Abstract
Components that comprise our brain parenchymal and cerebrovascular structures provide a homeostatic environment for proper neuronal function to ensure normal cognition. Cerebral insults (e.g. ischaemia, microbleeds and infection) alter cellular structures and physiologic processes within the neurovascular unit and contribute to cognitive dysfunction. COVID-19 has posed significant complications during acute and convalescent stages in multiple organ systems, including the brain. Cognitive impairment is a prevalent complication in COVID-19 patients, irrespective of severity of acute SARS-CoV-2 infection. Moreover, overwhelming evidence from in vitro, preclinical and clinical studies has reported SARS-CoV-2-induced pathologies in components of the neurovascular unit that are associated with cognitive impairment. Neurovascular unit disruption alters the neurovascular coupling response, a critical mechanism that regulates cerebromicrovascular blood flow to meet the energetic demands of locally active neurons. Normal cognitive processing is achieved through the neurovascular coupling response and involves the coordinated action of brain parenchymal cells (i.e. neurons and glia) and cerebrovascular cell types (i.e. endothelia, smooth muscle cells and pericytes). However, current work on COVID-19-induced cognitive impairment has yet to investigate disruption of neurovascular coupling as a causal factor. Hence, in this review, we aim to describe SARS-CoV-2's effects on the neurovascular unit and how they can impact neurovascular coupling and contribute to cognitive decline in acute and convalescent stages of the disease. Additionally, we explore potential therapeutic interventions to mitigate COVID-19-induced cognitive impairment. Given the great impact of cognitive impairment associated with COVID-19 on both individuals and public health, the necessity for a coordinated effort from fundamental scientific research to clinical application becomes imperative. This integrated endeavour is crucial for mitigating the cognitive deficits induced by COVID-19 and its subsequent burden in this especially vulnerable population.
Keywords: cognitive impairment, endothelium, SARS-CoV-2, neurovascular uncoupling, oxidative stress
Owens et al. determined SARS-CoV-2 as a driver of neurovascular unit disruption and cognitive impairment. In this review, they were the first to propose neurovascular uncoupling as a mechanism for COVID-19-induced cognitive deterioration.
Graphical Abstract
Graphical Abstract.
Introduction
As of February 2024, COVID-19 has affected over 770 million individuals with 7 million COVID-19 attributed deaths worldwide.1 Acute SARS-CoV-2 infection typically proceeds as a mild to moderate infection with common symptoms of fever, fatigue and dry cough.2 However, in older patients with vascular comorbidities [i.e. type 2 diabetes (T2D), hypertension], SARS-CoV-2 infection pathogenicity rises, involving multi-organ dysfunction and increased risk of death.3-5 The brain is a critical target organ in acute and recovered COVID-19 patients6 experiencing long-term symptoms (i.e. Long-COVID).7 Clinical evidence has indicated that up to 70% of patients enduring persistent COVID-19 symptoms after recovery from initial infection report cognitive deficits.8 Moreover, COVID-19 related cognitive sequela are more prominent in older adults with underlying complications,9 making prompt investigation into mechanisms of COVID-19-induced cognitive impairment of the utmost importance for this vulnerable patient population. However, pathophysiological mechanisms have yet to be uncovered for cognitive dysfunction preceded by COVID-19.
Neurovascular coupling (NVC) is a critical mechanism to ensure normal cognitive processing and involves the coordinated action of brain parenchymal (i.e. neurons and glia) and vascular cells [i.e. endothelia, smooth muscle (SMC), pericytes] that comprise the neurovascular unit (NVU).10 NVC is responsible for temporally and spatially precise adjustment of cerebromicrovascular blood flow to meet the energetic and nutritive needs of active neurons.11 Disruption of the NVU is documented in preclinical models and contributes to impaired NVC and resulting cognitive dysfunction.12 Moreover, clinical studies have replicated these findings, showing impaired NVC responses in aging13-15 and age-related disorders [i.e. T2D,16 peripheral artery disease,17-19 cerebral small vessel disease (CSVD)20 and dementia].21,22
The endothelium is an essential player in the NVC response. In direct communication with glial and neuronal cell types on the parenchymal side, and contractile pericytes and SMCs superficial to the vascular intima, the endothelium is in an ideal position to receive neuronal and glial signalling to mediate the vasodilatory response.10 Impairment of the endothelium in common age-related conditions disrupts the NVC response, contributing to cognitive dysfunction.23 In addition, clinical studies showed that widespread macro- and microvascular dysfunction strongly associated with NVC impairment and cognitive decline.17,24 SARS-CoV-2 has effects on all cells within the NVU; however, permissibility of cell types is variable, with the endothelium largely deemed as the most permissible, and likely involved in mediating the neuropathologic consequences associated throughout the NVU.25 Systemic vascular dysfunction is prevalent in acute and convalescent COVID-19.26 Moreover, evidence suggests that SARS-CoV-2 may have specific neuroanatomical predilection of CSVD pathology (i.e. cerebral microbleeds) that is associated with cognitive impairment.6 Hence, the SARS-CoV-2 virus is at the forefront of NVU disruption, but NVC impairment has yet to be investigated as a mechanism for COVID-19-induced cognitive sequela.
This review aims to (i) detail COVID-19 as a factor that contributes to, and exacerbates, cognitive impairment, (ii) review components of the NVC response and COVID-19's role in NVU and NVC disruption and (iii) provide evidence of preventative and therapeutic treatment to mitigate COVID-19-induced cognitive impairment through rescue of NVC. Neurovascular dysfunction is one of the most prominent, as well as a preceding feature, in cognitive decline and progression to dementia.27 Hence, we aim to unravel the COVID-19-induced effects on NVC and cognition to pave the way for bench to bedside research and clinical therapeutic intervention.
Cognitive impairment in COVID-19
COVID-19-induced cognitive impairment is highly prevalent, with nearly one in every four patients presenting with cognitive deficits up to 1 year following infection.28-30 Inability to critically think and remember past events is the one of leading causes of disability in older adults with ∼25% of individuals over 65 presenting with cognitive dysfunction in the United States.31 Moreover, this impairment worsens in pathological neurocognitive disorders such as mild cognitive impairment (MCI) and dementia. A looming question remains: how long do the effects of COVID-19 on cognition last? After 3 years post-pandemic, we have yet to reach this answer, as the risk horizon (i.e. no greater risk between COVID-19 and control) and time to equal incidence (i.e. equal cumulative incidence between groups) have not arrived.32 Hence, it is imperative to investigate mechanisms that contribute to COVID-19-induced cognitive impairment and develop therapeutic interventions to mitigate cognitive decline following recovery from acute SARS-CoV-2 infection. Here, we highlight information from systematic reviews and meta-analyses (Table 1) assessing COVID-19-induced cognitive impairment.
Table 1.
Meta-analysis evidence of long-term post-COVID-19 cognitive sequela
| Year | Area of focus | Evidence |
|---|---|---|
| 2023 | Executive function impairment in the post-COVID population compared to healthy controls | Velichkovsky et al. evaluated six studies involving 9411 participants using fluid cognitive tests and found that post-COVID-19 individuals demonstrated a reduction in executive functioning, including inhibition and set shifting, when compared with healthy controls [Standardized Mean Difference −0.35, 95% CI (−0.59, −0.11), P < 0.05]. |
| 2022 | Prevalence of neurologic sequelae of COVID-19 infection persisting for one year following diagnosis | Zeng et al. evaluated 68 studies and 738 430 participants and found that nearly 20% of patients recovering from COVID-19 experienced general cognitive impairment [19.7%, 95% CI (8.8–33.4%)] while ∼18% [17.5%, 95% CI (8.6–29.6%)] suffered from memory impairment in the post-COVID phase. Furthermore, cognitive impairment was found to be a persistent side effect of COVID-19 infection with ∼22% of individuals [22.2%, 95% CI (19.6–25.0%)] continuing to experience cognitive deficits 6–12 months following infection. |
| General cognitive impairment in the post-COVID population compared to healthy controls | Crivelli et al. evaluated 27 studies involving 2049 participants using primarily the Montreal Cognitive Assessment and found that individuals recovering from COVID-19 infection demonstrated a significant decline in general cognitive functioning at 6-month follow-up compared to healthy controls [−0.94, 95% CI (−1.59, −0.29); P = 0.0049]. Additionally, post-COVID-19 individuals demonstrated persistent decreased performance in executive functioning, memory, and attention when compared to healthy individuals. | |
| Long-term neurologic impact of COVID-19 infection in recovered adult patients | Premraj et al. evaluated 18 studies involving 10 530 participants and found that nearly 30% of individuals recovering from COVID-19 experienced memory impairment [28.44%, 95% CI (21.52–35.35%)], 21% experienced attention deficits [21.84%, 95% CI (7.30–36.38%)], and 32% reported a subjective sense of ‘brain fog’ [32.17%, 95% CI (10.31–54.03%)] presenting within 3 months following infection and persisting as a long-term sequalae (6 months or more) of the disease. | |
| 2021 | Prevalence of persistent subjective and objective cognitive impairment in individuals recovered from COVID-19 infection | Badenoch et al. evaluated 51 studies involving 18 917 and found that ∼20% of participants [20.2%, 95% CI (10.3–35.7%)] experienced cognitive impairment assessed by objective measurements such as Montreal Cognitive Assessment. It was determined that 15% of participants [15.3%, 95% CI (8.9–25%)] experienced subjective cognitive impairment assessed using self-reporting of memory impairment, concentration impairment, or ‘brain fog’. |
Multiple cognitive domains are impaired following SARS-CoV-2 infection. Executive function, controlled largely by the prefrontal cortex and critically involved in higher-level thinking and decision making, is one of these domains.33 Specific deficits of executive function include planning, abstraction, behavioural control and orientation.28,34,35 Other functions of the prefrontal cortex, including attention and working memory, are also commonly impaired after recovery from COVID-19.28,34,36 Visuospatial and episodic memory impairment are a consistent finding following SARS-CoV-2 infection.28,34 In recovered COVID-19 patients, visuospatial abnormalities are associated with increased burden of white matter hyperintensities,34 a common marker of ischaemic damage to the brain parenchyma due to CSVD.37 These findings are critical in understanding underlying aetiologies for COVID-19 induced cognitive impairment, as COVID-19 has a specific neuroanatomical predilection of CSVD and associated with cognitive dysfunction.6 Episodic memory, an area controlled largely by the medial temporal lobe and hippocampus, is also as an impaired cognitive domain following SARS-CoV-2 infection.34 Importantly, as these symptoms, along with COVID-19 neuropathology, have similar features to Alzheimer's disease,38,39 there is an urgent need to unravel a mechanism that contributes to this detrimental progression of cognitive impairment.
The impact of illness severity on cognitive impairment has been a significant focus of investigation. In multiple systematic reviews encompassing over 25 separate studies of COVID-19-induced cognitive dysfunction, severity of acute infection was not shown to chiefly contribute to onset of cognitive impairment28,40 Vascular comorbidities (i.e. T2D, hypertension and dyslipidemia) are the most common modifiable risk factors of dementia progression.41 Cognitive impairment post-COVID-19 is associated with concomitant vascular comorbidities; however, patients with a history of COVID-19 had an 18% increase in cognitive impairment prevalence compared with controls, even after adjusting for vascular comorbidities.33 Hence, COVID-19 is likely an independent factor in cognitive impairment development. Given COVID-19's classification as a vascular disease primarily affecting endothelial function,42 its recognition as an additional risk factor of cognitive impairment, particularly when combined with established vascular comorbidities, represents a defining moment in our ongoing understanding of long-term cognitive sequela.
In addition to the impact of SARS-CoV-2 on cognitive health, extrinsic factors from the COVID-19 pandemic and lock down procedures posed challenges that may have affected cognition. Prolonged indoor settings and limited social interaction have negative impacts on brain health and increases the risk of depression.43 During the peak of the COVID-19 pandemic there was an elevated incidence of depression.44 Depression itself an independent risk factor for development of cognitive impairment.45 Social isolation at the onset of the pandemic altered sleep/wake cycles, which is a predictor of depression46 and affects cardiovascular function47,48 and functional cognitive processes.49,50 Physical activity levels of individuals decreased world-wide during the height of the pandemic.51 Moderate physical exercise beginning in midlife has been showing to significantly reduce risk of cognitive decline.52 Hence, physical exercise could potentially aid in alleviating long-COVID brain fog in middle-aged to older adults.
Neurovascular coupling mechanism and COVID-19-induced cell-specific impairment
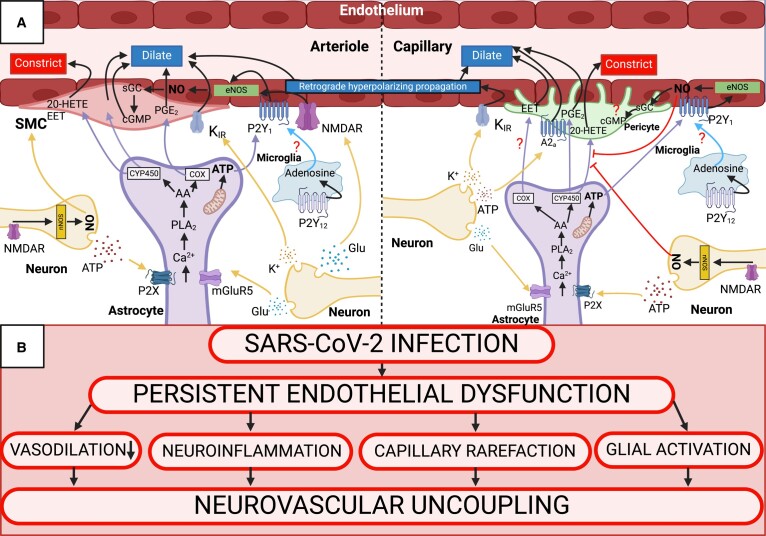
Cognitive function is intrinsically linked to the homeostatic mechanism of NVC. In its most simplistic form, increased local neuronal activity initiates the process of NVC, or functional hyperaemia, by eliciting spatially and temporally precise cerebromicrovascular vasodilation and increased blood flow through direct or indirect mechanisms. Due to a lack of neuronal energy reserves, this tight coupling mechanism is essential for efficient delivery of oxygen and glucose to meet local neuronal demand.11 Moreover, neurotoxic metabolites (e.g. CO2 and amyloid-β peptide) are cleared through NVC-mediated increase in blood flow, thereby creating a homeostatic microenvironment to ensure proper neuronal function.10,53 Acute disease and progression of long-term symptoms in COVID-19 are detrimental to brain function. As COVID-19 has been deemed an endothelial disease42,54-56 with prevalent cerebral small and large vessel complications arising from endothelial impairment, we describe COVID-19 mechanism of infection in the context of the cerebrovascular endothelium. Moreover, specific pathways within the NVU that contribute to NVC, along with hypothesized SARS-CoV-2-mediated effects on the NVC response, are represented in Fig. 1. Here, we will cover cell-specific interactions within the NVC response, and in vitro, preclinical, and clinical evidence of SARS-CoV-2 cell-specific impairment.
Figure 1.
NVC response and SARS-CoV-2-mediated neurovascular disruption. (A) NVC response at the arteriolar level (left) and capillary level (right). Arrows with question marks indicate hypothesized pathways yet to be uncovered. Sharp arrows indicate cellular signaling within the NVU. Flathead arrows indicate inhibitory action on NVU pathways. (B) Hypothesized SARS-CoV-2-mediated neurovascular uncoupling. SARS-CoV-2 known action of persistent endothelial dysfunction is hypothesized as a causal factor that underlies vasodilatory impairment, neuroinflammation, capillary rarefaction, and glial activation–all known factors that contribute to neurovascular uncoupling. Created with BioRender.com. 20-HETE = 20-hydroxyeicosatetraenoic acid; cGMP = cyclic guanosine monophosphate; sGC = soluble guanylate cyclase; nNOS = neuronal nitric oxide synthase; PGE2 = prostaglandin E2; P2X = purinergic ligand-gated ion channel; AA = arachidonic acid; CYP450 = cytochrome P450; P2Y1, P2Y12 = purinergic G protein-coupled receptors.
Mechanism of infection
Originally deemed as a respiratory epithelial infection,57 the systemic vasculature, with specificity to the endothelium, quickly came to the forefront of COVID-19 induced multi-organ impairment.42 The impact on the brain is of specific interest as cognitive dysfunction and cerebral macro- and microvascular pathologies are prevalent in acute and Long-COVID patients.6 Moreover, the endothelium is heterogeneous in structure and function at different areas the body,58 and SARS-CoV-2 utilizes region specific cellular characteristics of the cerebrovascular endothelium to elicit brain pathology.
At the onset of cerebrovascular endothelial infection, SARS-CoV-2 spike (S1) glycoprotein attaches to the angiotensin converting enzyme 2 (ACE2) receptor. ACE2 is a key contributor in the renin-angiotensin pathway, cleaving the leucyl residue from angiotensin (Ang) I and AngII, producing Ang1–9 and Ang1–7, respectively.59 Ang1–7 acting on the Mas receptor (ACE2-Ang1–7-Max axis) is neuroprotective,60 while ACE-Ang II-Ang II receptor type 1 (AT1R) binding contributes to endothelial activation [i.e. elevations in pro-inflammatory cytokines, reactive oxygen species (ROS), prothrombotic factors] and a persistent thromboinflammatory state.19,61
Following S1 binding, upregulation of proteases ADAM17 and TMPRSS2 (a disintegrin and metallopeptidase domain 17 and transmembrane serine protease 2) lead to ACE2 shedding,62 resulting in uninhibited binding of AngII to AT1R and loss of the neuroprotective ACE2-Ang1–7-Mas axis. The ensuing endothelial activation and loss of ACE2 lead to upregulation of leukocyte adhesion molecule expression19,59 and bradykinin binding to B2 receptors, all contributing to leukocyte transmigration and blood brain barrier disruption (BBB). Moreover, this state of endothelial activation triggers platelet activation, resulting in a fibrin clot and tissue plasminogen activator (tPA)-mediated plasmin formation with concomitant bradykinin production, further disrupting BBB integrity.18,19,63,64
After acute infection, there is strong clinical evidence of long term endothelial dysfunction,55 but the mechanism has not been uncovered. However, highly plausible mechanisms have been hypothesized with the theory of chronically elevated mitochondrial driven ROS generation and suppression of NO production as a mechanism for long-term endothelial dysfunction.65 NO is widely known for its roles as a gasotransmitter responsible for vasodilatory response. This molecule is also critically involved in maintaining the quiescent state of the endothelium,66 and when production is disrupted, is causally related to vascular pathologic consequences, including impairment of NVC and cognitive decline.27,67
Neurons
At initiation of NVC, neurons release glutamate, and activity at glutaminergic post-synaptic receptors, N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazol propionic acid (AMPA), lead to elevations in intracellular calcium ion (Ca2+) concentration that activate Ca2+ dependent enzymes [i.e. neuronal nitric oxide synthase (nNOS), cyclooxygenase 2 (COX-2)] and produce vasodilatory molecules [i.e. nitric oxide (NO), prostanoids].68,69 Moreover, glutamate can act at metabotropic glutamate receptors (mGluR) and ionotropic receptors (NMDAR) on glial and vascular cells, respectively, contributing to the NVC response.27 Elevations in extracellular potassium ion (K+) concentration, due to excitatory neuronal activity, may also facilitate the vascular response,70 and neuronal release of adenosine and adenosine triphosphate (ATP), potent vasodilators, have been reported to act on purinergic receptors and elicit increases in local cerebromicrovascular blood flow.10
There are many possibilities regarding how SARS-CoV-2 may disrupt normal neuronal function.71 The strongest possibility is COVID-19 induced cerebrovascular dysfunction contributing to BBB disruption, clotting cascade abnormalities and endothelial activation (i.e. elevated cytokine secretion and ROS, all of which are known to disrupt neuronal function and will be discussed in the Endothelia and Mural cell sections. Other mechanisms are also plausible and most likely work in tandem. In a mouse model of SARS-CoV-2 infection that was bioengineered to restrict SARS-CoV-2 infection to the respiratory system, the virus was cleared in a week and did not enter the brain. However, the infection-induced immune response resulted in elevated cerebral spinal fluid (CSF) cytokines and impaired hippocampal neurogenesis, most likely related to increased microglial reactivity seen following infection.72 Moreover, these findings have been replicated in other preclinical models and decreased hippocampal neurons and global brain atrophy have been seen at autopsy of COVID-19 patients.73,74 These findings highlight the immune-glial (discussed in Astrocyte and Microglial sections) interaction and the effect of respiratory specific infection on neuronal cells without spread to other organ systems. Autoimmunity may also contribute to SARS-CoV-2 induced neuronal dysfunction as COVID-19 patient CSF revealed auto-antibodies to neuronal and cerebrovascular cell types.75,76 Reactivation of latent pathogens (i.e. Epstein-Barr virus [EBV]) is another possibility77 for neuronal damage and has been postulated as one of the mechanisms of Long-COVID.55 EBV reactivation is typically associated with immunocompromised patients. However, in COVID-19, the physiological stress induced by acute infection has the potential to reactivate latent viruses. This, in turn, may contribute to the neurological sequela78 observed in Long-COVID, possibly through mechanisms involving direct neuronal infection and/or BBB disruption.79 Clinical evidence suggests that SARS-CoV-2 direct neuronal infection is rare and does not account for the neurological sequela associated with COVID-19.71 Autopsy and CSF studies from COVID-19 patients with neurological symptoms show no detection of SARS-CoV-2 in the brain or CSF.75,80-84 In addition, in vitro studies of SARS-CoV-2 original, Delta, and Omicron variants did not infect neurons or astrocytes, but microglia were highly permissive.85 The non-permissive nature of neurons to infection through the endocytic ACE2 pathway is due to low or absent ACE2 receptors. In contrast, others have reported SARS-CoV-2 in the post-mortem brain from COVID-19 patients.86 This may occur through alternate pathways of neuronal infection, such as tunnelling nanotubes, and provide a route for SARS-CoV-2 spreading from permissive to non-permissive cells.87 Based on these data collected since the onset of the pandemic, direct neuronal infection in humans may be plausible, but evidence suggests a greater likelihood of neuronal dysfunction arising from alternative, indirect pathways.
Astrocytes
With placement between neuronal synapses and the cerebromicrovasculature, astrocytes provide a logical spatial relationship for intermediate signalling within the NVC response. However, many of the investigated signalling pathways postulated to link neuronal activity to increased cerebral blood flow (CBF) have been called into question. Evidence of intracellular Ca2+ increases in astrocytes with concomitant increase in microvascular diameter is achieved through activation of mGluR on astrocytes and inositol triphosphate (IP3) signalling, leading to uncaging of intracellular stores of Ca2+, activating phospholipase A2 (PLA2), and producing vasodilatory molecules [epoxyeicosatrienoic acids (EET) and prostanoids] that act on vascular SMCs and pericytes.10,68 Neuronal ATP binding to the astrocytic P2X receptor, which leads to an increase in intracellular Ca2+, has also been shown to affect vasodilation of cerebral micro-vessels, and inhibition of astrocytic P2X receptor suppressed the NVC response.88 Studies that challenge intracellular Ca2+ increases in astrocytes and their contribution to the vascular response have reported that the increases seen are too slow for the rapid coupling response and were shown to occur after vasodilation.53,89 The mGluR-IP3-PLA2 pathway has received criticism as well. In mice lacking IP3 receptors, functional hyperaemia occurred without preceding astrocytic intracellular Ca2+ increase,90-92 and NVC was unaffected in the absence of prostaglandin synthesis.93-95 Moreover, astrocytic potassium siphoning from neuronal excitatory transmission was proposed by Paulson and Newman96 as a plausible mechanism to elicit the vascular response. However, this has been largely refuted, showing that glial K+ siphoning does not contribute to the NVC response.97,98 While there is mixed evidence regarding glial contribution to the haemodynamic response, astrocytes remain an area of active research in neuro-(glial)-vascular coupling.
Aside from their contribution to NVC, astrocytes perform essential action within the brain parenchyma to ensure a stable, homeostatic microenvironment for proper neuronal function. Astrocytes function to regulate neurotransmitter release, buffer extracellular K+ following neuronal excitatory transmission, form the parenchymal side of the BBB, and modulate immune responses.99 However, during an insult (e.g. ischaemia, infection) they take on a ‘reactive’ phenotype where astrocytes hypertrophy, release pro-inflammatory signalling molecules and in cases of neuronal injury form a glial scar to isolate and contain the damage. This can be beneficial to prevent spread of injury and inflammatory processes to healthy neural tissues. During persistent activation, this can be detrimental to neuronal function, ensuing in loss of neural network connection, neuronal degeneration and impaired regeneration.100 Reactive astrocytes are a common finding in post-mortem examination and elevations in serum levels of glial fibrillary acidic protein (GFAP) and S100B were seen COVID-19 patients.101,102 Although evidence has suggested that astrocytes themselves are non-permissive to SARS-CoV-2 infection due to low or absent primary viral entry receptor ACE2, in vitro studies have revealed an alternative route for SARS-CoV-2 entry via the receptor basigin (BSG/CD147).103 Whether astrocytes become reactive to pro-inflammatory stimuli secreted from immune and/or endothelial cells, or if SARS-CoV-2 crosses the BBB and enters into astrocytes through alternative pathways, reactive astrocytic consequences on neuronal cell populations and network dynamics largely depends on vascular cell types with high permissibility.104
Endothelia
The cerebromicrovascular endothelium is intimately related to the brain parenchymal cells. Direct interaction at the capillary level is made between astrocyte end feet and in small sections, neuronal synaptic bulbs. At the arteriole level, parenchymal vasculature is covered by SMC and astrocyte end feet, and penetrating arterioles are separated from the parenchyma by the perivascular space created by the glia limitans.10 Endothelial function within the peripheral vasculature is well known as a large contributor to vascular diameter and flow regulation. However, study of its role in the NVC response is relatively recent. Two predominant mechanisms are postulated to contribute to the vascular response of NVC, potassium inward rectifier (KIR) induced retrograde hyperpolarization signal propagation and local NO generation, both comprising endothelium-dependent models of NVC.105,106
Brain capillary endothelial cell KIR channels are activated by extracellular K+ following neuronal excitation. Activation of these channels result in a propagated hyperpolarizing electrical signal upstream through connexins-based endothelial gap junctions and locally through myoendothelial gap junctions between pericytes and/or SMCs to elicit vasodilation by intracellular Ca2+ reduction.105,107 Endothelial NOS (eNOS) is postulated to be activated directly by neuronal activity and through the traditional neuronal-astrocytic signalling of NVC. NO is the most potent vasorelaxant in the brain, irrespective of NO generated by nNOS or eNOS, and blocking the signalling pathway of either enzyme results in a greater than 50% decrease in the NVC response in vivo.108 Within the neuronal-astrocytic mediated signalling framework, neuronal activity induces calcium wave propagation in astrocytes, elevates astrocytic ATP and hydrolyzed forms [i.e. adenosine diphosphate (ADP) and adenosine] binding to P2Y receptors on the cerebromicrovascular endothelium, contributing to eNOS mediated increases in NO and resulting NVC response.53 Moreover, selective inhibition of endothelial P2Y receptor and eNOS−/− mice have a blunted NVC response, which provides evidence for the neuro-(glial)-vascular coupling hypothesis.67 Direct neuronal activation of endothelial NMDAR may also contribute to eNOS mediated dilation.109 Activity at NMDAR recruits eNOS through increased endothelial intracellular Ca2+ concentration. However, co-activation is required by astrocytic release of D-serine in response to neuronal activation,110 and future work is needed to determine how astrocytes promote D-serine release to favour endothelial NMDAR activation. Both models (i.e. retrograde hyperpolarizing signal and local eNOS mediated vasodilation) may work in tandem, as NMDARs are reported to be scarce in the capillaries but present in arterioles.111,112 Hence, at the capillary level extracellular increases in K+ concentration may initiate the start of the propagated vasodilatory response, with eNOS regulating upstream to increase precision of spatiotemporal CBF distribution, either through neuronal glutaminergic signalling or glial ATP release.
In contrast to neurons and astrocytes, the endothelium extensively expresses the ACE2 entry receptor for SARS-CoV-2. With the addition of common systemic comorbidities (i.e. T2D,113 hypertension114 and hypercholesterolaemia)115 that chronically disrupt vascular endothelial function, SARS-CoV-2 may be the tipping point to elicit multi-organ dysfunction, including brain pathologies contributing to cognitive and neuropsychiatric impairments. Indirect effects (i.e. leukocyte-driven cytokine storm mediated by neutrophils)116 are also a possibility in the pathogenesis of endothelial dysfunction. Both direct and indirect are likely to occur in tandem, as the SARS-CoV-2 virus has been seen within endothelial cells on histopathology reports54 and hypercytokinemia is known to cause deleterious effects on endothelial function.117
Following direct infection, S1 glycoprotein elicits elevated procoagulant and cytokine/chemokine markers118,119 and degradation of endothelial tight junctions making up the BBB.120 Moreover, the SARS-CoV-2 nucleocapsid protein (NP) can promote endothelial dysfunction, in contrast to NPs from other coronaviruses.121 The glycocalyx, a proteoglycan microstructure covering the endothelium essential for maintaining homeostatic function, is also damaged during SARS-CoV-2 infection.122 A healthy glycocalyx may prevent binding of S1 protein to endothelial ACE2; however, when damaged, the glycocalyx facilitates this interaction.123,124 Glycocalyx shedding is reported in both acute and recovered COVID-19 patients125-127 and glycocalyx treated with COVID-19 patient plasma triggered shedding and endothelial impairment,122 further exemplifying the persistent endothelial damage associated with COVID-19. Furthermore, endothelial infection by SARS-CoV-2 promotes endothelial cell injury and death,54 endothelial to mesenchymal transition,128,129 hyperpermeability,130 inflammation and elevations in leukocyte adhesion molecules,119,130-135 increased oxidative stress, decreased NO bioavailability,65 induction of endothelial senescence136,137 and impaired mitochondrial function.138 SARS-CoV-2-mediated mitochondrial dysfunction may also initiate a feedback-loop of excess mitochondrial ROS production and inflammation, which may contribute to the long-lasting endothelial dysfunction observed clinically.65
Clinically relevant cerebrovascular accidents, such as ischaemic and haemorrhagic stroke, are known complications of SARS-CoV-2. The risk of stroke from acute infection was proposed as a dependent consequence of COVID-19 severity.139 However, Veterans Affairs data showed post-COVID-19 patients (1 year after infection) have a significant increased risk of cardiovascular complications (including stroke) irrespective of acute severity of SARS-CoV-2 infection.140 Relative risk of stroke is 3–4 times higher in hospitalized COVID-19 patients compared to hospitalized patients without infection.139 Moreover, there is a 7–8 fold greater risk of stroke in patients with COVID-19 compared to patients with influenza.141 Cerebrovascular complications associated with COVID-19 are also more prevalent in patients with concomitant vascular risk factors.139 Aside from large vessel stroke, CSVD is a prevalent complication in both acute and recovered COVID-19 patients.6 CSVD is an often clinically silent disease affecting the small perforating arteries, arterioles, venules and capillaries.37 In our recently conducted systematic review of CSVD pathology in COVID-19, cerebral small vessel insults, both ischaemic lesions and microbleeds, were prevalent across studies, with microbleeds having a specific predilection to the corpus callosum, subcortical/deep white matter and juxtacortical white matter, differing from often comorbid conditions (i.e. acute respiratory distress syndrome, hypertension). Post-mortem studies also showed a high prevalence of subcortical microbleeds and increased burden of white matter hyperintensities (i.e. the most common marker of CSVD). In addition, histopathology reports from COVID-19 patients revealed CSVD pathology, including, endotheliitis, intravascular thrombosis, endothelial fibrosis and degeneration and BBB disruption.6 As the endothelium is the area of initial insult in large and small vessel stroke and CSVD pathology,19 this strengthens the ongoing consensus of COVID-19 as an endothelial disease.
Mural cells
SMCs and pericytes represent the destination for neuro-(glial)-vascular signalling in arterioles and capillaries, respectively, to induce vasodilation and resulting increases in CBF to meet the nutritive and energetic needs of neurons. SMC relaxation in the brain is mediated by a wide variety of signalling pathways generated from neurons, astrocytes, and endothelium (e.g. NO, prostaglandin E2, EETs, etc.) as previously discussed. Intrinsic to the SMC, penetrating arterioles participate in regulation of flow, independent of signalling from the NVU (i.e. myogenic tone), by the mechanism of autoregulation.142 Exceeding the autoregulatory limit of SMCs disrupts NVU integrity by elevating downstream pressure in distal small vessels.143 At the transition zone between parenchymal arterioles and capillaries, there is a switch in mural cell predominance from SMCs to pericytes. Like SMCs, capillary pericytes are contractile, and participate in regulation of blood flow to help maintain relatively constant blood supply to the brain parenchyma.144 The vascular response to NVC (i.e. blood vessel dilation and resulting increased CBF) has been shown to occur first at the capillary level, approximately one second prior to arteriolar dilation in mice,145 indicating a role for pericytes as the initial ‘effectors’ of the vasodilatory response. As with SMCs, pericytes can respond to a wide variety of signalling molecules from neurons, astrocytes, and endothelia to initiate vasodilation at the most local level of the NVC response.146,147
Endothelial activation can cause detrimental effects on the brain parenchymal cells not only through dysregulation of blood flow, but also by secretion of pro-inflammatory mediators, ROS, and transmigration of leukocytes.148,149 SMCs, lining the media of arteries and arterioles in the brain, can also experience deleterious consequences following endothelial activation.150,151 Most relevant in the context of NVC is COVID-19-induced decrease in endothelium-derived NO bioavailability.152 As described, NO is a potent regulator of SMC vasorelaxation through activation of guanylyl cyclase mediated production of cyclic GMP (guanosine monophosphate). Hence, COVID-19-induced endothelial dysfunction may impair SMC regulation of vascular tone. Another consequence of SMC dysfunction mediated by endothelial activation is progression, or pre-existing history, of atherosclerosis. There is a bidirectional relationship between atherosclerosis and COVID-19, as presence of vascular comorbidities increases the severity of COVID-19 and COVID-19 itself can induce atherosclerotic-type vascular injuries.153 In response to endothelial dysfunction, SMCs proliferate and ingest cholesterol, contributing to early atherosclerotic lesions. Moreover, SMC-produced collagen deposits contribute to fibrous cap formation, potentially obstructing the lumen, leading to hypoperfusion. This is especially prevalent in the brain, as the majority of CSVD cases are driven by atherosclerosis progression.154 As COVID-19 has a strong association with small vessel pathologies,6 SARS-CoV-2-mediated endothelial dysfunction and its contribution to SMC proliferation and extracellular matrix deposits may elicit the atherosclerotic phenotype associated with CSVD. Atherosclerosis also affects the SMC intrinsic mechanism of cerebral autoregulation.155 Evidence suggests that autoregulation may be impaired in COVID-19 patients and could contribute to cognitive dysfunction.156 Aside from endothelium-mediated SMC dysfunction, disruption of endothelial barriers may allow for direct infection of SMC. However, the results indicate that SMCs and pericytes may not express the SARS-CoV-2 entry receptor (i.e. ACE2) and host protease (TMPRSS2) needed for viral fusion.157
Evidence indicates that COVID-19 may specifically be a microvascular endothelial disease, which leads to further exploration of SARS-CoV-2-induced effects on other microvascular cell types (i.e. pericytes). The susceptibility of pericytes to SARS-CoV-2 infection is conflicting. Whereas some report that pericytes are not susceptible to infection,157 multiplexed immunostaining of human brains revealed high ACE2 expression in pericytes.158 Further, CSF levels of platelet-derived growth factor β, a pericyte biomarker, were lower in COVID-19 patients, indicating a disruption in pericyte homeostasis.158 Moreover, an ex vivo study of hamster cortical brain slices indicated SARS-CoV-2 infection of brain pericytes, decreased functional ACE2 membrane activity and AngII-mediated capillary constriction,159 thus causing decreased cerebromicrovascular blood flow.160 However, the question of how SARS-CoV-2 crosses the BBB to reach the abluminal pericyte ACE2 receptor remains unanswered. Rather, we hypothesize that if SARS-CoV-2 has an effect on pericytes, it may be due to movement of S1 protein across the BBB via transcytosis161 or through BBB breakdown mediated by direct endothelial dysfunction and/or cytokine storm as a result of initial respiratory infection.159,162 Whether capillary pericyte or endothelium, concomitant ischaemia and microvascular dysfunction elicit elevations in pro-inflammatory cytokines, ROS and BBB disruption, which contribute to neuronal and astrocytic dysfunction mentioned above, and microglial reactivity mentioned below.163-165
Microglia
Besides regulating immunological processes in the brain, microglia have also been linked to neurodegenerative diseases.166,167 While not until recently, evidence has suggested a role for microglia participation in the NVC response. Like astrocytes, microglia is well positioned between neurons and blood vessels and interact with both. Hence, critical appraisal of microglial role in the NVC response is an area of continued investigation. Mechanistically, microglia has direct purinergic connections between cellular components of the vasculature (i.e. endothelia, SMCs and pericytes). When purinergic microglial receptor (P2Y12R) was absent, vasodilation in response to CO2168 and functional hyperaemia in response to mouse whisker stimulation was diminished, suggesting microglial dynamic sensing of purines (e.g. release of neuronal ATP) may be critical for cerebromicrovascular vasodilatory response169 Moreover, microglia are a significant source of adenosine in the brain, potentially contributing to the NVC response through activation on purinergic receptors on the endothelium67 and/or modulation of neuronal transmission at synapses.170
In COVID-19 patients, post-mortem examinations reveal pronounced reactive microgliosis, with elevations in markers of phagocytosis.81,82,171-173 RNA-seq results have reported alterations of microglial transcriptome in COVID-19 patients compared to controls.173 Microglial differentially expressed genes in COVID-19 were related to inflammatory and cell death pathways.81,173 Hence, microglial activation is present in COVID-19 patients; however, how SARS-CoV-2 triggers this activation [e.g. direct infection, pattern recognition receptors (PRRs) endothelial mediated ROS/pro-inflammatory cytokines, etc.] is under investigation. Microglia respond to viral infection invasive to the parenchyma through PRRs, and elicit a pro-inflammatory response.174 Whether SARS-CoV-2 is neurotrophic is inconclusive, meaning that microglia activation may proceed through different pathways. Similar to pericytes, the S1 glycoprotein may cross the BBB and activate microglial PRRs. In vitro studies indicate that S1 glycoprotein induces elevations in pro-inflammatory cytokines produced by microglia.175 Danger-associated molecular patterns (DAMPs), such as extracellular matrix components and molecules released from damaged cells, may also activate microglia.176 In support of this postulation in COVID-19, studies of ischaemic stroke showed that BBB disruption and release of DAMPs rapidly activate microglia.177 As microglial activation is critical to respond to insults in the CNS (whether DAMPs or neurotrophic viruses), continued activation can prove to be detrimental to brain health.178 Little is known of the long-term complication regarding microglial activation following SARS-CoV-2 infection; however, some evidence does provide insight into the microglial response. Post-mortem analysis of cortical slices from COVID-19 patients has evidenced a transcriptomic profile of microglia similar to what is observed in neurodegenerative conditions.81 Preclinical models showed that S1 glycoprotein induced microglial activation associated with cognitive impairment.179 Also, a mild COVID-19 model resulted in microglial activation for up to seven weeks,72 thereby suggesting the possibility of microglial activation in the pathogenesis of long-term cognitive impairment. In addition, microglia may be directly infected if SARS-CoV-2 crosses the BBB, as evidenced by an in vitro study of the original, Delta and Omicron variants.85
Neurovascular uncoupling in COVID-19
NVC impairment, or neurovascular uncoupling, is a prevalent mechanism in cognitive impairment14 and dementia.53,180 Vascular diseases are the predominant modifiable risk factors in the progression from normal aging to MCI, and from MCI to dementia. Moreover, SARS-CoV-2 is the newest ‘vascular risk factor’ that contributes to onset of cognitive impairment28-30 or worsening of an already pathologic cognitive decrement.181 While SARS-CoV-2's effects on the NVU have been recognized, its role in NVC responses has yet to be uncovered. In this section, we describe how acute SARS-CoV-2 infection and history of COVID-19 may disrupt NVC through the endothelium-dependent model. This focus is supported by the vast literature on COVID-19 as an endothelial disease and leads to increased translatability into the clinic for therapeutic intervention of long-standing, validated treatments known to restore endothelial health systemically.
While ROS are critical for homeostatic signalling pathways, persistent excessive levels are detrimental to normal cellular function182 and can damage tissues and organs such as the endothelium.183 Moreover, one of the most potent effects of elevated levels of ROS is uncoupling of eNOS [i.e. oxidation of co-factors (tetrahydrobiopterin)] and/or formation of reactive nitrogen species (i.e. peroxynitrite). NO plays an essential role in the control of vasomotor function throughout the body.184 In addition, this gasotransmitter is critical for maintaining an anti-inflammatory and anti-thrombotic state, and regulating platelet aggregation, SMC cell proliferation and migration, apoptosis, and endothelial barrier function.185 In the cerebromicrovasculature, the deleterious effects of ROS are evident, as endothelium-mediated NVC responses are impaired,186-189 and are associated with cognitive decline in preclinical models.23,190 In humans, modifiable vascular risk factors (e.g. obesity, hypertension, peripheral artery disease and type 2 diabetes), in which elevations in ROS and oxidative damage are central to their pathogenesis, impair endothelial function and NVC responses.17,191,192 In COVID-19, elevations in ROS are highly prevalent acutely and long-term,193-195 plausibly contributing to endothelial dysfunction as evidenced through mechanistic study of AT1R-mediated ROS generation. In support, preclinical models showed AngII-induced NVC impairment,196,197 even in the presence of endothelium-dependent vasodilators. Moreover, these findings are owed to increased AngII-induced cerebromicrovascular endothelial ROS production. Importantly, deleterious effects of AngII on NVC were attenuated by angiotensin receptor blockers (ARBs),196 and therapeutics that block signalling within the renin-angiotensin pathway showed beneficial effects on endothelial function in COVID-19 patients54 (discussed further in Prevention and therapeutics in COVID-19-induced cognitive impairment). Patients with acute COVID-19 had higher total oxidant status, oxidative stress biomarkers and lower anti-oxidant capacity than healthy controls.193,194 The same pattern was observed in convalescent COVID-19 patients.195 Oxidative stress and inflammation mutually enforce one another117 and contribute to the acute and convalescent COVID-19 thromboinflammatory phenotype.198 Pro-inflammatory binding to receptors on the endothelium elicits elevations in cytoplasmic and mitochondrial ROS, and in turn, production of cellular ROS activates transcription factors (e.g. nuclear factor κ B), which upregulate the synthesis of pro-inflammatory mediators and adhesion molecules. Moreover, this compromises vasoprotective sirtuin-mediated signalling pathways due to nicotinamide adenine dinucleotide (NAD+) depletion from pro-inflammatory PARP-1 [poly (ADP-ribose) polymerase 1] activation, further decreasing eNOS transcription, impairing the quiescent state of the endothelium.117 In sum, SARS-CoV-2 infection brings about acute and chronic endothelial activation,54 potentially mediating impairment of NVC in convalescent COVID-19 patients. While studies have yet to uncover the NVC response in acute and convalescent COVID-19 patients, recent work from Kuchler et al. showed that retinal microvascular reactivity in response to flickering light stimulation was decreased in COVID-19 patients compared to controls.199 This represents a crucial finding as the retina shares embryologic origin with the central nervous system200 and dilation in retinal micro-vessels is a direct consequence of NO release.201 Moreover, retinal microvascular reactivity is predictive of cognitive impairment.202 Additionally, there is a strong association with CSVD—both ischaemic and haemorrhagic—and neurovascular uncoupling.203 With COVID-19's predilection of CSVD pathology to subcortical regions,6 this highlights the need for a detailed, mechanistic study of NVC in vulnerable patient populations. Hence, early clinical evidence supports mechanistic study of microvascular impairment, plausibly contributing to impaired NVC as a mechanism for COVID-19-induced cognitive impairment.
Prevention and therapeutics in COVID-19-induced cognitive impairment
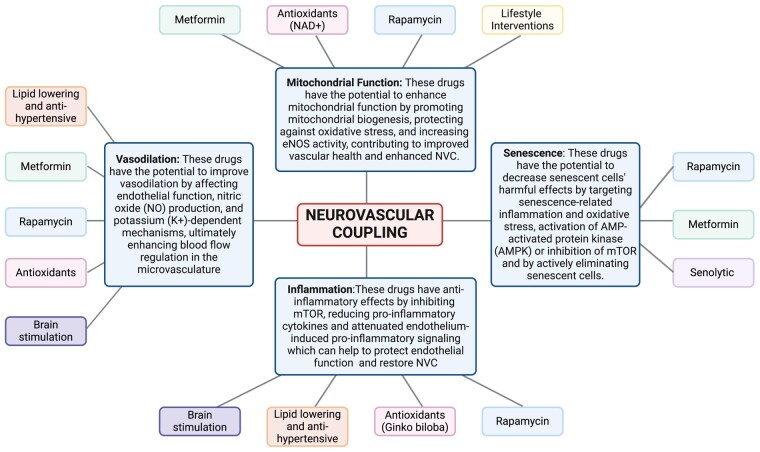
Pharmacological therapy improves endothelial function in patients with history of COVD-19-induced long-term vascular dysfunction.54 In this section we present current evidence of therapeutics known to benefit endothelial function systemically in the context of COVID-19-induced NVC impairment. In addition, we highlight potential avenues for treatment options to be investigated in COVID-19-induced vascular dysfunction, ranging from life-style interventions to transcranial magnetic brain stimulation (Fig. 2). With this evidence, we hope to provide valuable insight into the mechanisms and therapeutic potential of these interventions to mitigate cognitive impairment following COVID-19. For detailed information on other therapeutic strategies targeting endothelial and cerebromicrovascular function not contemplated in this section, please refer to the excellent review by Xu et al.54
Figure 2.
Therapeutics targeting neurovascular uncoupling in COVID-19 induced cognitive impairment. This schematic figure illustrates four key categories, each addressing specific aspects of neurovascular function affected by COVID-19: (i) Mitochondrial Function: Metformin, Anti-oxidants, Rapamycin and life-style interventions, such as time restricted eating; (ii) Senescence: Senolytics, Rapamycin and Metformin; (iii) Inflammation: Anti-oxidants Rapamycin, Statins, ACEI/ARBs and brain stimulation; (iv) Vasodilation Interventions: Anti-oxidants, Statins, ACEI/ARBs, brain stimulation, and metformin. While these interventions have shown promise in preclinical and clinical studies, further research is needed to fully elucidate their effects and potential benefits in the context of COVID-19-related cognitive decline. Created with BioRender.com.
Anti-oxidants
Vitamins C and E
Vitamins C and E both function as anti-oxidants through direct scavenging of free radicals, mitigating the detrimental consequences of cellular exacerbations of ROS.204,205 In addition, they have widely beneficial effects on the systemic vasculature, and at supraphysiological levels contribute to restoring endothelial function, partly through restoring eNOS function.206-208 As eNOS critically contributes to the NVC response, supplementation of these anti-oxidant vitamins may be useful to restore NVC and mitigate cognitive decrement. However, more studies are needed to determine their impact on the cerebromicrovascular endothelium-mediated response. In support of the beneficial effect of these vitamins on the brain, vitamin C supplementation has been associated with the reduction of free radicals, neuroinflammation, iron chelation, and amyloid-beta (Aβ) deposition.209 In vitamin C deficiency, preclinical models showed elevated oxidative stress markers and increased Aβ levels, emphasizing its role in mitigating neurodegenerative processes.210 Recent clinical trials have highlighted vitamin C's role in endothelial barrier integrity and redox homeostasis,211 providing further evidence of its potential as a therapeutic option in attenuating endothelium-dependent functional hyperaemic impairment. Moreover, plasma vitamin C concentrations were elevated in cognitively adept groups compared to individuals with cognitive impairment,212 highlighting the potential link between the known mechanistic aspects of vitamin C and cognitive function. Vitamin E, particularly in the form of α-tocopherol, has been proposed as a neuroprotective agent by demonstrating its ability to ameliorate learning and memory deficits, reduce oxidative stress, diminish Aβ deposits, and enhance cognition within Alzheimer’s disease models.213,214 Moreover, clinical trials show that 800–2000 IU/day of vitamin E to Alzheimer’s disease patients exhibited decreased functional decline compared to placebo, and oxidative stress was attenuated.213,215 Hence, both vitamin C and E may be beneficial in restoring NVC responses and cognition in acute and convalescent COVID-19 patients with cognitive impairment.
Ginkgo biloba
Ginkgo biloba special extract EGb 761® emerges as a promising contender for addressing NVC and mitigating COVID-19-induced cognitive impairment. EGb 761® has been proposed as a potential remedy to alleviate cognitive symptoms persisting beyond three months post-COVID-19 infection.216,217 This extract has demonstrated a multifaceted approach to combatting the complications arising from SARS-CoV-2 infection, particularly by targeting oxidative stress and inflammation, both integral to the pathogenesis of post-acute sequelae.218 Its active constituents, including flavonoids, biflavones, terpene trilactones and ginkgolic acids, contribute to its antiviral potential and ability to modulate various stages of the coronavirus life cycle.219 The neuroprotective properties of EGb 761® were elucidated through studies involving cerebral injury induced by ischaemia/reperfusion (I/R) in rats. These investigations demonstrated reductions in malondialdehyde (MDA) levels, pro-inflammatory cytokines (TNF-α and IL-1β) downregulation, increased expression of anti-inflammatory cytokines (IL-10) and enhanced enzymatic anti-oxidant activities. Notably, EGb 761's beneficial effects on I/R injury are attributed to its ability to inhibit inflammation, subsequently reducing oxidative stress.219 These mechanisms align with the potential to mitigate cognitive deficits experienced post-COVID-19 infection. EGb 761’s efficacy in treating cognitive impairment, coupled with its anti-inflammatory effects and enhancement of neuroplasticity, makes it a potential low-risk treatment option for patients experiencing persistent cognitive symptoms following COVID-19 infection.
Resveratrol
Resveratrol, a natural polyphenol abundant in red grapes and red wine, has established anti-oxidant, anti-inflammatory, and anti-tumorigenic properties.220 Increasing evidence suggests that redox balance plays a pivotal role in viral infections, with viruses capable of depleting glutathione levels, and interventions targeting oxidative stress showing potential.221,222 In the context of COVID-19, resveratrol has demonstrated antiviral activity against SARS-CoV-2 through inhibition of viral replication, while reducing cytotoxicity.223,224 Notably, resveratrol has been shown to selectively inhibit key proteases of SARS-CoV-2 in vitro.225 Clinical trials have also provided insights into resveratrol's potential in combating COVID-19-related complications. A randomized, double-blind, placebo-controlled proof-of-concept trial revealed that resveratrol supplementation was associated with a lower incidence of hospitalization, COVID-related emergency room visits, and pneumonia among mild COVID-19 outpatients.226 Resveratrol's impact on the renin-angiotensin system (RAS) and ACE-2 expression positions it as a candidate for attenuating SARS-CoV-2 entry and viral shedding.227 Resveratrol treatment in elderly mice restored NVC and acetylcholine-induced responses. This effect was linked to a decrease in NADPH (nicotinamide adenine dinucleotide phosphate) oxidase expression and reduced oxidative/nitrative stress markers in the cortex. Additionally, resveratrol mitigated age-related increases in ROS production in cultured cerebromicrovascular endothelial cells. This suggests that resveratrol's impact on cortical NVC in aged mice is likely due to the restoration of cerebromicrovascular endothelial function through downregulation of NADPH oxidase-derived ROS production. These cerebromicrovascular benefits of resveratrol could contribute to its protective role in preserving cognitive function during aging.228 Human studies and clinical trials have also explored resveratrol's effects on cognitive function. In a randomized trial, acute resveratrol administration increased CBF in a dose-dependent manner, though cognitive improvements were not immediate.229 Chronic resveratrol supplementation demonstrated improvements in cognitive function, specifically accuracy and reduction of fatigue.230 The benefits of resveratrol on cognitive performance were observed in overweight older adults,231 where memory retention and functional connectivity between the hippocampus and other brain areas improved. Similar effects were reported in patients with T2D, indicating potential cognitive benefits through enhanced cerebrovascular function.232 These findings collectively underscore the potential of resveratrol as a therapeutic option to prevent and treat COVID-19-induced cognitive impairment and emphasize its role in NVC.
Cocoa
Cocoa, in its natural unprocessed form, contains abundant catechins and epicatechins, which are flavanols belonging to the subclass of flavonoids.233 Cocoa intake holds the potential to mitigate cognitive decline by promoting improved cerebral vasodilation, blood flow, perfusion, and angiogenesis.234-236 Notably, epicatechin possesses the ability to be readily absorbed, cross the blood–brain barrier, and exert physiological effects in the brain.236 Studies showed that flavonoids, particularly in higher amounts (500–750 mg/day), have the potential to enhance memory and executive function in healthy older adults.237 Clinical trials have been conducted to explore the cognitive effects of cocoa consumption. One such trial, Cocoa Supplement and Multivitamin Outcomes Study for the Mind, assessed the impact of daily cocoa extract administration (containing 500 mg flavanols) over a period of 3 years in older adults. The results indicated no significant cognitive benefit from the cocoa extract. In contrast, another trial involving older participants revealed that 30 days of cocoa consumption238 led to increased NVC and improved cognitive performance, particularly in individuals with impaired NVC at baseline.239 While the potential of cocoa in vascular health and cognitive enhancement is evident, further research are needed to better understand its role in preventing and treating COVID-19-induced cognitive impairment.
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide has numerous roles in maintenance of cellular functional and metabolic homeostasis. Importantly, NAD+ is a rate-limiting substrate for sirtuin activity, which is critical for maintaining proper mitochondrial function (i.e. mitophagy and biogenesis). Activation of sirtuin 1 protects the cell from excessive ROS production240,241 and supplementation of sirtuin 1 activator SS-31 enhanced cerebromicrovascular endothelial function, functional hyperaemia, and cognitive performance. Moreover, NAD+ and/or precursor supplementation reduced cerebromicrovascular endothelial cell mitochondrial ROS production, improving NVC and cognition in a sirtuin-dependent manner.242 NAD+ bioavailability decreases with age and during viral infection, in part due to PARP1 consuming enzymes that interact with, and perpetuate pro-inflammatory and oxidative signalling.117,243 In addition, reduced NAD+ levels play a critical role in neurodegenerative disorders and vascular dementia. Preclinical models have evidenced that with supplementation of NAD+, cognitive impairment was significantly ameliorated.244 In light of low NAD+ levels association with poor outcomes in COVID-19 patients,243,245 NAD+ depletion may contribute to impairment of NVC, and cognition seen in preclinical models. Clinical trials are needed to assess the effects of NAD+ supplementation on NVC in convalescent COVID-19 patients.
Alpha-ketoglutarate
As an intermediate of the Krebs cycle, alpha-ketoglutarate (AKG) plays essential roles in metabolism and metabolic processes within the human body. Importantly, in the context of therapeutic treatment in COVID-19, AKG functions as an anti-oxidant, potentially alleviating the detrimental consequences of elevated oxidative stress,246 AKG's contribution to brain health is multifaceted. AKG deamination forms glutamate and can be further decarboxylated to GABA (gammaaminobutyric acid), the two main excitatory and inhibitory neurotransmitters in the brain, respectively. Moreover, AKG alleviates oxidative damage in the vasculature, reducing stiffness, and increasing NO production.247 In COVID-19, AKG supplementation inhibited SARS-CoV-2 replication, reduced inflammation and thrombosis, rescued lung function, and restored oxygen saturation in SARS-CoV-2 infected animals.248 Hence, with the benefits of enhanced neuronal function, neurotransmitter regulation, and vascular homeostasis, AKG positions itself as a prime supplement to alleviate NVC disruption and cognitive impairment in COVID-19.
Life-style and non-invasive interventions
Time-restricted eating
Intermittent fasting is a dietary pattern of eating that involves extended periods (typically 16–48 hours) of reduced or no food consumption, followed by regular eating intervals.249 Animal models’ studies showed that intermittent fasting can activate adaptive cellular stress signalling pathways, enhance mitochondrial metabolism, promote DNA repair, improve endothelial function and stimulate autophagy, resulting in benefits for chronic diseases including diabetes, cardiovascular disorders, cancer and neurological conditions like Alzheimer’s disease.250,251 As such, through these beneficial actions on endothelial cells it is highly plausible that NVC can be rescued through varying time-restricted eating paradigms. By leveraging these adaptive mechanisms, intermittent fasting enhances fat oxidation as an energy source, lowers serum glucose levels and preserves spatial memory function.252,253 Intermittent fasting holds promising potential for enhancing vascular health and addressing COVID-19-induced cognitive impairment. The connection between metabolic brain studies using FDG-PET (fluorodeoxyglucose-positron emission tomography) imaging and cognitive deterioration in post-COVID-19 patients underscores the importance of addressing cerebral glucose hypometabolism, a hallmark of conditions like MCI and Alzheimer’s disease.254-259 This hypometabolism can be mitigated through dietary interventions that elevate serum ketone bodies, such as intermittent fasting.260-262 In a study evaluating the outcomes of patients engaging in periodic fasting prior to COVID-19 diagnosis, a lower risk of hospitalization or mortality was observed among those practicing periodic fasting compared to non-fasters.263 Hence, further research and clinical trials are needed to elucidate whether intermittent fasting can prevent and treat COVID-19-induced cognitive impairment.
Transcranial direct current stimulation
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique that modulates brain vascular function, enhances synaptic plasticity and reduces pro-inflammatory cytokines,264 making it a viable candidate for treating COVID-19-induced cognitive dysfunction through NVC-mediated mechanism.265 Prior research has demonstrated the effectiveness of tDCS in non-COVID-19 samples and in combination with cognitive tasks for neurorehabilitation and cognitive enhancement.266,267 A pilot case series involving four patients with long-COVID cognitive symptoms showed promising trends. The patients underwent 20 daily sessions of bilateral prefrontal tDCS combined with online cognitive training. Although this pilot study lacked statistical power, it demonstrated several encouraging outcomes: improvement in depression symptoms, reduced self-reported cognitive and emotional symptoms, enhanced processing speed and executive functioning, and better delayed and immediate recall.268 In another case report, two patients demonstrated remarkable enhancements in physical, cognitive, emotional, and functional aspects, allowing them to successfully return to the workforce and resume their previous activities. In this case, tDCS was also delivered over left dorsolateral prefrontal cortex and combined with physical exercise, online adaptive computerized cognitive training and guided mindfulness meditation.269 However, preliminary results from an ongoing study of tDCS on cognitive impairment in post-COVID patients indicated that there was no significant difference between the active tDCS and sham groups in terms of cognitive test results after four weeks.270 Overall, tDCS emerges as a promising intervention where multiple ongoing studies are dedicated to unravelling the impact of tDCS on cognitive dysfunction subsequent to COVID-19 (NCT04944147; NCT05389592, NCT05965739). These studies exemplify the ongoing efforts to delve into tDCS's potential within the context of post-COVID-19.
Transcranial magnetic stimulation
Transcranial magnetic stimulation (TMS) is a non-invasive technique capable of directly modulating neuronal activity and synaptic plasticity within the cortex and interconnected brain regions.271 This modulation extends to a range of neural processes, encompassing NMDAR activity, GABA mediated inhibition, and the activation of neuromodulators like acetylcholine, dopamine, norepinephrine, and serotonin.272-274 Moreover, repetitive TMS also influences nonneuronal processes, including alterations in blood flow.273,275 While its impact on endothelial function is not extensively studied, TMS significantly contributes to the exploration of cognitive mechanisms and behavioural plasticity within the human brain. Additionally, TMS has demonstrated effectiveness in ameliorating cognitive deficits among patients with MCI and Alzheimer’s disease.276 In a recent case series involving 23 patients with Long-COVID, TMS mitigated subjective and objective depressive symptoms, as well as improvements in cognitive impairments such as brain fog, were observed.277 Moreover, executive function, as assessed by the Trail Making Test, showed notable improvement following TMS treatment. A recent case study, utilizing personalized TMS guided by EEG, reported a positive outcome in a 36-year-old patient experiencing lingering COVID-19-induced “brain fog”. The treatment yielded improvements in mood, olfactory function, and cognitive clarity.278 These findings highlight TMS's potential as a therapeutic path to tackle COVID-19-induced cognitive impairment. Nevertheless, further investigations are warranted to explore into the full scope of TMS's effectiveness in ameliorating cognitive deficits arising from COVID-19, as well as to explore whether these effects are contingent upon NVC.
Prebiotics, probiotics, fecal microbiota transplant
Microbial gut composition is strongly associated with clinical cognitive outcomes,279 and dysbiosis of the gut microbiome may contribute to neurocognitive disorders, such as Alzheimer’s disease.280 Gut dysbiosis has been described as a mechanism of long-COVID pathogenesis due to the direct and indirect links between SARS-CoV-2-induced NVU and intestinal barrier impairment that disrupts the gut-brain axis.281 A randomized, double-blinded, placebo-controlled, multicenter clinical trial determined that probiotics promoted increases in cognitive performance and elevations in serum brain-derived neurotrophic factor (BDNF) level in older adults.282 Additionally, preclinical models have shown that prebiotics may also provide promise in alleviating cognitive impairment through modulation of neurotransmitters, BDNF, and NMDAR subunits.283 Fecal microbiota transplantation (FMT) is a procedure to introduce a healthy donor fecal matter into the gastrointestinal tract of a patient. Recent evidence has shown that introduction of a healthy microbiome to patients with cognitive impairment and Clostridioides difficile infection improved cognition compared to the control group with antibiotic treatment.284 Moreover, FMT has been proposed to have a positive impact on the treatment of Alzheimer’s disease through mechanistic pathways involving anti-inflammation and synaptic plasticity.285 These therapies may have a positive influence on COVID-19 induced NVU disruption due to their promising neuromodulatory effects.
Lipid lowering and anti-hypertensive pharmaceuticals
Statins are first-line therapy for individuals with hypercholesterolaemia and atherosclerotic artery disease. These drugs have potent effects on the vasculature with lipid lowering, anti-inflammatory, anti-oxidant, and anti-thrombotic properties54 Hence, statin therapy may provide great benefit to the cerebrovasculature, and in turn, rescue endothelium-dependent vasodilatory regulation. While statins are known to increase the expression of ACE2, clinical data supports the use of statins in COVID-19 patients.286 Statins have been shown to lower the risk of mortality in COVID-19 patients287 and mechanistically, statins attenuated endothelium-induced inflammation and pro-inflammatory signalling.54 Statins also showed benefit in preclinical Alzheimer’s disease models, completely rescuing cerebrovascular reactivity, endothelial NO production, and NVC responses.288 Moreover, statins have improved cognitive function,289 exemplifying long-standing statin therapy as a potential mediator to attenuate cognitive decrement following SARS-CoV-2 infection. Like statins, angiotensin converting enzyme inhibitors (ACEI) and AngII receptor blockers (ARBs), were initially thought to increase vulnerability to SARS-CoV-2 infection due to their role in upregulation of ACE2. However, discontinuation of ACEI/ARBs has been associated with poor outcomes in COVID-19 patients.54 Hence, professionals from cardiovascular societies suggest continuation of ACEI/ARBs during COVID-19 infection.290 A beneficial effect of these lipid lowering and anti-hypertensive drugs could be their action in upregulation of ACE2, as virus-treated microvascular endothelial cells exposed to an ACE2 agonist reversed SARS-CoV-2 induced hyperpermeability, stabilizing endothelial barrier integrity.54,291 In addition, ARBs showed a positive effect on the NVU by rescuing NVC response through restoration of cerebral endothelial cell NO bioavailability in preclinical models,292 further strengthening the premise of lipid lowering and anti-hypertensives as treatment for NVC impairment in COVID-19.
Senolytics
Increases in cellular damage and oxidative stress can trigger cellular senescence, a complex stress response293 and potentially underlying pathophysiologic mechanism contributing to decreased cognitive function in cognitive impairment and dementia.294 In response to this stress, the endothelium can switch to a senescent phenotype, which contributes to loss of vascular tone regulation, and impairment of anti-coagulant, anti-oxidant, and anti-inflammatory quiescent properties, in part, by impairing NO production.295 Senolytic drugs have shown promise in preclinical models to restore the NVC response and hippocampus dependent learning and memory.293 In a preclinical chemotherapy model, paclitaxel induced endothelial senescence contributed to a decreased NVC response and cognitive impairment. When senescent endothelial cells were eliminated, microvascular density was increased, NVC was restored, and cognitive performance improved.296 Moreover, a senescent endothelial phenotype secretes pro-inflammatory molecules and contributes to oxidative damage, capable of disrupting the BBB and inducing neuroinflammation, which is associated with impaired NVC.296 Elimination of senescent endothelial cells in a preclinical model of whole brain irradiation restored BBB integrity and increased cortical capillarization.297 Clinically, virally induced senescence markers have been detected in lung endothelial cells298 and there is preclinical evidence of senolytic treatment reducing mortality rate in SARS-CoV-2 infected mice.299 Hence, the molecular and physiological consequences of elevations in pro-inflammatory cytokines and ROS may be due to phenotypic change of endothelial cells. Senolytic clinical trial to modulate the progression of Alzheimer’s disease (SToMP-Alzheimer’s disease) showed decreases in senescence associated secretory phenotype factors; however, cognition and neuroimaging endpoints showed no difference compared to baseline.300 Further clinical trials are needed to assess the effectiveness of senolytics, and how these drugs can potentially be used to mitigate cognitive deficits in COVID-19 patients.
Metformin
Metformin is a low cost and common diabetes drug worldwide. Aside from its beneficial effects of lowering blood glucose and increasing insulin sensitivity, metformin exhibits potent anti-inflammatory and antioxidative properties. Metformin showed benefit in decreasing endothelial oxidative stress and preserving eNOS phosphorylation in high fat diet fed rats.301 Preclinically, metformin also showed cognitive benefits and restoration of cerebrovascular function.302 In addition, clinical results conclude that metformin significantly reduces cardiovascular complications303 and a recent meta-analysis of 10 studies suggests that metformin reduces impairment of cognition in patients with T2D.304 In the context of COVID-19, metformin outpatient treatment reduced the incidence of Long-COVID by 41%.305 Metformin is a safe and globally available pharmacologic, and due to its favourable outcomes in COVID-19 and positive effects on the NVU and cognition, this drug may prove useful for restoration of the NVC response and cognitive function.
Rapamycin
Rapamycin is a macrolide antibiotic that exhibits extensive immunosuppressive activity, with clinical uses that span from preventing organ transplant rejection to cancer.306 Mechanistically, rapamycin inhibits the molecular target of rapamycin (mTOR), which plays a key role in viral replication and production of pro-inflammatory cytokines.307 Since 2009 when Harrison et. al. discovered rapamycin's use in extending life span,308 rapamycin has been at the forefront for treatment of age-related conditions, including cardiovascular and neurodegenerative diseases.306 Due to rapamycin's inhibitory effects on the mTOR pathways and potential as an anti-aging drug, rapamycin is currently being investigated in clinical trials to mitigate COVID-19-induced detrimental cellular and molecular consequences across organ systems.307 Importantly, rapamycin shows preclinical evidence of NVC and memory rescue in a preclinical Alzheimer’s disease model.309 Studies examining the effect of rapamycin in the rescue of NVC response revealed that inhibition of mTOR restored cerebrovascular function by rescuing eNOS function.310,311 In addition, rapamycin improved nNOS activation, all-in-all, indicating that the mTOR pathway has critical regulation on the most potent stimuli of the NVC response, NO.309 As we await clinical trial results of rapamycin efficacy for prevention of post-COVID-19 effects, mTOR inhibition seems to be a promising avenue to restore cerebromicrovascular and cognitive function in COVID-19 patients.
Conclusion
Over the past 3 years, the detrimental consequences of acute and long-term COVID-19 have been evident, with systemic spread of endothelial impairment as a major driver of brain pathologies. SARS-CoV-2-induced NVU impairment has perpetuated a cognitive sequela whereas of now, we lack the knowledge regarding the specific time point at which convalescence to acute infection will render individuals unsusceptible. In this review, we presented evidence of our hypothesis that long-term cognitive impairment is due to a compromise of the endothelium-mediated mechanism of NVC. Moreover, there is therapeutic potential of many low-risk treatments, non-invasive procedures, and life-style changes, which may provide benefit in COVID-19-induced cognitive impairment by rescuing the NVC response. Paths forward to achieve the goal of nullifying cognitive impairment associated with COVID-19 will take a combined effort from bench to bedside research, investigating mechanisms and pharmacological trials to restore cerebromicrovascular health and in turn, reconstitute cognitive processes.
Acknowledgements
Figures 1 and 2 were created with biorender.com
Contributor Information
Cameron D Owens, Oklahoma Center for Geroscience and Healthy Brain Aging, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73117, USA; Vascular Cognitive Impairment, Neurodegeneration and Healthy Brain Aging Program, Department of Neurosurgery, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA.
Camila Bonin Pinto, Oklahoma Center for Geroscience and Healthy Brain Aging, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73117, USA; Vascular Cognitive Impairment, Neurodegeneration and Healthy Brain Aging Program, Department of Neurosurgery, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA.
Sam Detwiler, Oklahoma Center for Geroscience and Healthy Brain Aging, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73117, USA.
Lauren Olay, Oklahoma Center for Geroscience and Healthy Brain Aging, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73117, USA.
Ana Clara da C Pinaffi-Langley, Oklahoma Center for Geroscience and Healthy Brain Aging, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73117, USA.
Peter Mukli, Oklahoma Center for Geroscience and Healthy Brain Aging, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73117, USA; Vascular Cognitive Impairment, Neurodegeneration and Healthy Brain Aging Program, Department of Neurosurgery, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA; International Training Program in Geroscience, Doctoral School of Basic and Translational Medicine/Departments of Public Health, Translational Medicine and Physiology, Semmelweis University, Budapest, 1089, Hungary.
Anna Peterfi, Oklahoma Center for Geroscience and Healthy Brain Aging, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73117, USA; Vascular Cognitive Impairment, Neurodegeneration and Healthy Brain Aging Program, Department of Neurosurgery, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA; International Training Program in Geroscience, Doctoral School of Basic and Translational Medicine/Departments of Public Health, Translational Medicine and Physiology, Semmelweis University, Budapest, 1089, Hungary.
Zsofia Szarvas, Oklahoma Center for Geroscience and Healthy Brain Aging, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73117, USA; Vascular Cognitive Impairment, Neurodegeneration and Healthy Brain Aging Program, Department of Neurosurgery, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA; International Training Program in Geroscience, Doctoral School of Basic and Translational Medicine/Departments of Public Health, Translational Medicine and Physiology, Semmelweis University, Budapest, 1089, Hungary.
Judith A James, Oklahoma Center for Geroscience and Healthy Brain Aging, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73117, USA; Arthritis & Clinical Immunology Research Program, Oklahoma Medical Research Foundation, Oklahoma City, OK 73104, USA; Department of Internal Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA; Department of Pathology, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA.
Veronica Galvan, Oklahoma Center for Geroscience and Healthy Brain Aging, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73117, USA; Department of Biochemistry and Molecular Biology, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA; Veterans Affairs Medical Center, Oklahoma City, OK 73104, USA.
Stefano Tarantini, Oklahoma Center for Geroscience and Healthy Brain Aging, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73117, USA; Vascular Cognitive Impairment, Neurodegeneration and Healthy Brain Aging Program, Department of Neurosurgery, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA; International Training Program in Geroscience, Doctoral School of Basic and Translational Medicine/Departments of Public Health, Translational Medicine and Physiology, Semmelweis University, Budapest, 1089, Hungary; The Peggy and Charles Stephenson Cancer Center, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA; Department of Health Promotion Sciences, College of Public Health, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA.
Anna Csiszar, Oklahoma Center for Geroscience and Healthy Brain Aging, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73117, USA; Vascular Cognitive Impairment, Neurodegeneration and Healthy Brain Aging Program, Department of Neurosurgery, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA; International Training Program in Geroscience, Doctoral School of Basic and Translational Medicine/Departments of Public Health, Translational Medicine and Physiology, Semmelweis University, Budapest, 1089, Hungary.
Zoltan Ungvari, Oklahoma Center for Geroscience and Healthy Brain Aging, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73117, USA; Vascular Cognitive Impairment, Neurodegeneration and Healthy Brain Aging Program, Department of Neurosurgery, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA; International Training Program in Geroscience, Doctoral School of Basic and Translational Medicine/Departments of Public Health, Translational Medicine and Physiology, Semmelweis University, Budapest, 1089, Hungary; Department of Health Promotion Sciences, College of Public Health, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA.
Angelia C Kirkpatrick, Veterans Affairs Medical Center, Oklahoma City, OK 73104, USA; Cardiovascular Section, Department of Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73117, USA.
Calin I Prodan, Veterans Affairs Medical Center, Oklahoma City, OK 73104, USA; Department of Neurology, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA.
Andriy Yabluchanskiy, Oklahoma Center for Geroscience and Healthy Brain Aging, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73117, USA; Vascular Cognitive Impairment, Neurodegeneration and Healthy Brain Aging Program, Department of Neurosurgery, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA; International Training Program in Geroscience, Doctoral School of Basic and Translational Medicine/Departments of Public Health, Translational Medicine and Physiology, Semmelweis University, Budapest, 1089, Hungary; Department of Health Promotion Sciences, College of Public Health, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA.
Funding
C.D.O.: National Institute on Aging-supported Geroscience Training Program in Oklahoma (T32AG052363). C.B.P.: American Heart Association (23DIVSUP1070991). J.A.J.: Oklahoma Shared Clinical and Translational Resources (U54GM104938). S.T.: American Heart Association (AHA941290), National Institute on Aging (R03AG070479, K01AG073614), National Cancer Institute (P30CA225520). AC: National Cancer Institute (R01CA255840), National Institute on Aging (R01AG068295). Z.U.: National Institute on Aging (RF1AG072295, R01AG055395), National Institute of Neurological Disorders and Stroke (R01NS100782). A.C.K.: US Department of Veterans Affairs (Merit award number CX002578). C.I.P.: US Department of Veterans Affairs (Merit award number CX000340). A.Y.: American Heart Association (AHA966924), National Institute on Aging (R01AG075834).
Competing interests
The authors report no competing interests.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1. WHO . WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int. 2023.
- 2. Hu B, Guo H, Zhou P, Shi Z-L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chatterjee S, Nalla LV, Sharma M, et al. Association of COVID-19 with comorbidities: An update. ACS Pharmacol Transl Sci. 2023;6:334–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shankar P, Singh J, Joshi A, et al. Organ involvement in COVID-19: A molecular investigation of autopsied patients. Microorganisms. 2022;10:1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao W, Li H, Li J, Xu B, Xu J. The mechanism of multiple organ dysfunction syndrome in patients with COVID-19. J Med Virol. 2022;94:1886–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Owens CD, Pinto CB, Detwiler S, et al. Cerebral small vessel disease pathology in COVID-19 patients: A systematic review. Ageing Res Rev. 2023;88:101962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mina Y, Enose-Akahata Y, Hammoud DA, et al. Deep phenotyping of neurologic postacute sequelae of SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. 2023;10:e200097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo P, Benito Ballesteros A, Yeung SP, et al. COVCOG 2: Cognitive and memory deficits in long COVID: A second publication from the COVID and cognition study. Front Aging Neurosci. 2022;14:804937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu Y-H, Wang Y-R, Wang Q-H, et al. Post-infection cognitive impairments in a cohort of elderly patients with COVID-19. Mol Neurodegener. 2021;16:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iadecola C. The neurovascular unit coming of age: A journey through neurovascular coupling in health and disease. Neuron. 2017;96:17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Csipo T, Lipecz A, Mukli P, et al. Increased cognitive workload evokes greater neurovascular coupling responses in healthy young adults. PLoS One. 2021;16:e0250043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tarantini S, Hertelendy P, Tucsek Z, et al. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35:1871–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lipecz A, Csipo T, Tarantini S, et al. Age-related impairment of neurovascular coupling responses: A dynamic vessel analysis (DVA)-based approach to measure decreased flicker light stimulus-induced retinal arteriolar dilation in healthy older adults. Geroscience. 2019;41:341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Csipo T, Mukli P, Lipecz A, et al. Assessment of age-related decline of neurovascular coupling responses by functional near-infrared spectroscopy (fNIRS) in humans. Geroscience. 2019;41:495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mukli P, Pinto CB, Owens CD, et al. Impaired neurovascular coupling and increased functional connectivity in the frontal cortex predict age-related cognitive dysfunction. Adv Sci 2023. Advance Access published on December 28, 2023, doi: 10.1002/advs.202303516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barloese MCJ, Bauer C, Petersen ET, Hansen CS, Madsbad S, Siebner HR. Neurovascular coupling in type 2 diabetes with cognitive decline. A narrative review of neuroimaging findings and their pathophysiological implications. Front Endocrinol (Lausanne). 2022;13:874007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Owens CD, Mukli P, Csipo T, et al. Microvascular dysfunction and neurovascular uncoupling are exacerbated in peripheral artery disease, increasing the risk of cognitive decline in older adults. Am J Physiol Heart Circ Physiol. 2022;322:H924–H935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu J, Sun PD. High affinity binding of SARS-CoV-2 spike protein enhances ACE2 carboxypeptidase activity. J Biol Chem. 2020;295:18579–18588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sashindranath M, Nandurkar HH. Endothelial dysfunction in the brain. Stroke. 2021;52:1895–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ruan Z, Sun D, Zhou X, et al. Altered neurovascular coupling in patients with vascular cognitive impairment: A combined ASL-fMRI analysis. Front Aging Neurosci. 2023;15:1224525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat Rev Neurosci. 2017;18:419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van der Heide FCT, van Sloten TT, Willekens N, Stehouwer CDA. Neurovascular coupling unit dysfunction and dementia: Retinal measurements as tools to move towards population-based evidence. Front Endocrinol (Lausanne). 2022;13:1014287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yabluchanskiy A, Nyul-Toth A, Csiszar A, et al. Age-related alterations in the cerebrovasculature affect neurovascular coupling and BOLD fMRI responses: Insights from animal models of aging. Psychophysiology. 2021;58:e13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Csipo T, Lipecz A, Fulop GA, et al. Age-related decline in peripheral vascular health predicts cognitive impairment. Geroscience. 2019;41:125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wenzel J, Lampe J, Müller-Fielitz H, et al. The SARS-CoV-2 main protease Mpro causes microvascular brain pathology by cleaving NEMO in brain endothelial cells. Nat Neurosci. 2021;24:1522–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ambrosino P, Sanduzzi Zamparelli S, Mosella M, et al. Clinical assessment of endothelial function in convalescent COVID-19 patients: A meta-analysis with meta-regressions. Ann Med. 2022;54:3234–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu WM, Neuhaus A, Beard DJ, Sutherland BA, DeLuca GC. Neurovascular coupling mechanisms in health and neurovascular uncoupling in Alzheimer's disease. Brain. 2022;145:2276–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bertuccelli M, Ciringione L, Rubega M, Bisiacchi P, Masiero S, Del Felice A. Cognitive impairment in people with previous COVID-19 infection: A scoping review. Cortex. 2022;154:212–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zeng N, Zhao Y-M, Yan W, et al. A systematic review and meta-analysis of long term physical and mental sequelae of COVID-19 pandemic: Call for research priority and action. Mol Psychiatry. 2023;28:423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Badenoch JB, Rengasamy ER, Watson C, et al. Persistent neuropsychiatric symptoms after COVID-19: A systematic review and meta-analysis. Brain Commun. 2022;4:fcab297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Manly JJ, Jones RN, Langa KM, et al. Estimating the prevalence of dementia and mild cognitive impairment in the US: The 2016 health and retirement study harmonized cognitive assessment protocol project. JAMA Neurol. 2022;79:1242–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harrison PJ, Taquet M. Neuropsychiatric disorders following SARS-CoV-2 infection. Brain. 2023;146:2241–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Crivelli L, Palmer K, Calandri I, et al. Changes in cognitive functioning after COVID-19: A systematic review and meta-analysis. Alzheimers Dement. 2022;18:1047–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perrottelli A, Sansone N, Giordano GM, et al. Cognitive impairment after post-acute COVID-19 infection: A systematic review of the literature. J Pers Med. 2022;12:2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Velichkovsky BB, Razvaliaeva AY, Khlebnikova AA, Manukyan PA, Kasatkin VN. Attention and memory after COVID-19 as measured by neuropsychological tests: Systematic review and meta-analysis. Acta Psychol (Amst). 2023;233:103838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Premraj L, Kannapadi NV, Briggs J, et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J Neurol Sci. 2022;434:120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Quick S, Moss J, Rajani RM, Williams A. A vessel for change: Endothelial dysfunction in cerebral small vessel disease. Trends Neurosci. 2021;44:289–305. [DOI] [PubMed] [Google Scholar]
- 38. Gallagher M, Koh MT. Episodic memory on the path to Alzheimer’s disease. Curr Opin Neurobiol. 2011;21:929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reiken S, Sittenfeld L, Dridi H, Liu Y, Liu X, Marks AR. Alzheimer’s-like signaling in brains of COVID-19 patients. Alzheimers Dement. 2022;18:955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Biagianti B, Di Liberto A, Nicolò Edoardo A, et al. Cognitive assessment in SARS-CoV-2 patients: A systematic review. Front Aging Neurosci. 2022;14:909661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prevention CoDCa . Dementia Risk Reduction. Accessed 14 September 2023. https://www.cdc.gov/aging/publications/features/dementia-risk-reduction-june-2022/index.html.
- 42. Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41:3038–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Holm-Hadulla RM, Wendler H, Baracsi G, Storck T, Möltner A, Herpertz SC. Depression and social isolation during the COVID-19 pandemic in a student population: The effects of establishing and relaxing social restrictions. Front Psychiatry. 2023;14:1200643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Collier Villaume S, Chen S, Adam EK. Age disparities in prevalence of anxiety and depression among US adults during the COVID-19 pandemic. JAMA Network Open. 2023;6:e2345073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cantón-Habas V, Rich-Ruiz M, Romero-Saldaña M, Carrera-González MDP. Depression as a risk factor for dementia and Alzheimer's disease. Biomedicines. 2020;8:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Escobar-Córdoba F, Ramírez-Ortiz J, Fontecha-Hernández J. Effects of social isolation on sleep during the COVID-19 pandemic. Sleep Sci. 2021;14:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rao A, Gupta A, Kain V, Halade GV. Extrinsic and intrinsic modulators of inflammation-resolution signaling in heart failure. Am J Physiol Heart Circ Physiol. 2023;325:H433–H448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Halade GV, Mat Y, Gowda SGB, et al. Sleep deprivation in obesogenic setting alters lipidome and microbiome toward suboptimal inflammation in acute heart failure. FASEB J. 2023;37:e22899. [DOI] [PubMed] [Google Scholar]
- 49. Csipo T, Lipecz A, Owens C, et al. Sleep deprivation impairs cognitive performance, alters task-associated cerebral blood flow and decreases cortical neurovascular coupling-related hemodynamic responses. Sci Rep. 2021;11:20994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mukli P, Csipo T, Lipecz A, et al. Sleep deprivation alters task-related changes in functional connectivity of the frontal cortex: A near-infrared spectroscopy study. Brain Behav. 2021;11:e02135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Park AH, Zhong S, Yang H, Jeong J, Lee C. Impact of COVID-19 on physical activity: A rapid review. J Glob Health. 2022;12:05003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc. 2011;86:876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: Contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2017;94:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xu S-W, Ilyas I, Weng J-P. Endothelial dysfunction in COVID-19: An overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol Sin. 2023;44:695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: Major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wu X, Xiang M, Jing H, Wang C, Novakovic VA, Shi J. Damage to endothelial barriers and its contribution to long COVID. Angiogenesis. 2023;27:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bridges JP, Vladar EK, Huang H, Mason RJ. Respiratory epithelial cell responses to SARS-CoV-2 in COVID-19. Thorax. 2022;77:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. [DOI] [PubMed] [Google Scholar]
- 59. Amraei R, Rahimi N. COVID-19, renin-angiotensin system and endothelial dysfunction. Cells. 2020;9:1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Regenhardt RW, Bennion DM, Sumners C. Cerebroprotective action of angiotensin peptides in stroke. Clin Sci. 2014;126:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Noureddine FY, Altara R, Fan F, Yabluchanskiy A, Booz GW, Zouein FA. Impact of the renin-angiotensin system on the endothelium in vascular dementia: Unresolved issues and future perspectives. Int J Mol Sci. 2020;21:4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xiao L, Sakagami H, Miwa N. ACE2: The key molecule for understanding the pathophysiology of severe and critical conditions of COVID-19: Demon or angel? Viruses. 2020;12:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gauberti M, Potzeha F, Vivien D, Martinez de Lizarrondo S. Impact of bradykinin generation during thrombolysis in ischemic stroke. Front Med (Lausanne). 2018;5:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Marcos-Contreras OA, Martinez de Lizarrondo S, Bardou I, et al. Hyperfibrinolysis increases blood–brain barrier permeability by a plasmin-and bradykinin-dependent mechanism. Blood. 2016;128:2423–2434. [DOI] [PubMed] [Google Scholar]
- 65. Chang R, Mamun A, Dominic A, Le N-T. SARS-CoV-2 mediated endothelial dysfunction: The potential role of chronic oxidative stress. Front Physiol. 2021;11:605908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Boughaleb H, Lobysheva I, Dei Zotti F, Balligand JL, Montiel V. Biological assessment of the NO-dependent endothelial function. Molecules. 2022;27:7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Toth P, Tarantini S, Davila A, et al. Purinergic glio-endothelial coupling during neuronal activity: Role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol. 2015;309:H1837–H1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lecrux C, Hamel E. Neuronal networks and mediators of cortical neurovascular coupling responses in normal and altered brain states. Philos Trans R Soc B Biol Sci. 2016;371:20150350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Filosa JA, Bonev AD, Straub SV, et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. [DOI] [PubMed] [Google Scholar]
- 71. Monje M, Iwasaki A. The neurobiology of long COVID. Neuron. 2022;110:3484–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fernández-Castañeda A, Lu P, Geraghty AC, et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell. 2022;185:2452–2468.e2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Frere JJ, Serafini RA, Pryce KD, et al. SARS-CoV-2 infection in hamsters and humans results in lasting and unique systemic perturbations after recovery. Sci Transl Med. 2022;14:eabq3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Soung AL, Vanderheiden A, Nordvig AS, et al. COVID-19 induces CNS cytokine expression and loss of hippocampal neurogenesis. Brain. 2022;145:4193–4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Song E, Bartley CM, Chow RD, et al. Divergent and self-reactive immune responses in the CNS of COVID-19 patients with neurological symptoms. Cell Rep Med. 2021;2:100288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wang EY, Mao T, Klein J, et al. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595:283–288. [DOI] [PubMed] [Google Scholar]
- 77. Chen B, Julg B, Mohandas S, Bradfute SB, Force RMPT. Viral persistence, reactivation, and mechanisms of long COVID. eLife. 2023;12:e86015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Manoharan S, Ying LY. Epstein barr virus reactivation during COVID-19 hospitalization significantly increased mortality/death in SARS-CoV-2(+)/EBV(+) than SARS-CoV-2(+)/EBV(-) patients: A comparative meta-analysis. Int J Clin Pract. 2023;2023:1068000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhang N, Zuo Y, Jiang L, Peng Y, Huang X, Zuo L. Epstein-Barr virus and neurological diseases. Front Mol Biosci. 2021;8:816098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lee MH, Perl DP, Nair G, et al. Microvascular injury in the brains of patients with COVID-19. N Engl J Med. 2021;384:481–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yang AC, Kern F, Losada PM, et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature. 2021;595:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Thakur KT, Miller EH, Glendinning MD, et al. COVID-19 neuropathology at Columbia university irving medical center/New York Presbyterian hospital. Brain. 2021;144:2696–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Alexopoulos H, Magira E, Bitzogli K, et al. Anti-SARS-CoV-2 antibodies in the CSF, blood-brain barrier dysfunction, and neurological outcome: Studies in 8 stuporous and comatose patients. Neurol Neuroimmunol Neuroinflamm. 2020;7:e893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Remsik J, Wilcox JA, Babady NE, et al. Inflammatory leptomeningeal cytokines mediate COVID-19 neurologic symptoms in cancer patients. Cancer Cell. 2021;39:276–283.e273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kase Y, Sonn I, Goto M, Murakami R, Sato T, Okano H. The original strain of SARS-CoV-2, the delta variant, and the omicron variant infect microglia efficiently, in contrast to their inability to infect neurons: Analysis using 2D and 3D cultures. Exp Neurol. 2023;363:114379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hashimoto K. Detrimental effects of COVID-19 in the brain and therapeutic options for long COVID: The role of Epstein-Barr virus and the gut-brain axis. Mol Psychiatry 2023. Advance Access published on July 4, 2023, doi: 10.1038/s41380-023-02161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pepe A, Pietropaoli S, Vos M, Barba-Spaeth G, Zurzolo C. Tunneling nanotubes provide a route for SARS-CoV-2 spreading. Sci Adv. 2022;8:eabo0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mishra A, Reynolds JP, Chen Y, Gourine AV, Rusakov DA, Attwell D. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat Neurosci. 2016;19:1619–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Takano T, Tian G-F, Peng W, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. [DOI] [PubMed] [Google Scholar]
- 90. Nizar K, Uhlirova H, Tian P, et al. In vivo stimulus-induced vasodilation occurs without IP3 receptor activation and may precede astrocytic calcium increase. J Neurosci. 2013;33:8411–8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jego P, Pacheco-Torres J, Araque A, Canals S. Functional MRI in mice lacking IP3-dependent calcium signaling in astrocytes. J Cereb Blood Flow Metab. 2014;34:1599–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bonder DE, McCarthy KD. Astrocytic Gq-GPCR-linked IP3R-dependent Ca2+ signaling does not mediate neurovascular coupling in mouse visual cortex in vivo. J Neurosci. 2014;34:13139–13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rosenegger DG, Tran CHT, Wamsteeker Cusulin JI, Gordon GR. Tonic local brain blood flow control by astrocytes independent of phasic neurovascular coupling. J Neurosci. 2015;35:13463–13474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Niwa K, Haensel C, Ross ME, Iadecola C. Cyclooxygenase-1 participates in selected vasodilator responses of the cerebral circulation. Circ Res. 2001;88:600–608. [DOI] [PubMed] [Google Scholar]
- 95. Liu X, Li C, Falck JR, Harder DR, Koehler RC. Relative contribution of cyclooxygenases, epoxyeicosatrienoic acids, and pH to the cerebral blood flow response to vibrissal stimulation. Am J Physiol Heart Circ Physiol. 2012;302:H1075–H1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Paulson OB, Newman EA. Does the release of potassium from astrocyte endfeet regulate cerebral blood flow? Science. 1987;237:896–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Metea MR, Kofuji P, Newman EA. Neurovascular coupling is not mediated by potassium siphoning from glial cells. J Neurosci. 2007;27:2468–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. MacVicar BA, Newman EA. Astrocyte regulation of blood flow in the brain. Cold Spring Harb Perspect Biol. 2015;7:a020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gee JR, Keller JN. Astrocytes: Regulation of brain homeostasis via apolipoprotein E. Int J Biochem Cell Biol. 2005;37:1145–1150. [DOI] [PubMed] [Google Scholar]
- 100. Patabendige A, Singh A, Jenkins S, Sen J, Chen R. Astrocyte activation in neurovascular damage and repair following ischaemic stroke. Int J Mol Sci. 2021;22:4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Steardo L Jr, Steardo L, Scuderi C. Astrocytes and the psychiatric sequelae of COVID-19: What we learned from the pandemic. Neurochem Res. 2023;48:1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Aceti A, Margarucci LM, Scaramucci E, et al. Serum S100B protein as a marker of severity in COVID-19 patients. Sci Rep. 2020;10:18665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Andrews MG, Mukhtar T, Eze UC, et al. Tropism of SARS-CoV-2 for human cortical astrocytes. Proc Natl Acad Sci USA. 2022;119:e2122236119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lee MH, Perl DP, Steiner J, et al. Neurovascular injury with complement activation and inflammation in COVID-19. Brain. 2022;145:2555–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Negri S, Faris P, Soda T, Moccia F. Endothelial signaling at the core of neurovascular coupling: The emerging role of endothelial inward-rectifier K+ (Kir2.1) channels and N-methyl-d-aspartate receptors in the regulation of cerebral blood flow. Int J Biochem Cell Biol. 2021;135:105983. [DOI] [PubMed] [Google Scholar]
- 106. Kaplan L, Chow BW, Gu C. Neuronal regulation of the blood–brain barrier and neurovascular coupling. Nat Rev Neurosci. 2020;21:416–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Longden TA, Dabertrand F, Koide M, et al. Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci. 2017;20:717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hosford PS, Gourine AV. What is the key mediator of the neurovascular coupling response? Neurosci Biobehav Rev. 2019;96:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hogan-Cann AD, Lu P, Anderson CM. Endothelial NMDA receptors mediate activity-dependent brain hemodynamic responses in mice. Proc Natl Acad Sci USA. 2019;116:10229–10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lu L, Hogan-Cann AD, Globa AK, et al. Astrocytes drive cortical vasodilatory signaling by activating endothelial NMDA receptors. J Cereb Blood Flow Metab. 2019;39:481–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Vanlandewijck M, He L, Mäe MA, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554:475–480. [DOI] [PubMed] [Google Scholar]
- 112. Kovacs-Oller T, Ivanova E, Bianchimano P, Sagdullaev BT. The pericyte connectome: Spatial precision of neurovascular coupling is driven by selective connectivity maps of pericytes and endothelial cells and is disrupted in diabetes. Cell Discov. 2020;6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dhananjayan R, Koundinya KS, Malati T, Kutala VK. Endothelial dysfunction in type 2 diabetes mellitus. Indian J Clin Biochem. 2016;31:372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gallo G, Volpe M, Savoia C. Endothelial dysfunction in hypertension: Current concepts and clinical implications. Front Med (Lausanne). 2021;8:798958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Landmesser U, Hornig B, Drexler H. Endothelial dysfunction in hypercholesterolemia: Mechanisms, pathophysiological importance, and therapeutic interventions. Semin Thromb Hemost. 2000;26:529–537. [DOI] [PubMed] [Google Scholar]
- 116. Chan L, Karimi N, Morovati S, et al. The roles of neutrophils in cytokine storms. Viruses. 2021;13:2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123:849–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Rotoli BM, Barilli A, Visigalli R, Ferrari F, Dall’Asta V. Endothelial cell activation by SARS-CoV-2 spike S1 protein: A crosstalk between endothelium and innate immune cells. Biomedicines. 2021;9:1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Jover E, Matilla L, Garaikoetxea M, et al. Beneficial effects of mineralocorticoid receptor pathway blockade against endothelial inflammation induced by SARS-CoV-2 spike protein. Biomedicines. 2021;9:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Raghavan S, Kenchappa DB, Leo MD. SARS-CoV-2 spike protein induces degradation of junctional proteins that maintain endothelial barrier integrity. Front Cardiovasc Med. 2021;8:687783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Qian Y, Lei T, Patel PS, et al. Direct activation of endothelial cells by SARS-CoV-2 nucleocapsid protein is blocked by simvastatin. J Virol. 2021;95:e0139621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Potje SR, Costa TJ, Fraga-Silva TFC, et al. Heparin prevents in vitro glycocalyx shedding induced by plasma from COVID-19 patients. Life Sci. 2021;276:119376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. du Preez HN, Aldous C, Hayden MR, Kruger HG, Lin J. Pathogenesis of COVID-19 described through the lens of an undersulfated and degraded epithelial and endothelial glycocalyx. FASEB J. 2022;36:e22052. [DOI] [PubMed] [Google Scholar]
- 124. Targosz-Korecka M, Kubisiak A, Kloska D, Kopacz A, Grochot-Przeczek A, Szymonski M. Endothelial glycocalyx shields the interaction of SARS-CoV-2 spike protein with ACE2 receptors. Sci Rep. 2021;11:12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Stahl K, Gronski PA, Kiyan Y, et al. Injury to the endothelial glycocalyx in critically ill patients with COVID-19. Am J Respir Crit Care Med. 2020;202:1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yamaoka-Tojo M. Vascular endothelial glycocalyx damage in COVID-19. Int J Mol Sci. 2020;21:9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Vollenberg R, Tepasse P-R, Ochs K, et al. Indications of persistent glycocalyx damage in convalescent COVID-19 patients: A prospective multicenter study and hypothesis. Viruses. 2021;13:2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Eapen MS, Lu W, Gaikwad AV, et al. Endothelial to mesenchymal transition: A precursor to post-COVID-19 interstitial pulmonary fibrosis and vascular obliteration? Eur Respir J. 2020;56:2003167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Falleni M, Tosi D, Savi F, Chiumello D, Bulfamante G. Endothelial-Mesenchymal transition in COVID-19 lung lesions. Pathol Res Pract. 2021;221:153419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yang RC, Huang K, Zhang HP, et al. SARS-CoV-2 productively infects human brain microvascular endothelial cells. J Neuroinflammation. 2022;19:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Tong M, Jiang Y, Xia D, et al. Elevated expression of Serum endothelial cell adhesion molecules in COVID-19 patients. J Infect Dis. 2020;222:894–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kumar N, Zuo Y, Yalavarthi S, et al. SARS-CoV-2 spike protein S1-mediated endothelial injury and pro-inflammatory state is amplified by dihydrotestosterone and prevented by mineralocorticoid antagonism. Viruses. 2021;13:2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Qin Z, Liu F, Blair R, et al. Endothelial cell infection and dysfunction, immune activation in severe COVID-19. Theranostics. 2021;11:8076–8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Won T, Wood MK, Hughes DM, et al. Endothelial thrombomodulin downregulation caused by hypoxia contributes to severe infiltration and coagulopathy in COVID-19 patient lungs. EBioMedicine. 2022;75:103812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ebihara T, Matsumoto H, Matsubara T, et al. Resistin associated with cytokines and endothelial cell adhesion molecules is related to worse outcome in COVID-19. Front Immunol. 2022;13:830061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. D'Agnillo F, Walters KA, Xiao Y, et al. Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19. Sci Transl Med. 2021;13:eabj7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Meyer K, Patra T, Vijayamahantesh, Ray R. SARS-CoV-2 spike protein induces paracrine senescence and leukocyte adhesion in endothelial cells. J Virol. 2021;95:e0079421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Costa TJ, Potje SR, Fraga-Silva TF, et al. Mitochondrial DNA and TLR9 activation contribute to SARS-CoV-2-induced endothelial cell damage. Vascul Pharmacol. 2022;142:106946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. De Michele M, Kahan J, Berto I, et al. Cerebrovascular complications of COVID-19 and COVID-19 vaccination. Circ Res. 2022;130:1187–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Merkler AE, Parikh NS, Mir S, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020;77:1366–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Koller A, Toth P. Contribution of flow-dependent vasomotor mechanisms to the autoregulation of cerebral blood flow. J Vasc Res. 2012;49:375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: Mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312:H1–H20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. You TY, Dong Q, Cui M. Emerging links between cerebral blood flow regulation and cognitive decline: A role for brain microvascular pericytes. Aging Dis. 2023;14:1276–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Grubb S, Lauritzen M, Aalkjær C. Brain capillary pericytes and neurovascular coupling. Comp Biochem Physiol A Mol Integr Physiol. 2021;254:110893. [DOI] [PubMed] [Google Scholar]
- 147. Hartmann DA, Coelho-Santos V, Shih AY. Pericyte control of blood flow across microvascular zones in the central nervous system. Annu Rev Physiol. 2022;84:331–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Hainsworth AH, Oommen AT, Bridges LR. Endothelial cells and human cerebral small vessel disease. Brain Pathol. 2015;25:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Fan LM, Geng L, Cahill-Smith S, et al. Nox2 contributes to age-related oxidative damage to neurons and the cerebral vasculature. J Clin Invest. 2019;129:3374–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Cao G, Xuan X, Hu J, Zhang R, Jin H, Dong H. How vascular smooth muscle cell phenotype switching contributes to vascular disease. Cell Commun Signal. 2022;20:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Lou M, Yuan D, Liao S, Tong L, Li J. Potential mechanisms of cerebrovascular diseases in COVID-19 patients. J Neurovirol. 2021;27:35–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Vassiliou AG, Zacharis A, Keskinidou C, et al. Soluble Angiotensin Converting Enzyme 2 (ACE2) is upregulated and soluble endothelial Nitric Oxide Synthase (eNOS) is downregulated in COVID-19-induced Acute Respiratory Distress Syndrome (ARDS). Pharmaceuticals (Basel). 2021;14:695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Vilaplana-Carnerero C, Giner-Soriano M, Dominguez À, et al. Atherosclerosis, cardiovascular disease, and COVID-19: A narrative review. Biomedicines. 2023;11:1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Li Q, Yang Y, Reis C, et al. Cerebral small vessel disease. Cell Transplant. 2018;27:1711–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Tian G, Ji Z, Lin Z, Pan S, Yin J. Cerebral autoregulation is heterogeneous in different stroke mechanism of ischemic stroke caused by intracranial atherosclerotic stenosis. Brain Behav. 2021;11:e01907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Caldas J, Passos R, Sancho L, Rosa Ramos JG, Panerai RB. Monitoring cerebral hemodynamics in COVID-19 patients in the prone position. J Crit Care. 2022;70:154055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Martínez-Salazar B, Holwerda M, Stüdle C, et al. COVID-19 and the vasculature: Current aspects and long-term consequences. Front Cell Dev Biol. 2022;10:824851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Bocci M, Oudenaarden C, Sàenz-Sardà X, et al. Infection of brain pericytes underlying neuropathology of COVID-19 patients. Int J Mol Sci. 2021;22:11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Hirunpattarasilp C, James G, Kwanthongdee J, et al. SARS-CoV-2 triggers pericyte-mediated cerebral capillary constriction. Brain. 2022;146:727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Vincent J-M, Kwan YW, Chan SL, Perrin-Sarrado C, Atkinson J, Chillon J-M. Constrictor and dilator effects of angiotensin II on cerebral arterioles. Stroke. 2005;36:2691–2695. [DOI] [PubMed] [Google Scholar]
- 161. Rhea EM, Logsdon AF, Hansen KM, et al. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat Neurosci. 2021;24:368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kumar A, Pareek V, Prasoon P, et al. Possible routes of SARS-CoV-2 invasion in brain: In context of neurological symptoms in COVID-19 patients. J Neurosci Res. 2020;98:2376–2383. [DOI] [PubMed] [Google Scholar]
- 163. Maddahi A, Edvinsson L. Cerebral ischemia induces microvascular pro-inflammatory cytokine expression via the MEK/ERK pathway. J Neuroinflammation. 2010;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Nian K, Harding IC, Herman IM, Ebong EE. Blood-brain barrier damage in ischemic stroke and its regulation by endothelial mechanotransduction. Front Physiol. 2020;11:605398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Wang Y, Wu J, Wang J, et al. Mitochondrial oxidative stress in brain microvascular endothelial cells: Triggering blood-brain barrier disruption. Mitochondrion. 2023;69:71–82. [DOI] [PubMed] [Google Scholar]
- 166. Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol. 2017;35:441–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Prinz M, Priller J. Microglia and brain macrophages in the molecular age: From origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–312. [DOI] [PubMed] [Google Scholar]
- 168. Bisht K, Okojie KA, Sharma K, et al. Capillary-associated microglia regulate vascular structure and function through PANX1-P2RY12 coupling in mice. Nat Commun. 2021;12:5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Császár E, Lénárt N, Cserép C, et al. Microglia modulate blood flow, neurovascular coupling, and hypoperfusion via purinergic actions. J Exp Med. 2022;219:e20211071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Badimon A, Strasburger HJ, Ayata P, et al. Negative feedback control of neuronal activity by microglia. Nature. 2020;586:417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Khan M, Yoo S-J, Clijsters M, et al. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell. 2021;184:5932–5949.e5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Lei Y, Zhang J, Schiavon CR, et al. SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE 2. Circ Res. 2021;128:1323–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Fullard JF, Lee H-C, Voloudakis G, et al. Single-nucleus transcriptome analysis of human brain immune response in patients with severe COVID-19. Genome Med. 2021;13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Vanderheiden A, Klein RS. Neuroinflammation and COVID-19. Curr Opin Neurobiol. 2022;76:102608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Clough E, Inigo J, Chandra D, et al. Mitochondrial dynamics in SARS-COV2 spike protein treated human microglia: Implications for neuro-COVID. J Neuroimmune Pharmacol. 2021;16(4):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Gülke E, Gelderblom M, Magnus T. Danger signals in stroke and their role on microglia activation after ischemia. Ther Adv Neurol Disord. 2018;11:1756286418774254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Dong R, Huang R, Wang J, Liu H, Xu Z. Effects of microglial activation and polarization on brain injury after stroke. Front Neurol. 2021;12:620948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Mistretta M, Farini A, Torrente Y, Villa C. Multifaceted nanoparticles: Emerging mechanisms and therapies in neurodegenerative diseases. Brain. 2023;146:2227–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Oh J, Cho W-H, Barcelon E, Kim KH, Hong J, Lee SJ. SARS-CoV-2 spike protein induces cognitive deficit and anxiety-like behavior in mouse via non-cell autonomous hippocampal neuronal death. Sci Rep. 2022;12:5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Kotliar K, Hauser C, Ortner M, et al. Altered neurovascular coupling as measured by optical imaging: A biomarker for Alzheimer’s disease. Sci Rep. 2017;7:12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Dubey S, Das S, Ghosh R, et al. The effects of SARS-CoV-2 infection on the cognitive functioning of patients with pre-existing dementia. J Alzheimers Dis Rep. 2023;7:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Shields HJ, Traa A, Van Raamsdonk JM. Beneficial and detrimental effects of reactive oxygen Species on lifespan: A comprehensive review of comparative and experimental studies. Front Cell Dev Biol. 2021;9:628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Alhayaza R, Haque E, Karbasiafshar C, Sellke FW, Abid MR. The relationship between reactive oxygen species and endothelial cell metabolism. Front Chem. 2020;8:592688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Katusic ZS, Austin SA. Endothelial nitric oxide: Protector of a healthy mind. Eur Heart J. 2013;35:888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Rosselli M, Keller PJ, Dubey RK. Role of nitric oxide in the biology, physiology and pathophysiology of reproduction. Hum Reprod Update. 1998;4:3–24. [DOI] [PubMed] [Google Scholar]
- 186. Costea L, Mészáros Á, Bauer H, et al. The blood-brain barrier and its intercellular junctions in age-related brain disorders. Int J Mol Sci. 2019;20:5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. 2007;27:1908–1918. [DOI] [PubMed] [Google Scholar]
- 188. Tarantini S, Giles CB, Wren JD, et al. IGF-1 deficiency in a critical period early in life influences the vascular aging phenotype in mice by altering miRNA-mediated post-transcriptional gene regulation: Implications for the developmental origins of health and disease hypothesis. Age (Dordr). 2016;38:239–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Toth P, Tucsek Z, Tarantini S, et al. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab. 2014;34:1887–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Tucsek Z, Toth P, Tarantini S, et al. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Balasubramanian P, Kiss T, Tarantini S, et al. Obesity-induced cognitive impairment in older adults: A microvascular perspective. Am J Physiol Heart Circ Physiol. 2021;320:H740–H761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Monteiro A, Castro P, Pereira G, et al. Neurovascular coupling is impaired in hypertensive and diabetic subjects without symptomatic cerebrovascular disease. Front Aging Neurosci. 2021;13:728007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Karkhanei B, Talebi Ghane E, Mehri F. Evaluation of oxidative stress level: Total antioxidant capacity, total oxidant status and glutathione activity in patients with COVID-19. New Microbes New Infect. 2021;42:100897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Avila-Nava A, Pech-Aguilar AG, Lugo R, Medina-Vera I, Guevara-Cruz M, Gutiérrez-Solis AL. Oxidative stress biomarkers and their association with mortality among patients infected with SARS-CoV-2 in Mexico. Oxid Med Cell Longev. 2022;2022:1058813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Al-Hakeim HK, Al-Rubaye HT, Al-Hadrawi DS, Almulla AF, Maes M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: A proof of concept and mechanism study. Mol Psychiatry. 2023;28:564–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcirculation through Nox-2–derived radicals. Arterioscler Thromb Vasc Biol. 2006;26:826–832. [DOI] [PubMed] [Google Scholar]
- 197. Boily M, Li L, Vallerand D, Girouard H. Angiotensin II disrupts neurovascular coupling by potentiating calcium increases in astrocytic endfeet. J Am Heart Assoc. 2021;10:e020608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Alam MS, Czajkowsky DM. SARS-CoV-2 infection and oxidative stress: Pathophysiological insight into thrombosis and therapeutic opportunities. Cytokine Growth Factor Rev. 2022;63:44–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Kuchler T, Günthner R, Ribeiro A, et al. Persistent endothelial dysfunction in post-COVID-19 syndrome and its associations with symptom severity and chronic inflammation. Angiogenesis. 2023;26:547–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Owens CD, Bonin Pinto C, Mukli P, et al. Vascular mechanisms leading to progression of mild cognitive impairment to dementia after COVID-19: Protocol and methodology of a prospective longitudinal observational study. PLoS One. 2023;18:e0289508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Hanssen H, Streese L, Vilser W. Retinal vessel diameters and function in cardiovascular risk and disease. Prog Retin Eye Res. 2022;91:101095. [DOI] [PubMed] [Google Scholar]
- 202. Czakó C, Kovács T, Ungvari Z, et al. Retinal biomarkers for Alzheimer's disease and vascular cognitive impairment and dementia (VCID): Implication for early diagnosis and prognosis. Geroscience. 2020;42:1499–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Yang S, Webb AJS. Associations between neurovascular coupling and cerebral small vessel disease: A systematic review and meta-analysis. Eur Stroke J. 2023;8:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Bieri JG, Corash L, Hubbard VS. Medical uses of vitamin E. N Engl J Med. 1983;308:1063–1071. [DOI] [PubMed] [Google Scholar]
- 205. Beyer RE. The role of ascorbate in antioxidant protection of biomembranes: Interaction with vitamin E and coenzyme Q. J Bioenerg Biomembr. 1994;26:349–358. [DOI] [PubMed] [Google Scholar]
- 206. Ülker S, McKeown PP, Bayraktutan U. Vitamins reverse endothelial dysfunction through regulation of eNOS and NAD(P)H oxidase activities. Hypertension. 2003;41:534–539. [DOI] [PubMed] [Google Scholar]
- 207. Hornig B, Arakawa N, Kohler C, Drexler H. Vitamin C improves endothelial function of conduit arteries in patients with chronic heart failure. Circulation. 1998;97:363–368. [DOI] [PubMed] [Google Scholar]
- 208. Kugiyama K, Motoyama T, Hirashima O, et al. Vitamin C attenuates abnormal vasomotor reactivity in spasm coronary arteries in patients with coronary spastic angina. J Am Coll Cardiol. 1998;32:103–109. [DOI] [PubMed] [Google Scholar]
- 209. Monacelli F, Acquarone E, Giannotti C, Borghi R, Nencioni A. Vitamin C, aging and Alzheimer’s disease. Nutrients. 2017;9:670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Dixit S, Bernardo A, Walker JM, et al. Vitamin C deficiency in the brain impairs cognition, increases amyloid accumulation and deposition, and oxidative stress in APP/PSEN1 and normally aging mice. ACS Chem Neurosci. 2015;6:570–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Morelli MB, Gambardella J, Castellanos V, Trimarco V, Santulli G. Vitamin C and cardiovascular disease: An update. Antioxidants (Basel). 2020;9:1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Travica N, Ried K, Sali A, Scholey A, Hudson I, Pipingas A. Vitamin C status and cognitive function: A systematic review. Nutrients. 2017;9:960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Gugliandolo A, Bramanti P, Mazzon E. Role of vitamin E in the treatment of Alzheimer’s disease: Evidence from animal models. Int J Mol Sci. 2017;18:2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Plascencia-Villa G, Perry G. Preventive and therapeutic strategies in Alzheimer’s disease: Focus on oxidative stress, redox metals, and ferroptosis. Antioxid Redox Signal. 2021;34:591–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Farina N, Llewellyn D, Isaac M, Tabet N. Vitamin E for Alzheimer’s dementia and mild cognitive impairment. Cochrane Database Syst Rev. 2017;4:CD002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Xiong Y, Zhu GH, Wang HN, et al. Discovery of naturally occurring inhibitors against SARS-CoV-2 3CL(pro) from Ginkgo biloba leaves via large-scale screening. Fitoterapia. 2021;152:104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Chen Z, Cui Q, Cooper L, et al. Ginkgolic acid and anacardic acid are specific covalent inhibitors of SARS-CoV-2 cysteine proteases. Cell Biosci. 2021;11:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Yang Y, Liu P, Chen L, et al. Therapeutic effect of Ginkgo biloba polysaccharide in rats with focal cerebral ischemia/reperfusion (I/R) injury. Carbohydr Polym. 2013;98:1383–1388. [DOI] [PubMed] [Google Scholar]
- 219. Akanchise T, Angelova A. Ginkgo Biloba and long COVID: In vivo and in vitro models for the evaluation of nanotherapeutic efficacy. Pharmaceutics. 2023;15:1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Ramírez-Garza SL, Laveriano-Santos EP, Marhuenda-Muñoz M, et al. Health effects of resveratrol: Results from human intervention trials. Nutrients. 2018;10:1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Palamara AT, Nencioni L, Aquilano K, et al. Inhibition of influenza A virus replication by resveratrol. J Infect Dis. 2005;191:1719–1729. [DOI] [PubMed] [Google Scholar]
- 222. Palamara AT, Perno CF, Aquaro S, Buè MC, Dini L, Garaci E. Glutathione inhibits HIV replication by acting at late stages of the virus life cycle. AIDS Res Hum Retroviruses. 1996;12:1537–1541. [DOI] [PubMed] [Google Scholar]
- 223. Yang M, Wei J, Huang T, et al. Resveratrol inhibits the replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in cultured Vero cells. Phytother Res. 2021;35:1127–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Pasquereau S, Nehme Z, Haidar Ahmad S, et al. Resveratrol inhibits HCoV-229E and SARS-CoV-2 coronavirus replication in vitro. Viruses. 2021;13:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Xu H, Li J, Song S, et al. Effective inhibition of coronavirus replication by Polygonum cuspidatum. Front Biosci (Landmark Ed). 2021;26:789–798. [DOI] [PubMed] [Google Scholar]
- 226. McCreary MR, Schnell PM, Rhoda DA. Randomized double-blind placebo-controlled proof-of-concept trial of resveratrol for outpatient treatment of mild coronavirus disease (COVID-19). Res Sq. 2021;12:10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Jang IA, Kim EN, Lim JH, et al. Effects of resveratrol on the renin-angiotensin system in the aging kidney. Nutrients. 2018;10:1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Toth P, Tarantini S, Tucsek Z, et al. Resveratrol treatment rescues neurovascular coupling in aged mice: Role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am J Physiol Heart Circ Physiol. 2014;306:H299–H308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Kennedy DO, Wightman EL, Reay JL, et al. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Am J Clin Nutr. 2010;91:1590–1597. [DOI] [PubMed] [Google Scholar]
- 230. Wightman EL, Reay JL, Haskell CF, Williamson G, Dew TP, Kennedy DO. Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: A randomised, double-blind, placebo-controlled, cross-over investigation. Br J Nutr. 2014;112:203–213. [DOI] [PubMed] [Google Scholar]
- 231. Wong RH, Berry NM, Coates AM, et al. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J Hypertens. 2013;31:1819–1827. [DOI] [PubMed] [Google Scholar]
- 232. Wong RH, Nealon RS, Scholey A, Howe PR. Low dose resveratrol improves cerebrovascular function in type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2016;26:393–399. [DOI] [PubMed] [Google Scholar]
- 233. Nehlig A. The neuroprotective effects of cocoa flavanol and its influence on cognitive performance. Br J Clin Pharmacol. 2013;75:716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Sorond FA, Lipsitz LA, Hollenberg NK, Fisher ND. Cerebral blood flow response to flavanol-rich cocoa in healthy elderly humans. Neuropsychiatr Dis Treat. 2008;4:433–440. [PMC free article] [PubMed] [Google Scholar]
- 235. Williams RJ, Spencer JP. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic Biol Med. 2012;52:35–45. [DOI] [PubMed] [Google Scholar]
- 236. Abd El Mohsen MM, Kuhnle G, Rechner AR, et al. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic Biol Med. 2002;33:1693–1702. [DOI] [PubMed] [Google Scholar]
- 237. Barrera-Reyes PK, de Lara JC, González-Soto M, Tejero ME. Effects of cocoa-derived polyphenols on cognitive function in humans. Systematic review and analysis of methodological aspects. Plant Foods Hum Nutr. 2020;75:1–11. [DOI] [PubMed] [Google Scholar]
- 238. Baker LD, Manson JE, Rapp SR, et al. Effects of cocoa extract and a multivitamin on cognitive function: A randomized clinical trial. Alzheimers Dement. 2023;19:1308–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Sorond FA, Hurwitz S, Salat DH, Greve DN, Fisher ND. Neurovascular coupling, cerebral white matter integrity, and response to cocoa in older people. Neurology. 2013;81:904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Wan W, Hua F, Fang P, et al. Regulation of mitophagy by sirtuin family proteins: A vital role in aging and age-related diseases. Front Aging Neurosci. 2022;14:845330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Singh CK, Chhabra G, Ndiaye MA, Garcia-Peterson LM, Mack NJ, Ahmad N. The role of sirtuins in antioxidant and redox signaling. Antioxid Redox Signal. 2018;28:643–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, et al. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17:e12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Zheng M, Schultz MB, Sinclair DA. NAD(+) in COVID-19 and viral infections. Trends Immunol. 2022;43:283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Campbell JM. Supplementation with NAD(+) and its precursors to prevent cognitive decline across disease contexts. Nutrients. 2022;14:3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Habeichi NJ, Tannous C, Yabluchanskiy A, et al. Insights into the modulation of the interferon response and NAD(+) in the context of COVID-19. Int Rev Immunol. 2022;41:464–474. [DOI] [PubMed] [Google Scholar]
- 246. Liu S, He L, Yao K. The antioxidative function of alpha-ketoglutarate and its applications. Biomed Res Int. 2018;2018:3408467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Gyanwali B, Lim ZX, Soh J, et al. Alpha-Ketoglutarate dietary supplementation to improve health in humans. Trends Endocrinol Metab. 2022;33:136–146. [DOI] [PubMed] [Google Scholar]
- 248. Agarwal S, Kaur S, Asuru TR, et al. Dietary alpha-ketoglutarate inhibits SARS CoV-2 infection and rescues inflamed lungs to restore O(2) saturation by inhibiting pAkt. Clin Transl Med. 2022;12:e1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Van Cauwenberghe C, Vandendriessche C, Libert C, Vandenbroucke RE. Caloric restriction: Beneficial effects on brain aging and Alzheimer's disease. Mamm Genome. 2016;27:300–319. [DOI] [PubMed] [Google Scholar]
- 250. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Shin BK, Kang S, Kim DS, Park S. Intermittent fasting protects against the deterioration of cognitive function, energy metabolism and dyslipidemia in Alzheimer's disease-induced estrogen deficient rats. Exp Biol Med (Maywood). 2018;243:334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Headland ML, Clifton PM, Keogh JB. Effect of intermittent energy restriction on flow mediated dilatation, a measure of endothelial function: A short report. Int J Environ Res Public Health. 2018;15:1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Antoni R, Johnston KL, Collins AL, Robertson MD. Investigation into the acute effects of total and partial energy restriction on postprandial metabolism among overweight/obese participants. Br J Nutr. 2016;115:951–959. [DOI] [PubMed] [Google Scholar]
- 254. Cani I, Barone V, D’Angelo R, et al. Frontal encephalopathy related to hyperinflammation in COVID-19. J Neurol. 2021;268:16–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Delorme C, Paccoud O, Kas A, et al. COVID-19-related encephalopathy: A case series with brain FDG-positron-emission tomography/computed tomography findings. Eur J Neurol. 2020;27:2651–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Daulatzai MA. Cerebral hypoperfusion and glucose hypometabolism: Key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer’s disease. J Neurosci Res. 2017;95:943–972. [DOI] [PubMed] [Google Scholar]
- 257. Guedj E, Million M, Dudouet P, et al. (18)F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: Substrate for persistent/delayed disorders? Eur J Nucl Med Mol Imaging. 2021;48:592–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. An Y, Varma VR, Varma S, et al. Evidence for brain glucose dysregulation in Alzheimer’s disease. Alzheimers Dement. 2018;14:318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Juby AG, Cunnane SC, Mager DR. Refueling the post COVID-19 brain: Potential role of ketogenic medium chain triglyceride supplementation: An hypothesis. Front Nutr. 2023;10:1126534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Lei E, Vacy K, Boon WC. Fatty acids and their therapeutic potential in neurological disorders. Neurochem Int. 2016;95:75–84. [DOI] [PubMed] [Google Scholar]
- 262. Cunnane SC, Trushina E, Morland C, et al. Brain energy rescue: An emerging therapeutic concept for neurodegenerative disorders of ageing. Nat Rev Drug Discov. 2020;19:609–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Horne BD, May HT, Muhlestein JB, et al. Association of periodic fasting with lower severity of COVID-19 outcomes in the SARS-CoV-2 prevaccine era: An observational cohort from the INSPIRE registry. BMJ Nutr Prev Health. 2022;5:e000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Suchting R, Colpo GD, Rocha NP, Ahn H. The effect of transcranial direct current stimulation on inflammation in older adults with knee osteoarthritis: A Bayesian residual change analysis. Biol Res Nurs. 2020;22:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Bahr-Hosseini M, Bikson M. Neurovascular-modulation: A review of primary vascular responses to transcranial electrical stimulation as a mechanism of action. Brain Stimul. 2021;14:837–847. [DOI] [PubMed] [Google Scholar]
- 266. Dedoncker J, Brunoni AR, Baeken C, Vanderhasselt MA. A systematic review and meta-analysis of the effects of transcranial Direct Current Stimulation (tDCS) over the dorsolateral prefrontal Cortex in healthy and neuropsychiatric samples: Influence of stimulation parameters. Brain Stimul. 2016;9:501–517. [DOI] [PubMed] [Google Scholar]
- 267. Mancuso LE, Ilieva IP, Hamilton RH, Farah MJ. Does transcranial direct current stimulation improve healthy working memory?: A meta-analytic review. J Cogn Neurosci. 2016;28:1063–1089. [DOI] [PubMed] [Google Scholar]
- 268. Cavendish BA, Lima A, Bertola L, et al. Combination of transcranial direct current stimulation with online cognitive training improves symptoms of post-acute sequelae of COVID-19: A case series. Brain Stimul. 2022;15:1375–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Eilam-Stock T, George A, Lustberg M, Wolintz R, Krupp LB, Charvet LE. Telehealth transcranial direct current stimulation for recovery from post-acute sequelae of SARS-CoV-2 (PASC). Brain Stimul. 2021;14:1520–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Biačková N, Klírová M, Voráčková V, et al. Treatment of cognitive symptoms in post-COVID-19 syndrome – a transcranial direct current stimulation (tDCS) approach. Brain Stimul. 2023;16:247. [Google Scholar]
- 271. Wassermann EM. The Oxford handbook of transcranial stimulation. Oxford University Press; 2021. [Google Scholar]
- 272. Demirtas-Tatlidede A, Vahabzadeh-Hagh AM, Pascual-Leone A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology. 2013;64:566–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Gazzaley A, D’Esposito M. Top-down modulation and normal aging. Ann N Y Acad Sci. 2007;1097:67–83. [DOI] [PubMed] [Google Scholar]
- 274. Lisanby SH, Luber B, Perera T, Sackeim HA. Transcranial magnetic stimulation: Applications in basic neuroscience and neuropsychopharmacology. Int J Neuropsychopharmacol. 2000;3:259–273. [DOI] [PubMed] [Google Scholar]
- 275. Gershon AA, Dannon PN, Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. Am J Psychiatry. 2003;160:835–845. [DOI] [PubMed] [Google Scholar]
- 276. Yan Y, Tian M, Wang T, Wang X, Wang Y, Shi J. Transcranial magnetic stimulation effects on cognitive enhancement in mild cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis. Front Neurol. 2023;14:1209205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Noda Y, Sato A, Shichi M, et al. Real world research on transcranial magnetic stimulation treatment strategies for neuropsychiatric symptoms with long-COVID in Japan. Asian J Psychiatr. 2023;81:103438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Zhang JX, Zhang JJ. Case report of improvement in long-COVID symptoms in an air force medic treated with transcranial magnetic stimulation using electro-magnetic brain pulse technique. Mil Med. 2023;188:e3711–e3715. [DOI] [PubMed] [Google Scholar]
- 279. Meyer K, Lulla A, Debroy K, et al. Association of the gut Microbiota with cognitive function in midlife. JAMA Network Open. 2022;5:e2143941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Liang X, Fu Y, Cao W-T, et al. Gut microbiome, cognitive function and brain structure: A multi-omics integration analysis. Transl Neurodegener. 2022;11:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Plummer AM, Matos YL, Lin HC, et al. Gut-brain pathogenesis of post-acute COVID-19 neurocognitive symptoms. Front Neurosci. 2023;17:1232480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Kim CS, Cha L, Sim M, et al. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: A randomized, double-blind, placebo-controlled, multicenter trial. J Gerontol A Biol Sci Med Sci. 2021;76:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Desmedt O, Broers VJV, Zamariola G, Pachikian B, Delzenne N, Luminet O. Effects of prebiotics on affect and cognition in human intervention studies. Nutr Rev. 2018;77:81–95. [DOI] [PubMed] [Google Scholar]
- 284. Park SH, Lee JH, Kim JS, et al. Fecal microbiota transplantation can improve cognition in patients with cognitive decline and Clostridioides difficile infection. Aging (Albany NY). 2022;14:6449–6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Xiang W, Xiang H, Wang J, et al. Fecal microbiota transplantation: A novel strategy for treating Alzheimer’s disease. Front Microbiol. 2023;14:1281233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Onorato D, Carpene G, Henry BM, Sanchis-Gomar F, Lippi G. Protective effects of statins administration in European and North American patients infected with COVID-19: A meta-analysis. Semin Thromb Hemost. 2021;47(4):392-399. [DOI] [PubMed] [Google Scholar]
- 287. Zhang X-J, Qin J-J, Cheng X, et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020;32:176–187.e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Tong XK, Lecrux C, Rosa-Neto P, Hamel E. Age-dependent rescue by simvastatin of Alzheimer's disease cerebrovascular and memory deficits. J Neurosci. 2012;32:4705–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Schultz BG, Patten DK, Berlau DJ. The role of statins in both cognitive impairment and protection against dementia: A tale of two mechanisms. Transl Neurodegener. 2018;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Tetlow S, Segiet-Swiecicka A, O'Sullivan R, et al. ACE inhibitors, angiotensin receptor blockers and endothelial injury in COVID-19. J Intern Med. 2021;289:688–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Joffre J, Rodriguez L, Matthay ZA, et al. COVID-19–associated lung microvascular endotheliopathy: A “from the bench” perspective. Am J Respir Crit Care Med. 2022;206:961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Royea J, Hamel E. Brain angiotensin II and angiotensin IV receptors as potential Alzheimer’s disease therapeutic targets. Geroscience. 2020;42:1237–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Tarantini S, Balasubramanian P, Delfavero J, et al. Treatment with the BCL-2/BCL-xL inhibitor senolytic drug ABT263/Navitoclax improves functional hyperemia in aged mice. Geroscience. 2021;43:2427–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Liu RM. Aging, cellular senescence, and Alzheimer’s disease. Int J Mol Sci. 2022;23:1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Han Y, Kim SY. Endothelial senescence in vascular diseases: Current understanding and future opportunities in senotherapeutics. Exp Mol Med. 2023;55:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Ahire C, Nyul-Toth A, DelFavero J, et al. Accelerated cerebromicrovascular senescence contributes to cognitive decline in a mouse model of paclitaxel (Taxol)-induced chemobrain. Aging Cell. 2023;22:e13832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Gulej R, Nyúl-Tóth Á, Ahire C, et al. Elimination of senescent cells by treatment with Navitoclax/ABT263 reverses whole brain irradiation-induced blood-brain barrier disruption in the mouse brain. Geroscience. 2023;45:2983–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Schmitt CA, Tchkonia T, Niedernhofer LJ, Robbins PD, Kirkland JL, Lee S. COVID-19 and cellular senescence. Nat Rev Immunol. 2023;23:251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Pastor-Fernández A, Bertos AR, Sierra-Ramírez A, et al. Treatment with the senolytics dasatinib/quercetin reduces SARS-CoV-2-related mortality in mice. Aging Cell. 2023;22:e13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Orr M, Gonzales M, Garbarino V, et al. Senolytic therapy to modulate the progression of Alzheimer’s disease (SToMP-AD)—Outcomes from the first clinical trial of senolytic therapy for Alzheimer’s disease. Res Sq. 2023;9(1):22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Liu J, Aylor KW, Chai W, Barrett EJ, Liu Z. Metformin prevents endothelial oxidative stress and microvascular insulin resistance during obesity development in male rats. Am J Physiol Endocrinol Metab. 2022;322(3):E293–E306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Zhu X, Shen J, Feng S, et al. Metformin improves cognition of aged mice by promoting cerebral angiogenesis and neurogenesis. Aging. 2020;12:17845–17862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Deng M, Su D, Xu S, et al. Metformin and vascular diseases: A focused review on smooth muscle cell function. Front Pharmacol. 2020;11:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Zhang QQ, Li WS, Liu Z, Zhang HL, Ba YG, Zhang RX. Metformin therapy and cognitive dysfunction in patients with type 2 diabetes: A meta-analysis and systematic review. Medicine (Baltimore). 2020;99:e19378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Bramante CT, Buse JB, Liebovitz DM, et al. Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): A multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial. Lancet Infect Dis. 2023;23:1119–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Selvarani R, Mohammed S, Richardson A. Effect of rapamycin on aging and age-related diseases—Past and future. GeroScience. 2021;43:1135–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Patocka J, Kuca K, Oleksak P, et al. Rapamycin: Drug repurposing in SARS-CoV-2 infection. Pharmaceuticals (Basel). 2021;14:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Van Skike CE, Hussong SA, Hernandez SF, Banh AQ, DeRosa N, Galvan V. mTOR attenuation with rapamycin reverses neurovascular uncoupling and memory deficits in mice modeling Alzheimer’s disease. J Neurosci. 2021;41:4305–4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Lin A-L, Zheng W, Halloran JJ, et al. Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer’s disease. J Cereb Blood Flow Metab. 2013;33:1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Van Skike CE, Galvan V. A perfect sTORm: The role of the mammalian target of rapamycin (mTOR) in cerebrovascular dysfunction of Alzheimer’s disease: A mini-review. Gerontology. 2018;64:205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.