Abstract
Pistacia chinensis is locally practiced for treating diabetes, pain, inflammation, and erectile dysfunction. Therefore, the current studies subjected the crude extract/fractions and the isolated compound (2-(3,4-dihydroxyphenyl)-7,8-dihydroxy-3-methoxy-4H-chromen-4-one) to α‐glucosidase inhibitor and anti-glycation activities. The development of long-term complications associated with diabetes is primarily caused by chronic hyperglycemia. Regarding α‐glucosidase, the most significant inhibitory effect was observed with compound 1 (93.09%), followed by the methanolic extract (80.87%) with IC50 values of 45.86 and 86.32 μM. The maximum anti-glycation potential was shown by an isolated compound 1 followed by methanolic extract with effect inhibition of 90.12 and 72.09, respectively. Compound 1 is expected to have the highest gastrointestinal absorption rate, with a predicted absorption rate of 86.156%. This indicates oral suitability. The compound 1 is expected to have no harmful effects on the liver. In addition, our docking results suggest that alpha-glucosidase and isolated compounds showed strong interaction with ILE821, GLN900, and ALA901 residues, along with a −11.95 docking score.
Keywords: Pistacia chinensis, flavonoid; α‐glucosides; Antiglycation; Docking analysis
Abbreviations
- ADMET
Absorption, Distribution, Metabolism, Excretion and Toxicity
- AGE
Advanced Glycation Endproduct
- BSA
Bovine Serum Albumins
- D.M
Diabetes Mellitus
- MGO
Methylglyoxal
- MOE
Molecular Operating Environment
- PDB
Protein Data Bank
- PLI
Protein-Ligand Interaction
- RAGE
Receptor for Advanced Glycation Endproduct
- SMILES
Simplified Molecular-Input Line-Entry System
- TLC
Thin Layer Chromatography
1. Introduction
The main factors contributing to mortality in diabetes mellitus (DM) are long-term complications caused by high blood sugar levels [1]. Maintaining vigilant and consistent control over one's blood glucose levels can help alleviate these symptoms. At the level of the arteries, injury to the micro- and macrovascular systems leads to heart disease, stroke, and other cardiovascular problems. Myocardial infarction and cerebral ischemia are potential outcomes of these conditions [2,3]. Non-enzymatic glycation of plasma proteins (albumin, collagen, globulin, and fibrinogen) is characteristic of glucose accumulation in chronic hyperglycemia [1,4]. Retinopathy, nephropathy, and cardiomyopathy are all consequences of diabetes that have strong links to protein glycation and the production of advanced glycation end products (AGEs) [1]. Recent research has shown that AGEs impact intracellular signaling, gene expression, and the production of pro-inflammatory chemicals and free radicals. These effects occur through interactions with specific plasma membrane receptors known as RAGE [5]. The brush-rim membrane of intestinal cells may contain the hydrolase beta-glucosidase. It contributes to the elevation of blood sugar levels after a meal by catalyzing the hydrolysis of dietary oligosaccharides into glucose molecules. By inhibiting enzyme activity, the postprandial glycemic peak can be reduced, and glycemia can be maintained [6], also in multi-drug combination therapy [7,8]. Consequently, the post-meal glucose surge is diminished.
For millennia, plants have been recognized as having direct therapeutic effects for treating common disorders [9,10]. Pistacia chinensis (P. chinensis) is an abundant species of Pistacia native to Asia. Traditional uses for P. chinensis include wood, seed oil, ornamental applications, and folk remedies for detoxification, pharyngitis, and diarrhea. According to De Pooter et al. [11], the primary chemical components of P. chinensis leaf essential oils cultivated in Egypt were trans-8-ocimene, limonene, and others. The twig extract of P. chinensis [12] yielded two 3-3″-dimeric 4-phenyl dihydro coumarin compounds with estrogen-like activity. In Ayurvedic medicine, the galls of P. chinensis are used to treat a variety of conditions, including but not limited to hiccups, asthma, chronic bronchitis, phthisis, fever, vomiting in neonates, skin diseases, psoriasis, serpent bite, scorpion sting, increased hunger, and removal of bed humor [13]. There is significant medicinal promise due to the plant's pharmacological action and active components. The plant P. integerrima has piqued the interest of researchers, and a vast amount of literature is already available [14]. Few studies have examined specific aspects of P. chinensis. In Pakistan, P. chinensis galls treat hepatitis and liver disorders. Leishmanicidal [15], depressant [16], analgesic and anti-inflammatory [17], spasmolytic [18], and hyperuricemic [19] properties have been reported. Noureen et al. concluded that the bark of P. chinensis could be a suitable source for isolating potent antioxidant compounds [20]. This study aims to investigate the potential inhibitory effects of compound 1 isolated from Pistacia chinensis on the proliferation of α-glucosides and glycation through in vitro screenings.
2. Material and methods
2.1. Collection of plant material
Pistacia chinensis tree bark was collected from the Hostel 02 area at the University of Peshawar in KP, Pakistan. Dr. Muhammad Ilays, the director of the Botany Department at the University of Swabi, successfully ascertained the botanical classification of the plant specimen. Herbarium of this department currently contains the voucher specimen UOS/Bot-55.
2.2. Extractions and isolation
The barks of Pistacia chinensis were cleaned using water and dried in the shade. The 7.34 kg of shade-dried plant materials were processed in a grinder machine to create a finely powdered plant material. The ground plant materials (7.20 kg) were soaked in methanol for 16 days until the extraction was completed. The obtained extract was filtered and concentrated using a rotary evaporator under low temperatures and pressure, resulting in a crude extract weighing 81.29 gm. Using a separating funnel, the crude extract (81.29 g) was fractionated into hexane (5.01 g), chloroform (17.12 g), ethyl acetate (6.43 g), and methanolic fractions (32.98 g). Based on the TLC profile, the methanolic extract was subjected to repeated column chromatography using silica gel. The column was eluted with chloroform and methanol which afforded AF01-AF-104 sub-fractions. Based on the TLC profile the sub-fraction AF-13 was subjected to penical column chromatography; the column was eluted with methanol and chloroform (04:96), yielding compound 1. Furthermore, compound 1 was purified by recrystallization in a mixture of chloroform and methanol (1:1). yielding pure crystals of compound 1. The chemical structure of compound 1 was determined through analysis of physical and spectroscopic data [21,22].
2.3. Evaluation of α‐Glucosidase inhibitory activity
Uddin et al. (2012) outline a technique for assessing the efficacy of crude extracts and compound 1 in inhibiting α‐glucosidase [23]. On the enzyme α-glucosidase (EC3.2.1.20), the inhibitory effect of a phosphate buffer (0.1 M, pH 6.8) and 37 °C was determined. In this investigation, α-glucosidase enzyme (0.2/mL) was incubated for 15 min at 37 °C with (0.2 μg concentration) and compound 1 (0.2 μM) in buffered saline. After mixing the substrate (0.7 Mm, p-nitrophenyl-d-glucopyranoside), the spectrophotometer was used to measure the time-dependent change in 400 nm absorbance. 75% DMSO‑d6 was substituted for the examined extracts and compound 1 in the control. As a standard for α-glucosidase activity, acarbose was utilized. This equation was used to determine the percentage of effort expended.
2.4. Evaluation of antiglycation activity
This activity was evaluated using a modified version of the reported method [24]. Using aseptic conditions, three separate samples of bovine serum albumin (BSA) with a concentration of 10.0 mg/mL, 14.0 mM methylglyoxal (MGO), and 0.10 M phosphate buffer (pH 7.4) containing 30.0 mM NaN3 were placed in each well of a 96-well plate. 0.2 mg/mL of salicylalazine was added to 50 mL of bovine serum albumin solution and 50 mL of methyl guanidine octanoate. The plate was then incubated for nine days at 37 °C, with and without the presence of 0.2 μM of test compounds. Glycation of the protein was evaluated by measuring the specific fluorescence (excitation: 330 nm; emission: 440 nm) against a blank using a microtiter plate spectrophotometer (Spectra Max, Molecular Devices, California, United States). This was done after the protein had been incubated for nine days. The IC50 value for Rutin was determined to be 294 ± 1.50 mM ± standard error of the mean (SEM).
2.5. ADMET predictions
The ADMET predictions were performed using the online free software. The SMILES were found using “SwissADME (http://www.swissadme.ch/index.php)", and the SMILES were then entered in the “pkCMS (https://biosig.lab.uq.edu.au/pkcsm/prediction)" for the prediction of pharmacokinetics properties of like absorption, distribution, metabolism, and excretion.
2.6. Molecular docking
Molecular docking was performed to investigate how synthetic molecules bind with alpha-glucosidase using the Molecular Operating Environment (MOE). First, Chem Draw was used to create the 3D structure for the isolated compound 1. The isolated compound 1 was protonated and energy was reduced by using the MOE's default settings. Alpha glucosidase's structural coordinates were acquired from the protein databank with PDB code 3W37 [25]. The protonation process also used the default value of the MOE's structure preparation module, hydrogen atoms were added, and water molecules and other co-factors were removed. The energy was minimized for the selected coordinate to obtain the lowest energy conformation. In the docking parameter, we used MMFF94x force field and the triangle matcher placement algorithm. For scoring the poses, the London dG method was applied (Ajmal, A. et al., 2023). Finally, the docking investigation used the MOE default parameters [26]. Protein-ligand interaction (PLI) analysis was performed on the top-ranked conformations determined by docking score (S) and binding interaction.
2.6.1. Reference
Ajmal, A. et al., Computer-assisted drug repurposing for thymidylate kinase drug target in monkeypox virus. Frontiers in Cellular and Infection Microbiology, 2023. 13: p. 618.
2.7. Statistical analysis
The data was presented in the form of percentages and IC50 values.
3. Results
3.1. α‐glucosidase inhibitory effect
The effect of crude extract/fractions and isolated compound 1 of Pistacia chinensis against α‐glucosidase is presented in Table 1. The maximum percent inhibitory effect was noticed against compound 1 (93.09%), followed by methanolic extract (80.87%) with IC50 values of 45.86 and 86.32 μM. The standard α‐glucosides inhibitor was used as acorbose (95.98% inhibition).
Table 1.
α‐glucosides inhibitory screening of extracts and isolated compound (1) from Pistacia chinensis.
| Samples | Concentration | % inhibition | IC50 μM |
|---|---|---|---|
| n- hexane | 0.2 μg | 47.98 | – |
| Chloroform | 0.2 μg | 69.20 | 121.98 ± 2.09 |
| Ethyl acetate | 0.2 μg | 77.54 | 92.87 ± 2.44 |
| Methanol | 0.2 μg | 80.87 | 85.32 ± 2.00 |
| Compound (1) | 0.2 μM | 93.09 | 45.86 ± 1.98 |
| Acorbose | 0.2 μM | 95.98 | 42.09 ± 1.65 |
3.2. Antiglycation effect
The data presented in Table 2 showcases the antiglycation effect of the extract/fractions and isolated compound (1) derived from Pistacia chinensis. The isolated compound 1 demonstrated the maximum impact, followed by the methanolic extract, with 90.12% and 72.09% inhibitory effects, respectively.
Table 2.
An antiglycation screening was conducted on extracts and an isolated compound (1) obtained from Pistacia chinensis.
| Samples | Concentration | % inhibition | IC50 μM |
|---|---|---|---|
| n-hexane | 0.2 μg | 32.87 | – |
| Chloroform | 0.2 μg | 41.09 | – |
| Ethyl acetate | 0.2 μg | 49.09 | – |
| Methanol | 0.2 μg | 72.09 | 280.09 |
| Compound (1) | 0.2 μM | 90.12 | 228.09 ± 1.09 |
| Standard (Rutin) | 0.2 μM | 97.21 | 220.12 ± 2.09 |
3.3. ADMET predictions
The pharmacokinetic properties of compound 1 are outlined in Table 3. Compound 1 is expected to have the highest gastrointestinal (GI) absorption rate, estimated at 86.156%. The following is an indication of the oral suitability. The observed low BBB permeability could be attributed to the presence of hydroxyl groups. The compound is anticipated to exhibit no hepatotoxicity.
Table 3.
Pharmacokinetic properties of compound 1 isolated from Pistacia chinensis.
| SMILES = “COC1=C(OC2=C(C=CC(O)=C2O)C1=O)C1=CC=C(O)C(O)=C1” | ||
|---|---|---|
|
Absorption | ||
| Properties | Numeric with unit | |
| 01 | Water solubility | −3.424 log mol/L |
| 02 | Caco2 permeability | −0.149 log Papp in 10−6 cm/s |
| 03 | Intestinal absorption (human) | 86.156 % |
| 04 | Skin Permeability | −2.735 log kp |
| 05 | P-glycoprotein (substrate) | Yes |
| 06 | P-glycoprotein I (inhibitor) | No |
| 07 | P-glycoprotein II (inhibitor) | No |
| Distribution | ||
| 01 | BBB permeability | −1.411 log bb |
| 02 | CNS permeability | −3.499 log PS. |
| Biotransformation | ||
| 01 | CYP2D6 (substrate) | No |
| 02 | CYP3A4 (substrate) | No |
| 03 | CYP1A2 (inhibitor) | Yes |
| Excretion | ||
| 01 | Total Clearance | 0.449 log ml/min/kg |
| 02 | Renal OCT2 (substrate) | No |
| Toxicity | ||
| 01 | AMES toxicity | Yes |
| 02 | Max. Tolerated dose (human) | 0.804 log mg/kg/day |
| 03 | Oral Rat Acute Toxicity (LD50) | 2.546 mol/kg |
| 04 | Oral Rat Chronic Toxicity (LOAEL) | 2.178 log mg/kg_bw/day |
| 05 | Hepatotoxicity | No |
| 06 | Skin Sensitisation | No |
| 07 | Tetrahymena pyriform toxicity | 0.298 log ug/L |
| 08 | Minnow toxicity | 1.354 log Mm |
| Physicochemical properties | ||
| 01 | Number of rotatable bonds | 02 |
| 02 | Number of H-bond (acceptors) | 07 |
| 03 | Number of H-bond (donors) | 04 |
| Druglikeness | ||
| 01 | Lipinski | Yes (No violation) |
3.4. Docking studies
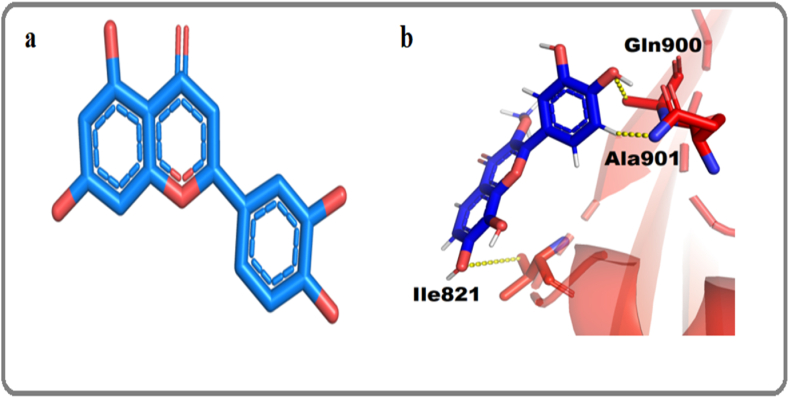
During the investigation, the isolated compound 1 was discovered to form two hydrogen bond donor contacts with ILE821 and GLN900 in the docking results against alpha-glucosidase. At the same time, ALA901 forms one pi-H interaction bond with the isolated compound. As shown in Table 4, the anticipated value for the docking score of the isolated molecule was found to be −11.95 kcal/mol. Fig. 1a indicates the three-dimensional structure of optimized compound 1 and the binding interactions of compound 1 with alpha-glucosidase are indicated in Fig. 1b. The overall result indicates that the isolated molecule (1; Fig. 2) strongly interacts with the alpha-glucosidase active site, showing the isolated compound has considerable inhibitory potential against alpha-glucosidase.
Table 4.
Provides a detailed illustration of the binding interaction and docking score between the isolated compound and alpha-glucosidase.
| Compounds | Receptor | Interaction | Distance | E (kcal/mol) | Docking Score |
|---|---|---|---|---|---|
| OE1 | GLN 900 | H-donor | 3.02 | −1.0 | −11.95 kcal/mol |
| CB | ALA 901 | pi-H | 3.68 | −0.5 | |
| O6 | ILE 821 | H-donor | 3.12 | −0.6 |
Fig. 1.
(a). Three-dimensional structure of optimized compound 1. (b) Indicate the interaction between the isolated compound 1 and alpha-glucosidase.
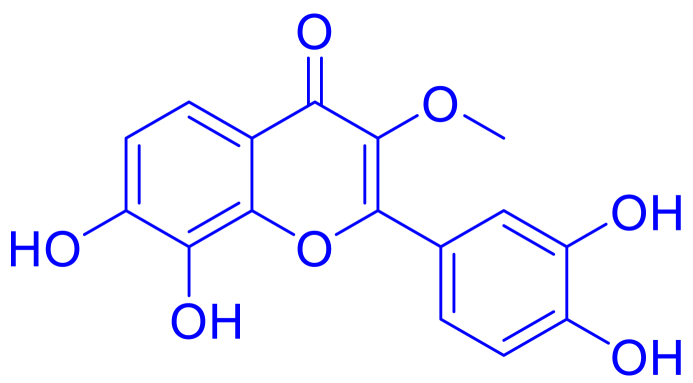
Structure of Compound 1.
Fig. 2.
The chemical structure of compound 1, which has been isolated from Pistacia chinensis.
4. Discussion
Natural products are gaining popularity and acceptance by the world population of both developing and non-developing countries. This upsurge in natural product consumption is due to its high safety profile and economical nature. The general public considers plant-based medicines free of side effects. However, natural products are not entirely free of any side effects. They are safer as compared to synthetic drugs. In the current research studies, the crude extract/fractions and isolated compound of Pistacia chinensis were subjected to evaluate the anti-diabetic effect in the highest regard for discovering a secure and efficacious anti-diabetic agent. The treatment of diabetes is an enduring therapy associated with different side effects.
Moreover, the use of synthetic drugs leads to poor patient compliance and is associated with multiple adverse effects [27]. These factors compel the patient to switch the therapeutic plane from synthetic to alternative medicines. Diabetes is one of the chronic disorders that affects multiple biological systems with time. Therefore, searching for safe and effective therapeutic agents is the need of the day. Different medicinal plant extracts and compounds have been shown to have significant anti-diabetic effects [28]. Therefore, the Pistacia chinensis extract and nanoparticles were subjected to investigate the anti-diabetic impact on the current research. The Pistacia chinensis and the genus Pistacia are locally practiced for treating and managing diabetes in different parts of the world. In addition to the anti-diabetic effect, it is also used to reduce pain and inflammation.
The significant inhibition of α‐glucosidase indicates the anti-hyperglycemic property of extract and compound. The α‐glucosidase antagonistic molecules attenuate the blood glucose level by inhibiting the metabolism of complex carbohydrates. Once this digestion is delayed, simple carbohydrate moieties will not be available for absorption so the blood glucose level will decrease. The anti-diabetic effect of the extract or isolated compound might be involved in inhibiting the metabolism of complex carbohydrates. This inhibition of carbohydrate metabolism converts the hyperglycemic situation to normoglycemia. However, the induction of hypoglycemia might be a side effect of this extract or plant. The excess of sugar leads to the glycation of protein and forming advanced glycation end products (AGPs). These AGPs are notorious for different harmful effects like inflammation, etc. Our tested samples significantly inhibited the glycation, leading to anti-diabetic and anti-inflammatory effects. Chronic hyperglycemia primarily contributes to developing long-term complications associated with diabetes by inducing protein glycation and the sluggish production of advanced glycation end products (AGEs) in numerous body tissues. So, the anti-glycation drug will reduce AGEs. These endogenous molecules are biomarkers implicated in developing or worsening diabetes, nephrotic problems, atherosclerosis, etc. These complexes are also notorious for developing inflammatory cascades. To stop these complications, the anti-glycation process is of utmost importance. The isolated compound 1 and extract significantly blocked the glycation, indicating the above-mentioned benefits. Diabetic patients have problems with body aches, especially peripheral neuropathy retinopathy and erectile dysfunction. Interestingly, the tested plant is an analgesic and anti-inflammatory for erectile dysfunction. Molecular modeling or docking facilitates the analysis of inhibitor interactions with active site residues by predicting correct binding poses and affinity through consideration of factors such as electrostatic, steric hindrance, and hydrogen bonding analysis. The molecular docking interaction typically involves the formation of stable complexes through binding to key sides of protein, disrupting the normal function and leading to observed inhibition. The specificity and strength of these interactions play a crucial role in determining the effectiveness of the inhibitor in experimental settings. Therefore, we also carried out a molecular docking study for isolated compounds against alpha-glucosidase enzyme [29,30]. Our docking results revealed that the isolated compound 1 can strongly bond with alpha-glucosidase enzyme, with a docking score of −11.95 kcal/mol. The predicted active site residues were Ile821, Gln900, and Ala901 (Fig. 1b). The residue Ile821 was found to be the same as reported. Overall, results found that compound 1 has a maximum percent inhibitory effect, less IC50 value, also exhibits a good docking score, and showed strong interactions with alpha-glucosidase.
5. Conclusion
It was concluded that the extract/fractions and the isolated compound 1 of Pistacia chinensis resulted in a significant alpha-glucosidase and antiglycation effect. These anti-diabetic effects provide a strong scientific background to the anti-diabetic folklore of Pistacia chinensis. The molecular docking also showed a good interaction of isolated compounds with targeted enzymes.
Funding
None.
Data availability statement
The spectroscopic and other physical data of compounds mentioned in this publication can be obtained from the corresponding author upon request.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Tareq Abu-Izneid: Formal analysis, Conceptualization. Abdur Rauf: Data curation, Conceptualization. Zuneera Akram: Formal analysis, Data curation. Saima Naz: Data curation, Conceptualization. Abdul Wadood: Formal analysis, Data curation. Naveed Muhammad: Data curation, Conceptualization. Chandni Hayat: Methodology, Investigation. Yahya S. Al-Awthan: Methodology, Investigation. Omar S. Bahattab: Methodology, Investigation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We thank the Pakistan Science Foundation (PSF) in Islamabad, Pakistan, for assisting with this research through project number PSF/NSLP/KP-UoS (892).
Contributor Information
Tareq Abu-Izneid, Email: tizneid@gmail.com.
Abdur Rauf, Email: mashaljcs@yahoo.com.
Zuneera Akram, Email: dr.zunaira@baqai.edu.pk.
Saima Naz, Email: saima_khan201164@yahoo.com.
Abdul Wadood, Email: awadood@awkum.edu.pk.
Naveed Muhammad, Email: drnaveedrph@gmail.com.
Chandni Hayat, Email: chandnihayatkp@gmail.com.
Yahya S. Al-Awthan, Email: alawthan@ut.edu.sa.
Omar S. Bahattab, Email: obahattab@ut.edu.sa.
References
- 1.Paul S., Ali A., Katare R. Molecular complexities underlying the vascular complications of diabetes mellitus–A comprehensive review. J. Diabet. Complicat. 2020;34(8) doi: 10.1016/j.jdiacomp.2020.107613. [DOI] [PubMed] [Google Scholar]
- 2.Katakami N. Mechanism of development of atherosclerosis and cardiovascular disease in diabetes mellitus. J. Atherosclerosis Thromb. 2018;25:27–39. doi: 10.5551/jat.RV17014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ceriello A., Catrinoiu D., Chandramouli C., Cosentino F., Dombrowsky A.C., Itzhak B., Lalic N N.M., Prattichizzo F., Schnell O., Seferović P.M., et al. Heart failure in type 2 diabetes: current perspectives on screening, diagnosis and management. Cardiovasc. Diabetol. 2021;20:1–19. doi: 10.1186/s12933-021-01408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fournet M., Bonté F., Desmoulière A. Glycation damage: a possible hub for major pathophysiological disorders and aging. Aging Dis. 2018;9:880–900. doi: 10.14336/AD.2017.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen C.Y., Lu C.H., Wu C.H., Li K.J., Kuo Y.M., Hsieh S.C., Yu C.L. The development of Maillard reaction, and advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling inhibitors as novel therapeutic strategies for patients with age-related diseases. Molecules. 2020;25:5591. doi: 10.3390/molecules25235591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padhi S., Nayak A.K., Behera A. Type II diabetes mellitus: a review on recent drug based therapeutics, Biomed. Pharma. 2020;131 doi: 10.1016/j.biopha.2020.110708. [DOI] [PubMed] [Google Scholar]
- 7.Kusunoki M., Wakazono N., Wakazono N., Tsutsumi K., Tsutsumi K., Oshida Y., Oshida Y., Miyata T., Miyata T. Combination therapy of α-glucosidase inhibitor, thiazolidinedione and sodium glucose co-transporter-2 inhibitor in Japanese type 2 diabetes patients. J. Endocrinol. Metab. 2020;10:6. [Google Scholar]
- 8.Blahova J., Martiniakova M., Babikova M., Kovacova V., Mondockova V., Omelka R. Pharmaceutical drugs and natural therapeutic products for the treatment of type 2 diabetes mellitus. Pharmaceuticals. 2021;14:806. doi: 10.3390/ph14080806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma V., Gautam D.N., Radu A.F., Behl T., Bungau S.G., Vesa C.M. Reviewing the traditional/modern uses, phytochemistry, essential oils/extracts and pharmacology of embelia ribes burm. Antioxidants. 2022;1311(7):1359. doi: 10.3390/antiox11071359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai W., Long L., Wang X., Li S., Xu H. Phytochemicals targeting Toll-like receptors 4 (TLR4) in inflammatory bowel disease. Chin. Med. 2022;17(1):53. doi: 10.1186/s13020-022-00611-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Pooter H.L., Schamp N.M., Aboutabl E.A., El Tohamy S.F., Doss S.L. Essential oils from the leaves of three Pistacia species grown in Egypt. Flavour Fragrance J. 1991;6(3):229–232. [Google Scholar]
- 12.Nishimuta S., Taki M., Takaishi S., Iijima Y., Akiyama T. Structures of 4-aryl-coumarin (neoflavone) dimers isolated from Pistacia chinensis BUNGE and their estrogen-like activity. Chem. Pharm. Bull. 2000;48(4):505–508. doi: 10.1248/cpb.48.505. [DOI] [PubMed] [Google Scholar]
- 13.Chopra R.N., Badhwar R.K., Ghosh S. Indian Council of Agricultural Research; New Delhi: 1965. Poisonous Plants of India; p. 270. [Google Scholar]
- 14.Bibi Y., Zia M., Qayyum A. An overview of Pistacia integerrima a medicinal plant species: ethnobotany, biological activities and phytochemistry. Pak. J. Phrama. Sci. 2015;28(3):1009–1013. [PubMed] [Google Scholar]
- 15.Rauf A., Rashid U., Shbeer A.M., Al-Ghorbani M., Muhammad N., Khalil A.A., Naz H., Sharma R., Ribaudo G G. Flavonoids from Pistacia chinensis subsp. integerrima with leishmanicidal activity: computational and experimental evidence. Nat. Prod. Res. 2023;20:1–6. doi: 10.1080/14786419.2023.2228459. [DOI] [PubMed] [Google Scholar]
- 16.Ansari S.H., Ali M., Qadry J.S. Essential oils of Pistacia integerrima galls and their effect on the central nervous system. Int. J. Pharmacogn 1. 1993;31(2):89–95. [Google Scholar]
- 17.Ahmad N.S., Waheed A., Farman M., Qayyum A. Analgesic and anti-inflammatory effects of Pistacia integerrima extracts in mice. J. Ethnophacol. 2010;27(2):250–253. doi: 10.1016/j.jep.2010.03.017. 129. [DOI] [PubMed] [Google Scholar]
- 18.Shirole R.L., Shirole N.L., Saraf M.N. In vitro relaxant and spasmolytic effects of essential oil of Pistacia integerrima Stewart ex Brandis Galls. J. Ethnopharmacol. 2015;20(168):61–65. doi: 10.1016/j.jep.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Huang C.Y., Chang Y.Y., Chang S.T., Chang H.T. Xanthine oxidase inhibitory activity and chemical composition of Pistacia chinensis leaf essential oil. Pharmaceutics. 1982;20(10) doi: 10.3390/pharmaceutics14101982. (2022), 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noureen F., Khan M.R., Shah N.A., Khan R.A., Naz K., Sattar S S. Pistacia chinensis: strong antioxidant and potent testicular toxicity amelioration agent. Asian Pac. J. Tropical Med. 2017;10(4):380–389. doi: 10.1016/j.apjtm.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 21.Rauf A., Akram Z., Naveed M., AlMasoud N., Alomar T.S., Saleem M., Ribaudo G. Studies on the inhibition of ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) by 2-(3, 4-dihydroxyphenyl)-7, 8-dihydroxy-3-methoxychromen-4-one, a flavonoid from Pistacia chinensis. Chemistry. 2023;5(4):2094–2103. [Google Scholar]
- 22.Kang W.Y., Wang J.M., Ji Z.Q. Flavonoids in luculia pinceana. Chem. Nat. Compd. 2008;44:644–645. [Google Scholar]
- 23.Uddin G., Rauf A., Al-Othman A.M., Collina S., Arfan M., Ali G., Khan I. Pistagremic acid, a glucosidase inhibitor from Pistacia integerrima. Fitoterapia. 2012;83(8):1648–1652. doi: 10.1016/j.fitote.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Lee E.J., Kim J.Y., Oh S.H. Advanced glycation end products (AGEs) promote melanogenesis through receptor for AGEs. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep27848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tagami T., Yamashita K., Okuyama M., Mori H., Yao M., Kimura A. Molecular basis for the recognition of long-chain substrates by plant α-glucosidases. J. Biol. Chem. 2013;288(26):19296–19303. doi: 10.1074/jbc.M113.465211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Babatunde O., Hameed S., Mbachu K.A., Saleem F., Chigurupati S., Wadood A., Rehman A.U., Venugopal V., Khan K.M., Taha M., Ekundayo O. Evaluation of derivatives of 2, 3-dihydroquinazolin-4 (1H)-one as inhibitors of cholinesterases and their antioxidant activity: in vitro, in silico and kinetics studies. J. Serb. Chem. Soc. 2023;88(9) [Google Scholar]
- 27.Taha N., Abd El-Azeaz M.A., Abd El-Razik B.G. Factors affecting compliance of diabetic patients toward therapeutic management. Diabetes. 2005;5:365–368. [Google Scholar]
- 28.Majeed S., Abidin N.B.Z., Muthukumarasamy R., Danish M., Mahmad A., Ibrahim M.N.M., Sisinthy S.P. Wound healing and anti-diabetic properties of green synthesized silver nanoparticles in 3T3-L1 mouse embryo fibroblast cells through 2-NBDG expression. Inorg. Chem. Commun. 2024;159 [Google Scholar]
- 29.Ajmal A., Mahmood C., Hayat M.A., Hakami B.S., Alotaibi M., Umair A.N., Abdalla, Li P., He P., Wadood A., Hu J. Computer-assisted drug repurposing for thymidylate kinase drug target in monkeypox virus. Front. Cell. Infect. 2023;13:618. doi: 10.3389/fcimb.2023.1159389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adelusi T.I., Oyedele A.Q.K., Boyenle I.D., Ogunlana A.T., Adeyemi R.O., Ukachi C.D., Idris M.O., Olaoba O.T., Adedotun I.O., Kolawole O.E., Xiaoxing Y. Molecular modeling in drug discovery. Inform. Med. Unlocked. 2022;29 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The spectroscopic and other physical data of compounds mentioned in this publication can be obtained from the corresponding author upon request.