Abstract
Introduction
Cardiac fibres are affected invariably in myocardial infarction, with longitudinal strain being the earliest to be detected in the ischaemic cascade. The present study aims to assess strain imaging in acute myocardial infarction (AMI) patients admitted to the cardiology department at our institute and correlate GLS and DESL findings with other markers for myocardial function.
Methods and material
This augmented cross-sectional study was conducted amongst the patients admitted with diagnosis of AMI. During the study period, 157 subjects were sampled through convenience sampling, and examined as well as tested with routine investigations at baseline. The subjects were then followed through at first, third and six months, and findings noted. Chi-square was used to assess the crude association between sample characteristics. Pearson correlation and student t-test were used to find association between continuous variables.
Results
After screening 564 patients, 157 patients were included in the study after fulfilment of inclusion and exclusion criteria. A significant difference was found in baseline GLS scores and NTproBNP levels at 6 months in alive patients with STEMI, t (21.728) = −5.717, p < .001. Out of the 50 NSTEMI patients, 35 (70 %) were positive for ESL, similarly out of 43 STEMI patients without any RWMA, ESL was positive in 39 (90.02 %) patients.
Conclusions
GLS by STE has good correlation with LVEF, WMSI and NT pro-BNP and it is an independent predictor of mortality and heart failure among patients with AMI.
Keywords: Global longitudinal strain, Early systolic lengthening, Post systolic contraction, Infarction, Speckle tracking echocardiography
1. Introduction
Chest pain, ECG, cardiac biomarkers, and echocardiography is being used in the diagnosis and prognosis of acute myocardial infarction (AMI) but with limitations like low sensitivity of ECG in situations like bundle branch block, posterior wall AMI, NSTEMI and early STEMI; Lack of AMI specificity for cardiac biomarkers in sepsis, chronic kidney disease, myocarditis; and inter-observer variation as well as confounding by various preload and afterload situation for 2D-Echocardiography. Nevertheless, echocardiography may provide a quick overview of the status of the myocardium, but common indexes such as the left ventricular ejection fraction (LVEF) fail to detect minimal changes.1
The use of speckle tracking echocardiography (STE) now has expanded the spectrum to detect and quantify patterns of myocardial ischemia. Longitudinal strain is one of the earliest to be affected due to the sub-endocardial location of the longitudinal fibers. STE is less operator dependent. Studies have shown that STE predicts cardiovascular outcomes such as new onset heart failure and cardiovascular death after AMI.2,3 Among STE-related parameters, global longitudinal strain (GLS) most reliably predicted outcomes.4 Prognosis is known to be related to the extent of infarction and GLS was associated with infarction size.5,6
STE also helps to identify post systolic contraction (PSC) and early systolic lengthening (ESL) a sensitive indicator of acute myocardial ischemia and stunning.7, 8, 9 PSC is defined as myocardial contraction occurring after the closure of the aortic valve. Passive recoil of dyskinetic segments and active contraction of hypokinetic and akinetic segments, with altered fatty acid metabolism due to ischemia are the mechanism of PSC.10,11
Ischaemic myocardium tends to lengthen before the onset of shortening, probably due to its reduced ability to generate adequate active force as the LV pressure rises steeply during the isovolumic contraction phase (IVC); this is known as ESL. The duration of ESL (DESL) is proportional to the infarct size in AMI patients. During IVC, the non-ischaemic segments with a preserved contractile force will cause ischaemic segments to lengthen as these segments are incapable of increasing contractile force at a similar rate as the non-ischaemic segments.12, 13, 14, 15
The present study aims to assess strain imaging in AMI patients admitted to the cardiology department at our institute and correlate GLS and DESL findings with other markers for myocardial function including LVEF, WMSI, etc. The present study will not only add to the available evidence but will also provide insight into the unique settings prevalent in our study area.
2. Materials and methods
The present augmented cross-sectional study was conducted during January 2019 to December 2019 on admitted participants in the cardiology department of a tertiary care institute in West Bengal. The ethical approval for the study was obtained from Institutional Ethics Committee.
All patients with diagnosis of acute myocardial infarction based on presenting symptoms, new ECG changes and cardiac biomarker positivity as per fourth universal definition of myocardial infarction were included in the present study.16 Patients with old myocardial infarction (MI), post coronary artery bypass graft (CABG), NSTEMI with regional wall motion abnormality(RWMA), with ECG features of left main equivalent, those with bundle branch block, brady–or tachy-arrhythmia, those with cardiomyopathies and those with poor echo window where endocardium was not properly visible were excluded from the study.
A total of 157 patients were recruited for the study after fulfilment of inclusion and exclusion criteria and therefore constituted our final sample size. After taking detailed history with the help of a semi structured questionnaire, proper clinical examination of the patients was performed. Thereafter, echocardiography using 2-D, M-Mode, and Colour Doppler followed by speckle tracking echocardiography with Philips EPIQ 7 with ∂CMQ technology was done. Baseline laboratory examinations were also ordered at the outset.
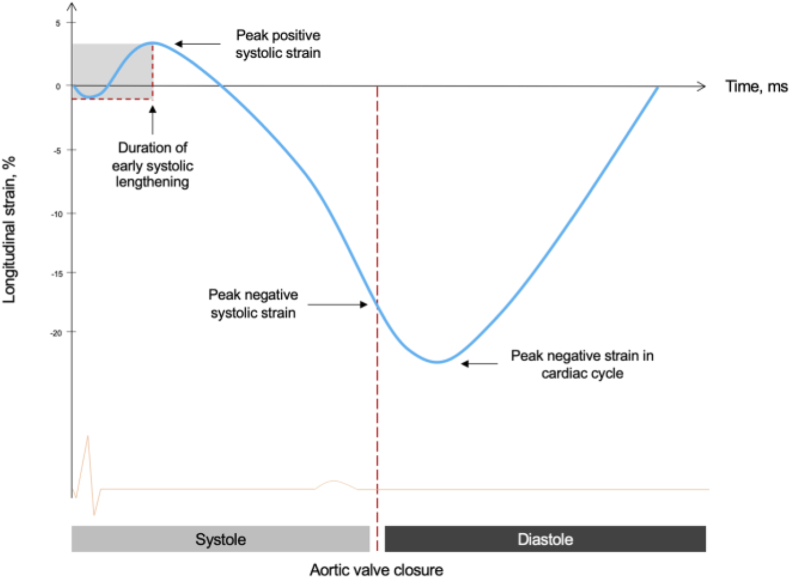
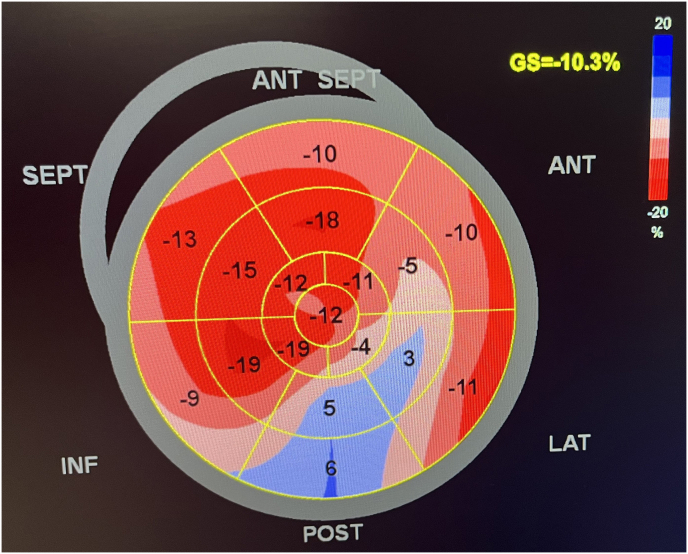
Study participants were then followed up at the end of one, three and six months after the index hospitalization and their NT-pro BNP levels and other biomarkers were measured; participants were also evaluated for clinical or subclinical heart failure at each follow up including for LVEF. Global longitudinal strain was calculated using ECG gated apical four, three and two chamber view of left ventricle. Q wave in ECG as initiation and aortic valve closure has been taken as end of systole; region of interest was traced and assessed for tracking quality. After segmental longitudinal strain, each was integrated into global longitudinal strain using Bulls eye (Fig. 1). GLS was measured in values and its value above −18 % was taken as normal while those below −16 % as abnormal.17 Early systolic lengthening (Fig. 2) was marked as positive if duration from q wave or initial R wave to peak of first positive contractile wave in speckle tracking was more than 50 msec.12,13 Post systolic contraction is defined as systolic contraction coming after closure of aortic valve in any segment and taken as positive if post systolic index (maximum strain in cardiac cycle –peak systolic strain/maximum strain in cardiac cycle) was greater than 20 % (Fig. 2). Peak systolic strain in this case is the maximum negative deflection before the closure of aortic valve, while maximum negative deflection in cardiac cycle is total negative deflection from baseline occurring after the closure of aortic valve.7, 8, 9
Fig. 1.
GLS Bull's eye.
Fig. 2.
Esl & PSC.
In the present study GLS was compared with WMSI, LVEF% and NT pro BNP at baseline and with LVEF% and NT pro BNP in the follow up. The purpose of baseline comparison was to look for appearance of abnormality in GLS value before changes in ejection fraction (EF %) and WMSI so as to evaluate its early prediction ability.
The data were analysed using Statistical Package of Social Science (SPSS Version 20). Frequencies (with percentages) and means (with standard deviations) were used to describe sample variable. An independent sample t-test and Pearson's r correlation analysis was used for analysis of continuous variables and univariate analysis using Chi-square (χ2) was used to assess the crude association between the different categorical variables. For analysis p < .05 was considered statistically significant for all tests of hypothesis.
3. Results
All patients underwent thorough physical examination, blood sample were taken and bedside echocardiography done immediately after hospitalization. Review echocardiography was done at the end of one month, three month and six month along with NT pro BNP to evaluate the prognostic significance of baseline strain imaging. 100 patients remained in follow up till 6 months while 37 were lost to follow up and 20 patients died during hospitalization.
3.1. Baseline characteristics
The mean ± SD age of the participants was found to be 55.69 ± 9.87 years. Majority, 70.7 % (111) of the study subjects were male and more than half, 55.4 % (87) of the study subjects were Hindu.
Almost half 49 % (77) of the study participants had BMI within the range of 18.6–24.9, whereas 15.9 % (25) of the study participants had BMI greater than 30. 55.4 % (87) of the study participants had past history of hypertension and 51.6 % (81) of the study participants had past history of diabetes while more than half 59.9 % (94) reportedly had past history of smoking. ST elevation on electrocardiogram was present in 107 (68.2 %) of the subjects, Among STEMI, most 37.6 % (37) study subjects were diagnosed with AWMI, 8.3 % LWMI, while IWMI was diagnosed in 22.3 % (35) of the participants. NSTEMI accounted for 31.8 % (50) cases. among whom at least 59.8 % (64) had wall motion score index (WMSI) ≥ 1.1. ESL was present in 138 (87.9 %) and PSC was present in 74 (47.1 %) of the subjects respectively, Table-2. All the STEMI patients included in the study presented within 6 h of onset of symptoms.
Table-2.
Pearson correlations for study variables at baseline.
| GLS | LVEF | WMSI | NTproBNP | |
|---|---|---|---|---|
| LVEF | −.881* | |||
| WMSI | .883* | −.999* | ||
| NTproBNP | .892* | −.837* | .837* | |
| Time of Presentation | −.352* | .244* | −.244* | −.244* |
* = statistically significant at p < .05.
Demographic and echocardiographic findings according to the outcome:
There was no significant difference in age at baseline in patients who were alive and those who died during the follow up period. However, the baseline NTproBNP levels at baseline were significantly different among the two groups of patients. Those subjects who remained alive, 772.94 ± 663.27 (mean ± SD) at the end of follow up period had significantly lower NTproBNP levels than those who died 2783.50 ± 839.38 (mean ± SD) during the follow up period, Table-1.
Table-1.
Distribution of various demographic and echocardiographic findings insubjects according to the outcome(n = 120).
| PARAMETERS | ALIVE AT FOLLOW UP (n = 100) | DEAD AT FOLLOW UP (n = 20) | p-value |
|---|---|---|---|
| AGE (in years) | 55.79 ± 9.324 | 53.70 ± 12.990 | .501 |
| NTproBNP | 772.94 ± 663.269 | 2783.50 ± 839.380 | <.001 |
| LVEF % | 55.08 ± 7.484 | 38.05 ± 6.345 | <.001 |
| WMSI | 1.24 ± .375 | 2.08 ± .311 | <.001 |
| E/e' | 10.36 ± 4.237 | 16.25 ± 4.141 | <.001 |
| GLS | −12.78 ± 3.389 | −4.90 ± 3.998 | <.001 |
| ESL | χ2 = 2.66 (1), .214* | ||
| PRESENT | 88 (73.3 %) | 20 (16.7 %) | |
| ABSENT | 12 (10 %) | 00 (0 %) | |
| PSC | χ2 = 6.06 (1), .014# | ||
| PRESENT | 50 (41.7 %) | 4 (3.3 %) | |
| ABSENT | 50 (41.7 %) | 16 (13.3 %) | |
Values are in mean ± SD*Values are for Fisher's exact test#Values are for Chi square test.
(ESL-early systolic lengthening, PSC- post systolic contraction).
All the Conventional Echocardiographic and 2-Dimensional speckle tracking parameter included were significantly different among patients who were alive and those who had died during the follow up period except for early systolic lengthening (ESL). Left ventricular ejection fraction (LVEF) in living subjects was significantly higher (55.08 ± 7.484) while values for E/e', a marker for diastolic dysfunction (10.36 ± 4.24) and mean WMSI (1.24 ± .37) were significantly lower in patients alive at the end of follow up, i.e., at 6 months, Table-1.
Global longitudinal strain (GLS) was also significantly different between the two groups; with subjects alive at the end of the period of follow up (−12.78 ± 3.389) having better mean scores than subjects who were dead (−4.90 ± 3.998). Similarly post systolic contraction was also significantly different between the two groups; greater number of patients who were alive at the end of follow up showed post systolic contraction than the patients who were dead, Table-1. Early systolic lengthening however did not vary significantly between the two groups.
Relationship between NTproBNP levels, Conventional echocardiographic and Speckle tracking echocardiography (STE) findings:
A Pearson's correlation was run to assess the relationship between GLS, NTproBNP levels, WMSI scores and LVEF at baseline. Data for all the 157 participants were available.
The result of Pearson correlation showed that there was a strong positive and statistically significant relationship between GLS and NT proBNP levels {r (155) = .892, p < .001} and this relationship couldaccount for 79.6 % of variation scores (r2 = .796). Similar results were obtained for Pearson correlation between GLS and WMSI scores wherein a statistically significant, strong positive relationship between GLS and WMSI{r (155) = .883, p < .001} was found. This relationship could account for 77.9 % of variation scores (r2 = .779) in between the two variables. Table-2.
However, the result of Pearson correlation between GLS and LVEF showed that there was a statistically significant but strong negative relationship between GLS and LVEF {r (155) = - .881, p < .001} and that this relationship could account for 77.6 % of the variation between the two variables. Pearson correlation between GLS and time of presentationalso showed that there was a statistically significant and moderate negative relationship between the variables {r (155) = - .352, p < .001} and 12.39 % of the variation could be explained by this relationship. Table-2.
Similarly a statistically significant, strong positive and strong negative correlation was found between GLS at baseline and NTproBNP {r (98) = .619, p < .001}, LVEF {r (98) = - .813, p < .001} at the end of 6 months respectively.
Moreover an independent sample t test reported a significant difference in GLS scores between alive and dead patients with STEMI, t (79) = −8.765, p < .001, 95 % C.I [−8.5787 to −5.4035]. The dead patients had on an average higher GLS (M = −4.211, SD = 2.6157) as compared to alive patients (M = −11.202, SD = 3.1564).
GLS as an early predictor of Acute Myocardial Infarction.
GLS scores ≥ −16 were considered abnormal for univariate analysis, whereas WMSI scores ≥1.1 indicated the presence of regional wall motion abnormality (RWMA) of any kind. Similarly a cut off value of NTproBNP levels ≥125 pg/ml and LVEF ≤35 % was taken as severe for the purposes of analysis.
On univariate analysis it was found that GLS was observed in 90.4 % of the subjects where as WMSI could identify 59.2 % of the subjects at baseline. This finding was statistically significant with χ2 = 11.41 (1), p = .001. Similarly there was a statistically significant difference, χ2 = 20.34 (1), p= <.001 between NTproBNP at presentation and GLS score with the latter found in more number of subjects.
The area under the ROC curve for GLS was .915, which represents an excellent level of discrimination according to Hosmer et al (2013).
4. Discussion
Comparing echocardiographic parameters between alive and dead, found statistically significant value. The mean LVEF% among alive group is 55.08 ± 7.484, while in dead group it is significantly lower 38.05 ± 6.345. It is statistically significant (p < .001). The mean WMSI among alive group is 1.24 ± .375 while the mean among dead group is significantly higher 2.08 ± .311(p < .001). the E/e’ a marker of diastolic dysfunction is 10.36 ± 4.237, while it is 16.25 ± 4.141. in dead group (p < .001). the mean value of GLS among alive group is −12.78 ± 3.389 while those of dead group is markedly diminished −4.90 ± 3.998 (p < .001). the mean NT pro BNP level in alive and dead group is 772.94 ± 663.269 & 2783.50 ± 839.380 respectively. This value is statistically significant (p < .001). Prognostic value of ejection fraction in patients admitted with acute coronary syndrome: A real world study by PerelshteinBrezinov O et al;18 showed that Mortality rates were highest among patients with severe LV dysfunction, intermediate among those with mild-moderate LV dysfunction, and lowest among those with preserved LV function. Echocardiography can be useful for risk estimation and assessment of prognosis after myocardial infarction, which is the objective of this study. Various echocardiographic parameters are providing prognostic information, left ventricular volumes and ejection fraction and introduction of tissue Doppler imaging and speckle-tracking strain imaging has resulted in additional prognostic parameters, such as left ventricular strain (rate) and dys-synchrony. Moller et al19 reported that wall motion score index (WMSI) had a greater prognostic power, although both LVEF and WMSI provided prognostic information. Carluccio et al20 also reported that WMSI independently predicted cardiac events in patients with acute MI. Park et al21 reported that both GLS and segmental LS of left anterior descending coronary artery territory were independent predictors of all-cause mortality in patients with anterior-wall MI. The prognostic value of GLS was reported by Hung et al22 in 603 high-risk patients with MIs from the Valsartan in Acute Myocardial Infarction study and both Munk et al23 (n = 425) and Antoni et al24 demonstrated the prognostic value in STEMI. MadsErsbøllMD et al;25 in their study ‘Prediction of All-Cause Mortality and Heart Failure Admissions From Global Left Ventricular Longitudinal Strain in Patients With Acute Myocardial Infarction and Preserved Left Ventricular Ejection Fraction” showed that assessment of GLS us ing a semiautomatic algorithm and a cut off of −14 % enabled the identification of a smaller subgroup with 3 times higher risk for the combined endpoint, 12 times higher risk for cardiac death, and 5 times higher risk for HF hospitalization, and, importantly, the remaining large group had very favourable outcomes.
4.1. Association of WMSI with ESL among study subjects with STEMI and NSTEMI
Moreover ESL is present in all cases of RWMA; however even patients with no RWMA, ESL was positive in 90.7 % (n = 39) patients. Recent studies26,27 have shown that ischaemic myocardium tends to lengthen before the onset of systolic shortening, probably due to its reduced ability to generate adequate active force as the LV pressure rises steeply during the isovolumic contraction phase (IVC).28 The duration of early systolic lengthening (ESL) has been shown to be proportional to the infarct size in STEMI patients.29 The rationale for assessment of stretch during early systole is that during IVC, when the mitral valve has moved to its final closing position and LV volume is constrained, there is a substantial ‘tug-of-war’ effect between the different LV segments. This effect is enforced as the volume has to remain fixed, i.e. if one segment shortens, another has to lengthen, which is not a necessity when volume can change during ejection.12 During IVC, the non-ischaemic segments with a preserved contractile force will cause ischaemic segments to lengthen as the ischaemic segments are incapable of increasing contractile force at a similar rate as the non-ischaemic segments. With LV ejection the constant LV volume constraint is not active, dP/dt decreases and ischaemic segments are also able to shorten to some extent, and it dependent on the degree of preserved myocardium. So, smaller infarcts would have a shorter DESL, while larger would have longer DESL.13 This may be partly explained by the afterload dependency of GLS, reduced shortening in patients with no visible scar could be due to loading factors such as hypertension, rather than ischemia. In contrast, DESL may potentially be less dependent on afterload, as it takes place during IVC.
P Brainin et al;28in their study ‘early systolic lengthening in patients with ST-segment elevation myocardial infarction: a novel predictor of cardiovascular events” showed that Assessment of ESL in patients with STEMI yields independent and significant prognostic information on the future risk of cardiovascular events. Wasim Zahid, et al;30 ischaemic myocardial segments tend to stretch as the intraventricular pressure rises steeply during the iso-volumic contraction phase before they contract during ejection. ESL could identify patients with minimal myocardial damage, differentiate between occlusion and non-occlusion, and may be helpful in the risk stratification of patients with NSTE-ACS. DESL was significantly better than GLS, WMSI, and LVEF. With above discussion that very short, or lack of, DESL is associated with minimal myocardial damage, while prolonged DESL is associated with larger infarcts and occluded arteries, and hence higher risk in patients with NSTE-ACS.
5. Conclusion
GLS by speckle tracking echocardiography has good correlation with LVEF, WMSI and NT pro-BNP, as well as it is a strong predictor of mortality and heart failure among patients with acute myocardial infarction. Early systolic lengthening appears in a minimally damaged myocardium well before the appearance of regional wall motion abnormality and derangement of ejection fraction. It can be utilized as an early diagnostic tool in cases of NSTEMI as well as amongst STEMI without appreciable wall motion abnormality. Although bigger studies is required to come to conclusion but Its low cost, less time consumption, portability, no added risk and wider availability should be given consideration. No other single test in the management of acute myocardial infarction has such diagnostic, myocardial viability detection as well as prognostic values at such a lower cost.
The limitations of our study was small population size, shorter follow up period, unavailability of grading system in GLS, no standard recommended value of DESL for early systolic lengthening and post systolic index for post systolic contraction were the limitation of this study.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We appreciate and thanks to all individuals who involve directly or indirectly to support this study including the study participants.
Footnotes
Institute of Cardiovascular sciences, R G Kar Medical College and Hospital, Kolkata, West Bengal- 700,004.
Contributor Information
Dhananjay Kumar, Email: djvishainya@gmail.com.
Manish Saha, Email: drmanishsaha@gmail.com.
Santanu Guha, Email: guhas55@gmail.com.
Tirthankar Roy, Email: hopefultiro@gmail.com.
Rohit Kumar, Email: rohit.mbbs.md.dm@gmail.com.
Abhirup K. Sinha, Email: dr.abhirup.sinha@gmail.com.
Abbreviation
GLS- global longitudinal strain.
ESL- Early systolic lengthening.
PSC- Post systolic contraction.
STEMI- ST segment elevation myocardial infarction.
NSTEMI- Non ST segment elevation myocardial infarction.
WMSI- Wall motion score index.
RWMA- Regional wall motion abnormality.
LVEF- Left ventricular ejection fraction.
AMI – acute myocardial infarction.
References
- 1.Bansal M., Kasliwal R.R. How do I do it? Speckle-tracking echocardiography. Indian Heart. 2013;65:117–123. doi: 10.1016/j.ihj.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ersbøll M., Valeur N., Mogensen U.M., et al. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cariol. 2013;61:2365–2373. doi: 10.1016/j.jacc.2013.02.061. [DOI] [PubMed] [Google Scholar]
- 3.Wang N., Hung C.L., Shin S.H., et al. Regional cardiac dysfunction and outcome in patients with left ventricular dysfunction, heart failure, or both after myocardial infarction. Eur Heart J. 2016;37:466–472. doi: 10.1093/eurheartj/ehv558. [DOI] [PubMed] [Google Scholar]
- 4.Kalam K., Otahal P., Marwick T.H. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100:1673–1680. doi: 10.1136/heartjnl-2014-305538. [DOI] [PubMed] [Google Scholar]
- 5.Dandel M., HetzerR Echocardiographic strain and strain rate imaging--clinical applications. Int J Cardiol. 2008;132:11–24. doi: 10.1016/j.ijcard.2008.06.091. [DOI] [PubMed] [Google Scholar]
- 6.Bière L., Donal E., Terrien G., et al. Longitudinal strain is a marker of microvascular obstruction and infarct size in patients with acute ST-segment elevation myocardial infarction. PLoS One. 2014;9 doi: 10.1371/journal.pone.0086959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brainin P., Biering-Sørensen S.R., Møgelvang R., Sogaard P., Jensen J.S., Biering-Sorensen T. Postsystolic shortening by speckle tracking echocardiography is an independent predictor of cardiovascular events and mortality in the general population. JAm Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi J.O., ChoSW, Song Y.B., et al. Longitudinal 2D strain at rest predicts the presence of left main and three vessel coronary artery disease in patients without regional wall motion abnormality. Eur J Echocardiogr. 2009;10:695–701. doi: 10.1093/ejechocard/jep041. [DOI] [PubMed] [Google Scholar]
- 9.Voigt J.U., Lindenmeier G., Exner B., et al. Incidence and characteristics of segmental postsystolic longitudinal shortening in normal, acutely ischemic, and scarred myocardium. J Am Soc Echocardiogr. 2003;16:415–423. doi: 10.1016/s0894-7317(03)00111-1. [DOI] [PubMed] [Google Scholar]
- 10.Skulstad H., Edvardsen T., Urheim S., et al. Postsystolic shortening in ischemic myocardium: active contraction or Passive recoil? Circulation. 2002;106:718–724. doi: 10.1161/01.cir.0000024102.55150.b6. [DOI] [PubMed] [Google Scholar]
- 11.Claus P., Weidemann F., Dommke C., et al. Mechanisms of postsystolic thickening in ischemic myocardium: mathematical modelling and comparison with experimental ischemic substrates. Ultrasound Med Biol. 2007;33:1963–1970. doi: 10.1016/j.ultrasmedbio.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Brainin P., Haahr-Pedersen S., Olsen F.J., et al. Early systolic lengthening in patients with ST-segment-elevation myocardial infarction: a novel predictor of cardiovascular events. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.013835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyseggen E., Skulstad H., Helle-Valle T., et al. Myocardial strain analysis in acute coronary occlusion: a tool to assess myocardial viability and reperfusion. Circulation. 2005;112:3901–3910. doi: 10.1161/CIRCULATIONAHA.105.533372. 2005. [DOI] [PubMed] [Google Scholar]
- 14.Russell K., Smiseth O.A., Gjesdal O., et al. Mechanism of prolonged electromechanical delay in late activated myocardium during left bundle branch block. Am J Physiol Heart Circ Physiol. 2011;301:H2334–H2343. doi: 10.1152/ajpheart.00644.2011. [DOI] [PubMed] [Google Scholar]
- 15.Yingchoncharoen T., Agarwal S., Popović Z.B., Marwick T.H. Normal ranges of left ventricular strain: AMeta-Analysis. J Am Soc Echocardiogr. 2013;26:185–191. doi: 10.1016/j.echo.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Thygesen Kristian, Alpert Joseph S., Jaffe Allan S., et al. Fourth universal definition of myocardial infarction. J Am Coll Cardiol. 2018;72(18):2231–2264. doi: 10.1016/j.jacc.2018.08.1038. 2018. [DOI] [PubMed] [Google Scholar]
- 17.Yang Hong, Wright Leah, Negishi Tomoko, et al. Research to practice: assessment of left ventricular global longitudinal strain for surveillance of cancer chemotherapeutic-related cardiac dysfunction. J Am Coll Cardiol Img. 2018;11(8):1196–1201. doi: 10.1016/j.jcmg.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brezinov O.P., KlempfnerR Zekry. SB, Goldenberg I, Kuperstein R . Prognostic value of ejection fraction in patients admitted with acute coronary syndrome: a real world study. Medicine (Baltim) 2017;96:e6226. doi: 10.1097/MD.0000000000006226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Møller J.E., Hillis G.S., Oh J.K., Reeder G.S., Gersh B.J., Pellikka P.A. Wall motion score index and ejection fraction for risk stratification after acute myocardial infarction. Am Heart J. 2006;151:419–425. doi: 10.1016/j.ahj.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 20.Carluccio E., Tommasi S., Bentivoglio M., Buccolieri M., Prosciutti L., Corea L. Usefulness of the severity and extent of wall motion abnormalities as prognostic markers of an adverse outcome after a first myocardial infarction treated with thrombolytic therapy. Am J Cardiol. 2000;85:411–415. doi: 10.1016/s0002-9149(99)00764-x. [DOI] [PubMed] [Google Scholar]
- 21.Park Y.H., Kang S.J., Song J.K., et al. Prognostic value of longitudinal strain after primary reperfusion therapy in patients with anterior-wall acute myocardial infarction. J Am Soc Echocardiogr. 2008;21:262–267. doi: 10.1016/j.echo.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 22.HungCL VermaA., Uno H., Shin S.H., Bourgoun M., Hassanein A.H., et al. Longitudinal and circumferential strain rate, left ventricular remodeling, and prognosis after myocardial infarction. J Am Coll Cardiol. 2008;56:1812–1822. doi: 10.1016/j.jacc.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 23.MunkK AndersenNH., Terkelsen C.J., Bibby B.M., Johnsen S.P., Botker H.E., et al. Global left ventricular longitudinal systolic strain for early risk assessment in patients with acute myocardial infarction treated with primary percutaneous intervention. J Am Soc Echocardiogr. 2008;25:644–651. doi: 10.1016/j.echo.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Antoni M.L., Mollema S.A., Delgado V., Atary J.Z., BorleffsCJW, Boersma E., et al. Prognostic importance of strain and strain rate after acute myocardial infarction. Eur Heart J. 2008;31:1640–1647. doi: 10.1093/eurheartj/ehq105. [DOI] [PubMed] [Google Scholar]
- 25.Prediction of All-Cause Mortality and Heart Failure Admissions from Global Left Ventricular Longitudinal Strain in Patients with Acute Myocardial Infarction and Preserved Left Ventricular Ejection Fraction. 2020. [DOI] [PubMed] [Google Scholar]
- 26.Lyseggen E., Skulstad H., Helle-Valle T., et al. Myocardial strain analysis in acute coronary occlusion a tool to assess myocardial viability and reperfusion. Circulation. 2005;112:3901–3910. doi: 10.1161/CIRCULATIONAHA.105.533372. [DOI] [PubMed] [Google Scholar]
- 27.Smedsrud M.K., Sarvari S., Haugaa K.H., et al. Duration of myocardial early systolic lengthening predicts the presence of significant coronary artery disease. J Am Coll Cardiol. 2012;60:1086–1093. doi: 10.1016/j.jacc.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 28.Brainin P., Haahr-Pedersen S., Olsen F.J., et al. Early systolic lengthening in patients with ST-segment elevation myocardial infarction: a novel predictor of cardiovascular events. Eur Heart J. 2019;40:40. doi: 10.1161/JAHA.119.013835. ehz748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeiher A.M., Wollschlaeger H., Bonzel T., Kasper W., Just H. Hierarchy of levels of ischemia‐induced impairment in regional left ventricular systolic function in man. 1987;76:768–776. doi: 10.1161/01.cir.76.4.768. Circulation. [DOI] [PubMed] [Google Scholar]
- 30.Zahid W., Eek C.H., Remme E.W., Skulstad H., Fosse E., Edvardsen T. Early systolic lengthening may identify minimal myocardial damage in patients with non-ST-elevation acute coronary syndrome. Eur Heart J - Cardiovasc Imaging. 2014;15:1152–1160. doi: 10.1093/ehjci/jeu101. [DOI] [PubMed] [Google Scholar]