Abstract
Background
To inform clinical practice, we sought to identify racial and ethnic differences in the medical management of common poststroke complications.
Methods and Results
A cohort of acutely hospitalized, first‐time non‐Hispanic White (NHW), non‐Hispanic Black, and Hispanic patients with stroke was identified from electronic medical records of 51 large health care organizations (January 1, 2003 to December 5, 2022). Matched propensity scores were used to account for baseline differences. Primary outcomes included receipt of medication(s) associated with the management of the following poststroke complications: arousal/fatigue, spasticity, mood, sleep, neurogenic bladder, neurogenic bowel, and seizure. Differences were measured at 14, 90, and 365 days. Subgroup analyses included differences restricted to patients with ischemic stroke, younger age (<65 years), and stratified by decade (2003–2012 and 2013–2022). Before matching, the final cohort consisted of 348 286 patients with first‐time stroke. Matching resulted in 63 722 non‐Hispanic Black–NHW pairs and 24 009 Hispanic–NHW pairs. Non‐Hispanic Black (versus NHW) patients were significantly less likely to be treated for all poststroke complications, with differences largest for arousal/fatigue (relative risk (RR), 0.58 [95% CI, 0.54–0.62]), spasticity (RR, 0.64 [95% CI, 0.0.62–0.67]), and mood disorders (RR, 0.72 [95% CI, 0.70–0.74]) at 14 days. Hispanic–NHW differences were similar, albeit with smaller magnitudes, with the largest differences present for spasticity (RR, 0.67 [95% CI, 0.63–0.72]), arousal/fatigue (RR, 0.77 [95% CI, 0.70–0.85]), and mood disorders (RR, 0.79 [95% CI, 0.77–0.82]). Subgroup analyses revealed similar patterns for ischemic stroke and patients aged <65 years. Disparities for the current decade remained significant but with smaller magnitudes compared with the prior decade.
Conclusions
There are significant racial and ethnic disparities in the treatment of poststroke complications. The differences were greatest at 14 days, outlining the importance of early identification and management.
Keywords: health care quality, access, and evaluation; health inequities; racial disparities; stroke; stroke rehabilitation
Subject Categories: Complications, Disparities, Health Equity, Health Services, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- NHB
non‐Hispanic White
- NHW
non‐Hispanic Black
- NIHSS
National Institutes of Health Stroke Scale
- RD
risk difference
Clinical Perspective.
What Is New?
There are significant racial and ethnic disparities in the medical management of common poststroke sequelae (fatigue, spasticity, depression, insomnia, seizure, and neurogenic bladder/bowel).
Disparities were largest at 2 weeks, persisted at 1 year, and may be due to patient–provider interactions (eg, health literacy, implicit bias, cultural competence) rather than access to care.
What Are the Clinical Implications?
Clinicians who routinely care for stroke survivors (eg, neurologists, physiatrists, primary care providers) should proactively screen for poststroke complications such as depression, fatigue, and spasticity, as opportunities to initiate medical management for poststroke complications are routinely being missed for racial and ethnic minority patients.
Significant racial and ethnic disparities in stroke outcomes are well‐known among patients in the United States. 1 Much of the work to date has focused on differences in risk factors associated with stroke incidence and death. 2 However, a significant proportion of patients with stroke survive and are at a considerable risk of debility due to poststroke complications. 3 The cumulative impact of these complications contributes to stroke being one of the leading causes of preventable disability. Unfortunately, racial and ethnic disparities in stroke extend beyond incidence and mortality to effect poststroke function among surviors. 1 , 3 , 4 Identification of the specific causes that contribute to racial and ethnic differences in poststroke function remains unclear, as prior studies have not identified clear racial and ethnic differences in prestroke function, the amount of rehabilitation therapies that patients receive, or access to specialized postacute care at inpatient rehabilitation facilities or skilled nursing facilities. 3 , 4 , 5 , 6
Prior studies on racial and ethnic differences in poststroke function have primarily assessed differences at single time points, yet stroke recovery is highly dynamic. 4 The dynamic nature of stroke recovery is partially due to differing natural histories of various complications. For example, bowel/bladder dysfunction often improves early, whereas issues such as spasticity may develop weeks to months after the stroke and worsen over time. 7 In addition, functional differences in recovery may be mediated through a variety of biopsychosocial domains. 7 , 8 Our objectives were to (1) identify racial and ethnic differences in the medical management of common poststroke complications and (2) identify time points during poststroke recovery when magnitudes of differences were greatest.
METHODS
Our methods and results adhere to Strengthening the Reporting of Observational Studies in Epidemiology guidelines. 9 Study data were acquired from the TriNetX Analytics network platform, which provides near real‐time access to patient data from electronic medical records identified from health care organizations that actively participate within their research division. Data are harmonized by the TriNetX informatics team, which leverages standard terminologies across multiple data domains to construct internally verified data maps. Additional details on TriNetX data, as well as its harmonization process have previously been published. 10 The data used in this study were considered exempt by the Institutional Review Board as they were deidentified and do not involve intervention or interaction with human subjects. Additional information on the privacy principles employed by TriNetX are publicly available. 11 All data and materials can be accessed via the online TriNetX platform.
For this study, the cohort included patients with first‐time stroke hospitalized with either an ischemic or hemorrhagic stroke between January 1, 2003 and December 5, 2022. Patients with stroke were identified using the International Classification of Diseases, Ninth Revision (ICD‐9) and Tenth Revision (ICD‐10). Patients with ischemic stroke were identified using ICD‐9 codes 433 and 434 or ICD‐10 code I63, whereas patients with hemorrhagic stroke were identified using ICD‐9 codes 430 and 431or ICD‐10 codes I60 to I62. Prior studies have shown similar accuracy of ICD‐9 and ICD‐10 codes where 85% and 97% of patients with ischemic and hemorrhagic stroke, respectively, were accurately identified. 12 Race and ethnicity were self‐identified. Per the TriNetX data dictionary, possible values for patient race were American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or other Pacific Islander, White, or Unknown. Ethnicity or cultural background was classified as Hispanic or Latino, Not Hispanic or Latino, or Unknown. Patients were excluded for the following reasons: (1) race and ethnicity not documented as non‐Hispanic White (NHW), non‐Hispanic Black (NHB), or Hispanic; (2) aged <18 years; and (3) prior history of stroke.
Primary Outcomes
All outcomes were measured at 14, 90, and 365 days from the indexed stroke. Primary outcomes included the use of medication(s) associated with the treatment of 7 poststroke complications: arousal/fatigue, spasticity, mood disorder, sleep disturbance, neurogenic bladder, neurogenic bowel, and seizure. Medical management for each complication were based on the Department of Veterans Affairs Drug Classification System and consisted of the following:
Arousal/fatigue (use of orally formulated central nervous system stimulants, modafinil/ armodafinil, or amantadine)
Spasticity (use of orally formulated baclofen, tizanidine, dantrolene, diazepam, or intramuscular botulinum toxin)
Depressed mood (use of orally formulated selective serotonin reuptake inhibitors or serotonin–norepinephrine reuptake inhibitors)
Sleep disturbance (use of orally formulated melatonin, trazodone, zolpidem, suvorexant, or ramelteon)
Neurogenic bladder (use of orally formulated α agonists or urinary antispasmodics)
Neurogenic bowel (use of orally formulated or rectally administered laxatives)
Seizure (use of orally formulated anticonvulsants)
Secondary Outcomes
Secondary outcomes included stroke severity, acute lifesaving treatments, radiographic or symptomatic complications, and ambulatory follow‐up visits. Stroke severity was classified by the National Institutes of Health Stroke Scale (NIHSS) score; with strokes categorized as mild (NIHSS score <10), moderate (NIHSS score 10–20), or severe (NIHSS score >20). Acute lifesaving treatments included (1) receipt of either intravenous tissue plasminogen activator or mechanical thrombectomy, (2) neurosurgical procedures (craniotomy, endovascular coiling, and Burr hole trephination), and (3) intubation or mechanical ventilation. Radiographic and symptomatic complications included the following: (1) cerebral edema or brain compression, (2) hemiplegia, (3) aphasia, and (4) 14‐day mortality rate. Ambulatory follow‐up visits included the number of ambulatory care visits at 90 days. Procedures and ambulatory follow‐up visits were identified by Current Procedural Terminology codes detailed in Table S1.
Independent Variable
Race and ethnicity was the independent variable that was operationalized as a single categorical variable with 3 mutually exclusive categories: NHW, NHB, or Hispanic.
Study Variables
Baseline variables (clinical comorbidities, prior medications, demographics, prior acute health care use) included potential confounders that were collected in the year before the indexed stroke and selected a priori on the basis of prior studies or perceived clinical importance. 5 , 13 Demographic factors includes age (per year), and sex. Clinical comorbidities were identified by ICD‐9 and ICD‐10 codes and included absence or presence of the following conditions: hypertension, chronic ischemic heart disease, acute myocardial infarction, hyperlipidemia, atherosclerosis, arterial thrombosis, seizures, Parkinson disease, multiple sclerosis, Alzheimer disease, hemiplegia, paraplegia, peripheral neuropathy, neurogenic bladder and bowel, constipation, type 1 and type 2 diabetes, obesity, depression, anxiety, sleep disorders, chronic obstructive pulmonary disease, asthma, acute kidney injury, chronic kidney disease, benign prosthetic hyperplasia, and prior neoplasm. Prior medications included use of anticonvulsants, central nervous system stimulants, selective norepinephrine reuptake inhibitors/serotonin–norepinephrine reuptake inhibitors, laxatives, α blockers, urinary antispasmodic medications, melatonin, tizanidine, baclofen, and insulin. When available, the most recent values recorded for systolic blood pressure (<140, 140–180, 180–200, >220 mm Hg), hemoglobin A1c (<5.7%, ≥5.7 to <6.5%, ≥ 6.5 to <9%, ≥9%), and low‐density lipoprotein cholesterol (<70, 70–189, >189 mg/dL) were used. Prior health care use was identified by any acute inpatient hospitalization, excluding emergency department visits.
Statistical Analysis
Data were analyzed using an emulated trial framework under principles of randomized trial design (eg, clearly defined time 0 and intention‐to‐treat protocol) to mimic a hypothetical randomized controlled trial allowing results to be interpreted under the same counterfactual framework as randomized controlled trials. 14 , 15 To emulate random treatment allocation, matched propensity scores were calculated using a multivariable logistic regression model that adjusted for all available baseline variables described above. Patients were matched 1:1 using a greedy nearest neighbor matching algorithm with a caliper width of 0.25 times the SD. 16 This approach assumes missing at random, that is, the propensity for a data point to be missing is not related to unobserved data, but it is related to some of the observed data. 17 Separate propensity score matches were made for the 2 separate comparisons (ie, NHB versus NHW and Hispanic versus NHW). Under this analysis, results are interpreted as the average treatment effect among the treated (ATT), which is ATT=E [Y 1 − Y 0∣D=1], where E is the expected outcome, Y is the counterfactual outcome for medical treatment (Y 1) or no medical treatment (Y 0), and D (1 or 0) is NHW or NHB/Hispanic depending on the specific comparision. 15 This counterfactual framework is analogous to that used by randomized controlled trials. 18 Due to the large sample sizes, absolute standardized differences were calculated in lieu of P values to identify systematic differences in baseline covariates between populations for both unmatched and matched patients, with absolute standardized differences >0.1 considered clinically important. 19 Thus, analysis is analogous to intention‐to‐treat as patients were matched on factors collected before the indexed stroke, which was considered time 0. 20
For comparative binary outcomes, relative risks (RRs) and risk differences (RDs) with corresponding 95% CIs were calculated for both matched and unmatched patients. Matched pairs were analyzed as stratified pairs, and differences were independently calculated at each time point for the 2 racial and ethnic comparisons. Results for matched pairs were considered adjusted for all covariates that were included within the propensity score model. Racial and ethnic differences in the number of ambulatory visits at 90 days were assessed using t‐tests. All analyses were performed within a built‐in statistical platform within TriNetX. Statistical tests were 2‐sided with significance set at P<0.05.
Subgroup Analyses
Three separate subgroup analyses were performed. For more homogenous groups, we restricted our analysis to patients with ischemic stroke and younger age (<65 years). 13 Next, we explored the effects of time, by stratifying groups by decade (January 1, 2003, to December 31, 2012) and (January 1, 2013, to December 5, 2022) with a focus on differences at 90 days. All the subgroup analyses mimicked the primary analysis, with the exception that results were only reported as RRs for matched pairs.
RESULTS
We initially identified 565 348 hospitalized patients with stroke. Patients were then excluded for the following reasons: race and ethnicity other than NHW, NHB, or Hispanic (n=71 489), aged <18 years (n=63 391), or prior history of stroke (n=82 182). Thus, our final cohort consisted of 348 286 patients who were identified from 51 health care organizations. Of these patients, 259 805 were NHW, 64456 were NHB, and 24 025 were Hispanic (Figure 1).
Figure 1. Consolidated Standards of Reporting Trials diagram for patient selection.
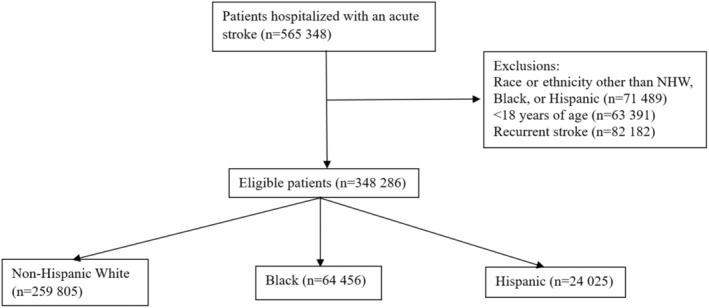
NHW indicates non‐Hispanic White.
Table 1 shows the distribution of selected baseline covariates by race and ethnicity. Compared with both NHB and Hispanic patients, NHW patients were older, more likely to have a prior history of neoplasm, anxiety disorders, and use of selective norepinephrine reuptake inhibitor/serotonin–norepinephrine reuptake inhibitor medications. Significant differences for the NHB–NHW comparison included NHB patients having increased odds of being diagnosed with type 2 diabetes and using insulin. Specific Hispanic–NHW differences included Hispanic patients being less likely to have prior history of chronic ischemic heart disease, chronic obstructive lung disease, and hyperlipidemia.
Table 1.
Racial and Ethnic Differences for Patient‐level Characteristics Among Hospitalized Patients With Acute Stroke
| NHW, % (n=259 805) | NHB, % (n=64 456) | Hispanic, % (n=24 025) | Absolute standardized differences | ||
|---|---|---|---|---|---|
| NHW vs NHB | NHW vs Hispanic | ||||
| Age, y, mean±SD | 64.5±14.9 | 59.6±14.3 | 56.4±16.5 | 0.33* | 0.52* |
| Female sex | 44.9 | 47.5 | 42.4 | 0.05 | 0.05 |
| Comorbidities | |||||
| Hypertensive diseases | 27.0 | 31.1 | 23.1 | 0.09 | 0.09 |
| Chronic ischemic heart disease | 11.3 | 8.6 | 7.1 | 0.09 | 0.14* |
| Acute myocardial infarction | 2.7 | 2.8 | 2.2 | 0.01 | 0.03 |
| Hyperlipidemia | 19.5 | 15.9 | 12.9 | 0.09 | 0.18* |
| Atherosclerosis | 2.6 | 2.3 | 1.9 | 0.02 | 0.04 |
| Arterial thrombosis | 0.8 | 0.7 | 0.6 | 0.01 | 0.03 |
| Seizures | 2.0 | 2.5 | 2.0 | 0.04 | <0.01 |
| Parkinson disease | 0.9 | 0.3 | 0.4 | 0.08 | 0.06 |
| Multiple sclerosis | 0.3 | 0.3 | 0.1 | 0.01 | 0.04 |
| Alzheimer disease | 0.6 | 0.4 | 0.4 | 0.02 | 0.03 |
| Hemiplegia | 0.5 | 0.6 | 0.6 | 0.01 | 0.02 |
| Paraplegia | 0.2 | 0.3 | 0.2 | 0.02 | <0.01 |
| Peripheral neuropathy | 2.9 | 2.2 | 2.2 | 0.03 | 0.045 |
| Neurogenic bladder | 0.4 | 0.3 | 0.4 | 0.02 | 0.01 |
| Neurogenic bowel | 0.1 | 0.1 | 0.1 | 0.01 | 0.01 |
| Constipation | 4.9 | 5.1 | 4.9 | 0.01 | <0.01 |
| Type 1 diabetes | 1.4 | 1.8 | 1.7 | 0.03 | 0.02 |
| Type 2 diabetes | 12.9 | 16.7 | 15.9 | 0.11* | 0.09 |
| Obesity | 6.4 | 6.7 | 6.1 | 0.01 | 0.01 |
| Depression | 7.8 | 5.6 | 6.0 | 0.09 | 0.07 |
| Anxiety | 7.8 | 4.3 | 5.4 | 0.15* | 0.10 |
| Sleep disorders | 7.5 | 5.9 | 5.6 | 0.06 | 0.07 |
| Chronic obstructive pulmonary disease | 6.1 | 4.8 | 2.3 | 0.06 | 0.12* |
| Asthma | 3.0 | 4.1 | 2.6 | 0.06 | 0.03 |
| Acute kidney failure | 5.3 | 7.5 | 5.3 | 0.09 | <0.01 |
| Chronic kidney disease | 7.2 | 10.6 | 7.3 | 0.12 | <0.01 |
| Benign prostatic hyperplasia | 3.5 | 2.2 | 2.2 | 0.08 | 0.08 |
| Neoplasm | 14.1 | 10.3 | 9.3 | 0.12* | 0.15* |
| Prior medication use | |||||
| Anticonvulsants | 11.1 | 11.4 | 10.6 | 0.01 | 0.02 |
| CNS stimulants | 2.0 | 1.2 | 2.0 | 0.07 | <0.01 |
| SSRI/SNRIs | 13.4 | 9.0 | 10.0 | 0.14* | 0.11* |
| Laxatives | 16.8 | 18.7 | 16.6 | 0.05 | 0.01 |
| α Blockers | 4.7 | 3.7 | 3.4 | 0.05 | 0.06 |
| Urinary antispasmodic | 7.2 | 10.6 | 7.3 | 0.12 | <0.01 |
| Melatonin | 3.4 | 2.7 | 3.4 | 0.04 | <0.01 |
| Tizanidine | 1.0 | 0.8 | 0.8 | 0.02 | 0.02 |
| Baclofen | 1.0 | 1.0 | 1.0 | 0.01 | 0.01 |
| Insulin | 9.6 | 12.7 | 12.3 | 0.10* | 0.09 |
| Prior clinical data | |||||
| Hemoglobin A1c | |||||
| 0%–5.70% | 3.7 | 4.0 | 3.1 | 0.02 | 0.04 |
| 5.70%–6.50% | 4.4 | 5.6 | 4.0 | 0.06 | 0.02 |
| 6.50%–9.0% | 4.6 | 5.8 | 4.5 | 0.05 | <0.01 |
| >9.0% | 2.0 | 3.3 | 3.1 | 0.08 | 0.07 |
| Systolic blood pressure, mm Hg | |||||
| <140 | 29.1 | 23.1 | 18.0 | 0.14* | 0.26* |
| 140–180 | 22.5 | 21.5 | 13.1 | 0.03 | 0.25* |
| 180–220 | 5.4 | 7.7 | 3.7 | 0.09 | 0.08 |
| >220 | 1.3 | 0.6 | 0.6 | 0.07 | 0.01 |
| LDL cholesterol in serum or plasma, mg/dL | 13.3 | 13.5 | 9.7 | 0.15 | 0.01 |
| 0–70 | 4.4 | 3.7 | 3.3 | 0.04 | 0.06 |
| 71–189 | 10.3 | 10.3 | 6.8 | 0.02 | 0.10* |
| >190 | 0.4 | 0.5 | 0.3 | 0.02 | <0.01 |
| Prior health care use | |||||
| Prior hospitalization | 23.3 | 19.6 | 21.3 | 0.09 | 0.04 |
Prior data were collected in the year preceding the indexed stroke. CNS indicates central nervous system; NHB, non‐Hispanic Black; NHW, non‐Hispanic White; SNRI, serotonin–norepinephrine reuptake inhibitor; and SSRI, selective serotonin reuptake inhibitor.
Clinically important differences based on absolute standardized differences ≥0.1.
Propensity score matching resulted in 63 722 matched pairs for the NHB–NHW comparison and 24 009 matched pairs for the Hispanic–NHW comparison. After matching, all covariates were well balanced (ie, absolute standardized differences were < 0.1) indicating that there were no significant differences across baseline covariates (Table S2).
NIHSS scores were available for ≈10% of patients. Among these patients, NHB (versus NHW) patients were more likely to have a moderate (20.4% versus 18.5%) or severe (12.6% versus 9.8%) stroke, whereas Hispanic (versus NHW) patients had fewer moderate (19.1% versus 16.4%) but more severe (9.7% versus 10.3%) strokes (data not shown). In Table 2, distributions of acute lifesaving treatments as well as radiographic or symptomatic complications among matched patients are shown. Regarding acute treatment for the NHB–NHW comparison, NHB patients were slightly less likely to receive either tissue plasminogen activator or mechanical thrombectomy (7.8% versus 9.4%) and a neurosurgical procedure (4.0% versus 5.6%) but slightly more likely to receive mechanical ventilation/intubation (12.2% versus 11.2%). For radiographic and symptomatic complications, slightly fewer NHB patients had cerebral edema (14.4% versus 16.5%), while slightly more had hemiparesis (20.6% versus 18.8%) and aphasia (15.0% versus 14.4%). The 14‐day mortality rates were slightly lower for NHB patients (5.4% versus 7.6%). For the Hispanic–NHW comparison, Hispanic patients were less likely to receive tissue plasminogen activator or mechanical thrombectomy (8.7% versus 9.3%) and mechanical ventilation/intubation (12.1% versus 12.9%), but more likely to receive a neurosurgical procedure (7.5% versus 6.1%). For radiographic and symptomatic complications, Hispanic patients were more likely to have cerebral edema (19.9% versus 17.5%) but less likely to have hemiparesis (16.9% versus 17.9%) or aphasia (10.2% versus 14.4%). There was no difference in 14‐day mortality rates.
Table 2.
Racial and Ethnic Differences in Acute Treatment, Radiography, and Symptoms Among Propensity Score–Matched Patients With Acute Stroke
| NHW vs NHB (n=63 722 PS‐matched pairs) | NHW vs Hispanic (n=24 009 PS‐matched pairs) | |||||
|---|---|---|---|---|---|---|
| NHW, % | NHB, % | P value | NHW, % | Hispanic, % | P value | |
| Acute lifesaving treatments | ||||||
| tPA/mechanical thrombectomy | 9.4 | 7.8 | <0.01 | 9.3 | 8.7 | 0.01 |
| Neurological surgical procedure | 5.6 | 4.0 | <0.01 | 6.1 | 7.5 | <0.01 |
| Ventilation or mechanical intubation | 11.2 | 12.2 | <0.01 | 12.9 | 12.1 | <0.01 |
| Radiographic or symptomatic complications | ||||||
| Cerebral edema | 16.5 | 14.4 | <0.01 | 17.5 | 19.9 | <0.01 |
| Hemiparesis | 18.8 | 20.6 | <0.01 | 17.9 | 16.9 | 0.03 |
| Aphasia | 14.4 | 15.0 | <0.01 | 14.4 | 10.2 | <0.01 |
| 14‐d All‐cause death | 7.6 | 5.4 | <0.01 | 7.2 | 7.5 | 0.24 |
Significance was set at P<0.05 using McNemar's tests. Neurological surgery procedure refers to craniotomy, endovascular coiling, or Burr hole trephination. Matched pairs: patients were matched using a multivariable propensity score. Separate propensity scores matches were performed for the NHW vs NHB and NHW vs Hispanic comparisons. NHB indicates non‐Hispanic Black; NHW, non‐Hispanic White; and tPA, tissue plasminogen activator.
In Figure 2, coefficient plots showing the RRs of receiving medical management of 7 common poststroke complications among matched pairs at 14, 90, and 365 days are shown. In the supplemental materials, a corresponding table of results (Table S3) as well as unadjusted results (Table S4) are shown. For the NHB–NHW comparison, NHB patients were significantly less likely to receive medical treatment for every condition at nearly every time point. Treatment differences were greatest at 14 days and slightly attenuated over time. The largest relative difference included NHB patients being 42% less likely to receive medications associated with the management of arousal/fatigue at 2 weeks (RR, 0.58 [95% CI, 0.54–0.62]). Other large differences included NHB patients being significantly less likely to receive medications for the treatment of spasticity (RR, 0.64 [95% CI, 0.62–0.67]), mood disorders (RR, 0.72 [95% CI, 0.70–0.74]), and sleep disturbances (RR, 0.78 [95% CI, 0.76–0.80]). Differences in the treatment of seizures (RR, 0.89 [95% CI, 0.88–0.90]) and neurogenic bowel (RR, 0.99 [95% CI, 0.97–0.99]) were smaller but statistically significant. Results for the Hispanic (versus NHW) comparison were largely similar with a couple of exceptions. First, the magnitudes of differences were slightly smaller. Second, Hispanic patients were slightly more likely to receive medications used to treat neurogenic bladder and bowel, and there was no statistically significant racial and ethnic difference for the treatment of seizures.
Figure 2. Relative risk for medical treatment of common poststroke complications at 14, 90, and 365 days.
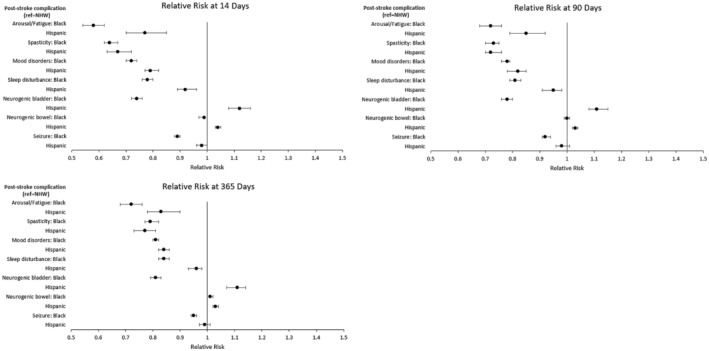
Time starts on day 1 of the acute hospitalization from the indexed stroke. Relative risks were estimated from matched pairs, and significance was set at P<0.05. Patients were matched 1:1 on the basis of a propensity score estimated from a multivariable logistic regression model. Separate propensity score matches were performed for the NHW vs Black (n=63 722 matched pairs) and NHW vs Hispanic (n=24 009 matched pairs) comparisons. Circles represent point estimates, and whiskers represent 95% CIs. NHW indicates non‐Hispanic White.
Figure 3 shows coefficient plots for results reported on the absolute scale as RDs, with a corresponding table shown in Table S5. RDs for the NHB (versus NHW) comparison remained significant for all compilations, but magnitudes were not always consistent with RRs. For example, the largest RR was for the treatment of arousal/fatigue, but the largest RD was for the treatment of mood disorders at 90 days (−9.6 [95% CI, −10.0 to −9.3]). The discordance is due to differences in the frequency of medication use, as substantially fewer patients received medications used to manage arousal/fatigue (NHW, 4.6% versus NHB, 3.3%) compared with mood disorders (NHW, 33.6% versus NHB, 24.0%). For the Hispanic (versus NHW) comparison, the largest RDs included differences for the treatment of mood disorders (RD, −8.2 [95% CI, −8.6 to −7.5]), spasticity (RD, −2.4 [95% CI, −2.7 to −2.1]), and sleep disturbances (RD, −2.3 [95% CI, −2.8 to −1.8]) at 14 days. Additional details on the frequency of medication use are shown in Table S6.
Figure 3. Risk differences for medical treatment of common poststroke complications at 14, 90, and 365 days.
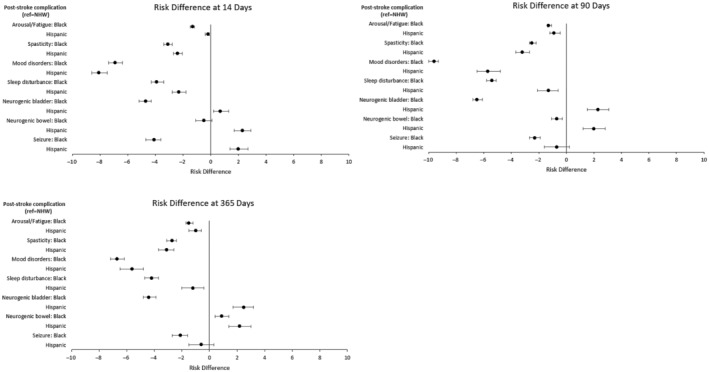
Time starts on day 1 of the acute hospitalization from the indexed stroke. Risk differences were estimated from matched pairs, and significance was set at P<0.05. Patients were matched 1:1 on the basis of a propensity score estimated from a multivariable logistic regression model. Separate propensity score matches were performed for the NHW vs Black (n=63 722 matched pairs) and NHW vs Hispanic (n=24 009 matched pairs) comparisons. Circles represent point estimates, and whiskers represent 95% CIs. NHW indicates non‐Hispanic White.
Regarding the number of ambulatory care follow‐up visits at 90 days, NHB patients had more outpatient clinic visits (μ=18) compared with NHW (μ=13) patients (P<0.01). For the Hispanic–NHW comparison, Hispanic patients had fewer ambulatory clinic visits (13 versus 11; P<0.01).
Figure 4 shows coefficient plots for results from the first subgroup analysis, detailing the RR for treatment of poststroke complications among patients with ischemic stroke. Overall, 47 907 pairs were matched for the NHB–NHW comparison, 18 569 pairs were matched for the Hispanic–NHW comparison, and covariates remained well balanced for each comparison. Subgroup analysis results were similar to the results for all patients with stroke, with several exceptions. For the NHB (versus NHW) comparison, the magnitudes of treatment differences were smaller for the management of mood disorders, sleep disturbances, and neurogenic bladder, while differences for seizure treatment were no longer statistically significant at 90 and 365 days. For the Hispanic (versus NHW) comparison, the magnitudes of treatment differences were larger for arousal/fatigue, sleep disturbance, and seizures (see Table S7).
Figure 4. Subgroup anaysis for relative risk for medical treatment of common poststroke complications at 14, 90, and 365 days among patients with ischemic stroke.
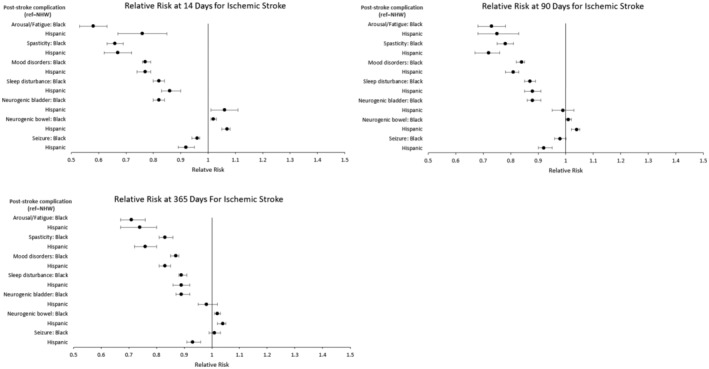
Time starts on day 1 of the acute hospitalization from the indexed stroke. Relative risks were estimated from matched pairs, and significance was set at P<0.05. Patients were matched 1:1 on the basis of a propensity score estimated from a multivariable logistic regression model. Separate propensity score matches were performed for the NHW vs Black (n=47 907 matched pairs) and NHW vs Hispanic (n=18 569 matched pairs) comparisons. Circles represent point estimates, and whiskers represent 95% CIs. NHW indicates non‐Hispanic White.
In Figure 5, coefficient plots from the second subgroup analysis in which racial and ethnic differences among patients with stroke aged <65 years were assessed are shown. After matching, there were 42 963 and 17 156 pairs for the NHB–NHW and Hispanic–NHW comparisons, respectively, with all covariates well balanced. Overall, results were similar to those from all patients with stroke, with the exception that for the NHB–NHW comparison, the magnitude of differences for arousal/fatigue was lower. Corresponding results are shown in Table S8.
Figure 5. Subgroup analysis for relative risk for medical treatment of common poststroke complications at 14, 90, and 365 days among patients aged <65 years.
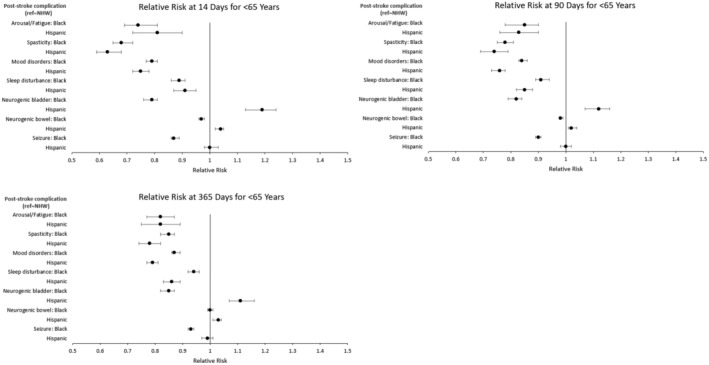
Time starts on day 1 of the acute hospitalization from the indexed stroke. Relative risks were estimated from matched pairs, and significance was set at P<0.05. Matches were based on a multivariable propensity score. Separate propensity score matches were performed for the NHW vs Black (n=42 963 matched pairs) and NHW vs Hispanic (n=17 156 matched pairs) comparisons. Circles represent point estimates, and whiskers represent 95% CIs. NHW indicates non‐Hispanic White.
Figure 6 details racial and ethnic differences in poststroke complications stratified by decade (2003–2012 and 2013–2022). For the NHB–NHW comparison, disparities for the most recent decade generally remained significant, albeit smaller for the treatment of spasticity (RR, 0.49 versus 0.75), neurogenic bladder (RR, 0.60 versus 0.80), and seizure (RR, 0.77 versus 0.94). For the Hispanic–NHW comparison, reductions in magnitudes of health disparities were observed for the treatment of arousal/fatigue (RR, 0.67 versus 0.88), spasticity (RR, 0.50 versus 0.72), and mood disorder (RR, 0.72 versus 0.84). Differences for sleep disturbances, neurogenic bladder, neurogenic bowel, and seizure were eliminated or reversed for the most recent decade. Corresponding results are shown in Table S9.
Figure 6. Subgroup analysis of racial and ethnic differences in the management of poststroke complications at 90 days stratified by decade.
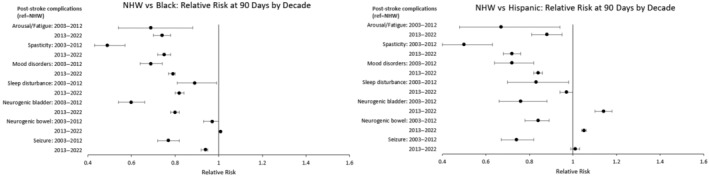
Time starts on day 1 of the acute hospitalization from the indexed stroke. Relative risks were estimated from matched pairs, and significance was set at P<0.05. Matches were based on a multivariable propensity score, with separate propensity score matches performed by decade for each racial and ethnic comparison. The number of matched patients for each comparison were as follows: NHW vs Black (2003–2012): n=5674 matched pairs; NHW vs Black (2013–2022): n=57 973 matched pairs; NHW vs Hispanic (2003–2012): n=2292 matched pairs; NHW vs Hispanic (2013–2022): n=21 693 matched pairs. Circles represent point estimates, and whiskers represent 95% CIs. NHW indicates non‐Hispanic White.
DISCUSSION
This large multicenter study of nearly 350 000 hospitalized patients with acute stroke identified significant racial and ethnic disparities in the medical management of common poststroke complications. Disparities were only marginally attenuated over time and remained significant at 1 year. Access to ambulatory follow‐up care is unlikely to explain differences in NHB–NHW disparities but may contribute to Hispanic–NHW disparities. Finally, our subgroup analysis showed that while significant disparities remain, positive progress has been made as the magnitude of racial and ethnic disparities has been reduced over time.
Identification that the magnitude of racial and ethnic differences in poststroke complications was largest at 2 weeks is important, as this corresponds with the time period during which patients were acutely hospitalized, and many of whom potentially would be receiving inpatient rehabilitation facility– or skilled nursing facility–based postacute care. Although the magnitude of disparities attenuated over time, absolute changes were small, indicating opportunities to initiate early treatment of many poststroke complications during the hospitalization, postacute rehabilitation care, and early follow‐up appointments.
Health care accessibility contributes to racial and ethnic disparities, but 2 aspects of our results demonstrate that accessibility alone is unlikely to explain treatment gaps. 21 First, NHB patients were the least likely to receive medical treatment, despite having the most ambulatory care follow‐up visits by 90 days. The finding corresponds with prior literature showing that NHB (versus NHW) patients were actually more likely to receive stroke rehabilitation care. 3 , 22 Second, the magnitudes of disparities were not consistent as treatment differences were smaller for complications with higher visibility (eg, neurogenic bowel/bladder) or clinical acuity (eg, seizures). The signs and symptoms of poststroke complications may be subtle and require a trusting physician–patient relationship (eg, fatigue/mood disorders) or specialized physical examination skills (eg, spasticity). We believe that clinicians who routinely take care of stroke survivors (eg, primary care providers, neurologists, and physiatrists) are missing these complications and may need to engage in more active surveillance of poststroke complications. This corresponds with a top 10 recommendation from the 2023 Subarachnoid Hemorrhage Clinical Practice Guidelines, which recommend the use of validated screening tools to identify physical, cognitive, behavioral, and quality‐of‐life deficits. 23
Other considerations to help close treatment gaps include structural changes to existing hospital standard performance measures (ie, quality metrics). Changes should focus on reforms to existing metrics; for example, stroke education could be expanded to improve patient and family awareness of the signs and symptoms of poststroke complications that impair recovery and negatively impact quality of life. Screening for poststroke depression at hospital discharge and 90 days as recommended by the 2019 Update to the 2018 Acute Ischemic Stroke Clinical Practice Guidelines is an example of this in clinical practice. 24 Stroke recovery is dynamic, and most functional gains occur during the first 90 days. 25 Thus, early identification and management of complications is essential to optimize recovery. This is particularly important for medications such as selective norepinephrine reuptake inhibitors/serotonin–norepinephrine reuptake inhibitors, which may take weeks to months to appropriately titrate. 8
While racial and ethnic disparities in poststroke function exist, prior studies have been unable to identify specific factors that contribute to these differences. 4 Our outcomes did not directly measure functional gains, but our results potentially move the timeline of racial and ethnic differences in medical management forward to the early subacute period. This slightly differs from a prior study that found that significant racial and ethnic disparities in function first emerged in the later subacute period after inpatient rehabilitation facility–based stroke rehabilitation. 13 However, in this study, function was directly captured using the Functional Independence Measure, an 18‐item scale that measures an individual's capability to complete tasks related to self‐care, mobility, continence, transfers, communication, and cognition. 13 , 26
Beyond stroke, our results are consistent with a host of other health‐related conditions such as cancer, 27 maternal death, 28 and more recently the 2019 coronavirus. 29 As our results outline, equitable recognition and management of health conditions is complex and requires enhanced engagement from patients and health care providers. As demonstrated by our results, access to care is not enough; mounting literature demonstrates that achieving equity requires addressing gaps in quality as well as quantity of care. 30 Improved quality requires a multifaceted response that targets patient‐, provider‐, and system‐level factors. 31 Interventions aimed at improving patient‐level constructs such as health literacy, self‐efficacy, and trust improve patient–provider relationships. 32 On the provider level, implicit bias and lack of cultural competency negatively impact patient care. 31 Implicit bias refers to attitudes or stereotypes that affect the perceived actions of others. 33 Cultural competency refers to contextual understanding of the manner in which personal, cultural, and environmental factors interact to influence patient health and health behaviors of their patients. 1 At the level of health systems, policies that address social determinants of health, promote transparency, and improve trust while maintaining consistent access to care have the promise of ensuring that patients receive not only high‐quality health care but maintenance of health vitality. 34
Our subgroup analysis indicating that, over time, positive progress has been made in the reduction of the magnitude of racial and ethnic disparities aligns with prior studies. 35 , 36 Progress in this realm demonstrates the positive cumulative effects of efforts that have been made to address inequities within stroke and health care more broadly. 37 While some of these trends are positive, the goal of true health equity has not been achieved, particularly in reference to individual‐ and system‐level factors that contribute to racial and ethnic disparities within health care. 31
Several limitations should be considered when interpreting our results. First, our results are subject to residual confounding, as we were unable to adjust for important socioeconomic confounders such as health insurance, education, and income. Given the importance of sociodemographic determinants of health, we hope for more routine collection of such variables by both electronic medical records and administrative databases. Second, our primary outcomes captured only receipt of medication and did not account for treatment indications and medication refusal or directly capture changes in patient‐level function. Notably, providers may overlook treatment indications, and clinical experience demonstrates that perceived treatment effects can be highly individualized. Thus, our results most closely correspond with measurable, real‐world racial and ethnic differences in opportunities to trial medications that are associated with functional recovery. Fourth, within the TriNetX platform, we were unable to adjust for either repeated measures or for clustering within hospitals. Thus, our results are prone to multicollinearity and overestimated standard errors with increases the risk of a type 1 error (ie, false positive). 38 Finally, the scope of our analysis was limited to difference among 3 racial and ethnic groups (ie, NHW, NHB, and Hispanic) and did not include medical management of other complications such as infection and pain. These conditions were not included due to concern for misclassification bias from the challenge of attributing medical management directly to the indexed stroke.
Our study also has several strengths. First, our data had strong generalizability as they captured outcomes for a large sample of patients with stroke across 51 health care organizations. Second, our models adjusted for many demographic and clinical confounders, and there was excellent balance between matched pairs. Third, the follow‐up time of 1 year captured differences at various time points, allowing for substantial functional recovery. 25 Fourth, our 3 subgroup analyses provide a more granular contextual assessment of our data and provide evidence toward positive progress in racial and ethnic disparities among patients with stroke. Finally, the TriNetX platform provides real‐world differences in racial and ethnic differences in the medical management of poststroke complications for non‐Hispanic Black and Hispanic patients, encompassing the 2 largest racial and ethnic minority groups in the United States. 21
In conclusion, this large multicenter study identified significant racial and ethnic disparities in the medical management of common poststroke complications. Disparities were generally largest at 14 days, with only small improvements observed by 1 year. Disparities were larger for complications with more subtle signs/symptoms (eg, arousal/fatigue and mood disorders), which demonstrates the need for active surveillance and improved relationships between patients and health care providers. Our subgroup analysis demonstrated that progress has been made, given that the magnitudes of disparities declined with time. However, significant disparities remain, and more work is needed to implement interventions aimed at closing these equity gaps.
Sources of Funding
Dr Atem has received support from 3 National Institutes of Health–funded research grants. He is a coinvestigator and lead statistician for an RO1 (1R01MD011686‐01A1), a diversity supplemental investigator on a separate RO1 (1R01CA234205‐01A1), and the lead statistician on an R21 (R21HD101854). Dr Welch has received research support from Stryker and MicroVention. Dr Ifejika's current work is supported by the University of Texas Southwestern/Texas Health Resources Clinical Scholar Award (No. 4). Dr Ifejika's previous work was supported by the Center for Clinical and Translational Sciences at the McGovern Medical School at the University of Texas Health Science Center at Houston, funded by the National Institutes of Health/National Center for Advancing Translational Sciences Clinical and Translational Awards UL1 TR000371 and KL2 TR000370. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Dr Ifejika's preliminary work was supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke Diversity Supplement to P50 NS 044227, the University of Texas Specialized Program of Translational Research in Acute Stroke.
Disclosures
Dr Welch has worked as a consultant for MicroVention, Stryker Corporation, and Medtronic. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S9
Acknowledgments
We would like to thank Rohan Shankar for his help with the creation of the figures.
This manuscript was sent to Mahasin S. Mujahid, PhD, MS, FAHA, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.030537
For Sources of Funding and Disclosures, see page 12.
References
- 1. Stansbury JP, Jia H, Williams LS, Vogel WB, Duncan PW. Ethnic disparities in stroke: epidemiology, acute care, and postacute outcomes. Stroke. 2005;36:374–386. doi: 10.1161/01.STR.0000153065.39325.fd [DOI] [PubMed] [Google Scholar]
- 2. Morgenstern LB, Kissela BM. Stroke disparities: a large global problem that must be addressed. Stroke. 2015;46:3560–3563. doi: 10.1161/STROKEAHA.115.009533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chavez AA, Simmonds KP, Venkatachalam AM, Ifejika NL. Health care disparities in stroke rehabilitation. Phys Med Rehabil Clin. 2023. doi: 10.1016/j.pmr.2023.06.030 [DOI] [PubMed] [Google Scholar]
- 4. Skolarus LE, Burke JF. Towards an understanding of racial differences in post‐stroke disability. Curr Epidemiol Rep. 2015;2:191–196. doi: 10.1007/s40471-015-0047-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Skolarus LE, Feng C, Burke JF. No racial difference in rehabilitation therapy across all post‐acute care settings in the year following a stroke. Stroke. 2017;48:3329–3335. doi: 10.1161/STROKEAHA.117.017290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ross JS, Halm EA, Bravata DM. Use of stroke secondary prevention services: are there disparities in care? Stroke. 2009;40:1811–1819. doi: 10.1161/STROKEAHA.108.539619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, Deruyter F, Eng JJ, Fisher B, Harvey RL, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47:e98–e169. doi: 10.1161/STR.0000000000000098 [DOI] [PubMed] [Google Scholar]
- 8. McDougall J, Wright V, Rosenbaum P. The ICF model of functioning and disability: incorporating quality of life and human development. Dev Neurorehabil. 2010;13:204–211. doi: 10.3109/17518421003620525 [DOI] [PubMed] [Google Scholar]
- 9. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. PLoS Med. 2007;4:1623–1627. doi: 10.1371/journal.pmed.0040296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Topaloglu U, Palchuk MB. Using a federated network of real‐world data to optimize clinical trials operations. JCO Clin Cancer Inform. 2018;2:1–10. doi: 10.1200/CCI.17.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malin B. Summary of the assessment of triNetX data privacy principles through an empirical analysis. TriNetX. 2020. Accessed on August 17, 2023. https://trinetx.com/wp‐content/uploads/2021/12/TriNetX‐Empirical‐Summary‐by‐Brad‐Malin‐2020.pdf
- 12. Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005;36:1776–1781. doi: 10.1161/01.STR.0000174293.17959.a1 [DOI] [PubMed] [Google Scholar]
- 13. Simmonds KP, Luo Z, Reeves M. Race/ethnic and stroke subtype differences in poststroke functional recovery after acute rehabilitation. Arch Phys Med Rehabil. 2021;102:1473–1481. doi: 10.1016/j.apmr.2021.01.090 [DOI] [PubMed] [Google Scholar]
- 14. Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183:758–764. doi: 10.1093/aje/kwv254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33:1057–1069. doi: 10.1002/sim.6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Little R, Rubin DB. Statisical Analysis with Missing Data. Vol 798. John Wiley & Sons; 2019. doi: 10.1002/9781119482260 [DOI] [Google Scholar]
- 18. Simmonds KP, Burke J, Kozlowski AJ, Andary M, Luo Z, Reeves MJ. Emulating 3 clinical trials that compare stroke rehabilitation at inpatient rehabilitation facilities with skilled nursing facilities. Arch Phys Med Rehabil. 2022;103:1311–1319. doi: 10.1016/j.apmr.2021.12.029 [DOI] [PubMed] [Google Scholar]
- 19. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wong TC, Ng KK, Fung JY, Chan AA, Cheung TT, Chok KS, Dai JW, Lo CM. Long‐term survival outcome between living donor and deceased donor liver transplant for hepatocellular carcinoma: intention‐to‐treat and propensity score matching analyses. Ann Surg Oncol. 2019;26:1454–1462. doi: 10.1245/s10434-019-07206-0 [DOI] [PubMed] [Google Scholar]
- 21. Cruz‐Flores S, Rabinstein A, Biller J, Elkind MS, Griffith P, Gorelick PB, Howard G, Leira EC, Morgenstern LB, Ovbiagele B, et al. Racial‐ethnic disparities in stroke care: the American experience a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2091–2116. doi: 10.1161/STR.0b013e3182213e24 [DOI] [PubMed] [Google Scholar]
- 22. Hong I, Karmarkar A, Chan W, Kuo YF, Mallinson T, Ottenbacher KJ, Goodwin JS, Andersen CR, Reistetter TA. Discharge patterns for ischemic and hemorrhagic stroke patients going from acute care hospitals to inpatient and skilled nursing rehabilitation. Am J Phys Med Rehabil. 2018;97:636–645. doi: 10.1097/PHM.0000000000000932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoh BL, Ko NU, Amin‐Hanjani S, Chou SH, Cruz‐Flores S, Dangayach NS, Derdeyn CP, Du R, Hänggi D, Hetts SW, et al. 2023 Guideline for the management of patients with aneurysmal subarachnoid hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2023;54:e314–e370. doi: 10.1161/STR.0000000000000436 [DOI] [PubMed] [Google Scholar]
- 24. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 25. Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377:1693–1702. doi: 10.1016/S0140-6736(11)60325-5 [DOI] [PubMed] [Google Scholar]
- 26. Ottenbacher KJ, Hsu Y, Granger CV, Fiedler RC. The reliability of the functional independence measure: a quantitative review. Arch Phys Med Rehabil. 1996;77:1226–1232. doi: 10.1016/S0003-9993(96)90184-7 [DOI] [PubMed] [Google Scholar]
- 27. Zahnd WE, Murphy C, Knoll M, Benavidez GA, Day KR, Ranganathan R, Luke P, Zgodic A, Shi K, Merrell MA, et al. The intersection of rural residence and minority race/ethnicity in cancer disparities in the United States. Int J Environ Res Public Health. 2021;18:1384. doi: 10.3390/ijerph18041384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. MacDorman MF, Thoma M, Declcerq E, Howell EA. Racial and ethnic disparities in maternal mortality in the United States using enhanced vital records, 2016–2017. Am J Public Health. 2021;111:1673–1681. doi: 10.2105/AJPH.2021.306375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Magesh S, John D, Li WT, Li Y, Mattingly‐App A, Jain S, Chang EY, Ongkeko WM. Disparities in COVID‐19 outcomes by race, ethnicity, and socioeconomic status: a systematic review and meta‐analysis. JAMA Netw Open. 2021;4:e2134147. doi: 10.1001/jamanetworkopen.2021.34147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trivedi AN, Zaslavsky AM, Schneider EC, Ayanian JZ. Relationship between quality of care and racial disparities in Medicare health plans. JAMA. 2006;296:1998–2004. doi: 10.1001/jama.296.16.1998 [DOI] [PubMed] [Google Scholar]
- 31. Towfighi A, Boden‐Albala B, Cruz‐Flores S, El Husseini N, Odonkor CA, Ovbiagele B, Sacco RL, Skolarus LE, Thrift AG. Strategies to reduce racial and ethnic inequities in stroke preparedness, care, recovery, and risk factor control: a scientific statement from the American Heart Association. Stroke. 2023;54:e371–e388. doi: 10.1161/STR.0000000000000437 [DOI] [PubMed] [Google Scholar]
- 32. Gutierrez J, Williams OA. A decade of racial and ethnic stroke disparities in the United States. Neurology. 2014;82:1080–1082. doi: 10.1212/WNL.0000000000000237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saluja B, Bryant Z. How implicit bias contributes to racial disparities in maternal morbidity and mortality in the United States. J Womens Health. 2021;30:270–273. doi: 10.1089/jwh.2020.8874 [DOI] [PubMed] [Google Scholar]
- 34. Levine DA, Duncan PW, Nguyen‐Huynh MN, Ogedegbe OG. Interventions targeting racial/ethnic disparities in stroke prevention and treatment. Stroke. 2020;51:3425–3432. doi: 10.1161/STROKEAHA.120.030427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bhattacharya P, Mada F, Salowich‐Palm L, Hinton S, Millis S, Watson SR, Chaturvedi S, Rajamani K. Are racial disparities in stroke care still prevalent in certified stroke centers? J Stroke Cerebrovasc Dis. 2013;22:383–388. doi: 10.1016/j.jstrokecerebrovasdis.2011.09.018 [DOI] [PubMed] [Google Scholar]
- 36. Howard G, Howard VJ. Twenty years of progress toward understanding the stroke belt. Stroke. 2020;51:742–750. doi: 10.1161/STROKEAHA.119.024155 [DOI] [PubMed] [Google Scholar]
- 37. Towfighi A, Ovbiagele B. Health equity and actionable disparities in stroke: 2021 update. Stroke. 2022;29:636–642. doi: 10.1161/STROKEAHA.122.035816 [DOI] [PubMed] [Google Scholar]
- 38. Ntani G, Inskip H, Osmond C, Coggon D. Consequences of ignoring clustering in linear regression. BMC Med Res Methodol. 2021;21:139. doi: 10.1186/s12874-021-01333-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S9


