Abstract
Introduction
Cauda equina syndrome (CES), conus medullaris syndrome (CMS), and sciatica-like syndromes or “sciatica mimics” (SM) may present as diagnostic and/or therapeutic dilemmas for the practicing spine surgeon. There is considerable controversy regarding the appropriate definition and diagnosis of these entities, as well as indications for and timing of surgery. Our goal is to formulate the most current, evidence-based recommendations for the definition, diagnosis, and management of CES, CMS, and SM syndromes.
Methods
We performed a systematic literature search in PubMed from 2012 to 2022 using the keywords “cauda equina syndrome”, “conus medullaris syndrome”, “sciatica”, and “sciatica mimics”. Standardized screening criteria yielded a total of 43 manuscripts, whose data was summarized and presented at two international consensus meetings of the World Federation of Neurosurgical Societies (WFNS) Spine Committee. Utilizing the Delphi method, we generated seven final consensus statements.
Results and conclusion
s: We provide standardized definitions of cauda equina, cauda equina syndrome, conus medullaris, and conus medullaris syndrome. We advocate for the use of the Lavy et al classification system to categorize different types of CES, and recommend urgent MRI in all patients with suspected CES (CESS), considering the low sensitivity of clinical examination in excluding CES. Surgical decompression for CES and CMS is recommended within 48 h, preferably within less than 24 h. There is no data regarding the role of steroids in acute CES or CMS. The treating physician should be cognizant of a variety of other pathologies that may mimic sciatica, including piriformis syndrome, and how to manage these.
Keywords: Cauda equina syndrome, Conus medullaris syndrome, Sciatica, Sciatica like syndromes, Sciatica mimics, Pyriformis syndrome, Evaluation of cauda equina syndrome, Management of cauda equina syndrome
Abbreviations
- WFNS
World Federation of Neurosurgical Societies
- MRI
Magnetic resonance imaging
- CT
Computer tomography
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- CES
Cauda equina syndrome
- CMS
Conus medularis syndrome
- SM
Sciatica mimics
1. Introduction
A major clinical challenge for treating physicians, cauda equina syndrome (CES) is a rare condition, with an annual reported incidence between 0.3 and 0.6 patients per 100,000 per year.1 It may be missed at the time of presentation, especially when motor and sensory symptoms are mild or unnoticeable by the patient, resulting in progressive and sometimes permanent bowel and bladder dysfunction. A recent review reported as many as seventeen distinct definitions for cauda equina syndrome,2 and there is still no consensus regarding the standard definition of CES. The most common cause for CES is acute lumbar disc herniation (45%); other etiologies include trauma, neoplasia, infection, vascular pathology, hematoma, and iatrogenic (i.e. post-lumbar surgery or post-procedure, occurring in up to 0.2%–1.2% of patients).3 Conus medullaris syndrome (CMS) is another rare and unique entity presenting with features of both spinal cord and cauda equina compression. Different studies have described various levels of distal spinal cord and root involvement, with no clear-cut consensus on its definition. Sciatica-like syndromes or sciatic mimics (SM) may further confound the correct diagnosis of the pathology at hand.
Delayed diagnosis and intervention for CES and CMS can lead to permanent neurologic morbidity, including permanent loss of bowel and bladder function. Given the controversy surrounding their definition/diagnosis and the potential for major disability, there has been an ever-increasing occurrence of litigation and medicolegal cases internationally against healthcare workers and hospitals regarding CES and CMS.
The goal of this study is to perform a systematic literature review of all relevant recent studies related to CES, CMS, and SM. We then used a Delphi method during two meetings to generate seven consensus statements from the World Federation of Neurosurgical Societies (WFNS) Spine Committee. These guidelines provide the latest evidence-based recommendations on the definition, classification, diagnosis, and management of CES, CMS, and SM.
2. Methods
2.1. Literature review
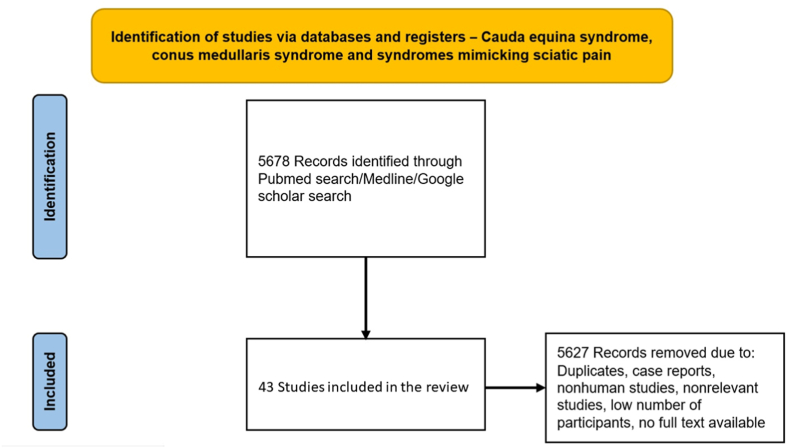
We performed a comprehensive online search using the PubMed/Medline database from 2012 to 2012 using the following keywords: “cauda equina syndrome”, “conus medullaris syndrome”, “sciatica”, “sciatica mimics”. Initial search yielded 5678 articles. Case reports, non-human studies, non-English language articles, articles without full text, and non-relevant articles were excluded, yielding 43 relevant articles for final review (Fig. 1). We focused specifically on prospective and retrospective studies with >50 patients, randomized controlled trials, systematic reviews, and meta-analyses and adhered to PRISMA guidelines.
Fig. 1.
We summarized the relevant literature in order to address the following questions.
-
1)
What are the most precise definitions for cauda equina, cauda equina syndrome (CES), conus medullaris, and conus medullaris syndrome (CMS)?
-
2)
What is the role of MRI in CES and CMS diagnosis?
-
3)
Is there a role for digital rectal examination and/or ultrasound examination of bladder in diagnosing or excluding CES or CMS?
-
4)
What are current recommendations for use of steroids in CES?
-
5)
What is the optimal timeframe for surgical decompression in acute CES, and how does this correlate with outcomes?
-
6)
What are non-spinal origins of sciatic syndrome, also known as sciatica mimics (SM)?
-
7)
How do we diagnose and manage piriformis syndrome?
2.2. Consensus meetings
Summarized literature data was presented at two consensus meetings, the first in Karachi, Pakistan, in May 2022, followed by a second meeting in Istanbul, Turkey in Sep 2022. During both meetings, the consensus statements were discussed and voted on by XX members of the World Federation of Neurosurgical Societies (WFNS) Spine Committee, who are attending neurosurgeons and international experts in spine care.
We utilized the Delphi method, and each participant voted independently and anonymously using a Likert scale from 1 to 5 (1 = strongly agree, 2 = agree, 3 = somewhat agree, 4 = disagree, 5 = strongly disagree). Results were presented as a percentage of respondents who scored each item as 1, 2 or 3 (agreement) or as 4 or 5 (disagreement). Consensus was achieved when the sum for disagreement or agreement was ≥66%.
3. Results and discussion
3.1. Definition: Cauda equina and cauda equina syndrome (CES)
The term cauda equina was first described by the French anatomist Andre du Laurens in 1595 as the rope-like tail of fibers at the distal end of the spinal cord. He described this bunch of axons which represent the bundle of lumbar, sacral and coccygeal roots surrounding the filum terminale and caudal to the spinal cord as “cauda equina” which is Latin for “horse's tail”.4
We propose the following as the most precise definition of cauda equina. The cauda equina is the bundle of axons arising from the distal part of the spinal cord, usually around L1 vertebra level, which comprises the sensory and motor axons of all the lumbar, sacral and coccygeal nerve roots. These nerve roots resemble a thick bundle of fibers mimicking a horse’s tail.
Mixter and Barr first described cauda equina syndrome due to a ruptured intervertebral disc in 1934. Since then, various authors have described CES, and up to seventeen different definitions of CES have been reported in the literature.2,5 Todd and colleagues described five characteristic features of CES, including bilateral sciatica, reduced perineal sensation, loss of anal tone, sexual dysfunction, and altered bladder function ultimately leading to painless urinary retention. They further note that not all symptoms will be present in any individual patient and that no symptom or sign individually or in combination can reliably diagnose or exclude CES. Furthermore, the diagnostic accuracy of digital rectal examination/anal tone has been questioned multiple times in literature. Tabrah and colleagues in a meta-analysis report DRE of minimal clinical value in diagnosing CES and that it may give false reassurance to the examiner.6
Building upon the work of Fraser5 and Lavy,2 we propose the following definition for CES: Cauda equina syndrome is a clinical diagnosis resulting from dysfunction of one or more of the sacral nerve roots from S2 and below. One or more of the following symptoms or signs must be present: (1) Bladder and/or bowel dysfunction; (2) Reduced sensation in the saddle area; (3) Sexual dysfunction. Back and leg pain, as well as lower limb motor/sensory changes or radiculopathy are often present but are not essential for the diagnosis of CES.
3.2. Definition: conus medullaris (CM) and conus medullaris syndrome (CMS)
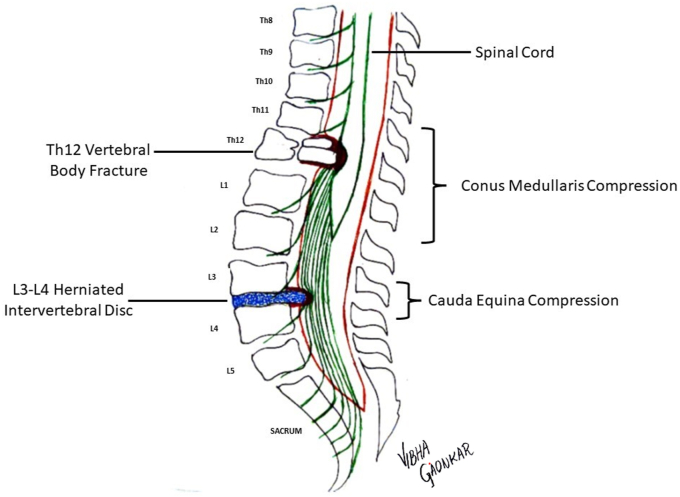
The conus medullaris (CM) anatomically represents the terminal part of the spinal cord which ends at a mean level of L1. Cadaveric and MRI studies report the anatomical level of the CM ranging from the lower third of vertebrae T11 to the upper third of L3.7 The difference between the conus medullaris and the more inferiorly located cauda equina is demonstrated in Fig. 2.
Fig. 2.
We propose the following as the most precise definition of conus medullaris: The conus medullaris is defined as the terminal part of the spinal cord ending at a mean level of L1 with individual variations between lower third of T11 to upper third of L2. At this level, there are exiting lower motor neuron cauda equina roots adjacent to the upper motor neurons of the spinal cord.
There is significant overlap in the literature between the definitions of conus medullaris syndrome (CMS) and CES. Jefferson first described traumatic injury to the CM and CE and that injury to vertebral level Th12 or L1 might affect the CM and injuries below the level may affect CE.8 Lesions at the level of CM affect both the upper and lower motor neurons (as compared to involvement of only lower motor neurons in CES) and may therefore present with mixed signs. As compared to CES, the involvement of motor and sensory dermatomyotomes is often symmetrical in CMS.7,9, 10, 11 However, clinical examination alone may not be sufficient to label a compressive spinal lesion as CMS or CES, and MRI imaging is usually needed to identify the correct level for appropriate diagnosis.
Building upon the work of Brouwers and colleagues,7 we propose the following as the most precise definition of conus medullaris syndrome (CMS): CMS is defined by the presence of one or more of the following: (1) Bladder and/or bowel dysfunction; (2) Reduced sensation in the saddle area; (3) Sexual dysfunction. Motor weakness may or may not be present; if present, it is predominantly symmetrical. The radiological level of compression must correspond to T12-L1 vertebral level. The neurological level of injury is between T12 and S5.
3.3. Classification systems for CES and CMS
There are multiple classification systems for CES and CMS that have been proposed in the literature, each with their own advantages/disadvantages. Importantly, no classification system can reliably predict the history of the disease. For instance, some patients with complete CES with urinary retention may benefit from surgery even in a delayed fashion,12, 13, 14 while other patients with early CES may worsen over the next few hours and end up with irreversible bladder dysfunction.15 Other patients with incomplete CES may remain stable for prolonged periods after presentation.
The Tandon and Sankaran classification system16 was first proposed in 1967. It focuses primarily on prior history of back problems, which is not felt to be as relevant today. In particular, this classification system does not stratify partial, incomplete, or impending cauda equina syndrome and is rarely used today.
The more recent Gleave and McFarlane classification system focuses on the importance of incomplete bladder dysfunction (early urinary difficulties such as altered urinary sensation, loss of desire to void, poor stream, or straining when voiding) versus complete urinary dysfunction (defined as painless urinary retention with overflow). These authors state that once CES with retention is established, the timing of surgery may not significantly impact patient outcome. In contrast, they argue that incomplete CES warrants urgent treatment. This classification system is used more widely in recent literature, with many authors modifying and adding appropriate suffixes to enhance the applicability of this system. Additional recent and comprehensive classification CES/CMS systems include the 2010 Shi classification system and 2018 Cauda Equina Scoring systems (Table 1).
Table 1.
Cauda Equina Syndrome (CES) Classification Systems. *indicates preferred classification system of WFNS Spine Committee.
| Tandon and Sankaran classification (1967)16 | Type 1- A rapid onset of CES symptoms with no history of back problems Type 2- Acute bladder/CES symptoms with a history of back problems and sciatia Type 3- Long-standing back problems and gradually progressive CES, often with spinal stenosis |
|---|---|
| Gleave and McFarlane (2002)17 |
CES-I– Incomplete- this represents urinary difficulties of neurogenic origin such as altered urinary sensation, loss of desire to void, poor stream or the need to strain, but there is still executive control of bladder function and voiding is possible even if difficult. CES-R– Retention- this occurs when the bladder is no longer under executive control and there is painless retention of urine with overflow. |
| Shi et al (2010)18 |
Group 1 (preclinical) - low-back pain with only bulbocavernosus reflex (BCR) and ischiocavernosus reflex (ICR) abnormalities and no typical symptoms of CES. Group 2 (early) saddle sensory disturbance and bilateral sciatica. Group 3 (middle): saddle sensory disturbance, bowel or bladder dysfunction, motor weakness of the lower extremity, and reduced sexual function. Group 4 (late): absence of saddle sensation and sexual function in addition to uncontrolled bowel function. |
| Cauda Scoring System (2018)19 | (1) Perineal sensation: Graded 0 to 3, where 0 is absent sensation and 3 is normal perineal sensation (2) Anal tone/squeeze: Graded 0 (absent), 1 (reduced), 2 (present) (3) Bladder function: Graded 0 (painless retention >500 mL or painless incontinence), 1 (straining to pass urine with reduced bladder or urethral sensation), 2 (reduced bladder or urethral sensation with normal micturition control), 3 (urgency or hesitancy, but normal bladder sensation and control), 4 (normal bladder function) |
| Lavy et al modified CESI/CESR system (2022)* | Suspected CES (CESS): No bladder/bowel/genital/perineal symptoms, but bilateral sciatica or motor/sensory loss in legs (clinical CESS) Or Known large disc herniation on existing MRI (radiological CESS) Symptom only CES (Early CES, CESE)- Normal bladder, bowel and sexual function but some sensory loss in perineum or change in micturition frequency Incomplete CES (CESI)- Alteration in bladder/urethral sensation or function, but maintenance of executive bladder control. ± perineal sensory changes, or sexual or bowel sensory or functional changes CES with urinary retention (CESR)- As in CESI, but with painless bladder retention and overflow Complete CES (CESC)- Insensate bladder with overflow incontinence, no perineal perianal or sexual sensation, no anal tone |
We recommend the use of the Lavy et al modified CESI/CESR classification system (Table 1). This classification system is particularly relevant because it includes the entity of suspected cauda equina syndrome. First described in a paper by Todd,20 suspected CES is seen in patients with bilateral radiculopathy and/or subjective sphincter problems without any objective evidence of CES. The Lavy system also defines early symptom-only CES as an entity separate from incomplete CES. These patients have some subjective and/or objective evidence of perineal sensory impairment without any bladder problems. These patients would not fall under the category of CESI (incomplete CES), which also includes alteration in bladder/urethral sensation or function. Finally, the Lavy system includes a late stage CES (complete CES), where there is loss of all motor and sensory and autonomic functions below the affected level. It may present as an insensate bladder with overflow incontinence, bowel dysfunction, a patulous anus, and complete loss of muscle power. This situation is more extreme than CESR, where some genital/perineal sensation may be maintained.2 In patients with difficulties in micturition but preserved perineal sensation, a neurogenic origin of micturition dysfunction could be delayed. Such patients fall under the category of Early CES.21
3.4. Evaluation of CES
The diagnosis of cauda equina syndrome requires a high index of suspicion. CES patients usually present with sciatic pain symptoms. It is often difficult to pinpoint the exact time frame, but subtle changes in urinary habits and perineal sensation must be elicited from the patient. Urinary retention with overflow incontinence indicates late stage CES and by this time, bowel/bladder damage may be irreversible.3
Todd described potential red and white flags which may aid the emergency physician or outpatient physician to take necessary steps to refer or treat patients before permanent neurological morbidity occurs. Red flags are signs of caution which must alert the physician to evaluate further and triage patients as CESS (suspected CES) until proven otherwise, while white flags constitute potentially irreversible situations. Definite red flags include bilateral radiculopathy and progressive neurological deficits in the legs. Possible red or white flags include impaired perineal sensation and/or anal tone and/or unspecified urinary disturbance. Definite white flags correspond to signs of CESR/CESC—namely, perineal anesthesia, urinary retention/incontinence, and fecal incontinence.
3.5. Role and timing of MRI
A non-contrast MRI of the lumbar spine is the preferred and most important investigation to diagnose compression of the cauda equina structures and to determine the cause of compression including disc herniation, hematoma, tumor, etc.22 A vast majority of patients who present with symptoms of CES and they turn out scan negative for CE compression. Hoeritzauer reported 61% patients presenting with symptoms of CES as Scan Negative.23 Acute severe pain, affecting the brain bladder feedback has been postulated as a possible cause for the development of symptoms.24 We recommend urgent MRI in all patients with suspected CES (CESS), considering the low sensitivity of clinical examination in excluding CES.6,15,25, 26, 27, 28, 29
3.6. Role of bladder ultrasound
Many authors advocate for the routine use of bladder ultrasound for detection of post void residual (PVR) urine volume as a surrogate marker for assessment of bladder function.30 Ultrasound is a more widely available diagnostic tool as compared to MRI, which is expensive and may not be available at all centers or at all hours. Venkatesan et al reported a sensitivity of 94%, specificity of 72%, and positive predictive value of 43% for CES when a PVR cutoff of >200 mL was used.31 In a study of 260 patients, Katzouraki et al found that a PVR cutoff >200 mL had a 94% sensitivity, 66.8% specificity, 29.9% positive predictive value, and 98.7% negative predictive value for CES.30 Another study reported that a PVR volume >500 mL had a sensitivity of 100% and a specificity of 93% in detecting cauda equina syndrome.25
While ultrasound can help detect changes in bladder function, it may not replace an MRI scan even in an emergency setting. Its use is encouraged where there is unavailability of urgent MRI service and patient has a suspected CES in order to document bladder function. A PVR <200 mL may then be considered for earliest possible MRI, while PVR>200 mL should be considered for transfer to a center with emergency MRI service. It should be noted, however, that in a review of 50 medicolegal cases of CES, 50% (13/26) patients with a Clinical/MRI positive CES had a PVR of <200 mL. We therefore recommend that bladder scan/PVR does not dictate the decision to get an early MRI scan.
3.7. Management and timing of surgery for acute CES
Surgery to halt progression and potentially reverse neurologic deficit is the mainstay of treatment for MRI-proven cauda equina syndrome.17 The goal of surgery is decompression of the neural structures. Once diagnosis is established, there is no reason to delay spinal decompression. Animal studies have shown that not only the duration of compression but also the degree of compression play a major role in final outcome.32
Ahn et al (2000) observed superior outcomes in recovery of sensory and motor function when surgical intervention was performed before 48 h.33 This time window was challenged in future studies, with some authors stating no difference in outcomes irrespective of timing and that initial severity of symptoms dictated outcomes,34 while others proposed a 24-h time window as the ideal time for surgical decompression.35,36 In a recent prospective cohort study of 621 patients with acute CES, there was no correlation observed between timing of surgery and neurological outcomes. However, it must be noted that the time of onset can be highly arbitrary with patient reported timings and different time reporting methodologies between papers. The authors also noted that patients requiring catheterization preoperatively or those who were deteriorating rapidly were taken for surgery earlier.37 A review of major studies highlighting the timing of surgery and outcomes for CES is shown in Table 2.
Table 2.
Major studies highlighting the timing of surgery and outcomes for CES.
| Study (Ref) | Year | Design | Methodology | Conclusion |
|---|---|---|---|---|
| Srikandarajah et al37 | 2015 | Retrospective, single centre | 200 patients (61 CESR, 139 CESI) different timepoints of symptom onset (24h, 48h, 72h), all with MRI confirmed compressive origin, all underwent decompression within 48h after admission 3 months follow-up |
Rate of improvement of bladder function deteriorated continuously depending on time between symptom onset and surgery with CESI patients (88% at <24h to 50% at >72h), With CESR surgery timing had no sig. Influence was roughly 26% in total |
| Thakur et al36 | 2017 | Retrospective multi centre | 4066 patients all grades of CES, Nationwide inpatient Sample (NIS, USA) database 2005–2011, timing of decompression surgery after hospital admission <24h, 24–48h, later | Patients operated within 24h of admission showed sig. Better outcome regarding neurological deficits, hospitality charges, hospitality stay and mortality than those operated 24–48 and > 48h. |
| Heyes et al38 | 2018 | Retrospective, single centre | 136 patients, all grades of CES), all with MRI confirmed compressive origin, all underwent decompression within 48h after admission, 2 y follow-up | Independent of surgery timing sig. Improvement to preop baseline. Surgery <24h of symptom onset not favourable vs Surgery at 24–48h. After 6 months no further improvement of bladder function. |
| Hogan et al35 | 2019 | Retrospective multi centre | 20,924 patients all grades of CES, Nationwide Inpatient Sample (NIS, USA) database 2000–2014. Timing of surgery, two groups <48 and later. | Day 0 and 1 surgery 0 (<48h after admission) is superior to later performed surgery. |
| Planty-Bonjour, Alexia et al34 | 2022 | Prospective, single center | 140 Consecutive patients undergoing surgery for CES | Poor functional prognosis overall, initial severity of symptoms – highly prognostic of outcome, No difference between early or late decompression (<24 or 48 h or later) |
Taking this data together, we recommend early surgery, preferably within 24 h or less than 48 h from onset of acute CES symptoms. In case the diagnosis or presentation is beyond the 48-h time frame, surgery should be considered as early as possible.
3.8. Management and timing of surgery for CMS
There is a lack of studies specifically discussing the management of acute conus medullaris compression. There is therefore a need for further research on the approach and management of CMS. Recommendations and guidelines may be extrapolated from data available for CES. However, CMS represents a distinct entity with involvement of spinal cord along with the nerve roots. Recommended imaging (specifically, MRI) and clinical evaluation remain similar for CMS as they are for CES. Timing of surgery for acute CMS may also be considered similar to CES, i.e. preferably within 48 h.
3.9. Outcomes after surgical decompression of CES
The outcomes following surgery for CES are variable and depend on the symptoms at the time of presentation.38, 39, 40, 41, 42 In a cohort study of 621 patients, Woodfield et al reported complete symptom resolution at hospital discharge in 45% (273/608) patients, with ongoing back pain in 18% (110/608) and ongoing sciatica in 8% (46/608). Pain scores were lower at discharge compared to admission. 66% (188/284) patients reported leg weakness, and 51% (125/244) had altered sensation at one year post-op.37 49% (117/241) participants had bladder symptoms at the time of hospital discharge. At one year, 50% (122/245) patients reported abnormal bladder function, with 34% (83/242) not always able to tell when their bladder was full. Of those who were catheterized pre-operatively, 30% (57/190) required a catheter at discharge, and 26% (22/84) at one year.37 Preoperative catheterization and absent perianal sensation has been reported as a marker for poor bladder function postoperatively and essentially signifies complete CES.
3.10. Role of steroids in CES/CMS
The role of steroids in the management of acute CES not well established. A literature review yields no significant results mentioning use of steroids for acute CES. Some authors recommend use of high dose corticosteroids in the emergency department while awaiting evaluation and/or surgery.43 Given potential significant side effects of steroids, there is need for further studies to better elucidate their role in acute CES. The role of steroids in CMS may be extrapolated from the data on steroid use in acute spinal cord injury.
3.11. Role of intraoperative-neuromonitoring
A literature survey did not yield any results pertaining to the regular use of intraoperative neuromonitoring (IONM) in the management of patients with acute CES/CMS. Extrapolating from the established use of IONM in neoplastic pathologies and in spinal dysraphism surgery, it may be used as an added adjunct in the surgical management of acute CMS.
3.12. Syndromes mimicking sciatica
The evaluation of a patient with lumbosacral radiculopathy requires that the physician understand other conditions and extraspinal causes of “sciatic-type” pain. The term “sciatica” or ischias (Greek ἰσχιάς) was used by ancient Greeks to describe pain around the hip or thigh.44 Sciatica technically means any pain transmitted along the sciatic nerve (SN) roots and along the course of the SN. That being said, “sciatica” is commonly used to describe radiculopathic leg symptoms that may be in the distribution of any of the lumbosacral nerve roots. Lumbar disc herniations are responsible for approximately 90% of radiculopathy cases. History and physical examination form the mainstay of diagnosis. However, no combination of physical and clinical characteristics have a high sensitivity and specificity.45 The straight leg raise test (Lasegue's sign) is the most commonly used physical test to detect lumbosacral radiculopathy, with reported sensitivity of 91% and specificity of 26%. The crossed straight leg raise test has a reported pooled specificity of 88% and sensitivity of 29%.46
Other major causes of sciatica include spinal stenosis, spondylolisthesis, as well as piriformis syndrome, neoplastic pelvic lesions, and pregnancy.44,47 Other sciatica mimics (SM) include cyclical or catamenial sciatica, wallet neuritis, superior cluneal nerve disorders, referred pain from the quadratus lumborum (QL), gluteal medius tendinitis, myofascial pain syndrome (MPS), and post injection sciatica.47,48 The various pathologies which can present with sciatic-like pain are listed in Table 3.
Table 3.
Sciatica like syndromes and their mimics.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3.13. Pyriformis syndrome
One of the more common sciatica mimics (SM), piriformis syndrome arises from sciatic nerve entrapment at the level of the ischial tuberosity. It can mimic classical sciatic pain of spinal origin. Patients complain of pain in the gluteal/buttock region that may radiate down to the lower extremities. This may be accompanied by numbness in the buttocks and tingling along the distribution of the sciatic nerve. Piriformis syndrome is a clinical diagnosis after exclusion of other pain syndromes. It includes patients with chronic buttock and posterior hip pain without correlation on the neuroimaging.63,64 Positive clinical examination including the pyriformis stretch test, active piriformis test, Beatty Test, FAIR Test, Pace Test, and/or tenderness upon palpation of the greater sciatic notch.50,65 For diagnosis of piriformis syndrome, at least one of the piriformis stretch tests should be positive.
Management of pyriformis syndrome initially includes rest, NSAIDs, muscle relaxants, gabapentin and/or physical therapy. Second-line therapy may include steroid or local anesthetic injections directly into the body of the piriformis muscle.66 Botulinum toxin injections into the muscle can also be considered for pain reduction.50,67 For cases refractory to these conservative treatments, surgical intervention via cutting of the piriformis muscle tendon and sciatic nerve decompression may be considered.68, 69,70
4. WFNS spine committee recommendations
Taking this literature in summary, and via the two rounds of voting outlined in our methods section, the WFNS Spine Committee proposed the following seven consensus statements on the definition, diagnosis, and management of cauda equina syndrome (CES), conus medullaris syndrome (CMS), and sciatica mimics (SM). See Table 4 for final voting on each statement.
-
1.
The optimal treatment for MRI- proven cauda equina syndrome and conus medullaris syndrome is decompression surgery.
-
2.
Studies regarding cauda equina and conus medullaris syndrome showed improvement after surgery compared to baseline. Surgery should be performed as soon as possible. Better outcomes are expected if the surgery is performed within 24 h.
-
3.
The outcome in cauda equina and conus medullaris syndrome depends on multiple factors, especially preoperative neurological severity.
-
4.
Evidence for the benefit of steroids in cauda equina and conus medullariis syndrome is lacking.
-
5.
Piriformis syndrome is a clinical diagnosis after exclusion of other pain syndromes. It includes patients with chronic buttock and posterior hip pain without correlation on neuroimaging.
-
6.
For the diagnosis of piriformis syndrome, at least one of the piriformis stretch tests should be positive.
-
7.
Conservative therapy with pain medication and physical therapy is the first line treatment for piriformis syndrome. Second line treatment is local lidocaine and botulinum injections. Surgery (decompression with/without piriformis muscle resection) is reserved for selected refractory cases, which showed good but short-term relief after injections.
Table 4.
Statements voted after “Lumbar disc herniation: Prevention and Treatment of Recurrence” statements.
| Statement | Likert type scale | No of respondents |
|---|---|---|
| Statement 1: The optimal treatment modality for Cauda equina syndrome and Conus medularis syndrome with MRI confirmed compression is decompression surgery. |
1. Strongly agree 2. Agree 3. Somewhat agree 4. Disagree 5. Strongly disagree |
9 (100%) |
| Statement 2: Studies regarding Cauda equina- and Conus medularis syndrome showed improvement after surgery compared to baseline. Surgery should be performed as soon as possible. We can expect better outcomes if the surgery is performed within 24h. |
1. Strongly agree 2. Agree 3. Somewhat agree 4. Disagree 5. Strongly disagree |
6 (66.7%) 3 (33.3%) |
| Statement 3: The outcome in Cauda equina- and Conus medularis syndrome highly depends on various factors, especially its preoperative neurological severity. |
1. Strongly agree 2. Agree 3. Somewhat agree 4. Disagree 5. Strongly disagree |
6 (66.7%) 2 (22.2%) 1 (11.1%) |
| Statement 4: Evidence for the benefit of steroids in Cauda equina- and Conus medularis syndrome is lacking. |
1. Strongly agree 2. Agree 3. Somewhat agree 4. Disagree 5. Strongly disagree |
7 (77.8%) 2 (22.2%) |
| Statement 5: Piriformis syndrome is a clinical diagnosis after exclusion of other pain syndromes. It includes patients with chronic buttock and posterior hip pain without correlation in the neuroimaging. |
1. Strongly agree 2. Agree 3. Somewhat agree 4. Disagree 5. Strongly disagree |
6 (66.7%) 2 (22.2%) 1 (11.1%) |
| Statement 6: For the diagnosis of piriformis syndrome, at least one of the piriformis stretching tests needs to be positive. |
1. Strongly agree 2. Agree 3. Somewhat agree 4. Disagree 5. Strongly disagree |
6 (66.7%) 2 (22.2%) 1 (11.1%) |
| Statement 7: Conservative therapy with pain medication and physical therapy is the first line treatment for piriformis syndrome. Second line treatment is local lidocaine and botulinum injections. Surgery (decompression with/without piriformis muscle resection) is reserved for selected refractory cases, which showed good but short-term effect of injections. |
1. Strongly agree 2. Agree 3. Somewhat agree 4. Disagree 5. Strongly disagree |
6 (66.7%) 3 (33.3%) |
5. Conclusion
Cauda equina syndrome and conus medullaris syndrome are medical emergencies which mandate a timely and appropriate diagnosis. MRI scanning is essential for confirming radiological cauda equina compression and need for surgical decompression. Early surgical decompression within 48 h and preferably within 24 h is recommended once diagnosis of CES/CMS is established. There is no data on the role of steroids in treatment of acute CES/CMS. The treating physician must be cognizant of a variety of other pathologies which may mimic sciatica, including piriformis syndrome.
CRediT authorship contribution statement
Sandeep Vaishya: Writing – original draft, Data curation, Conceptualization. Mirza Pojskic: Data curation, Conceptualization, Writing – review & editing. Manbachan Singh Bedi: Data curation, Visualization, Writing – original draft, Writing – review & editing. Joachim Oertel: Conceptualization, Data curation, Writing – review & editing. Christoph Sippl: Writing – review & editing, Conceptualization, Data curation. Scott Robertson: Data curation, Conceptualization, Writing – original draft. Corinna Zygourakis: Validation, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
- CM –
Conus medullaris
- CMS –
Conus medullaris syndrome
- CES –
Cauda equina syndrome
- SM –
Sciatica mimics
- WFNS –
World Federation of Neurosurgical Societies
- CESI –
Cauda equina syndrome incomplete
- CESC -
Cauda equina syndrome complete
- CESR –
Cauda equina syndrome with retention
- CESS –
Suspected cauda equina syndrome
- PVR –
Post void residual urine
- IONM –
Intraoperative neuromonitoring
- SN –
Sciatic Nerve
- MPS –
Myofascial pain syndrome
References
- 1.Hoeritzauer I., Wood M., Copley P.C., Demetriades A.K., Woodfield J. What is the incidence of cauda equina syndrome? A systematic review. J Neurosurg Spine. 2020:1–10. doi: 10.3171/2019.12.SPINE19839. [DOI] [PubMed] [Google Scholar]
- 2.Lavy C., Marks P., Dangas K., Todd N. Cauda equina syndrome-a practical guide to definition and classification. Int Orthop. 2022 Feb;46(2):165–169. doi: 10.1007/s00264-021-05273-1. Epub 2021 Dec 4. PMID: 34862914; PMCID: PMC8782783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bulloch L., Thompson K., Spector L. Cauda equina syndrome. Orthop Clin N Am. 2022;53:247–254. doi: 10.1016/j.ocl.2021.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Olry R., Haines D.E. Between André Du Laurens' horse tail and William Cadogan's pony tail. J Hist Neurosci. 2012;21:327–331. doi: 10.1080/0964704X.2012.678135. [DOI] [PubMed] [Google Scholar]
- 5.Fraser S., Roberts L., Murphy E. Cauda equina syndrome: a literature review of its definition and clinical presentation. Arch Phys Med Rehabil. 2009;90:1964. doi: 10.1016/j.apmr.2009.03.021. –8. [DOI] [PubMed] [Google Scholar]
- 6.Tabrah J., Wilson N., Phillips D., Böhning D. Can digital rectal examination be used to detect cauda equina compression in people presenting with acute cauda equina syndrome? A systematic review and meta-analysis of diagnostic test accuracy studies. Musculoskelet Sci Pract. 2022;58 doi: 10.1016/j.msksp.2022.102523. [DOI] [PubMed] [Google Scholar]
- 7.Brouwers E., van de Meent H., Curt A., Starremans B., Hosman A., Bartels R. Definitions of traumatic conus medullaris and cauda equina syndrome: a systematic literature review. Spinal Cord. 2017;55:886–890. doi: 10.1038/sc.2017.54. [DOI] [PubMed] [Google Scholar]
- 8.Jefferson G. Discussion on spinal injuries. Proc Roy Soc Med. 1928;21:625–648. [PMC free article] [PubMed] [Google Scholar]
- 9.Orendácová J., Cízková D., Kafka J., et al. Cauda equina syndrome. Prog Neurobiol. 2001;64 doi: 10.1016/s0301-0082(00)00065-4. 613–37. [DOI] [PubMed] [Google Scholar]
- 10.Podnar S. Epidemiology of cauda equina and conus medullaris lesions. Muscle Nerve. 2007;35:529–531. doi: 10.1002/mus.20696. [DOI] [PubMed] [Google Scholar]
- 11.Wagner R., Jagoda A. Spinal CORD syndromes. Emerg Med Clin. 1997;15:699–711. doi: 10.1016/s0733-8627(05)70326-6. [DOI] [PubMed] [Google Scholar]
- 12.Jha V., Deep G., Pandita N., Ahuja K., Ifthekar S., Kandwal P. Factors affecting urinary outcome after delayed decompression in complete cauda equina syndrome: “A regression model study.”. Eur J Trauma Emerg Surg. 2022;48:1009–1016. doi: 10.1007/s00068-020-01589-6. [DOI] [PubMed] [Google Scholar]
- 13.Lai X.-W., Li W., Wang J.-X., Zhang H.-J., Peng H.-M., Yang D.-H. [Delayed decompression for cauda equina syndrome secondary to lumbar disc herniation: long-term follow-up results] Nan Fang Yi Ke Da Xue Xue Bao. 2017;37:1143–1148. doi: 10.3969/j.issn.1673-4254.2017.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarkar S., Hossen M.K., Mazumder U., Dey A. Surgical outcome of cauda equina syndrome secondary to disc herniation presenting late in developing countries. Mymensingh Med J. 2022;31(4):1121–1127. [PubMed] [Google Scholar]
- 15.Todd N.V., Dickson R.A. Standards of care in cauda equina syndrome. Br J Neurosurg. 2016;30:518–522. doi: 10.1080/02688697.2016.1187254. [DOI] [PubMed] [Google Scholar]
- 16.Tandon P.N., Sankaran B. Cauda equina syndrome due to lumbar disc prolapse. Indian J Orthop. 1967;1(2):112–119. ISSN 0019-5413. [Google Scholar]
- 17.Gleave J.R.W., Macfarlane R. Cauda equina syndrome: what is the relationship between timing of surgery and outcome? Br J Neurosurg. 2002;16:325–328. doi: 10.1080/0268869021000032887. [DOI] [PubMed] [Google Scholar]
- 18.Shi J., Jia L., Yuan W., et al. Clinical classification of cauda equina syndrome for proper treatment. Acta Orthop. 2010;81:391–395. doi: 10.3109/17453674.2010.483985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Todd N.V. Quantifying the clinical aspects of the cauda equina syndrome - the Cauda Scale (TCS) Br J Neurosurg. 2018;32:260–263. doi: 10.1080/02688697.2018.1441975. [DOI] [PubMed] [Google Scholar]
- 20.Todd N.V. Guidelines for cauda equina syndrome. Red flags and white flags. Systematic review and implications for triage. Br J Neurosurg. 2017;31:336–339. doi: 10.1080/02688697.2017.1297364. [DOI] [PubMed] [Google Scholar]
- 21.Todd N.V. Early cauda equina syndrome (CESE) Br J Neurosurg. 2017;31:400. doi: 10.1080/02688697.2017.1322175. [DOI] [PubMed] [Google Scholar]
- 22.Quaile A. Cauda equina syndrome-the questions. Int Orthop. 2019;43:957–961. doi: 10.1007/s00264-018-4208-0. [DOI] [PubMed] [Google Scholar]
- 23.Hoeritzauer I., Carson A., Statham P., et al. Scan-negative cauda equina syndrome: a prospective cohort study. Neurology. 2021;96:e433–e447. doi: 10.1212/WNL.0000000000011154. [DOI] [PubMed] [Google Scholar]
- 24.Hoeritzauer I., Stanton B., Carson A., Stone J. “Scan-negative” cauda equina syndrome: what to do when there is no neurosurgical cause. Practical Neurol. 2022;22:6–13. doi: 10.1136/practneurol-2020-002830. [DOI] [PubMed] [Google Scholar]
- 25.Gooding B.W.T., Higgins M.A., Calthorpe D.A.D. Does rectal examination have any value in the clinical diagnosis of cauda equina syndrome? Br J Neurosurg. 2013;27:156–159. doi: 10.3109/02688697.2012.732715. [DOI] [PubMed] [Google Scholar]
- 26.Bell D.A., Collie D., Statham P.F. Cauda equina syndrome: what is the correlation between clinical assessment and MRI scanning? Br J Neurosurg. 2007;21:201–203. doi: 10.1080/02688690701317144. [DOI] [PubMed] [Google Scholar]
- 27.Domen P.M., Hofman P.A., van Santbrink H., Weber W.E.J. Predictive value of clinical characteristics in patients with suspected cauda equina syndrome. Eur J Neurol. 2009;16:416–419. doi: 10.1111/j.1468-1331.2008.02510.x. [DOI] [PubMed] [Google Scholar]
- 28.Balasubramanian K., Kalsi P., Greenough C.G., Kuskoor Seetharam M.P. Reliability of clinical assessment in diagnosing cauda equina syndrome. Br J Neurosurg. 2010;24:383–386. doi: 10.3109/02688697.2010.505987. [DOI] [PubMed] [Google Scholar]
- 29.Curtis Lopez C., Berg A.J., Clayton B., et al. Evaluation of the role of anal tone and perianal sensation examination in the assessment of suspected cauda equina syndrome. Br J Neurosurg. 2021:1–5. doi: 10.1080/02688697.2021.2005775. [DOI] [PubMed] [Google Scholar]
- 30.Katzouraki G., Zubairi A.J., Hershkovich O., Grevitt M.P. A prospective study of the role of bladder scanning and post-void residual volume measurement in improving diagnostic accuracy of cauda equina syndrome. Bone Joint Lett J. 2020;102-B:677–682. doi: 10.1302/0301-620X.102B6.BJJ-2020-0195.R1. [DOI] [PubMed] [Google Scholar]
- 31.Venkatesan M., Nasto L., Tsegaye M., Grevitt M. Bladder scans and postvoid residual volume measurement improve diagnostic accuracy of cauda equina syndrome. Spine. 2019;44:1303–1308. doi: 10.1097/BRS.0000000000003152. [DOI] [PubMed] [Google Scholar]
- 32.Pronin S., Koh C.H., Bulovaite E., Macleod M.R., Statham P.F. Compressive pressure versus time in cauda equina syndrome: a systematic review and meta-analysis of experimental studies. Spine. 2019;44:1238–1247. doi: 10.1097/BRS.0000000000003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahn U.M., Ahn N.U., Buchowski J.M., Garrett E.S., Sieber A.N., Kostuik J.P. Cauda equina syndrome secondary to lumbar disc herniation: a meta-analysis of surgical outcomes. Spine. 2000;25:1515–1522. doi: 10.1097/00007632-200006150-00010. [DOI] [PubMed] [Google Scholar]
- 34.Planty-Bonjour A., Kerdiles G., François P., et al. Cauda equina syndrome: poor recovery prognosis despite early treatment. Spine. 2022;47:105–113. doi: 10.1097/BRS.0000000000004170. [DOI] [PubMed] [Google Scholar]
- 35.Hogan W.B., Kuris E.O., Durand W.M., Eltorai A.E.M., Daniels A.H. Timing of surgical decompression for cauda equina syndrome. World Neurosurg. 2019;132:e732–e738. doi: 10.1016/j.wneu.2019.08.030. [DOI] [PubMed] [Google Scholar]
- 36.Thakur J.D., Storey C., Kalakoti P., et al. Early intervention in cauda equina syndrome associated with better outcomes: a myth or reality? Insights from the Nationwide Inpatient Sample database (2005-2011) Spine J. 2017;17:1435–1448. doi: 10.1016/j.spinee.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 37.Woodfield J., Hoeritzauer I., Jamjoom A.A.B., et al. Presentation, management, and outcomes of cauda equina syndrome up to one year after surgery, using clinician and participant reporting: a multi-centre prospective cohort study. Lancet Reg Health Eur. 2023;24 doi: 10.1016/j.lanepe.2022.100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srikandarajah N., Boissaud-Cooke M.A., Clark S., Wilby M.J. Does early surgical decompression in cauda equina syndrome improve bladder outcome? Spine. 2015;40:580–583. doi: 10.1097/BRS.0000000000000813. [DOI] [PubMed] [Google Scholar]
- 39.Heyes G., Jones M., Verzin E., McLorinan G., Darwish N., Eames N. Influence of timing of surgery on Cauda equina syndrome: outcomes at a national spinal centre. J Orthop. 2018;15:210–215. doi: 10.1016/j.jor.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar V., Baburaj V., Rajnish R.K., Dhatt S.S. Outcomes of cauda equina syndrome due to lumbar disc herniation after surgical management and the factors affecting it: a systematic review and meta-analysis of 22 studies with 852 cases. Eur Spine J. 2022;31:353–363. doi: 10.1007/s00586-021-07001-0. [DOI] [PubMed] [Google Scholar]
- 41.Seidel H., Bhattacharjee S., Pirkle S., et al. Long-term rates of bladder dysfunction after decompression in patients with cauda equina syndrome. Spine J. 2021;21:803–809. doi: 10.1016/j.spinee.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Reddy A.P., Mahajan R., Rustagi T., Chhabra H.S. Bladder recovery patterns in patients with complete cauda equina syndrome: a single-center study. Asian Spine J. 2018;12:981–986. doi: 10.31616/asj.2018.12.6.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levis J.T. Cauda equina syndrome. West J Emerg Med. 2009 Feb;10(1):20. PMID: 19561762; PMCID: PMC2672294. [PMC free article] [PubMed] [Google Scholar]
- 44.Stafford M.A., Peng P., Hill D.A. Sciatica: a review of history, epidemiology, pathogenesis, and the role of epidural steroid injection in management. Br J Anaesth. 2007;99:461–473. doi: 10.1093/bja/aem238. [DOI] [PubMed] [Google Scholar]
- 45.Valat J.-P., Genevay S., Marty M., Rozenberg S., Koes B. Sciatica. Best Pract Res Clin Rheumatol. 2010;24:241–252. doi: 10.1016/j.berh.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Devillé W.L.J.M., van der Windt D.A.W.M., Dzaferagić A., Bezemer P.D., Bouter L.M. The test of lasègue. Spine. 2000;25:1140–1147. doi: 10.1097/00007632-200005010-00016. [DOI] [PubMed] [Google Scholar]
- 47.Siddiq M.A.B., Clegg D., Hasan S.A., Rasker J.J. Extra-spinal sciatica and sciatica mimics: a scoping review. Korean J Pain. 2020;33:305–317. doi: 10.3344/kjp.2020.33.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park C.-W., Cho W.-C., Son B.-C. Iatrogenic injury to the sciatic nerve due to intramuscular injection: a case report. Korean J Nutr. 2019;15:61–66. doi: 10.13004/kjnt.2019.15.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park J.W., Lee Y.-K., Lee Y.J., Shin S., Kang Y., Koo K.-H. Deep gluteal syndrome as a cause of posterior hip pain and sciatica-like pain. Bone Joint Lett J. 2020;102-B:556–567. doi: 10.1302/0301-620X.102B5.BJJ-2019-1212.R1. [DOI] [PubMed] [Google Scholar]
- 50.Probst D., Stout A., Hunt D. Piriformis syndrome: a narrative review of the anatomy, diagnosis, and treatment. Pharm Manag PM R. 2019;11(suppl 1) doi: 10.1002/pmrj.12189. S54–63. [DOI] [PubMed] [Google Scholar]
- 51.Siddiq M.A.B., Jahan I., Masihuzzaman S. Wallet neuritis - an example of peripheral sensitization. Curr Rheumatol Rev. 2018;14:279–283. doi: 10.2174/1573397113666170310100851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aldashash F., Elraie M. Solitary osteochondroma of the proximal femur causing sciatic nerve compression. Ann Saudi Med. 2017;37:166–169. doi: 10.5144/0256-4947.2017.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Solomons J.N.T., Sagir A., Yazdi C., Paresthetica Meralgia. Curr Pain Headache Rep. 2022;26:525–531. doi: 10.1007/s11916-022-01053-7. [DOI] [PubMed] [Google Scholar]
- 54.Siddiq M.A.B., Rasker J.J. Piriformis pyomyositis, a cause of piriformis syndrome-a systematic search and review. Clin Rheumatol. 2019;38:1811–1821. doi: 10.1007/s10067-019-04552-y. [DOI] [PubMed] [Google Scholar]
- 55.Mannan K., Altaf F., Maniar S., Tirabosco R., Sinisi M., Carlstedt T. Cyclical sciatica: endometriosis of the sciatic nerve. J Bone Joint Surg Br. 2008;90:98–101. doi: 10.1302/0301-620X.90B1.19832. [DOI] [PubMed] [Google Scholar]
- 56.Wadhwa V., Thakkar R.S., Maragakis N., et al. Sciatic nerve tumor and tumor-like lesions - uncommon pathologies. Skeletal Radiol. 2012;41:763–774. doi: 10.1007/s00256-012-1384-7. [DOI] [PubMed] [Google Scholar]
- 57.Visser L.H., Nijssen P.G.N., Tijssen C.C., van Middendorp J.J., Schieving J. Sciatica-like symptoms and the sacroiliac joint: clinical features and differential diagnosis. Eur Spine J. 2013;22:1657–1664. doi: 10.1007/s00586-013-2660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pacult M.A., Henderson F.C., Jr., Wooster M.D., Varma A.K. Sciatica caused by venous varix compression of the sciatic nerve. World Neurosurg. 2018;117:242–245. doi: 10.1016/j.wneu.2018.06.058. [DOI] [PubMed] [Google Scholar]
- 59.Mahmoudi S.F., Layeb M., Layouni S., et al. The Tarlov cyst: a cause of sciatica. Ann Phys Rehabil Med. 2016;59:e95. [Google Scholar]
- 60.Naber W.J., 2nd, Netzloff C.L., Bakshi R.R. Atypical cause for back pain and sciatica in a 35-year-old female with fibromyalgia. Am J Phys Med Rehabil. 2023 doi: 10.1097/PHM.0000000000002203. published online Feb 3. [DOI] [PubMed] [Google Scholar]
- 61.Koh E. Imaging of peripheral nerve causes of chronic buttock pain and sciatica. Clin Radiol. 2021;76:626.e1–626.e11. doi: 10.1016/j.crad.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 62.Goddyn C., Passuti N., Leconte R., Redon H., Gouin F. Sciatic nerve compression related to ossification of the sacrospinous ligament secondary to pelvic balance abnomalities. Orthop Traumatol Surg Res. 2009;95:645–648. doi: 10.1016/j.otsr.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 63.Cassidy L., Walters A., Bubb K., Shoja M.M., Tubbs R.S., Loukas M. Piriformis syndrome: implications of anatomical variations, diagnostic techniques, and treatment options. Surg Radiol Anat. 2012 Aug;34(6):479–486. doi: 10.1007/s00276-012-0940-0. [DOI] [PubMed] [Google Scholar]
- 64.Siddiq M.A.B. Piriformis syndrome and wallet neuritis: are they the same? Cureus. 2018 May 10;10(5) doi: 10.7759/cureus.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin H.D., Kivlan B.R., Palmer I.J., Martin R.L. Diagnostic accuracy of clinical tests for sciatic nerve entrapment in the gluteal region. Knee Surg Sports Traumatol Arthrosc. 2014;22:882–888. doi: 10.1007/s00167-013-2758-7. [DOI] [PubMed] [Google Scholar]
- 66.Misirlioglu To M.D. Piriformis syndrome: comparison of the effectiveness of local anesthetic and corticosteroid injections: a double-blinded, randomized controlled study. Pain Physician. 2015;2(18):163–171. [PubMed] [Google Scholar]
- 67.Al-Al-Shaikh M., Michel F., Parratte B., Kastler B., Vidal C., Aubry S. An MRI evaluation of changes in piriformis muscle morphology induced by botulinum toxin injections in the treatment of piriformis syndrome. Diagn Interv Imaging. 2015;96:37–43. doi: 10.1016/j.diii.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 68.Ilizaliturri V.M., Arriaga R., Villalobos F.E., Suarez-Ahedo C. Endoscopic release of the piriformis tendon and sciatic nerve exploration. J Hip Preserv Surg. 2018;5:301–306. doi: 10.1093/jhps/hny018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Indrekvam K., Sudmann E. Piriformis muscle syndrome in 19 patients treated by tenotomy--a 1- to 16-year follow-up study. Int Orthop. 2002;26:101–103. doi: 10.1007/s00264-001-0319-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ilizaliturri V.M., Jr., Arriaga R., Villalobos F.E., Jr., Suarez-Ahedo C. Endoscopic release of the piriformis tendon and sciatic nerve exploration by the lateral decubitus approach through an incision on the iliotibial band. Arthrosc Tech. 2018;7:e785–e790. doi: 10.1016/j.eats.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]