ABSTRACT
Introduction:
There is growing interest in the efficacy of orexin receptor antagonists (ORA), one of the new psychopharmacological agents used in the treatment of insomnia, in other psychiatric disorders such as depression.
Methods:
This meta-analysis was conducted in accordance with PRISMA requirements. Literature searches were conducted using PubMed, Scopus and EBSCO (Medline) databases. Search words were (depression OR mood disorder OR affective disorder) AND (orexin OR orx OR hypocretin OR orx1 OR orx2 OR orexin receptor antagonist OR almorexant OR suvorexant OR lemborexant OR daridorexant OR seltorexant OR vornorexant OR filorexant). No date restrictions were used. The random effects model was used for analyses with I2 values above 50% and fixed effects model was used for analyses with I2 values below 50%.
Results:
In the acute phase, ORAs had no significant effect on core, sleep-adjusted and total symptoms of depression respectively; Standardized Mean Difference (SMD) for random effect -0.422, 95% CI [-0.90; 0.06], p=0.089, I2=62.4%; SMD for random effect -0.375, 95% CI [-1.24; 0.49], p=0.400; I2=66.6% and SMD for random effect -0.477, 95% CI [-0.97; 0.01], p=0.059; I2=83.1%). However, they had a significant effect on core and total symptoms of depression in the early period respectively; SMD for fixed effect=-0.228, 95% CI [-0.44; -0.01], p=0.036, I2=9.1%; and SMD for fixed effect=-0.186, 95% CI [-0.37; -0.001], p=0.048, I2=0.0%, respectively).
Conclusion:
The results of this meta-analysis suggest that ORAs may provide direct antidepressant efficacy when added to existing antidepressant treatment and may also have indirect antidepressant effects through improvement in sleep symptoms. Considering the physiological effects of orexin on behaviors, ORAs may be promising new treatment modalities in the treatment of many psychiatric disorders other than insomnia. However, these results are preliminary and further studies with different ORAs at different doses and with different samples are needed.
Keywords: Depression, filorexant, orexin receptor antagonist, seltorexant, suvorexant
INTRODUCTION
Orexin is involved in the functioning of many behavioural processes as a mediator of a system that sends out extensive projections from the lateral hypothalamus to the brain. These processes include wakefulness, emotional behaviour, feeding and foraging, locomotion, metabolism, reward and addiction systems, sexual behaviour, vigilance and alertness (1–4).
These are observable similarities between the behavioural and autonomic functions of orexin and the symptoms of major depressive disorder (MDD). In animal studies, orexin has been shown to increase psychological resilience by stimulating Gamma-Aminobutyric Acid (GABA) activity in the pallidum, decreasing the response to foot shock in orexin knockout mice, increasing fear in open spaces, and increasing the fear response to an unpleasant odour (5,6). Acute stress increases orexinergic activity, whereas chronic stress suppresses it (7,8).
It has been suggested that the main effect of antidepressants is to increase hippocampal Brain-Derived Neurotrophic Factor (BDNF) messenger ribonucleic acid (mRNA) (9). This leads to an increase in long-term potentiation (LTP) and synaptic connections in this region. Studies suggest that orexin also increases LTP in the hippocampus. Although this has also been shown with orexin A, it has been suggested that orexin B specifically increases LTP (10). Orexin may also have antidepressant effects through its effects on the dopaminergic pathway. 20% of the projections from the lateral hypothalamus to the ventral tegmental area (VTA) are orexinergic and stimulate the VTA→nucleus accumbens (NAc) and VTA→medial prefrontal cortex (mPFC) pathways (11). The locus coeruleus and dorsal raphe also receive orexin-rich fibres. Orexin A and B provide 5-hydroxytryptamine (5-HT) stimulation from the dorsal raphe. The dorsal raphe is also important in patients with narcolepsy and depression is common in these patients. At the same time, REM latency was shortened and rapid eye movement (REM) intensity was increased in patients with both depression and narcolepsy. Again, the improvement of depression in ligin mice with therapy supports this relationship (12,13).
Highlights
Orexin receptor antagonists (ORAs) may reduce depressive symptoms when added to antidepressant treatment.
It is not clear whether the antidepressant effect of ORAs is secondary to the improvement in sleep.
Further studies with different doses of different ORAs are needed.
There is a negative correlation between cerebrospinal fluid (CSF) and serum orexin levels and depression, and depressed patients have been shown to have decreased orexin levels in the VTA, mPFC and hypothalamus (14). Clinical studies have shown a negative correlation between Hamilton Depression Rating Scale (HAMD) scores and orexin A levels (15). CSF orexin A levels have also been found to be lower in patients with depression than in patients with adjustment disorder and dysthymia. Some associations have also been found between CSF orexin levels and suicidality. Studies have found orexin A levels of 74–195 pg/ml in patients with depression (16). However, there are also studies with conflicting results. One study found no difference between orexin A levels in mania, depression and controls (17).
However, preclinical and clinical studies have produced controversial results regarding the effects of the orexin system on depression. For example, some studies have reported that inhibition of orexin neurons increases social interaction and decreases depressive behavior in susceptible individuals (18). In animal models of depression, orexin neurons have been found to be reduced in number and volume (14). However, there are also publications with opposite results. These studies found hyperactivity in the orexin system in depressed patients. In mice with experimental depression models treated with clomipramine, an increase in orexin A and B levels was observed in the hypothalamus, while a decrease was observed in multiple brain regions (19). In the forced swim test and tail suspension test, immobility decreased in orexin 1 knockout mice, whereas immobility increased in orexin 2 knockout mice. This suggests that orexin 2 has anti-depressive effects and orexin 1 has pro-depressive effects (20).
Dual orexin receptor antagonists (DORA) developed for insomnia antagonise orexin 1 and orexin 2 receptors. These drugs have been shown to increase sleep onset time and sleep duration (21). They also reduce the level of stress hormones, cortisol and norepinephrine (NE), and anxiety and depression in psychiatric patients with depression. It is thought that DORAs may have a role in the treatment of addiction and hypnotic responses (22). In addition, orexin antagonists have been shown to increase social interaction time, reduce blood pressure in hypertensive rats, and reduce the startle response to conditioned fear stimuli without muscle relaxation in rats in which high CO2 levels induce a panic-like state (23–25). Almorexant has been shown to produce similar results to fluoxetine treatment in anxiety and depression developed in animals using mild stress models (26). While these results may apply to orexin 1 antagonists, this may not be the case for orexin 2 antagonists. In a dialysis patient with insomnia, depression and generalised anxiety disorder, suvorexant treatment was reported to worsen symptoms while it could be successfully treated with antidepressants (27). In a study of filorexant in patients with depression, no beneficial change in depression scores was observed and the study was discontinued due to an increase in suicidal ideation (28). A trial of almorexant was also stopped due to similar results (29).
Studies of orexin antagonists have shown improvement in cognitive symptoms of depression as well as improvement in sleep. However, the doses used in these studies were ten times higher than those used for sleep (30). Antipanic and anxiolytic effects have also been shown at sleep doses (23). However, the evidence for the use of orexin receptor antagonists in anxiety and depression is still limited, and there are also conflicting results regarding the role of orexin receptor antagonists in the treatment of depression. In light of this information, it can be said that the orexin system plays a role in the regulation of mood and behaviour, but this role has not yet been clearly established. The clinical effects of orexin agonists and antagonists are not always predictable. The fact that the orexin 1 and 2 receptor systems have different behavioural and emotional effects further complicates the picture. Although studies have consistently shown the effects of orexin antagonists on sleep, studies of their efficacy and safety in the treatment of psychiatric disorders such as depression are inconclusive. Although significant progress has been made in the treatment of depression, there are still cases that are resistant to treatment or patients with residual symptoms despite treatment. Therefore, there is a great need for research on the clinical relevance of drugs with different mechanisms of action, such as ORAs, which have important physiological roles in behavioural and emotional regulation and support the traditional psychopharmacodynamic approach to the treatment of depression. This meta-analysis of the literature on the efficacy of orexin antagonists in treatment of depression aims to bring together the evidence on whether these drugs can help improve symptoms of depression and provide more reliable evidence on this issue.
METHOD
Protocol
We have conducted this systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (31).
Inclusion and Exclusion Criteria
Patients with depression receiving antidepressant therapy, augmentation with orexin receptor antagonists, Randomized Controlled Trials (RCTs) or uncontrolled studies with a repeated measures design and written in English were included.
Studies with missing outcome variables, studies with methods other than RCT or designs with uncontrolled repeated measures, duplicate studies, case reports and reviews, studies in a language other than English, and studies including patients with depression with psychotic symptoms were excluded.
Literature Search and Data Extraction
The literature search was conducted using PubMed, Scopus, and EBSCO (Medline). All studies were searched in the databases without any date limitation. Keywords were selected as follows; “depression OR mood disorder OR affective disorder AND orexin OR orx OR hypocretin OR orx1 OR orx2 OR orexin receptor antagonist OR almorexant OR suvorexant OR lemborexant OR daridorexant OR seltorexant OR vornorexant OR filorexant”. We also searched the references of systematic reviews on this topic. Finally, the literature was independently reviewed. After the literature was selected, the author extracted data from appropriate studies. Extracted data included the author’s name, year of publication, sample size, age, intervention, control group, the time of assessment, and outcomes. Data were recorded in acute (2–3 weeks) vs early (4–6 weeks) period outcomes*core symptoms vs total symptoms vs adjusted symptoms (2*3 design).
Outcome Measures
In this meta-analysis, the outcome measure was depression. When the trials were analysed, the effects of orexin antagonists were assessed in three ways, depending on the method of measurement. In order to evaluate the effects of ORAs on depression independently of their effects on sleep, the outcome variables were analysed separately as core symptoms of depression, sleep-adjusted symptoms of depression and total symptoms of depression. In the second phase, the acute (11 days and three weeks) and early (28 days and six weeks) results of the orexin antagonists were analysed separately. Depression was assessed using the HAMD and the Montgomery Asberg Depression Rating Scale (MADRS).
The MADRS-6 consists of a subscale focusing on core symptoms of depression, assessed by six items: overt sadness, reported sadness, inner tension, lassitude, inability to feel, and pessimistic thoughts. The sleep-adjusted MADRS (adj-MADRS) consisted of items that did not include sleep items. The sleep-adjusted subscale is also derived from the HAMD17 scale, excluding the three insomnia questions from the total score. The six core symptoms of depression in the HAMD6 are depressed mood, guilt, work and interests, psychomotor retardation, psychic anxiety and overall somatic symptoms.
Risk of Bias and Assessment of the Quality of the Evidence
The author assessed the risk of bias using the Cochrane Handbook for Systematic Reviews of Interventions to assess the methodological quality of the included literature (32). For all assessment items, the quality of each study was rated as “low risk of bias (green)”, “high risk of bias (red)”, or “some concern (yellow)”. The assessment items included randomisation process (3 questions), departure from the intended interventions (7 questions), missing outcome data (4 questions), measurement of the outcome (5 questions), selection of the reported outcome (3 questions), and overall bias. All assessments were scored using risk-of-bias tool for randomized trials (ROB2) (33).
Data Synthesis and Analysis
We calculated the mean difference (MD) with 95% confidence interval (CI) for continuous outcomes. Primary and secondary outcomes were all continuous variables, and we used the MD and its 95% CI to present them. Heterogeneity was assessed by the magnitude of tau2 and I2 statistics. A fixed effect model was used for minor heterogeneity if the I2 was lower than 50%. A random effect model with high heterogeneity was used if the I2 was above 50% (34). In subgroup analyses, the effect of orexin antagonists was analysed separately in two periods according to the time of assessment (acute vs. early outcomes). Data analysis was performed using the meta package in the R software (35,36).
RESULTS
Literature Search and Its Flow Chart
The search yielded 247 studies. After excluding duplicate studies, case reports, case series, reviews, animal studies and pharmaceutical studies, six studies were retrieved. One of them was not included in the meta-analysis because of insufficient data (only mean difference and confidence interval were reported, mean and standard deviation were not included and depression was assessed with Quick Inventory of Depressive Symptomatology, self-reported version (QIDS-SR)) (37) and the other was not included in the meta-analysis because of the presence of patients with different diagnoses in the sample (one with mild cognitive impairment, seven with alcoholic psychosis, seven with schizophrenia/schizoaffective disorder, seven with mood disorder, and one with neurotic/stress-related disorder) (22). As a result, four trials were analysed (28,38–40) (Figure 1).
Figure 1.
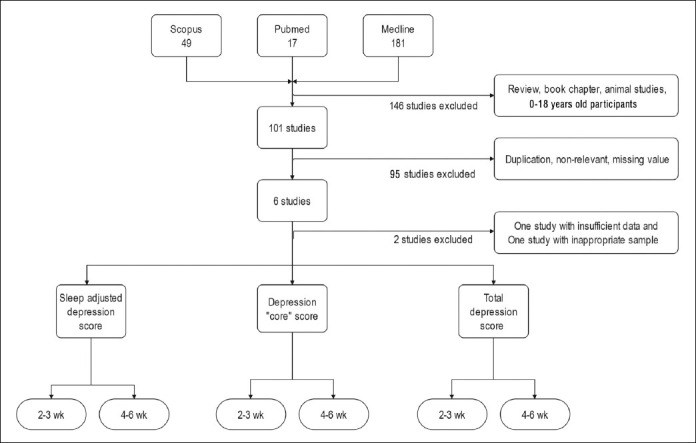
Flowchart for meta-analysis.
Characteristics of the Included Studies
Six studies were included in this meta-analysis. Three of these were randomised controlled trials. The total number of patients who received orexin receptor antagonists was 241. Of these, 120 (49.8%) were female and 121 (50.2%) were male. The mean age of the participants in this group was 48.53 (13.1). There were 90 participants in the control group, of whom 70 (77.8%) were male and 20 (22.2%) were female. The total number of controls was 213. Of these, 110 (51.6%) were female and 103 (48.4%) were male. The mean age of the control group was 47.43 (11.55).
The trial included patients with MDD. These were patients with moderate to severe depression who did not respond adequately to available antidepressant monotherapies. Patients with psychotic symptoms or active suicidal ideation were excluded. Orexin receptor antagonists were used at doses of 10 mg for filorexant, 10, 20 and 40 mg for seltorexant and 15–20 mg/g for suvorexant. Three trials had a placebo control group, while the control group in one trial consisted of patients receiving eszopiclone. The duration of treatment with orexin receptor antagonists ranged from 11 days to six weeks. Depression was assessed using the MADRS, HAMD and Beck Depression Inventory-II (BDI-II). Depression was assessed using the total scores of these scales, as well as the sleep-adjusted and modified forms with items covering the core symptoms of depression.
These trials also assessed the safety of orexin receptor antagonists. The most common side effects were headache, somnolence and nausea. No deaths, suicidal behaviour or plans were observed with the use of orexin receptor antagonists. However, one study reported an increase in suicidal thoughts (Table 1).
Table 1.
Summary of studies included in meta-analysis
| Conner 2017 | Recourt 2019 | Savitz 2021 | Shigetsura 2022 | |
|---|---|---|---|---|
| Study design | 6-week, double-blind, placebo-controlled, randomized, parallel-group study | An exploratory double-blind, placebo-controlled, multicentre study | Randomized, double-blind, parallel-group, placebo-controlled, 6-week adaptive dose-finding, multi-center phase 2b study | All investigators remained blinded to treatment allocation during the run-in period and were unblinded after randomization. |
|
| ||||
| Sample | 61 filorexant, 62 placebo. Persistence of moderate to severe, non-psychotic depressive symptoms, despite an adequate trial (dose and duration of treatment) | 24 Seltorexant, 12 placebo. MDD without psychotic symptoms, Patients were either antidepressant-naive or treated with a maximum of two concurrent monoaminergic antidepressants. | 146 Seltorexant, 137 placebo. MDD who had an inadequate response to ≥1 but ≤3 antidepressants, Under antidepressant treatment for at least 4 week to 12 week. | 9 suvorexant, 9 eszopiclone. MDD, No suicide history or high-risk patients |
|
| ||||
| Antidepressant | SSRI, SNRI or Bupropion | Nine patients SSRI, one patient Duloxetine | SSRI or SNRI monotherapy | SSRI, SNRI and Trazodone |
|
| ||||
| Adverse events | 27 (42%) filorexant, 17 (27%) placebo. Somnolence and suicidal ideation; filorexant [7, (10.9%)] > placebo [3, (4.7%)]. No death, no suicidal behavior or plan. | 16 (72%) Seltorexant, 7 (58%) placebo. Headache [2 (8.4%)] vs [2 (16.6%)] Somnolence [4 (18.1%)] vs [0 (0%)] Nausea [1 (4.1%)] vs [0 (0%)] The suicidal ideation scores either improved or were maintained from screening through the end of the study | 55 (37.7%) Seltorexant, 56 (40.9) Placebo. Headache [9 (6.2%)] vs [9 (6.6%)] Somnolence [9 (6.2%)] vs [7 (5.1%)] Nausea [8 (5.5%)] vs [4 (2.9%)] No suicidal ideation or behavior | Headache [5 (55.6%)] vs [4 (44.4%)] Somnolence [4 (44.4%)] vs [6 (66.7%)] Fatique [8 (88.9%)] vs [5 (55.6%)] No patients experienced suicidal ideation |
|
| ||||
| Orexin receptor antagonist | Filorexant 10 mg | Seltorexant 20 mg | Seltorexant 10 mg, 20 mg, 40 mg | Suvorexant 15/20 mg |
|
| ||||
| Suicide assessment | MADRS or Columbia Suicidality Severity Rating Scale | Columbia Suicide Severity Rating Scale | Columbia Suicide Severity Rating Scale | Face-to-face interview |
|
| ||||
| Outcome | MADRS (Total, sleep adjusted) HAMD17 (Total, Bech-item 1, 2, 7, 8, 10, 13) | HAMD17, HAMD6 (depressed mood, guilt feelings, work and interests, psychomotor retardation, psychic anxiety, and general somatics) | MADRS (total) and MADRS-6 (apparent sadness, reported sadness, inner tension, lassitude, inability to feel, pessimistic thoughts) | BDI-II total score |
|
| ||||
| Measurement time point | Baseline, 3 week, and 6 week | Baseline, Day 1–2, Day 11, and Day 28 | Baseline, 1, 3, and 6 week | Baseline, 2 week, and 4 week |
BDI-II: Beck Depression Inventory-II; HAMD: Hamilton Depression Rating Scale; MADRS: Montgomery Asberg Depression Rating Scale; MDD: Major depressive disorder; SNRI: Serotonin and Norepinephrine Reuptake Inhibitors; SSRI: Selective Serotonin Reuptake Inhibitors.
Methodological Quality Assessment
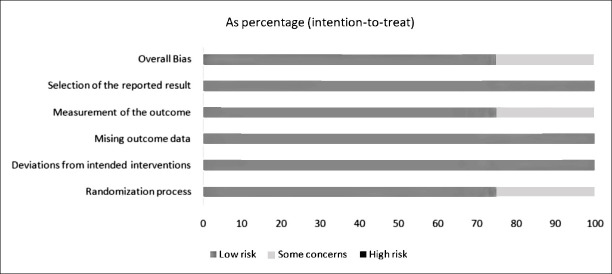
The quality of publications was assessed using ROB2. Accordingly, the data quality ranged from “low risk” to “some risk”. Only one study used eszopiclone instead of placebo at randomisation, and in this study the difference in baseline depression scores appeared to be significant. There were no significant problems in other areas. Therefore, the results of this meta-analysis are consistent (Figure 2).
Figure 2.
Risk of bias graph of studies included in meta-analysis.
Results of Meta-Analysis
The results were processed in 2 stages depending on the time of measurement. Firstly, they were processed according to the time at which the effects of the orexin antagonists were assessed, i. e. measurements taken around 2–3 weeks after treatment (acute orexin antagonist results) and assessments made at least 4–6 weeks after treatment (early orexin antagonist results). Secondly, they were processed depending on outcome variables (core, total and sleep-adjusted depression scores).
Acute Outcomes of Orexin Receptor Antagonists
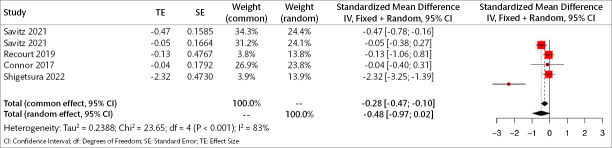
Two studies (168 patients) were included in the meta-analysis to examine the acute results of orexin antagonists on core symptoms of depression. Analyses show that, in the acute period, orexin antagonists have a significant effect on core symptoms of depression in the fixed model but not the random model (Standardized mean difference [SMD] for fixed effect=-0.369, 95% CI [-0.62; -0.10], p=0.005; SMD for random effect -0.422, 95% CI [-0.90; 0.06], p=0.089; I2=62.4%) (Figure 3).
Figure 3.
Forest plot for core depression symptoms in the acute period.
Two trials (86 patients) were included in the meta-analysis to examine the acute effects of orexin antagonists on sleep-adjusted depressive symptoms. Analyses show that in the acute period, orexin antagonists have no significant effect on sleep-adjusted depressive symptoms in both fixed and random models (SMD for fixed effect=-0.137, 95% CI [-0.46; -0.19], p=0.416; SMD for random effect -0.375, 95% CI [-1.24; 0.49], p=0.400; I2=66.6%) (Figure 4).
Figure 4.
Forest plot for sleep-adjusted depression symptoms in the acute period.
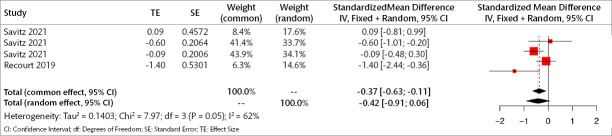
Four studies (241 patients) were included in the meta-analysis to examine the acute effects of orexin antagonists on total depression symptoms. Analyses show that, in the acute period, orexin antagonists have a significant effect on total depression score in the fixed model but not the random model (SMD for fixed effect=-0.283, 95% CI [-0.46; -0.10], p=0.023; SMD for random effect -0.477, 95% CI [-0.97; 0.01], p=0.059; I2=83.1%) (Figure 5).
Figure 5.
Forest plot for total depression symptoms in the acute period.
Early Outcomes of Orexin Receptor Antagonists
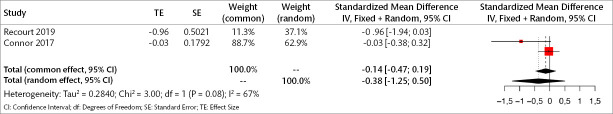
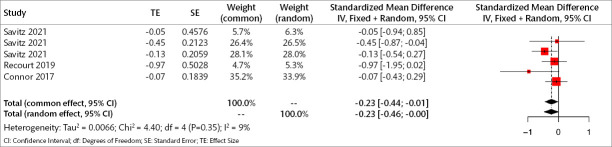
Three trials (232 patients) were included in the meta-analysis to examine the early results of orexin antagonists on core symptoms of depression. The analyses show that in the early period, orexin antagonists have a significant effect on core symptoms of depression in both fixed and random effects (SMD for fixed effect=-0.228, 95% CI [-0.44; -0.01], p=0.036; SMD for random effect -0.234, 95% CI [-0.46; -0.004], p=0.045; I2=9.1%) (Figure 6).
Figure 6.
Forest plot for core depression symptoms in the early period.
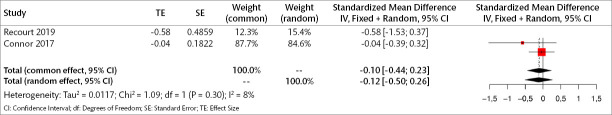
Two studies (86 patients) were included in the meta-analysis to examine the early results of orexin antagonists on sleep-adjusted depressive symptoms. Analyses show that in the early period, orexin antagonists have no significant effect on sleep-adjusted depressive symptoms in both fixed and random models (SMD for fixed effect=-0.104, 95% CI [-0.43; -0.23], p=0.540; SMD for random effect -0.120, 95% CI [-0.50; 0.26], p=0.536; I2=8.0%) (Figure 7).
Figure 7.
Forest plot for sleep-adjusted depression symptoms in the early period.
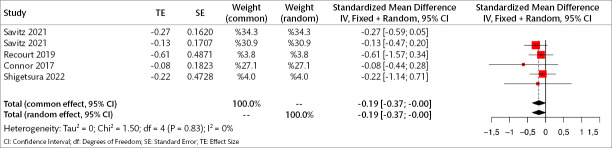
Four studies (241 patients) were included in the meta-analysis to examine the early results of orexin antagonists on total depression symptoms. Analyses show that in the early period, orexin antagonists have a significant effect on total depression score in both fixed and random effect models (SMD for fixed effect=-0.186, 95% CI [-0.37; -0.001], p=0.048; SMD for random effect -0.186, 95% CI [-0.37; -0.001], p=0.048; I2=0.0%) (Figure 8).
Figure 8.
Forest plot for total depression symptoms in the early period.
Publication Bias
Because the number of included studies for each of these outcomes was lower than 10, we could not create an inverted funnel plot to assess the influence of publication bias of the included studies.
DISCUSSION
The results of this study show that the efficacy of orexin receptor antagonists in the treatment of patients with depression in the acute phase is controversial. The interpretation of the antidepressant efficacy of orexin receptor antagonists in the acute period seems to change depending on the chosen meta-analytic method (fixed effects or random model). However, although the significance thresholds are different in both models, the fact that the SMD values are close to each other in favor of orexin receptor antagonists suggests that these drugs may have antidepressant efficacy in the acute period. Of course, there are not enough studies on this subject yet and more data are needed to interpret the results. On the other hand, although the acute effects of orexin receptor antagonists on sleep are well known, the fact that the antidepressant effects of orexin receptor antagonists occur in the early period (4–6 weeks) rather than in the acute period may be interpreted as the antidepressant effects of orexin receptor antagonists not being dependent on their effects on sleep, but direct. However, the possibility that the positive effects of the acute improvement in sleep health with orexin receptor antagonists on depression may be delayed should also be taken into consideration. In order to clarify this point, randomized-controlled and crossover studies requiring longer follow-up are needed.
The efficacy of orexin receptor antagonists in the early period (4–6 weeks) appears to be important when considering core and global symptoms of depression. Although orexin receptor antagonists have previously been shown to have antidepressant effects, studies suggest that these agents may increase depressive symptoms such as suicidal ideation (23–28). This meta-analysis suggests that orexin receptor antagonists may help reduce core symptoms and overall symptoms of depression. Secondly, it is unclear whether the beneficial effects of orexin receptor antagonists on depression symptoms are directly due to their ‘antidepressant’ properties or indirectly ‘secondary’ to their beneficial effects on sleep. However, this study suggests that orexin receptor antagonists may have beneficial effects on core symptoms of depression independent of their beneficial effects on sleep. In addition, although not included in the meta-analysis, none of the four trials found risky clinical conditions such as suicidal behaviour or planning among participants. Although more research and analysis are needed, this preliminary information suggests that at least the orexin receptor antagonists included in this meta-analysis may be safe.
One of the advantages of this study is that the trials included in the meta-analysis were randomised controlled trials with similar samples. This reduces the risk of bias in the trials. But there are also some limitations. One of them is the small number of trials included in the meta-analysis. Orexin receptor antagonists are one of the newer molecules in psychopharmacology. At the moment, their use in the treatment of insomnia is at the forefront, but it can be said that more studies will be carried out with future clinical observations and trials. Another limitation is that the trials included in the meta-analysis used different methods to assess depression. These include the HAMD17, the MADRS and the BDI-II. Although these scales are different, they have high validity and reliability in people with depression and are often used in research.
Orexin 1 and 2 are known to be involved in different physiological processes. In general, orexin 1 activity is anxiogenic, whereas orexin 2 activity is thought to be anxiolytic (41,42). Orexin 2 receptor signalling plays a role in mediating the arousal from sleep and orexin-2 receptor antagonists may have antidepressant-like activity (43). This meta-analysis pooled the efficacy of three different orexin receptor antagonists in the treatment of depression and the results suggest that orexin receptor antagonists are effective. However, these results are preliminary and trials and meta-analyses of the efficacy of orexin 1 receptor antagonists, orexin 2 receptor antagonists and DORAs are needed.
In addition, questions concerning the efficacy of different doses of orexin receptor antagonists, the responses seen in depression samples other than those with moderate to severe depression who do not respond adequately to antidepressant treatment, and the results of longer-term follow-up remain unanswered.
This study provides a consolidated account of the antidepressant or reinforcing properties of orexin receptor antagonists in depressed patients. Although it does not provide strong evidence for the efficacy of orexin antagonists in depressed patients, it may provide a rationale for further research to further investigate their importance in the treatment of depression.
Footnotes
Peer-review: Externally peer-reviewed.
Conflict of Interest:The authors declared that there is no conflict of interest.
Financial Disclosure: This work was not supported by any financial institution.
REFERENCES
- 1.Giardino WJ, de Lecea L. Hypocretin (orexin) neuromodulation of stress and reward pathways. Curr Opin Neurobiol. 2014;29:103–108. doi: 10.1016/j.conb.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James MH, Mahler SV, Moorman DE, Aston-Jones G. A decade of orexin/hypocretin and addiction:where are we now? Curr Top Behav Neurosci. 2016:247–281. doi: 10.1007/7854_2016_57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G. Motivational activation:a unifying hypothesis of orexin/hypocretin function. Nat Neurosci. 2014;17(10):1298–1303. doi: 10.1038/nn.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakurai T. The role of orexin in motivated behaviours. Nat Rev Neurosci. 2014;15(11):719–731. doi: 10.1038/nrn3837. [DOI] [PubMed] [Google Scholar]
- 5.Nocjar C, Zhang J, Feng P, Panksepp J. The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neurosci. 2012;218:138–153. doi: 10.1016/j.neuroscience.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 6.Ji M-J, Zhang X-Y, Chen Z, Wang J-J, Zhu J-N. Orexin prevents depressive-like behavior by promoting stress resilience. Mol Psychiatry. 2019;24(2):282–293. doi: 10.1038/s41380-018-0127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furlong TM, Vianna DM, Liu L, Carrive P. Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur J Neurosci. 2009;30(8):1603–1614. doi: 10.1111/j.1460-9568.2009.06952.x. [DOI] [PubMed] [Google Scholar]
- 8.Lutter M, Krishnan V, Russo SJ, Jung S, McClung CA, Nestler EJ. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J Neurosci. 2008;28(12):3071–3075. doi: 10.1523/JNEUROSCI.5584-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada N, Katsuura G, Tatsuno I, Kawahara S, Ebihara K, Saito Y, et al. Orexins increase mRNA expressions of neurotrophin-3 in rat primary cortical neuron cultures. Neurosci Lett. 2009;450(2):132–135. doi: 10.1016/j.neulet.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 10.Selbach O, Bohla C, Barbara A, Doreulee N, Eriksson K, Sergeeva O, et al. Orexins/hypocretins control bistability of hippocampal long-term synaptic plasticity through co-activation of multiple kinases. Acta Physiol. 2010;198(3):277–285. doi: 10.1111/j.1748-1716.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- 11.Baimel C, Borgland SL. Hypocretin modulation of drug-induced synaptic plasticity. Prog. Brain Res. 2012;198:123–131. doi: 10.1016/B978-0-444-59489-1.00008-2. [DOI] [PubMed] [Google Scholar]
- 12.Adidharma W, Leach G, Yan L. Orexinergic signaling mediates light-induced neuronal activation in the dorsal raphe nucleus. Neurosci. 2012;220:201–207. doi: 10.1016/j.neuroscience.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasegawa E, Maejima T, Yoshida T, Masseck OA, Herlitze S, Yoshioka M, et al. Serotonin neurons in the dorsal raphe mediate the anticataplectic action of orexin neurons by reducing amygdala activity. Proc Natl Acad Sci U S A. 2017;114(17):E3526–E3535. doi: 10.1073/pnas.1614552114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allard JS, Tizabi Y, Shaffery JP, Trouth CO, Manaye K. Stereological analysis of the hypothalamic hypocretin/orexin neurons in an animal model of depression. Neuropeptides. 2004;38(5):311–315. doi: 10.1016/j.npep.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Rotter A, Asemann R, Decker A, Kornhuber J, Biermann T. Orexin expression and promoter-methylation in peripheral blood of patients suffering from major depressive disorder. J Affect Disord. 2011;131(1-3):186–192. doi: 10.1016/j.jad.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt FM, Arendt E, Steinmetzer A, Bruegel M, Kratzsch J, Strauß M, et al. CSF-hypocretin-1 levels in patients with major depressive disorder compared to healthy controls. Psychiatry Res. 2011;190(2-3):240–243. doi: 10.1016/j.psychres.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt FM, Brügel M, Kratzsch J, Strauß M, Sander C, Baum P, et al. Cerebrospinal fluid hypocretin-1(orexin A) levels in mania compared to unipolar depression and healthy controls. Neurosci Lett. 2010;483(1):20–22. doi: 10.1016/j.neulet.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 18.Grafe LA, Eacret D, Dobkin J, Bhatnagar S. Reduced orexin system function contributes to resilience to repeated social stress. Eneuro. 2018;5(2) doi: 10.1523/ENEURO.0273-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng P, Vurbic D, Wu Z, Hu Y, Strohl K. Changes in brain orexin levels in a rat model of depression induced by neonatal administration of clomipramine. J Psychophar. 2008;22(7):784–791. doi: 10.1177/0269881106082899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott MM, Marcus JN, Pettersen A, Birnbaum SG, Mochizuki T, Scammell TE, et al. Hcrtr1 and 2 signaling differentially regulates depression-like behaviors. Behav Brain Res. 2011;222(2):289–294. doi: 10.1016/j.bbr.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owen RT. Suvorexant:efficacy and safety profile of a dual orexin receptor antagonist in treating insomnia. Drugs Today (Barcelona, Spain:|y1998) 2016;52(1):29–40. doi: 10.1358/dot.2016.52.1.2439940. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura M, Nagamine T. Neuroendocrine, autonomic, and metabolic responses to an orexin antagonist, suvorexant, in psychiatric patients with insomnia. Innov Clin Neurosci. 2017;14(3-4):30. https://pubmed.ncbi.nlm.nih.gov/28584695/ [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson PL, Federici LM, Fitz SD, Renger JJ, Shireman B, Winrow CJ, et al. Orexin 1 and 2 receptor involvement in CO2-induced panic-associated behavior and autonomic responses. Depress Anxiety. 2015;32(9):671–683. doi: 10.1002/da.22403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li A, Hindmarch CC, Nattie EE, Paton JF. Antagonism of orexin receptors significantly lowers blood pressure in spontaneously hypertensive rats. J Physiol. 2013;591(17):4237–4248. doi: 10.1113/jphysiol.2013.256271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steiner MA, Lecourt H, Jenck F. The brain orexin system and almorexant in fear-conditioned startle reactions in the rat. Psychopharmacology. 2012;223(4):465–475. doi: 10.1007/s00213-012-2736-7. [DOI] [PubMed] [Google Scholar]
- 26.Nollet M, Gaillard P, Tanti A, Girault V, Belzung C, Leman S. Neurogenesis-independent antidepressant-like effects on behavior and stress axis response of a dual orexin receptor antagonist in a rodent model of depression. Neuropsychopharmacology. 2012;37(10):2210–2221. doi: 10.1038/npp.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrous J, Furmaga K. Adverse reaction with suvorexant for insomnia:acute worsening of depression with emergence of suicidal thoughts. BMJ Case Rep. 2017;2017:222037. doi: 10.1136/bcr-2017-222037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Connor KM, Ceesay P, Hutzelmann J, Snavely D, Krystal AD, Trivedi MH, et al. Phase II proof-of-concept trial of the orexin receptor antagonist filorexant (MK-|y6096)in patients with major depressive disorder. Int J Neuropsychopharmacol. 2017;20(8):613–618. doi: 10.1093/ijnp/pyx033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cruz HG, Hay JL, Hoever P, Alessi F, te Beek ET, van Gerven JM, et al. Pharmacokinetic and pharmacodynamic interactions between almorexant, a dual orexin receptor antagonist, and desipramine. Eur Neuropsychopharmacol. 2014;24(8):1257–1268. doi: 10.1016/j.euroneuro.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Li S-B, Nevárez N, Giardino WJ, de Lecea L. Optical probing of orexin/hypocretin receptor antagonists. Sleep. 2018;41(10):141. doi: 10.1093/sleep/zsy141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement:an updated guideline for reporting systematic reviews. BMJ. 2021;372:71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011] Cochrane Collaboration, 2011. https://handbook-5-1.cochrane.org/
- 33.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2:a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 34.Borenstein M, Hedges L, Rothstein H. Meta-analysis:fixed effect vs. random effects. Meta-analysis.com. 2007. https://www.meta-analysis.com/downloads/M-a_f_e_v_r_e_sv.pdf. [DOI] [PubMed]
- 35.R Core Team R. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. http://www. R-project org/ [Google Scholar]
- 36.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R. a practical tutorial. Evid Based Ment Health. 2019;22(4):153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brooks S, Jacobs GE, de Boer P, Kent JM, Van Nueten L, van Amerongen G, et al. The selective orexin-2 receptor antagonist seltorexant improves sleep:an exploratory double-blind, placebo controlled, crossover study in antidepressant-treated major depressive disorder patients with persistent insomnia. J Psychopharmacol. 2019;33(2):202–209. doi: 10.1177/0269881118822258. [DOI] [PubMed] [Google Scholar]
- 38.Savitz A, Wajs E, Zhang Y, Xu H, Etropolski M, Thase ME, et al. Efficacy and safety of seltorexant as adjunctive therapy in major depressive disorder:a phase 2b, randomized, placebo-controlled, adaptive dose-finding study. Int J Neuropsychopharmacol. 2021;24(12):965–976. doi: 10.1093/ijnp/pyab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Recourt K, de Boer P, Zuiker R, Luthringer R, Kent J, van der Ark P, et al. The selective orexin-2 antagonist seltorexant (JNJ-42847922/MIN-202) shows antidepressant and sleep-promoting effects in patients with major depressive disorder. Transl Psychiatry. 2019;9(1):1–10. doi: 10.1038/s41398-019-0553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shigetsura Y, Imai S, Endo H, Shimizu Y, Ueda K, Murai T, et al. Assessment of Suvorexant and Eszopiclone as Alternatives to Benzodiazepines for Treating Insomnia in Patients With Major Depressive Disorder. Clin Neuropharmacol. 2022;45(3):52–60. doi: 10.1097/WNF.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 41.Johnson PL, Samuels BC, Fitz SD, Federici LM, Hammes N, Early MC, et al. Orexin 1 receptors are a novel target to modulate panic responses and the panic brain network. Physiol Behav. 2012;107(5):733–742. doi: 10.1016/j.physbeh.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staton CD, Yaeger JD, Khalid D, Haroun F, Fernandez BS, Fernandez JS, et al. Orexin 2 receptor stimulation enhances resilience, while orexin 2 inhibition promotes susceptibility, to social stress, anxiety and depression. Neuropharmacology. 2018;143:79–94. doi: 10.1016/j.neuropharm.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fitch TE, Benvenga MJ, Jesudason CD, Zink C, Vandergriff AB, Menezes MM, et al. LSN2424100:a novel, potent orexin-2 receptor antagonist with selectivity over orexin-1 receptors and activity in an animal model predictive of antidepressant-like efficacy. Front Neurosci. 2014;8:5. doi: 10.3389/fnins.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]