Abstract
Patients undergoing cardiopulmonary bypass procedures require inotropic support to improve hemodynamic function and cardiac output. Current inotropes such as dobutamine, can promote arrhythmias, prompting a demand for improved inotropes with little effect on intracellular Ca2+ flux. Low‐dose carbon monoxide (CO) induces inotropic effects in perfused hearts. Using the CO‐releasing pro‐drug, oCOm‐21, we investigated if this inotropic effect results from an increase in myofilament Ca2+ sensitivity. Male Sprague Dawley rat left ventricular cardiomyocytes were permeabilized, and myofilament force was measured as a function of ‐log [Ca2+] (pCa) in the range of 9.0–4.5 under five conditions: vehicle, oCOm‐21, the oCOm‐21 control BP‐21, and levosimendan, (9 cells/group). Ca2+ sensitivity was assessed by the Ca2+ concentration at which 50% of maximal force is produced (pCa50). oCOm‐21, but not BP‐21 significantly increased pCa50 compared to vehicle, respectively (pCa50 5.52 vs. 5.47 vs. 5.44; p < 0.05). No change in myofilament phosphorylation was seen after oCOm‐21 treatment. Pretreatment of cardiomyocytes with the heme scavenger hemopexin, abolished the Ca2+ sensitizing effect of oCOm‐21. These results support the hypothesis that oCOm‐21‐derived CO increases myofilament Ca2+ sensitivity through a heme‐dependent mechanism but not by phosphorylation. Further analyses will confirm if this Ca2+ sensitizing effect occurs in an intact heart.
Keywords: calcium sensitivity, carbon monoxide, heme, myofilament
1. INTRODUCTION
Acute myocardial infarction occurs from an absence of oxygen delivery to the myocardium due to severe coronary artery occlusion and often results in pathological left ventricular (LV) remodeling (Reed et al., 2017). To correct these vascular defects, patients undergo cardiopulmonary bypass procedures, which can impose prolonged ischemic periods resulting in perioperative complications such as stunned myocardium, LV dysfunction and ultimately, heart failure (Kloner et al., 1994; Monaco et al., 2020). A substantial proportion of these cardiac surgical patients will receive inotropes, to reduce the development of low‐output syndromes and sustain adequate organ perfusion (Karami et al., 2020). Inotropes, such as dobutamine or milrinone, increase heart rate and augment the cytosolic Ca2+ transient, saturating the Ca2+ binding subunit of the myofilament, troponin C (TnC), to increase myofilament cross‐bridge formation (DesJardin & Teerlink, 2021). However, this increase in heart rate and Ca2+ flux is associated with a significant increase in myocardial oxygen demand (Bailey et al., 1994; Fellahi et al., 2008; Shabana et al., 2020). Additionally, the enhanced re‐uptake of Ca2+ into the sarcoplasmic reticulum may promote store overload induced Ca2+ release, resulting in an increased risk of arrhythmias (Said et al., 2008; Zhang & Zhang, 2020).
Myotropes, such as omecamtiv mecarbil and levosimendan, represent a newer class of inotropes that increase LV contractility via direct interaction with the sarcomere to enhance Ca2+ sensitivity and actin‐myosin interactions (Edes et al., 1995; Nagy et al., 2015; Psotka et al., 2019). By increasing LV contractility without influencing intracellular Ca2+, it has been proposed that these drugs will avoid adverse increases in myocardial oxygen demand and subsequent arrhythmias. However, while the direct myosin activator omecamtiv mecarbil does not alter intracellular Ca2+ transients, this myotrope can induce diastolic dysfunction and electrical alternans within therapeutic concentrations (200 μg/kg) (Fülöp et al., 2021). Similarly, the Ca2+ sensitizer levosimendan also inhibits phosphodiesterase III, resulting in an increased intracellular Ca2+ flux in addition to sensitizing the myofilament to Ca2+ (Bokník et al., 1997; Ørstavik et al., 2014).
Carbon monoxide (CO) administered either in gaseous form (<500 ppm) or using low micromolar concentrations of CO‐releasing molecules (CORMs), is a cardioprotective agent that interacts with heme elements to exert vasodilatory, anti‐apoptotic, anti‐inflammatory, and positive inotropic effects (Brouard et al., 2000; Collman et al., 1976; Dugbartey et al., 2021; Otterbein et al., 2000; Sammut et al., 1998). While CORMs have been extensively tested both in vivo and in vitro studies, CORMs such as CORM‐2 and CORM‐3 however, contain a ruthenium transitional metal center which is cytotoxic in cardiomyocyte, renal, and bacterial cell studies (Southam et al., 2018; Winburn et al., 2012), bringing their utility into question. To combat these cytotoxic effects, boron‐ and manganese‐based CORMs were developed (Motterlini et al., 2005) however, these compounds have unpredictable CO release rates and chemical reactivity associated with the parent compound (Bauer, Yang, et al., 2023; Bauer, Yuan, et al., 2023). A new organic CO‐releasing prodrug, oCOm‐21, has been shown to rapidly release CO at physiological conditions (t 1/2 = 19 min; pH 7.4) and is non‐cytotoxic at concentrations <20 μM (Kueh et al., 2017; Kueh et al., 2020). Low concentrations of oCOm‐21 (1–10 μM) increase vasodilation in isolated aortic rings and LV systolic pressure in a Langendorff‐perfused rat heart model (Thwaite et al., 2021; Kueh et al., 2017) with no evidence of arrhythmia formation or a change in heart rate. While the positive inotropic mechanism of oCOm‐21 is still uncertain, the absence of arrhythmogenicity and chronotropic effect suggests that oCOm‐21 may be directly interacting with the myofilament.
Given the evident binding affinity of CO for heme elements within various hemoproteins (Collman et al., 1976), we hypothesized that oCOm‐21 increases Ca2+ sensitivity via heme interactions. The myotropic effects of oCOm‐21 in a permeabilized cardiomyocyte preparation were compared to the established Ca2+ sensitizer levosimendan. To determine if oCOm‐21 derived‐CO directly interacts with the myofilament, this study tested the Ca2+ sensitivity of permeabilized cardiomyocytes in the presence of oCOm‐21 (3 and 10 μM) and the spent CO by‐product (BP‐21). Potential mechanisms behind alterations in Ca2+ sensitivity were also investigated. Sex as a biological variable was considered in this myofilament study, however prior work has shown no sex differences in either the baseline LV contractile function in isolated hearts, or in myofilament protein phosphorylation, and Ca2+ sensitivity of permeabilized myofilaments of mice (≤12 months of age) (Kane et al., 2020).
2. MATERIALS AND METHODS
2.1. Materials
oCOm‐21 and BP‐21 (CO‐depleted oCOm‐21 byproduct), were synthesized by the Larsen group, Department of Chemistry, University of Otago (Kueh et al., 2017). Levosimendan (L5545) and hemopexin (H9291), phenylmethylsulfonyl fluoride (78830), Protease Inhibitor Cocktail (P8340), Phosphatase Inhibitor Cocktail (P5726), oxalic acid, and Spurr's epoxy resin were purchased from Sigma‐Aldrich. HALT protease and phosphatase inhibitor cocktail 100× (78430), Triton X‐100 (28314), Pro‐Q™ Diamond Phosphoprotein gel stain (P33300), SYPRO® Ruby Protein Gel Stain (S12000), and PeppermintStick™ Phosphoprotein molecular weight Standard (P27167) were purchased from Thermo Fisher Scientific.
2.2. Animal ethics and care
Male Sprague Dawley rats (320–360 g, n = 9 animals) were obtained from the University of Otago's Biological Research Facility. All procedures described were carried out under institutional approval granted (AUP 21–63) in accordance with the “Guidelines on the Care and Use of Laboratory Animals” set out by the University of Otago Animal Ethics Committee. Hearts were excised under deep anesthesia using 5% isoflurane, and the LV immediately separated and frozen in liquid nitrogen.
2.3. Cardiomyocyte force‐pCa measurements
Permeabilized LV cardiomyocytes isolated from male Sprague Dawley rats were used to examine changes in Ca2+ sensitivity as described previously (Greenman et al., 2022; Ng et al., 2022). Briefly, frozen LV tissue was homogenized in a Relax buffer (100 mM KCl, 1.75 mM EGTA, 10 mM imidazole, 4 mM ATP, 5 mM MgCl2, pH 7) with HALT protease and phosphatase inhibitor cocktail. The myocyte suspension was subsequently treated with 1% Triton X‐100 for 8 min to yield a pool of permeabilized cardiomyocytes. Permeabilized cardiomyocytes were then washed twice with Relax buffer to remove the detergent and cytosolic/membrane‐bound components and stored on ice. Individual permeabilized cardiomyocytes were mounted to an inverted phase contrast microscope (Nikon Eclipse Ts2R, Coherent, Australia) and adhered to 100 μm pins connected to a force transducer and a piezoelectric motor arm (Aurora Scientific, 406a model, Aurora, ON, Canada) using silicone glue within a micro‐organ bath containing relax buffer at 15°C. Each cardiomyocyte preparation was adjusted to a sarcomere length of 2.2 μm using micromanipulators while the cells were immersed in a well containing pCa 9.0 (7 mM EGTA, 20 mM imidazole, 5.42 mM MgCl2, 79.16 mM KCl, 10−9 M free Ca2+, 14.5 mM creatine phosphate, 4.74 mM ATP, pH 7). Cell width, length, and sarcomere length were monitored throughout the experiment using an IDS camera and VSL 900B software (Aurora Scientific) (Figure 1a).
FIGURE 1.
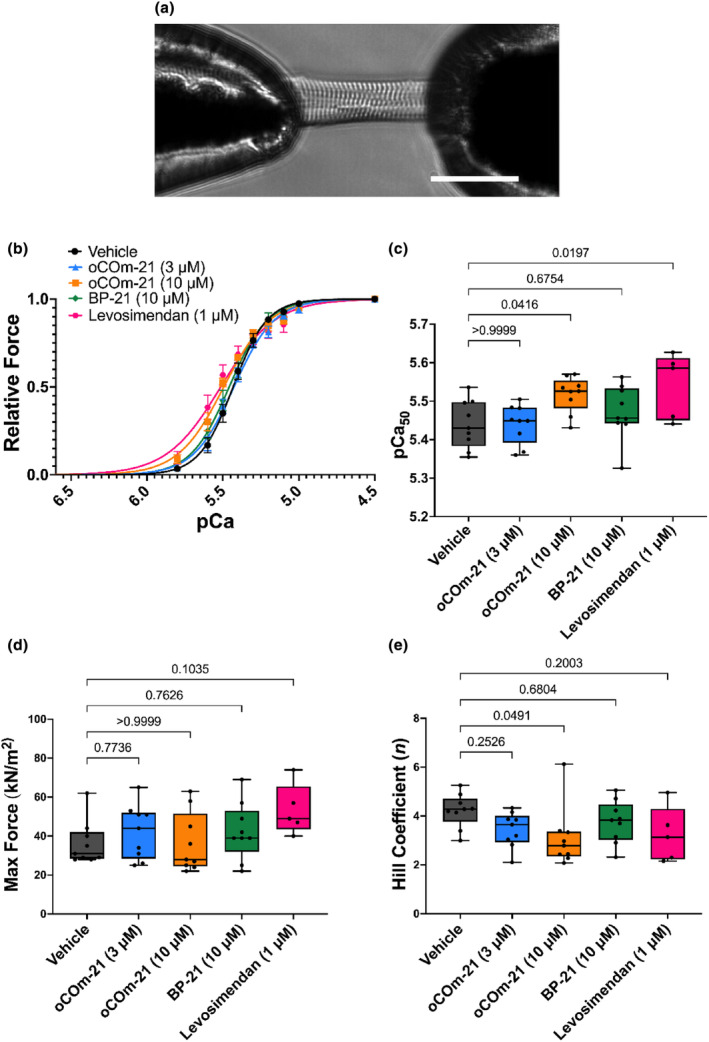
oCOm‐21 (10 μM) increases myofilament Ca2+ sensitivity. (a) Example of a permeabilized cardiomyocyte, glued to two micro‐pins connected to a force transducer and piezoelectric motor arm at 20× magnification. Scale bar shown represents 50 μm. (b) Force‐pCa relationships of permeabilized cardiomyocytes treated with either vehicle, oCOm‐21 (3, 10 μM), BP‐21 (10 μM), or levosimendan (1 μM). (c) pCa50 values for each treatment group. (d) Maximal active force values for each treatment group. (e) Hill coefficient (n) values for each treatment group. Individual cell data points in (c–e) are denoted using black solid circles. Data in 1B is expressed as mean ± S.E.M while data in (c–e) are expressed as Box and Whiskers plots representing the median and interquartile range, Experimental repeat n = 5–9 cells/group obtained from three individual hearts. Significance was determined using a One‐way ANOVA with Dunnett post hoc test and p is represented on each experimental group.
Cardiomyocytes were immersed in a randomly‐ordered series of submaximal pCa solutions (pCa 5.8–5.0) and allowed to generate steady state force until a plateau was established. The cardiomyocytes were then slackened by 20% of their initial length to measure force production before being transferred back to the relaxing pCa 9.0 solution to allow myofilament relaxation. Force was measured as a function of pCa (−log [Ca2+]) in the range of pCa 9.0–4.5 under 5 test conditions: vehicle control, oCOm‐21 at 3 or 10 μM, the CO‐depleted control BP‐21 (10 μM), and levosimendan (1 μM). The force generated in pCa 4.5 (7 mM EGTA, 20 mM imidazole, 5.26 mM MgCl2, 64 mM KCl, 10−4.5 M free Ca2+, 14.5 mM creatine phosphate, 4.81 mM ATP, pH 7) was used to assess maximal force production. This was followed by eight, random selected submaximal pCa solutions (pCa 5.8–5.0), with a pCa 4.5 measurement conducted after every fourth measurement to assess the performance of the cardiomyocyte. The Ca2+ concentration at which 50% of maximal force is produced (pCa50), was used to measure Ca2+ sensitivity. Cardiomyocytes which declined in force by 15% from the beginning of the experiment were discarded. In a separate set of experiments, permeabilized cardiomyocytes were incubated with the heme scavenger hemopexin (HPX) (1 μM) in pCa 9.0. HPX‐treated cells were then incubated with oCOm‐21 (10 μM) to determine whether heme was required for the observed Ca2+ sensitizing effect.
Maximal active force was normalized to the cross‐sectional area of the cell based on a circular structure (Cross‐sectional area = 3.14 × (1/2cell width)2). Force generation at each pCa was expressed as a fraction of the maximal force obtained for that cell under the same conditions and data was analyzed using the Hill equation:
P rel is force expressed as a fraction of maximal force, n is the Hill coefficient, and k is the intercept of the fitted line with the x‐axis corresponding to the pCa50 (Hofmann et al., 1991). Using constants derived from the Hill equation, force data was fitted using Prism software (GraphPad Software Inc., La Jolla, CA, USA) with the following equation (Diffee et al., 2001):
2.4. Pro‐Q™ diamond phosphoprotein gel staining
Pro‐Q™ Diamond Phosphoprotein gel stain was used to determine the effect of oCOm‐21 on myofilament protein phosphorylation in the permeabilized cardiomyocyte. Permeabilized cardiomyocytes were homogenized in RIPA buffer (50 mM NaCl, 1% SDS, 1% Triton X‐100, 1 mM edetate disodium, pH 7.4 with HCl) containing phenylmethylsulfonyl fluoride, protease inhibitor cocktail and phosphatase inhibitors. Permeabilized cardiomyocytes were treated with either vehicle or oCOm‐21 (3, 10 μM) for 20 minutes and protein samples were then prepared for gel electrophoresis and denatured at 70°C for 10 minutes. Protein concentrations were determined by a Lowry assay (Sammut et al., 2001) and 20 μg of protein was loaded into each well onto a gradient (4%–20%) gel (Mini‐PROTEAN® TGX™ Precast Gels, Bio‐Rad # 4561096) and run with 25 mM Tris Base, 192 mM glycine, 1% SDS buffer. PeppermintStick™ Phosphoprotein Molecular Weight Standard (Invitrogen) was also added on neighboring lanes to act as both a molecule weight marker and a positive control. Electrophoresis was performed at 80 V for an initial 5 min, followed by an increase to 120 V (1 h). After gel electrophoresis, gels were fixed overnight in 50% methanol and 10% acetic acid to remove SDS. Gels were then washed to remove the fixing solution and subsequently stained with Pro‐Q™ Diamond gel stain. Gels were protected from light and stained for 60 min. Gels were de‐stained using the Pro‐Q™ Diamond Phosphoprotein Gel Destaining Solution for 90 min to reduce background staining. Gels were then imaged using an Amersham ImageQuant 800 (Cytiva, Auckland, NZ) at 535 nm with a 0.2 s exposure.
To quantify total protein, gels were stained with SYPRO® Ruby Protein Gel Stain overnight and subsequently washed with 10% and 7% acetic acid before imaging. SYPRO® Ruby‐stained gels were imaged using the Amersham ImageQuant 800 at 460 nm with a 0.2 s exposure.
Protein band intensities were quantified using Image Lab Software (Bio‐Rad v.6.1). Phosphorylation was normalized to total protein using the ratio of the signal intensity of the phospho‐stained band divided by the SYPRO®‐stained band.
2.5. Microscopy
To investigate the presence of mitochondria, permeabilized cardiomyocytes were prepared for transmission electron microscopy (Sammut et al., 2001). Briefly, permeabilized cardiomyocytes were incubated in relax buffer with 2.5% glutaraldehyde for 2 h at 4°C and for a further 30 min at room temperature before being pelleted at 15,000 g for 2 min. The pellet was resuspended in Relax buffer and washed before pelleting at 5000 g and embedded in 3% low gelling‐temperature agarose at 4°C for 10 min. The pellet was then immersed in 1% osmium tetroxide in relax buffer for 1.5 h and subsequently dehydrated through an ethanol series (50%–100%) and transferred to propylene oxide. The pellet was then immersed in Spurr's epoxy resin and allowed to cure for 24 h at 60°C before being sectioned. Ultra‐thin sections were stained with uranyl acetate and lead citrate and examined using a Phillips CM100 TEM.
2.6. Cardiomyocyte heme content
Heme content in permeabilized LV cardiomyocytes was determined by an oxalic acid based fluorescent measurement of the heme precursor protoporphyrin IX (PPIX). Samples were either pre‐treated with HPX (1 μM) or vehicle (0.15 M NaCl in phosphate buffered solution). Briefly, sample protein content was determined using the Lowry assay as previously described (Sammut et al., 2001). Oxalic acid (2 M) was added to 10 μg of protein and heated to 100°C for 30 min to generate fluorescent PPIX from heme. Protein samples were subsequently centrifuged at 3630 g for 5 min at 4°C and added to a 96‐well plate. Fluorescence excitation and emission were set at 405 and 631 nm, respectively, and measured using the i3X SpectraMax (Molecular Devices, San Jose, USA). Background fluorescence was evaluated in parallel in non‐boiled protein samples.
2.7. Statistical analysis
The researchers conducting these experiments were blinded to the identity of the experimental agent until all data had been analyzed and graphed.
Results are expressed as means ± S.E.M and were analyzed either using an unpaired t‐test or a one‐way ANOVA with a Dunnett or Bonferroni post hoc as appropriate to determine significance between groups where p < 0.05 was considered statistically significant. All statistical analysis were performed using GraphPad Prism v.9 (GraphPad Software, La Jolla, CA).
3. RESULTS
3.1. oCOm‐21 increases Ca2+ sensitivity of the myofilament
Ca2+ sensitivity was assessed in permeabilized cardiomyocytes treated with either vehicle, oCOm‐21 (3, 10 μM), BP‐21 (10 μM) or levosimendan (1 μM). Incubation with oCOm‐21 at 10 μM, increased pCa50 compared to vehicle (5.52 vs. 5.44 respectively; p < 0.05) while reducing (p < 0.05) the Hill Coefficient (n), (Figure 1b,c,e) while 3 μM oCOm‐21 did not alter pCa50 (5.44 vs. 5.44; p > 0.05) (Figure 1b,c). BP‐21 did not increase pCa50 compared to vehicle (5.47 vs. 5.44 respectively; p > 0.05). Comparably, levosimendan increased pCa50 compared to vehicle (5.44 vs. 5.54 respectively; p < 0.05). No other treatment groups increased maximal active force of the cardiomyocyte or altered co‐operativity of Ca2+ binding compared to vehicle (p > 0.05) (Figure 1d,e).
3.2. oCOm‐21 does not impact the phosphorylation status of the myofilament
In order to assess a potential mechanism by which oCOm‐21 alters Ca2+ sensitivity, the total phosphorylation status and specifically, the phosphorylation of cardiac troponin I (cTnI) of permeabilized cardiomyocytes were assessed. No change in total protein phosphorylation or cTnI phosphorylation was observed with oCOm‐21 at either concentration compared to vehicle (p > 0.05) (Figure 2a–d).
FIGURE 2.
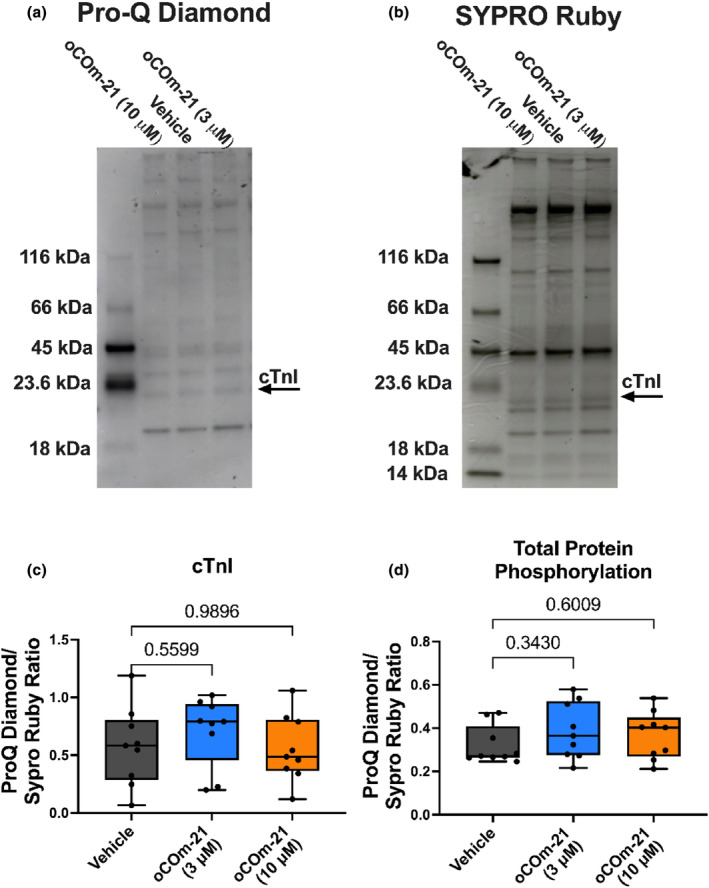
oCOm‐21 does not alter myofilament phosphorylation in a permeabilized cardiomyocyte. (a) Representative Pro‐Q™ Diamond protein phosphorylation‐stained gradient gel and, (b) subsequent SYPRO® Ruby total protein staining. (c) Ratio of Pro‐Q™ Diamond and SYPRO® Ruby signal for cTnI. (d) Ratio of Pro‐Q™ Diamond and SYPRO® Ruby signal for total protein phosphorylation. Individual data points in (c, d) are denoted using black solid circles. Data are expressed as a Box and Whiskers plot representing the median and interquartile range, n = 9 hearts/group. Significance was determined using a One‐way ANOVA with Dunnett post hoc test and p is represented on each experimental group.
3.3. Hemopexin abolishes the Ca2+ sensitizing effect of oCOm‐21
Electron microscopical studies confirmed the presence of mitochondrial remnants throughout the myofilament (Figure 3a), providing evidence for an intracellular store of free heme within the permeabilized cardiomyocyte. To confirm this ultrastructural finding, heme content was quantified in permeabilized cardiomyocytes fluorometrically. Heme content was significantly reduced to the lowest limit of detection with the heme scavenger HPX, indicating free heme exists within these permeabilized cells (Figure 3b). Removal of free heme, the binding target for CO, using HPX before incubation with oCOm‐21 prevented the Ca2+ sensitizing effect and dropped pCa50 (5.52–5.42; p < 0.01) indicating a heme‐dependent mechanism of oCOm‐21 (Figure 3b,c). Additionally, HPX pretreatment increased maximal force of the cardiomyocyte compared to vehicle and oCOm‐21 (p < 0.05) (Figure 3d–f).
FIGURE 3.
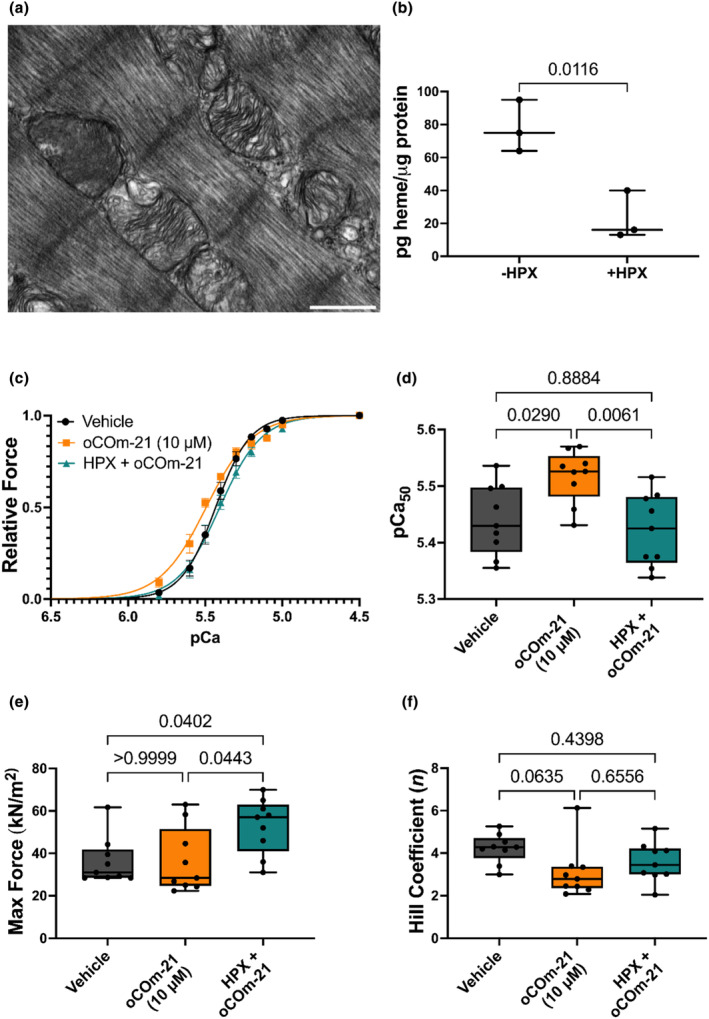
HPX prevents the Ca2+ sensitizing effect of oCOm‐21 (10 μM). (a) Electron micrograph of interfibrillar mitochondrial structures in a permeabilized cardiomyocyte at 33,000× magnification; scale bar shown represents 500 nm. (b) Fluorescence analysis of heme content in both untreated and HPX treated (1 μM) permeabilized cardiomyocytes. (c) Force‐pCa relationships of permeabilized cardiomyocytes treated with vehicle, oCOm‐21 (10 μM) or HPX (1 μM) + oCOm‐21 (10 μM). (d) pCa50 values for each treatment. (e) Max force values for each treatment. (f) Hill coefficient values for each treatment group. Individual cell data points in (d–f) are denoted using black solid circles. Data in (b) is expressed as a Box and Whiskers plot representing the median and interquartile range, n = 3 hearts/group, significance determined by an unpaired t‐test. Data in (c) is expressed as the means ± S.E.M while data in (d–f) is expressed as a Box and Whiskers plot representing the median and interquartile range, n = 9 cells/group obtained from three hearts. Significance was determined using a One‐way ANOVA with Bonferroni post hoc test and p is represented on each experimental group.
4. DISCUSSION
This study investigated the Ca2+ sensitizing effects of a CO‐releasing prodrug, oCOm‐21. The addition of oCOm‐21 to a permeabilized cardiomyocyte preparation increased pCa50 to the same degree as the clinically approved drug levosimendan, an established Ca2+ sensitizer. Importantly BP‐21, the CO‐depleted by‐product of oCOm‐21, did not alter pCa50. Moreover, when CO binding targets were removed with the free‐heme scavenger HPX, the Ca2+ sensitizing effects of oCOm‐21 were abolished. These findings support our hypothesis that oCOm‐21 increases Ca2+ sensitivity through a CO‐heme‐dependent mechanism.
Our group (Greenman et al., 2021; Ng et al., 2022) and others (Budde et al., 2021; Sevrieva et al., 2023; van der Velden et al., 2003) have shown that phosphorylation of myofilament proteins (including cTnI and myosin binding protein C) is associated with changes in Ca2+ sensitivity. CO activates the soluble guanylate cyclase (sGC) pathway to phosphorylate cytosolic (e.g., L‐type Ca2+ channel, ryanodine receptor) and myofilament proteins such as TnI at Ser23/24 (Layland et al., 2002; Stone & Marletta, 1994). Inhibition of sGC activity with (1H‐[1,2,4]oxadiazolo‐[4,3‐a]quinoxaline‐1‐one) (ODQ) in perfused rat hearts inhibited the inotropic effect of the earlier CO prodrug CORM‐3 (Musameh et al., 2006), suggesting that CO elicited an inotropic effect via transient phosphorylation of cytosolic and myofilament proteins. However, the present study showed that oCOm‐21 increased pCa50 in permeabilized cardiomyocyte preparations, where the cytosolic enzyme sGC should not be present (Gao et al., 2012; Solaro et al., 1971; van der Velden et al., 2003). Indeed, Pro‐Q diamond staining showed that oCOm‐21 did not change the phosphorylation status of the myofilament proteome in permeabilized cell homogenates. These discrepancies may indicate that oCOm‐21 induces protein phosphorylation affecting contractility and myofilament Ca2+ sensitivity within intact preparations. The novel finding of this study is that oCOm‐21 increased Ca2+ sensitivity without affecting phosphorylation of the myofilament.
Direct binding of CO to myofilament proteins, as seen with other myotropes including TA1 (He et al., 2022), levosimendan (Edes et al., 1995; Haikala et al., 1995) and EMD 57033 (Li et al., 2000) may explain the increased pCa50 identified with oCOm‐21. The present study did not confirm a direct binding mechanism of oCOm‐21, however the established heme dependency for the Ca2+ sensitization effect may provide insight. Leclerc et al. (1993), identified a direct interaction of the CO‐heme complex with skeletal TnC and other Ca2+ binding proteins. It is possible that CO derived from oCOm‐21 binds heme and interacts with TnC to increase Ca2+ sensitivity. Permeabilization of cardiomyocytes, removes cytosolic, and membrane‐bound components (Solaro et al., 1971), however, it was unclear whether residual fragments of interfibrillar mitochondria which contain heme (Lange et al., 1999) were present. Therefore, transmission electron microscopy was used to determine whether the permeabilized cardiomyocytes in these preparations retained mitochondria. Our micrographs visually confirmed the presence of mitochondrial components in the permeabilized cardiomyocyte preparations, as established sources of free heme for CO interactions (Barth et al., 1992; Medlock et al., 2015; Swenson et al., 2020). Heme content in permeabilized cardiomyocytes was reduced upon treatment with HPX, further indicating pools of free heme for CO interactions are retained following cardiomyocyte permeabilization (Detzel et al., 2021). Moreover, HPX abolished the Ca2+ sensitizing effect of oCOm‐21, demonstrating that the elimination of free heme from these myofilament preparations, nullifies the effect of oCOm‐21 on myofilament contractility. These findings suggest that CO interacts with mitochondrial‐derived heme stores in a permeabilized cardiomyocyte preparation to increase pCa50. It is however impossible to speculate whether heme is derived from the mitochondrial hemoprotein degeneration or through heme generation by mitochondrial remnants.
We were surprised to find that HPX also increased the maximal active force of the cardiomyocyte. Maximal active force produced is determined by a combination of the maximal number of actin‐myosin cross bridges formed and the rigidity of the cross‐bridges (Linari et al., 2007). Alvarado et al. ( 2015) showed that the addition of heme to myofilaments reduced maximal active force of cardiomyocytes due to oxidation on myosin light chain 1 which could be reversed upon HPX treatment. In this context, the addition of HPX in our experiments may have removed basal concentrations of heme from the myofilament thereby improving maximal active force.
Interestingly, oCOm‐21 reduced myofilament co‐operativity as identified by the decreased Hill slope. Co‐operativity for thin filament activation is described as the result of a specific event (e.g., Ca2+ binding to TnC), nonlinearly favoring the occurrence of that same event (Razumova et al., 2000). In other words, as more Ca2+ binds to TnC, the affinity of neighboring TnC proteins for Ca2+ increases, therefore, force production is dependent on co‐operativity (Farman et al., 2010). Ca2+ sensitizers such as levosimendan have no impact on cooperativity (Szilágyi et al., 2004; Van Hees et al., 2011) however, omecamtiv mecarbil increases Ca2+ sensitivity while decreasing cooperativity (Nagy et al., 2015), comparable to oCOm‐21. Both omecamtiv mecarbil and oCOm‐21 increase force production at [Ca2+] below the pCa50 but produce similar force at [Ca2+] above the pCa50, resulting in the ascending portion of the force‐pCa relationship becoming less steep, reducing co‐operativity. The observed increase in force production at lower [Ca2+] may prolong contractions, consequently impairing diastolic function. Therefore, further studies should investigate the impact of oCOm‐21 on diastolic function and if the Ca2+ sensitizing effect still occurs at lower [Ca2+] in an intact heart preparation.
5. LIMITATIONS
A limitation to this study was that only permeabilized cardiomyocyte preparations were utilized to examine the Ca2+ sensitizing effects of oCOm‐21. CO is highly pleiotropic and interacts with a variety of heme‐containing proteins such as hemoglobin, myoglobin, and mitochondrial complexes (Coletta et al., 1985; Dugbartey et al., 2021). By using a permeabilized preparation, these components were removed (Solaro et al., 1971), suggesting that CO interacts with the myofilament. The micrographical evidence provided (Figure 3) however showed that mitochondrial elements were still present in these cells and that heme was implicated in this CO response. It is unclear however, from the use of this permeabilised cell model whether CO is still able to preferentially interact with the myofilament over other hemoproteins within an intact cell. While heme levels could be quantified in permeabilized cells, incubation with HPX, reduced heme concentrations to the lowest limit of detection making it difficult to accurately determine concentrations of free heme in the HPX treated cardiomyocytes. This finding however, confirms that HPX treatment, significantly reduced the availability of free heme in the subsequent Force‐pCa experiments provided in Figure 3.
Consideration was also given to the inclusion of myofilaments isolated from female hearts in this study. Similar work conducted by Howlett's group in a mouse model however, found no difference in Ca2+ sensitivity or myofilament protein phosphorylation as a consequence of sex (Kane et al., 2020). This lack of dimorphism in myofilament contractility is supported by earlier work conducted in myofilaments isolated from a cat cardiac model (Petre et al., 2007) and in wild type exercise‐trained or sedentary mice (Najafi et al., 2015). Schuldt et al. (2021) also recently found that tubulin was the only sarcomeric protein whose expression was affected by sex in cardiomyocytes harvested from patients receiving LV outflow tract obstruction reduction surgeries.
In summary, these results indicate that in the presence of heme, myofilament Ca2+ sensitivity is increased when oCOm‐21 is applied directly to a permeabilized cardiomyocyte preparation. This suggests that liberated CO interacts with myofilament proteins through a heme‐dependent mechanism to elicit this effect rather than through a posttranslational modification. This interaction will be examined using mass‐spectrophotometric analysis. Further investigations will also establish whether this Ca2+ sensitizing effect is conserved in treated hearts and if oCOm‐21 alters Ca2+ cycling. These findings add to our understanding of the clinically valuable effects of low dose CO administration. While high concentrations of CO are clearly cardiotoxic, this study suggests that low concentrations of the oCOms have positive valuable myotropic effects which may be used to safely improve myocardial contractility in cardiac injury.
AUTHOR CONTRIBUTIONS
Fergus M. Payne, Gary M. Diffee, James C. Baldi, and Ivan A. Sammut designed the experiments. Fergus M. Payne performed the experiments for Figures 1 and 3 and wrote the manuscript draft. Samantha Nie performed the experiments for Figure 2. Fergus M. Payne, Samantha Nie and James C. Baldi analyzed and interpreted data. Joanne C. Harrison and Ivan A. Sammut, ensured that the test conditions were blinded. David S. Larsen synthesized oCOm‐21 and BP‐21 in collaboration with Joanne C. Harrison and Ivan A. Sammut. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
FUNDING INFORMATION
This work was supported by a University of Otago Research Grant, Grant/Award Number: ORG0122‐0323 (to I.A.S, J.C.B. and J.C.H.) and a Health Research Council of New Zealand Grant, Grant/Award No: 20/274 (to I.A.S, J.C.H, G.T.W. and D.S.L.).
CONFLICT OF INTEREST STATEMENT
The authors declare no competing financial interests.
ACKNOWLEDGMENTS
The authors would like to thank Richard Easingwood and the OMNI unit at Otago for preparing samples used for transmission electron microscopy. Open access publishing facilitated by University of Otago, as part of the Wiley ‐ University of Otago agreement via the Council of Australian University Librarians.
Payne, F. M. , Nie, S. , Diffee, G. M. , Wilkins, G. T. , Larsen, D. S. , Harrison, J. C. , Baldi, J. C. , & Sammut, I. A. (2024). The carbon monoxide prodrug oCOm‐21 increases Ca2+ sensitivity of the cardiac myofilament. Physiological Reports, 12, e15974. 10.14814/phy2.15974
DATA AVAILABILITY STATEMENT
All data are presented in Figures 1, 2, 3 but are also available upon request.
REFERENCES
- Alvarado, G. , Jeney, V. , Tóth, A. , Csősz, É. , Kalló, G. , Huynh, A. T. , Hajnal, C. , Kalász, J. , Pásztor, E. T. , & Édes, I. (2015). Heme‐induced contractile dysfunction in human cardiomyocytes caused by oxidant damage to thick filament proteins. Free Radical Biology & Medicine, 89, 248–262. 10.1016/j.freeradbiomed.2015.07.158 [DOI] [PubMed] [Google Scholar]
- Bailey, J. M. , Levy, J. H. , Kikura, M. , Szlam, F. , & Hug, C. C. (1994). Pharmacokinetics of intravenous milrinone in patients undergoing cardiac surgery. Anesthesiology, 81, 616–622. 10.1097/00000542-199409000-00014 [DOI] [PubMed] [Google Scholar]
- Barth, E. , Stämmler, G. , Speiser, B. , & Schaper, J. (1992). Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. Journal of Molecular and Cellular Cardiology, 24, 669–681. 10.1016/0022-2828(92)93381-S [DOI] [PubMed] [Google Scholar]
- Bauer, N. , Yang, X. , Yuan, Z. , & Wang, B. (2023). Reassessing CORM‐A1: Redox chemistry and idiosyncratic CO‐releasing characteristics of the widely used carbon monoxide donor. Chemical Science, 14, 3215–3228. 10.1039/D3SC00411B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, N. , Yuan, Z. , Yang, X. , & Wang, B. (2023). Plight of CORMs: The unreliability of four commercially available CO‐releasing molecules, CORM‐2, CORM‐3, CORM‐A1, and CORM‐401, in studying CO biology. Biochemical Pharmacology, 214, 115642. 10.1016/j.bcp.2023.115642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokník, P. , Neumann, J. , Kaspareit, G. , Schmitz, W. , Scholz, H. , Vahlensieck, U. , & Zimmermann, N. (1997). Mechanisms of the contractile effects of levosimendan in the mammalian heart. The Journal of Pharmacology and Experimental Therapeutics, 280, 277–283. https://jpet.aspetjournals.org/content/280/1/277 [PubMed] [Google Scholar]
- Brouard, S. , Otterbein, L. E. , Anrather, J. , Tobiasch, E. , Bach, F. H. , Choi, A. M. , & Soares, M. P. (2000). Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. The Journal of Experimental Medicine, 192, 1015–1026. 10.1084/jem.192.7.1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde, H. , Hassoun, R. , Tangos, M. , Zhazykbayeva, S. , Herwig, M. , Varatnitskaya, M. , Sieme, M. , Delalat, S. , Sultana, I. , & Kolijn, D. (2021). The interplay between S‐glutathionylation and phosphorylation of cardiac troponin I and myosin binding protein C in end‐stage human failing hearts. Antioxidants (Basel, Switzerland), 10, 1134. 10.3390/antiox10071134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coletta, M. , Ascenzi, P. , Traylor, T. , & Brunori, M. (1985). Kinetics of carbon monoxide binding to monomeric hemoproteins. Role of the proximal histidine. The Journal of Biological Chemistry, 260, 4151–4155. 10.1016/S0021-9258(18)89244-4 [DOI] [PubMed] [Google Scholar]
- Collman, J. P. , Brauman, J. I. , Halbert, T. R. , & Suslick, K. S. (1976). Nature of O2 and CO binding to metalloporphyrins and heme proteins. Proceedings of the National Academy of Sciences of the United States of America, 73, 3333–3337. 10.1073/pnas.73.10.3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesJardin, J. T. , & Teerlink, J. R. (2021). Inotropic therapies in heart failure and cardiogenic shock: An educational review. European Heart Journal Acute Cardiovascular Care, 10, 676–686. 10.1093/ehjacc/zuab047 [DOI] [PubMed] [Google Scholar]
- Detzel, M. S. , Schmalohr, B. F. , Steinbock, F. , Hopp, M.‐T. , Ramoji, A. , Paul George, A. A. , Neugebauer, U. , & Imhof, D. (2021). Revisiting the interaction of heme with hemopexin. Biological Chemistry, 402, 675–691. 10.1515/hsz-2020-0347 [DOI] [PubMed] [Google Scholar]
- Diffee, G. M. , Seversen, E. A. , & Titus, M. M. (2001). Exercise training increases the Ca2+ sensitivity of tension in rat cardiac myocytes. Journal of Applied Physiology, 91, 309–315. 10.1152/jappl.2001.91.1.309 [DOI] [PubMed] [Google Scholar]
- Dugbartey, G. J. , Alornyo, K. K. , Luke, P. P. , & Sener, A. (2021). Application of carbon monoxide in kidney and heart transplantation: A novel pharmacological strategy for a broader use of suboptimal renal and cardiac grafts. Pharmacological Research, 173, 105883. 10.1016/j.phrs.2021.105883 [DOI] [PubMed] [Google Scholar]
- Edes, I. , Kiss, E. , Kitada, Y. , Powers, F. M. , Papp, J. G. , Kranias, E. G. , & Solaro, R. J. (1995). Effects of levosimendan, a cardiotonic agent targeted to troponin C, on cardiac function and on phosphorylation and Ca2+ sensitivity of cardiac myofibrils and sarcoplasmic reticulum in Guinea pig heart. Circulation Research, 77, 107–113. 10.1161/01.RES.77.1.107 [DOI] [PubMed] [Google Scholar]
- Farman, G. P. , Allen, E. J. , Schoenfelt, K. Q. , Backx, P. H. , & De Tombe, P. P. (2010). The role of thin filament cooperativity in cardiac length‐dependent calcium activation. Biophysical Journal, 99, 2978–2986. 10.1016/j.bpj.2010.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellahi, J.‐L. , Parienti, J.‐J. , Hanouz, J.‐L. , Plaud, B. , Riou, B. , & Ouattara, A. (2008). Perioperative use of dobutamine in cardiac surgery and adverse cardiac outcome: Propensity‐adjusted analyses. Anesthesiology, 108, 979–987. 10.1097/ALN.0b013e318173026f [DOI] [PubMed] [Google Scholar]
- Fülöp, G. Á. , Oláh, A. , Csipo, T. , Kovács, Á. , Pórszász, R. , Veress, R. , Horváth, B. , Nagy, L. , Bódi, B. , & Fagyas, M. (2021). Omecamtiv mecarbil evokes diastolic dysfunction and leads to periodic electromechanical alternans. Basic Research in Cardiology, 116, 1–16. 10.1007/s00395-021-00866-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, W. D. , Murray, C. I. , Tian, Y. , Zhong, X. , DuMond, J. F. , Shen, X. , Stanley, B. A. , Foster, D. B. , Wink, D. A. , & King, S. B. (2012). Nitroxyl‐mediated disulfide bond formation between cardiac myofilament cysteines enhances contractile function. Circulation Research, 111, 1002–1011. 10.1161/CIRCRESAHA.112.270827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenman, A. C. , Diffee, G. M. , Power, A. S. , Wilkins, G. T. , Gold, O. M. , Erickson, J. R. , & Baldi, J. C. (2021). Increased myofilament calcium sensitivity is associated with decreased cardiac troponin I phosphorylation in the diabetic rat heart. Experimental Physiology, 106, 2235–2247. 10.1113/EP089730 [DOI] [PubMed] [Google Scholar]
- Greenman, A. C. , Diffee, G. M. , Power, A. S. , Wilkins, G. T. , Gold, O. M. , Erickson, J. R. , & Baldi, J. C. (2022). Treadmill running increases the calcium sensitivity of myofilaments in diabetic rats. Journal of Applied Physiology, 132, 1350–1360. 10.1152/japplphysiol.00785.2021 [DOI] [PubMed] [Google Scholar]
- Haikala, H. , Kaivola, J. , Nissinen, E. , Pi, W. , Levijoki, J. , & Lindén, I.‐B. (1995). Cardiac troponin C as a target protein for a novel calcium sensitizing drug, levosimendan. Journal of Molecular and Cellular Cardiology, 27, 1859–1866. 10.1016/0022-2828(95)90009-8 [DOI] [PubMed] [Google Scholar]
- He, H. , Baka, T. , Balschi, J. , Motani, A. S. , Nguyen, K. K. , Liu, Q. , Slater, R. , Rock, B. , Wang, C. , & Hale, C. (2022). Novel small‐molecule troponin activator increases cardiac contractile function without negative impact on energetics. Circulation. Heart Failure, 15, e009195. 10.1161/CIRCHEARTFAILURE.121.009195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann, P. , Hartzell, H. , & Moss, R. (1991). Alterations in Ca2+ sensitive tension due to partial extraction of C‐protein from rat skinned cardiac myocytes and rabbit skeletal muscle fibers. The Journal of General Physiology, 97, 1141–1163. 10.1085/jgp.97.6.1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane, A. E. , Bisset, E. S. , Keller, K. M. , Ghimire, A. , Pyle, W. G. , & Howlett, S. E. (2020). Age, sex and overall health, measured as frailty, modify myofilament proteins in hearts from naturally aging mice. Scientific Reports, 10, 10052. 10.1038/s41598-020-66903-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karami, M. , Hemradj, V. V. , Ouweneel, D. M. , den Uil, C. A. , Limpens, J. , Otterspoor, L. C. , Vlaar, A. P. , Lagrand, W. K. , & Henriques, J. P. (2020). Vasopressors and inotropes in acute myocardial infarction related cardiogenic shock: A systematic review and meta‐analysis. Journal of Clinical Medicine, 9, 2051. 10.3390/jcm9072051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloner, R. A. , Przyklenk, K. , & Kay, G. L. (1994). Clinical evidence for stunned myocardium after coronary artery bypass surgery. Journal of Cardiac Surgery, 9, 397–402. 10.1111/jocs.1994.9.3s.397 [DOI] [PubMed] [Google Scholar]
- Kueh, J.T.B. , Seifert‐Simpson, J. M. , Thwaite, S. H. , Rodgers, G. D. , Harrison, J. C. , Sammut, I. A. , & Larsen, D. S. (2020). Studies towards non‐toxic, water soluble, vasoactive norbornene organic carbon monoxide releasing molecules. Asian . The Journal of Organic Chemistry, 9, 2127–2135. 10.1002/ajoc.202000546 [DOI] [Google Scholar]
- Kueh, J. T. B. , Stanley, N. J. , Hewitt, R. J. , Woods, L. M. , Larsen, L. , Harrison, J. C. , Rennison, D. , Brimble, M. A. , Sammut, I. A. , & Larsen, D. S. (2017). Norborn‐2‐en‐7‐ones as physiologically‐triggered carbon monoxide‐releasing prodrugs. Chemical Science, 8, 5454–5459. 10.1039/C7SC01647F [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange, H. , Kispal, G. , & Lill, R. (1999). Mechanism of iron transport to the site of heme synthesis inside yeast mitochondria. The Journal of Biological Chemistry, 274, 18989–18996. 10.1074/jbc.274.27.18989 [DOI] [PubMed] [Google Scholar]
- Layland, J. , Li, J. M. , & Shah, A. M. (2002). Role of cyclic GMP‐dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. The Journal of Physiology, 540, 457–467. 10.1113/jphysiol.2001.014126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc, E. , Leclerc, L. , Cassoly, R. , Derterrossian, E. , Wajcman, H. , Poyart, C. , & Marden, M. (1993). Heme binding to calmodulin, troponin C, and parvalbumin, as a probe of calcium‐dependent conformational changes. Archives of Biochemistry and Biophysics, 306, 163–168. 10.1006/abbi.1993.1495 [DOI] [PubMed] [Google Scholar]
- Li, M. X. , Spyracopoulos, L. , Beier, N. , Putkey, J. A. , & Sykes, B. D. (2000). Interaction of cardiac troponin C with Ca2+ sensitizer EMD 57033 and cardiac troponin I inhibitory peptide. Biochemistry, 39, 8782–8790. 10.1021/bi000473i [DOI] [PubMed] [Google Scholar]
- Linari, M. , Caremani, M. , Piperio, C. , Brandt, P. , & Lombardi, V. (2007). Stiffness and fraction of myosin motors responsible for active force in permeabilized muscle fibers from rabbit psoas. Biophysical Journal, 92, 2476–2490. 10.1529/biophysj.106.099549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlock, A. E. , Shiferaw, M. T. , Marcero, J. R. , Vashisht, A. A. , Wohlschlegel, J. A. , Phillips, J. D. , & Dailey, H. A. (2015). Identification of the mitochondrial heme metabolism complex. PLoS ONE, 10, e0135896. 10.1371/journal.pone.0135896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco, F. , Di Prima, A. L. , Kim, J. H. , Plamondon, M.‐J. , Yavorovskiy, A. , Likhvantsev, V. , Lomivorotov, V. , Hajjar, L. A. , Landoni, G. , & Riha, H. (2020). Management of challenging cardiopulmonary bypass separation. Journal of Cardiothoracic and Vascular Anesthesia, 34, 1622–1635. 10.1053/j.jvca.2020.02.038 [DOI] [PubMed] [Google Scholar]
- Motterlini, R. , Sawle, P. , Bains, S. , Hammad, J. , Alberto, R. , Foresti, R. , & Green, C. J. (2005). CORM‐A1: A new pharmacologically active carbon monoxide‐releasing molecule. The FASEB Journal, 19, 1–24. 10.1096/fj.04-2169fje [DOI] [PubMed] [Google Scholar]
- Musameh, M. , Fuller, B. , Mann, B. , Green, C. , & Motterlini, R. (2006). Positive inotropic effects of carbon monoxide‐releasing molecules (CO‐RMs) in the isolated perfused rat heart. British Journal of Pharmacology, 149, 1104–1112. 10.1038/sj.bjp.0706939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy, L. , Kovács, Á. , Bódi, B. , Pásztor, E. , Fülöp, G. Á. , Tóth, A. , Édes, I. , & Papp, Z. (2015). The novel cardiac myosin activator omecamtiv mecarbil increases the calcium sensitivity of force production in isolated cardiomyocytes and skeletal muscle fibres of the rat. British Journal of Pharmacology, 172, 4506–4518. 10.1111/bph.13235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi, A. , Schlossarek, S. , van Deel, E. D. , van den Heuvel, N. , Güçlü, A. , Goebel, M. , Kuster, D. W. , Carrier, L. , & van der Velden, J. (2015). Sexual dimorphic response to exercise in hypertrophic cardiomyopathy‐associated MYBPC3‐targeted knock‐in mice. Pflügers Archiv, 467, 1303–1317. 10.1007/s00424-014-1570-7 [DOI] [PubMed] [Google Scholar]
- Ng, Y. H. , Lamberts, R. R. , Jones, P. P. , Sammut, I. A. , Diffee, G. M. , Wilkins, G. T. , & Baldi, J. C. (2022). Sarco/endoplasmic reticulum calcium ATPase activity is unchanged despite increased myofilament calcium sensitivity in Zucker type 2 diabetic fatty rat heart. Scientific Reports, 12, 16904. 10.1038/s41598-022-20520-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørstavik, Ø. , Ata, S. , Riise, J. , Dahl, C. , Andersen, G. Ø. , Levy, F. , Skomedal, T. , Osnes, J. B. , & Qvigstad, E. (2014). Inhibition of phosphodiesterase‐3 by levosimendan is sufficient to account for its inotropic effect in failing human heart. British Journal of Pharmacology, 171, 5169–5181. 10.1111/bph.12647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterbein, L. E. , Bach, F. H. , Alam, J. , Soares, M. , Lu, H. T. , Wysk, M. , Davis, R. J. , Flavell, R. A. , & Choi, A. M. (2000). Carbon monoxide has anti‐inflammatory effects involving the mitogen‐activated protein kinase pathway. Nature Medicine, 6, 422–428. 10.1038/74680 [DOI] [PubMed] [Google Scholar]
- Petre, R. E. , Quaile, M. P. , Rossman, E. I. , Ratcliffe, S. J. , Bailey, B. A. , Houser, S. R. , & Margulies, K. B. (2007). Sex‐based differences in myocardial contractile reserve. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 292, R810–R818. 10.1152/ajpregu.00377.2006 [DOI] [PubMed] [Google Scholar]
- Psotka, M. A. , Gottlieb, S. S. , Francis, G. S. , Allen, L. A. , Teerlink, J. R. , Adams, K. F. , Rosano, G. M. , & Lancellotti, P. (2019). Cardiac calcitropes, myotropes, and mitotropes: JACC review topic of the week. Journal of the American College of Cardiology, 73, 2345–2353. 10.1016/j.jacc.2019.02.051 [DOI] [PubMed] [Google Scholar]
- Razumova, M. V. , Bukatina, A. E. , & Campbell, K. B. (2000). Different myofilament nearest‐neighbor interactions have distinctive effects on contractile behavior. Biophysical Journal, 78, 3120–3137. 10.1016/S0006-3495(00)76849-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, G. W. , Rossi, J. E. , & Cannon, C. P. (2017). Acute myocardial infarction. Lancet, 389, 197–210. 10.1016/S0140-6736(16)30677-8 [DOI] [PubMed] [Google Scholar]
- Said, M. , Becerra, R. , Palomeque, J. , Rinaldi, G. , Kaetzel, M. , Diaz‐Sylvester, P. , Copello, J. A. , Dedman, J. R. , Mundiña‐Weilenmann, C. , & Vittone, L. (2008). Increased intracellular Ca2+ and SR Ca2+ load contribute to arrhythmias after acidosis in rat heart. Role of Ca2+/calmodulin‐dependent protein kinase II. American Journal of Physiology. Heart and Circulatory Physiology, 295, H1669–H1683. 10.1152/ajpheart.00010.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammut, I. A. , Foresti, R. , Clark, J. E. , Exon, D. J. , Vesely, M. J. , Sarathchandra, P. , Green, C. J. , & Motterlini, R. (1998). Carbon monoxide is a major contributor to the regulation of vascular tone in aortas expressing high levels of haeme oxygenase‐1. British Journal of Pharmacology, 125, 1437–1444. 10.1038/sj.bjp.0702212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammut, I. A. , Jayakumar, J. , Latif, N. , Rothery, S. , Severs, N. J. , Smolenski, R. T. , Bates, T. E. , & Yacoub, M. H. (2001). Heat stress contributes to the enhancement of cardiac mitochondrial complex activity. The American Journal of Pathology, 158, 1821–1831. 10.1038/sj.bjp.0702212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldt, M. , Pei, J. , Harakalova, M. , Dorsch, L. M. , Schlossarek, S. , Mokry, M. , Knol, J. C. , Pham, T. V. , Schelfhorst, T. , & Piersma, S. R. (2021). Proteomic and functional studies reveal detyrosinated tubulin as treatment target in sarcomere mutation‐induced hypertrophic cardiomyopathy. Circulation. Heart Failure, 14, e007022. 10.1161/CIRCHEARTFAILURE.120.007022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevrieva, I. R. , Ponnam, S. , Yan, Z. , Irving, M. , Kampourakis, T. , & Sun, Y.‐B. (2023). Phosphorylation‐dependent interactions of myosin‐binding protein C and troponin coordinate the myofilament response to protein kinase a. The Journal of Biological Chemistry, 299, 102767. 10.1016/j.jbc.2022.102767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabana, A. , Dholoo, F. , & Banerjee, P. (2020). Inotropic agents and vasopressors in the treatment of cardiogenic shock. Current Heart Failure Reports, 17, 438–448. 10.1007/s11897-020-00493-9 [DOI] [PubMed] [Google Scholar]
- Solaro, R. J. , Pang, D. C. , & Briggs, F. N. (1971). The purification of cardiac myofibrils with triton X‐100. Biochimica et Biophysica Acta‐Bioenergetics, 245, 259–262. 10.1016/0005-2728(71)90033-8 [DOI] [PubMed] [Google Scholar]
- Southam, H. M. , Smith, T. W. , Lyon, R. L. , Liao, C. , Trevitt, C. R. , Middlemiss, L. A. , Cox, F. L. , Chapman, J. A. , El‐Khamisy, S. F. , & Hippler, M. (2018). A thiol‐reactive Ru (II) ion, not CO release, underlies the potent antimicrobial and cytotoxic properties of CO‐releasing molecule‐3. Redox Biology, 18, 114–123. 10.1016/j.redox.2018.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone, J. R. , & Marletta, M. A. (1994). Soluble guanylate cyclase from bovine lung: Activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry, 33, 5636–5640. 10.1021/bi00184a036 [DOI] [PubMed] [Google Scholar]
- Swenson, S. A. , Moore, C. M. , Marcero, J. R. , Medlock, A. E. , Reddi, A. R. , & Khalimonchuk, O. (2020). From synthesis to utilization: The ins and outs of mitochondrial heme. Cells, 9, 579. 10.3390/cells9030579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilágyi, S. , Pollesello, P. , Levijoki, J. , Kaheinen, P. , Haikala, H. , Édes, I. , & Papp, Z. (2004). The effects of levosimendan and OR‐1896 on isolated hearts, myocyte‐sized preparations and phosphodiesterase enzymes of the Guinea pig. European Journal of Pharmacology, 486, 67–74. 10.1016/j.ejphar.2003.12.005 [DOI] [PubMed] [Google Scholar]
- Thwaite, S. H. , Read, M. I. , Larsen, D. S. , Harrison, J. C. , & Sammut, I. A. (2021). Can carbon monoxide protect hypertrophic hearts against ischaemia‐reperfusion injury? New Zealand Medical Journal, 134(1535), 98. https://journal.nzma.org.nz/journal‐articles/proceedings‐of‐the‐252nd‐otago‐medical‐school‐research‐society‐masters‐and‐honours‐student‐speaker‐awards 34239167 [Google Scholar]
- van der Velden, J. , Papp, Z. , Zaremba, R. , Boontje, N. , de Jong, J. W. , Owen, V. , Burton, P. , Goldmann, P. , Jaquet, K. , & Stienen, G. (2003). Increased Ca2+−sensitivity of the contractile apparatus in end‐stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovascular Research, 57, 37–47. 10.1016/S0008-6363(02)00606-5 [DOI] [PubMed] [Google Scholar]
- Van Hees, H. , Andrade Acuña, G. , Linkels, M. , Dekhuijzen, P. , & Heunks, L. (2011). Levosimendan improves calcium sensitivity of diaphragm muscle fibres from a rat model of heart failure. British Journal of Pharmacology, 162, 566–573. 10.1111/j.1476-5381.2010.01048.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winburn, I. C. , Gunatunga, K. , McKernan, R. D. , Walker, R. J. , Sammut, I. A. , & Harrison, J. C. (2012). Cell damage following carbon monoxide releasing molecule exposure: Implications for therapeutic applications. Basic & Clinical Pharmacology & Toxicology, 111, 31–41. 10.1111/j.1742-7843.2012.00856.x [DOI] [PubMed] [Google Scholar]
- Zhang, C. , & Zhang, Y. (2020). Caffeine and dobutamine challenge induces bidirectional ventricular tachycardia in normal rats. Heart Rhythm O2, 1(5), 359–367. 10.1016/j.hroo.2020.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are presented in Figures 1, 2, 3 but are also available upon request.


