Abstract
As a permanent state of cell cycle arrest, cellular senescence has become an important factor in aging and age-related diseases. As a central regulator of physiology and pathology associated with cellular senescence, the senescence associated secretory phenotype can create an inflammatory and catabolic environment through autocrine and paracrine ways, ultimately affecting tissue microstructure. As an age-related disease, the correlation between intervertebral disc degeneration and cellular senescence has been confirmed by many studies. Various pathological factors in the microenvironment of intervertebral disc degeneration promote senescent cells to produce and accumulate and express excessive senescence associated secretory phenotype. In this case, senescence associated secretory phenotype has received considerable attention as a potential target for delaying or treating disc degeneration. Therefore, we reviewed the latest research progress of senescence associated secretory phenotype, related regulatory mechanisms and intervertebral disc cell senescence treatment strategies. It is expected that further understanding of the underlying mechanism between cellular senescence pathology and intervertebral disc degeneration will help to formulate reasonable senescence regulation strategies, so as to achieve ideal therapeutic effects.
The translational potential of this article
Existing treatment strategies often fall short in addressing the challenge of repairing intervertebral disc Intervertebral disc degeneration(IVD) degeneration. The accumulation of senescent cells and the continuous release of senescence-associated secretory phenotype (SASP) perpetually impede disc homeostasis and hinder tissue regeneration. This impairment in repair capability presents a significant obstacle to the practical clinical implementation of strategies for intervertebral disc degeneration. As a result, we present a comprehensive overview of the latest advancements in research, the associated regulatory mechanisms, and strategies for treating SASP in IVD cells. This article aims to investigate effective interventions for delaying the onset and progression of age-related intervertebral disc degeneration. In an era where the aging population is becoming increasingly prominent, this endeavor holds paramount practical and translational significance.
Keywords: Cell senescence, Intervertebral disc degeneration, SASP, Senotherapeutics
Graphical abstract

1. Introduction
Low back pain (LBP) is one of the most common and important causes of disability, with approximately 60%–80% of people worldwide experiencing symptoms of LBP throughout their lifetime. And the incidence rate is increasing year by year worldwide [1]. Low back pain can be caused by many factors, including intervertebral disc degeneration (IVDD), spinal stenosis, vertebral body displacement, etc [2]. Scholars have continued and in-depth exploration of the pathogenic factors and mechanisms of intervertebral disc degeneration [3,4]. In recent years, cellular senescence, as one of the emerging important causes, has received continued attention from researchers.
The accumulated research results showed that cell senescence increased with the degree of IVDD progression [5], while exhibiting typical tissue characteristics of matrix degeneration. Senescent cells (SNCs) in degenerated human intervertebral discs are distributed in cell clusters, and the expression of the senescence marker p16INK4a increases in an age-dependent manner [6]. The accumulation of SNCs and the decrease in the density of healthy nucleus pulposus cells (NPCs) lead to the imbalance of catabolism and anabolism [7]. Inflammatory catabolism is characterized by the secretion of inflammatory mediators, matrix metalloproteinases (MMP), A Disintegrin and Metalloproteinase with Thrombospondin motifs (ADAMTS), and chemokines. It leads to the reduction of collagen and glucosaminoglycan, dehydration fibrosis of nucleus pulposus, vascular nerve ingrowth of annulus fibrosus, cartilage endplate calcification and other matrix changes [8]. On the other hand, the specific microenvironment of intervertebral disc degeneration includes avascular, acidic, hypertonic, abnormal mechanical load, oxidative stress and other stressors [9,10]. These factors in turn promote the generation of stress-induced premature senescence of cells, and show different expression profiles of Senescence-associated secretory phenotype (SASP). Over time, the accumulation of molecular and cellular damage by SASP leads to abnormal tissue balance, which ultimately leads to the decline of intervertebral disc tissue structure and function.
Cellular senescence denotes the enduring arrest of the cell cycle subsequent to exposure to various stresses, concomitantly giving rise to a secretory ensemble termed the “senescence-related secretory phenotype" (SASP) [11]. However, the intervertebral disc's microenvironment happens to harbor an array of stressors, including vascular insufficiency, hypoxia, acidity, mechanical load, and high osmotic pressure. These stressors induce DNA damage in intervertebral disc cells, hastening the generation and accumulation of SNCs [12]. SNCs react to diverse stressors and secrete distinct SASP components. SASP modulates cellular conditions by releasing inflammatory factors, MMP, growth factors, chemokines, epigenetic factors, and extracellular vesicles, while also engaging in communication with neighboring cells [13]. Consequently, the aging of adjacent healthy cells, depletion of stem cells, inflammation, and disruption in the balance of metabolic decomposition/synthesis occur. The catabolic effects exemplified by MMP remodeling the intervertebral disc matrix, resulting in fibrosis, vascularization, structural and functional impairment, and the compromise of immune privilege [14]. The progressive deterioration of the intervertebral disc microenvironment accelerates the progression of intervertebral disc degeneration.
As an important challenge in anti-aging strategies, the pathogenic mediators, substrates, and effective intervention strategies involved in the influence of SNCs on the process of intervertebral disc degeneration are receiving increasing attention. In this review, we provide an updated overview of the key mediators responsible for the pathological and physiological activities of SNCs remodeling the intervertebral disc matrix - SASP and its bioactive components, as well as related pathogenic mechanisms. In addition, we summarized the selective elimination of aging cells and potential strategies that SASP can follow.
2. .Composition of senescence related secret phenotype
SASP has heterogeneity, tissue specificity, and pleiotropy, mainly including a heterogeneous mixture of inflammatory factors, MMP, growth factors, chemokines, epigenetic factors, and other components [15](Fig. 1). Its composition and intensity vary depending on the stressors. These components can regulate their own phenotype and mediate the aging of adjacent cells through autocrine or paracrine forms [16].
Fig. 1.
Schematic model of senescence associated secretory phenotype (SASP) and their biological functions during intervertebral disc degeneration. SASP exerts an inflammatory cascade reaction through pro-inflammatory factors to maintain the chronic inflammatory state of the intervertebral disc. Intervertebral disc immune privilege is broken concurrently with the progression of autophagy, vascularization and neuroinvasion.MMP further aggravate catabolism and promote the destruction of intervertebral disc tissue structure and fibrotic degeneration. Chemokines primarily stimulate cellular migration and assume crucial roles in immune surveillance, inflammation, and tissue regeneration by recruiting beneficial cells from immune cell populations, stem cells, or other tissues. Excessive and persistent growth factors promote fibrosis of the disc matrix and induce vascular and neural invasion. Epigenetic factors coordinate DNA methylation, histone-related epigenetic machinery, chromatin remodeling and accelerate cellular senescence.
2.1. Inflammatory cytokines
The obvious characteristic of intervertebral disc degeneration is low-level chronic inflammation, and its main effect comes from the inflammatory response caused by pro-inflammatory factors. The secretion of inflammatory factors can amplify its harmful biological effects through a cascade, and the occurrence of vascularization and nerve ingrowth also breaks the immune privilege of the intervertebral disc, further enriching the source of immune cells [17]. Chronic inflammation of the intervertebral disc mediated by key cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, and IL-8 has been widely studied and confirmed [18]. Increases in SASP components such as TNF-α, IL-8, and IL-1β can be observed in non-degenerated intervertebral disc cells and degenerated intervertebral cells. As pleiotropic cytokines, both TNF-α and IL-1β can induce senescence in healthy cells, as evidenced by the upregulation of β-galactosidase and p16 [19]. Some other studies have shown that IL-1β and TNF-α significantly inhibit cell proliferation and telomerase activity and promote G0/1 cell cycle arrest [20]. Natural products such as curcumin and o-vanillin can reduce SASP factors (IL-1, IL-6, IL-8) of senescent IVD cells related to inflammation and back pain [21]. α-Ketoglutarate (α-KG) can reduce the levels of IL-6, phosphorylated JAK2 and STAT3 in IL-1β-induced senescent NPCs and the nuclear translocation of p-STAT3, and increased synthesis of aggrecan and type II collagen in NPCs and significantly attenuated senescence and MMP-13 protein expression [22]. In addition, IL-1β and TNF-α mediate various pathological processes of intervertebral disc degeneration, including autophagy, matrix destruction, apoptosis, pyroptosis, and proliferation. Previous studies have shown that NPCs can adapt to long-term residence in tissue ecological niches with nutrient scarcity and maintain high levels of autophagy activity independent of nutrient flux to achieve a certain level of survival [23]. Upon short-term stimulation with IL-1β, activation of the PI3K/AKT/mTOR pathway in NP cells leads to attenuation of autophagy, resulting in NP degeneration [24].
2.2. Matrix metalloproteinases
As an important manifestation of intervertebral disc degeneration, catabolic and synthetic imbalances persist in extracellular matrix remodeling (ECM). Matrix metalloproteinases (MMPs) are key enzymes involved in aging-related ECM remodeling. Promotes reduced expression of key structural components including collagen, aggrecan, versican, decorin and elastin, significantly promoting catabolic processes [25]. Among them, MMP-1 shows high expression through various mechanisms that induce nucleus pulposus or annulus fibrosus cell senescence, such as replicative senescence, ionizing radiation, and p16INK4a over expression [14]. MMP-1, -2, -3 and 13, the major aging-associated ECM-degrading enzymes, persist in the aging disc environment [26]. MMP-2 functions primarily as a gelatinase, targeting denatured collagen, gelatin, and laminin. There is evidence that MMP-2 activity is higher in senescent nucleus pulposus (NP) cells compared with young NPCs under both classic and intervertebral disc conditions [15]. In addition, increased levels of p16INK4a were associated with increased gene expression of the degrading enzymes MMP-13 and ADAMTS-5 [5]. Furthermore, abnormal MMP activity is associated with nucleus pulposus fibrosis, and MMP-2 and MMP-12 are two major profibrotic markers of NPCs [27]. MMP-2 dissociates the E-cadherin-β-catenin complex and increases β-catenin nuclear translocation and binding to lymphoid enhancer binding factor 1 (LEF1), thereby promoting the high expression of fibrosis genes α-SMA and collagen I [28]. MMP-12 was significantly up-regulated in the rat injury-induced NP fibrosis model and co-localized with α-SMA in NP cells, indicating that it is related to the generation of myofibroblasts [29].
2.3. Growth factors
SASP includes several growth factors, including transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and epidermal growth factor (EGF). TGF- β is a multifaceted cytokine that exerts powerful growth inhibitory and pro-apoptotic effects in different cell types [16]. Its growth inhibitory effect mainly depends on the induction of cyclin-dependent kinase (CDK) inhibitors, namely p16INK4b, p21 and p27, as well as other factors that hinder proliferation [30]. Recent research shows that TGF-β induces a deeper state of aging under hypoxic conditions than under normoxic conditions [31]. Excessive and sustained TGF-β activation is observed in the progression of nucleus pulposus fibrosis. TGF-β1 treatment can induce the expression of α-SMA and collagen I in human NP cells [32]. VEGF and p53 genes are cooperatively expressed in degenerated intervertebral disc tissue, and jointly participate in the formation and infiltration of new blood vessels, accelerating the degeneration of intervertebral disc tissue [33]. In addition, studies have shown that VEGF levels are reduced in P16 KO mice [34].
2.4. Chemokines
Chemokines primarily stimulate cellular migration and assume crucial roles in immune surveillance, inflammation, and tissue regeneration by recruiting beneficial cells from immune cell populations, stem cells, or other tissues. Senescent cells exhibit an augmented production of chemokines, thereby reshaping the microenvironment of the tissue and exerting influence on neighboring normal cells through autocrine and paracrine signaling [35]. Aging NPCs, when exposed to TLR-2/6 agonists, demonstrate a substantial increase in the secretion of SASP factors, namely CCL2, CCL5, CCL7, and CCL8. Remarkably, the utilization of o-vanillin and RG-7112 as therapeutic interventions for aging yields a significant reduction in the secretion of these chemokines [36]. In the context of replicative aging of nucleus pulposus cells, which characterizes decomposition and inflammatory metabolic changes, SASP manifests a modest upregulation of chemokines, including CCL5, CXCL10, and CCL2. Furthermore, the introduction of N-acetylated Proline Glycine Proline (N-Ac-PGP) into the matrix not only promotes the secretion of aforementioned chemokines but also enhances the sustained destructive impact of aging NPCs on the structure and function of intervertebral discs [37]. Chemokines can also attract immune cells to perform immune monitoring on intervertebral discs, especially after the loss of immune immunity in the nucleus pulposus tissue. The expression of CC motif chemokine 2 (CCL2) and CC motif chemokine 3 (CCL3) in NPC activates CCR1+and CCR2+M1 macrophages and promotes macrophage migration [38].
2.5. Epigenetic factors
Epigenetic factors orchestrate DNA methylation, histone-associated epigenetic mechanisms, and chromatin remodeling, intricately interconnecting with the aging network and giving rise to diverse aging phenotypes [39]. Yang et al. found that WTAP increased in aging NPCs and significantly promoted the modification of NORAD m6A due to KDM5a-mediated epigenetic increase in promoter H3K4me3. NORAD deficiency leads to reduced sequestration of PUMILIO protein, resulting in enhanced PUM1/2 activity, thereby inhibiting the expression of target E2F3 mRNA and promoting senescence of NPCs [40]. Silent mating type information regulator 2 homolog-1 (SIRT1), a member of the NAD+-dependent deacetylase family, engages in the regulation of cell cycle, DNA repair, autophagy, aging, and numerous other vital processes. It directly participates in the negative regulation of SASP expression. Remarkably, SIRT1 overexpression significantly impedes cellular senescence in nucleus pulposus (NP) cells, promoting cell proliferation and suppressing apoptosis [41]. Moreover, the utilization of its agonist, Resveratrol, exerts an anti-aging effect by modulating the oxidative stress-induced aging of rat NP cells via the Akt-Foxo1 pathway [42]. METTL14's involvement in TNF-α-induced miR-34a-5p m6A modification facilitates cellular senescence in human NP cells and NP cells from intervertebral disc degeneration (IVDD) patients. SIRT1 serving as an effective target of miR-34a-5p, counteracts the cellular senescence promoted by its overexpression [43]. Numerous studies have shown that SIRT1 can mediate tissue physiological functions such as stress resistance, apoptosis, senescence, and inflammation through deacetylation of histones, transcription factors, or coactivators [44]. SIRT1 exists as a protective mediator in IVD degeneration, and its expression is reduced in severely degenerated intervertebral discs [45,46]. Enhanced expression of SIRT1 also significantly ameliorates senescence of nucleus pulposus cells due to oxidative stress [42,47]. For cellular senescence and mitochondrial dysfunction in NP cells due to high-intensity mechanical loading, SIRT1 attenuates cellular senescence and mitochondrial dysfunction in NP cells through the regulation of PINK1 (a mitochondrial autophagy regulator)-dependent mitochondrial autophagy [48].
3. Mechanisms associated with cellular senescence
3.1. p53/p21 and p16/Rb
In addition to regulating the basic feature of the cell cycle, the p53/p21 pathway also plays an important role in the composition and intensity of SASP(Fig. 2). The p53 transcription factor plays a key role in cellular responses to stress. It is activated in response to DNA damage, leading to cell growth arrest, allowing for DNA repair, or driving cellular senescence or apoptosis, ultimately maintaining genome integrity. The levels and activity of p53 are strongly regulated primarily by post-transcriptional mechanisms, including MDM2-mediated ubiquitination and proteasomal degradation. Inhibitors developed against MDM2 have been shown to significantly inhibit SASP. USP7 inhibition stabilizes p53 by promoting MDM2 autoubiquitination and degradation and abolishes doxorubicin-induced increases in IL-1α, IL-1β, IL-6, and Tnfsf11 mRNA levels [49]. Similarly, treatment with the MDM2 inhibitors nutlin-3a or MI-63 significantly reduced the expression of the hallmark SASP factors IL-6 and IL-1α in cells senescent by genotoxic stimulation [50]. Regarding p53 and its Ubiquitin ligase MDM2, the SIRT1-dependent inhibition of p53 deacetylation can mitigate cellular senescence and SASP through the P53/P21 pathway [51]. As a downstream target gene of p53, p21 has been shown to mediate p53-induced G1 cell cycle arrest. It has also been shown to produce SASP through retinoblastoma protein (Rb)-dependent transcription and activate immune surveillance of senescent cells [52]. Targeted elimination of p21Cip1+ reduces senescent cells with telomere dysfunction, as well as several radiation-induced pro-inflammatory senescence-related secreted phenotypic factors, such as IL-6, MMP-12, CCL2, and CCL7 [53].
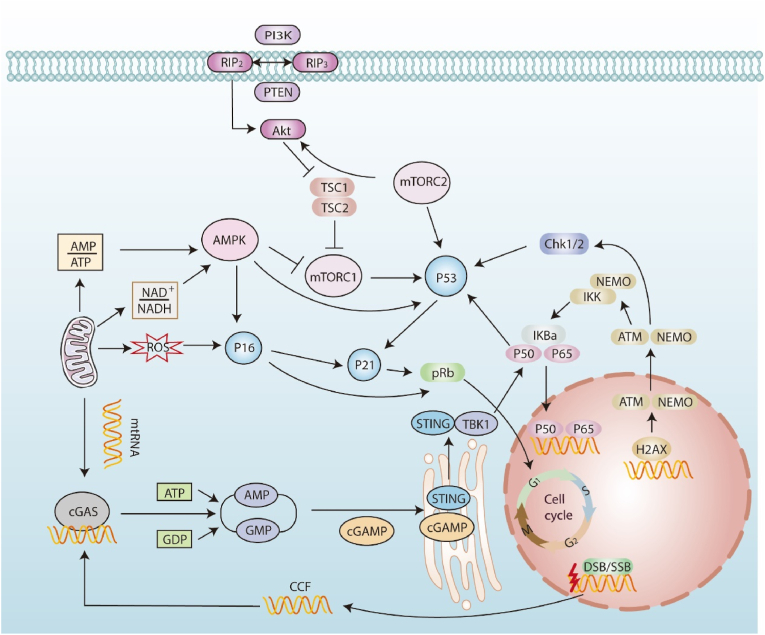
Fig. 2.
SASP regulation of aging intervertebral disc cells converges on the p53/p21 and p16/Rb pathways. NF-κB: NF-κB is a major factor regulating the transcription of many SASP-related components. ATM autophosphorylates and promotes phosphorylation, SUMOylation, and monoubiquitination of NEMO. As a result, monoubiquitinated NEMO translocates to the cytoplasm together with ATM, activating the IKK complex. Phosphorylation of IκB leads to the release of p65, which is then transferred to the nucleus and upregulates the transcriptional program of SASP factors. AMPK: Mitochondria-derived reactive oxygen species (ROS) replenish DNA damage foci and DDR signaling after initial senescence. and mediates changes in metabolic status low NAD+/NADH and increases in AMP: ATP ratios. AMP-activated protein kinase (AMPK) is activated by high AMP and ADP levels and can affect SASP by activating p53 and stabilizing p21 mRNA. cGAS/STING: After cytoplasmic chromatin fragments and mitochondrial DNA are damaged, they are released into the cytoplasm and then activate the cGAS/STING signaling pathway to produce the second messenger cGAMP. STING dimerizes and is excreted from the endoplasmic reticulum to the Golgi apparatus. It then binds to TBK1 to form a complex. TBK1 then phosphorylates IRF3 and IκB kinase in parallel. IKK phosphorylates inhibitor of NFκB (IκB), resulting in the release of NFκB. mTOR: Phosphatidylinositol 3-kinase (PI3K)/AKT activates class I PI3K. This process generates PIP3 and leads to PKB/Akt activation. Active PKB/Akt activates mTOR indirectly by inhibiting its negative regulator [tuberous sclerosis complex (TSC1/2)]. Activated mTORC1 enhances p53 function.
The role of p21CIP1 may be limited to the onset of senescence, whereas p16INK4a maintains a long-lasting growth arrest, suggesting the existence of differentially regulated stages of senescence [54]. As a CDK inhibitor, p16 selectively binds directly to CDK4-cyclin D and CDK6-cyclin D complexes. As a result, it blocks the phosphorylation of pRB, thereby blocking E2F-mediated transcription of genes related to cell proliferation. Levels of p16 INK4a are elevated in aged and stressed tissues compared to young, healthy tissues. Further studies have shown that the level of expression of p16 is positively correlated with the severity of IVDD degeneration in humans. The BubR1 progeria transgenic mice constructed by Darren et al. were able to induce the elimination of p16Ink4a-positive senescent cells after administration. The results showed that lifelong elimination of p16INK4A cells could significantly delay age-related diseases, while elimination of p16INK4A cells later in life would alleviate these age-related pathologies [55]. Che et al. found that compared with healthy mice, the intervertebral discs of mice whose p16 encoding gene was deleted were less likely to degenerate. Selective elimination of P16-positive senescent cells can attenuate IVDD by promoting cell cycle and inhibiting SASP, cellular senescence, and oxidative stress [7]. Studies have shown that long-term intervention with the drug combination of dasatinib and quercetin as a protective factor can attenuate dependent disc degeneration by significantly reducing the aging markers p16INK4a, p19ARF and the SASP molecules IL-6 and MMP13 [56].
3.2. mTOR
The mechanistic target of rapamycin (mTOR) is a serine–threonine kinase. Studies have shown that mTOR-dependent signaling can regulate SASP by controlling mRNA transcription and protein synthesis. mTOR-dependent signaling is necessary to maintain or implement different aspects of cellular senescence. In particular, regulation with SASP reflects complex changes in the activity of mTOR and other metabolic pathways, such as autophagy [57]. A large number of studies have shown that rapamycin can significantly reduce SASP secretion, mainly through mTOR complex 1 (mTORC1). Inhibition of mTORC1 can also selectively block the translation of membrane-bound IL-1α and reduce the transcriptional activity of NF-κB to inhibit the expression and secretion of inflammatory cytokines in senescent cells. Both are important factors in autocrine amplification loops that regulate the levels of other SASP factors. It can reduce the expression and secretion of IL-6 and IL-8 [58]. The effects of mTORC1 inhibition on the secretory phenotype may reflect, at least in part, the ability of 4EBP1, one of the substrates of mTORC1, to regulate phosphorylation of the RNA-binding protein ZFP36L1 during aging, thereby inhibiting its transcript degradation of SASP components [59]. What is even more interesting is that mTOR can serve as both an upstream regulator and a downstream effector of autophagy. Autophagy has been shown to produce high fluxes of recycled amino acids and other metabolites. mTORC1, which is subsequently bound by lysosomes, is used to synthesize SASP factors, such as the cytokines IL-6 and IL-8 [60]. Narita and other scholars discovered that the TOR-autophagy spatial coupling compartment (TASCC), that is, the catabolic process (autophagy) can be coupled with the anabolic process (mTORC1-dependent protein synthesis) to effectively coordinate the production of SASP proteins [61].
3.3. AMPK
AMP activated protein kinase (AMPK) serves as a sensor of the cell's energy-reduced state. As the NAD+/NADH ratio decreases, the AMP:ATP and ADP:ATP ratios increase, and AMPK is dephosphorylated and activated [62]. Activation of AMPK can lead to direct phosphorylation of p53 and increase in p21 transcription, thereby releasing SASP, which is involved in the cellular aging process [10]. Specifically, the decrease in the NAD+/NADH ratio and the depletion of NAD + increase the secretion of some of the SASPs and also contribute to the development of degenerative diseases associated with aging. NAD levels in most tissues are maintained through the NAD salvage pathway and its rate-limiting enzyme nicotinamide phosphoribosyltransferase (NAMPT) [63]. At the same time, NAMPT can regulate pro-inflammatory SASP (IL-1β, IL-6, and IL-8) through the high-mobility group A protein (HMGA)–NAMPT–NAD + signaling axis, independent of growth arrest. AMPK activation enhances p53-mediated inhibition of p38MAPK, thereby reducing NF-kB-mediated inflammatory signaling [64]. Sun et al. found that small extracellular vesicles derived from NAMPT-enriched adipocytes could improve the SASP(TNF-α, IL-6 and IL-8) of nucleus pulposus cells and cartilage endplate cells by activating NAD + biosynthesis and rejuvenate them to attenuate IVDD [65]. Du et al. found that selective agonism of AMPK can reduce the expression of p16INK4a and SASP proteins (including MMP9, MMP13 and HMGB1) and significantly delay NPC senescence [66].
3.4. NF-κB
Accumulating studies indicate that NF-κB is an important regulator of pro-inflammatory SASP expression in multiple modes of aging, and that both direct and indirect inhibition of NF-κB exhibit attenuation of the SASP transcriptional program [67]. NF-κB is activated during normal and accelerated aging. Inhibition of NF-κB delays the onset of premature aging symptoms in Ercc1 –/Δ mice. At the same time, late senescent cells exert a bystander effect through NF-κB activation, secreting IL-1α and IL-8 to synergistically promote the cellular senescence of early MSCs. This effect can be eliminated by shRNA transfection or the use of NF-κB inhibitors in early cells [68]. As an important component of SASP, activation of the NF-κB signaling pathway directly or indirectly induces the expression of pro-inflammatory mediators, chemokines, MMP and other related factors. Studies have shown that activation of NF-κB can regulate the expression of CXCL1, CXCL2 and CXCL3 and induce the accumulation of inflammatory factors in NPCs. And activation of NF-κB response elements located in the promoters of MMP-3, MMP-9, MMP-13, ADAMTS- 4 and ADAMTS-5 genes can induce the expression of matrix metalloproteinase genes [69]. Shusuke et al. found that bleomycin-induced cell senescence can increase the activity of NF-κB p65 subunit and increase the mRNA expression of IL-6, IL-8 and CCL20. Administration of the NF-κB inhibitor BAY 11–7082 can inhibit NF-κB activity and reduce the secretion of IL-6 and IL-8 [70].
3.5. cGAS-STING
The cyclic GMP-AMP synthase (cGAS)-interferon gene stimulator (STING) signaling pathway plays a crucial role in the innate immune response. Various cellular stresses, such as DNA damage, can promote the production of cytosolic DNA, including cytoplasmic chromatin fragments (CCF), mtDNA, cDNA, etc. Recognition of such cytosolic DNA by the cGAS-STING pathway leads to the production of SASP [71].
The cytosolic DNA sensor cGAS is responsible for connecting DNA damage to the activation of the SASP. This process involves cGAS catalyzing the production of the second messenger cGMP, which in turn activates the adaptor protein STING. The activation of STING leads to the activation of both IRF3 and NF- κB [72]. Additionally, a non-canonical pathway can trigger the activation of STING through cGAS-independent DNA damage, involving p53 and the ubiquitin E3 ligase TRAF6. This pathway preferentially activates NF- κB rather than IRF3. The γH2AX and STING markers associated with DNA damage demonstrated an increase in NP cells treated with tert-butyl hydroperoxide (TBHP). The activation of IFN regulatory factor 3 (IRF3) via STING is known to mediate ECM degradation, senescence, and apoptosis in NP cells. In vivo studies have shown that silencing of STING attenuates puncture-induced intervertebral disc degeneration (IVDD) in rats [73]. Accumulated oxidative damage to the proteins and lipids of the mitochondrial membrane eventually leads to increased permeability or even rupture of the membrane, allowing mtDNA to leak into the cytoplasm [74]. Moreover, under oxidative stress, the mitochondrial permeability transition pore (mPTP) can open in human nucleus pulposus cells as a result of cytoplasmic release of mitochondrial DNA (mtDNA) [75]. The binding of cGAS to cellular DNA fragments can activate the NF-κB signal transduction pathway, leading to the downstream expression of proinflammatory cytokines and the induction of SASP in senescent cells [76].
4. Cellular senescence treatment strategies
Accumulating preclinical evidence suggests that therapeutic regimens that inhibit inflammatory cytokines or selectively eliminate SNCs are promising candidates for strategies to slow disc degeneration. However, there is still controversy over the choice of senostatics and senolytics [77]. Senolytics are a class of drugs or compounds that can selectively induce apoptosis (programmed cell death) in SNCs. By selectively eliminating SNCs, senolytics effectively prevent the accumulation of SNCs. Unlike senolytics, senostatics do not kill SNCs but inhibit paracrine signaling. For example, by regulating the SASP regulatory network in SNCs, including the aforementioned therapeutic targets such as p53/p21 and p16/Rb, mTOR, AMPK, NF-κB and cGAS-STING. This prevents the increase in SNCs caused by the bystander effect.
Although drugs or compounds involving both therapeutic strategies have shown promising anti-aging efficacy, the lack of cell selectivity and potential side effects still provide challenges to anti-aging strategies.
4.1. Natural products and their analogues
Many natural compounds have been reported to have anti-aging or age-related effects (Table .1). Studies have shown that quercetin, a plant-derived product with antioxidant and anti-apoptotic properties, can modulate the expression of SASP in NP cells [78]. Quercetin might inhibit the NF-κB signaling pathway by binding to the Keap1-Nrf2 complex. This complex is involved in the activation of the antioxidant response elements (AREs), which can further help in mitigating the inflammation caused by oxidative stress, thus reducing SASP in NP cells [79]. Further study, Novais et al. discovered that prolonged administration of dasatinib and quercetin mitigates age-related degeneration of intervertebral discs in mice. Moreover, the combination of dasatinib and quercetin significantly reduces aging markers (p16INK4a and p19ARF) and SASP molecules (IL-6 and MMP-13) [80]. Curcumin is a natural product with various biological activities, including anti-inflammatory, antioxidant, and other effects. Curcumin and its metabolite, o-vanillin, exhibit anti-aging properties on aging human intervertebral disc cells. These compounds have been validated as potential agents for eliminating SNCs via the Nrf2 and NF-κB pathways [21]. Dehydrocostus lactone (DHE) is a natural sesquiterpene lactone that possesses potent anti-inflammatory properties, mediated by the NF-κB and MAPK signaling pathways, as established by various studies. It has been shown that DHE can also effectively inhibit TNF-α-induced cellular senescence through the STING pathway [81].
Table 1.
Senotherapeutics related to intervertebral disc degeneration.
| Drugs | Mechanism | Outcomes | Reference(s) |
|---|---|---|---|
| Quercetin | Nrf2/NF-kB axis | Influencing SASP factor expression and senescence phenotype in NPCs | [82] |
| Dasatinib and Quercetin | Significantly reduced the senescence markers p16INK4a and p19ARF and the SASP molecules IL-6 and MMP13 | [83] | |
| Curcumin and O-vanillin | Expression of SASP factors was decreased, and matrix synthesis increased | [84] | |
| Dehydrocostus lactone | TBK1/NF-κB and MAPK Signaling | Partially attenuated TNF- α Induced ECM degradation and NP cell senescence | [85] |
| ABT-263/Navitoclax | Bcl-2 family | Significant decrease in intervertebral disc SASP (IL-6 and MMP-13) secretion and diminished ECM degradation | [86] |
| 17-DMAG | Hsp90 | Increased PG levels | [87] |
| RG7112(RO5045337) | p53 | Selectively kill senescent intervertebral disc (IVD) cells through apoptosis and reduce the expression of SASP factors in culture, including IFN- γ, IL-6 and CCL24 | [88] |
| SIRT1 | Regulating autophagy as well as counteracting oxidative stress | [89] | |
| Ganciclovir | Selectively remove p16 Ink4a positive senescent cells | Reduction in the number of senescent cells ameliorates multiple age-associated changes within the disc tissue | [90] |
| Rapamycin | mTOR signaling pathway | Reduce p16 expression and reverse the senescent phenotype of human NPCs | [91] |
| Amiloride | non-specific ASIC inhibitor | Reversed the decline in NP-MSC proliferative capacity and cell cycle arrest together with blocking cells from becoming senescent | [92] |
| Klotho | Inhibitor of Wnt/β-catenin | Reduce age-related SASP protein secretion and maintain NPC phenotype and viability | [93] |
| KU55933 | Inhibitor of ATM | Ameliorates myeloid cell senescence and stromal GAG loss | [94,95] |
| SSK1/JHB75B/NaV-Gal | β-gal-targeted prodrug | Selective killing of SNCs | [[96], [97], [98]] |
Nrf2/NF-kB axis: NF-E2-related factor 2/Nuclear factorkappa B; SASP: Senescence-associated secretory phenotype; NPCs: Nucleus pulposus cells;
PG: Proteoglycan; ABT-263: A Bad-like BH3 mimetic; Bcl-2 family: B cell CLL/lymphoma-2 (BCL-2) and its relatives comprise the BCL-2 family of proteins;
17-DMAG: 17-dimethylaminoethylamino-17-demethoxygeldanamycin, a heat shock protein 90 (Hsp90) inhibitor; RG7112: MDM2 Small-Molecule Antagonist;
SIRT1: Sirtuin 1; IFN- γ: Interferon-γ; CCL24: C–C motif chemokine ligand 24; ASIC: Acid-sensing ion channels; NP-MSC: Nucleus pulposus mesenchymal stem cells;
ATM: The ataxia-telangiectasia mutated; GAG: Glycosaminoglycan; SNCs: Senescent cells
4.2. Bcl-2 family inhibitors
3.3 As negative regulators of cell apoptosis, members of the Bcl-2 family encompass proteins such as pro-apoptotic Bax, Bak, and Bok, as well as the BH3 protein. In addition, they include anti-apoptotic proteins Bcl-2, Bcl-W, and Bcl-XL [99]. Among these, anti-apoptotic proteins (including Bcl-2, Bcl-w, and Bcl-XL) are typically upregulated in aged cells and exhibit resistance to cell apoptosis induction signals [100]. Aged cells exhibit resistance to apoptosis due to the transcription expression of anti-apoptotic Bcl-2 proteins. The efficacy of a series of developed Bcl-2 family inhibitors demonstrates the feasibility of promoting apoptosis in aged cells. Bcl-2 family members can combat mitochondrial outer membrane permeability (MOMP) by binding to the BH3 motif of Bax or Bak5, which is a key step in cell apoptosis [101]. To this end, some researchers have developed BH3 motif mimics that promote apoptosis in aged cells by binding to Bcl-2, such as ABT-263 (navitoclax). Lin et al. treated degenerated intervertebral discs with ABT-263 loaded polylactic-co-glycolic acid nanoparticles (PLGA-ABT). The study found that ABT-263 significantly reduced the secretion of aging-associated secretory phenotype (SASP) markers, including IL-6 and MMP-13, and inhibitedECM degradation [102]. Similar small molecule inhibitors have been developed for proteins BCL-2, BCL-W, and BCL-XL (ABT-737). ABT-737 effectively eliminates SNCs induced by DNA damage in the lungs when treated with mice [103]. Both ABT-737 and ABT-263 exhibit high affinity for BCL-2, BCL-XL, and BCL-W. By binding to these anti-apoptotic proteins, ABT-737 and ABT-263 essentially mimic the presence of BH3 pro-apoptotic proteins, and both compete with and replace them, promoting apoptosis in aged cells. Hsp90 inhibitors.
Heat shock protein 90 (HSP90) is a type of molecular chaperone that functions with the assistance of co-chaperones and ATP. The chaperone function involves binding the client protein with HSP90 and ATP, followed by the hydrolysis of ATP into ADP and inorganic phosphate. The energy released by this cleavage is then utilized to repair the client protein, aided by co-chaperones [104]. The role of heat shock protein 90 and its homologues in cellular aging is still a complex topic with various research findings. For instance, HSP90's client protein phosphorylated AKT (p-AKT) can stabilize aging cells by preventing them from undergoing apoptosis [105]. Another example is that Hsp90α contributes to the lysosomal degradation of p14ARF, which is related to the C-terminus of the Hsp70 interacting protein. P14ARF can trigger aging via multiple pathways such as activating p53 signaling, Myc protein, E2F1 protein, and ATM/ATR/CHK DNA repair [106]. The HSP90 inhibitors, such as 17-DMAG (alvespimycin), geldanamycin, 17-AAG (tanespimycin), and ganetespib were assessed by bioinformatics and high-throughput sequencing to demonstrate senescence activity in a cell type-specific manner across a range of SNCs. Treatment of Ercc1 -/Δ mice with 17-DMAG, not only extended their healthy lifespan but also boosted their PGs levels [107]. 17-AAG inhibited M1 polarization of macrophages by targeting MAPK and NF-κB pathways, improved M1-CM induced pro-inflammatory and catabolic phenotypes of NPC, and ultimately attenuated NP fibrosis and macrophage-induced pathological angiogenesis [108,109].
4.3. p53
The vital role of p53, a tumor suppressor factor functioning as a stress response transcription factor, in age-related cell cycle arrest is evidenced by a vast body of research. Telomere erosion and DNA damage are significant factors that impact the core pathway of p53/p21 cip1 cell aging. The activation of ATM/ATR by DDR subsequently triggers the p53/p21cip1 axis by means of the phosphorylation of p53 and its ubiquitin ligase MDM2 (Mouse double minute 2) [110]. Its activity as a transcription factor is regulated by post-translational modifications or stability. MDM2 serves as an upstream regulator of P53 by regulating its levels through degradation [111]. RG7112 (RO5045337) is a small molecule inhibitor of MDM2/p53 interaction that has the ability to revive p53 activity. Recent reports indicate that apoptosis-mediated selective killing of senescent IVD cells is achievable, which also leads to a reduction in the expression of culture-based SASP factors including IFN-γ, IL-6, and CCL24. Similarly, in vitro treatment of intact human intervertebral discs with RG7112 may improve the stability of intervertebral disc matrix through its aging effect on aging IVD cells [112]. Second, due to the synergistic action of SIRT1-dependent p53 deacetylation control activity for ubiquitination, the accumulation of SNCs can be effectively avoided by preventing SIRT1-dependent deacetylation [113]. SIRT1 has been shown to reduce oxidative stress-induced senescence in human CEP cells via the p53/p21 pathway, while also playing a role in attenuating senescence by regulating autophagy as well as counteracting oxidative stress [114].
4.5. Other studies on anti-aging of intervertebral discs
In addition to the P53 elimination strategy mentioned above, clearing p16Ink4a positive aging cells can also delay aging related diseases. Patil et al. used ganciclovir to selectively remove p16 Ink4a positive SNCs in p16-3MR transgenic mice. The experimental results showed that the proteolytic degradation of aggregating proteoglycans in intervertebral discs was significantly reduced, the total proteoglycan matrix content was increased, and the histological characteristics of intervertebral discs were improved [115]. Rapamycin has the ability to significantly diminish the expression of the aging marker p16 gene. The downregulation of p16, in turn, may deliver antioxidant effects, reverse the aging-related phenotype of human NP cells, and consequently hinder the progression of IVDD [116]. An equally fascinating discovery is that the activities of ASIC1 and ASIC3 have been recognized as upstream to the aging pathway, encompassing p53 p21/p27 and p16-Rb signal transduction, as opposed to ASIC2 and ASIC4. By inhibiting ASIC1 and ASIC3, selective acid ion channel inhibitors such as Amir, PcTx1, and APETx2 effectively reverse NP-MSC proliferation and cell cycle arrest, thereby halting the process of cell aging [117]. Meanwhile, it has been shown that activation of the Wnt/β-catenin signaling pathway promotes NPC apoptosis and mediates the process of disc degeneration [118]. Klotho, a natural inhibitor of Wnt/β-catenin, can reduce age-related SASP protein secretion and maintain NPC phenotype and viability [93]. Ataxia-telangiectasia (ATM) signaling is the primary pathway used by cells to respond to genomic damage and to resist constant attack by endogenous and environmental factors. Activation of ATM leads to cell cycle arrest, senescence and/or apoptosis. Targeting ATM inhibition ameliorates myeloid cell senescence and stromal glycosaminoglycan loss. In turn, this behavior may drive disc cell senescence and matrix perturbation through the ATM-p53-p21 axis [119,120].
5. Conclusions and perspectives
The evidence linking cellular senescence, SASP, and a key cause of age-related intervertebral disc degeneration (IDD) is compelling. However, the study of disc cellular senescence is still in its initial stages of exploration. SASP, regulatory signaling pathways, and resulting phenotypes of cellular senescence in the Intervertebral Disc (IVD) are gradually being uncovered through in vitro and in vivo experiments; Each of these processes may lead to progressive alterations in cellular metabolism, morphology, as well as function, with further progression to loss of structural and functional homeostasis of disc tissue. Therefore, a deeper understanding of the underlying mechanisms of the pathology of cellular senescence with respect to therapeutic strategies against disc degeneration seems to be beneficial for subsequent corresponding treatments.
Anti-aging therapies for age-related degenerative diseases have been partially investigated in preclinical studies with remarkable success. However, intervention strategies targeting senescence-related pathways have also demonstrated worrisome side effects. Therefore, in order to realize the translation of novel drugs into clinical trials, it is necessary to explore the mechanisms of cellular senescence and screen anti-aging drugs more deeply. Meanwhile, the SNCs accumulated in different organs or tissues are highly heterogeneous. Therefore, to monitor the efficacy of anti-aging drugs, it is also necessary to screen senescent cell-specific biomarkers for a series of SASPs in order to monitor the number of SNCs and phenotypic changes. A more profound comprehension of the molecular mechanisms that drive the multi-stage advancement of senescence, as well as the development and operation of acute versus chronic SNCs, has the potential to usher in innovative therapeutic approaches for age-related pathologies and increase longevity.
Credit author statement
Conceptualization: QY; Visualization: QY, XS; Writing – original draft: YL; Writing – review and editing: YL, YMD, XS, QY; Supervision: QY.
Funding
The authors acknowledge financial support from the National Key R&D Program of China (2023YFC2416900), National Natural Science Foundation of China (82372419, 81871782), Applied Basic Research Multi-input Foundation of Tianjin (21JCZDJC01040).
Data availability
No data was used for the research described in the article.
Declaration of competing interest
None declared.
Contributor Information
Xun Sun, Email: sunxun_tju@yeah.net.
Qiang Yang, Email: yangqiang1980@126.com.
References
- 1.Knezevic N., Candido K., Vlaeyen J., Van Zundert J., Cohen S.J.L. Low back pain. 2021;398(10294):78–92. doi: 10.1016/S0140-6736(21)00733-9. [DOI] [PubMed] [Google Scholar]
- 2.Chen S., Chen M., Wu X., Lin S., Tao C., Cao H., et al. Global, regional and national burden of low back pain 1990–2019: a systematic analysis of the Global Burden of Disease study 2019. Journal of Orthopaedic Translation. 2022;32:49–58. doi: 10.1016/j.jot.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tryfonidou M.A., de Vries G., Hennink W.E., Creemers L.B. “Old Drugs, New Tricks” – Local controlled drug release systems for treatment of degenerative joint disease. Adv Drug Deliv Rev. 2020;160:170–185. doi: 10.1016/j.addr.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Jia H., Lin X., Wang D., Wang J., Shang Q., He X., et al. Injectable hydrogel with nucleus pulposus-matched viscoelastic property prevents intervertebral disc degeneration. Journal of Orthopaedic Translation. 2022;33:162–173. doi: 10.1016/j.jot.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Maitre C.L., Freemont A.J., Hoyland J.A. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2007;9(3):R45. doi: 10.1186/ar2198. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novais E.J., Diekman B.O., Shapiro I.M., Risbud M.V. p16Ink4a deletion in cells of the intervertebral disc affects their matrix homeostasis and senescence associated secretory phenotype without altering onset of senescence. Matrix Biol. 2019;82:54–70. doi: 10.1016/j.matbio.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Che H., Ma C., Li H., Yu F., Wei Y., Chen H., et al. Rebalance of the polyamine metabolism suppresses oxidative stress and delays senescence in nucleus pulposus cells. Oxid Med Cell Longev. 2022:2022. doi: 10.1155/2022/8033353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L., Zhang Y., Fu J., Ai X., Long D., Leng X., et al. Gli1 depletion induces oxidative stress and apoptosis of nucleus pulposus cells via Fos in intervertebral disc degeneration. Journal of Orthopaedic Translation. 2023;40:116–131. doi: 10.1016/j.jot.2023.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vadalà G., Ambrosio L., Russo F., Papalia R., Denaro V. Interaction between mesenchymal stem cells and intervertebral disc microenvironment: from cell therapy to tissue engineering. Stem Cell Int. 2019;2019 doi: 10.1155/2019/2376172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang F., Liu W., Huang Y., Yang S., Shao Z., Cai X., et al. Regulated cell death: implications for intervertebral disc degeneration and therapy. Journal of Orthopaedic Translation. 2022;37:163–172. doi: 10.1016/j.jot.2022.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gasek N.S., Kuchel G.A., Kirkland J.L., Xu M. Strategies for targeting senescent cells in human disease. Nat Aging. 2021;1(10):870–879. doi: 10.1038/s43587-021-00121-8. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vo N.V., Hartman R.A., Patil P.R., Risbud M.V., Kletsas D., Iatridis J.C., et al. Molecular mechanisms of biological aging in intervertebral discs. J Orthop Res. 2016;34(8):1289–1306. doi: 10.1002/jor.23195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y., Yang B., Wang J., Cheng F., Shi K., Ying L., et al. Cell senescence: a nonnegligible cell state under survival stress in pathology of intervertebral disc degeneration. Oxid Med Cell Longev. 2020;2020 doi: 10.1155/2020/9503562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ngo K., Patil P., McGowan S.J., Niedernhofer L.J., Robbins P.D., Kang J., et al. Senescent intervertebral disc cells exhibit perturbed matrix homeostasis phenotype. Mech Ageing Dev. 2017;166:16–23. doi: 10.1016/j.mad.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young D., Barter M., Soul J. Osteoarthritis year in review: genetics, genomics, epigenetics. Osteoarthritis Cartilage. 2022;30(2):216–225. doi: 10.1016/j.joca.2021.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borodkina A., Deryabin P., Nikolsky N. “Social life” of senescent cells: what is SASP and why study it? Acta Naturae (англоязычная версия) 2018;10(1):4–14. 36. [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X., Tan Y., Liu F., Wang J., Liu F., Zhang Q., et al. Pharmacological network analysis of the functions and mechanism of kaempferol from Du Zhong in intervertebral disc degeneration (IDD) Journal of Orthopaedic Translation. 2023;39:135–146. doi: 10.1016/j.jot.2023.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Risbud M.V., Shapiro I.M. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10(1):44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashraf S., Santerre P., Kandel R. Induced senescence of healthy nucleus pulposus cells is mediated by paracrine signaling from TNF-α–activated cells. Faseb J. 2021;35(9) doi: 10.1096/fj.202002201R. [DOI] [PubMed] [Google Scholar]
- 20.Li X., Lin F., Wu Y., Liu N., Wang J., Chen R., et al. Resveratrol attenuates inflammation environment-induced nucleus pulposus cell senescence in vitro. Biosci Rep. 2019;39(5) doi: 10.1042/BSR20190126. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Cherif H., Bisson D.G., Jarzem P., Weber M., Ouellet J.A., Haglund L. Curcumin and o-vanillin exhibit evidence of senolytic activity in human IVD cells in vitro. J Clin Med. 2019;8(4):433. doi: 10.3390/jcm8040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu H, Fang X, Liu X, Zhang S, Yi Y, Chang S, et al. α-ketoglutaric acid ameliorates intervertebral disc degeneration by blocking the IL-6/JAK2/STAT3 pathway. Am J Physiol Cell Physiol. 2023;325(4):C1119–C1130. doi: 10.1152/ajpcell.00280.2023. [DOI] [PubMed] [Google Scholar]
- 23.Kritschil R., Li V., Wang D., Dong Q., Silwal P., Finkel T., et al. Impact of autophagy inhibition on intervertebral disc cells and extracellular matrix. JOR Spine. 2024;7(1) doi: 10.1002/jsp2.1286. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu R., Xu H., Deng X., Wang Y., He Z., Xu S., et al. Physalin A alleviates intervertebral disc degeneration via anti-inflammatory and anti-fibrotic effects. J Orthop Translat. 2023;39:74–87. doi: 10.1016/j.jot.2023.01.001. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vamvakas S.-S., Mavrogonatou E., Kletsas D. Human nucleus pulposus intervertebral disc cells becoming senescent using different treatments exhibit a similar transcriptional profile of catabolic and inflammatory genes. Eur Spine J. 2017;26(8):2063–2071. doi: 10.1007/s00586-017-5198-0. [DOI] [PubMed] [Google Scholar]
- 26.Kouroumalis A., Mavrogonatou E., Savvidou O.D., Papagelopoulos P.J., Pratsinis H., Kletsas D. Major traits of the senescent phenotype of nucleus pulposus intervertebral disc cells persist under the specific microenvironmental conditions of the tissue. Mech Ageing Dev. 2019;177:118–127. doi: 10.1016/j.mad.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Sun Y., Lyu M., Lu Q., Cheung K., Leung V. Current perspectives on nucleus pulposus fibrosis in disc degeneration and repair. Int J Mol Sci. 2022;23(12):6612. doi: 10.3390/ijms23126612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng G., Zha Z., Huang Y., Li J., Wang Y., Ke W., et al. Sustained and bioresponsive two‐stage delivery of therapeutic miRNA via polyplex micelle‐loaded injectable hydrogels for inhibition of intervertebral disc fibrosis. Adv Healthcare Mater. 2018;7(21) doi: 10.1002/adhm.201800623. [DOI] [PubMed] [Google Scholar]
- 29.Lv F.-J., Peng Y., Lim F., Sun Y., Lv M., Zhou L., et al. Matrix metalloproteinase 12 is an indicator of intervertebral disc degeneration co-expressed with fibrotic markers. Osteoarthritis Cartilage. 2016;24(10):1826–1836. doi: 10.1016/j.joca.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y., Alexander P.B., Wang X.F. TGF-Β family signaling in the control of cell proliferation and survival. Cold Spring Harbor Perspect Biol. 2017;9(4) doi: 10.1101/cshperspect.a022145. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuda S., Revandkar A., Dubash T.D., Ravi A., Wittner B.S., Lin M., et al. TGF-β in the microenvironment induces a physiologically occurring immune-suppressive senescent state. Cell Rep. 2023;42(3) doi: 10.1016/j.celrep.2023.112129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng G., Zhang Z., Dang M., Zhang X., Doleyres Y., Song Y., et al. Injectable nanofibrous spongy microspheres for NR4A1 plasmid DNA transfection to reverse fibrotic degeneration and support disc regeneration. Biomaterials. 2017;131:86–97. doi: 10.1016/j.biomaterials.2017.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu X.-Y., Ding X.-H., Zhong L.-J., Xia H., Chen X.-D., Huang H. Expression and significance of VEGF and p53 in degenerate intervertebral disc tissue. Asian Pac J Tropical Med. 2013;6(1):79–81. doi: 10.1016/S1995-7645(12)60206-5. [DOI] [PubMed] [Google Scholar]
- 34.Che H., Li J., Li Y., Ma C., Liu H., Qin J., et al. p16 deficiency attenuates intervertebral disc degeneration by adjusting oxidative stress and nucleus pulposus cell cycle. Elife. 2020;9 doi: 10.7554/eLife.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng H., Fang J., Lu W., Liu Y., Chen S., Huang G., et al. TCF12 regulates the TGF-β/Smad2/3 signaling pathway to accelerate the progression of osteoarthritis by targeting CXCR4. Journal of Orthopaedic Translation. 2024;44:35–46. doi: 10.1016/j.jot.2023.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mannarino M., Cherif H., Li L., Sheng K., Rabau O., Jarzem P., et al. Toll-like receptor 2 induced senescence in intervertebral disc cells of patients with back pain can be attenuated by o-vanillin. Arthritis Res Ther. 2021;23(1):117. doi: 10.1186/s13075-021-02504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng C., Zhang Y., Yang M., Lan M., Liu H., Wang J., et al. The matrikine N-acetylated proline-glycine-proline induces premature senescence of nucleus pulposus cells via CXCR1-dependent ROS accumulation and DNA damage and reinforces the destructive effect of these cells on homeostasis of intervertebral discs. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2017;1863(1):220–230. doi: 10.1016/j.bbadis.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 38.Nakawaki M., Uchida K., Miyagi M., Inoue G., Kawakubo A., Kuroda A., et al. Sequential CCL2 expression profile after disc injury in mice. J Orthop Res : official publication of the Orthopaedic Research Society. 2020;38(4):895–901. doi: 10.1002/jor.24522. [eng] [DOI] [PubMed] [Google Scholar]
- 39.Cheng L.-Q., Zhang Z.-Q., Chen H.-Z., Liu D.-P. Epigenetic regulation in cell senescence. J Mol Med. 2017;95(12):1257–1268. doi: 10.1007/s00109-017-1581-x. [DOI] [PubMed] [Google Scholar]
- 40.Li G., Ma L., He S., Luo R., Wang B., Zhang W., et al. WTAP-mediated m6A modification of lncRNA NORAD promotes intervertebral disc degeneration. Nat Commun. 2022;13(1):1469. doi: 10.1038/s41467-022-28990-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo J., Shao M., Lu F., Jiang J., Xia X. Role of Sirt1 plays in nucleus pulposus cells and intervertebral disc degeneration. Spine (Phila Pa. 2017;42(13):E757. doi: 10.1097/BRS.0000000000001954. 1976. e66. [eng] [DOI] [PubMed] [Google Scholar]
- 42.He J., Zhang A., Song Z., Guo S., Chen Y., Liu Z., et al. The resistant effect of SIRT1 in oxidative stress-induced senescence of rat nucleus pulposus cell is regulated by Akt-FoxO1 pathway. Biosci Rep. 2019;39(5) doi: 10.1042/BSR20190112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu H., Sun B., Zhu L., Zou G., Shen Q. N6-Methyladenosine induced miR-34a-5p promotes TNF-α-induced nucleus pulposus cell senescence by targeting SIRT1. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.642437. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hwang J-w, Yao H., Caito S., Sundar I.K., Rahman I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radical Biol Med. 2013;61:95–110. doi: 10.1016/j.freeradbiomed.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo J., Shao M., Lu F., Jiang J., Xia X. Role of Sirt1 plays in nucleus pulposus cells and intervertebral disc degeneration. Spine. 2017;42(13) doi: 10.1097/BRS.0000000000001954. [DOI] [PubMed] [Google Scholar]
- 46.Wang P., Yang C., Lu J., Ren Y., Goltzman D., Miao D. Sirt1 protects against intervertebral disc degeneration induced by 1,25-dihydroxyvitamin D insufficiency in mice by inhibiting the NF-κB inflammatory pathway. Journal of Orthopaedic Translation. 2023;40:13–26. doi: 10.1016/j.jot.2023.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi P.Z., Wang J.W., Wang P.C., Han B., Lu X.H., Ren Y.X., et al. Urolithin a alleviates oxidative stress-induced senescence in nucleus pulposus-derived mesenchymal stem cells through SIRT1/PGC-1α pathway. World J Stem Cell. 2021;13(12):1928–1946. doi: 10.4252/wjsc.v13.i12.1928. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y., Wang H., Zhuo Y., Hu Y., Zhang Z., Ye J., et al. SIRT1 alleviates high-magnitude compression-induced senescence in nucleus pulposus cells via PINK1-dependent mitophagy. Aging (Albany NY) 2020;12(16):16126–16141. doi: 10.18632/aging.103587. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He Y., Li W., Lv D., Zhang X., Zhang X., Ortiz Y.T., et al. Inhibition of USP7 activity selectively eliminates senescent cells in part via restoration of p53 activity. Aging Cell. 2020;19(3) doi: 10.1111/acel.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiley C.D., Schaum N., Alimirah F., Lopez-Dominguez J.A., Orjalo A.V., Scott G., et al. Small-molecule MDM2 antagonists attenuate the senescence-associated secretory phenotype. Sci Rep. 2018;8(1):2410. doi: 10.1038/s41598-018-20000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cherif H., Bisson D.G., Mannarino M., Rabau O., Ouellet J.A., Haglund L. Senotherapeutic drugs for human intervertebral disc degeneration and low back pain. Elife. 2020;9 doi: 10.7554/eLife.54693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sturmlechner I., Zhang C., Sine C.C., van Deursen E.-J., Jeganathan K.B., Hamada N., et al. p21 produces a bioactive secretome that places stressed cells under immunosurveillance. Science. 2021;374(6567) doi: 10.1126/science.abb3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chandra A., Lagnado A.B., Farr J.N., Doolittle M., Tchkonia T., Kirkland J.L., et al. Targeted clearance of p21- but not p16-positive senescent cells prevents radiation-induced osteoporosis and increased marrow adiposity. Aging Cell. 2022;21(5) doi: 10.1111/acel.13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharpless N.E., Sherr C.J. Forging a signature of in vivo senescence. Nat Rev Cancer. 2015;15(7):397–408. doi: 10.1038/nrc3960. [DOI] [PubMed] [Google Scholar]
- 55.Baker D.J., Wijshake T., Tchkonia T., LeBrasseur N.K., Childs B.G., van de Sluis B., et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–236. doi: 10.1038/nature10600. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Novais E.J., Tran V.A., Johnston S.N., Darris K.R., Roupas A.J., Sessions G.A., et al. Long-term treatment with senolytic drugs Dasatinib and Quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat Commun. 2021;12(1):5213. doi: 10.1038/s41467-021-25453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen D., Jiang X., Zou H. hASCs-derived exosomal miR-155-5p targeting TGFβR2 promotes autophagy and reduces pyroptosis to alleviate intervertebral disc degeneration. Journal of Orthopaedic Translation. 2023;39:163–176. doi: 10.1016/j.jot.2023.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Orjalo A.V., Bhaumik D., Gengler B.K., Scott G.K., Campisi J. Cell surface-bound IL-1α is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci USA. 2009;106(40):17031–17036. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herranz N., Gallage S., Mellone M., Wuestefeld T., Klotz S., Hanley C.J., et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol. 2015;17(9):1205–1217. doi: 10.1038/ncb3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carroll B., Nelson G., Rabanal-Ruiz Y., Kucheryavenko O., Dunhill-Turner N.A., Chesterman C.C., et al. Persistent mTORC1 signaling in cell senescence results from defects in amino acid and growth factor sensing. J Cell Biol. 2017;216(7):1949–1957. doi: 10.1083/jcb.201610113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Narita M., Young A.R., Arakawa S., Samarajiwa S.A., Nakashima T., Yoshida S., et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science. 2011;332(6032):966–970. doi: 10.1126/science.1205407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hernandez-Segura A., Nehme J., Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. 2018;28(6):436–453. doi: 10.1016/j.tcb.2018.02.001. [eng] [DOI] [PubMed] [Google Scholar]
- 63.Kuehnemann C., Hu K.-Q., Butera K., Patel S.K., Bons J., Schilling B., et al. Extracellular nicotinamide phosphoribosyltransferase is a component of the senescence-associated secretory phenotype. Front Endocrinol. 2022;13 doi: 10.3389/fendo.2022.935106. [English] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nacarelli T., Lau L., Fukumoto T., Zundell J., Fatkhutdinov N., Wu S., et al. NAD+ metabolism governs the proinflammatory senescence-associated secretome. Nat Cell Biol. 2019;21(3):397–407. doi: 10.1038/s41556-019-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun Y., Li X., Yang X., Chen B., Zhang W. Small extracellular vesicles derived from adipocytes attenuate intervertebral disc degeneration in rats by rejuvenating senescent nucleus pulposus cells and endplate cells by delivering exogenous NAMPT. Oxid Med Cell Longev. 2021;2021 doi: 10.1155/2021/9955448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Du J., Xu M., Kong F., Zhu P., Mao Y., Liu Y., et al. CB2R attenuates intervertebral disc degeneration by delaying nucleus pulposus cell senescence through AMPK/GSK3β pathway. Aging and disease. 2022;13(2):552–567. doi: 10.14336/AD.2021.1025. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Glaeser J.D., Salehi K., Kanim L.E.A., NaPier Z., Kropf M.A., Cuéllar J.M., et al. NF-κB inhibitor, NEMO-binding domain peptide attenuates intervertebral disc degeneration. Spine J. 2020;20(9):1480–1491. doi: 10.1016/j.spinee.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chou L.-Y., Ho C.-T., Hung S.-C. Paracrine senescence of mesenchymal stromal cells involves inflammatory cytokines and the NF-κB pathway. Cells. 2022;11(20):3324. doi: 10.3390/cells11203324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhongyi S., Sai Z., Chao L., Jiwei T. Effects of nuclear factor kappa B signaling pathway in human intervertebral disc degeneration. Spine. 2015;40(4):224–232. doi: 10.1097/BRS.0000000000000733. [DOI] [PubMed] [Google Scholar]
- 70.Yasuda S., Horinaka M., Iizumi Y., Goi W., Sukeno M., Sakai T. Oridonin inhibits SASP by blocking p38 and NF-κB pathways in senescent cells. Biochem Biophys Res Commun. 2022;590:55–62. doi: 10.1016/j.bbrc.2021.12.098. [DOI] [PubMed] [Google Scholar]
- 71.Loo T.M., Miyata K., Tanaka Y., Takahashi A. Cellular senescence and senescence-associated secretory phenotype via the cGAS-STING signaling pathway in cancer. Cancer Sci. 2020;111(2):304–311. doi: 10.1111/cas.14266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang H., Wang H., Ren J., Chen Q., Chen Z.J. cGAS is essential for cellular senescence. Proc Natl Acad Sci USA. 2017;114(23):E4612–E4620. doi: 10.1073/pnas.1705499114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo Q., Zhu D., Wang Y., Miao Z., Chen Z., Lin Z., et al. Targeting STING attenuates ROS induced intervertebral disc degeneration. Osteoarthritis Cartilage. 2021;29(8):1213–1224. doi: 10.1016/j.joca.2021.04.017. [DOI] [PubMed] [Google Scholar]
- 74.Wu H.-C., Rérolle D., Berthier C., Hleihel R., Sakamoto T., Quentin S., et al. Actinomycin D targets NPM1c-primed mitochondria to restore PML-driven senescence in AML therapy. Cancer Discov. 2021;11(12):3198–3213. doi: 10.1158/2159-8290.CD-21-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang W., Li G., Luo R., Lei J., Song Y., Wang B., et al. Cytosolic escape of mitochondrial DNA triggers cGAS-STING-NLRP3 axis-dependent nucleus pulposus cell pyroptosis. Exp Mol Med. 2022;54(2):129–142. doi: 10.1038/s12276-022-00729-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loo T.M., Miyata K., Tanaka Y., Takahashi A. Cellular senescence and senescence-associated secretory phenotype via the cGAS-STING signaling pathway in cancer. Cancer Sci. 2020;111(2):304–311. doi: 10.1111/cas.14266. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kang C. Senolytics and senostatics: a two-pronged approach to target cellular senescence for delaying aging and age-related diseases. Mol Cell. 2019;42(12):821–827. doi: 10.14348/molcells.2019.0298. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Özsoy Gökbilen S., Becer E., Vatansever H.S. Senescence-mediated anticancer effects of quercetin. Nutr Res (NY) 2022;104:82–90. doi: 10.1016/j.nutres.2022.04.007. [eng] [DOI] [PubMed] [Google Scholar]
- 79.Shao Z., Wang B., Shi Y., Xie C., Huang C., Chen B., et al. Senolytic agent Quercetin ameliorates intervertebral disc degeneration via the Nrf2/NF-κB axis. Osteoarthritis Cartilage. 2021;29(3):413–422. doi: 10.1016/j.joca.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 80.Novais E.J., Tran V.A., Johnston S.N., Darris K.R., Roupas A.J., Sessions G.A., et al. Long-term treatment with senolytic drugs Dasatinib and Quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat Commun. 2021;12(1):1–17. doi: 10.1038/s41467-021-25453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen Z., Yang X., Zhou Y., Liang Z., Chen C., Han C., et al. Dehydrocostus lactone attenuates the senescence of nucleus pulposus cells and ameliorates intervertebral disc degeneration via inhibition of STING-TBK1/NF-κB and MAPK signaling. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.641098. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shao Z., Wang B., Shi Y., Xie C., Huang C., Chen B., et al. Senolytic agent Quercetin ameliorates intervertebral disc degeneration via the Nrf2/NF-κB axis. Osteoarthritis Cartilage. 2021;29(3):413–422. doi: 10.1016/j.joca.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 83.Novais E.J., Tran V.A., Johnston S.N., Darris K.R., Roupas A.J., Sessions G.A., et al. Long-term treatment with senolytic drugs Dasatinib and Quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat Commun. 2021;12(1):1–17. doi: 10.1038/s41467-021-25453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cherif H., Bisson D.G., Jarzem P., Weber M., Ouellet J.A., Haglund L. Curcumin and o-vanillin exhibit evidence of senolytic activity in human IVD cells in vitro. J Clin Med. 2019;8(4):433. doi: 10.3390/jcm8040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Z., Yang X., Zhou Y., Liang Z., Chen C., Han C., et al. Dehydrocostus lactone attenuates the senescence of nucleus pulposus cells and ameliorates intervertebral disc degeneration via inhibition of STING-TBK1/NF-κB and MAPK signaling. Front Pharmacol. 2021:12. doi: 10.3389/fphar.2021.641098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lim S., An S.B., Jung M., Joshi H.P., Kumar H., Kim C., et al. Local delivery of senolytic drug inhibits intervertebral disc degeneration and restores intervertebral disc structure. Adv Healthcare Mater. 2022;11(2) doi: 10.1002/adhm.202101483. [DOI] [PubMed] [Google Scholar]
- 87.Fuhrmann-Stroissnigg H., Ling Y.Y., Zhao J., McGowan S.J., Zhu Y., Brooks R.W., et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun. 2017;8(1):422. doi: 10.1038/s41467-017-00314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cherif H., Bisson D.G., Mannarino M., Rabau O., Ouellet J.A., Haglund L. Senotherapeutic drugs for human intervertebral disc degeneration and low back pain. Elife. 2020:9. doi: 10.7554/eLife.54693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou N., Lin X., Dong W., Huang W., Jiang W., Lin L., et al. SIRT1 alleviates senescence of degenerative human intervertebral disc cartilage endo-plate cells via the p53/p21 pathway. Sci Rep. 2016;6(1) doi: 10.1038/srep22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Patil P., Dong Q., Wang D., Chang J., Wiley C., Demaria M., et al. Systemic clearance of p16INK4a-positive senescent cells mitigates age-associated intervertebral disc degeneration. Aging Cell. 2019;18(3) doi: 10.1111/acel.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Che H., Li J., Li Y., Ma C., Liu H., Qin J., et al. p16 deficiency attenuates intervertebral disc degeneration by adjusting oxidative stress and nucleus pulposus cell cycle. Elife. 2020;9 doi: 10.7554/eLife.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ding J., Zhang R., Li H., Ji Q., Cheng X., Thorne R.F., et al. ASIC1 and ASIC3 mediate cellular senescence of human nucleus pulposus mesenchymal stem cells during intervertebral disc degeneration. Aging (Albany NY) 2021;13(7):10703–10723. doi: 10.18632/aging.202850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yi Y.-Y., Chen H., Zhang S.-B., Xu H.-W., Fang X.-Y., Wang S.-J. Exogenous Klotho ameliorates extracellular matrix degradation and angiogenesis in intervertebral disc degeneration via inhibition of the Rac1/PAK1/MMP-2 signaling axis. Mech Ageing Dev. 2022;207 doi: 10.1016/j.mad.2022.111715. [DOI] [PubMed] [Google Scholar]
- 94.Han Y., Zhou C.-M., Shen H., Tan J., Dong Q., Zhang L., et al. Attenuation of ataxia telangiectasia mutated signalling mitigates age-associated intervertebral disc degeneration. Aging Cell. 2020;19(7) doi: 10.1111/acel.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao J., Zhang L., Lu A., Han Y., Colangelo D., Bukata C., et al. ATM is a key driver of NF-κB-dependent DNA-damage-induced senescence, stem cell dysfunction and aging. J]. Aging (Albany NY) 2020;12(6):4688–4710. doi: 10.18632/aging.102863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cai Y., Zhou H., Zhu Y., Sun Q., Ji Y., Xue A., et al. Elimination of senescent cells by β-galactosidase-targeted prodrug attenuates inflammation and restores physical function in aged mice. Cell Res. 2020;30(7):574–589. doi: 10.1038/s41422-020-0314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guerrero A., Guiho R., Herranz N., Uren A., Withers D.J., Martínez‐Barbera J.P., et al. Galactose‐modified duocarmycin prodrugs as senolytics. Aging Cell. 2020;19(4) doi: 10.1111/acel.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.González‐Gualda E., Pàez‐Ribes M., Lozano‐Torres B., Macias D., Wilson J.R., III, González‐López C., et al. Galacto‐conjugation of Navitoclax as an efficient strategy to increase senolytic specificity and reduce platelet toxicity. Aging Cell. 2020;19(4) doi: 10.1111/acel.13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cao Y., Li Y., Fu S.C., Shen J., Zhang H., Jiang C., et al. Platelet-rich plasma pretreatment protects anterior cruciate ligament fibroblasts correlated with PI3K-Akt-mTOR pathway under hypoxia condition. Journal of Orthopaedic Translation. 2022;34:102–112. doi: 10.1016/j.jot.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martin N., Popgeorgiev N., Ichim G., Bernard D. BCL-2 proteins in senescence: beyond a simple target for senolysis? Nat Rev Mol Cell Biol. 2023:1–2. doi: 10.1038/s41580-023-00594-y. [DOI] [PubMed] [Google Scholar]
- 101.Luna-Vargas M.P.A., Chipuk J.E. Physiological and pharmacological control of BAK, BAX, and beyond. Trends Cell Biol. 2016;26(12):906–917. doi: 10.1016/j.tcb.2016.07.002. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lim S., An S.B., Jung M., Joshi H.P., Kumar H., Kim C., et al. Local delivery of senolytic drug inhibits intervertebral disc degeneration and restores intervertebral disc structure. Adv Healthcare Mater. 2022;11(2) doi: 10.1002/adhm.202101483. [DOI] [PubMed] [Google Scholar]
- 103.Shahverdi M., Amini R., Amri J., Karami H. Gene therapy with MiRNA-mediated targeting of mcl-1 promotes the sensitivity of non-small cell lung cancer cells to treatment with ABT-737. Asian Pac J Cancer Prev APJCP : Asian Pac J Cancer Prev APJCP. 2020;21(3):675–681. doi: 10.31557/APJCP.2020.21.3.675. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dutta Gupta S., Pan C.H. Recent update on discovery and development of Hsp90 inhibitors as senolytic agents. Int J Biol Macromol. 2020;161:1086–1098. doi: 10.1016/j.ijbiomac.2020.06.115. [DOI] [PubMed] [Google Scholar]
- 105.Fuhrmann-Stroissnigg H., Ling Y.Y., Zhao J., McGowan S.J., Zhu Y., Brooks R.W., et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun. 2017;8(1):422. doi: 10.1038/s41467-017-00314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Han S.Y., Ko A., Kitano H., Choi C.H., Lee M.-S., Seo J., et al. Molecular chaperone HSP90 is necessary to prevent cellular senescence via lysosomal degradation of p14ARFHSP90-mediated p14ARF degradation in NSCLC. Cancer Res. 2017;77(2):343–354. doi: 10.1158/0008-5472.CAN-16-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fuhrmann-Stroissnigg H., Ling Y.Y., Zhao J., McGowan S.J., Zhu Y., Brooks R.W., et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun. 2017;8(1):422. doi: 10.1038/s41467-017-00314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang S., Wang P., Hu B., Liu W., Lv X., Chen S., et al. HSP90 inhibitor 17-AAG attenuates nucleus pulposus inflammation and catabolism induced by M1-polarized macrophages. Front Cell Dev Biol. 2022;9 doi: 10.3389/fcell.2021.796974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang S., Wang P., Hu B., Lv X., Liu W., Chen S., et al. Inhibiting HSP90 attenuates nucleus pulposus fibrosis and pathological angiogenesis induced by macrophages via downregulating CEMIP. Am J Pathol. 2023;193(7):960–976. doi: 10.1016/j.ajpath.2023.03.014. [DOI] [PubMed] [Google Scholar]
- 110.Harris S.L., Levine A.J. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24(17):2899–2908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- 111.Jimenez G.S., Nister M., Stommel J.M., Beeche M., Barcarse E.A., Zhang X.-Q., et al. A transactivation-deficient mouse model provides insights into Trp53 regulation and function. Nat Genet. 2000;26(1):37–43. doi: 10.1038/79152. [DOI] [PubMed] [Google Scholar]
- 112.Cherif H., Bisson D.G., Mannarino M., Rabau O., Ouellet J.A., Haglund L. Senotherapeutic drugs for human intervertebral disc degeneration and low back pain. Elife. 2020;9 doi: 10.7554/eLife.54693. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tang Y., Zhao W., Chen Y., Zhao Y., Gu W. Acetylation is indispensable for p53 activation. Cell. 2008;133(4):612–626. doi: 10.1016/j.cell.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhou N., Lin X., Dong W., Huang W., Jiang W., Lin L., et al. SIRT1 alleviates senescence of degenerative human intervertebral disc cartilage endo-plate cells via the p53/p21 pathway. Sci Rep. 2016;6(1) doi: 10.1038/srep22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Patil P., Dong Q., Wang D., Chang J., Wiley C., Demaria M., et al. Systemic clearance of p16INK4a-positive senescent cells mitigates age-associated intervertebral disc degeneration. Aging Cell. 2019;18(3) doi: 10.1111/acel.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Che H., Li J., Li Y., Ma C., Liu H., Qin J., et al. p16 deficiency attenuates intervertebral disc degeneration by adjusting oxidative stress and nucleus pulposus cell cycle. Elife. 2020;9 doi: 10.7554/eLife.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ding J., Zhang R., Li H., Ji Q., Cheng X., Thorne R.F., et al. ASIC1 and ASIC3 mediate cellular senescence of human nucleus pulposus mesenchymal stem cells during intervertebral disc degeneration. Aging (Albany NY) 2021;13(7):10703–10723. doi: 10.18632/aging.202850. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu Z.-L., Xie Q.-Q., Liu T.-C., Yang X., Zhang G.-Z., Zhang H.-H. Role of the Wnt pathway in the formation, development, and degeneration of intervertebral discs. Pathol Res Pract. 2021;220 doi: 10.1016/j.prp.2021.153366. [DOI] [PubMed] [Google Scholar]
- 119.Han Y., Zhou C.-M., Shen H., Tan J., Dong Q., Zhang L., et al. Attenuation of ataxia telangiectasia mutated signalling mitigates age-associated intervertebral disc degeneration. Aging Cell. 2020;19(7) doi: 10.1111/acel.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhao J., Zhang L., Lu A., Han Y., Colangelo D., Bukata C., et al. ATM is a key driver of NF-κB-dependent DNA-damage-induced senescence, stem cell dysfunction and aging. Aging (Albany NY) 2020;12(6):4688–4710. doi: 10.18632/aging.102863. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.