Abstract
Introduction & importance
Invasive ductal carcinoma is the commonest primary breast carcinoma to metastasize to the axillary nodes. Squamous carcinoma (SCC) of the breast is seen rarely as a primary breast malignancy. Breast SCC with coexistent invasive ductal/lobular carcinoma as a ‘collision tumour’ is rare.
Case presentation
A 52-year-old Sri Lankan female presented with a right sided breast lump and ipsilateral cystic axillary mass. She was diagnosed with locally advanced invasive breast carcinoma and underwent neoadjuvant chemotherapy followed by mastectomy and axillary clearance where tumour infiltration of the brachial plexus was observed. Histology revealed two separate carcinomas; an invasive carcinoma of the breast and squamous carcinoma in the axilla. A squamous primary was not found despite evaluation. The patient developed recurrent axillary ulceration due to residual tumour and was transferred for oncological care.
Clinical discussion
This patient had a biopsy-proven invasive breast carcinoma with a cystic axillary mass with lymphadenopathy. This was concluded as locally advanced breast cancer. Pathological examination of the specimen indicated the presence of two separate malignancies of the breast and axilla. No evidence of squamous metaplasia or carcinoma of the breast was seen on histology, neither was a squamous primary identified on imaging or endoscopy. Neoadjuvant therapy may have caused resolution of the squamous component.
Conclusion
The presence of two separate cancers of varied histology in the breast and ipsilateral axilla in close proximity to each other is a rare phenomenon. Clinicians must be cautious not to misinterpret it as evidence of lymphatic spread.
Keywords: Squamous cell carcinoma, Breast cancer, Collision tumour, Two cancers, Case report
Highlights
-
•
Squamous cell carcinoma (SCC) of the breast is a rare primary breast cancer and metastasis of SCC to the breast is more common.
-
•
Invasive breast carcinoma has been described in combination with primary breast SCC and SCC of the breast skin as collision tumours.
-
•
Invasive breast carcinoma with SCC of unknown primary in the ipsilateral axilla as two separate distinct tumours has not been described before.
1. Introduction
Invasive ductal carcinoma is the commonest type of breast cancer and metastasizes commonly to the axillary nodes. Squamous cell carcinoma (SCC) of the breast is a rare primary breast cancer which accounts for <0.2 % of all breast malignancies with only a few reported cases [[1], [2], [3]]. It has been described in combination with invasive ductal carcinoma as a collision tumour. More rarely it has been seen in combination with invasive lobular carcinoma of the same breast.
This is a case report of a patient with an invasive breast carcinoma but with a concurrent axillary squamous carcinoma of unknown primary. This case has been reported in line with SCARE criteria [4].
2. Case presentation
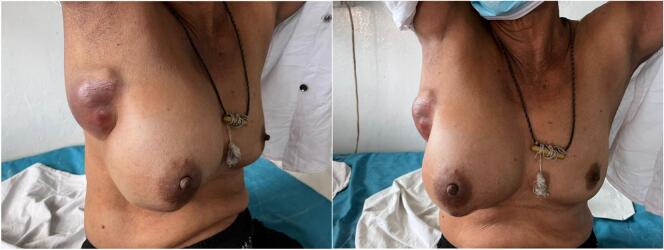
A 52-year-old, postmenopausal, previously healthy, Sri Lankan Sinhalese mother of 3 children presented to the surgical clinic with a progressively enlarging right axillary lump of 2 months' duration. She had no significant personal or familial risk factors for breast carcinoma. On clinical examination, she had a 3x3cm clinically malignant right breast lump in the 3–5 o'clock position with skin tethering and nipple retraction, and a large 10x15cm cystic axillary lump with overlying erythematous skin changes (Fig. 1). Bilateral mammography and breast ultrasound revealed a BIRADS 5 lesion at the 3 o'clock position of the right breast 1 cm from the nipple and multiple core biopsies were taken. 240 ml of straw-colored particulate fluid was aspirated from the cystic axillary lump and sent for cytological analysis. Core biopsy of the breast lesion revealed a Nottingham grade II invasive breast carcinoma (NST), which was Estrogen receptor (ER) +, Progesterone Receptor (PR) +, Human Epidermal Growth Factor receptor (Her2Neu) -, Ki67–38 % on immunohistochemical analysis. The fine needle aspirate of the axillary lump was reported as a C2 benign smear and negative for malignant cells. Staging contrast enhanced CT scan of the chest, abdomen and pelvis revealed a locally advanced breast carcinoma with enlarged ipsilateral axillary nodes without evidence of distant metastasis in the thorax or abdomen. (T4N1M0).
Fig. 1.
Initial presentation with clinically malignant breast lump and cystic axillary mass.
Following a multi-disciplinary team discussion, she was referred for neoadjuvant chemotherapy (NACT). She underwent 8 cycles of NACT over a course of 6 months during which she developed ulceration of the axillary lump. Restaging with CECT after completion of NACT revealed a right sided locally advanced breast carcinoma in lower inner quadrant measuring 3.8 cm, with ipsilateral axillary and internal mammary lymphadenopathy without evidence of distant metastasis.
Right total mastectomy and level II axillary clearance was performed by the General Surgeon under general anesthesia in the supine position. Intraoperatively a large tumour mass was seen encasing the brachial plexus and axillary vessels and was scooped out carefully. A suction drain was inserted to the axilla and routine closure was performed. The drain was removed on postoperative day 5 and the early postoperative period was uncomplicated.
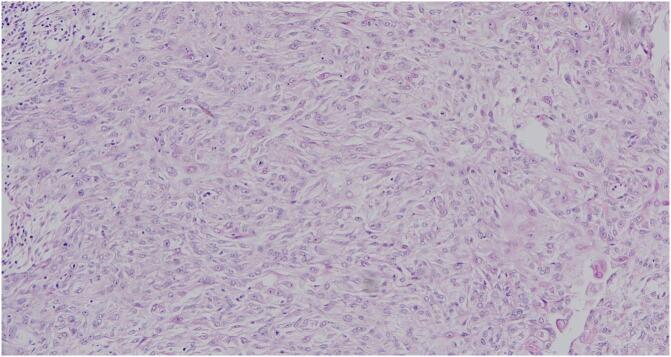
Histological examination of the specimen revealed a 25x22x15mm Nottingham grade II invasive carcinoma of the breast no specific type (NST) with high grade DCIS and perineural invasion but absent lymphovascular invasion with R0 resection margins, two reactive lymph nodes and E cadherin membrane positivity on immunochemistry (Fig. 2). The axillary mass did not contain lymph nodes as suspected but was found to be a primary invasive keratinizing squamous cell carcinoma with skin ulceration and focal involvement of the resection margins (Fig. 3). The axillary cavity was re-excised which revealed only reactive reparative changes without residual tumour.
Fig. 2.
Post NACT residual invasive carcinoma of the breast NST with high grade DCIS and perineural invasion.
Fig. 3.
H&E of the axillary mass showing keratinizing squamous cell carcinoma with skin ulceration and focal involvement of the resection margins.
Search for a squamous primary did not yield fruitful results. Macroscopic skin inspection of head, neck and thorax was normal, upper gastro intestinal endoscopy and endoscopic evaluation of the nasopharynx, oropharynx, hypopharynx and larynx did not reveal suspicious lesions. Transvaginal ultrasound scan revealed a slightly thickened endometrium but the histology from the curettage was of only atrophic endometrium. A relook of the post neoadjuvant CECT chest, abdomen and pelvis was performed by the Consultant Radiologist in view of scanning for a primary squamous lesion but was without success.
Re-excision of the axillary cavity, was complicated by the development of a non-healing wound primarily due to residual and regrowth of tumour locally. Further excisions were not possible due to risk of damage to the brachial plexus. The patient was transferred to an oncology unit 2 months after surgery for continuation of care and adjuvant therapy.
3. Discussion
Invasive ductal carcinoma is the commonest type of breast cancer and metastasizes commonly to the axillary nodes. Primary squamous carcinoma (SSC) of the breast is very rare, and thought to originate from mammary duct epithelium or from foci of squamous metaplasia within a preexisting adenocarcinoma [5]. Approximately two thirds of these tumours are cystic or have cystic components with central necrosis [6,7]. Diagnosis of SCC is reached when there are no other neoplastic components such as ductal or mesenchymal elements in the tumour, the tumour origin is independent of the nipple and absence of an associated primary squamous cell carcinoma in a second site (oral cavity bronchus, esophagus, renal pelvis, bladder, ovary, and cervix) [3]. Metastasis of squamous cell carcinoma to the breast from other sites is more common than primary breast SCC [8].
Squamous cell carcinoma of the breast has been described in combination with invasive ductal carcinoma as a collision tumour but more rarely it has been seen in combination with invasive lobular carcinoma of the same breast [9]. There are a few case reports of squamous cell carcinoma of the breast skin with invasive ductal carcinoma occurring as a collision tumour [10].
In the above case, although the patient initially presented with a cystic axillary lump, she was found to have an ipsilateral clinically malignant breast lump and was diagnosed with an invasive breast carcinoma following core biopsy. Even though the aspirate of the axillary lump was negative for malignant cells it was assumed that the axillary lump was due to axillary lymph node metastasis from the primary breast carcinoma. As the tumour was locally advanced, she was referred for NACT and developed ulceration of the axillary lesion on completion of therapy which was again thought to be due to poor treatment response and progression of the locally advanced tumour.
Intraoperatively, the axilla contained what was assumed to be a lymph node mass that encased the brachial plexus and axillary vessels which had to be scooped out in order to preserve these structures. It was only during histological examination of the surgical specimen that it was noted that the axillary mass did not contain lymph nodes but was rather a primary axillary squamous carcinoma. The mastectomy specimen was confirmed as invasive breast carcinoma NST without evidence of metaplastic squamous cell carcinoma. It contained 2 lymph nodes which were free of tumour. The two tumours were separate with no evidence of collision or connection between the two components.
Since she was post NACT, the microscopic architecture of the carcinoma had varied and there was difficulty in differentiating if it was ductal or lobular carcinoma with the appearance more in keeping with lobular carcinoma. E-cadherin immunochemistry is useful in classifying cases of breast cancer with indeterminate histological features. Negative E-cadherin stain is a sensitive and specific biomarker to confirm invasive lobular carcinoma [11]. Since the specimen stained positive for E-cadherin, the breast lesion was reported as invasive breast carcinoma NST.
Although rare, there are several case reports that describe pure squamous cell carcinoma of the breast, squamous cell carcinoma elsewhere metastasizing to the breast and squamous carcinoma occurring concurrently with primary breast ductal or lobular carcinoma [[1], [2], [3],[6], [7], [8], [9]]. This patient had two separate tumours presumably of two separate origins occurring concurrently adjacent to each other in the breast and axilla which has not been reported previously.
Pathological examination of the mastectomy specimen did not reveal and area of squamous metaplasia or squamous carcinoma. Investigations such as contrast enhanced CT of the chest, abdomen, pelvis, rhinoscopy, laryngoscopy, bronchoscopy, Oro gastroduodenoscopy and transvaginal ultrasound failed to demonstrate a possible primary squamous lesion.
Since this patient had 8 cycles of neoadjuvant chemotherapy, it is possible that there had been primary squamous lesion in the breast or elsewhere that responded to the therapy. Nonetheless, the presence of two separate primary tumours in the breast and ipsilateral axilla, of two separate origins occurring close to each other within the territory of lymphatic drainage can be easily mistaken for and mimics lymphatic spread from a breast primary. This is a unique situation that has not been reported in literature previously.
4. Conclusion
The presence of two separate cancers of different histology, in the breast and ipsilateral axilla in close proximity to each other is a rare phenomenon and has not been reported. It must be kept in mind as it can be easily misinterpreted as evidence of lymphatic spread.
Consent for publication
Written informed consent was obtained from the patient for their anonymized information to be published in this article. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
Ethical approval was deemed unnecessary by the institutional ethics committee as the paper reports a single case that emerged during normal practice.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution
Deshan Gomez: Conceptualization, Writing – original draft, Writing – review and editing, Visualization.
Sanjeewa Seneviratne: Writing – review and editing.
Guarantor
Deshan Gomez
Declaration of competing interest
The author(s) declare(s) that there is no conflict of interest.
Acknowledgments
Not applicable.
Contributor Information
Deshan Gomez, Email: deshan.gomez@yahoo.com.
Sanjeewa Seneviratne, Email: sanjeewa@srg.cmb.ac.lk.
References
- 1.Konuk, E., Arpaci, E., Ergen, S., Isik, E., & Yurdakan, G. (2017). Primary squamous cell carcinoma of the breast: a case report in a review of current literature. J. Oncol. Sci., 3(1), 29–31. Available from: https://www.sciencedirect.com/science/article/pii/S2452336416300371 [Accessed on 30 December 2023].
- 2.Yoneto, T., Hasumi, K., Yoshimoto, T., Takahashi, N., & Takeda, Y. (2018). Case report: two cases of extremely rare primary pure squamous cell carcinoma of the breast. Medicine, 97(37). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6156038/ [Accessed on 30 December 2023]. [DOI] [PMC free article] [PubMed]
- 3.Youssef, S., Brahmi, S. A., & Said, A. (2015). Primary squamous cell carcinoma of the breast: a case report and review of literature. Pan Afr. Med. J., 20. Available from: https://www.proquest.com/openview/4524312dee80c6e7e18fafb9b94d8390/1?pq-origsite=gscholar&cbl=2045576 [Accessed on 30 December 2023]. [DOI] [PMC free article] [PubMed]
- 4.Sohrabi C, Mathew G, Maria N, Kerwan A, Franchi T, Agha RA. The SCARE 2023 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. Lond. Engl. 2023;109(5):1136. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10389401/ [Accessed on 2 January 2024]. [DOI] [PMC free article] [PubMed]
- 5.Mitra, B., Pal, M., Debnath, S., Paul, B., Saha, T. N., & Maiti, A. (2011). Primary squamous cell carcinoma of breast with ipsilateral axillary lymph node metastasis: an unusual case. Int. J. Surg. Case Rep., 2(7), 194–197.6. Available from: https://www.sciencedirect.com/science/article/pii/S2210261211000630 [Accessed on 30 December 2023]. [DOI] [PMC free article] [PubMed]
- 6.Shigekawa, T., Tsuda, H., Sato, K., Ueda, S., Asakawa, H., Shigenaga, R., … & Mochizuki, H. (2007). Squamous cell carcinoma of the breast in the form of an intracystic tumor. Breast Cancer, 14, 109–112. Available from: 10.2325/jbcs.14.109 [Accessed on 30 December 2023]. [DOI] [PubMed]
- 7.Weigel, R. J., Ikeda, D. M., & Nowels, K. W. (1996). Primary squamous cell carcinoma of the breast. South. Med. J., 89(5), 511–515.8. Available from: https://europepmc.org/article/med/8638180 [Accessed on 30 December 2023]. [DOI] [PubMed]
- 8.Anne, N., Sulger, E., & Pallapothu, R. (2019). Primary squamous cell carcinoma of the breast: a case report and review of the literature. J. Surg. Case. Rep., 2019(6), rjz182. Available from: https://academic.oup.com/jscr/article/2019/6/rjz182/5518407 [Accessed on 7 February 2024]. [DOI] [PMC free article] [PubMed]
- 9.Harb, O. A., Baiomy, T. A., Abdelhamid, M. I., Gertallah, L. M., Obaya, A. A., & Basha, M. A. A. (2019). Combination of both primary pure squamous cell carcinoma (SCC) and conventional invasive lobular carcinoma (ILC) in the same breast; a rare case report and review of the literature. Human Pathol. Case Rep., 18, 200333. Available from: https://www.sciencedirect.com/science/article/pii/S2214330019300872 [Accessed on 30 December 2023].
- 10.Alawami, H. A., Al-Faraj, Z. H., Al Duhileb, M. A., AlOmran, H. A., & El Sayed, A. A. (2021). Unusual collision tumor with infiltrating ductal carcinoma and breast skin squamous cell carcinoma: a case report and literature review. Int. J. Surg. Case Rep., 78, 167–171. Available from: https://www.sciencedirect.com/science/article/pii/S2210261220311998 [Accessed on 7 February 2024]. [DOI] [PMC free article] [PubMed]
- 11.Singhai, R., Patil, V. W., Jaiswal, S. R., Patil, S. D., Tayade, M. B., & Patil, A. V. (2011). E-cadherin as a diagnostic biomarker in breast cancer. N. Am. J. Med. Sci., 3(5), 227. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3337742/ [Accessed on 7 February 2024]. [DOI] [PMC free article] [PubMed]