Abstract
Background
Vector surveillance is among the World Health Organization global vector control response (2017–2030) pillars. Human landing catches are a gold standard but difficult to implement and potentially expose collectors to malaria infection. Other methods like light traps, pyrethrum spray catches and aspiration are less expensive and less risky to collectors.
Methods
Three mosquito sampling methods (UV light traps, CDC light traps and Prokopack aspiration) were evaluated against human landing catches (HLC) in two villages of Rarieda sub-county, Siaya County, Kenya. UV-LTs, CDC-LTs and HLCs were conducted hourly between 17:00 and 07:00. Aspiration was done indoors and outdoors between 07:00 and 11:00 a.m. Analyses of mosquito densities, species abundance and sporozoite infectivity were performed across all sampling methods. Species identification PCR and ELISAs were done for Anopheles gambiae and Anopheles funestus complexes and data analysis was done in R.
Results
Anopheles mosquitoes sampled from 608 trapping efforts were 5,370 constituting 70.3% Anopheles funestus sensu lato (s.l.), 19.7% Anopheles coustani and 7.2% An. gambiae s.l. 93.8% of An. funestus s.l. were An. funestus sensu stricto (s.s.) and 97.8% of An. gambiae s.l. were Anopheles arabiensis. Only An. funestus were sporozoite positive with 3.1% infection prevalence. Indoors, aspiration captured higher An. funestus (mean = 6.74; RR = 8.83, P < 0.001) then UV-LT (mean = 3.70; RR = 3.97, P < 0.001) and CDC-LT (mean = 1.74; RR = 1.89, P = 0.03) compared to HLC. UV-LT and CDC-LT indoors captured averagely 0.18 An. arabiensis RR = 5.75, P = 0.028 and RR = 5.87, P = 0.028 respectively. Outdoors, UV-LT collected significantly higher Anopheles mosquitoes compared to HLC (An. funestus: RR = 5.18, P < 0.001; An. arabiensis: RR = 15.64, P = 0.009; An. coustani: RR = 11.65, P < 0.001). Anopheles funestus hourly biting indoors in UV-LT and CDC-LT indicated different peaks compared to HLC.
Conclusions
Anopheles funestus remains the predominant mosquito species. More mosquitoes were collected using aspiration, CDC-LTs and UV-LTs indoors and UV-LTs and CD-LTs outdoors compared to HLCs. UV-LTs collected more mosquitoes than CDC-LTs. The varied trends observed at different times of the night suggest that these methods collect mosquitoes with diverse activities and care must be taken when interpreting the results.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12936-024-04907-0.
Keywords: UV light trap, Human landing catches, Anopheles, Trapping methods
Background
The Global Vector Control Response 2017–2030 (GVCR) provides a framework to enhance vector control through improved capacity and surveillance, and through better coordination and integrated action across sectors and diseases. One of the four pillars of this strategy is enhanced vector surveillance [1]. Robust vector surveillance is critical for monitoring currently recommended vector control tools as well as to evaluate novel control strategies [2]. The objectives for vector surveillance of the World Health Organization (WHO) include: characterizing receptivity (to evaluate vector presence and density to enable selection and stratification of interventions), tracking of malaria vector densities (for selection and timing of vector control deployment by biting time or seasonality of transmission), monitoring of insecticide resistance (IR) for selecting insecticides to be used by programmes, and identifying other threats to vector control efficacy including detecting gaps in intervention coverage [3, 4]. However, the range of entomological surveillance methods currently available may lack the sensitivity to detect subtle changes in vector behaviours or may not be adequate to evaluate the performance of novel vector control tools that may target a greater diversity of adult mosquito behaviours [5, 6].
The most common vector control tools—long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS)—target indoor biting mosquitoes that are most active when people are in bed (LLINs) or that spend time resting on walls inside the house (IRS). The effectiveness of these vector control tools is threatened by changes in vector biting and resting behaviour and the diversity of their vectorial system [7]. For example, some mosquitoes either bite outdoors [8] or indoors at times that people are not under the protection of their bed nets [9]. LLINs and IRS have a direct impact on vector bionomics [10] and, historically, have been monitored using human landing catches (HLC), CDC light traps, pyrethrum spray catches, and aspiration techniques.
The HLC technique is a method in which human volunteers sit indoors and/or outdoors and collect mosquitoes that land on them throughout the night. It is considered the gold standard for sampling host-seeking mosquitoes [11] and for the estimation of entomological exposure rates [12, 13] for the evaluation of vector control interventions, and for the study of mosquito behaviour [12, 14, 15]. However, HLC is labour-intensive, exposes human collectors to potentially infectious mosquito bites, and is subjected to collector bias [16]. Other surveillance tools like light traps, pyrethrum spray catches and aspiration are less expensive and can be implemented more widely, but the information they provide is generally limited to indoor abundance. These methods either cannot be implemented outdoors at all or are thought to be inefficient in capturing mosquitoes outdoors. Furthermore, they generally do not provide information on mosquito behaviour, particularly the time and location of mosquito biting. Additionally, in the context of evaluating novel vector control tools, it is prudent assess surveillance tools that can provide information on subtle changes in mosquito behaviour, hence the inclusion of two outdoor locations in this study.
This study evaluated CDC light traps (CDC-LT), UV light traps (UV-LT) and Prokopack® aspiration (hereafter referred to as aspiration) against HLC conducted either inside or outside houses as potential tools for monitoring mosquito populations.
Methods
Study area
The study was conducted in Memba (− 0.16118, 34.36639) and Mabinju (− 0.17966, 34.37003) villages in Rarieda sub-county, Siaya county, western Kenya. Residents of the area live in scattered compounds which consist of an average of 3 houses occupied by closely related family members and interspersed with farmlands. The area immediately around the house structures is usually delineated from the surrounding farmland by a fence or hedges. The area experiences intense, year-round malaria transmission [17] with Plasmodium falciparum as the predominant malaria parasite species and Anopheles funestus, Anopheles arabiensis and Anopheles gambiae sensu stricto (s.s.) the main vectors [18, 19]. Historically, malaria transmission in western Kenya was very high with an estimated 300 infectious bites per person per year in the late 1980s and early 1990s [20]. Transmission has declined substantially since then, largely due to the scale up of insecticide treated nets through mass distributions targeting universal coverage (1 net for every 2 people) supplemented with routine distribution to pregnant women and children < 1 year [21]. However, the burden of malaria remains high with parasite prevalence at 19% in children aged 6 months to 14 years in the region [22]. Additionally, the deployment of malaria vector control tools such as ITNs has been accompanied by shifts in vector populations in this region beginning with the near complete disappearance of An. funestus [23], followed by a decline in An. gambiae s.s [19]. and a return of An. funestus [18]. Additionally, deployment of anti-vector interventions may lead to adaptive modifications in vector behavioural patterns [24–27]. These shifts in mosquito populations and vector behaviours necessitate frequent evaluation of trapping tools.
Mosquito trapping methods
Light traps
CDC light traps (CDC-LTs) (model 512) and UV light traps (UV-LTs) (model 912) (John W. Hock Company, Gainesville, Florida, U. S. A), without artificial attractants, were used. The CDC light trap uses an incandescent light, while the UV light traps are similar to CDC-LTs in design but with an ultraviolet light bulb. The traps were installed by hanging them approximately 1.5 m above the ground either indoors or outdoors. The indoor traps were placed next to a person sleeping under a bed net whereas the outdoor traps were placed either 10 m from the structure (referred to hereafter as outdoors close) or 10 m outside the compound (referred to hereafter as outdoors far) where indoor sampling was conducted. The outdoor traps were not baited and relied only on source from the CDC and UV light traps to attract mosquitoes. All light traps were powered by a rechargeable 12-V battery and were switched on at 17:00. Collection cups for the traps were replaced every hour of the night by field staff in all instances of outdoor traps and a subset of indoor light traps. Twelve light traps out of 163 indoor collections were not picked from houses during hourly trapping as the compound owners refused entry after they had gone to bed. In those houses, light traps were collected at 07:00 a.m. the next morning. Data analysis from these houses included hourly mosquito activity up to the last time entry was granted; the rest of the collections till morning were excluded in the analysis of hourly biting but aggregate numbers of mosquitoes collected throughout the night were included in comparisons of trap efficiency.
Human landing catches
To reduce the risk of transmission of Covid-19, collectors were recruited from the compounds in which they lived. Collectors were males above the age of 18 years, organized into teams comprised of 6 volunteers. The team of six volunteers per compound were split into one indoor and two outdoor locations and they worked in two shifts. The first shift ran from 17:00 until 00:00 p.m. when the next team took over until 07:00 a.m. The volunteers were trained in HLC and provided with a flashlight, a mouth aspirator, mosquito collection cups and a hurricane lamp. The hurricane lamp was placed on the ground, approximately 1 m from the HLC collectors and turned as low as possible to allow for observation of mosquitoes landing on the collector’s legs [28]. The collectors sat on a chair with their legs exposed from foot to knee and captured mosquitoes as soon as they landed on the exposed leg [29]. Collections were conducted over 45 min within each hour with a 15-min break to allow collectors to rest and to change collection cups. Each hour’s collection was kept separately in labelled paper cups with the labels bearing unique hourly codes generated by the tablet and taking into account the village code, house number, collection method, collection location, collection day and collection time. The date of collection was also written on the paper cups. Supervisors were assigned to coordinate the collection activities and ensure volunteers were consistently engaged in mosquito collections throughout each collection night. HLC data was collected using tablets, with the forms programmed in CommCare® (Dimagi, Inc, Massachusetts, USA).
Resting collections (Aspiration)
Prokopack aspirators (Model 1419, John W Hock Company, Gainesville, Florida, USA) were used to collect mosquitoes resting indoors and outdoors from 07:00 to 11:00 a.m. A total of ten sleeping structures from different compounds nearest to the light trap and HLC houses were conveniently selected for aspiration. Sampling was done by moving the aspirator along the walls and the roof, in dark corners, and underneath furniture in the house to collect indoor resting mosquitoes for 10 min in each structure. Outdoor sampling was performed by aspirating from clay pots and other water collection containers that were already present in the compound and located within 5 m of each sampled house [30]. After every collection, the samples were released into an adult mosquito cage for sorting. The sampled mosquitoes from each collection were transferred to labeled paper cups per collection separating the outdoor and indoor catches. All aspirations were conducted by trained entomology staff for exactly 10 min per structure, timed using a stopwatch.
House selection and rotation scheme
A community household survey was conducted in the two villages outlined in the months of September to October 2020. Prior to the start of the study, compound selection was done and houses with similar housing characteristics such as roof type, wall type and open eaves were recruited to participate in the study. Compounds from which mosquitoes were to be collected each night were randomly selected from the database of houses that had been identified and in the event that there were more than one inhabited houses in the compound, the primary house of the head of household was selected. A total of 160 houses were eligible for sampling during the study period. Different compounds were selected every night where the HLC, CDC-LT and UV-LT were rotated among 10 compounds in each village following a rotation schedule such that each house was sampled by each collection method the same number of times by the end of the study period. Aspiration was conducted the morning preceding HLC, CDC-LT and UV-LT in 10 compounds but close to those sampled the previous night. Each compound had only one trap type placed in three different positions: indoors, outdoors close and outdoors far. These locations were selected to enable a comprehensive assessment of the most efficient trapping method when collecting natural sugar fed mosquitoes that were likely to be captured as has been described in detail in a separate article [31]. The mosquito collections were done for 4 days every week over the two months’ study duration.
Mosquito processing
Samples were transported in cooler boxes to the field laboratory in Lwak, Asembo for morphological assessment. Entomology field supervisors and a driver collected the paper cups with mosquitoes from the HLC collectors and light trap collection cups every hour of the night and placed them in cooler boxes containing ice packs for transport to the field laboratory. Upon reception at the field laboratory, the samples were immobilized by freezing at − 20 °C for a period of 10 min and for longer storage prior to processing. In the case of power outages or when the number of samples received from the field overwhelmed the available freezer space, mosquitoes were immobilized by exposing to chloroform in a killing jar for 1 min. The mosquitoes were separated by species, sex, and the abdominal status (unfed, half-fed, fed and gravid) for females and numbers collected per trap recorded. The mosquitoes were identified morphologically using taxonomic keys [32] to differentiate between An. funestus s.l. and An. gambiae s.l. and other secondary malaria vectors.
Molecular assays
Polymerase chain reaction (PCR) was used to differentiate between mosquitoes of the An. gambiae species complex following the protocols described by Scott et al. [33] and between sibling species of the An. funestus complex using the protocols described by Koekemoer et al. [34]. All non-amplified samples were processed twice and the samples that were morphologically identified as An. gambiae, but failed to amplify were run using An. funestus primers and vice versa. Sporozoite infection rates were determined by enzyme linked immunosorbent assay (ELISA) using the protocol adapted from Wirtz et al. as described in the MR4 Methods in Anopheles Research [35, 36].
Data analysis
Vector abundance was assessed using descriptive statistics (means, SD, proportions, and 95% CI). Separate analyses of trap comparisons were conducted on the three most common female species collected: An. funestus sensu lato (s.l.), An. gambiae s.l. and Anopheles coustani s.l. Aggregated numbers of mosquitoes collected each night were estimated for the primary analyses. For HLCs, no adjustments were made for the fact that collectors were operating for only 45 min within each hour. Since the data were over-dispersed, generalized linear mixed models (GLMM) using Template Model Builder (glmmTMB) were fitted using negative binomial distribution for analysis of daily mosquito numbers by various collection methods. Daily numbers of female Anopheles mosquitoes were assessed as a function of collection method as a fixed effect while collection compound and collection day were treated as random factors apart from when evaluating An. arabiensis outdoors where mosquito counts variation was not sufficient with both collection compound and collection day as random effect. Only collection compound was treated as a random effect while modelling An. arabiensis outdoors. Pairwise comparisons of the mean numbers of each Anopheles species collected by the different trapping methods were done by Tukey’s test. For assessment of hourly trap catches, data only included structures that had at least 12–14 collections during the night; structures/nights that did not achieve this threshold were excluded from these analyses of hourly collections. All data analyses were performed using R statistical software version 4.1.2 while all figures and graphs were fitted using ggplot2 package in R. Statistical significance level was set at α = 0.05.
Results
Abundance of Anopheles mosquitoes
A total of 5,370 male and female Anopheles mosquitoes were sampled during the study period from a total of 608 trapping efforts: CDC-LT (165), UV-LT (152), aspiration (158) and HLC (133). Anopheles funestus constituted more than half (n = 3780; 70.4%) of the sampled Anopheles mosquitoes with the rest being An. gambiae s.l. (n = 385; 7.2%), An. coustani (n = 1061; 19.7%) and other Anopheles species (n = 144; 2.7%) including Anopheles pharoensis (n = 120), Anopheles rufipes (n = 16), Anopheles gibbinsi (n = 5), Anopheles maculipalpis (n = 1), Anopheles chrysti (n = 1), and Anopheles tenebrosus (n = 1). Only An. rufipes (n = 3), An. pharoensis (n = 1) and An. parensis (n = 1) of the secondary Anophelines, other than An. coustani were collected indoors; the rest were trapped outdoors (Table 1). A total of 3,562 female mosquitoes were collected during the study (2169 An. funestus, 284 An. gambiae s.l., 973 An. coustani, 136 other Anopheles). All subsequent analyses included only females.
Table 1.
Summary of mosquito numbers collected by each trapping method
| Species | Trapping location | CDC-LT | UV-LT | HLC | Resting collections | Total Females | Total Males | Total Anopheles | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | Males | Females | Males | Females | Males | Females | Males | |||||
| An. funestus | Indoor | 249 | 308 | 456 | 560 | 61 | 28 | 997 | 567 | 1763 | 1463 | 3777 |
| Outdoor | 125 | 42 | 248 | 82 | 26 | 2 | 7 | 19 | 406 | 145 | ||
| An. gambiae | Indoor | 26 | 15 | 51 | 16 | 2 | 0 | 53 | 28 | 132 | 59 | 385 |
| Outdoor | 30 | 9 | 99 | 25 | 1 | 0 | 15 | 15 | 152 | 50 | ||
| An. coustani | Indoor | 38 | 2 | 9 | 4 | 5 | 0 | 0 | 0 | 52 | 6 | 1061 |
| Outdoor | 304 | 34 | 577 | 37 | 20 | 0 | 20 | 11 | 921 | 82 | ||
| Other Anopheles Species* | Indoor | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 147 |
| Outdoor | 27 | 3 | 107 | 4 | 2 | 0 | 0 | 0 | 136 | 7 | ||
*Other Anopheles species included: An. pharoensis (N = 120), An. rufipes (N = 16), An. gibbinsi (N = 5), An. parensis (N = 3), An. maculipalpis (N = 1), An. chrysti (N = 1), and An. tenebrosus (N = 1). Only An. rufipes (N = 3) and An. parensis (N = 1) were trapped indoors. The rest were trapped outdoors
A proportion of the sampled mosquitoes (51% of An. funestus and 53% of An. gambiae) were processed for species identification by PCR and sporozoite detection using ELISA. Out of the 1760 An. funestus s.l. samples analysed by PCR, 1650 (93.8%) were confirmed to be An. funestus s.s. and 45 (2.6%) An. leesoni, while 65 (3.7%) did not amplify. A total of 214 An. gambiae s.l. were processed through PCR out of which 209 (97.8%) were confirmed to be An. arabiensis and the remaining 5 (2.3%) samples did not amplify. A sample of the three predominant species An. funestus s.l. (862/2169, 39.7%), An. arabiensis (168/284, 59.2%) and An. coustani (358/973, 36.8%) were analysed for P. falciparum sporozoite infection. Only An. funestus samples were positive, with a species specific sporozoite infection prevalence of 3.1% (27/862).
Comparison of mean numbers of Anopheles mosquitoes caught per trapping method each night/day
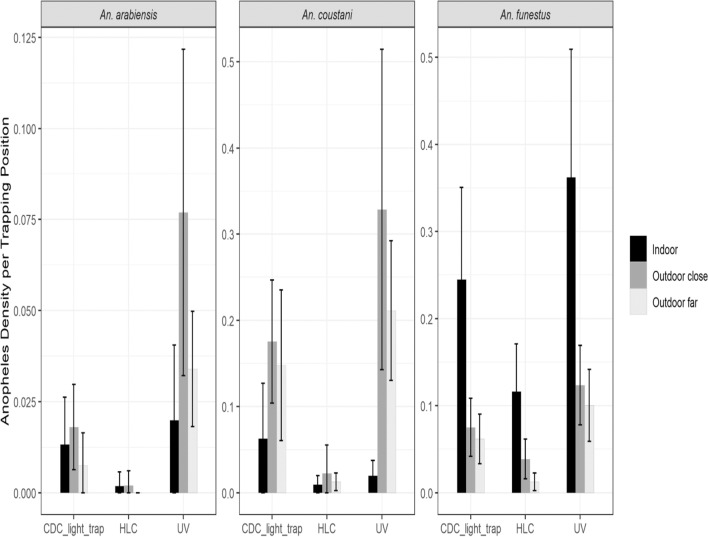
The average number of mosquitoes collected indoors each night by HLC was 0.97 for An. funestus, 0.03 for An. arabiensis, and 0.08 for An. coustani. When compared to indoor HLC, indoor aspiration method captured the highest number of An. funestus with a mean of 6.74, (RR = 8.83, 95% CI 4.72–16.52, p < 0.001) followed by indoor UV-LT with a mean of 3.70, (RR = 3.97, 95% CI 2.28–6.92, p < 0.001) then indoor CDC-LT with a mean of 1.74 (RR = 1.89, 95% CI 1.07–3.34, p = 0.03). Compared to HLC, significantly higher numbers of An. arabiensis were collected indoors by UV-LT (RR = 5.87, 95% CI 1.22–28.34) and CD-LT (RR = 5.75, 95% CI 1.20–27.48) with a mean of 0.18 each, followed by aspiration and HLC, although these were not statistically different in pairwise comparisons. For An. coustani, the CDC-LT collected the highest number of mosquitoes indoors mean of 0.29 although this difference was not statistically different compared to HLC (RR = 2.01, 95% CI 0.50–8.03, p = 0.325). The indoor UV-LT collected a mean of 0.08 An. coustani per trap-night (RR = 0.89, 95% CI 0.18–4.47, p = 0.887) while no An. coustani were collected by indoor aspiration.
Outdoors, when data was aggregated to night of collection or day of collection (for aspiration), there were no differences in the means for the two outdoor locations (outdoor far and outdoor close) for either the CDC-LT, the UV-LT or HLCs for any of the species. The data sets for the two outdoor locations were therefore combined as outdoors in the daily mean analysis. Outdoor UV-LT collected significantly higher numbers of Anopheles mosquitoes across all species analysed (An. funestus mean = 1.69, RR = 5.18, 95% CI 2.68–10.00, p < 0.001; An. arabiensis mean = 0.22, RR = 15.64, 95% CI 1.97–124.36, p = 0.009; An. coustani mean = 3.74, RR = 11.65, 95% CI 5.18–26.20, p < 0.001) when compared to outdoor HLC. Outdoor CDC-LT also collected higher mosquitoes compared to outdoor HLC for all three species (An. funestus mean = 1.00, RR = 3.09, 95% CI 1.62–5.90, p < 0.001; An. arabiensis mean = 0.15, RR = 10.81, 95% CI 1.34–87.35, p = 0.026; An. coustani mean = 2.14, RR = 11.22, 95% CI 4.95–25.43, p < 0.001 (Table 2). For outdoor aspiration, significantly fewer An. funestus were collected per sampling effort compared to HLC (mean = 0.06, RR = 0.21, 95% CI 0.07–0.67, p = 0.008).
Table 2.
Comparison of mean numbers of Anopheles mosquitoes caught by UV-LT, CDC-LT and aspiration to HLC
| Species | Collection position | Collection method* | Mean daily trap catch | RR (95% CI) | P-value |
|---|---|---|---|---|---|
| An. funestus | Indoors | CDC-LTa | 1.74 (1.02–2.45) | 1.89 (1.07–3.34) | 0.028 |
| UV-LTb | 3.70 (2.59–4.82) | 3.97 (2.28–6.92) | < 0.001 | ||
| Aspirationc | 6.74 (4.69–8.78) | 8.83 (4.72–16.52) | < 0.001 | ||
| HLCd (Ref) | 0.97 (0.61–1.39) | Ref | Ref | ||
| Outdoors | CDC-LTa | 1.00 (0.74–1.40) | 3.09 (1.62–5.90) | < 0.001 | |
| UV-LTb | 1.69 (1.06–2.32) | 5.18 (2.68–10.00) | < 0.001 | ||
| Aspirationc | 0.06 (0.01–0.12) | 0.21 (0.07–0.67) | 0.008 | ||
| HLCd (Ref) | 0.37 (0.15–0.60) | Ref | Ref | ||
| An. arabiensis | Indoors | CDC_LTa | 0.18 (0.06–0.29) | 5.75 (1.20–27.48) | 0.028 |
| UV-LTa | 0.18 (0.07–0.30) | 5.87 (1.22–28.34) | 0.028 | ||
| Aspirationab | 0.10 (0.03–0.17) | 3.38 (0.64–17.90) | 0.152 | ||
| HLCb (Ref) | 0.03 (0–0.08) | Ref | Ref | ||
| Outdoors | CDC-LTab | 0.15 (0.05–0.26) | 10.81 (1.34–87.35) | 0.026 | |
| UV-LTb | 0.22 (0.11–0.33) | 15.64 (1.97–124.36) | 0.009 | ||
| Aspirationac | 0.05 (0–0.11) | 3.59 (0.38–34.28) | 0.267 | ||
| HLCc (Ref) | 0.01 (0–0.04) | Ref | Ref | ||
| An. coustani | Indoors | CDC-LTa | 0.29 (0–0.64) | 2.01 (0.50–8.03) | 0.325 |
| UV-LTa | 0.08 (0.02–0.15) | 0.89 (0.18–4.47) | 0.887 | ||
| Aspiration | 0 | – | – | ||
| HLCa (Ref) | 0.08 (0–0.18) | Ref | Ref | ||
| Outdoors | CDC-LTa | 2.14 (0.46–3.82) | 11.22 (4.95–25.43) | < 0.001 | |
| UV-LTa | 3.74 (1.28–6.20) | 11.65 (5.18–26.20) | < 0.001 | ||
| Aspirationb | 0.23 (0.05–0.41) | 1.25 (0.30–5.17) | 0.755 | ||
| HLCb (Ref) | 0.29 (0–0.58) | Ref | Ref |
*Post hoc comparison of the trapping methods. Methods bearing the same letter do not differ significantly at 5% level. Note that for An. coustani indoors, pairwise comparisons with aspiration could not be done as there were no females of this species collected by aspiration
All variables with the same letter implies that the trapping methods do not differ significantly at 5% level based on mean daily trap catch for each mosquito species. If two variables have different letters, they are significantly different at 5% level
Pairwise comparisons of mean densities of different Anopheles species by collection methods and location are presented in Additional file 1: Table S1. When a post hoc analysis was done to compare the performance in mean mosquito collection between traps, aspiration collected statistically more An. funestus indoors mean = 6.74, RR = 8.83, 95% CI 4.72–5.16.52, p < 0.001 than UV-LT which in turn collected significantly more An. funestus (mean = 1.74; RR = 1.89, 95% CI 1.07–3.34, P = 0.03) compared to indoor CDC-LT (mean = 3.70; RR = 3.97, 95% CI 2.28–6.92, P < 0.001).
Outdoors, UV-LT collected significantly more An. funestus (mean = 1.69, RR = 5.18, 95% CI 2.68–10.00, p < 0.001) compared to CDC-LT (mean = 1.00, RR = 3.09, 95% CI 1.62–5.90, p < 0.001), which collected significantly more An. funestus compared to outdoor aspiration (mean = 0.06, RR = 0.21, 95% CI 0.07–0.67, p = 0.008). Indoors, UV-LT and CDC-LT collected significantly higher numbers of An. arabiensis compared to HLC but no other pairwise comparisons were significantly different. Outdoors, UV-LT and CDC-LT collected significantly more An. arabiensis compared to HLC while UV-LT collected significantly more An. arabiensis compared to aspiration. Outdoor UV-LT and CDC-LT collected significantly more An. coustani compared to HLC and aspiration collections but there were no differences in mean numbers of An. coustani by the different trapping methods indoors (Table 2).
Comparison of hourly mosquito collections by trapping method
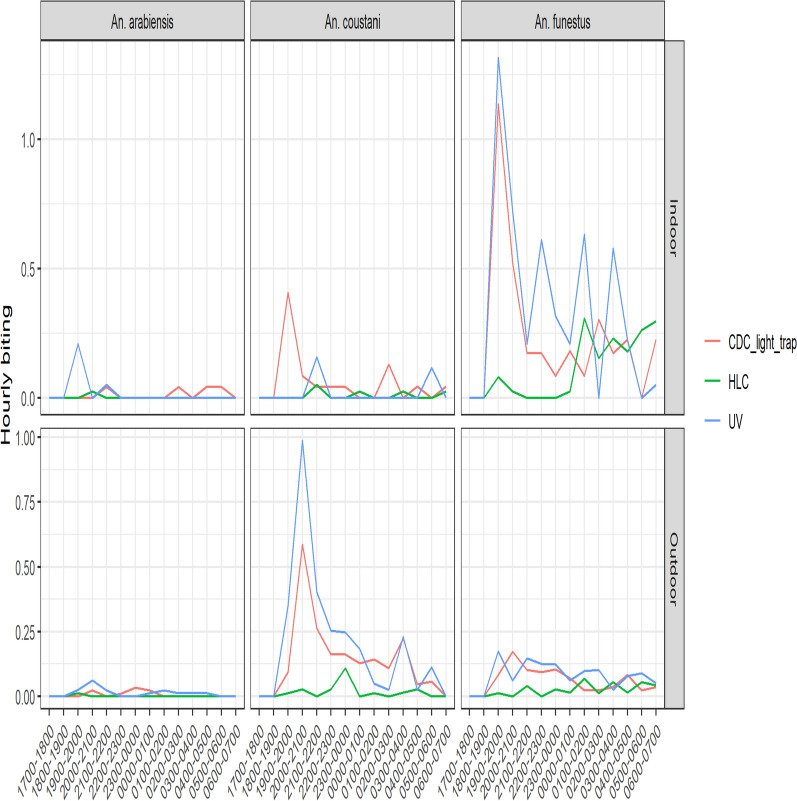
The mean number of mosquitoes captured by hour and by location using the three different collection methods are presented in Fig. 1. The hourly biting patterns are shown in Fig. 2. By HLC, biting by An. funestus was low from the start of collections until midnight when there was increased biting reaching a plateau that remained consistent throughout the remainder of the night. In contrast, a peak of activity for An. funestus was observed by both CDC-LT and UV-LT between 7 and 8 p.m. which diminished rapidly but activity was still observed throughout the night by both methods. Outdoors, An. funestus showed similar patterns although they were less distinct given the lower number of mosquitoes collected. For An. coustani outdoors, a peak in activity was observed by CDC-LT and UV-LT between 8 and 9 p.m. which declined rapidly though some activity was still observed throughout the night. Numbers of An. coustani collected by HLC outdoors or by any collection method indoors were too low to discern a pattern. Similarly, the numbers of An. arabiensis collected by the three methods both indoors and outdoors were too low to detect a clear pattern of activity throughout the night.
Fig. 1.
Comparison of UV-LT, CDC-LT and HLC at three different locations
Fig. 2.
Comparison of hourly trap catches from indoor and outdoor locations
Discussion
This study compared the efficiency of different trapping methods, placed at different locations around the peridomestic space to identify the most suitable method or set of methods to use as potential alternatives to HLCs. Anopheles funestus was predominantly caught resting indoors with aspiration being the most effective method of collection. Based on the mean numbers collected, UV-LT outperformed the CDC-LT in trapping An. funestus indoors and outdoors. The UV-LT also collected more An. arabiensis and An. coustani compared to the CDC-LT except for sampling An. coustani indoors; however, other than for An. funestus indoors, none of these observed differences were statistically significant. The UV-LT and CDC-LT trapped more mosquitoes than HLC both indoors and outdoors. Hourly biting rates in UV-LT and CDC-LT indicated different peaks in biting from that of HLC which raises questions about the physiological state and behaviour of mosquitoes captured by the different collection methods.
The observation of An. funestus as the primary vector collected during the evaluation of these trapping methods coupled with the fact that these mosquitoes were mostly captured indoors demonstrates the resilience in this vector species after years of high coverage of ITNs in the study area. Anopheles funestus reemerged [18] after almost being eliminated in the study area following the distribution of ITNs in 2000s [37]. Multiple research groups have reported resurgences of An. funestus despite sustained control efforts in multiple countries [18, 38]. The reemergence of An. funestus is likely associated with high levels of pyrethroid resistance that developed in this species [39, 40]. The fact that only indoor collected An. funestus were positive for sporozoites indicates that the bulk of malaria transmission in this area is likely propagated indoors by this species and complementary indoor vector control tools are needed to achieve malaria elimination.
All the An. gambiae s.l. caught by the different trapping methods were An. arabiensis. The predominance of An. arabiensis compared to An. gambiae s.s. following the scale up of ITNs was previously reported [19, 41–43] indicating that An gambiae s.s. has not responded in the same way as An. funestus despite the presence of phenotypic and genotypic resistance in An. gambiae s.s [44]. Anopheles arabiensis were mostly collected outdoors by light traps and aspiration from clay pots, consistent with the species’ exophilic and exophagic behaviour previously reported in in the region [45–47]. This likely has enabled them to avoid indoor deployed interventions, such as LLINs and IRS [14, 48, 49]. Despite not being detected in the current study, sporozoite positive An. arabiensis have been reported previously albeit at lower rates compared to An. funestus [18]. Given their tendency to feed and rest outdoors, An. arabiensis may contribute to residual transmission of malaria [50]. The presence of An. coustani in the peri-domestic space has been observed previously [51] but their importance for malaria transmission remains to be elucidated.
Comparison of different mosquito trapping methods indicates that mechanical aspirations indoors and UV-LT outdoors captured high numbers An. funestus mosquitoes. UV-LT performed well outdoors and indoors, second only to aspiration in the number of An. funestus mosquitoes collected indoors. UV-LTs generally collected more mosquitoes than CDC-LTs, although the difference was statistically significant only for the collection of An. funestus indoors and outdoors. It is possible that the efficacy of incandescent light in CDC-LTs may be affected by other light sources in the night including moonlight [52]. Also, mosquitoes have diverse response to different light spectra as previously reported where mosquito response to artificial light indicated that blue and green light is often more attractive than that in the yellow-orange and red regions of the visible spectrum [53, 54]. UV-LT is a largely unexplored trapping technique that could be useful for both indoor and outdoor trapping of mosquitoes especially when evaluating outdoor deployed vector control methods such as ATSBs as was recently done in Mali [55].
Fewer mosquitoes were collected by HLCs compared to both UV-LT and CDC-LT both indoors and outdoors. Previous comparisons of HLCs versus CDC LTs have resulted in diverse outcomes [56] with some indicating greater efficiency of HLCs [57, 58] and others indicating higher efficiency of CDC LTs [46, 59]. In western Kenya, HLCs were also less efficient in collecting the primary vectors compared to the Furvela tent trap, the host decoy trap, mosquito electrocuting traps and outdoor CDC light traps [52]. While HLCs are considered the gold standard for monitoring entomological measures of malaria transmission, the low numbers collected suggest they may underestimate entomological inoculation rates. The reason for the low numbers captured by HLCs is not clear as the collectors were provided adequate training and supervision. It is possible that light traps and aspiration collections capture more than just host-seeking mosquitoes [16, 56, 60].
Human landing catches remains the gold standard method for monitoring the abundance and host-seeking behaviour of mosquitoes because they elicit the natural host-seeking activity of malaria vectors using the same cues such as carbon dioxide, host odors, body heat and images. Other traps such as CDC light trap deploy light cues or artificial odors to attract mosquitoes and as such may not be used to accurately study the host-seeking activity of malaria vectors with the precision seen in HLC [61–64]. Furthermore, HLCs are easy to standardize and can be conducted in rural settings with limited access to electricity. However, HLCs are labour-intensive and potentially expose collectors to infectious mosquito bites. Therefore, CDC-LT, and less frequently UV-LT, are routinely used in monitoring Anopheles abundance during entomological surveillance. These traps are usually set up in the evening and left to run uninterrupted the whole night and therefore are unable to account for the specific hours at which mosquitoes were trapped as an indicator of host-seeking behaviour [12, 56, 65, 66]. Rotator light traps have been used to assess diel mosquito activity in studies of Aedes mosquitoes [67–69] and less frequently to monitor Anopheles activity [41, 61, 70]. In this study, CDC-LT and UV-LT bags were collected every hour through the night. Despite being labour-intensive and intrusive, this method allowed a direct comparison of the mosquito host-seeking behaviour patterns to those usually depicted by HLC. In western Kenya, previous HLC collections demonstrated a single peak in biting by An. funestus that extended from midnight until around 6 a.m. [71] similar to what was observed in this study. CDC-LT and UV-LT identified high mosquito activity early in the evening when people are unlikely to be under the protection of their bed nets. This differed from the HLC collections which is consistent with previous observations where biting was observed primarily when people were in bed and under their bed nets. Similar observations have been reported in the highlands of western Kenya [9] where it was suggested that transmission could occur at times when people were not under the protection of nets. However, the differences in collection times by the different methods raises questions about mosquito behaviour in the peridomestic space including those unrelated to host-seeking. Observations from this study suggest that while CDC-LTs and UVLTs may be useful as proxy indicators of the total mosquito population, they may not represent the host-seeking population of mosquitoes and care should be taken in interpreting the results of CDC-LTs and UVLTs as proxies for HLC in estimating EIRs. An. arabiensis densities were too low to derive any meaningful inferences on their behaviour indoors but like An. coustani, were observed to peak early in the evening outdoors.
Some limitations in this study included low number of An. funestus samples collected using HLC and limited number of An. arabiensis in all trapping methods therefore reducing the statistical power. Comparisons of the times of collection by the various methods were limited by the fact that some households refused entry while they were asleep and this may have biased the activity patterns of mosquitoes collected by CDC-LTs and UV-LTs. However, this was accounted for by limiting analyses of biting times to only those households with at least 12 hourly collections over the course of the night.
Conclusion
Anopheles funestus was the predominant malaria vector in this study with lower numbers of An. arabiensis and An. coustani. This study indicates that aspiration, CDC-LTs and UV-LTs are efficient methods for trapping Anopheles mosquitoes indoors and outdoors and often collect more mosquitoes than HLCs. Although not statistically significant, UV-LTs generally collected more mosquitoes than CDC-LTs. Different trends in collection times were observed for An. funestus when collected by CDC-LT and UV-LTs compared to HLCs. This suggests that the different collection methods are capturing mosquitoes engaged in different behaviours throughout the night.
Supplementary Information
Additional file 1: Table S1. Pairwise comparisons of means of different Anopheles species between collection methods.
Acknowledgements
We are sincerely grateful to the communities where the study was conducted, and especially the household owners for giving us access to their houses for mosquito collection. Our thanks also go to the entomology field and laboratory teams for their dedicated efforts collecting and processing the samples. We express our appreciation to Siaya county for the technical support. We also greatly appreciate the BEI resources for their assistance with the ELISA kits that was used in the sporozoite detection assays.
Disclaimer
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Kenya Medical Research Institute or the US Centers for Disease Control and Prevention.
Abbreviations
- CDC
Centers for disease control
- ELISA
Enzyme linked immunosorbent assay
- GVCR
Global Vector Control Response
- HLC
Human landing catch
- ID
Identification
- IRS
Indoor residual spray
- LLINs
Long lasting insecticidal nets
- KEMRI
Kenya Medical Research Institute
- PCR
Polymerase chain reaction
- CDC-LTs
Center for disease control light traps
- UV-LTs
Ultra violet light traps
- WHO
World Health Organization
Author contributions
JK, SO, participated in study design, coordinated sample collection and processing and drafted the manuscript, VM, BA performed statistical analysis and interpretation and manuscript reviews, DPM, MD, CO, participated in data interpretation, manuscript reviews and revisions, JEG, and EO conceptualized the study, supervised its implementation and analysis and offered technical support. All authors read and approved the final manuscript.
Funding
This study was funded by the Bill and Melinda Gates Foundation through the Innovative Vector Control Consortium (IVCC), UK, BMGF grant number (INV-007509) and the Foreign Commonwealth and Development Office (FCDO) grant number (30041–105) and the funders had no role in the study design, data collection, analysis, the decision to publish, or the preparation of the manuscript as part of the entomology validation studies preceding the epidemiological evaluation of the Attractive Targeted Sugar Baits.
Data availability
This is not applicable, however, the source document has been referenced.
Declarations
Ethics approvals and consent to participate
The study was approved by the scientific and ethics review unit (SERU) of the Kenya Medial Research Institute (protocol number KEMRI/SERU/CGHR/123/3613) and by Institutional Review Board of the US Centers for Disease Control and Prevention (IRB# 7112). Written informed consent was obtained from all HLC collectors. The HLC collectors were given mefloquine malaria prophylaxis (Cheplapharm Arzneimittel GmbH, Ziegelhof 24, 17,489 Greifswald, Germany) and regularly tested for malaria. The prophylaxis treatment started 1 week before collections began and were given repeat doses once every week through the collection period, until 4 weeks after collections ended. Verbal consent was sought from the compound head to use light traps and Prokopack aspiration in their compounds.
Competing interests
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jackline Kosgei, Email: jackieruto@yahoo.com.
Eric Ochomo, Email: ericochomo@yahoo.com.
References
- 1.WHO. Global Vector Control Response 2017–2030. Geneva: World Health Organization; 2017.
- 2.Burkot TR, Farlow R, Min M, Espino E, Mnzava A, Russell TL. A global analysis of National Malaria Control Programme vector surveillance by elimination and control status in 2018. Malar J. 2019;18:399. doi: 10.1186/s12936-019-3041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farlow R, Russell TL, Burkot TR. Nextgen Vector Surveillance Tools: sensitive, specific, cost-effective and epidemiologically relevant. Malar J. 2020;19:432. doi: 10.1186/s12936-020-03494-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Malaria surveillance, monitoring & evaluation: a reference manual. Geneva: World Health Organization; 2018.
- 5.Sougoufara S, Ottih EC, Tripet F. The need for new vector control approaches targeting outdoor biting Anopheline malaria vector communities. Parasit Vectors. 2020;13:295. doi: 10.1186/s13071-020-04170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Killeen GF, Chaki PP, Reed TE, Moyes CL, Govella NJ. Entomological surveillance as a cornerstone of malaria elimination: a critical appraisal. In: Towards malaria elimination: a leap forward. Manguin S, Dev V (eds.). IntechOpen. 2018.
- 7.Bamou R, Mbakop LR, Kopya E, Ndo C, Awono-Ambene P, Tchuinkam T, et al. Changes in malaria vector bionomics and transmission patterns in the equatorial forest region of Cameroon between 2000 and 2017. Parasit Vectors. 2018;11:464. doi: 10.1186/s13071-018-3049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherrard-Smith E, Skarp JE, Beale AD, Fornadel C, Norris LC, Moore SJ, et al. Mosquito feeding behavior and how it influences residual malaria transmission across Africa. Proc Natl Acad Sci USA. 2019;116:15086–15095. doi: 10.1073/pnas.1820646116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooke MK, Kahindi SC, Oriango RM, Owaga C, Ayoma E, Mabuka D, et al. ‘A bite before bed’: exposure to malaria vectors outside the times of net use in the highlands of western Kenya. Malar J. 2015;14:259. doi: 10.1186/s12936-015-0766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abongo B, Gimnig JE, Torr SJ, Longman B, Omoke D, Muchoki M, et al. Impact of indoor residual spraying with pirimiphos-methyl (Actellic 300CS) on entomological indicators of transmission and malaria case burden in Migori County, western Kenya. Sci Rep. 2020;10:4518. doi: 10.1038/s41598-020-61350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maliti DV, Govella NJ, Killeen GF, Mirzai N, Johnson PCD, Kreppel K, et al. Development and evaluation of mosquito-electrocuting traps as alternatives to the human landing catch technique for sampling host-seeking malaria vectors. Malar J. 2015;14:502. doi: 10.1186/s12936-015-1025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mboera LE. Sampling techniques for adult Afrotropical malaria vectors and their reliability in the estimation of entomological inoculation rate. Tanzania Health Res Bull. 2005;7:117–124. doi: 10.4314/thrb.v7i3.14248. [DOI] [PubMed] [Google Scholar]
- 13.Kilama M, Smith D, Hutchinson R, Kigozi R, Yeka A, Lavoy G, et al. Estimating the annual entomological inoculation rate for Plasmodium falciparum transmitted by Anopheles gambiae s.l. using three sampling methods in three sites in Uganda. Malar J. 2014;13:111. doi: 10.1186/1475-2875-13-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sougoufara S, Diédhiou SM, Doucouré S, Diagne N, Sembène PM, Harry M, et al. Biting by Anopheles funestus in broad daylight after use of long-lasting insecticidal nets: a new challenge to malaria elimination. Malar J. 2014;13:125. doi: 10.1186/1475-2875-13-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bockarie MJ, Alexander N, Bockarie F, Ibam E, Barnish G, Alpers M. The late biting habit of parous Anopheles mosquitoes and pre-bedtime exposure of humans to infective female mosquitoes. Trans R Soc Trop Med Hyg. 1996;90:23–25. doi: 10.1016/S0035-9203(96)90465-4. [DOI] [PubMed] [Google Scholar]
- 16.Kenea O, Balkew M, Tekie H, Gebre-Michael T, Deressa W, Loha E, et al. Comparison of two adult mosquito sampling methods with human landing catches in south-central Ethiopia. Malar J. 2017;16:30. doi: 10.1186/s12936-016-1668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong J, Bayoh N, Olang G, Killeen G, Hamel M, Vulule J, et al. Standardizing operational vector sampling techniques for measuring malaria transmission intensity: evaluation of six mosquito collection methods in western Kenya. Malar J. 2013;12:143. doi: 10.1186/1475-2875-12-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCann RS, Ochomo E, Bayoh MN, Vulule JM, Hamel MJ, Gimnig JE, et al. Reemergence of Anopheles funestus as a vector of Plasmodium falciparum in Western Kenya after long-term implementation of insecticide-treated bed nets. Am J Trop Med Hyg. 2014;90:597–604. doi: 10.4269/ajtmh.13-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province. Kenya Malar J. 2010;9:62. doi: 10.1186/1475-2875-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beier JC, Perkins PV, Onyango FK, Gargan TP, Oster CN, Whitmire RE, et al. Characterization of malaria transmission by Anopheles (Diptera, Culicidae) in western Kenya in preparation for malaria vaccine trials. J Med Entomol. 1990;27:570–577. doi: 10.1093/jmedent/27.4.570. [DOI] [PubMed] [Google Scholar]
- 21.NMCP. Towards a Malaria free Kenya; Kenya Malaria Strategy 2019–2023. Ministry of Health, Nairobi, Kenya, 2019.
- 22.DNMP, ICF. Kenya Malaria Indicator Survey 2020. Nairobi, Kenya and Rockville, USA: 2021.
- 23.Gimnig JE, Vulule JM, Kamau L, Kolczak MS, Phillips-Howard PA, et al. Impact of permethrin-treated bednets on entomological indices in an area of intense year-round malaria transmission. Am J Trop Med Hyg. 2003;68:16–22. doi: 10.4269/ajtmh.2003.68.16. [DOI] [PubMed] [Google Scholar]
- 24.Forson AO, Hinne IA, Dhikrullahi SB, Sraku IK, Mohammed AR, Attah SK, et al. The resting behavior of malaria vectors in different ecological zones of Ghana and its implications for vector control. Parasit Vectors. 2022;15:246. doi: 10.1186/s13071-022-05355-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy MR, Overgaard HJ, Abaga S, Reddy VP, Caccone A, Kiszewski AE, et al. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island. Equatorial Guinea Malar J. 2011;10:184. doi: 10.1186/1475-2875-10-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odero J, Abongo B, Moshi V, Ekodir S, Harvey S, Ochomo E, et al. Early morning anopheline mosquito biting, a potential driver of malaria transmission in Busia County, western Kenya. Malar J. 2024;23:66. doi: 10.1186/s12936-024-04893-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omondi S, Kosgei J, Musula G, Muchoki M, Abongo B, Agumba S, et al. Late morning biting behaviour of Anopheles funestus is a risk factor for transmission in schools in Siaya, western Kenya. Malar J. 2023;22:366. doi: 10.1186/s12936-023-04806-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Service MW. Mosquito Ecology: Field Sampling Methods. Elsevier Applied Science, 1973.
- 29.Service MW A critical review of procedures for sampling populations of adult mosquitoes. Bull Entomol Res. 1993;67:343–382. doi: 10.1017/S0007485300011184. [DOI] [Google Scholar]
- 30.Odiere M, Bayoh M, Gimnig J, Vulule J, Irungu L, Walker E. Sampling outdoor, resting Anopheles gambiae and other mosquitoes (Diptera: Culicidae) in western Kenya with clay pots. J Med Entomol. 2007;44:14–22. doi: 10.1093/jmedent/41.5.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Omondi S, Kosgei J, Agumba S, Polo B, Yalla N, Moshi V, et al. Natural sugar feeding rates of Anopheles mosquitoes collected by different methods in western Kenya. Sci Rep. 2022;12:20596. doi: 10.1038/s41598-022-25004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coetzee M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae) Malar J. 2020;19:70. doi: 10.1186/s12936-020-3144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 34.Koekemoer LL, Kamau L, Hunt RH, Coetzee M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg. 2002;66:804–811. doi: 10.4269/ajtmh.2002.66.804. [DOI] [PubMed] [Google Scholar]
- 35.Wirtz RA, Zavala F, Charoenvit Y, Campbell GH, Burkot T, Schneider I, et al. Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull World Health Organ. 1987;65:39–45. [PMC free article] [PubMed] [Google Scholar]
- 36.Wirtz R, Avery M, Benedict M. Specific Anopheles techniques. 3.3 Plasmodium sporozoite ELISA. Malaria Research and Reference Reagent Resource Center. MR4. 2007.
- 37.Gimnig J, Kolczak M, Hightower A, Vulule J, Schoute E, Kamau L, et al. Effect of permethrin-treated bed nets on the spatial distribution of malaria vectors in Western Kenya. Am J Trop Med Hyg. 2003;68:115–120. doi: 10.4269/ajtmh.2003.68.115. [DOI] [PubMed] [Google Scholar]
- 38.Machani MG, Ochomo E, Amimo F, Kosgei J, Munga S, Zhou G, et al. Resting behaviour of malaria vectors in highland and lowland sites of western Kenya: implication on malaria vector control measures. PLoS ONE. 2020;15:e0224718. doi: 10.1371/journal.pone.0224718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinda PG, Eichenberger C, Ngowo HS, Msaky DS, Abbasi S, Kihonda J, et al. Comparative assessment of insecticide resistance phenotypes in two major malaria vectors, Anopheles funestus and Anopheles arabiensis in south-eastern Tanzania. Malar J. 2020;19:408. doi: 10.1186/s12936-020-03483-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matowo NS, Martin J, Kulkarni MA, Mosha JF, Lukole E, Isaya G, et al. An increasing role of pyrethroid-resistant Anopheles funestus in malaria transmission in the Lake Zone. Tanzania Sci Rep. 2021;11:13457. doi: 10.1038/s41598-021-92741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ototo EN, Mbugi JP, Wanjala CL, Zhou G, Githeko AK, Yan G. Surveillance of malaria vector population density and biting behaviour in western Kenya. Malar J. 2015;14:244. doi: 10.1186/s12936-015-0763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitau J, Oxborough RM, Tungu PK, Matowo J, Malima RC, Magesa SM, et al. Species shifts in the Anopheles gambiae complex: do LLINs successfully control Anopheles arabiensis? PLoS ONE. 2012;7:e31481. doi: 10.1371/journal.pone.0031481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lwetoijera DW, Harris C, Kiware SS, Dongus S, Devine GJ, McCall PJ, et al. Increasing role of Anopheles funestus and Anopheles arabiensis in malaria transmission in the Kilombero Valley. Tanzania Malar J. 2014;13:331. doi: 10.1186/1475-2875-13-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Githinji EK, Irungu LW, Ndegwa PN, Machani MG, Amito RO, Kemei BJ, et al. Species composition, phenotypic and genotypic resistance levels in major malaria vectors in Teso North and Teso South Subcounties in Busia County. Western Kenya J Parasitol Res. 2020;2020:3560310. doi: 10.1155/2020/3560310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charlwood JD, Kessy E, Yohannes K, Protopopoff N, Rowland M. Studies on the resting behaviour and host choice of Anopheles gambiae and An arabiensis from Muleba. Tanzania. Med Vet Entomol. 2018;32:263–270. doi: 10.1111/mve.12299. [DOI] [PubMed] [Google Scholar]
- 46.Fornadel CM. Analysis of Anopheles arabiensis blood feeding behavior in southern Zambia during the two years after introduction of insecticide-treated bed nets. Am J Trop Med Hyg. 2010;83:848–853. doi: 10.4269/ajtmh.2010.10-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Githeko AK, Adungo NI, Karanja DM, Hawley WA, Vulule JM, Seroney IK, et al. Some observations on the biting behavior of Anopheles gambiae s.s., Anopheles arabiensis, and Anopheles funestus and their implications for malaria control. Exp Parasitol. 1996;82:306–315. doi: 10.1006/expr.1996.0038. [DOI] [PubMed] [Google Scholar]
- 48.Sanou A, Nelli L, Guelbéogo WM, Cissé F, Tapsoba M, Ouédraogo P, et al. Insecticide resistance and behavioural adaptation as a response to long-lasting insecticidal net deployment in malaria vectors in the Cascades region of Burkina Faso. Sci Rep. 2021;11:17569. doi: 10.1038/s41598-021-96759-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Musiime AK, Smith DL, Kilama M, Rek J, Arinaitwe E, Nankabirwa JI, et al. Impact of vector control interventions on malaria transmission intensity, outdoor vector biting rates and Anopheles mosquito species composition in Tororo. Uganda Malar J. 2019;18:445. doi: 10.1186/s12936-019-3076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carnevale P, Manguin S. Review of issues on residual malaria transmission. J Infect Dis. 2021;223 Suppl_2:S61-S80. [DOI] [PMC free article] [PubMed]
- 51.Mwangangi JM, Muturi EJ, Muriu SM, Nzovu J, Midega JT, Mbogo C. The role of Anopheles arabiensis and Anopheles coustani in indoor and outdoor malaria transmission in Taveta District. Kenya Parasit Vectors. 2013;6:114. doi: 10.1186/1756-3305-6-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abongo B, Gimnig JE, Longman B, Odongo T, Wekesa C, Wewile A, et al. Comparison of four outdoor mosquito trapping methods as potential replacements for human landing catches in western Kenya. Parasit Vectors. 2021;14:320. doi: 10.1186/s13071-021-04794-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Y-N, Liu Y-J, Chen Y-C, Ma H-Y, Lee H-Y. Enhancement of mosquito trapping efficiency by using pulse width modulated light emitting diodes. Sci Rep. 2017;7:40074. doi: 10.1038/srep40074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burkett DA, Butler JF, Kline DL. Field evaluation of colored light-emitting diodes as attractants for woodland mosquitoes and other Diptera in north central Florida. J Am Mosq Control Assoc. 1998;14:186–195. [PubMed] [Google Scholar]
- 55.Traore MM, Junnila A, Traore SF, Doumbia S, Revay EE, Kravchenko VD, et al. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali. West Africa Malar J. 2020;19:72. doi: 10.1186/s12936-020-3132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Briët OJ, Huho BJ, Gimnig JE, Bayoh N, Seyoum A, Sikaala CH, et al. Applications and limitations of Centers for Disease Control and Prevention miniature light traps for measuring biting densities of African malaria vector populations: a pooled-analysis of 13 comparisons with human landing catches. Malar J. 2015;14:247. doi: 10.1186/s12936-015-0761-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hii JLK, Smith T, Mai A, Ibam E, Alpers MP. Comparison between anopheline mosquitoes (Diptera: Culicidae) caught using different methods in a malaria endemic area of Papua New Guinea. Bull Entomol Res. 2000;90:211–219. doi: 10.1017/S000748530000033X. [DOI] [PubMed] [Google Scholar]
- 58.Lines J, Curtis C, Wilkes T, Njunwa K. Monitoring human-biting mosquitoes (Diptera: Culicidae) in Tanzania with light-traps hung beside mosquito nets. Bull Entomol Res. 1991;81:77–84. doi: 10.1017/S0007485300053268. [DOI] [Google Scholar]
- 59.Davis JR, Hall T, Chee EM, Majala A, Minjas J, Shiff CJ. Comparison of sampling anopheline mosquitoes by light-trap and human-bait collections indoors at Bagamoyo. Tanzania Med Vet Entomol. 1995;9:249–255. doi: 10.1111/j.1365-2915.1995.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 60.Lima JB, Rosa-Freitas MG, Rodovalho CM, Santos F, Lourenço-de-Oliveira R. Is there an efficient trap or collection method for sampling Anopheles darlingi and other malaria vectors that can describe the essential parameters affecting transmission dynamics as effectively as human landing catches? - A Review. Mem Inst Oswaldo Cruz. 2014;109:685–705. doi: 10.1590/0074-0276140134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Degefa T, Yewhalaw D, Zhou G, Atieli H, Githeko AK, Yan G. Evaluation of human-baited double net trap and human-odour-baited CDC light trap for outdoor host-seeking malaria vector surveillance in Kenya and Ethiopia. Malar J. 2020;19:174. doi: 10.1186/s12936-020-03244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meza FC, Kreppel KS, Maliti DF, Mlwale AT, Mirzai N, Killeen GF, et al. Mosquito electrocuting traps for directly measuring biting rates and host-preferences of Anopheles arabiensis and Anopheles funestus outdoors. Malar J. 2019;18:83. doi: 10.1186/s12936-019-2726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sikaala CH, Killeen GF, Chanda J, Chinula D, Miller JM, Russell TL, et al. Evaluation of alternative mosquito sampling methods for malaria vectors in Lowland South - East Zambia. Parasit Vectors. 2013;6:91. doi: 10.1186/1756-3305-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abongo B, Yu X, Donnelly MJ, Geier M, Gibson G, Gimnig J, et al. Host Decoy Trap (HDT) with cattle odour is highly effective for collection of exophagic malaria vectors. Parasit Vectors. 2018;11:533. doi: 10.1186/s13071-018-3099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y, Su X, Zhou G, Zhang H, Puthiyakunnon S, Shuai S, et al. Comparative evaluation of the efficiency of the BG-Sentinel trap, CDC light trap and Mosquito-oviposition trap for the surveillance of vector mosquitoes. Parasit Vectors. 2016;9:446. doi: 10.1186/s13071-016-1724-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sriwichai P, Karl S, Samung Y, Sumruayphol S, Kiattibutr K, Payakkapol A, et al. Evaluation of CDC light traps for mosquito surveillance in a malaria endemic area on the Thai-Myanmar border. Parasit Vectors. 2015;8:636. doi: 10.1186/s13071-015-1225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anderson JF, McKnight S, Ferrandino FJ. Aedes japonicus japonicus and associated woodland species attracted to Centers for Disease Control and Prevention miniature light traps baited with carbon dioxide and the Traptech mosquito lure. J Am Mosq Control Assoc. 2012;28:184–191. doi: 10.2987/12-6260R.1. [DOI] [PubMed] [Google Scholar]
- 68.Chao S, Zhu D, Dixon D, Khater E, Xue RD. Diel activity patterns of adult female mosquitoes (Diptera: Culicidae) determined by a novel rotated trap in northeastern Florida, USA. J Vector Ecol. 2019;44:149–153. doi: 10.1111/jvec.12339. [DOI] [PubMed] [Google Scholar]
- 69.Smith M, Dixon D, Bibbs C, Autry D, Xue RD. Diel patterns of Aedes aegypti (Diptera: Culicidae) after resurgence in St. Augustine, Florida as collected by a mechanical rotator trap. J Vector Ecol. 2018;43:201–204. doi: 10.1111/jvec.12302. [DOI] [PubMed] [Google Scholar]
- 70.Kakilla C, Manjurano A, Nelwin K, Martin J, Mashauri F, Kinung'hi SM, et al. Malaria vector species composition and entomological indices following indoor residual spraying in regions bordering Lake Victoria. Tanzania Malar J. 2020;19:383. doi: 10.1186/s12936-020-03452-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bayoh MN, Walker ED, Kosgei J, Ombok M, Olang GB, Githeko AK, et al. Persistently high estimates of late night, indoor exposure to malaria vectors despite high coverage of insecticide treated net. Parasit Vectors. 2014;7:380. doi: 10.1186/1756-3305-7-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Pairwise comparisons of means of different Anopheles species between collection methods.
Data Availability Statement
This is not applicable, however, the source document has been referenced.