Abstract
The axon-initial-segment (AIS) of mature neurons contains microtubule (MT) fascicles (linear bundles) implicated as retrograde diffusion barriers in the retention of MT-associated protein (MAP) tau inside axons. Tau dysfunction and leakage outside of the axon is associated with neurodegeneration. We report on the structure of steady-state MT bundles in varying concentrations of Mg2+ or Ca2+ divalent cations in mixtures containing αβ-tubulin, full-length tau, and GTP at 37 °C in a physiological buffer. A concentration-time kinetic phase diagram generated by synchrotron SAXS reveals a wide-spacing MT bundle phase (Bws), a transient intermediate MT bundle phase (Bint), and a tubulin ring phase. SAXS with TEM of plastic-embedded samples provides evidence of a viscoelastic intervening network (IN) of complexes of tubulin oligomers and tau stabilizing MT bundles. In this model, αβ-tubulin oligomers in the IN are crosslinked by tau’s MT binding repeats, which also link αβ-tubulin oligomers to αβ-tubulin within the MT lattice. The model challenges whether the cross-bridging of MTs is attributed entirely to MAPs. Tubulin-tau complexes in the IN or bound to isolated MTs are potential sites for enzymatic modification of tau, promoting nucleation and growth of tau fibrils in tauopathies.
Subject terms: Intrinsically disordered proteins, Supramolecular assembly, SAXS
X-ray scattering and electron microscopy are used in concert to show that complexes of tubulin oligomers and tau are building blocks of an intervening network that cross-bridge microtubules into bundles with the same linear geometry observed in neurons.
Introduction
Microtubules (MTs) are hollow protein nanotubes resulting from GTP-mediated assembly of αβ-tubulin heterodimers, which stack to form curved GTP-tubulin oligomers and protofilaments (PFs). PFs interact laterally to form the MT wall1, with these interactions hypothesized to stabilize their straight conformation2,3. Once incorporated in an MT wall, GTP at the β-tubulin subunit can hydrolyze, forming GDP-tubulin PFs that tend to adopt a higher curvature conformation often leading to MT disassembly4. These distinct PF conformations underlie MT dynamic instability (DI) and enable the stochastic switching between periods of slow growth (polymerization) and rapid depolymerization2–6, regulated in part by the relative abundance of GTP- and GDP-tubulin. In cells, MT-associated proteins (MAPs) can also regulate DI and are implicated in the formation of MT bundles7–11, which are involved in numerous cellular functions8,12,13. Linear MT bundles (fascicles, Supplementary Fig. 1) with large wall-to-wall spacing are found in the central core of the axon initial segment (AIS) of mature neurons14–16, which form a retrograde diffusion barrier for MAP tau, compartmentalizing tau in the axon17.
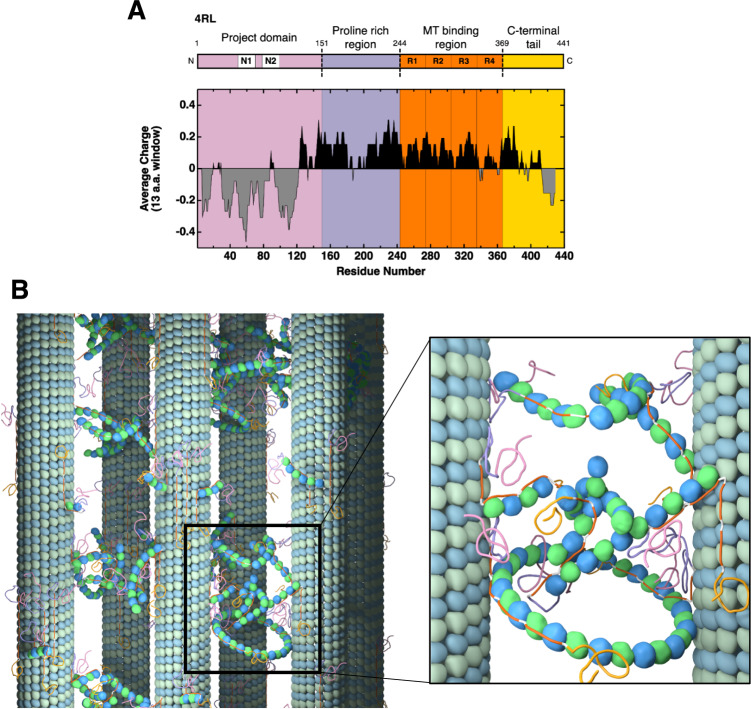
In our study, we focused on mixtures of αβ-tubulin and the canonical, full-length isoform (4RL) of MAP tau, an intrinsically disordered protein that binds to MTs, partially suppresses DI, and facilitates the transport of cargo along MTs in axons8,18–23. Tau dysfunction is implicated in neurodegenerative tauopathies, which include Alzheimer’s disease24, FTDP-1725, and chronic traumatic encephalopathy26. Humans express six wild-type tau isoforms resulting from alternative splicing of exons 2, 3, and 10 of the MAPT gene (Fig. 1A)27. The N-terminal projection domain (PD) and the C-terminal tail of tau protrude radially outward when bound to MTs, while tau’s proline-rich region and MT binding region (MTBR) are enriched with cationic residues thought to interact with negatively charged residues at the carboxyl-terminal end of αβ-tubulin28–30.
Fig. 1. Tau charge distribution and tau-tubulin network stabilizing bundled MTs.
A Schematic and average charge profile of full length 4RL tau with major features labeled, including inserts 1 and 2, encoded by exons 2 and 3, respectively, and all four MT-binding repeats (R1-R4). 4RL tau is depicted with labeled domains: N-terminal tail consisting of the projection domain (PD) and proline rich region, the microtubule (MT) binding region (MTBR), and the C-terminal tail28–30. The charge distribution is calculated using a rolling sum over thirteen residues. Despite anionic regions at the N- and C-termini, tau isoforms have a net cationic charge, which contributes to tau’s binding (via MTBR) to negatively charged residues of αβ-tubulin. Data are from the National Center for Biotechnology Information Protein Database (accession number NP_005901.2). B Cartoon of a microtubule bundle (left) with blow-up (right) showing an intervening network of complexes of tubulin oligomers (curved short protofilaments and rings) and tau, which stabilizes MT bundles in the bundled wide-spacing (Bws) and bundled intermediate (Bint) phases. The cationic MT binding repeats of tau (orange sections) link αβ-tubulin oligomers both to other free tubulin oligomers and to αβ-tubulin dimers within the MT lattice, creating the intervening network that cross-bridges neighboring MTs. Tau is depicted to bind either side of curved tubulin oligomers and rings consistent with studies that show tau may bind (via the MT binding repeats) either the outside surface or lumen of the MT38,69,70.
In-vitro experiments of paclitaxel-stabilized MTs in the absence of tau show that divalent cations induce ion-specific MT bundling31 and depolymerization32. In follow-up studies excluding paclitaxel, tau-stabilized MTs reproduced linear bundles mimicking MT fascicles found in the AIS33. Building on those results, the current study seeks to elucidate ion-specific effects on the structure and stability of tau-stabilized MT bundles by adding Mg2+ or Ca2+ to minimal dissipative reaction mixtures at 37 °C containing αβ-tubulin, 4RL-tau, and GTP in standard PIPES buffer at pH 6.834. The mixtures contained a 1/20 tau/tubulin-dimer molar ratio, which corresponds to sub-monolayer coverage of tau on MTs in the mushroom regime35. Divalent cation concentrations were in the millimolar range, approximating average cellular Mg2+ content36. Thus, a motivation for the study was to reveal ion-specific effects between metal ions at physiological Mg2+ concentrations in a minimal, cell-free model of MT fascicles of the AIS.
Time-dependent synchrotron small-angle X-ray scattering (SAXS) was used to monitor samples over 33 h (in the presence of excess GTP), revealing three distinct tubulin structural states. Kinetic phase diagrams generated from this data show that the tau-tubulin structural state is a function of both time and Mg2+ or Ca2+ concentration (added to the PIPES buffer, Methods). Phase diagrams guided the selection of specific time points for parallel transmission electron microscopy (TEM) of plastic-embedded samples, providing real-space images for comparison to reciprocal-space SAXS data. In agreement with previous measurements by Chung et al.33, widely-spaced MT bundles (labeled Bws with MT wall-to-wall spacing dw-w ≈ 40 to 45 nm), were stable below critical lower divalent cation concentrations clower (≈ 0.8 mM CaCl2 and ≈1.6 mM MgCl2 at 2 mM GTP). MT bundled structures also remained stable against added monovalent cations (up to 125 mM added KCl, above which SAXS data suggests KCl dramatically lowers tau tubulin binding affinity).
Here, we show that over a narrow range of added Ca2+ or Mg2+ concentrations (clower < c<cupper cupper ≈ 1.4 mM CaCl2 and ≈2.4 mM MgCl2 at 2 mM GTP), the Bws state undergoes a complex structural evolution over a period of hours. The onset of this structural evolution is signaled by the depolymerization of a fraction of MTs, which leads to a sudden increase in the formation of tubulin rings and curved tubulin oligomers (observed in SAXS). During this period, remaining bundled MTs enter a transient intermediate bundle state (Bint), with a more ordered lattice and smaller dw-w (≈ 30–35 nm). In agreement with these SAXS findings, TEM images of MTs fixed in the Bint state show larger, more tightly packed bundles, with smaller dw-w, and significantly more cross-bridges between MTs (observed in both states as ≈ 5 nm wide flexible filaments and rings), suggesting that the increased tubulin oligomer products created from depolymerized MTs distributed within the bundles, directly participate in the structural rearrangement of MT bundles by increasing the number of MT-MT cross-links. Taken together, SAXS and TEM data are consistent with MTs in both the Bws and Bint states bundled by an intervening network of tubulin oligomers complexed with tau. This suggests a significant revision to current dogma where cross-bridges between MTs were attributed solely to MAPs7–12,37. In our model, MT bundle formation is due to coded assembly by tau’s MT binding repeats (orange sections in Figs. 1A and 1B) acting as the glue linking αβ-tubulin oligomers in the intervening network. In this role, tau links αβ-tubulin oligomers within the intervening network to one another and to αβ-tubulin within the MT lattice (Fig. 1B). Large MT wall-to-wall spacings observed in both bundled states (much larger than the size of tau’s PD) are set by the average size of tau-coated, curved tubulin oligomers and rings.
Results
Time-dependent SAXS reveal three distinct tau-tubulin phases
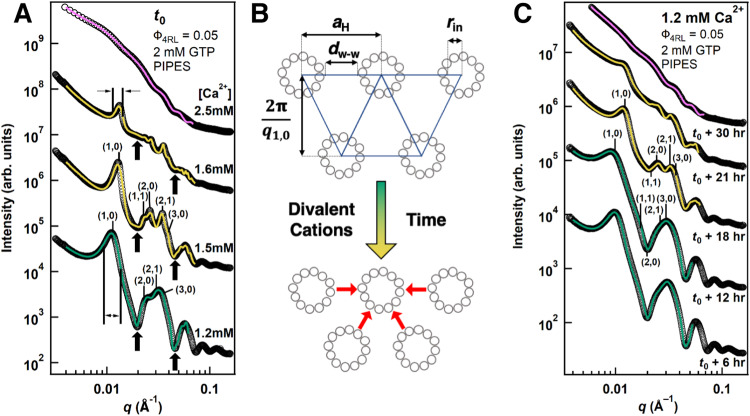
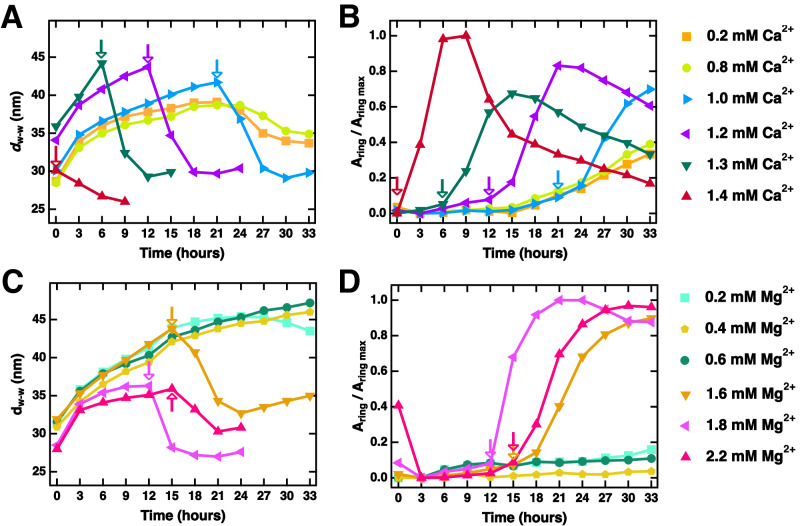
To understand how divalent cation content modulates the stability and structural features of tau-mediated MT bundles, time-dependent synchrotron SAXS measurements (Supplementary Table 1) were performed on tubulin reaction mixtures (40 µM) containing 2 mM GTP, co-assembled at 37 °C with 4RL-tau (tau to tubulin-dimer molar ratio, Φ4RL = 0.05) at varying CaCl2 or MgCl2 concentrations (0 to 5 mM added to standard PIPES buffer containing 1 mM of Mg2+ (Methods)). Reactions mixtures underwent 30 min of polymerization at 37 °C, followed by 30 min of centrifugation at 37 °C, and were then loaded onto the x-ray diffractometer with initial data points taken approximately 15 min after centrifugation (referred to here as timepoint t0). Samples were exposed to 1 s of synchrotron radiation once every 3 h for a 33-hour period. Analysis of all azimuthally averaged SAXS profiles reveals three distinct concentration-dependent tau-tubulin structural phases outlined in Fig. 2A.
Fig. 2. Time-dependent synchrotron SAXS data with increasing divalent cations in PIPES buffer at pH 6.8 reveals an intermediate bundled (Bint) microtubule state between the bundled wide-spacing (Bws) and the tubulin ring states.
A SAXS data (open circles) and corresponding fits (solid lines) for increased Ca2+ concentrations at t0, where t0 is the time of the first measurement. Color of the fitted line represents the identified tubulin phase with green, yellow, and magenta, corresponding to profiles in the wide-spacing bundled (Bws), intermediate bundled (Bint), and depolymerized tubulin ring states respectively. Indexing of two lower profiles is for a 2D hexagonal lattice of MTs. The Bint (profiles at 1.5 mM and 1.6 mM Ca2+) is differentiated from the Bws (profile at 1.2 mM Ca2+) by peaks shifted to larger q (i.e. smaller lattice spacing), decreased peak widths (compare 1,0 peak widths for 1.6 mM and 1.2 mM Ca2+), and increased scattering intensity at local minima of the MT form factor (solid arrows at 1.2 mM, 1.5 mM, and 1.6 mM Ca2+). At 2.5 mM of added Ca2+, the SAXS is dominated by tubulin rings and curved oligomers. B Cartoon of hexagonal MT bundles in the Bws (top) and Bint, (bottom) states highlights changes in MT-MT spacing. C Time-dependent SAXS data (open circles) and corresponding fits (solid lines) of a sample with 1.2 mM added Ca2+. Evolution of SAXS profiles show that phase transitions occur from Bws to Bint (between t0 + 12 and t0 + 18 hrs) and from Bint to the tubulin ring state (between t0 + 18 and t0 + 30 hrs). Source data for A and C are provided in the Source Data file.
At initial timepoint t0, all samples below a threshold divalent concentration of 1.4 mM added Ca2+ or 2.4 mM added Mg2+ exhibited MT bundling characteristics indistinguishable from controls with no added divalent cations (Supplementary Fig. 2). A typical SAXS profile representing the Bws phase is plotted in Fig. 2A and displays scattering characteristics indicative of strong MT polymerization and registers Bragg peaks consistent with 2D hexagonal packing of MTs (q10, q11 = 31/2q10, q20 = 2q10, q21 = 71/2q10, q30 = 3q10, q22 = 121/2q10). Wall-to-wall distances (dw-w, Fig. 2B) calculated from the location of q10 (Methods) ranged between dw-w = 29.5 and 33.1 nm for all samples, consistent with values of the widely-spaced MT bundle state reported previously33.
Above threshold concentrations tau-tubulin reaction mixtures produced scattering profiles at initial timepoints that were markedly different from the Bws phase and are indicative of a new intermediate bundled state (Bint) (Fig. 2A, profiles at 1.5 mM and 1.6 mM Ca2+). One major difference between the two states is the characteristic shift in the location of all Bragg peaks to higher q values, indicating that the wall-to-wall distance is smaller in the Bint than in the Bws phase (dintw-w = 24.5 to 26.7 nm). The Bragg peaks are also sharper in the Bint state (Fig. 2A, full-width at half maximum (FWHM) for q10, vertical lines at 1.2 mM and 1.6 mM Ca2+), implying that the coherent domain size of the MT lattice is much larger, despite having a smaller lattice parameter and decreased scattering from bundled MTs (Fig. 2A, scattering intensity of (1,0) peak diminishes with increased Ca2+). Unlike the Bws phase, scattering data shows that, within the Bint phase, Ca2+ and Mg2+ inhibit MT polymerization and increase the prevalence of depolymerized tubulin products in a concentration-dependent manner, indicated by the decrease in bundled MT scattering and the increase in scattering at the form factor minima (Fig. 2A, arrows), respectively.
At higher Ca2+ and Mg2+ concentrations (1.9 - 3.0 mM Ca2+ or 2.6 - 3.0 mM Mg2+) MT polymerization was substantially inhibited, and scattering was dominated by single tubulin rings (Fig. 2A, broad oscillations at 2.5 mM Ca2+) and curved tubulin oligomers. Similar to previously reported free tau-tubulin heterodimer and oligomer complexes38–40 and tubulin spiral structures under non-assembly-promoting conditions41, the SAXS data suggests that the curved tubulin structures in the three distinct states (Bws, Bint, Ring) are coated with tau (discussed below).
Mg2+- or Ca2+-induced structural changes (smaller lattice size, larger bundle domain size, increased depolymerization) also occur as a function of time for samples with intermediate concentrations of added Ca2+ or Mg2+ (clower ≈ 0.8 to cupper ≈ 1.4 mM Ca2+ or clower ≈ 1.2 to cupper ≈ 2.2 mM Mg2+). As shown for 1.2 mM Ca2+ (Fig. 2C), each of these samples originates in the Bws state but abruptly transitions to the Bint state after several hours. This transition is accompanied by increased scattering intensity at the form factor minima over time due to the rapid increase in MT depolymerization rate and tubulin ring proliferation, despite excess GTP remaining in the system for up to 72 h (Supplementary Fig. 2). Depolymerization of nearly all MTs and the structural evolution to the ring state is typically observed between 6 to 9 h after the Bws to Bint transition. Figure 2C (1.2 mM Ca2+) shows a typical example, where the Bws phase is observed at t0 + 6 hrs and t0 + 12 hrs, the Bint phase at t0 + 18 hrs and t0 + 21 hrs, and the ring state at t0 + 30 hrs. We note that the observed depolymerization is not occurring due to denaturation from multiple one-second synchrotron exposures and that separate experiments testing the effects of prolonged X-ray radiation were unable to produce the intermediate state (Supplementary Fig. 3).
Kinetic phase diagrams for Ca2+ (Fig. 3A) and Mg2+ (Fig. 3B) summarize the SAXS data and visualize distinct regions where the Bws (green), Bint (yellow), and (tau-coated) tubulin rings (magenta) are dominant. This data reveals a clear decrease in the lifetime of the Bws with increased divalent cation content, which is likely related to a similar effect by tetra-valent spermine on paclitaxel-stabilized MTs42, where ion-induced depolymerization occurs due to disruption of the lateral bond between the M-loop on one β-tubulin and the H1-S2-loop on the neighboring β-tubulin. The intermediate state was not observed with increased monovalent cations (instead of divalent ions) added to the standard buffer, instead showing that dw-w remained constant over the tested range of added KCl (up to 150 mM KCl added to the PIPES buffer, Fig. 4).
Fig. 3. Kinetic phase diagrams of tau/tubulin mixtures as a function of Ca2+ or Mg2+ concentration and time.
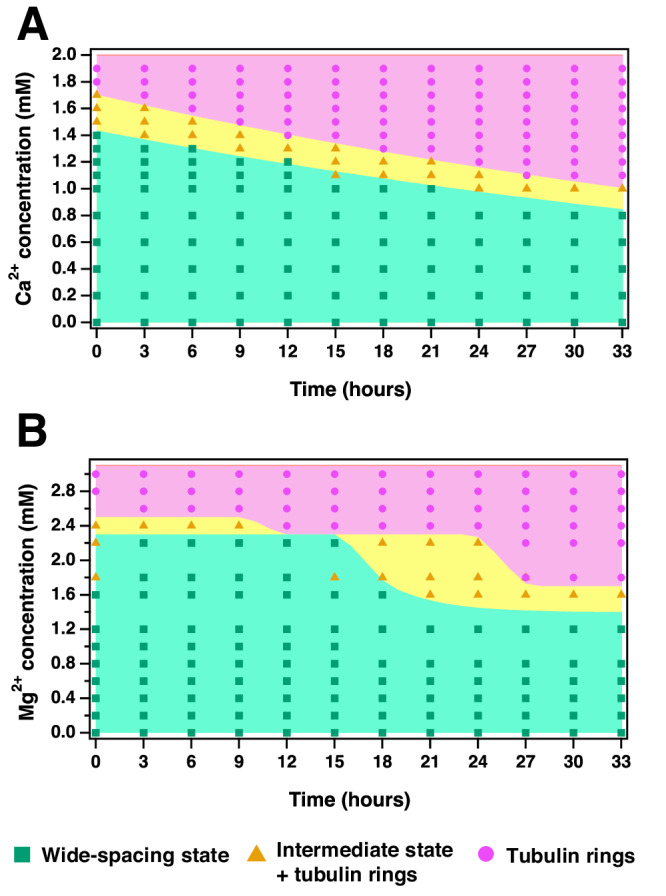
Markers denote the identified phase from line shape fitting analysis of SAXS experiments with green squares, yellow triangles, and pink circles corresponding to the Bws, Bint, and (tau-coated) tubulin ring states respectively. Data shows distinct regions for each phase and highlights that the bundled microtubule state is a function of time and A Ca2+ or B Mg2+ concentration. Source data of all points is provided in the Source Data file.
Fig. 4. Synchrotron SAXS data reveals the wall-to-wall distance of bundled microtubules is not dependent on KCl concentration.
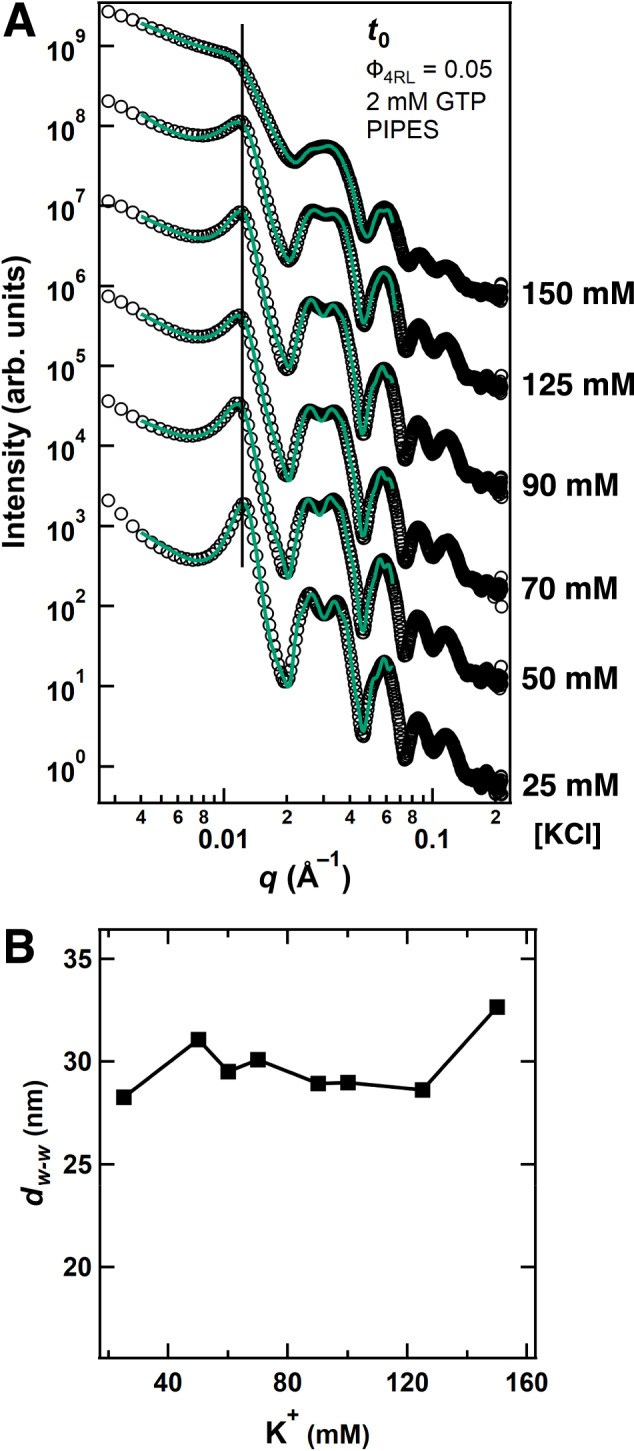
A SAXS data (open circles) corresponding fits (solid green lines) of tubulin/tau/GTP mixtures at 37 °C and 4RL-tau to tubulin-dimer molar ratio Φ4RL = 0.05 with increasing KCl concentration. SAXS scans are offset for clarity. B Plot of the fitted wall-to-wall distance (dw-w) from SAXS data in A highlights the lack of change in dw-w with increasing KCl concentration. Samples contained the stated KCl concentrations added to PIPES buffer at pH 6.8, which includes 1 mM of Mg2+. All data is provided in the Source Data file.
We note that curved tubulin oligomers exist to some degree in all three labeled phases with a larger fraction in the Bint state compared to the Bws state. Additionally, in the Bint state, significantly increased scattering contributions from closed rings are observed. The ring state in Fig. 3 describes SAXS profiles where the BMT q10 peaks are unable to be resolved. We also note that while the time of transition to the intermediate state was reproducible when comparing samples from the same batches of tau and tubulin, variability was observed when comparing the time of transition across different protein batches (Supplementary Fig. 4). Because of this, all time-based experiments presented here were conducted using the same batches of tau and tubulin.
Increasing (or decreasing) the initial GTP concentration for a prepared sample also effectively shifts the kinetic phase diagrams shown in Fig. 3 by either increasing (or decreasing) the time delay before the intermediate phase transition and by lowering (or increasing) the minimum divalent ion concentration necessary for the intermediate phase to be observed by 33 h. Figure 5 shows scattering profiles for samples prepared with 1.5 mM added Mg2+ (Fig. 5A) and Ca2+ (Fig. 5B) at time t0 and varying GTP concentrations. Data shows clear examples of all three labeled tubulin phases with varying GTP concentrations and highlights that the concentration necessary to induce the intermediate phase is lower for Ca2+ compared to Mg2+.
Fig. 5. Synchrotron SAXS data reveals that the tubulin-tau assembly state is GTP dependent.
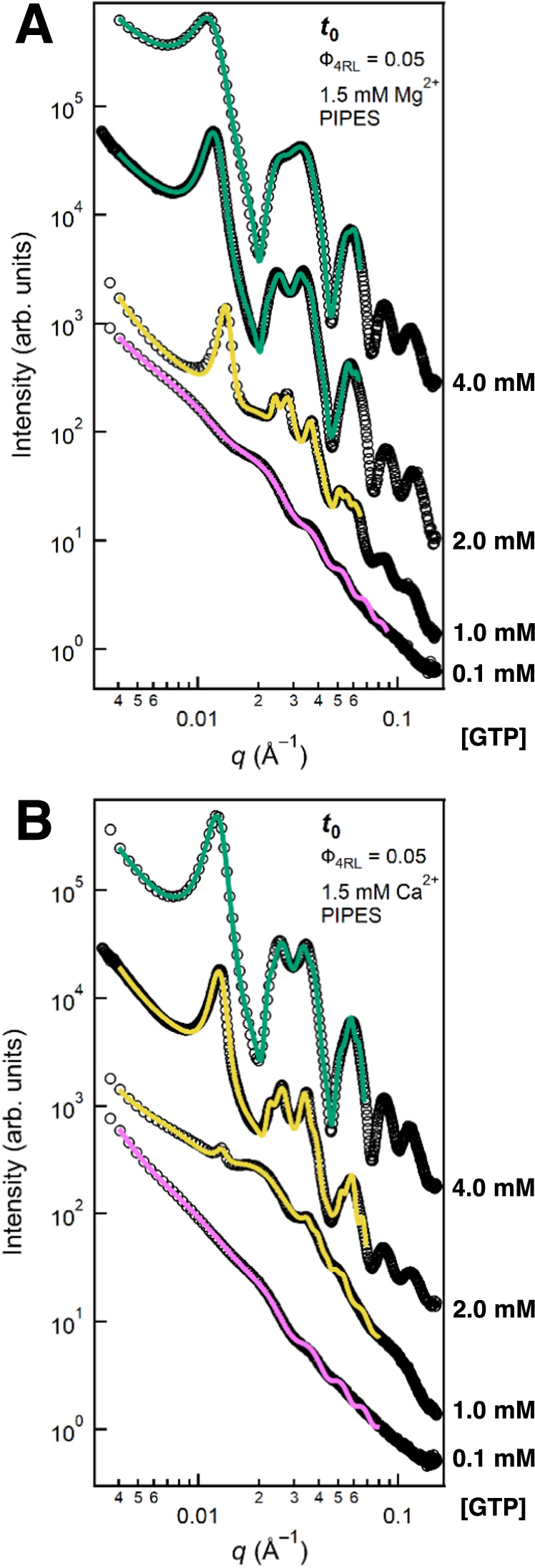
SAXS data (open circles) and fit lines (colored solid lines) of tubulin/tau/GTP mixtures at 37 °C and 4RL-tau to tubulin-dimer molar ratio Φ4RL = 0.05 with increasing GTP concentration and 1.5 mM MgCl2 (A) or CaCl2 (B). SAXS scans are offset for clarity, and fit lines are color-coded to the dominant scattering phase for each sample with green, yellow, and magenta, corresponding to the wide-spacing bundled (Bws), intermediate bundled (Bint), and depolymerized tubulin ring states respectively. Raw data and corresponding fits are provided in the Source Data file.
While the time delay of the intermediate phase transition can be modulated with GTP concentration, it is important to note that the changes in the time of transition shown in Fig. 3 are primarily driven by the increased concentration in divalent cations and not due to differences in depletion or availability of GTP. This is apparent when considering that GTPase activity at the β-tubulin subunit occurs primarily for GTP-tubulin incorporated in an MT lattice43–46, limiting the rate of GTP hydrolysis proportional to the ratio of lattice-bound GTP-tubulin to free GTP-tubulin. SAXS data indicates this ratio is highest (most GTPase activity) at low divalent cation concentrations—where the scattering intensity from MTs is the highest. SAXS line-shape analysis of samples with low divalent cation concentrations (below 1.2 mM Mg2+ and 0.8 mM Ca2+) indicates that MTs are stable at late time points (up to 72 h, Supplementary Fig. 1), despite high lattice-bound GTP-tubulin:free GTP-tubulin ratios, implying that GTP is present well beyond experimental timeframes, even when the rate of hydrolysis is maximal. Thus, reaction mixtures with higher divalent cation content – where the Bint state and the ring state are observed – must also contain excess GTP, even as MT depolymerization occurs.
TEM confirms structural differences between Bint and Bws
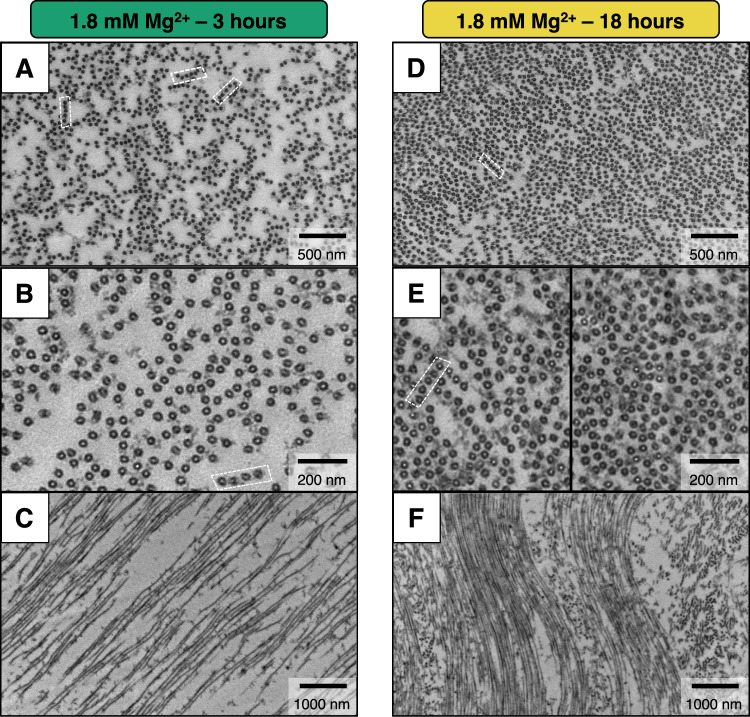
To better understand structural differences between the two bundled states, TEM experiments were performed on plastic-embedded tau/tubulin/2 mM GTP reaction mixtures in standard buffer containing 1.8 mM of added Mg2+ at 37 °C (Methods). Based on the phase diagram above, samples were individually prepared and fixed at 3 h (Fig. 6A–C) and 18 h (Fig. 6D–F) after polymerization to capture the Bws and Bint states, respectively. Consistent with SAXS line-shape analysis, cross-sectional images at lower (Fig. 6A, D) and higher (Fig. 6B, E) magnification show distinct phase-separated bundled domains at both time points. Larger domain sizes (bundle widths) are seen at 18 h (Fig. 6D, E in Bint state) compared to 3 h (Fig. 6A, B in Bws state), and measurements from higher magnification images also reveal an average interaxial spacing (aH) between bundled MTs that is 7.1 nm larger in the Bws phase (Fig. 6B) compared to the Bint phase (Fig. 6E), comparable to values measured via SAXS (ΔaH = 6.7 nm, ΔaH for TEM was determined by comparing the Pair-Distance-Distribution-Function of n = 4,843 MTs and n = 2,912 MTs for t0 + 3 h and t0 + 18 h respectively). Similarly, TEM reveals MT pairs at 18 h have more cross-linkages and lower heterogeneity of wall-to-wall distances compared to those at 3 h, which coincides with narrower SAXS peak widths (i.e. larger coherent domain sizes) observed for the Bint phase. Low-magnification side-view TEM images (Fig. 6C, F) also reveal structural features on length scales beyond the resolution of SAXS, showing that MT bundles in the intermediate state form larger extended arrays than in the wide-spacing state.
Fig. 6. Plastic-embedded TEM confirms the existence of the Bws and Bint bundled phases.
Electron microscopy of microtubule assemblies prepared at 37 °C with mixtures of tau, tubulin, and 2 mM GTP in PIPES buffer at pH 6.8 with 1.8 mM added Mg2+ and fixed after 3 h (A–C) and 18 h (D–F). Time points for sample fixation were selected based on SAXS data for samples prepared with identical conditions, where the wide-spacing Bws and the intermediate Bint bundled phases were present at 3 and 18 h, respectively. A, D Top panels depicting cross-sections at low magnification show the extent of MT bundling for both phases (Bws state in A and Bint state in D). The images further show the propensity for MT bundles to arrange in linear arrays (dashed white boxes). B, E Higher magnification cross-sections at 3-hour and 18-hour timepoints highlight the larger MT-MT spacing in the Bws compared to Bint phase and the larger number of MT-MT cross-bridges per MT (and larger average number of neighbors for each MT) in the Bint compared to the Bws phase. C, F Low magnification side views at 3-hour and 18-hour timepoints show that the width of the MT bundles (i.e. bundle size) is much larger in the Bint compared to the Bws phase, and the spacing between MTs is smaller in the Bint compared to the Bws phase, consistent with trends observed in SAXS data. All samples contained the stated Mg2+ concentrations added to standard PIPES buffer at pH 6.8, which includes 1 mM of Mg2+ (see Methods). Raw TEM images are provided in the Source data file.
In agreement with plastic-embedded TEM measurements of Chung et al.33 (with no added Mg2+ to the buffer), few well-defined hexagonal arrays are observed, especially in the wide-spacing state. Instead, there is an apparent preference for MTs to form linear, string-like bundles (Fig. 6A, B, D, E white boxes) reminiscent of fascicles found within the axon-initial-segment (Supplementary Fig. 1). Even within regions of high microtubule density (Fig. 6D), where the probability of MT-MT interactions is higher, stacks of linear arrays or branched chains of MTs are more abundant than true hexagonal bundles.
Increased tubulin ring concentration in Bint phase
To quantify the spike in MT depolymerization corresponding to the Bws to Bint transition observed via SAXS (i.e. filling in of local minima, Fig. 2A, C), scattering data was fit to a model profile, I(q) (Methods, Supplementary Code 1), which consists of three separate terms modeling hexagonally bundled microtubules31,33,42, tubulin rings, and scattering from unpolymerized tau-tubulin mixtures and their aggregates plus a constant scattering background47. Separating the fit model into these three terms allowed us to differentiate the relative abundance of depolymerized tubulin in the ring configuration compared to unpolymerized tau-tubulin oligomers and aggregates (Methods). Comparing fit data from all of our samples revealed that the proportion of tubulin mass in the ring state is much higher in the Bint compared to the Bws state.
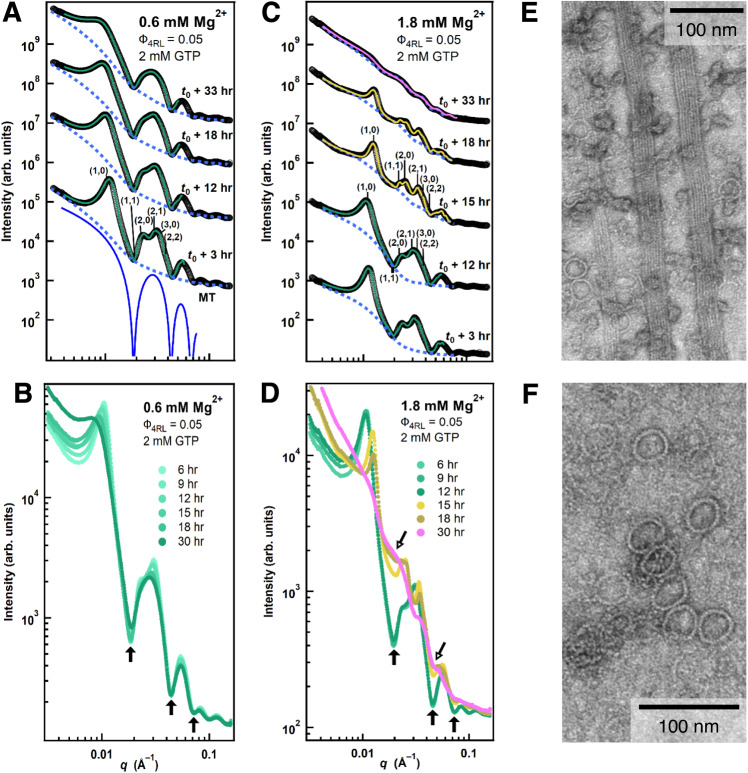
Fits to the time-dependent SAXS data of reaction mixtures polymerized with 0.6 and 1.8 mM added Mg2+ (Fig. 7A–D) highlight the differences in scattering from unpolymerized tubulin oligomers between the Bws and Bint states, similar to behavior found for Ca2+ samples (Fig. 2A, C). Solid lines through the SAXS profiles in Fig. 7A, C are fits of the data to I(q) (Methods). For 0.6 mM Mg2+ (below clower ≈ 1.6 mM Mg2+) the Bws state is stable for the duration of the experiment, despite indications of gradual MT depolymerization due to ongoing partially suppressed dynamic instability. Overlapping the SAXS profiles without offset shows a slight increase in scattering intensity at the first local minimum over time (Fig. 7B, solid arrows) but not at all other minima, indicating that tubulin rings were not formed (Methods) and that the tubulin depolymerization products are instead forming larger size tubulin oligomers and aggregates.
Fig. 7. Time-dependent synchrotron SAXS data at 0.6 and 1.8 mM Mg2+ in the wide-spacing (Bws) and intermediate (Bint) microtubule (MT) bundle states reveal prevalence of rings in the Bint state.
A SAXS data (open circles, profiles offset for clarity) and corresponding fits (solid lines) of a sample at 0.6 mM Mg2+ (below clower ≈ 1.6 mM Mg2+) in the Bws state (2D Hexagonal peaks indexed for profile at t0 + 3 hrs). Dashed lines are non-MT scattering contribution obtained from fits. Bottom profile (solid blue curve) depicts the form factor of a MT. B Data from A plotted without offset. Solid arrows point to MT form factor minima, highlighting nominal change in non-MT scattering over time. (C) SAXS data (open circles, profiles offset for clarity) and corresponding fits (solid lines) at 1.8 mM Mg2+ (between clower ≈ 1.6 mM Mg2+ and cupper ≈ 2.4 mM Mg2+) show a transition with increasing time from the Bws (profiles at t0 + 3 hrs and t0 + 12 hrs) to the Bint (profiles at t0 + 15 hrs and t0 + 18 hrs) and to the tubulin ring state (profile at t0 + 33 hrs). 2D Hexagonal peaks indexed for profiles at t0 + 12 hrs (Bws) and t0 + 15 hrs (Bint). D Data from C without offset. An abrupt increase in tubulin ring scattering fills in the minima of the MT Form Factor (open arrows) at the transition between t0 + 12 and t0 + 15 h. The fit lines in A–D are color-coded (green, yellow, and magenta represent the Bws, Bint, and tubulin ring states, respectively). Parallel whole-mount TEM image taken at t0 + 18 hrs of a sample prepared with 1.8 mM Mg 2+ added, showing E coexistence of tubulin rings with bundled MTs and F approximate dimensions of single tubulin rings. Samples contained the stated Mg2+ concentrations added to PIPES buffer at pH 6.8, which includes 1 mM of Mg2+. All data shown is provided in the Source Data file.
For Mg2+ concentrations between clower and cupper (≈ 1.6 to ≈ 2.4 mM Mg2+), the Bws state is stable for many hours with characteristically little tubulin ring formation (Fig. 7C, D, <15 h). However, transitioning to the intermediate bundled state coincides with a sudden spike in scattering intensity at all form factor minima (Fig. 7D, solid arrows). This distinct change in scattering (between 12 and 15 h for 1.8 mM Mg2+) is well described by an increase in scattering from tubulin rings (Methods) and smaller curved tubulin oligomers. Plots of the raw scattering data for 1.8 mM Mg2+ without offset (Fig. 7D) show a remarkable overlap of scattering intensity around the local minima at t0 + 18 and t0 + 30 hrs, with oscillations in SAXS at t0 + 30 hrs well-described by the theoretical scattering profile of a single tubulin ring (rin = 17.3 nm, Methods). Whole-mount TEM images prepared at 18 h (Fig. 7E, F) confirm this observation made with SAXS, showing the prevalence and coexistence of tubulin rings with MT bundles (Fig. 7E) which contain similar dimensions to those determined by SAXS (Fig. 7F). Together, our SAXS and TEM data directly show that changes to the bundled MT lattice strongly correlate with the sudden proliferation of tubulin rings and small curved tubulin oligomers (due to the dramatic increase in the rate of MT depolymerization).
SAXS data suggests that the coverage of tau on the MT surface remains roughly constant during MT depolymerization and thus implies that tau remains bound to the depolymerized tubulin products. This is inferred by measuring changes in the average MT’s radius with time. Tau was previously shown to increase the average MT diameter in a concentration-dependent manner48. This effect was also observed through our own SAXS experiments designed to test the effect of tau coverage on MT wall-to-wall spacing (Supplementary Fig. 4), where tau-tubulin dimer ratios ranging from Φ4RL = 1/100 (mushroom regime) to Φ4RL = 1/5 (brush regime) showed only small differences in dw-w between samples (Supplementary Fig. 4), but a monotonic increase in the MT radius. Conversely, throughout all time-based SAXS experiments where MT bundles were observed to transition from the wide-spacing to the intermediate state, the measured radius of the MT never increased but instead slightly decreased. Together this shows that the dw-w of the Bint state cannot be explained by changes in the density of tau on the MT surface during MT depolymerization.
Bws to Bint transition coincides with tubulin ring formation
Figure 8 summarizes the results obtained from fitting SAXS data to I(q). Within the Bws state (Fig. 8, arrows indicate the last time point where Bws was observed), dw-w increased rapidly at early time points due to relaxation from sample centrifugation and more slowly thereafter, reaching values up to dw-w ≈ 49 nm at 33 h (Fig. 8A, C). Unlike in the Bws state, dw-w spacings are comparatively stable in the Bint state. The average stabilized wall-to-wall distance in the Bint state for samples that transitioned during the experiment was 29.8 nm and was independent of dw-w before the time of transition. Similarly, samples initially observed in the intermediate Bint state at t0 (yellow regions at time = 0, Fig. 3A, B) show no relaxation from centrifugation over time (unlike the Bws state), instead converging to average spacing dw-w = 30.4 nm for Mg2+ and 26.4 nm for Ca2+.
Fig. 8. Change in the microtubule (MT) wall-to-wall spacing, upon transitioning from the wide-spacing (Bws) to the intermediate (Bint) MT bundle state, correlates with tubulin ring proliferation.
Wall-to-wall spacings (dw-w = ah – 2[rin + w]) plotted as a function of time for the series of Ca2+ (A) and Mg2+ (C) samples (SAXS data shown in Figs. 2 and 7, respectively). Some data points omitted for clarity. Arrows indicate the latest time point at which the Bws state is observed before the sample transitions to the Bint state. Fitted tubulin ring scattering amplitude plotted as a function of time for the same series of Ca2+ (B) and Mg2+ (D) samples. Amplitudes are normalized by the maximum ring scattering measured. Arrows indicate the latest time point at which the bundled wide-spacing (Bws) state is observed before the sample transitions to the bundled intermediate (Bint) state, with increases in the tubulin ring scattering amplitude observed after. Comparisons between A and B for the Ca2+ series and between C and D for the Mg2+ series show that the onset of tubulin ring proliferation occurs concurrently with the transition from the Bws to the Bint state. Time “0” on the x-axis corresponds to t0, as defined in Fig. 2. All samples contained the stated Mg2+ or Ca2+ concentrations added to standard PIPES buffer at pH 6.8, which includes 1 mM of Mg2+ (see Methods). Data is provided in the Source Data file.
The stability of dw-w values measured in the Bint state relative to those in the Bws state implies that stronger interactions dictate MT-spacing in the Bint compared to the Bws state. This implication is further substantiated by the co-occurrence of the drop in dw-w and the substantial increase in coherent domain size (≈41 percent growth in the 6 h following the transition Supplementary Fig. 5) upon transitioning to the Bint state (i.e. time between data collected at the arrows in Fig. 7A, C and the next data point).
For each sample, comparing dw-w to the amplitude of scattering from tubulin rings (Aring, Fig. 8B, D) highlights the striking synchronization between the proliferation of tubulin rings and the abrupt drop in dw-w. This reduced MT-MT spacing, together with the increase in MT bundle domain size, precisely when increasing amounts of rings and smaller curved tubulin oligomers begin proliferating, suggests that tubulin oligomers directly affect the bundling of MTs and drive the Bws to Bint transition observed through SAXS and TEM.
The phase of bundled MTs is tunable with temperature
To directly test the effect of enhancing unpolymerized tubulin oligomer content on MT bundling, time-dependent SAXS experiments were devised to modulate dynamic instability. Protofilaments (tubulin oligomers) in excess GTP are known to adopt a higher curvature conformation at low temperatures49,50. Therefore, lowering the temperature of the sample environment below a critical temperature for polymerization pushes the tubulin dynamic equilibrium toward depolymerization, effectively increasing the tubulin oligomer content in the solution.
A sample polymerized with 2.0 mM added Mg2+ was monitored while the temperature was cycled for 30-minute periods at 37 °C, 21 °C, and 37 °C again. SAXS data from this temperature cycling experiment (Fig. 9) revealed that the otherwise stable wide-spacing state rapidly transitioned to the Bint state immediately following the drop in temperature to 21 °C. The characteristic shift in hexagonal Bragg peaks to higher q was observed, coinciding with a sudden increase in tubulin ring scattering. At 21 °C, dw-w decreased to a minimum value of 21.8 nm (change in wall-to-wall distance, Δdw-w = 8.5 nm), and scattering from tubulin rings increased over the 30-minute incubation at 21 °C. Increasing the temperature back up to 37 °C following the 30-minute incubation at 21 °C, the microtubule bundles reverted to the wide-spacing state, as indicated by the increase in dw-w to 30.5 nm, decrease in the coherent bundle size, abrupt decrease in scattering from tubulin rings, and corresponding increase in BMT scattering. This result further demonstrates the correlation between free tubulin oligomer content and MT bundle architecture as the system transitions between the Bws and the Bint states.
Fig. 9. Synchrotron SAXS data reveal the stability of the bundled wide-spacing state depends on temperature.
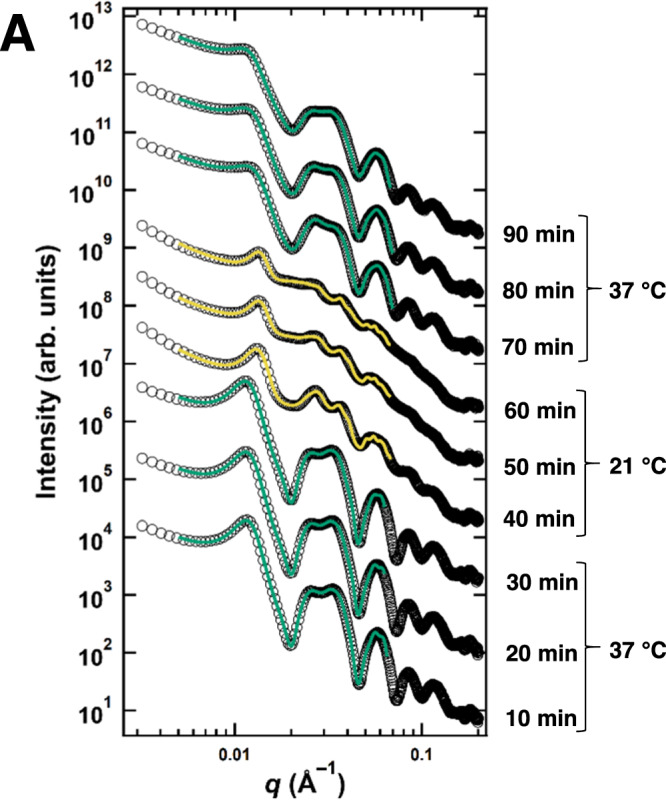
A SAXS data (open circles) and corresponding fits (solid lines) for a sample prepared with 2.0 mM MgCl2 added to the standard PIPES buffer at pH 6.8. The sample temperature was held at 37 °C for 30 min, quickly reduced to 21 °C and held for 30 min, then quickly cycled back up to 37 °C. Data was taken via 1-second x-ray exposures every 10 min. Fit lines are color-coded for the wide-spacing bundled (green) and the intermediate state (yellow). Raw data and corresponding fits are provided in the Source Data file.
TEM images show tubulin-tau complexes cross-bridging MTs
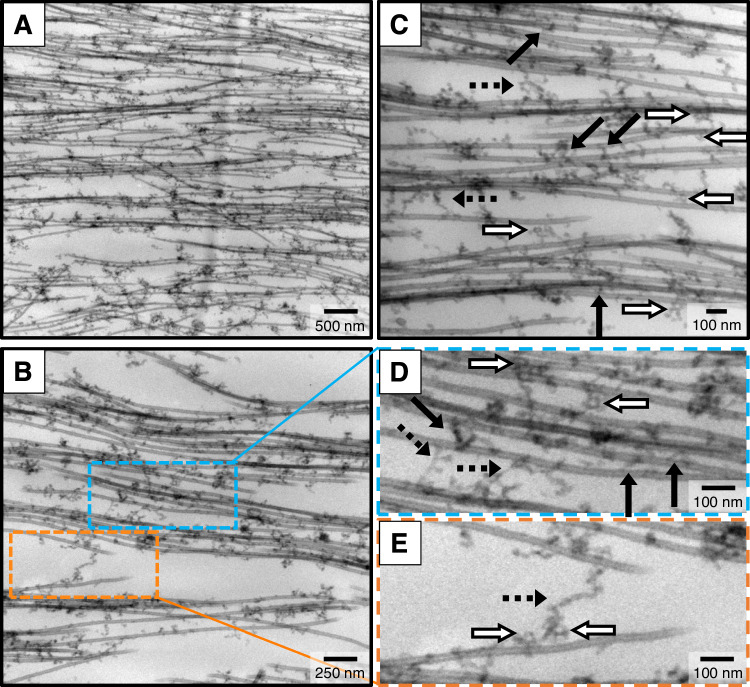
Plastic-embedded TEM side-views along the MT length provide potential insight into the structural components cross-bridging neighboring MTs and reveal a possible explanation for the Bint phase observed through SAXS. Close inspection of bundles in the Bws phase at 3 h at lower (Fig. 10A, B) and higher (Fig. 10C–E) magnifications reveals an extended intervening network of crosslinked proteins connecting MTs (Fig. 10C–E). Remarkably, cross-bridges are seen not only connecting MTs within bundles (Fig. 10C–E, solid black arrows) but also connecting neighboring bundles (Fig. 10C–E, dashed black arrows). Evidence of these tethers is also seen within the high magnification cross-sectional views shown in Fig. 6, with more crosslinks present in the Bint state (Fig. 6E) compared to the Bws state (Fig. 6B).
Fig. 10. Plastic-embedded TEM provides evidence that complexes of tubulin oligomers and tau cross-bridge bundled microtubules.
Electron microscopy with increasing magnification of microtubule assemblies prepared from mixtures of tau, tubulin, and 2 mM GTP in standard buffer with 1.8 mM added Mg2+ at 37 °C and fixed after 3 h. A, B At low and intermediate magnification, TEM images provide evidence that a network of filamentous proteins between bundled MTs is the linking medium that stabilizes MT bundles. C–E At higher magnifications the filamentous proteins of the network are seen to form MT-MT cross-bridges both within (black arrows) and between (black dashed arrows) bundled domains. The morphology of these cross-bridges is consistent with ≈ 5 nm wide semi-flexible tubulin oligomers. Tubulin ring structures (white arrows) are also present within the protein network. (D, E are expanded views of sections in B with blue and orange outlines). Complexes of tubulin oligomers and tau are also observed coating MTs but not forming MT-MT cross-bridges. All samples contained 1.8 mM Mg2+ added to standard PIPES buffer at pH 6.8, which includes 1 mM of Mg2+ (see Methods). Raw TEM images are provided in the Source data file.
While cross-bridges between bundled MTs in cells have been previously reported but attributed only to tau7,8,51, intrinsically disordered proteins such as tau (≈0.5 nm in width) are too thin to be visualized by TEM in our plastic-embedded preparations, implying that tau alone cannot make up the MT cross-bridge. In contrast, the morphology and dimension of the cross-bridges observed in TEM are consistent with tubulin oligomers, existing both as semi-flexible filaments (black solid and dashed arrows in Fig. 10C–E) and as tubulin ring structures (white arrows in Fig. 10C–E) with the same radius measured with SAXS and seen in whole-mount TEM (Fig. 7E, F). Thus, TEM data suggests that tubulin oligomers (which may include tubulin rings) are a central component of the observed intervening network between MTs and suggests that tau’s role in MT bundling is to act as the glue (through the binding repeats) which connects tubulin oligomers.
Discussion
The combined SAXS and TEM data lead us to propose a model where complexes of tubulin oligomers and tau form a viscoelastic intervening network that cross-bridges MTs into bundles. In this model bundling occurs due to coded assembly where tau’s MT binding repeats link together αβ-tubulin oligomers in the intervening network and tubulin oligomers near the MT surface to tubulin in the MT lattice (Fig. 1B). The model presents an important revision to current dogma where cross-bridging between MTs is attributed entirely to tau7,8,11,33,51–53.
This model is consistent with SAXS data for both the Bws and Bint GTP-stabilized MT bundled states and the transition between them. The abrupt influx of (tau-coated) tubulin rings and curved tubulin oligomers (due to Mg2+ or Ca2+ mediated MT depolymerization of a fraction of MTs) drives the transition from Bws to Bint by increasing the average number of tubulin-tau cross-bridges in remaining MT bundles (seen in TEM). Increased cross-bridging would explain the observed increase in bundle domain size of the Bint state.
The model reconciles years of contradicting reports regarding tau’s role in bundling tau7,8,11,51,54. While numerous early publications pointed to MT bundling as one of many roles of tau7,8,11,51, several cell-free studies of MTs containing the MT-stabilizing drug paclitaxel pointed to tau’s apparent inability to mediate MT bundles19,55. More recent SAXS studies show that paclitaxel, at paclitaxel/tubulin-dimer molar ratios of Λpaclitaxel = 1/1, suppresses MT bundling by all six tau isoforms56. Follow-up SAXS and TEM experiments showed that reducing paclitaxel below Λpaclitaxel ≈ 1/8 restores tau-mediated MT bundles48. These findings are consistent with our central discovery that bundling of MTs by tau requires free tubulin oligomers as Λpaclitaxel = 1/156 severely reduces free tubulin oligomers and MT bundles, and reducing paclitaxel below Λpaclitaxel ≈ 1/848 restores free tubulin and MT bundles.
At high osmotic pressures, tau-coated MTs stabilized by paclitaxel form tightly packed bundles with dw-w spacings in the range of ≈ 3.5 - 4 nm—close to the radius of gyration of tau’s PD35 and consistent with polyelectrolyte theory57. In contrast, to account for the average large dw-w observed GTP stabilized MTs, tau-only models for MT bundling currently require a highly extended conformation for the projection domain (PD) of tau (Fig. 1A). Proposals of short-range charge-charge interactions between weakly penetrating tau PDs33 and electrostatic zippers53 based on the dipolar nature of tau’s PD require extensions of the PD by factors of two and four, respectively, (Methods). Such extensions of tau’s PD are inconsistent with traditional polyelectrolyte theories57 for chain stretching in the mushroom regime (at low tau/tubulin dimer molar ratio) and would require sequence-specific theories of highly stretched polypeptide chains at physiological salt concentrations ( ≈ 150 mM 1:1 salt).
In our model, the wall-to-wall spacing is largely set by the average radius of a mixture of (tau-coated) curved tubulin oligomers and tubulin rings (outer diameter ≈ 40 nm). However, the relative fraction of curved tubulin oligomers and rings are different in the Bws state versus the Bint state, which contains significantly more rings. Quantitative SAXS line shape analysis indicates that the average size of free curved tubulin oligomers present in the Bws state is larger than in the Bint state. This is consistent with the smaller wall-to-wall spacing of the Bint state.
Because the binding interactions between tau and tubulin oligomers are specifically encoded by the MT binding repeats of tau, MT bundling is expected to be only weakly dependent on added monovalent salts. Indeed, dw-w is found to be essentially constant over a wide range of salt concentrations up to ≈ 150 mM KCl added to the buffer, which already contains ≈ 100 mM of 1:1 electrolytes (Fig. 4).
Together our results draw attention to the essential role of unpolymerized tubulin on MT bundling and highlight the significant impact of varied experimental conditions on tau’s bundling function. Our finding that divalent cations near average physiological Mg2+ concentrations can destabilize dynamic, steady-state MT bundles at 37 °C, despite the presence of tau and excess GTP, is unexpected. In particular, at concentrations above a critical divalent concentration clower (≈1.6 mM Mg2+), MT bundles undergoing suppressed dynamic instability abruptly become unstable, favoring the formation of tau-coated tubulin rings and effectively halting dynamic instability. This suggests a mechanism where MT growth and stability can be modulated within cells through fluctuations in local divalent cation concentrations, with potential consequences for cargo transport in axons. Additionally, we expect the value of clower to be modulated by disease-relevant post-translational modifications and truncations of tau.
We expect tau’s role in MT bundling to be synergistic with other proteins that promote MT bundle formation in neurons, such as TRIM4658–60, and other members of the vertebrate family of MAPs with similar MT binding regions61. The implications of our discovery of an intervening network of complexes of tubulin oligomer and tau should spur further studies on tau and other MAPs, such as MAP2, that mediate bundle formation with different wall-to-wall spacings7,8,54,55.
A major function for bundled MT fascicles in the axon-initial-segment (AIS) is related to their role as a component of the filter for MT-based cargo trafficked between the soma and axon17. An intervening tubulin-tau network stabilizing MT fascicles should lead to a more efficient retrograde diffusion barrier that captures and prevents tau leakage outside of the axon. Biochemical alterations to tau (as happens in Alzheimer’s disease and other tauopathies) could further modulate the barrier properties in the AIS, with significant implications for missorting of tau to the somatodendritic compartment and neurodegeneration. Finally, complexes of tubulin oligomers and tau of the intervening network, or those bound to MTs not in bundles, may represent an important site of chemical modification or fragmentation of tau by enzymes leading to aberrant tau behavior and nucleation and growth of tau fibrils in tauopathies.
Methods
Tubulin and Tau Purification
Tubulin was purified from bovine brain obtained at a commercial slaughterhouse from Mr. Ramero Carlos of Manning Beef LLC in Pico Rivera, CA. Purified tubulin was filtered into PEM 50 buffer containing 50 mM PIPES, 1 mM MgSO4, 1 mM EGTA, and 0.1 mM GTP, pH 6.8 with ≈ 75 mM NaOH, and drop frozen into liquid nitrogen34. First, meninges, superficial blood vessels, and blood clots were removed at the slaughterhouse. Brains were then blended for 30 s at a low setting in a Waring Commercial Blender at a ratio of 1.5 mL of L-GNPEM buffer, pH 6.85, per gram of wet brain weight and subsequently homogenized in a motor-driven Teflon pestle/glass homogenizer operated at maximum speed (Tri R Stir R motor). Brain homogenate was then centrifuged at 32,500 x g for 40 min at 4 °C using 50 mL centrifuge tubes, supernatant collected, and GTP was added to supernatant in dry form for 2.5 mM total concentration. This crude brain extract was subjected to one purification cycle of the following steps: glycerol-free polymerization (incubation at 30 °C for 30 min), centrifugation (at 45,000 x g for 30 min at 30 °C, pellet containing MTs collected), depolymerization (dilution of collected pellet into cold L-GNPEM buffer and incubation for 40 min at 4 °C), and clarification (centrifugation at 45,000 x g for 45 min at 4 °C, supernatant collected). 2.5 mM GTP was then added to the resulting tubulin-rich supernatant, and the supernatant was incubated at 30 °C for 30 min. The resulting suspension of microtubules was centrifuged at 45,000 x g for 60 min at 30 °C, with the pellet collected and frozen in liquid nitrogen and stored at –70 °C. To remove any microtubule-associated proteins from the purified tubulin, frozen pellets were quick thawed at room temperature, then cooled on ice, resuspended in PEM50 buffer, pH 6.8, homogenized in a glass pestle, and incubated on ice for 20 min. The solubilized pellet solution was then centrifuged at 105,000 x g for 1 h at 4 °C. The supernatant was collected, and 100 μM GTP was added to the solution, which was subsequently passed through a phosphocellulose column and eluted into 2–3 mL fractions. Fractions containing purified tubulin were pooled, placed on ice, and concentrated to between 7 and 14 mg/mL as measured by Bradford assay using BSA as a standard, then drop-frozen in liquid nitrogen to be stored as frozen beads at –70 °C.
cDNA expression vectors (pRK) encoding full-length (4RL) tau was gifted by Dr. Kenneth Kosik (University of California, Santa Barbara). Following standard procedures62, Tau was expressed in BL21(DE3) pLacI cells (Invitrogen) with 18 h incubation in 250 mL of Luria broth (10 g of tryptone, 5 g of yeast extract, and 10 g of NaCl per liter of DI water) followed by 24 h incubation in 6 L of auto-induction media (10 g of tryptone, 5 g of yeast extract, 0.5 g of dextrose, 2 g of α-D-lactose and 5 mL of glycerol per liter of 25 mM NaHPO4, 25 mM KH2PO4, 50 mM NH4Cl, 5 mM Na2SO4 in DI water). Bacteria were harvested by centrifugation in a Sorvall RC-5B Plus centrifuge at 4,200 x g for 10 min maintained between 4 °C and 10 °C. Bacteria resuspended in BRB80 buffer (80 mM PIPES, 1 mM EGTA and 1 mM MgSO4) pH 6.8 with ≈120 mM NaOH, total 1:1 ion equivalent of 160 mM, Debye length, λD ≈ 7.6 Å63) were lysed by passing through a French pressure cell three times at >900 PSI, subsequently boiled for 10 min, and then centrifuged at 20,200 x g for 40 min. The supernatant was collected and passed over a phosphocellulose anionic exchange column and eluted with increasing concentration of (NH4)2SO4 (up to 1 M) in BRB80. Tau-containing fractions were subsequently pooled and brought to 1.25 M (NH4)2SO4, then further purified using hydrophobic interaction column chromatography (HisTrap Phenyl HP, GE Healthcare), eluted with decreasing concentration of (NH4)2SO4 in BRB80. Fractions containing pure tau were pooled, then concentrated and buffer-exchanged into BRB80 by successive centrifugation cycles using Amicon Ultra-15 Centrifugal Units with MWCO = 10,000 (EMD Millipore, Darmstadt, Germany). Final tau stocks were stored at −80 °C until needed for experiments. Concentration was determined by SDS-PAGE comparison with a tau mass standard, the concentration of which had been established by protein mass spectrometry and stored at – 80 °C in BRB80 (80 mM PIPES, 1 mM EGTA, 1 mM MgCl2, pH 6.8 with ≈ 120 mM NaOH, total 1:1 ion equivalent of 160 mM, Debye length, λD ≈ 7.6 Å64).
SAXS and TEM (Whole-mount and plastic-embedded) sample preparation
Reaction mixtures were prepared on ice in the following way. Purified tubulin (92 uM stock) was thawed, diluted into PEM50 (50 mM PIPES, 1 mM EGTA, 1 mM MgCl2, pH 6.8 with ≈75 mM NaOH, λD ≈9.6 Å), and mixed with solutions of GTP in PEM50 (100 mM stock), tau (37 uM stock in BRB80), and PEM50 buffer with additional MgCl2 or CaCl2 content (5–50 mM stocks). Final reaction mixtures contained 4.0 mg/mL tubulin in 50 uL of buffer (λD ≈9.5 Å). Sample tubes containing reaction mixtures were placed in a 37 °C water bath for 30 min of polymerization to reach dynamic equilibrium. The MT-tau reaction mixtures were then prepared for experiments as follows.
For SAXS, reaction mixtures were directly loaded into 1.5-mm diameter quartz mark capillaries (Hilgenberg GmbH, Malsfeld, Germany) after polymerization. Capillaries were subsequently spun in a capillary rotor in a Universal 320R centrifuge (Hettich, Kirchlengern, Germany) at 9500 x g and 37 °C for 30 min to form protein-dense pellets suitable for SAXS. Pelleted capillaries were then sealed and held at 37 °C in a custom-made, temperature-controlled sample holder for data acquisition.
Sample preparations for whole-mount and plastic-embedded TEM mirrored methods previously described31,33,42 and were as follows. For whole-mount TEM, reaction mixtures were diluted into a warm buffer to 0.2 mg/mL tubulin and loaded onto highly stable Formvar carbon-coated copper grids (Ted Pella, Redding, CA), with excess solution wicked with Whatmann paper after 2 min. 1% uranyl acetate was added to the grid for 20 s and wicked off. Then, five drops of Milipore H2O (18.2 MΩ) were added and wicked off. Grids were allowed to dry for 24 h at room temperature before imaging.
For plastic-embedded TEM, reaction mixtures were centrifuged to a pellet in microcentrifuge tubes at 9500 x g at 37 °C for 30 min. The supernatant was removed, and pellets were fixed with 2% glutaraldehyde and 4% tannic acid overnight. Pellets were stained with 0.8% OsO4 in PEM50 buffer for 1 h and subsequently rinsed four times with PEM50. Another stain of 1% uranyl acetate stain was applied for 1 h and rinsed with DI water. Fixed and stained pellets were subsequently dehydrated with 25/50/75/100% solutions of acetone in DI water for 15 min each. Pellets were then embedded in an epoxy-based low viscosity embedding media prepared by mixing 5 g of ERL 4221 (3,4 Epoxy Cyclohexyl Methyl 3,4 epoxy Cyclohexyl Carboxylate), 4 g of D.E.R. 736 (diglycidyl ether of propylene glycol), 13 g of NSA (nonenyl succinic anhydride), 0.2 g of EASE (PolyCut-Ease), and 0.2 g of DMAE (dimethylaminoethanol). Dehydrated sample pellets were infiltrated with embedding media, poured into flat embedding molds, held at 65 °C for 48 h to polymerize, and then cooled overnight before sectioning. Plastic-embedded samples were then cut to ~ 50-nm slices with a microtome (Ted Pella, Redding, CA) and transferred to Formvar carbon-coated copper EM grids.
X-ray scattering and analysis
SAXS experiments were performed at beamline 4-2 of the Stanford Synchrotron Radiation Lightsource at 9 keV using a custom-made temperature-controlled sample holder. Experiments were performed at 37 °C unless otherwise noted. For the temperature ramp-down experiment, initial data was taken at 37 °C. Then the temperature controller was set to 5 °C, which was reached over <7 min. A needle temperature probe was inserted into a water-filled quartz capillary placed in the sample holder for instantaneous temperature recordings during the ramp down, approximating the apparent temperature for the samples. Additional information on data collection and experimental setup is provided in Supplementary Table 1.
Data from 2D scattering images was obtained with a Pilatus3 X 1 M 2D-detector and azimuthally averaged to create 1D scattering profiles. Data reduction was performed using Nika SAS65, and quantitative line-shape analysis was performed using a custom-written function in C-plot (Supplementary Table 1). Scattering data was fit to the model profile, I(q):
| 1 |
The first term in I(q) consists of the structure factor S(qr) of the bundled MT lattice multiplied by the form factor of a MT (|FMT(qz,qr)|2) and is averaged over all orientations in q-space (qr, qz are wavevectors perpendicular and parallel to the MT cylinder axis). The structure factor of the bundled MT state was modeled as the sum of square Lorentzians at every 2D reciprocal lattice vector qhk = q10(h2 + k2+hk)1/2 with amplitude Ahk and peak width κhk:
| 2 |
The first three Bragg peaks (q10, q11 = 31/2q10, q20 = 2q10) were individually fit, while all other peaks were thereafter fit simultaneously. To limit the number of fitting parameters, all simultaneously fit peaks were assumed to have the same peak width as κ20 (the highest-order peak that was individually fit). The center-to-center distance between microtubules is given by ah = 4π/(q10√3), and the coherent domain size of the MT lattice (i.e. the hexagonal bundle width) is inversely related to the width of the structure factor peaks and is given by Llattice = 2(πln4)1/2/κ10)42.
The form factors (F) of both MTs and tubulin rings were calculated by modeling them each as hollow cylinders with uniform electron density35,56, wall width set to w = 49 Å63, and lengths fixed at LMT = 20,000 Å (larger than the resolution of our wavevector) and Lring = 42 Å (consistent with electron microscopy data for single tubulin rings)56:
| 3 |
| 4 |
Here, J1 is the Bessel function of order 1, rin and rring is the ensemble-averaged inner radius of the MT and tubulin ring in Eq3. and Eq.4, respectively, and Aring is the scattering amplitude of the ring state. The MT cylinder’s inner radius, rin, was the only fit parameter in the MT form factor. The tubulin ring inner radius and length was fit for samples identified to be in the ring state. For samples in the Bint or Bws states, the ring’s inner radius and length was held at values fitted for that sample at later time points (i.e. if it transitioned into the tubulin ring state), or set to literature values (rring = 16.3 nm and Lring = 42 A) if the sample never fully depolymerized.
Since the first order Bessel functions of the MT form factor (J1 terms in Eq3.) equal 0 for distinct values of qr (given rin), the scattering contribution from MTs (both bundled and unbundled) to the total raw scattering profile will also approach zero at these points, resulting in the deep local minima observed in the theoretical scattering from a single MT (Fig. 6A, bottom blue curve). Therefore, since scattering from all tubulin within the MT lattice is suppressed at these distinct values of qr, the measured intensity at these points within the raw data must be almost entirely due to the scattering from unpolymerized tubulin oligomers plus the q-independent background (|FRing|2 and BG(q), Eq. 1). Because of this, an approximation for the concentration of tubulin rings can be made as: 1) any increase in the concentration of tubulin rings would require the intensity of all Bessel function minima to increase by a linear amount and 2) the exponential Porod scattering from larger tubulin structures decays much quicker than tubulin ring scattering, meaning that observed changes in the scattering intensity at higher q (>0.04) are dominated by changes in Aring.
The third term in Eq.1, BG(q), which accounts for scattering from unpolymerized tau-tubulin mixtures and their aggregates plus a constant scattering background, was modeled simply as a two-layer, unified fit function47 at the Bessel function minima for samples at initial timepoints (t0):
| 5 |
The first term, G, is Guiner’s law, the second and third terms, B1 and B2, are power-law scattering terms which describe the scattering at lengths where qRg »1 and qRg «1 (Rg defined below), respectively, and the fourth term, BG0, is a flat scattering term independent of q. Scattering from the third and fourth term in the equation above are only appreciable to the total scattering profile (I(q), Eq. 1) at very low-q (<0.005 Å −1) and high-q (>0.15 Å −1) respectively, and thus were individually fit within these two domains. Fitting bounds for Rg were determined by fits of the unified scattering function to unpolymerized tau tubulin samples consisting of a distribution of tubulin oligomers and their aggregates resulting from the presence of tau (with minimal scattering contributions from tubulin rings) and were fit simultaneously with the first Bragg peak, as its scattering was most prominent at this length scale (Rg = 15–20 nm). Lastly, the fitting parameters of the second term dominate the BG scattering signal for our samples between q = 0.03–0.09 Å−1, and were thus determined by fitting it to the set of points measured at time t0 where the scattering contribution of the bundled MT state to the raw data was approximately zero (i.e. at the Bessel function minima).
Transmission Electron Microscopy
All data were taken at 80 kV using the turn key JEM 1230 (JEOL) Transmission Electron Microscope at the University of California, Santa Barbara.
Calculation of RG and projection domain extensions
Previously, the radius of gyration (RG) of wild-type tau in solution was found66,67 to scale as an unstructured protein with random coil behavior, with RG = 0.1927N0.588 nm, which was subsequently used to calculate RGPD ≈ 3.7 nm and the physical diameter DPhys = 2RPhys = 2(5/3)1/2Rg ≈ 9.5 nm68 for the PD of 4RL tau. For the weakly penetrating tau-tau interaction model, DPhys is doubled to account for each opposing tau PD, giving a predicted dw-w of 19 nm, roughly half the dw-w we observed via SAXS. Similarly, for the electrostatic zipper model that proposes complete overlap of two opposing tau molecules, the predicted dw-w would just be DPhys, requiring significant extensions beyond previously reported RG values.
Statistics and reproducibility
As described in the results section, the time of transition from the Bws to Bint phase was consistent for samples prepared from the same batch of tau and tubulin. The time of transition at a given concentration across different batches however could differ by multiple hours (Supplementary Fig. 4). The general trends outlined within this paper however were consistent with all batches used. All data presented with the exception to the temperature experiments were performed using the same batch of tau and tubulin.
The number of times each of the SAXS experiments were independently conducted (across different batches of tau and tubulin) are as follows: thrice for Figs. 2, 3, 7, and 8, twice for Fig. 4, and once for Figs. 5 and 9.
For the plastic-embedded TEM, images came from 3 unique plastic-embedded slices for each time point. From them, 136 unique images of the Bws state were taken down the cylindrical MT axis and 34 unique images along the length of the MT. For the Bint state, 57 unique images were taken along the length of the MT and 209 unique images were taken down the cylindrical axis.
Whole-mount TEM images presented are from a single independent experiment, however the experiment was independently conducted on five different occasions with similar results. From the experiment presented 46 unique images were taken at 3 h and 57 unique images were taken at 18 h.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
This work was supported by the Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering, under award DE-FG02-06ER46314 (CRS and YL, charged bio-assemblies inspired by nature) and, in part, by the US National Science Foundation, Division of Materials Research, under award DMR-1807327 (CRS, phase behavior in biomaterials). PJC acknowledges support from the US National Science Foundation under award DMR-2104854. SCF acknowledges support from the US National Institutes of Health grant NS-35010 and the Academic Senate of the University of California at Santa Barbara. MCC was supported by Korea Dementia Research Center KDRC HU23C0094, KAIST Grand Challenge KC30 N11230021, Korea Basic Science Institute KBSI 2021R1A6C103B422, and National Research Foundation of Korea NRF RS202300277142 and NRF 2020M3A7B6026565.
Author contributions
C.R.S., C.S., B.J.F., P.A.K. and Y.L. designed research; H.P.M. purified tubulin; C.S., B.J.F. and C.T. provided tau protein isoforms from plasmid preparations; C.S., B.J.F., P.A.K. and X.G.A. performed research; P.A.K. analyzed SAXS data; P.A.K., C.R.S., B.J.F. and Y.L. modelled data; P.A.K., B.J.F. and C.R.S. wrote the paper; Y.L., P.J.C., R.L.B., S.C.F., M.C.C., L.W., C.T. and H.P.M. provided additional writing input; and Y.L., S.C.F., M.C.C. and L.W. provided critical suggestions on the presentation of X-ray and TEM microscopy data.
Peer review
Peer review information
Nature Communications thanks Eva Nogales and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The uncropped TEM images, SAXS data, and all resulting fits generated in this study are provided in the Source Data excel file. Source data are provided with this paper.
Code availability
The code used for the fitting of the SAXS data can be found in the Supplementary Code 1 file.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Phillip A. Kohl, Chaeyeon Song, Bretton J. Fletcher.
Contributor Information
Youli Li, Email: youli@mrl.ucsb.edu.
Cyrus R. Safinya, Email: cyrussafinya@ucsb.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-46438-x.
References
- 1.Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev. Cell Dev. Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- 2.Alushin GM, et al. High-resolution microtubule structures reveal the structural transitions in alphabeta-tubulin upon GTP hydrolysis. Cell. 2014;157:1117–1129. doi: 10.1016/j.cell.2014.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rice LM, Montabana EA, Agard DA. The lattice as allosteric effector: structural studies of alphabeta- and gamma-tubulin clarify the role of GTP in microtubule assembly. Proc. Natl Acad. Sci. USA. 2008;105:5378–5383. doi: 10.1073/pnas.0801155105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyman AA, Chretien D, Arnal I, Wade RH. Structural changes accompanying GTP hydrolysis in microtubules: information from a slowly hydrolyzable analogue guanylyl-(alpha,beta)-methylene-diphosphonate. J. Cell Biol. 1995;128:117–125. doi: 10.1083/jcb.128.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller-Reichert T, Chretien D, Severin F, Hyman AA. Structural changes at microtubule ends accompanying GTP hydrolysis: information from a slowly hydrolyzable analogue of GTP, guanylyl (alpha,beta)methylenediphosphonate. Proc. Natl Acad. Sci. USA. 1998;95:3661–3666. doi: 10.1073/pnas.95.7.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chretien D, Fuller SD, Karsenti E. Structure of growing microtubule ends: two-dimensional sheets close into tubes at variable rates. J. Cell Biol. 1995;129:1311–1328. doi: 10.1083/jcb.129.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Kanai Y, Cowan NJ, Hirokawa N. Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature. 1992;360:674–677. doi: 10.1038/360674a0. [DOI] [PubMed] [Google Scholar]
- 8.Pollard, T. D., Earnshaw, W. C., Lippincott-Schwartz, J., Johnson, G. Cell biology E-book. (Elsevier Health Sciences, 2016).
- 9.Hirokawa N, Hisanaga S, Shiomura Y. MAP2 is a component of crossbridges between microtubules and neurofilaments in the neuronal cytoskeleton: quick-freeze, deep-etch immunoelectron microscopy and reconstitution studies. J. Neurosci. 1988;8:2769–2779. doi: 10.1523/JNEUROSCI.08-08-02769.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takeuchi M, Hisanaga S, Umeyama T, Hirokawa N. The 72-kDa microtubule-associated protein from porcine brain. J. Neurochem. 1992;58:1510–1516. doi: 10.1111/j.1471-4159.1992.tb11372.x. [DOI] [PubMed] [Google Scholar]
- 11.Conde C, Caceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- 12.Drubin DG, Feinstein SC, Shooter EM, Kirschner MW. Nerve growth factor-induced neurite outgrowth in PC12 cells involves the coordinate induction of microtubule assembly and assembly-promoting factors. J. Cell Biol. 1985;101:1799–1807. doi: 10.1083/jcb.101.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drubin DG, Kirschner MW. Tau protein function in living cells. J. Cell Biol. 1986;103:2739–2746. doi: 10.1083/jcb.103.6.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters, A. S., Palay, L. H., Webster, d. F. (Oxford University Press, 1991).
- 15.Palay SL, Sotelo C, Peters A, Orkand PM. The axon hillock and the initial segment. J. Cell Biol. 1968;38:193–201. doi: 10.1083/jcb.38.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasband MN. The axon initial segment and the maintenance of neuronal polarity. Nat. Rev. Neurosci. 2010;11:552–562. doi: 10.1038/nrn2852. [DOI] [PubMed] [Google Scholar]
- 17.Li X, et al. Novel diffusion barrier for axonal retention of Tau in neurons and its failure in neurodegeneration. EMBO J. 2011;30:4825–4837. doi: 10.1038/emboj.2011.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drechsel DN, Hyman AA, Cobb MH, Kirschner MW. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol. Biol. Cell. 1992;3:1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gustke N, Trinczek B, Biernat J, Mandelkow EM, Mandelkow E. Domains of Tau-protein and interactions with microtubules. Biochemistry. 1994;33:9511–9522. doi: 10.1021/bi00198a017. [DOI] [PubMed] [Google Scholar]
- 20.Panda D, Goode BL, Feinstein SC, Wilson L. Kinetic stabilization of microtubule dynamics at steady state by tau and microtubule-binding domains of tau. Biochemistry. 1995;34:11117–11127. doi: 10.1021/bi00035a017. [DOI] [PubMed] [Google Scholar]
- 21.Trinczek B, Biernat J, Baumann K, Mandelkow EM, Mandelkow E. Domains of tau protein, differential phosphorylation, and dynamic instability of microtubules. Mol. Biol. Cell. 1995;6:1887–1902. doi: 10.1091/mbc.6.12.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panda D, Samuel JC, Massie M, Feinstein SC, Wilson L. Differential regulation of microtubule dynamics by three- and four-repeat tau: implications for the onset of neurodegenerative disease. Proc. Natl Acad. Sci. USA. 2003;100:9548–9553. doi: 10.1073/pnas.1633508100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cleveland DW, Hwo SY, Kirschner MW. Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J. Mol. Biol. 1977;116:207–225. doi: 10.1016/0022-2836(77)90213-3. [DOI] [PubMed] [Google Scholar]
- 24.Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl Acad. Sci. USA. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutton M, et al. Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 26.McKee AC, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Himmler A, Drechsel D, Kirschner MW, Martin DW., Jr. Tau consists of a set of proteins with repeated C-terminal microtubule-binding domains and variable N-terminal domains. Mol. Cell Biol. 1989;9:1381–1388. doi: 10.1128/mcb.9.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butner KA, Kirschner MW. Tau protein binds to microtubules through a flexible array of distributed weak sites. J. Cell Biol. 1991;115:717–730. doi: 10.1083/jcb.115.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee G, Neve RL, Kosik KS. The microtubule binding domain of Tau-protein. Neuron. 1989;2:1615–1624. doi: 10.1016/0896-6273(89)90050-0. [DOI] [PubMed] [Google Scholar]
- 30.Goode BL, Feinstein SC. Identification of a novel microtubule binding and assembly domain in the developmentally regulated inter-repeat region of tau. J. Cell Biol. 1994;124:769–782. doi: 10.1083/jcb.124.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Needleman DJ, et al. Higher-order assembly of microtubules by counterions: from hexagonal bundles to living necklaces. Proc. Natl Acad. Sci. USA. 2004;101:16099–16103. doi: 10.1073/pnas.0406076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Needleman DJ, et al. Ion specific effects in bundling and depolymerization of taxol-stabilized microtubules. Faraday Discuss. 2013;166:31–45. doi: 10.1039/c3fd00063j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung PJ, et al. Tau mediates microtubule bundle architectures mimicking fascicles of microtubules found in the axon initial segment. Nat. Commun. 2016;7:12278. doi: 10.1038/ncomms12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller, H. P. & Wilson, L. Methods in Cell Biology, Wilson, L. C. J. J. Ed. (Elsevier Inc., 2010), vol. 95, pp. 3–15. [DOI] [PubMed]
- 35.Chung PJ, et al. Direct force measurements reveal that protein Tau confers short-range attractions and isoform-dependent steric stabilization to microtubules. Proc. Natl Acad. Sci. USA. 2015;112:E6416–6425. doi: 10.1073/pnas.1513172112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milo, R. & Phillips, R. Cell biology by the numbers. (Garland Science, 2015).
- 37.Kanai Y, Chen J, Hirokawa N. Microtubule bundling by tau proteins in vivo: analysis of functional domains. EMBO J. 1992;11:3953–3961. doi: 10.1002/j.1460-2075.1992.tb05489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kellogg EH, et al. Near-atomic model of microtubule-tau interactions. Science. 2018;360:1242–1246. doi: 10.1126/science.aat1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li XH, Culver JA, Rhoades E. Tau binds to multiple tubulin dimers with helical structure. J. Am. Chem. Soc. 2015;137:9218–9221. doi: 10.1021/jacs.5b04561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li XH, Rhoades E. Heterogeneous Tau-Tubulin complexes accelerate microtubule polymerization. Biophys. J. 2017;112:2567–2574. doi: 10.1016/j.bpj.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Best RL, et al. Tau isoform-specific stabilization of intermediate states during microtubule assembly and disassembly. J. Biol. Chem. 2019;294:12265–12280. doi: 10.1074/jbc.RA119.009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ojeda-Lopez MA, et al. Transformation of taxol-stabilized microtubules into inverted tubulin tubules triggered by a tubulin conformation switch. Nat. Mater. 2014;13:195–203. doi: 10.1038/nmat3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dorléans A, et al. Variations in the colchicine-binding domain provide insight into the structural switch of tubulin. Proc. Natl Acad. Sci. USA. 2009;106:13775–13779. doi: 10.1073/pnas.0904223106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chattopadhyaya S, Chakravorty D, Basu G. A collective motion description of tubulin βT7 loop dynamics. Biophys. Physicobiol. 2019;16:264–273. doi: 10.2142/biophysico.16.0_264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsui T, Han X, Yu J, Yao M, Tanaka I. Structural change in FtsZ Induced by intermolecular interactions between bound GTP and the T7 loop. J. Biol. Chem. 2014;289:3501–3509. doi: 10.1074/jbc.M113.514901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carlier MF, Didry D, Pantaloni D. Hydrolysis of GTP associated with the formation of tubulin oligomers is involved in microtubule nucleation. Biophys. J. 1997;73:418–427. doi: 10.1016/S0006-3495(97)78081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beaucage G. Approximations leading to a unified exponential/power-law approach to small-angle scattering. J. Appl. Crystallogr. 1995;28:717–728. doi: 10.1107/S0021889895005292. [DOI] [Google Scholar]
- 48.Choi MC, et al. Paclitaxel suppresses Tau-mediated microtubule bundling in a concentration-dependent manner. Biochim Biophys. Acta Gen. Subj. 2017;1861:3456–3463. doi: 10.1016/j.bbagen.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 49.Bray, D. Cell movements: from molecules to motility. (Garland Science, 2000).
- 50.Shemesh A, Ginsburg A, Levi-Kalisman Y, Ringel I, Raviv U. Structure, assembly, and disassembly of tubulin single rings. Biochemistry. 2018;57:6153–6165. doi: 10.1021/acs.biochem.8b00560. [DOI] [PubMed] [Google Scholar]
- 51.Harada A, et al. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature. 1994;369:488–491. doi: 10.1038/369488a0. [DOI] [PubMed] [Google Scholar]
- 52.George-Hyslop PHS. Piecing together Alzheimer’s. Sci. Am. 2000;283:76–83. doi: 10.1038/scientificamerican1200-76. [DOI] [PubMed] [Google Scholar]
- 53.Rosenberg KJ, Ross JL, Feinstein HE, Feinstein SC, Israelachvili J. Complementary dimerization of microtubule-associated tau protein: Implications for microtubule bundling and tau-mediated pathogenesis. Proc. Natl Acad. Sci. USA. 2008;105:7445–7450. doi: 10.1073/pnas.0802036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brandt R, Lee G. Orientation, assembly, and stability of microtubule bundles induced by a fragment of tau protein. Cell Motil. Cytoskeleton. 1994;28:143–154. doi: 10.1002/cm.970280206. [DOI] [PubMed] [Google Scholar]
- 55.Brandt R, Lee G. Functional organization of microtubule-associated protein tau. Identification of regions which affect microtubule growth, nucleation, and bundle formation in vitro. J. Biol. Chem. 1993;268:3414–3419. doi: 10.1016/S0021-9258(18)53710-8. [DOI] [PubMed] [Google Scholar]
- 56.Choi MC, et al. Human microtubule-associated-protein tau regulates the number of protofilaments in microtubules: a synchrotron x-ray scattering study. Biophys. J. 2009;97:519–527. doi: 10.1016/j.bpj.2009.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rubinstein, M. Colby, R. H. Polymer physics. (Oxford University Press, New York, 2003), vol. 23.
- 58.Curcio M, Bradke F. Microtubule organization in the Axon: TRIM46 determines the orientation. Neuron. 2015;88:1072–1074. doi: 10.1016/j.neuron.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 59.Harterink M, et al. TRIM46 organizes microtubule fasciculation in the axon initial segment. J. Neurosci. 2019;39:4864–4873. doi: 10.1523/JNEUROSCI.3105-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Beuningen SFB, et al. TRIM46 controls neuronal polarity and axon specification by driving the formation of parallel microtubule arrays. Neuron. 2015;88:1208–1226. doi: 10.1016/j.neuron.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 61.Dehmelt L, Halpain S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005;6:204. doi: 10.1186/gb-2004-6-1-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Best RL, et al. Expression and isolation of recombinant tau. Methods Cell Biol. 2017;141:3–26. doi: 10.1016/bs.mcb.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 63.Andreu JM, et al. Low-resolution structure of microtubules in solution. Synchrotron X-ray scattering and electron microscopy of taxol-induced microtubules assembled from purified tubulin in comparison with glycerol and MAP-induced microtubules. J. Mol. Biol. 1992;226:169–184. doi: 10.1016/0022-2836(92)90132-4. [DOI] [PubMed] [Google Scholar]
- 64.Israelachvili, J. N. Intermolecular and surface forces. (Academic Press, Burlington, Massachusetts, ed. 3rd, 2011).
- 65.Ilavsky J. Nika: software for two-dimensional data reduction. J. Appl. Crystallogr. 2012;45:324–328. doi: 10.1107/S0021889812004037. [DOI] [Google Scholar]
- 66.Kohn JE, et al. Random-coil behavior and the dimensions of chemically unfolded proteins. Proc. Natl Acad. Sci. USA. 2004;101:12491–12496. doi: 10.1073/pnas.0403643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mylonas E, et al. Domain conformation of tau protein studied by solution small-angle X-ray scattering. Biochemistry. 2008;47:10345–10353. doi: 10.1021/bi800900d. [DOI] [PubMed] [Google Scholar]
- 68.Fagherazzi G. Small angle X-ray scattering edited by O. Glatter and O. Kratky. Acta Crystallogr. Sect. A: Found. Crystallogr. 1983;39:500–500. doi: 10.1107/S0108767383000926. [DOI] [Google Scholar]
- 69.Kar S, Fan J, Smith MJ, Goedert M, Amos LA. Repeat motifs of tau bind to the insides of microtubules in the absence of taxol. EMBO J. 2003;22:70–77. doi: 10.1093/emboj/cdg001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Makrides V, Massie MR, Feinstein SC, Lew J. Evidence for two distinct binding sites for tau on microtubules. Proc. Natl Acad. Sci. USA. 2004;101:6746–6751. doi: 10.1073/pnas.0400992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The uncropped TEM images, SAXS data, and all resulting fits generated in this study are provided in the Source Data excel file. Source data are provided with this paper.
The code used for the fitting of the SAXS data can be found in the Supplementary Code 1 file.