Abstract
Among the numerous complications of diabetes mellitus, diabetic wounds seriously affect patients’ quality of life and result in considerable psychological distress. Promoting blood vessel regeneration in wounds is a crucial step in wound healing. Lonicerin (LCR), a bioactive compound found in plants of the Lonicera japonica species and other honeysuckle plants, exhibits anti-inflammatory and antioxidant activities, and it recently has been found to alleviate ulcerative colitis by enhancing autophagy. In this study we investigated the efficacy of LCR in treatment of diabetic wounds and the underlying mechanisms. By comparing the single-cell transcriptomic data from healing and non-healing states in diabetic foot ulcers (DFU) of 5 patients, we found that autophagy and SIRT signaling activation played a crucial role in mitigating inflammation and oxidative stress, and promoting cell survival in wound healing processes. In TBHP-treated human umbilical vein endothelial cells (HUVECs), we showed that LCR alleviated cell apoptosis, and enhanced the cell viability, migration and angiogenesis. Furthermore, we demonstrated that LCR treatment dose-dependently promoted autophagy in TBHP-treated HUVECs by upregulating Sirt1 expression, and exerted its anti-apoptotic effect through the Sirt1-autophagy axis. Knockdown of Sirt1 significantly decreased the level of autophagy, and mitigated the anti-apoptotic effect of LCR. In a STZ-induced diabetic rat model, administration of LCR significantly promoted wound healing, which was significantly attenuated by Sirt1 knockdown. This study highlights the potential of LCR as a therapeutic agent for the treatment of diabetic wounds and provides insights into the molecular mechanisms underlying its effects.
Keywords: diabetic wounds, wound healing, lonicerin, oxidative stress, angiogenesis, autophagy, Sirt1
Introduction
Diabetes mellitus (DM) is a metabolic disorder characterized by high blood sugar levels, and it represents a significant global health concern with an increasing number of diabetic patients worldwide. The projected number of diabetic individuals is expected to exceed 629 million by 2045 [1]. One of the most severe complications of diabetes is the development of persistent non-healing wounds, which can lead to disability and death, causing reduced quality of life and substantial healthcare costs [2–4]. Diabetic wounds can significantly affect patients’ quality of life and result in considerable psychological distress [5, 6]. Current treatments include dressing changes and surgical debridement, but results have not been satisfactory [7, 8]. Normal wound healing is a complex biological process, and it requires the coordinated actions of multiple cells, cytokines and extracellular matrix (ECM) [9]. However, poor wound healing in diabetes is associated with pathological processes such as hypoxia [10], impaired angiogenesis [11], reactive oxygen species (ROS) damage [12] and neuropathy [13], although the underlying mechanisms are still not fully understood.
Cutaneous wound healing is a multifaceted process that repairs and regenerates tissue structure and function. This process is traditionally classified into four interrelated and overlapping phases: coagulation, inflammation, proliferation, and remodeling [14, 15]. The process of angiogenesis, involving the regeneration of blood vessels, actively contributes to the entire process of wound healing, providing significant assistance [16, 17]. During the healing process, the microvascular network, which comprises angiogenic capillaries, provides the wound site with the nutrition and oxygen necessary for tissue repair [18]. However, oxygen is utilized for energy production via oxidative phosphorylation, it also results in the production of reactive oxygen species (ROS), which can lead to oxidative stress and further injury to the endothelial cells at the wound site [19–21]. Evidence suggests that diabetes aggravates oxidative stress in wound healing and delays wound healing [22, 23]. Therefore, protecting endothelial cells from oxidative stress-induced damage may be a promising therapeutic target for expediting the healing of the cutaneous wound.
In recent years, therapeutic approaches aimed at targeting angiogenesis have become the prevailing strategy for treating diabetic wound [24–26]. Within these approaches, strategies that focus on alleviating oxidative stress of endothelial cells, reducing endothelial cell apoptosis, and promoting angiogenesis have demonstrated a significant therapeutic effect. Therefore, it is crucial to explore ways to further improve vascular endothelial cell homeostasis, alleviate oxidative stress damage and reduce endothelial cell apoptosis in order to enhance the treatment of diabetic wounds.
Autophagy is an intracellular degradation process that sequesters cytosolic constituents, misfolded proteins, and damaged organelles into double-membrane vesicles, which are then delivered to the lysosome for degradation [27]. The conversion of LC3B-I to LC3B-II by conjugation with phosphatidylethanolamine is critical for cargo recruitment, autophagosome biogenesis, and completion [28, 29], with the LC3B-II/LC3B-I ratio serving as an indicator of autophagic activity. P62, which binds to LC3-II and is incorporated into autophagosomes, is degraded during the autophagy process and thus is negatively correlated with autophagy [30]. Accumulating evidence has demonstrated that autophagy is essential for maintaining cellular homeostasis and improving survival, and it has been implicated in the pathogenesis of various diseases [31, 32], including cardiovascular disease [31], liver disease [33] and kidney disease [34, 35]. Meanwhile, a large number of studies have shown that the delayed healing of diabetic wounds is related to the autophagy disorder of the wounds. One study found that a hyperglycemic environment inhibits autophagy in keratinocytes, delaying the healing of diabetic wound [36]. Advanced glycation end products (AGEs), which are end products of non-enzymatic reaction between amino and aldehyde groups of proteins, nucleotides, and nucleic acids under continuous hyperglycemia [37], polarize macrophages to an M1 phenotype through autophagy activation, leading to refractory wounds [38]. Studies have shown that keratinocyte autophagy can promote wound healing in mice [39]. Recent studies have found that certain materials can inhibit the production of reactive oxygen species (ROS) by activating autophagy, thus shielding cells from damage caused by oxidative stress and facilitating skin wound healing, indicating that the regulation of autophagy can promote wound healing [40]. Overall, the potential for promoting autophagy flow to facilitate the recovery of diabetic wounds holds significant promise for future applications.
Silent information regulator 1 (Sirt1) is a highly conserved nicotinamide adenine dinucleotide (NAD+) dependent histone deacetylase and has been implicated in a variety of intracellular signals, such as senescence, inflammation, apoptosis, and autophagy [41–43]. Studies have shown that diabetic rats and nerve cells exposed to high levels of glucose exhibit reduced SIRT1 expression, which is associated with reduced mitochondrial biosynthesis and autophagy [44]. Previous studies have shown that Sirt1 regulates mitochondrial function and biogenesis, oxidative stress, inflammation, apoptosis, and cell aging [45]. Furthermore, patients with diabetes have been found to exhibit low expression of Sirt1 and severe oxidative stress in their skin tissue, indicating that the disorder of Sirt1 promoted the onset of diabetes and its complications. The latest evidence shows that Sirt1 can accelerate wound healing in diabetic mice by promoting angiogenesis, which is achieved by protecting vascular endothelial cells from oxidative stress damage [46].
Natural sources have long been utilized in clinical settings to discover therapeutic agents, making them an invaluable tool in the field of medicine [47]. Lonicerin, a flavonoid glycoside isolated from Lonicera japonica Thunb, has been traditionally used in East-Asian historical prescriptions for treating inflammatory and infectious diseases, with its flower bud being the most frequently recorded source [48]. A bunch of studies have demonstrated that lonicerin is one of the predominant constituents that possess anti-inflammatory and immunomodulatory activities [49–51]. However, the effect of lonicerin on wound healing under diabetes and the specific mechanism of lonicerin’s effect has not been covered by the current research.
Our aim is to delve into the pivotal targets involved in wound healing and endeavor to achieve effective treatment through the utilization of natural compounds. Initially, employing single-cell data analysis, we unveil the crucial role of endothelial cells in the intricate process of wound healing. Through the utilization of the PCA algorithm, we have confirmed the significant association between the autophagy levels, SIRT signaling within endothelial cells and the healing outcomes of the wounds. Subsequently, upon administering LCR treatment to diabetic wound rats, we ascertain its ability to alleviate oxidative stress and mitigate cellular apoptosis by facilitating the Sirt1-autophagy pathway, thereby expediting the healing process of the wound in vivo and in vitro. In aggregate, our research sheds light on the critical targets and pathways implicated in the diabetic wound healing process, concurrently providing an efficacious avenue for treatment.
Materials and methods
Confocal microscopy scanning
Laser confocal experiments were acquired using a Zeiss LSM 800 confocal microscope equipped with a 63 × 1.4 numerical aperture oil objective. Airyscan microscopy was performed using a Zeiss LSM 800 confocal microscope, equipped with Plan-Apochromat 63×1.4 numerical aperture oil objective and pixel size of 8.7 nm. Images were subjected to post-acquisition Airyscan processing. Image acquisition and processing were performed with Zen Blue software.
Reagents and antibodies
Lonicerin (purity >99%) was purchased from MedChemExpress (HY-N4136, NJ, USA) and dissolved in DMSO (D8371, Solarbio Life Science, Beijing, China). TBHP was purchased from Sigma-Aldrich (458139, WI, USA). Primary antibodies against BCL-2 (#3498), BAX (#14796), Cleave caspase-3 (#9661), Caspase-3 (#9662), ATG5 (#12994) and β-actin (#3700) were purchased from Cell Signaling Technologies (MA, USA). Primary antibodies against P62 (ab109012), Sirt1 (ab110304), Beclin1 (ab302669), PARP1 (ab191217), Cleaved PARP1 (ab32064) and LC3B (ab192890) were acquired from Abcam (Cambridge, UK). LAMP1 (21997-1-AP) was purchased from Proteintech (Rosemont, USA).
Cell culture and treatment protocols
Human umbilical vein endothelial cells (HUVECs) were obtained from Procell Life Science & Technology (CP-H082, Wuhan, China) and grown in DMEM/F12 supplemented with 10% heat-inactivated FBS and 1% penicillin and streptomycin at 37 °C in a humidified atmosphere of 5% CO2. HUVECs were treated with TBHP solution (IC50: 35 μM) for 24 h to mimic oxidative stress damage [52].
To examine the effect of LCR on HUVECs viability, cells were treated with different concentrations of LCR for 24 h. After the treatment concentration of TBHP was determined, cells were pre-treated with TBHP for 24 h before different concentrations of LCR (20, 40, and 80 μM) addition to investigate the effects of LCR on cell apoptosis and dysfunction.
Cell viability assay
Cell viability was determined using a Cell Counting Kit-8 (C0038, Beyotime Biotechnology, Shanghai, China) assay according to the manufacturer’s protocol. HUVECs were seeded in 96-well plates (3 × 103 cell/well) and incubated with DMEM/F12 at 37 °C for 24 h. Then, the cells were treated with various concentrations of lonicerin. After 24 h of treatment, the cells were washed with PBS. Then, 100 μL of non-FBS (DMEM/F12) containing 10 μL of Cell Counting Kit-8 solution was added to each well, and the plate was incubated for an additional 2 h. The absorbance of the wells at 450 nm was then measured by a microplate reader (Thermo Fisher, MA, USA).
Western blotting
Western blotting was performed using routine protocols. Total protein was extracted from treated HUVECs with RIPA with 1 mM PMSF, and the protein concentrations were measured using a BCA Protein Assay Kit (Beyotime, Shanghai, China). Then, a total of 40 μg protein was divided with sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) and transferred onto a polyvinylidene difluoride membrane (Bio-Rad, USA). After 1.5 h blocking with 5% nonfat milk, membranes were incubated with primary antibodies solutions overnight at 4 °C: BCL-2 (1:1000), BAX (1:1000), PARP1 (1:1000), Cleaved PARP1 (1:1000), Caspase 3 (1:500), Cleave-caspase 3 (1:500), β-actin (1:3000), P62 (1:1000), Beclin1 (1:800), LC3B (1:800), ATG5 (1:1000) and Sirt1 (1:1000) at 4 °C. Subsequently, the membranes were incubated with the corresponding secondary antibodies for 2 h at room temperature. The protein bands were then visualized using a ChemiDicTM XRS + Imaging System (Bio-Rad, USA). Eventually, the software of Image Lab (Bio-Rad, USA) was used to analyze the expression of each group of proteins.
Quantitative real-time PCR assay
After the cells were treated according to the experimental design, total RNA was extracted from NPCs with TRIzol reagent (Invitrogen, Grand Island, NY, USA). CDNA (MBI Fermantas, Germany) was synthesized with 1 μg of total RNA. Specific primers for each gene are shown in Supplementary Table S1. Quantitative real-time PCR (quantitative real-time PCR, qPCR) uses a reaction volume of 10 μL. The qPCR parameters are 10 min 95 °C, 15 s 95 °C, 1 min 60 °C, a total of 40 cycles. The reaction was performed using the CFX96 Real-Time PCR system (BioRad Laboratories, CA, USA). The cycle threshold (Ct) value was collected and normalized to the GAPDH level. The mRNA level relative to each target gene is calculated using the 2−ΔΔCt method.
TUNEL staining
The TUNEL staining is used for measuring apoptotic DNA fragmentation. HUVECs were seeded on slides in a six-well plate and allowed to attach for 24 h. According to the experimental design, after a certain treatment, the HUVECs were washed gently with PBS three times and fixed with freshly prepared 4% paraformaldehyde for 30 min before being incubated with 0.1% Triton X-100 for 5 min. Then, the cells were stained with the TUNEL Apoptosis Detection Kit (Yeasen Biochemical, Shanghai, China) for 30 min at 37 °C according to the manufacturer’s instructions, and the nuclei were stained with DAPI. The images were captured by a Nikon ECLIPSE Ti microscope (Nikon, Tokyo, Japan).
Transwell assay
HUVEC migration was assessed using transwell apparatus (Costar, Cambridge, MA, USA) with an 8-μm-pore polycarbonate membrane Boyden chamber. HUVECs were treated with TBHP and LCR as described above. Then, the cells were detached using trypsin/EDTA, centrifuged, and re-suspended as single-cell solutions. In total, 4 × 104 cells in 200 μL of non-FBS containing DMEM/F12 were seeded on a transwell apparatus, and 700 μL of culture medium containing 1% FBS was added to the lower chamber. Following incubation of the cells for 16 h at 37 °C in a 5% CO2 incubator, the membranes were washed with PBS three times and fixed with 4% paraformaldehyde. The transwell apparatus was then stained using a hematoxylin solution, and the cells on the top surface of the insert were wiped away with cotton wool. Cells that migrated to the bottom surface of the insert were counted manually in three random microscopic fields (100×).
Scratch wound healing assay
For scratch wound healing assay, HUVECs were seeded in 6-well plates, and after a certain treatment, they were cultured and reached a density of 90%. A sterile pipette tip was usded to create a clean scratch in the center of the cell layer. Photographs were taken under the microscope after 24 h, and the cell migration distance was estimated by ImageJ software.
Tube formation assay
HUVEC tube formation was carried out on Matrigel-coated chamber slides using an in vitro angiogenesis assay kit (Chemicon, CA, USA). An ECMatrix gel solution was mixed with ECMatrix diluent buffer and placed in a μ-Slide plate at 37 °C for 1 h to allow the matrix solution to solidify. HUVECs were pre-treated as described above and then harvested with trypsin/EDTA. Then, HUVECs (2 × 104 cells) were seeded on the layer of previously polymerized Matrigel. The Matrigel culture was incubated at 37 °C for 6 h. Tube formation was evaluated using a phase contrast microscope (40×) and quantified by counting the number of connected cells in randomly selected fields of each well.
Single-cell bioinformatics analysis
The single-cell transcriptomic data were obtained from the GEO public database (GSE165816). Single-cell analysis was performed using the R programming language. Standardized analysis was performed using the Seurat package. The t-Distributed Stochastic Neighbor Embedding (t-SNE) function was employed to cluster the single cells into seven groups based on the original reference [53]: Fibroblasts, Keratinocytes, Endothelial cells, Pericytes, Immune cells, and others. Subsequently, the Endothelial cells were further clustered based on their autophagy status. The R package Cellchat was utilized to analyze the intercellular interactions between different cell types. Gene ontology enrichment analysis was conducted employing the R package Clusterprofile. Pseudotime analysis was carried out utilizing the R package Monocle.
Bioinformatic analysis
To calculate the metabolism score, we identified genes of interest in each metabolism pathway from the Kyoto Encyclopedia of Genes and Genomes (KEGG), GSEA-Hallmark, or GO databases and then used the RMA normalization method to convert gene expression of each identified gene to a z-score to obtain the relative expression of the two groups of samples as previously described [54]. The principal components analysis (PCA) algorithm assigns different weighted coefficients according to the contribution of different genes in the metabolism pathway and finally yields the metabolism score by adding the z-score of each single gene in the pathway with its paired coefficient. For the analytic code and introduction of the algorithm, please refer to the GitHub IOBR package (version 0.99.9, date of access 17 June 2021) (https://github.com/IOBR/IOBR) [55]. Briefly, PCA used the following method to calculate the metabolism score of each sample
In this equation, i isthe z-score of the gene whose Cox coefficient is positive, and j is the z-score of the gene whose Cox coefficient is negative. The GSEA analysis was carried out on the GSEA website (https://www.gsea-msigdb.org/gsea/index.jsp). The relevant biological processes and genes were obtained from KEGG, GSEA, GO, and published literature.
Conjugation Kit (FAST)-lighting-link
Because the LAMP1 and LC3B antibodies we used were both from a rabbit source, we could not perform conventional cell fluorescence double-staining experiments. According to the experimental requirements, we used the Conjugation Kit (Fast)-Lightning-Link (Abcam Inc., USA). Equilibrate all materials and prepare reagents to room temperature prior to use. Add 1 µL of Modifier reagent to each 10 µL of antibody to be labeled and mix the solution gently. Remove the cap from the vial of DyLight® 594 or 488 Conjugation Mix and pipette the antibody sample (with added Modifier reagent) directly into the lyophilized solution. Resuspend the above mixture gently by withdrawing and re-dispensing the liquid once or twice using a pipette. Replace the cap on the vial and leave it standing for 15 min in the dark at room temperature (20–25 °C). Longer incubation time, such as overnight, exerts no negative effect on the conjugation. After incubation for 15 min (or more), 1 µL of Quencher reagent was added for every 10 µL of antibody used and mixed gently. The conjugates can be used after 5 min and do not require purification.
Transmission electron microscopy
After being fixed in 2.5% glutaraldehyde overnight, rat HUVECs were fixed in 2% osmium tetroxide for 1 h and stained with 2% uranyl acetate for 1 h. Before embedded into araldite and cut into semithin sections, these samples were dehydrated in an ascending series of acetone. Then, semithin sections were stained with toluidine blue to locate cells before being observed with a transmission electron microscope (Hitachi, Tokyo, Japan).
STZ-induced diabetes
Seven-week-old male Sprague-Dawley (SD) rats were purchased from the Animal Center of the Chinese Academy of Sciences in Shanghai, China. All SD rats were housed in a specific pathogen-free room with a 12-h light/dark cycle and provided regular food and water for 1 week prior to any experimental procedures. All animal experimental procedures were performed in accordance with the policies of the Southern Medical University Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals of the National Institute of Health in China and the current NIH guidelines. All animal studies complied with the ARRIVE guidelines.
SD rats were randomly divided into three groups: Control group (n = 12), LCR + LV-NC group (n = 12) and LCR + LV-shSirt1 (n = 12). Diabetes was induced using a single intraperitoneal injection of 100 mg/kg STZ (Sigma-Aldrich) in citrate buffer (pH 4.5). Hyperglycemia was confirmed if the glucose level exceeded 250 mg/dL in heparinized tail vein blood (measured by a glucometer) 2 weeks after treatment with STZ. The rats with a blood glucose level above 300 mg/dL were considered diabetic and used in our experiment. Blood glucose and body weight were measured for all animals before injection and 7 and 14 days after injection. Two rats both in the Control group and LCR + LV-shSirt1 group died within 14 days of injection.
Establishment of a wound model
Thirty-six normal rats were randomly divided into either the Control group (n = 12), LCR + LV-NC group (n = 12), or LCR + LV-shSirt1 group (n = 12). All rats were subjected to induced surgery 2 weeks after STZ injection. One percent (w/v) pentobarbital sodium (50 mg/kg) was intraperitoneally injected into the mice for anesthesia. After shaving and sterilization, two full-thickness wounds (20 mm in diameter) were made on each side of the rat’s back. LCR was then intragastrically delivered at a dose of 50 mg·kg−1·d−1 until the rats were sacrificed (days 10 and 20), while equivalent normal saline was administered for vehicle control. All treated rats were housed in individual cages, and images of the wounds were obtained in days 5, 10 and 20. The wound area was calculated by tracing the wound margins and evaluated as a percent area of the original wound using Image-Pro Plus 6.0 software.
Assessment of blood flow in the wound area
On days 5, 10 and 20 after surgery, a laser Doppler instrument (MoorLDI-2, Moor Instruments Limited, UK) was used to assess microvascular network reconstruction in back wounds. The blood flow and the results were measured using the MoorLDI Review software.
lentivirus use in vivo
One week prior to surgery, mice in the LV-shSirt1 group and LV-NC group received 3 µL viral vectors with 5 × 108 TU/mL or empty vector via subcutaneous injections in 3 areas using a microsyringe. Getting lentivirus every 2 days, a total of three times.
Histological analysis
Skin tissues were fixed in 4% paraformaldehyde overnight. Then, gross specimens were paraffin-embedded and sectioned to 5-mm thickness with a microtome. Slides for each specimen were stained with hematoxylin and eosin (H&E). Images were captured with an optical microscope. Then, the length of the wound closure and the number of capillaries in the wound bed were analyzed.
Molecular docking
The complex’s crystal structure was acquired from the Protein Data Bank and documented in Supplementary Table S2, while the molecular structure of LCR was sourced from the PubChem database. To refine the structure, PyMOL (Version 2.4, New York, NY, USA) was used to eliminate water and hetero molecules from the proteins and include hydrogen atoms and charges. Using AutoDockTools (Autodock 1.5.7, Scripps Research Institute, San Diego, USA), the LCR molecule and Sirt protein family were formatted in pdbqt and used for molecular docking via Autodock Vina (version 1.1.2) [56]. The resulting visualization was generated with PyMOL, and the grid center and size were established in accordance with the previous research, as documented in Supplementary Table S3.
Cellular thermal shift assay (CETSA)
Cellular thermal shift assay (CETSA) was conducted according to Jafari et al. [57]. with minor adjustments. The HUVECs were pretreated with 80 μmol/L lonicerin or DMSO. HUVEC cells were washed and resuspended with PBS, divided into six aliquots in an equal volume, and heated individually at different temperatures (37, 42, 47, 52, 57, 62 °C) for 5 min and then cooled at room temperature for 3 min. They were lysed with RIPA solution. Following centrifugation at 14,000 × g for 15 min at 4 °C, the supernatants were transferred to new tubes and stored at −80 °C until immunoblotting was performed.
Immunohistochemical examination
Immunohistochemical staining. Tissue sections were dewaxed in xylene for 30 min and rehydrated as previously described. The sections were then incubated at 4 °C overnight with the primary antibodies of anti-Sirt1 (1:200), anti-BAX (1:200), anti-BCL-2 (1:200) or anti-C-C3 (1:200). The following day, the slides were washed three times and incubated for 2 h with secondary antibodies of goat anti-rabbit IgG or goat anti-mouse IgG (ZSGB-Bio, Beijing, China) for 2 h. DAB (ZSGB-Bio, Beijing, China) was used as a chromogen to reveal the reaction.
Small interfering ribonucleic acid transfection
Double-stranded siRNA for human Sirt1 gene silencing was designed and chemically synthesized (RiboBio, Guangzhou, China). When the cell density reaches about 60%, HUVECs were transfected with 50 nM siRNA and Lipofectamine 2000 Reagent (Thermo Fisher, UT, USA) for 36 h according to the manufacturer’s instructions. Then, the cells were treated with LCR and TBHP as described previously, and the cells were harvested for Western blot analysis after these procedures.
Statistical analysis
All experiments were performed at least three times. The data obtained are expressed as the mean ± standard error of the mean (SEM). Statistical analyses were performed using GraphPad Prism version 8.0 software (GraphPad Software, CA, USA). Comparisons between two groups were analyzed by unpaired Student’s t test. For multiple-group comparisons, we used one-way analysis of variance (ANOVA) as a parametric method. Probability values of P < 0.05 were considered statistically significant.
Results
Lonicerin treatment decreases TBHP-induced apoptosis in HUVECs
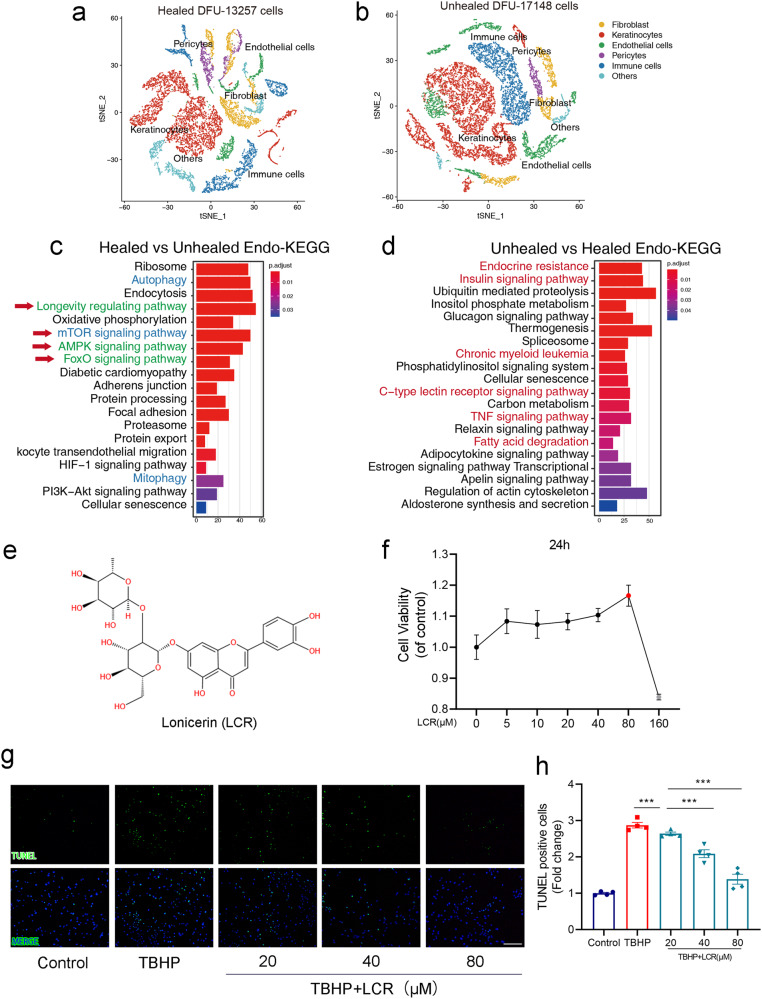
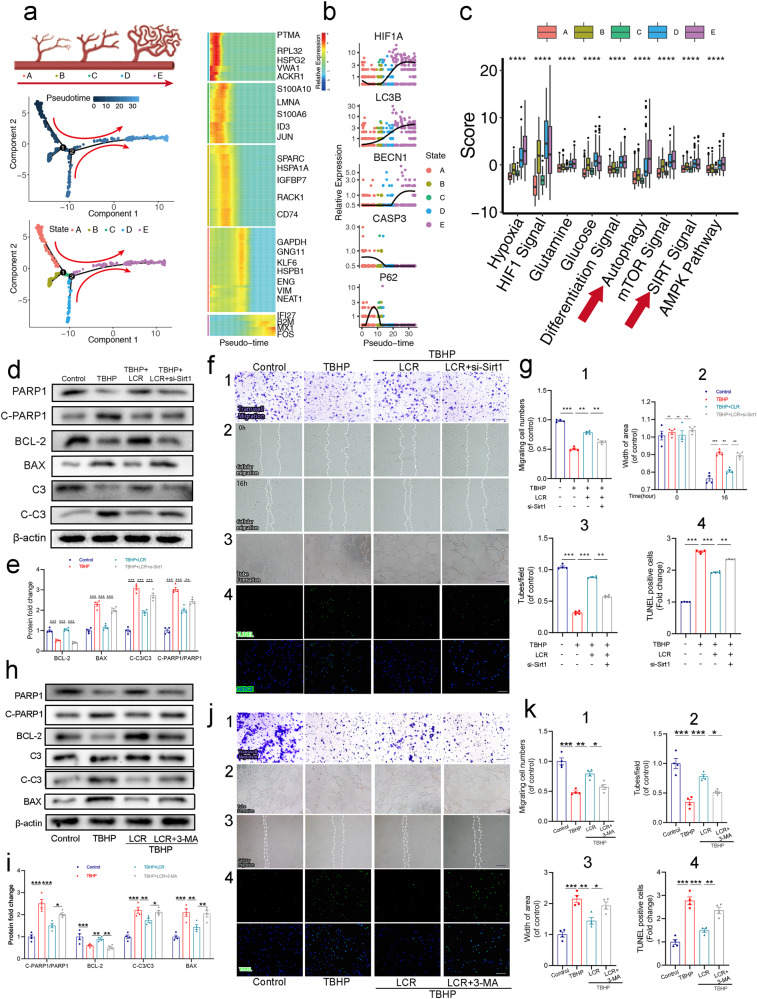
We initially performed a comparative analysis of single-cell omics data between healed and unhealed states in five patients with diabetic foot ulcers (DFUs) to investigate the underlying mechanisms of wound repair and explore potential therapeutic strategies (Fig. 1a, b). A total of 13,257 cells from healed DFU and 17,148 cells from unhealed DFU were classified into seven distinct clusters by biomarkers, including Endothelial cells, Fibroblasts, Keratinocytes, Pericytes, Immune cells, and others.
Fig. 1. Lonicerin treatment decreases TBHP-induced apoptosis in HUVECs.
a, b tSNE plot showing clusters and annotations of cells identified in patients with Healed DFU and Unhealed DFU, respectively. Cells from different patients gather together. Different cell types are shown in different colors; (c, d) KEGG enrichment analysis of the genes in endothelial cells in patients with Healed DFU and Unhealed DFU, respectively; (e) The chemical structure for LCR; (f) CCK8 assay of HUVECs was conducted to measure relative cell viability after being treated with different concentrations of LCR; (g, h) Representative images demonstrating terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling-positive nuclei (green color). Scale bar = 100 μm. HUVECs treated with or without LCR for 24 h. The values presented are the means ± SEM (n = 4). < 0.05, P < 0.01, ***P < 0.001.
As shown in Fig. 1a, b, endothelial cells were significantly different between the two groups. Meanwhile, endothelial cells play a crucial role in providing nutritional support during wound healing, and the preservation of their integrity is paramount to successful tissue repair. Therefore, we conducted a comprehensive screening and analysis of the endothelial cells.
We aim to gain a comprehensive understanding of the specific pathways involved in determining the outcome of wound healing. The KEGG enrichment analysis of endothelial cells in the healed group (Fig. 1c) revealed increased expressions of longevity signals (green) and autophagy signals (blue), along with significant activation of the AMPK and FOXO pathways. In contrast, the unhealed group (Fig. 1d) exhibited activation of multiple pathways including endocrine resistance, insulin signaling, chronic myeloid leukemia, C-type lectin receptor signaling, TNF signaling, and fatty acid degradation. Based on the single-cell data presented above, it is evident that autophagy plays a crucial role in mitigating inflammation, oxidative stress, and promoting cell survival in wound healing processes [58].
Lonicerin (LCR), a bioactive compound found in plants of the Lonicera japonica species and other honeysuckle plants, possesses a rich medicinal history and remarkable therapeutic value. It exhibits remarkable anti-inflammatory and antioxidant potential, and recent studies have reported its potential to enhance autophagy [51]. We attempted to investigate the therapeutic potential of LCR in chronic wounds.
Figure 1e shows the chemical structure of LCR. To evaluate the therapeutic potential of LCR in treating diabetic wounds, the toxicity of Lonicerin on HUVECs was initially evaluated using CCK-8, as described in the methods section. Figure 1f demonstrates that Lonicerin concentrations below 80 μM did not exhibit obvious toxicity to HUVECs. However, concentrations of 160 μM significantly affected HUVEC activity. Since TBHP is a potent inducer of oxidative stress-apoptosis [52], we utilized TUNEL staining, a specific method for apoptotic cell staining [59], to further study the effect of LCR on apoptotic HUVECs. As depicted in Fig. 1g, h, TBHP treatment led to a significant increase in TUNEL-positive cells, indicating increased HUVEC apoptosis. Conversely, LCR intervention decreased the level of HUVEC apoptosis. These results suggest that LCR has a protective effect on HUVECs and can combat apoptosis caused by TBHP.
Lonicerin combats TBHP-induced apoptosis and promotes the function of HUVECs
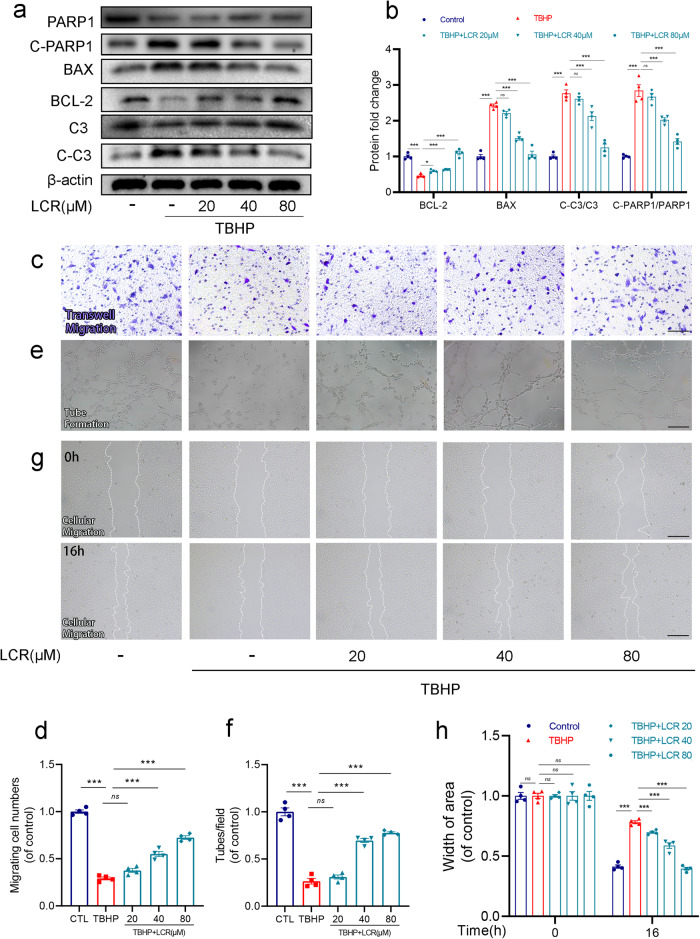
To further demonstrate the protective effect of LCR on HUVECs, we employed Western blotting to investigate the expression levels of apoptosis-related proteins BCL-2, BAX, Caspase 3, Cleave-caspase3 (C-C3), PARP1, Cleave-PARP1. As shown in Fig. 2a, b, the expression of BCL-2 was found to be elevated, while the expressions of BAX, C-PARP1/PARP1 and C-C3/C3 were decreased upon the addition of LCR, compared to the TBHP-treated group. These findings suggest that LCR can effectively reduce the level of apoptosis in HUVECs.
Fig. 2. Lonicerin combats TBHP-induced apoptosis and promotes the function of HUVECs.
a, b The protein expression of BCL-2, BAX, C-PARP1/PARP and C-C3/C3 was detected by Western blot in the HUVECs; (c, d) Transwell migration assay results demonstrate the effect of LCR on HUVEC migration. Scale bar = 100 μm; (e, f) Tube formation assay results demonstrate the effect of LCR on HUVEC neovascularization. Scale bar = 100 μm; (g, h) Representative images of scratch wound healing assay of HUVECs in vitro at 0 and 16 h posttreatment and its quantitative analysis. Scale bar = 100 μm. HUVECs treated with or without 20, 40, 80 μM LCR for 24 h. The values presented are the means ± SEM (n = 4). **P < 0.01, ***P < 0.001. ns indicates no significant difference.
Subsequently, we evaluated the viability of HUVECs using Transwell, tube formation, and scratch wound healing assays. As can be seen from Fig. 2c, f, TBHP significantly reduced the cell migration of HUVECs, indicating that the migration capacity of HUVECs is reduced. After adding LCR, this phenomenon is significantly weakened, and the effect is enhanced with the increase in LCR concentration. As shown in the scratch wound healing assay, compared to the TBHP-treated group, the distance between the scratches was significantly shortened after the addition of LCR, which indicated the enhancement of cell migration ability (Fig. 2e, h). The number of capillary-like structures in HUVECs reflects the cells’ ability to form blood vessels. In Fig. 2d, g, tube formation assay showed that LCR can promote the tube-form ability of HUVECs under oxidative stress, indicating that LCR can significantly promote angiogenesis.
Taken together, all the results indicate that LCR can effectively alleviate oxidative stress-induced apoptosis in HUVECs, thereby improving the cell viability, migration, and angiogenic abilities of HUVECs.
Lonicerin promotes autophagy in TBHP-treated HUVECs
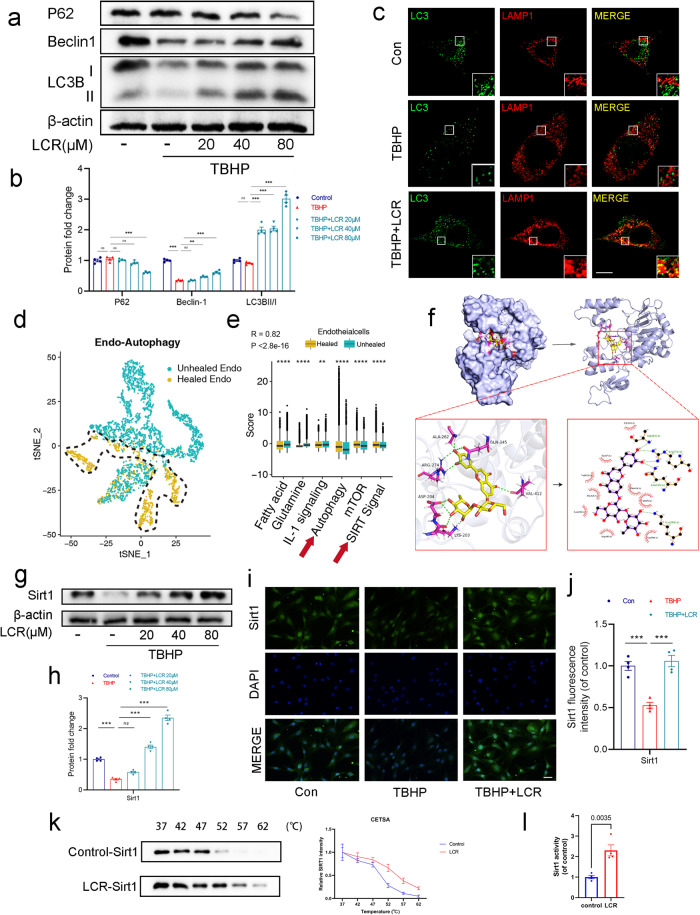
After confirming the ability of LCR to reduce apoptosis and protect HUVECs, we aimed to further investigate whether LCR improves wound healing through the promotion of autophagy. Based on our previous analysis of a single-cell data set (Fig. 1c), we observed higher levels of autophagy in wounds that exhibited good wound healing outcomes. Our hypothesis is that autophagy plays a crucial role in promoting accelerated wound healing. We assessed the expression of Beclin-1 and the ratio of LC3B-II/LC3B-I, which are classical indicators of autophagy levels and observed an increase in their levels upon LCR treatment, accompanied by a decrease in P62 expression (Fig. 3a, b). Additionally, co-localization of LC3B and LAMP1 (the lysosome), as determined by cellular immunofluorescence, further confirmed the promotion of autophagy by LCR (Fig. 3c).
Fig. 3. Lonicerin promotes autophagy in TBHP-treated HUVECs.
a, b The protein expression of P62, Beclin-1, LC3B in HUVECs treated with different concentrations of LCR was visualized by Western blotting; (c) The double immunofluorescence staining of LC3B and LAMP1 in TBHP-exposed HUVECs treated with LCR (green signals represent LC3B), red signals represent LAMP1, scale bar = 10 μm; (d) tSNE plot showing clusters of endothelial cells in autophagy. Different patient groups are shown in different colors. Blue represents the endothelial cells of unhealed patients, yellow represents the endothelial cells of healed patients; (e) Scores for autophagy, SIRT signal, fatty acid and glutamine metabolism, and IL-1 signaling calculated by PCA method in keratinocytes comparing healed patients versus unhealed patients; (f) The 3D and 2D results of the optimal conformation of LCR binding to Sirt1 showed that the linked amino acids were ALA-204, LYS-203, ARG-274, ALA-262, GLN-345 and VAL-412, respectively; (g, h) The protein expression of Sirt1 was detected by Western blot in the HUVECs; (i) Immunofluorescence staining of Sirt1 (green). Nuclei were counterstained with DAPI (blue) scale bar = 20 μm; (j) Quantification data of mean fluorescence intensity of Sirt1; (k) In the CETSA assay, the degree of protein expression of Sirt1 in HUVECs in the presence and absence of LCR; (l) The effect of LCR on Sirt1 activity was detected by Sirt1 activity assay kit. HUVECs treated with or without 20, 40, 80 μM LCR for 24 h. The values presented are the means ± SEM (n = 4). **P < 0.01, ***P < 0.001.
To explore the specific mechanism by which LCR promotes autophagy, we performed clustering and scoring of autophagy-related genes in single cells. Figure 3d demonstrates the successful separation of cell populations into Unhealed Endothelial cells and Healed Endothelial cells based on differentially expressed autophagy genes. Although some cells were not clearly distinguishable, possibly due to their transitional state, it indicates the presence of differential expression of autophagy-related genes in endothelial cells. Additionally, higher expression of Fatty acid, Glutamine, and IL-1 signaling was observed in the Unhealed group, while autophagy, mTOR, and SIRT signaling showed higher expression in the Healed group (Fig. 3e), suggesting the crucial role of the Sirt protein family. The Sirt protein family is known to mediate autophagy, maintain cellular homeostasis, integrate cellular energy metabolism, and remove damaged and wasted cells [60]. As mentioned in Fig. 1c, longevity signals, AMPK, and FOXO were highly activated in the Healed group, and Sirt1, a protein from the Sirt family, was identified as a key mediator of these signals [61, 62]. We employed molecular docking techniques to investigate the interaction between LCR and Sirt1–7. Interestingly, the results revealed the highest binding affinity and favorable hydrophobic interactions between Sirt1 and LCR (Fig. 3f, Supplementary Table S2). Therefore, we speculate that the mechanism by which LCR promotes autophagy and protects HUVECs is associated with the involvement of Sirt1.
The results of the Western blot showed that LCR could effectively promote the expression of Sirt1 (Fig. 3g, h). The PCR results indicate that treatment with LCR leads to an increase in Sirt1 mRNA expression (Supplementary Fig. S1b). Meanwhile, the cellular immunofluorescence showed that TBHP could inhibit the expression of Sirt1, while LCR could reverse the effect of TBHP and promote the expression of Sirt1 (Fig. 3i, j). We employed the Cellular Thermal Shift Assay (CETSA) to examine the binding between LCR and Sirt1. We observed that following LCR treatment and gradient heating, Western blotting revealed an increased thermal stability of Sirt1 (Fig. 3k). Following this, we assessed the enzymatic activity of Sirt1 under the influence of LCR, and observed a significant enhancement in Sirt1 activity due to LCR treatment. Therefore, we infer that LCR can bind to Sirt1 and enhance its activity.
All in all, these results indicate that LCR can promote autophagy in HUVECs by increasing the expression and enhancing the activity of the autophagy-related protein Sirt1. This is the first time to find that LCR can promote Sirt1 expression and enhance its activity. These findings provide new insights into the potential therapeutic effects of LCR on conditions associated with reduced autophagy and decreased Sirt1 expression.
Lonicerin promotes autophagy by increasing Sirt1 expression
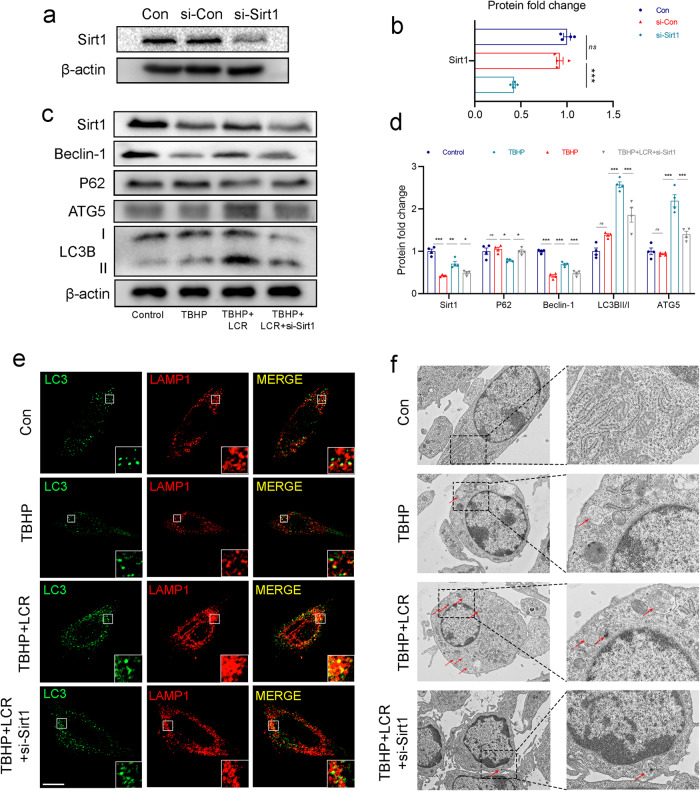
After demonstrating that LCR can promote autophagy levels and Sirt1 expression, we sought to investigate the relationship between LCR and the Sirt1-autophagy axis. Since sirt1 expression increases under the influence of LCR, we silenced Sirt1 using siRNA to assess its impact on autophagy. The knockdown efficiency of si-Sirt1 was detected by Western blot, and the results are shown in Fig. 4a, b. We then used cellular immunofluorescence and Western blot analysis to assess the changes in autophagy levels in HUVECs following si-Sirt1 administration. As can be seen from Fig. 4c, d, the addition of siRNA resulted in a significant reduction in Sirt1 expression in HUVECs treated with LCR. Moreover, the expression of Beclin-1, ATG5 and the ratio of LC3B-II/LC3B-I were significantly reduced, indicating a decrease in the level of autophagy. These findings were further supported by cellular immunofluorescence analysis, which revealed a decrease in the degree of co-localization between LC3B and LAMP1 after si-Sirt1 administration compared to LCR-treated HUVECs (Fig. 4e). As shown in Fig. 4f, LCR induced a remarkable accumulation of autophagosomes with double membranes in HUVECs. However, Sirt1 knockdown decreased the number of autophagosomes significantly.
Fig. 4. Lonicerin promotes autophagy by increasing Sirt1 expression.
a, b The knockdown effect of si-RNA was tested by Western blot; (c, d) The Western blot results of Sirt1, Beclin-1, P62, ATG5 and LC3B under LCR treatment with si-Sirt1 before TBHP addition; (e) The representative images of double immunofluorescence staining in HUVECs treated as above (green signals represent LC3B), red signals represent LAMP1, scale bar = 10 μm; (f) TEM images of autophagic vesicles in HUVECs treated as indicated (red arrow: autophagic vesicles). Scale bar = 1 μm. HUVECs treated with or without 80 μM LCR for 24 h. The values presented are the means ± SEM (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001. ns indicates no significant difference.
Taken together, these results suggest that LCR may promote autophagy through Sirt1.
Anti-apoptotic effects of LCR are related to Sirt1 in HUVECs
Since Sirt1 is implicated in the autophagy-promoting effect of LCR, we aim to further understand the role of Sirt1 in the development of vascular endothelial cells. Used pseudotime to simulate the developmental progression of vascular endothelial cells (Fig. 5a), we observed a correlation between the progression of pseudotime and the upregulation of HIF1A and autophagy-related gene expression (LC3B, BECN1, CASP3, P62) at the individual gene level (Fig. 5b). Additionally, as cells progressed in development, there was a stronger association with hypoxia, differentiation, autophagy, and Sirt signaling (Fig. 5c). This can be attributed to the increased energy demand of more mature cells, whereas less energy is required during the early differentiation stages of stem cells. These findings collectively suggest the involvement of Sirt1 in regulating the progression and apoptosis of endothelial cells.
Fig. 5. Anti-apoptotic effects of Lonicerin (LCR) are related to Sirt1 in HUVECs.
a Pseudotime analysis showed the endothelial cell development pattern; (b) HIF1A, LC3B, and BECN1 increased, whereas CASP3 of endothelial cells decreased, but p62 increased firstly and then down with pseudotime; (c) The gene expression scores for hypoxia, glutamine, and glucose metabolism, and autophagy, AMPK, SIRT, HIF1, differentiation signaling pathways as divided by wound healing stage. Box plot graphs indicated the value of minimum, first, quartile, median, third quartile, and maximum; (d, e) The Western blot results of BCL-2, BAX, C-PARP1/PARP1 and C-C3/C3 under LCR treatment with si-Sirt1 pretreatment before TBHP addition; (f, g, 1) Representative transwell migration assay images of HUVECS treated with LCR and si-Sirt1 pretreatment before TBHP addition. Scale bar = 100 μm; (f, g, 2) Representative images of scratch wound healing assay of HUVECs in vitro at 0 and 16 h posttreatment and its quantitative analysis. Scale bar = 100 μm; (f, g, 3) Tube formation assay results demonstrate the effect of LCR on HUVEC neovascularization. Scale bar, 100 μm; (f, g, 4) Apoptotic chondrocytes were examined using TUNEL fluorescence immunocytochemistry (green). Nuclei were counterstained with DAPI (blue). Scale bar = 100 μm; (h, i) The Western blot results of BCL-2, BAX, C-PARP1/PARP1 and C-C3/C3 under LCR treatment with 3-MA pretreatment before TBHP addition; (j, k, 1) Representative transwell migration assay images of HUVECs treated with LCR and 3-MA pretreatment before TBHP addition. Scale bar = 100 μm; (j, k, 2) Tube formation assay results demonstrate the effect of LCR on HUVEC neovascularization. Scale bar, 100 μm; (j, k, 3) Representative images of scratch wound healing assay of HUVECs in vitro at 16 h posttreatment and its quantitative analysis. Scale bar = 100 μm; (j, k, 4) Apoptotic chondrocytes were examined using TUNEL fluorescence immunocytochemistry (green). Nuclei were counterstained with DAPI (blue). Scale bar = 100 μm; HUVECs treated with or without 80 μM LCR for 24 h. The values presented are the means ± SEM (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001. ns indicates no significant difference.
To address this hypothesis, we treated HUVECs with si-Sirt1 and assessed the expression of C-C 3/C3, C-PARP1/PARP1, BCL-2, and BAX using Western blotting. As shown in Fig. 5d, e, compared with TBHP-induced apoptotic HUVECs cells treated with LCR, the expression of C-C3/C3, C-PARP1/PARP1 and BAX was increased after the addition of si-Sirt1, while the expression of BCL-2 was decreased, indicating the weakening of the anti-apoptotic effect of LCR.
To further examine the effect of Sirt1 on HUVECs cell function, we used the related experimental techniques such as Transwell assay, Tube formation assay, TUNEL staining, scratch wound healing assay and etc. The results of Transwell assay and scratch wound healing assay, after the addition of si-Sirt1, the cell migration ability of HUVECs was significantly decreased compared with the TBHP group added with LCR (Fig. 5f, g (1&2)). Meanwhile, because angiogenesis is an important process in wound healing and is responsible for transporting oxygen, nutrients, and metabolic waste, we evaluated whether LCR improves the tube formation ability of HUVECs in vitro. In this manner, we used the tube formation assay to assess and quantify tube formation ability. Images of Matrigel tube formation are shown in Fig. 5f, g (3), demonstrating that the tube-forming ability of HUVECs was substantially increased following treatment with LCR. However, the use of si-Sirt1 inhibited the angiogenic effect of LCR.
Moreover, TUNEL staining showed that knocking down Sirt1 increased the proportion of apoptosis-positive cells compared to the LCR treated group, further confirming the important role of Sirt1 in regulating the cell viability and apoptosis level of HUVECs (Fig. 5f, g (4)). Collectively, these results further prove that Sirt1 is closely related to the cell viability and apoptosis level of HUVECs, and LCR exerts its protective effect by acting on Sirt1.
Subsequently, in order to further validate the role of autophagy in LCR treatment, we treated LCR-treated HUVECs with the classic autophagy inhibitor 3-MA. We observed that 3-MA treatment inhibited the therapeutic effects of LCR, including its anti-apoptotic, pro-angiogenic, and migratory effects on HUVECs (Fig. 5h, k). Therefore, we speculate that during the LCR treatment process, the sirt1-autophagy axis plays a crucial role.
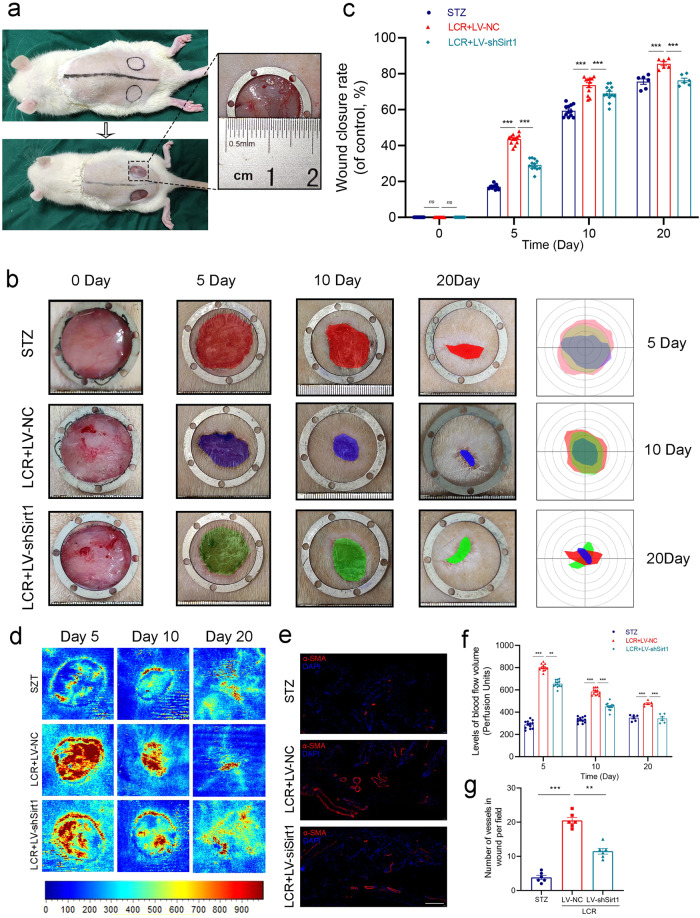
Lonicerin accelerates cutaneous wound healing in rats
Figure 6a illustrates the surgical procedure. Wound closure was significantly reduced in the LCR-treated group compared to the STZ group on days 5, 10, and 20 (Fig. 6b, c). To investigate the angiogenic effects of LCR in vivo, we evaluated blood flow at the wound site and newly formed vascular networks. Microvascular network reconstruction in the wound area was visualized using LDBF, which indicated the blood flow intensity: redder color indicating greater blood flow. The blood flow intensity in the LCR group was significantly higher than that in the STZ group, which was consistent with the in vitro experiments. Besides, the knockdown of Sirt1 can inhibit the vasotropism effect of LCR (Fig. 6d, f). Subsequently, we employed immunofluorescence staining of tissues, labeling α-SMA (α-smooth muscle actin), to observe the extent of vascular formation in different groups. Consistently, we obtained similar conclusions (Fig. 6e, g).
Fig. 6. Lonicerin accelerates cutaneous wound healing in rats.
a As the animal experimental schematics show, rats were anesthetized and the dorsal fur was shaved and then two full-thickness wounds were made with biopsy punch per mouse; (b) Representative images of the mice skin wound healing in the STZ group, LCR + LV-NC group, and LCR + LV-shSirt1 groups. The rulers in the pictures are shown in millimeters; (c) Wound area closure rate at different time points (days 0, 5, 10 and 20) in groups mentioned above. n = 12 or 6 independent animals per group; (d, f) Representative color laser Doppler images are taken to value subcutaneous blood flow on postsurgery days 5, 10 and 20. n = 12 or 6 independent animals per group. n = 12 or 6 independent animals per group; (e, g) Immunofluorescence staining for α-SMA (red) and their quantified results (n = 6). Scale bar = 100 μm; The values presented are the means ± SEM. **P < 0.01, ***P < 0.001. ns indicates no significant difference. ns indicates no significant difference.
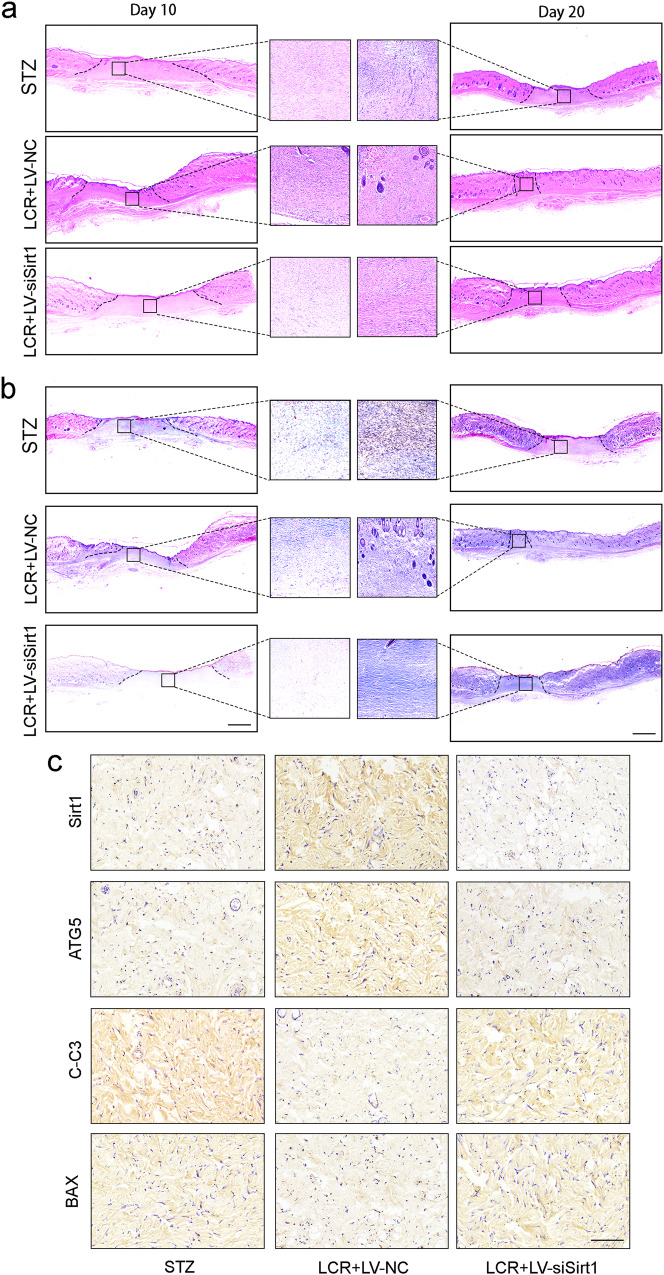
H&E staining and Masson staining showed that LCR promoted ECM generation, especially collagen secretion. Further analysis indicated that delayed re-epithelialization was observed in the untreated diabetic wounds, and LCR facilitated the re-epithelialization (Fig. 7a, b). Meanwhile, we detected the expressions of Sirt1, ATG5, BAX and C-C3 in tissues. Immunohistochemical staining showed that LCR could promote the expression of Sirt1 and ATG5, promote the enhancement of autophagy, and inhibit the expression of apoptosis-related proteins BAX and C-C3 (Fig. 7c).
Fig. 7. Lonicerin accelerates cutaneous wound healing in rats (continue).
a Representative H&E-stained sections of dorsal skin wound samples from different groups on day 10 and day 20. Black solid line indicated an epidermal gap. Scale bar = 500 μm; (b) Representative Masson’s trichrome-stained sections of dorsal skin wound samples from different groups on day 10 and day 20. Scale bar = 500 μm; (c) Immunohistochemistry staining images of Sirt1, ATG5, C-C3 and BAX of the control, LCR + LV-NC, and LCR + LV-siSirt1 groups. Scale bar = 100 μm. n = 6 independent animals per group.
These results suggest that LCR can promote wound healing in diabetic rats in vivo, and it acts through the Sirt1-autophagy axis.
Discussion
The efficacious management of chronic wounds has persistently posed a substantial obstacle in clinical practice [63, 64]. These types of wounds are frequently complicated by a variety of comorbidities, which can significantly prolong healing time and even result in non-healing [65]. Angiogenesis, the growth of new blood vessels, plays a critical role in the healing process of chronic wounds and is an effective therapeutic approach for reducing oxidative stress injury in vascular endothelial cells as well as decreasing cell apoptosis during the healing process [66, 67].
Single-cell transcriptomic analysis is of significant importance in identifying distinct cell populations, revealing informative cellular features, and elucidating critical intercellular relationships [68]. In this study, we performed an analysis of single-cell transcriptomic data from public databases, focusing on the differential expression of autophagy in endothelial cells. Our aim was to explore the effects of a natural compound regulating autophagy on the recovery of diabetic wounds (Fig. 1). Lonicerin (LCR), a flavonoid found in Lonicerae Flos, has been shown to possess various beneficial properties including antibacterial [49], anti-inflammatory [69], and anti-oxidative stress effects [70]. Previous investigations have demonstrated the potential efficacy of LCR in treating conditions such as osteoarthritis [71] and bacterial pneumonia [50]. Importantly, recent research has indicated that LCR can alleviate ulcerative colitis by modulating autophagy-mediated inflammation [51]. However, the therapeutic effectiveness of LCR in diabetic wound treatment remains uncertain.
Next, we determined the optimal concentration of LCR and found that it effectively reduces apoptosis of HUVECs under oxidative stress in vivo (Fig. 1). The proliferation of vascular endothelial cells is the initial step in angiogenesis, followed by migration and tube formation, ultimately culminating in the creation of novel blood vessels [72, 73]. To determine the effect of LCR on angiogenesis, we evaluated its ability to facilitate HUVEC migration and tube formation. Our results indicate for the first time that LCR has a powerful pro-angiogenic effect (Fig. 2).
Subsequently, we aimed to further investigate whether LCR improves wound healing through the promotion of autophagy. Autophagy plays a crucial role in the healing process of chronic diabetic wounds, acting as a regulator of wound healing throughout the hemostasis/inflammatory, proliferative, and remodeling phases. Autophagy facilitates the survival, proliferation, and migration of neutrophils, macrophages, endothelial cells, keratinocytes, and fibroblasts, thus supporting their biological functions and promoting wound healing [58]. Despite its established role, the connection between LCR and autophagy remains unclear. To explore this relationship, we performed clustering and scoring of autophagy-related genes in single cells. We found that autophagy and Sirt signaling showed higher expression in the Healed group and Sirt1 is the core gene in this process. Then we conducted experiments using a diabetic wound rat model to demonstrate it. Concur with speculation, we discovered for the first time that LCR augments autophagic flux in HUVECs (Fig. 3). Specifically, we found that LCR suppresses P62 accumulation and relieves the inhibition of autophagic flux.
Furthermore, multiple upstream mechanisms can enhance autophagy. In order to more accurately understand the specific mechanism by which LCR exerts its function, we attempted to identify potential drug targets for LCR. Sirtuins (SIRTs) are nicotine adenine dinucleotide (+)-dependent histone deacetylases that regulate critical signaling pathways in both prokaryotes and eukaryotes and are involved in numerous biological processes [60]. Many studies have confirmed the importance of the Sirt in anti-inflammation, anti-oxidative stress, and anti-apoptosis [74, 75]. At the same time, our previous research has focused on the Sirt family, exploring their function in multiple disease models [76–78]. Therefore, we also examined the interaction between LCR and some classic proteins in the Sirt family. Our results showed that LCR significantly increased the expression of Sirt1, a member of the sirtuin family, thereby promoting autophagy. The CETSA results indicate a potential direct interaction between LCR and SIRT1. Additionally, we found that knocking out Sirt1 led to a decrease in the therapeutic effect of LCR, which may be related to the apoptosis process. This is the first instance where a possible interaction between LCR and Sirt1 has been identified (Figs. 3, 4, 5). The relationship between LCR and SIRT1 appears to be multifaceted, involving both direct and indirect interactions. We observed that LCR treatment increases Sirt1 mRNA expression and enhances SIRT1 activity (Fig. 3i and Supplementary Fig. S1b). SIRT1 exerts its functions through various mechanisms, including increasing protein expression and deacetylation processes. Whether there is a bridge between LCR and SIRT1, as well as how LCR enhances SIRT1 activity, are questions worthy of further investigation in the future.
Finally, we established a chronic wound model in diabetic rats and treated them with LCR and found that LCR significantly promoted wound healing in diabetic rats. However, when we knocked down Sirt1 with a lentivirus during drug treatment, the therapeutic effect of LCR was significantly suppressed (Figs. 6, 7). This suggests that the interaction between LCR and Sirt1 plays a critical role in the healing process of chronic diabetic wounds.
The healing process of diabetic wounds is divided into four stages: coagulation, inflammation, proliferation, and remodeling [16]. This study primarily focuses on the proliferation and remodeling stages of diabetic wound healing, which are critical to the regeneration of vascular tissue. We investigated the effects of LCR on restoring autophagy flow in HUVECs from the perspective of vascular regeneration and found that it effectively reduces cell apoptosis and promotes vascular regeneration. However, other aspects of wound healing, such as LCR’s anti-inflammatory effects, have not been fully explored and warrant further investigation in future experiments.
Conclusions
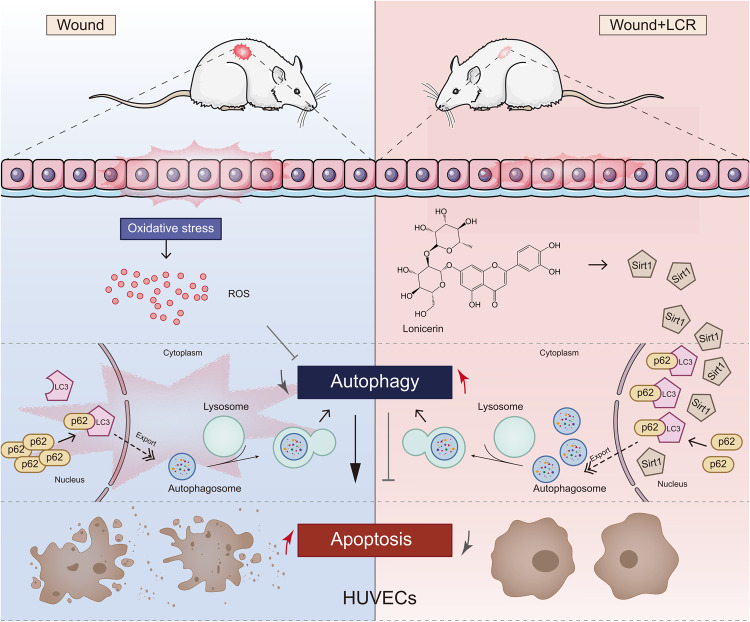
In summary, our study investigated the therapeutic effects and potential mechanisms of LCR in a diabetic wound rat model (Fig. 8). We discovered for the first time that LCR has a potent ability to promote cell autophagy and identified the upstream regulatory protein sirt1 as a potential target for LCR. Additionally, our results indicate that LCR can promote angiogenesis, cell migration, and anti-apoptosis, which may have implications for other research areas such as skin flap survival, cerebral ischemia and reperfusion, and intestinal epithelial injury healing.
Fig. 8.
Lonicerin promotes diabetic wound healing by enhancing blood vessel regeneration through Sirt1-mediated autophagy.
Supplementary information
Acknowledgements
This study is funded by the National Natural Science Foundation of China (82202468, 82372532), Natural Science Foundation of Guangdong Province (2023A1515012227), the Foundation of Guangzhou Municipal Science and Technology Bureau, the Basic Research Program - Basic and Applied Basic Research Project (2023A04J2355), High Level Introduction of Talent Research Startup Fund Southern Medical University (Gaofeng Wang) and Nanfang Hospital Distinguished Young Cultivation Program (2022J003).
Author contributions
ZL and LYL performed experiments, drafted and critically revised the manuscript. ZL, CJ, JRZ, CYQ and PW analyzed, interpreted the data and critically revised the manuscript. LY, CZL, YYG, LC, YL and LBN performed statistical analysis. LBN and GFW conceived, designed the study, discussed the experiments and critically revised the manuscript. All authors have approved the final manuscript and have agreed to be accountable for all the aspects of the work.
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The Single cell RNA-seq data were obtained in the NCBI GEO: https://ncbi.nlm.nih.gov/geo/ under accession numbers GSE165816.
Competing interests
The authors declare no competing interests.
Contributor Information
Li-bin Ni, Email: nlb1209@163.com.
Gao-feng Wang, Email: gwang45@jhmi.edu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-023-01193-5.
References
- 1.The Lancet Diabetes E. Diabetes: mapping the titanic struggle ahead. The Lancet Diabetes Endocrinol. 2018;6:1. doi: 10.1016/S2213-8587(17)30414-X. [DOI] [PubMed] [Google Scholar]
- 2.Long Y, Wei H, Li J, Yao G, Yu B, Ni D, et al. Effective wound healing enabled by discrete alternative electric fields from wearable nanogenerators. ACS Nano. 2018;12:12533–40. doi: 10.1021/acsnano.8b07038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiekh PA, Singh A, Kumar A. Exosome laden oxygen releasing antioxidant and antibacterial cryogel wound dressing oxoband alleviate diabetic and infectious wound healing. Biomaterials. 2020;249:120020. doi: 10.1016/j.biomaterials.2020.120020. [DOI] [PubMed] [Google Scholar]
- 4.Martí-Carvajal AJ, Gluud C, Nicola S, Simancas-Racines D, Reveiz L, Oliva P, et al. Growth factors for treating diabetic foot ulcers. Cochrane Database Syst Rev. 2015;2015:Cd008548. doi: 10.1002/14651858.CD008548.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang M, Nguyen TT. Strategy for treatment of infected diabetic foot ulcers. Acc Chem Res. 2021;54:1080–93. doi: 10.1021/acs.accounts.0c00864. [DOI] [PubMed] [Google Scholar]
- 6.Wu H, Li F, Shao W, Gao J, Ling D. Promoting angiogenesis in oxidative diabetic wound microenvironment using a nanozyme-reinforced self-protecting hydrogel. ACS Cent Sci. 2019;5:477–85. doi: 10.1021/acscentsci.8b00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.An T, Chen Y, Tu Y, Lin P. Mesenchymal stromal cell-derived extracellular vesicles in the treatment of diabetic foot ulcers: Application and challenges. Stem Cell Rev Rep. 2021;17:369–78. doi: 10.1007/s12015-020-10014-9. [DOI] [PubMed] [Google Scholar]
- 8.Deng L, Du C, Song P, Chen T, Rui S, Armstrong DG, et al. The role of oxidative stress and antioxidants in diabetic wound healing. Oxid Med Cell Longev. 2021;2021:8852759. doi: 10.1155/2021/8852759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 10.Schreml S, Szeimies RM, Prantl L, Karrer S, Landthaler M, Babilas P. Oxygen in acute and chronic wound healing. Br J Dermatol. 2010;163:257–68. doi: 10.1111/j.1365-2133.2010.09804.x. [DOI] [PubMed] [Google Scholar]
- 11.Guan Y, Niu H, Liu Z, Dang Y, Shen J, Zayed M, et al. Sustained oxygenation accelerates diabetic wound healing by promoting epithelialization and angiogenesis and decreasing inflammation. Sci Adv. 2021;7:eabj0153. [DOI] [PMC free article] [PubMed]
- 12.Panday A, Sahoo MK, Osorio D, Batra S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell Mol Immunol. 2015;12:5–23. doi: 10.1038/cmi.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–29. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosemann W, Wigand ME, Göde U, Länger F, Dunker I. Normal wound healing of the paranasal sinuses: Clinical and experimental investigations. Eur Arch Otorhinolaryngol. 1991;248:390–4. doi: 10.1007/BF01463560. [DOI] [PubMed] [Google Scholar]
- 15.Komi DEA, Khomtchouk K, Santa Maria PL. A review of the contribution of mast cells in wound healing: Involved molecular and cellular mechanisms. Clin Rev Allergy Immunol. 2020;58:298–312. doi: 10.1007/s12016-019-08729-w. [DOI] [PubMed] [Google Scholar]
- 16.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–21. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 17.Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19:134–48. doi: 10.1111/j.1524-475X.2011.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiPietro LA. Angiogenesis and wound repair: When enough is enough. J Leukoc Biol. 2016;100:979–84. doi: 10.1189/jlb.4MR0316-102R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez PG, Felix FN, Woodley DT, Shim EK. The role of oxygen in wound healing: A review of the literature. Dermatol Surg. 2008;34:1159–69. doi: 10.1111/j.1524-4725.2008.34254.x. [DOI] [PubMed] [Google Scholar]
- 20.Schäfer M, Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol Res. 2008;58:165–71. doi: 10.1016/j.phrs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Kvietys PR, Granger DN. Role of reactive oxygen and nitrogen species in the vascular responses to inflammation. Free Radic Biol Med. 2012;52:556–92. doi: 10.1016/j.freeradbiomed.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang P, Li T, Wu X, Nice EC, Huang C, Zhang Y. Oxidative stress and diabetes: antioxidative strategies. Front Med. 2020;14:583–600. doi: 10.1007/s11684-019-0729-1. [DOI] [PubMed] [Google Scholar]
- 23.Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–67. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 24.Okonkwo UA, DiPietro LA. Diabetes and wound angiogenesis. Int J Mol Sci. 2017;18:1419. [DOI] [PMC free article] [PubMed]
- 25.Yang L, Zhang L, Hu J, Wang W, Liu X. Promote anti-inflammatory and angiogenesis using a hyaluronic acid-based hydrogel with mirna-laden nanoparticles for chronic diabetic wound treatment. Int J Biol Macromol. 2021;166:166–78. doi: 10.1016/j.ijbiomac.2020.10.129. [DOI] [PubMed] [Google Scholar]
- 26.Barcelos LS, Duplaa C, Kränkel N, Graiani G, Invernici G, Katare R, et al. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of Wnt signaling. Circ Res. 2009;104:1095–102. doi: 10.1161/CIRCRESAHA.108.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doherty J, Baehrecke EH. Life, death and autophagy. Nat Cell Biol. 2018;20:1110–7. doi: 10.1038/s41556-018-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen ZH, Lam HC, Jin Y, Kim HP, Cao J, Lee SJ, et al. Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc Natl Acad Sci USA. 2010;107:18880–5. doi: 10.1073/pnas.1005574107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen S, Jiang YZ, Huang L, Zhou RJ, Yu KD, Liu Y, et al. The residual tumor autophagy marker LC3B serves as a prognostic marker in local advanced breast cancer after neoadjuvant chemotherapy. Clin Cancer Res. 2013;19:6853–62. doi: 10.1158/1078-0432.CCR-13-1617. [DOI] [PubMed] [Google Scholar]
- 30.Salazar G, Cullen A, Huang J, Zhao Y, Serino A, Hilenski L, et al. SQSTM1/p62 and PPARGC1A/PGC-1alpha at the interface of autophagy and vascular senescence. Autophagy. 2020;16:1092–110. doi: 10.1080/15548627.2019.1659612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bravo-San Pedro JM, Kroemer G, Galluzzi L. Autophagy and mitophagy in cardiovascular disease. Circ Res. 2017;120:1812–24. doi: 10.1161/CIRCRESAHA.117.311082. [DOI] [PubMed] [Google Scholar]
- 32.Wang P, Shao BZ, Deng Z, Chen S, Yue Z, Miao CY. Autophagy in ischemic stroke. Prog Neurobiol. 2018;163-164:98–117. doi: 10.1016/j.pneurobio.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Ueno T, Komatsu M. Autophagy in the liver: functions in health and disease. Nat Rev Gastroenterol Hepatol. 2017;14:170–84. doi: 10.1038/nrgastro.2016.185. [DOI] [PubMed] [Google Scholar]
- 34.Mizushima N, Levine B. Autophagy in human diseases. N Engl J Med. 2020;383:1564–76. doi: 10.1056/NEJMra2022774. [DOI] [PubMed] [Google Scholar]
- 35.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–62. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 36.Li L, Zhang J, Zhang Q, Zhang D, Xiang F, Jia J, et al. High glucose suppresses keratinocyte migration through the inhibition of p38 MAPK/autophagy pathway. Front Physiol. 2019;10:24. doi: 10.3389/fphys.2019.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaikh-Kader A, Houreld NN, Rajendran NK, Abrahamse H. The link between advanced glycation end products and apoptosis in delayed wound healing. Cell Biochem Funct. 2019;37:432–42. doi: 10.1002/cbf.3424. [DOI] [PubMed] [Google Scholar]
- 38.Guo Y, Lin C, Xu P, Wu S, Fu X, Xia W, et al. Ages induced autophagy impairs cutaneous wound healing via stimulating macrophage polarization to M1 in diabetes. Sci Rep. 2016;6:36416. doi: 10.1038/srep36416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiang L, Yang S, Cui YH, He YY. Keratinocyte autophagy enables the activation of keratinocytes and fibroblastsand facilitates wound healing. Autophagy. 2021;17:2128–43. doi: 10.1080/15548627.2020.1816342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Z, Tang W, Liu J, Han Y, Yan Q, Dong Y, et al. A novel sprayable thermosensitive hydrogel coupled with zinc modified metformin promotes the healing of skin wound. Bioact Mater. 2023;20:610–26. doi: 10.1016/j.bioactmat.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arunachalam G, Samuel SM, Marei I, Ding H, Triggle CR. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through sirt1. Br J Pharmacol. 2014;171:523–35. doi: 10.1111/bph.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hwang JW, Yao H, Caito S, Sundar IK, Rahman I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic Biol Med. 2013;61:95–110. doi: 10.1016/j.freeradbiomed.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vachharajani VT, Liu T, Brown CM, Wang X, Buechler NL, Wells JD, et al. Sirt1 inhibition during the hypoinflammatory phenotype of sepsis enhances immunity and improves outcome. J Leukoc Biol. 2014;96:785–96. doi: 10.1189/jlb.3MA0114-034RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yerra VG, Kalvala AK, Kumar A. Isoliquiritigenin reduces oxidative damage and alleviates mitochondrial impairment by SIRT1 activation in experimental diabetic neuropathy. J Nutr Biochem. 2017;47:41–52. doi: 10.1016/j.jnutbio.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Xu C, Wang L, Fozouni P, Evjen G, Chandra V, Jiang J, et al. Sirt1 is downregulated by autophagy in senescence and ageing. Nat Cell Biol. 2020;22:1170–9. doi: 10.1038/s41556-020-00579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Wu G, Han F, Wang K, Bai X, Jia Y, et al. Sirt1 activation promotes angiogenesis in diabetic wounds by protecting endothelial cells against oxidative stress. Arch Biochem Biophys. 2019;661:117–24. doi: 10.1016/j.abb.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 47.Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 48.Shang X, Pan H, Li M, Miao X, Ding H. Lonicera japonica thunb.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional chinese medicine. J Ethnopharmacol. 2011;138:1–21. doi: 10.1016/j.jep.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Z, Li K, Pan T, Liu J, Li B, Li C, et al. Lonicerin, an anti-alge flavonoid against Pseudomonas aeruginosa virulence screened from Shuanghuanglian formula by molecule docking based strategy. J Ethnopharmacol. 2019;239:111909. doi: 10.1016/j.jep.2019.111909. [DOI] [PubMed] [Google Scholar]
- 50.Gu LZ, Sun H. Lonicerin prevents inflammation and apoptosis in LPS-induced acute lung injury. Front Biosci (Landmark Ed). 2020;25:480–97. [DOI] [PubMed]
- 51.Lv Q, Xing Y, Liu J, Dong D, Liu Y, Qiao H, et al. Lonicerin targets EZH2 to alleviate ulcerative colitis by autophagy-mediated NLRP3 inflammasome inactivation. Acta Pharm Sin B. 2021;11:2880–99. doi: 10.1016/j.apsb.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lou J, Wang X, Zhang H, Yu G, Ding J, Zhu X, et al. Inhibition of PLA2G4E/cPLA2 promotes survival of random skin flaps by alleviating lysosomal membrane permeabilization-induced necroptosis. Autophagy. 2022;18:1841–63. doi: 10.1080/15548627.2021.2002109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Theocharidis G, Thomas BE, Sarkar D, Mumme HL, Pilcher WJR, Dwivedi B, et al. Single cell transcriptomic landscape of diabetic foot ulcers. Nat Commun. 2022;13:181. doi: 10.1038/s41467-021-27801-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang G, Miao Y, Kim N, Sweren E, Kang S, Hu Z, et al. Association of the psoriatic microenvironment with treatment response. JAMA Dermatol. 2020;156:1057–65. doi: 10.1001/jamadermatol.2020.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang G, Sweren E, Andrews W, Li Y, Chen J, Xue Y, et al. Commensal microbiome promotes hair follicle regeneration by inducing keratinocyte HIF-1α signaling and glutamine metabolism. Sci Adv. 2023;9:eabo7555. doi: 10.1126/sciadv.abo7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trott O, Olson AJ. Autodock vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–61. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jafari R, Almqvist H, Axelsson H, Ignatushchenko M, Lundbäck T, Nordlund P, et al. The cellular thermal shift assay for evaluating drug target interactions in cells. Nat Protoc. 2014;9:2100–22. doi: 10.1038/nprot.2014.138. [DOI] [PubMed] [Google Scholar]
- 58.Ren H, Zhao F, Zhang Q, Huang X, Wang Z. Autophagy and skin wound healing. Burns Trauma. 2022;10:tkac003. doi: 10.1093/burnst/tkac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lozano GM, Bejarano I, Espino J, Gonzalez D, Ortiz A, Garcia JF, et al. Relationship between caspase activity and apoptotic markers in human sperm in response to hydrogen peroxide and progesterone. J Reprod Dev. 2009;55:615–21. doi: 10.1262/jrd.20250. [DOI] [PubMed] [Google Scholar]
- 60.Wu QJ, Zhang TN, Chen HH, Yu XF, Lv JL, Liu YY, et al. The sirtuin family in health and disease. Signal Transduct Target Ther. 2022;7:402. doi: 10.1038/s41392-022-01257-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shen F, Shi Y. Recent advances in single-cell view of mesenchymal stem cell in osteogenesis. Front Cell Dev Biol. 2021;9:809918. doi: 10.3389/fcell.2021.809918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sadria M, Layton AT. Interactions among mtorc, ampk and sirt: a computational model for cell energy balance and metabolism. Cell Commun Signal. 2021;19:57. doi: 10.1186/s12964-021-00706-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Powers JG, Higham C, Broussard K, Phillips TJ. Wound healing and treating wounds: Chronic wound care and management. J Am Acad Dermatol. 2016;74:607–25. doi: 10.1016/j.jaad.2015.08.070. [DOI] [PubMed] [Google Scholar]
- 64.Jones RE, Foster DS, Longaker MT. Management of chronic wounds-2018. JAMA. 2018;320:1481–2. doi: 10.1001/jama.2018.12426. [DOI] [PubMed] [Google Scholar]
- 65.Morton LM, Phillips TJ. Wound healing and treating wounds: Differential diagnosis and evaluation of chronic wounds. J Am Acad Dermatol. 2016;74:589–605. doi: 10.1016/j.jaad.2015.08.068. [DOI] [PubMed] [Google Scholar]
- 66.Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173:370–8. doi: 10.1111/bjd.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Veith AP, Henderson K, Spencer A, Sligar AD, Baker AB. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv Drug Deliv Rev. 2019;146:97–125. doi: 10.1016/j.addr.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stuart T, Satija R. Integrative single-cell analysis. Nat Rev Genet. 2019;20:257–72. doi: 10.1038/s41576-019-0093-7. [DOI] [PubMed] [Google Scholar]
- 69.Shen CY, Jiang JG, Huang CL, Zhu W, Zheng CY. Polyphenols from blossoms of Citrus aurantium l. Var. Amara Engl. show significant anti-complement and anti-inflammatory effects. J Agric Food Chem. 2017;65:9061–8. doi: 10.1021/acs.jafc.7b03759. [DOI] [PubMed] [Google Scholar]
- 70.Kim NM, Kim J, Chung HY, Choi JS. Isolation of luteolin 7-O-rutinoside and esculetin with potential antioxidant activity from the aerial parts of artemisia montana. Arch Pharmacal Res. 2000;23:237–9. doi: 10.1007/BF02976451. [DOI] [PubMed] [Google Scholar]
- 71.Lee JH, Han Y. Antiarthritic effect of lonicerin on candida albicans arthritis in mice. Arch Pharmacal Res. 2011;34:853–9. doi: 10.1007/s12272-011-0520-6. [DOI] [PubMed] [Google Scholar]
- 72.Marziano C, Genet G, Hirschi KK. Vascular endothelial cell specification in health and disease. Angiogenesis. 2021;24:213–36. doi: 10.1007/s10456-021-09785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pasut A, Becker LM, Cuypers A, Carmeliet P. Endothelial cell plasticity at the single-cell level. Angiogenesis. 2021;24:311–26. doi: 10.1007/s10456-021-09797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang X, Yao Z, Wang K, Lou L, Xue K, Chen J, et al. MDL-800, the Sirt6 activator, suppresses inflammation via the NF-κB pathway and promotes angiogenesis to accelerate cutaneous wound healing in mice. Oxid Med Cell Longev. 2022;2022:1619651. doi: 10.1155/2022/1619651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kurundkar D, Kurundkar AR, Bone NB, Becker EJ Jr, Liu W, Chacko B, et al. Sirt3 diminishes inflammation and mitigates endotoxin-induced acute lung injury. JCI Insight 2019;4:e120722. [DOI] [PMC free article] [PubMed]
- 76.Lin Z, Teng C, Ni L, Zhang Z, Lu X, Lou J, et al. Echinacoside upregulates sirt1 to suppress endoplasmic reticulum stress and inhibit extracellular matrix degradation in vitro and ameliorates osteoarthritis in vivo. Oxid Med Cell Longev. 2021;2021:3137066. doi: 10.1155/2021/3137066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Z, Wu J, Teng C, Wang J, Yu J, Jin C, et al. Orientin downregulating oxidative stress-mediated endoplasmic reticulum stress and mitochondrial dysfunction through AMPK/Sirt1 pathway in rat nucleus pulposus cells in vitro and attenuated intervertebral disc degeneration in vivo. Apoptosis. 2022;27:1031–48. doi: 10.1007/s10495-022-01770-9. [DOI] [PubMed] [Google Scholar]
- 78.Lu J, Miao Z, Jiang Y, Xia W, Wang X, Shi Y, et al. Chrysophanol prevents IL-1β-induced inflammation and ecm degradation in osteoarthritis via the sirt6/nf-κb and nrf2/nf-κb axis. Biochem Pharmacol. 2023;208:115402. doi: 10.1016/j.bcp.2022.115402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The Single cell RNA-seq data were obtained in the NCBI GEO: https://ncbi.nlm.nih.gov/geo/ under accession numbers GSE165816.