Abstract
To generate transcriptionally targeted vectors, tissue-specific elements of the human tyrosinase promoter were exchanged with corresponding viral elements in the Moloney murine leukemia virus long terminal repeat (LTR). From these experiments, a vesicular stomatitis virus type G pseudotyped, hybrid LTR vector that contained three tyrosinase enhancer elements and gave high-level, tightly tissue-specific expression at high titers (3 × 107 CFU/ml) was constructed.
The use of Moloney murine leukemia virus (Mo-MLV)-derived retroviral vectors for in vivo gene therapy has been limited so far by low titers, inactivation by human complement, and the possibility of insertional mutagenesis (12, 31). Despite strategies to overcome these difficulties (6, 17, 39), successful in vivo gene therapy of most genetic diseases also requires the targeted delivery of therapeutic genes to target cells. For example, this is of particular importance in cancer gene therapy for the delivery of suicide genes (16), in which the restriction of gene expression to the malignant cells is required to avoid nonspecific toxicity (25, 33). Several approaches have been considered to target viral vectors to specific tumor cell types (5, 15, 33), including the use of tissue- or tumor cell-specific promoters to regulate expression of therapeutic genes preferentially in malignant cells, without affecting the surrounding normal cells (25). To this end, we have previously described both plasmid and retroviral vectors which incorporate promoter/enhancer elements of the murine tyrosinase gene (27–30). When these regulatory sequences were incorporated as internal promoters, the resulting vectors expressed cytokine genes, or the suicide gene HSVtk, preferentially in murine melanoma cells (29) and were effective in the treatment of both primary and metastatic deposits of B16 melanoma after in vivo delivery (30). However, partial loss of specific gene expression from these vectors was observed due to promoter interference by the strong promoter/enhancer elements in the U3 region of the viral long terminal repeat (LTR) (8) and also by an additional simian virus 40 (SV40) internal promoter which directed expression of the marker gene (29). Therefore, to restore the specificity of the tyrosinase promoter elements and to overcome the loss of titers associated with the use of internal promoters, we reasoned that we could simplify these vectors by replacing the viral enhancer elements with tissue-specific regulatory sequences.
The U3 region in the LTR contains viral transcriptional control elements, including the TATA sequence between −23 and −30, the CCAAT element at −78, and repeated CGCTT motifs, which contribute to basal promoter activity (13). Further upstream are a GC-rich region and a sequence of 75 bp which is present as a tandem repeat and provides viral enhancer activity. Both elements are required for maximal LTR activity (13). At the 5′ end of the LTR there is a negative upstream control element that can inhibit LTR promoter activity under the appropriate conditions (11). The U3 region in the LTR of murine retroviruses acts as a constitutive enhancer/promoter in many different cells. However, in cases in which viral strains show differential tropisms for different cell types it is often the viral enhancer which confers this property (3, 20, 23). In addition, this differential cell tropism can be transferred between viral strains as shown by experiments in which Mo-MLV retroviral vectors engineered to contain the SL3-3 U3 region generated high levels of gene expression in transduced human T cells (7). In a similar strategy, we replaced the viral enhancer in the U3 region with either 2.5 or 0.7 kbp of the murine tyrosinase promoter. In those constructs, the promoter interference effects seen with the internal promoters were abolished but the titers obtained were low (103 PFU/ml). We observed that, although high levels of specific gene expression required the use of the longer promoter sequence (2.5 kbp), the reduction in viral titers correlated with the size of the inserted promoter, probably reflecting decreased efficiency of reverse transcription (26).
It has recently been reported that a 380-bp fragment of the human tyrosinase promoter contains the sequences required for cell type-specific expression in melanoma cells (1). In addition, an enhancer element, located between −2.0 and −1.8 kb upstream from the transcriptional start site of the human tyrosinase promoter, has also been identified (22, 24). The latter sequence contains a tyrosinase distal element (TDE) (positions −1861 to −1842), which enhances tissue-specific transcription of the human tyrosinase gene, via binding of the microphthalmia-associated transcription factor (38). Therefore, we reasoned that the use of these small defined elements of the human tyrosinase promoter/enhancer sequences would overcome the problems associated with loss of titer due to the large fragments of the murine promoter. In addition, several reports have shown that expression from heterologous promoters incorporated into retroviral vectors can be shut off with time (18, 19). We have seen gradual loss of expression from the murine tyrosinase promoter in human melanoma cells, but less so in murine cells (24a). Therefore, it seemed likely that optimal levels of expression in human melanomas would require the use of the human promoter/enhancer sequences. Finally, by using these small defined cellular elements we could investigate how their interchange with the similarly sized viral promoter and enhancer elements could be best achieved to generate high-level, targeted expression from a hybrid LTR while retaining the integrity of retroviral functions such as reverse transcription.
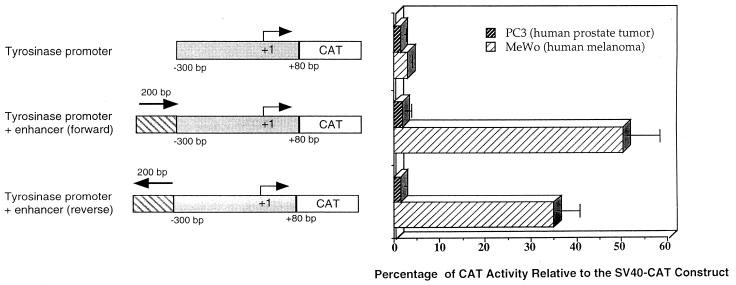
Initially, we studied the ability of the 380-bp fragment of the human tyrosinase promoter to drive chloramphenicol acetyltransferase (CAT) gene expression either alone or in combination with the 200-bp tyrosinase enhancer element. (Fig. 1). No significant CAT activity was detected after transient transfection of human melanoma (MeWo) or prostate (PC3) cell lines with pHT300-CAT (2 μg) containing 380 bp of the human tyrosinase promoter upstream of the CAT gene (1) (Fig. 1). Therefore, we cloned the enhancer element, located between −2.0 and −1.8 kbp from the transcriptional start site of the human tyrosinase promoter (22), upstream of the 380-bp minimal promoter. This enhancer/promoter construct, in either orientation relative to the promoter, directed high levels of CAT expression in MeWo cells (at about 50% of that of the control SV40-CAT plasmid) but not in the prostatic cell line (Fig. 1). Therefore, we decided to incorporate these sequences into hybrid LTRs to generate transcriptionally targeted retroviral vectors.
FIG. 1.
Tissue-specific activity of the human tyrosinase promoter/enhancer elements. The plasmids containing the CAT reporter gene under the control of regions of the tyrosinase promoter are shown to the left. The direction of transcription is indicated by a bent arrow, and the transcription initiation site is numbered +1. The data shown to the right are the means + standard deviations (error bars) of three independent experiments.
Therefore, a range of modifications were engineered into the shuttle vector pSK, which contains a single copy of the wild-type Mo-MLV 3′ LTR (WT) (a kind gift of R. Jaggar, Oxford, United Kingdom) (Fig. 2). As a negative control to assess basal promoter activity, the viral enhancer was deleted between the NheI and XbaI restriction sites to generate dLTR. (All of the vectors in which the viral enhancer was deleted lack the negative upstream control element). The modified LTRs were cloned into the parental retroviral vector plasmid pBabe Puro granulocyte-macrophage colony-stimulating factor (GM-CSF) (Fig. 2) and transfected into the AM12 packaging cell line (14), and supernatants from puromycin-resistant pools of producer cells were used to transduce melanoma or nonmelanoma target cells. The only vector in which a significant reduction in titer was observed relative to the wild-type pBabe Puro GM-CSF vector (4 × 105 CFU/ml) was TPE+80 (4 × 104 CFU/ml), in which the viral R region was interrupted by the insertion of 80 bp of tyrosinase sequence. Representative titers on MeWo and HeLa cells are shown in Fig. 2.
FIG. 2.
Mo-MLV-tyrosinase hybrid LTRs. Modifications to the 3′ LTR of the parental retroviral vector pBabe Puro GM-CSF were carried out as described in the text. The structures of the hybrid LTRs are shown with the appropriate cloning sites. The direction of transcription is indicated by a bent arrow, and the transcription initiation site is numbered +1. Viral titers from AM12 pooled populations are expressed as puromycin-resistant CFU per milliliter. Full details of sequences are available on request.
Modifications to the 3′ LTR of the retroviral vector plasmid form the template for production of the 5′ LTR in the proviral DNA in the infected target cell following viral packaging, reverse transcription, and integration (34). Therefore, the hybrid LTRs direct transcription of the murine GM-CSF gene in retrovirally infected cells (Fig. 2). Tissue-specific gene expression from each hybrid LTR vector was determined by assaying the production of GM-CSF secreted by 5 × 105 cells from pooled populations of infected cells by enzyme-linked immunosorbent assay (ELISA) with antibody pairs obtained from Pharmingen (Cambridge BioScience, Cambridge, United Kingdom).
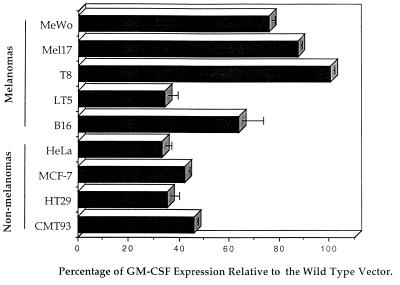
In the construct TE, the 270-bp viral enhancer was replaced by the 200-bp tyrosinase enhancer while retaining the Mo-MLV viral promoter sequences. This straight exchange of enhancers produced significantly enhanced levels of GM-CSF production relative to the same cells transduced with the control vector with the enhancer deleted (dLTR) (between 3.5- and 6-fold increases) (Fig. 3) in four of the five transduced melanoma cell lines. The exception, LT5, is a human amelanotic cell line which we have previously shown has very low levels of gene expression from the mouse tyrosinase promoter (27), possibly due to defects in the melanin biosynthetic pathway. Otherwise, the lowest level of GM-CSF production was in the murine melanoma line B16, consistent with our hypothesis that human elements are best for expression in human cells. In contrast, GM-CSF production in nonmelanoma cells was not significantly different (0- to 0.5-fold increase) relative to the same cells transduced with the vector with the LTR deleted (Fig. 3). These data show that tissue specificity can be engineered into the LTR of a retroviral vector, while maintaining viral titer, by replacing the viral enhancer with the tyrosinase enhancer.
FIG. 3.
GM-CSF production from melanoma and nonmelanoma cell lines following infection with the TE hybrid LTR retroviral vector. Lines of melanoma and nonmelanoma cells were transduced with the TE and dLTR vectors. After selection in puromycin, the amount of GM-CSF released from 106 cells in 48 h was measured by ELISA. MeWo, Mel17, and T8 are human melanoma cell lines. LT5 is an amelanotic human melanoma cell line. B16 is a murine melanoma cell line GM-CSF levels from transduced cells are expressed as fold induction relative to cells transduced with the dLTR vector (with the viral enhancer deleted). The data shown are representative of three separate experiments. Error bars, standard deviations.
Despite the introduction of tissue specificity into the viral LTR, the levels of gene expression generated with the TE vector in all cell lines were comparatively low (10-fold reduction) relative to those with the wild-type vector (WT) (data not shown), reflecting the weakness of the tyrosinase enhancer relative to viral enhancers. To investigate the effects of combining enhancer elements, WT+TE (forward) and WT+TE (reverse) were constructed such that the tyrosinase enhancer was inserted in either orientation next to the viral enhancer, without any deletion of viral sequences from the LTR (Fig. 2). Melanoma cells transduced with WT+TE (forward) produced GM-CSF at levels between 60 and 100% of those with the wild-type vector (WT) (Fig. 4). In contrast, in nonmelanoma cells, the secreted GM-CSF levels were typically only 45% of those with the wild-type vector. Similar results were obtained when the tyrosinase enhancer was placed in the reverse orientation [WT+TE (reverse)] (data not shown). These results suggest that the tyrosinase enhancer has a weak suppressive effect over the strong viral enhancer in transduced nonmelanoma cells, consistent with the results of Ferrari and coworkers (9), who found that the muscle creatinine kinase enhancer can partially repress the viral enhancer in nonmyogenic cells. However, in the case of the tyrosinase enhancer here, it seems that the viral enhancer is clearly the more powerful of the two elements and its influence predominates in the enhancer combination vectors.
FIG. 4.
GM-CSF production from melanoma and nonmelanoma cell lines following infection with the WT+TE (forward) hybrid LTR retroviral vector in which the tyrosinase enhancer is added to an intact retroviral LTR. Panels of melanoma and nonmelanoma cells were transduced with the WT+TE (forward) vector. After selection in puromycin, the amount of GM-CSF released from 106 cells in 48 h was measured by ELISA. The levels of GM-CSF from transduced cells are expressed as percentages relative to the same cell lines transduced with the wild-type retroviral vector (WT). The data shown are representative of three separate experiments. Error bars, standard deviations.
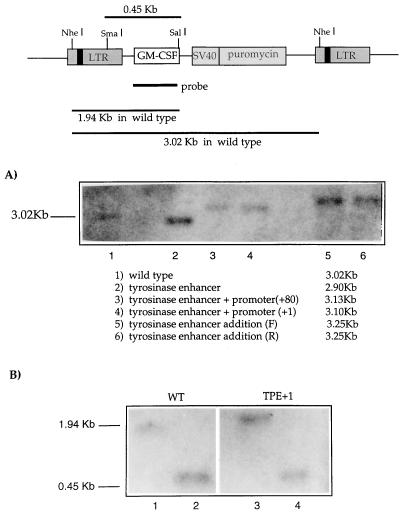
Southern blotting and PCR were used to look for the presence of rearranged proviruses in cells transduced with the different hybrid LTR vectors. Genomic DNA (5 μg) from pooled populations of puromycin-resistant HeLa cells was digested with NheI and hybridized with a 32P-labelled murine GM-CSF probe. For each vector, bands of the sizes predicted for each of the intact proviruses were obtained in both infected HeLa (Fig. 5A) and MeWo (data not shown) cells, with no alternative bands or smears, indicative of proviruses of rearranged structures (Fig. 5A). Similarly, amplification of genomic DNA from transduced HeLa cells, using primers spanning the enhancer/promoter insertions, generated the expected bands for each of the constructs tested, with no evidence of major rearrangements in the LTR (data not shown). These results indicate that for each hybrid LTR vector, the tyrosinase enhancer/promoter elements have been incorporated successfully and stably into the proviral DNA in transduced cells.
FIG. 5.
(A) Hybrid tyrosinase-LTR structures are correctly and stably transferred into the viral 5′ LTR following reverse transcription. The integrity of proviral structure in transduced cells was analyzed by Southern blotting (see text for details). (B) Lack of expression from TPE+1 is not due to increased methylation in the LTR. Genomic DNA prepared from MeWo cells infected with WT or TEP+1 vectors was digested with NheI and then with either SmaI (lanes 2 and 4) or SalI (lanes 1 and 3) as a control. SmaI is a methylation-sensitive restriction enzyme, so in the presence of methylated CpG sequences, this enzyme would not cut or would cut very inefficiently within the LTR compared to SalI. In contrast, a strong band of 0.45 kbp, indicative of SmaI activity, was present in cells transduced with both the WT and TEP+1 retroviruses, suggesting that no major methylation has taken place in this sequence.
In TEP+80 and TEP+1, both the viral enhancer and basal promoter were replaced with the corresponding combination of tyrosinase enhancer and promoter (Fig. 2). To preserve the structure of the promoter required for optimal expression as shown in the plasmid experiments (Fig. 1), TEP+80 retains the first 80 bp downstream of the transcriptional start site cloned into the KpnI site of the LTR, thereby deleting 33 bp of the viral R region. To control for the disruption of the R region in reverse transcription and to preserve the viral transcriptional start site, TEP+1, in which an intact R region is retained, was constructed. Replacement of the viral enhancer/promoter combination with either of the tyrosinase enhancer/promoter fragments (TPE+80 or TPE+1) gave no detectable gene expression in either MeWo or HeLa cell lines (data not shown). This lack of expression was not due to rearrangements of the viral genome, as shown by PCR and Southern blots (data not shown), nor was it due to increased methylation of the provirus in infected cells, since methylation-dependent SmaI digestion of integrated proviruses was not inhibited relative to digestion with a methylation-independent neighboring SalI site (Fig. 5B). It may be, therefore, that the tyrosinase promoter/enhancer combination is too weak to function efficiently in the context of the LTR.
The results from the WT+TE vectors suggested that addition of further repressive elements from the tyrosinase enhancer may be beneficial for obtaining high levels of specificity, combined with high levels of gene expression. Hence, we constructed a synthetic enhancer element containing three copies of the TDE (positions −1861 to −1842; GGAGATCATGTGATGACTTC) responsible for tissue-specific transcription of the human tyrosinase gene (38). In plasmid constructs, this element (3×TDE) conferred high-level, tissue-specific expression (data not shown). Our previous results also suggested that tissue specificity is best incorporated into a hybrid LTR by exchanging viral and cellular enhancer elements but retaining a strong viral promoter. Therefore, we hypothesized that the levels of tissue-specific expression could be increased by including a basal promoter stronger than the Mo-MLV promoter, which is relatively weak in human cells.
Hence, we constructed three new vectors, in which we replaced the Mo-MLV promoter with the basal promoter of SV40 and either retained the Mo-MLV enhancer (SV40) or replaced the Mo-MLV enhancer with the TDE (3×TDE-SV40-1) or added the TDE to the LTR still containing the Mo-MLV enhancer (3×TDE-SV40-2) (Fig. 6A). Viral stocks from all three of these vectors had reduced titers relative to wild-type LTR vectors (Fig. 6), consistent with the loss of a small part of the R region in the LTR as described above for construct TPE+80. As for constructs WT+TE (forward) and WT+TE (reverse), simple addition of the TDE to the LTR retaining the Mo-MLV enhancer showed only very slight suppression of expression in nonmelanoma cells (Fig. 6B). However, when cells were infected with 3×TDE-SV40-1, levels of GM-CSF expression approaching that of the WT vector were obtained from infected melanoma cells, but no detectable expression was observed in infected HeLa cells (Fig. 6B). (Southern blotting, PCR, and subsequent sequencing of the hybrid LTRs confirmed that the triplet TDEs were intact in both the melanoma and nonmelanoma cells following infection.) These data confirm that insertion of cellular and heterologous viral elements within the viral LTR can generate targeted retroviral vectors which confer highly tissue-specific expression.
FIG. 6.
A double-hybrid LTR vector containing both heterologous viral and cellular regulatory sequences can confer tightly tissue-specific gene expression. (A) Double-hybrid LTR vectors were constructed as shown, by incorporating the SV40 promoter in place of the Mo-MLV promoter and using the restriction sites as indicated. Viral titers from AM12 pooled populations are expressed as puromycin-resistant CFU per milliliter. Full details and sequences of the constructs are available on request. (B) GM-CSF production from melanoma and nonmelanoma cell lines following infection with double-hybrid LTR vectors. MeWo and HeLa cells were transduced with the double-hybrid LTR vectors. After selection in puromycin, the amount of GM-CSF released from 106 cells in 48 h was measured by ELISA. The levels of GM-CSF from transduced cells are expressed as percentages relative to the same cell lines transduced with the wild-type retroviral vector (WT). The data shown are representative of three separate experiments. Error bars, standard deviations.
The titers of the 3×TDE-SV40-1 vector from pooled populations of transfected AM12 cells are too low to be realistically considered for use in clinical trials (5 × 103 CFU/ml). Based on the data from the TPE+1 and TPE+80 vectors, we predict that restoring to the 3×TDE-SV40-1 vector the Mo-MLV bases which have been removed from R would restore the titer of this vector to near wild-type levels. Moreover, further deletional analysis of the SV40 promoter sequences used in this vector may further improve viral titers and/or levels of tissue-specific expression. These experiments are currently under way in the laboratory. In the meantime, we cloned individual producer clones of the AM12 transfectants and were able to isolate clones in which the titer was increased by at least an order of magnitude. However, to be of value for in vivo gene delivery, we prepared viral stocks by cotransfection of the producer cells by the 3×TDE-SV40-1 vector and the vesicular stomatitis virus type G envelope gene as described previously (4). The pseudotyping of the Mo-MLV core particles allows improved concentration of viral particles, and we were able to generate titers of 3 × 107 CFU/ml. Consistent with our previous results, however, this titer was reduced relative to that with the wild-type retroviral vector (5 × 108 CFU/ml), although it is high enough to justify use for in vivo delivery.
In the current absence of effective methods to target the envelopes of retroviral particles specifically to melanoma cells, the first generation of targeted retroviral vectors will probably have to be transcriptionally targeted. Recently, complementary studies using the tyrosinase enhancer to target tissue specific gene expression in adenoviral vectors have been reported (24). The high titers achievable with adenoviral vectors make these attractive candidates for in vivo delivery, but the immunological properties of the different vector systems (35–37) may favor the use of one over the other, depending upon the protocol used. Therefore, it will be of great interest to compare transcriptionally targeted retroviral and adenoviral vectors in in vivo protocols. In addition, to overcome the limitations of titers of currently available recombinant viral delivery systems, the use of replication-competent vectors has been proposed (21). Any such consideration would require several combined, effective targeting strategies to restrict the infections to target cells (32). In this respect, the generation of replicating viruses with altered transcriptional properties has already been described (10). Although development of such targeted replicating vectors remains a prospect for the future, the use of replicating vectors for the gene therapy of cancer is already in clinical trials (2).
In summary, we have described a series of Mo-MLV-based vectors in which the viral enhancer and/or promoter sequences have been interchanged with the corresponding minimal cellular regulatory elements from the human tyrosinase promoter. These retroviral particles were produced at high titers (105 CFU/mL), showing that swapping of viral LTR domains with functionally equivalent cellular sequences does not adversely affect reverse transcription. Although, some vectors demonstrated complete loss of expression from the hybrid LTR in infected cells, cells transduced with vectors in which the 270-bp viral enhancer was replaced with a 200-bp fragment of the human tyrosinase enhancer expressed GM-CSF in the expected tissue-specific manner. Since this vector gave relatively low levels of expression, we also constructed vectors in which tandem repeats of the core element of the tyrosinase enhancer element replaced the viral enhancer and in which the Mo-MLV promoter was replaced by the stronger SV40 viral promoter. The vector in which the SV40-TDE cassette replaced both the Mo-MLV enhancer and promoter generated levels of GM-CSF at about 60% of those with the wild-type LTR in melanoma cells but gave no detectable expression in nonmelanoma cells, significantly better than the simple enhancer exchange vector (TE). Moreover, using a packaging protocol in which retroviral core particles were pseudotyped with the vesicular stomatitis virus type G envelope protein, we generated vector stocks, at relatively high titers, which would be suitable for direct in vivo delivery. Therefore, we have shown that it is possible to engineer tissue specificity into the viral LTR by the simple exchange of Mo-MLV regulatory sequences with both heterologous cellular and viral sequences and are currently developing these targeted retroviral vectors for protocols for the in vivo gene therapy of melanoma.
Acknowledgments
This work was supported by the Imperial Cancer Research Fund (R.G.V.) and I.R.H.), The Richard Dimbleby Cancer Fund (I.R.H.), and CONICIT (R.M.D.).
REFERENCES
- 1.Bentley N J, Eisen T, Goding R C. Melanocyte-specific expression of the human tyrosinase promoter: activation by the microphthalmia gene product and role of the initiator. Mol Cell Biol. 1994;14:7996–8006. doi: 10.1128/mcb.14.12.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bischoff J, Kirn D H, Williams A, Heise C, Horn S, Muna M, Ng L, Nye J, Sampson-Johannes A, Fattaey A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 3.Boral A L, Okenquist S A, Lenz J. Identification of the SL3-3 virus enhancer core as a T-lymphoma cell-specific element. J Virol. 1989;63:76–84. doi: 10.1128/jvi.63.1.76-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns J C, Friedmann T, Driever W, Burrascano M, Yee J K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosset F-L, Russell S J. Targeting retrovirus entry. Gene Ther. 1996;3:946–956. [PubMed] [Google Scholar]
- 6.Cosset F-L, Takeuchi Y, Battini J-L, Weiss R A, Collins M K L. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J Virol. 1995;69:7430–7436. doi: 10.1128/jvi.69.12.7430-7436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couture L A, Mullen C A, Morgan R A. Retroviral vectors containing chimeric promoter/enhancer elements exhibit cell-type-specific gene expression. Hum Gene Ther. 1994;5:667–677. doi: 10.1089/hum.1994.5.6-667. [DOI] [PubMed] [Google Scholar]
- 8.Emerman M, Temin H M. Genes with promoters in retroviral vectors can be independently suppressed by an epigenic mechanism. Cell. 1984;39:459–467. [PubMed] [Google Scholar]
- 9.Ferrari G, Salvatori G, Rossi C, Cossu G, Mavilio F. A retroviral vector containing a muscle-specific enhancer drives gene expression only in differentiated muscle fibers. Hum Gene Ther. 1995;6:733–742. doi: 10.1089/hum.1995.6.6-733. [DOI] [PubMed] [Google Scholar]
- 10.Feuer G, Fan H. Substitution of murine transthyretin (prealbumin) regulatory sequences into the Moloney murine leukemia virus long terminal repeat yields infectious virus with altered biological properties. J Virol. 1990;64:6130–6140. doi: 10.1128/jvi.64.12.6130-6140.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flanagan J R, Krieg A M, Max E E, Khan A S. Negative control region at the 5′ end of the murine leukemia virus long terminal repeats. Mol Cell Biol. 1989;8:739–746. doi: 10.1128/mcb.9.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jolly D. Viral vector systems for gene therapy. Cancer Gene Ther. 1994;1:51–64. [PubMed] [Google Scholar]
- 13.Majors J. Retroviruses, strategies of replication. The structure and function of retroviral long terminal repeats. In: Swanstrom R, Vogt P K, editors. Berlin, Germany: Springer-Verlag; 1990. pp. 49–92. [DOI] [PubMed] [Google Scholar]
- 14.Markowitz D, Goff S, Bank A. Construction and use of a safe and efficient amphotropic packaging cell line. Virology. 1988;167:400–406. [PubMed] [Google Scholar]
- 15.Miller N, Vile R G. Targeted vectors for gene therapy. FASEB J. 1995;9:190–199. doi: 10.1096/fasebj.9.2.7781922. [DOI] [PubMed] [Google Scholar]
- 16.Moolten F L. Drug sensitivity (“suicide”) genes for selective cancer chemotherapy. Cancer Gene Therapy. 1994;1:279–287. [PubMed] [Google Scholar]
- 17.Ory D S, Neugeboren B A, Mulligan R C. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci USA. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer T D, Rosman G J, Osborne W R A, Miller A D. Genetically modified skin fibroblasts persist long after transplantation but gradually inactivate introduced genes. Proc Natl Acad Sci USA. 1991;88:1330–1334. doi: 10.1073/pnas.88.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richards C A, Huber B E. Generation of a transgenic model for retrovirus-mediated gene therapy for hepatocellular carcinoma is thwarted by the lack of transgene expression. Hum Gene Therapy. 1993;4:143–150. doi: 10.1089/hum.1993.4.2-143. [DOI] [PubMed] [Google Scholar]
- 20.Rosen C A, Haseltine W A, Lenz J, Ruprecht R, Cloyd M W. Tissue selectivity of murine leukemia virus infection is determined by long terminal repeat sequences. J Virol. 1985;55:862–866. doi: 10.1128/jvi.55.3.862-866.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell S J. Replicating vectors for cancer therapy: a question of strategy. Semin Cancer Biol. 1994;5:437–443. [PubMed] [Google Scholar]
- 22.Shibata K, Muraosa Y, Tomita Y, Tagami H, Shibahara S. Identification of a cis-acting element that enhances the pigment cell-specific expression of the human tyrosinase gene. J Biol Chem. 1992;267:20584–20588. [PubMed] [Google Scholar]
- 23.Short M K, Okenquist S A, Lenz J. Correlation of leukemogenic potential of murine retroviruses with transcriptional tissue preference of the viral long terminal repeats. J Virol. 1987;61:1067–1072. doi: 10.1128/jvi.61.4.1067-1072.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siders W M, Halloran P J, Fenton R G. Transcriptional targeting of recombinant adenoviruses to human and murine melanoma cells. Cancer Res. 1996;56:5638–5646. [PubMed] [Google Scholar]
- 24a.Vile, R. G. Unpublished observations.
- 25.Vile R G. Tumour specific gene expression. Semin Cancer Biol. 1994;5:429–436. [PubMed] [Google Scholar]
- 26.Vile R G, Diaz R M, Miller N, Mitchell S, Tuszynski A, Russell S J. Tissue specific gene expression from Mo-MLV retroviral vectors with hybrid LTRs containing the murine tyrosinase enhancer/promoter. Virology. 1995;214:307–313. doi: 10.1006/viro.1995.9923. [DOI] [PubMed] [Google Scholar]
- 27.Vile R G, Hart I R. In vitro and in vivo targeting of gene expression to melanoma cells. Cancer Res. 1993;53:962–967. [PubMed] [Google Scholar]
- 28.Vile R G, Hart I R. Use of tissue-specific expression of the herpes simplex virus thymidine kinase gene to inhibit growth of established murine melanomas following direct intratumoral injection of DNA. Cancer Res. 1993;53:3860–3864. [PubMed] [Google Scholar]
- 29.Vile R G, Miller N, Chernajovsky Y, Hart I R. A comparison of the properties of different retroviral vectors containing the murine tyrosinase promoter to achieve transcriptionally targeted expression of the HSVtk or IL-2 genes. Gene Ther. 1994;1:307–316. [PubMed] [Google Scholar]
- 30.Vile R G, Nelson J A, Castleden S, Chong H, Hart I R. Systemic gene therapy of murine melanoma using tissue specific expression of the HSVtk gene involves an immune component. Cancer Res. 1994;54:6228–6234. [PubMed] [Google Scholar]
- 31.Vile R G, Russell S J. Retroviruses as vectors. Br Med Bull. 1995;51:12–30. doi: 10.1093/oxfordjournals.bmb.a072941. [DOI] [PubMed] [Google Scholar]
- 32.Vile, R. G., K. Sunassee, and R. M. Diaz. Strategies for achieving multiple layers of selectivity in gene therapy. Mol. Med. Today, in press. [DOI] [PubMed]
- 33.Walther W, Stein U. Targeted vectors for gene therapy of cancer and retroviral infections. Mol Biotechnol. 1996;6:267–286. doi: 10.1007/BF02761707. [DOI] [PubMed] [Google Scholar]
- 34.Whitcomb J M, Hughes S H. Retroviral reverse transcription and integration: progress and problems. Annu Rev Cell Biol. 1992;8:275–306. doi: 10.1146/annurev.cb.08.110192.001423. [DOI] [PubMed] [Google Scholar]
- 35.Wilson C, Kay M A. Immunomodulation to enhance gene therapy. Nat Med. 1995;1:890–893. doi: 10.1038/nm0995-887. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Nunes F A, Berencsi K, Furth E E, Gonczol E, Wilson J M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y, Trinchieri G, Wilson J M. Recombinant IL-12 prevents formation of blocking IgA antibodies to recombinant adenovirus and allows repeated gene therapy to mouse lung. Nat Med. 1995;1:890–893. doi: 10.1038/nm0995-890. [DOI] [PubMed] [Google Scholar]
- 38.Yasumoto K-I, Yokoyama K, Shibata K, Tomita Y, Shibahara S. Microphthalmia-associated transcription factor as a regulator for melanocyte-specific transcription of the human tyrosinase gene. Mol Cell Biol. 1994;14:8058–8070. doi: 10.1128/mcb.14.12.8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu S-F, Von Ruden T, Kantoff P W, Garber C, Seiberg M, Ruther U, French Anderson W, Wagner E F, Gilboa E. Self inactivating retroviral vectors designed for transfer of whole genes into mammalian cells. Proc Natl Acad Sci USA. 1986;83:3194–3198. doi: 10.1073/pnas.83.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]