Abstract
Radioactive iodine therapy and posttreatment scanning are essential components of differentiated thyroid carcinoma treatment and detection of metastatic disease. False-positive results can be seen on an I-131 scan and are important for clinicians to be aware of. Here, we present a case of a 33-year-old female with follicular thyroid carcinoma who was noted to have an area of moderate uptake in the chest on a whole-body scan following remnant ablation with 30 mCi of I-131 (1.11GBq) concerning for a metastatic hilar lymph node. This was determined to be a mediastinal bronchogenic cyst on surgical pathology. It has been previously proposed that the expression of sodium iodide symporters in some bronchogenic cysts could be the mechanism by which iodine uptake is seen within them. We were able to demonstrate positive immunohistochemical staining for both sodium iodide symporter and the associated paired box gene 8 transcription factor in the cyst sample, which supports the proposed theory.
Keywords: follicular thyroid carcinoma, bronchogenic cyst, radioactive iodine, sodium iodide symporter
Introduction
Radioactive Iodine (RAI) therapy and posttreatment whole-body scans have long been a part of the management of differentiated thyroid carcinoma. Surgical pathology, disease status after total thyroidectomy, and patient preference are factors that inform the decision for either remnant ablation or adjuvant therapy with RAI. After treatment, radioisotope scanning provides valuable information on the location of remaining RAI-avid disease (1-3). Despite its utility, on rare occasions uptake outside the expected biodistribution of iodine can cause false-positive results on I-131 whole-body scintigraphy. Here we present a case of a bronchogenic cyst that appeared as a false positive on a posttherapy whole-body scan following remnant ablation with 30 mCi of I-131 (1.11GBq) for follicular thyroid carcinoma. To our knowledge, this is the first case where immunohistochemical (IHC) staining of the bronchogenic cyst for sodium iodide symporter (NIS) was performed, demonstrating the mechanism by which bronchogenic cysts may appear positive on thyroid scintigraphy.
Case Presentation
A 33-year-old female patient presented with palpable swelling in the right side of her neck and intermittent dysphagia for 3 months. She denied difficulty breathing, voice changes, or symptoms indicative of hypothyroidism or hyperthyroidism. She was a lifelong nonsmoker. Her family history was significant for thyroid cancer in her paternal aunt and paternal first cousin who were treated with total thyroidectomy and RAI therapy. She denied a history of head and neck radiation exposure preceding her thyroid cancer diagnosis.
A neck ultrasound done to evaluate her neck mass revealed a 5.5 cm right-sided solid, isoechoic thyroid nodule. This was classified as an American College of Radiology TIRADS TR3 thyroid nodule and met the criteria for fine-needle aspiration (FNA). FNA showed clusters of follicular cells with crowding, overlapping, and enlarged nuclei without colloid. This was classified as atypia of undetermined significance, Bethesda Classification Category III. Subsequent molecular testing performed due to indeterminate FNA results revealed an H-RAS Q61R mutation and microRNA expression that was classified as “moderate risk” with an overall estimated risk of malignancy of 75% to 85% in the nodule.
She elected to proceed with total thyroidectomy for management of the thyroid nodule. Preoperative neck ultrasonography revealed no abnormal lymphadenopathy. Surgical pathology showed a 7.5 × 4.4 × 4.0 cm encapsulated follicular carcinoma of the thyroid. The margins were negative, and no extrathyroidal extension was noted. There was 1 focus of angioinvasion noted, without any lymphatic invasion. One central compartment lymph node was sampled and tested negative for malignancy. This would be classified as pT3aN0Mx (American Joint Committee on Cancer, 8th edition), stage I disease.
Diagnostic Assessment
The patient transferred her care to our institution roughly 1 year following surgery. At her initial visit with us, she was doing well overall and was clinically euthyroid on 150 mcg of levothyroxine daily. Laboratory testing revealed a serum TSH that was suppressed at 0.18 µIU/L (0.18 mIU/mL) (normal reference range: 0.35–4.94 µIU/mL; 0.35–4.94 mIU/mL). Thyroglobulin (Tg) by chemiluminescent immunoassay (CIA) was <0.1 ng/mL (<0.1 µg/L) (normal reference range: 1.3–31.8 ng/mL; 1.3–31.8 µg/L) and Tg antibody (TgAb) was <0.9 IU/mL (<0.9 kIU/L) (normal reference range: 0.0–4.0 IU/mL; 0.0–4.0 kIU/L). A neck ultrasound revealed no masses in the thyroidectomy bed or suspicious lymphadenopathy.
The risks vs benefits of remnant ablation with RAI were discussed with the patient given the size of the tumor and the presence of angioinvasion on surgical pathology, although her overall risk of recurrence was thought to be low. She elected to proceed with remnant ablation and received 30 mCi of I-131 (1.11GBq) after being prepared with 2 consecutive doses of recombinant human TSH (rhTSH). Approximately 72 hours after receiving the second dose of rhTSH, her TSH rose to 5.17 µIU/mL (5.17 mIU/L) (normal reference range: 0.35–4.94 µIU/mL; 0.35–4.94 mIU/mL). While TgAb remained at <0.9 IU/mL (<0.9 kIU/L) (normal reference range: 0.0–4.0 IU/mL; 0.0–4.0 kIU/L), Tg by CIA rose to a low level of 0.6 ng/mL (0.6 µg/L) (normal reference range: 1.3–31.8 ng/mL; 1.3–31.8 µg/L). It is unclear why her TSH level was lower than would be expected after receiving rhTSH. I-131 whole body scintigraphy following 30 mCi of RAI (1.11GBq) showed uptake within the neck, thought to represent remnant thyroid tissue. There was an additional focus of moderate uptake in the posterior left lower chest also noted (Fig. 1).
Figure 1.
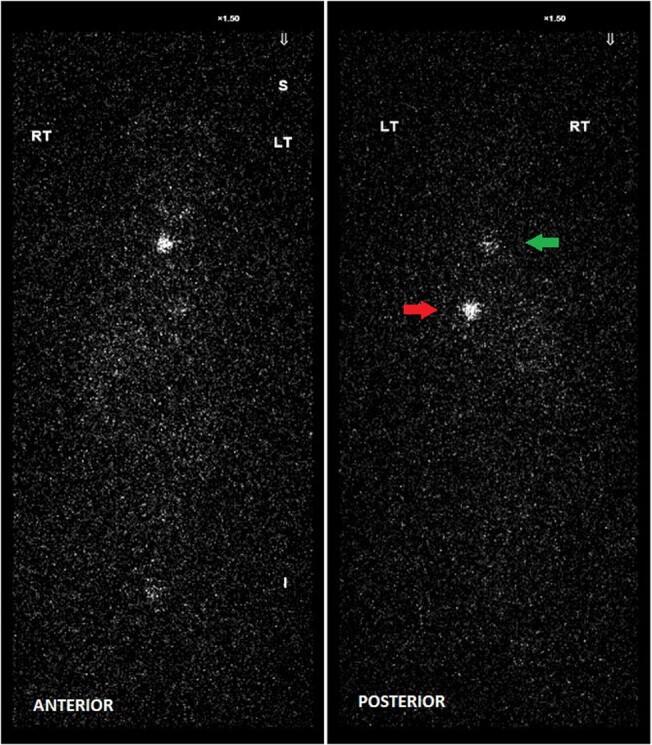
I-131 Whole-body scan after administration of 30 mCi of radioactive iodine (1.11GBq) demonstrating uptake within the neck (green arrow) and an additional focus of moderate uptake in the posterior left lower chest (red arrow).
A computed tomography (CT) chest with intravenous contrast done to further evaluate this area of uptake revealed a 2.2 cm mass, thought to be a left mediastinal lymph node, near the left atrium corresponding to the area of uptake noted on the I-131 scan (Fig. 2). A subsequent fludeoxyglucose positron emission tomography CT showed mildly avid cervical lymph nodes without any corresponding activity to match the iodine-avid mediastinal mass seen on CT chest. Bronchoscopy and endobronchial ultrasound guided biopsy of the 2.2 cm presumed mediastinal lymph node was attempted but had to be aborted due to the vasculature surrounding the mass making it a high-risk biopsy. Despite her Tg levels being undetectable, there was concern that this area of iodine uptake in the chest could represent metastatic thyroid cancer.
Figure 2.
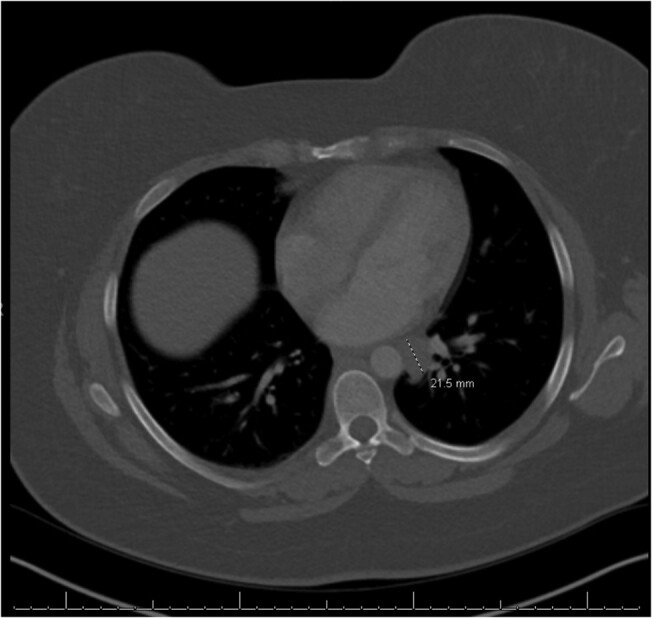
Computed tomography image of 2.2 cm presumed left mediastinal lymph node near the left atrium corresponding to the area of uptake noted on the I-131 whole-body scan.
Treatment
The options of continued surveillance or surgical resection were discussed with the patient. She elected to proceed with surgery and underwent a robot-assisted thoracoscopic resection of the mediastinal mass. Intraoperatively, the target lesion was visualized, and, during dissection, there was an eruption of purulent material from the mass. The erupted material, remaining cyst, and surrounding lymph nodes sampled were sent for evaluation. Surgical pathology results showed a 1.4 × 0.6 × 0.5 cm previously disrupted benign bronchogenic cyst.
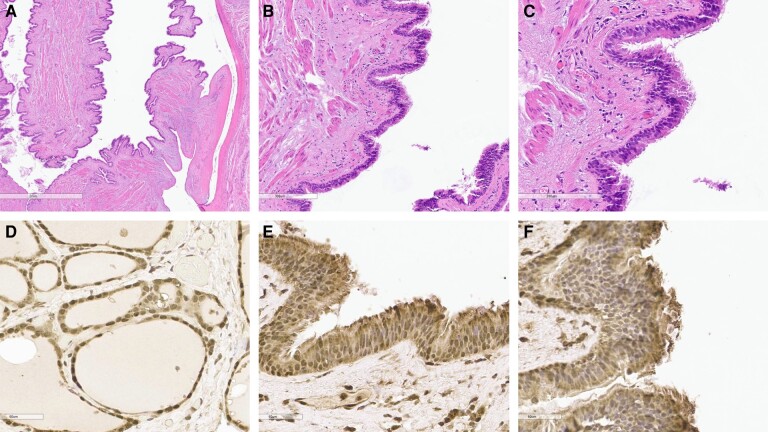
Immunohistochemical (IHC) staining was also performed to confirm the diagnosis. The cyst stained negative for transcription termination factor 1 (TTF1) and positive for paired box gene 8 (PAX8). TTF1 is a tissue-specific protein expressed in thyroid follicular cells to maintain differentiation and function of the thyroid gland and type II alveolar epithelial cells to regulate surfactant proteins. Lack of staining for TTF1 ruled out ectopic functioning thyroid tissue (4, 5). IHC staining for NIS was also pursued using a rabbit-specific NIS polyclonal antibody (Fig. 3). A multinodular goiter specimen was also stained with NIS to serve as a positive control. Staining for NIS was positive in the bronchogenic cyst wall supporting the theory that expression of NIS in the cyst could be the explanation of I-131 uptake. The lining of the bronchogenic cyst additionally stained positive for PAX8.
Figure 3.
Immunohistochemical staining of bronchogenic cyst wall. Hematoxylin and eosin; magnification 2x (A), 10x (B), and 40x (C) Positive immunohistochemical stain for sodium iodide symporter transporter in the control sample of a multinodular goiter (D) and in bronchogenic cyst using a rabbit specific sodium iodide symporter polyclonal antibody: magnification 10x (E) and 40x (F).
Outcome and Follow-up
The patient recovered well postoperatively, and lab work showed a Tg by CIA of 0.1 ng/mL (0.1 µg/L) (normal reference range: 1.3–31.8 ng/mL; 1.3–31.8 µg/L), TgAb of <0.9 IU/mL (<0.9 kIU/L) (normal reference range: 0.0–4.0 IU/mL; 0.0–4.0 kIU/L), and TSH of 46.88 µIU/mL (46.88 mIU/mL) (normal reference range: 0.35–4.94 µIU/mL; 0.35–4.94 mIU/mL). She was counseled on the importance of compliance with levothyroxine therapy following these results.
Discussion
We report a rare case of a bronchogenic cyst that presented as an iodine-avid lesion in the chest on a posttherapy whole-body scan following RAI therapy for follicular thyroid carcinoma, raising concern for metastatic thyroid cancer. There have been only 5 other such reported cases in the literature, all of which were discovered incidentally on whole-body scans after the patient received RAI for papillary thyroid carcinoma (3, 6-9). The expression of NIS in the cyst wall was first proposed as the mechanism for iodine uptake within a bronchogenic cyst in a case report published in the year 2000 but was not substantiated (9).
Bronchogenic cysts are derived from abnormal budding of the primitive foregut, with their location dependent on when the budding occurs during development. While these cysts are usually found in the mediastinum or lung parenchyma, they can occur anywhere from the neck to the lower lumbar spine. Bronchogenic cysts are usually discovered incidentally on radiography or due to compressive symptoms, cough, fever, pain, dyspnea, or postobstructive infection (10, 11). Despite being found incidentally, imaging alone is not diagnostic due to varying appearance and ability to appear like neoplasms. Surgical resection is often recommended due to reports of malignancy and high rates of complications such as pneumothorax, pneumonia, pleurisy, and tracheal compression arising from the cysts (10, 12).
Iodine scintigraphy relies on the high avidity iodine has for functional thyroid tissue due to enhanced expression of NIS. This increased symporter expression allows for trapping, organification, and storage of iodine for future thyroid hormone production (13). RAI is known to physiologically accumulate in the stomach, liver, kidneys, and urinary tract as it is absorbed, metabolized, and excreted (3). Other types of extrathyroidal tissues have been shown to have NIS expression through conventional whole-tissue sections and high-density tissue microarrays, notably breast, ovarian, prostate, and lung malignancies. Additionally, NIS expression has been identified in normal bronchial epithelium, breast tissue, and salivary glands (14, 15). Because bronchogenic cysts are embryologically derived from the foregut, it is plausible that they could express NIS, leading to uptake of I-131. This has been suggested as a mechanism by authors of previous case reports but has not been definitively demonstrated prior to our case. However, the expression of NIS in the bronchial epithelium alone may not be sufficient to cause avidity on iodine scintigraphy.
In our patient, the lining of the bronchogenic cyst stained positive for PAX8 in addition to NIS. PAX8 is a transcription factor that regulates the development of thyroid, kidney, and Müllerian tissues (16). Additionally, it is well established to upregulate transcription of the NIS in healthy thyroid tissue (17). While it is not typical of lung or bronchogenic cyst epithelium, there have been documented cases of PAX8 positivity in pulmonary tissue samples before (18). It is possible that expression of PAX8 in our bronchogenic cyst case, which in turn upregulates NIS expression, is the underlying cause of the iodine uptake seen.
RAI uptake outside of thyroid tissue and expected physiologic locations can cause undue stress. With our case, we wanted to highlight a rare, though potential, source of nonmalignant iodine uptake on a whole-body scan. It is important for clinicians to familiarize themselves with the various causes of false-positive results on thyroid scintigraphy and interpret imaging results in conjunction with the patient's overall clinical picture and risk for recurrence of thyroid cancer.
Learning Points
Bronchogenic cysts can be a rare cause of uptake seen on iodine scintigraphy.
Expression of NIS in the bronchogenic cyst wall, as shown by IHC staining in our case, is the predicted cause of iodine uptake in bronchogenic cysts.
PAX8 is a transcription factor that regulates the development of thyroid tissue and transcription of the NIS in healthy thyroid tissue. Expression of PAX8 in the bronchogenic cyst wall could possibly be the underlying cause of NIS expression in our patient.
Acknowledgments
We would like to thank Dr. Carl Malchoff for the suggestion to stain the surgical specimen for NIS and Dr. Kevin Claffey for performing the IHC staining.
Contributor Information
Martha Dillon, Primary Care Internal Medicine Residency, University of Connecticut Health Center: UConn Health, Farmington, CT 06030, USA.
Rachel Zielinski, Primary Care Internal Medicine Residency, University of Connecticut Health Center: UConn Health, Farmington, CT 06030, USA.
Jennifer Worth, Thoracic Surgery, Hartford Healthcare, Norwich, CT 06360, USA.
Melinda Sanders, Pathology and Laboratory Medicine, University of Connecticut Health Center: UConn Health, Farmington, CT 06030, USA.
Omar Ibrahim, Interventional Pulmonology, University of Connecticut Health Center: UConn Health, Farmington, CT 06030, USA.
Tarunya Vedere, Endocrine Neoplasia, Endocrinology, University of Connecticut Health Center: UConn Health, Farmington, CT 06030, USA.
Contributors
All authors made individual contributions to authorship. T.V., O.I., and J.W. were involved in the diagnosis and management of this patient. T.V., M.D., and R.Z. were involved in writing and editing the manuscript. M.S. was responsible for interpreting the pathology specimens.
Funding
No public or commercial funding.
Disclosures
None declared.
Informed Patient Consent for Publication
Signed informed consent was obtained directly from the patient.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Sawka AM, Thephamongkhol K, Brouwers M, Thabane L, Browman G, Gerstein HC. A systematic review and metaanalysis of the effectiveness of radioactive iodine remnant ablation for well-differentiated thyroid cancer. J Clin Endocrinol Metab. 2004;89(8):3668‐3676. [DOI] [PubMed] [Google Scholar]
- 2. Haugen BR, Alexander EK, Bible KC, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agriantonis D, Hall L, Wilson M. Pitfalls of I-131 whole body scan interpretation: bronchogenic cyst and mucinous cystadenoma. Clin Nucl Med. 2008;33(5):325‐327. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&NEWS=n&CSC=Y&PAGE=fulltext&D=ovft&AN=00003072-200805000-00003. [DOI] [PubMed] [Google Scholar]
- 4. Guan L, Zhao X, Tang L, et al. Thyroid transcription factor-1: structure, expression, function and its relationship with disease. Biomed Res Int. 2021;2021:9957209‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johansson L. Histopathologic classification of lung cancer: relevance of cytokeratin and TTF-1 immunophenotyping. Ann Diagn Pathol. 2004;8(5):259‐267. [DOI] [PubMed] [Google Scholar]
- 6. Turba UC, Sildiroglu O, Rehm PK. Radioiodine (131 I) accumulation in bronchogenic cyst in the setting of thyroid carcinoma remission. Clin Imaging. 2012;36(3):224‐227. https://www.clinicalkey.es/playcontent/1-s2.0-S0899707111001896. [DOI] [PubMed] [Google Scholar]
- 7. Shah Mohammadi MR, Norouzi G, Pirayesh E. Radioiodine-avid paravertebral bronchogenic cyst mimicking neurofibroma: a diagnostic pitfall. Clin Nucl Med. 2022;47(4):e370‐e371. https://www.ncbi.nlm.nih.gov/pubmed/35143456. [DOI] [PubMed] [Google Scholar]
- 8. Jiang X, Zeng H, Gong J, Huang R. Unusual uptake of radioiodine in a retroperitoneal bronchogenic cyst in a patient with thyroid carcinoma. Clin Nucl Med. 2015;40(5):435‐436. https://www.ncbi.nlm.nih.gov/pubmed/25546201. [DOI] [PubMed] [Google Scholar]
- 9. Lejeune M, Héron C, Tenenbaum F, et al. Iodine 131 uptake by a bronchogenic cyst in a patient with differentiated carcinoma of the thyroid gland. Presse Méd. 2000;29(24):1345‐1347. https://www.ncbi.nlm.nih.gov/pubmed/10938686. [PubMed] [Google Scholar]
- 10. Sarper A, Ayten A, Golbasi I, Demircan A, Isin E. Bronchogenic cyst. Tex Heart Inst. J. 2003;20(2):105‐108. [PMC free article] [PubMed] [Google Scholar]
- 11. Aktogu S, Yuncu G, Halilcolar H, Ermete S, Buduneli T. Bronchogenic cysts: clinicopathological presentation and treatment. Eur Respir J. 1996;9(10):2017‐2021. http://erj.ersjournals.com/cgi/content/abstract/9/10/2017. [DOI] [PubMed] [Google Scholar]
- 12. Jiang C, Wang H, Chen G, Jiang G, Zhang P. Intradiaphragmatic bronchogenic cyst. Ann Thorac Surg. 2013;96(2):681‐683. https://www.clinicalkey.es/playcontent/1-s2.0-S0003497512023624. [DOI] [PubMed] [Google Scholar]
- 13. Oh J, Ahn B. False-positive uptake on radioiodine whole-body scintigraphy: physiologic and pathologic variants unrelated to thyroid cancer. Am J Nucl Med Mol Imaging. 2012;2(3):362‐385. https://www.ncbi.nlm.nih.gov/pubmed/23133823. [PMC free article] [PubMed] [Google Scholar]
- 14. Wapnir IL, van de Rijn M, Nowels K, et al. Immunohistochemical profile of the sodium/iodide symporter in thyroid, breast, and other carcinomas using high density tissue microarrays and conventional sections. J Clin Endocrinol Metab. 2003;88(4):1880‐1888. [DOI] [PubMed] [Google Scholar]
- 15. Ozcan Kara P, Gunay EC, Erdogan A. Radioiodine contamination artifacts and unusual patterns of accumulation in whole-body I-131 imaging: a case series. Int J Endocrinol Metab. 2014;12(1):e9329. https://www.ncbi.nlm.nih.gov/pubmed/24696698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xiang L, Kong B. PAX8 is a novel marker for differentiating between various types of tumor, particularly ovarian epithelial carcinomas. Oncol Lett. 2013;5(3):735‐738. https://www.ncbi.nlm.nih.gov/pubmed/23425942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Riesco-Eizaguirre G, Wert-Lamas L, Perales-Patón J, Sastre-Perona A, Fernández LP, Santisteban P. The miR-146b-3p/PAX8/NIS regulatory circuit modulates the differentiation phenotype and function of thyroid cells during carcinogenesis. Cancer Res. 2015;75(19):4119‐4130. https://www.ncbi.nlm.nih.gov/pubmed/26282166. [DOI] [PubMed] [Google Scholar]
- 18. McHugh KE, Arrossi AV, Farver CF, Mukhopadhyay S. Does strong and diffuse PAX-8 positivity occur in primary lung carcinoma? An immunohistochemical study of 418 cases and review of the literature. Appl Immunohistochem Mol Morphol. 2019;27(2):140‐146. https://www.ncbi.nlm.nih.gov/pubmed/28777151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.