Abstract
The present study was designed to investigate the endocytic pathway involved in canine parvovirus (CPV) infection. Reduced temperature (18°C) or the microtubule-depolymerizing drug nocodazole was found to inhibit productive infection of canine A72 cells by CPV and caused CPV to be retained in cytoplasmic vesicles as indicated by immunofluorescence microscopy. Consistent with previously published results, these data indicate that CPV enters a host cell via an endocytic route and further suggest that microtubule-dependent delivery of CPV to late endosomes is required for productive infection. Cytoplasmic microinjection of CPV particles was used to circumvent the endocytosis and membrane fusion steps in the entry process. Microinjection experiments showed that CPV particles which were injected directly into the cytoplasm, thus avoiding the endocytic pathway, were unable to initiate progeny virus production. CPV treated at pH 5.0 prior to microinjection was unable to initiate virus production, showing that factors of the endocytic route other than low pH are necessary for the initiation of infection by CPV.
Before viruses can replicate in the host cell and infection can occur, they must cross the plasma membrane barrier and then target their genome and accessory proteins to the right organelle. To begin a successful infection, a virus binds to the surface of a receptive cell via surface proteins of the virion interacting with structures on the target cell. The enveloped viruses bound to the cell surface enter the host either by direct fusion or by receptor-mediated endocytosis (21, 25, 26). In the endosomes they encounter an acidic pH (25, 37). The role of conformational changes in the viral capsid proteins, which are induced either by interaction with the receptor and/or by an acidic endosomal environment, is very important in the delivery of viral particles or genome from endosomes (17, 23–25). The low pH in the endocytic pathway is not always needed for the initiation of infection (18, 22, 24, 31, 34, 39). The process of entry for several enveloped animal viruses is fairly well known. For nonenveloped viruses, however, the mechanism of viral entry into a host cell is far from understood. Some nonenveloped viruses are known to use receptor-mediated endocytosis to gain access to the interior of a cell (13, 27, 32). Some nonenveloped viruses can even penetrate cells directly through the plasma membrane. This kind of direct penetration has been suggested, e.g., for rotaviruses (20). However, the mechanism of the penetration of the endosomal membrane barrier by nonenveloped viral particles or viral genome is not well understood.
Canine parvovirus (CPV) is a nonenveloped DNA virus of the autonomous Parvoviridae family that causes enteritis and myocarditis in canidae (10, 33). CPV emerged in 1978 as a new virus infecting dogs, and it was probably derived as a variant of the feline panleucopenia virus (35, 36). The infectious entry pathway in A72 cells starts with the binding of CPV to specific attachment molecules, identified as 40- to 42-kDa glycoproteins (5). Early events in the entry of the virus are not completely understood, but on the basis of ultrastructural studies, CPV particles have been localized inside endosome-like structures in infected A72 cells (4). CPV has also been shown to pass through an acidic compartment to initiate a productive infection (4). CPV replicates in the nucleus. The uncoating process and the release of DNA have been suggested to take place in the nucleus, and an incoming capsid is believed to be involved in the initiation of viral gene expression (10).
In this study we focus on the endocytic route of CPV by using two complementary approaches. First we studied the effects of two endocytic transport-blocking factors, reduced temperature and nocodazole, on CPV entry. Second, we microinjected CPV particles directly into the cell cytoplasm in order to assess the importance of the endocytic pathway to productive infection (11, 12). To further elucidate the possible role of the acidic endosomal environment in the nuclear transport of the virus, which is needed for successful infection of the cell, injection experiments were performed after pretreatment of viral particles with acidic buffers (18). The intracellular location of viral antigens was analyzed immunocytochemically. Both endocytic transport-blocking factors used in the present study interfere with the endosomal traffic between peripheral-early and perinuclear-late endosomes. Inhibition of virus proliferation in the presence of these agents would support a role for the endocytic pathway in CPV entry. Nocodazole blocks the endocytic pathway by causing the depolymerization of microtubules, a process which mediates the endosomal delivery between early and late endosomes (3, 16). A reduction in the temperature to 16 to 22°C also interrupts the endocytic membrane traffic between peripheral-early and perinuclear-late endosomes (14, 40).
Our primary aim was to determine whether transport from early to late endosomes is required for CPV to start a productive infection. In this study we were able to show that microtubule-dependent delivery of CPV from early to late endosomes is required for productive infection. Furthermore, the cytoplasmic microinjection experiments showed that even when pretreated in acidic buffers prior to microinjection, viral particles are unable to start a productive infection without travelling through the endocytic pathway.
The CPV strain used was serotype CPV-2, a wild-type strain isolated in 1980 from a clinically ill dog and then passaged six times. CPV was propagated in canine fibroma cell line A72 (6) and grown (for 72 h at 37°C in 5% CO2) in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum (Gibco, Paisley, United Kingdom). CPV was purified according a modification of the procedure of Paradiso (28, 29). The ratio of infectious to empty viral particles was estimated to be 40:60 from the sedimentation profile of hemagglutinating CPV in CsCl gradients (1). The hemagglutination inhibition titer of the stock was 60,000 to 80,000 (8). Experiments were carried out at a relatively high multiplicity of infection (MOI) in order to visualize the input of viral particles. Purified viruses were concentrated and used at an MOI of 4 to 5.
A72 cells were treated with nocodazole {methyl-[5-(2-thienylcarbonyl)-1H-benzimidazole-2-yl]-carbamate (20 μg/ml); Sigma, St. Louis, Mo.} for various periods of time, and the viral infection was carried out in either its presence or absence at 37°C. Cells were preincubated with nocodazole 60 min before the virus was introduced.
Polyclonal rabbit antiserum to raccoon parvovirus was a gift from Pirjo Veijalainen (National Veterinary and Food Research Institute, Helsinki, Finland). The antibody was used at a dilution of 1:100 (38). Mouse monoclonal antibody to α-tubulin was purchased from the Radiochemical Center (Amersham, United Kingdom). Goat anti-mouse immunoglobulin G conjugated with fluorescein and goat anti-rabbit immunoglobulin G conjugated with rhodamine were purchased from Organon Teknika Corporation (Durham, N.C.).
For the endocytic transport-blocking experiments, cell cultures were inoculated with 1.5 × 104 to 2 × 104 cells/cm2, and cells were grown for 1 day in 8-mm-diameter wells of eight-well Teflon-coated coverslips (CML, Nemours Cedex, France). Four sets of experiments were performed, and all variations of blocking experiments were conducted on four wells of the coverslips in each set. We scored the results for 100 cells from each well. Cells were synchronized by following the procedure of Cotmore and Tattersall (10). Cells were infected with CPV as described above, and mock infections were carried out for controls with phosphate-buffered saline (PBS). The coverslips were dipped after an appropriate cultivation time in PBS (pH 7.4) and fixed with methanol for 6 min at −20°C. After being rinsed with PBS, the cells were incubated with the primary antibodies diluted in 3% bovine serum albumin in PBS for 45 min and rinsed with PBS. The cells were incubated with fluorescein isothiocyanate- or rhodamine-conjugated second antibodies for 45 min and rinsed several times with PBS. The immunolabeling was carried out at room temperature. The monolayers were mounted in glycerol containing 10% PBS and 1 mg of para-phenylenediamine per ml. The cells were viewed under a fluorescence microscope.
For cytoplasmic microinjection of CPV particles, a model 5246 microinjector and a model 5171 micromanipulator (Eppendorf, Hamburg, Germany) were used, the latter being mounted on an IMT-2 inverted microscope (Olympus Optical Co., Tokyo, Japan). Capillaries for injections were prepared from glass tubing (GC 120 F-15; Clark Electromedical Instruments, Pangbourne, United Kingdom) with a model P97 capillary puller from Sutter Instruments (Novato, Calif.). For the microinjection experiments, cell cultures were inoculated with 2 × 104 to 3 × 104 cells/cm2, and the cells were grown for 2 days on round microgrid coverslips (diameter, 12 mm; grid size, 175 μm; Eppendorf), which were placed in a 35-mm-diameter dish (Nunc, Roskilde, Denmark). During microinjection, the cells were covered with 5 ml of cell culture medium. The cells were pretreated with chloroquine (200 μM) for 1 h, and microinjection was carried out in the presence of this reagent. After injection, the medium was removed and the cells were extensively washed with PBS (pH 7.4) before 2 ml of new medium was added. Approximately 100 cells were injected per coverslip. For injection, CPV particles were purified as described earlier. For some experiments, an acidic pretreatment of CPV was done before microinjection. The viral particles were treated with citrate buffer at pH 5.0 or 5.5 (100 mM citric acid, 200 mM Na2HPO3) for 30 min and then neutralized with 0.5 M Na2HPO4. After the cells were fixed with methanol, viral antigens were detected by immunofluorescence as described above.
CPV DNA synthesis was quantitated in cell monolayers at 90 min or 9 h postinfection by measuring [3H]thymidine incorporation. Briefly, cells were seeded at 2.5 × 104/cm2 into 3.2-cm-diameter culture plates, allowed to attach at 37°C for 2 h, and then inoculated with 0.4 ml of virus at an MOI of 1 with [3H]thymidine (20 μCi/ml; specific activity, 22.0 Ci/mmol; Amersham). DNA was extracted from cell cultures inoculated with virus according to the method of Hirt (19). CPV DNA was separated from host DNA on agarose gels (4, 30), and the gel slices were counted to measure the radioactivity associated with CPV DNA. The assays were run with triplicate samples.
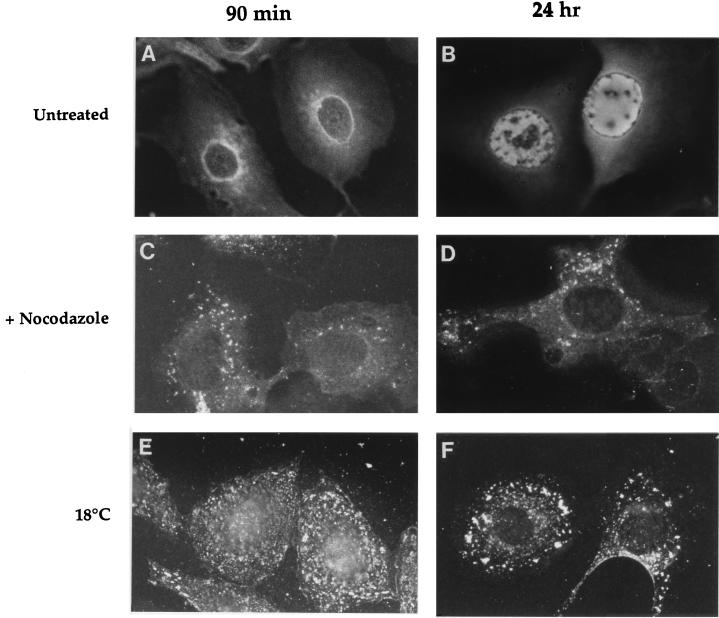
Immunofluorescence staining of nocodazole-treated cells for tubulin showed extensive disruption of microtubules within 30 min of drug addition compared with nontreated cells (not shown). At 90-min postinfection in the presence of nocodazole (Fig. 1C), immunofluorescence staining for viral antigens showed intense fluorescence which appeared to be restricted to small cytoplasmic vacuole-like structures. These fluorescent vacuoles were scattered around the cytoplasm and perinucleus. In contrast, in untreated infected cells, viral antigens were concentrated around the periphery of the nucleus (Fig. 1A). At 24 h postinfection in the presence of nocodazole (Fig. 1D), some immunofluorescence was observed in small cytoplasmic vacuoles, together with diffuse immunofluorescence in the cytoplasm. As shown in Fig. 1B, nuclear fluorescence was observed in the absence of the drug at 24-h postinfection. This nuclear fluorescence was due to the newly synthesized viral proteins. CPV infection is blocked by nocodazole when the compound is present only during the first hour of infection, suggesting that nocodazole affects the endocytic membrane traffic phase during the process of virus infection. In this assay nocodazole was added either together with the virus or at different times after virus infection. With the compound present at time 0 or added 1 h after the addition of the virus, the virus entry route was blocked. However, no protection against the virus infection was observed if nocodazole was added 2 h after the virus (not shown). Viral DNA synthesis, as measured at 9 h postinfection, was almost totally inhibited by nocodazole when the drug was added 30 min before infection and maintained thereafter. The percentage of [3H]thymidine incorporation measured from untreated CPV-infected cells was 3.7.
FIG. 1.
Immunofluorescence of CPV-infected cells in the presence and absence of factors blocking endocytic transport. Untreated A72 cells infected with CPV showing specific fluorescence in the nuclear periphery at 90-min postinfection (A) and indicating CPV proliferation at 24-h postinfection (B). Cells infected in the presence of 20 μg of nocodazole per ml showing only cytoplasmic and perinuclear fluorescence at 90-min (C) and 24-h (D) postinfection. Cells infected at 18°C showing cytoplasmic and perinuclear fluorescence at 90-min (E) and 24-h (F) postinfection.
Immunofluorescence analysis of infected cells that were held at 18°C for 90 min revealed that viral antigens were predominantly localized in numerous small vesicle-like structures throughout the cytoplasm (Fig. 1E), indicating a failure of the viral antigen to reach the nuclear membrane. Parallel control cells grown at 37°C for 90 min showed accumulation of viral antigens around the nucleus (Fig. 1A). Figure 1F shows CPV antigen immunofluorescence at 18°C at 24-h postinfection. Viral antigens were located in cytoplasmic vacuoles, whereas the specific nuclear fluorescence that was seen in control cells (Fig. 1B) was not observed.
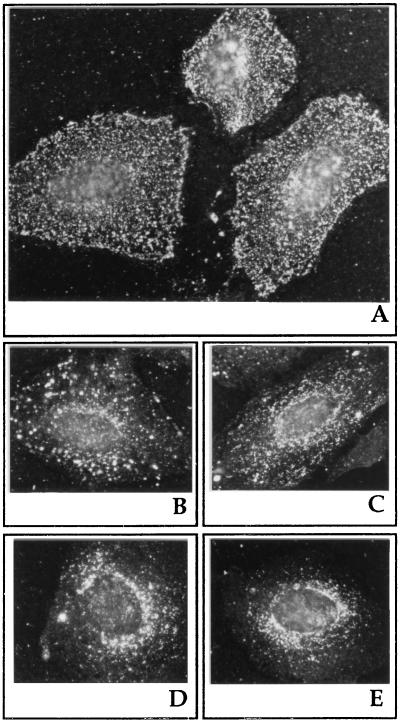
When the temperature block (90 min at 18°C) was released by incubating cells at 37°C, the viral antigens were found mostly in small cytoplasmic and perinuclear vacuolar structures at time 0 (counting from the release of the block, Fig. 2A) or 15 min (Fig. 2B). At 30 min (Fig. 2C) or 60 min (Fig. 2D) after the block was released, some viral antigens still appeared in cytoplasmic vacuoles, while most of the antigens showed a pattern of staining around the rim of the nucleus. With increasing time after the removal of the block, more and more cells were observed with viral antigens located around the nucleus. Finally, 90 min after the release of the block, most of the viral antigens were located around the nucleus (Fig. 2E). The viral DNA synthesis after the 9-h incubation of cells at low temperature (18°C) was significantly inhibited, showing only 4.2% of the amount of [3H]thymidine incorporation for untreated CPV-infected cells.
FIG. 2.
Reversibility of low-temperature block. Immunofluorescence of CPV-infected cells in the presence of the temperature block (18°C) (A) and 15 (B), 30 (C), 60 (D), and 90 (E) min after the temperature block was released (37°C). A72 cells infected with CPV were incubated for 90 min at 18°C before the block was removed.
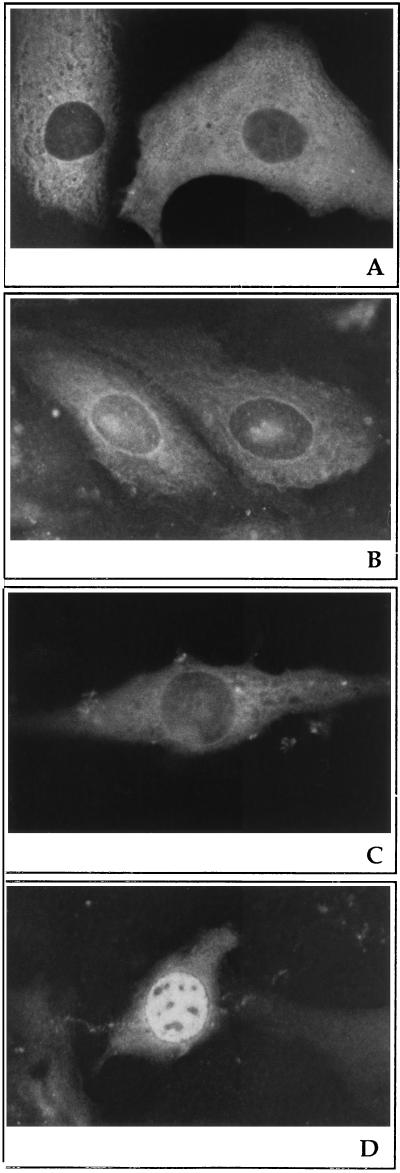
CPV microinjected into cytoplasm was found after 60 min to be distributed throughout the cytoplasm (Fig. 3A). After 20 h, viral antigens remained in the cytoplasm and displayed a circular staining pattern around the nucleus (Fig. 3B). To prevent the productive infection caused by virions internalized via the endocytic route, injections were carried out in the presence of chloroquine, which neutralizes acidic endosomal vesicles and blocks the penetration of virions from endocytic organelles to the cytoplasm. Viral DNA synthesis was almost totally inhibited by chloroquine at 90 min and at 9-h postinfection when the drug was added 30 min before infection and maintained thereafter (not shown). As shown in Fig. 3D, nuclear fluorescence due to the newly synthesized viral proteins was observed in the absence of chloroquine at 20-h postinjection. When acid-treated virus particles were microinjected into cells, viral antigens were observed by immunofluorescence microscopy to be located cytoplasmically after 60 min (not shown) and after 20 h of injection (Fig. 3C).
FIG. 3.
Sites of CPV antigen accumulation after cytoplasmic microinjection of viral particles. A72 cells were microinjected with a solution containing purified CPV particles (A and B) or low-pH-treated (pH 5.5), purified CPV particles (C) in the presence of chloroquine. After injection, cells were immediately transferred into new medium at 37°C and incubated for 60 min (A) or 20 h (B and C). Cells microinjected with CPV in the absence of chloroquine after 20 h of incubation (D). Viral antigens were visualized with anti-raccoon parvovirus antibody and rhodamine-conjugated goat anti-rabbit antibody.
Productive infection of CPV has been shown to be initiated by the absorption of virions into specific cell surface receptors identified as 40- to 42-kDa glycoproteins (5). The entry of CPV particles after binding to the receptor occurs mainly via small noncoated vesicles at 15-min postinfection (4). Then, virions are taken up in small, endosome-like vesicles. Ultrastructural studies have shown that these small vesicles containing virions fuse with larger vacuoles at 1- to 1.5-h postinfection (4). Moreover, Basak and Turner (4) have shown that infection of A72 cells by CPV was prevented by lysosomotropic bases raising the intracellular pH (NH4Cl and chloroquine), indicating that the infectious entry pathway of CPV requires the virus to pass through an acidic intracellular compartment.
Materials taken up by endocytosis generally pass through discrete compartments characterized as early and late endosomes (15). Transport from early to late endosomes is microtubule dependent and mediated by vesicular intermediates known as endosomal carrier vesicles (2, 9, 16). Disruption of the microtubule network allows the formation of endosomal carrier vesicles from peripheral-early endosomes but not their delivery to perinuclear-late endosomes (16). Carrier vesicle formation is inhibited at a reduced temperature (2, 7, 16) and by inhibition of the vacuolar H+-ATPase pump (9).
The present study was designed to investigate whether the microtubule-mediated transfer to late endosomes is a necessary step in the productive infection of CPV. We used two endocytic transport-blocking factors, reduced temperature and nocodazole. Without these treatments, CPV antigens were seen to concentrate around or close to the nuclear membrane at 90-min postinfection. Productive infection was observed 24-h postinfection with the appearance of CPV antigens in the nuclei. Nocodazole caused extensive disruption of the microtubule network in A72 cells. In the presence of nocodazole, CPV antigens were observed in small cytoplasmic vacuoles but not in the nuclear membrane at 90-min postinfection or thereafter. Maximum inhibition of CPV productive infection was observed when nocodazole was administered 60 min before the cells were treated with CPV and maintained thereafter. No significant inhibition was observed when the above reagents were added 2-h postinfection, suggesting that the virions had already passed the microtubule-dependent early step of CPV entry. Our results indicated that when CPV infection was carried out at a reduced temperature (18°C), the virus remained confined to endocytic vacuoles and a productive infection was not initiated. The blocking effect of the reduced temperature was reversible; if the temperature was restored to 37°C, infection proceeded normally. The inhibition of viral DNA synthesis in the presence of two endocytic transport-blocking factors, reduced temperature and nocodazole, supports the view that microtubule-mediated uptake is important in CPV replication. These results unequivocally demonstrate the involvement of microtubule-linked membrane traffic in CPV entry and suggest that CPV passes through late endosomes.
An attempt to circumvent the endocytic and membrane fusion steps in the entry process was made by microinjecting CPV particles into the cytoplasm. The microinjection experiments showed that CPV particles injected directly into the cytoplasm were unable to initiate progeny virus production. Delivery of virus particles into the cytoplasm resulted in the localization of viral antigens around the nucleus 20-h postinjection. Although injected viruses could reach the nucleus, they were unable to enter it. Pretreatment of CPV particles with an acidic buffer at pH 5.0 or 5.5, imitating the acidic conditions of late endosomes, did not result in a productive infection. Obviously, factors other than low pH are required for a productive infection by CPV. We cannot rule out the possibility that, after leaving the late endosomes, CPV continues to pass some other compartment before reaching the nuclear membrane. Thus, we conclude that endocytic entry, involving the exposure of virions to a low pH, is necessary but not sufficient for the initiation by CPV of a successful infection. Besides the putative low-pH-induced conformational changes of virions in the endocytic pathway, there may be conformational changes in the viral capsid proteins caused by other factors, such as interaction with the cell surface receptor.
Acknowledgments
We are grateful to Pirjo Veijalainen for the generous gift of anti-raccoon parvovirus antibodies. Many thanks to Pirjo Kauppinen for excellent technical assistance and to Paavo Niutanen and Pasi Purhonen for assistance in photography.
This study was supported by grants from the Academy of Finland (NR 29783), the Artturi and Ellen Nyyssönen foundation, and the Finnish Foundation for Research on Viral Diseases.
REFERENCES
- 1.Agbandje M, McKenna R, Rossmann M G, Strassheim M L, Parrish C R. Structure determination of feline panleucopenia virus empty particles. Proteins. 1993;16:155–171. doi: 10.1002/prot.340160204. [DOI] [PubMed] [Google Scholar]
- 2.Aniento F, Emans N, Griffiths G, Gruenberg J. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J Cell Biol. 1993;123:1373–1387. doi: 10.1083/jcb.123.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avitable E, di Gaeta S, Torrisi M R, Ward P L, Roizman B, Campadelli-Fiume G. Redistribution of microtubules and Golgi apparatus in herpes simplex virus-infected cells and their role in viral exocytosis. J Virol. 1995;69:7472–7482. doi: 10.1128/jvi.69.12.7472-7482.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basak S, Turner H. Infectious entry pathway for canine parvovirus. Virology. 1992;186:368–376. doi: 10.1016/0042-6822(92)90002-7. [DOI] [PubMed] [Google Scholar]
- 5.Basak S, Turner H, Parr S. Identification of a 40- to 42-kDa attachment polypeptide for canine parvovirus in A72 cells. Virology. 1994;205:7–16. doi: 10.1006/viro.1994.1614. [DOI] [PubMed] [Google Scholar]
- 6.Binn L N, Marchwicki R H, Stephenson E H. Establishment of a canine cell line: derivation, characterization, and viral spectrum. Am J Vet Res. 1980;41:855–860. [PubMed] [Google Scholar]
- 7.Bomsel M, Parton R, Kuznetsov S A, Schroer T A, Gruenberg J. Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell. 1990;62:719–731. doi: 10.1016/0092-8674(90)90117-w. [DOI] [PubMed] [Google Scholar]
- 8.Carmichael L E, Joubert J C, Pollock R V H. Hemagglutination by canine parvovirus: serologic studies and diagnostic applications. Am J Vet Res. 1980;41:784–791. [PubMed] [Google Scholar]
- 9.Clague M J, Urbé S, Aniento F, Gruenberg J. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J Biol Chem. 1994;269:21–24. [PubMed] [Google Scholar]
- 10.Cotmore S F, Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- 11.Diacumakos E G, Gershey E L. Uncoating and gene expression of simian virus 40 in CV-1 cell nuclei inoculated by microinjection. J Virol. 1977;24:903–906. doi: 10.1128/jvi.24.3.903-906.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliot G, O’Hare P. Intracellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 13.Greber U F, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 14.Griffiths G, Hoflavk B, Simons K, Mellman I, Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988;52:329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- 15.Gruenberg J, Howell K E. Membrane traffic in endocytosis: insights from cell-assays. Annu Rev Cell Biol. 1989;5:453–481. doi: 10.1146/annurev.cb.05.110189.002321. [DOI] [PubMed] [Google Scholar]
- 16.Gruenberg J, Griffiths G, Howell K E. Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J Cell Biol. 1989;108:1301–1316. doi: 10.1083/jcb.108.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guinea R, Carrasco L. Requirement for vacuolar proton-ATPase activity during entry of influenza virus into cells. J Virol. 1995;69:2306–2312. doi: 10.1128/jvi.69.4.2306-2312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagelstein J, Fathinejad F, Stremmel W, Galle P R. pH-independent uptake of hepatitis B virus in primary human hepatocytes. Virology. 1997;229:292–294. doi: 10.1006/viro.1996.8376. [DOI] [PubMed] [Google Scholar]
- 19.Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 20.Kaljot K T, Shaw R D, Rubin D H, Greenberg H B. Infectious rotavirus enters cells by direct cell membrane penetration, not by endocytosis. J Virol. 1988;62:1136–1144. doi: 10.1128/jvi.62.4.1136-1144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kartenbeck J, Stukenbrok H, Helenius A. Endocytosis of simian virus 40 into the endoplasmic reticulum. J Cell Biol. 1989;109:2721–2729. doi: 10.1083/jcb.109.6.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kooi C, Cervin M, Anderson R. Differentiation of acid-pH-dependent and -nondependent entry pathways for mouse hepatitis virus. Virology. 1991;180:108–119. doi: 10.1016/0042-6822(91)90014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanzrein M, Schlegel A, Kempf C. Entry and uncoating of enveloped viruses. Biochem J. 1994;302:313–320. doi: 10.1042/bj3020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsh M, Pelchen-Matthews A. Entry of animal viruses into cells. Rev Med Virol. 1993;3:173–185. [Google Scholar]
- 25.Marsh M, Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marsh M. The entry of enveloped viruses into cells by endocytosis. Biochem J. 1984;218:1–10. doi: 10.1042/bj2180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neubauer C, Frasel L, Kuechler E, Blaas D. Mechanism of entry of human rhinovirus 2 into HeLa cells. Virology. 1987;158:255–258. doi: 10.1016/0042-6822(87)90264-9. [DOI] [PubMed] [Google Scholar]
- 28.Paradiso P R. Infectious process of the parvovirus H-1: correlation of protein content, particle density and viral infectivity. Virology. 1981;39:800–807. doi: 10.1128/jvi.39.3.800-807.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paradiso P R, Rhode S L, Singer I I. Canine parvovirus: a biochemical and ultrastructural characterization. J Gen Virol. 1982;62:113–125. doi: 10.1099/0022-1317-62-1-113. [DOI] [PubMed] [Google Scholar]
- 30.Parrish C R, Carmichael L E. Characterization and recombination mapping of an antigenic and host range mutation of canine parvovirus. Virology. 1986;148:121–132. doi: 10.1016/0042-6822(86)90408-3. [DOI] [PubMed] [Google Scholar]
- 31.Pérez L, Carrasco L. Entry of poliovirus into cells does not require a low-pH step. J Virol. 1993;67:4543–4548. doi: 10.1128/jvi.67.8.4543-4548.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prchla E, Kuechler E, Blaas D, Fuchs R. Uncoating of human rhinovirus serotype 2 from late endosomes. J Virol. 1994;68:3713–3723. doi: 10.1128/jvi.68.6.3713-3723.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reed A P, Jones E V, Miller T J. Nucleotide sequence and genome organization of canine parvovirus. J Virol. 1988;62:266–276. doi: 10.1128/jvi.62.1.266-276.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rigg R J, Schaller H. Duck hepatitis B virus infection of hepatocytes is not dependent on low pH. J Virol. 1992;66:2829–2836. doi: 10.1128/jvi.66.5.2829-2836.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Truyen U, Evermann J F, Vielker E, Parrish C R. Evolution of canine parvovirus involved loss and gain of feline host range. Virology. 1996;215:186–189. doi: 10.1006/viro.1996.0021. [DOI] [PubMed] [Google Scholar]
- 36.Truyen U, Gruenberg A, Chang S F, Obermaier B, Veijalainen P, Parrish C R. Evolution of feline-subgroup parvoviruses and the control of canine host range in vivo. J Virol. 1995;69:4702–4710. doi: 10.1128/jvi.69.8.4702-4710.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varga M J, Weibull C, Everitt E. Infectious entry pathway of adenovirus type 2. J Virol. 1991;65:6061–6070. doi: 10.1128/jvi.65.11.6061-6070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vihinen-Ranta M, Lindfors E, Heiska L, Veijalainen P, Vuento M. Detection of canine parvovirus antigens with antibodies to synthetic peptides. Arch Virol. 1996;141:1741–1748. doi: 10.1007/BF01718296. [DOI] [PubMed] [Google Scholar]
- 39.Wittels M, Spear P G. Penetration of herpes simplex virus does not require a low pH-dependent endocytic pathway. Virus Res. 1990;18:271–290. doi: 10.1016/0168-1702(91)90024-p. [DOI] [PubMed] [Google Scholar]
- 40.Wolkoff A W, Klausner R D, Ashwell G, Harford J. Intracellular segregation of asialoglycoproteins and their receptor: a prelysosomal event subsequent to dissociation of the ligand-receptor complex. J Cell Biol. 1984;98:375–381. doi: 10.1083/jcb.98.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]