Abstract
Kirsten rat sarcoma 2 viral oncogene homolog (KRAS) is the most frequently mutated oncogene in human cancers with mutations predominantly occurring in codon 12. These mutations disrupt the normal function of KRAS by interfering with GTP hydrolysis and nucleotide exchange activity, making it prone to the GTP-bound active state, thus leading to sustained activation of downstream pathways. Despite decades of research, there has been no progress in the KRAS drug discovery until the groundbreaking discovery of covalently targeting the KRASG12C mutation in 2013, which led to revolutionary changes in KRAS-targeted therapy. So far, two small molecule inhibitors sotorasib and adagrasib targeting KRASG12C have received accelerated approval for the treatment of non-small cell lung cancer (NSCLC) harboring KRASG12C mutations. In recent years, rapid progress has been achieved in the KRAS-targeted therapy field, especially the exploration of KRASG12C covalent inhibitors in other KRASG12C-positive malignancies, novel KRAS inhibitors beyond KRASG12C mutation or pan-KRAS inhibitors, and approaches to indirectly targeting KRAS. In this review, we provide a comprehensive overview of the molecular and mutational characteristics of KRAS and summarize the development and current status of covalent inhibitors targeting the KRASG12C mutation. We also discuss emerging promising KRAS-targeted therapeutic strategies, with a focus on mutation-specific and direct pan-KRAS inhibitors and indirect KRAS inhibitors through targeting the RAS activation-associated proteins Src homology-2 domain-containing phosphatase 2 (SHP2) and son of sevenless homolog 1 (SOS1), and shed light on current challenges and opportunities for drug discovery in this field.
Keywords: KRAS mutation, KRASG12C inhibitor, Pan-KRAS inhibitor, SHP2 allosteric inhibitor, SOS1 inhibitor, combination therapy
Introduction
Kirsten rat sarcoma 2 viral oncogene homolog (KRAS) is the most notorious oncogene, which is frequently mutated in various cancers, mainly lung cancer, colorectal cancer, and pancreatic cancer. KRAS is a small GTPase with slow intrinsic GTP hydrolysis activity, prompting KRAS to switch from the GTP-bound active state to the GDP-bound inactive state. Regulated by GTPase activating proteins (GAPs) that stimulate GTP hydrolysis and guanine exchange factors (GEFs) that promote the exchange of GDP to GTP, KRAS is in the cycle between GTP- and GDP-bound state [1]. The balance of GTP hydrolysis and guanine exchange determines the level of KRAS activation. In the GTP-bound state, KRAS interacts with and activates downstream effector molecules such as RAF kinase mediating the MAPK pathway or PI3K-AKT pathway. However, various KRAS mutations elicit impaired GTP hydrolysis or enhanced nucleotide exchange, compelling KRAS to be in the GTP-bound active state [2].
Long-term efforts to target KRAS to develop GTP-competitive inhibitors failed due to its picomolar affinity for GTP and lack of an obvious binding pocket. Nevertheless, in 2013, Kevan Shokat proposed the concept of covalent inhibitors that trap KRASG12C into an inactive GDP-bound conformation, which led to the breakthrough discovery of covalent inhibitors as the most prevailing means of targeting the KRASG12C-specific mutation [3]. Since then, numerous covalent inhibitors have been developed and some of them are in clinical trials [4–6]. Among them, sotorasib (also known as AMG510) and adagrasib (MRTX849) have been accelerated approval by the U.S. Food and Drug Administration (FDA) in May 2021 and December 2022 for KRASG12C mutated non-small cell lung cancer (NSCLC), respectively [7, 8].
Although the success of targeting the KRASG12C mutation broke the curse of KRAS “undruggable”, drug discovery targeting the entire field of KRAS still faces grim challenges. In particular, drug discovery strategies like KRASG12C covalent inhibitors may not apply to other mutations, and the inevitable drug resistance problems are gradually exposed in clinical trials of those drugs targeting KRASG12C [9, 10]. In this review, we look back at the development process of KRASG12C covalent inhibitors from bench to bedside in the past decade and the recent advances in targeting KRAS, as well as look forward to the future development of KRAS inhibitors (Fig. 1). Firstly, we describe the structural and biochemical characteristics of KRAS and the occurrence frequency of different KRAS mutations in various tumors. Secondly, we summarize the current status and existing problems of KRASG12C covalent inhibitors. Finally, we outline and highlight the emerging potential drug development strategies that target other KRAS mutations beyond KRASG12C, and approaches to indirectly inhibit KRAS by targeting RAS pathway-associated proteins, with emphasis on the non-receptor protein tyrosine phosphatase SHP2 and the GEF son of sevenless homolog 1 (SOS1).
Fig. 1. Overview of advances in KRAS-targeted therapy inhibitors.
At present, KRAS-targeted therapy is mainly through direct or indirect strategies to inhibit RAS activation and the downstream MAPK and PI3K pathways, thereby suppressing cell proliferation and survival. Direct inhibition of KRAS involves mutation-specific inhibitors (such as KRASG12C inhibitors and KRASG12D inhibitors), RAS(ON) inhibitors, and other emerging KRAS inhibitors. Indirect inhibition of KRAS including SHP2 and SOS1 inhibitors, affects the conversion of KRAS-GDP to KRAS-GTP by disrupting the guanylate exchange process of the KRAS cycle.
Structure and function of KRAS
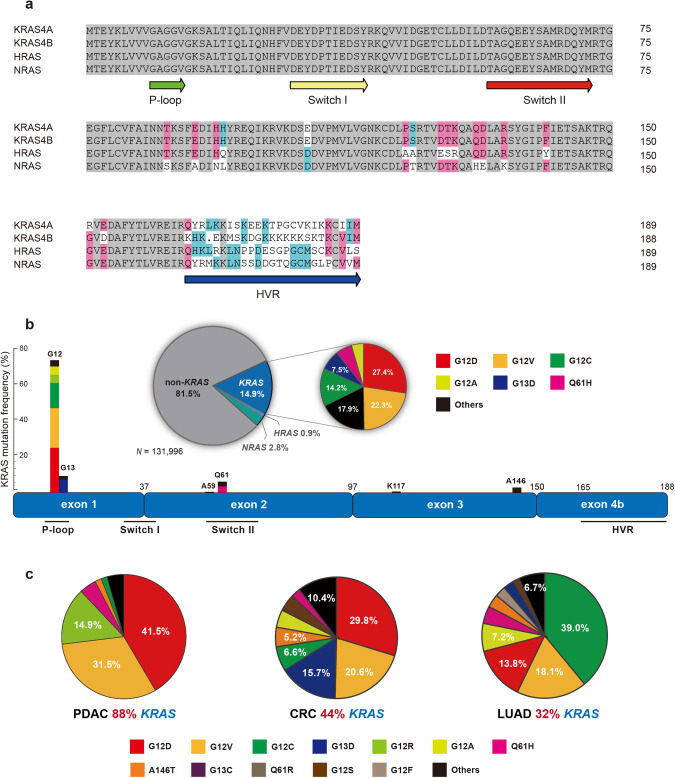
The KRAS gene is a member of the mammalian RAS gene family and encodes two different isoforms, KRAS4A, and KRAS4B, through alternative splicing of the fourth exon. The N-terminal amino acid sequences (1–164 aa) of KRAS4A and KRAS4B are almost identical and are highly homologous to other members of the RAS family (NRAS and HRAS), but the alternative fourth exon results in a hypervariable region (HVR) in C-terminus (165–188/189 aa) [11] (Fig. 2a). The HVR includes a CAAX (here, C refers to cysteine, A refers to an aliphatic amino acid, X means any amino acid) motif, where the farnesylation of the cysteine and the palmitoylation of one or two cysteine residues upstream of the CAAX motif together affect the membrane orientation of KRAS, which is essential for KRAS signal activation [1, 12]. Due to structural differences, KRAS4B does not undergo palmitoylation, and its membrane orientation is determined by the interaction between the positively charged lysine-rich region in the HVR and the negatively charged lipid membrane [1, 13]. Generally considered, KRAS4B is the predominant isoform, but several recent studies have shown that KRAS4A is widely expressed in RAS mutant tumors as well [12, 14]. However, the difference in function and expression level of the two splice variants is still less clear. A recent intriguing study found that due to different post-translational modifications, the palmitoylation of KRAS4A enables it to co-localize with hexokinase 1 (HK1) on the outer mitochondrial membrane, thereby directly regulating the metabolic activity of HK1, but KRAS4B does not [15].
Fig. 2. Structure of KRAS protein and mutation frequency in human cancer.
a Sequence homology comparison of RAS family proteins, analyzed by DNAMAN software. b Structure of KRAS protein, and the location and relative frequency of various KRAS mutations. Pie chart showing the mutation frequencies of the three RAS isoforms in human cancers and the proportion of each mutant in the whole KRAS missense mutations. c Mutation type and frequency of KRAS in pancreatic adenocarcinoma (PDAC), colorectal cancer (CRC), and lung adenocarcinoma (LUAD). Data from GENIE Cohort 13.0-public (https://genie.cbioportal.org/).
Functionally, KRAS protein belongs to the small GTPases family, which is inseparable from its N-terminal structure. The N-terminal 164 amino acids comprise the G domain that binds and hydrolyzes GTP, which is divided into two parts: the effector lobe and the allosteric lobe [16, 17]. The effector lobe contains P-loop (phosphate-binding loop, 10–14 aa), switch I (30–40 aa), and switch II (58–72 aa) regions [17]. The P-loop is composed of glycine-rich sequences and is a common motif for GTP binding [18]. The switch regions are the interface where KRAS interacts with effector proteins and regulatory proteins (GAP and GEF), and they are relatively flexible for the RAS conformation switch [17]. The P-loop and switch II regions are the most frequently mutated sites of KRAS, and many current KRAS inhibitors bind to these sites (Fig. 2b).
KRAS cycles between GTP-bound and GDP-bound states by GTP hydrolysis and nucleotide exchange. In the GTP-bound state, the γ-phosphate of GTP forms hydrogen bonds with the threonine (T35) and glycine (G60) residues in the switch I and switch II regions, respectively [19]. KRAS has a slow intrinsic GTP hydrolysis activity, which is greatly increased when stimulated by GAPs [20, 21]. When GTP is hydrolyzed into GDP, γ-phosphate is released, and the switch regions relax to a flexible conformation, which is described as a loaded spring mechanism [19]. Mg2+ also plays a critical role in this process [19, 21]. At this point, GEFs spatially interact with the switch regions driving out GDP and Mg2+ from the active site, thereby giving GTP a chance to rebound [19]. Due to the picomolar affinity of KRAS for GTP/GDP, the fact that intracellular GTP levels are higher than GDP, and the catalytic effect of GEFs collectively promotes the conversion from GDP to GTP-bound state [19, 22]. The conformational switch to GTP-bound KRAS interacts more tightly with downstream effectors, such as RAF kinase or PI3K, and then activates downstream MAPK or AKT-mTOR signaling pathways, affecting cell proliferation and survival [19, 23, 24].
Frequency of KRAS mutations in cancer and biochemical characteristics
Approximately 20% of cancer patients harbor RAS mutations, of which KRAS mutations account for approximately 80% (Fig. 2b). Based on cBioPortal database analysis (https://genie.cbioportal.org/) [25], KRAS mutations are the most prevalent in pancreatic adenocarcinoma (PDAC; 88%), colorectal cancer (CRC; 44%), and lung adenocarcinoma (LUAD; 32%) in terms of tumor types (Fig. 2c). In contrast, NRAS and HRAS mutations are less frequent across all tumor types.
KRAS mutations are mainly base mutations that lead to missense substitution of coding amino acids, and the mutation at codon 12 bears the brunt, followed by codons 13 and 61. G12D, G12V, G12C, G12A, and the other two mutations G13D and Q61H accounted for more than 80% of all KRAS mutations (Fig. 2b). G12D and G12V mutations are far more prevalent in PDAC than other mutations and relatively less in CRC, while G13D mutation is particularly in CRC. In LUAD, 80% of KRAS mutations are at G12, and the dominant mutation is G12C (Fig. 2c). Understanding the variation in the frequency of KRAS mutations in different tumors is extremely necessary for the development of RAS-targeted therapeutics, especially for selecting KRASG12C inhibitor-sensitive populations in clinical trials.
Whether KRAS is activated or not depends on the nucleotides bound to it, and KRAS mutations disrupt the normal KRAS GTPase cycle. Hunter JC and colleagues systematically compared the biochemical properties of different KRAS mutants [2]. Compared with wild-type (WT) KRAS, almost all KRAS mutations impaired their intrinsic GTP hydrolysis ability, while only the G12C mutation had a minimal impact [2]. Since G12C mutation endows KRAS with relatively strong hydrolysis ability, the development of KRASG12C-specific inhibitors that trap the KRASG12C mutation in its GDP-bound inactive state has been feasible and successful [3, 26]. In addition, GAP-stimulated KRAS-mutant GTPase activity was significantly attenuated relative to WT KRAS without exception [2]. And the intrinsic nucleotide exchange of mutant KRAS is almost the same, except for the G13D mutation, which has a faster nucleotide exchange rate than WT KRAS [2]. Collectively, KRAS mutations result in a higher likelihood of KRAS in the GTP-bound state, regardless of impaired GTP hydrolysis or enhanced guanylate exchange. This also reminds us that different KRAS alleles may have distinct physiological functions, including affinity with GAP, dependence on GEF, and changes in affinity with downstream effectors should be painstakingly considered during drug development.
KRASG12C targeted therapy
In view that GTP-bound KRAS is in its active state, initially targeting KRAS pinned their hopes on GTP-competitive inhibitors, but it was considered impractical due to the picomolar affinity of KRAS for GTP while the intracellular GTP concentration in the micromolar range [27]. For the sake of conquering KRAS mutations, medicinal chemists have turned their attention to the KRASG12C special mutation, where glycine at position 12 mutates to cysteine, allowing for the development of cysteine-reactive small molecules. Kevan Shokat and colleagues were the first to put this idea into practice through the tethering screen approach to identify a class of disulfide-based compounds that bind in the allosteric pocket of KRASG12C protein, termed the switch-II pocket (S-IIP) [3]. Further screening of irreversibly binding compounds found that compound 12 was the most potent inhibitor that binds to and stabilizes the GDP-bound state of KRAS, impairing SOS-catalyzed nucleotide exchange and the interaction with effector protein RAF [3]. Since then, these milestone discoveries have been the starting point for KRASG12C inhibitors, opening up a novel path for KRAS-targeted therapy (Fig. 3a).
Fig. 3. Chemical structures of representative KRAS-targeted therapeutic inhibitors.
a KRASG12C covalent inhibitors. b–f Direct KRAS inhibitors beyond KRASG12C mutation, including KRASG12D inhibitors (b), KRASG12S inhibitor (c), KRASG12R inhibitor (d), and pan-KRAS inhibitors that bind to the switch I/II or switch II pocket (e), and RAS(ON) inhibitor (f) that could form a tri-complex with GTP-bound RAS. g, h Indirect KRAS inhibitors, including SHP2 allosteric inhibitors (g) and SOS1::KRAS protein–protein interaction inhibitors (h).
KRASG12C covalent inhibitors
ARS-1620
Building on the work of pioneers, Piro Lito et al. reported a novel compound, ARS-853, trapped KRASG12C in the GDP-bound conformation [26]. ARS-853 significantly inhibited the proliferation of KRASG12C mutant tumor cells and reduced intracellular KRAS-GTP levels at micromolar concentrations in vitro. Further construction of co-mutations in KRASG12C mutant cells found that those mutations that damage the GTP hydrolysis activity of KRASG12C (A59G) or increase its nucleotide exchange activity (Y40A) reduced the efficacy of ARS-853, while mutations that decrease nucleotide exchange (Y32S) slightly increased its effect, indicating that the GTPase activity of KRASG12C is crucial to the inhibitory activity of ARS-853 [26]. This work strengthened the feasibility of targeting the KRASG12C mutation, that is, trapping KRASG12C in a GDP-bound inactive state by covalent inhibitors may be a promising and effective strategy for anti-KRAS. Meanwhile, Wellspring Biosciences scientists also confirmed that ARS-853 is cell-active and can be exploited as the first pharmacological tool for KRASG12C targeted therapy [28].
Optimization of the potency and pharmacokinetic properties of the ARS-853 series compounds, the compound ARS-1620 with rigid quinazoline scaffold was identified, which interacts with the H95 of the KRASG12C protein with improved efficacy [29]. In KRASG12C mutant cell lines, ARS-1620 selectively covalently bound to KRASG12C and suppressed KRAS downstream signaling network [29]. As expected, ARS-1620 exhibited extensive in vivo anti-tumor activity on a variety of cell-line-derived xenograft (CDX) and patient-derived xenograft (PDX) models harboring KRASG12C mutant allele. They also proposed that in vitro cell proliferation experiments under 3D-spherical culture conditions can better predict the in vivo anti-tumor activity of KRASG12C inhibitors, rather than 2D-adhesion culture, which provided great guidance for subsequent screening of candidate clinical compounds [29]. Most notably, this is the first reported KRASG12C inhibitor with in vivo activity, and subsequent inhibitor JNJ-74699157 (ARS-3248) jointly developed by Wellspring Biosciences and Janssen Biotech has entered a phase 1 study in 2019 (NCT04006301) (Table 1). However, the clinical trial was reported to have been stopped due to phase 1 results showing dose-limiting skeletal muscle toxicity and lack of efficacy [30].
Table 1.
Clinical development of KRAS targeted therapy inhibitors.
| Drug | Company | Monotherapy or combination | Disease indications tested in trials | Study phase | Clinical trials identifier |
|---|---|---|---|---|---|
| KRASG12C INHIBITORS | |||||
| AMG510 (SOTORASIB) | Amgen | Monotherapy | KRAS-G12C mutant advanced solid tumors | Phase 1/2 | NCT03600883a |
| + PD-1/L1 inhibitor | |||||
| Monotherapy | KRAS-G12C mutant advanced solid tumors | Phase 1b/2 | NCT04185883 | ||
| + pembrolizumab (PD-1 inhibitor) | |||||
| + trametinib (MEK inhibitor) | |||||
| + trametinib (MEK inhibitor) and panitumumab (EGFR antibody) | |||||
| + RMC-4630 (SHP2 inhibitor) | |||||
| + afatinib (pan-ErbB inhibitor) | |||||
| + atezolizumab (PD-L1 inhibitor) | |||||
| + panitumumab (EGFR antibody) +/- FOLFIRI (chemotherapy) | |||||
| + carboplatin and pemetrexed or docetaxel (chemotherapy) | |||||
| + AMG 404 (PD-1 inhibitor) | |||||
| + everolimus (mTOR inhibitor) | |||||
| + palbociclib (CDK 4/6 inhibitor) | |||||
| + MVASI (VEGF inhibitor) and FOLFIRI or FOLFOX (chemotherapy) | |||||
| + TNO155 (SHP2 inhibitor) | |||||
| + BI 1701963 (SOS1 inhibitor) | |||||
| + avutometinib (RAF/MEK inhibitor) | KRAS-G12C mutant NSCLC | Phase 1/2 | NCT05074810 | ||
| + MVASI (VEGF inhibitor) | KRAS-G12C mutant NSCLC | Phase 1/2 | NCT05180422 | ||
| Monotherapy | KRAS-G12C mutant non-squamous NSCLC | Phase 2 | NCT04625647 | ||
| Monotherapy | KRAS-G12C mutant stage III NSCLC | Phase 2 | NCT05398094 | ||
| Monotherapy | KRAS-G12C mutant stage IV NSCLC | Phase 2 | NCT04933695a | ||
| Monotherapy | KRAS-G12C mutant NSCLC | Phase 3 | NCT04303780a | ||
| + panitumumab (EGFR inhibitor) | KRAS-G12C mutant CRC | Phase 3 | NCT05198934a | ||
| MRTX849 (ADAGRASIB) | Mirati Therapeutics | Monotherapy | KRAS-G12C mutant advanced solid tumors | Phase 1/2 | NCT03785249 |
| + pembrolizumab (PD-1 inhibitor) | |||||
| + cetuximab (EGFR antibody) | |||||
| + afatinib (pan-ErbB inhibitor) | |||||
| + TNO155 (SHP2 inhibitor) | KRAS-G12C mutant advanced solid tumors | Phase 1/2 | NCT04330664a | ||
| Monotherapy | KRAS-G12C mutant advanced NSCLC | Phase 2/3 | NCT04613596 | ||
| + pembrolizumab (PD-1 inhibitor) | |||||
| + cetuximab (EGFR antibody) | KRAS-G12C mutant advanced CRC | Phase 3 | NCT04793958 | ||
| Monotherapy | KRAS-G12C mutant previously treated NSCLC | Phase 3 | NCT04685135 | ||
| + BI 1701963 (SOS1 inhibitor) | KRAS-G12C mutant advanced solid tumors | Phase 1 | NCT04975256 | ||
| + palbociclib (CDK 4/6 inhibitor) | KRAS-G12C mutant advanced solid tumors | Phase 1 | NCT05178888a | ||
| Monotherapy | KRAS-G12C mutant PDAC | Phase 1b | NCT05634525b | ||
| + cetuximab (EGFR antibody) and irinotecan | KRAS-G12C mutant CRC | Phase 1 | NCT05722327b | ||
| JNJ-74699157 (ARS-3248) | Janssen | Monotherapy | KRAS-G12C mutant advanced solid tumors | Phase 1 | NCT04006301c |
| LY3499446 | Lilly | Monotherapy | KRAS-G12C mutant advanced solid tumors | Phase 1/2 | NCT04165031c |
| JDQ443 | Novartis | Monotherapy | KRAS-G12C mutant advanced solid tumors | Phase 1b/2 | NCT04699188 |
| + TNO155 (SHP2 inhibitor) | |||||
| + tislelizumab (PD-1 inhibitor) | |||||
| + TNO155 and tislelizumab | |||||
| Monotherapy | KRAS-G12C mutant advanced NSCLC | Phase 3 | NCT05132075 | ||
| + trametinib (MEK inhibitor) | KRAS-G12C mutant advanced solid tumors | Phase 1b/2 | NCT05358249 | ||
| + ribociclib (CDK4/6 inhibitor) | |||||
| + cetuximab (EGFR antibody) | |||||
| LY3537982 | Lilly | Monotherapy | KRAS-G12C mutant solid tumors | Phase 1 | NCT04956640 |
| + abemaciclib (CDK4/6 inhibitor) | |||||
| + erlotinib (EGFR inhibitor) | |||||
| + pembrolizumab (PD-1 inhibitor) | |||||
| + temuterkib (ERK inhibitor) | |||||
| + LY3295668 (Aurora-A kinase) | |||||
| + cetuximab (EGFR antibody) | |||||
| + TNO155 (SHP2 inhibitor) | |||||
| D-1553 | InventisBio | Monotherapy | KRAS-G12C mutant advanced solid tumor | Phase 1/2 | NCT04585035 |
| + other | |||||
| Monotherapy | KRAS-G12C mutant advanced solid tumor | Phase 1/2 | NCT05383898 | ||
| + IN10018 (FAK inhibitor) | KRAS-G12C mutant advanced solid tumor | Phase 1/2 | NCT05379946b | ||
| + other | KRAS-G12C mutant advanced solid tumor | Phase 1/2 | NCT05492045b | ||
| GDC-6036 | Genentech | Monotherapy | KRAS-G12C mutant advanced solid tumor | Phase 1 | NCT04449874 |
| + atezolizumab (PD-L1 inhibitor) | |||||
| + cetuximab (EGFR antibody) | |||||
| + bevacizumab (VEGF inhibitor) | |||||
| + erlotinib (EGFR inhibitor) | |||||
| + GDC-1971 (SHP2 inhibitor) | |||||
| + inavolisib (PI3Kα inhibitor) | |||||
| + pembrolizumab (PD-1 inhibitor) | KRAS-G12C mutant NSCLC | Phase 1b/2 | NCT05789082 | ||
| BI 1823911 | Boehringer Ingelheim | Monotherapy | KRAS-G12C mutant advanced solid tumor | Phase 1 | NCT04973163 |
| + BI 1701963 (SOS1 inhibitor) | |||||
| JAB-21822 | Jacobio Pharmaceuticals | Monotherapy | KRAS-G12C mutant advanced solid tumors | Phase 1/2 | NCT05002270 |
| + cetuximab (EGFR antibody) | |||||
| Monotherapy | KRAS-G12C mutant advanced solid tumors | Phase 1/2 | NCT05009329 | ||
| + cetuximab (EGFR antibody) | KRAS-G12C mutant advanced solid tumors | Phase 1/2 | NCT05194995 | ||
| Monotherapy | KRAS-G12C mutant NSCLC | Phase 1/2 | NCT05276726 | ||
| + cetuximab (EGFR antibody) | KRAS-G12C mutant CRC | Phase 2 | NCT05002270 | ||
| GFH925 | Genfleet Therapeutics | Monotherapy | KRAS-G12C mutant advanced solid tumors | Phase 1/2 | NCT05005234 |
| + cetuximab (EGFR antibody) | KRAS-G12C mutant NSCLC | Phase 1b/2 | NCT05756153b | ||
| GH35 | Suzhou Genhouse Bio | Monotherapy | KRAS-G12C mutant advanced solid tumors | Phase 1 | NCT05010694 |
| BPI-421286 | Betta Pharmaceuticals | Monotherapy | Advanced solid tumors | Phase 1 | NCT05315180 |
| RAS(ON) INHIBITORS | |||||
| RMC-6236 | Revolution Medicines | Monotherapy | KRAS mutant advanced solid tumors | Phase 1 | NCT05379985 |
| RMC-6291 | Revolution Medicines | Monotherapy | KRAS-G12C mutant advanced solid tumors | Phase 1 | NCT05462717 |
| KRASG12D INHIBITORS | |||||
| MRTX1133 | Mirati Therapeutics | Monotherapy | KRAS-G12D mutant advanced solid tumors | Phase 1 | NCT05737706 |
| HRS-4642 | Jiangsu HengRui Medicine | Monotherapy | KRAS-G12D mutant advanced solid tumors | Phase 1 | NCT05533463 |
| ASP3082 | Astellas Pharma | Monotherapy | KRAS-G12D mutant advanced solid tumors | Phase 1 | NCT05382559 |
| + cetuximab (EGFR antibody) | |||||
| SHP2 INHIBITORS | |||||
| TNO155 | Novartis | Monotherapy | KRAS-G12C mutant advanced solid tumors | Phase 1 | NCT03114319 |
| + EGF816 (EGFR inhibitor) | Advanced EGFR mutant NSCLC | ||||
| + spartalizumab (PD-1 inhibitor) | Selected malignancies (NSCLC, HNSCC, ESCC, GISTs, CRC) | Phase 1b | NCT04000529a | ||
| + ribociclib (CDK4/6 inhibitor) | |||||
| + dabrafenib (BRAF inhibitor) and LTT462 (ERK inhibitor) | BRAF V600-mutant CRC | Phase 1 | NCT04294160 | ||
| + dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor) | |||||
| + lorlatinib (ALK inhibitor) | ALK-positive or ROS1-positive lung cancer | Phase 1/2 | NCT04292119 | ||
| RMC-4630 (SAR442720) | Revolution Medicines | Monotherapy | Relapsed or refractory solid tumors | Phase 1 | NCT03634982a |
| + AMG510 (KRASG12C inhibitor) | KRAS G12C-mutant NSCLC | Phase 2 | NCT05054725a | ||
| + LY3214996 (ERK inhibitor) | Metastatic KRAS mutant cancers | Phase 1 | NCT04916236 | ||
| + cobimetinib (MEK inhibitor) | Relapsed/refractory solid tumors | Phase 1b/2 Phase 1b | NCT03989115 | ||
| + osimertinib (EGFR inhibitor) | EGFR mutant NSCLC | ||||
| + pembrolizumab (PD-1 inhibitor) | Advanced solid tumors | Phase 1/2 | NCT04418661a | ||
| + MRTX849 (KRASG12C inhibitor) | KRAS mutant NSCLC | ||||
| JAB-3068 | Jacobio Pharmaceuticals | Monotherapy | Advanced solid tumors | Phase 1 | NCT03518554 |
| Monotherapy | NSCLC, ESCC, and HNSCC | Phase 1/2a | NCT03565003 | ||
| + JS001 (PD1 inhibitor) | Advanced solid tumors | Phase 1b/2a | NCT04721223 | ||
| JAB-3312 | Jacobio Pharmaceuticals | Monotherapy | Advanced solid tumors | Phase 1 | NCT04045496 |
| Monotherapy | Advanced solid tumors | Phase 1 | NCT04121286 | ||
| + pembrolizumab (PD-1 inhibitor) | Advanced solid tumors | Phase 1/2a | NCT04720976 | ||
| + binimetinib (MEK inhibitor) | |||||
| + JAB-21822 (KRASG12C inhibitor) | KRAS-G12C mutant advanced solid tumors (NSCLC, CRC, PDAC) | Phase 1/2a | NCT05288205 | ||
| BBP-398 (IACS-15509) | Navire Pharma | Monotherapy | Advanced solid tumors | Phase 1/1b | NCT04528836 |
| + nivolumab (PD-1 inhibitor) | KRAS mutant advanced NSCLC | Phase 1 | NCT05375084 | ||
| + AMG510 (KRASG12C inhibitor) | KRAS-G12C mutant advanced solid tumors | Phase 1 | NCT05480865 | ||
| Monotherapy | Advanced solid tumors | Phase 1 | NCT05621525 | ||
| GDC-1971 (RLY-1971) | Relay Therapeutics | Monotherapy | Advanced or metastatic solid tumors | Phase 1 | NCT04252339 |
| + atezolizumab (PD-L1 inhibitor) | Advanced or metastatic solid tumors | Phase 1b | NCT05487235 | ||
| ERAS-601 | Erasca | Monotherapy | Advanced or metastatic solid tumors | Phase 1 | NCT04670679 |
| + cetuximab (EGFR antibody) | |||||
| + pembrolizumab (PD-1 inhibitor) | |||||
| + ERAS-007 (ERK inhibitor) | Advanced or metastatic solid tumors | Phase 1 | NCT04866134 | ||
| SH3809 | Nanjing Sanhome Pharmaceutical | Monotherapy | Advanced solid tumors | Phase 1 | NCT04843033 |
| HBI-2376 | HUYABIO International | Monotherapy | Advanced solid tumors harboring KRAS or EGFR mutations | Phase 1 | NCT05163028 |
| PF-07284892 | Pfizer | Monotherapy | Advanced solid tumors | Phase 1 | NCT04800822 |
| + lorlatinib (ALK inhibitor) | |||||
| + encorafenib (BRAF inhibitor) + cetuximab (EGFR antibody) | |||||
| + binimetinib (MEK inhibitor) | |||||
| ET0038 | Etern BioPharma | Monotherapy | Advanced solid tumors | Phase 1 | NCT05354843 |
| Monotherapy | Advanced solid tumors | Phase 1 | NCT05525559b | ||
| ICP-189 | Beijing InnoCare Pharma Tech | Monotherapy | Advanced solid tumors | Phase 1 | NCT05370755b |
| BPI-442096 | Betta Pharmaceuticals | Monotherapy | Advanced solid tumors | Phase 1 | NCT05369312b |
| SOS1 INHIBITORS | |||||
| BI 1701963 | Boehringer Ingelheim | Monotherapy | KRAS mutated advanced solid tumors | Phase 1 | NCT04111458a |
| + trametinib (MEK inhibitor) | |||||
| + BI 3011441 (MEK inhibitor) | KRAS mutated advanced solid tumors | Phase 1 | NCT04835714c | ||
| + irinotecan (chemotherapy) | KRAS mutated advanced bowel cancer | Phase 1 | NCT04627142c | ||
| MRTX0902 | Mirati Therapeutics | Monotherapy | KRAS/MAPK pathway mutated solid tumors | Phase 1 | NCT05578092 |
| + MRTX849 (KRASG12C inhibitor) | KRAS-G12C mutant advanced solid tumors | Phase 2 | |||
Data from clinicaltrials.gov, as of June 2023.
NSCLC non-small-cell lung cancer, CRC colorectal cancer, PDAC pancreatic ductal adenocarcinoma, HNSCC head and neck squamous cell carcinoma, ESCC esophageal SCC, GISTs gastrointestinal stromal tumors.
aActive, but not recruiting;
bNot yet recruiting;
cTerminated.
Sotorasib
Amgen’s sotorasib (AMG510) is the first clinically active KRASG12C inhibitor reported in 2019 [31]. Compared with ARS-1620, sotorasib with a larger volume of the isopropyl-methylpyridine substituent can better occupy the S-II pocket, especially exploiting the unique H95 groove markedly improved binding and potency [4, 32]. Sotorasib selectively inhibited the proliferation and phospho-ERK levels of KRASG12C mutant cells at nanomolar concentrations, whether heterozygous or homozygous, while is not effective against KRAS non-G12C mutant or WT KRAS [4]. In preclinical models, sotorasib significantly suppressed KRASG12C-mutant tumors as a single agent, and in combination with cytotoxic (carboplatin) or targeted drugs (MEK inhibitor or anti-PD-1 antibody) exhibited enhanced efficacy [4]. Even more interesting is that sotorasib caused durable cures of tumors in immune-competent mice, but only tumor regression in immunodeficient mice lacking T cells, suggesting sotorasib may have some effect on the immune system. Immunophenotyping analysis revealed that sotorasib induced a pro-inflammatory tumor microenvironment and synergized with anti-PD-1 immunotherapy [4]. These exciting findings, combined with the striking positive phase 1 early results, have greatly boosted people’s confidence in KRASG12C inhibitors [33].
One year later, the phase 1 results of sotorasib showed encouraging anticancer activity in NSCLC patients with KRASG12C mutation with a confirmed objective response rate (ORR) of 32.2% (19/59) and disease control rate (DCR) of 88.1% (52/59) and no dose-limiting toxic effects (NCT03600883) [34]. In December 2020, the FDA granted Breakthrough Therapy Designation to sotorasib for the treatment of patients with locally advanced or metastatic NSCLC with KRASG12C mutation. Soon after, on May 28, 2021, sotorasib received FDA accelerated approval for the treatment of KRASG12C-mutated NSCLC. Based on the results of a single-arm phase 2 clinical trial, sotorasib showed durable clinical benefit for KRASG12C mutant NSCLC patients with ORR of 37.1% (46/126) and a median duration of response (DoR) of 11.1 months at a dose of 960 mg once daily (NCT03600883) [35]. It must be mentioned here that the approval of sotorasib is a remarkable milestone and subversively breaks the curse of KRAS as “undruggable”.
Long-term analysis of phase 1 and phase 2 study (CodeBreaK100), updated ORR was 40.7%, median DoR was 12.3 months and 2-year overall survival (OS) was observed in 30% of 174 KRASG12C mutated NSCLC patients [36]. More recently, results from the phase 3 trial showed that sotorasib significantly improved the median PFS (5.6 vs 4.5 months) of KRASG12C-mutant NSCLC patients compared with intravenous docetaxel and had a more favorable safety profile, but no advantage in OS (10.6 vs 11.3 months) (NCT04303780) [37]. Other studies of sotorasib in unresectable stage III NSCLC ineligible for chemo-radiotherapy or stage IV NSCLC whose tumors need first-line treatment are also ongoing (NCT05398094 and NCT04933695).
Meanwhile, sotorasib demonstrated clinically meaningful anticancer activity with ORR of 21.1% (8/38) and DCR of 84.2% (32/38) in KRASG12C-mutated pancreatic cancer (NCT03600883) [38]. However, ORR was only 9.7% (6/62) in the phase 2 colorectal cancer cohort of sotorasib monotherapy and in combination with EGFR inhibitor panitumumab significantly improved efficacy with ORR of 30% (12/40) and DCR of 90% (36/40) in an ongoing phase 1b study (NCT04185883) [39, 40].
Adagrasib
Adagrasib (MRTX849) developed by Mirati Therapeutics is a competitive KRASG12C inhibitor that almost goes hand in hand with sotorasib [41]. The co-crystal structure showed that adagrasib was bound to the S-II pocket and formed a covalent bond with the Cys12 of the KRASG12C protein [42]. Notably, adagrasib employed a substituted 2-fluoroacrylamide warhead, which greatly reduced glutathione (GSH) metabolism and the half-life (t1/2) of whole blood stability by over 50 h in all species [42]. Adagrasib selectively targeted KRASG12C mutant and suppressed KRAS-dependent signal transduction and cell survival in vitro experiments. In a panel of KRASG12C mutant human tumor xenograft tumor models (CDX and PDX), adagrasib broadly inhibited tumor growth or induced regression, despite the heterogeneity [5]. Combination with other targeted therapies, regardless of inhibition of upstream (EGFR family or SHP2 inhibitors) or downstream (CDK4/6 or mTOR inhibitors) of KRAS, exhibited synergistic anti-tumor effects [5]. More recently, adagrasib has also been reported to reshape the tumor microenvironment and sensitize tumors to checkpoint inhibitor therapy, which is similar to sotorasib [43].
Supported by the preliminary results of the phase 1/2 multi-cohort KRYSTAL-01 trial with ORR of 45% (23/51) and DCR of 96% in NSCLC (NCT03785249), Mirati Therapeutics announced that FDA had granted Breakthrough Therapy Designation to adagrasib for the treatment of NSCLC with KRASG12C mutations in June 2021 [44]. As expected, based on the phase 2 registration-enabling cohort of KRASG12C-mutated NSCLC with ORR of 42.9% (48/112) and media DoR of 8.5 months, FDA granted accelerated approval of adagrasib on December 12, 2022 (NCT03785249) [45]. Phase 3 study of adagrasib versus docetaxel in previously treated patients with KRASG12C-mutated NSCLC is underway (NCT04685135). Meanwhile, those trials including neoadjuvant KRASG12C-directed therapy (NCT05118854 and NCT05472623) are recruiting.
More recently, it was reported that adagrasib monotherapy in other advanced solid tumors with KRASG12C mutations (excluding NSCLC and CRC) had an ORR of 35.1% (20/57) from a phase 2 cohort data analysis, including PDAC (33.3%; 7/21), biliary tract cancers (41.7%; 5/12), gynecologic cancers (57.1%; 4/7) (NCT03785249) [46]. For the CRC cohorts, adagrasib monotherapy showed a certain efficacy with ORR of 19% (8/43) and media DoR of 4.3 months, while combined with cetuximab (anti-EGFR monoclonal antibody) significantly improved ORR of 46% (13/28) and media DoR of 7.6 months (NCT03785249) [47]. Given these, in combination with EGFR antibodies perhaps a promising treatment strategy, the FDA has granted Breakthrough Therapy Designation for adagrasib plus cetuximab for the treatment of advanced KRASG12C-mutated CRC in December 2022.
JDQ443
JDQ443, developed by Novartis, is a structurally unique KRASG12C covalent inhibitor that forms novel interactions with the S-II pocket avoiding direct interaction with H95 [6, 48]. JDQ443 selectively inhibited KRASG12C-mutated tumor cells in vitro and displayed antitumor effects in multiple KRASG12C-dependent CDX and PDX models, synergistically with SHP2 inhibitors [6]. Currently, JDQ443 is undergoing a phase 1/2 study (KontRASt-01) as a single agent or in combination with the SHP2 inhibitor TNO155 in advanced solid tumors harboring KRASG12C mutation (NCT04699188) and has demonstrated early signs of clinical activity in NSCLC patients [49].
D-1553
D-1553 (garsorasib) developed by InventisBio was well tolerated with no dose-limiting toxicities in an ongoing international multicenter phase 1 study (NCT04585035) [50–52]. Among 21 evaluable KRASG12C-mutated patients, confirmed ORR was 19.0% (4/21) and DCR was 85.7% (18/21). In another study of D-1553 in NSCLC patients with KRASG12C mutation, early results demonstrated significant anti-tumor activity with ORR of 38.7% (24/62) and DCR of 90.3% (56/62) at the recommended phase 2 dose (NCT05383898) [53].
Other KRASG12C covalent inhibitors
LY3499446 is a KRASG12C inhibitor of Eli Lilly, but the trial was terminated in 2020 due to unexpected toxicity in the phase 1 study (NCT04165031). LY3537982 is another new generation of KRASG12C inhibitor from Eli Lilly with potent preclinical activity and high target occupancy, and the phase 1 trial has been initiated (NCT04956640) [54]. GDC-6036, developed by Genentech, is currently in phase 1 trial and promising responses have been observed in NSCLC and CRC patients with ORR of 53.4% (31/58) and 29.1% (16/55), respectively (NCT04449874) [55, 56]. More slightly redundant inhibitors are currently in clinical studies, such as BI 1823911 (NCT04973163), JAB-21822 (NCT05009329), GFH925 (NCT05005234), GH35 (NCT05010694), and BPI-421286 (NCT05315180) or preclinical studies, such as APG-1842 and AZD4625 [57–62].
KRASG12C PROTACs
Proteolysis targeting chimeras (PROTACs) is an emerging drug development technology, that is, a small molecule assembled by an E3 ubiquitin ligase ligand and a target-protein ligand through a “linker” to achieve the degradation of the target protein through the ubiquitin-proteasome system, especially popular when targeting some “intractable” targets [63, 64]. Over the past few years, the idea of PROTAC-mediated degradation of KRAS protein has been reported by many scientists. The first patent mentioned the KRAS PROTAC compound, but no details [65]. Zeng M et al. employed cell-based methods and TR-FRET-based biochemical assay to identify a compound, XY-4-88, based on ARS-1620 and CRBN ligand, that could degrade exogenously transferred GFP-KRASG12C in reporter cells but had no degradation effect on endogenous KRASG12C in tumor cells [66]. LC-2 is the first PROTAC reported to be capable of degrading endogenous KRASG12, based on the modification of MRTX849 and a VHL E3 ligase ligand [67]. Comparing XY-4-88 and LC-2, besides the difference in the partial structure of the targeted KRASG12C, the key lies in the selection of the E3 ubiquitin ligase ligand, which suggests that the recruitment of VHL in these cells may degrade KRAS more effectively, which was also proved by other studies of VHL-fused monobody that can degrade endogenous KRAS [68, 69]. These studies together imply that targeted degradation of KRAS is feasible, but still needs to be cautious, especially when it is advanced to the clinic, a suitable E3 ligase is the first consideration [70, 71].
Inevitable resistance of KRASG12C inhibitors
Although KRASG12C inhibitors are just emerging in the clinic, inescapable resistance has begun to surface. Piro Lito et al. were the first to report the non-uniform rapid adaptation to KRASG12C inhibitors after short-term treatment [72]. Performing single-cell RNA sequencing (scRNA-seq) revealed divergent single-cell fates and identified EGFR signaling and AURKA involved in the adaptive response of KRAS inhibitors [72]. Further study also found that the newly synthesized KRASG12C protein caused KRAS reactivation because KRASG12C inhibitors are conformation-specific inhibitors, and only bind in the GDP-bound state [72]. Furthermore, another study observed that multiple receptor tyrosine kinases (RTKs) activation mediated the rapid adaptive resistance of KRASG12C inhibitors, in combination with SHP2 inhibitors through vertical pathway inhibition sustained KRAS pathway inhibition and improved in vitro and in vivo efficacy [73, 74]. Vito Amodio and colleagues investigated the mechanisms underlying the differential response of KRASG12C mutant NSCLC and CRC to KRASG12C inhibitors [75, 76]. In CRC cells, high basal RTK activation was associated with feedback reactivation of the MAPK pathway after KRAS inhibition, and EGFR signaling was the dominant resistance mechanism. KRASG12C inhibitor combined with anti-EGFR antibody cetuximab could increase the sensitivity of AMG510 on CRC cells, patient-derived organoids, and PDX models [76]. Epithelial-to-mesenchymal transition (EMT) has also been reported as related to KRASG12C inhibitor resistance, especially in elucidating the heterogeneity of NSCLC cell lines in response to KRASG12C inhibition [77, 78].
Although the above-mentioned resistance mechanisms are primarily laboratory results, they are almost consistent with clinical practice. Recently, the mechanism of conferring acquired resistance to adagrasib in clinical trials has been elaborated, mainly in terms of acquired KRAS alterations, bypass activation (RTK/RAS/MAPK pathway alterations), and histopathological transformation [9, 10, 79]. Especially prominently, the acquired secondary mutations of KRAS include G12D/R/V/W, G13D, Q61H, R68S, H95D/Q/R, and Y96C, which are completely unexpected and yet somehow inevitable [9]. Similarly, second-site mutations of KRASG12C occurred in cis (mutation on the same allele as G12C) or trans (mutation on a different allele from G12C) [9]. Of note, G12, G13, and Q61 mutations are related to KRAS activation, while the H95 and Y96 mutations directly alter the drug-binding site. Constructing these co-mutations on cells observed that the Y96 mutation consistently caused adagrasib or sotorasib resistance, while the H95 mutation had almost no effect on sotorasib [9, 10]. This can be explained by the binding mode of sotorasib different from adagrasib, that is, sotorasib exploits a cryptic His95-groove in KRASG12C, which also provides a reference for the subsequent development of KRASG12C covalent inhibitors [32]. A newly published article also highlighted those secondary mutations of KRAS conferred resistance to KRASG12C inhibitors sotorasib and adagrasib in in vitro experiments [80].
Emerging novel KRAS inhibitors beyond KRASG12C
Targeting the specific KRASG12C mutation has made remarkable progress, especially the approval of the KRASG12C covalent inhibitor sotorasib. Nonetheless, we should also be soberly aware that targeting the KRAS field as a whole still faces severe challenges. In other words, the successful experience of targeting KRASG12C cannot be replicated to other mutations without a hitch, and the resistance mutation of the KRASG12C covalent inhibitor itself may be the next concern [10, 64]. The outstanding troubles at the moment include: (i) KRASG12C covalent inhibitor is not a panacea, and it is not always effective in all KRASG12C mutant tumors; (ii) a considerable number of NSCLC patients indeed harbor the KRASG12C mutation, but G12D and G12V mutations are the most prevalent in terms of the entire KRAS mutations; (iii) other mutations lack the advantages of being targeted like G12C mutation, such as active cysteine residues, reserved relative GTP hydrolysis activity, and targetable S-II pocket; (iv) mutations in the drug-binding site exposed in current clinical trials may be endemic to other KRASG12C covalent inhibitors, although few evaluable samples. Consensus and deep comprehension of these existing problems, novel KRAS inhibitors beyond KRASG12C that are still in their infancy have gradually gained our attention, as we will elaborate on the following based on the limited literature available.
Targeting KRASG12D mutation
Mirati Therapeutics innovatively discovered a reversible KRASG12D-selective inhibitor through a salt-bridge-based strategy, MRTX-1133, with more than 1000-fold selectivity over WT KRAS and a half-life of up to 50 h [81] (Fig. 3b). They first modified the substituted acrylamide electrophile of MRTX-849 to piperazine to interact with the aspartate side chain of the KRASG12D mutant, and then optimized the structure based on surface plasmon resonance (SPR) binding and TR-FRET displacement assay, and finally MRTX-1133 with nanomolar affinity for KRASG12D mutation was identified [81]. The crystal structure revealed that MRTX1133 occupied the S-II pocket similar to the binding mode of adagrasib to KRASG12C. MRTX-1133 demonstrated broad, potent inhibitory activity in a panel of KRASG12D mutant cell lines in vitro. In addition, MRX-1133 dose-dependently inhibited KRAS signaling and demonstrated potent anti-tumor activity in various KRASG12D mutant pancreatic and CRC xenograft models in vivo. Further pharmacological studies and CRISPR-based screening found that the combination of PI3K or EGFR inhibitors to address the putative feedback and resistance pathways significantly improved antitumor efficacy [82]. In multiple immunocompetent mouse models of PDAC, the treatment of MRTX-1133 led to sustained tumor regression by remodeling the tumor microenvironment [83]. More recently, the phase 1/2 study of MRTX-1133 has been initiated, which will be a vital proof-of-concept (NCT05737706).
Notably, MRTX-1133 demonstrated proof-of-principle for the development of selective and non-covalent inhibitors to target KRASG12D mutation with potent preclinical activity, and another study has also confirmed the feasibility of the salt-bridge strategy [84]. HRS-4642 is another KRASG12D inhibitor entering the clinic with fewer details (NCT05533463). ASP3082 is a KRASG12D PROTAC developed by Astellas Pharma and the phase 1 study is ongoing (NCT05382559) [85, 86].
Targeting other KRAS mutation
With great success in targeting the KRASG12C mutation, recently, Kevan Shokat’s lab is also exploring covalent strategies to target other KRAS hotspot mutations. For the KRASG12S mutation, they employed a potent electrophile, β-lactone-containing molecules, to covalently modify the less reactive serine residue [87]. By contrast, the strategy for covalent targeting reactive cysteine residue with acrylamide warheads has been particularly well established in the case of the G12C mutation. Several lead compounds were synthesized through the structural modification of adagrasib and bound to the S-II pocket of KRAS similarly. Among them, the most active one is G12Si-5, which reduced the GTP-bound RAS level and inhibited RAS downstream signaling and the growth of KRASG12S mutant cells (Fig. 3c). In addition, they covalently targeted the G12R mutation using the α,β-diketoamides system, and detected the covalent modification of the KRAS protein in in vitro assays and at the cellular level [88] (Fig. 3d). Apart from the covalent strategy, Kevan Shokat et al. also investigated whether small-molecule inhibitors could reversibly bind to the S-IIP of various KRAS hotspot mutants. By virtue of the KRASG12D inhibitor MRTX-EX185, a compound from Mirati Therapeutics’ patent, they found that many KRAS mutants other than G12C are also vulnerable to S-IIP engagement with the preference of the GDP-bound inactive state [89].
In addition to the S-II pocket, Genentech researchers and Fesik’s group at Vanderbilt simultaneously identified another small-molecule binding pocket on KRAS through fragment-based screening, located between switch I and II regions (termed SI/II-pocket) [90, 91]. Later, Boehringer Ingelheim researchers in collaboration with Fesik et al. reported a chemical probe bound to this pocket with nanomolar affinity, BI-2852 [92] (Fig. 3e). BI-2852 inhibited both active and inactive states of KRAS, unlike the G12C inhibitors described above that target the S-II pocket, which are only effective against GDP-bound KRAS. Therefore, the SI/II-pocket compounds are promising pan-KRAS inhibitors but require improved potency and selectivity for other RAS isoforms [93].
Fesik et al. also proposed covalently blocking the SI/II-pocket to identify second-site ligands through fragment screening, which directly led to the discovery of Boehringer Ingelheim’s G12C covalent inhibitor BI-0474 (an analog of the clinical compound BI 1823911) [94, 95]. Recently, Piro Lito et al. and Boehringer Ingelheim’s researchers reported a pan-KRAS inhibitor, BI-2865, based on structure optimizations of BI-0474, bound to various KRAS mutants with high affinity in the GDP-bound state (Fig. 3e) [96]. High-resolution cocrystal structure revealed that BI-2865 bound at the distal portion of the switch II motif without extension to the P-loop near the G12 residue, partially overlapping with the binding mode of current covalent inhibitors. BI-2865 significantly prevented the guanylate exchange activation process of GDP-bound KRAS variants for in vitro biochemical assays albeit with no selectivity for WT KRAS; but selectively inhibited oncogenic signaling in a panel of KRAS mutant cancer cells, possibly because of the dependence of these cells on the KRAS oncogene. Its structural analog BI-2493 attenuated the growth of KRAS-mutant xenograft tumors for in vivo models [96]. Collectively, independent studies by these two groups (Kevan Shokat et al. and Piro Lito et al.) suggest that inhibition of KRAS in the inactive state (or S-IIP as the binding site) is not a privilege of the G12C mutation and that this approach can also be applied to target a broad range of KRAS mutations [89, 96].
Tri-complex KRAS inhibitors
Chemically induced proximity (CIP) through the specific affinity of small molecules to induce neo-protein-protein interactions has become an important technical means for drug discovery, also well-known as “molecular glue” [97, 98]. Revolution Medicines is a pioneer and the first to adopt this theory to tackle the “untargetable” KRAS mutation. Inspired by mTOR inhibitors rapamycin, they established a technology platform that could screen tri-complex KRAS inhibitors, which was first developed by Warp Drive Bio termed Small Molecule-Assisted Receptor Targeting (SMARTTM) and acquired by Revolution Medicines in 2018 [99, 100]. Since then, they have employed this technology to develop a series of so-called RAS(ON) inhibitors, that bound to activated KRAS and chaperone protein Cyclophilin A (CYPA) simultaneously to form a ternary complex to sterically hinder the binding of downstream effector proteins, which were distinct in mechanism from KRASG12C covalent inhibitors that bind KRAS in the GDP-bound state [101].
RMC-6291, a tri-complex KRASG12C(ON) inhibitor, has been shown to rapidly covalently bind to KRASG12C to disrupt the interaction with RAF and interrupt the RAS signaling [102] (Fig. 3f). RMC-6236, another KRASmulti(ON) inhibitor, demonstrated profound anti-tumor activity in KRASG12V mutant preclinical models, despite the potential to inhibit multiple KRAS mutations [103, 104]. Other tri-complex RAS(ON) inhibitors targeting KRAS-G12D and G13C mutations have also been reported [105, 106]. Based on these preclinical profiles, whether this novel inhibitor could achieve the selectivity for KRAS mutations, especially sparing WT KRAS, remains to be deliberated, although it seemed tempting. Interestingly, RM-018, a representative RAS(ON) inhibitor from Revolution Medicines, was able to overcome covalent inhibitor resistance caused by drug-binding site mutations reported in the recent literature [10]. Given that RM-018 specifically binds to and inhibits GTP-bound activated KRASG12C, it is not surprising that it could overcome the resistance of previous covalent inhibitors theoretically.
Have to be convinced, these RAS(ON) inhibitors provide a new idea for the development of KRAS inhibitors. If selectivity and safety can be ensured, it will undoubtedly bring a new revolution to KRAS-targeted drug discovery, not only targeting KRASG12C. Zhang Z and Shokat KM exploited a similar approach to directly link ARS1620 derivatives and immunophilin ligands to synthesize bifunctional small molecules confirming that both FK506 (an FKBP12 ligand)- and cyclosporin (a CYPA ligand)-derived compounds could covalently react with the cysteine of KRASG12C, while had limited cellular activity [107]. Currently, RMC-6236 and RMC-6291 have begun to be evaluated in clinical trials, and preliminary evidence is on the way (NCT05379985 and NCT05462717).
Targeting KRAS indirectly
KRAS mutations have long been considered refractory, and attempts have been made to inhibit KRAS activation by indirectly inhibiting its upstream and downstream signaling, such as EGFR inhibitors, MAPK pathway inhibitors, and PI3K pathway inhibitors [108]. Some of these inhibitors as single agents or in combination are even in clinical development, however, the results are less than satisfactory, and none has been approved for clinical use so far. In recent years, SHP2 inhibitors and SOS1 inhibitors have sprung up as promising KRAS-targeted therapies, whose inhibition leads to the impairment of the KRAS nucleotide exchange process (Fig. 3g, h).
SHP2 inhibitors
SHP2, encoded by the PTPN11 gene, is a non-receptor protein tyrosine phosphatase (PTP) and scaffolding protein that mediates the activation of RAS signals downstream of multiple RTKs [109–111]. SHP2 protein contains two N-terminal SH2 domains (termed N-SH2 and C-SH2), a classical catalytic domain (PTP), and a C-terminal tail (C-tail) [112]. The activity of SHP2 is regulated by the interaction between its N-SH2 domain and PTP domain. In the inactive state, the hydrophobic interaction between the amino acid residues in the N-SH2 domain and the PTP domain stabilizes SHP2 at its “auto-inhibited” conformation; when RTKs signaling is activated, various phosphorylated peptides bind to the N-SH2 domain to relieve this autoinhibition, exposing the PTP domain and then activating the SHP2 signal [113, 114].
TNO155
Novartis is not the first to evolve an idea on SHP2, but it is the most innovative and breakthrough. In 2016, Novartis scientists masterly exploited the natural regulation mechanism of SHP2 to design a bisphosphorylated peptide to mimic the activation process of SHP2 under physiological conditions and established an SHP2 allosteric inhibitor screening platform [115]. Through the screening of a compound library, the lead compound SHP836 was identified and then optimized as SHP099, a representative SHP2 allosteric inhibitor tool drug [116]. SHP099 was validated with SHP2-dependent anti-tumor activity in a variety of RTKs-driven cancer cells and significantly suppressed the growth of KYSE520 (amplification of EGFR) xenograft tumors in vivo [115]. This pioneering work confirmed SHP2 inhibition as a viable strategy for targeting RTK-driven cancers and set off a wave of SHP2 allosteric inhibitor development.
Although Novartis’s work did not intentionally link SHP2 to KRAS, several subsequent studies revealed the dependence of KRAS mutant tumors on SHP2, and SHP099 was widely utilized as a chemical tool in these studies [117–119]. However, due to the phototoxicity, phospholipidosis, and hERG channel inhibition of SHP099 in the preclinical safety evaluation, Novartis optimized a new generation of SHP2 allosteric inhibitor, TNO155 [120]. TNO155 in combination with KRASG12C inhibitors exhibited a synergistic effect in KRASG12C mutant cells and shrank tumors in the KRASG12C NSCLC PDX preclinical model [121]. According to initial clinical results, TNO155 showed favorable pharmacokinetic properties and safety, but limited efficacy as a single agent in various advanced solid tumors (NCT03114319) [122]. Currently, TNO155 is undergoing clinical trials in combination with other KRASG12C inhibitors adagrasib (NCT04330664) or JDQ443 (NCT04699188) in advanced solid tumors harboring KRASG12C mutation [123, 124].
RMC-4630
Researchers at Revolution Medicines identified a potent and selective SHP2 allosteric inhibitor RMC-4550 [125, 126]. They found that beyond RTK alterations, SHP2 inhibition is also effective against RAS/MAPK pathway oncogenic mutations, including class 3 BRAF mutations, RAS GAP neurofibromin 1 (NF1) loss, and certain KRAS mutations [126]. RMC-4550 inhibited diverse aforementioned mutant cell proliferation and RAS/MAPK signaling and suppressed tumor growth in CDX and PDX models in vivo [126]. Subsequently, an SHP2 inhibitor code-named RMC-4630 entered a phase 1 clinical trial (NCT03634982) [127]. According to the latest clinical results, RMC-4630 is safe and tolerable as a single agent for the treatment of RAS-addicted solid tumors with DCR of 61% (23/38) in KRASMUT NSCLC patients and 80% (12/15) in KRASG12C NSCLC, but more case is still needed to confirm the results [128]. Based on RMC-4630, and other structurally related SHP2 inhibitors demonstrated combinatorial benefit with multiple inhibitors in preclinical models, the combination of RMC-4630 with MEK inhibitor cobimetinib, EGFR inhibitor osimertinib, and KRASG12C inhibitor sotorasib is being evaluated in clinical trials (NCT03989115 and NCT05054725) [129]. Furthermore, sotorasib in combination with RMC-4630 has shown preliminary positive results and further study is on the way [130].
JAB-3068 and JAB-3312
Jacobio Pharmaceuticals Co. Ltd is another company that targets SHP2 to develop inhibitors and its JAB-3068 is the second SHP2 drug candidate to enter clinical development with an Investigational New Drug (IND) application reviewed by FDA. Currently, JAB-3068 is undergoing a phase 1/2a trial in China (NCT04721223) and a phase 1 trial in the United States (NCT03518554). According to the latest information, phase 2a enrollment has been closed to evaluate the efficacy of JAB-3068 monotherapy on three types of solid tumors (ESCC, NSCLC, and HNSCC).
JAB-3312 is another SHP2 inhibitor of Jacobio in clinical research. Jacobio claimed that JAB-3068 and JAB-3312 exhibited different chemical characteristics and effects in preclinical and clinical studies, and their clinical development plans were designed to focus on different indications and combination therapies. Phase 1 trials to evaluate the safety and tolerability of JAB-3312 in patients with advanced solid tumors are underway (NCT04045496 and NCT04121286). At present, a phase 1/2 study of JAB-3312 in combination with KRASG12C inhibitor sotorasib or MEK inhibitor binimetinib is ongoing (NCT04720976), and in combination with another KRASG12C inhibitor, JAB-21822, is also underway (NCT05288205) [131].
BBP-398 (IACS-15509)
IACS-13909 is a preclinical SHP2 allosteric inhibitor discovered by Navire Pharma [132]. In vivo and in vitro data demonstrated that IACS-13909 exerted anti-tumor activity by inhibiting MAPK pathway signaling in RTKs-driven tumors [133]. It was worth mentioning that IACS-13909 could also overcome EGFR-dependent (C797S) and EGFR-independent (RTK bypass activation) osimertinib resistance, suggesting that the combination of SHP2 allosteric inhibitors might be a potential treatment strategy to overcome EGFR inhibitors resistance [133]. However, IACS-13909 exhibited strong hERG activity, which was further optimized to yield a potent, orally bioavailable SHP2 inhibitor, IACS-15414 [134]. And BBP-398 (formerly known as IACS-15509), a clinical development candidate, entered a phase 1/1b clinical trial for the treatment of tumors driven by RAS and RTK mutations in September 2020 (NCT04528836) [135].
Other SHP2 inhibitors
GDC-1971 (also known as RLY-1971) is an SHP2 inhibitor developed by Relay Therapeutics and entered a phase 1 clinical trial in February 2020 (NCT04252339) [136]. In December 2020, Relay Therapeutics announced that it has reached a worldwide license and cooperation agreement with Genentech to develop and commercialize RLY-1971, and a phase 1 study in combination with Genentech’s KRASG12C inhibitor GDC-6036 is ongoing (NCT04449874). PF-07284892 (ARRY-558) is a novel SHP2 inhibitor designed to overcome bypass-pathway-mediated resistance to targeted therapies, developed by Pfizer [137]. Early phase 1 data showed that the combination of PF-07284892 resensitized drug-resistant patients to targeted therapy, including those patients with ALK/ROS fusion, BRAFV600E, and KRASG12D mutation (NCT04800822) [137, 138]. Apart from that, there are numerous potential SHP2 inhibitors in the early clinical stage or preclinical studies, such as ERAS-601 (NCT04670679), SH3809 (NCT04843033), HBI-2376 (NCT05163028), ET0038 (NCT05354843), ICP-189 (NCT05370755), and BPI-442096 (NCT05369312) [139–142].
SOS1::KRAS inhibitor
The RAS activation process is regulated by GEF, thus in theory KRAS inhibition could be achieved by disrupting the KRAS-GEF interaction, albeit the nucleotide exchange of certain KRAS mutations may not rely on GEF [2]. The GEF family proteins all have the CDC25 homologous catalytic domain, and SOS is the most studied RAS GEF among them [143]. Previous attempts to develop RAS-SOS interaction inhibitors to inhibit RAS activation have failed due to low cellular activity [144–147]. Surprisingly, during the study of targeting RAS, Fesik’s group reported some small molecule inhibitors bound to the Ras:SOS:Ras complex instead of inhibiting but activating SOS-catalyzed nucleotide exchange [148–151]. Here, we summarized several SOS1-targeting compounds that inhibit KRAS signaling, two of which have entered phase 1 clinical trials.
BAY-293
In 2019, Bayer scientists reported a cell-active compound based on a dual screening approach and structure-guided design, BAY-293, which selectively inhibited the KRAS-SOS1 interaction [147]. Fragment hits obtained by fragment screening stabilized KRAS-SOS1 interaction, and their binding site on SOS1 was the same as the previously reported activator pocket [147, 148]. Through high-throughput screening (HTS) identifying initial SOS1 inhibitors and linking HTS-derived inhibitor series and fragment hits resulted in optimized disruptors of KRAS-SOS1 interaction rather than activators [147]. BAY-293 exhibited submicromolar growth inhibition activity at the cellular level and had a synergistic anti-proliferative activity with KRASG12C covalent inhibitor ARS-853 [147]. However, BAY-293 lacked selectivity to KRAS mutant over wild-type cells, as well as bioavailability issues, thus difficult to verify its activity in in vivo experiments. Even so, BAY-293 was the first reported cell-active SOS1 inhibitor and served as an important chemical tool for the follow-up investigation of KRAS-SOS1 interaction.
BI-3406 (BI 1701963)
BI-3406 is a potent, orally bioavailable KRAS-SOS1 interaction inhibitor discovered by researchers at Boehringer Ingelheim [152]. Utilizing the orthogonal high-throughput screening of AlphaScreen and TR-FRET assay, they identified BI-68BS as the leading hit of SOS1 and KRASG12D protein-protein interaction inhibitor [152, 153]. However, BI-68BS was originally synthesized as an EGFR kinase inhibitor, and its structure was further modified and optimized to eliminate kinase activity, leading to BI-3406 [153]. At the molecular assay, BI-3406 not only inhibited the interaction between KRASG12C and SOS1 but also KRASG12D and SOS1, while not its paralog SOS2. The SOS1 inhibition of BI-3406 was verified in various KRAS-driven cells, accompanied by RAS-GTP and phospho-ERK reduction. In xenograft tumor models, BI-3406 single administration was sufficient to suppress tumor growth. When combined with MEK inhibitors trametinib, it blocked adaptive resistance, resulting in long-term MAPK pathway inhibition, and induced tumor regression in multiple KRAS mutant tumors [152]. In addition, BI-3406 combined with afatinib, an EGFR inhibitor, showed anti-tumor activity in a DLD-1 KRASG13D mutant colorectal xenograft tumor [153]. Recently, it has also been reported that in NSCLC and CRC models, combination with SOS1 inhibitor enhanced drug efficacy and delayed acquired resistance compared with KRASG12C inhibitor alone [154]. BI 1701963, an analog of BI-3406, is the first and most advanced SOS1::pan-KRAS inhibitor, and phase 1 clinical trials of a single agent or in combination with other inhibitors are underway, including MEK inhibitors and KRASG12C inhibitors (NCT04111458, NCT04973163, and NCT04975256) [155]. However, the combination of MEK inhibitor BI 3011441 in advanced cancer and irinotecan in CRC has been terminated for some reason (NCT04835714 and NCT04627142).
MRTX0902
MRTX0902, developed by Mirati Therapeutics, is a brain-penetrant and highly selective phthalazine-based SOS1 inhibitor [156]. In the preclinical KRASG12C tumor models, MRTX0902 exhibited enhanced suppression of the RAS-MAPK signaling and induced tumor regression when co-administration with adagrasib [157]. Recently, MRTX0902 was introduced into the clinic, and a phase 1/2 study evaluating MRTX0902 alone and in combination with adagrasib is ongoing (NCT05578092).
Other SOS1 inhibitors
Revolution Medicines presented a potent and selective SOS1 inhibitor, RMC-0331 (RM-023), as an in vivo tool compound, and they have disclosed several related patent applications [158, 159]. He et al. reported that based on the structural optimization of BI-3406, tetracyclic quinazoline compounds were identified as potent SOS1 inhibitors with limited CYP inhibition and cardiac toxicity [160, 161]. Moreover, there were also studies extending the design concept of PROTAC to SOS1 in the literature, including SOS1 agonist-based and inhibitor-based, that exhibited antitumor activity in KRAS-mutated CDX and patient-derived organoid models [162–165].
Conclusions and future perspective
For a considerable period, KRAS has been the most prominent target in the field of tumor-targeted therapy. The scientific community and pharmaceutical companies have developed an endless stream of development strategies targeting KRAS. Undoubtedly, so far, the KRASG12C covalent inhibitor is the most successful paradigm of RAS-targeted therapy. In particular, the approval of sotorasib completely broke the “undruggable” curse of KRAS and revolutionized the treatment landscape of KRASG12C-mutated NSCLC. With the announcement of the phase 3 study results, sotorasib significantly improved PFS, although no OS benefit. Nevertheless, it has provided a new oral agent for patients with advanced/metastatic NSCLC with a more favorable safety profile. Notably, although KRASG12C mutation is the most frequent in NSCLC, this oncogenic mutation is also related to other cancers. Several G12C inhibitors have shown preliminary promising clinical activity in KRASG12C-mutated CRC, as well as other solid tumors such as PDAC [40, 46, 47, 56]. At present, sotorasib or adagrasib in combination with an anti-EGFR agent is being further evaluated in phase 3 studies in CRC patients with KRASG12C mutation, respectively (NCT05198934 and NCT04793958) [166, 167]. Taken together, it is worth expecting that expanding KRASG12C inhibitors to other KRASG12C-positive malignancies, especially those with limited treatment options and poor prognosis, while exploring rational clinical combinations is necessary.
However, there is no one-size-fits-all approach to addressing all KRAS mutations. Given that KRASG12C mutation accounts for only a small proportion of the total KRAS mutations, mainly NSCLC, tumors with other KRAS mutations are still helpless [168]. More deadly, there have been gradually increasing reports of acquired drug resistance mediated by KRAS secondary mutations, although many G12C covalent inhibitors are still in clinical trials. Therefore, selective inhibitors targeting other KRAS mutations or pan-KRAS inhibitors are promising KRAS-targeted therapy strategies. MRTX-1133, a selective non-covalent KRASG12D inhibitor, has demonstrated promising anti-tumor activity in multiple KRASG12D-mutated preclinical models, and its clinical trials are currently recruiting (NCT05737706) [82, 83]. Revolution Medicines’ RAS(ON) inhibitors completely differ from the currently approved G12C RAS(OFF) inhibitors mechanistically, which may bring new hope to KRAS precision therapy. Furthermore, clinically unmet pan-KRAS inhibitors are on the horizon, which might once again change the targeted therapy landscape for all KRAS mutant patients rather than a specific subset [96, 169]. Nevertheless, these studies are only early attempts, and their feasibility urgently needs further confirmation, particularly in clinical studies.
Targeting SHP2 or SOS1 to indirectly inhibit KRAS activation is also considered a promising pan-KRAS therapy strategy. Among these strategies, SHP2 allosteric inhibitors are extremely attractive research and development hotspots of anti-tumor drugs, but their clinical activities need to be further investigated. Revolution Medicines’ clinical compound RMC-4630 has shown initial efficacy in KRAS mutant tumors, especially those with KRASG12C mutation [128]. However, RMC-4630 was ineffective in most patients with KRASG12D mutation, which is consistent with a recent report that SHP2 inhibition showed a modest combined effect with MRTX1133 [128]. This may be partly attributable to that SHP2 inhibition disrupts the SOS1-mediated KRAS nucleotide cycling, while KRASG12D-mutated tumors are less susceptible to SOS1 inhibition [82, 126]. Perhaps, this can be better explained by an in-depth study of the function of SHP2 in tumors and its regulatory mechanism in the RTK-RAS-MAPK pathway, with a focus on the distinct roles of scaffolding and phosphatase activity. On the other hand, since the initial results of another SHP2 allosteric inhibitor in advanced solid tumors were not outstanding, it is necessary to find suitable biomarkers in preclinical studies or clinical trials to guide the design of clinical trials [122]. And the combination with other targeted drugs might be an excellent choice, whether to enhance the efficacy or delay drug resistance [122]. In addition, SHP2 is a key downstream effector of PD-1 signaling in T cells and several studies have confirmed that SHP2 allosteric inhibitors not only directly inhibit KRAS-driven tumors but also exert anti-tumor immunity in the tumor microenvironment [121, 170, 171]. Based on these encouraging preclinical results, clinical trials evaluating the efficacy of the combination of SHP2 allosteric inhibitors and PD-1 antibodies are planned or underway (NCT04418661, NCT04000529, NCT04721223 and NCT04720976).
SOS1 is a key regulator of RAS activation, and the GTP exchange process of most mutant KRAS depends on SOS1. The SOS1::KRAS interaction inhibitor developed on this theoretical basis holds great potential for the treatment of KRAS mutant tumors, and BI-3406 is the best proof-of-concept at the moment [171]. The rational combination of MEK inhibitors and other targeted drugs may bring new options for KRAS-targeted therapy. From the in vitro biochemical assay, the G13D mutation is much stronger than wild-type and other KRAS mutations in terms of the intrinsic nucleotide exchange of KRAS. However, the dependence of different KRAS mutations on SOS1 within the cell is still unclear [2, 108]. BI-3406 seems to be effective on colorectal cancer cells and xenografts with KRASG13D mutation, suggesting that our in-depth understanding of these issues may better guide the development of SOS1 inhibitors across KRAS-driven tumors [152, 153, 172]. Two SOS1 inhibitors, BI 1701963 and MRTX0902, are being evaluated in phase 1 trials for the treatment of KRAS-mutated tumors either alone or in combination with other targeted drugs.
Previous efforts to target the MAPK pathway, the key downstream effector of KRAS, have been unsatisfactory in the treatment of KRAS mutant tumors due to the low treatment window and adaptive feedback reactivation [108]. With the emergence of small molecule inhibitors that directly target KRAS mutations or target key upstream regulators of KRAS to indirectly inhibit KRAS activation, the combination with MEK/ERK inhibitors has gradually risen in clinical trials. At present, it is believed that SHP2 or SOS1 inhibitors are the best partners for KRAS combination therapy, and the combination of sotorasib/RMC4630 and adagrasib/TNO155 has attracted much attention [123, 130]. Perhaps soon, KRAS-targeted drugs or combination therapies will become a reality in patients with KRAS mutations, not just KRASG12C mutations.
Acknowledgements
This research was supported by grants from the National Natural Science Foundation of China (82273948), High-level Innovative Research Institute (2021B0909050003), State Key Laboratory of Drug Research (SKLDR-2023-TT-01 and SIMM2205KF-09).
Competing interests
The authors declare no competing interests.
Contributor Information
Hua Xie, Email: hxie@simm.ac.cn.
Jian Ding, Email: jding@simm.ac.cn.
References
- 1.Simanshu DK, Nissley DV, McCormick F. RAS proteins and their regulators in human disease. Cell. 2017;170:17–33. doi: 10.1016/j.cell.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunter JC, Manandhar A, Carrasco MA, Gurbani D, Gondi S, Westover KD. Biochemical and structural analysis of common cancer-associated KRAS mutations. Mol Cancer Res. 2015;13:1325–35. doi: 10.1158/1541-7786.MCR-15-0203. [DOI] [PubMed] [Google Scholar]
- 3.Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548–51. doi: 10.1038/nature12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575:217–23. doi: 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- 5.Hallin J, Engstrom LD, Hargis L, Calinisan A, Aranda R, Briere DM, et al. The KRAS(G12C) inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov. 2020;10:54–71. doi: 10.1158/2159-8290.CD-19-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss A, Lorthiois E, Barys L, Beyer KS, Bomio-Confaglia C, Burks H, et al. Discovery, preclinical characterization, and early clinical activity of JDQ443, a structurally novel, potent, and selective covalent oral inhibitor of KRASG12C. Cancer Discov. 2022;12:1500–17. doi: 10.1158/2159-8290.CD-22-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FDA grants accelerated approval to sotorasib for KRAS G12C mutated NSCLC, https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-sotorasib-kras-g12c-mutated-nsclc.
- 8.FDA grants accelerated approval to adagrasib for KRAS G12C-mutated NSCLC, https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-adagrasib-kras-g12c-mutated-nsclc.
- 9.Awad MM, Liu S, Rybkin II, Arbour KC, Dilly J, Zhu VW, et al. Acquired resistance to KRAS(G12C) inhibition in cancer. N Engl J Med. 2021;384:2382–93. doi: 10.1056/NEJMoa2105281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka N, Lin JJ, Li C, Ryan MB, Zhang J, Kiedrowski LA, et al. Clinical acquired resistance to KRAS(G12C) inhibition through a novel KRAS switch-II pocket mutation and polyclonal alterations converging on RAS-MAPK reactivation. Cancer Discov. 2021;11:1913–22. doi: 10.1158/2159-8290.CD-21-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hobbs GA, Der CJ, Rossman KL. RAS isoforms and mutations in cancer at a glance. J Cell Sci. 2016;129:1287–92. doi: 10.1242/jcs.182873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai FD, Lopes MS, Zhou M, Court H, Ponce O, Fiordalisi JJ, et al. K-Ras4A splice variant is widely expressed in cancer and uses a hybrid membrane-targeting motif. Proc Natl Acad Sci USA. 2015;112:779–84. doi: 10.1073/pnas.1412811112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hancock JF, Cadwallader K, Marshall CJ. Methylation and proteolysis are essential for efficient membrane binding of prenylated p21K-ras(B) EMBO J. 1991;10:641–6. doi: 10.1002/j.1460-2075.1991.tb07992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salmon M, Paniagua G, Lechuga CG, Fernandez-Garcia F, Zarzuela E, Alvarez-Diaz R, et al. KRAS4A induces metastatic lung adenocarcinomas in vivo in the absence of the KRAS4B isoform. Proc Natl Acad Sci USA. 2021;118:e2023112118. doi: 10.1073/pnas.2023112118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amendola CR, Mahaffey JP, Parker SJ, Ahearn IM, Chen WC, Zhou M, et al. KRAS4A directly regulates hexokinase 1. Nature. 2019;576:482–6. doi: 10.1038/s41586-019-1832-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bryant KL, Mancias JD, Kimmelman AC, Der CJ. KRAS: feeding pancreatic cancer proliferation. Trends Biochem Sci. 2014;39:91–100. doi: 10.1016/j.tibs.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pantsar T. The current understanding of KRAS protein structure and dynamics. Comput Struct Biotechnol J. 2020;18:189–98. doi: 10.1016/j.csbj.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saraste M, Sibbald PR, Wittinghofer A. The P-loop—a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990;15:430–4. doi: 10.1016/0968-0004(90)90281-F. [DOI] [PubMed] [Google Scholar]
- 19.Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–304. doi: 10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- 20.Settleman J, Albright CF, Foster LC, Weinberg RA. Association between GTPase activators for Rho and Ras families. Nature. 1992;359:153–4. doi: 10.1038/359153a0. [DOI] [PubMed] [Google Scholar]
- 21.Mondal S, Hsiao K, Goueli SA. A homogenous bioluminescent system for measuring GTPase, GTPase activating protein, and guanine nucleotide exchange factor activities. Assay Drug Dev Technol. 2015;13:444–55. doi: 10.1089/adt.2015.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.John J, Sohmen R, Feuerstein J, Linke R, Wittinghofer A, Goody RS. Kinetics of interaction of nucleotides with nucleotide-free H-ras p21. Biochemistry. 1990;29:6058–65. doi: 10.1021/bi00477a025. [DOI] [PubMed] [Google Scholar]
- 23.Pacold ME, Suire S, Perisic O, Lara-Gonzalez S, Davis CT, Walker EH, et al. Crystal structure and functional analysis of Ras binding to its effector phosphoinositide 3-kinase gamma. Cell. 2000;103:931–43. doi: 10.1016/S0092-8674(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 24.Lavoie H, Therrien M. Regulation of RAF protein kinases in ERK signalling. Nat Rev Mol Cell Biol. 2015;16:281–98. doi: 10.1038/nrm3979. [DOI] [PubMed] [Google Scholar]
- 25.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lito P, Solomon M, Li LS, Hansen R, Rosen N. Allele-specific inhibitors inactivate mutant KRAS G12C by a trapping mechanism. Science. 2016;351:604–8. doi: 10.1126/science.aad6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter JC, Gray NS, Westover KD. GTP-competitive inhibitors of RAS family members. In: Azmi AS, editor. Conquering RAS. Boston: Academic Press; 2017. p 155–174.
- 28.Patricelli MP, Janes MR, Li LS, Hansen R, Peters U, Kessler LV, et al. Selective inhibition of oncogenic KRAS output with small molecules targeting the inactive state. Cancer Discov. 2016;6:316–29. doi: 10.1158/2159-8290.CD-15-1105. [DOI] [PubMed] [Google Scholar]
- 29.Janes MR, Zhang J, Li LS, Hansen R, Peters U, Guo X, et al. Targeting KRAS mutant cancers with a covalent G12C-specific inhibitor. Cell. 2018;172:578–89.e17. doi: 10.1016/j.cell.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Martin-Romano P, Cassier P, Johnson M, Haura E, Lenox L, et al. Phase I study of JNJ-74699157 in patients with advanced solid tumors harboring the KRAS G12C mutation. Oncologist. 2022;27:536–e53. doi: 10.1093/oncolo/oyab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fakih M, O’Neil B, Price TJ, Falchook GS, Desai J, Kuo J, et al. Phase 1 study evaluating the safety, tolerability, pharmacokinetics (PK), and efficacy of AMG 510, a novel small molecule KRASG12C inhibitor, in advanced solid tumors. J Clin Oncol. 2019;37:3003. doi: 10.1200/JCO.2019.37.15_suppl.3003. [DOI] [Google Scholar]
- 32.Lanman BA, Allen JR, Allen JG, Amegadzie AK, Ashton KS, Booker SK, et al. Discovery of a covalent inhibitor of KRAS(G12C) (AMG 510) for the treatment of solid tumors. J Med Chem. 2020;63:52–65. doi: 10.1021/acs.jmedchem.9b01180. [DOI] [PubMed] [Google Scholar]
- 33.Govindan R, Fakih MG, Price TJ, Falchook GS, Desai J, Kuo JC, et al. Phase I study of AMG 510, a novel molecule targeting KRAS G12C mutant solid tumours. Ann Oncol. 2019;30:163. doi: 10.1093/annonc/mdz244.008. [DOI] [Google Scholar]
- 34.Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, et al. KRAS(G12C) inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383:1207–17. doi: 10.1056/NEJMoa1917239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skoulidis F, Li BT, Dy GK, Price TJ, Falchook GS, Wolf J, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384:2371–81. doi: 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dy GK, Govindan R, Velcheti V, Falchook GS, Italiano A, Wolf J, et al. Abstract CT008: long-term outcomes with sotorasib in pretreated KRASp.G12C-mutated NSCLC: 2-year analysis of CodeBreaK100. Cancer Res. 2022;82:CT008. doi: 10.1158/1538-7445.AM2022-CT008. [DOI] [Google Scholar]
- 37.de Langen AJ, Johnson ML, Mazieres J, Dingemans AC, Mountzios G, Pless M, et al. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRAS(G12C) mutation: a randomised, open-label, phase 3 trial. Lancet. 2023;401:733–46. doi: 10.1016/S0140-6736(23)00221-0. [DOI] [PubMed] [Google Scholar]
- 38.Strickler JH, Satake H, George TJ, Yaeger R, Hollebecque A, Garrido-Laguna I, et al. Sotorasib in KRAS p.G12C-mutated advanced pancreatic cancer. N Engl J Med. 2023;388:33–43. doi: 10.1056/NEJMoa2208470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fakih MG, Kopetz S, Kuboki Y, Kim TW, Munster PN, Krauss JC, et al. Sotorasib for previously treated colorectal cancers with KRAS(G12C) mutation (CodeBreaK100): a prespecified analysis of a single-arm, phase 2 trial. Lancet Oncol. 2022;23:115–24. doi: 10.1016/S1470-2045(21)00605-7. [DOI] [PubMed] [Google Scholar]
- 40.Kuboki Y, Yaeger R, Fakih M, Strickler JH, Masuishi T, Kim EJH, et al. 45MO Sotorasib in combination with panitumumab in refractory KRAS G12C-mutated colorectal cancer: Safety and efficacy for phase Ib full expansion cohort. Ann Oncol. 2022;33:S1445–S6. doi: 10.1016/j.annonc.2022.10.077. [DOI] [Google Scholar]
- 41.Papadopoulos KP, Ou SHI, Johnson ML, Christensen J, Velastegui K, Potvin D, et al. A phase I/II multiple expansion cohort trial of MRTX849 in patients with advanced solid tumors with KRAS G12C mutation. J Clin Oncol. 2019;37:TPS3161. doi: 10.1200/JCO.2019.37.15_suppl.TPS3161. [DOI] [Google Scholar]
- 42.Fell JB, Fischer JP, Baer BR, Blake JF, Bouhana K, Briere DM, et al. Identification of the clinical development candidate MRTX849, a covalent KRAS(G12C) inhibitor for the treatment of cancer. J Med Chem. 2020;63:6679–93. doi: 10.1021/acs.jmedchem.9b02052. [DOI] [PubMed] [Google Scholar]
- 43.Briere DM, Li S, Calinisan A, Sudhakar N, Aranda R, Hargis L, et al. The KRAS(G12C) inhibitor MRTX849 reconditions the tumor immune microenvironment and sensitizes tumors to checkpoint inhibitor therapy. Mol Cancer Ther. 2021;20:975–85. doi: 10.1158/1535-7163.MCT-20-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riely GJ, Ou SHI, Rybkin I, Spira A, Papadopoulos K, Sabari JK, et al. 99O_PR KRYSTAL-1: activity and preliminary pharmacodynamic (PD) analysis of adagrasib (MRTX849) in patients (Pts) with advanced non–small cell lung cancer (NSCLC) harboring KRASG12C mutation. J Thorac Oncol. 2021;16:S751–S2. doi: 10.1016/S1556-0864(21)01941-9. [DOI] [Google Scholar]
- 45.Janne PA, Riely GJ, Gadgeel SM, Heist RS, Ou SI, Pacheco JM, et al. Adagrasib in non-small-cell lung cancer harboring a KRAS(G12C) mutation. N Engl J Med. 2022;387:120–31. doi: 10.1056/NEJMoa2204619. [DOI] [PubMed] [Google Scholar]
- 46.Bekaii-Saab TS, Yaeger R, Spira AI, Pelster MS, Sabari JK, Hafez N, et al. Adagrasib in advanced solid tumors harboring a KRAS(G12C) mutation. J Clin Oncol. 2023;41:4097–106. doi: 10.1200/JCO.23.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yaeger R, Weiss J, Pelster MS, Spira AI, Barve M, Ou SI, et al. Adagrasib with or without cetuximab in colorectal cancer with mutated KRAS G12C. N Engl J Med. 2023;388:44–54. doi: 10.1056/NEJMoa2212419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lorthiois E, Gerspacher M, Beyer KS, Vaupel A, Leblanc C, Stringer R, et al. JDQ443, a structurally novel, pyrazole-based, covalent inhibitor of KRAS(G12C) for the treatment of solid tumors. J Med Chem. 2022;65:16173–203. doi: 10.1021/acs.jmedchem.2c01438. [DOI] [PubMed] [Google Scholar]
- 49.Tan DS, Shimizu T, Solomon B, Heist RS, Schuler M, Luken MJDM, et al. Abstract CT033: KontRASt-01: a phase Ib/II, dose-escalation study of JDQ443 in patients (pts) with advanced, KRAS G12C-mutated solid tumors. Cancer Res. 2022;82:CT033. doi: 10.1158/1538-7445.AM2022-CT033. [DOI] [Google Scholar]
- 50.Shi Z, Weng J, Fan X, Wang E, Zhu Q, Tao L, et al. Abstract 932: discovery of D-1553, a novel and selective KRas-G12C inhibitor with potent anti-tumor activity in a broad spectrum of tumor cell lines and xenograft models. Cancer Res. 2021;81:932. doi: 10.1158/1538-7445.AM2021-932. [DOI] [Google Scholar]
- 51.Price T, Grewal J, Abed A, Moore M, Yeh Y-M, Gadgeel S, et al. Abstract CT504: a phase 1 clinical trial to evaluate safety, tolerability, pharmacokinetics (PK) and efficacy of D-1553, a novel KRASG12C inhibitor, in patients with advanced or metastatic solid tumor harboring KRASG12C mutation. Cancer Res. 2022;82:CT504. doi: 10.1158/1538-7445.AM2022-CT504. [DOI] [Google Scholar]
- 52.Shi Z, Weng J, Niu H, Yang H, Liu R, Weng Y, et al. D-1553: a novel KRAS(G12C) inhibitor with potent and selective cellular and in vivo antitumor activity. Cancer Sci. 2023;114:2951–60. doi: 10.1111/cas.15829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Z, Song Z, Zhao Y, Wang P, Jiang L, Gong Y, et al. D-1553 (Garsorasib), a potent and selective inhibitor of KRAS(G12C) in patients with NSCLC: phase 1 study results. J Thorac Oncol. 2023;18:940–51. doi: 10.1016/j.jtho.2023.03.015. [DOI] [PubMed] [Google Scholar]
- 54.Peng S-B, Si C, Zhang Y, Van Horn RD, Lin X, Gong X, et al. Abstract 1259: preclinical characterization of LY3537982, a novel, highly selective and potent KRAS-G12C inhibitor. Cancer Res. 2021;81:1259. doi: 10.1158/1538-7445.AM2021-1259. [DOI] [Google Scholar]
- 55.Purkey H. Abstract ND11: discovery of GDC-6036, a clinical stage treatment for KRAS G12C-positive cancers. Cancer Res. 2022;82:ND11. doi: 10.1158/1538-7445.AM2022-ND11. [DOI] [Google Scholar]
- 56.Sacher A, LoRusso P, Patel MR, Miller WH, Jr., Garralda E, Forster MD, et al. Single-agent divarasib (GDC-6036) in solid tumors with a KRAS G12C mutation. N Engl J Med. 2023;389:710–21. doi: 10.1056/NEJMoa2303810. [DOI] [PubMed] [Google Scholar]
- 57.Waizenegger IC, Lu H, Thamer C, Savarese F, Gerlach D, Rudolph D, et al. Abstract 2667: trial in progress: phase 1 study of BI 1823911, an irreversible KRASG12C inhibitor targeting KRAS in its GDP-loaded state, as monotherapy and in combination with the pan-KRAS SOS1 inhibitor BI 1701963 in solid tumors expressing KRASG12C mutation. Cancer Res. 2022;82:2667. doi: 10.1158/1538-7445.AM2022-2667. [DOI] [Google Scholar]
- 58.Li J, Zhao J, Cao B, Fang J, Li X, Wang M, et al. A phase I/II study of first-in-human trial of JAB-21822 (KRAS G12C inhibitor) in advanced solid tumors. J Clin Oncol. 2022;40:3089. doi: 10.1200/JCO.2022.40.16_suppl.3089. [DOI] [Google Scholar]
- 59.Zhou Q, Yang N, Zhao J, Dong X, Wang H, Yuan Y, et al. Phase I dose-escalation study of IBI351 (GFH925) monotherapy in patients with advanced solid tumors. J Clin Oncol. 2022;40:3110. doi: 10.1200/JCO.2022.40.16_suppl.3110. [DOI] [Google Scholar]
- 60.Zhu X, Lu Y, Guo J, Yan D, Li B, Chen X, et al. Abstract 5443: BPI-421286: a highly potent small molecule inhibitor targeting KRASG12C mutation. Cancer Res. 2022;82:5443. doi: 10.1158/1538-7445.AM2022-5443. [DOI] [Google Scholar]
- 61.Gu S, Li Q, Li C, Wang Q, Fang DD, Wang S, et al. Abstract 2664: development of covalent KRASG12C inhibitor APG-1842 for the treatment of solid tumors. Cancer Res. 2022;82:2664. doi: 10.1158/1538-7445.AM2022-2664. [DOI] [Google Scholar]
- 62.Kettle JG, Bagal SK, Bickerton S, Bodnarchuk MS, Boyd S, Breed J, et al. Discovery of AZD4625, a covalent allosteric inhibitor of the mutant GTPase KRAS(G12C) J Med Chem. 2022;65:6940–52. doi: 10.1021/acs.jmedchem.2c00369. [DOI] [PubMed] [Google Scholar]
- 63.Bond MJ, Crews CM. Proteolysis targeting chimeras (PROTACs) come of age: entering the third decade of targeted protein degradation. RSC Chem Biol. 2021;2:725–42. doi: 10.1039/D1CB00011J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rudolph J, Settleman J, Malek S. Emerging trends in cancer drug discovery-from drugging the “undruggable” to overcoming resistance. Cancer Discov. 2021;11:815–21. doi: 10.1158/2159-8290.CD-21-0260. [DOI] [PubMed] [Google Scholar]
- 65.Kargbo RB. PROTAC-mediated degradation of KRAS protein for anticancer therapeutics. ACS Med Chem Lett. 2020;11:5–6. doi: 10.1021/acsmedchemlett.9b00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zeng M, Xiong Y, Safaee N, Nowak RP, Donovan KA, Yuan CJ, et al. Exploring targeted degradation strategy for oncogenic KRAS(G12C) Cell Chem Biol. 2020;27:19–31.e6. doi: 10.1016/j.chembiol.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 67.Bond MJ, Chu L, Nalawansha DA, Li K, Crews CM. Targeted degradation of oncogenic KRAS(G12C) by VHL-recruiting PROTACs. ACS Cent Sci. 2020;6:1367–75. doi: 10.1021/acscentsci.0c00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roth S, Macartney TJ, Konopacka A, Chan KH, Zhou H, Queisser MA, et al. Targeting endogenous K-RAS for degradation through the affinity-directed protein missile system. Cell Chem Biol. 2020;27:1151–63.e6. doi: 10.1016/j.chembiol.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Teng KW, Tsai ST, Hattori T, Fedele C, Koide A, Yang C, et al. Selective and noncovalent targeting of RAS mutants for inhibition and degradation. Nat Commun. 2021;12:2656. doi: 10.1038/s41467-021-22969-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schneider M, Radoux CJ, Hercules A, Ochoa D, Dunham I, Zalmas LP, et al. The PROTACtable genome. Nat Rev Drug Discov. 2021;20:789–97. doi: 10.1038/s41573-021-00245-x. [DOI] [PubMed] [Google Scholar]
- 71.Bekes M, Langley DR, Crews CM. PROTAC targeted protein degraders: the past is prologue. Nat Rev Drug Discov. 2022;21:181–200. doi: 10.1038/s41573-021-00371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xue JY, Zhao Y, Aronowitz J, Mai TT, Vides A, Qeriqi B, et al. Rapid non-uniform adaptation to conformation-specific KRAS(G12C) inhibition. Nature. 2020;577:421–5. doi: 10.1038/s41586-019-1884-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ryan MB, Fece de la Cruz F, Phat S, Myers DT, Wong E, Shahzade HA, et al. Vertical pathway inhibition overcomes adaptive feedback resistance to KRAS(G12C) inhibition. Clin Cancer Res. 2020;26:1633–43. doi: 10.1158/1078-0432.CCR-19-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yaeger R, Solit DB. Overcoming adaptive resistance to KRAS inhibitors through vertical pathway targeting. Clin Cancer Res. 2020;26:1538–40. doi: 10.1158/1078-0432.CCR-19-4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Amodio V, Yaeger R, Arcella P, Cancelliere C, Lamba S, Lorenzato A, et al. EGFR blockade reverts resistance to KRAS(G12C) inhibition in colorectal cancer. Cancer Discov. 2020;10:1129–39. doi: 10.1158/2159-8290.CD-20-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koleilat MK, Kwong LN. Same name, different game: EGFR drives intrinsic KRAS(G12C) inhibitor resistance in colorectal cancer. Cancer Discov. 2020;10:1094–6. doi: 10.1158/2159-8290.CD-20-0612. [DOI] [PubMed] [Google Scholar]
- 77.Adachi Y, Ito K, Hayashi Y, Kimura R, Tan TZ, Yamaguchi R, et al. Epithelial-to-mesenchymal transition is a cause of both intrinsic and acquired resistance to KRAS G12C inhibitor in KRAS G12C-mutant non-small cell lung cancer. Clin Cancer Res. 2020;26:5962–73. doi: 10.1158/1078-0432.CCR-20-2077. [DOI] [PubMed] [Google Scholar]
- 78.Solanki HS, Welsh EA, Fang B, Izumi V, Darville L, Stone B, et al. Cell type-specific adaptive signaling responses to KRAS(G12C) inhibition. Clin Cancer Res. 2021;27:2533–48. doi: 10.1158/1078-0432.CCR-20-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li BT, Velcheti V, Price TJ, Hong DS, Fakih M, Kim D-W, et al. Largest evaluation of acquired resistance to sotorasib in KRAS p.G12C-mutated non–small cell lung cancer (NSCLC) and colorectal cancer (CRC): Plasma biomarker analysis of CodeBreaK100. J Clin Oncol. 2022;40:102. doi: 10.1200/JCO.2022.40.16_suppl.102. [DOI] [Google Scholar]
- 80.Koga T, Suda K, Fujino T, Ohara S, Hamada A, Nishino M, et al. KRAS secondary mutations that confer acquired resistance to KRAS G12C inhibitors, sotorasib and adagrasib, and overcoming strategies: insights from in vitro experiments. J Thorac Oncol. 2021;16:1321–32. doi: 10.1016/j.jtho.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 81.Wang X, Allen S, Blake JF, Bowcut V, Briere DM, Calinisan A, et al. Identification of MRTX1133, a noncovalent, potent, and selective KRAS(G12D) inhibitor. J Med Chem. 2022;65:3123–33. doi: 10.1021/acs.jmedchem.1c01688. [DOI] [PubMed] [Google Scholar]
- 82.Hallin J, Bowcut V, Calinisan A, Briere DM, Hargis L, Engstrom LD, et al. Anti-tumor efficacy of a potent and selective non-covalent KRAS(G12D) inhibitor. Nat Med. 2022;28:2171–82. doi: 10.1038/s41591-022-02007-7. [DOI] [PubMed] [Google Scholar]
- 83.Kemp SB, Cheng N, Markosyan N, Sor R, Kim IK, Hallin J, et al. Efficacy of a small-molecule inhibitor of KrasG12D in immunocompetent models of pancreatic cancer. Cancer Discov. 2023;13:298–311. doi: 10.1158/2159-8290.CD-22-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mao Z, Xiao H, Shen P, Yang Y, Xue J, Yang Y, et al. KRAS(G12D) can be targeted by potent inhibitors via formation of salt bridge. Cell Discov. 2022;8:5. doi: 10.1038/s41421-021-00368-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nagashima T, Inamura K, Nishizono Y, Suzuki A, Tanaka H, Yoshinari T, et al. ASP3082, a first-in-class novel KRAS G12D degrader, exhibits remarkable anti-tumor activity in KRAS G12D mutated cancer models. Eur J Cancer. 2022;174:S30. doi: 10.1016/S0959-8049(22)00881-4. [DOI] [Google Scholar]
- 86.Tolcher AW, Park W, Wang JS, Spira AI, Janne PA, Lee H-J, et al. Trial in progress: a phase 1, first-in-human, open-label, multicenter, dose-escalation and dose-expansion study of ASP3082 in patients with previously treated advanced solid tumors and KRAS G12D mutations. J Clin Oncol. 2023;41:TPS764. doi: 10.1200/JCO.2023.41.4_suppl.TPS764. [DOI] [Google Scholar]
- 87.Zhang Z, Guiley KZ, Shokat KM. Chemical acylation of an acquired serine suppresses oncogenic signaling of K-Ras(G12S) Nat Chem Biol. 2022;18:1177–83. doi: 10.1038/s41589-022-01065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Z, Morstein J, Ecker AK, Guiley KZ, Shokat KM. Chemoselective covalent modification of K-Ras(G12R) with a small molecule electrophile. J Am Chem Soc. 2022;144:15916–21. doi: 10.1021/jacs.2c05377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vasta JD, Peacock DM, Zheng Q, Walker JA, Zhang Z, Zimprich CA, et al. KRAS is vulnerable to reversible switch-II pocket engagement in cells. Nat Chem Biol. 2022;18:596–604. doi: 10.1038/s41589-022-00985-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maurer T, Garrenton LS, Oh A, Pitts K, Anderson DJ, Skelton NJ, et al. Small-molecule ligands bind to a distinct pocket in Ras and inhibit SOS-mediated nucleotide exchange activity. Proc Natl Acad Sci USA. 2012;109:5299–304. doi: 10.1073/pnas.1116510109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun Q, Burke JP, Phan J, Burns MC, Olejniczak ET, Waterson AG, et al. Discovery of small molecules that bind to K-Ras and inhibit sos-mediated activation. Angew Chem Int Ed. 2012;51:6140–3. doi: 10.1002/anie.201201358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kessler D, Gmachl M, Mantoulidis A, Martin LJ, Zoephel A, Mayer M, et al. Drugging an undruggable pocket on KRAS. Proc Natl Acad Sci USA. 2019;116:15823–9. doi: 10.1073/pnas.1904529116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kessler D, Bergner A, Bottcher J, Fischer G, Dobel S, Hinkel M, et al. Drugging all RAS isoforms with one pocket. Future Med Chem. 2020;12:1911–23. doi: 10.4155/fmc-2020-0221. [DOI] [PubMed] [Google Scholar]
- 94.Sun Q, Phan J, Friberg AR, Camper DV, Olejniczak ET, Fesik SW. A method for the second-site screening of K-Ras in the presence of a covalently attached first-site ligand. J Biomol NMR. 2014;60:11–4. doi: 10.1007/s10858-014-9849-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Broker J, Waterson AG, Smethurst C, Kessler D, Bottcher J, Mayer M, et al. Fragment optimization of reversible binding to the switch II pocket on KRAS leads to a potent, in vivo active KRAS(G12C) inhibitor. J Med Chem. 2022;65:14614–29. doi: 10.1021/acs.jmedchem.2c01120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim D, Herdeis L, Rudolph D, Zhao Y, Bottcher J, Vides A, et al. Pan-KRAS inhibitor disables oncogenic signalling and tumour growth. Nature. 2023;619:160–6. doi: 10.1038/s41586-023-06123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stanton BZ, Chory EJ, Crabtree GR. Chemically induced proximity in biology and medicine. Science. 2018;359:eaao5902. doi: 10.1126/science.aao5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schreiber SL. The rise of molecular glues. Cell. 2021;184:3–9. doi: 10.1016/j.cell.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 99.Stewart ML, Perl NR, Lee S-J, Xue L, Zhou M, Simon J, et al. Abstract B37: development of inhibitors of the activated form of KRAS G12C. Mol Cancer Res. 2020;18:B37. doi: 10.1158/1557-3125.RAS18-B37. [DOI] [Google Scholar]
- 100.Zhou M, Yuzhakov A, Benod CC, Xue L, Silver AD, Iyer G, et al. Abstract A06: biophysical and biochemical characterization of KRAS G12C inhibition through the SMARTTM platform. Mol Cancer Res. 2020;18:A06. doi: 10.1158/1557-3125.RAS18-A06. [DOI] [Google Scholar]
- 101.Schulze CJ, Bermingham A, Choy TJ, Cregg JJ, Kiss G, Marquez A, et al. Abstract PR10: tri-complex inhibitors of the oncogenic, GTP-bound form of KRASG12C overcome RTK-mediated escape mechanisms and drive tumor regressions in vivo. Mol Cancer Ther. 2019;18:PR10. doi: 10.1158/1535-7163.TARG-19-PR10. [DOI] [Google Scholar]
- 102.Nichols RJ, Yang YC, Cregg J, Schulze CJ, Wang Z, Dua R, et al. Abstract 3595: RMC-6291, a next-generation tri-complex KRASG12C(ON) inhibitor, outperforms KRASG12C(OFF) inhibitors in preclinical models of KRASG12C cancers. Cancer Res. 2022;82:3595. doi: 10.1158/1538-7445.AM2022-3595. [DOI] [Google Scholar]
- 103.Koltun E, Cregg J, Rice MA, Whalen DM, Freilich R, Jiang J, et al. Abstract 1260: first-in-class, orally bioavailable KRASG12V(ON) tri-complex inhibitors, as single agents and in combinations, drive profound anti-tumor activity in preclinical models of KRASG12V mutant cancers. Cancer Res. 2021;81:1260. doi: 10.1158/1538-7445.AM2021-1260. [DOI] [Google Scholar]
- 104.Koltun ES, Rice MA, Gustafson WC, Wilds D, Jiang J, Lee BJ, et al. Abstract 3597: direct targeting of KRASG12X mutant cancers with RMC-6236, a first-in-class, RAS-selective, orally bioavailable, tri-complex RASMULTI(ON) inhibitor. Cancer Res. 2022;82:3597. doi: 10.1158/1538-7445.AM2022-3597. [DOI] [Google Scholar]
- 105.Knox JE, Jiang J, Burnett GL, Liu Y, Weller CE, Wang Z, et al. Abstract 3596: RM-036, a first-in-class, orally-bioavailable, Tri-Complex covalent KRASG12D(ON) inhibitor, drives profound anti-tumor activity in KRASG12D mutant tumor models. Cancer Res. 2022;82:3596. doi: 10.1158/1538-7445.AM2022-3596. [DOI] [Google Scholar]
- 106.Schulze CJ, Cregg J, Seamon KJ, Yang YC, Wang Z, Garrenton LS, et al. Abstract 3598: a first-in-class tri-complex KRASG13C(ON) inhibitor validates therapeutic targeting of KRASG13Cand drives tumor regressions in preclinical models. Cancer Res. 2022;82:3598. doi: 10.1158/1538-7445.AM2022-3598. [DOI] [Google Scholar]
- 107.Zhang Z, Shokat KM. Bifunctional small-molecule ligands of K-Ras induce its association with immunophilin proteins. Angew Chem Int Ed Engl. 2019;58:16314–9. doi: 10.1002/anie.201910124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moore AR, Rosenberg SC, McCormick F, Malek S. RAS-targeted therapies: is the undruggable drugged? Nat Rev Drug Discov. 2020;19:533–52. doi: 10.1038/s41573-020-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Frankson R, Yu ZH, Bai Y, Li Q, Zhang RY, Zhang ZY. Therapeutic targeting of oncogenic tyrosine phosphatases. Cancer Res. 2017;77:5701–5. doi: 10.1158/0008-5472.CAN-17-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu Q, Qu J, Zhao M, Xu Q, Sun Y. Targeting SHP2 as a promising strategy for cancer immunotherapy. Pharmacol Res. 2020;152:104595. doi: 10.1016/j.phrs.2019.104595. [DOI] [PubMed] [Google Scholar]
- 111.Yuan X, Bu H, Zhou J, Yang CY, Zhang H. Recent advances of SHP2 inhibitors in cancer therapy: current development and clinical application. J Med Chem. 2020;63:11368–96. doi: 10.1021/acs.jmedchem.0c00249. [DOI] [PubMed] [Google Scholar]
- 112.Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–93. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 113.Barford D, Neel BG. Revealing mechanisms for SH2 domain mediated regulation of the protein tyrosine phosphatase SHP-2. Structure. 1998;6:249–54. doi: 10.1016/S0969-2126(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 114.Hof P, Pluskey S, Dhe-Paganon S, Eck MJ, Shoelson SE. Crystal structure of the tyrosine phosphatase SHP-2. Cell. 1998;92:441–50. doi: 10.1016/S0092-8674(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 115.Chen YN, LaMarche MJ, Chan HM, Fekkes P, Garcia-Fortanet J, Acker MG, et al. Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature. 2016;535:148–52. doi: 10.1038/nature18621. [DOI] [PubMed] [Google Scholar]
- 116.Garcia Fortanet J, Chen CH, Chen YN, Chen Z, Deng Z, Firestone B, et al. Allosteric inhibition of SHP2: identification of a potent, selective, and orally efficacious phosphatase inhibitor. J Med Chem. 2016;59:7773–82. doi: 10.1021/acs.jmedchem.6b00680. [DOI] [PubMed] [Google Scholar]
- 117.Wong GS, Zhou J, Liu JB, Wu Z, Xu X, Li T, et al. Targeting wild-type KRAS-amplified gastroesophageal cancer through combined MEK and SHP2 inhibition. Nat Med. 2018;24:968–77. doi: 10.1038/s41591-018-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ruess DA, Heynen GJ, Ciecielski KJ, Ai J, Berninger A, Kabacaoglu D, et al. Mutant KRAS-driven cancers depend on PTPN11/SHP2 phosphatase. Nat Med. 2018;24:954–60. doi: 10.1038/s41591-018-0024-8. [DOI] [PubMed] [Google Scholar]
- 119.Mainardi S, Mulero-Sanchez A, Prahallad A, Germano G, Bosma A, Krimpenfort P, et al. SHP2 is required for growth of KRAS-mutant non-small-cell lung cancer in vivo. Nat Med. 2018;24:961–7. doi: 10.1038/s41591-018-0023-9. [DOI] [PubMed] [Google Scholar]
- 120.LaMarche MJ, Acker M, Argintaru A, Bauer D, Boisclair J, Chan H, et al. Identification of TNO155, an allosteric SHP2 inhibitor for the treatment of cancer. J Med Chem. 2020;63:13578–94. doi: 10.1021/acs.jmedchem.0c01170. [DOI] [PubMed] [Google Scholar]
- 121.Liu C, Lu H, Wang H, Loo A, Zhang X, Yang G, et al. Combinations with allosteric SHP2 inhibitor TNO155 to block receptor tyrosine kinase signaling. Clin Cancer Res. 2021;27:342–54. doi: 10.1158/1078-0432.CCR-20-2718. [DOI] [PubMed] [Google Scholar]
- 122.Brana I, Shapiro G, Johnson ML, Yu HA, Robbrecht D, Tan DSW, et al. Initial results from a dose finding study of TNO155, a SHP2 inhibitor, in adults with advanced solid tumors. J Clin Oncol. 2021;39:3005. doi: 10.1200/JCO.2021.39.15_suppl.3005. [DOI] [Google Scholar]
- 123.Sabari JK, Park H, Tolcher AW, Ou S-HI, Garon EB, George B, et al. KRYSTAL-2: a phase I/II trial of adagrasib (MRTX849) in combination with TNO155 in patients with advanced solid tumors with KRAS G12C mutation. J Clin Oncol. 2021;39:TPS146. doi: 10.1200/JCO.2021.39.3_suppl.TPS146. [DOI] [Google Scholar]
- 124.Brachmann SM, Weiss A, Guthy DA, Beyer K, Voshol J, Maira M, et al. Abstract P124: JDQ443, a covalent irreversible inhibitor of KRAS G12C, exhibits a novel binding mode and demonstrates potent anti-tumor activity and favorable pharmacokinetic properties in preclinical models. Mol Cancer Ther. 2021;20:P124. doi: 10.1158/1535-7163.TARG-21-P124. [DOI] [Google Scholar]
- 125.Koltun ES, Aay N, Buckl A, Jogalekar AS, Kiss G, Marquez A, et al. Abstract 4878: RMC-4550, an allosteric inhibitor of SHP2: Synthesis, structure, and anti-tumor activity. Cancer Res. 2018;78:4878. doi: 10.1158/1538-7445.AM2018-4878. [DOI] [Google Scholar]
- 126.Nichols RJ, Haderk F, Stahlhut C, Schulze CJ, Hemmati G, Wildes D, et al. RAS nucleotide cycling underlies the SHP2 phosphatase dependence of mutant BRAF-, NF1- and RAS-driven cancers. Nat Cell Biol. 2018;20:1064–73. doi: 10.1038/s41556-018-0169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ou SI, Koczywas M, Ulahannan S, Janne P, Pacheco J, Burris H, et al. A12 The SHP2 inhibitor RMC-4630 in patients with KRAS-mutant non-small cell lung cancer: preliminary evaluation of a first-in-man phase 1 clinical trial. J Thorac Oncol. 2020;15:S15–S6. doi: 10.1016/j.jtho.2019.12.041. [DOI] [Google Scholar]
- 128.Koczywas M, Haura E, Janne PA, Pacheco JM, Ulahannan S, Wang JS, et al. Abstract LB001: anti-tumor activity and tolerability of the SHP2 inhibitor RMC-4630 as a single agent in patients with RAS-addicted solid cancers. Cancer Res. 2021;81:LB001. doi: 10.1158/1538-7445.AM2021-LB001. [DOI] [Google Scholar]
- 129.Bendell J, Ulahannan S, Koczywas M, Brahmer J, Capasso A, Eckhardt SG, et al. Intermittent dosing of RMC-4630, a potent, selective inhibitor of SHP2, combined with the MEK inhibitor cobimetinib, in a phase 1b/2 clinical trial for advanced solid tumors with activating mutations of RAS signaling. Eur J Cancer. 2020;138:S8–9. doi: 10.1016/S0959-8049(20)31089-3. [DOI] [Google Scholar]
- 130.Falchook G, Li BT, Marrone KA, Bestvina CM, Langer CJ, Krauss JC, et al. OA03.03 Sotorasib in combination with RMC-4630, a SHP2 inhibitor, in KRAS p.G12C-mutated NSCLC and other solid tumors. J Thorac Oncol. 2022;17:S8. doi: 10.1016/j.jtho.2022.07.022. [DOI] [Google Scholar]
- 131.Wang P, Zheng Q, Kang D, Sun X, Zhu S, Wang Y, et al. 30P Investigation of KRAS G12C inhibitor JAB-21822 as a single agent and in combination with SHP2 inhibitor JAB-3312 in preclinical cancer models. Ann Oncol. 2022;33:S1441. doi: 10.1016/j.annonc.2022.10.040. [DOI] [Google Scholar]
- 132.Sun Y, Meyers BA, Johnson SB, Harris AL, Czako B, Cross JB, et al. Abstract C036: siscovery of IACS-13909, an allosteric SHP2 inhibitor that overcomes multiple mechanisms underlying osimertinib resistance. Mol Cancer Ther. 2019;18:C036. doi: 10.1158/1535-7163.TARG-19-C036. [DOI] [Google Scholar]
- 133.Sun Y, Meyers BA, Czako B, Leonard P, Mseeh F, Harris AL, et al. Allosteric SHP2 inhibitor, IACS-13909, overcomes EGFR-dependent and EGFR-independent resistance mechanisms toward osimertinib. Cancer Res. 2020;80:4840–53. doi: 10.1158/0008-5472.CAN-20-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Czako B, Sun Y, McAfoos T, Cross JB, Leonard PG, Burke JP, et al. Discovery of 6-[(3S,4S)-4-amino-3-methyl-2-oxa-8-azaspiro[4.5]decan-8-yl]-3-(2,3-dichlorophenyl)-2-methyl-3,4-dihydropyrimidin-4-one (IACS-15414), a potent and orally bioavailable SHP2 inhibitor. J Med Chem. 2021;64:15141–69. doi: 10.1021/acs.jmedchem.1c01132. [DOI] [PubMed] [Google Scholar]
- 135.Stice JP, Donovan S, Sun Y, Kohl N, Czako B, Mseeh F, et al. Abstract P207: BBP-398, a potent, small molecule inhibitor of SHP2, enhances the response of established NSCLC xenografts to KRASG12C and mutEGFR inhibitors. Mol Cancer Ther. 2021;20:P207. doi: 10.1158/1535-7163.TARG-21-P207. [DOI] [Google Scholar]
- 136.Williams B, Taylor A, Orozco O, Owen C, Kelley E, Lescarbeau A, et al. Abstract 3327: discovery and characterization of the potent, allosteric SHP2 inhibitor GDC-1971 for the treatment of RTK/RAS driven tumors. Cancer Res. 2022;82:3327. doi: 10.1158/1538-7445.AM2022-3327. [DOI] [Google Scholar]
- 137.Drilon A, Sharma MR, Johnson ML, Yap TA, Gadgeel S, Nepert D, et al. SHP2 inhibition sensitizes diverse oncogene-addicted solid tumors to re-treatment with targeted therapy. Cancer Discov. 2023;13:1789–801. doi: 10.1158/2159-8290.CD-23-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Drilon AE, Johnson ML, Gadgeel SM, Nepert D, Feng G, Golmakani M, et al. A first-in-human, phase 1 study of the SHP2 inhibitor PF-07284892 as monotherapy and in combination with different targeted therapies in oncogene-driven, treatment-resistant solid tumors. J Clin Oncol. 2023;41:3020. doi: 10.1200/JCO.2023.41.16_suppl.3020. [DOI] [Google Scholar]
- 139.Martin L, Patel R, Zhang J, Nevarez R, Congdon T, Brail L, et al. Abstract 2670: ERAS-601, a potent allosteric inhibitor of SHP2, synergistically enhances the efficacy of sotorasib/adagrasib and cetuximab in NSCLC, CRC, and HNSCC tumor models. Cancer Res. 2022;82:2670. doi: 10.1158/1538-7445.AM2022-2670. [DOI] [Google Scholar]
- 140.Shojaei F, Shojaei F, Ricono JM, Fang C, Kabbinavar F, Goodenow B, et al. Abstract 1041: HBI-2376, HUYABIO clinical stage SHP2 inhibitor, possess robust in vitro potency and in vivo efficacy in several preclinical tumor models carrying KrasG12C or EGFR mutations. Cancer Res. 2022;82:1041. doi: 10.1158/1538-7445.AM2022-1041. [DOI] [Google Scholar]
- 141.Xia X, Du L, Zhuge H, Zheng Q, Cui W, Zhu J, et al. Abstract 1475: discovery of ETS-001, a highly potent allosteric SHP2 inhibitor to treat RTK/RAS-driven cancers. Cancer Res. 2021;81:1475. doi: 10.1158/1538-7445.AM2021-1475. [DOI] [Google Scholar]
- 142.Li L, Fu B, Han H, Sun Z, Zhao X, Jv X, et al. Abstract 5463: BPI-442096: a potent and selective inhibitor of SHP2 for the treatment of multiple cancers. Cancer Res. 2022;82:5463. doi: 10.1158/1538-7445.AM2022-5463. [DOI] [Google Scholar]
- 143.Vigil D, Cherfils J, Rossman KL, Der CJ. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Nat Rev Cancer. 2010;10:842–57. doi: 10.1038/nrc2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Patgiri A, Yadav KK, Arora PS, Bar-Sagi D. An orthosteric inhibitor of the Ras-Sos interaction. Nat Chem Biol. 2011;7:585–7. doi: 10.1038/nchembio.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Leshchiner ES, Parkhitko A, Bird GH, Luccarelli J, Bellairs JA, Escudero S, et al. Direct inhibition of oncogenic KRAS by hydrocarbon-stapled SOS1 helices. Proc Natl Acad Sci USA. 2015;112:1761–6. doi: 10.1073/pnas.1413185112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Winter JJ, Anderson M, Blades K, Brassington C, Breeze AL, Chresta C, et al. Small molecule binding sites on the Ras:SOS complex can be exploited for inhibition of Ras activation. J Med Chem. 2015;58:2265–74. doi: 10.1021/jm501660t. [DOI] [PubMed] [Google Scholar]
- 147.Hillig RC, Sautier B, Schroeder J, Moosmayer D, Hilpmann A, Stegmann CM, et al. Discovery of potent SOS1 inhibitors that block RAS activation via disruption of the RAS-SOS1 interaction. Proc Natl Acad Sci USA. 2019;116:2551–60. doi: 10.1073/pnas.1812963116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Burns MC, Sun Q, Daniels RN, Camper D, Kennedy JP, Phan J, et al. Approach for targeting Ras with small molecules that activate SOS-mediated nucleotide exchange. Proc Natl Acad Sci USA. 2014;111:3401–6. doi: 10.1073/pnas.1315798111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hodges TR, Abbott JR, Little AJ, Sarkar D, Salovich JM, Howes JE, et al. Discovery and structure-based optimization of benzimidazole-derived activators of SOS1-mediated nucleotide exchange on RAS. J Med Chem. 2018;61:8875–94. doi: 10.1021/acs.jmedchem.8b01108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Abbott JR, Patel PA, Howes JE, Akan DT, Kennedy JP, Burns MC, et al. Discovery of quinazolines that activate SOS1-mediated nucleotide exchange on RAS. ACS Med Chem Lett. 2018;9:941–6. doi: 10.1021/acsmedchemlett.8b00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sarkar D, Olejniczak ET, Phan J, Coker JA, Sai J, Arnold A, et al. Discovery of sulfonamide-derived agonists of SOS1-mediated nucleotide exchange on RAS using fragment-based methods. J Med Chem. 2020;63:8325–37. doi: 10.1021/acs.jmedchem.0c00511. [DOI] [PubMed] [Google Scholar]
- 152.Hofmann MH, Gmachl M, Ramharter J, Savarese F, Gerlach D, Marszalek JR, et al. BI-3406, a potent and selective SOS1–KRAS interaction inhibitor, is effective in KRAS-driven cancers through combined MEK inhibition. Cancer Discov. 2021;11:142–57. doi: 10.1158/2159-8290.CD-20-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ramharter J, Kessler D, Ettmayer P, Hofmann MH, Gerstberger T, Gmachl M, et al. One atom makes all the difference: getting a foot in the door between SOS1 and KRAS. J Med Chem. 2021;64:6569–80. doi: 10.1021/acs.jmedchem.0c01949. [DOI] [PubMed] [Google Scholar]
- 154.Thatikonda V, Lu H, Jurado S, Kostyrko K, Bristow CA, Bosch K, et al. Combined KRAS (G12C) and SOS1 inhibition enhances and extends the anti-tumor response in KRAS (G12C) -driven cancers by addressing intrinsic and acquired resistance. bioRxiv. 2023. 2023.01.23.525210.
- 155.Hofmann MH, Lu H, Duenzinger U, Gerlach D, Trapani F, Machado AA, et al. Abstract CT210: trial in process: Phase 1 studies of BI 1701963, a SOS1::KRAS Inhibitor, in combination with MEK inhibitors, irreversible KRASG12C inhibitors or irinotecan. Cancer Res. 2021;81:CT210. doi: 10.1158/1538-7445.AM2021-CT210. [DOI] [Google Scholar]
- 156.Ketcham JM, Haling J, Khare S, Bowcut V, Briere DM, Burns AC, et al. Design and discovery of MRTX0902, a potent, selective, brain-Penetrant, and orally bioavailable inhibitor of the SOS1:KRAS protein-protein interaction. J Med Chem. 2022;65:9678–90. doi: 10.1021/acs.jmedchem.2c00741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ketcham JM, Khare S, Sudhakar N, Briere DM, Yan L, Laguer J, et al. Abstract ND02: MRTX0902: a SOS1 inhibitor for therapeutic intervention of KRAS-driven cancers. Cancer Res. 2022;82:ND02. doi: 10.1158/1538-7445.AM2022-ND02. [DOI] [Google Scholar]
- 158.Buckl A, Quintana E, Lee GJ, Shifrin N, Zhong M, Garrenton LS, et al. Abstract 1273: discovery of a potent, selective, and orally bioavailable SOS1 inhibitor, RMC-023, an in vivo tool compound that blocks RAS activation via disruption of the RAS-SOS1 interaction. Cancer Res. 2021;81:1273. doi: 10.1158/1538-7445.AM2021-1273. [DOI] [Google Scholar]
- 159.Thompson SK, Buckl A, Dossetter AG, Griffen E, Gill A. Small molecule Son of Sevenless 1 (SOS1) inhibitors: a review of the patent literature. Expert Opin Ther Pat. 2021;31:1189–204. doi: 10.1080/13543776.2021.1952984. [DOI] [PubMed] [Google Scholar]
- 160.Zhang S, Zhang Y, Chen X, Xu J, Fang H, Li Y, et al. Design and structural optimization of orally bioavailable SOS1 inhibitors for the treatment of KRAS-driven carcinoma. J Med Chem. 2022;65:15856–77. doi: 10.1021/acs.jmedchem.2c01517. [DOI] [PubMed] [Google Scholar]
- 161.He H, Zhang Y, Xu J, Li Y, Fang H, Liu Y, et al. Discovery of orally bioavailable SOS1 inhibitors for suppressing KRAS-driven carcinoma. J Med Chem. 2022;65:13158–71. doi: 10.1021/acs.jmedchem.2c00986. [DOI] [PubMed] [Google Scholar]
- 162.Zhou C, Fan Z, Zhou Z, Li Y, Cui R, Liu C, et al. Discovery of the first-in-class agonist-based SOS1 PROTACs effective in human cancer cells harboring various KRAS mutations. J Med Chem. 2022;65:3923–42. doi: 10.1021/acs.jmedchem.1c01774. [DOI] [PubMed] [Google Scholar]
- 163.Bian Y, Alem D, Beato F, Hogenson TL, Yang X, Jiang K, et al. Development of SOS1 inhibitor-based degraders to target KRAS-mutant colorectal cancer. J Med Chem. 2022;65:16432–50. doi: 10.1021/acs.jmedchem.2c01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhou Z, Zhou G, Zhou C, Fan Z, Cui R, Li Y, et al. Discovery of a potent, cooperative, and selective SOS1 PROTAC ZZ151 with In vivo antitumor efficacy in KRAS-mutant cancers. J Med Chem. 2023;66:4197–214. doi: 10.1021/acs.jmedchem.3c00075. [DOI] [PubMed] [Google Scholar]
- 165.Hamilton G, Stickler S, Rath B. Targeting of SOS1: from SOS1 activators to proteolysis targeting chimeras. Curr Pharm Des. 2023;29:1741–6. doi: 10.2174/1381612829666230418114520. [DOI] [PubMed] [Google Scholar]
- 166.Paez D, Meriggi F, Cremolini C, Folprecht G, Korantzis I, Chan E, et al. 437TiP Trial in progress: a phase III global study of sotorasib, a specific KRAS G12C inhibitor, in combination with panitumumab versus investigator’s choice in chemorefractory metastatic colorectal cancer (CodeBreaK 300) Ann Oncol. 2022;33:S734. doi: 10.1016/j.annonc.2022.07.575. [DOI] [Google Scholar]
- 167.Weiss J, Yaeger RD, Johnson ML, Spira A, Klempner SJ, Barve MA, et al. LBA6 KRYSTAL-1: Adagrasib (MRTX849) as monotherapy or combined with cetuximab (Cetux) in patients (Pts) with colorectal cancer (CRC) harboring a KRASG12C mutation. Ann Oncol. 2021;32:S1294. doi: 10.1016/j.annonc.2021.08.2093. [DOI] [Google Scholar]
- 168.Nassar AH, Adib E, Kwiatkowski DJ. Distribution of KRAS (G12C) somatic mutations across race, sex, and cancer type. N Engl J Med. 2021;384:185–7. doi: 10.1056/NEJMc2030638. [DOI] [PubMed] [Google Scholar]
- 169.Hofmann MH, Gerlach D, Misale S, Petronczki M, Kraut N. Expanding the reach of precision oncology by drugging all KRAS mutants. Cancer Discov. 2022;12:924–37. doi: 10.1158/2159-8290.CD-21-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Quintana E, Schulze CJ, Myers DR, Choy TJ, Mordec K, Wildes D, et al. Allosteric inhibition of SHP2 stimulates antitumor immunity by transforming the immunosuppressive environment. Cancer Res. 2020;80:2889–902. doi: 10.1158/0008-5472.CAN-19-3038. [DOI] [PubMed] [Google Scholar]
- 171.Fedele C, Li S, Teng KW, Foster CJR, Peng D, Ran H, et al. SHP2 inhibition diminishes KRASG12C cycling and promotes tumor microenvironment remodeling. J Exp Med. 2021;218:e20201414. doi: 10.1084/jem.20201414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Moghadamchargari Z, Shirzadeh M, Liu C, Schrecke S, Packianathan C, Russell DH, et al. Molecular assemblies of the catalytic domain of SOS with KRas and oncogenic mutants. Proc Natl Acad Sci USA. 2021;118:e2022403118. doi: 10.1073/pnas.2022403118. [DOI] [PMC free article] [PubMed] [Google Scholar]