Abstract
Unlike those of the S and the L envelope proteins, the functional role of the related M protein in the life cycle of the hepatitis B virus (HBV) is less understood. We now demonstrate that a single N glycan, specific for M, is required for efficient secretion of M empty envelope particles. Moreover, this glycan mediates specific association of M with the chaperone calnexin. Conversely, the N glycan, common to all three envelope proteins, is involved neither in calnexin binding nor in subviral particle release. As proper folding and trafficking of M need the assistance of the chaperone, the glycan-dependent association of M with calnexin may thus play a crucial role in the assembly of HBV. Beyond being modified by N glycosylation, M is modified by O glycosylation occurring within its amino acid sequence at positions 27 to 47. The O glycans, however, were found to be dispensable for secretion of M but may rather support viral infectivity. Surprisingly, nonglycosylated M localizes exclusively to the cytosol, either for degradation or for a yet-unknown function.
The hepatitis B virus (HBV) is a double-shelled sphere with an inner nucleocapsid and an outer lipoprotein envelope containing three related viral proteins, the small (S), middle (M), and large (L) proteins. The S and L envelope proteins are essential for the outcome of a viral infection, whereas the function of the M protein is less clear (4, 6, 14, 23). Virion assembly is initiated by insertion of the envelope proteins into the endoplasmic reticulum (ER) membrane and is thought to proceed at pre-Golgi membranes, where cytosolic capsids are packaged by transmembrane envelope proteins. Virions then bud into intraluminal cisternae and leave the cell via the constitutive pathway of secretion (9, 11). An excess of envelope proteins, however, is not incorporated into virion envelopes but self-assembles into secreted subviral lipoprotein particles (9, 17). Virion formation requires the S and L proteins, while the contribution of the M protein to this process is still a subject of debate (4, 6, 23). Subviral particle production is solely driven by the S protein but is also sustained by the M protein (9, 15, 17).
The S, M, and L proteins are translated from a single open reading frame of the viral genome by means of three different start codons that are spaced at intervals of 108 (or 119, depending on the subtype) and 55 codons. Therefore, the 226-amino-acid sequence of the S protein is repeated at the C termini of the M and L proteins, which carry the additional pre-S2 domain or pre-S2 and pre-S1 domains, respectively. All three proteins are found in two forms, either glycosylated at Asn146 of the S sequence or unglycosylated at this site. The M protein is additionally glycosylated at Asn4 within its pre-S2 region (9, 15).
Although the role of the M protein is in doubt, all mammalian hepadnaviruses contain the M protein and conserve its specific N glycosylation motif (15), which might reflect an important function of this sequence. In support of this notion, recent studies provide evidence for a role of N glycosylation and glycan trimming in the secretion of HBV virions, thereby highlighting the particular significance of the N glycan attached to Asn4 of M (3, 13, 14). However, the mechanism involved in the retention of glycan-defective HBV has yet to be determined. A major role in this process may be played by the quality control system of the ER, which retains incorrectly or incompletely folded (glyco)proteins within this organelle. Central to this pathway is the ER chaperone calnexin, which binds to many but not all glycoproteins to ensure their proper folding (1, 8). In this study, we have investigated whether calnexin assists in the formation of the HBV envelope. To this end, we have examined the association of the envelope proteins with the chaperone and have analyzed the requirements for this association and its impacts on the assembly and secretion of subviral particles. Moreover, we provide evidence for O glycosylation of the M protein and for selective exclusion of nonglycosylated M protein from subviral particles.
Secretion of M subviral particles requires the N glycan at Asn4.
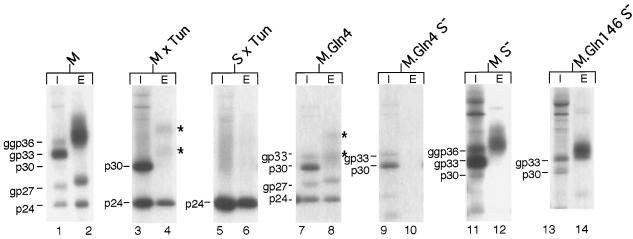
To study the structure and function of carbohydrates attached to the HBV envelope proteins, the M and S genes were transiently expressed in COS-7 cells (19). Transfectants were pulse-labeled for 4 h with [35S]methionine-cysteine and chased for 24 h, as described previously (18). Cellular lysates and supernatants were immunoprecipitated with polyclonal S-specific antiserum recognizing antigenic determinants common to the M and S proteins (12) and were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). In cellular lysates, the M protein appeared predominantly in the single-glycosylated form (gp33), modified at Asn4 in pre-S2, and, to a lesser extent, in the twice-glycosylated form (ggp36), partially modified at Asn146 in S also (Fig. 1, lane 1). A nonglycosylated form of M (p30) was also faintly visible, but only when total cellular extracts were analyzed (Fig. 1, lane 1; see also lane 11). We will return to this observation later. In addition to the triplet of M species, the nonglycosylated (p24) and glycosylated (gp27) forms of S were obtained due to internal initiation of translation (Fig. 1, lane 1). Both glycosylated forms of M were efficiently released from the cells together with S (Fig. 1, lane 2). During export, the N glycans are known to be modified by Golgi processing, leading to an increase in apparent molecular weight relative to intracellular counterparts (9, 17). The molecular mass of the gp27 form of S increased thereby by about 1 kDa, while the secreted M forms appeared as a broad smear (Fig. 1, lane 2).
FIG. 1.
Synthesis, glycosylation, and secretion of wild-type and mutant M and S envelope proteins. For transient expression, plasmids pNI2.M and pNI2.S, which have been previously described (19), were used. COS-7 cells were transfected with the indicated constructs and were metabolically pulse-chase-labeled 48 h after transfection. After cell lysis, intracellular lysates (I) and extracellular supernatants (E) were immunoprecipitated with an S-specific antiserum and were subjected to SDS-PAGE. Where indicated, tunicamycin (Tun) (10 μg/ml; Sigma) was added during the pulse-chase-labeling. Nonglycosylated (p) and glycosylated (gp or ggp) forms of wild-type and mutant envelope proteins are indicated to the left of each panel. Stars to the right of lanes 4 and 8 mark the faintly visible proteins.
To analyze whether N glycans play a role in the secretion of M subviral particles, transfected cells were metabolically labeled in the presence of tunicamycin, which prevents N glycosylation. As expected, the M protein was now synthesized in the nonglycosylated p30 form only, along with the nonglycosylated version of S (Fig. 1, lane 3). In the presence of tunicamycin, the secretion of M was found to be severely impaired (Fig. 1, lane 4). Nonetheless, two forms with surprisingly higher molecular masses, rather than the expected p30 form of M, were released (Fig. 1, lane 4), suggesting a further modification(s) beyond N glycosylation. Conversely, but consistent with previous studies (14, 17, 21), secretion of the S protein did not require N glycosylation, as shown by tunicamycin treatment of cells producing S alone (Fig. 1, lanes 5 and 6).
To rule out the possibility that tunicamycin affected secretion of M simply due to nonspecific toxicity, we inactivated the M-specific glycosylation motif by substituting glutamine for asparagine at position 4 of M by site-directed mutagenesis with the antisense oligonucleotide 5′-GTGGAAGGTTGTGGACTGCCACTGC-3′ (mutations are in boldface type). In cellular lysates, this mutant (M.Gln4) appeared in the nonglycosylated p30 form and, less prominently, in the single-glycosylated gp33 form, due to partial modification at Asn146, along with both forms of S (Fig. 1, lane 7). Importantly, mutant M.Gln4 displayed a secretory phenotype similar to that of wild-type M released from tunicamycin-treated cells: again, secretion was depressed, and two bands, migrating more slowly than expected in SDS gels, appeared (Fig. 1, lane 8). Efficient secretion of M subviral particles thus depends on the N glycan linked to Asn4.
We wondered why knocking out the Asn4-linked glycosylation inhibited but did not totally block secretion of M. Since the HBV envelope proteins have the characteristic of forming mixed oligomers upon biosynthesis (9, 18), we hypothesized that the residual export of the glycosylation-deficient M protein can be rescued by interaction with the internally initiated S protein. Therefore, the internal translational start codon for the S protein was inactivated by mutagenesis using oligonucleotide 5′-GATGTTATCCGTGTTCAGCG-3′. As shown in Fig. 1, this mutation abolished concomitant S protein expression in both the mutant M.Gln4 (lane 9) and the wild-type M (lane 11) (the remaining band in the 24-kDa range was also present in nontransfected cells [data not shown] and was attributed to nonspecific immunoprecipitation). Indeed, secretion of the glycan mutant M.Gln4S− was entirely blocked when S protein coexpression was abolished (Fig. 1, lane 10). In contrast, the wild-type M (i.e., MS−) did not require the helper function of S for particle assembly and extracellular release (Fig. 1, lane 12).
As secretion of M requires N glycans, we finally analyzed whether its N glycan at Asn146 is also needed for trafficking of M. However, the glycan mutant M.Gln146S−, which was constructed with the oligonucleotide 5′-GGAATACATGTGCACTGTCCGTCCGAAG-3′, was efficiently secreted (Fig. 1, lanes 13 and 14), thus demonstrating that the Asn146 glycan is dispensable for the secretion of M.
M associates with calnexin.
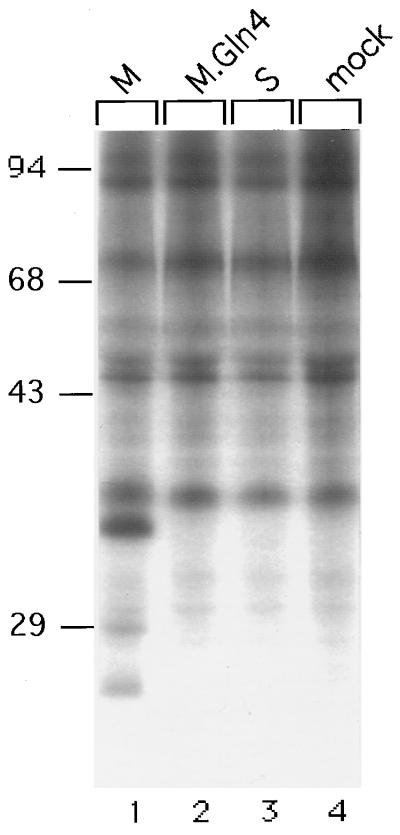
The N-glycan-dependent secretion of M indicated that the Asn4-linked carbohydrate may aid in productive folding and maturation of M. Therefore, the involvement of molecular chaperones is suggested. A candidate for such a chaperone is calnexin, a resident 90-kDa protein of the ER membrane that transiently binds to a variety of newly synthesized glycoproteins (1, 8). This lectin-like interaction is thought to detain monoglucosylated proteins in the ER until they are properly folded (1, 8). To examine whether the M protein associates with calnexin, transfected cells were pulse-labeled for 30 min, lysed with 2% CHAPS {3-[(3-cholamidopropyl) - dimethylammonio] - 1 - propanesulfonate}–HBS (50 mM HEPES-KCl [pH 7.5]–200 mM NaCl), and reacted overnight with polyclonal anticalnexin rabbit serum (1:100 dilution; Biomol). Immune complexes were collected with protein A-Sepharose and were washed three times with 0.5% CHAPS–HBS and once with 125 mM Tris-HCl (pH 6.8) prior to SDS-PAGE. As shown in Fig. 2, the M protein was efficiently coimmunoprecipitated with the calnexin antiserum (lane 1). Its gp33 form was clearly present, while its twice-glycosylated ggp36 form could not be detected because of comigrating coprecipitates (Fig. 2, compare lanes 1 and 4). Both forms of S appeared in addition, possibly due to their intermolecular oligomerization with calnexin-complexed M (Fig. 2, lane 1). In contrast, the mutant M.Gln4 failed to coprecipitate with calnexin, as neither its p30 nor gp33 forms could be detected (Fig. 2, lane 2). When synthesized alone, the S protein also did not interact with the chaperone (Fig. 2, lane 3). Taken together, these data demonstrate a specific association between wild-type M and calnexin. Strikingly, this interaction depends strictly on the glycan at Asn4 of M, while the glycan at Asn146 is not involved.
FIG. 2.
Specific association between M and calnexin requires the N-glycan at Asn4 of M. Mock-transfected cells or cells transfected with the indicated constructs were pulse-labeled, solubilized with the detergent CHAPS, and were reacted with anticalnexin antiserum. Immunoprecipitates were then analyzed by SDS-PAGE. Numbers to the left show positions of molecular mass standards (in kilodaltons).
M is modified by O glycosylation.
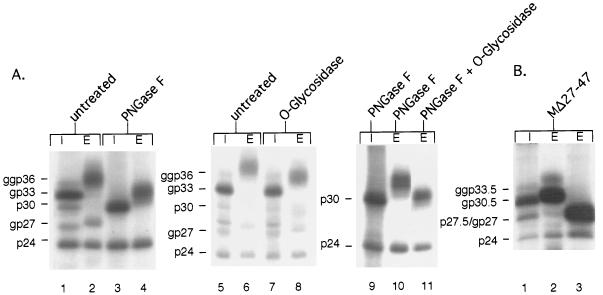
As suggested by the tunicamycin experiment (Fig. 1, lanes 3 and 4), M is subjected to additional posttranslational modification(s) unrelated to N glycosylation. To confirm these results, we used enzymatic N deglycosylation rather than inhibition of N glycosylation. Immunoprecipitated lysates and supernatants of cells producing M either were left untreated or were treated with peptide:N-glycosidase F (PNGase F; New England BioLabs), which cleaves all N-linked glycans, irrespective of their type. After digestion with PNGase F, the three intracellular forms of M and the two forms of S both migrated in a single band, corresponding to unglycosylated M and S, respectively (Fig. 3A, compare lanes 1 and 3). Conversely, PNGase F diminished the apparent molecular weight of the secreted M but not entirely to its nonglycosylated p30 form, while the secreted S was completely deglycosylated under these conditions (Fig. 3A, compare lanes 2 and 4). To investigate the modifications involved, we then studied the effect of O-glycosidase (Boehringer Mannheim), since the C-terminal half of the pre-S2 region of M (i.e., amino acids 27 to 47) is remarkably rich in serine and threonine residues (10 out of 21), which are known to serve as potential attachment sites for O glycans (7). The intracellular M protein was found to be O-glycosidase resistant, while the extracellular form was indeed sensitive, as judged by its mobility shift (Fig. 3A, lanes 7 and 8, respectively) (for comparison, a mock-treated sample was run on the gel in adjacent lanes 5 and 6). When PNGase F and O-glycosidase were used together, the electrophoretic mobility of the secreted M was further diminished, compared to that with PNGase F treatment alone (Fig. 3A, lanes 11 and 10, respectively). Nonetheless, the deglycosylated M thus obtained still had a slightly greater molecular weight than intracellular nonglycosylated p30 (Fig. 3A, lanes 11 and 9, respectively). This might be indicative of heterogeneous O glycans linked to M, of which some structures are resistant to O-glycosidase cleavage.
FIG. 3.
(A) Characterization of carbohydrates attached to M by digestion with glycosidases. Immunoprecipitated lysates (I) and supernatants (E) of M-expressing cells either were left untreated or were treated with the indicated enzymes. Digestions with PNGase F and O-glycosidase were performed on denatured proteins, according to the instructions of the manufacturers. (B) Glycosylation and secretion of mutant MΔ27-47, devoid of the proposed O glycan attachment site. Immunoprecipitated lysate (I) and supernatant (E) of transfected and labeled cells either were left untreated (lanes 1 and 2) or were digested with PNGase F (lane 3). Nonglycosylated (p) and glycosylated (gp or ggp) forms of wild-type and mutant M and internally initiated S are indicated to the left of each panel.
To map the O glycan attachment site(s) of M, we constructed a deletion mutant lacking the serine- and threonine-rich amino acid sequence at positions 27 to 47, using oligonucleotide 5′-GTCGCGTCCCAGGGGATTAGGGCCACCAGCAGGAAGATACAG-3′. This mutant (MΔ27-47) was syn-thesized in nonglycosylated p27.5 and in single (gp30.5)- and double (ggp33.5)-N-glycosylated forms (Fig. 3B, lane 1). During export of the glycosylated forms, their molecular weights increased (Fig. 3B, lane 2), but they increased less than is typical for M. Most importantly, PNGase F alone was sufficient to completely deglycosylate the secreted polypeptides (Fig. 3B, lane 3), thus demonstrating that mutant MΔ27-47 lacks O-linked sugars. As mutant MΔ27-47 was secreted as efficiently as wild-type M (Fig. 3B, lane 2), the O glycans attached to the C-terminal pre-S2 region of M are clearly dispensable for assembly and secretion.
Nonglycosylated M localizes to the cytosol.
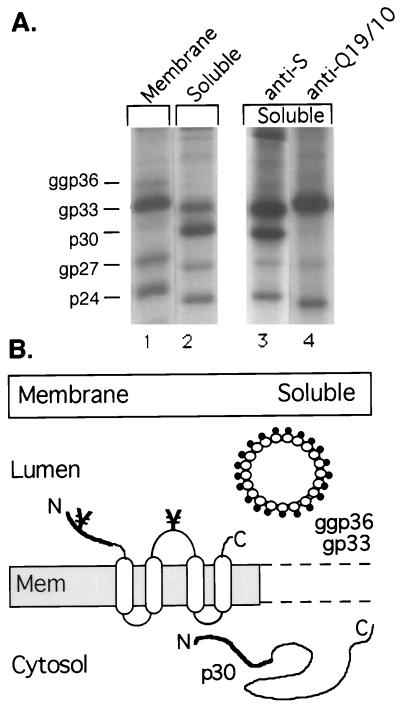
The O glycosylation of M is likely to occur during transit through the Golgi stacks. This may explain the absence of nonglycosylated p30 from secreted viral and subviral particles (9, 15). To study the intracellular fate of nonglycosylated p30, we carried out subcellular fractionation experiments. Pulse-labeled cells were subjected to extensive Dounce homogenization by 40 strokes in 5 mM Tris-HCl (pH 7.5)–15 mM NaCl to disrupt cells and intracellular organelles. Unbroken cells and nuclei were removed by low-speed centrifugation, and the postnuclear supernatant was ultracentrifuged, exactly as described previously (19), in order to yield a membrane pellet and a supernatant fraction. While the membrane fraction should contain integral and peripheral membrane proteins, the supernatant fraction should harbor soluble cytosolic and soluble intraluminal proteins leaked from intracellular cisternae during the harsh homogenization. The M protein should be distributed between the membrane and the soluble fraction, because the HBV envelope proteins have been shown to behave as intraluminal soluble proteins rather than membrane-bound proteins after budding of subviral particles into the intracellular cisternae (18, 25). As shown in Fig. 4A, the glycosylated M along with both forms of S was distributed between both fractions, as expected (lanes 1 and 2). The nonglycosylated M (p30), however, was almost exclusively found in the soluble fraction (Fig. 4A, lane 2). Hence, p30 might fail to integrate into intracellular membranes, thereby being excluded from subviral particle assembly. The failure of membrane insertion, however, is not due to full translocation of p30 into the ER lumen, since we could never identify p30 within microsomal vesicles (12, 19). Alternatively, a rapid assembly of p30 into subviral particles might prevent its detection in the membrane fraction. To distinguish between these possibilities, we probed whether p30 was incorporated into intraluminal particles present in the soluble fraction. Therefore, we used (co)immunoprecipitation with the monoclonal antibody (MAb) Q19/10, which only recognizes N-glycosylated M (9). As shown in Fig. 4A, p30 failed to coprecipitate with the gp33 and ggp36 forms, recognized by Q19/10, while both forms of the internally initiated S did (lane 4). We conclude from these data that nonglycosylated M has no access to the assembly and secretion of subviral particles.
FIG. 4.
(A) Subcellular localization of M. Transfected cells were pulse-labeled for 1 h and were disrupted by Dounce homogenization. Membrane-associated (Membrane) and soluble (Soluble) proteins were separated by centrifugation, adjusted to 0.5% Nonidet-P40, and subjected to immunoprecipitation with the anti-S antiserum and SDS-PAGE. For (co)immunoprecipitation, the soluble fraction was reacted with the M-specific MAb Q19/10, which only recognizes N-glycosylated M (lane 4), or with the S-specific antibody as a control (lane 3). Nonglycosylated (p) and glycosylated (gp or ggp) forms of M and internally initiated S are indicated on the left. (B) Schematic presentation of the fractionation experiment. On the left, the membrane fraction containing the transmembrane form of M in the ER membrane (Mem) is shown. The pre-S2 region of M (thick line) faces the ER lumen, while its S region (open boxes and thin lines) traverses the membrane four times. Sites of N glycosylation at Asn4 and Asn146 are indicated (¥). Upon assembly, subviral spherical M particles containing glycosylated gp33 and ggp36 bud into intraluminal cisternae (top right), where they behave as soluble proteins. Nonglycosylated p30 localizes to the cytosol (bottom right), as it appeared neither in the membrane fraction nor in subviral particles.
Discussion.
Although many viral envelope and most mammalian surface proteins carry carbohydrates, a universal role for glycosylation has remained obscure. Recent studies, however, have revealed a major function for N glycosylation as a folding device within the ER. Central to this pathway is the chaperone calnexin, which binds to a broad but limited range of glycoproteins and anchors the polypeptides to the ER until they have achieved their correct folding conformation (references 1 and 8 and references therein). Here, we show that the M envelope protein of HBV is an addition to this group of proteins whose maturation requires the assistance of calnexin. The specific association of M with calnexin was seen upon synthesis in transfected cells and was found to depend strictly on the Asn4-linked glycan, specific for M, rather than on the Asn146-linked glycan, shared by all three HBV envelope proteins. Consistent with this, the M-specific but not the common N glycan proved to be essential for the secretion of M subviral particles from COS-7 cells and also from HepG2 cells (our unpublished observation). The strict correlation between glycan-dependent chaperone binding and secretion of M and vice versa indicates that calnexin promotes folding and thus trafficking of M. Interestingly, recent studies have demonstrated that secretion of HBV virions requires N glycosylation and processing, with the M-specific glycosylation motif being most crucial (3, 13, 14). In these works, viral secretion was shown to be impaired upon tunicamycin treatment, inhibition of glucosidase I, and mutational inactivation of the M-specific glycan attachment site, which all prevent the formation of monoglucosylated M, a prerequisite for glycan-dependent calnexin binding (1, 8). The data described here might explain those observations by demonstrating that proper folding and transport of M need the interaction with calnexin. We therefore propose an essential role for calnexin in chaperoning the assembly of HBV. As an attractive model, transient retention of M by calnexin may be an important step to facilitate proper contacts between individual S, M, and L envelope chains needed for envelopment of the viral capsid (4, 23). Calnexin might thus act as a scaffold on which assembly of the viral envelope occurs. In support of this view, calnexin has recently been suggested to be also involved in intracellular retention of the HBV L envelope protein (25).
Nonetheless, the crucial role both for the M-specific glycan and for calnexin is surprising, as there is evidence that HBV virions can be secreted in the absence of M. The M protein has been reported to be dispensable for virion formation and even for infectivity in permissive HepG2 cells transfected with mutant viral genomes (4) or in a chronically infected host carrying an HBV variant defective in M protein expression (6). Conversely, however, in another report the M protein was claimed to be essential for virus formation in vitro (23). Whether or not the M protein plays a vital role in the viral life cycle, the M protein may nonetheless regulate virus assembly. Such an interpretation was recently suggested by Metha et al. (14) to explain why HBV secretion requires the M-specific glycan but may not need the M protein. Nonglycosylated aberrant M would thus act in a dominant negative manner, thereby destabilizing the viral envelope and hindering HBV secretion.
Beyond N glycosylation, O glycosylation was identified as a further modification of M. The C-terminal pre-S2 region of M serves as the O-glycan attachment site, which is consistent with the observation that O-linked sugars are typically clustered in distinct protein domains in which their acceptor serine and threonine residues are frequently present (7). O glycosylation of M was previously described for a modified envelope protein containing parts of pre-S2 preceeding S but was attributed to the particular expression system used (22). O glycosylation of M has also been observed upon expression in yeast cells (2). Since mammalian cells likewise add sugars in O linkage to M, as described here, we consider O glycosylation to be a natural modification occurring during replication of HBV. The biological significance of the O glycosylation of M, however, is unrelated to the secretion of subviral particles, as shown by the efficient release of mutant MΔ27-47, lacking O-linked sugars. As O-linked glycans often function at the (cell) surface (7), the O glycan(s) of M may rather provide specific recognition structures supporting HBV infectivity.
Although intriguing, O glycosylation of M does not explain the absence of its nonglycosylated p30 form from secreted viral and subviral particles (9, 15). Rather, we found that p30 exclusively localizes to the cytosol, thereby being excluded from subviral particle assembly. To account for the cytosolic localization of p30, inefficient, facultative, or aborted mechanisms of translocation into the ER membrane may be considered, as cotranslational membrane insertion of M is mediated by the signal sequences located downstream in its S region (5). Nonetheless, these signals govern efficient membrane integration of the even-larger L envelope protein (16). Alternatively, p30 might reach the cytosol by dislocation from the ER. Such retrograde processes of transport from the ER to the cytosol have recently been reported to occur, e.g., in the course of destruction of major histocompatibility class I molecules in the cytosol of cytomegalovirus-infected cells, or during endocytosis of toxins like ricin (20, 24). Whether nonglycosylated M localizes to the cytosol just to be degraded or to serve a special function remains to be determined. Strikingly, C-terminally truncated M proteins have been shown to act as transcriptional transactivators when their pre-S2 region is oriented to the cytosol (10). Accordingly, cytosolic p30 of wild-type M might be similarly involved in enhancing the pathogenicity of HBV.
To conclude, the elucidation of a critical role both of the M-specific glycan and of calnexin in the assembly and secretion of the HBV envelope may be a first step in the design of antiviral agents. Whether and how O glycosylation and cytosolic localization of M contribute to virogenesis of HBV await further investigation.
Acknowledgments
We thank Rolf E. Streeck for critical reading of the manuscript and for helpful discussions. We are grateful to K. H. Heermann and W. H. Gerlich for providing MAb Q19/10.
This work was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 311).
REFERENCES
- 1.Bergeron J J M, Brenner M B, Thomas D Y, Williams D B. Calnexin: a membrane-bound chaperone of the endoplasmic reticulum. Trends Biochem Sci. 1994;19:124–128. doi: 10.1016/0968-0004(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 2.Biemans R, Thines D, Rutgers T, De Wilde M, Cabezon T. The large surface protein of hepatitis B virus is retained in the yeast endoplasmic reticulum and provokes its unique enlargement. DNA Cell Biol. 1991;10:191–200. doi: 10.1089/dna.1991.10.191. [DOI] [PubMed] [Google Scholar]
- 3.Block T M, Lu X, Platt F M, Foster G R, Gerlich W H, Blumberg B S, Dwek R A. Secretion of human hepatitis B virus is inhibited by the imino sugar N-butyldeoxynojirimycin. Proc Natl Acad Sci USA. 1994;91:2235–2239. doi: 10.1073/pnas.91.6.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruss V, Ganem D. The role of envelope proteins in hepatitis B virus assembly. Proc Natl Acad Sci USA. 1991;88:1059–1063. doi: 10.1073/pnas.88.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eble B E, Lingappa V R, Ganem D. The N-terminal (pre-S2) domain of a hepatitis B virus surface glycoprotein is translocated across membranes by downstream signal sequences. J Virol. 1990;64:1414–1419. doi: 10.1128/jvi.64.3.1414-1419.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernholz D, Galle P R, Stemler M, Brunetto M, Bonino F, Will H. Infectious hepatitis B virus variant defective in pre-S2 protein expression in a chronic carrier. Virology. 1993;194:137–148. doi: 10.1006/viro.1993.1243. [DOI] [PubMed] [Google Scholar]
- 7.Gahmberg C G, Tolvanen M. Why mammalian cell surface proteins are glycoproteins. Trends Biochem Sci. 1996;21:308–311. [PubMed] [Google Scholar]
- 8.Hebert D N, Foellmer B, Helenius A. Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell. 1995;81:425–433. doi: 10.1016/0092-8674(95)90395-x. [DOI] [PubMed] [Google Scholar]
- 9.Heermann K H, Gerlich W H. Surface proteins of hepatitis B viruses. In: McLachlan A, editor. Molecular biology of the hepatitis B virus. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 109–143. [Google Scholar]
- 10.Hildt E, Urban S, Hofschneider P H. Characterization of essential domains for the functionality of the MHBst transcriptional transactivator and identification of a minimal MHBst activator. Oncogene. 1995;11:2055–2066. [PubMed] [Google Scholar]
- 11.Huovila A J, Eder A M, Fuller S D. Hepatitis B surface antigen assembles in a post-ER, pre-Golgi compartment. J Cell Biol. 1992;118:1305–1320. doi: 10.1083/jcb.118.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Löffler-Mary H, Werr M, Prange R. Sequence-specific repression of cotranslational translocation of the hepatitis B virus envelope proteins coincides with binding of heat shock protein Hsc70. Virology. 1997;235:144–152. doi: 10.1006/viro.1997.8689. [DOI] [PubMed] [Google Scholar]
- 13.Lu X, Metha A, Dwek R, Butters T, Block T. Evidence that N-linked glycosylation is necessary for hepatitis B virus secretion. Virology. 1995;213:660–665. doi: 10.1006/viro.1995.0038. [DOI] [PubMed] [Google Scholar]
- 14.Metha A, Lu X, Block T M, Blumberg B S, Dwek R A. Hepatitis B virus (HBV) envelope glycoproteins vary drastically in their sensitivity to glycan processing: evidence that alteration of a single N-linked glycosylation site can regulate HBV secretion. Proc Natl Acad Sci USA. 1997;94:1822–1827. doi: 10.1073/pnas.94.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neurath A R, Kent S B H. The pre-S region of hepadnavirus envelope proteins. Adv Virus Res. 1988;34:65–142. doi: 10.1016/s0065-3527(08)60516-3. [DOI] [PubMed] [Google Scholar]
- 16.Ostapchuk P, Hearing P, Ganem D. A dramatic shift in the transmembrane topology of a viral envelope glycoprotein accompanies hepatitis B viral morphogenesis. EMBO J. 1994;13:1048–1058. doi: 10.1002/j.1460-2075.1994.tb06353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patzer E J, Nakamura G R, Yaffe A. Intracellular transport and secretion of hepatitis B surface antigen in mammalian cells. J Virol. 1984;51:346–353. doi: 10.1128/jvi.51.2.346-353.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prange R, Nagel R, Streeck R E. Deletions in the hepatitis B virus small envelope protein: effect on assembly and secretion of surface antigen particles. J Virol. 1992;66:5832–5841. doi: 10.1128/jvi.66.10.5832-5841.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prange R, Streeck R E. Novel transmembrane topology of the hepatitis B virus envelope proteins. EMBO J. 1995;14:247–256. doi: 10.1002/j.1460-2075.1995.tb06998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rapak A, Falnes P Ø, Olsnes S. Retrograde transport of mutant ricin to the endoplasmic reticulum with subsequent translocation to cytosol. Proc Natl Acad Sci USA. 1997;94:3783–3788. doi: 10.1073/pnas.94.8.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheu S Y, Lo S J. Biogenesis of the hepatitis B viral middle (M) surface protein in a human hepatoma cell line: demonstration of an alternate secretion pathway. J Gen Virol. 1994;75:3031–3039. doi: 10.1099/0022-1317-75-11-3031. [DOI] [PubMed] [Google Scholar]
- 22.Shiraki K, Ochiai H, Matsui S, Aiba N, Yoshida Y, Okuno T, Yamanishi K, Takahashi M. Processing of hepatitis B virus surface antigen expressed by recombinant Oka varicella vaccine virus. J Gen Virol. 1992;73:1401–1407. doi: 10.1099/0022-1317-73-6-1401. [DOI] [PubMed] [Google Scholar]
- 23.Ueda K, Tsurimoto T, Matsubara K. Three envelope proteins of hepatitis B virus: large S, middle S, and major S proteins needed for the formation of Dane particles. J Virol. 1991;65:3521–3529. doi: 10.1128/jvi.65.7.3521-3529.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiertz E J H J, Tortorella D, Bogyo M, Yu J, Mothes W, Jones T R, Rapoport T A, Ploegh H L. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature (London) 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- 25.Xu Z, Bruss V, Yen T S B. Formation of intracellular particles by hepatitis B virus large surface protein. J Virol. 1997;71:5487–5494. doi: 10.1128/jvi.71.7.5487-5494.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]