Abstract
The bovine papillomavirus type 1 E1 protein is important for viral DNA replication and transcriptional repression. It has been proposed that the full-length E1 protein consists of a small N-terminal and a larger C-terminal domain. In this study, it is shown that an E1 polypeptide containing residues 132 to 605 (which represents the C-terminal domain) is able to support transient viral DNA replication, although at a level lower than that supported by the wild-type protein. This domain can also repress E2-mediated transactivation from the P89 promoter as well as the wild-type E1 protein can.
The bovine papillomavirus type 1 (BPV-1) genome replicates as a stable nuclear episome. In addition to cellular proteins, the viral origin of replication and the E1 and E2 proteins are necessary for DNA replication (31). The origin consists of an AT-rich region and an E1 and E2 binding site (16, 30, 31, 37). The E1 protein initiates DNA replication by binding to the origin (6, 33, 36), and the E2 protein is a transcriptional transactivator that cooperatively binds to the origin with E1 (22, 25, 36). E1 also represses viral transformation (8, 20) and can regulate viral gene expression (9, 18). The E2 protein can activate transcription from several viral promoters (24). The P89 promoter is located just downstream from the replication origin, and E1 can significantly repress E2-mediated transactivation of this promoter (9, 18, 24).
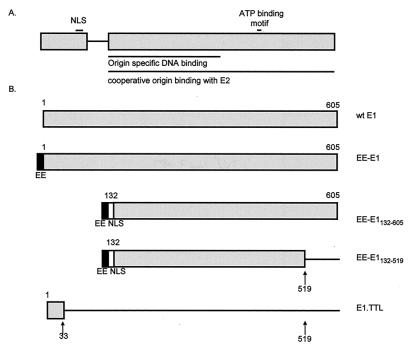
The E1 proteins are well-conserved among papillomaviruses. There is moderate homology of the N-terminal 120 amino acids and high homology of the C-terminal 450 amino acids among E1 proteins. A short nonconserved sequence links these regions (19). This fact suggests that E1 might consist of two separate structural domains linked by a short spacer region. The E1 protein also has minimal sequence homology with simian virus 40 (SV40) large T antigen. Homology between these proteins exists primarily in the nuclear localization sequence (NLS) in the N-terminal region of both proteins and in the ATP binding motif in the C-terminal region (2, 11). A second protein, E1-M, is encoded by the E1 open reading frame (ORF) and consists of the putative N-terminal domain (residues 1 to 129) linked to 13 amino acids of a downstream ORF (28). No function has been assigned to E1-M, but its existence lends support to the hypothesis that the N-terminal region of E1 constitutes a separate domain. The putative N-terminal domain of E1 contains the NLS (11) and multiple phosphorylation sites (11, 40). There are reports that polypeptides containing the N-terminal region can interact and cooperatively bind to the origin with E2 (1, 10, 29). However, other studies have shown that E1 polypeptides containing the putative C-terminal domain can interact with E2 and cooperatively bind to the origin as efficiently as wild-type E1 (17, 19, 39). Based on these findings, we have postulated that the E1 protein is comprised of two distinct functional domains (see Fig. 1).
FIG. 1.
(A) Diagram of the two putative functional domains of the BPV-1 E1 protein. The regions of E1 required for nuclear localization (NLS) (11), ATP binding (26), origin binding, and cooperative origin binding with E2 (19) have been previously reported. (B) E1 proteins used in this study. The filled rectangles represent EE epitopes, and the open rectangles represent the SV40 T-antigen NLS. Arrows indicate the positions of TTLs. wt, wild type.
EE-E1132-605 can cooperatively bind to the origin with the E2 protein.
Our previous studies have shown that E1 residues 162 to 605 (E1162-605) are required for cooperative origin binding with E2 (19). Thus, E1132-605 with the EE epitope (EE-E1132-605) should specifically bind the origin and this binding should be enhanced by E2. This hypothesis was tested by a DNA-protein coimmunoprecipitation assay. 35S-labeled E1 and E2 proteins were expressed by TNT coupled transcription and translation (Promega) from plasmids containing a T7 RNA polymerase promoter. The E1 proteins contain a short EE epitope (5) fused to their N termini to enable immunoprecipitation of the truncated E1 protein. Plasmid p5′EE-pTM1E1 encodes the entire E1 polypeptide with the EE epitope (19). pTZEE-E1132-605 was generated by placing the NruI-to-BglII (nucleotides 840 to 1515) fragment of pTZE1 (which contains BPV-1 nucleotides 840 to 2766 downstream from the T7 promoter in pTZ18R [U.S. Biochemicals]) with an NruI-to-BglII fragment that encodes the EE epitope, the SV40 T antigen NLS (MGEEEEYMPMEGPKKKRKV), and sequences of E1. Full-length E2 was expressed from pTZkzE2 (15). A diagram of the E1 proteins used in this study is shown in Fig. 1.
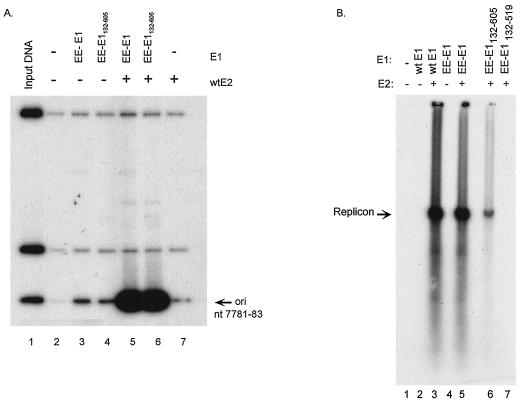
The E1 and/or E2 protein was added to a mixture of three 32P-labeled DNA fragments derived from plasmid KS+/origin (25), one of which contained origin sequences (BPV-1 nucleotides 7781 to 7946 and 1 to 83). DNA-protein complexes were immunoprecipitated with an antibody against the EE epitope (19). All incubations were performed at room temperature. The coprecipitated protein and DNA present in the complexes was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Both the DNA and the protein components can be observed if the samples are not heated. The gels were exposed to two films; the film closest to the gel contains the 35S and 32P signals, and the second film has only the 32P DNA signal. These experiments showed that E1 proteins coprecipitated much greater amounts of the origin-containing fragment than they did of the two larger nonspecific fragments. As shown in Fig. 2A, EE-E1132-605 (lane 4) was able to bind specifically to the origin as efficiently as EE-E1 (lane 3). Addition of E2 increased origin-specific binding of EE-E1 and EE-E1132-605 approximately 100-fold (compare lanes 5 and 6 to lanes 3 and 4). These results confirm that E1 residues 132 to 605 are sufficient for origin-specific binding and cooperative binding to the origin with E2.
FIG. 2.
Origin binding properties of truncated E1 proteins. The EE-E1132-605 protein was tested for DNA binding activity in a DNA-protein coimmunoprecipitation assay. Lane 1 contains 1 ng (1/200) of the input 32P-labeled DNA probe. The origin-containing fragment (ori) is indicated by an arrow. Lane 2 contains 35 μl of unprogrammed lysate; lanes 3 to 6 contain 35 μl of in vitro-translated E1 protein, as described above each lane; and lanes 5 to 7 contain 25 μl of lysate containing E2. The amount of total lysate per assay was kept constant by addition of control lysate. In all lanes the DNA-protein complexes were immunoprecipitated with the EE antibody. Percentages of origin binding were quantitated with a PhosphorImager, and levels of cooperative origin binding are expressed relative to the amount of binding found when only EE-E1 was added, which was given a value of 1. Only the 32P signal is shown. wt, wild-type; nt, nucleotides. (B) Transient-replication properties of the truncated E1 proteins in CHO cells. Results of a representative transient-replication assay are shown. In each lane, cells were electroporated with replicon DNA; pCGE2 expression vector (where indicated); and pUC18 (lane 1), pCGMluE1 (lanes 2 and 3), pCGEE-E1 (lanes 4 and 5), pCGEE-E1132-605 (lane 6), or pCGEE-E1132-519 (lane 7). Replication activity was quantitated with a PhosphorImager and is expressed relative to wild-type E2–plus–EE-E1 activity, which was given a value of 100%.
EE-E1132-605 can support transient viral replication.
To determine if the truncated E1 protein could support transient viral DNA replication, E1 expression plasmids were transfected into CHO cells by electroporation (31) together with the E2 expression vector pCGE2 (31) and a plasmid containing the replication origin (p716; nucleotides 4786 to 7946 and 1 to 83) (34). pCGMluE1 expresses the E1 protein from the cytomegalovirus early promoter (31) and contains an MluI (nucleotide 7352)-to-BamHI (nucleotide 4451) BPV-1 fragment with a deletion from AvrII (nucleotide 2766) to BstXI (nucleotide 3881). pCGEE-E1 was derived from pCGMluE1 and encodes the full-length E1 gene with the EE epitope fused to its N terminus. pCGEE-E1132-605 encodes the C-terminal region of E1 (amino acids 132 to 605) with the EE epitope and NLS fused to its N terminus. pCGEE-E1132-519 was derived from pCGEE-E1132-605 by insertion of a translation termination linker (TTL) at the BstEII site (nucleotide 2405). Five days posttransfection, low-molecular-weight DNA was isolated, digested with both DpnI and HindIII or MboI and HindIII, and analyzed by Southern blot hybridization with a 32P-labeled long control region (LCR) DNA fragment (nucleotides 6987 to 7946 and 1 to 36). DNA that has undergone replication in eukaryotic cells is resistant to cleavage by DpnI but sensitive to MboI digestion. The linearized DpnI-resistant replicon migrates as a 3.2-kb fragment.
As shown in Fig. 2A, EE-E1132-605 was able to support viral DNA replication (lane 6) when it was expressed with E2, although to a much lesser extent than the wild-type E1 and EE-E1 proteins did (lanes 3 and 5, respectively). The level of replication observed ranged between 6 and 16% of that of the wild type (based on results from eight experiments). This low level of replication was confirmed by MboI digestion (data not shown). EE-E1132-519 (lane 7) was not able to support detectable replication. These results indicate that the C-terminal region of E1 (EE-E1132-605) is able to support low-level DNA replication and therefore contains all basic functions required for initiation of DNA synthesis.
To confirm that the truncated E1 proteins were stably expressed, the E1 expression vectors (pCGEE-E1, pCGEE-E1132-605, and pCGEE-E1132-519) were transfected into COS-7 cells. The E1 proteins were labeled with [35S]methionine and isolated by immunoprecipitation with the EE antibody. This analysis showed that the truncated EE-E1 proteins were expressed at least as well as the full-length EE-E1 protein (data not shown). Therefore, the reduced ability of the EE-E1132-605 protein to support replication is not due to protein instability.
Although the putative C-terminal domain of E1 contains the basic functions necessary to support replication, the N-terminal domain must have an important auxiliary function(s) needed for efficient replication. Similar results have been found with SV40 large T antigen. The analogous C-terminal region of T antigen (residues 83 to 708) retains helicase activity and binds SV40 origin DNA with reduced affinity but can support reduced levels of DNA replication in vitro (32). The truncated polypeptide also oligomerizes incorrectly on SV40 DNA. Thus, the first 82 residues of T antigen are not strictly required for DNA replication but may play a role in correct hexamer assembly and efficient origin binding (32). A similar region of polyomavirus large T antigen can also support reduced amounts of viral replication in vivo (4). The E1 protein initially binds the origin as a complex with E2 and then undergoes a transition to a trimeric or hexameric form that no longer contains E2 (3, 13, 21). In this study, EE-E1132-605 bound the replication origin as well as the wild-type protein; however, its ability to form hexamers has not been investigated. The EE-E1132-605 protein may be deficient in efficient oligomerization and therefore may not be able to support wild-type levels of replication.
The 5′ region of the E1 ORF also encodes the E1-M protein. This protein has been detected in virally transformed cells, but its function is unknown (28). A BPV-1 genome that does not express E1-M can replicate with a stability and copy number similar to those of wild-type DNA (7). Therefore, E1-M does not appear to be essential for stable replication. However, E1-M may have a regulatory function and its existence strengthens the hypothesis that full-length E1 is comprised of two domains.
EE-E1132-605 is sufficient for repression of the viral P89 promoter.
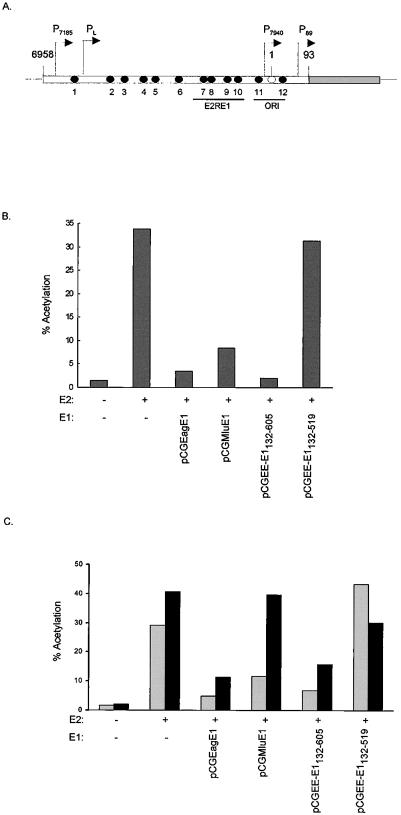
The E1 protein can repress E2-transactivated chloramphenicol acetyltransferase (CAT) expression from the P89 promoter (9, 18). To determine if the C-terminal region of E1 can support this function, primary BEF (bovine embryo fibroblast) cells were cotransfected with the E1 and E2 expression plasmids and the p1066 reporter plasmid, as described previously (18). p1066 contains the LCR and P89 promoter upstream from the CAT gene (Fig. 3A) (24). In this experiment we used plasmids pCGMluE1 and pCGEagE1 (which was derived from pCGMluE1 by deletion of sequences between MluI [nucleotide 7352] and EagI [nucleotide 619]) (31). Plasmid C59 was used to express E2 (38). E2 transactivates P89 by binding to E2 sites in the LCR (24), and as expected, CAT expression from p1066 was greatly increased (approximately 23-fold) in the presence of E2 (Fig. 3B). Cotransfection with pCGEagE1 or pCGMluE1 resulted in dramatic repression of E2-mediated CAT production from P89. However, pCGEagE1 repressed P89 expression to a greater extent than pCGMluE1 (approximately 10-fold versus 4-fold repression). This may be due to different levels of expression of E1 from the two plasmid backgrounds. Plasmid pCGEagE1 encodes only the E1 ORF, but pCGMluE1 also encodes the E6 and E7 ORFs (31). Coexpression of EE-E1132-605 (which is in the pCGMluE1 background) also resulted in severely repressed levels of CAT production from P89 (approximately 17-fold repression). The C-terminal region of E1 consistently inhibited P89 activity to a greater extent than wild-type E1 expressed from pCGEagE1 or pCGMluE1 did. This may be due to the higher levels of this protein that were detected by immunoprecipitation. EE-E1132-519 repressed P89 minimally, even though it contains the E1 DNA binding domain. However, E1132-519 is unable to cooperatively bind to the origin with the E2 protein. These results indicate that the entire C-terminal region of E1 is necessary and sufficient for repression of E2-mediated transactivation of P89.
FIG. 3.
(A) Structure of the p1066 reporter plasmid. The open rectangle represents the LCR sequences, and the shaded rectangle represents the CAT gene. Filled circles represent the 12 E2 binding sites (12), and an open circle represents the E1 binding site. The E2-responsive element (E2RE1) (23), BPV-1 promoters, and the origin of replication (ORI) are also shown. (B) Repression by E1 of E2-transactivated P89 promoter activity. Results from a representative CAT assay are shown. The reporter plasmid p1066 was cotransfected into BEF cells with the indicated E1 and/or E2 (C59) expression vector. Each plasmid was tested in approximately 10 experiments. CAT activities are expressed as percentages of acetylation. (C) E1 repression of E2-transactivated CAT expression from heterologous promoters. The values were averaged from results of three experiments. Reporter plasmids p964 (shaded bars) and pTKM6 (filled bars) were cotransfected into BEF cells with the indicated E1 and E2 (C59) expression vectors. CAT activities are expressed as percentages of acetylation.
EE-E1132-605 can repress heterologous E2-responsive promoters.
The ability of E1 to repress E2-transactivated CAT expression from two heterologous E2-responsive promoters was tested to determine if the observed repression was specific to P89. It has been previously shown that E2-responsive heterologous promoters are also repressed by E1. One study found minimal repression of E2-responsive heterologous promoters (18), while another reported more significant repression (9). p964 contains E2-responsive element 1 (E2RE1) upstream from the SV40 promoter (24) and is transactivated by E2 (Fig. 3C). Wild-type E1 proteins (expressed from pCGEagE1 and pCGMluE1) and EE-E1132-605 were able to repress the E2-responsive SV40 promoter (approximately 6-, 3.5-, and 4.5-fold repression, respectively); however, repression was less than that observed with p1066. Plasmid pTKM6 contains six E2 binding sites upstream of the thymidine kinase (TK) promoter (27). E2 also efficiently transactivated expression from this plasmid. The E2-responsive TK promoter was repressed approximately 3.5-fold by pCGEagE1 but was not repressed by pCGMluE1. The ability of these wild-type E1 constructs to repress transcription may be due to different levels of E1 expression. EE-E1132-605 repressed E2 transactivation from the TK promoter approximately 2.6-fold; however, this repression was also less than that seen for P89 (approximately 17-fold repression) (Fig. 3B). EE-E1132-519 did not significantly repress either heterologous promoter. These results indicate that the C-terminal region of the E1 protein can repress E2-transactivated transcription from heterologous promoters. However, as seen with wild-type E1, the level of repression is reduced compared to that of the P89 promoter.
The DNA binding function of E1 is not absolutely required for repression of viral transcription.
A previous study indicated that binding of E1 to the origin is crucial for repression of transcription (18), while another study found that an E1 binding site is not necessary (9). To investigate if E1 DNA binding is necessary for repression of E2-mediated transactivation, E1 proteins defective for DNA binding were tested in the repression assay (Fig. 4). PCGEagE1-based plasmids LPM4, LPM5, and LPM6 containing these mutations were obtained from Michael Botchan (29). XmaCI-to-BstEII fragments from these plasmids were subcloned into pT7E1 to generate plasmids p1588, p1589, and p1585, respectively.
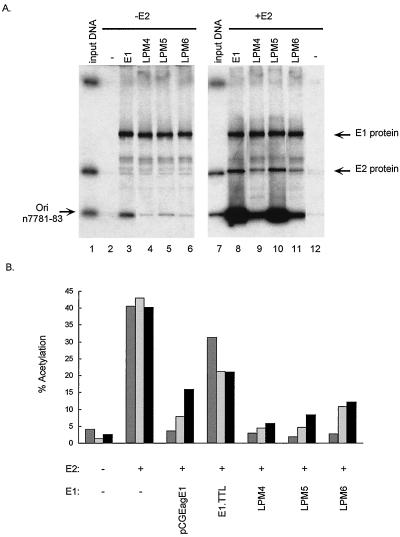
FIG. 4.
(A) DNA binding activity of E1 proteins containing mutations in the DNA binding domain. The left gel demonstrates E1 origin-specific binding, and the right gel demonstrates E1-E2 cooperative origin binding. Lanes 1 and 7 contain 1 ng (1/200) of input probe DNA, and the origin-containing fragment is indicated (Ori); lane 2 contains unprogrammed lysate; lanes 3 to 6 and 8 to 11 contain 35 μl of in vitro-translated E1 proteins, as described above each lane; and lanes 8 to 12 contain 25 μl of E2 protein lysate. The amount of total lysate per assay was kept constant by addition of control lysate. The positions of the E1 and E2 proteins are indicated. In each lane the DNA-protein complexes were immunoprecipitated with the E1-specific antibody SSQN (25), eluted from beads, and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The percentages of origin binding were quantitated with a PhosphorImager, and levels of cooperative origin binding were expressed relative to the amount of binding found when only E1 was added, which was given a value of 1. The gel shows both 35S and 32P signals. n7781-83, nucleotides 7781 to 7783. (B) Effects of point mutations in the DNA binding domain of E1 on the protein’s ability to repress E2-mediated transactivation. The values were averaged from results of three experiments. Reporter plasmids p1066 (darkly shaded bars), p964 (lightly shaded bars), and pTKM6 (filled bars) were cotransfected into BEF cells with the indicated E1 (pCGEagE1) and/or E2 (C59) expression vector. Each plasmid was tested in several independent experiments. CAT activities are expressed as percentages of acetylation.
The ability of these mutated E1 proteins to bind the replication origin was tested by the DNA-protein coimmunoprecipitation assay described above. The results are shown in Fig. 4A. E1 was able to bind the origin alone, and addition of E2 enhanced its origin-specific binding 30-fold. LPM4 and LPM6 E1 proteins were defective in origin binding in the absence of E2 (lanes 4 and 6), and binding was only minimally enhanced in the presence of E2 (five- and fourfold, respectively) (lanes 9 and 11). LPM5 was not able to significantly bind the origin alone; however, E2 rescued its ability to bind the origin (compare lanes 5 and 10). These results confirm the findings of Thorner et al. (29), although the levels of origin binding in the presence of E2 were found to be slightly higher in our study. Correspondingly, LPM4 and LPM6 proteins are unable to support DNA replication and LPM5 is able to support reduced levels of DNA replication (29).
As shown in Fig. 4B, all of the E1 proteins defective for DNA binding were able to repress the P89 and heterologous promoters at least as well as the wild-type E1 protein did (Fig. 4A). Cotransfection with an E1.TTL control plasmid (18), which should not express any functional E1 protein, did not significantly repress P89. The SV40 and TK promoters were slightly repressed by the E1.TTL construct. This repression could be due to competition between the cytomegalovirus promoters in pCGEagE1 and the E1.TTL plasmid for binding cellular transcription factors. Even LPM4 and LPM6, which are deficient in origin binding even in the presence of E2 (29), repressed transcription of all three promoters as well as wild-type E1 did. The very low level of cooperative origin binding observed with the mutated E1 proteins may be sufficient for repression. However, this possibility does not explain the observed repression of pTKM6, which does not contain a known E1 binding site. Alternatively, these results imply that the DNA binding function of the E1 protein is not necessary for repression of viral transcription and that this repression is not specific to promoters containing an E1 binding site. LMP4 and LMP6, which are defective for replication, are able to efficiently repress transcription. Therefore, these results confirm previous findings that DNA replication is not correlated with transcriptional repression (9, 18).
There are several mechanisms by which E1 may repress transactivation. Repression may be due to an E1-E2 complex that binds the origin region upstream from P89 and blocks binding of essential transcription factors. Alternatively, E1 might block the regions of the E2 protein that interact with and activate the basal transcriptional machinery (independent of E1 DNA binding). Both mechanisms may act to repress E2-mediated transactivation from the origin-containing plasmid p1066, which is most efficiently repressed by E1. pTKM6 contains no known E1 binding site and is the least repressed of the promoters. Repression of this promoter may be due to E1 blocking the ability of E2 to activate transcription. p964 shows intermediate repression; this plasmid contains E2RE1 (which contains four high-affinity E2 binding sites) upstream from the SV40 early promoter. Yang and Botchan have reported that E1 binds sequences within E2RE1 in the presence of E2 (35). Binding of E1 to the E2RE1 may increase repression of E2-mediated transactivation. Unbound E1 or E1-E2 proteins may also indirectly repress transcription by sequestering essential cellular transcription factors. Nonspecific repression may be due to squelching, since the E1.TTL construct (which should not express functional E1 protein) decreased E2-mediated transactivation somewhat (Fig. 4B). Nonspecific repression may also be due to cellular toxicity of the E1 protein. This study did not attempt to define the mechanism of E1-mediated transcriptional repression but attempted to determine which functions of E1 were supported by the putative C-terminal domain. These experiments show that EE-E1132-605 repressed E2-mediated transactivation from the P89 promoter and two heterologous promoters in a manner similar to that of the full-length E1 protein.
Wild-type E1 (pCGEagE1) and EE-E1132-605 were able to repress E2-mediated transactivation of heterologous promoters that lacked an E1 binding site (pTKM6). In addition, E1 proteins defective for DNA binding and cooperative DNA binding (LPM4 and LPM6) repressed transcription of all three promoters, indicating that origin-specific binding by E1 is not necessary for transcriptional regulation. A recent study by Mansky et al. also found that efficient DNA binding is not required for repression (14). This result suggests that repression of transcription can occur by direct protein-protein interaction and does not necessarily require E1-E2 complex binding to DNA. Indirect evidence that protein-protein interaction is necessary for transcriptional repression comes from EE-E1132-519. This truncated protein was not able to support detectable viral replication, significantly repress E2-mediated transactivation from P89, or bind the origin with E2 (19). The inability of this protein to interact with E2 may explain why it did not significantly repress transcription.
In summary, an E1 polypeptide containing residues 132 to 605 of the E1 protein was able to support origin-specific DNA binding, cooperative origin binding with the E2 protein, reduced transient viral DNA replication, and repression of E2-mediated transactivation. These results indicate that the C-terminal region of the E1 protein can act as a functional domain. Although the N-terminal region was not absolutely required for the transient-replication functions of E1, it must have some properties that are important for efficient viral DNA replication.
Acknowledgments
We thank Jodi Vogel and Carl Baker for critical reviews of the manuscript.
REFERENCES
- 1.Benson J D, Howley P M. Amino-terminal domains of the bovine papillomavirus type 1 E1 and E2 proteins participate in complex formation. J Virol. 1995;69:4364–4372. doi: 10.1128/jvi.69.7.4364-4372.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clertant P, Seif I. A common function for polyoma virus large-T and papillomavirus E1 proteins. Nature. 1984;311:276–279. doi: 10.1038/311276a0. [DOI] [PubMed] [Google Scholar]
- 3.Fouts, E., E. Egelman, and M. Botchan. Unpublished data.
- 4.Gjorup O V, Rose P E, Holman P S, Bockus B J, Schaffhausen B S. Protein domains connect cell cycle stimulation directly to initiation of DNA replication. Proc Natl Acad Sci USA. 1994;91:12125–12129. doi: 10.1073/pnas.91.25.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grussenmeyer T, Scheidtmann K H, Hutchinson M A, Eckart W, Walter G. Complexes of polyoma virus medium T antigen and cellular proteins. Proc Natl Acad Sci USA. 1985;82:7952–7954. doi: 10.1073/pnas.82.23.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holt S E, Schuller G, Wilson V G. DNA binding specificity of the bovine papillomavirus E1 protein is determined by sequences contained within an 18-base-pair inverted repeat element at the origin of replication. J Virol. 1994;68:1094–1102. doi: 10.1128/jvi.68.2.1094-1102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hubert W G, Lambert P F. The 23-kilodalton E1 phosphoprotein of bovine papillomavirus type 1 is nonessential for stable plasmid replication in murine C127 cells. J Virol. 1993;67:2932–2937. doi: 10.1128/jvi.67.5.2932-2937.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambert P F, Howley P M. Bovine papillomavirus type 1 E1 replication-defective mutants are altered in their transcriptional regulation. J Virol. 1988;62:4009–4015. doi: 10.1128/jvi.62.11.4009-4015.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Moal M A, Yaniv M, Thierry F. The bovine papillomavirus type 1 (BPV1) replication protein E1 modulates transcriptional activation by interacting with BPV1 E2. J Virol. 1994;68:1085–1093. doi: 10.1128/jvi.68.2.1085-1093.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leng X, Ludes-Meyers J H, Wilson V G. Isolation of an amino-terminal region of bovine papillomavirus type 1 E1 protein that retains origin binding and E2 interaction capacity. J Virol. 1997;71:848–852. doi: 10.1128/jvi.71.1.848-852.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lentz M R, Pak D, Mohr I, Botchan M R. The E1 replication protein of bovine papillomavirus type 1 contains an extended nuclear localization signal that includes a p34cdc2 phosphorylation site. J Virol. 1993;67:1414–1423. doi: 10.1128/jvi.67.3.1414-1423.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li R, Knight J, Bream G, Stenlund A, Botchan M. Specific recognition nucleotides and their DNA context determine the affinity of E2 protein for 17 binding sites in the BPV-1 genome. Genes Dev. 1989;3:510–526. doi: 10.1101/gad.3.4.510. [DOI] [PubMed] [Google Scholar]
- 13.Lusky M, Hurwitz J, Seo Y S. The bovine papillomavirus E2 protein modulates the assembly of but is not stably maintained in a replication-competent multimeric E1-replication origin complex. Proc Natl Acad Sci USA. 1994;91:8895–8899. doi: 10.1073/pnas.91.19.8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mansky K C, Batiza A, Lambert P F. Bovine papillomavirus type 1 E1 and simian virus 40 large T antigen share regions of sequence similarity required for multiple functions. J Virol. 1997;71:7600–7608. doi: 10.1128/jvi.71.10.7600-7608.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McBride A A, Bolen J B, Howley P M. Phosphorylation sites of the E2 transcriptional regulatory proteins of bovine papillomavirus type 1. J Virol. 1989;63:5076–5085. doi: 10.1128/jvi.63.12.5076-5085.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohr I J, Clark R, Sun S, Androphy E J, MacPherson P, Botchan M R. Targeting the E1 replication protein to the papillomavirus origin of replication by complex formation with the E2 transactivator. Science. 1990;250:1694–1699. doi: 10.1126/science.2176744. [DOI] [PubMed] [Google Scholar]
- 17.Muller F, Sapp M. Domains of the E1 protein of human papillomavirus type 33 involved in binding to the E2 protein. Virology. 1996;219:247–256. doi: 10.1006/viro.1996.0242. [DOI] [PubMed] [Google Scholar]
- 18.Sandler A B, Vande Pol S B, Spalholz B A. Repression of BPV-1 transcription by the E1 replication protein. J Virol. 1993;67:5079–5087. doi: 10.1128/jvi.67.9.5079-5087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarafi T R, McBride A A. Domains of the BPV-1 E1 replication protein required for origin-specific DNA binding and interaction with the E2 transactivator. Virology. 1995;211:385–396. doi: 10.1006/viro.1995.1421. [DOI] [PubMed] [Google Scholar]
- 20.Schiller J T, Kleiner E, Androphy E J, Lowy D R, Pfister H. Identification of bovine papillomavirus E1 mutants with increased transforming and transcriptional activity. J Virol. 1989;63:1775–1782. doi: 10.1128/jvi.63.4.1775-1782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sedman J, Stenlund A. The initiator protein E1 binds to the bovine papillomavirus origin of replication as a trimeric ring-like structure. EMBO J. 1996;15:5085–5092. [PMC free article] [PubMed] [Google Scholar]
- 22.Seo Y S, Muller F, Lusky M, Hurwitz J. Bovine papilloma virus (BPV)-encoded E1 protein contains multiple activities required for BPV DNA replication. Proc Natl Acad Sci USA. 1993;90:702–706. doi: 10.1073/pnas.90.2.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spalholz B A, Baker C C, Lambert P F, Howley P M. Bovine papillomavirus type-1 E2 trans-activation: characterization of the enhancers and promoters in the long control region. In: Steinberg B M, Brandsma J L, Taichman L B, editors. Papillomaviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1987. pp. 5–13. [Google Scholar]
- 24.Spalholz B A, Lambert P F, Yee C L, Howley P M. Bovine papillomavirus transcriptional regulation: localization of the E2-responsive elements of the long control region. J Virol. 1987;61:2128–2137. doi: 10.1128/jvi.61.7.2128-2137.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spalholz B A, McBride A A, Sarafi T, Quintero J. Binding of bovine papillomavirus E1 to the origin is not sufficient for DNA replication. Virology. 1993;193:201–212. doi: 10.1006/viro.1993.1116. [DOI] [PubMed] [Google Scholar]
- 26.Sun S, Thorner L, Lentz M, MacPherson P, Botchan M. Identification of a 68-kilodalton nuclear ATP-binding phosphoprotein encoded by bovine papillomavirus type 1. J Virol. 1990;64:5093–5105. doi: 10.1128/jvi.64.10.5093-5105.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thierry F, Dostatni N, Arnos F, Yaniv M. Cooperative activation of transcription by bovine papillomavirus type 1 E2 can occur over a large distance. Mol Cell Biol. 1990;10:4431–4437. doi: 10.1128/mcb.10.8.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorner L, Bucay N, Choe J, Botchan M. The product of the bovine papillomavirus type 1 modulator gene (M) is a phosphoprotein. J Virol. 1988;62:2474–2482. doi: 10.1128/jvi.62.7.2474-2482.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorner L K, Lim D A, Botchan M R. DNA-binding domain of bovine papillomavirus type 1 E1 helicase: structural and functional aspects. J Virol. 1993;67:6000–6014. doi: 10.1128/jvi.67.10.6000-6014.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ustav E, Ustav M, Szymanski P, Stenlund A. The bovine papillomavirus origin of replication requires a binding site for the E2 transcriptional activator. Proc Natl Acad Sci USA. 1993;90:898–902. doi: 10.1073/pnas.90.3.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ustav M, Stenlund A. Transient replication of BPV-1 requires two viral polypeptides encoded by the E1 and E2 open reading frames. EMBO J. 1991;10:449–457. doi: 10.1002/j.1460-2075.1991.tb07967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weisshart K, Bradley M K, Weiner B M, Schneider C, Moarefi I, Fanning E, Arthur A K. An N-terminal deletion mutant of simian virus 40 (SV40) large T antigen oligomerizes incorrectly on SV40 DNA but retains the ability to bind to DNA polymerase α and replicate SV40 DNA in vitro. J Virol. 1996;70:3509–3516. doi: 10.1128/jvi.70.6.3509-3516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson V G, Ludes-Meyers J. A bovine papillomavirus E1-related protein binds specifically to bovine papillomavirus DNA. J Virol. 1991;65:5314–5322. doi: 10.1128/jvi.65.10.5314-5322.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winokur P L, McBride A A. Separation of the transcriptional activation and replication functions of the bovine papillomavirus-1 E2 protein. EMBO J. 1992;11:4111–4118. doi: 10.1002/j.1460-2075.1992.tb05504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang L, Botchan M. Replication of bovine papillomavirus type 1 DNA initiates within an E2-responsive enhancer element. J Virol. 1990;64:5903–5911. doi: 10.1128/jvi.64.12.5903-5911.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang L, Mohr I, Fouts E, Lim D A, Nohaile M, Botchan M. The E1 protein of bovine papillomavirus 1 is an ATP-dependent DNA helicase. Proc Natl Acad Sci USA. 1993;90:5086–5090. doi: 10.1073/pnas.90.11.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang L, Mohr I, Li R, Nottoli T, Sun S, Botchan M. Transcription factor E2 regulates BPV-1 DNA replication in vitro by direct protein-protein interaction. Cold Spring Harbor Symp Quant Biol. 1991;LVI:335–346. doi: 10.1101/sqb.1991.056.01.040. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y C, Okayama H, Howley P M. Bovine papillomavirus contains multiple transforming genes. Proc Natl Acad Sci USA. 1985;82:1030–1034. doi: 10.1073/pnas.82.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yasugi T, Benson J D, Sakai H, Vidal M, Howley P M. Mapping and characterization of the interaction domains of human papillomavirus type 16 E1 and E2 proteins. J Virol. 1997;71:891–899. doi: 10.1128/jvi.71.2.891-899.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zanardi T A, Stanley C M, Saville B M, Spacek S M, Lentz M R. Modulation of bovine papillomavirus DNA replication by phosphorylation of the viral E1 protein. Virology. 1997;228:1–10. doi: 10.1006/viro.1996.8375. [DOI] [PubMed] [Google Scholar]