Abstract
The promyelocytic leukemia protein (PML) forms nuclear bodies which are altered in some disease conditions. We report that the cytoplasmic RNA virus lymphocytic choriomeningitis virus (LCMV) influences the distribution of PML bodies. In cells infected with LCMV, the Z protein and PML form large bodies primarily in the cytoplasm. Transient transfection studies indicate that Z alone is sufficient to redistribute PML to the cytoplasm and that PML and Z colocalize. Coimmunoprecipitation studies show specific interaction between PML and Z proteins. A similar result was observed with a Z protein from another arenavirus, Lassa virus, suggesting that this is a general feature of the Arenaviridae. Genetically engineered mutations in PML were used to show that the Z protein binds the N-terminal region of PML and does not need the PML RING or the nuclear localization signal to colocalize. The Z protein acts dominantly to overcome the diffuse phenotype observed in several PML mutants. The interaction between PML and Z may influence certain unique characteristics of arenavirus infection.
The promyelocytic leukemia (PML) protein was first described as part of a fusion protein present in acute promyelocytic leukemia (APL). PML nuclear bodies are multiprotein complexes distinct from small nuclear RNPs and nucleoli and are also known as PML oncogenic domains, ND10, or Kr bodies (1, 11, 21, 25, 26, 47). PML bodies contain at least six proteins, including SP100, an autoantigen of primary biliary cirrhosis (43); NDP52 (22); PIC1, a ubiquitin-like molecule (4); NDP55 (1); PML-associated factor (17); and PML (11, 21, 46). The PML protein contains three cysteine-rich zinc-binding domains, which are known as RING and B boxes (B1 and B2), and a leucine coiled-coil domain forming a tripartite RBCC motif (14, 33).
APL involves changes in the PML protein. A portion of the PML protein, including the RING finger, is fused to retinoic acid receptor alpha (RARα) by a chromosomal translocation (8a, 14, 18, 19, 30) and makes cells less prone to apoptosis (35). PML-RARα no longer generates nuclear bodies but forms a microparticulate pattern in the cytoplasm (21). Cytoplasmic redistribution of PML also occurs in liver carcinomas (45). Mutations in the PML RING finger or adjacent B boxes create a diffuse nuclear distribution (6, 7); deletion of the nuclear localization signal also leads to a cytoplasmic redistribution and loss of growth suppressor activity (23).
PML nuclear bodies are affected by some double-stranded DNA viruses. Adenovirus type 5 infection converts spherical nuclear bodies to filamentous structures (10, 17, 32). Nuclear bodies are unchanged during simian virus 40 replication but are found adjacent to virus replication sites (17). Herpes simplex virus type 1 (HSV-1), cytomegalovirus (CMV), and Epstein-Barr virus (EBV) affect PML bodies. HSV-1 infection redistributes nuclear bodies (27) that become associated with viral DNA (17, 24). CMV infection leads to a diffuse nuclear pattern or an increased number of PML bodies coincident with the onset of immediate-early gene expression (20). Redistribution of PML protein coincides with the onset of CMV immediate-early gene expression. EBV protein EBNA-5 accumulates in PML bodies but does not disrupt them (42). Thus, DNA viruses have multiple types of interactions with PML.
Lymphocytic choriomeningitis virus (LCMV) is a negative-stranded RNA virus in the arenavirus family. Arenaviruses such as Lassa virus, Junin virus, and Machupo virus cause hemorrhagic fevers in human beings (reviewed in references 31 and 36). Platelet dysfunction and thrombocytopenia leading to bleeding are common in arenaviral hemorrhagic fevers and are also features of APL (44). LCMV is carried as an inapparent chronic infection by rodents, although nonhuman primates can develop clinical signs similar to those of human beings with Lassa fever (28).
Arenaviruses have two single-stranded RNA genome segments, no introns, and no DNA intermediates during replication (36). Arenaviruses encode five different products: a nucleocapsid protein (NP), an envelope glycoprotein (GP) that is processed into GP1 and GP2, an RNA polymerase (L), and an 11-kDa protein (Z) containing a RING finger domain with unknown function (38). Biochemical studies of Z protein from Tacaribe virus demonstrated its role in genome and mRNA synthesis (13). Z protein is packaged into virions in both LCMV and Tacaribe virus and may be involved in replication immediately after infection (13, 37).
PML protein and the arenavirus Z proteins contain RING domains. The RING is a 60-residue zinc-binding motif that uses conserved cysteines and a histidine to bind two zinc atoms (5, 39). High-resolution solution structures showed that the RING motif is unlike any other zinc finger structure (3, 6). The RING motif appears in more than 80 plant, animal, and virus proteins, including several proto-oncoproteins such as PML and the breast cancer gene product BRCA1 (5, 39). Proteins containing RING motifs are found in the nucleus or cytoplasm and are more likely to bind other proteins via the RING domain than to bind nucleic acid (39).
The interaction between PML and Z is the first reported interaction between an arenavirus protein and a host protein and may have some bearing on the unique characteristics of arenavirus infections such as the noncytopathicity of arenaviruses and the ability to cause hemorrhagic fever. PML and Z colocalize in both infected and transfected cells and form specific complexes in vitro. Z protein from Lassa fever virus also binds PML in vitro, and this interaction might be a general feature of arenavirus Z proteins. We speculate that arenaviruses acquired a RING finger protein as a molecular mimic for the PML RING domain.
MATERIALS AND METHODS
Producing recombinant Z and PML proteins and raising Z-specific antisera.
The cDNAs for the entire Z protein (38) and PML (69-kDa isoform [6]) were cloned into mammalian expression vectors with either the simian virus 40 (for PML) or Moloney leukemia virus (for Z) enhancers (8). All constructs were sequenced to ensure that no mutations occurred during amplification and subcloning. The Z gene was inserted between BamHI/XhoI sites. Z-pGEX was produced by insertion at the BamHI site of pGEX-20T to obtain a Z–glutathione S-transferase (GST) fusion protein that was cleaved with thrombin (40) to yield Z protein. The first 20 amino acids of Z protein were N terminally sequenced to confirm accurate thrombin cutting. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis indicated that the protein was 11 kDa, as expected. This material was used to produce antiserum that was affinity purified. The Z (Lassa virus)-GST construct contained the full coding region of Z from Lassa fever virus.
Virus infection.
NIH 3T3 or HeLa cells were grown on coverslips and infected with LCMV Armstrong at a multiplicity of infection of 1 PFU per cell. At 70, 90, or 100 h after infection, the coverslips were washed in phosphate-buffered saline and fixed in acetone for 5 min. The fixed cells were stained with hyperimmune guinea pig anti-LCMV serum to confirm infection. Guinea pig serum was heated for 1 h at 78°C to inactivate virus and was used at a 1:500 dilution with a 1:200 fluorescein isothiocyanate (FITC)-conjugated goat anti-guinea pig secondary antibody (Jackson Immunoresearch).
Transient transfection studies.
NIH 3T3 cells were transiently transfected by the calcium phosphate method with 5 to 8 μg of the appropriate mammalian expression construct. Transfecting with Lipofectamine (Gibco) and 1 μg of the appropriate construct yielded identical results. At 40 h after transfection, cells were prepared for immunofluorescence as described elsewhere (6, 7). Briefly, cells were washed twice in phosphate-buffered saline, followed by fixation in methanol for 10 min at −20°C prior to application of antibodies.
Immunofluorescence studies.
PML polyclonal antibody, a kind gift of K. Howe (4, 6), was used at a dilution of 1:200, and the monoclonal antibody 5E10 (MAb 5E10), a kind gift of L. de Jong, was used at 1:100. MAb 5E10 is specific to human PML protein (41). Antibodies were detected with either a 1:200 dilution of goat anti-rabbit FITC (Jackson Immunoresearch) antiserum or a 1:100 dilution of horse anti-mouse Texas Red (Vector) antiserum, and immunofluorescence testing was performed as described previously (6). When the cells were stained with rabbit anti-PML and guinea pig anti-LCMV sera, a donkey anti-rabbit Texas Red secondary antibody was used at a dilution of 1:100 (Jackson Immunoresearch) and a goat anti-guinea pig FITC (Jackson Immunoresearch) antibody was used at a dilution of 1:100. Fluorescence was observed with a confocal laser microscope (Zeiss or Leica) that independently recorded red (568-nm excitation) or green (488-nm excitation) fluorescence.
Coimmunoprecipitation studies.
Protein-protein interactions were demonstrated by coimmunoprecipitation assays. PML protein was produced from the pLINK-pml construct in reticulocyte lysates (Promega) as described previously (7). The Z-GST fusion protein was eluted from glutathione agarose beads with 15 mM glutathione. The lysate was mixed with either Z-GST or Z (Lassa virus)-GST or negative control protein GAPDH, and the mixture was incubated with agitation for approximately 12 h at 4°C followed by coimmunoprecipitation. Immunoprecipitations were carried out as described elsewhere (7) in the presence of the GST antibody (Sigma) and bovine serum albumin (0.5 mg/ml) in buffer E (50 mM Tris, 150 mM NaCl, 1 mM EDTA; pH 7.5). The samples were incubated at 4°C for 1.5 h with protein A-Sepharose (Pharmacia). The samples were washed twice in buffer E and 1% Nonidet P-40. The samples were boiled in reducing buffer, subjected to 20% PAGE–SDS, and blotted onto polyvinylidene difluoride (PVDF) membranes (16). MAb 5E10 detected PML on immunoblots. Western blots used the ECL method (Amersham) to visualize bound antibodies. Converse experiments were also carried out with Z protein, not fusion protein, to incubate with PML. The samples were immunoprecipitated with anti-PML polyclonal antibody and were immunoblotted with Z antibody (see below).
Mapping experiments with deletion mutants.
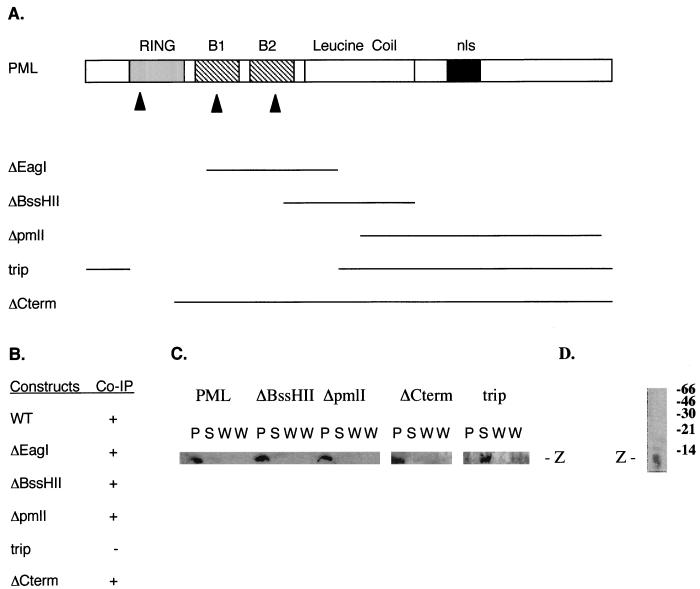
A series of mutations were made in PML to map regions that bind Z. Deletions were made in pLINK-pml with restriction enzymes EagI, BssHII, and pmlI, and a double cut was made with AvrII/SpeI; the corresponding mutations are referred to as ΔEagI, ΔBssHII, ΔpmlI, and ΔCterm, respectively. Deletions were confirmed by in vitro translation in reticulocyte lysates as described elsewhere (7). Mutant pLINK-pml constructs were also confirmed by restriction digestion. An additional construct was made by PCR. This construct (referred to as trip) contained the tripartite region of PML from nucleotides 150 to 840 inserted at the BamHI site of pGEX-20T. The fragment was cloned into a GST fusion vector and sequenced to confirm the absence of mutations; the protein was produced in Escherichia coli (40).
Z-GST was cleaved with thrombin to obtain Z protein, as was PML-GST in the case of the trip construct. The resulting Z protein was mixed with either the translation product from the reticulocyte lysate or pure PML protein and left to incubate overnight at 4°C. Identical results were obtained after 1 h of incubation. The mixtures were immunoprecipitated as described above with the rabbit anti-PML, immunoblotted, and probed with rabbit anti-Z serum.
Construction of point mutations in PML and Z.
Double-point mutations in PML were made by PCR stitching methods (29) as described for the site 1 RING mutation Cys9Cys12ΔAla9Ala12 (6) and for the B1 B box Cys17Cys20ΔAla17Ala20 and B2 B box Cys21Cys24ΔAla21Ala24 mutations (7). The coilless mutation was made in the PML mammalian expression construct by deletion of the BssHII fragment as described for the pLINK-pml construct. This results in loss of both the leucine coil and part of the B2 B box.
A double-point mutation in Z site 2 of the RING domain was also made by PCR stitching methods. The Z gene was inserted into pMLV at the BamHI/XhoI site. The mutation resulted in the production of a HindIII site. Thus, the presence of the mutation was easily determined by restriction digestion and confirmatory sequencing. The mutation was Cys32Cys35ΔPhe32Gly35. This change has been shown to abrogate the metal binding ability of other RINGs, and the introduction of phenylalanine should be structurally destabilizing. The PML RING requires zinc for its structure (6); therefore, mutation of the zinc-binding ligands should destroy the structure of the RING and at least partially unfold the Z protein.
RESULTS
LCMV infection affects PML distribution.
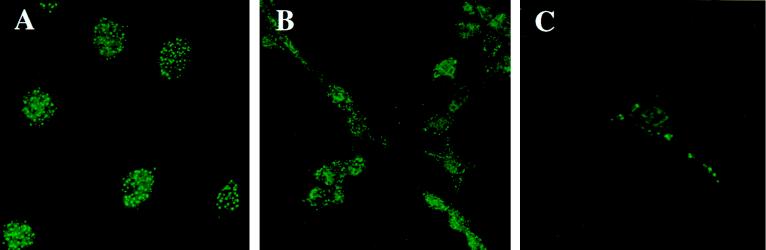
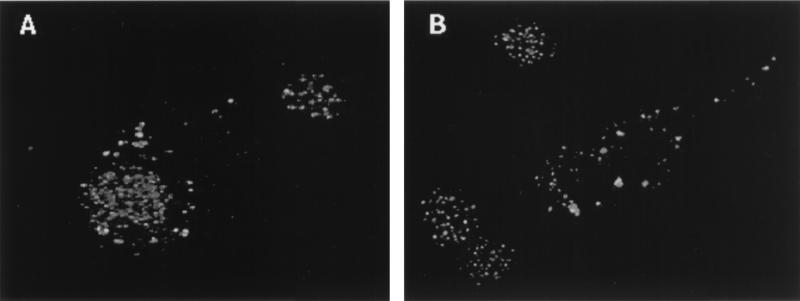
LCMV infection affects PML distribution in NIH 3T3 cells (Fig. 1). Cells were stained with polyclonal sera to monitor endogenous mouse PML nuclear bodies. Confocal microscopy was used to focus on a narrow optical plane and differentiate between nuclear and cytoplasmic localizations. Uninfected cells (Fig. 1A) show the punctate nuclear pattern characteristic of PML staining (see reference 7 and references therein). Figure 1B shows cells infected for 70 h with LCMV. A higher-magnification view of a cell infected for 90 h is shown (Fig. 1C). There were no differences between cells infected for 70 or 90 h. PML appears after infection as punctate cytoplasmic staining with a minor punctate nuclear component on a diffuse background. Infected cells stained with affinity-purified Z antibody gave a pattern similar to that for PML, with predominant punctate cytoplasmic staining and some nuclear staining. Cells were stained with anti-LCMV to confirm infection. When the cells were incubated with heat-inactivated virus, PML retained its uninfected pattern with its punctate nuclear distribution.
FIG. 1.
Effect of LCMV infection on PML nuclear bodies. (A) Uninfected NIH 3T3 cells; (B) 70-h LCMV-infected NIH 3T3 cells; (C) 90-h LCMV-infected cells. LCMV infection is described in Materials and Methods. Cells were stained with the PML polyclonal antibody and FITC-conjugated secondary antibody as described in Materials and Methods. Immunofluorescence was observed by confocal laser microscopy. Magnification, ×40 objective with zooms of 2.6 (A), 1.7 (B), and 3.9 (C).
LCMV infection caused similar changes for PML bodies in NIH 3T3 and HeLa cells (data not shown). The use of HeLa cells instead of NIH 3T3 cells enabled double-staining experiments to be carried out with the affinity-purified Z antibody and MAb 5E10, which recognizes human but not mouse PML (41). Cells were infected with LCMV for 100 h. When cells were stained with either the 5E10 or Z antibody, the resulting pattern was predominantly cytoplasmic, with bodies surrounding the nucleus. Double-staining experiments indicated that the PML and Z bodies colocalized. Colocalization was observed for cytoplasmic and nuclear bodies.
Transient transfection of the LCMV Z gene recapitulates effects of virus infection.
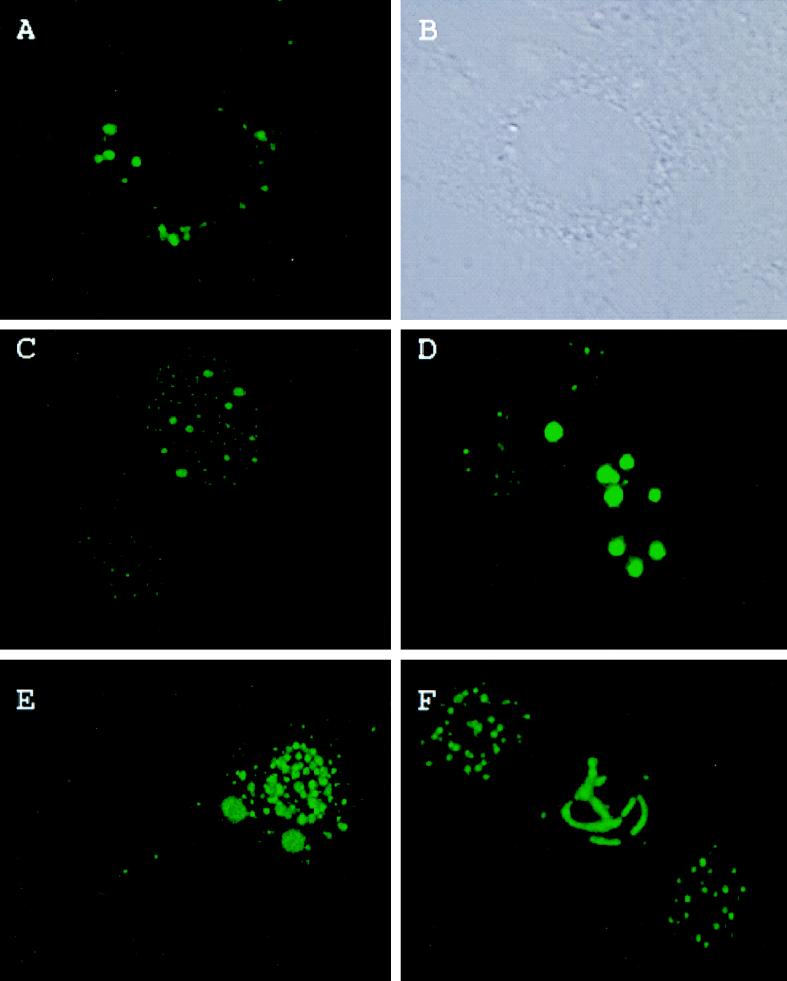
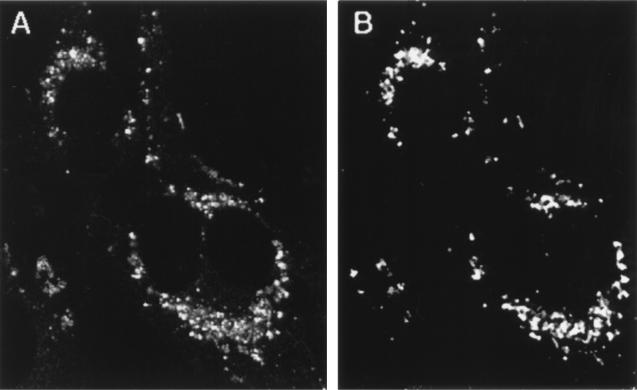
Z protein produced in transiently transfected NIH 3T3 cells distributed in a manner similar to that of Z protein in infected cells. Z protein is associated with large cytoplasmic bodies in transfected cells, often adjacent to the nucleus and with some bodies present in the nucleus (Fig. 2A and B). In cells stained with affinity-purified Z antibody, Z protein sometimes forms a ring around the nucleus (Fig. 2A). There is one body that is localized in the nucleus compared to the phase-contrast view (Fig. 2B). Our observations are consistent with biochemical fractionation experiments that detected Z protein mainly in the cytoplasm of infected cells (37) and specifically in the polysomal fraction (35a).
FIG. 2.
Subcellular localization of the Z protein, PML, and the effect of Z protein expression on PML subcellular distribution. Experiments were carried out as described in Materials and Methods. (A) Cell transfected only with the Z construct and stained with the affinity-purified Z antibody followed by FITC; (B) phase-contrast view of the same cell; (C) two cells, one of which was transfected with the PML construct to show the normal PML phenotype for the 69-kDa isoform which is found in the nucleus (see reference 7 and references therein). See text for further details. (D to F) Cells transfected with both the expression constructs for Z and PML but stained only with the PML polyclonal antibody followed by FITC. Immunofluorescence was observed with confocal laser microscopy. FITC was excited at 488 nm. Magnifications: ×40 objective with zooms of 3.5 (A and B), 3.5 (C), 3.0 (D), and 3.0 (E) and ×25 objective with a zoom of 6.0 (F).
Z protein affects the subcellular distribution of PML (Fig. 2D to F). Z and human PML constructs were cotransfected into NIH 3T3 cells, and PML-specific immunofluorescence was evaluated. In Fig. 2C to F, the cells were stained only with the PML polyclonal antibody to observe the effects of the Z protein both on exogenous human PML and the endogenous mouse PML. PML transfected by itself is given as a comparison in Fig. 2C. Two cell nuclei were present; the larger one on the upper right was transfected, and the smaller one on the lower left was not transfected. The smaller nucleus typifies the punctate nuclear pattern expected for endogenous PML in NIH 3T3 cells. In the transfected cell, bright larger nuclear bodies were transfected human PML and numerous smaller nuclear bodies were endogenous mouse protein (7).
Several different phenotypes were observed in cells cotransfected with PML and Z (Fig. 2D to F). Figure 2D shows cells with very large cytoplasmic bodies some of which form a ring around the nucleus (center of the field in panel D). Figure 2E shows PML bodies more densely packed in the nucleus than in cytoplasm. There are two large cytoplasmic bodies with smaller bodies located further down the cell processes. This pattern with both nuclear and cytoplasmic staining is predominant in these transfection studies. In Fig. 2F, the middle cell is transfected while the cell nuclei on either side display the typical untransfected, nuclear punctate staining of endogenous PML. The middle cell in Fig. 2F, which stained almost exclusively in the nucleus, demonstrates an interesting phenotype, in which PML appears to be threaded around nucleoli that are visible under phase contrast (data not shown). In transfected cells, there was none of the normal pattern for endogenous PML (e.g., Fig. 2C). The absence of normal punctate PML distribution indicated that Z protein affects both endogenous and exogenous PML. As expected, transfected cells with overexpressed protein have larger PML bodies. These experiments indicate that Z protein alone is sufficient to redistribute PML from its normal nuclear localization into the cytoplasm.
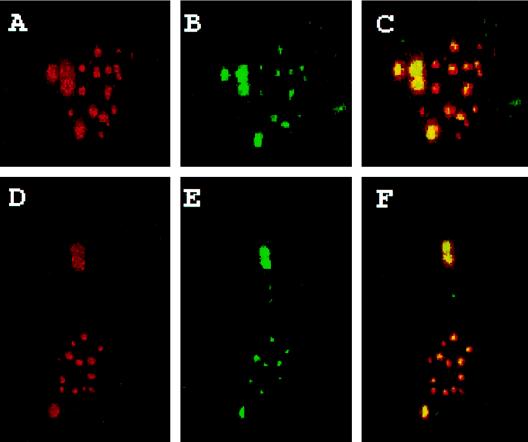
PML and Z proteins colocalize in transfected cells.
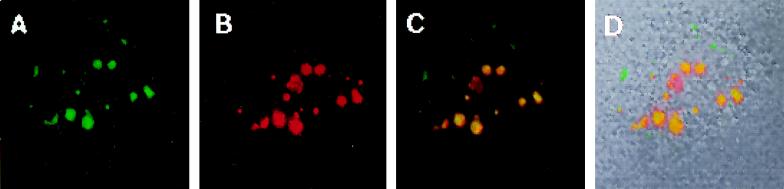
Indirect-immunofluorescence studies showed that PML and Z proteins colocalized (Fig. 3). The cells were stained with the affinity-purified Z antibody (green) and the human PML-specific MAb 5E10 (red); this antibody does not stain the endogenous mouse PML. Colocalization of PML and Z generates yellow for cytoplasmic or nuclear colocalization (Fig. 3C and F). Z-PML bodies are larger when located in the cytoplasm. The cells (second row) showed smaller bodies in the nucleus and larger cytoplasmic bodies in the same cell. Additional Z bodies (in green) were also observed. This is probably a result of the Z protein colocalizing with endogenous PML which was not stained by MAb 5E10. However, most of the endogenous PML was incorporated with Z (Fig. 2). Other antibodies were tested to determine whether they also colocalized with PML-Z bodies. Bax, heat shock protein 70, and p53 do not colocalize to PML-Z bodies (5a). These results indicate that in the absence of LCMV infection, the Z protein colocalizes with PML.
FIG. 3.
Colocalization studies of the Z protein and PML. Cells were transfected as described in Materials and Methods with equivalent amounts of the Z- and PML-containing mammalian expression vectors. Upper panels correspond with lower panels. (A and D) Cells stained with the PML MAb 5E10 with Texas Red-labeled anti-mouse secondary antibodies; (B and E) cells stained with affinity-purified Z antibody with FITC-labeled anti-rabbit secondary antibodies; (C and F) overlay (colocalization is in yellow). FITC was excited at 488 nm, and Texas Red was excited at 568 nm. The two channels were recorded independently. Images were overlaid in Photoshop. Magnification, ×40 objective with a zoom of 2. Images were further enlarged in Photoshop for presentation.
Coimmunoprecipitation studies indicate that PML and Z interact directly.
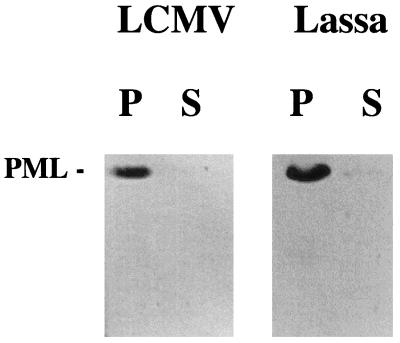
Coimmunoprecipitation assays were performed to determine whether the interaction between PML and Z is direct (Fig. 4). The same 69-kDa isoform of PML used in transfection studies was produced in reticulocyte lysate as described previously (7). Reticulocyte lysates were mixed with purified Z-GST. Samples were coimmunoprecipitated with anti-GST antibody followed by immunoblotting with MAb 5E10 (Fig. 4). Figure 4, lane P (precipitate), indicates that the two proteins coprecipitated; lane S (supernatant) shows that there were only trace amounts of PML present in the supernatant after precipitation. PML mixed with Z protein alone also formed a complex that was precipitated with Z antibody and was detected with 5E10. This complex was precipitated by anti-PML and was detected with Z antibody (see below; Fig. 5C). GAPDH mixed with Z-GST did not form a complex, and GST alone did not interact with PML. Z-GST fusion formed a complex with PML (Fig. 4). The complex was precipitated with anti-GST antibody and was detected with 5E10.
FIG. 4.
Direct protein-protein interaction between PML and Z shown by coimmunoprecipitation. Z proteins from both LCMV and Lassa virus were studied. The respective Z-GST fusion proteins were mixed with the PML protein and coimmunoprecipitated with an anti-GST antibody as described in Materials and Methods. Samples were run on SDS-20% PAGE gels and then blotted onto PVDF membranes. The Western analysis was carried out with the enhanced chemiluminescence detection system (ECL; Amersham) and MAb 5E10 to detect any PML that was immunoprecipitated. S, supernatant; P, precipitate. Lane P of the autoradiograph indicates that PML coprecipitates with LCMV Z and Lassa virus Z fusion proteins. Lane S shows that supernatant after coimmunoprecipitation retains only trace amounts of PML.
FIG. 5.
Mapping the interaction between PML and Z. (A) Summary of the constructs used in these studies. The boxes indicate the various motifs found in PML; B1 and B2, the respective B boxes; nls, nuclear localization signal (see text). Lines indicate the deleted regions of PML. See Materials and Methods for details of constructs. (B) Summary of the coimmunoprecipitation (Co-IP) results with PML and Z. The Z protein and not Z-GST was used for these studies. + and −, PML and Z did and did not immunoprecipitate, respectively. Mutations are as described for panel A. WT, wild type. (C) The relevant autoradiographs used for the data in panel B. P, precipitate; S, supernatant; W, wash. Two washes were done for each immunoprecipitation. Experiments were carried out as described in Materials and Methods. (D) Specificity of the affinity-purified Z antibody. The S100 fraction of the cell lysate was immunoblotted and probed with the affinity-purified Z antibody. Molecular weight markers are shown. See text for further details.
The N terminus of PML is necessary for colocalization with Z.
Mapping experiments to identify regions of PML that bind Z protein were performed. Deletion constructs of pLINK-pml, were prepared, with lines indicating deleted regions (Fig. 5A). The effects of these mutations were ascertained by coimmunoprecipitation studies with mixtures of the Z protein and the translation product of the appropriate PML construct. Subsequent immunoblots were probed with Z antibody (Fig. 5B). Figure 5C shows the relevant autoradiographs. Only the trip construct eliminated coprecipitation. Deleting the rest of PML but leaving the N terminus intact (ΔCterm) confirmed that the N-terminal region was sufficient for binding. The interaction site was between amino acids 5 and 50 of PML, a region with high proline content (∼36%). Interestingly, neither RING nor B1 or B2 B box was involved in binding, and Z protein did not bind the nuclear localization signal region in vitro. The results from in vivo immunolocalization mapping studies with mutant forms of PML and wild-type Z support the coimmunoprecipitation results (see below).
Figure 5D shows that the affinity-purified Z antibody used in Fig. 5C was specific for Z. The S100 fraction of a cell lysate from Z gene-transfected NIH 3T3 cells was subjected to gel electrophoresis and immunoblotted. The gel was probed with the Z antibody, and a band corresponding to the expected size for the Z protein was observed. In separate experiments, the corresponding band was cut out and N-terminally sequenced to confirm that it corresponded to Z. There was no staining in cells treated similarly but not transfected with the Z gene. Preincubation of purified Z protein with the Z antibody decreased the observed signal, whereas preincubation with bacterial lysate not expressing the Z protein did not alter the signal. The majority of Z was found in the S100 (polysomal) fraction of the transfected cells (6a), in agreement with the finding that the majority of Z is found in this fraction in infected cells (37).
The Z protein changes the diffuse nuclear phenotype observed with PML mutants.
Some mutations in the PML protein disrupt nuclear body formation and cause a diffuse nuclear pattern: double-point mutations of zinc-binding residues in the RING finger (6), double-point mutations in any of the metal-ligating residues in either the B1 or the B2 B box (7), and deletion of the leucine coiled-coil region (23). Arrows indicate the positions of the double-point mutations in Fig. 5A. The ΔBssHII construct has a deletion of the coiled-coil (Fig. 5A). All of these mutations destroy the structure of the given domain without affecting the ability of PML to bind Z (Fig. 5). Double-point mutants were preferable to deletion mutants such as trip, ΔCterm, and Δpml that had no nuclear localization signal (Fig. 5A).
In cotransfection studies of the Z gene with PML mutants (the diffuse nuclear RING and B-box point mutations and leucine coiled-coil deletion mutants), PML bodies were present in both the cytoplasm and the nucleus. There was no evidence of diffuse nuclear staining in any of the cells. Figure 6 is an example of PML and Z transfected into NIH 3T3 cells and stained only with the PML polyclonal antibody to monitor the appearance of endogenous and transfected PML. In Fig. 6A, a RING mutant of PML was transfected with Z, and in Fig. 6B, a B1 B-box mutant was transfected with Z. In both cases, the pattern of PML expression was like that observed when wild-type PML and Z were cotransfected (Fig. 2D and E). In Fig. 6A and B, there are also untransfected cells. The transfected cell in panel A reveals dense nuclear staining with additional cytoplasmic staining (similar to that in Fig. 2D). An untransfected cell is shown in the upper right hand corner of the micrograph. Figure 6B, in which two transfected cells with predominantly cytoplasmic punctate staining are seen, is similar to Fig. 2D. Three untransfected cells are presented for comparison, two in the lower left corner and one in the upper left corner, all displaying the normal nuclear punctate pattern. Identical results were observed for the coilless mutant and mutations in the B2 B box (data not shown). In all cases, double-staining experiments indicated that PML and Z colocalized as they did in the wild-type transfection experiment (Fig. 3), a result consistent with our coimmunoprecipitation results (Fig. 5).
FIG. 6.
PML mutants cotransfected with LCMV Z gene. Mutants stained with the PML polyclonal antibody with FITC secondary antibody. Images were collected by confocal laser microscopy. (A) PML RING mutant cotransfected with Z; (B) PML B1 B box mutant cotransfected with Z. See Materials and Methods for description of mutations. Magnification, ×40 with zooms of 1.7 (A) and 2.8 (B).
Similar experiments were carried out with a double-point mutation in metal-binding residues of the Z protein RING domain. In this case, mutant Z was transfected into NIH 3T3 cells and appeared similar in distribution to wild-type Z (as seen in Fig. 2A), accumulating in the cytoplasm adjacent to the nucleus (data not shown). In cotransfection studies, the mutant Z protein and wild-type PML still colocalized (Fig. 7). As seen by the phase picture (Fig. 7D), PML-Z bodies were again in both the cytoplasm and the nucleus.
FIG. 7.
Cotransfection of wild-type PML and the Z gene with a double-point mutation in the RING region. The cells were stained with affinity-purified anti-Z antibody with an FITC secondary (A) or with the PML MAb 5E10 with a Texas Red secondary antibody (B). (C) Overlay showing colocalization in yellow; (D) the phase view and fluorescence overlay. Images were collected on a confocal laser microscope exciting each channel independently. Magnification, ×40 with a zoom of 1.9.
We cotransfected the PML RING and Z RING mutants (Fig. 8). Although the PML-Z bodies still form, they are no longer in the nucleus. The two mutant proteins still colocalize (Fig. 8) as one would expect from our mapping studies (Fig. 5). Thus, it appears that at least one of the proteins must have an intact RING domain in order to form nuclear bodies but not cytoplasmic ones. These immunolocalization studies are consistent with the in vitro coimmunoprecipitation studies (Fig. 5).
FIG. 8.
Effects of transient transfection when both PML and Z have mutations in the RING domain. Cells were stained with the PML MAb 5E10 with Texas Red (A) or the affinity-purified Z antibody with an FITC secondary antibody (B). See Materials and Methods for description of mutants. Images were collected on a confocal laser microscope by exciting each channel independently. Magnification, ×100.
DISCUSSION
LCMV infection or transfection with the LCMV Z gene alone alters the appearance of PML from punctate nuclear to punctate nuclear-cytoplasmic. Endogenous murine and transfected human PML proteins are highly homologous (15) and are affected similarly. PML distribution in the presence of Z varies from mostly cytoplasmic to cytoplasmic and nuclear (Fig. 2D to F) and may reflect the fact that cells are at different stages in the cell cycle during transfection. Colocalization of PML and Z by indirect immunofluorescence microscopy is corroborated by coimmunoprecipitation studies in vitro. Antibodies to either PML or Z could precipitate both recombinant Z and PML, respectively, from a mixture of the two (Fig. 4 and 5), while no precipitation was seen with negative controls. The exact region of PML protein necessary for binding Z was determined by deletion mapping to be within the first 50 amino acids of PML.
Point mutations in the PML RING and B boxes and deletions in the PML coiled-coil region do not affect the ability of PML to homodimerize or to bind Z but do affect the intracellular distribution of PML (6, 23) (Fig. 5 to 7). When these PML mutants were transfected into NIH 3T3 cells and stained with PML antibody, a diffuse nuclear pattern rather than a punctate phenotype was observed. Cotransfection with the LCMV Z gene converted the diffuse phenotype to the nuclear-cytoplasmic punctate pattern normally seen in LCMV-infected and Z-transfected cells (Fig. 6 and 8).
Z protein RING finger mutations did not affect its normal cytoplasmic distribution or its ability to colocalize with PML or to convert the diffuse phenotype of PML mutants to a punctate one (Fig. 7). Even with cotransfection of mutations in both the PML RING and the Z RING, PML and Z still colocalized and the Z mutant converted the nuclear diffuse phenotype of the PML RING mutant to a punctate one (Fig. 8). However, Z RING mutations did not prevent the appearance of PML-Z bodies in the nucleus. Thus, we propose that Z and PML bind each other outside the RING finger domains and that the RING domains have a common docking site in the nucleus that facilitates their colocalization even when the RING domain of one (either PML or Z) is altered.
Our proposal of a common RING-docking site in the nucleus is complicated by the fact that several RING proteins are predominantly cytoplasmic (5, 39). Thus, there may be more than one RING-docking site involved in the distribution of RING proteins or more than one feature of the protein responsible for distribution. Several other RING proteins are known to form macromolecular assemblages, including BRCA1 and transforming factor 18, and neither assemblage colocalizes with PML (5, 39). Previous data suggest that the RING and B-box domains of PML are involved in macromolecular assembly and that this may be common to RING and B-box proteins (7). Since so many proteins contain these domains, selectivity of targeting would come from other contextual cues, e.g., the presence of other domains and the precise sequence of the given RING domain.
The 11-kDa Z proteins of several arenaviruses have been sequenced: Lassa virus, Tacaribe virus, Pichinde virus, and LCMV. Since the RING domains of the Z proteins are highly conserved (9), it is likely that they will all have similar effects on PML nuclear bodies. PML is prominently expressed in reticuloendothelial cells (12), the most frequent targets of arenavirus infection (36). The fact that arenaviruses require the cell nucleus for replication even though they replicate in the cytoplasm (2) makes PML a candidate for the nuclear component. PML distribution to cytoplasm is often an antiapoptotic event (23, 35), suggesting a simple mechanism to explain the noncytopathic nature of arenaviruses. The arenaviruses may have acquired the Z RING finger to act as a molecular mimic of the PML RING in order to commandeer the host cell machinery needed to replicate. The direct interaction between PML and Z could also be essential for the virulence of these viruses and, as such, could present a novel drug target for arenavirus infection. In this regard, LCMV virulence in the guinea pig has been mapped to the RNA segment coding for the Z protein, but this segment also encodes the virus polymerase which could mediate virulence (34). Further studies of the interaction of Z protein with PML should provide valuable insights into both arenavirus diseases and APL.
ACKNOWLEDGMENTS
We thank Lynne Maillet-Frotten for assistance with confocal microscopy. We are very grateful for the PML polyclonal antibody from K. Howe, M. N. Boddy, P. Freemont, and E. Solomon and for the PML MAb 5E10 from L. de Jong. We are indebted to M. Djavani for the kind gift of the Z (Lassa virus)-GST construct. We thank L. Etkin and P. Freemont for helpful discussions and C. D. Pauza, G. W. Carlile, M. Dobson, and I. Lukashevich for critical comments on the manuscript.
K.L.B.B. acknowledges financial support from MRC (Canada) MT-13608 and the Imperial Cancer Research Fund, London, United Kingdom, and M.S.S. acknowledges support from NIH (grants R01 AI32107 and R29 AI25522).
REFERENCES
- 1.Ascoli C A, Maul G G. Identification of a novel nuclear domain. J Cell Biol. 1991;112:785–795. doi: 10.1083/jcb.112.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee S N, Buchmeier M, Rawls W E. Requirement of a cell nucleus for the replication of an arenavirus. Intervirology. 1976;6:190–196. doi: 10.1159/000149472. [DOI] [PubMed] [Google Scholar]
- 3.Barlow P N, Luisi B, Milner A, Elliot M, Everett R. Structure of the C3HC4 domain by 1H-nuclear magnetic resonance spectroscopy. J Mol Biol. 1994;237:201–211. doi: 10.1006/jmbi.1994.1222. [DOI] [PubMed] [Google Scholar]
- 4.Boddy M N, Howe K, Etkin L D, Solomon E, Freemont P S. PIC1, a novel ubiquitin-like protein which interacts with the PML component of a multiprotein complex that is disrupted in acute promyelocytic leukaemia. Oncogene. 1996;13:971–982. [PubMed] [Google Scholar]
- 5.Borden K L B, Freemont P S. The RING finger: an example of a sequence structure family. Curr Opin Struc Biol. 1996;6:395–401. doi: 10.1016/s0959-440x(96)80060-1. [DOI] [PubMed] [Google Scholar]
- 5a.Borden, K. L. B. Unpublished observations.
- 6.Borden K L B, Boddy M N, Lally J, O’Reilly N J, Martin S, Howe K, Solomon E, Freemont P S. The solution structure of the RING finger domain from the acute promyelocytic leukaemia proto-oncoprotein, pml. EMBO J. 1995;14:1532–1541. doi: 10.1002/j.1460-2075.1995.tb07139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Borden, K. L. B., E. J. Campbell-Dwyer, and M. S. Salvato. Unpublished data.
- 7.Borden K L B, Lally J M, Martin S R, O’Reilly N J, Solomon E, Freemont P S. In vivo and in vitro characterisation of the B1 and B2 zinc-binding domains from the acute promyelocytic leukemia protein PML. Proc Natl Acad Sci USA. 1996;93:1601–1606. doi: 10.1073/pnas.93.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalton S, Treisman R. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell. 1992;68:597–612. doi: 10.1016/0092-8674(92)90194-h. [DOI] [PubMed] [Google Scholar]
- 8a.de The H C, Lavau C A, Marchio C, Chrornr L, Degos L, Dejean A. The PML-RARα fusion mRNA generated by t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991;66:675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- 9.Djavani M, Lukashevich I S, Sanchez A, Nichol S T, Salvato M S. Completion of the Lassa fever virus sequence and identification of a RING finger open reading frame at the L RNA 5′ end. Virology. 1997;235:414–418. doi: 10.1006/viro.1997.8722. [DOI] [PubMed] [Google Scholar]
- 10.Doucas V, Ishov A M, Romo A, Juguilon J, Weitzman M, Evans R M, Maul G G. Adenovirus replication is coupled with the dynamic properties of the PML nuclear structure. Genes Dev. 1996;10:196–207. doi: 10.1101/gad.10.2.196. [DOI] [PubMed] [Google Scholar]
- 11.Dyck J A, Maul G G, Miller W H, Chen D J, Kakizuka A, Evans R M. A novel macromolecular structure is a target of the promyelocyte-retinoic acid receptor oncoprotein. Cell. 1994;76:333–343. doi: 10.1016/0092-8674(94)90340-9. [DOI] [PubMed] [Google Scholar]
- 12.Flenghi L, Fagioli M, Tomassoni L, Pileri S, Gambacorta M, Pacini R, Grignani F, Casini T, Ferrucci P F, Martelli M F, Pelicci P G, Falini B. Characterization of a new monoclonal antibody (PG-M3) directed against the aminoterminal portion of the PML gene product: immuncytochemical evidence for high expression of PML proteins on activated macrophages, endothelial cells and epithelia. Blood. 1995;85:1871–1880. [PubMed] [Google Scholar]
- 13.Garcin D, Rochat S, Kolakofsky D. The Tacaribe arenavirus small zinc finger protein is required for initiation of Tacaribe genome replication. J Virol. 1993;67:807–812. doi: 10.1128/jvi.67.2.807-812.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goddard A D, Borrow J, Freemont P S, Solomon E. Characterization of a zinc finger gene disrupted by the t(15:17) in acute promyelocytic leukemia. Science. 1991;254:1371–1373. doi: 10.1126/science.1720570. [DOI] [PubMed] [Google Scholar]
- 15.Goddard A D, Yuan J Q, Fairbairn L, Dexter D M, Borrow J, Kozak C, Solomon E. Cloning of the murine homologue of the leukemia associated PML gene. Mamm Genome. 1995;6:732–737. doi: 10.1007/BF00354296. [DOI] [PubMed] [Google Scholar]
- 16.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1988. [Google Scholar]
- 17.Ishov A M, Maul G G. The periphery of nuclear domain 10 (ND10) as site of DNA virus deposition. J Cell Biol. 1996;134:815–826. doi: 10.1083/jcb.134.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kakizuka A, Miller W, Umensono K, Warrell R, Frankel S R, Murty V V, Dmitrovsky E, Evans R M. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RARa with a novel putative transcription factor PML. Cell. 1991;66:663–674. doi: 10.1016/0092-8674(91)90112-c. [DOI] [PubMed] [Google Scholar]
- 19.Kastner P, Perez A, Lutz Y, Rochette-Egly C, Gaub B, Durnad M, Lanotte M, Berger R, Chambon P. Structure, localization and transcriptional properties of two classes of retinoic acid receptor alpha fusion proteins in acute promyelocytic leukemia (APL): structural similarities with a new family of oncoproteins. EMBO J. 1992;11:629–642. doi: 10.1002/j.1460-2075.1992.tb05095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly C, Van Driel R, Wilkinson G W G. Disruption of PML-associated nuclear bodies during human cytomegalovirus infection. J Gen Virol. 1995;76:2887–2893. doi: 10.1099/0022-1317-76-11-2887. [DOI] [PubMed] [Google Scholar]
- 21.Koken M H M, Puvion-Dutilleul F, Guillemin M C, Viron V, Linares-Cruz G, Stuurman N, de Jong L, Szostecki C, Calvo F, Chomienne C, de The H. The t(15;17) retranslocation alters a nuclear body in a retinoic acid reversible fashion. EMBO J. 1994;13:1073–1083. doi: 10.1002/j.1460-2075.1994.tb06356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korioth F, Gieffers C, Maul G G, Frey J. Molecular characterization of NDP52, a novel protein of the nuclear domain 10, which is redistributed upon virus infection and interferon treatment. J Cell Biol. 1995;130:1–13. doi: 10.1083/jcb.130.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le X F, Yang P, Chang K S. Analysis of the growth and transformation suppressor domains of promyelocytic leukemia gene PML. J Biol Chem. 1996;271:130–135. doi: 10.1074/jbc.271.1.130. [DOI] [PubMed] [Google Scholar]
- 24.Maul G G, Ishov A M, Everett R D. Nuclear domain 10 as preexisting potential replication start sites of herpes simplex virus type-1. Virology. 1996;217:67–75. doi: 10.1006/viro.1996.0094. [DOI] [PubMed] [Google Scholar]
- 25.Maul G G, Yu E, Ishov A M, Epstein A L. Nuclear domain 10 (ND10) associated proteins are present in nuclear bodies and redistribute to hundreds of nuclear sites after stress. J Cell Biochem. 1995;59:499–514. doi: 10.1002/jcb.240590410. [DOI] [PubMed] [Google Scholar]
- 26.Maul G G, Guldner H H, Spivack J G. Modification of discrete nuclear domains induced by herpes simplex virus type-1 immediate early gene 1 product (ICP0) J Gen Virol. 1993;74:2679–2690. doi: 10.1099/0022-1317-74-12-2679. [DOI] [PubMed] [Google Scholar]
- 27.Maul G G, Everett R D. The nuclear location of PML, a cellular member of the C3HC4 zinc-binding domain protein family, is rearranged during herpes simplex virus infection by the C3HC4 viral protein ICP0. J Gen Virol. 1994;75:1223–1233. doi: 10.1099/0022-1317-75-6-1223. [DOI] [PubMed] [Google Scholar]
- 28.Montali R J, Connolly B M, Armstrong D L, Scanga C A, Holmes K V. Pathology and immunohistochemistry of callitrichid hepatitis, an emerging disease of captive new world primates caused by lymphocytic choriomeningitis virus. Am J Pathol. 1995;148:1441–1449. [PMC free article] [PubMed] [Google Scholar]
- 29.Mullis K, Faloona F, Scharf S, Saiki R, Horn G, Erlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harbor Symp Quant Biol. 1986;51:263–273. doi: 10.1101/sqb.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- 30.Pandolfi P P, Grignani F, Alcalay M, Mencarelli A, Biondi A, LoCoco F, Grignani F, Pellicci P G. Structure and origin of the acute promyelocytic leukemia myl/RARa cDNA and characterization of its retinoid-binding and transactivation properties. Oncogene. 1991;6:1285–1292. [PubMed] [Google Scholar]
- 31.Peters C J. Viral hemorrhagic fevers. In: Nathanson N, editor. Viral pathogenesis. Philadelphia, Pa: Lippincott-Raven Publishers; 1995. pp. 779–799. [Google Scholar]
- 32.Puvion-Dutilleul F, Chelbi-Alix M K, Koken M, Quignon F, Puvion E, de The H. Adenovirus infection induces rearrangements in the intranuclear distribution of the nuclear body associated PML protein. Exp Cell Res. 1995;218:9–16. doi: 10.1006/excr.1995.1125. [DOI] [PubMed] [Google Scholar]
- 33.Reddy B A, Etkin L D, Freemont P S. A novel zinc finger coiled-coil domain in a family of nuclear proteins. Trends Biochem Sci. 1992;17:344–345. doi: 10.1016/0968-0004(92)90308-v. [DOI] [PubMed] [Google Scholar]
- 34.Riviere Y. Mapping arenavirus gene causing virulence. Curr Top Microbiol Immunol. 1986;133:59–66. doi: 10.1007/978-3-642-71683-6_5. [DOI] [PubMed] [Google Scholar]
- 35.Rogaia D, Grignani F, Grignani F, Nicoletti I, Pelicci P G. The acute promyelocytic leukemia specific PML/RARa fusion protein reduces the frequency of commitment to apoptosis upon growth factor deprivation of GM-CSF-dependent myeloid cells. Leukemia. 1995;9:1467–1472. [PubMed] [Google Scholar]
- 35a.Salvato, M. S. Unpublished observations.
- 36.Salvato M S, Rai K S. Arenaviruses. In: Collier L H, Mahy B W J, editors. Topley and Wilson’s microbiology and microbial infec tions. 9th ed. London, United Kingdom: Arnold Publishing; 1997. [Google Scholar]
- 37.Salvato M S, Schweighofer K J, Burns J, Shimomaye E M. Biochemical and immunological evidence that the 11 kDa zinc-binding protein of lymphocytic choriomeningitis virus is a structural component of the virus. Virus Res. 1992;22:185–198. doi: 10.1016/0168-1702(92)90050-j. [DOI] [PubMed] [Google Scholar]
- 38.Salvato M S, Shimomaye E M. The completed sequence of lymophocytic choriomeningitis virus reveals a unique RNA structure and a gene for a zinc finger protein. Virology. 1989;173:1–10. doi: 10.1016/0042-6822(89)90216-x. [DOI] [PubMed] [Google Scholar]
- 39.Saurin A J, Borden K L B, Boddy M N, Freemont P S. Does this have a familiar RING? Trends in Biochem Sci. 1996;246:208–213. [PubMed] [Google Scholar]
- 40.Smith D B, Johnson K S. Single step purification of polypeptides expressed in Escherichia coli as fusions with glutathione transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 41.Stuurman N, De Graaf A, Floore A, Josso A, Humbel B, De Jong L, Van Driel R. A monoclonal antibody recognizing nuclear matrix associated nuclear bodies. J Cell Sci. 1992;101:773–784. doi: 10.1242/jcs.101.4.773. [DOI] [PubMed] [Google Scholar]
- 42.Szekely L, Pokrovskaja K, Jiang W-A, de The H, Ringertz N, Klein G. The Epstein-Barr virus-encoded nuclear antigen EBNA-5 accumulates in PML-containing bodies. J Virol. 1996;70:2562–2568. doi: 10.1128/jvi.70.4.2562-2568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szostecki J, Guldner H H, Netter H J, Will H. Isolation and characterization of cDNA encoding a human nuclear antigen predominantly recognized by autoantibodies from patients with primary biliary cirrhosis. J Immunol. 1990;145:4338–4347. [PubMed] [Google Scholar]
- 44.Tallman M S, Kwann H C. Reassessing the hemostatic disorder associated with acute promyelocytic leukemia. Blood. 1992;79:543–553. [PubMed] [Google Scholar]
- 45.Terris B, Baldin V, Dubois S, Degott C, Flejou J-F, Henin D, Dejean A. PML nuclear bodies are general targets for inflammation and cell proliferation. Cancer Res. 1995;55:1590–1597. [PubMed] [Google Scholar]
- 46.Weiss K, Rambaud S, Lavau C, Jansen J, Carvalho T, Carmo-Foneseca M, Lamond A, Dejean A. Retinoic acid regulates aberrant nuclear localization of PML-RARα in acute promyelocytic leukemia cells. Cell. 1994;76:345–356. doi: 10.1016/0092-8674(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 47.Zhu Z, Cai W, Schaffer P A. Cooperativity among herpes simplex virus type 1 immediate-early regulatory proteins: ICP4 and ICP27 affect the intracellular localization of ICP0. J Virol. 1994;68:3027–3040. doi: 10.1128/jvi.68.5.3027-3040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]