Abstract
Background
Hypertension is the leading modifiable cardiovascular risk factor with recognized sex‐ and gender‐based differences. We assessed the incorporation of sex and gender reporting in the antihypertensive medication literature informing hypertension guidelines.
Methods and Results
Literature cited in the International Society of Hypertension (2020), European Society of Cardiology/European Society of Hypertension (2018), American College of Cardiology/American Heart Association (2017), Latin American Society of Hypertension (2017), Pan‐African Society of Cardiology (2020), and Hypertension Canada (2020) guidelines was systematically reviewed. Observational studies, randomized controlled trials, and systematic reviews involving antihypertensive medications were included. Studies with participants of a single sex, guidelines, and commentaries were excluded. Data on study participation‐to‐prevalence ratio by sex, analysis of baseline demographics and study outcomes by sex, and stratification of adverse events by sex were extracted. Of 1659 unique citations, 331 studies met inclusion criteria. Of those, 81% reported the sex of participants, and 22% reported a male‐to‐female participation‐to‐prevalence ratio of 0.8 to 1.2. Three percent of studies stratified baseline characteristics by sex, and 20% considered sex during analysis through statistical adjustment or stratification. Although 32% of studies reported adverse events, only 0.6% stratified adverse events by sex. Most (58%) studies reporting sex/gender used sex and gender terms interchangeably.
Conclusions
Incorporation of sex‐ and gender‐based considerations in study population, analysis, or reporting of results and adverse events is not common in the antihypertensive medication literature informing international hypertension guidelines. Greater attention to sex‐ and gender‐based factors in research is required to optimally inform management of hypertension.
Keywords: adverse events, gender, guidelines, hypertension, sex
Subject Categories: Hypertension
Nonstandard Abbreviations and Acronyms
- AE
adverse event
- PPR
participation‐to‐prevalence ratio
Clinical Perspective.
What Is New?
Approximately 1 in 5 antihypertensive medication studies informing hypertension guidelines do not incorporate any sex‐ and gender‐based reporting or analysis.
Fewer than 1 in 4 antihypertensive medication studies have appropriate sex‐based representation in study participants, and sex‐stratified analysis of results is not common.
Sex‐stratified adverse events are rarely reported.
What Are the Clinical Implications?
Despite an emphasis on precision medicine and mandates from journals and funding agencies, antihypertensive medication studies informing hypertension guidelines rarely incorporate sex‐ and gender‐based reporting and analysis.
Greater attention to sex‐ and gender‐based factors in research is required to optimally inform clinical practice and improve management of hypertension in all individuals.
Globally, hypertension is the leading modifiable risk factor for cardiovascular mortality in men and women. 1 , 2 , 3 , 4 Although the number of adults living with hypertension doubled between 1990 and 2019 to 1.3 billion, 4 of 5 individuals are not adequately treated. 5 Underscoring blood pressure control as 1 of the most important determinants of cardiovascular and kidney health, the World Health Organization has recently set a global target of a 25% relative reduction in the prevalence of increased blood pressure by 2025. 5
However, despite increasingly recognized sex (biological) and gender (sociocultural) differences in the prevalence, cause, and control of hypertension as well as antihypertensive effects of interventions, 6 , 7 clinical recommendations for hypertension management endorse a 1‐size‐fits‐all approach. We assessed the incorporation of sex and gender reporting in the antihypertensive medication literature informing hypertension guidelines.
METHODS
The authors declare that all supporting data are available within the article and its online supplementary files. We systematically reviewed all literature cited in the International Society of Hypertension (2020), 8 Latin American Society of Hypertension (2017), 9 European Society of Cardiology/European Society of Hypertension (2018), 10 Pan‐African Society of Cardiology (2020), 11 American College of Cardiology/American Heart Association (2017),12 and Hypertension Canada (2020) 13 guidelines (Table S1). The terms sex and gender are not synonymous. However, recognizing these terms are often used interchangeably in studies, we assumed women to mean female sex and men to mean male sex. The inclusion criteria were observational studies, randomized controlled trials, and systematic reviews involving antihypertensive medications. The exclusion criteria were single‐sex studies, guidelines, and commentaries (Table S2). Two reviewers (N.G. and K.T.M.) independently extracted data using a standardized data abstraction form, with data items including ratio of male‐to‐female participants, analysis of baseline demographics and study outcomes by sex, reporting of adverse events (AEs), and stratification of AEs by sex. Any event reported using terminology such as “adverse effects, side effects, adverse outcomes, or safety outcomes” was defined an “AE." The participation‐to‐prevalence ratio (PPR) is a measure of the representation of a specific group in the study population relative to the prevalence of the condition of interest in the same group. In the context of sex and hypertension, this metric can be calculated by dividing the proportion of men or women in the study population by the proportion of men or women, respectively, with hypertension in the general population, 14 which, given that the global prevalence of hypertension is roughly equal in men and women, 3 , 6 , 15 is 0.5. As a PPR approximating 1 suggests a representative study population composition, a PPR <0.8 and >1.2 indicates the underrepresentation and overrepresentation of male or female participants. Calculation of the PPR is an important metric in the development of sex‐specific guidelines. 14 , 16 Institutional review board approval and informed consent were not required for this study as all data were publicly available.
RESULTS
Of 1659 unique articles cited in the 6 guidelines, 331 studies met inclusion criteria (Figure 1). Of the 331 studies that met the inclusion criteria, 267 (81%) reported the sex of participants (Table S3), with only 73 (22%) reporting a male‐to‐female PPR of 0.8 to 1.2, 140 (42%) with a PPR of >1.2 (overrepresentation of men), and 40 (12%) with a PPR of <0.8 (overrepresentation of women), whereas the PPR could not be determined for 14 studies (4%) (Figures 2 and 3). Baseline characteristics were stratified by sex in 11 studies (3%), and 67 (20%) considered sex in analysis through statistical adjustment (n=18 [5%]) or stratification (n=49 [15%]). Although 105 studies (32%) reported AEs, only 2 (0.6%) stratified AEs by sex. Of the 267 studies that reported the sex or gender of participants, 87 (33%) used sex‐based terms (eg, male or female) to describe their participants, 24 (9%) studies used gender‐based terms (eg, men or women), and 156 (58%) used sex‐ and gender‐based terms interchangeably. No study reported how the sex or gender of participants was determined. 17
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flow diagram.
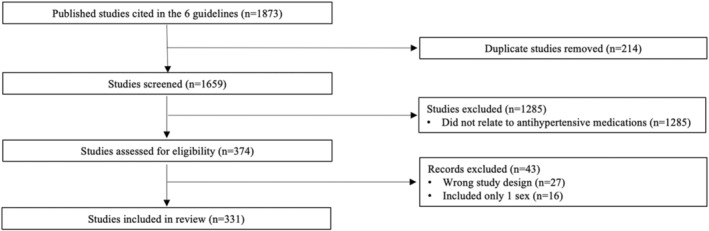
Figure 2. Flowchart of sex‐based analysis and reporting in antihypertensive medication studies informing clinical hypertension guidelines.
*Studies that were systematic reviews. PPR indicates population‐to‐prevalence ratio.
Figure 3. Bar chart displaying the number of studies looking at sex‐based analysis and reporting in antihypertensive literature informing clinical hypertension guidelines.
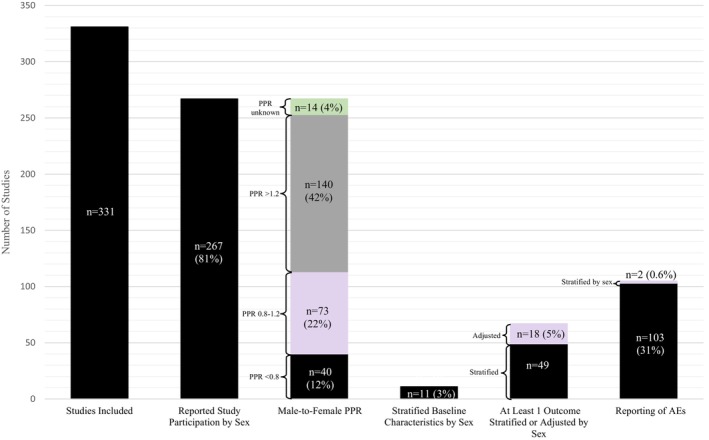
AE indicates adverse event; and PPR, population‐to‐prevalence ratio.
DISCUSSION
Our key findings were as follows: (1) approximately 1 in 5 antihypertensive medication studies informing hypertension guidelines did not incorporate any sex‐ and gender‐based reporting or analysis; (2) <1 in 4 studies had appropriate sex‐based representation in study participants; (3) approximately15% of studies reported sex‐stratified outcomes; (4) sex‐stratified AEs were rarely reported; and (5) sex‐ and gender‐based terminology was commonly used interchangeably. The results highlight that despite the increasing emphasis on precision health and personalized cardiovascular care, 18 , 19 , 20 , 21 , 22 few antihypertensive medication studies informing commonly used guidelines for hypertension management incorporated principles of sex‐ and gender‐based analysis, including targeting a study PPR of 0.8 to 1.2, reporting baseline participant demographics by sex and gender, or analyzing or reporting study outcomes and AEs stratified by sex and gender. 23 These findings are concerning given the recognized sex and gender differences in the pathophysiology and cardiovascular and kidney risks of hypertension, 3 , 6 , 24 , 25 , 26 , 27 , 28 as well as access, 29 , 30 adherence, 31 and AEs 32 , 33 , 34 , 35 related to antihypertensive agents. 6
The National Institutes of Health launched the Precision Medicine Initiative and instituted the Sex as a Biological Variable Policy in 2015 36 , 37 ; and although the guidelines included in this review were published between 2017 and 2020, the design of this study does not capture more recently published research. However, a subanalysis of literature informing the guidelines published during or after 2015 demonstrated a similar pattern to our overall results (Figure S1). Our findings are also consistent with a recent scoping review of antihypertensive medication studies published between 1964 and 2020 that showed substantial underrepresentation of female participants in clinical trials, with only 3.7% of studies stratifying results by sex. 38 Our results are also in keeping with previous work highlighting the underrepresentation of women in cardiovascular and kidney trials. 14 , 38 , 39 , 40 , 41 Pharmacokinetics and pharmacodynamics of drugs differ by sex, 42 which may account for greater AEs and lower adherence in women compared with men using antihypertensive medications, 32 , 33 , 34 , 35 underscoring the importance of reporting sex‐stratified AEs.
The incomplete reporting of sex and gender and an emphasis on sex rather than gender noted in this study have also been observed in other research settings. 43 , 44 , 45 Similar to our findings showing most studies used the terms sex and gender interchangeably, only 35% of Canadian clinical practice guidelines published between 2013 and 2015 for noncommunicable health conditions that included “sex” and/or “gender” used the terms correctly 46 according to the Sex and Gender Equity in Research guidelines. 47 This may partially reflect a lack of integration of sex, as a biological attribute, and gender, as a socially constructed identity, in health research reporting guidelines. In a systematic review of 407 reporting guidelines listed on the Equator Network registry and published between 1995 and 2018, only 1 reporting guideline met the criteria of the correct use of sex and gender concepts. 48
The fact that no study reported both the sex and the gender of participants deserves mention. The assumption that sex assigned at birth always aligns with gender identity does not take into account the growing global transgender, gender‐diverse, and nonbinary populations; moreover, these populations are impacted by disparities across a variety of cardiovascular risk factors compared with their cisgender peers. 49 Most research on hypertension in transgender or nonbinary adults has focused on the impact of gender‐affirming hormone therapy on blood pressure, which to date has been overall inconclusive. 50 , 51 Application of frameworks 52 , 53 to improve incorporation of sex and gender considerations in blood pressure research has the potential to create new knowledge in the management of hypertension.
Our study provides evidence that literature informing guidelines for management of hypertension poorly incorporates sex and gender considerations in study design, analysis, and reporting despite mandates from funders, 36 , 54 , 55 journals, 47 and governments. 56 , 57 , 58 Structured frameworks exist to determine whether sex‐specific recommendations should be made in clinical guidelines. 23 However, research informing guidelines first needs to systematically incorporate sex‐ and gender‐related considerations to achieve the goal of optimizing health outcomes for all.
Sources of Funding
N. Gulamhusein was supported by a Canada Graduate Scholarship–Master's through the Canadian Institutes of Health Research.
Disclosures
None.
Supporting information
Tables S1–S3
Figure S1
This article was sent to Tochukwu M. Okwuosa, DO, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.030613
For Sources of Funding and Disclosures, see page 4.
References
- 1. Collaborators GBDRF . Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285–292. doi: 10.1161/HYPERTENSIONAHA.119.14240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colafella KMM, Denton KM. Sex‐specific differences in hypertension and associated cardiovascular disease. Nat Rev Nephrol. 2018;14:185–201. doi: 10.1038/nrneph.2017.189 [DOI] [PubMed] [Google Scholar]
- 4. Global report on hypertension: the race against a silent killer. Accessed September 23, 2023. https://www.who.int/teams/noncommunicable‐diseases/hypertension‐report.
- 5. World health organization: hypertension. 2023. Accessed September 23, 2023. https://www.who.int/news‐room/fact‐sheets/detail/hypertension.
- 6. Gerdts E, Sudano I, Brouwers S, Borghi C, Bruno RM, Ceconi C, Cornelissen V, Dievart F, Ferrini M, Kahan T, et al. Sex differences in arterial hypertension. Eur Heart J. 2022;43:4777–4788. doi: 10.1093/eurheartj/ehac470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meinert F, Thomopoulos C, Kreutz R. Sex and gender in hypertension guidelines. J Hum Hypertens. 2023;37:654–661. doi: 10.1038/s41371-022-00793-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026 [DOI] [PubMed] [Google Scholar]
- 9. Task Force of the Latin American Society of H . Guidelines on the management of arterial hypertension and related comorbidities in Latin America. J Hypertens. 2017;35:1529–1545. doi: 10.1097/HJH.0000000000001418 [DOI] [PubMed] [Google Scholar]
- 10. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 11. Jones ES, Damasceno A, Ogola EN, Ojji DB, Dzudie A, Rayner BL. PASCAR commentary on the International Society of Hypertension global guidelines 2020: relevance to sub‐Saharan Africa. Cardiovasc J Afr. 2020;31:325–329. doi: 10.5830/CVJA-2020-055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and Management of High Blood Pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 13. Rabi DM, McBrien KA, Sapir‐Pichhadze R, Nakhla M, Ahmed SB, Dumanski SM, Butalia S, Leung AA, Harris KC, Cloutier L, et al. Hypertension Canada's 2020 comprehensive guidelines for the prevention, diagnosis, risk assessment, and treatment of hypertension in adults and children. Can J Cardiol. 2020;36:596–624. doi: 10.1016/j.cjca.2020.02.086 [DOI] [PubMed] [Google Scholar]
- 14. Norris CM, Tannenbaum C, Pilote L, Wong G, Cantor WJ, McMurtry MS. Systematic incorporation of sex‐specific information into clinical practice guidelines for the management of ST‐segment‐elevation myocardial infarction: feasibility and outcomes. J Am Heart Assoc. 2019;8:e011597. doi: 10.1161/JAHA.118.011597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, Chen J, He J. Global disparities of hypertension prevalence and control: a systematic analysis of population‐based studies from 90 countries. Circulation. 2016;134:441–450. doi: 10.1161/CIRCULATIONAHA.115.018912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keuken DG, Haafkens JA, Hellema MJ, Burgers JS, Moerman CJ. Incorporating a gender perspective into the development of clinical guidelines: a training course for guideline developers. Implement Sci. 2007;2:35. doi: 10.1186/1748-5908-2-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clayton JA, Tannenbaum C. Reporting sex, gender, or both in clinical research? JAMA. 2016;316:1863–1864. doi: 10.1001/jama.2016.16405 [DOI] [PubMed] [Google Scholar]
- 18. Precision health: Improving health for each of us and all of us. Accessed September 23, 2023. https://www.cdc.gov/genomics/about/precision_med.htm.
- 19. Leopold JA, Loscalzo J. Emerging role of precision medicine in cardiovascular disease. Circ Res. 2018;122:1302–1315. doi: 10.1161/CIRCRESAHA.117.310782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Currie G, Delles C. Precision medicine and personalized medicine in cardiovascular disease. Adv Exp Med Biol. 2018;1065:589–605. doi: 10.1007/978-3-319-77932-4_36 [DOI] [PubMed] [Google Scholar]
- 21. Antman EM, Loscalzo J. Precision medicine in cardiology. Nat Rev Cardiol. 2016;13:591–602. doi: 10.1038/nrcardio.2016.101 [DOI] [PubMed] [Google Scholar]
- 22. Califf RM. Future of personalized cardiovascular medicine: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2018;72:3301–3309. doi: 10.1016/j.jacc.2018.09.079 [DOI] [PubMed] [Google Scholar]
- 23. Tannenbaum C, Norris CM, McMurtry MS. Sex‐specific considerations in guidelines generation and application. Can J Cardiol. 2019;35:598–605. doi: 10.1016/j.cjca.2018.11.011 [DOI] [PubMed] [Google Scholar]
- 24. Azizi Z, Alipour P, Raparelli V, Norris CM, Pilote L. The role of sex and gender in hypertension. J Hum Hypertens. 2022;37:589–595. doi: 10.1038/s41371-022-00789-4 [DOI] [PubMed] [Google Scholar]
- 25. Satoh M, Hirose T, Nakayama S, Murakami T, Takabatake K, Asayama K, Imai Y, Ohkubo T, Mori T, Metoki H. Blood pressure and chronic kidney disease stratified by Gender and the use of antihypertensive drugs. J Am Heart Assoc. 2020;9:e015592. doi: 10.1161/JAHA.119.015592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ji H, Niiranen TJ, Rader F, Henglin M, Kim A, Ebinger JE, Claggett B, Merz CNB, Cheng S. Sex differences in blood pressure associations with cardiovascular outcomes. Circulation. 2021;143:761–763. doi: 10.1161/CIRCULATIONAHA.120.049360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maranon R, Reckelhoff JF. Sex and gender differences in control of blood pressure. Clin Sci (Lond). 2013;125:311–318. doi: 10.1042/CS20130140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ji H, Kim A, Ebinger JE, Niiranen TJ, Claggett BL, Bairey Merz CN, Cheng S. Sex differences in blood pressure trajectories over the life course. JAMA Cardiol. 2020;5:19–26. doi: 10.1001/jamacardio.2019.5306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heise L, Greene ME, Opper N, Stavropoulou M, Harper C, Nascimento M, Zewdie D, Gender Equality N, Health SC. Gender inequality and restrictive gender norms: framing the challenges to health. Lancet. 2019;393:2440–2454. doi: 10.1016/S0140-6736(19)30652-X [DOI] [PubMed] [Google Scholar]
- 30. Mosca L, Linfante AH, Benjamin EJ, Berra K, Hayes SN, Walsh BW, Fabunmi RP, Kwan J, Mills T, Simpson SL. National study of physician awareness and adherence to cardiovascular disease prevention guidelines. Circulation. 2005;111:499–510. doi: 10.1161/01.CIR.0000154568.43333.82 [DOI] [PubMed] [Google Scholar]
- 31. Hamrahian SM, Maarouf OH, Fulop T. A critical review of medication adherence in hypertension: barriers and facilitators clinicians should consider. Patient Prefer Adherence. 2022;16:2749–2757. doi: 10.2147/PPA.S368784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bots SH, den Ruijter HM. Recommended heart failure medications and adverse drug reactions in women. Circulation. 2019;139:1469–1471. doi: 10.1161/CIRCULATIONAHA.118.037585 [DOI] [PubMed] [Google Scholar]
- 33. Os I, Franco V, Kjeldsen SE, Manhem K, Devereux RB, Gerdts E, Hille DA, Lyle PA, Okin PM, Dahlof B, et al. Effects of losartan in women with hypertension and left ventricular hypertrophy: results from the losartan intervention for endpoint reduction in hypertension study. Hypertension. 2008;51:1103–1108. doi: 10.1161/HYPERTENSIONAHA.107.105296 [DOI] [PubMed] [Google Scholar]
- 34. Turnbull F, Woodward M, Neal B, Barzi F, Ninomiya T, Chalmers J, Perkovic V, Li N, MacMahon S; Blood pressure lowering treatment trialists C . Do men and women respond differently to blood pressure‐lowering treatment? Results of prospectively designed overviews of randomized trials. Eur Heart J. 2008;29:2669–2680. doi: 10.1093/eurheartj/ehn427 [DOI] [PubMed] [Google Scholar]
- 35. Bots SH, Groepenhoff F, Eikendal ALM, Tannenbaum C, Rochon PA, Regitz‐Zagrosek V, Miller VM, Day D, Asselbergs FW, den Ruijter HM. Adverse drug reactions to guideline‐recommended heart failure drugs in women: a systematic review of the literature. JACC Heart Fail. 2019;7:258–266. doi: 10.1016/j.jchf.2019.01.009 [DOI] [PubMed] [Google Scholar]
- 36. Consideration of sex as a biological variable in NIH‐funded research. 2015. Accessed September 23, 2023. https://grants.nih.gov/grants/guide/notice‐files/not‐od‐15‐102.html.
- 37. Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mohseni‐Alsalhi Z, Vesseur MAM, Wilmes N, Laven S, Meijs DAM, van Luik EM, Vaes EWP, Dikovec CJR, Wiesenberg J, Almutairi MF, et al. The representation of females in studies on antihypertensive medication over the years: a scoping review. Biomedicines. 2023;11:11. doi: 10.3390/biomedicines11051435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vinson AJ, Collister D, Ahmed S, Tennankore K. Underrepresentation of women in recent landmark kidney trials: the gender gap prevails. Kidney Int Rep. 2022;7:2526–2529. doi: 10.1016/j.ekir.2022.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vinson AJ, Ahmed SB. Representation of women in contemporary kidney transplant trials. Transpl Int. 2023;36:11206. doi: 10.3389/ti.2023.11206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jin X, Chandramouli C, Allocco B, Gong E, Lam CSP, Yan LL. Women's participation in cardiovascular clinical trials from 2010 to 2017. Circulation. 2020;141:540–548. doi: 10.1161/CIRCULATIONAHA.119.043594 [DOI] [PubMed] [Google Scholar]
- 42. Rosano GM, Lewis B, Agewall S, Wassmann S, Vitale C, Schmidt H, Drexel H, Patak A, Torp‐Pedersen C, Kjeldsen KP, et al. Gender differences in the effect of cardiovascular drugs: a position document of the working group on pharmacology and drug therapy of the ESC. Eur Heart J. 2015;36:2677–2680. doi: 10.1093/eurheartj/ehv161 [DOI] [PubMed] [Google Scholar]
- 43. Collister D, Pyne L, Bhasin AA, Ahmed SB, Smyth B, Herrington W, Jardine M, Walsh M. Sex and gender in randomized controlled trials of adults receiving maintenance dialysis: a meta‐epidemiologic study. Am J Kidney Dis. 2022;81:575–582.e1. doi: 10.1053/j.ajkd.2022.10.015 [DOI] [PubMed] [Google Scholar]
- 44. Laprise C, Cole K, Sridhar VS, Marenah T, Crimi C, West L, Foster BJ, Pilote L, Sapir‐Pichhadze R. Sex and Gender considerations in transplant research: a scoping review. Transplantation. 2019;103:e239–e247. doi: 10.1097/TP.0000000000002828 [DOI] [PubMed] [Google Scholar]
- 45. Carcel C, Woodward M, Balicki G, Koroneos GL, Sousa DA, Cordonnier C, Lukaszyk C, Thompson K, Wang X, Davies L, et al. Trends in recruitment of women and reporting of sex differences in large‐scale published randomized controlled trials in stroke. Int J Stroke. 2019;14:931–938. doi: 10.1177/1747493019851292 [DOI] [PubMed] [Google Scholar]
- 46. Tannenbaum C, Clow B, Haworth‐Brockman M, Voss P. Sex and gender considerations in Canadian clinical practice guidelines: a systematic review. CMAJ Open. 2017;5:E66–E73. doi: 10.9778/cmajo.20160051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Heidari S, Babor TF, De Castro P, Tort S, Curno M. Sex and Gender Equity in research: rationale for the SAGER guidelines and recommended use. Res Integr Peer Rev. 2016;1:2. doi: 10.1186/s41073-016-0007-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gogovor A, Zomahoun HTV, Ekanmian G, Adisso EL, Deom Tardif A, Khadhraoui L, Rheault N, Moher D, Legare F. Sex and gender considerations in reporting guidelines for health research: a systematic review. Biol Sex Differ. 2021;12:62. doi: 10.1186/s13293-021-00404-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Streed CG Jr, Beach LB, Caceres BA, Dowshen NL, Moreau KL, Mukherjee M, Poteat T, Radix A, Reisner SL, Singh V, et al. Assessing and addressing cardiovascular Health in people who are transgender and gender diverse: a scientific statement from the American Heart Association. Circulation. 2021;144:e136–e148. doi: 10.1161/CIR.0000000000001003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Connelly PJ, Clark A, Touyz RM, Delles C. Transgender adults, gender‐affirming hormone therapy and blood pressure: a systematic review. J Hypertens. 2021;39:223–230. doi: 10.1097/HJH.0000000000002632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Banks K, Kyinn M, Leemaqz SY, Sarkodie E, Goldstein D, Irwig MS. Blood pressure effects of gender‐affirming hormone therapy in transgender and gender‐diverse adults. Hypertension. 2021;77:2066–2074. doi: 10.1161/HYPERTENSIONAHA.120.16839 [DOI] [PubMed] [Google Scholar]
- 52. Rytz CL, Beach LB, Saad N, Dumanski SM, Collister D, Newbert AM, Peace L, Lett E, Greene D, Connelly P, et al. Improving the inclusion of transgender and nonbinary individuals in the planning, completion, and mobilization of cardiovascular research. Am J Physiol Heart Circ Physiol. 2023;324:H366–H372. doi: 10.1152/ajpheart.00494.2022 [DOI] [PubMed] [Google Scholar]
- 53. Chang DH, Dumanski SM, Ahmed SB. Female sex‐specific considerations to improve rigor and reproducibility in cardiovascular research. Am J Physiol Heart Circ Physiol. 2023;324:H279–H287. doi: 10.1152/ajpheart.00462.2022 [DOI] [PubMed] [Google Scholar]
- 54. Gender equality in research and innovation. Accessed September 23, 2023. https://research‐and‐innovation.ec.europa.eu/strategy/strategy‐2020‐2024/democracy‐and‐rights/gender‐equality‐research‐and‐innovation_en#gender‐mainstreaming‐through‐the‐integration‐of‐the‐gender‐dimension‐in‐research‐and‐innovation‐content.
- 55. How to integrate sex and gender into research. Accessed September 23, 2023. https://cihr‐irsc.gc.ca/e/50836.html.
- 56. Sex‐ and gender‐based analysis plus in action at health Canada. Accessed September 23, 2023. https://www.canada.ca/en/health‐canada/corporate/transparency/sex‐gender‐based‐analysis‐action.html.
- 57. Gender equality strategy. Accessed September 23, 2023. https://commission.europa.eu/strategy‐and‐policy/policies/justice‐and‐fundamental‐rights/gender‐equality/gender‐equality‐strategy_en#:~:text=The%20goal%20is%20a%20Union,and%20lead%20our%20European%20society.
- 58. National strategy on gender equity and equality. Accessed September 23, 2023. https://www.whitehouse.gov/wp‐content/uploads/2021/10/National‐Strategy‐on‐Gender‐Equity‐and‐Equality.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figure S1


