 gene were subcloned into the luciferase reporter vector (Fig. 4a). Luciferase activities were tested in N2a cells at 12 h after OSMI-1 treatment. Compared to DMSO, OSMI-1 treatment significantly reduced the reporter signal of all the truncated fragments (Fig. 4b). In addition to gene transcription, protein expression can also be regulated by mRNA stability. The generation of new mRNA in N2a cells was inhibited by the administration of Actinomycin D, and the changes of the half-life of Stat3 and Foxo1 mRNA were measured. Compared with the control, OSMI-1 treatment did not affect mRNA stability of all these truncations (Fig. 4c). qPCR results showed that OSMI-1 treatment significantly decreased the mRNA level of Foxo1, but increased the mRNA level of Stat3 (Fig. 4d). However, OGT overexpression increased the Stat3 promoter activity and mRNA level without affecting the mRNA stability, whereas had no significant effect on the Foxo1 mRNA (Fig. 4e–h). The above results suggest that besides gene transcription, O-GlcNAcylation may regulate the expression of STAT3 by post-transcriptional mechanism, and the promoter of STAT3 is more sensitive to the up and downregulation of O-GlcNAcylation.
Fig. 4. The promoter activities of STAT3 and FOXO1 were decreased by OSMI-1, while the promoter activities of STAT3 was increased by OGT overexpression.
a Schematic of different truncated fragments of the promoter. Green squares: possible transcription factor binding sequences on the STAT3 and FOXO1 promoters. b OSMI-1 (60 μM) treatment markedly reduced the reporter signal containing different fragments with promoters of Stat3 or Foxo1 (n = 3/4). c qPCR showed that OSMI-1 treatment did not affect mRNA stability of both Stat3 and Foxo1 (n = 3). d OSMI-1 treatment affected mRNA level of both Stat3 and Foxo1 (n = 3). e OGT overexpression increased mRNA levels of ogt (n = 3). f OGT overexpression elevated the promoter activity of Stat3 but had no effect on Foxo1 (n = 3). g qPCR showed that OGT overexpression did not affect mRNA stability of both Stat3 and Foxo1 (n = 3). h OGT overexpression increased mRNA levels of Stat3 without effect on Foxo1 (n = 3). Data are presented as mean ± SEM. **P < 0.01; ***P < 0.001.
Overexpression of STAT3 or FOXO1 attenuated OSMI-1-induced apoptosis in N2a cells
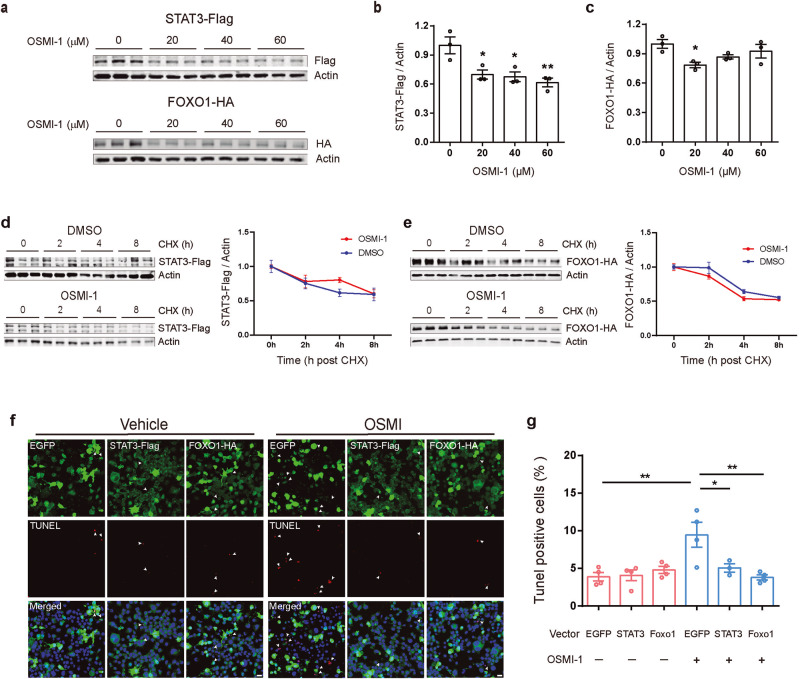
After demonstrating that the protein levels of STAT3 and FOXO1 are regulated by O-GlcNAcylation, we speculated whether such regulation participates in apoptosis induced by O-GlcNAcylation inhibition. STAT3-Flag or FOXO1-HA was transfected into N2a cells to overexpress STAT3 or FOXO1. At 36 h after transfection, OSMI-1 was administered to inhibit intracellular O-GlcNAcylation for another 12 h. Consistent with the results of endogenous STAT3 or FOXO1 expression, OSMI-1 also decreased the exogenous protein levels of STAT3 and FOXO1 (Fig. 5a-c). However, OSMI-1 treatment did not significantly affect the degradation of exogenous STAT3 and FOXO1 overexpressed in N2a cells (Fig. 5d-e). Next, we assessed apoptosis in DMSO or OSMI-1 treated cells. Immunofluorescence analysis showed that OSMI-1-induced apoptosis was significantly attenuated in cells overexpressing STAT3 or FOXO1 compared to the control cells overexpressing EGFP (Fig. 5f, g). Taken together, these results suggest that upregulation of STAT3 or FOXO1 expression exerts protective effect against OSMI-1-induced apoptosis.
Fig. 5. Overexpression of exogenous STAT3 or FOXO1 resists apoptosis induced by OSMI-1.
a–c N2a cells were transfected with STAT3-Flag or FOXO1-HA plasmid for 36 h and subsequently treated with the indicated concentrations of OSMI-1 for 12 h. Western blotting showed that OSMI-1 (20, 40 and 60 μM) treatment inhibited STAT3 and FOXO1 overexpression (n = 3). d, e Western blotting showed that OSMI-1 did not affect the degradation of exogenous protein level of STAT3-Flag (d) and FOXO1-HA (e). f Representative immunofluorescence images showing the co-labeling of TUNEL and anti-HA or anti-Flag antibodies in the N2a cells under DMSO or OSMI-1 treatment. Green, EGFP/ Flag/ HA; red, TUNEL; blue, DAPI; White arrows, co-labeled cells; scale bars, 20 μm. g Overexpression of STAT3 or FOXO1 protein significantly attenuated the apoptosis induced by OSMI-1 treatment without causing any changes in basal apoptosis levels. Data are presented as mean ± SEM (n = 3–4 per group). *P < 0.05; **P < 0.01.
STAT3 and FOXO1 are involved in the protective effect of Thiamet-G (TG) in H2O2-induced apoptosis
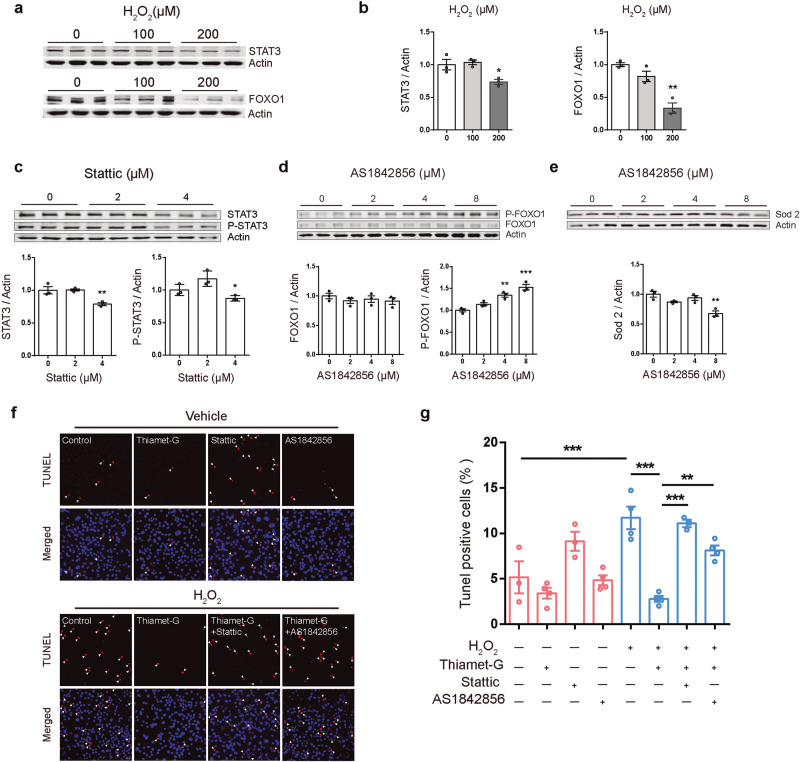
To assess whether apoptosis induced by H2O2 oxidative stress is mediated by STAT3 and FOXO1 pathways, the protein levels of STAT3 and FOXO1 following H2O2 treatment were assessed. H2O2 200 μM treatment significantly downregulated STAT3 and FOXO1 protein levels in N2a cells (Fig. 6a, b). To assess the role of STAT3 and FOXO1 in H2O2-induced apoptosis, the effect of Stattic [54], a STAT3 inhibitor, and AS1842856 [55, 56], a FOXO1 inhibitor were used (Fig. 6c–e). The results showed that 4 μM Stattic treatment significantly inhibited the expression of STAT3 and its phosphorylation (Fig. 6c), while 8 μM AS1842856 treatment significantly promoted the phosphorylation of FOXO1 and reduced the protein level of Sod2, a downstream gene of FOXO1 (Fig. 6d, e). In addition, the anti-apoptotic effect of TG was abolished by Stattic or AS1842856 treatment (Fig. 6f, g) (5.15% ± 1.76% in the control group, 3.40% ± 0.60% in the TG group, 9.13% ± 1.05% in the Stattic group, 4.85% ± 0.55% in the AS1842856 group, 11.70% ± 1.25% in the H2O2 group, 2.78% ± 0.33% in the TG + H2O2 group, 11.09% ± 0.40% in the TG + H2O2+Stattic group, and 8.13% ± 0.53% in the TG + H2O2 + AS1842856 group).
Fig. 6. The protective effect of thiamet-G (TG) against H2O2-induced apoptosis was alleviated by STAT3 or FOXO1 inhibitors.
a, b Western blot showing that STAT3 and FOXO1 protein levels were both significantly downregulated when cells were treated with 200 μM H2O2 (n = 3). c N2a cells were treated with the indicated concentrations of Stattic (0, 2 and 4 μM) for 12 h. Whole-cell extracts were then prepared and the levels of P-STAT3 (S727) and STAT3 were examined by Western blot analysis. β-actin was used as internal control. Stattic 4 μM treatment significantly inhibited the expression of STAT3 (n = 3). d, e N2a cells were treated with the indicated concentrations of AS1842856 (0, 2, 4 and 8 μM) for 12 h. Whole-cell extracts were then prepared and the levels of P-FOXO1 (S256), Foxo1 and Sod2 were examined by Western blot analysis. β-actin was used as internal control. AS1842856 8 μM treatment significantly promoted the phosphorylation of FOXO1 and reduced the protein level of Sod2 (n = 3). f Cells pre-treated with 5 μM Thiamet-G were incubated with 4 μM Stattic or 8 μM AS1842856 for 12 h, then stimulated of 200 μM H2O2 for 12 h. Representative immunofluorescence images showing the apoptosis rate in the N2a cells under different treatment (Red, TUNEL; Blue, DAPI; White arrows indicate TUNEL positive cells; scale bars, 20 μm). g TG resisted H2O2-induced apoptosis, whereas the anti-apoptotic effect of TG is blocked when the specific inhibitors were presented (n = 3/4). Data are presented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
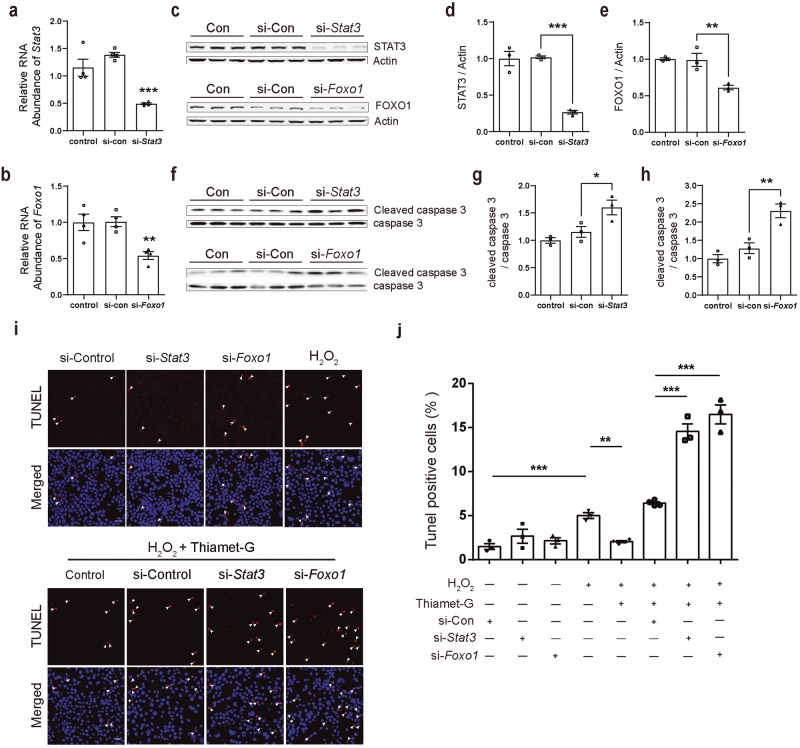
To exclude non-specific effects of inhibitors, siRNAs were used to specifically knock down the endogenous Stat3 and Foxo1 in N2a cells (Fig. 7a–e). Compared with the cells transfected with control siRNA, the expression of pro-apoptotic cleaved caspase 3 protein was significantly increased in cells transfected with Stat3 or Foxo1 siRNA (Fig. 7f, h). TUNEL assay showed that, Stat3- or Foxo1-siRNA did not induce significant apoptosis in N2a cells, whereas blocked the protective effect of TG against H2O2-induced apoptosis (Fig.7i, j) (1.51% ± 0.34% in the si-Control group, 2.68% ± 0.80% in the si-stat3 group, 2.15% ± 0.36% in the si-foxo1 group, 5.00% ± 0.33% in the H2O2 group, 2.07% ± 0.22% in the TG + H2O2 group, 6.43% ± 0.16% in the TG + H2O2 + si-Control group, 14.57% ± 0.86% in the TG + H2O2 + si-Stat3 group and 16.50% ± 1.08% in the TG + H2O2+ si-Foxo1 group). The above results suggest that STAT3 and FOXO1 are involved in the protective effect of TG against H2O2-induced apoptosis.
Fig. 7. Knockdown of Stat3 or Foxo1 promotes caspase 3 activation and abolishes the protective effect of Thiamet-G against H2O2-induced apoptosis.
a, b qPCR showed that specific siRNA inhibits mRNA level ofStat3 and Foxo1 (n = 3). c–e Western blotting showed that Stat3- or Foxo1- siRNA treatment inhibited endogenous STAT3 or FOXO1 protein level (n = 3). f–h Western blotting showed that si-Stat3 or si-Foxo1 treatment promoted caspase 3 activation (n = 3). i, j Representative immunofluorescence images showing the labeling of TUNEL in the N2a cells under different treatment. (red, TUNEL; blue, DAPI; white arrows, co-labeled cells; scale bars, 50 μm). Data are presented as mean ± SEM (n = 3–5 per group). *P < 0.05, **P < 0.01, ***P < 0.001.
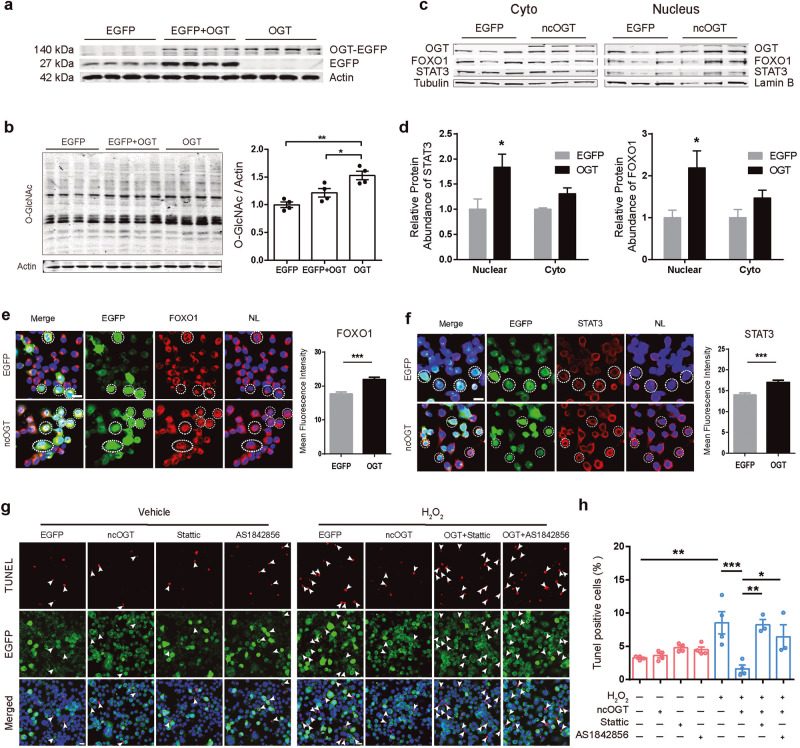
Overexpression of OGT increases STAT3 and FOXO1 in the nucleus and attenuates H2O2-induced apoptosis
To exclude the non-specific effect of TG, OGT was overexpressed by transfection with pEGFP-C1-ncOGT. Overexpression of OGT significantly increased intracellular O-GlcNAc levels compared to the EGFP control group (Fig. 8a, b). The protein levels of STAT3 and FOXO1 were determined by WB and immunostaining. The protein expression of STAT3 and FOXO1 in cytoplasm and nucleus components was analyzed. Western blot showed that the protein levels of STAT3 and FOXO1 were significantly increased in the nucleus component of the OGT-overexpressing cells (Fig. 8c, d). Consistent with the results from TG treatment, the average cellular fluorescent intensity of both STAT3 and FOXO1 were significantly higher in OGT-overexpressing cells, compared with EGFP control group (Fig. 8e, f). The effect of OGT overexpression on H2O2-induced apoptosis was further assessed. The results showed that following H2O2 treatment, the percentage of TUNEL+ signal was decreased in OGT-EGFP positive cells compared with EGFP control cells, indicating that OGT overexpression was able to resist H2O2-induced apoptosis (Fig. 8g, h). In addition, this protective effect against oxidative stress was also blocked when treated with STAT3 or FOXO1 inhibitors (Fig. 8g, h) (3.25% ± 0.14% in the EGFP group, 3.61% ± 0.36% in the OGT group, 4.79% ± 0.30% in the Stattic group, 4.50% ± 0.41% in the AS1842856 group, 8.54% ± 1.68% in the H2O2 group, 1.63% ± 0.55% in the H2O2 + OGT group, 8.26% ± 0.77% in the H2O2 + OGT + Stattic group and 5.98% ± 1.45% in the H2O2 + OGT + AS1842856 group). These above results suggest that STAT3 and FOXO1 are involved in the protective effect of OGT in H2O2-induced apoptosis.
Fig. 8. STAT3 and FOXO1 are involved in the protective effect of OGT against H2O2-induced apoptosis.
a, b N2a cells were transfected with pEGFP-ncOGT or EGFP plasmid for 48 h. Whole-cell extracts were then prepared and the levels of EGFP and O-GlcNAc were examined by Western blot analysis, β-actin was used as internal control. c, d Western blotting showed that ncOGT overexpression increased the STAT3 and FOXO1 protein level in the nucleus extracts (n = 3) e, f Left: Representative immunofluorescence images showing STAT3 and FOXO1 protein level in the N2a cells under different transfections (green, ncOGT/EGFP; red, STAT3/FOXO1; blue, DAPI; white dashed circles, co-labeled cells; scale bars, 20 μm). Right: ncOGT overexpression increased the overall STAT3 and FOXO1 protein level (n = 254 cells). g N2a cells were transfected with pEGFP-ncOGT or EGFP plasmid for 36 h and 4 μM Stattic or 8 μM AS1842856 was added for 12 h, then stimulated with 200 μM H2O2 for another 12 h. Representative immunofluorescence images showing the apoptosis rate in the N2a cells under different treatment (Green, ncOGT/EGFP; Red, TUNEL; Blue, DAPI; White arrows indicate TUNEL positive cells; scale bars, 20 μm). h ncOGT overexpression was able to resist apoptosis promoted by H2O2, and this protective effect against oxidative stress is also blocked when acting in parallel with STAT3 or FOXO1 inhibitors. Data are presented as mean ± SEM (n = 3–4 per group). *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
Previous studies have shown that the level of O-GlcNAcylation in cells is critical for coping with energy imbalance and stress-induced biological response processes, and this regulation may be coordinated by multiple levels of intracellular signaling pathways. However, the molecular bridges of O-GlcNAcylation playing important roles in controlling apoptosis and oxidative stress response remain to be revealed.
Although H2O2 can act as a signaling molecule, elevated levels of H2O2 and excessive ROS production will have deleterious effect on cells [57, 58]. H2O2 is usually considered as a natural inducer of oxidative stress and is involved in a variety of pathological conditions, including cancer, inflammatory diseases, type 2 diabetes, neurodegenerative diseases, and aging [59–61]. Significant ROS production was detected in cells when the H2O2 concentration was only 25 μM [62]. In contrast to superoxide, H2O2 can diffuse into cells and activate many defense systems, including PCD (programmed cell death). Previous studies indicate that different H2O2 concentration treatments triggered distinct cellular effects, lower concentration (0–25 μM) promoting cell growth, while medium concentration (50–150 μM) promoted premature cell death with little effect on cell activity, and high concentration (>200 μM) induced significant apoptosis [62]. In our study, 200 μM H2O2 significantly induced apoptosis and activated the clipping of caspase, decreased cell viability, consistent with previous study.
Oxidative stress is usually accompanied with changes in energy metabolism through adaptive processes [63]. Since a variety of metabolites such as amino acids, fatty acids, nucleotides and glucose metabolites enter the HBP pathway to generate UDP-GlcNAc, the level of O-GlcNAc reflects the energy metabolic state of the cell to some extent. In our study, the O-GlcNAc level of N2a cells was significantly inhibited by H2O2 treatment, suggesting that the metabolic homeostasis of the cells was disrupted. O-GlcNAcylation, as crucial post-translational modifications of proteins in cells, has been shown to play an important regulatory role in the cellular response to oxidative stress. Despite general trends demonstrating global increases in O-GlcNAc levels in response to injury, some stressors decrease O-GlcNAc (for instance, traumahemorrhage [64]), some display complex dynamics in O-GlcNAc cycling (for instance, oxidative stress in cardiomyocytes [65]), and, in some cell models, O-GlcNAc levels appear to increase globally, but protein specific analysis revealed nuanced results (for instance, oxidative stress in mouse embryonic fibroblasts (MEFs) [66]. In the proteomic analysis, detoxification enzymes such as superoxide dismutase and peroxiredoxin 3 were found to be regulated by O-GlcNAcylation [67]. In addition, mitochondria, an important site of energy metabolism, are also a source of ROS production, several studies have shown that O-GlcNAcylation partly regulates mitochondrial bioenergetics and homeostasis, including mitochondrial membrane potential and respiration rate by affecting the expression of respiratory complex components, ATP synthase and various metabolic enzymes, thereby regulating ROS levels [67–71]. Furthermore, mOGT, as one of the spliced variants of OGT, is localized to mitochondria [72]. Recent studies have shown that overexpression of mOGT leads to increased mitochondrial ROS production and it may act as a pro-apoptotic helper molecule to induce apoptosis in cancer cell lines [68]. Thus, intracellular O-GlcNAc levels also reflect the response pattern of cells to oxidative stress.
Apoptosis induced by oxidative stress involves the interaction of numerous signaling pathways. STAT3 and FOXO1 are critical signaling molecules regulated by many physiological cues and pathological stress stimuli that are often associated with oxidative stress and apoptosis. They can not only perform the most classical transcriptional regulatory functions as transcription factors to activate its downstream related targets, such as magnesium superoxide dismutase (MnSOD), catalases and HIF1α [73–76], also have other physiological roles involved in the regulation of oxidative stress. Mitochondrial STAT3 inhibits ROS production by regulating electron transfer complex (ETC) activity and the formation of respiratory chain complexes [77, 78]. In addition, constitutively activated STAT3 participates in endoplasmic reticulum calcium homeostasis and inhibits the intrinsic apoptotic program [79, 80]. It is worth noting that since FOXO1 can also act on pro-apoptotic element (e.g., Bim, Bcl-6, TRAIL and Fas ligands) [81–83], the physiological roles of FOXO1 in ROS homeostasis are complex and largely depend on the cell type and the damage degree [84, 85], alterations in FOXO protein levels synchronously balance cell survival state, but the mechanisms regulating this signaling transition are currently unclear. The relationship between STAT3 and FOXO1 is very complicated, they can not only bind to the nearby region of the same gene promoter to respond to different upstream signals [44], but also can adjust each other’s gene transcription and functions synergistically or antagonistically [41, 86, 87]. In our result, the expression levels of both STAT3 and FOXO1 were significantly downregulated in N2a cells by H2O2 treatment (Fig. 6a, b) and the caspase 3 was cleaved in N2a cells knockdown of endogenous STAT3 or FOXO1 (Fig. 7f–h), indicating that STAT3 and FOXO1 may synergistically regulate the classic apoptotic pathway induced by H2O2. Overexpression of STAT3 and FOXO1 resists apoptosis induced by downregulation of O-GlcNAcylation, while STAT3 and FOXO1 inhibitors or siRNA abolished anti-apoptosis effects of TG treatment and OGT overexpression in H2O2-induced apoptosis, indicating their potential therapeutic roles in oxidative stress-related diseases.
Many data support the hypothesis that OGT and OGA-mediated O-GlcNAcylation cycle responds appropriately to different stimuli through the precise regulation of gene expression. Almost all RNAP II-related transcription factors have O-GlcNAcylation sites, and their O-GlcNAcylation affects their activity, localization, stability and the ability to bind DNA [88]. Besides, OGT can interact with TET protein at the TSS to enhance the O-GlcNAcylation of histones [89], or be targeted to chromatin to regulate the DNA methylation [9, 10] and other epigenetic regulators [5–8]. In our study, the promoter activity of Stat3 and Foxo1 was disturbed by downregulating O-GlcNAcylation, indicating there may be some binding motifs in the promoter regions of Stat3 and Foxo1 to be regulated by O-GlcNAcylation. In addition to regulation of specific transcription factors that bind to target genes, O-GlcNAcylation has also been shown to regulate the functions of RNA polymerases by coordinating with the different types of PTM on the carboxy-terminal structural domain (CTD) of RNAP (RNA polymerase), and thereby affecting the overall transcriptional activity [90, 91]. The altered STAT3 and FOXO1 protein levels in our experiments may also be related to the cross-check between O-GlcNAcylation and other post-translational modifications. Several different post-translational modification sites, such as phosphorylation and ubiquitination were identified on both STAT3 and FOXO1, which affect not only their activation status but also their structural stability [92–97]. Therefore, alteration of STAT3 and FOXO1 induced by changed O-GlcNAcylation level in N2a probably trigger multiple signaling pathways in respond to oxidative stress.
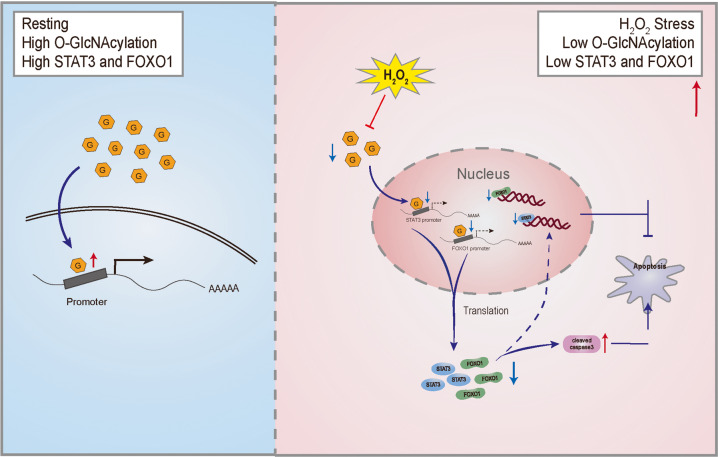
In summary, our results reveal that the O-GlcNAcylation in N2a cells plays an important role in anti-apoptosis response after oxidative stress (Fig. 9). The specific mode in which O-GlcNAcylation acts on the expression and function of STAT3 and FOXO1, and whether there are other synergistic mechanisms to trigger downstream signal transduction remains to be further investigated.
Fig. 9. O-GlcNAcylation-regulated expression of STAT3 and FOXO1 is involved in H2O2-induced apoptosis in N2a cells.
Left (blue): Intracellular protein O-GlcNAcylation level is stable and involved in the regulation of gene expression and signal transduction which is necessary to maintain cellular physiological processes. Right (red): After chronic H2O2 induced oxidative stress, intracellular level of O-GlcNAcylation is downregulated, resulting in decreased promoter activity of STAT3 and FOXO1, which inhibits their protein expression, increases the activation of caspase 3, and thereby exacerbating the redox imbalance and driving apoptosis.
Conclusion
Studies with pharmacological and genetic manipulations of O-GlcNAcylation level in N2a cells indicate that the protein O-GlcNAcylation is essential to the oxidative stress-induced apoptosis via regulating the expression of transcription factors STAT3 and FOXO1.
Supplementary information
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (32222033 to FFW, 32330041 and 31930046 to LM, 32271064 to CYJ, 32270660 to QML, 32171041 to XL), the Science Technology Innovation 2030 Project of China (2021ZD0203500 to FFW and LM, 2021ZD0202104 to XL, 2022ZD0214500 to CYJ), the CAMS Innovation Fund for Medical Sciences (2021-I2M-5-009 to LM and XL).
Author contributions
CCZ designed and performed the experiments. YL, CYJ, QML and XL analyzed the data and provided critical consumables. CCZ drafted the manuscript. LM and FFW supervised the project and revised the manuscript.
Competing interests
The authors declare no competing interests.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-023-01218-z.
References
- 1.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–99. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 2.Alpay M, Backman LR, Cheng X, Dukel M, Kim WJ, Ai L, et al. Oxidative stress shapes breast cancer phenotype through chronic activation of ATM-dependent signaling. Breast Cancer Res Treat. 2015;151:75–87. doi: 10.1007/s10549-015-3368-5. [DOI] [PubMed] [Google Scholar]
- 3.Harris IS, Denicola GM. The complex interplay between antioxidants and ROS in cancer. Trends Cell Biol. 2020;30:440–51. doi: 10.1016/j.tcb.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Bai R, Guo J, Ye XY, Xie Y, Xie T. Oxidative stress: the core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res Rev. 2022;77:101619. doi: 10.1016/j.arr.2022.101619. [DOI] [PubMed] [Google Scholar]
- 5.Chu CS, Lo PW, Yeh YH, Hsu PH, Peng SH, Teng YC, et al. O-GlcNAcylation regulates EZH2 protein stability and function. Proc Natl Acad Sci USA. 2014;111:1355–60. doi: 10.1073/pnas.1323226111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu D, Cai Y, Jin J. Potential coordination role between O-GlcNAcylation and epigenetics. Protein Cell. 2017;8:713–23. doi: 10.1007/s13238-017-0416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrer CM, Lu TY, Bacigalupa ZA, Katsetos CD, Sinclair DA, Reginato MJ. O-GlcNAcylation regulates breast cancer metastasis via SIRT1 modulation of FOXM1 pathway. Oncogene. 2017;36:559–69. doi: 10.1038/onc.2016.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu G, Tao T, Zhang D, Liu X, Qiu H, Han L, et al. O-GlcNAcylation of histone deacetylases 1 in hepatocellular carcinoma promotes cancer progression. Glycobiology. 2016;26:820–33. doi: 10.1093/glycob/cww025. [DOI] [PubMed] [Google Scholar]
- 9.Hardiville S, Hart GW. Nutrient regulation of gene expression by O-GlcNAcylation of chromatin. Curr Opin Chem Biol. 2016;33:88–94. doi: 10.1016/j.cbpa.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X, Qian K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat Rev Mol Cell Biol. 2017;18:452–65. doi: 10.1038/nrm.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma J, Li Y, Hou C, Wu C. O-GlcNAcAtlas: a database of experimentally identified O-GlcNAc sites and proteins. Glycobiology. 2021;31:719–23. doi: 10.1093/glycob/cwab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wulff-Fuentes E, Berendt RR, Massman L, Danner L, Malard F, Vora J, et al. The human O-GlcNAcome database and meta-analysis. Sci Data. 2021;8:25. doi: 10.1038/s41597-021-00810-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gewinner C, Hart G, Zachara N, Cole R, Beisenherz-Huss C, Groner B. The coactivator of transcription CREB-binding protein interacts preferentially with the glycosylated form of Stat5. J Biol Chem. 2004;279:3563–72. doi: 10.1074/jbc.M306449200. [DOI] [PubMed] [Google Scholar]
- 14.Lamarre-Vincent N, Hsieh-Wilson LC. Dynamic glycosylation of the transcription factor CREB: a potential role in gene regulation. J Am Chem Soc. 2003;125:6612–3. doi: 10.1021/ja028200t. [DOI] [PubMed] [Google Scholar]
- 15.Deplus R, Delatte B, Schwinn MK, Defrance M, Mendez J, Murphy N, et al. TET2 and TET3 regulate GlcNAcylation and H3K4 methylation through OGT and SET1/COMPASS. EMBO J. 2013;32:645–55. doi: 10.1038/emboj.2012.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slawson C, Zachara NE, Vosseller K, Cheung WD, Lane MD, Hart GW. Perturbations in O-linked beta-N-acetylglucosamine protein modification cause severe defects in mitotic progression and cytokinesis. J Biol Chem. 2005;280:32944–56. doi: 10.1074/jbc.M503396200. [DOI] [PubMed] [Google Scholar]
- 17.Yoo TY, Mitchison TJ. O-GlcNAc modification of nuclear pore complexes accelerates bidirectional transport. J Cell Biol. 2021;220:e202010141. [DOI] [PMC free article] [PubMed]
- 18.Yang X, Su K, Roos MD, Chang Q, Paterson AJ, Kudlow JE. O-linkage of N-acetylglucosamine to Sp1 activation domain inhibits its transcriptional capability. Proc Natl Acad Sci USA. 2001;98:6611–6. doi: 10.1073/pnas.111099998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toleman CA, Schumacher MA, Yu SH, Zeng W, Cox NJ, Smith TJ, et al. Structural basis of O-GlcNAc recognition by mammalian 14-3-3 proteins. Proc Natl Acad Sci USA. 2018;115:5956–61. doi: 10.1073/pnas.1722437115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pathak S, Borodkin VS, Albarbarawi O, Campbell DG, Ibrahim A, van Aalten DM. O-GlcNAcylation of TAB1 modulates TAK1-mediated cytokine release. EMBO J. 2012;31:1394–404. doi: 10.1038/emboj.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarrant MK, Rho HS, Xie Z, Jiang YL, Gross C, Culhane JC, et al. Regulation of CK2 by phosphorylation and O-GlcNAcylation revealed by semisynthesis. Nat Chem Biol. 2012;8:262–9. doi: 10.1038/nchembio.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zachara NE, O’Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem. 2004;279:30133–42. doi: 10.1074/jbc.M403773200. [DOI] [PubMed] [Google Scholar]
- 23.Zachara NE, Hart GW. O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim Biophys Acta. 2004;1673:13–28. doi: 10.1016/j.bbagen.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Yi W, Clark PM, Mason DE, Keenan MC, Hill C, Goddard WR, et al. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science. 2012;337:975–80. doi: 10.1126/science.1222278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao X, Duan X, Mao W, Li X, Li Z, Li Q, et al. O-GlcNAcylation of G6PD promotes the pentose phosphate pathway and tumor growth. Nat Commun. 2015;6:8468. doi: 10.1038/ncomms9468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levy DE, Darnell JJ. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–62. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 27.Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109:1139–42. [DOI] [PMC free article] [PubMed]
- 28.Fukada T, Hibi M, Yamanaka Y, Takahashi-Tezuka M, Fujitani Y, Yamaguchi T, et al. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity. 1996;5:449–60. [DOI] [PubMed]
- 29.Ho KK, Myatt SS, Lam EW. Many forks in the path: cycling with FoxO. Oncogene. 2008;27:2300–11. doi: 10.1038/onc.2008.23. [DOI] [PubMed] [Google Scholar]
- 30.Kim DH, Park MH, Lee EK, Choi YJ, Chung KW, Moon KM, et al. The roles of FoxOs in modulation of aging by calorie restriction. Biogerontology. 2015;16:1–14. doi: 10.1007/s10522-014-9519-y. [DOI] [PubMed] [Google Scholar]
- 31.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–21. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 32.Nakae J, Biggs WR, Kitamura T, Cavenee WK, Wright CV, Arden KC, et al. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet. 2002;32:245–53. doi: 10.1038/ng890. [DOI] [PubMed] [Google Scholar]
- 33.Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, et al. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168:5024–31. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol. 2000;20:8969–82. doi: 10.1128/MCB.20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–9. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 36.Klotz LO, Sanchez-Ramos C, Prieto-Arroyo I, Urbanek P, Steinbrenner H, Monsalve M. Redox regulation of FoxO transcription factors. Redox Biol. 2015;6:51–72. doi: 10.1016/j.redox.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang F, Tong Q. SIRT2 suppresses adipocyte differentiation by deacetylating FOXO1 and enhancing FOXO1’s repressive interaction with PPARgamma. Mol Biol Cell. 2009;20:801–8. doi: 10.1091/mbc.e08-06-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salih DA, Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 2008;20:126–36. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X, Greer C, Secombe J. KDM5 interacts with Foxo to modulate cellular levels of oxidative stress. PLoS Genet. 2014;10:e1004676. doi: 10.1371/journal.pgen.1004676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Araujo J, Breuer P, Dieringer S, Krauss S, Dorn S, Zimmermann K, et al. FOXO4-dependent upregulation of superoxide dismutase-2 in response to oxidative stress is impaired in spinocerebellar ataxia type 3. Hum Mol Genet. 2011;20:2928–41. doi: 10.1093/hmg/ddr197. [DOI] [PubMed] [Google Scholar]
- 41.Yang G, Lim CY, Li C, Xiao X, Radda GK, Li C, et al. FoxO1 inhibits leptin regulation of pro-opiomelanocortin promoter activity by blocking STAT3 interaction with specificity protein 1. J Biol Chem. 2009;284:3719–27. doi: 10.1074/jbc.M804965200. [DOI] [PubMed] [Google Scholar]
- 42.Xu M, Huang J, Zhu F, Shen K, Liu F, Deng X. FOXO1 inhibits FSL-1 regulation of integrin beta6 by blocking STAT3 binding to the integrin beta6 gene promoter. Front Cell Infect Microbiol. 2022;12:998693. doi: 10.3389/fcimb.2022.998693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oh HM, Yu CR, Golestaneh N, Amadi-Obi A, Lee YS, Eseonu A, et al. STAT3 protein promotes T-cell survival and inhibits interleukin-2 production through up-regulation of Class O Forkhead transcription factors. J Biol Chem. 2011;286:30888–97. doi: 10.1074/jbc.M111.253500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kitamura T, Feng Y, Kitamura YI, Chua SJ, Xu AW, Barsh GS, et al. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–40. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- 45.Abell K, Bilancio A, Clarkson RW, Tiffen PG, Altaparmakov AI, Burdon TG, et al. Stat3-induced apoptosis requires a molecular switch in PI3K subunit composition. Nat Cell Biol. 2005;7:392–8. [DOI] [PubMed]
- 46.Xu Q, Briggs J, Park S, Niu G, Kortylewski M, Zhang S, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24:5552–60. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- 47.Ochodnicka-Mackovicova K, Bahjat M, Bloedjes TA, Maas C, de Bruin AM, Bende RJ, et al. NF-kappaB and AKT signaling prevent DNA damage in transformed pre-B cells by suppressing RAG1/2 expression and activity. Blood. 2015;126:1324–35. doi: 10.1182/blood-2015-01-621623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 2011;80:825–58. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brimble S, Wollaston-Hayden EE, Teo CF, Morris AC, Wells L. The role of the O-GlcNAc modification in regulating eukaryotic gene expression. Curr Signal Transduct Ther. 2010;5:12–24. doi: 10.2174/157436210790226465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeidan Q, Wang Z, De Maio A, Hart GW. O-GlcNAc cycling enzymes associate with the translational machinery and modify core ribosomal proteins. Mol Biol Cell. 2010;21:1922–36. doi: 10.1091/mbc.e09-11-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fathi N, Rashidi G, Khodadadi A, Shahi S, Sharifi S. STAT3 and apoptosis challenges in cancer. Int J Biol Macromol. 2018;117:993–1001. doi: 10.1016/j.ijbiomac.2018.05.121. [DOI] [PubMed] [Google Scholar]
- 52.Baek SH, Ko JH, Lee H, Jung J, Kong M, Lee JW, et al. Resveratrol inhibits STAT3 signaling pathway through the induction of SOCS-1: role in apoptosis induction and radiosensitization in head and neck tumor cells. Phytomedicine. 2016;23:566–77. [DOI] [PubMed]
- 53.Guan XH, Liu XH, Hong X, Zhao N, Xiao YF, Wang LF, et al. CD38 deficiency protects the heart from ischemia/reperfusion injury through activating SIRT1/FOXOs-mediated antioxidative stress pathway. Oxid Med Cell Longev. 2016;2016:7410257. doi: 10.1155/2016/7410257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol. 2006;13:1235–42. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 55.Nagashima T, Shigematsu N, Maruki R, Urano Y, Tanaka H, Shimaya A, et al. Discovery of novel forkhead box O1 inhibitors for treating type 2 diabetes: improvement of fasting glycemia in diabetic db/db mice. Mol Pharmacol. 2010;78:961–70. [DOI] [PubMed]
- 56.Qi Y, Chen S, Lu Y, Zhang Z, Wang S, Chen N, et al. Grape seed proanthocyanidin extract ameliorates ionizing radiation-induced hematopoietic stem progenitor cell injury by regulating Foxo1 in mice. Free Radic Biol Med. 2021;174:144–56. doi: 10.1016/j.freeradbiomed.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 57.Gerber PA, Rutter GA. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid Redox Signal. 2017;26:501–18. doi: 10.1089/ars.2016.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spahis S, Delvin E, Borys JM, Levy E. Oxidative stress as a critical factor in nonalcoholic fatty liver disease pathogenesis. Antioxid Redox Signal. 2017;26:519–41. doi: 10.1089/ars.2016.6776. [DOI] [PubMed] [Google Scholar]
- 59.Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6:456–80. doi: 10.4239/wjd.v6.i3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stadtman ER. Role of oxidant species in aging. Curr Med Chem. 2004;11:1105–12. doi: 10.2174/0929867043365341. [DOI] [PubMed] [Google Scholar]
- 61.Ortiz GG, Pacheco MF, Mireles-Ramirez M, Flores-Alvarado LJ, Gonzalez-Usigli H, Sanchez-Gonzalez VJ, et al. Oxidative stress: love and hate history in central nervous system. Adv Protein Chem Struct Biol. 2017;108:1–31. doi: 10.1016/bs.apcsb.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 62.Yang J, Chatterjee-Kishore M, Staugaitis SM, Nguyen H, Schlessinger K, Levy DE, et al. Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation. Cancer Res. 2005;65:939–47. doi: 10.1158/0008-5472.939.65.3. [DOI] [PubMed] [Google Scholar]
- 63.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. [DOI] [PubMed]
- 64.Zou L, Yang S, Hu S, Chaudry IH, Marchase RB, Chatham JC. The protective effects of PUGNAc on cardiac function after trauma-hemorrhage are mediated via increased protein O-GlcNAc levels. Shock. 2007;27:402–8. doi: 10.1097/01.shk.0000245031.31859.29. [DOI] [PubMed] [Google Scholar]
- 65.Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, et al. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation. 2008;117:1172–82. doi: 10.1161/CIRCULATIONAHA.107.730515. [DOI] [PubMed] [Google Scholar]
- 66.Lee A, Miller D, Henry R, Paruchuri VD, O’Meally RN, Boronina T, et al. Combined antibody/lectin enrichment identifies extensive changes in the O-GlcNAc sub-proteome upon oxidative stress. J Proteome Res. 2016;15:4318–36. doi: 10.1021/acs.jproteome.6b00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma J, Liu T, Wei AC, Banerjee P, O’Rourke B, Hart GW. O-GlcNAcomic profiling identifies widespread O-linked beta-N-acetylglucosamine modification (O-GlcNAcylation) in oxidative phosphorylation system regulating cardiac mitochondrial function. J Biol Chem. 2015;290:29141–53. doi: 10.1074/jbc.M115.691741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jozwiak P, Ciesielski P, Zakrzewski PK, Kozal K, Oracz J, Budryn G, et al. Mitochondrial O-GlcNAc transferase interacts with and modifies many proteins and its up-regulation affects mitochondrial function and cellular energy homeostasis. Cancers. 2021;13:2956. [DOI] [PMC free article] [PubMed]
- 69.Akinbiyi EO, Abramowitz LK, Bauer BL, Stoll M, Hoppel CL, Hsiao CP, et al. Blocked O-GlcNAc cycling alters mitochondrial morphology, function, and mass. Sci Rep. 2021;11:22106. doi: 10.1038/s41598-021-01512-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ngoh GA, Watson LJ, Facundo HT, Jones SP. Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino Acids. 2011;40:895–911. doi: 10.1007/s00726-010-0728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dontaine J, Bouali A, Daussin F, Bultot L, Vertommen D, Martin M, et al. The intra-mitochondrial O-GlcNAcylation system rapidly modulates OXPHOS function and ROS release in the heart. Commun Biol. 2022;5:349. doi: 10.1038/s42003-022-03282-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Love DC, Kochan J, Cathey RL, Shin SH, Hanover JA. Mitochondrial and nucleocytoplasmic targeting of O-linked GlcNAc transferase. J Cell Sci. 2003;116:647–54. doi: 10.1242/jcs.00246. [DOI] [PubMed] [Google Scholar]
- 73.Higuchi M, Dusting GJ, Peshavariya H, Jiang F, Hsiao ST, Chan EC, et al. Differentiation of human adipose-derived stem cells into fat involves reactive oxygen species and Forkhead box O1 mediated upregulation of antioxidant enzymes. Stem Cells Dev. 2013;22:878–88. doi: 10.1089/scd.2012.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rached MT, Kode A, Xu L, Yoshikawa Y, Paik JH, Depinho RA, et al. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab. 2010;11:147–60. doi: 10.1016/j.cmet.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen L, Fu L, Kong X, Xu J, Wang Z, Ma X, et al. Jumonji domain-containing protein 2B silencing induces DNA damage response via STAT3 pathway in colorectal cancer. Br J Cancer. 2014;110:1014–26. doi: 10.1038/bjc.2013.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jung CH, Kim EM, Song JY, Park JK, Um HD. Mitochondrial superoxide dismutase 2 mediates gamma-irradiation-induced cancer cell invasion. Exp Mol Med. 2019;51:1–10. doi: 10.1038/s12276-019-0207-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T, et al. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–7. [DOI] [PMC free article] [PubMed]
- 78.Yang R, Lirussi D, Thornton TM, Jelley-Gibbs DM, Diehl SA, Case LK, et al. Mitochondrial Ca2+ and membrane potential, an alternative pathway for Interleukin 6 to regulate CD4 cell effector function. Elife. 2015;4:e06376. [DOI] [PMC free article] [PubMed]
- 79.Yang R, Rincon M. Mitochondrial Stat3, the need for design thinking. Int J Biol Sci. 2016;12:532–44. doi: 10.7150/ijbs.15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Avalle L, Camporeale A, Morciano G, Caroccia N, Ghetti E, Orecchia V, et al. STAT3 localizes to the ER, acting as a gatekeeper for ER-mitochondrion Ca2+ fluxes and apoptotic responses. Cell Death Differ. 2019;26:932–42. [DOI] [PMC free article] [PubMed]
- 81.Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EB, et al. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. [DOI] [PubMed]
- 82.Modur V, Nagarajan R, Evers BM, Milbrandt J. FOXO proteins regulate tumor necrosis factor-related apoptosis inducing ligand expression. Implications for PTEN mutation in prostate cancer. J Biol Chem. 2002;277:47928–37. doi: 10.1074/jbc.M207509200. [DOI] [PubMed] [Google Scholar]
- 83.Park SJ, Sohn HY, Yoon J, Park SI. Down-regulation of FoxO-dependent c-FLIP expression mediates TRAIL-induced apoptosis in activated hepatic stellate cells. Cell Signal. 2009;21:1495–503. doi: 10.1016/j.cellsig.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 84.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–39. [DOI] [PubMed]
- 85.Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–23. [DOI] [PMC free article] [PubMed]
- 86.Ma W, Fuentes G, Shi X, Verma C, Radda GK, Han W. FoxO1 negatively regulates leptin-induced POMC transcription through its direct interaction with STAT3. Biochem J. 2015;466:291–8. doi: 10.1042/BJ20141109. [DOI] [PubMed] [Google Scholar]
- 87.Oh HM, Yu CR, Dambuza I, Marrero B, Egwuagu CE. STAT3 protein interacts with Class O Forkhead transcription factors in the cytoplasm and regulates nuclear/cytoplasmic localization of FoxO1 and FoxO3a proteins in CD4+ T cells. J Biol Chem. 2012;287:30436–43. [DOI] [PMC free article] [PubMed]
- 88.Andrali SS, Qian Q, Ozcan S. Glucose mediates the translocation of NeuroD1 by O-linked glycosylation. J Biol Chem. 2007;282:15589–96. doi: 10.1074/jbc.M701762200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mendel M, Chen KM, Homolka D, Gos P, Pandey RR, Mccarthy AA, et al. Methylation of structured RNA by the m6A writer METTL16 is essential for mouse embryonic development. Mol Cell. 2018;71:986–1000. [DOI] [PMC free article] [PubMed]
- 90.Lewis BA, Burlingame AL, Myers SA. Human RNA polymerase II promoter recruitment in vitro is regulated by O-Linked N-Acetylglucosaminyltransferase (OGT) J Biol Chem. 2016;291:14056–61. doi: 10.1074/jbc.M115.684365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ranuncolo SM, Ghosh S, Hanover JA, Hart GW, Lewis BA. Evidence of the involvement of O-GlcNAc-modified human RNA polymerase II CTD in transcription in vitro and in vivo. J Biol Chem. 2012;287:23549–61. doi: 10.1074/jbc.M111.330910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matsuzaki H, Daitoku H, Hatta M, Tanaka K, Fukamizu A. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc Natl Acad Sci USA. 2003;100:11285–90. doi: 10.1073/pnas.1934283100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang F, Su K, Yang X, Bowe DB, Paterson AJ, Kudlow JE. O-GlcNAc modification is an endogenous inhibitor of the proteasome. Cell. 2003;115:715–25. [DOI] [PubMed]
- 94.Housley MP, Rodgers JT, Udeshi ND, Kelly TJ, Shabanowitz J, Hunt DF, et al. O-GlcNAc regulates FoxO activation in response to glucose. J Biol Chem. 2008;283:16283–92. doi: 10.1074/jbc.M802240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Housley MP, Udeshi ND, Rodgers JT, Shabanowitz J, Puigserver P, Hunt DF, et al. A PGC-1alpha-O-GlcNAc transferase complex regulates FoxO transcription factor activity in response to glucose. J Biol Chem. 2009;284:5148–57. doi: 10.1074/jbc.M808890200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wen Z, Zhong Z, Darnell JJ. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–50. [DOI] [PubMed]
- 97.Wong GL, Manore SG, Doheny DL, Lo HW. STAT family of transcription factors in breast cancer: pathogenesis and therapeutic opportunities and challenges. Semin Cancer Biol. 2022;86:84–106. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.