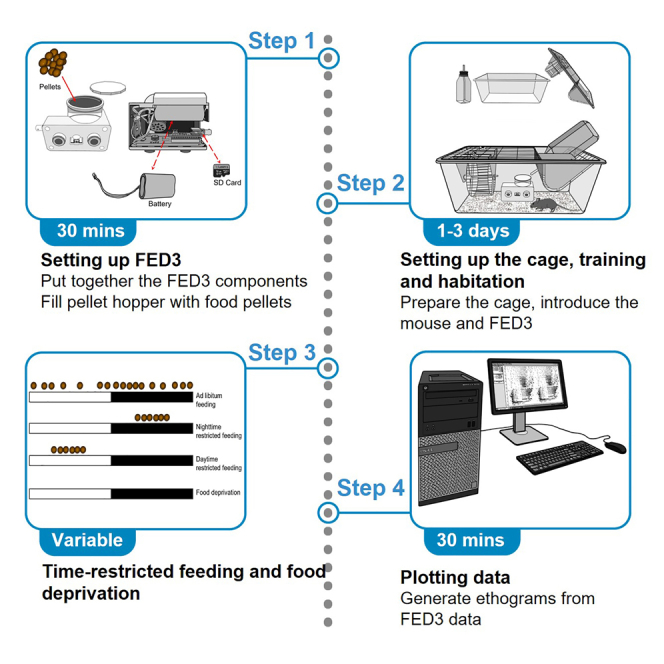
Summary
Food-anticipatory nose poking is a unique food-seeking behavior driven by the food-entrainable oscillator. Here, we present a protocol to record a novel food-seeking nose poking behavior in mice under temporally restricted feeding followed by food deprivation using the open-source feeding experimentation device version 3 (FED3). We describe steps for setting up the FED3 and cage, training, and habituation. We then detail procedures for setting up the schedule for time-restricted feeding and food deprivation and for generating ethograms from FED3 data.
For complete details on the use and execution of this protocol, please refer to Ehichioya et al.1
Subject areas: Neuroscience, Behavior
Graphical abstract
Highlights
-
•
FED3 is a powerful operant feeding device for measuring food-seeking circadian behavior
-
•
Food-anticipatory nose poking can be measured at night without being masked by activity
-
•
With modified codes, time-restricted feeding and food deprivation can be performed
-
•
This protocol provides step-by-step instructions for recording food-seeking behavior
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Food-anticipatory nose poking is a unique food-seeking behavior driven by the food-entrainable oscillator. Here, we present a protocol to record a novel food-seeking nose poking behavior in mice under temporally restricted feeding followed by food deprivation using the open-source feeding experimentation device version 3 (FED3). We describe steps for setting up the FED3 and cage, training, and habituation. We then detail procedures for setting up the schedule for time-restricted feeding and food deprivation and for generating ethograms from FED3 data.
Before you begin
When rodents are exposed to a temporally restricted feeding schedule, allowing only a few hours of food access during the daytime, animals exhibit food anticipatory activity which starts a few hours prior to the daily mealtime.2,3,4 Traditionally, researchers record wheel-running activity, ambulatory activity, and occasionally core body temperature to measure the circadian rhythm of food anticipation. When measured this way, food anticipatory activity is masked by nocturnal activity, which limited prior studies to performing timed restricted feeding during the daytime when nocturnal rodents are inactive. Recently, Mistlberger and colleagues showed that lever pressing and nose poking are outputs of the food entrainable oscillator (FEO).5 This motivated us to use an open-source feeding experimentation device version 3 (FED3),6 to measure food-seeking nose-poking behavior during temporally restricted feeding. Food anticipatory nose poking behavior, which the FEO controls, is distinguishable from nocturnal activity controlled by the primary central circadian pacemaker, the suprachiasmatic nucleus. The FED3 allows researchers to measure food anticipatory poking behavior at any time of the day, including at night. This protocol describes the steps for recording food-seeking nose pokes using the FED3 device.
The FED3 is a stand-alone, battery-powered device that runs on the Adafruit Adalogger MO feather board. FED3 is specifically designed to feed and train mice on operant tasks. The Kravitz Lab developed the device to address the previous difficulties and time constraints in quantifying animals’ food intake and temporal feeding patterns.6 The FED3 features two nose poke holes, a pellet hopper, a dispenser, a speaker for auditory stimuli, eight multi-color LEDs for visual stimuli, and an LCD screen for experimenter feedback. The screen displays information such as battery life, date and time, the number of left and right pokes, and the count of dispensed pellets. To dispense food pellets, mice must poke the designated activated nose poke hole. Food pellets are stored in the pellet hopper, and the pellets are released one at a time through the pellet dispenser. Before another food pellet can be dispensed, the mouse must first retrieve the previously dispensed pellet from the pellet dispenser and then perform a nose poke. For this experiment, we modified the code to disable the LEDs and buzzer. With this modified code, the FED3 dispenses one pellet when the left-nose-poke hole is poked, but not when the right-nose-poke hole is. The FED3 records the times of pellet intake, rewarded left nose pokes, and unrewarded right nose pokes.
Institutional permissions
All animal studies were carried out in accordance with the National Institutes of Health Guidelines regarding the care and use of animals for experimental procedures and were approved by the Institutional Animal Care and Use Committee (IACUC) at UT Southwestern Medical Center (Protocol #: 2016-10376-G).
Appropriate institutional approvals for use of animals should be obtained before starting this protocol. Our approved protocol allows us to conduct up to 48 h food deprivation. However, allowable food deprivation duration may vary by each IACUC and animal protocol.
Setting up the FED3
Timing: 30 min
-
1.
Charge a rechargeable lithium-ion battery (ICR18650, 3.7 V, 4.4 Ah) using the charger and place the charged battery inside of the FED3.
-
2.
The FED3 comes with a micro-SD card. If there is no micro-SD card inside of the FED3, insert a micro-SD card (16 GB or larger).
-
3.
Install Arduino IDE software to a computer and start the Arduino IDE.
-
4.
While the FED3 Arduino board (Adafruit feather M0) is turned off, connect the board to a computer using the included USB data cable.
-
5.
From the Arudio IDE, open FED3.cpp, FED3.h, and the FED3 run code (FED3_1.16.1_RF.ino) in the FED3_1.16.1_RF folder.
-
6.
Compile all of the code and upload it onto the FED3 Arduino board.
Note: The original Arduino IDE run code for FED3 (“Timed_FR1.ino.ino” developed with FED3_1.16.1 libraries) was written by the Kravitz group specifically for controlling FED3 hardware, such as the stepper motor which rotates the disk attached to dispense a pellet. It is written using Arduino Integrative Development Environment (Arduino IDE), an open-source platform that uses a programming language based on C++. “FED3_1.16.1_RF.ino” is a modified version of the original code, modified to enable the feeding schedule to cross midnight when the date change occurs. For instance, a FED3 feeding schedule from 22:00 to 2:00 cannot be programmed in the original code. With this modified code, the feeding schedule can be programmed as 22:00 to 26:00 and the FED3 will then provide a continuous feeding window from 22:00 to 2:00. "FED3.cpp" is a code written in C++ which contains user-defined functions that are declared within a class (FED3) in the "FED3.h" header file. The "FED3_1.16.1_RF.ino" file calls the class FED3. The “FED3.cpp” and “FED3.h” files are written separately from “FED3_1.16.1_RF.ino" but are compiled as a single ino file. Arduino IDE compiles all three files and uploads them onto the FED3 Arduino board. We deactivated the buzzer and LED lights in the modified FED3.cpp. We also modified FED3.h to display a FED3 version with an “RF” extension.
When the run code is uploaded, the clock time on the Arduino board is initially synchronized to a PC real-time clock (RTC). However, because the clock runs with a speed slightly faster or slower than 24h, the current time on the FED3 display needs to periodically be adjusted (see CRITICAL below).
-
7.
Turn on the FED3 by switching on the power button on the board.
-
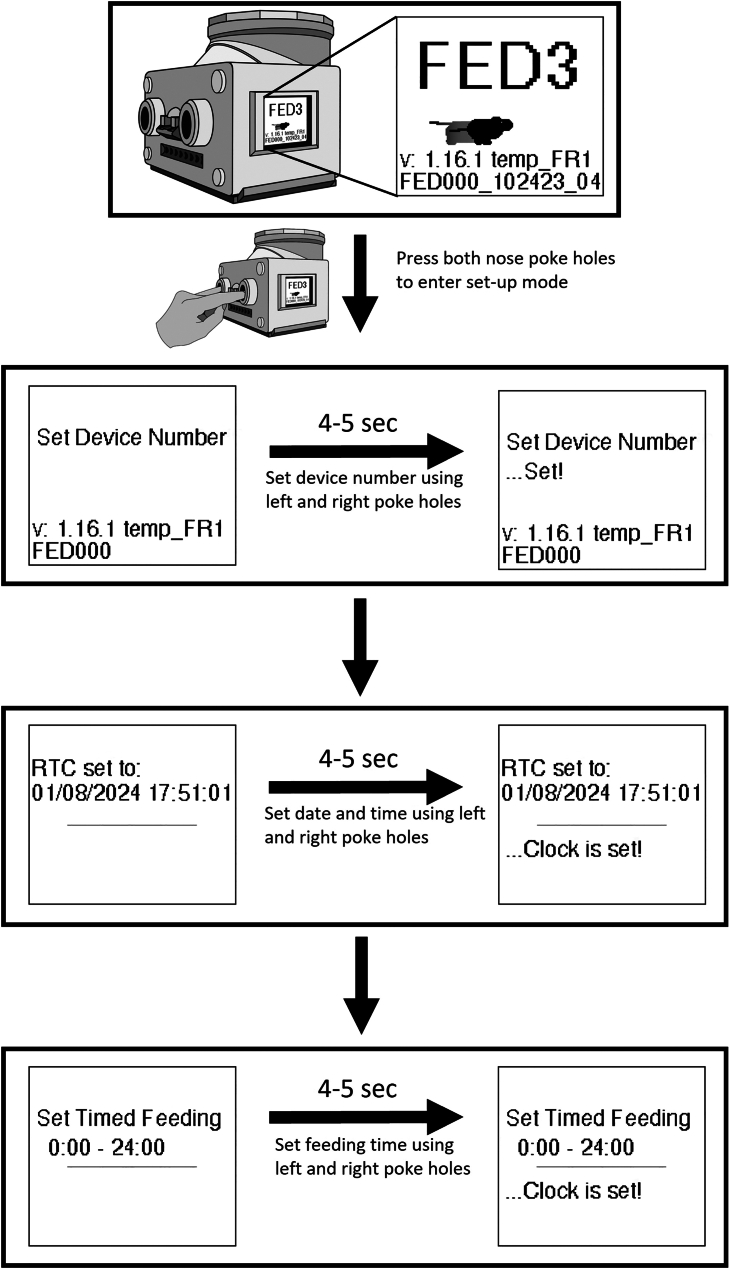
8.Access the FED3 settings (Figure 1).
-
a.When the FED3 starts up, the display screen shows a video of a running mouse.
-
b.Simultaneously put your fingers in both the left and right nose poke holes during this time, and the device will enter settings edit mode.
-
c.In the device settings, the screen initially shows "set device number".
-
d.After a brief delay of about 4–5 s, “set” will briefly appear at the bottom of the screen.
-
e.In the settings edit mode, the device number, RTC- date and time, and feeding time can be modified by using the left and right nose poke holes.
-
a.
CRITICAL: The clock on the Arduino board runs slightly faster or slower than 24 h. The speed is different in each FED3. We occasionally see ∼2 min difference of the FED3 clock time compared to local time one week after we start running the FED3. It is important to check RTC on the FED3 LCD screen regularly and adjust the FED3 clock to the local time if the FED3 clock differs from the actual time by more than 1 min. Abrupt large changes of the FED3 clock time (e.g. 5 min) will create an artificial phase shift (advance or delay) of recorded circadian rhythms.
Figure 1.
The step-by-step process of setting up the FED3
Setting up the cage
Timing: 1 h
-
9.
Load 20 mg pellets into the pellet hopper.
Note: Within the pellet hopper, there is a designed “max fill” mark that indicates the maximum level to which the food hopper should be filled with food pellets.
-
10.
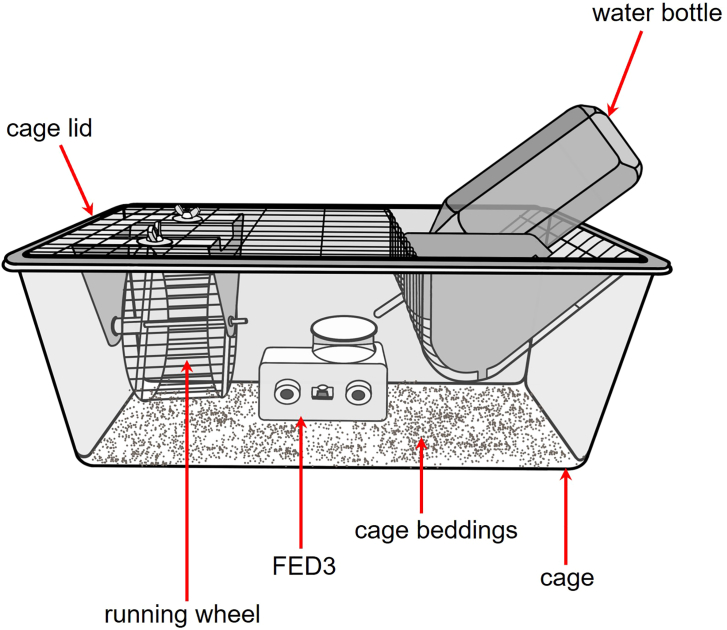
Place the FED3 inside of a cage (16.0 cm W, 32.0 cm L, 12.5 cm H) with woodchip bedding (Figure 2).
-
11.
Attach a running wheel (11.0 cm diameter) to the cage lid (Figure 2).
CRITICAL: At the beginning of the experiment, it is important to manually mark all 3 events (left poke, right poke, and pellet intake) by poking the left nose poke hole (which dispenses a pellet), removing the pellet, and then poking the right nose poke hole. Repeat this a few times to create a mark at the start of the data. This is done because, when the data is converted to ClockLab format, ClockLab plots the events separately and will not start a plot with blank days. Instead, ClockLab will start each event’s plot from the day of the first instance of that particular event. As a result, if the mouse does not trigger all of the events on the day the experiment starts, the experiment days can become misaligned between the different events.
Note: Use ClockLab or other circadian data acquisition systems to record magnetic switch-activated wheel-running revolutions. We recommend recording wheel revolutions every 1 min because the data conversion code converts FED3 data in 1 min bins.
Optional: A small running wheel cage (11.5 cm W, 29.5 cm L, 12.0 cm H, wheel diameter 11.0 cm) can be used. However, in this case, the FED3 needs to be attached to the outside of the cage using the magnets on the FED3, and the cages need to each have a hole on the side for the mice to access the nose poke holes and pellet dispenser. The FED3 can be magnetically attached to each cage by gluing metal washers, corresponding with the magnets on the FED3, to the side of the cage. This FED3 outside configuration causes less disturbance for mice than the FED3 inside configuration does, as the routine of refilling the pellets, changing the battery, downloading the data, and checking the daily pellet intake on the FED3 LCD screen can be done without opening the cage lid.
-
12.
Place the cage inside of a ventilated light tight box.
-
13.
Place a water bottle in the cage lid (Figure 2).
Optional: The experiments can be done inside of the home cages. However, changing the light dark cycle cannot be done in the home cages in the animal room. A passive infra-red motion detector may be used instead of a running wheel to monitor ambulatory activity.
Figure 2.
Typical cage setting
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental models: Organisms/strains | ||
| Mouse: C57BL/6J | The Jackson Laboratory | JAX: 000664 |
| Software and algorithms | ||
| ClockLab Data Collection (ver. 3.604) | Actimetrics | https://actimetrics.com/products/clocklab |
| ClockLab Analysis software (version 6.1.15) | Actimetrics | https://actimetrics.com/products/clocklab/ |
| Python (version 3.11.4) | N/A | https://www.python.org/ |
| Arduino IDE (version 2.1.1) | N/A | https://www.arduino.cc/en/software |
| Other | ||
| FED3 – feeding device | LABmaker | FED3 |
| 20 mg food pellet | Bio-Serv | F0163 |
| Woodchip bedding | PJ Murphy Forest Products | Sani-Chips |
| Lithium ion battery pack 3.7V 4,400 mAh | Adafruit | Product ID: 354 |
| Micro Lipo - USB LiIon/LiPoly charger - v2 | Adafruit | Product ID: 304 |
| 16 GB Micro SD card | KIOXIA, Kingston | |
| FED3 code | This paper | https://doi.org/10.17632/cr5hkkdtx2.1 |
| Python code for FED3 data conversion | This paper | https://doi.org/10.17632/cr5hkkdtx2.1 |
Step-by-step method details
Adaptation period and training to dispense food pellets
Timing: 1–3 days
-
1.
Introduce mice to the cage with the FED3.
CRITICAL: The mouse must learn to dispense the pellets by left nose poking. Shortly after introducing the mouse to the FED3, the mouse will explore the FED3. Within the first 2 days, most of the mice learn how to dispense a pellet by a left poke. It is important to do the training in the ad libitum setting, as food deprivation or food restriction being introduced during training may impact food anticipatory activity. If the mouse only takes a few pellets on the first day, provide one piece of chow in the food hopper of the cage. If the mouse doesn’t learn to dispense the pellet by the second day, remove the mouse from experiment. (In our experiments, which we conducted with 89 mice, 3 mice didn’t learn to dispense pellets and ate only a few pellets on the first day. Two of those mice learned to dispense pellets on the second day. Only one mouse was unable to learn the pellet dispense by the second day and was removed from the experiment.)
Timed restricted feeding and food deprivation
Timing: a few weeks
This step describes how to set up the feeding schedule. To observe food anticipatory nose poking behavior, food access needs to be restricted to a few hours per day. To adapt mice to timed restricted feeding, reduce food windows gradually over a period of several days (8 h for 4 days, 6 h for 4 days, then 4 h thereafter). To measure anticipatory poking controlled by autonomous circadian clock, monitor nose pokes under the food deprivation.
Note: If the mouse loses more than 20% of its body weight during the experiment, the mouse needs to be removed from the study to comply with our approved animal protocol. The criteria for removing mice from the experiment and for allowable duration of timed restricted feeding may vary in each protocol and IACUC. Mice can also adapt to a shortened schedule of 8 h for 2 days, 6 h for 2 days, followed by 4 h.
-
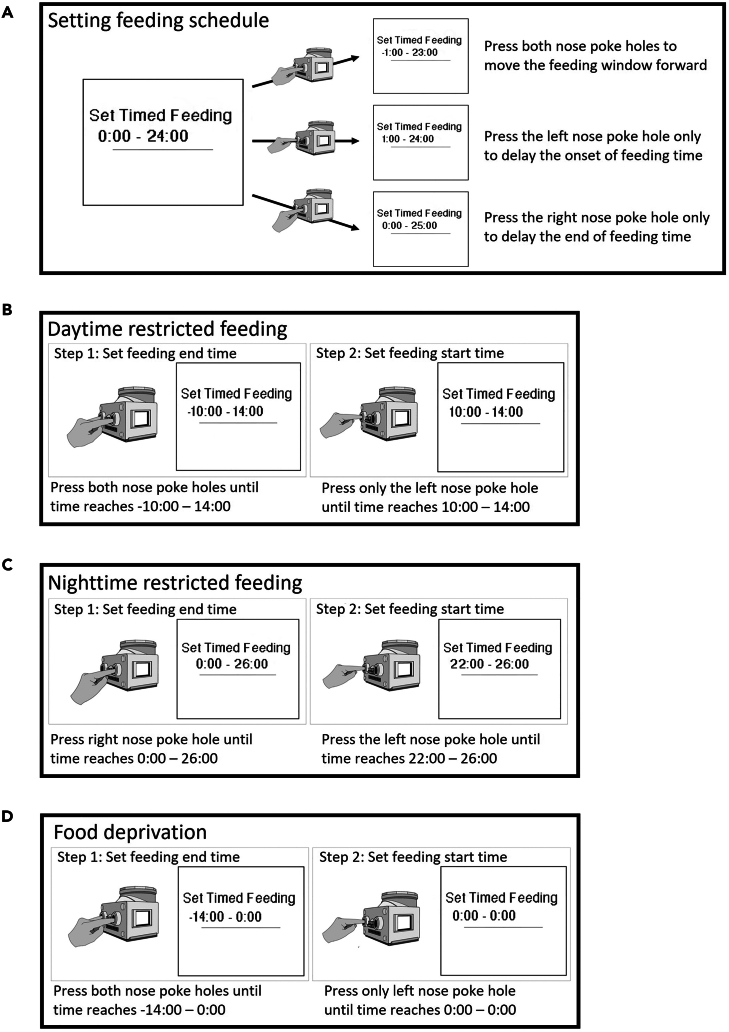
2.Set the feeding period (Figure 3A).
-
a.After about 4–5 s from setting the date and time, the FED3 screen will display “clock is set” at the bottom.
-
b.Then “set timed feeding” will be displayed on the screen. By default, the feeding time range is set from 0 to 24.
-
c.Simultaneously hold both the left and right nose poke holes to shift the entire time range earlier 1 h at a time (e.g., changing 0 to −1 and changing 24 to 23).
-
d.Press only the left nose poke to shift the start time forward 1 h at a time (e.g., changing 0 to 1).
-
e.Press only the right nose poke to shift the end time forward 1 h at a time (e.g., changing 24 to 25).
-
a.
Note: The numbers will keep changing as long as either the left or right nose pokes, or both, are held.
-
3.
To set a daytime restricted feeding, adjust the feeding period to a time range within the daytime (e.g., between lights-on at 06:00 and lights-off at 18:00, Figure 3B).
Note: For example, for a timed restricted feeding of 4 h, adjust the time of food availability to 10:00 - 14:00. To accomplish this, hold both the left and right nose pokes until the feeding end time “24” moves to an earlier time of “14”. This will also shift the start time to a negative value. Once the upper time point reaches “14”, immediately switch to holding only the left nose poke to adjust the start time to “10”.
-
4.
To set a nighttime restricted feeding, adjust the feeding period to a time range within the nighttime (e.g., between lights-off at 18:00 and lights-on at 06:00, Figure 3C).
Note: For example, for a timed restricted feeding of 4 h, adjust time of food availability to 22:00 - 26:00, where 26:00 is 2 AM. To achieve this, hold the right nose poke hole until the feeding end time reaches "26". Once it reaches "26″, immediately switch to holding only the left nose poke hole to adjust the start time from "0″ to "22".
-
5.
To set food deprivation, set the time of food availability to 0:00 to 0:00 (Figure 3D).
Note: To achieve this, hold both the left and right nose poke holes until the feeding end time reaches “0”. Once it reaches “0”, immediately switch to holding only the left nose poke to adjust the start time from “−14” to “0”.
Note: Record daily pellet intake if required by animal protocol.
CRITICAL: Due to the size of the pellet hopper and depending on the mice’s daily pellet consumption, it is necessary to add food pellets to the hopper on an almost daily basis. While it may be tedious, this routine is crucial to maintain a continuous food supply. Typically, the battery change interval in our experiment is 4–5 days during ad libitum feeding. Since the nose poking number drastically increases during the restricted feeding, the FED3 consumes more power so the battery needs to be changed every 3–4 days during restricted feeding. To avoid providing mice with time cues, those daily checks must be done at different times each day. We experienced the feeding schedule resetting when the year changed in some (but not all) FED3 devices. We recommend checking the feeding schedule on New Year’s Day and reprogramming the feeding schedule if it has reset.
Figure 3.
The step-by-step process of setting up feeding schedule
Downloading data from FED3
Timing: 1 h
With the FED3 clock, right nose poke, left nose poke, and pellet intake events are time-stamped and stored on the FED3’s micro SD card in a comma-separated value (CSV) format. This step describes how to convert time-stamped events to each event # / min bin, which is readable with the commonly used circadian analysis software, ClockLab.
-
6.
Install Python (current version) onto a computer.
-
7.
Create a folder in the desktop of the computer and label it as “FED3data”. Copy the FED3 analysis code (FED3_analysis_v1.4.3.py) into the “FED3 data” folder.
Note: Set a “PATH” for the FED3 analysis code if FED3data are not in the same folder as the code.
-
8.
Turn off the FED3. Remove the micro SD card from the FED3 and copy data files into the “FED3data” folder where FED3 analysis code (FED3_analysis_v1.4.3.py) is located.
-
9.
Start Command Prompt (Windows) or Terminal (Mac).
-
10.
Using a command, select a drive where the data folder is located (e.g., to change c drive, type “c:” and enter).
-
11.
Using a command, change the prompt to the directory where the folder has FED3 analysis code and FED3data (e.g., “cd Users” then enter, “cd Smith”, enter, “cd desktop”, enter, “cd FED3data”, enter). To return to root, enter “cd..”.
-
12.
Input the command:
> python3 ./FED3_analysis_v1.4.3.py [LOCATION of data (e.g. /Users/username/desktop/FED3data)] [MM DD YYYY when data conversion starts] [MM DD YYYY when data conversion ends]
For example.
> python3 ./FED3_analysis_v1.4.3.py /Users/Smith/desktop/FED3data 9 8 2023 10 23 2023
Note: “FED3_analysis_v1.4.3.py” is a Python code written for the analysis of data downloaded from the FED3 SD card. It is written using Python 3.11.4. This code converts the FED3 data to the text file format that can be opened by ClockLab. For data conversion, both FED3 analysis code and FED3 data need to be in the same folder, but the location of the folder can be anywhere in the computer and the folder name can be anything. However, if using an alternate location and/or name, the command needs to be altered accordingly. If FED3 is stopped and restarted again using a different SD card, there will be two data files. For data conversion, merge them to make one FED3 data file before converting the file.
Expected outcomes
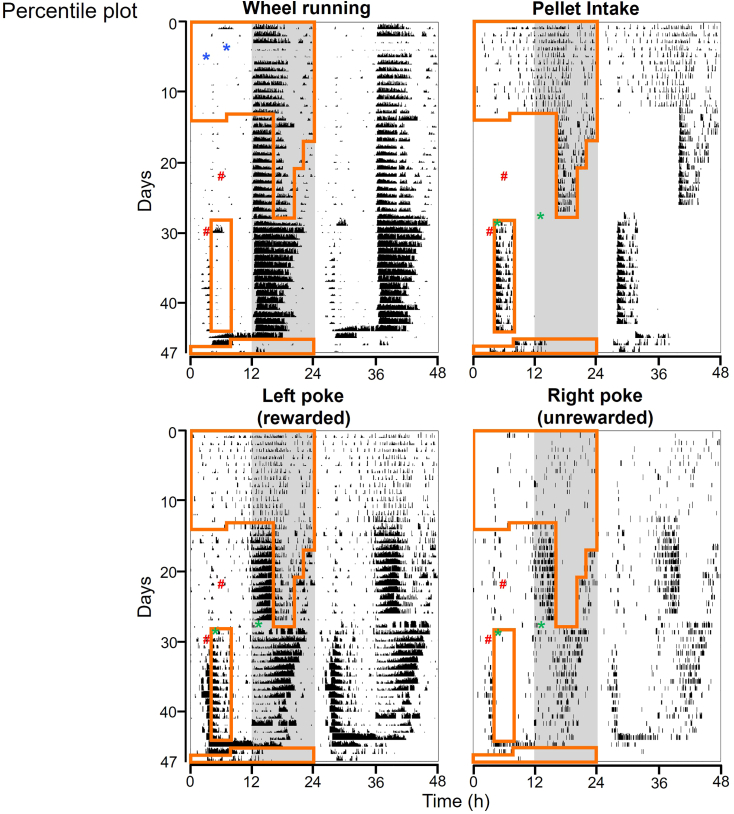
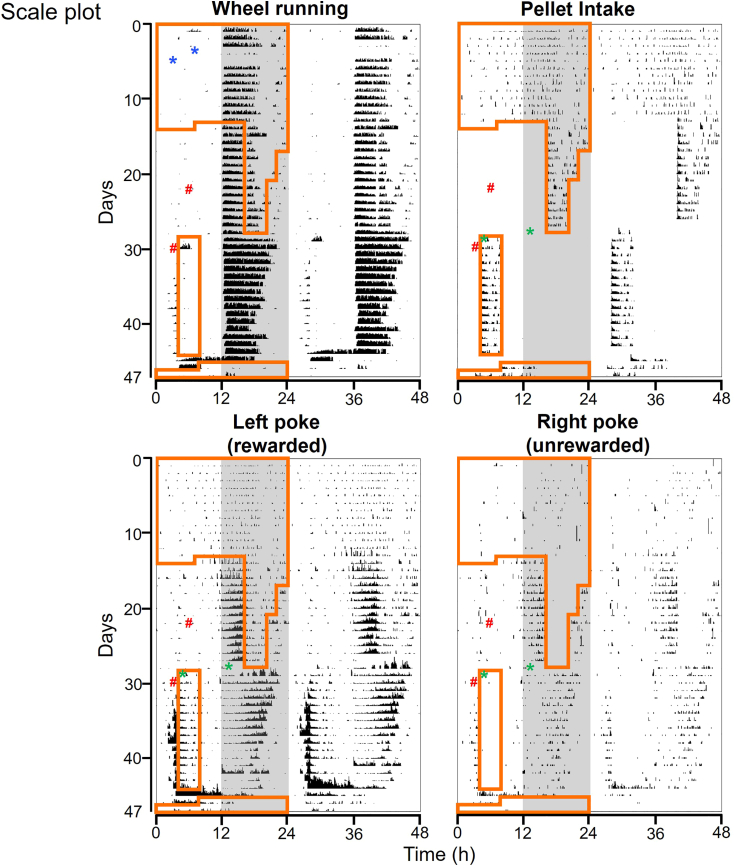
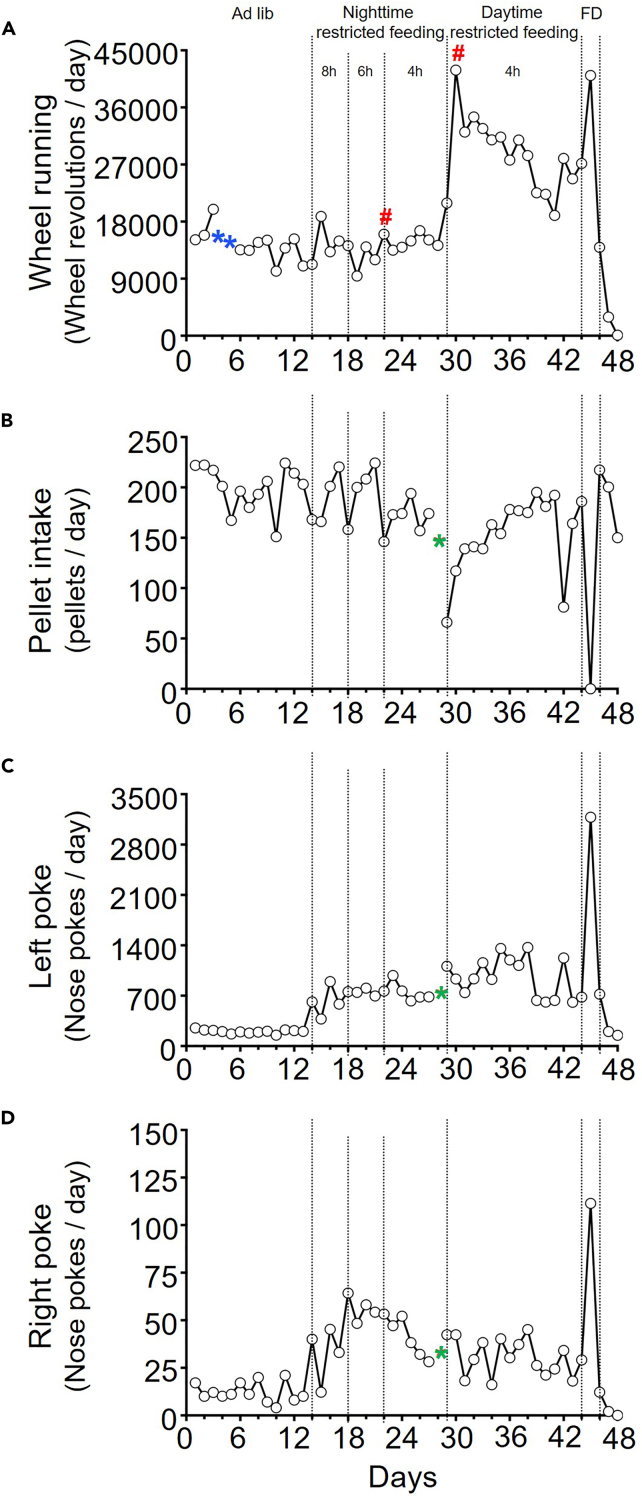
Our very first pilot experiment data using one 16-week-old C57BL/6J male mouse is shown in Figures 4, 5, and 6. Those are the typical behavioral rhythms we have seen.1 Double-plotted ethograms for wheel running activity, pellet intake, rewarded-left-nose-poking and unrewarded-right-pokes are plotted in Figure 4 (percentile plot with quantiles 50) and Figure 5 (scaled plot, 0–100/min for wheel running, 0–4/min for pellet intake, 0–6/min for left poke, 0–1 for right poke). 6 min bins are used for those ethograms, but 10 min bins are also commonly used. A percentile plot is preferred for seeing complicated rhythmic patterns. However, scaled plots have the advantage of detecting changes in absolute numbers (e.g., drastic increase of left pokes during restricted feeding and food deprivation). Under ad libitum feeding, mice typically consumed ∼70% of food at night. When the mouse was only given food access at night, the mouse exhibited very strong food anticipatory left pokes starting ∼4 h before food access. This anticipatory poking is also visible in the unrewarded-right pokes. However, anticipatory activity is not detectable in wheel-running because this is masked by the mouse’s nocturnal activity, which is controlled by the suprachiasmatic nucleus. When food access was moved to daytime, the mouse continued to poke during nighttime, even though pellets weren’t dispensing at night. In addition, the mouse started showing anticipatory poking for phase-advanced daytime feeding window. Therefore, two bouts of anticipatory poking are present, anticipating both the (unrewarded) previous meal schedule and the rewarded current feeding schedule. During food deprivation, the mouse expressed both daytime and nighttime food anticipatory poking.
Figure 4.
Representative double-plotted ethograms for wheel-running activity, food intake, rewarded-left-nose poking activity, and unrewarded-right-nose poking activity are shown with percentile plot
Data are plotted in 6 min bins, quantiles = 50. Light and dark condition is presented on the left half of the panel (white: light, gray: dark). Time when food was available is presented with an orange line on the left half of the panel. First blue asterisk: time when a microswitch position was fixed after it broke. Second blue asterisk: time when a microswitch was replaced. The times when cages were changed are indicated as #.
Figure 5.
Representative double-plotted ethograms shown with scaled plot
The same data presented in Figure 4 are re-plotted with scaled plot (as counts/min with scale; wheel-running: 0–100, pellet intake: 0–4, left poke 0–6, right poke 0–1). See others in Figure 4 legend.
Figure 6.
Daily quantification of wheel-running activity, food intake, rewarded left poke, and unrewarded right poke
FD: food deprivation. Green asterisk: FED3 jam. Blue asterisk: microswitch failure. #: cage change.
Daily changes in wheel-running activity, pellet intake, rewarded left poke, and unrewarded right poke are shown in Figure 6. The pellet intake per day stayed at ∼150–200 over the course of experiment, but whenever feeding conditions were changed, it temporarily dropped before gradually recovering.
During the 47 days of the experiment, we experienced one FED3 pellet jam on Day 29. This is indicated in each figure with a green asterisk. Reduced wheel running activity on days 4–5 is due to a microswitch position issue (indicated with blue asterisks).
Limitations
A limitation of this protocol is the necessity of daily visual inspection of the FED3 and the number of pellets dispensed. To avoid providing mice a daily cue, the visual inspection needs to be done different time during light phase each day. Other limitations are that pellets typically need to be re-filled daily and battery changes need to be done every 3–5 days.
Troubleshooting
Problem 1
The mouse doesn’t learn to dispense the pellet (step 1).
Potential solution
As described above, it is important to introduce the FED3 to the mouse in ad libitum setting. If the mouse doesn’t dispense pellets on the first night, place a piece of chow on the food hopper. If the mouse doesn’t take pellets on the second night, remove the mouse from the study.
Problem 2
The pellet does not dispense (step 1).
Potential solution
The occurrence of FED3 jams is typically caused by broken food pellets obstructing the passage connecting the pellet hopper and the pellet dispenser. Remove the broken pellets and clean the pellet dispenser. A pipe brush can be used to clear general debris and a smaller pipe brush (8 inches long, ⅕ inch in diameter) can be used to clean any debris stuck in hard-to-reach spots around the pellet dispenser. Because the FED3 jams also lead to rapid battery drainage, check and replace the battery whenever a jam occurs.
Problem 3
The rotating disc doesn’t rotate after a left nose poke (step 1).
Potential solution
The sensors of the nose poke holes or the pellet dispenser may be blocked by debris. Clean nose poke holes and pellet dispenser using a cleaning duster.
Problem 4
Card reader does not store information (step 1 and step 8).
Potential solution
If the micro SD card is inserted incorrectly, the FED3 fails to store the data. To avoid this, after inserting the micro SD into the FED3, switch the FED3 off and back on again. If the micro SD card is not properly inserted, “insert SD card” will appear on the FED3 screen. If the micro SD is properly inserted, the FED3 will display the normal start up screen. This should be done every time the micro SD card is inserted, including after it is removed to copy the data.
Problem 5
The FED3 data doesn’t convert to a ClockLab readable file (step 12).
Potential solution
Check that the prompt is under the folder which has the FED3 analysis code and FED3 data. Check the data export path and be sure it directs to the folder which has the FED3 analysis code and FED3 data.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Shin Yamazaki (shin.yamazaki@utsouthwestern.edu).
Technical contact
Further information about the FED3 operation should be directed to David Ehichioya (David.Ehichioya@UTSouthwestern.edu) or S K Tahajjul Taufique (SheikhTahajjul.Taufique@UTSouthwestern.edu).
Further information about the codes should be directed to Byeongha Jeong (Byeongha.Jeong@UTSouthwestern.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The codes generated during this study are available at Mendeley Data, https://doi.org/10.17632/cr5hkkdtx2.1.
The data presented in expected outcomes section is available upon request to the lead contact.
Acknowledgments
This work was supported by grants from the National Institutes of Health R01NS114527 and the National Science Foundation IOS-1931115 awarded to S.Y. D.E.E. is a postdoc scholar supported by the National Institutes of Health T32 training grant (T32HL139438). I.M. was a high school student supported by the UT Southwestern STARS Summer Research Program. A.E. was an undergraduate student supported by the Multi-Institutional Summer Undergraduate Research Program to Promote Diversity and Excellence in Sleep and Circadian Research Careers (National Institutes of Health R25 NS 125603). J.S.T. was an Investigator in the Howard Hughes Medical Institute.
Author contributions
Conceptualization, S.Y.; methodology, D.E.E., S.K.T.T., B.J., and S.Y., software, B.J.; investigation, D.E.E. and S.K.T.T., writing – original draft, D.E.E., B.J., I.M., S.F., A.E., K.B., and S.Y.; writing – review and editing, S.K.T.T., S.F., M.S. , J.S.T., and S.Y.; visualization, D.E.E., S.K.T.T, M.S., and S.F.; supervision, J.S.T. and S.Y.; funding acquisition, J.S.T. and S.Y.
Declaration of interests
The authors declare no competing interests.
References
- 1.Ehichioya D.E., Taufique S.K.T., Farah S., Yamazaki S. A time memory engram embedded in a light-entrainable circadian clock. Curr. Biol. 2023;33:5233–5239.e3. doi: 10.1016/j.cub.2023.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pendergast J.S., Yamazaki S. The Mysterious Food-Entrainable Oscillator: Insights from Mutant and Engineered Mouse Models. J. Biol. Rhythms. 2018;33:458–474. doi: 10.1177/0748730418789043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mistlberger R.E. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci. Biobehav. Rev. 1994;18:171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 4.Stephan F.K. The "other" circadian system: food as a Zeitgeber. J. Biol. Rhythms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- 5.Petersen C.C., Cao F., Stinchcombe A.R., Mistlberger R.E. Multiple entrained oscillator model of food anticipatory circadian rhythms. Sci. Rep. 2022;12:9306. doi: 10.1038/s41598-022-13242-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matikainen-Ankney B.A., Earnest T., Ali M., Casey E., Wang J.G., Sutton A.K., Legaria A.A., Barclay K.M., Murdaugh L.B., Norris M.R., et al. An open-source device for measuring food intake and operant behavior in rodent home-cages. Elife. 2021;10 doi: 10.7554/eLife.66173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The codes generated during this study are available at Mendeley Data, https://doi.org/10.17632/cr5hkkdtx2.1.
The data presented in expected outcomes section is available upon request to the lead contact.