Abstract
We have investigated the entry pathway of Borna disease virus (BDV). Virus entry was assessed by detecting early viral replication and transcription. Lysosomotropic agents (ammonium chloride, chloroquine, and amantadine), as well as energy depletion, prevented BDV infection, indicating that BDV enters host cells by endocytosis and requires an acidic intracellular compartment to allow membrane fusion and initiate infection. Consistent with this hypothesis, we observed that BDV-infected cells form extensive syncytia upon low-pH treatment. Entry of enveloped viruses into animal cells usually requires the membrane-fusing activity of viral surface glycoproteins (GPs). BDV GP is expressed as two products of 84 and 43 kDa (GP-84 and GP-43, respectively). We show here that only GP-43 is present at the surface of BDV-infected cells and therefore is likely the viral polypeptide responsible for triggering fusion events. We also present evidence that GP-43, which corresponds to the C terminus of GP-84, is generated by cleavage of GP-84 by the cellular protease furin. Hence, we propose that BDV GP-84 is involved in attachment to the cell surface receptor whereas its furin-cleaved product, GP-43, is involved in pH-dependent fusion after internalization of the virion by endocytosis.
Borna disease virus (BDV) causes central nervous system disease in several vertebrate species manifested by behavioral abnormalities and diverse pathology (18, 29, 36). Besides, there is accumulating evidence that BDV can infect humans and is possibly implicated in certain human neuropsychiatric disorders (1, 3, 13, 14, 25, 37, 38, 49), providing further impetus for the study of this novel neurotropic agent.
BDV is a nonsegmented, negative-stranded (NNS) RNA virus, the prototype of a new family in the Mononegavirales order (12, 40). The recent cloning and sequencing of the BDV genome have provided tools to study its molecular biology. BDV has a genomic organization characteristic of Mononegavirales (6, 10). However, as with plant nucleorhabdoviruses, BDV replicates and transcribes in the nucleus (5, 8), which is unique among known animal NNS RNA viruses.
Previous studies have suggested that a cellular receptor is required for BDV infection (15). However, little is known about the mechanism by which BDV enters the host cell. After adsorption at the cell surface, which is a receptor-mediated event, enveloped viruses enter cells by fusing with cell membranes (reviewed in references 32 and 51). This fusion event can occur at the plasma membrane, or alternatively, the virus can be internalized by receptor-mediated endocytosis and delivered to endosomes. The acidic interior of the endosome will trigger fusion events, usually related to a conformational change in a viral glycoprotein (GP) with fusogenic properties, that will allow the viral genome to be released into the cytoplasm. Among Mononegavirales, entry by members of the Paramyxoviridae occurs by fusion at the plasma membrane and is pH independent (28), whereas Rhabdoviridae and Filoviridae (31) fuse only when the pH is acidic along the endocytotic pathway. Entry of acid-dependent viruses can be inhibited by treatment with lysosomotropic agents that raise the intracellular pH and hence prevent endosomal acidification (32).
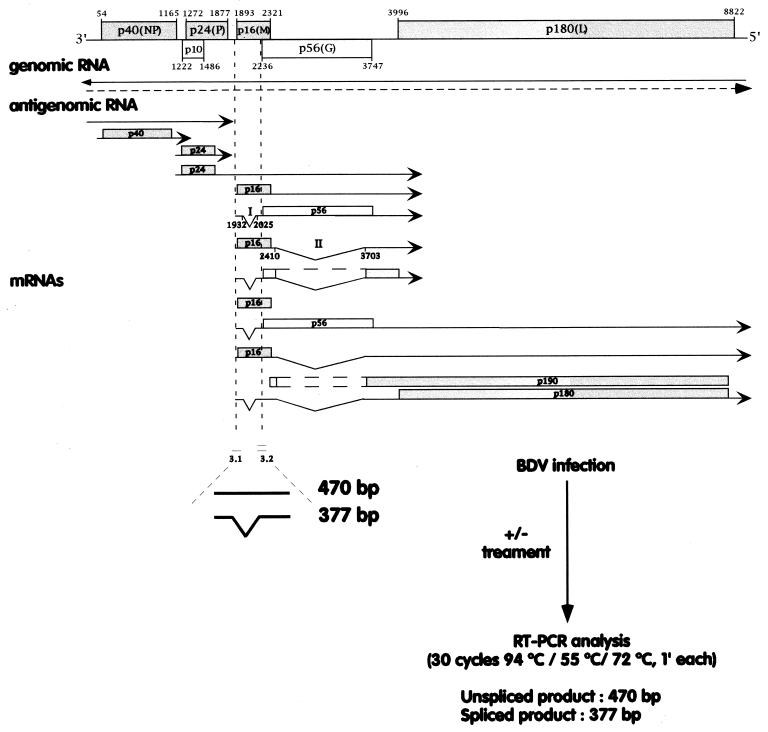
To examine the mechanisms of BDV entry, we developed an assay that allows the detection of the early steps of BDV replication and transcription. C6 rat glioma cells (ATCC CCL 107) were seeded in 24-well plates and infected at a multiplicity of infection of approximately 0.5 focus-forming unit per cell with cell-free BDV prepared as described previously (16). RNA was extracted at 0, 4, and 8 h postinfection, and BDV RNA species were detected by reverse transcriptase (RT)-PCR. We used the previously described primers 3.1 and 3.2, which flank intron I of the BDV transcription map (11). These primers allow the detection of products of 470 and 377 bp, corresponding to unspliced mRNA/antigenomic RNA and spliced mRNA, respectively (Fig. 1). In pilot experiments, we determined those experimental conditions, including multiplicity of infection, number of cycles of PCR, and time postinfection, that would allow a semiquantitative determination of BDV RNA levels (data not shown).
FIG. 1.
Overview of the RT-PCR assay used to detect early BDV replication and transcription. The positions of primers 3.1 and 3.2 relative to the BDV transcription map are indicated.
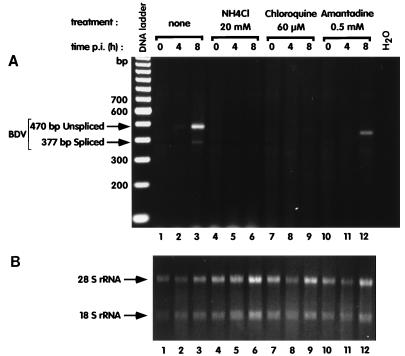
Cells were left untreated or were treated 1 h prior and during infection with three well-characterized lysosomotropic agents, i.e., ammonium chloride at 20 mM, chloroquine at 60 μM, and amantadine (AD) at 0.5 mM. These concentrations have been shown by others as being optimal in preventing endosomal acidification and having low short-term toxicity (35). Under these conditions, entry of viruses such as lymphocytic choriomeningitis virus and rabies virus is effectively inhibited (4, 46). All three lysosomotropic agents effectively inhibited or greatly decreased BDV RNA synthesis (Fig. 2). This effect was not merely virucidal, since treatment of the virus with these agents during the adsorption phase did not block subsequent infection upon removal of drugs (data not shown). Therefore, we conclude that entry of BDV is pH dependent. Importantly, AD was used in our assay in the millimolar range of concentrations. At these concentrations, AD is an established pH-raising compound and has a nonspecific antiviral activity on a number of viruses by blocking their endosomal release (23). In contrast, at lower concentrations (micromolar range), AD has a specific antiviral effect against influenza A viruses, due to a specific blockade of the ion channel activity of the influenza M2 protein (22). Although AD has been proposed to have antiviral activity against BDV at micromolar concentrations (2), results from our group (9) and others (20) have contradicted such findings.
FIG. 2.
Lysosomotropic agents block BDV infection. C6 cells were seeded in 24-well plates and infected with cell-free BDV. The cells were pretreated for 1 h with the different agents indicated at the top of the figure; this treatment was maintained throughout the experiment. RNA was extracted from the cells at 0, 4, and 8 h after the beginning of infection and analyzed by RT-PCR. (A) Results with primers designed to amplify a region of the BDV genome that contains intron I. The use of these primers allows the detection of BDV antigenomic and unspliced mRNA (top arrow) as well as spliced mRNA (bottom arrow). Due to the electrophoresis conditions, the band in lane 12 appears to migrate faster than in that in lane 3 but, however, it corresponds to the 470-bp BDV amplimer. (B) Amounts of RNA recovered for each time point. Pictures of gels were taken with a Stratagene Eagle Eye and scanned with an Agfa Studiostar scanner, and the composite image was generated with Adobe Photoshop and Canvas softwares.
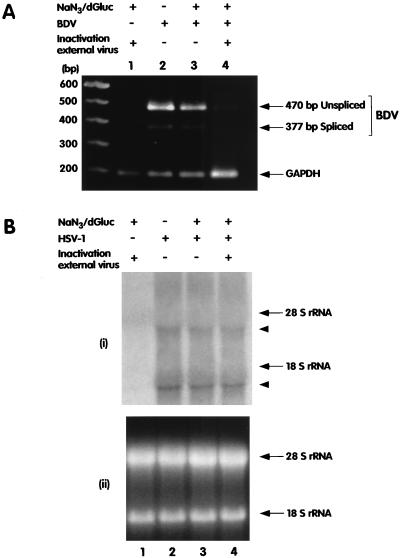
The requirement of a low-pH-dependent step for BDV entry suggests that the virion is internalized by endocytosis. Endocytosis is an energy-driven, ATP-dependent process and therefore can be blocked with inhibitors of ATP synthesis. We examined the effect of energy depletion on BDV entry by treating cells with sodium azide (NaN3) and 2-deoxy-d-glucose (dGluc), which block oxidative phosphorylation and glycolysis, respectively, by using procedures described before (27). Briefly, Vero cells were preincubated with 0.1% NaN3 and 50 mM dGluc for 1 h. The virus was then adsorbed at 37°C for 90 min, and infection was carried out for 2 h with the inhibitors still present. Extracellular virus was inactivated by incubation for 90 s with acid glycine buffer (pH 2.2). Fresh medium without inhibitors was added, and 6 h later, RNA was extracted and BDV RNA species were detected as described above. Energy blockade prevented BDV infection (Fig. 3A, lane 4). However, omission of the inactivation of extracellular virus (Fig. 3A, lane 3), which could then be taken up during the 6-h incubation phase in the absence of the inhibitors, resulted in a productive infection and demonstrated that the treatment with the inhibitors of cellular energy production was reversible. Also, infection with herpes simplex virus type 1 (HSV-1), which penetrates cells by direct fusion at the plasma membrane and does not require cellular energy for this process (43), was not affected by the treatment (Fig. 3B). Sodium azide and dGluc also inhibit transport of nuclear proteins and RNA. Although highly unlikely, our results could also be due to differences between the nuclear import of HSV-1 DNA and that of BDV RNA. Nevertheless, our data support the hypothesis that BDV is taken up by the host cell by receptor-mediated endocytosis and that fusion is pH dependent.
FIG. 3.
(A) Entry of BDV requires cellular energy. Vero cells were infected for 2 h with BDV with or without the addition of 0.1% sodium azide (NaN3) and 50 mM dGluc. After the infection period, the cells were washed and subjected to a brief low-pH treatment to inactivate external virus (lanes 1 and 4), new medium without inhibitors was added, and the cells were harvested and analyzed 6 h later. BDV replication and transcription were assessed by RT-PCR as illustrated in Fig. 1. In a separate RT-PCR, we used primers specific for GAPDH (bottom arrow) to monitor for the quality of the recovered RNA. Aliquots of both RT-PCR products were combined and loaded on the same gel for analysis. (B) Entry of HSV-1 is not affected by energy depletion. (i) Vero cells were infected with HSV-1 by the same protocol described for panel A. RNA was harvested 24 h postinfection and analyzed by Northern blotting with a probe spanning the entire immediate-early gene region of HSV-1. Arrowheads indicate the positions of the HSV-1 transcripts. (ii) The loading of each lane in the gel is illustrated. Images were processed as described in the legend to Fig. 2.
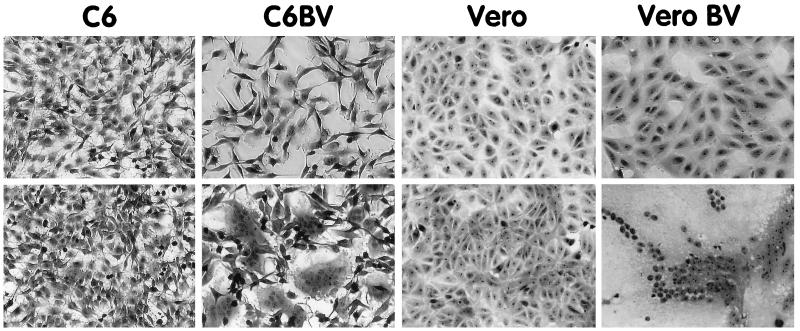
This hypothesis was further supported by the finding that BDV-infected cell lines formed extensive syncytia when subjected to a low-pH treatment (Fig. 4). Cells were washed briefly in phosphate-buffered saline and incubated for 5 min with prewarmed 20 mM citrate-phosphate buffer at pH 5 or 7. The cells were washed and returned to normal medium. One hour later, the cells were fixed for 10 min with 0.25% glutaraldehyde in phosphate-buffered saline, counterstained with 1% methylene blue–0.25% basic fuchsin in methanol, rinsed in water, and examined by light microscopy. Formation of giant multinucleated cells was observed with several BDV-infected cell lines, such as C6 or Vero, when the cell lines were treated at pH 5 but not when they were treated at pH 7. Fusion was not observed in the acid-treated uninfected control cell lines (Fig. 4). Since fusion was complete within 1 h following the pH drop, it is likely that fusion is mediated by a viral protein(s) already present at the cell surface. Fusion was not observed in 100% of the infected cells. This is likely due to the fact that the expression of BDV GP is restricted in persistently infected cells. Only a fraction of cells express levels of BDV GP detectable by immunofluorescence (16).
FIG. 4.
BDV-infected cells form syncytia when treated at low pH. C6 and Vero cells persistently infected with BDV (C6BV and Vero BV), as well as noninfected control cells, were treated for 5 min with 20 mM citrate-phosphate buffer at pH 7 (top row) or pH 5 (bottom row). After the cells were washed and incubated for 1 h in normal medium, they were fixed, counterstained, and examined by light microscopy. Pictures were taken with a video camera and processed as described in the legend to Fig. 2.
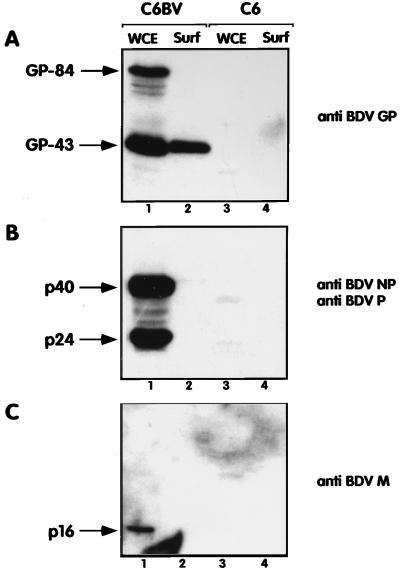
Virus surface GPs are usually involved in the penetration of enveloped viruses by triggering fusion of the viral envelope with cell membranes (39). We and others have recently characterized BDV GP (16, 41). We have shown that BDV GP is expressed as two glycosylated products of 84 and 43 kDa (GP-84 and GP-43, respectively). GP-84 corresponds to the full length of BDV GP, and GP-43 corresponds to the C terminus of BDV GP. Using a cell surface biotinylation assay described elsewhere (16), we studied which BDV proteins are present at the surface of infected cells. Consistent with our previous results, we found that among the four major BDV structural proteins, only GP-43 is present at the cell surface (Fig. 5). Therefore, we postulate that GP-43 is likely to mediate the acid-triggered fusion of BDV-infected cells and, consequently, of virus particles upon infection. To investigate whether BDV GP was sufficient to induce fusion, we transfected Vero cells with a plasmid vector expressing the BDV GP open reading frame under the control of a cytomegalovirus immediate-early promoter by using lipofectamine as described previously (17). After acid treatment, the transfected cells were processed as described above and the number of syncytia was counted. We found that cells transfected with the BDV GP expression vector displayed about 10 times more syncytia (defined as foci of three or more nuclei) than cells transfected with BDV nucleoprotein (NP) or matrix (M) protein expression constructs or with a vector without an insert (data not shown). However, the extent of fusion observed was lower than that found in BDV-infected cells. This could be due to experimental conditions. In particular, we observed that BDV GP expression was low and restricted to a limited number of cells, despite transfection efficiencies of 40 to 60%, as monitored by transfection with a vector expressing lacZ under the control of the same cytomegalovirus promoter (data not shown). The reasons for this low expression are presently unknown, but recent evidence suggests that expression of BDV GP is tightly regulated (42). Alternatively, another BDV protein(s), as well as cellular proteins induced by the infection, could play a potentiating role in fusion. BDV p16 protein, the counterpart of the M protein found in other NNS RNA viruses, could play a role in virus entry. It has been shown that BDV M protein is glycosylated and that it might be present at the surface of the virion (21, 26, 45). Nevertheless, we did not detect BDV M protein at the surface of infected cells, suggesting that this protein is unlikely to be involved in triggering fusion of BDV-infected cells.
FIG. 5.
GP-43 is the only BDV polypeptide present at the cell surface of infected cells. BDV-infected C6 cells (C6BV) or uninfected C6 cells (C6) were biotinylated with a lipid-impermeable reagent, and cell surface proteins were immunoprecipitated with streptavidin-agarose beads as described previously (16). Aliquots of the surface fraction (Surf) as well as equivalent amounts of whole-cell extracts (WCE) were analyzed by Western blotting with rabbit antisera raised against the BDV GP protein (A), the BDV p40 (NP) and BDV p24 (P) proteins (B), both antisera being combined in this assay, or the BDV p16 (M) protein (38) (C). Arrows indicate the positions of the expected polypeptides. Images were processed as described in the legend to Fig. 2.
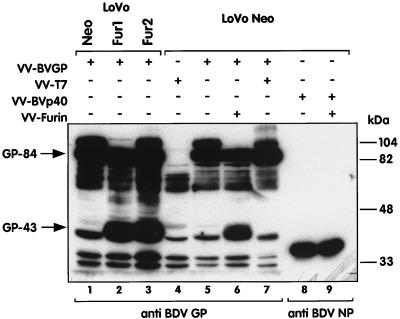
We have proposed that GP-43 is generated by cleavage of GP-84 by a cellular protease (16). At the expected cleavage site of GP-84, at positions 245 to 249 of the protein, there is a motif of 5 arginine residues (RRRRR). This sequence conforms to the minimal consensus sequence R(K/R)(K/R)R recognized by furin, a cellular calcium-dependent, subtilisin-like endoprotease. Furin cleaves many cellular and viral GP precursors, including the measles virus fusion (F) protein (50) and the gp160 protein of human immunodeficiency virus type 1 (19). It also cleaves the hemagglutinin (H) protein of avian influenza virus (44), and it has been shown that cleavability of this polypeptide is one main determinant of virus pathogenicity (24). To test whether furin is responsible for the cleavage of BDV GP, we infected LoVo cells (LoVo Neo) with a recombinant vaccinia virus expressing the BDV GP (VV-BVGP). LoVo cells, a human colon carcinoma cell line, lacks functional furin because of a single base deletion in the furin gene causing a translational frameshift (47). Cells were harvested 16 h after infection and analyzed by Western blotting for the expression of BDV GP products. Only GP-84 was detected in VV-BVGP-infected LoVo cells. In contrast, production of GP-43 was restored in two VV-BVGP-infected LoVo cell clones stably transfected with the mouse furin gene (LoVo Fur1 and Fur2, a gift from N. Kitamura [48]) (Fig. 6, compare lane 1 with lanes 2 and 3). Furthermore, infection of LoVo Neo cells with both VV-BVGP and a vaccinia vector expressing furin (VV-Furin, a gift from G. Thomas), but not with a control vaccinia virus expressing the T7 RNA polymerase (VV-T7), also restored the production of GP-43 (Fig. 6, lane 6). In contrast, production of the BDV p40 protein was not modified upon infection with VV-Furin. These results demonstrate unequivocally that furin is the major cellular protease responsible for cleavage of GP-84 and generation of GP-43. However, we cannot rule out that other subtilisin-like endoproteases are also able to cleave BDV GP.
FIG. 6.
BDV GP-43 is generated by cleavage of GP-84 by the cellular protease furin. LoVo Neo cells, which lack functional furin, and LoVo Fur1 and Fur2 cells, two clones stably transfected with the mouse furin gene, were infected with VV-BVGP (lanes 1 to 3). LoVo Neo cells were also infected with different combinations of VV-BVGP, VV-T7, recombinant vaccinia virus expressing the BDV p40 (NP) protein (VV-BVp40), and VV-Furin (lanes 4 to 9). Sixteen hours after infection of the cells, whole-cell extracts were prepared and analyzed by Western blotting with the antisera indicated at the bottom of the figure. Arrows indicate the positions of the expected GP-84 and GP-43 polypeptides. Additional bands, including one close to GP-43, were detected in cells infected with VV-T7 alone (lane 4) and were considered the result of nonspecific binding of the BDV GP antiserum. Images were processed as described in the legend to Fig. 2.
Members of the Mononegavirales exhibit a variety of strategies to enter cells. Paramyxoviridae, such as measles virus, have two surface GPs, the H and F proteins (28). The H protein is responsible for the interaction with the viral cellular receptor, e.g., CD46 in the case of measles virus (30, 34). The F protein, whose precursor is cleaved by furin into the F1 and F2 subunits along the constitutive secretory pathway, induces pH-independent cell fusion and allows entry and cell-to-cell transmission of the virus (33). In some paramyxoviruses, interactions between the H and F proteins are required for fusion (33). In contrast, members of the Rhabdoviridae, such as rabies or vesicular stomatitis virus, express only a single GP at the surface of the virion. This protein is responsible for both viral attachment and fusion upon endocytosis and endosomal acidification (7). From our previous results and those presented herein, we would like to propose an alternate mechanism for the processing of BDV GP and its role in virus entry. BDV expresses a single GP as a full-length product of 84 kDa. We have reported that a rabbit antiserum raised against BDV GP, which recognizes only the GP-84 product under native conditions, can neutralize BDV infectivity (16). This leads us to hypothesize that GP-84 is responsible for the interaction with the cellular receptor for BDV. GP-84 is cleaved by furin to generate GP-43, which is also associated with BDV infectious particles. After endocytosis, acidification in the endosome will likely induce a conformational change of GP-43 that will consequently trigger fusion, a situation mimicked by acid treatment of BDV-infected cells. Interestingly, sequence analysis indicates that the new N terminus of GP-43, which is exposed after GP-84 cleavage by furin, is highly hydrophobic. This is reminiscent of the fusogenic domain described for surface GPs of other viruses, such as the H protein of influenza virus or the F protein of paramyxoviruses (51). Nevertheless, there is no significant similarity between the BDV GP sequence and the fusogenic peptide consensus sequence derived from F1 proteins of paramyxoviruses (28).
The BDV genome (8.9 kb) is significantly smaller than those of the other known Mononegavirales (11 to 20 kb). Likely as a result of this smaller size, BDV has developed a variety of concurrent strategies for its gene expression regulation. These include an overlap of transcription units and transcriptive signals, a read-through of transcription termination signals, and RNA splicing (12, 40). BDV GP expression and function represent a new situation in NNS RNA viruses that is somewhat intermediate between the strategies adopted by paramyxoviruses and rhabdoviruses. The strategy adopted by BDV for the expression of its GP might be related to its exquisite ability to establish persistence. Animals chronically infected with BDV have high levels of viral antigen and RNA in the central nervous system. However, only extremely low levels of enveloped infectious virions are detected, and viral budding has not yet been observed in BDV-infected tissues and cells (18). This might be related to the restriction of BDV GP expression in infected cells. As a consequence, BDV GP is poorly recognized by serum antibodies from infected animals.
The cleavage of GP-84 by furin is likely required for exposure of a new hydrophobic N terminus in GP-43. This new N terminus will, in turn, be responsible for the pH-dependent fusion event necessary for a BDV-productive infection. Despite the localization of BDV GP products in separate cellular compartments, both products are associated with infectious virions (16). Hence, the accumulation of GP-84 and GP-43 in different cellular compartments may impose additional constraints on the production of infectious particles. The low level of virus production may contribute to escape from the host immune response and may favor the establishment of persistent infection.
Acknowledgments
This work was supported by grants NS 32355-02 (to J.C.T.) and from the Institut Pasteur (to D.G.-D.).
We thank N. Kitamura and G. Thomas for advice and the gift of reagents used in this study. C. Sauder is gratefully acknowledged for critically reading the manuscript.
Footnotes
Publication 11049-NP from the Scripps Research Institute.
REFERENCES
- 1.Bode L. Human infections with Borna disease virus and potential pathogenic implications. In: Koprowski H, Lipkin I, editors. Borna disease. Berlin, Germany: Springer-Verlag KG; 1995. pp. 103–130. [DOI] [PubMed] [Google Scholar]
- 2.Bode L, Dietrich D E, Stoyloff R, Emrich H M, Ludwig H. Amantadine and human Borna disease virus in vitro and in vivo in an infected patient with bipolar depression. Lancet. 1997;349:178–179. doi: 10.1016/S0140-6736(05)60979-8. [DOI] [PubMed] [Google Scholar]
- 3.Bode L, Zimmermann W, Ferszt R, Steinbach F, Ludwig H. Borna disease virus genome transcribed and expressed in psychiatric patients. Nat Med. 1995;1:232–236. doi: 10.1038/nm0395-232. [DOI] [PubMed] [Google Scholar]
- 4.Borrow P, Oldstone M B. Mechanism of lymphocytic choriomeningitis virus entry into cells. Virology. 1994;198:1–9. doi: 10.1006/viro.1994.1001. [DOI] [PubMed] [Google Scholar]
- 5.Briese T, de la Torre J C, Lewis A, Ludwig H, Lipkin W I. Borna disease virus, a negative-strand RNA virus, transcribes in the nucleus of infected cells. Proc Natl Acad Sci USA. 1992;89:11486–11489. doi: 10.1073/pnas.89.23.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briese T, Schneemann A, Lewis A J, Park Y S, Kim S, Ludwig H, Lipkin W I. Genomic organization of Borna disease virus. Proc Natl Acad Sci USA. 1994;91:4362–4366. doi: 10.1073/pnas.91.10.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coll J M. The glycoprotein G of rhabdoviruses. Arch Virol. 1995;140:827–851. doi: 10.1007/BF01314961. [DOI] [PubMed] [Google Scholar]
- 8.Cubitt B, de la Torre J C. Borna disease virus (BDV), a nonsegmented RNA virus, replicates in the nuclei of infected cells where infectious BDV ribonucleoproteins are present. J Virol. 1994;68:1371–1381. doi: 10.1128/jvi.68.3.1371-1381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cubitt, B., and J. C. de la Torre. Amantadine does not have antiviral activity against Borna disease virus. Arch. Virol., in press. [DOI] [PubMed]
- 10.Cubitt B, Oldstone C, de la Torre J C. Sequence and genome organization of Borna disease virus. J Virol. 1994;68:1382–1396. doi: 10.1128/jvi.68.3.1382-1396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cubitt B, Oldstone C, Valcarcel J, de la Torre J C. RNA splicing contributes to the generation of mature mRNAs of Borna disease virus, a non-segmented negative strand RNA virus. Virus Res. 1994;34:69–79. doi: 10.1016/0168-1702(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 12.de la Torre J C. Molecular biology of Borna disease virus: prototype of a new group of animal viruses. J Virol. 1994;68:7669–7675. doi: 10.1128/jvi.68.12.7669-7675.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de la Torre J C, Bode L, Dürrwald R, Cubitt B, Ludwig H. Sequence characterization of human Borna disease virus. Virus Res. 1996;44:33–44. doi: 10.1016/0168-1702(96)01338-x. [DOI] [PubMed] [Google Scholar]
- 14.de la Torre J C, Gonzalez-Dunia D, Cubitt B, Mallory M, Mueller-Lantzsch N, Grässer F A, Hansen L A, Masliah E. Detection of Borna disease virus antigen and RNA in human autopsy brain samples from neuropsychiatric patients. Virology. 1996;223:272–282. doi: 10.1006/viro.1996.0479. [DOI] [PubMed] [Google Scholar]
- 15.Duchala C S, Carbone K M, Narayan O. Preliminary studies on the biology of Borna disease virus. J Gen Virol. 1989;70:3507–3511. doi: 10.1099/0022-1317-70-12-3507. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Dunia D, Cubitt B, Grässer F A, de la Torre J C. Characterization of Borna disease virus p56 protein, a surface glycoprotein involved in virus entry. J Virol. 1997;71:3208–3218. doi: 10.1128/jvi.71.4.3208-3218.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Dunia D, Eddleston M, Mackman N, Carbone K, de la Torre J C. Expression of tissue factor is increased in astrocytes within the central nervous system during persistent infection with Borna disease virus. J Virol. 1996;70:5812–5820. doi: 10.1128/jvi.70.9.5812-5820.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gosztonyi G, Ludwig H. Borna disease—neuropathology and pathogenesis. In: Koprowski H, Lipkin I, editors. Borna disease. Berlin, Germany: Springer-Verlag KG; 1995. pp. 39–73. [PubMed] [Google Scholar]
- 19.Hallenberger S, Bosch V, Angliker H, Shaw E, Klenk H D, Garten W. Inhibition of furin-mediated cleavage activation of HIV-1 glycoprotein gp160. Nature. 1992;360:358–361. doi: 10.1038/360358a0. [DOI] [PubMed] [Google Scholar]
- 20.Hallensleben, W., M. Zocher, and P. Staeheli. Borna disease virus is not sensitive to amantadine. Arch. Virol., in press. [DOI] [PubMed]
- 21.Hatalski C G, Kliche S, Stitz L, Lipkin W I. Neutralizing antibodies in Borna disease virus-infected rats. J Virol. 1995;69:741–747. doi: 10.1128/jvi.69.2.741-747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hay A. The action of amantadines against influenza A viruses: inhibition of the M2 ion channel protein. Semin Virol. 1992;3:21–30. [Google Scholar]
- 23.Hirsch M S, Kaplan J C, D’Aquila R. Antiviral agents. In: Fields B N, Knipe D M, et al., editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 431–466. [Google Scholar]
- 24.Horimoto T, Kawaoka Y. Reverse genetics provides direct evidence for a correlation of hemagglutinin cleavability and virulence of an avian influenza A virus. J Virol. 1994;68:3120–3128. doi: 10.1128/jvi.68.5.3120-3128.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kishi M, Nakaya T, Nakamura Y, Zhong Q, Ikeda K, Senjo M, Kakinuma M, Kato S, Ikuta K. Demonstration of human Borna disease virus RNA in human peripheral blood mononuclear cells. FEBS Lett. 1995;364:293–297. doi: 10.1016/0014-5793(95)00406-y. [DOI] [PubMed] [Google Scholar]
- 26.Kliche S, Briese T, Henschen A H, Stitz L, Lipkin W I. Characterization of a Borna disease virus glycoprotein, gp18. J Virol. 1994;68:6918–6923. doi: 10.1128/jvi.68.11.6918-6923.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kock J, Borst E M, Schlicht H J. Uptake of duck hepatitis B virus into hepatocytes occurs by endocytosis but does not require passage of the virus through an acidic intracellular compartment. J Virol. 1996;70:5827–5831. doi: 10.1128/jvi.70.9.5827-5831.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamb R A, Kolakofsky D. Paramyxoviridae: the viruses and their replication. In: Fields B N, Knipe D M, et al., editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1177–1204. [Google Scholar]
- 29.Ludwig H, Bode L, Gosztonyi G. Borna disease: a persistent virus infection of the central nervous system. Prog Med Virol. 1988;35:107–151. [PubMed] [Google Scholar]
- 30.Manchester M, Liszewski M K, Atkinson J P, Oldstone M B. Multiple isoforms of CD46 (membrane cofactor protein) serve as receptors for measles virus. Proc Natl Acad Sci USA. 1994;91:2161–2165. doi: 10.1073/pnas.91.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mariankova R F, Glushakova S E, Pyzhik E V, Lukashevich I S. The penetration of the Marburg virus into eukaryotic cells. Vopr Virusol. 1993;38:74–76. [PubMed] [Google Scholar]
- 32.Marsh M, Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison T, Portner A. Structure, function and intracellular processing of the glycoproteins of paramyxoviridae. In: Kingsbury D W, editor. The paramyxoviruses. New York, N.Y: Plenum Press; 1991. pp. 347–382. [Google Scholar]
- 34.Naniche D, Varior-Krishnan G, Cervoni F, Wild T F, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez E, Everitt E. Adenovirus uncoating and nuclear establishment are not affected by weak base amines. J Virol. 1996;70:3470–3477. doi: 10.1128/jvi.70.6.3470-3477.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rott R, Becht H. Natural and experimental Borna disease in animals. In: Koprowski H, Lipkin I, editors. Borna disease. Berlin, Germany: Springer-Verlag KG; 1995. pp. 17–30. [DOI] [PubMed] [Google Scholar]
- 37.Salvatore M, Morzunov S, Schwemmle M, Lipkin W I the Borna Virus Study Group. Borna disease virus in brains of North American and European people with schizophrenia and bipolar disorder. Lancet. 1997;349:1813–1814. doi: 10.1016/s0140-6736(05)61693-5. [DOI] [PubMed] [Google Scholar]
- 38.Sauder C, Müller A, Cubitt B, Mayer J, Steinmetz J, Trabert W, Ziegler B, Wanke K, Mueller-Lantzsch N, de la Torre J C, Grässer F A. Detection of Borna disease virus (BDV) antibodies and BDV RNA in psychiatric patients: evidence for high sequence conservation of human blood-derived BDV RNA. J Virol. 1996;70:7713–7724. doi: 10.1128/jvi.70.11.7713-7724.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlesinger M J, Schlesinger S. Domains of virus glycoproteins. Adv Virus Res. 1987;33:1–44. doi: 10.1016/S0065-3527(08)60315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneemann A, Schneider P A, Lamb R A, Lipkin W I. The remarkable coding strategy of Borna disease virus: a new member of the nonsegmented negative strand RNA viruses. Virology. 1995;210:1–8. doi: 10.1006/viro.1995.1311. [DOI] [PubMed] [Google Scholar]
- 41.Schneider P A, Hatalski C G, Lewis A J, Lipkin W I. Biochemical and functional analysis of Borna disease virus G protein. J Virol. 1997;71:331–336. doi: 10.1128/jvi.71.1.331-336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider P A, Kim R, Lipkin W I. Evidence for translation of the Borna disease virus G protein by leaky ribosomal scanning and ribosomal reinitiation. J Virol. 1997;71:5614–5619. doi: 10.1128/jvi.71.7.5614-5619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spear P G. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–180. [Google Scholar]
- 44.Stieneke-Grober A, Vey M, Angliker H, Shaw E, Thomas G, Roberts C, Klenk H D, Garten W. Influenza virus hemagglutinin with multibasic cleavage site is activated by furin, a subtilisin-like endoprotease. EMBO J. 1992;11:2407–2414. doi: 10.1002/j.1460-2075.1992.tb05305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stoyloff R, Strecker A, Bode L, Franke P, Ludwig H, Hucho F. The glycosylated matrix protein of Borna disease virus is a tetrameric membrane-bound viral component essential for infection. Eur J Immunol. 1997;246:252–257. doi: 10.1111/j.1432-1033.1997.t01-2-00252.x. [DOI] [PubMed] [Google Scholar]
- 46.Superti F, Derer M, Tsiang H. Mechanism of rabies virus entry into CER cells. J Gen Virol. 1984;65:781–789. doi: 10.1099/0022-1317-65-4-781. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi S, Kasai K, Hatsuzawa K, Kitamura N, Misumi Y, Ikehara Y, Murakami K, Nakayama K. A mutation of furin causes the lack of precursor-processing activity in human colon carcinoma LoVo cells. Biochem Biophys Res Commun. 1993;195:1019–1026. doi: 10.1006/bbrc.1993.2146. [DOI] [PubMed] [Google Scholar]
- 48.Tsuneoka M, Nakayama K, Hatsuzawa K, Komada M, Kitamura N, Mekada E. Evidence for involvement of furin in cleavage and activation of diphtheria toxin. J Biol Chem. 1993;268:26461–26465. [PubMed] [Google Scholar]
- 49.Waltrip R W, II, Buchanan R W, Summerfelt A, Breier A, Carpenter W T, Bryant N, Rubin S A, Carbone K M. Borna disease virus and schizophrenia. Psych Res. 1995;56:33–44. doi: 10.1016/0165-1781(94)02600-n. [DOI] [PubMed] [Google Scholar]
- 50.Watanabe M, Hirano A, Stenglein S, Nelson J, Thomas G, Wong T C. Engineered serine protease inhibitor prevents furin-catalyzed activation of the fusion glycoprotein and production of infectious measles virus. J Virol. 1995;69:3206–3210. doi: 10.1128/jvi.69.5.3206-3210.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White J M. Viral and cellular membrane fusion proteins. Annu Rev Physiol. 1990;52:675–697. doi: 10.1146/annurev.ph.52.030190.003331. [DOI] [PubMed] [Google Scholar]