Dear Editor,
Plants have evolved intricate mechanisms to recognize fluctuating environments, relay signals, and ultimately recover from the cellular and tissue damage imposed by environmental stresses. Upon wounding, mechanical damage is recognized via cellular compounds released from damaged cells (Vega-Munoz et al., 2020) or changes in turgor pressure and cell-wall properties (Hoermayer et al., 2020). Subsequently, transcription factors and signaling components stimulate tissue repair and wound healing. A representative signaling axis includes the transcription factor ETHYLENE RESPONSE FACTOR115 (ERF115), which was first identified for its role in quiescent center cell divisions and stem cell regeneration upon wounding (Heyman et al., 2013). ERF115 is induced in cells neighboring damaged cells and promotes cell expansion and division to replenish damaged regions. ERF115, acting with PHYTOCHROME A SIGNAL TRANSDUCTION1 (PAT1), regulates genes including PHYTOSULFOKINE5, which possibly promotes cell expansion, and WOUND-INDUCED DEDIFFERENTIATION1, which is involved in wound-induced cell proliferation.
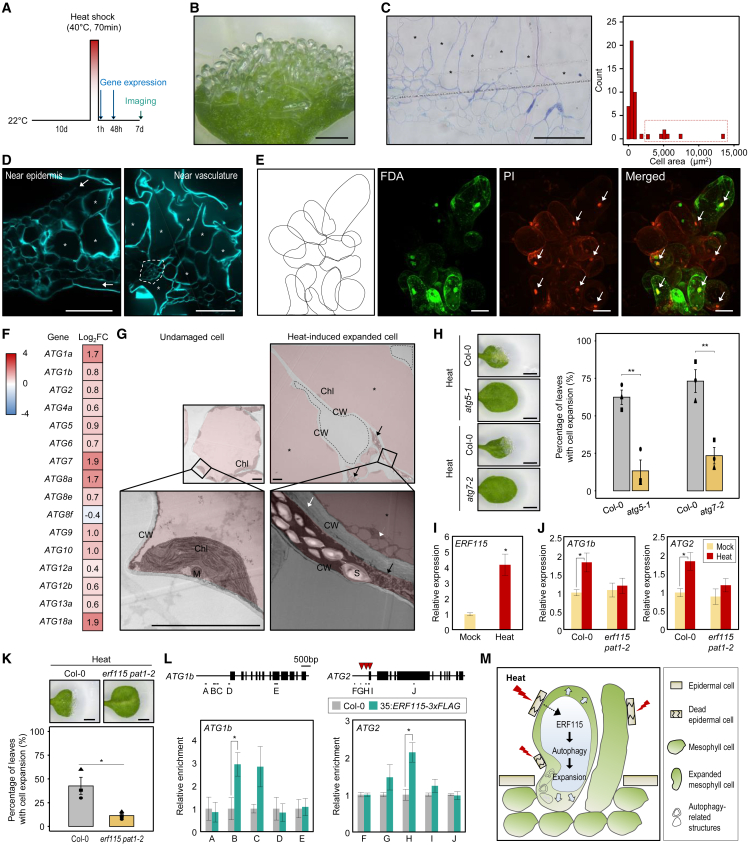
We noticed elongated cells near heat-damaged cells (Supplemental Figure 1) in Arabidopsis (Arabidopsis thaliana) leaves upon heat treatment. When we exposed Arabidopsis young seedlings to severe heat shock at 40°C for 70 min and allowed them to recover for 7 days under normal growth conditions (Figure 1A), about 50% of the heat-stressed seedlings showed a transparent cell mass at the leaf surface (Figure 1B). Closer examination revealed that the mass was composed of a single rod-shaped cell reaching around 5000 μm2 on the surface (Figure 1C). Cell expansion was primarily observed in mesophyll cells beneath the heat-damaged epidermis (Figure 1D).
Figure 1.
Heat stress promotes cell expansion in an autophagy-dependent manner.
(A) Diagram of heat stress treatment. Ten-day-old seedlings grown at 22°C under long-day conditions were subjected to heat shock at 40°C by immersing sealed plates into a water bath for 70 min, followed by 7 days of recovery at 22°C.
(B) Representative image of expanded cells from heat-treated leaves. Scale bar, 500 μm.
(C) Semi-thin cross-section of a leaf after heat stress. The leaf tissues were stained with toluidine blue. Right, distribution of mesophyll cell area. Asterisks (left) and dashed box (right) indicate cells with area larger than 2000 μm2. Scale bar, 100 μm.
(D) Cross-section of leaves with expanded cells after heat stress. Sections were stained with calcofluor white. Expanded mesophyll cells (asterisks), vasculature cells (dashed lines), and epidermal cells (arrow) are indicated. Scale bars, 100 μm.
(E) Representative confocal images of expanded cells labeled with fluorescein diacetate (green) and propidium iodide (red). Schematic diagram of cell arrangement for florescence imaging is shown at left. Arrows indicate propidium-iodide-stained nuclei. Scale bars, 100 μm.
(F) Expression of autophagy-related genes in heat-treated leaves based on RNA sequencing data. The seedlings were harvested at 48 h after heat treatment. Log2-normalized fold-change values in heat-treated leaves relative to mock-treated leaves are shown.
(G) Representative TEM micrographs of undamaged and heat-induced expanded mesophyll cells. Small vacuoles (arrowhead), cell-wall deposits (white arrow), damaged cells (black arrows), and expanded cells (asterisks) are indicated. All cytoplasmic regions are indicated with a red background. Dashed regions indicate empty space. M, mitochondrion; Chl, chloroplast; S, starch granule; CW, cell wall. Scale bars, 5 μm.
(H) Heat-induced cell expansion in atg5-1 and atg7-2 mutants. Percentage of 1st and 2nd rosette leaves with expanded cells after heat stress was calculated. Data represent mean ± SEM of three biological replicates (n > 60 leaves for each biological replicate). Statistically significant differences were analyzed by Student’s t-test (∗∗p < 0.01). Scale bars, 1 mm.
(I)ERF115 expression upon heat treatment, as determined by reverse transcription quantitative PCR analysis. Data represent the mean ± SEM. Asterisks indicate statistically significant differences (Student’s t-test, ∗p < 0.05).
(J) Relative transcript levels of ATG1b and ATG2 in erf115 pat1-2 in response to heat stress, as determined by reverse transcription quantitative PCR analysis. Data represent the mean ± SEM of three biological replicates. Asterisks indicate statistically significant differences (Student’s t-test, ∗p < 0.05).
(K) Heat-induced cell expansion phenotype of the erf115 pat1-2 double mutant. Percentage of 1st and 2nd rosette leaves with expanded cells after heat stress was calculated. Data represent mean ± SEM of three biological replicates (n > 70 leaves for each biological replicate). Statistically significant differences were analyzed by Student’s t-test (∗p < 0.05). Scale bars, 1 mm.
(L) Chromatin immunoprecipitation assays using anti-FLAG antibody. Gene structures with putative ERF115-binding sites (arrowheads; top) are shown. Chromatin immunoprecipitation signal of 35S:ERF115-3FLAG was quantified as fold enrichment relative to Col-0 after normalizing against eIF4a (bottom). Letters and bars beneath the gene diagrams indicate the regions of PCR amplification. Data represent the mean ± SEM of three biological replicates (Student’s t-test, ∗p < 0.05).
(M) Proposed model showing heat-induced cell expansion. Heat stress causes severe damage to epidermal cells. Neighboring mesophyll cells upregulate ERF115, which then promotes autophagic activity and enables cell expansion to compensate for cell damage at the leaf surface.
Because cell size and genome ploidy levels are generally positively correlated, we hypothesized that the expanded cells might form by endoreduplication. However, flow cytometry analysis revealed that overall ploidy levels of heat-treated leaves were comparable to those of mock-treated leaves (Supplemental Figure 2). Consistent with this result, the mutants siamese related1-1 (smr1-1), cell cycle switch protein52 a1-1 (ccs52a1-1), ccs52a1-2, ccs52a2-1, and suppressor of gamma response1-1 (sog1-1) and sog1-6 with lower endoreduplication levels exhibited normal cell expansion in response to heat stress (Supplemental Figure 3). In addition, because damage-induced cell proliferation is also frequently regulated by cytokinin signaling (Ikeuchi et al., 2017), we tested whether heat-stress-induced cell expansion might be accompanied by cytokinin biosynthesis and signaling. However, cytokinin signaling was unchanged in heat-treated seedlings compared with mock-treated seedlings (Supplemental Figures 4 and 5); moreover, a cytokinin biosynthetic mutant did not show defects in heat-induced cell expansion (Supplemental Figure 6). Auxin signaling was also not responsible for heat-induced cell expansion (Supplemental Figures 7 and 8).
Embryo suspensor cells of Norway spruce (Picea abies) form similar transparent, dramatically expanded cells with a rod shape during embryogenesis (Minina et al., 2013). Given that expansion of embryo suspensor cells is linked to programmed cell death (PCD) (Minina et al., 2013), we hypothesized that heat-induced cell expansion might involve PCD, possibly to degrade and recycle cellular materials under severe stress conditions to maintain energy balance (Su et al., 2020). We therefore performed cell viability assays on heat-expanded cells by dual staining with fluorescein diacetate (which stains live cells) and propidium iodide (which stains dead cells). Propidium iodide stained the nuclei of the expanded cells (arrow, Figure 1E), whereas expanded cells showed little or no staining with fluorescein diacetate (Figure 1E). The expanded cells remained after leaf senescence (Supplemental Figure 9), indicating that these cells were dead. Furthermore, RNA sequencing analysis indicated that genes upregulated by heat stress (p < 0.05, fold change > 2) were enriched for Gene Ontology terms related to “response to oxidative stress,” “innate immune response,” and “defense response” (Supplemental Figure 10), which are related to cellular stress and PCD defense responses.
Among several PCD programs in plants, autophagy mediates the degradation of cellular components via the vacuole or lysosome (Su et al., 2020). Sequential actions of AUTOPHAGY-RELATED (ATG) proteins enable lipid delivery, membrane expansion, and closure, resulting in autophagosome formation. Autophagy maintains plant fitness by removing protein aggregates and damaged cellular components after stress exposure (Wang et al., 2015). Indeed, autophagy genes were significantly upregulated in response to heat stress (Figure 1F; Supplemental Table 1). Transmission electron microscopy revealed a cluster of small vacuoles in the enlarged mesophyll cells, which may reflect autophagic activity (arrowhead, Figure 1G). Cells in contact with these enlarged cells frequently showed no clear organelles and dense cytosol, which may be associated with cell damage (Figure 1G; Supplemental Figure 11). The fully expanded cells usually had a clear interior devoid of cellular structures and surrounded by cell walls (Supplemental Figure 11). Notably, stress-induced cell expansion was impaired in the atg5-1 and atg7-2 mutants (Figure 1H), which are defective in autophagosome formation (Thompson et al., 2005). Our results demonstrate that heat-stress-induced cell expansion requires autophagy and is reminiscent of the cell expansion observed during P. abies embryo development, which is important for suspensor cell expansion and embryonic cell division (Minina et al., 2013). Although it is currently unclear how autophagy leads to cell expansion, we suspect that the autophagy process accelerates trafficking of cytoplasmic materials to vacuoles, which is followed by cell swelling.
ERF115 is induced in cells that contact damaged cells (Heyman et al., 2016); importantly, ERF115, but not its interacting protein-encoding gene PAT1, was responsive to heat treatment (Figure 1I; Supplemental Figure 12). We reasoned that epidermal cell death may activate ERF115 in neighboring mesophyll cells upon exposure to heat stress, triggering autophagy-dependent cell expansion. Indeed, heat-induced cell expansion was impaired in the erf115 pat1-2 double mutant with low autophagic activity (Figures 1J and 1K; Supplemental Figure 13), unlike other mutants related to callus formation and regeneration (Supplemental Figure 14). Given that ERF115-overexpressing transgenic plants did not exhibit a significant change in heat-induced cell expansion (Supplemental Figure 15), ERF115 is necessary, but not sufficient, for the cell expansion. Chromatin immunoprecipitation–qPCR analysis revealed that ERF115 directly binds to the ATG1b and ATG2 promoters (Figure 1L; Supplemental Figure 16). To confirm the direct binding of ERF115 to the ATG loci, we also performed transient expression analysis using Arabidopsis protoplasts. The 35S:ERF115 effector plasmid was co-transfected with the pATG:GUS reporter construct into Arabidopsis mesophyll protoplasts. As a result, ERF115 directly bound to the ATG promoters and promoted GUS activity (Supplemental Figure 17). Our results indicate that ERF115-induced autophagy is responsible for heat-stress-induced cell expansion (Figure 1M).
Heat-induced cell expansion may accompany epidermal cell death where temperature changes are first imposed. Indeed, genes upregulated by heat stress largely overlapped with those induced by wounding (Supplemental Figure 18; Supplemental Table 1). In addition, neighboring cells near the wounded region also produced elongated cells (Supplemental Figure 19). Thus, heat-induced cell expansion in mesophyll cells adjacent to epidermal cells is similar to wound-induced expansion of neighboring cells to fill in gaps left by dead cells. During heat-induced cell expansion, because the outer cell layer is absent, cell expansion is exceptionally robust, generating super-expanded cells. Notably, heat stress did not induce cell proliferation, unlike wounding stress (Supplemental Figure 20). Moreover, expanded cells were distinct from the callus that results from cell proliferation. Unlike callus, expanded cells are dead and thus cannot proliferate or regenerate into new organs and plantlets. Instead, expanded cells shield the underlying proliferative cells while sealing damaged regions to confer physical support and prevent desiccation, which is frequently observed during grafting and wound healing (Ikeuchi et al., 2022). The causal relationship between cell expansion and plant survival should be investigated to further confirm the physiological relevance of cell expansion upon neighboring cell damage.
Data and code availability
All RNA sequencing data generated in the current study are available in the NCBI Gene Expression Omnibus (GEO) database under accession number GEO: GSE247997. Other relevant materials are available from the corresponding authors upon reasonable request.
Funding
This work was supported by the Samsung Science and Technology Foundation under project number SSTF-BA2201-10.
Author contributions
P.J.S. managed the project. Y.N. and H.G.L. performed the experiments. H.L. analyzed the bioinformatics data. P.J.S. and Y.N. wrote and revised the manuscript.
Acknowledgments
We thank Dr. Ohkmae K. Park for providing atg5-1 and atg7-2 seeds; Dr. Lieven De Veylder for providing erf115 pat1-2, ccs52a2-1, ccs52a1-1, ccs52a1-2, and smr1-1 seeds; Dr. Lin Xu for providing wox11-2 wox12-1 seeds; Dr. Tatsuo Kakimoto for providing ipt3-2 ipt5-2 ipt7-1 seeds; Dr. Yoshikatsu Matsubayash for providing pskr1-2 pskr2-1 seeds; Dr. Yoo-Sun Noh for providing wox5-3 wox7-1 wox14-1 seeds; Dr. Masaaki Umeda for providing sog1-1 seeds; Dr. Jose Alonso for providing YUC1-OX seeds; Dr. Ben Scheres for providing plt3-1 plt5-2 plt7-1 seeds; Dr. Jungmook Kim for providing pLBD16:LBD16-SRDX seeds; and Dr. Hyo-Jun Lee for providing ein3-1 eil1-3 seeds. No conflict of interest is declared.
Published: November 20, 2023
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
Supplemental information is available at Plant Communications Online.
Supplemental information
References
- Heyman J., Cools T., Vandenbussche F., Heyndrickx K.S., Van Leene J., Vercauteren I., Vanderauwera S., Vandepoele K., De Jaeger G., Van Der Straeten D., De Veylder L. ERF115 controls root quiescent center cell division and stem cell replenishment. Science. 2013;342:860–863. doi: 10.1126/science.1240667. [DOI] [PubMed] [Google Scholar]
- Heyman J., Cools T., Canher B., Shavialenka S., Traas J., Vercauteren I., Van den Daele H., Persiau G., De Jaeger G., Sugimoto K., De Veylder L. The heterodimeric transcription factor complex ERF115-PAT1 grants regeneration competence. Nat. Plants. 2016;2 doi: 10.1038/nplants.2016.165. [DOI] [PubMed] [Google Scholar]
- Hoermayer L., Montesinos J.C., Marhava P., Benková E., Yoshida S., Friml J. Wounding-induced changes in cellular pressure and localized auxin signalling spatially coordinate restorative divisions in roots. Proc. Natl. Acad. Sci. USA. 2020;117:15322–15331. doi: 10.1073/pnas.2003346117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi M., Iwase A., Rymen B., Lambolez A., Kojima M., Takebayashi Y., Heyman J., Watanabe S., Seo M., De Veylder L., et al. Wounding Triggers Callus Formation via Dynamic Hormonal and Transcriptional Changes. Plant Physiol. 2017;175:1158–1174. doi: 10.1104/pp.17.01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi M., Iwase A., Ito T., Tanaka H., Favero D.S., Kawamura A., Sakamoto S., Wakazaki M., Tameshige T., Fujii H., et al. Wound-inducible WUSCHEL-RELATED HOMEOBOX 13 is required for callus growth and organ reconnection. Plant Physiol. 2022;188:425–441. doi: 10.1093/plphys/kiab510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minina E.A., Filonova L.H., Fukada K., Savenkov E.I., Gogvadze V., Clapham D., Sanchez-Vera V., Suarez M.F., Zhivotovsky B., Daniel G., et al. Autophagy and metacaspase determine the mode of cell death in plants. J. Cell Biol. 2013;203:917–927. doi: 10.1083/jcb.201307082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T., Li X., Yang M., Shao Q., Zhao Y., Ma C., Wang P. Autophagy: An Intracellular Degradation Pathway Regulating Plant Survival and Stress Response. Front. Plant Sci. 2020;11:164. doi: 10.3389/fpls.2020.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A.R., Doelling J.H., Suttangkakul A., Vierstra R.D. Autophagic nutrient recycling in Arabidopsis directed by the ATG8 and ATG12 conjugation pathways. Plant Physiol. 2005;138:2097–2110. doi: 10.1104/pp.105.060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Muñoz I., Duran-Flores D., Fernández-Fernández Á.D., Heyman J., Ritter A., Stael S. Breaking Bad News: Dynamic Molecular Mechanisms of Wound Response in Plants. Front. Plant Sci. 2020;11 doi: 10.3389/fpls.2020.610445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Cai S., Yin L., Shi K., Xia X., Zhou Y., Yu J., Zhou J. Tomato HsfA1a plays a critical role in plant drought tolerance by activating ATG genes and inducing autophagy. Autophagy. 2015;11:2033–2047. doi: 10.1080/15548627.2015.1098798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All RNA sequencing data generated in the current study are available in the NCBI Gene Expression Omnibus (GEO) database under accession number GEO: GSE247997. Other relevant materials are available from the corresponding authors upon reasonable request.