Abstract
Organotypic cultures of human keratinocytes provide a useful model system to study human papillomavirus (HPV)-host cell interactions. In this study, we analyzed organotypic cultures of two HPV type 16 (HPV16) (FK16A and FK16B)- and two HPV18 (FK18A and FK18B)-transfected keratinocyte cell lines through the process of immortalization in vitro. For FK16A and FK18B cells, passages of both mortal cells in their extended life span and subsequent immortal stages were studied. Mortal cells of FK16A and FK18B showed a morphology reminiscent of mild to moderate dysplasia, whereas in their immortal descendants, severely dysplastic features were observed. Immortal FK18A cells were mildly to moderately dysplastic, while FK16B cells were severely dysplastic. The increasing degrees of dysplasia were associated with a decreasing expression of differentiation markers cytokeratin 10 and profilaggrin. All raft cultures expressed E6-E7 mRNAs in the basal layer, while the amount of viral transcripts in the suprabasal cells was in general proportional to the degree of dysplasia. In all cases, E6-E7 transcription and dysplastic features were highly correlated with cellular proliferation, as assessed by Ki-67 (MIB-1) antigen expression. Moreover, high levels of E6-E7 transcription and expression of p21cip1 protein in the basal layer seemed to be mutually exclusive. We conclude that expression of E6-E7 in the basal cells associated with increased proliferation in the absence of detectable p21cip1 protein is apparently necessary but not sufficient for immortalization, or for the loss of terminal differentiation, for which yet to be discovered additional events are required. The model system described in this study provides a valuable tool to analyze alterations in viral transcription regulation during HPV-mediated cell transformation.
Infections with mucosotropic human papillomavirus (HPV) genotypes are related to the development of both benign and malignant mucosal epithelial lesions (55). Low-risk HPV types typically cause condylomata and papillomas, which rarely undergo neoplastic progression, whereas infections with high-risk HPV types, in particular HPV type 16 (HPV16) and HPV18, can progress to high-grade dysplasias and invasive carcinomas. In benign and low-grade lesions, the viral genome is maintained as extrachromosomal plasmids in basal cell nuclei, while vegetative DNA amplification occurs only in squamous epithelia undergoing terminal differentiation. Usually, only very low levels of viral mRNA can be detected in the infected basal cells, whereas viral transcription is markedly increased in the differentiated layers (4, 15, 46–48). Moreover, expression of the viral genes is associated with reactivation of the DNA replication machinery in the differentiating spinous cells in condylomata and in low-grade intraepithelial neoplasias (12, 13). This observation is supported by retrovirus-mediated gene transfer, which shows that expression of the high-risk or low-risk viral oncoprotein E7, which inactivates the tumor suppressor protein pRB (49), under the control of the native viral enhancer-promoter can induce proliferating cell nuclear antigen (PCNA) in a differentiation-dependent manner in primary keratinocytes grown as raft cultures. Furthermore, that the high-risk HPV E7 alone can cause host DNA replication in these differentiated cells (6). Therefore, it has been suggested that the natural function of the viral oncoprotein is to reactivate the host DNA replication machinery, thereby facilitating viral DNA replication in noncycling, differentiated cells. When expressed from a constitutive promoter, the high-risk HPV E7 together with E6, which inactivates another tumor suppressor protein p53 (28), can immortalize primary human keratinocytes in culture (22, 34). In addition, E7 specifically induces hyperproliferation of keratinocytes both in vitro and in vivo (3, 9). It is evident that the epithelial raft culture of human keratinocytes that are infected with recombinant retroviruses containing HPV sequences or transfected with cloned viral DNA is a valuable tool for studying the functions and regulation of HPV genes (2, 6, 18, 33) and enhancer and promoter elements (37, 53; reviewed in reference 8). The results correlate well with HPV infections in vivo. Thus organotypic cultures also provide a means for studying alterations in the virus-host interactions which underlie high-risk HPV-mediated transformation of epithelial cells.
We recently described a model system of HPV-mediated immortalization in which two distinct stages were defined during monolayer culturing of primary human foreskin keratinocytes transfected with HPV16 (cell lines FK16A and FK16B) or HPV18 (cell lines FK18A and FK18B) (45). The first stage was represented by cells that exhibited an extended but still finite life span, whereas the second stage corresponded with immortality. Transition from a mortal to an immortal stage has previously been correlated with a strong telomerase activity, postcrisis growth, or both. In cell lines FK16B and FK18B, mortal and immortal stages were clearly demarcated by a period of crisis at passages 13 and 20, respectively. No crisis period was observed in cell lines FK16A and FK18A. Moreover, immortal stages of cell lines FK16A, FK16B, and FK18B were marked by clonal allelic losses at one or more chromosomal loci.
In this study, passages of these cell lines representing mortal and immortal stages were cultured on collagen rafts and analyzed for morphology, E6-E7 transcription, and the patterns of cellular proliferation and differentiation. Our results show that the different cell lines at these states are reminiscent of various degrees of dysplasia, ranging from mild and moderate to severe dysplasia. However, independent of their morphological appearance, all raft cultures derived from mortal and immortal cells expressed E6-E7 mRNAs in the basal layer, while the amount of viral transcripts in the suprabasal cells was generally proportional to the degree of dysplasia. Moreover, in all cases, diffused viral oncogene transcription was correlated with dysplastic morphology and with cellular proliferation, as assessed by Ki-67 (MIB-1) antigen expression (1), but inversely related to expression of the universal cyclin-dependent kinase (CDK) inhibitor p21cip1 (43).
MATERIALS AND METHODS
Cell lines.
The cell lines FK16A, FK16B, FK18A, and FK18B were established by transfection of primary human foreskin keratinocytes (EK94-2) with the entire HPV16 and HPV18 genome (45). Cells were grown in serum-free keratinocyte growth medium (Life Technologies, Breda, The Netherlands) supplemented with bovine pituitary extract (50 μg/ml), epidermal growth factor (5 ng/ml), penicillin (100U/ml), streptomycin (100 μg/ml), and l-glutamine (2 mM) (Life Technologies).
Organotypic culture on collagen rafts.
Organotypic raft cultures were prepared as described previously (29, 50), with modifications (6, 37). For all four cell lines, duplicate rafts were developed from each passage number. The dermal equivalent contained Swiss 3T3 J2 fibroblasts (a gift from Elaine Fuchs, University of Chicago, Chicago, Ill.). Briefly, raft culture medium was modified from that of McCance et al. (32) and contained Dulbecco modified Eagle medium–Ham’s F-12 (3:1) supplemented with 10% fetal calf serum (Life Technologies, Bethesda, Md.), hydrocortisone (0.4 μg/ml), 0.1 nM cholera toxin, transferrin (5 μg/ml; Sigma), insulin (5 μg/ml; Sigma), and human epidermal growth factor (0.5 ng/ml; Life Technologies). Cultures were harvested after 9 days, fixed in 10% buffered formalin, and embedded in paraffin. Four-micrometer sections were stained with hematoxylin and eosin for histological examination.
RNA in situ hybridization.
In situ hybridization with 35S-labeled riboprobes was carried out as described previously (6, 13, 48). The sense- and antisense-strand HPV16 E6-E7 (spanning nucleotides 24 to 654) and HPV18 E6-E7 (6) probes had a specific activity of 1.17 × 108 cpm/μg and were applied at 50 to 70% saturation. After hybridization and a stringent wash at 63°C in 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), the sections were dipped into Kodak NTE liquid emulsion and exposed for 7 days before photographical development with D19. The slides were then photographed under dark-field illumination, using an Olympus BH2 microscope equipped with a dark-field and bright-field dual condensor.
Immunohistochemical analysis.
Immunohistochemical staining was performed on deparaffinized sections. Endogenous peroxidase was inactivated by incubation with 0.3% H2O2 in methanol for 30 min. The anti-cytokeratin 10 (K10) monoclonal antibody (1:100 dilution; Biogenex, San Ramon, Calif.) and the anti-profilaggrin/filaggrin antibody (1:100 dilution; Biomedical Technologies, Stoughton, Mass.) were detected by using a histostain-SP kit (Zymed Laboratories, South San Francisco, Calif.) in which aminoethyl carbazole was used as the chromogen. To detect profilaggrin, partial proteolytic digestion of tissue was performed with 0.1% trypsin in phosphate-buffered saline (pH 7.2) for 10 min at 37°C (50). For staining with monoclonal antibodies specific for MIB-1 (Ki-67; 1:40 dilution; Dianova, Germany) and p21cip1 (1:500 dilution; Pharmingen, San Diego, Calif.), antigen retrieval was performed by treating the slides in a 10 mM citrate buffer (pH 6.0) in a microwave oven set at 800 W for 15 min. Sections were incubated with primary antibodies at 4°C overnight, followed by incubation with a biotinylated rabbit anti-mouse polyclonal antibody (diluted 1:500; DAKO, Glostrup, Denmark). Antibody reactivity was detected by using a peroxidase-conjugated streptavidin-biotin complex (sABC, diluted 1:200; DAKO) and visualized by a 3-min reaction with diaminobenzidine (0.4 mg/ml; Sigma, St. Louis, Mo.)–0.002% H2O2 in 50 mM Tris-HCl (pH 7.6). The slides were counterstained lightly with hematoxylin to reveal tissue morphology.
RESULTS
Raft cultures of HPV16- and HPV18-transfected keratinocytes display a range of dysplastic changes during and following immortalization.
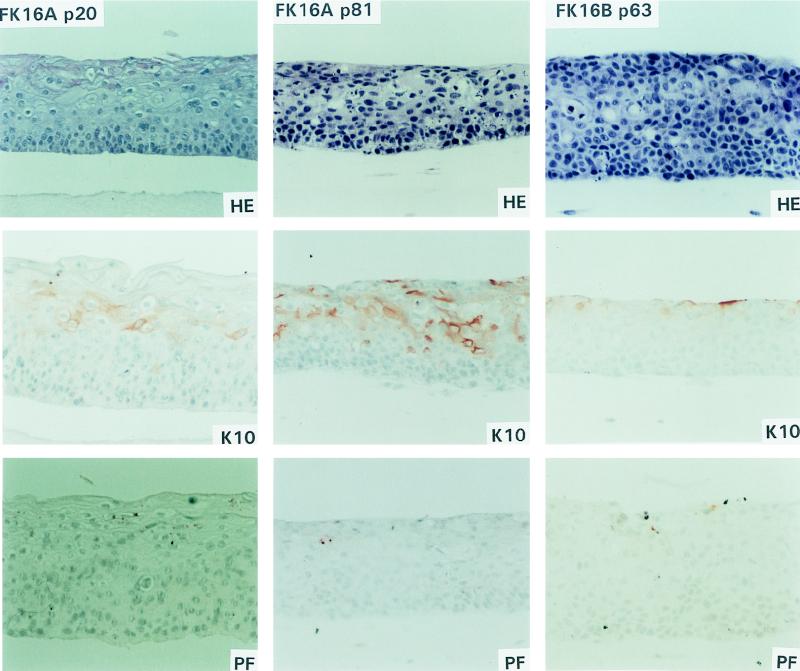
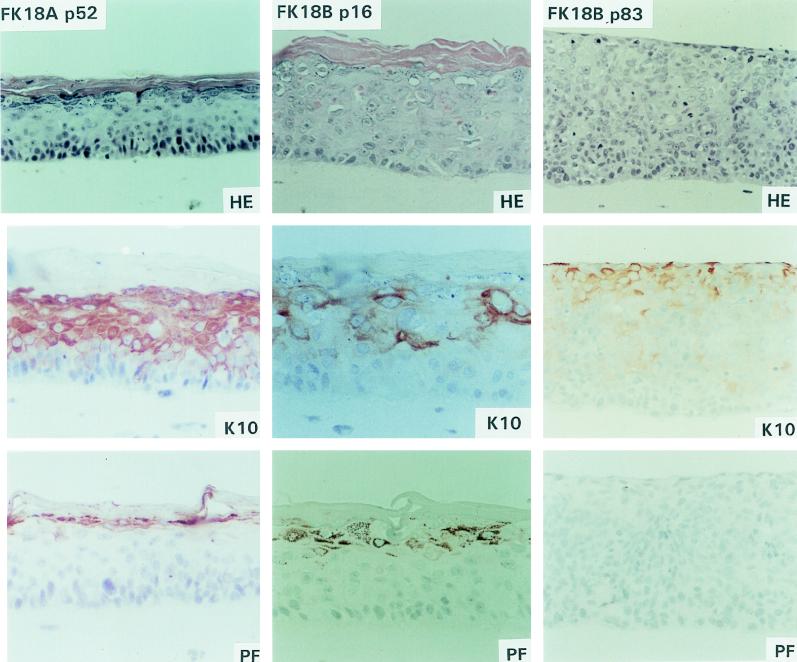
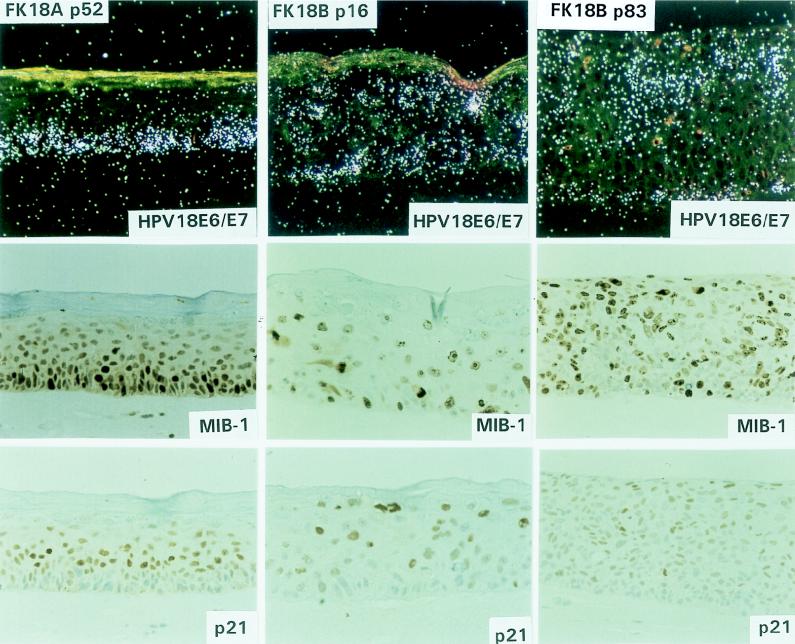
Epithelial raft cultures were developed from the primary donor keratinocytes (EK94-2) at passage 3 and from various passages of the HPV16 (cell lines FK16A and FK16B)- and HPV18 (cell lines FK18A and FK18B)-transfected descendants on a dermal equivalent containing mouse Swiss 3T3 J2 fibroblasts. Organotypic cultures of the primary donor keratinocytes closely resembled normal epithelium in vivo and contained morphologically distinct basal, spinous, granular, and cornified layers (Fig. 1). Of the four HPV- immortalized cell lines examined, mortal cells of FK16A and FK18B in the extended life span were also analyzed. Raft cultures of mortal FK16A cells at passages 17 and 20 showed abnormal differentiation, characterized by the presence of stratified squamous layers that resembled mild to moderate dysplasia in vivo (Fig. 2, FK16A p20). Cultures of the mortal FK18B cells (passages 16 and 18) were also reminiscent of mild to moderate dysplasia in vivo but appeared to be more differentiated than FK16A cells (passages 17 and 20) (Fig. 3, FK18B p16). Raft cultures of the subsequent immortal stages of FK16A (passage 81) and FK18B (passages 38 and 83) as well as immortal FK16B (passage 63) showed a loss of morphological differentiation and a histology consistent with severe dysplasia in vivo, characterized by increased layers of basal-like cells and a loss of granular cells (Fig. 2, FK16A p81 and FK16B p63; Fig. 3, FK18B p83). In contrast, immortal FK18A cells, at passages 27, 52, and 77, were all capable of terminal differentiation and exhibited defined spinous and granular layers and acellular squames (Fig. 3, FK18A p52); morphologically, no major differences were observed among these three passages, and all resembled mild dysplasia in vivo.
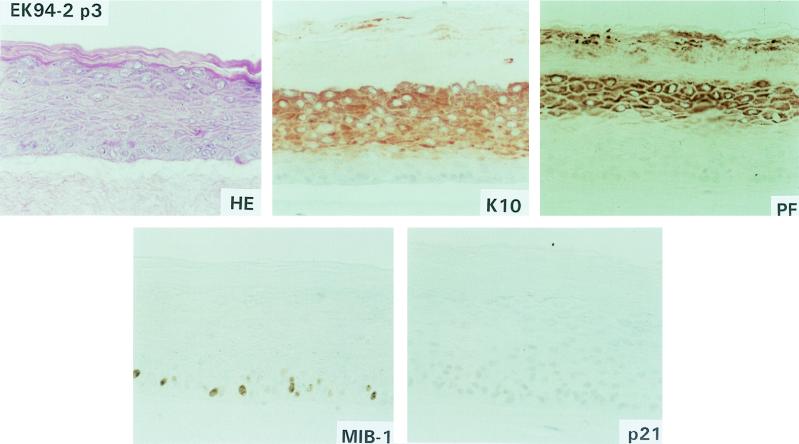
FIG. 1.
Epithelial raft cultures of donor primary keratinocytes (EK94-2). The same abbreviations are used in this figure and in Fig. 2 and 3. HE, hematoxylin-eosin staining; Immunohistochemical staining: K10, cytokeratin 10 expression; PF, profilaggrin expression; p21, p21cip1 protein expression; MIB, Ki-67 antigen expression.
FIG. 2.
Epithelial raft cultures of FK16A cells at passages 20 and 81 and FK16B cells at passage 63.
FIG. 3.
Epithelial raft cultures of FK18A cells at passage 52 and FK18B cells at passages 16 and 83.
To assess further the state of epithelial differentiation, immunostaining for K10 and profilaggrin was performed. The differentiation marker K10, which is normally expressed in cells that have left the basal layer and are committed to differentiation (20), was expressed in the spinous and granular layers of the primary donor keratinocytes (Fig. 1). In addition, normal expression of profilaggrin, a marker for terminal differentiation which is associated with keratohyalin granules (11), was detected in the granular layer of the primary keratinocytes (Fig. 1). That morphological differentiation was maintained in early stage FK18B cells as well as in all FK18A rafts was supported by K10 and profilaggrin staining patterns (Fig. 2 and 3). All of these cultures showed clear K10 and profilaggrin expression in the differentiated cell layers, although there are some differences in signal distribution and strength among different cultures. In contrast, loss of terminal differentiation in immortal FK16A and FK18B raft cultures was associated with weak antibody reactivity to K10 and sporadic or no profilaggrin expression (Fig. 2, FK16A p81; Fig. 3, FK18B p83). Interestingly, in FK16A cells at passage 20, K10 was greatly reduced whereas profilaggrin was virtually absent. FK16B showed an almost complete loss of K10 and profilaggrin expression in the immortal state (Fig. 2, FK16B p63).
Viral oncogene expression is closely correlated to cellular proliferation.
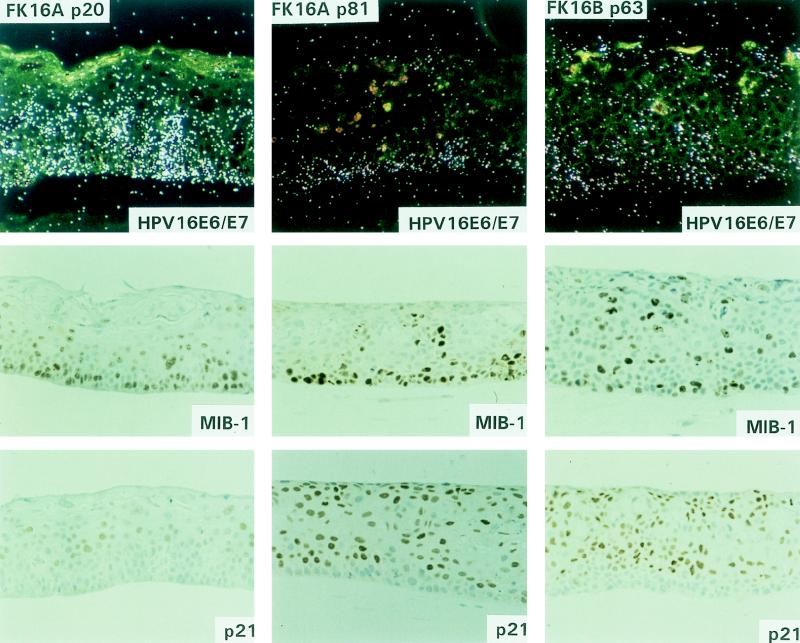
To analyze viral oncogene expression, RNA in situ hybridization was performed with radiolabeled HPV16 E6-E7- and HPV18 E6-E7-specific antisense riboprobes. The specificity of both probes was confirmed by the absence of signals in raft cultures of HPV-negative primary keratinocytes (data not shown). In mortal FK16A cells (passages 17 and 20), the viral oncogenes were primarily expressed in the lower strata, up to the mid-spinous cell layers, and signals tended to reduce in the upper differentiated layers (Fig. 4, FK16A p20). Following immortalization, FK16A cells showed a sustained transcription in the basal layer with much reduced signals in the suprabasal layers, whereas transcription in immortalized FK16B cells was mostly basal, with moderate intensity in suprabasal cells (Fig. 4, FK16A p81 and FK16B p63). Mortal FK18B cells (at passages 16 and 18) showed diffused E6-E7 mRNA signals throughout the epithelium, with strongly positive cells scattered in both basal and parabasal layers (Fig. 5, FK18B p16). Immortal FK18B cells, at passages 38 and 83, exhibited diffused signals throughout the thickness of the epithelium (Fig. 5, FK18B p83). All passages of the well-differentiated FK18A cells revealed transcription of the viral oncogenes primarily in the basal and parabasal cells (Fig. 5, FK18A p52). Signals were greatly reduced in the spinous cells, but occasional spinous cells had strong signals.
FIG. 4.
Molecular characterization of epithelial raft cultures of FK16A cells at passages 20 and 81 and FK16B cells at passage 63. HPV16 E6/E7, in situ hybridization with 35S-labeled antisense riboprobes to reveal HPV16 E6-E7 transcripts. Hybridization of FK16 p20 was performed in a separate experiment. The signal intensities should not be compared between the two experiments, but relative signal strengths in cells at different strata within each culture represent valid comparisons. Immunohistochemical staining: MIB, Ki-67 expression; p21, p21cip1 protein expression.
FIG. 5.
Molecular characterization of epithelial raft cultures of FK18A cells at passage 52 and FK18B cells at passages 16 and 83. HPV18 E6/E7, in situ hybridization with 35S-labeled antisense riboprobes to reveal HPV18 E6-E7 transcripts. Hybridization of FK18B p16 was performed in a separate experiment. Immunohistochemical staining: MIB, Ki-67 expression; p21, p21cip1 protein expression.
To correlate viral transcription with cell proliferation, consecutive sections were stained for Ki-67, using the MIB-1 antibody. As expected, raft cultures of the primary donor keratinocytes expressed Ki-67 primarily in a subset of basal cells. A higher percentage of HPV-transfected cells were positive. In general, MIB-1 antibody staining was located in the same areas that were morphologically dysplastic and had relatively strong viral transcription. Hence, immortal FK18A cells (passages 27, 52, and 77) showed Ki-67 expression mainly in the lower strata (Fig. 5, FK18A p52). In mortal FK16A (passages 17 and 20) and FK18B (passages 16 and 18) cells and immortal FK16A (passage 81), FK16B (passage 63), and FK18B (passages 38 and 83) cells, Ki-67 expression was observed in the basal and parabasal layers, but signals were also detected in the upper strata (Fig. 4, FK16A p20, FK16A p81, and FK16B p63; Fig. 5, FK18B p16 and FK18B p83).
p21cip1 protein is an inhibitor of cyclin-dependent kinases and PCNA, and it is transcriptionally activated by p53-dependent mechanisms in cycling cells and by p53-independent mechanisms during differentiation of a wide range of cell types and tissues (reviewed in reference 43). Recent observations indicate that p21cip1 protein is at or below the sensitivity of detection in formalin-fixed and paraffin-embedded squamous epithelium but was unexpectedly induced strongly in differentiated cells of HPV-infected warts and of mild and moderate dysplasias (41). In epithelial raft cultures, the expression of HPV18 E7 from the homologous viral enhancer-promoter in the differentiated keratinocytes recapitulated this protein induction (27). In vivo and in vitro, this induction was largely posttranscriptional and p53 independent. Furthermore, host and viral DNA replication and p21cip1 protein induction took place in separate populations of PCNA-positive cells. Consequently, it has been hypothesized that the induction of p21cip1 protein is a host physiological response resulting in the inhibition of unscheduled DNA synthesis in the differentiated cells activated by viral E7 protein. To analyze whether such a response was present during the process of immortalization, immunohistochemistry was performed for p21cip1 protein expression. The primary donor keratinocytes showed an extremely weak positivity for p21cip1 protein, primarily confined to the basal and parabasal cells, as has been observed in the previous study (27). In contrast, neither the raft cultures of FK16A or FK18B in the mortal stage nor any of the immortal cell lines stained positive for p21cip1 protein in the proliferating lower strata. However, various degrees of p21cip1 protein positivity were observed in some differentiated spinous regions of all the HPV-containing cultures (Fig. 4, FK16A p20, FK16A p81, and FK16B p63; Fig. 5, FK18B p16, FK18B p83, and FK18A p52).
DISCUSSION
In vitro studies have revealed the capability of high-risk HPVs, by means of their transformation proteins E6 and E7, to induce immortalization of their natural target cells, the primary human epithelial cells (22, 34). This process, considered an important step toward malignancy, has been extensively studied and shown to require host gene alterations in addition to the expression of HPV oncoproteins (5, 42). To identify the genetic and cellular changes involved in this process, we developed an in vitro model system, using HPV16- and HPV18-transfected human foreskin keratinocytes, in which the transition from a mortal to an immortal phenotype has been well characterized (45). In monolayer cultures, both HPV16 and HPV18 initially induced an extended but still finite life span. At this stage, cells displayed a remarkable level of cytogenetic instability, manifested as numerical and structural chromosome alterations (44). This genome instability has similarly been reported in human dermal fibroblasts that have been transduced with HPV16 E6 and E7 genes and have an extended life span (52). However, these epithelial cells were devoid of any specific allelic losses or strong telomerase activity and exhibited telomere shortening upon passaging. In contrast, the immortal descendants have a strong telomerase activity accompanied by telomere restoration. Three of these cell lines, FK16A, FK16B, and FK18B, showed specific clonal allelic losses (45). In addition, the level of cytogenetic instability was comparable to that of their mortal ancestors (44).
Here, we have applied the organotypic culture system to study the characteristics of these cells at different passages before and after immortalization with respect to squamous differentiation, viral transcription, and cellular proliferation. All raft cultures transfected with either HPV16 or HPV18 presented various degrees of abnormal differentiation patterns with dysplastic features. In particular, raft cultures of FK16A (HPV16) or FK18B (HPV18) cells at the stage of extended life span already exhibited mild to moderate dysplastic characteristics. Therefore, it can be concluded that a disruption of the normal differentiation program can occur prior to immortalization. Although three of the cell lines (FK16A, FK16B, and FK18B) became severely dysplastic after immortalization, this progression in phenotype is not obligatory. This is best illustrated by FK18A cells, which maintained a mild dysplastic phenotype and the ability to undergo terminal differentiation even at or beyond passage 77 in the immortal state. Consequently, in vitro immortalization can be associated with a wide range of morphological changes, as has been shown by other groups as well (2, 32, 33, 51).
In warts and low-grade cervical lesions, E6-E7 transcription is tightly linked to terminal differentiation (4, 15, 47, 48). The fact that differentiated cells already have lost the ability to divide explains that immortalization and transformation do not invariably occur despite reactivation of host DNA replication (6). In contrast, high-grade lesions and cervical carcinomas often display elevated E6-E7 transcripts in the basal-like cells that occupy much or all of the epithelium (4, 15, 46). Hence, immortalization and transformation apparently require alterations affecting intracellular control mechanisms of HPV expression in the proliferating cell compartment (4, 54). Indeed, in situ hybridization analysis revealed that all passages of the cell lines that we examined actively transcribed E6-E7 genes in the basal proliferating cell layer, whereas E6-E7 transcription was down-regulated in the differentiated suprabasal cells. A similar down-regulation of viral oncogene expression during differentiation has also been observed upon implantation of HPV16-immortalized keratinocytes in nude mice (14). This pattern of expression is different from the differentiation-dependent expression in warts and in primary foreskin keratinocytes acutely infected with retroviruses in which the viral oncogenes are under the control of the homologous viral enhancer and promoter (6). Interestingly, mortal FK18B cells showed an E6-E7 transcriptional pattern suggesting the coexistence of both basal and differentiation-dependent expression. Therefore, these cells may represent an intermediate state between a productive infection and cell transformation. At late passages, immortal descendants of FK18B cells showing a severely dysplastic phenotype exhibited E6-E7 expression throughout the culture, resembling high-grade lesions in vivo. The distinction between primary foreskin keratinocytes and the HPV-transfected cell lines described here is not completely surprising given the fact that these latter cells were selected for proliferation in monolayer cultures, while keratinocytes that underwent terminal differentiation were lost during passage. Nevertheless, our data underscore the idea that up-regulation of E6-E7 transcription in proliferating cells is essential but not sufficient for immortalization and subsequent progression since the vast majority of mortal cells in their extended life span did not reach an immortal state. One caveat to these conclusions is that effects of other HPV genes that are also expressed cannot be excluded as these cell lines harbor the entire HPV genome.
Thus, an essential step during immortalization apparently includes an altered regulation of the viral enhancer-promoter in the basal layer, which might be conferred by a loss of repressor(s), a gain of activator(s), or both. In this aspect, viral DNA integration into the host chromosomes in the E1 or E2 gene, an event which occurs frequently in squamous carcinomas of the exocervix and in carcinomas in situ and carcinomas of the endocervix associated with HPV16 and HPV18 (10, 16, 46), has been suggested to be an important determinant resulting in the up-regulation of viral oncogene expression in the basal layer. Integration in the E1 or E2 gene eliminates the capability to express viral E2 proteins that can down-regulate the promoter for the viral oncogenes in cultured keratinocytes or epithelial cell lines (reviewed in reference 7). However, a recent study using the bacterial lacZ gene as a reporter showed that integrated viral enhancer-promoter is active only in the differentiated cells in raft cultures of primary foreskin keratinocytes even in the absence of E1 and E2 proteins (37, 53). Integration in the E1 or E2 gene necessitates the utilization of host polyadenylation sites for functional E6-E7 mRNAs. It has been shown that upon integration, the E6-E7 messages are greatly stabilized relative to those transcribed from extrachromosomal plasmids, conferring to these cells a growth advantage (26). However, it does not explain how viral oncogenes are up-regulated in dyplasias prior to integration, which is usually a late event in viral carcinogenesis. Mutations in the binding site for transcription factor YY1 located in the viral enhancer-promoter have been detected in cancers and were suggested to be a contributing factor (31). Lastly, integrated viral DNA might experience altered transcription regulation due to influences from local chromatin structures or enhancer elements present in the host DNA near the integration site. In the four cell lines examined in this study, stable integration of the viral DNA was demonstrated at the earliest passages analyzed (45). We believe that the up-regulation of E6 and E7 transcription in the basal cells is due to both a cis and a trans effect. The expression of the bacterial lacZ gene driven by the HPV18 or HPV11 upstream regulatory region in raft cultures of the FK18A cell line is also more prominent in the basal cells than in the upper, more differentiated strata, whereas it is expressed only in the differentiated upper spinous and lower granular layers in raft cultures of primary foreskin keratinocytes (37, 53). This difference signifies a trans effect on both endogenous viral genes and the exogenous transgene. In addition, there is a more dramatic difference in E6-E7 signals in the basal versus suprabasal cells compared to the lacZ expression in the same compartments (25). This observation suggests a cis effect on the expression of endogenous viral genes.
A significant increase in Ki-67 antigen-positive cells indicates that immortalization by high-risk HPV types is correlated with increased proliferation. A similar observation has been described for high-grade cervical dysplasias harboring high-risk HPV DNA (1, 24). Our data also show that cellular proliferation (Ki-67 positivity) was directly linked to viral E6-E7 transcription. However, it appeared that the extent of neither E6-E7 nor Ki-67 expression was directly correlated to immortality or the degree of dysplasia that these cells displayed in raft cultures.
p21cip1 protein expression has been found to be up-regulated in a variety of differentiated glandular tissues (36, 38). In the present study, raft cultures of primary keratinocytes showed very weak positivity for p21cip1 protein, primarily in some of the basal and occasionally parabasal cells, in agreement with our recent observations in vitro (27). This result can be contrasted to previous studies of adult cutaneous skin, where weak p21cip1 antibody reactivity was detected in suprabasal squamous cells (19, 23, 30, 38). Additionally, p21cip1 positivity was shown to be strongly induced in UV-irradiated skin, in psoriatic lesions, as well as in skin treated with irritants (23, 38). In another study, little p21cip1 protein was detectable in non-UV-irradiated normal skin, but upon UV irradiation, a marked induction was observed (17). The differences in the topographic distribution of cells demonstrating detectable p21cip1 protein levels among various studies may be due to differences in the body sites where the skin specimens were removed, to the developmental stage, or physiological state of the tissues. Neonatal foreskin has more growth potential than normal adult skin, especially when the cells are grown in raft culture media that promote rapid basal cell proliferation and when the cells also express the growth-promoting viral oncogenes. Studies of normal human fibroblasts and epithelial cells, in which p21cip1 protein expression was followed through the different phases of the cell cycle, found that p21cip1 was first up-regulated after addition of growth factors to growth-arrested cells. This was followed by a down-regulation as cells move into S phase. These studies suggested that p21cip1 was an immediate-early response gene to growth stimuli perhaps to prevent premature activation of CDKs (21, 35). Our detection of p21cip1 protein in raft cultures of untransfected donor primary foreskin keratinocytes only in the rapidly dividing basal cells is not inconsistent with this interpretation.
In the present study, p21cip1 protein was observed in the more differentiated upper cell layers in raft cultures of all passages of HPV16 or HPV18 DNA-transfected cells. Whether this induction represents a posttranscriptional response similar to that observed in benign lesions, papillomas, condylomata, mild and moderate dysplasias, and fully differentiated E7-transduced raft cultures remains to be determined (27, 41). Also remarkable is the observation that in contrast to the untransfected donor cells, no p21cip1 protein was detected in the basal cycling cells of any of the HPV-transfected cells. This loss of p21cip1 protein correlated with the high levels of viral oncogene transcription and, by inference, viral oncoprotein expression. Since the high-risk HPV E6 protein causes a rapid degradation of p53 (40), the absence of p21cip1 protein expression in the basal layers might indicate that the p53-dependent transcription activation of the p21cip1 gene which normally occurs in cycling cells was abrogated (43). This interpretation would be in agreement with a previous study of human fibroblasts expressing HPV16 E6 (52). Furthermore, the same study additionally showed that when both HPV16 E6 and E7 were expressed, p21cip1 protein was no longer associated with CDKs/cyclins. We hypothesize that a high level of HPV E6-E7 in the basal layer is an important event during HPV-mediated immortalization. In essence, when constitutively expressed in the basal cycling cells, E7 bypasses the cell cycle control by pRB, E6 eliminates the function of p53, and E6 and E7 together abrogate the function of p21cip1 protein, resulting in a loss of normal control of cell proliferation (52).
In conclusion, basal expression of E6-E7, resulting in an increased proliferation in the absence of p21cip1 protein expression, is a likely prerequisite for HPV-mediated cell transformation in vitro. However, although necessary, this phenomenon is apparently insufficient for immortalization and the loss of terminal differentiation. These observations support the hypothesis that additional events are required for the latter processes. To analyze whether this phenomenon also represents an important step during transformation of HPV-infected cells in vivo, biopsies of women showing progressive cervical intraepithelial neoplasias in a follow-up study of a cohort of women (39) are currently being analyzed for p21cip1 protein expression. Finally, our data show that a model system as described in this study provides a valuable tool to analyze alterations in viral transcription regulation during HPV-mediated cell transformation.
ACKNOWLEDGMENTS
We thank Henri Schrijnemakers, Ge Jin, and Liesbeth van der Raaij-Helmer for excellent technical assistance.
This work was supported by grants VU 93-605 and VU 96-1151 from the Dutch Cancer Society and by USPHS grants CA36200 and AI 34574. J.N.P. and S.I. were partially supported by training grants T32CA09467 and T32AI07493. The research of P.J.F.S. was made possible by a fellowship from the Royal Netherlands Academy of Arts and Sciences.
REFERENCES
- 1.Al-Saleh W, Delvenne P, Greimers R, Fridman V, Doyen J, Boniver J. Assessment of Ki-67 antigen immunostaining in squamous intraepithelial lesions of the uterine cervix. Anat Pathol. 1995;104:154–160. doi: 10.1093/ajcp/104.2.154. [DOI] [PubMed] [Google Scholar]
- 2.Blanton R A, Perez R N, Merrick D T, McDougall J K. Epithelial cells immortalized by human papillomaviruses have premalignant characteristics in organotypic culture. Am J Pathol. 1991;138:673–685. [PMC free article] [PubMed] [Google Scholar]
- 3.Blanton R A, Coltrera M D, Gown A M, Halbert C L, McDougall J K. Expression of the HPV16 E7 gene generates proliferation in stratified squamous cell cultures which is independent of endogenous p53 levels. Cell Growth Differ. 1992;3:791–802. [PubMed] [Google Scholar]
- 4.Broker T R, Chow L T, Chin M T, Rhodes C R, Wolinsky S M, Whitbeck A, Stoler M H. A molecular portrait of human papillomavirus carcinogenesis. Cancer Cells. 1989;7:197–208. [Google Scholar]
- 5.Chen T M, Pecoraro G, Defendi V. Genetic analysis of in vitro progression of human papillomavirus-transfected human cervical cells. Cancer Res. 1993;53:1167–1171. [PubMed] [Google Scholar]
- 6.Cheng S, Schmidt-Grimminger D-C, Murant T, Broker T R, Chow L T. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev. 1995;9:2335–2349. doi: 10.1101/gad.9.19.2335. [DOI] [PubMed] [Google Scholar]
- 7.Chow L T, Broker T R. Small DNA tumor viruses. In: Nathanson N, editor. Viral pathogenesis. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 267–302. [Google Scholar]
- 8.Chow L T, Broker T R. In vitro experimental systems for HPV: epithelial raft cultures for investigations of viral reproduction and pathogenesis and for genetic analyses of viral proteins and regulatory sequences. Clin Dermatol. 1997;15:217–227. doi: 10.1016/s0738-081x(97)00069-2. [DOI] [PubMed] [Google Scholar]
- 9.Coussens L M, Hanahan D, Arbeit J M. Genetic predisposition and parameters of malignant progression in K14-HPV16 transgenic mice. Am J Pathol. 1996;149:1899–1917. [PMC free article] [PubMed] [Google Scholar]
- 10.Cullen A P, Reid R, Campion M, Lörincz A T. Analysis of the physical state of different human papillomavirus DNAs in intraepithelial and invasive cervical neoplasm. J Virol. 1991;65:606–612. doi: 10.1128/jvi.65.2.606-612.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dale B A, Resing K A, Lonsdale-Eccles J D. Filaggrin: a keratin filament associated protein. Ann N Y Acad Sci. 1985;455:330–342. doi: 10.1111/j.1749-6632.1985.tb50420.x. [DOI] [PubMed] [Google Scholar]
- 12.Demeter L M, Stoler M H, Broker T R, Chow L T. Induction of proliferating cell nuclear antigen in differentiated keratinocytes of human papillomavirus-infected lesions. Hum Pathol. 1994;25:343–348. doi: 10.1016/0046-8177(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 13.Dollard S C, Wilson J L, Demeter L M, Bonnez W, Reichman R C, Broker T R, Chow L T. Production of human papillomavirus and modulation of the infectious program in epithelial raft cultures. Genes Dev. 1992;6:1131–1142. doi: 10.1101/gad.6.7.1131. [DOI] [PubMed] [Google Scholar]
- 14.Dürst M, Bosch F X, Glitz D, Schneider A, zur Hausen H. Inverse relationship between human papillomavirus (HPV) type 16 early gene expression and cell differentiation in nude mouse epithelial cysts and tumors induced by HPV-positive human cell lines. J Virol. 1991;65:796–804. doi: 10.1128/jvi.65.2.796-804.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dürst M, Glitz D, Schneider A, zur Hausen H. Human papillomavirus type 16 (HPV 16) gene expression and DNA replication in cervical neoplasia: analysis by in situ hybridisation. Virology. 1992;189:132–140. doi: 10.1016/0042-6822(92)90688-l. [DOI] [PubMed] [Google Scholar]
- 16.Dürst M, Kleinheinz A, Holtz M, Gissmann L. The physical state of human papillomavirus type 16 DNA in benign and malignant tumors. J Gen Virol. 1985;66:1515–1522. doi: 10.1099/0022-1317-66-7-1515. [DOI] [PubMed] [Google Scholar]
- 17.El-Deiry W S, Tokino T, Waldman T, Oliner J D, Velculescu V E, Burrell M, Hill D E, Healy E, Rees J L, Hamilton S R, Kinzler K W, Vogelstein B. Topological control of p21WAF1/CIP1 expression in normal and neoplastic tissues. Cancer Res. 1995;55:2910–2919. [PubMed] [Google Scholar]
- 18.Frattini M G, Lim H B, Laimins L A. In vitro synthesis of oncogenic human papillomaviruses requires episomal genomes for differentiation-dependent late gene expression. Proc Natl Acad Sci USA. 1996;93:3062–3067. doi: 10.1073/pnas.93.7.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fredersdorf S, Milne A W, Hall P A, Lu X. Characterization of a panel of novel anti-p21waf1/cip1 monoclonal antibodies and immunochemical analysis of p21waf1/cip1 expression in normal human tissues. Am J Pathol. 1996;148:825–835. [PMC free article] [PubMed] [Google Scholar]
- 20.Fuchs E, Tyner A L, Giudice G J, Marchuk D, RayChaudhury A, Rosenberg M. The human keratin genes and their differential expression. Curr Top Dev Biol. 1987;22:5–34. doi: 10.1016/s0070-2153(08)60097-6. [DOI] [PubMed] [Google Scholar]
- 21.Gudas J, Nguyen H, Li T, Hill D, Cowan K H. Effects of cell cycle, wild-type p53 and DNA damage on p21cip1/waf1 expression in human breast epithelial cells. Oncogene. 1995;11:253–261. [PubMed] [Google Scholar]
- 22.Hawley-Nelson P, Vousden K H, Hubbert N L, Lowy D R, Schiller J T. HPV 16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989;8:3905–3910. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Healy E, Reynolds N J, Smith M D, Harrison D, Doherty E, Campbell C, Rees J L. Up-regulation of p21WAF1/CIP1 in psoriasis and after the application of irritants and tape stripping. J Invest Dermatol. 1995;105:274–279. doi: 10.1111/1523-1747.ep12318430. [DOI] [PubMed] [Google Scholar]
- 24.Heselmeyer K, Schrock E, du Manoir S, Blegen H, Shah K, Steinbeck R, Auer G, Ried T. Gain of chromosome 3q defines the transition from severe dysplasia to invasive carcinoma of the uterine cervix. Proc Natl Acad Sci USA. 1996;93:479–484. doi: 10.1073/pnas.93.1.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isern, S., J. N. Parker, R. D. M. Steenbergen, P. J. F. Snijders, J. M. M. Walboomers, C. J. L. M. Meijer, T. R. Broker, and L. T. Chow. Unpublished data.
- 26.Jeon S, Lambert P F. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: implications for cervical carcinogenesis. Proc Natl Acad Sci USA. 1995;92:1654–1658. doi: 10.1073/pnas.92.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jian, Y., D.-C. Schmidt-Grimminger, X. Wu, T. R. Broker, and L. T. Chow. Unpublished data.
- 28.Kastan M B, Canman C E, Leonard C J. p53, cell cycle control and apoptosis: implications for cancer. Cancer Metastasis Rev. 1995;14:3–15. doi: 10.1007/BF00690207. [DOI] [PubMed] [Google Scholar]
- 29.Kopan R, Traska G, Fuchs E. Retinoids as important regulators of terminal differentiation: examining keratin expression in individual epidermal cells at various states of keratinization. J Cell Biol. 1987;105:427–440. doi: 10.1083/jcb.105.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuta M, Kon S, Sasaki K, Matsuta M. Immunohistochemical detection of p21waf1/cip1 and p53 proteins in formalin-fixed paraffin-embedded tissue sections of squamous cell carcinoma of the skin. J Dermatol Sci. 1997;14:233–239. doi: 10.1016/s0923-1811(96)00579-8. [DOI] [PubMed] [Google Scholar]
- 31.May M, Dong X-P, Beyer-Finkler E, Stubenrauch F, Fuchs P G, Pfister H. E6/E7 promoter of extrachromosomal HPV16 DNA in cervical cancers escapes from cellular repression by mutation of target sequences for YY1. EMBO J. 1994;6:1460–1466. doi: 10.1002/j.1460-2075.1994.tb06400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCance D J, Kopan R, Fuchs E, Laimins L A. Human papillomavirus type 16 alters human epithelial cell differentiation in vitro. Proc Natl Acad Sci USA. 1988;85:7169–7173. doi: 10.1073/pnas.85.19.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merrick D T, Gown A M, Halbert C L, McDougall J K. Altered expression of proliferation and differentiation markers in human papillomavirus 16 and 18 immortalized epithelial cells grown in organotypic culture. Am J Pathol. 1992;140:167–177. [PMC free article] [PubMed] [Google Scholar]
- 34.Münger K, Phelps W C, Bubb V, Howley P M, Schlegel R M. The E6 and E7 genes of human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63:4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakanishi M, Adami G R, Robetorye R S, Noda A, Venable S F, Dimitrov D, Pereira-Smith O M, Smith J R. Exit from G0 and entry into the cell cycle of cells expressing p21sdi1 antisense RNA. Proc Natl Acad Sci USA. 1995;92:4352–4356. doi: 10.1073/pnas.92.10.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palazzo J P, Mercer W E, Kovatich A J, McHugh M. Immunohistochemical localization of p21waf1/cip1 in normal, hyperplastic, and neoplastic uterine tissues. Hum Pathol. 1997;28:60–66. doi: 10.1016/s0046-8177(97)90280-x. [DOI] [PubMed] [Google Scholar]
- 37.Parker J N, Zhao W, Askins K J, Broker T R, Chow L T. Mutational analyses of differentiation-dependent human papillomavirus type 18 enhancer elements in epithelial raft cultures of neonatal foreskin keratinocytes. Cell Growth Differ. 1997;8:751–762. [PubMed] [Google Scholar]
- 38.Pontén F, Berne B, Ren Z P, Nister M, Ponten J. Ultraviolet light induces expression of p53 and p21 in human skin: effect of sunscreen and constitutive p21 expression in skin appendages. J Invest Dermatol. 1995;105:402–406. doi: 10.1111/1523-1747.ep12321071. [DOI] [PubMed] [Google Scholar]
- 39.Remmink A J, Walboomers J M M, Helmerhorst T J M, Voorhorst F J, Rozendaal L, Risse E K J, Meijer C J L M, Kenemans P. The presence of persistent high-risk HPV genotypes in dysplastic cervical lesions is associated with progressive disease: natural history up to 36 months. Int J Cancer. 1995;61:306–311. doi: 10.1002/ijc.2910610305. [DOI] [PubMed] [Google Scholar]
- 40.Scheffner M, Romanczuk H, Münger K, Huibregtse J M, Mietz J A, Howley P M. Functions of human papillomavirus proteins. Curr Top Microbiol Immunol. 1994;186:83–99. doi: 10.1007/978-3-642-78487-3_5. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt-Grimminger, D.-C., X. Wu, Y. Jian, T. R. Broker, and L. T. Chow. Post-transcriptional induction of p21cip1 protein in warts and dysplasias is inversely related to human papillomavirus activities. Am. J. Pathol., in press. [PMC free article] [PubMed]
- 42.Seagon S, Dürst M. Genetic analysis of an in vitro model system for human papillomavirus type 16-associated tumorigenesis. Cancer Res. 1994;54:5593–5598. [PubMed] [Google Scholar]
- 43.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 44.Steenbergen, R. D. M., A. B. Oostra, H. Joenje, F. Arwert, J. M. M. Walboomers, C. J. L. M. Meijer, and P. J. F. Snijders. Cytogenetic instability following transfection of human keratinocytes with HPV 16 and HPV 18 DNA. Submitted for publication.
- 45.Steenbergen R D M, Walboomers J M M, Meijer C J L M, van der Raaij-Helmer, Parker J N, Chow L T, Broker T R, Snijders P J F. Transition of human papillomavirus type 16 and 18 transfected human foreskin keratinocytes towards immortality: activation of telomerase and allele losses at 3p, 10p, 11q and/or 18q. Oncogene. 1996;13:1249–1257. [PubMed] [Google Scholar]
- 46.Stoler M H, Rhodes C R, Whitbeck A, Wolinsky S M, Chow L T, Broker T R. Human papillomavirus type 16 and 18 gene expression in cervical neoplasia. Hum Pathol. 1992;23:117–128. doi: 10.1016/0046-8177(92)90232-r. [DOI] [PubMed] [Google Scholar]
- 47.Stoler M H, Whitbeck A, Wolinsky S M, Broker T R, Chow L T, Howett M K, Kreider J W. Infectious cycle of human papillomavirus type 11 in human foreskin xenografts in nude mice. J Virol. 1990;64:3310–3318. doi: 10.1128/jvi.64.7.3310-3318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stoler M H, Wolinsky S M, Whitbeck A, Broker T R, Chow L T. Differentiation-linked human papillomavirus types 6 and 11 transcription in genital condylomata revealed by in situ hybridization with message-specific RNA probes. Virology. 1989;172:331–340. doi: 10.1016/0042-6822(89)90135-9. [DOI] [PubMed] [Google Scholar]
- 49.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 50.Wilson J L, Dollard S C, Chow L T, Broker T R. Epithelial specific gene expression during differentiation of stratified primary human keratinocyte cultures. Cell Growth Differ. 1992;3:471–483. [PubMed] [Google Scholar]
- 51.Woodworth C D, Waggoner S, Barnes W, Stoler M H, DiPaolo J A. Human cervical and foreskin epithelial cells immortalized by human papillomavirus DNAs exhibit dysplastic differentiation in vivo. Cancer Res. 1990;50:3709–3715. [PubMed] [Google Scholar]
- 52.Xiong Y, Kuppuswamy D, Li Y, Livanos E M, Hixon M, White A, Beach D, Tlsty T D. Alteration of cell cycle kinase complexes in human papillomavirus E6- and E7-expressing fibroblasts precedes neoplastic transformation. J Virol. 1996;70:999–1008. doi: 10.1128/jvi.70.2.999-1008.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao W, Chow L T, Broker T R. Transcription activities of human papillomavirus type 11 E6 promoter-proximal elements in raft and submerged cultures of foreskin keratinocytes. J Virol. 1997;71:8832–8840. doi: 10.1128/jvi.71.11.8832-8840.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.zur Hausen H. Molecular pathogenesis of cancer of the cervix and its causation by specific human papillomavirus types. Curr Top Microbiol Immunol. 1994;186:131–156. doi: 10.1007/978-3-642-78487-3_8. [DOI] [PubMed] [Google Scholar]
- 55.zur Hausen H, de Villiers E-M. Human papillomaviruses. Annu Rev Microbiol. 1994;48:427–447. doi: 10.1146/annurev.mi.48.100194.002235. [DOI] [PubMed] [Google Scholar]