Abstract
Introduction
Indirect carotid-cavernous fistula (CCF) can lead to secondary glaucoma, posing significant treatment challenges. This paper discusses a case where standard embolization failed, and an Ahmed FP7 valved glaucoma tube shunt was crucial for managing the increased intraocular pressure (IOP), highlighting the necessity for individualized surgical approaches.
Case presentation
A 48-year-old female presented in the emergency department with conjunctival hyperemia, proptosis and elevated IOP; initial imaging findings were indicative of orbital inflammatory disease. Further evaluation with cerebral CT angiography revealed a possible CCF. Subsequent angiography confirmed an indirect CCF type D, leading to the patient undergoing endovascular embolization. Final monitoring revealed a subtotal occlusion of the fistula. Although there was some improvement post-procedure, IOP remained elevated despite medication, and subsequent attempts of embolization were unsuccessful. Surgical intervention with a tube shunt was performed, allowing IOP to decreased to a normal range. Optic nerve head optical coherence tomography, standard automated perimetry, and best-corrected visual acuity remained stable during the 33-month follow-up.
Discussion
In managing glaucoma linked to CCF, a multidisciplinary approach is critical. Conservative methods are often adequate, with spontaneous CCF closure observed in a significant percentage. Endovascular embolization is reserved for refractory cases, with embolization showing a higher rate of IOP normalization compared to medication alone. Yet, when fistula closure is challenging or contraindicated, individualized management strategies like glaucoma surgery may be employed.
Conclusions
When fistula closure is not achievable, the Ahmed FP7 valved tube shunt can successfully regulate IOP with minimal complications, providing an effective alternative for refractory cases.
Keywords: Secondary Glaucoma, Indirect carotid-cavernous fistula, Surgical management, Tube-shunt
Highlights
-
•
Indirect carotid-cavernous fistula (ICCF) induced glaucoma demands specialized treatment.
-
•
Surgery necessary when embolization fails to control ICCF induced glaucoma
-
•
Ahmed valve favored for lower complication rates compared to other surgical methods
-
•
Ahmed FP7 valve ensures IOP control, vital for glaucoma from Carotid-Cavernous Fistula.
-
•
Case highlights multidisciplinary approach in complex glaucoma management.
1. Introduction
Carotid-cavernous fistulas (CCFs) are acquired pathological shunts characterized by the redirection of blood flow from a high-flow arterial system, such as the internal carotid artery (ICA) or external carotid artery (ECA), into a low-flow venous system, the cavernous sinus (CS). Ophthalmologic symptoms frequently present first, typically prompting diagnosis by ophthalmologists before neurological evaluation.
The most widely accepted classification focuses on angiographic arterial architecture. Type A fistulas are direct communications between the ICA and the CS. Type B, C, and D, are indirect CCFs between dural branches of the ICA, ECA, or both, and the CS, respectively [1]. Arterialized pressures within the CS impede venous drainage and leads to vascular congestion upstream. Direct CCF presents with acute and marked symptoms [2]. However, in indirect CCF, symptoms may be mild and difficult to interpret [1]. While both CCF types can lead to ocular hypertension (OHT), is more common in indirect cases [[3], [4], [5], [6]].
There are three distinct mechanisms that can cause CCF-related OHT: open-angle glaucoma (OAG), closed-angle glaucoma, and neovascular glaucoma [1,7]. An increase in episcleral venous pressure (EVP) and venous congestion can impair the drainage of aqueous humor through the trabecular meshwork and Schlemm's canal, resulting OHT and potentially leading to secondary OAG [3]. In contrast, elevated orbital vein pressure may cause choroidal and ciliary body congestion, edema, and detachment, pushing the lens-iris forward, shallowing the anterior chamber and potentially leading to angle-closure glaucoma [[8], [9], [10]]. Alternatively, hemodynamic shifts in CCF may lower orbital blood flow, causing ocular ischemia, neovascularization, and potentially neovascular glaucoma [8,10]. OAG accounts for about 85 % of cases [3,7].
Low-flow fistulas may need only non-surgical management, but symptomatic CCFs often require embolization endovascular embolization to restore the pressure differential between the cavernous sinus and arterial systems and lower IOP [6,11,12]. Nevertheless, there is no definitive consensus regarding surgical glaucoma treatment for indirect CCF when embolization and medication fail to manage IOP.
This report was compiled in adherence to the SCARE criteria [13]. Written consent was obtained from the patient, which can be consulted by the Editor-in-Chief if required. Institutional Review Board is not required for case reports in our institute according to the Research/Human Ethics Committee.
2. Case presentation
A 48-year-old woman presented in the emergency department with a red left eye persisting for a week, without other symptoms, trauma, or significant medical history. Best correct visual acuity (BCVA) was 20/20 in right eye (OD) and 20/25 in the left eye (OS). Left proptosis and conjunctival chemosis, with episcleral vessels engorgement and tortuosity, were visible. Pupillary reflexes and extra-ocular motility were normal. IOP was 12 mmHg in OD and 32 mmHg in OS. Fundoscopy revealed a cup to disc (C/D) ratio of 0.5 bilaterally. Orbital CT scan revealed extraocular muscles enlargement, optic nerve thickening, and dilated SOV on the left, suggesting orbital inflammatory disease. Enlarged eye muscles and a dilated superior ophthalmic vein on imaging plus the clinical signs suggested a CCF.
CT angiography showed a potential left CCF, confirmed by cerebral angiography, leading to embolization and a subtotal occlusion of the fistula. Two weeks post-procedure, episcleral vessel engorgement persisted, IOP was 10 mmHg in the OD and 16 mmHg in the OS under timolol 5 mg/ml and brimonidine 2 mg/ml b.i.d, with no fundus abnormalities. Hertel exophthalmometry measured 15 mm in OD and 19 mm in OS, with a 106 mm inter-orbital margin distance. Gonioscopy revealed a Shaffer IV 360° bilaterally, without neovascularization. BCVA was 20/20 in OD and 20/25 in OS. Optical coherence tomography (OCT) of the optic nerve head (ONH), revealed a significant decrease in temporal-superior thickness of the retinal nerve fiber layer (RNFL) bilaterally (Fig. 1). Standard automated perimetry (SAP) showed a diffuse defect in OD and a paracentral, fixation threatening, field defect in OS (Fig. 2).
Fig. 1.
OCT of the ONH showing a decrease in the temporal-superior RNFL thickness.
Fig. 2.
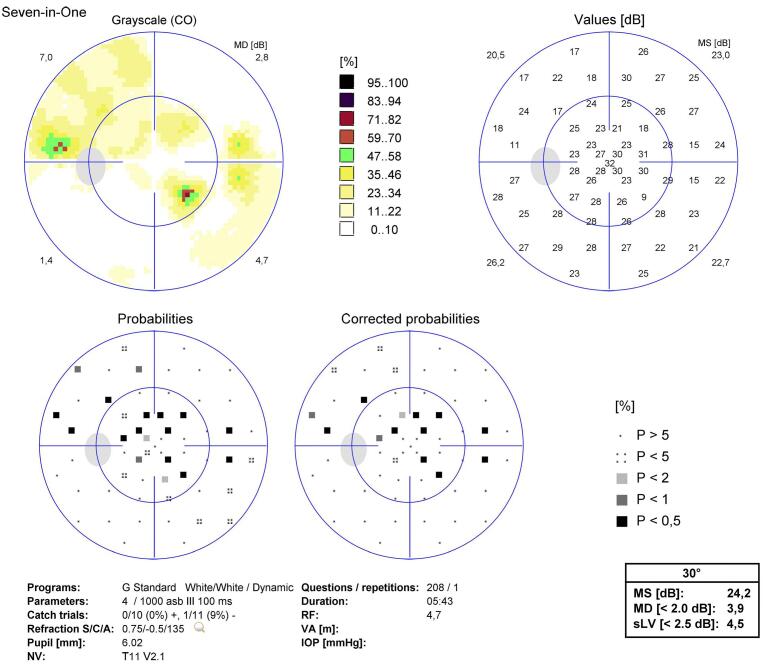
SAP demonstrating a paracentral field defect in OS.
Due to clinical deterioration, two more embolization attempts at 2 and 5 months post-initial procedure were made, both unsuccessful. Eight months after the initial evaluation, despite triple topical medication and oral acetazolamide 250 mg t.i.d., the OS IOP remained at 28 mmHg. Conjunctival chemosis and episcleral vessels engorgement worsened; rubeosis iridis was not observed. Gonioscopy revealed an open angle, without neovascularization. Fundoscopy revealed an increased OS C/D ratio of 0.6. Further neuroradiological intervention was contraindicated. It was decided to implant an Ahmed FP7 valved TS. During surgery, ophthalmic visco-surgical device (OVD) was used to fill the anterior chamber (AC), and it was intentionally left in place after the procedure to prevent abrupt decompression. No complications were observed. One month after surgery, the patient had an OS IOP of 10 mmHg with timolol 5 mg/ml and dorzolamide 20 mg/ml b.i.d. BCVA was 20/20 bilaterally. In the 33-month post-op follow-up, the patient showed improved but mild CCF signs, including OS exophthalmometry of 18 mm, and episcleral vessel engorgement. OS IOP remained below 15 mmHg on topical treatment with timolol 5 mg/ml plus dorzolamide 20 mg/ml b.i.d. OCT assessment of ONH revealed constant OS RNFL thickness during follow-up (Fig. 3). OS GCL thickness was within reference values, but GCL thickness map indicated glaucomatous damage (Fig. 4). SAP exhibited a diffuse defect bilaterally (Fig. 5). The patient maintained a BCVA of 20/20 during all evaluations.
Fig. 3.
RNFL thickness progression throughout follow-up.
Fig. 4.
GCL thickness map demonstrating of glaucomatous damage of the ON.
Fig. 5.
SAP 30 months after diagnosis, with a diffuse field defect.
3. Discussion
The optimal management of glaucoma in the setting of indirect CCF can be complex and requires a multidisciplinary approach involving ophthalmologists, neurologists and interventional radiologists.
Observation or conservative methods are preferred in low-risk cases [14,15], as spontaneous CCF closure occurs in 11–70 %, especially post-angiography [5,16,17]. Carotid compression therapy achieves closure rates of 17 % (direct CCFs) and 30 % (indirect CCFs).
Topical agents are first-line for CCF-induced OHT. However, due to elevated EVP, aqueous suppressants may lack efficacy. Prostaglandin receptor agonists, enhancing uveoscleral outflow, may be limited by arterial blood backup [14,18]. Similarly, pilocarpine and laser trabeculoplasty, targeting trabecular outflow, show low effectiveness [19]. Nonetheless, aqueous suppressants performing better compared to trabecular outflow enhancers [20,21].
In Khurana et al.'s study [4], 64.1 % of patients with CCF had OHT or glaucoma, 75.6 % by an open-angle mechanism. Effective IOP control was achieved in 70.73 % of cases using various methods, including endovascular treatment, IOP-lowering medications, or a combination thereof. In her study, Concepcion et al. [22] observed elevated IOP in 78 % of CCF cases. 36.5 % achieved control with medication while 37.5 % remained uncontrolled. Only 13.5 % underwent definitive procedures like embolization. Preechawat et al. [23] found elevated IOP in 51 % of CCF patients, with 13 % experiencing optic neuropathy. Endovascular treatment was performed in 75 % of cases and led to complete symptom resolution in 57 %, improvement in 33 %, and stable/aggravated symptoms in 10 %.
When maximal medical therapy can't control IOP, definitive CCF treatment is preferred over glaucoma surgery. In fact, refractory glaucoma is a major indication for definitive CCF treatment [6,16,24]. Options include surgery or minimally invasive methods like endovascular embolization, with success rates of 70–100 % [25,26]. CCF occlusion normalizes CS blood flow and pressure, reducing ophthalmic vein congestion and enhancing eye drainage, thereby lowering EVP and IOP [27].
In Saiegh et al.'s [27] retrospective analysis of patients with both direct and indirect CCF undergoing endovascular embolization, total occlusion was achieved in 83.3 % of cases. Furthermore, 72.0 % of patients achieved normalized IOP, while 19.0 % experienced increased or stable IOP post-procedure. Notably, 43 % of patients with partially occluded fistulas had increased IOP compared to 14 % with complete occlusion.
In our case, despite three embolization attempts, complete fistula closure wasn't achieved. Since dural CCF doesn't affect overall survival, further intervention was contraindicated [14]. However, managing symptoms and ensuring IOP control can be challenging when closure isn't possible. Glaucoma surgery is a viable option in such cases.
In Concepcion et al.'s study [22], 13 eyes receiving embolization had used IOP-lowering medication before. Postoperatively, 33 % had normal IOP, 27 % used medication for control, and 40 % had OHT despite medication. None underwent filtering surgery while one direct CCF patient underwent diode cyclophotocoagulation.
In Ishijima et al.'s [3] study, 64 % had OHT. Among the 5 eyes with confirmed CCF closure, 3 achieved effective IOP control without meds, 1 needed topical treatment, and another had trabeculectomy, all with good IOP control. However, in 4 cases with open/uncertain CCF, only 1 eye achieved good IOP control without meds. The others received medical/surgical treatment (cyclocryotherapy or cyclophotocoagulation) but didn't achieve successful IOP control. Overall, CCF closure proved more effective in IOP control than antiglaucoma treatments.
Patients with elevated EVP face increased risks during surgery, such as choroidal effusion post-penetrating glaucoma surgery [18,28] Hence, selecting appropriate surgical techniques is crucial to prevent hypotony during the procedure [19,29].
In trabeculectomy, adjustable sutures on the scleral flap, along with prophylactic sclerectomies, reduce likelihood of excessive filtering. However, choroidal detachment risk remains [18,28]. Non-penetrating filtration surgery is unlikely to be effective due to elevated EVP. Aqueous humor outflow across the trabecular meshwork is pressure-dependent and the pressure differential in this condition is low. TS provides an alternative drainage route for aqueous humor, bypassing the conventional pathway [30]. It allows direct flow from the AC into periocular capillaries and lymphatics, avoiding outflow resistance due to elevated EVP and venous congestion from CCFs.
Furthermore, valved TS, like Ahmed TS, regulate aqueous humor flow, opening at IOP of 8-12 mmHg. In contrast, trabeculectomy can cause post-op hypotony, worsening choroidal detachment, whereas valved TS maintain stable IOP, reducing hypotony-related complications [31]. Due to the design of the Ahmed TS, the valve to stay open even in the presence of a small pressure difference between the AC and the surrounding subconjunctival spaces [31]. Based on this, we opted for the implantation of the Ahmed FP7. Additional measures can be taken during surgery to maintain appropriate IOP, such as injecting OVD into the AC. Postoperatively, cycloplegic, oral and topical glucocorticoids may be used to alleviate inflammation and reduce the risk of choroidal effusion.
4. Conclusion
Managing glaucoma in indirect CCF is complex, often needing a multidisciplinary approach. Closure of CCF is the most effective treatment but not always feasible. If topical agents fail to control IOP, surgery may be necessary, yet CCF patients face added risks. This case shows that valved TS can be a suitable surgical option.
Patient consent
Consent to publish this case report has been obtained from the patient(s) in writing.
Ethical approval
Ethics committee is not required for case reports in our institute according to the Research/Human Ethics Committee. However, information was obtained and reported in a manner that was compliant with the standards set forth by the Declaration of Helsinki as amended in 2013. Written consent was obtained from the patient for this case report, which can for reviewed by the Editor-in-Chief if required.
Funding
No funding was received for this work.
Author contribution
Edgar Lopes – data collection, data analysis or interpretation, writing – original draft, review.
Ines Ludovico – writing – original draft.
Catarina Mota – writing – original draft.
Ana Xavier – study concept or design.
Ana Duarte – writing - review and editing.
Joana Cardigos – study concept or design, writing - review and editing.
Guarantor
Edgar Lopes and Joana Cardigos.
Declaration of competing interest
No conflicts of interest exist for any author.
Acknowledgments
No funding or grant support.
The following authors have no financial disclosures: EL, IL, CM, AX, AD, JC.
All authors attest that they meet the current ICMJE criteria for Authorship.
References
- 1.Barrow D.L., Spector R.H., Landman J.A., Tindall S.C., Tindall G.T. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J. Neurosurg. 1985;62:248–256. doi: 10.3171/jns.1985.62.2.0248. [DOI] [PubMed] [Google Scholar]
- 2.Phelps C.D., Stanley Thompson H., Ossoinig K.C. The diagnosis and prognosis of atypical carotid-cavernous fistula (red-eyed shunt syndrome) Am J. Ophthalmol. 1982;93(4):423–436. doi: 10.1016/0002-9394(82)90132-5. [DOI] [PubMed] [Google Scholar]
- 3.Ishijima K., Kashiwagi K., Nakano K., Shibuya T., Tsumura T., Tsukahara S. Ocular manifestations and prognosis of secondary glaucoma in patients with carotid-cavernous fistula. Jpn. J. Ophthalmol. 2003;47(6):603–608. doi: 10.1016/j.jjo.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Khurana M., Alam M.S., Balekudaru S., et al. Intraocular pressure in the eyes of patients with carotid-cavernous fistulas: profile, Intereye asymmetry, and treatment outcomes. J. Glaucoma. 2019;28(12):1074–1078. doi: 10.1097/IJG.0000000000001392. [DOI] [PubMed] [Google Scholar]
- 5.De Keizer R.J.W. Carotid-cavernous and orbital arteriovenous fistulas: ocular features, diagnostic and hemodynamic considerations in relation to visual impairment and morbidity. Orbit. 2003;22(2):121–142. doi: 10.1076/ORBI.22.2.121.14315. [DOI] [PubMed] [Google Scholar]
- 6.Kirsch M., Henkes H., Liebig T., et al. Endovascular management of dural carotid-cavernous sinus fistulas in 141 patients. Neuroradiology. 2006;48(7):486–490. doi: 10.1007/S00234-006-0089-9. [DOI] [PubMed] [Google Scholar]
- 7.Weinreb R.N., Karwatowski W.S.S. In: The Glaucomas. Ritch R., Shields B., Krupin T., editors. 1966. Glaucoma associated with elevated episcleral venous pressure; pp. 1143–1155. [Google Scholar]
- 8.Fiore P.M., Latina M.A., Shingleton B.J., Rizzo J.F., Ebert E., Bellows A.R. The dural shunt syndrome. I. Management of glaucoma. Ophthalmology. 1990;97(1):56–62. doi: 10.1016/S0161-6420(90)32641-6. [DOI] [PubMed] [Google Scholar]
- 9.Calafiore S., Perdicchi A., Scuderi G., Contestabile M.T., Abdolrahimzadeh S., Recupero S.M. Glaucoma management in carotid cavernous fistula. Case Rep Ophthalmol. 2016;7(2):296–302. doi: 10.1159/000446151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris G.J., Rice P.R. Angle closure in carotid-cavernous fistula. Ophthalmology. 1979;86(8):1521–1529. doi: 10.1016/S0161-6420(79)35367-2. [DOI] [PubMed] [Google Scholar]
- 11.Henderson A.D., Miller N.R. Carotid-cavernous fistula: current concepts in aetiology, investigation, and management. Eye (Lond.) 2018;32(2):164–172. doi: 10.1038/EYE.2017.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zanaty M., Chalouhi N., Tjoumakaris S.I., Hasan D., Rosenwasser R.H., Jabbour P. Endovascular treatment of carotid-cavernous fistulas. Neurosurg. Clin. N. Am. 2014;25(3):551–563. doi: 10.1016/J.NEC.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Sohrabi C., Mathew G., Maria N., Kerwan A., Franchi T., Agha R.A. The SCARE 2023 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surg Lond Engl. 2023;109(5):1136. doi: 10.1097/JS9.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller N.R. Diagnosis and management of dural carotid–cavernous sinus fistulas. Neurosurg. Focus. 2007;23(5):E13. doi: 10.3171/foc-07/11/e13. [DOI] [PubMed] [Google Scholar]
- 15.Korkmazer B., Kocak B., Tureci E., Islak C., Kocer N., Kizilkilic O. Endovascular treatment of carotid cavernous sinus fistula: a systematic review. World J. Radiol. 2013;5(4):143. doi: 10.4329/WJR.V5.I4.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis J.A., Goldstein H., Connolly E.S., Meyers P.M. Carotid-cavernous fistulas. Neurosurg. Focus. 2012;32:5. doi: 10.3171/2012.2.FOCUS1223. [DOI] [PubMed] [Google Scholar]
- 17.Liu H., Wang Y., Chen Y., Cheng J., Yip P., Tu Y. Long-term clinical outcome of spontaneous carotid cavernous sinus fistulae supplied by dural branches of the internal carotid artery. Neuroradiology. 2001;43(11):1007–1014. doi: 10.1007/S002340100621. [DOI] [PubMed] [Google Scholar]
- 18.Parikh R.S., Desai S., Kothari K. Dilated episcleral veins with secondary open angle glaucoma. Indian J. Ophthalmol. 2011;59(2):153. doi: 10.4103/0301-4738.77045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rong X., Li M. Advanced glaucoma secondary to bilateral idiopathic dilated episcleral veins - a case report. BMC Ophthalmol. 2018;18(1) doi: 10.1186/S12886-018-0892-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nassr M.A., Morris C.L., Netland P.A., Karcioglu Z.A. Intraocular pressure change in orbital disease. Surv. Ophthalmol. 2009;54(5):519–544. doi: 10.1016/J.SURVOPHTHAL.2009.02.023. [DOI] [PubMed] [Google Scholar]
- 21.Allingham P. In: Shields’ Textbook of Glaucoma. 6th ed. Allingham R.R., Damji K.F., Freedman S., Moroi S.E., Rhee D.J., Shields M.B., editors. Lippincott Williams & Wilkins; 2011. Glaucomas associated with elevated episcleral venous pressure. [Google Scholar]
- 22.Concepcion P.A., FlorCruz V. Increased intraocular pressure in patients with carotid-cavernous fistula seen at a tertiary eye care center. Philippine Academy of Ophthalmology. 2022;47:70–75. [Google Scholar]
- 23.Preechawat P., Narmkerd P., Jiarakongmun P., Poonyathalang A., Pongpech S. Dural carotid cavernous sinus fistula: ocular characteristics, endovascular management and clinical outcome. Medical journal of the Medical Association of Thailand. 2008;91(6):852. [PubMed] [Google Scholar]
- 24.Miller N.R. Dural carotid-cavernous fistulas: epidemiology, clinical presentation, and management. Neurosurg. Clin. N. Am. 2012;23(1):179–192. doi: 10.1016/J.NEC.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Leibovitch I., Modjtahedi S., Duckwiler G.R., Goldberg R.A. Lessons learned from difficult or unsuccessful cannulations of the superior ophthalmic vein in the treatment of cavernous sinus dural fistulas. Ophthalmology. 2006;113(7):1220–1226. doi: 10.1016/J.OPHTHA.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 26.Santos D. dos, Monsignore L.M., Nakiri G.S., Cruz A.A.V., e, Colli BO, Abud DG. Imaging diagnosis of dural and direct cavernous carotid fistulae. Radiol. Bras. 2014;47(4):251–255. doi: 10.1590/0100-3984.2013.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al Saiegh F., Baldassari M.P., Sweid A., et al. Onyx embolization of carotid-cavernous fistulas and its impact on intraocular pressure and recurrence: a case series. Oper Neurosurg (Hagerstown). 2021;20(2):174–182. doi: 10.1093/ONS/OPAA308. [DOI] [PubMed] [Google Scholar]
- 28.Bellows A.R., Chylack L.T., Epstein D.L., Hutchinson B.T. Choroidal effusion during glaucoma surgery in patients with prominent episcleral vessels. Arch. Ophthalmol. 1979;97(3):493–497. doi: 10.1001/ARCHOPHT.1979.01020010243011. [DOI] [PubMed] [Google Scholar]
- 29.Libre P.E. Nonpenetrating filtering surgery and goniopuncture (staged trabeculectomy) for episcleral venous pressure glaucoma. Am J. Ophthalmol. 2003;136(6):1172–1174. doi: 10.1016/S0002-9394(03)00669-X. [DOI] [PubMed] [Google Scholar]
- 30.Aref A.A., Gedde S.J., Budenz D.L. Glaucoma drainage implant surgery. Dev. Ophthalmol. 2017;59:43–52. doi: 10.1159/000458485. [DOI] [PubMed] [Google Scholar]
- 31.Riva I., Roberti G., Oddone F., Konstas A.G.P., Quaranta L. Ahmed glaucoma valve implant: surgical technique and complications. Clin. Ophthalmol. 2017;11:357. doi: 10.2147/OPTH.S104220. [DOI] [PMC free article] [PubMed] [Google Scholar]