Abstract
Background: Pro-inflammatory cytokines are implicated in depression caused by both environmental- and alcohol-induced stress. The purpose of the study was to investigate the cytokine levels in serum and hippocampus following induction of depression-like behaviors (DLB) by either forced swimming test (FST) or ethanol-induced DLB (EID). We also investigated the effect of prior administration of antidepressant drug fluoxetine on cytokines in animals exposed to both models of DLB. Methods: Animals were pretreated with fluoxetine before inducing DLB, while DLB was induced in some animals using FST and ethanol in different groups of rats without fluoxetine pretreatment. The ELISA was used to detect changes in cytokine (IL-1β, IL-6, and TNF-α) levels in serum and hippocampus. Results: The mean levels of IL-1β and IL-6 measured in serum and hippocampus were significantly higher in FST and EID models when compared to the control group. The serum concentrations of IL-1β and IL-6 were significantly reduced in animals pre-treated with 5 mg/kg and 10 mg/kg of fluoxetine in both FST and EID models when compared to the untreated FST and EID groups respectively. Conclusions: In conclusion, both environment and alcohol can induce stress and DLB in rats with similar intensity, and their mechanisms of DLB induction involve activation of pro-inflammatory cytokines. Moreover, fluoxetine can prevent stress-induced inflammation in models of DLB.
Keywords: Stressors, depression, fluoxetine, hippocampus, inflammation, cytokines
Introduction
Cytokines like IL-1. IL-6, and IFN-γ have been shown in human and experimental animals to produce behavioral changes and symptoms similar to those found in depression, such as anorexia, inability to feel pleasure, withdrawal from social situations, weight loss, irritability, lack of energy, and sleep disorders1–3 Depression is a significant global public health concern with over 268 million people of all ages living with the disease. 4 Depression is a mental disorder that presents with low mood, loss of interest or pleasure, feelings of guilt or low self-worth, disturbed sleep, appetite, low energy, poor concentration and suicidal attempts. 5 High rates of suicidal attempts and tragic fatality associated with the loss of about 850,000 lives every year have been reported to be related to severe depression.6,7 Although depression affects millions, limited studies have been conducted on the prophylactic approach to its treatment. Several factors precipitate depressive behavior from physical, emotional or environmental (external) stressors such as fatigue, occupational hazards, loss of loved ones or internal stressors, like diseases, inflammation8–10 and alcohol especially in genetically-predisposed individuals. 11
Ethanol increases levels of reactive oxygen species (ROS), which diminish cellular detoxification and repair capacity causing oxidative stress.12,13 Oxidative stress is an imbalance between the cellular production of ROS and the counteracting antioxidant mechanisms, which leads to the damage of cellular macromolecules. 14 The ROS generated in oxidative stress following alcohol intake include abnormal malondialdehyde (MDA), 8-F2-isoprostane (8-iso-PGF2α) levels, protein carbonyl (PC), 8-hydroxy-2-deoxyguanosine (8-OHdG) and 8-oxo-7, 8-dihydroguanosine (8-oxoGuo).15,16 Oxidative stress activates the immune-inflammatory pathways where pro-inflammatory cytokines are released.1,16 Pro-inflammatory cytokines; Interleukin (IL) −1β, IL-6 and tumor necrosis factor (TNF) α, are associated with the pathogenesis of depression. 17
It has also been reported that psychological stress can translate into immune system activation and release of pro-inflammatory cytokines, through a process termed “sterile inflammation”.18,19 In sterile inflammation, the immune system can detect danger signals in the absence of a pathogen through the release of danger-associated molecular patterns (DAMPs) and microbial-associated molecular patterns (MAMPs).20,21 The DAMPS are released during stress and elicit an immune response through the nucleotide-binding oligomerisation (NOD)-, leucine-rich repeat (LRR)- and pyrin domain-containing protein 3 (NLRP3) inflammasome.20,22 On the other hand, when MAMPs are released, they activate NF-κB, which in turn stimulates the release of pro-IL-1β. 20 The DAMPs stimulate the inflammasome to activate caspase-1, which cleaves pro-IL-1beta into its mature IL-1β.21,22 Active IL-1β stimulates the synthesis of IL-6 and TNF-α to potentiate its effects. 20
Ethanol induces depression by causing oxidative stress. 13 Alcohol-induced oxidative stress is mediated by cellular stress proteins called heat shock proteins (HSPs). 22 HSPs, particularly HSP 70 (also known as hsp72) and HSP 90, play a prominent role in inflammatory responses through their interaction with the immune signaling pathways. 23 HSP 90 activity is required for constitutive and inducible IκB kinase (IKK) and NF-κB activation, and increased pro-inflammatory cytokine production whereas HSP 70 inhibits intranuclear accumulation of NF-κB and prevents amplification of the inflammatory response. 23 Despite evidence that IL-1β, IL-6 and TNF-α play a pivotal role during the pathogenesis of depression, limited information is available on variation in their levels when depression is induced by environmental stressors or ethanol.
Fluoxetine is a selective serotonin reuptake inhibitor that is commonly prescribed for the treatment of major depression due to its safer profile, few side effects, and greater tolerability. Its neuroprotective function against microglial activation due to neurotoxicity in neurons 24 and anti-oxidant property against stress-induced oxidative cell damage25,26 have been reported. A possible antidepressant mechanism of fluoxetine is by decreasing depression-associated elevation of cytokines, e.g. IL-1β, IL-6, and TNF-α. 27 However, it has been reported that fluoxetine increases inflammatory cytokines and inflammasome activities in an enriched condition while decreasing these parameters in stressful conditions,28,29 suggesting that a pro- or anti-inflammatory effect of fluoxetine is possible, depending on the prevailing condition.
It is not presently understood if fluoxetine can prevent or ameliorate inflammation in either the environmental- or alcohol-induced stress model of depression. In this study, we compared serum and hippocampal cytokine levels in environmental- and alcohol-induced stress models of depression-like behaviors (DLB) in Wistar rats. Also, we investigated if pre-treatment with fluoxetine will prevent a DLB-induced increase in inflammation in rats. Information from this study will enable us to know if fluoxetine can prevent inflammatory response to stress-induced DLB. This study is the first to examine if fluoxetine will prevent an increase in pro-inflammatory cytokines induced by different stress models of DLB. We hypothesize that fluoxetine will abolish the stress-induced increase in pro-inflammatory cytokines in animal models of DLB.
Materials and methods
Experimental animals
A group of 54 male Wistar rats with median age and weight of 15.4 weeks and 157.3 g respectively were used for the study. Animals were housed under standard laboratory conditions at 24 ± 2°C, exposed to 12-h light-dark cycles (light from 7.00 h to 19:00 h) and had ad libitum access to food and water.
Experimental procedure
Two experiments were done with DLB induced using animal stress models as previously described by Petit-Demouliere et al. 30 and Nagy. 31 Animals were divided into seven groups. Group I served as the control (n = 6 rats), and the rats in this group were not exposed to any method of stress. Groups II - IV (n = 8 rats each) were exposed to DLB using a forced-swimming test (FST) as described by Petit-Demouliere et al. 30 However, groups III and IV were pre-treated with 5 and 10 mg/kg fluoxetine (Medreich PLC, UK) respectively before induction of DLB with FST. Groups V – VII were exposed to DLB using ethanol in different groups of rats as described by Nagy 31 respectively. Similarly, groups VI and VI were pre-treated with 5 and 10 mg/kg fluoxetine respectively before induction of DLB with ethanol. Inflammatory cytokine levels (IL 1β, IL 6 and TNFα) were determined in serum and hippocampus in both models.
External stress – induced DLB
External stress was induced in groups II - IV using FST as elaborately explained by Fraga-Junior. 32 Briefly, FST was done by immersing rats individually in a transparent bucket (30 cm in diameter × 60 cm high) containing water (25°C) to a depth of 20 cm from which they couldn’t escape and couldn’t touch the bottom. The induction process was done in two sessions, the first session was done for 15 min, and the second session was done after 24 h, for five minutes. The movements of the rats were observed in the 5-min session; rats initially swam energetically but gradually became immobile; floating in the water with minimum movements of paws and legs to keep their head above the water level; which are the behavioral signs of DLB for FST. At the end of stress induction, the rats were anesthetized using 60 mg/kg of sodium pentobarbital intraperitoneally, and then placed in the stereotaxic frame.
Ethanol – induced DLB
Ethanol– induced DLB model as elaborately explained by Kesic and Fraga-Junior32,33 was used for this model. Briefly, ethanol (0.07 g/kg) was injected to the rats intraperitoneally and 30 min after the administration, they were anesthetized using 60 mg/kg of sodium pentobarbital, also intraperitoneally, and then placed in the stereotaxic frame. Animals in the control group were similarly anesthetized using 60 mg/kg of sodium pentobarbital, also intraperitoneally, and then placed in the stereotaxic frame but without prior exposure to ethanol.
Administration of fluoxetine in animals
Our choice of two doses of fluoxetine (5 and 10 mg/kg) was inspired by a previous report of Kostadinov et al. (2015) 34 that established dose-dependent alterations in the depression-associated behavior and neural plasticity in female mice following administration of 5 and 10 mg/kg fluoxetine. We adopted these same doses in this study to see whether the possible anti-depression and anti-inflammatory effect of fluoxetine would also show any dose-dependence as previously noted by Kostadinov et al. (2015).
In both experiments, antidepressant, Fluoxetine was administered as a pre-treatment to the animals in groups III, IV, VI and VII.33,35 In the FST model, both treatment doses were administered three times as follows: the first dose was administered immediately after the first FST session, the second dose was given 4 h after the first dose and the last dose was given 30 min before the last session of induction of FST. For the ethanol model, both treatment doses were administered two times as follows: the first and the second doses of fluoxetine were administered at 30-min intervals, i.e. before administration of ethanol.
Sample collection
At the end of both models of stress induction, the rats were anesthetized using 60 mg/kg sodium pentobarbital. Blood from each animal was collected in a non-heparinized bottle through cardiac puncture. Serum was separated from blood by centrifugation at 4°C and 3000 rpm for 15 min and stored −20°C until analysis and each animal was decapitated and its brain was removed quickly from the skull; briefly washed in ice cold saline, and laid on cooled (4°C) metal plate.
The brain was dissected on iced plate to separate the hippocampus, and homogenized, contents washed out with a buffer and stored at −80°C, elaborately explained by. 36 Briefly, the hippocampi tissues were homogenized in ice-cold lysis buffer containing HEPES 25 mM, pH 7.4, 3-[(3-cholamidopropyl) dimethyl-ammonio]1-propanesulfonate 0.1%, MgCl2 5 mM, EDTA 1.3 mM, EGTA 1 mM, 10 μg/mL pepstatin, aprotinin, and leupeptin, and 1 mM PMSF. The homogenates were centrifuged (15 min at 50,000 rpm) and stored at −80°C until analysis. The control animals from Group 1 were similarly treated but without prior exposure to stress.
Cytokine assay
Inflammatory cytokines’ (IL-6, IL-1β and TNF-α) levels in serum and hippocampi tissues were quantified using an antibody capture ELISA, elaborately explained by. 37 For each cytokine, 96-well microplates were coated with recombinant capture antibody, i.e. either IL-6, IL-1β, and TNF-α (BD OptEIA™, UK) in a dilution of 1:250 in the coating buffer (0.1 M sodium bicarbonate pH 9.5). Plates were covered with aluminum foil and incubated overnight at room temperature. Unbound antibody was washed off with 100 µl phosphate buffered saline (PBS), (pH 7.0), containing 0.05% (v/v) Tween 20. The plates were then incubated with the assay samples in triplicate (serum or hippocampus) and incubated for one hour at room temperature. The plates were washed using 200 µl PBS with 0.05% tween-20. After washing three times, the plates were coated with 100 µl of the secondary antibody Biotinylated Anti-human cytokines i.e. IL-10, IL-6, IL-1β, TNF-α, and IFN-γ. After thorough washing, the substrate solution (Tetramethylbenzidine, BD pharmigen™, UK) was added to each well and the reaction was stopped after 30 min by the addition of 2 M H2SO4. Plates were read using a microplate reader (BioTek®, UK) at 450 nm.
Statistical analyses
All data were expressed as mean ± SEM because the data passed the Kolmogorov-Smirnov normality test. Statistical analysis was performed using GraphPad Prism version 6. The effect of DLB on serum and hippocampal cytokine levels and the effect of fluoxetine on pro-inflammatory cytokines were analyzed using a one-way ANOVA, followed by Tukey's multiple comparison test. Similarly, comparisons between the 2 DLB models were done using a T-test. In all cases, differences between groups were considered significant at p < 0.05.
Ethical considerations
The Food and Drug Authority (FDA) and OECD guidelines for testing of chemicals in laboratory animals were strictly adhered to. The study was approved by Mbarara University of Science & Technology, Research Ethics Committee with an approval number 05/03-14. Animals were acclimatized to laboratory conditions and weighed daily before the experiment. All efforts were used to minimize both suffering and the number of animals used. They were treated humanely as per the National Academy of Sciences guidelines and sacrificed under sodium pentobarbital, at the end of the experiment.
Results
To determine whether serum pro-inflammatory cytokines are up-regulated in DLB, the mean concentrations of IL-1β, IL-6 and TNF-α in the serum and hippocampus of rats were compared between the DLB models and the normal control. In order to establish whether pre-treatment of animals with fluoxetine before inducing DLB would have an effect on inflammatory markers, the mean concentrations of IL-1β and IL-6 in the serum and hippocampus of pre-treated and untreated animals were analyzed in the two stress models.
Fluoxetine attenuates stress-induced increase in serum pro-inflammatory cytokines in rats exposed to depression-like behaviors
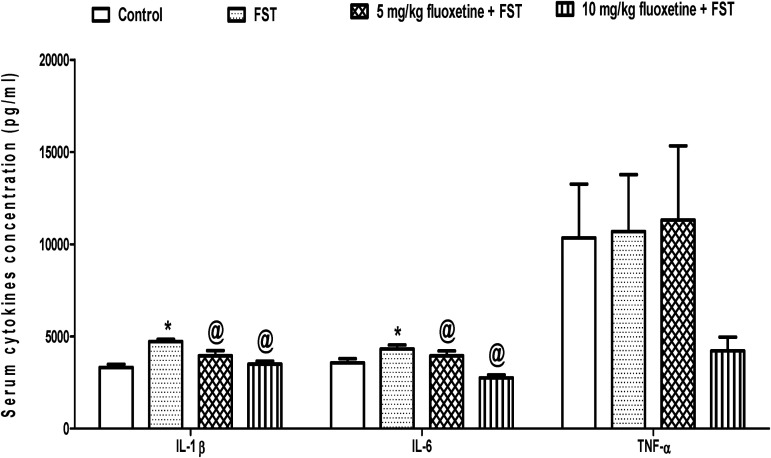
The mean serum concentration of IL-1β was significantly higher (p < 0.05) in FST (4715.4 ± 126.2 pg/ml) than control (3321.1 ± 164.7 pg/ml), but was significantly reduced (p < 0.05) in animals pre-treated with 5 mg/kg (3942.1 ± 288.9 pg/ml) and 10 mg/kg (3500.2 ± 158.4 pg/ml) of fluoxetine when compared to untreated FST group (4715.4 ± 126.2 pg/ml). Similarly, the mean serum concentration of IL-6 was significantly higher (p < 0.05) in FST (4319.1 ± 211.5 pg/ml) than control (3561.3 ± 233.8 pg/ml) but was significantly reduced (p < 0.05) in animals pre-treated with 5 mg/kg (3950.4 ± 260.8 pg/ml) and 10 mg/kg (2755.1 ± 162.3 pg/ml) of fluoxetine when compared to untreated FST group (4319.1 ± 211.5 pg/ml). However, the serum concentration of TNF-α did not change (p > 0.05) across the experimental groups (Figure 1).
Figure 1.
Effect of fluoxetine on serum cytokines in rats exposed to FST-induced depression-like behavior. FST, forced swimming test; *p < 0.05 vs. control; @p < 0.05 vs. FST; IL-1β, interleukin-1 beta; IL-6, interleukin 6; TNF-α, tumor necrotic factor – alpha.
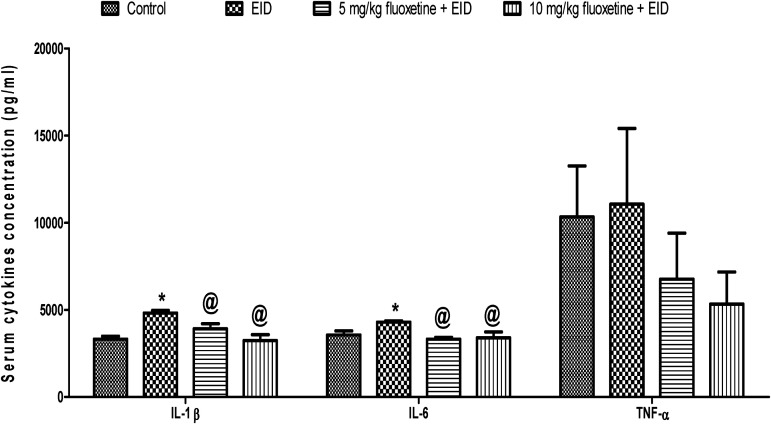
The pattern of the result in the EID model was similar to that of the FST model. The mean serum concentration of IL-1β was significantly higher (p < 0.05) in EID (4819.3 ± 154.8 pg/ml) than control (3321.1 ± 164.7 pg/ml) but was significantly reduced (p < 0.05) in animals pre-treated with 5 mg/kg (3920.4 ± 291.9 pg/ml) and 10 mg/kg (3242.2 ± 331.6 pg/ml) of fluoxetine when compared to untreated EID group (4819.3 ± 154.8 pg/ml). Similarly, the mean serum concentration of IL-6 was significantly higher (p < 0.05) in EID (4997.2 ± 72.9 pg/ml) than control (3561.3 ± 233.8 pg/ml) but was significantly reduced (p < 0.05) in animals pre-treated with 5 mg/kg (3322.4 ± 110.2 pg/ml) and 10 mg/kg (3395.1 ± 345.2 pg/ml) of fluoxetine when compared to untreated EID group (4297.2 ± 72.9 pg/ml). However, the serum concentration of TNF-α did not change (p > 0.05) across the experimental groups (Figure 2).
Figure 2.
Effect of fluoxetine on serum cytokines in rats exposed to EID-induced depression-like behavior. EID, ethanol-induced depression-like behavior model; *p < 0.05 vs. control; @p < 0.05 vs. EID; IL-1β, interleukin-1 beta; IL-6, interleukin 6; TNF-α, tumor necrotic factor – alpha.
Fluoxetine attenuates stress-induced increase in hippocampal pro-inflammatory cytokines in rats exposed to depression-like behaviors
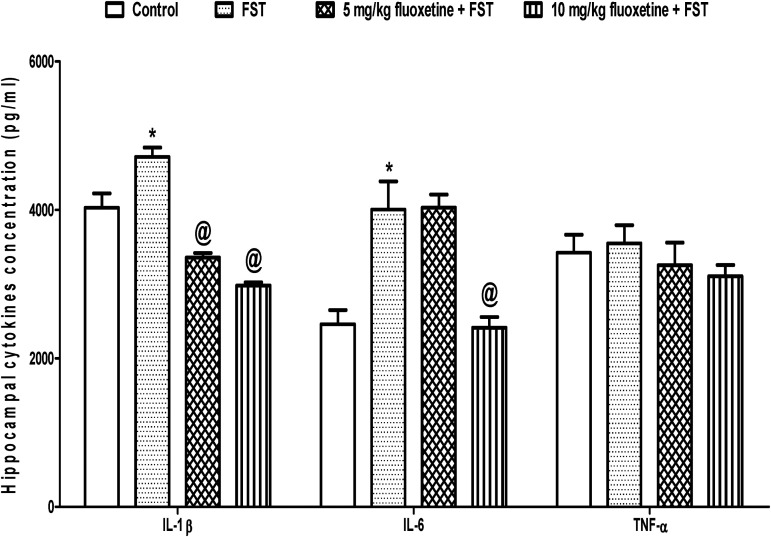
The mean hippocampal concentration of IL-1β was significantly higher (p < 0.05) in FST (4715.0 ± 126.2 pg/ml) than control (4030.2 ± 194.5 pg/ml), but was significantly reduced (p < 0.05) in animals pre-treated with 5 mg/kg (3360.4 ± 59.9 pg/ml) and 10 mg/kg (2982.4 ± 42.9 pg/ml) of fluoxetine when compared to untreated FST group (4715.0 ± 126.2 pg/ml). Similarly, the mean hippocampal concentration of IL-6 was significantly higher (p < 0.05) in FST (4005.4 ± 380.7 pg/ml) than control (2460.3 ± 190.3 pg/ml) but was significantly reduced (p < 0.05) in animals pre-treated with 5 mg/kg (4034.4 ± 174.3 pg/ml) and 10 mg/kg (2416.2 ± 142.9 pg/ml) of fluoxetine when compared to untreated FST group (4005.4 ± 380.7 pg/ml). However, the hippocampal concentration of TNF-α did not change (p > 0.05) across the experimental groups (Figure 3).
Figure 3.
Effect of fluoxetine on hippocampal cytokines in rats exposed to FST-induced depression-like behavior. FST, forced swimming test; *p < 0.05 vs. control; @p < 0.05 vs. FST; IL-1β, interleukin-1 beta; IL-6, interleukin 6; TNF-α, tumor necrotic factor - alpha.
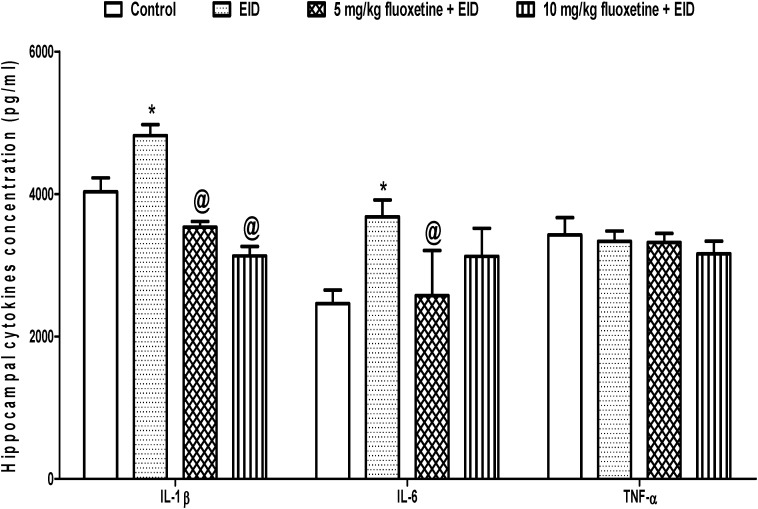
The pattern of the result in the EID model was similar to that of the FST model. The mean hippocampal concentration of IL-1β was significantly higher (p < 0.05) in EID (4819.4 ± 154.8 pg/ml) than control (4030.2 ± 194.5 pg/ml) but was significantly reduced (p < 0.05) in animals pre-treated with 5 mg/kg (3535.2 ± 78.3 pg/ml) and 10 mg/kg (3131.1 ± 133.1 pg/ml) of fluoxetine when compared to untreated EID group (4819.4 ± 154.8 pg/ml). Similarly, the mean hippocampal concentration of IL-6 was significantly higher (p < 0.05) in EID (3680.1 ± 237.7 pg/ml) than control (2460.3 ± 190.3 pg/ml) but was significantly reduced (p < 0.05) in animals pre-treated with 5 mg/kg (2572.2 ± 634.10 pg/ml) and 10 mg/kg (3123.3 ± 394.1 pg/ml) of fluoxetine when compared to untreated FST group (3680.1 ± 237.7 pg/ml). However, the hippocampal concentration of TNF-α did not change (p > 0.05) across the experimental groups (Figure 4).
Figure 4.
Effect of fluoxetine on hippocampal cytokines in rats exposed to EID-induced depression-like behavior. EID, ethanol-induced depression-like behavior model; *p < 0.05 vs. control; @p < 0.05 vs. EID.
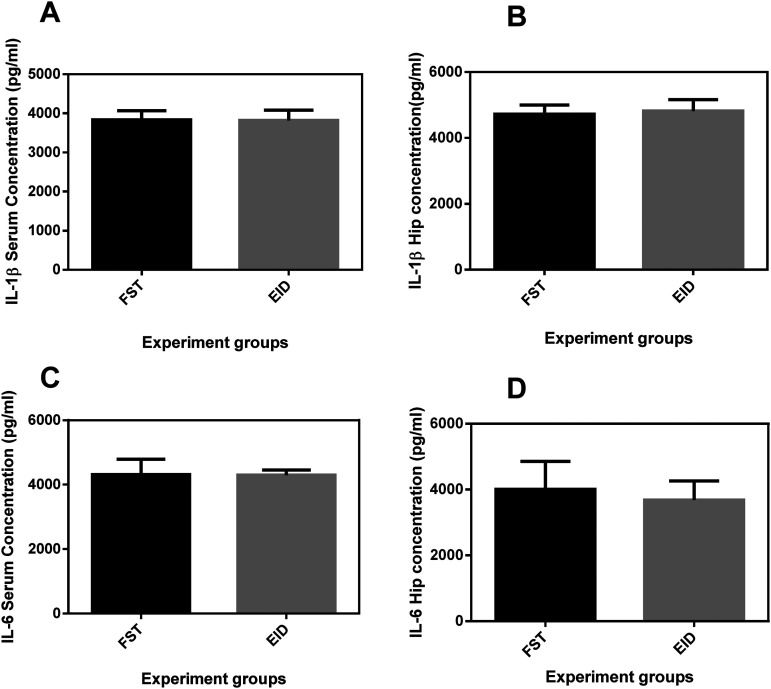
Comparison between cytokine levels in forced swimming test and EID models
In order to identify which mouse model would induce DLB better, we compared the mean concentrations of IL-1β and IL-6 in the serum and hippocampus in the two models. The results showed that the mean cytokine levels did not significantly differ (p > 0.05) across the two models (Figure 5).
Figure 5.
Comparison of serum and hippocampus levels of IL-1β and IL-6 in FST and EID models of DLB. Graphs A & B reflect serum and hippocampal concentrations (pg/ml) of IL-1β respectively while graphs C&D reflect serum and hippocampal concentrations (pg/ml) of IL-6, respectively. FST, forced swimming test; EID, ethanol-induced depression-like behavior model; Hip, hippocampus.
Discussion
Pro-inflammatory cytokines are a focal platform in the pathogenesis of depression in both extrinsic and intrinsic oxidative stress-induced depression. 38 An increase in the production of pro-inflammatory cytokines like IL-1β, IL-6, and interferon-γ have been observed in depression.2,13 However, scanty information is available regarding cytokine variations in serum and hippocampus when depression or DLB is induced. Fluoxetine, an antidepressant drug with an anti-inflammatory activity, 27 has been reported to decrease TNFα, IL-1β and IL-6 levels in plasma of patients and in animal models of depression. 39 Still, there is scanty information showing its effects on inflammatory cytokines when administered before stress induction is available. In the current study, we determined the cytokine levels in serum and hippocampus following induction of DLB by either FST or EID. We also investigated the effect of prior administration of the antidepressant drug fluoxetine on cytokines in male animals exposed to both models of DLB. Our finding is similar to the previous report of Caiaffo and Huang et al.40,41 that found an increase in the production of IL-1 and IL-2 in the supernatant of mitogen-stimulated splenocyte cultures from rats submitted to the chronic stress depression model. Kostadinov et al. (2015) 34 also reported an increase in the mean concentration of IL-1β and IL-6 in blood and brain when they induced DLB using the tail shock stress method in rats. The increase in the levels of IL-β and IL-6 observed in serum and hippocampus of FST models is possible because psychological stress can induce their release through sterile inflammation by activating the DAMPs and MAMPs pathways.20,22 In addition, acute psychological stress increases ATP levels in the hippocampus resulting in P2X7 receptor dependent elevation of IL-1β, and subsequent release of TNFα and IL-6.42,43
Similarly, Cargnelutti et al. 44 reported an increase in serum levels of IL-1β and IL-6 when rats were exposed to ethanol. In the same line, Gómez et al. 45 reported an increase in hippocampus levels of IL-1β and IL-6 when rats were exposed to ethanol. The increase in the levels of IL-1β and IL-6 observed in serum and hippocampus in EID models may be due to the concentration of ethanol (18 mM) used in the study and the time (25 min) it took in the body before the samples were harvested. Ethanol blood concentration of 0.07 g/dl used in the study is below the legal limits of alcohol blood concentration (0.08 g/dl), this maintains high levels of HSP 90, the constitutive HSP which activates NF-κB23,46 The activated NF-kB stimulates the release of pro-IL-1β, which is then cleaved by caspase-1 into mature IL 1β. 23 Active IL-1β stimulates the synthesis of IL-6 to potentiate its effects. 20
The study showed no significant increase in the level of TNF-α across all the experiments as compared to the controls both in serum and hippocampus. On the contrary, Liu et al. 46 and Yang et al. 47 reported an increase in the levels of TNF-α in serum and hippocampus when the rats were exposed to chronic mild stress. In the same line, Gómez and Fraga-Junior45,32 reported an increase in serum and hippocampus levels of TNF-α in rats following ethanol administration respectively. Possibly by the time the samples were harvested, an insignificant amount of TNF-α had been released both in the serum and hippocampus.
On comparison of IL-1β and IL-6 levels in FST and EID in serum and hippocampus, the mean concentration of inflammatory markers did not significantly differ. The results suggest that both extrinsic and intrinsic oxidative stress have equal chances of inducing depression in an individual. This possibly implies that the two stressors cause depression via the same pathways; which involves the activation of inflammasomes and NF-κB.19,48
A study had reported an anti-inflammatory effect of fluoxetine in carrageenan-induced inflammation, and the study found that the anti-inflammatory response of fluoxetine was comparable to that of standard anti-inflammatory drugs.27,40 Another study reported that fluoxetine promotes a decrease in the production of IL-6, TNF-α, and nitric oxide in microglia subjected to activation by lipopolysaccharide. 49 The authors suggested that fluoxetine's anti-inflammatory mechanism is through a decrease in gene expression of IL-6 and TNF-α mRNAs. In the present study, the animals pre-treated with 5 and 10 mg/kg of fluoxetine showed a significant decrease in the levels of IL-1β and IL-6 in serum across the experiments. The study of Qiu et al. 50 also reported a decrease in levels of IL-1β and IL-6 in the serum of humans pre-treated with antidepressants before inducing major depression. This suggests that an inflammatory pathway is implicated in the induction of DLB by both stress models used in this study, but we are not sure of any influence of the sex of animals since we used only male animals. The IL-6 and IL-1β levels significantly reduced in EID models in serum is possible because fluoxetine inactivates nuclear factor kappaB (NF-κB), a significant inflammatory signaling molecule that is activated by ethanol to stimulate the synthesis of pro-IL-1β.49,51 This finding is related to the previous observation of Park et al. 49 that fluoxetine inhibits the activation of NF-κB and the phosphorylation of mitogen-activated protein kinase, an important cytokine in the pro-inflammatory signaling pathway. It may thus be deduced that fluoxetine prevents inflammation induced by FST and EID models of DLB through suppression of the production of pro-inflammatory cytokines.
In the hippocampus, pretreatment with 5 mg/kg fluoxetine abolished an increase in IL-1 β in FST and EID models but only decreased IL-6 in the EID model of DLB. Moreover, pretreatment with 10 mg/kg fluoxetine decreased IL-1 β in FST and EID models of DLB but IL-6 in FST model of DLB only. While there is currently no available data for comparison, it was previously reported that IL-1β plays a major role in neurogenesis in the hippocampus, thus inactivating NF-κB stimulated by ethanol has a significant effect on its levels in the compartment. 51 This possibly explains why we had a significant decrease in IL-1β and not with IL-6 when the EID model was pretreated with 10 mg/kg of fluoxetine in the hippocampus, but the dose may still be appropriate for prophylaxis against DLB.
There was no significant decrease in the levels of TNF-α on pretreatment of the models with both 5 and 10 mg/kg of fluoxetine in serum and hippocampus. A study done by Hannestad et al. 52 also didn’t report any decrease in levels of TNF-α in serum of humans pre-treated with antidepressants before inducing major depression. The effect of fluoxetine on levels of TNF-α may not be immediate, thus it may not be an appropriate marker to establish a prophylactic dose against depression.
This study has some limitations, which include a lack of corticosterone levels to establish levels of stress, a lack of molecular immunoblotting, RT-PCR and immunohistochemistry assays of some inflammatory biomarkers that could have been more confirmatory, and a lack of an assay of reactive oxygen species that could have established the role of free radicals in the models. A lack of a sample size calculation could also be another limitation. Our future studies would build on these limitations to expand our understanding of the mechanisms anti-depressant and anti-inflammatory effects of fluoxetine on stress-induced DLB.
Conclusion
Induction of DLB by both FST and EID significantly increases the mean concentrations of IL-1β and IL-6 in serum and hippocampus of rats, which was attenuated by fluoxetine. On comparison, there was no significant difference in serum and hippocampus levels of IL-1β and IL-6 both in EID and FST. Possibly the two stress models cause depression via the same pathway.
Acknowledgments
The authors acknowledged Fogarty International Center of the National Institutes of Health, U.S Global AIDS Coordinator and Health Diplomacy (S/GAC), and President's Emergency Plan for AIDS Relief (PEPFAR) under Award Number IR25TW011213 for funding support. Great thanks to Dr Silver Ocho, Molecular biology laboratory manager, College of Veterinary Medicine and Biosafety, Makerere University, for laboratory assistance. Also, Mr Gakunga Jimmy, and Mr Boobo Alex for the assistance.
Author biographies
Ritah Nabirumbi, holds a Masters of Science in Medical Pharmacology. Her area of research is Neuropharmacology and Natural products.
Hope Onohuean, holds a PhD in biochemistry. His area of research is the molecular biology of infectious pathogens, cancer, and nano precision medicine.
Kato Charles Drago, holds a PhD in clinical immunology and molecular genetics. His area of research is Immunology and Molecular Biology.
Abdullateef Isiaka Alagbonsi, is an associate professor in Physiology. His area of research is in Neuroendocrinology, reproduction, and metabolism.
Ahmed A. Adedeji, is a professor of pharmacology. His research focus is on disease programming in humans and neurobehavioral imprints following exposure to diseases and drugs.
Footnotes
Authors’ contributions: RN and AAA conceived and designed the study. RN carried out the study. RN, HO, and KCD analysed and interpreted the data. AAA supervised the study. RN and HO drafted the manuscript. HO, KCD, and IAA revised the manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Medical Education Programme Initiative of Medical Education Support for Ugandan (research grant number 5R24TW008886), and Office of the US Global Aids Coordinator, National Institute of Health and Health Resource Services Administration.
ORCID iD: Hope Onohuean https://orcid.org/0000-0002-1890-6324
References
- 1.Wang H, Li P, Zhang Y, et al. Cytokine changes in different types of depression: specific or general? Neurology Psychiatry and Brain Research 2020; 36: 39–51. [Google Scholar]
- 2.Caroleo M, Carbone EA, Greco M, et al. Brain-behavior-immune interaction: serum cytokines and growth factors in patients with eating disorders at extremes of the body mass index (bmi) spectrum. Nutrients 2019; 11: 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onohuean H, Alagbonsi AI, Usman IM, et al. Annona muricata linn and khaya grandifoliola C.DC. Reduce oxidative stress in vitro and ameliorate plasmodium berghei-induced parasitemia and cytokines in BALB/c mice. J Evidence-Based Integr Med 2021; 26: 2515690X2110366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018; 392: 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu Y, Tang C, Liow CS, et al. A regressional analysis of maladaptive rumination, illness perception and negative emotional outcomes in Asian patients suffering from depressive disorder. Asian J Psychiatr 2014; 12: 69–76. [DOI] [PubMed] [Google Scholar]
- 6.Onohuean H, Oosthuizen F. Multinational appraisal of the epidemiological distribution of opioid fatalities: a systematic review and meta-analysis. Front Psychiatry 2023; 14: 1290461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachmann S. Epidemiology of suicide and the psychiatric perspective. Int J Environ Res Public Health 2018; 15: 1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Connor DB, Thayer JF, Vedhara K. Stress and health: a review of psychobiological processes. Annu Rev Psychol 2021; 72: 663–688. [DOI] [PubMed] [Google Scholar]
- 9.Szymkowicz SM, Gerlach AR, Homiack D, et al. Biological factors influencing depression in later life: role of aging processes and treatment implications. Transl Psychiatry 2023; 13: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onohuean H, Akiyode AO, Akiyode O, et al. Epidemiology of neurodegenerative diseases in the East African region: A meta-analysis. Front Neurol 2022; 13: 1024004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simon L, Souza-Smith FM, Molina PE. Alcohol-Associated tissue injury: current views on pathophysiological mechanisms. Annu Rev Physiol 2022; 84: 87–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jomova K, Raptova R, Alomar SY, et al. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch Toxicol 2023; 97: 2499–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsermpini EE, Plemenitaš Ilješ A, Dolžan V. Alcohol-Induced oxidative stress and the role of antioxidants in alcohol use disorder: a systematic review. Antioxidants 2022; 11: 1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onohuean H, Onohuean FE, Igbinoba SI, et al. Elucidation of chemical profiles and molecular targets of Mondia whitei leave fractions bioactive as novel therapeutics: an in vitro and in silico assay. J Genet Eng Biotechnol 2022; 20: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salim S. Oxidative Stress and Psychological Disorders. Curr Neuropharmacol 2014; 12: 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakunina N, Pariante CM, Zunszain PA. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 2015; 144: 365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ting EYC, Yang AC, Tsai SJ. Role of interleukin-6 in depressive disorder. Int J Mol Sci 2020; 21: 2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson JD, Barnard DF, Kulp AC, et al. Neuroendocrine regulation of brain cytokines after psychological stress. J Endocr Soc 2019; 3: 1302–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleshner M, Frank M, Maier SF. Danger signals and inflammasomes: stress-evoked Sterile inflammation in mood disorders. Neuropsychopharmacology 2017; 42: 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleshner M. Stress-evoked sterile inflammation, danger associated molecular patterns (DAMPs), microbial associated molecular patterns (MAMPs) and the inflammasome. Brain Behav Immun 2013; 27: –7. [DOI] [PubMed] [Google Scholar]
- 21.Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun 2013; 31: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maslanik T, Mahaffey L, Tannura K, et al. The inflammasome and danger associated molecular patterns (DAMPs) are implicated in cytokine and chemokine responses following stressor exposure. Brain Behav Immun 2013; 28: 54–62. [DOI] [PubMed] [Google Scholar]
- 23.Muralidharan S, Mandrekar P. Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation. J Leukoc Biol 2013; 94: 1167–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia N, Madore V, Albalakhi A, et al. Microglia-dependent neuroprotective effects of 4-octyl itaconate against rotenone-and MPP+-induced neurotoxicity in Parkinson’s disease. Sci Rep 2023; 13: 15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klran TR, Otlu O, Karabulut AB. Oxidative stress and antioxidants in health and disease. Journal of Laboratory Medicine 2023; 47: 1–11. [Google Scholar]
- 26.Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discovery 2021; 20: 689–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-García ML, Tovilla-Zárate CA, Villar-Soto M, et al. Fluoxetine modulates the pro-inflammatory process of IL-6, IL-1β and TNF-α levels in individuals with depression: a systematic review and meta-analysis. Psychiatry Res 2022; 307: 114317. [DOI] [PubMed] [Google Scholar]
- 28.Cheng Y, Pardo M, Armini R de S, et al. Stress-induced neuroinflammation is mediated by GSK3-dependent TLR4 signaling that promotes susceptibility to depression-like behavior. Brain Behav Immun 2016; 53: 207–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alboni S, Poggini S, Garofalo S, et al. Fluoxetine treatment affects the inflammatory response and microglial function according to the quality of the living environment. Brain Behav Immun 2016; 58: 261–271. [DOI] [PubMed] [Google Scholar]
- 30.Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology 2005; 177: 245–255. [DOI] [PubMed] [Google Scholar]
- 31.Nagy LE. Alcohol: methods and protocols. Springer Protocols 2008; 449: XX–414.
- 32.Fraga-Junior EB, Fernandes IL, Rohden CAH, et al. Attenuation of the levels of pro-inflammatory cytokines prevents depressive-like behavior during ethanol withdrawal in mice. Brain Res Bull 2022; 191: 9–19. [DOI] [PubMed] [Google Scholar]
- 33.Kesić M, Mokrović G, Tvrdeić A, et al. Constitutive serotonin tone modulates molecular and behavioral response to chronic fluoxetine treatment: a study on genetic rat mode. Front Psychiatry 2021; 12: 741222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kostadinov I, Delev D, Petrova M, et al. Study on anti-inflammatory and immunomodulatory effects of fluoxetine in rat models of inflammation. Eur J Inflamm 2015; 13: 173–182. [Google Scholar]
- 35.Kinoshita M, Hirayama Y, Fujishita K, et al. Anti-Depressant fluoxetine reveals its therapeutic effect via astrocytes. EBioMedicine 2018; 32: 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paul R, Borah A. Global loss of acetylcholinesterase activity with mitochondrial complexes inhibition and inflammation in brain of hypercholesterolemic mice. Sci Rep 2017; 7: 17922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hulse RE, Kunkler PE, Fedynyshyn JP, et al. Optimization of multiplexed bead-based cytokine immunoassays for rat serum and brain tissue. J Neurosci Methods 2004; 136: 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zakaria FH, Samhani I, Mustafa MZ, et al. Pathophysiology of depression: stingless bee honey promising as an antidepressant. Molecules 2022; 27: 5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baune BT, Eyre H. Anti-inflammatory effects of antidepressant and atypical antipsychotic medication for the treatment of major depression and comorbid arthritis: a case report. J Med Case Rep 2010; 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caiaffo V, Oliveira BDR, de Sá FB, et al. Anti-inflammatory, antiapoptotic, and antioxidant activity of fluoxetine. Pharmacol Res Perspect 2016; 4: e00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang C, Zhang F, Li P, et al. Low-Dose IL-2 attenuated depression-like behaviors and pathological changes through restoring the balances between IL-6 and TGF-β and between Th17 and treg in a chronic stress-induced mouse model of depression. Int J Mol Sci 2022; 23: 13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iwata M, Ota KT, Li XY, et al. Psychological stress activates the inflammasome via release of adenosine triphosphate and stimulation of the purinergic type 2X7 receptor. Biol Psychiatry 2016; 80: 12–22. [DOI] [PubMed] [Google Scholar]
- 43.Ferrari D, Pizzirani C, Adinolfi E, et al. The P2X 7 receptor: a key player in IL-1 processing and release. J Immunol 2007; 179: 8569–8569. [DOI] [PubMed] [Google Scholar]
- 44.Cargnelutti LO, Bitencourt PER, Bochi G, et al. Syzygium cumini leaf extract protects against ethanol-induced acute injury in rats by inhibiting adenosine deaminase activity and proinflammatory cytokine production. Res J Phytochem 2015; 9: 56–67. [Google Scholar]
- 45.Gómez GI, Falcon RV, Maturana CJ, et al. Heavy alcohol exposure activates astroglial hemichannels and pannexons in the hippocampus of adolescent rats: effects on neuroinflammation and astrocyte arborization. Front Cell Neurosci 2018; 12: 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu YN, Peng YL, Lei L, et al. TNFα mediates stress-induced depression by upregulating indoleamine 2,3-dioxygenase in a mouse model of unpredictable chronic mild stress. Eur Cytokine Netw 2015; 26: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang P, Gao Z, Zhang H, et al. Changes in proinflammatory cytokines and white matter in chronically stressed rats. Neuropsychiatr Dis Treat 2015: 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muralidharan S, Ambade A, Fulham MA, et al. Moderate alcohol induces stress proteins HSF1 and hsp70 and inhibits proinflammatory cytokines resulting in endotoxin tolerance. J Immunol 2014; 193: 1975–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park SH, Lee YS, Yang HJ, et al. Fluoxetine potentiates phagocytosis and autophagy in microglia. Front Pharmacol 2021; 12: 770610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qiu T, Li X, Chen W, et al. Prospective study on Maresin-1 and cytokine levels in medication-naïve adolescents with first-episode major depressive disorder. Front Psychiatry 2023; 14: 1132791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zou J, Crews FT. Inflammasome-IL-1β signaling mediates ethanol inhibition of hippocampal neurogenesis. Front Neurosci 2012; 6: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hannestad J, Dellagioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology 2011; 36: 2452–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El-Taukhy MA, Salama SM, Abou-Shousha SA, et al. Effects of chronic ethanol and vitamin C administration on production of tumor necrosis factor-alpha and interleukin-6 in rats. Egypt J Immunol. 2006; 13: 1–10. [PubMed] [Google Scholar]