Abstract
Objective
Studies focusing on bone and joint infections (BJIs) in young infants are rare. Some cases of BJI are accompanied by sepsis. This study aimed to identify the clinical and bacteriological features of sepsis in neonates and young infants with BJIs.
Methods
Neonates and infants younger than 3 months diagnosed with BJI in the present institution from 2014 to 2021 were retrospectively reviewed. Patient characteristics, clinical data, and outcomes were documented and compared between those with and without sepsis.
Results
Twenty-five patients with a mean age of 34.8 days were included. Nine BJI cases had concomitant sepsis (group A), and 16 had BJI without sepsis (group B). Within group A, staphylococcus aureus was the major pathogenic germ (5 cases, of which 4 were of the methicillin-resistant staphylococcus aureus (MRSA) type). There was no statistical difference in male-to-female ratio, age, history of hospitalization, anemia, birth asphyxia, peripheral leukocyte counts, C-reactive protein on admission, and sequelae between groups. Univariate analyses indicated a significant difference in the incidence of septic arthritis (SA) combined with osteomyelitis (OM) (88.9% vs 37.5%), congenital deformities (44.4% vs 0%), and mean duration of symptoms (2.83 days vs 9.21 days) in comparisons between groups A and B.
Conclusion
Staphylococcus aureus is the main pathogenic bacteria in BJI cases complicated with sepsis in neonates and young infants. Among infants younger than 3 months diagnosed with BJI, those with concurrent SA and OM, MRSA infection, or congenital deformities are more likely to develop sepsis.
Keywords: Neonate, Septic arthritis, Osteomyelitis, Sepsis
Introduction
Bone and joint infections (BJIs) are uncommon disorders in children and can be clinically classified as osteomyelitis (OM) or septic arthritis (SA). Acute OM is more likely concurrent with SA in young infants and newborns.1 The most common etiology of pediatric BJIs is hematogenous spread from concomitant bacteremia.2 Staphylococcus aureus and Streptococcus agalactiae were the most common pathogens underlying BJIs in young infants and newborns.2,3 Previous studies indicated that 9–11༅ of children with SA and 17.6༅ of those with acute OM developed sepsis,3,4 with co-occurrence of sepsis being more common in infants younger than 3 months than in older children.5 To date, few studies had focused on neonates and young infants with BJI accompanied by sepsis. Sepsis accounts for 6.8༅ of neonatal deaths and is an important cause of neurocognitive sequelae.6,7 The aim of this retrospective study was to identify the clinical and bacteriological features and factors associated with sepsis in neonates and infants younger than 3 months diagnosed with BJIs.
Methods
Study site
This retrospective study was carried out from January 2014 to January 2021 at the Children's Hospital of Soochow University, a tertiary teaching hospital in East China. The study was approved by the Institutional Ethics Committee, and all procedures were in accordance with the ethical standards.
Patients
The clinical data of neonates and infants younger than 3 months with acute BJIs who were hospitalized in the neonatal intensive care unit (NICU), neonatology department and orthopedic department were collected. All patient records were reviewed by two orthopedic surgeons and a neonatologist to confirm the diagnoses of OM, SA and sepsis. Exclusion criteria included immune compromise, malignancy, brachial plexus injuries, fractures, bone syphilis and mycobacterium tuberculosis arthritis. The enrolled patients with BJI were divided according to whether they also had sepsis (group A) or not (group B).
The diagnosis of BJI was based on medical files, imaging and clinical features. The inclusion criteria were: 1) clinical symptoms: joint or limb pain, limited range of motion, pseudo paralysis, edema and erythema, warmth, tenderness; 2) characteristic changes revealed by radiologic or ultrasound examination; 3) positive bone or joint cultures; 4) aspiration of pus from bone or joint; 5) serologic data, specifically white blood cell (WBC) counts, C-reactive protein (CRP) levels, ESR and blood culture. The criterion 1 above was the necessary prerequisite, and at least two additional criteria had to be met.7
Sepsis is a clinical syndrome characterized by a potentially fatal organ dysfunction due to systemic infection.8 Isolation of a pathogen from peripheral blood or cerebrospinal fluid is the standard diagnostic criterion for sepsis in children. If organism culture is negative, systemic sepsis is recognized based on: 1) whole-body toxicity symptoms, including abnormal core temperature, chills, apnea, food intolerance, lethargy, bradycardia, and severe respiratory distress7,9; and 2) symptoms of more than two systems of systemic inflammation caused by infection, such as abnormalities in the circulatory system and liver and kidney function.7
Patient data collected included: age, sex, birth weight, history of hospitalization, history of pregnancy and delivery, symptoms duration, core temperature on admission, extremity showing evidence consistent with BJI, serum WBC count, CRP level, accompanied infection, pathogen culture results, magnetic resonance diffusion tension imaging (MRI), radiographs (X-rays, computed tomography), methods of treatment, clinical outcomes and sequelae.
Statistical analysis
Categorical variables were expressed as proportions, and continuous variables were expressed as means. For data analysis and comparisons, univariate analysis with ordinal logistic regression, χ2, Fisher exact, and Wilcoxon rank-sum test were used. Mann-Whitney tests were used for data that were not normally distributed .IBM SPSS Version 25.0 (IBM Corp, Armonk, NY) was utilized for statistical analyses. 4A P-value ≤ 0.05 was considered significant.
Results
The incidence rate of BJI diagnosis in neonates was in the institution about 1.5 cases per 1000 admissions. During the study period, a total of 30 patients with BJI younger than 3 months were admitted. Of these 30 cases, clinical data were incomplete in 3 cases, and 2 cases had fatal chromosome-related malformations. Therefore, 25 cases were included in the study (Table 1). For this cohort of 25 cases, the male-to-female ratio was 0.79, the mean age was 34.8 days (range 10–87 days). The mean duration of symptoms at presentation was 6.7 days (range 0.5–30 days). Pseudoparalysis was the most common symptom (23 cases), followed by pain during movements (20 cases). The infection site was localized in the lower limbs in 9 cases, upper extremities in 14 cases, and in multiple sites in 2 cases. Hip (7 cases) and shoulder (7 cases) were the most common sites for SA, humerus (7 cases) and femur (8 cases) were the most common sites for OM (6 patients suffered from OM, 5 from SA and 14 from both).
Table 1.
Baseline patient demographics.
| Variables | Bone and Joint Infections (N = 25) |
|---|---|
| Mean Age, day (range) | 34.8 (10–87) |
| Sex (male: female) | 11:14 |
| Mean duration of symptoms, days (range) | 6.7 (0.5–30) |
| Main Symptoms (cases) | |
| Pseudoparalysis | 23 |
| pain | 20 |
| irritability | 17 |
| fever | 7 |
| edema | 5 |
| Involved site (cases) | |
| Femur | 8 |
| Hip | 7 |
| Humerus | 7 |
| Shoulder | 7 |
| Knee | 4 |
| Tibia | 1 |
| Type of BJIs (cases) | |
| SA | 5 |
| OM | 6 |
| SA combined with OM | 14 |
| Positive blood/ CSF culture (cases) | 8 |
| Positive pus culture (cases) | 4 |
BJIs, bone and joint infections; SA, septic arthritis; OM, osteomyelitis; CSF, Cerebrospinal Fluid.
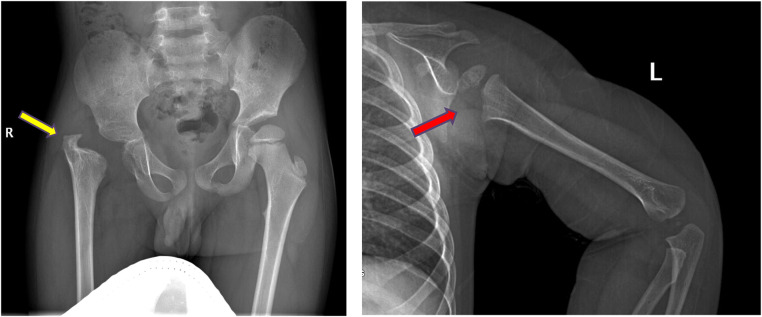
Skeletal sequelae were detected in 7 cases, of whom 6 had both SA and OM. Hip (3 cases) and shoulder (3 cases) were the main affected sites (Supplemental Material – Figure 1). Pathogen culture showed staphylococcus aureus infection in 5 cases (Table 2).
Figure 1.
Sequelae of hip and shoulder in the follow-up. a, AP radiograph of the pelvis demonstrating severe deformity of the hip, including complete loss of femoral head and neck with no articulation of the hip, limb-length discrepancy and acetabular dysplasia (yellow arrow). b, Lateral-view radiograph of humerus showing the epiphysis of the proximal humerus are partially absent (red arrow).
Table 2.
Patients with sequelae in the follow-up.
| Cases | Age (days) | Sex (f / m) |
Diagnosis | Concurrent sepsis (y/ n) | Blood culture | Pathogen isolation from bone and joint | Sequelae |
|---|---|---|---|---|---|---|---|
| 1 | 30 | m | Septic shoulder, Osteomyelitis of humerus |
Y | MRSA | – | Limit ROM of shoulder |
| 2 | 61 | f | Septic hip, Osteomyelitis of femur and humerus |
Y | MRSA | MRSA | Hip dislocation, discrepancy of femur, postencephalitis |
| 3 | 49 | f | Septic shoulder, Osteomyelitis of humerus |
Y | MSSA | – | Humerus shortening |
| 4 | 26 | m | Septic knee, Osteomyelitis of femur |
N | – | – | Discrepancy of femur |
| 5 | 25 | m | Septic hip, Osteomyelitis of femur |
N | – | – | Hip dislocation, discrepancy |
| 6 | 21 | f | Septic shoulder, Osteomyelitis of humerus |
N | – | MSSA | Limit ROM of shoulder, Hhumerus shortening |
| 7 | 20 | f | Septic hip, Osteomyelitis of femur |
N | – | MSSA | Hip dislocation, discrepancy |
ROM, range of motion; MRSA, Methicillin-resistant Staphylococcus aureus; MSSA, Methicillin-sensitive Staphylococcus aureus.
Nine patients who had BJI with sepsis were assigned to group A (Table 3), and sixteen patients without sepsis were assigned to group B. In group A, 8/9 cases had positive blood cultures; of those 2 also had positive cerebrospinal fluid (CSF) cultures. In this group, staphylococcus aureus accounted for the largest portion of positive cultures (5 cases), followed by klebsiella pneumoniae (2 cases) and bacillus paratyphosus (1 case). Of the 5 cases with sepsis associated with staphylococcus aureus, MRSA was isolated in 4 cases. Early-onset neonatal sepsis, which is defined as an infection appearing at less than 7 days of age,7 was identified in one patient. Other concomitant infections included pneumonia (4 cases), enteritis (1 case), cephalitis (3 cases), and endocarditis (2 cases). In group B, synovial fluid culture showed 2 cases of methicillin-sensitive staphylococcus aureus infection. There were 4 cases of congenital deformities in group A, including atrial septal defect (ASD, 2 cases), patent ductus arteriosus (PDA, 1 case), and subependymal cyst (1 case).
Table 3.
Patients’ data in Group A (with sepsis).
| Case | Age (days) |
Sex | Congenital malformation | Infection site | General Symptoms | WBC counts (x10^9) |
CRP (mg/L) |
Organism in Blood culture | Concurrent systemic infection | Open drainage |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | M | PDA | Knee, femur | Fever | 18.73 | 50.01 | MRSA | / | No |
| 2 | 32 | F | / | Shoulder, humerus | Irritability, polypnea | 29.01 | 188.02 | MRSA | Pneumonia, cerebritis | No |
| 3 | 27 | F | Subependymal cyst | Elbow, humerus, knee | Polycardia | 20.93 | 17.25 | / | myocarditis | No |
| 4 | 61 | F | ASD | Hip, femur, humerus | Poor feeding, lethargy, bradycardia | 34.51 | 185.4 | MRSA | Myocarditis, Pneumonia, cerebritis | yes |
| 5 | 25 | M | ASD | Shoulder, humerus | Fever, polypnea | 12.29 | 72 | MRSA | Pneumonia, bronchitis | yes |
| 6 | 27 | F | / | Knee, femur | Lethargy, Diarrhea, polypnea |
23.79 | 96.9 | Klebsiella pneumoniae | Pneumonia, cerebritis, enteritis | No |
| 7 | 23 | F | / | Shoulder, humerus | Fever | 16.62 | 30.6 | Bacillus paratyphosus | / | No |
| 8 | 16 | F | / | Shoulder, humerus | Fever | 18.52 | 11.1 | MSSA | / | yes |
| 9 | 19 | M | / | Femur | Fever | 11.6 | 65.42 | Klebsiella pneumoniae | / | No |
PDA, patent ductus arteriosus; ASD, atrial septal defect.
There were no statistically significant differences in the location of the affected site, male-to-female ratio, age on admission, history of birth asphyxia, birth weight, prematurity, cesarean section, previous hospitalization, peripheral leukocyte counts on admission, CRP, ratio of anemia, the proportion of patients with sequelae between groups A and B (Table 4). Univariate analysis revealed significant differences in the ratio of SA combined with OM (88.9% vs 37.5%, p=0.017), concomitant congenital deformity (44.4% vs 0%, p = 0.01), and duration of symptoms (2.83 vs 9.21, p = 0.027) between groups A and B. The parameters used as the diagnostic criteria for sepsis, including core temperature and positive blood culture, were excluded from this analysis.
Table 4.
Comparison of patient characteristics between groups.
| Parameters | The group A (9 cases) |
The group B (16 cases) |
P value |
|---|---|---|---|
| Mean age (days) | 34 | 37 | 0.894 |
| Sex (male/ female) | 3/6 | 8/8 | 0.352 |
| Preterm birth (cases) | 3 | 1 | 0.116 |
| Cesarean section (cases) | 5 | 7 | 0.44 |
| Low birth weight (cases) | 4 | 3 | 0.181 |
| Multiple pregnancies (cases) | 2 | 0 | 0.12 |
| Birth asphyxia (cases) | 0 | 1 | 0.64 |
| Associated malformation (cases) | 4 | 0 | 0.01 |
| Anemia (cases) | 7 | 11 | 0.648 |
| History of hospitalization (cases) | 3 | 4 | 0.499 |
| Peripheral leukocyte (×10^9/L) | 20.67 | 22.53 | 0.357 |
| CRP (mg/L) | 79.33 | 68.97 | 0.846 |
| SA combined with OM (cases) | 8 | 6 | 0.017 |
| Duration of symptoms (days) | 2.83 | 9.21 | 0.027 |
| Sequelae (cases) | 3 | 5 | 0.626 |
CRP, C-reactive protein; SA, septic arthritis; OM, osteomyelitis.
Discussion
Osteomyelitis (OM) and septic arthritis (SA) of neonates and young infants are uncommon but serious infectious diseases that may lead to potentially disastrous sequelae. Based on data provided by three Parisian Pediatric teaching hospitals, among the children diagnosed with BJI, infants younger than 3 months accounted for about 1.4%.2 Due to the venous channels that pierce the growth plate in neonates and infants, OM is likely to be concurrent with contiguous SA, the proportion could be 44–71%.10,11 In the studied hospital, the incidence of BJI in neonates was 1.5 cases per 1000 admissions, lower than 7/1000 reported in Argentina12 and in France.2 Among the 25 cases included in the present study, OM combined with SA occurred in 14 cases (56%). The femur was the most common site of infection within the entire group of 25 cases. However, the ratio of upper limb infections to lower limb infections was 1.5, and the male-to-female ratio was nearly 0.79, which was inconsistent with the literature reports that lower limbs and males predominate in infants with BJIs.10,13
The mortality related to BJI is low and is mainly associated with the presence of sepsis.12 Indeed, sepsis in neonates is reported to be one of the risk factors for the development of BJI and a predictor of its adverse sequelae.12,14 A previous study identified factors associated with concurrent systemic sepsis in adults with septic arthritis,15 but there have been no similar studies to our knowledge in neonates or young infants. This is the first study that focuses on the clinical and bacteriological features of sepsis in neonates and infants younger than 3 months diagnosed with BJIs.
The “gold standard” of diagnosis of sepsis in children is an isolation of the underlying pathogen from a normally sterile body site,16 although this criterion has also been questioned due to the low organism detection rate in bacterial cultures.7,9,16,17 The current definition of sepsis based on the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) is a life-threatening organ dysfunction caused by a dysregulated host response to infection.18 However, the Sepsis-3 definition is derived from data in adults in whom the measures significantly vary with age. So, opinions differ as to whether this definition is appropriate for children.19,20 In the present study, the diagnosis of sepsis was based not only on etiological evidence but also on clinical manifestations of systemic infection. There were 9 cases diagnosed as sepsis; of those, 8 had positive blood culture or CSF culture with clinical signs and 1 case had negative organism culture but multiple BJIs and a respiratory infection. When blood and other sterile site cultures are negative, the key signs of sepsis are derived from clinical and laboratory signs.17
In pediatric patients, the bacterial etiology of BJIs often varies with age. A retrospective multi-center study in France has proven that Streptococcus agalactiae is the most commonly observed pathogen in infants younger than 3 months diagnosed with BJIs 2. Another single-center study in Turkey indicated that Staphylococcus aureus was the predominant causative organism of SA in neonatal intensive care units. Among the patients with sepsis in the present study, Staphylococcus aureus was most common, and MRSA was detected in 4 out of 5 Staphylococcus aureus-positive cases. The frequency of pediatric OM and SA caused by MRSA has been increasing.21,22 The present data highlight the association between MRSA and sepsis in neonates and young infants with BJIs. In a study comparing the results of 4 infants treated for SA caused by MRSA with those of 5 non-MRSA cases, all 4 cases with MRSA had preceding sepsis and exhibited severe sequelae.23 From an observational study by Praveen, in a group of 25 pediatric patients with osteoarticular infections and generalized sepsis, the majority were the cases with MRSA infection indicated by positive blood culture (7/17) and aspirate culture (7/13).24
Previous studies have indicated risk factors of sepsis in neonates, including prematurity, low birth weight and cesarean delivery.7,25,26 Due to the immature skin barrier, underdeveloped immune regulatory systems, delayed start of breastfeeding, the risk of nosocomial transmission in prolonged hospitalization, less skin-to-skin contact, and frequent need for invasive procedures, infants with these risk factors are susceptible to infection at an increased rate.27,28 The risk could be exacerbated further due to the underlying disease of the mother during pregnancy. However, the present study found no significant difference in the rates of prematurity, low birth weight, and history of cesarean delivery between groups. When infants and neonates suffer from infections, some present with localized inflammation while others with systemic sepsis. Genetic polymorphisms in the immune response to infection have been shown to be associated with sepsis and bacterial osteomyelitis; this needs further research.29,30
It was previously reported that septic hip with concomitant OM in infants is associated with more severe hip deformity.31 The present study has come to a similar conclusion. According to these findings, young infants and neonates with concurrent OM and SA had a greater chance of developing sepsis. Of the 7 cases with skeletal sequelae during the follow-up, 6 had bone and joint co-infections. In a retrospective study of 453 children with infectious osteoarthritis, 103 had concurrent SA and OM. Generalized infection, longer hospital stays, more intensive care required, longer duration of bacteremia, and higher odds of treatment failure were especially common in children with concurrent SA and OM.32
The present study demonstrated that congenital deformities, especially congenital heart disease (CHD), were associated with sepsis in infants with BJIs. Although the basis for the relationship between CHD and sepsis is unclear, epidemiologic studies have proven that CHD is a risk factor for sepsis in children. In a study of 11,638 infants with CHD from 250 neonatal intensive care units (NICU), 656 (6%) had sepsis, with the coincidence being most common in infants with pulmonary stenosis (11%) and ASD (8%).33 In a retrospective study of 49,153 pediatric patients with severe sepsis, those with cardiac deformities were the largest group (27%).34
In the present study, the duration of symptoms before admission seems to be inversely proportional to the development of sepsis in young infants with BJIs. Neonates and infants with BJIs may present in an atypical fashion; they may not exhibit skeletal symptoms, fever or other signs of toxicity.35 Patients with sepsis in this study had a higher incidence of abnormal core temperature, food intolerance and lethargy, and often had other symptoms of systemic infection which prompted the parents to bring the child to the hospital. This is in contrast to infants with signs of local infection and only mild systemic symptoms whose thorough diagnosis is often delayed.
The present study has several limitations. First, due to the low incidence of BJIs and low absolute case numbers, the present findings may not be generalized. The sample size could not be determined a priori due to the retrospective nature of the study. However, randomized controlled trials whose goal is to identify the risk factors as in this study are not feasible. Second, given the retrospective nature of the present study, potential bias caused by confounding factors is possible which is inherent to observational studies.
Conclusion
Staphylococcus aureus is the main pathogenic bacteria detected in neonates and young infants with BJIs complicated with sepsis. Patients younger than 3 months diagnosed with BJI who also have septic arthritis with osteomyelitis, MRSA infection, or congenital deformities are more likely to develop sepsis. The duration of symptoms seems to be inversely associated with the risk of developing sepsis.
Fundings
This study was funded by the Key Project of Jiangsu Province Commission of Health and Family Planning (CN), Grant Number K2019005
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jped.2023.09.003.
Appendix. Supplementary materials
References
- 1.Montgomery C.O., Siegel E., Blasier R.D., Suva L.J. Concurrent septic arthritis and osteomyelitis in children. J Pediatr Orthop. 2013;33:464–467. doi: 10.1097/BPO.0b013e318278484f. [DOI] [PubMed] [Google Scholar]
- 2.Mediamolle N., Mallet C., Aupiais C., Doit C., Ntika S., Vialle R., et al. Bone and joint infections in infants under three months of age. Acta Paediatr. 2019;108:933–939. doi: 10.1111/apa.14569. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb M., Holladay D., Rice M. Current approach to the evaluation and management of septic arthritis. Pediatr Emerg Care. 2019;35:509–513. doi: 10.1097/PEC.0000000000001874. [DOI] [PubMed] [Google Scholar]
- 4.Okubo Y., Nochioka K., Testa M. Nationwide survey of pediatric acute osteomyelitis in the USA. J Pediatr Orthop B. 2017;26:501–506. doi: 10.1097/BPB.0000000000000441. [DOI] [PubMed] [Google Scholar]
- 5.Yeh T.C., Chiu N.C., Li W.C., Chi H., Lee Y.J., Huang F.Y. Characteristics of primary osteomyelitis among children in a medical center in Taipei, 1984-2002. J Formos Med Assoc. 2005;104:29–33. [PubMed] [Google Scholar]
- 6.Liu L., Oza S., Hogan D., Chu Y., Perin J., Zhu J., et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388:3027–3035. doi: 10.1016/S0140-6736(16)31593-8. Erratum in: Lancet. 2017;389:1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shane A.L., Sánchez P.J., Stoll B.J. Neonatal sepsis. Lancet. 2017;390:1770–1780. doi: 10.1016/S0140-6736(17)31002-4. [DOI] [PubMed] [Google Scholar]
- 8.Fernández-Sarmiento J., De Souza D.C., Martinez A., Nieto V., López-Herce J., Soares Lanziotti V., et al. Latin American Consensus on the Management of Sepsis in Children: Sociedad Latinoamericana de Cuidados Intensivos Pediátricos [Latin American Pediatric Intensive Care Society] (SLACIP) task force: executive summary. J Intensive Care Med. 2022;37:753–763. doi: 10.1177/08850666211054444. [DOI] [PubMed] [Google Scholar]
- 9.Infection, Inflammation, Immunology and Immunisation (I4) section of the ESPR. McGovern M., Giannoni E., Kuester H., Turner M.A., van den Hoogen A., Bliss J.M., et al. Challenges in developing a consensus definition of neonatal sepsis. Pediatr Res. 2020;88:14–26. doi: 10.1038/s41390-020-0785-x. [DOI] [PubMed] [Google Scholar]
- 10.Rubin L.G., Shin J., Kaur I., Scheuerman O., Levy I., Long S.S. Frequency of multifocal disease and pyogenic arthritis of the hip in infants with osteoarticular infection in three neonatal intensive care units. J Pediatr. 2020;227:157–162. doi: 10.1016/j.jpeds.2020.07.055. [DOI] [PubMed] [Google Scholar]
- 11.Weissberg E.D., Smith A.L., Smith D.H. Clinical features of neonatal osteomyelitis. Pediatrics. 1974;53:505–510. [PubMed] [Google Scholar]
- 12.Berberian G., Firpo V., Soto A., Lopez Mañan J., Torroija C., Castro G., et al. Osteoarthritis in the neonate: risk factors and outcome. Braz J Infect Dis. 2010;14:413–418. [PubMed] [Google Scholar]
- 13.Narang A., Mukhopadhyay K., Kumar P., Bhakoo O.N. Bone and joint infection in neonates. Indian J Pediatr. 1998;65:461–464. doi: 10.1007/BF02761144. [DOI] [PubMed] [Google Scholar]
- 14.Kabak S., Halici M., Akcakus M., Cetin N., Narin N. Septic arthritis in patients followed-up in neonatal intensive care unit. Pediatr Int. 2002;44:652–657. doi: 10.1046/j.1442-200x.2002.01649.x. [DOI] [PubMed] [Google Scholar]
- 15.Jung S.W., Kim D.H., Shin S.J., Kang B.Y., Eho Y.J., Yang S.W. Septic arthritis associated with systemic sepsis. Int Orthop. 2018;42:1–7. doi: 10.1007/s00264-017-3565-4. [DOI] [PubMed] [Google Scholar]
- 16.Wynn J.L., Wong H.R., Shanley T.P., Bizzarro M.J., Saiman L., Polin R.A. Time for a neonatal-specific consensus definition for sepsis. Pediatr Crit Care Med. 2014;15:523–528. doi: 10.1097/PCC.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wynn J.L. Defining neonatal sepsis. Curr Opin Pediatr. 2016;28:135–140. doi: 10.1097/MOP.0000000000000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matics T.J., Sanchez-Pinto L.N. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the sepsis-3 definitions in critically ill children. JAMA Pediatr. 2017;171 doi: 10.1001/jamapediatrics.2017.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sankar J., Dhochak N., Kumar K., Singh M., Sankar M.J., Lodha R. Comparison of international pediatric sepsis consensus conference versus sepsis-3 definitions for children presenting with septic shock to a tertiary care center in India: a retrospective study. Pediatr Crit Care Med. 2019;20:e122–e129. doi: 10.1097/PCC.0000000000001864. [DOI] [PubMed] [Google Scholar]
- 21.Kitajima H. Prevention of methicillin-resistant Staphylococcus aureus infections in neonates. Pediatr Int. 2003;45:238–245. doi: 10.1046/j.1442-200x.2003.01719.x. [DOI] [PubMed] [Google Scholar]
- 22.Sankaran G., Zacharia B., Roy A., Purayil S.P. Current clinical and bacteriological profile of septic arthritis in young infants: a prospective study from a tertiary referral centre. Eur J Orthop Surg Traumatol. 2018;28:573–578. doi: 10.1007/s00590-018-2142-x. [DOI] [PubMed] [Google Scholar]
- 23.Mortia M., Nakamura H., Kitano T. Comparison of clinical outcome after treatment of hip arthritis caused by MRSA with that caused by non-MRSA in infants. J Pediatr Orthop B. 2009;18:1–5. doi: 10.1097/BPB.0b013e3283150659. [DOI] [PubMed] [Google Scholar]
- 24.Sodavarapu P., Sudesh P., Gopinathan N.R., Jayashree M., Kumar P., Rangasamy K. Characteristics of musculoskeletal involvement in pediatric patients with disseminated sepsis in a Tertiary Care Center. Indian J Orthop. 2021;56:345–352. doi: 10.1007/s43465-021-00488-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah J., Jefferies A.L., Yoon E.W., Lee S.K., Shah P.S., Canadian Neonatal Network Risk factors and outcomes of late-onset bacterial sepsis in preterm neonates born at < 32 weeks' gestation. Am J Perinatol. 2015;32:675–682. doi: 10.1055/s-0034-1393936. [DOI] [PubMed] [Google Scholar]
- 26.Bejitual K., Fikre R., Ashegu T., Zenebe A. Determinants of neonatal sepsis among neonates admitted to the neonatal intensive care unit of public hospitals in Hawassa City Administration, Sidama Region, Ethiopia, 2020: an unmatched, case-control study. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2021-056669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bizzarro M.J. Health care-associated infections in the neonatal intensive care unit: barriers to continued success. Semin Perinatol. 2012;36:437–444. doi: 10.1053/j.semperi.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Prado D.S., Mendes R.B., Gurgel R.Q., Barreto I.D.C., Cipolotti R., Gurgel R.Q. The influence of mode of delivery on neonatal and maternal short and long-term outcomes. Rev Saude Publica. 2018;52:95. doi: 10.11606/S1518-8787.2018052000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie X., Li J., Gu F., Zhang K., Su Z., Wen Q., et al. Genetic determinants for bacterial osteomyelitis: a focused systematic review of published literature. Front Genet. 2021;12 doi: 10.3389/fgene.2021.654792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arcaroli J., Fessler M.B., Abraham E. Genetic polymorphisms and sepsis. Shock. 2005;24:300–312. doi: 10.1097/01.shk.0000180621.52058.e1. [DOI] [PubMed] [Google Scholar]
- 31.Choi I.H., Pizzutillo P.D., Bowen J.R., Dragann R., Malhis T. Sequelae and reconstruction after septic arthritis of the hip in infants. J Bone Joint Surg Am. 1990;72:1150–1165. [PubMed] [Google Scholar]
- 32.Yi J., Wood J.B., Creech C.B., Williams D., Jimenez-Truque N., Yildirim I., et al. Clinical epidemiology and outcomes of pediatric musculoskeletal infections. J Pediatr. 2021;234:236–244. doi: 10.1016/j.jpeds.2021.03.028. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hadzimuratovic E., Dinarevic S.M., Hadzimuratovic A. Sepsis in premature newborns with congenital heart disease. Congenit Heart Dis. 2010;5:435–438. doi: 10.1111/j.1747-0803.2010.00406.x. [DOI] [PubMed] [Google Scholar]
- 34.Sepsis Prevalence, Outcomes, and Therapies (SPROUT) Study Investigators and Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Weiss S.L., Fitzgerald J.C., Pappachan J., Wheeler D., Jaramillo-Bustamante J.C., Salloo A., et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med. 2015;191:1147–1157. doi: 10.1164/rccm.201412-2323OC. Erratum in: Am J Respir Crit Care Med. 2016;193:223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung S.M., Pollis R.E. Diagnostic pitfalls in septic arthritis of the hip in infants and children. Clin Pediatr. 1975;14:758–761. doi: 10.1177/000992287501400812. passim. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.