Abstract
Background
Neonates with congenital heart disease are at risk for impaired brain development in utero, predisposing children to postnatal brain injury and adverse long‐term neurodevelopmental outcomes. Given the vital role of the placenta in fetal growth, we assessed the incidence of placental pathology in fetal congenital heart disease and explored its association with total and regional brain volumes, gyrification, and brain injury after birth.
Methods and Results
Placentas from 96 term singleton pregnancies with severe fetal congenital heart disease were prospectively analyzed for macroscopic and microscopic pathology. We applied a placental pathology severity score to relate placental abnormalities to neurological outcome. Postnatal, presurgical magnetic resonance imaging was used to analyze brain volumes, gyrification, and brain injuries. Placental analyses revealed the following abnormalities: maternal vascular malperfusion lesions in 46%, nucleated red blood cells in 37%, chronic inflammatory lesions in 35%, delayed maturation in 30%, and placental weight below the 10th percentile in 28%. Severity of placental pathology was negatively correlated with cortical gray matter, deep gray matter, brainstem, cerebellar, and total brain volumes (r=−0.25 to −0.31, all P<0.05). When correcting for postmenstrual age at magnetic resonance imaging in linear regression, this association remained significant for cortical gray matter, cerebellar, and total brain volume (adjusted R 2=0.25–0.47, all P<0.05).
Conclusions
Placental pathology occurs frequently in neonates with severe congenital heart disease and may contribute to impaired brain development, indicated by the association between placental pathology severity and reductions in postnatal cortical, cerebellar, and total brain volumes.
Keywords: brain development, congenital heart disease, fetus, magnetic resonance imaging, neonate, neuroplacentology, placenta
Subject Categories: Magnetic Resonance Imaging (MRI), Congenital Heart Disease, Pregnancy, Pediatrics, Preeclampsia
Nonstandard Abbreviations and Acronyms
- SV‐AO
single ventricle defect with aortic obstruction
Clinical Perspective.
What Is New?
In pregnancies with a fetal diagnosis of congenital heart disease, placental pathology is caused primarily by maternal vascular malperfusion, chronic inflammation, delayed villous maturation, and low placental weight.
Higher placental pathology severity is associated with reduced postnatal volumes of the cortical gray matter, cerebellum, and total brain.
What Are the Clinical Implications?
Placental pathology may exacerbate impaired brain growth in neonates with congenital heart disease.
Monitoring placental health and standardized pathological review of the placenta after birth is important in pregnancies with fetal congenital heart disease, because placental pathology may be an early biomarker for adverse neurodevelopment.
Targeting placental pathology may hold promise as a prenatal intervention for improving neurodevelopmental outcomes in congenital heart disease.
In recent decades, significant progress has been made in the treatment of children with severe congenital heart disease (CHD), as surgical techniques and perioperative care continually improve. 1 Nevertheless, these children remain at risk for neurodevelopmental sequelae, such as cognitive, motor, and behavioral problems, with reported incidence rates ranging from 20% to 60%. 2 Although these neurologic abnormalities have predominantly been attributed to cerebral vulnerability in the critical neonatal period surrounding cardiac surgery, 3 , 4 there is growing evidence that brain aberrations already develop in utero. Multiple fetal imaging studies have shown disrupted brain development in fetuses with CHD. 5 , 6 , 7 , 8 Altered fetal brain volume is associated with poorer neurodevelopmental outcomes, explaining up to 10% to 21% in the variance. 9 Disturbances in fetal cerebral perfusion and oxygenation, resulting from cardiac pathology, are thought to be the underlying cause of impaired brain growth and maturation. 5 , 10
However, during pregnancy, the developing brain is affected not only by fetal cardiac physiology but also by genetic factors and the intrauterine environment, which is shaped by maternal risk factors and placental health. 7 , 11 The placenta plays a crucial role in fetal brain development by supplying oxygen, nutrients, and hormones to the fetus and removing metabolic waste products. 12 In early gestation, the fetal heart and the placenta develop via shared regulatory pathways. Consequently, disruptions affecting these shared pathways may alter the development of both organs. 13 Numerous studies have linked CHD and abnormal placentation. Fetal CHD is strongly correlated with preeclampsia, a condition caused by inadequate spiral artery remodeling. 14 , 15 In addition, fetal CHD was found to correlate with low placental weight and pathologies such as thrombosis, infarction, delayed villous maturation, and chorangiosis. 16 , 17 , 18 , 19 , 20 , 21 , 22 Such pathologies alter the structure and function of the placenta, which in turn, might additionally affect the developing brain, next to the detrimental effects caused by CHD itself. 19 , 21 , 23 Yet, the pathophysiologic mechanisms of altered placental development in CHD and its impact on other organs, such as the brain, remain poorly understood.
Considering the potential contributing role of placental pathology to altered brain development in fetal CHD, this study aimed to assess the incidence of placental pathology in a cohort of neonates with CHD and explore the effect on total and regional brain volumes, gyrification, and brain injury after birth. With this study, we aspired to provide more insights into the placenta‐heart‐brain axis and identify potential early biomarkers for adverse neurodevelopment in children with CHD.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Clinical Cohort
This single‐center, prospective, observational study was performed in the Wilhelmina Children's Hospital, Utrecht, the Netherlands, between January 2016 and June 2021. We included term neonates who were antenatally diagnosed with severe CHD (as defined by Hoffman and Kaplan 24 ), requiring cardiac surgery or cardiac catheterization in the first 6 months of life. For eligibility, the placenta had to be available for histopathological assessment. Exclusion criteria included confirmed genetic or chromosomal disorders, multiple gestation pregnancies, gestational age at birth <36 weeks, or major extracardiac anomalies. Maternal comorbidity was assessed and categorized as pregnancy‐related comorbidities or preexisting comorbidities. The study was approved by the institutional review board (Medical Research Ethics Committee number 16–093), and written informed consent to use clinical data for research purposes was obtained from all parents or legal guardians.
Histological Assessment of the Placenta
The collected placentas were reviewed by 2 perinatal pathologists (L.E.v.d.M., P.G.J.N.) who were blinded to neonatal outcome except for gestational age at birth. Placental weight was measured without the umbilical cord and membranes, and classified according to the gestational age percentile, as previously described by Pinar et al. 25 Insertion of the umbilical cord was categorized as central, paracentral, marginal (<1 cm from the placental disk border), or velamentous (insertion into the fetal membranes). The cord coiling index was measured before fixation as the number of complete coils (360°) of the arteries divided by the umbilical cord length (centimeters). We defined hypocoiling as an index of <0.1, and hypercoiling as an index of >0.3. 26
Placentas were scored for the presence of the following microscopic pathologies according to the Amsterdam criteria 26 : (1) placental maturation and maternal vascular malperfusion, which included ischemic changes, infarcts and distal villous hypoplasia; (2) fetal vascular malperfusion (ie, signs of fetal thrombosis); (3) fetal hypoxia, defined as the presence of nucleated red blood cells in >2 capillaries in any ×10 field (magnification ×400); (4) chorangiosis and chorangiomatosis (eg, signs of increased fetal capillary proliferation); (5) chronic inflammation in the placental parenchyma, including chronic villitis of unknown cause, sustained villitis, chronic (histiocytic) intervillositis, or (massive) perivillous fibrin; (6) chorioamnionitis, assessed as the presence of neutrophilic granulocytes in the chorionic plate or the extraplacental membranes; and (7) meconium pigment membrane.
To measure the extent and severity of the placental abnormalities in each placenta, we adapted the placenta severity score from Harteman et al. 27 All placental abnormalities were assigned points based on their expected impact on placental function or loss of functional placental parenchyma and, in turn, fetal condition (Table 1). The placental pathology severity score was generated per subject by adding up assigned points.
Table 1.
Overview of the Placental Pathology Severity Score
| Variables | |
| Macroscopic abnormalities | Score |
| Placental weight | |
| <10th Percentile | 7 |
| >90th Percentile | 1 |
| Umbilical cord insertion | |
| Paracentral | 0 |
| Central | 0 |
| Marginal | 1 |
| Velamentous | 3 |
| Single umbilical artery | 1 |
| Umbilical cord coiling index | |
| Hypocoiling (index <0.1) | 1 |
| Hypercoiling (index >0.3) | 7 |
| Microscopic abnormalities | |
| Placental maturation | |
| Delayed | 7 |
| Normal | 0 |
| Accelerated | 3 |
| Maternal vascular malperfusion | |
| Placental ischemia | 5 |
| Placental infarct | <5%: 2, 5%−10%: 3, >10%: 5 |
| Distal villous hypoplasia | 3 |
| Fetal vascular malperfusion | <10%: 3, 10%−25%: 4, >25%: 6 |
| Nucleated red blood cells | 2 |
| Chorangiosis | 3 |
| Inflammation or reactive changes | |
| Villitis of unknown cause | <50%: 3, >50%: 7 |
| Intervillositis | <50%: 3, >50%: 7 |
| Perivillous fibrin | <50%: 3, >50%: 7 |
| Sustained villitis | 2 |
| Chorioamnionitis | 1 |
| Meconium pigment membrane | 0 |
The placental pathology severity score was adapted from Harteman et al. 27
Cerebral Imaging Data
Neonates underwent cerebral magnetic resonance imaging (MRI) preoperatively per routine clinical protocol on a 3.0 T magnetic resonance (MR) system (Philips Medical Systems, Best, the Netherlands). Neonates in whom the MRI scan was performed <14 days after birth were included in the imaging analyses. For the brain injury analysis, we excluded neonates who participated in a currently ongoing, double‐blinded, postnatal neuroprotective trial (NCT04217421). 28 The imaging protocol included volumetric coronal 3‐dimensional T1‐weighted imaging, coronal T2‐weighted imaging, axial diffusion‐weighted imaging, axial susceptibility‐weighted imaging, axial diffusion tensor imaging, flow quantification imaging, and 3‐dimensional MR venography.
We reviewed the MR images for the presence of ischemic and hemorrhagic lesions. Hemorrhagic injuries included intraparenchymal, intraventricular, subdural, or cerebellar hemorrhages. Ischemic injuries were defined as white matter injury, stroke (involving cortical gray matter, basal ganglia, or thalamus), and hypoxic–ischemic watershed injury, as previously described. 29
We applied the structural processing pipeline of the developing Human Connectome Project to the coronal T2‐weighted images. 30 To summarize, T2‐weighted images were reconstructed and underwent bias correction and brain extraction. Following this, volumetric tissue segmentation was performed using an automated segmentation algorithm for the neonatal brain. 31 We visually checked all segmentations on quality and manually corrected minor errors with ITK‐SNAP 3.6.0 32 ; scans with significant segmentation errors or motion artifacts were excluded. The cortical gray matter, white matter, deep gray matter, cerebellum, brainstem, and hippocampus‐amygdala labels were included in the current study (Figure 1). After extracting the regional tissue volumes, total brain volume was calculated as the sum of these 6 tissue labels. To assess cortical folding, we included the gyrification index in our analysis, which is calculated by the developing Human Connectome Project pipeline as the ratio between the pial surface area and the simulated surface area of a smooth curved hull around the pial surface. 30 , 33
Figure 1. Volumetric segmentation of brain structures.
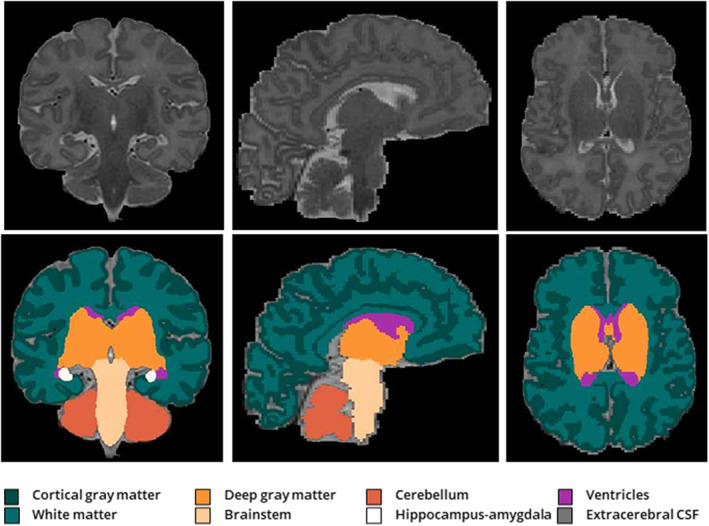
Volumetric tissue segmentation 30 , 31 of the cortical gray matter, white matter, deep gray matter, brainstem, hippocampus‐amygdala, cerebellum, ventricles (not included in analysis), and extracerebral CSF (not included in analysis) on T2‐weighted magnetic resonance imaging in coronal (left), sagittal (middle), and axial (right) planes. CSF indicates cerebrospinal fluid.
Statistical Analysis
The statistical analyses were conducted with SPSS version 27.0 (IBM). Normality was examined with histograms, Q‐Q plots, and Kolmogorov‐Smirnov tests. Descriptive statistics were used to summarize the data. Categorical data are presented as counts (percentage), whereas continuous data are presented as mean (SD) or median (interquartile range [IQR]), depending on their data distribution.
We applied Pearson χ2 or Fisher exact tests to identify associations between maternal comorbidities and placental pathologies, as well as connections between CHD subtype and placental pathologies. However, due to the limited sample size of the CHD groups with pulmonary obstruction, we only conducted these CHD subtype analyses for neonates with transposition of the great arteries, single ventricle defects with aortic obstruction (SV‐AO), and biventricular defects with aortic obstruction.
Mann‐Whitney U tests were used to test the relationship between hemorrhagic and ischemic brain injury and the placental pathology severity score. To explore relationships between the placental pathology severity score and brain volumes and gyrification index, we conducted correlational analyses using Pearson correlation or Spearman correlation tests, depending on the linearity between variables. Cerebral variables showing a significant correlation with the placental pathology severity score were entered as a dependent variable in linear regression models, with the placental pathology severity score as independent variable, while controlling for postmenstrual age at MRI. Considering the explorative nature of the study, a 2‐tailed significance level of P<0.05 was set.
Results
Study Population
The placentas of 123 neonates were collected for histological examination. Of those, 96 neonates were included in the study, because we excluded cases with confirmed chromosomal or genetic disorders (n=12), twin pregnancies (n=6), premature birth (n=5), major extracardiac anomalies (n=3), and without severe CHD (n=1). Table 2 summarizes the infant and maternal characteristics of the included study population. The means of gestational age at birth, birth weight, and birth weight Z score were 38.8 weeks (±1.4 weeks), 3205 g (±549 g), and −0.3 (±1.3), respectively. The majority of cases had a transposition of the great arteries (31%), SV‐AO (24%), or biventricular defect with aortic arch obstruction (23%).
Table 2.
Clinical Characteristics of the Included Study Population
| Characteristics | |
|---|---|
| Infant characteristics | Value |
| Male sex, n (%) | 65 (67.7) |
| Gestational age, wk, mean (SD) | 38.8 (1.4) |
| Birth weight, g, mean (SD) | 3205.2 (549.4) |
| Z score birth weight, mean (SD) | −0.3 (1.3) |
| Apgar score 1 min, median (IQR) | 8.0 (8.0−9.0) |
| Apgar score 5 min, median (IQR) | 9.0 (8.0−10.0) |
| Modality, n (%) | |
| Spontaneous vaginal delivery | 27 (28.1) |
| Induced vaginal delivery | 51 (53.1) |
| Elective caesarean section | 10 (10.4) |
| Secondary caesarean section | 8 (8.3) |
| Genetic analysis performed, n (%) | 68 (70.8) |
| Distribution of CHD subtypes | |
| Transposition of the great arteries, n (%) | 30 (31.3) |
| Transposition of the great arteries with an intact VSD | 16 (16.7) |
| Transposition of the great arteries with a VSD | 14 (14.6) |
| Single ventricle defects with aortic obstruction, n (%) | 23 (24.0) |
| Hypoplastic left heart syndrome | 19 (19.8) |
| Double inlet left ventricle with aortic coarctation or arch hypoplasia | 2 (2.1) |
| Unbalanced AVSD with aortic arch hypoplasia | 2 (2.1) |
| Biventricular defects with aortic obstruction, n (%) | 22 (22.9) |
| Aortic coarctation | 1 (1.0) |
| Aortic coarctation with arch hypoplasia | 7 (7.3) |
| Interrupted aortic arch | 3 (3.1) |
| Borderline left heart | 8 (8.3) |
| Aortic valve stenosis with arch hypoplasia | 1 (1.0) |
| AVSD with aortic arch hypoplasia | 2 (2.1) |
| Single ventricle defects with pulmonary obstruction, n (%) | 5 (5.2) |
| Pulmonary atresia | 2 (2.1) |
| Tricuspid atresia | 1 (1.0) |
| Complex single ventricle with pulmonary atresia | 1 (1.0) |
| Complex single ventricle with pulmonary stenosis | 1 (1.0) |
| Biventricular defects with pulmonary obstruction, n (%) | 10 (10.4) |
| Tetralogy of Fallot | 9 (9.4) |
| Pulmonary atresia without hypoplasia of right ventricle | 1 (1.0) |
| Other biventricular defects, n (%) | 6 (6.3) |
| Truncus arteriosus | 3 (3.1) |
| Total anomalous pulmonary venous connection | 1 (1.0) |
| Aortopulmonary window | 1 (1.0) |
| Large VSD | 1 (1.0) |
| Maternal characteristics | |
| Maternal age, y, mean (SD) | 31.1 (4.2) |
| Gravidity, median (IQR) | 3.0 (2.0−3.0) |
| Parity, median (IQR) | 1.0 (0.0−2.0) |
| Smoking, n (%) | 5 (5.2) |
| Pregnancy complications, n (%) | 14 (14.6) |
| Gestational diabetes | 8 (8.3) |
| Gestational hypertension | 3 (3.1) |
| Preeclampsia | 2 (2.1) |
| Intrahepatic cholestasis | 2 (2.1) |
| Preexisting comorbidities, n (%) | 13 (13.5) |
| Hypothyroidism | 4 (4.2) |
| Preexistent hypertensive disorder | 1 (1.0) |
| Preexistent diabetic disorder | 3 (3.1) |
| Autoimmune disorder | 4 (4.2) |
| Hepatitis B | 1 (1.0) |
| Essential thrombocytosis | 1 (1.0) |
AVSD indicates atrioventricular septal defect; CHD, congenital heart disease; IQR, interquartile range; and VSD, ventricular septal defect.
Histological Assessment of the Placenta
The placental characteristics of the study cohort are listed in Table 3. Gross analyses of the placenta revealed a placental weight below the 10th percentile in 28%, and abnormal umbilical cord insertion (ie, marginal or velamentous) in 15%. Microscopic placental pathology was observed in 90%. Maternal vascular malperfusion lesions were present in 46% and were the most prevalent type of pathology. The second most common abnormality was nucleated red blood cells, occurring in 37%. Other frequently observed pathologies were chronic parenchymal inflammation and chorangiosis, identified in 35% and 22%, respectively. Delayed maturation of the placenta was seen in 30%, and accelerated maturation in 4%. The median placental pathology severity score in the study cohort was 10 (IQR, 5−16), with scores ranging from 0 to 33.
Table 3.
Placenta Characteristics of the Included Subjects
| Placental characteristic | |
|---|---|
| Macroscopic evaluation | Value |
| Placental weight, g, median (IQR) | 452.0 (394.0−520.0) |
| <10th Percentile, n (%) | 27 (28.1) |
| >90th Percentile, n (%) | 10 (10.4) |
| Umbilical cord insertion, n (%) | |
| Paracentral | 71 (74.0) |
| Central | 11 (11.5) |
| Marginal | 11 (11.5) |
| Velamentous | 3 (3.1) |
| Single umbilical artery, n (%) | 5 (5.2) |
| Umbilical cord coiling index, mean (SD) | 0.13 (0.07) |
| Hypocoiling, n (%) | 39 (40.6) |
| Hypercoiling, n (%) | 2 (2.1) |
| Microscopic evaluation | |
| Presence of microscopic placental pathology, n (%) | 86 (89.6) |
| Placental maturation, n (%) | |
| Delayed | 29 (30.2) |
| Normal | 63 (65.6) |
| Accelerated | 4 (4.2) |
| Maternal vascular malperfusion, n (%) | 44 (45.8) |
| Placental ischemia | 39 (40.6) |
| Placental infarct | 12 (12.5) |
| Distal villous hypoplasia | 2 (2.1) |
| Fetal vascular malperfusion, n (%) | 10 (10.4) |
| Nucleated red blood cells, n (%) | 35 (36.5) |
| Chorangiosis, n (%) | 21 (21.9) |
| Inflammation or reactive changes, n (%) | 34 (35.4) |
| Villitis of unknown cause | 29 (30.2) |
| Intervillositis | 4 (4.2) |
| Perivillous fibrin | 16 (16.7) |
| Sustained villitis | 10 (10.4) |
| Chorioamnionitis, n (%) | 14 (14.6) |
| Meconium pigment membrane, n (%) | 3 (3.1) |
| Cumulative placental pathology severity score, median (IQR) | 10.0 (5.0−16.0) |
IQR indicates interquartile range.
Placental Pathology and Clinical Characteristics
Maternal vascular malperfusion in the placenta was associated with maternal pregnancy complications (χ2[1]=4.33, P=0.038) and chronic parenchymal inflammation with preexistent maternal comorbidities (χ2[1]=4.49, P=0.034). Other placental pathology rates were not related to preexistent maternal comorbidities or maternal pregnancy complications.
In regard to placental characteristics and CHD subtypes, no differences in the rates of placental lesions were identified, except for fetal vascular malperfusion. This pathology occurred in 10 neonates (10%), of whom 5 had an SV‐AO and 5 had another cardiac diagnosis. When comparing these 2 groups, we found that the prevalence of fetal thrombosis was significantly higher in neonates with SV‐AO (χ2[1]=4.16, P=0.042).
Placental Pathology and Cerebral Imaging
Within this cohort, 68 neonates (71%) underwent a postnatal, preoperative cerebral MRI at a median postnatal age of 4.0 days (IQR, 2.0−5.0) and postmenstrual age of 39.5 weeks (±1.2). Sixty‐five neonates had sufficient MRI quality for volumetric analysis and 54 neonates for quantification of the gyrification index.
The placental pathology severity score was negatively correlated with the volumes of the cortical gray matter (r[63]=−0.30, P=0.01), deep gray matter (r[63]=−0.28, P=0.02), cerebellum (r[63]=−0.31, P=0.01), brainstem (r[63]=− 0.25, P=0.04), and total brain (r[63]=−0.29, P=0.02). We did not discover any other associations between the remaining volumes and pathology severity score or between the gyrification index and pathology severity score.
Linear regression analyses indicated that the placental pathology severity score significantly accounted for a proportion of the variance in cerebellar volume (F[2,62]=29.3, β=−0.08, P=0.02), cortical gray matter volume (F[2,62]=25.8, β=−0.46, P=0.03), and total brain volume (F[2,62]=11.8, β=−0.99, P=0.04) when covarying for postmenstrual age at MRI (Table 4, Figure 2). This association was not seen between the placental pathology severity score and brainstem and deep gray matter volume.
Table 4.
Linear Regression Models to Study Associations Between the Placental Pathology Severity Score and Brain Volumes
| Tissue class | Mean (SD) | β Coefficient (95% CI) | P value | Adjusted R 2 |
|---|---|---|---|---|
| Total brain volume, mL | 323.52 (36.54) | 0.25 | ||
|
Placental pathology severity score Postmenstrual age at MRI, wk |
−0.99 (−1.96 to −0.03) 13.38 (6.74 to 20.02) |
0.044 <0.005 |
||
| Cortical gray matter volume, mL | 122.37 (18.22) | 0.44 | ||
|
Placental pathology severity score Postmenstrual age at MRI, wk |
−0.46 (−0.88 to −0.05) 9.22 (6.34 to 12.09) |
0.030 <0.005 |
||
| Cerebellar volume, mL | 21.94 (3.03) | 0.47 | ||
|
Placental pathology severity score Postmenstrual age at MRI, wk |
−0.08 (−0.15 to −0.01) 1.59 (1.13 to 2.06) |
0.023 <0.005 |
||
| Deep gray matter volume, mL | 23.81 (2.27) | 0.24 | ||
|
Placental pathology severity score Postmenstrual age at MRI, wk |
−0.06 (−0.12 to 0.00) 0.83 (0.41 to 1.24) |
0.058 <0.005 |
||
| Brainstem volume, mL | 5.97 (0.53) | 0.21 | ||
|
Placental pathology severity score Postmenstrual age at MRI, wk |
−0.01 (−0.03 to 0.00) 0.18 (0.08 to 0.28) |
0.102 <0.005 |
MRI indicates magnetic resonance imaging.
Figure 2. Linear regression analyses between the placental pathology severity score and postnatal brain volumes.
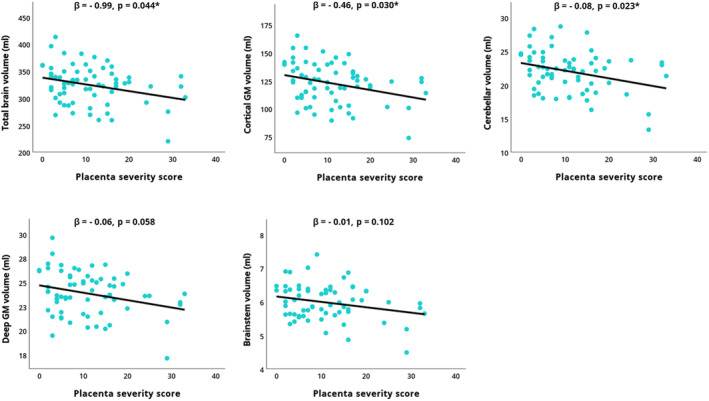
Cerebral variables showing a significant correlation with the placental pathology severity score were entered as a dependent variable in linear regression models, with the placental pathology severity score as independent variable, covarying for postmenstrual age at MRI. *P<0.05. GM indicates gray matter.
Among the 59 neonates included for brain injury analysis, 29% exhibited ischemic injury and 53% exhibited hemorrhagic injury on MRI. The predominant lesions comprised subdural hemorrhages (36%), intraventricular hemorrhages (24%), and white matter injury (20%). Both ischemic and hemorrhagic brain injury were not related to the placental pathology severity score.
Discussion
In CHD, there is an increased risk for impaired brain development in utero, predisposing children to brain injury and adverse neurodevelopmental outcomes. Given the vital role of the placenta in fetal growth, we aimed to assess the association between placental pathology and brain development in a prospective cohort of neonates with isolated CHD through standardized detailed review of the placenta and use of high‐quality postnatal MR imaging data. This prospective cohort study shows that placental lesions occur frequently in pregnancies with fetal CHD, with maternal vascular malperfusion lesions, increased fetal nucleated red blood cells, chronic parenchymal inflammation, and delayed villous maturation as the most commonly observed abnormalities. Higher placental pathology severity scores were associated with smaller postnatal volumes of the cortex, cerebellum, and total brain. These findings suggest that placental pathology may have a contributive effect on impaired brain growth in children with CHD.
The high rate of placental abnormalities in our cohort of neonates with CHD is in concordance with previous studies, which showed similar lesions, including maternal vascular malperfusion lesions, delayed villous maturation, chorangiosis, chronic inflammation, and reduced placental weight. 16 , 17 , 18 , 19 , 20 , 21 , 22 Comparable to these previous studies, maternal vascular malperfusion lesions were the most prevalent pathology in our cohort, occurring in 46% of cases. Maternal vascular malperfusion lesions are the result of inadequate spiral artery remodeling and affect the oxygen tension and perfusion of the intervillous space. 14 It can already develop early in gestation, supporting the hypothesis that placental abnormalities in CHD may arise from early disruptions in shared developmental pathways of the fetal heart and placenta. 13 Furthermore, we identified abnormalities that develop in response to a hypoxic intrauterine environment. Nucleated red blood cells were found in 37% of cases, indicating prolonged fetal hypoxia. 34 Similarly, chorangiosis, characterized by an increase of fetal capillaries within the placental villi in reaction to chronic hypoxia, occurred frequently. 35 These findings are important, because fetal hypoxia is a well‐documented phenomenon in fetuses with severe CHD. 5 , 7 Another prevalent finding was delayed villous maturation, which has been attributed in prior research to alterations in fetal hemodynamics, umbilical cord pathology, and maternal diabetes. 22 , 36 , 37 Lastly, we noted an increased incidence of fetal vascular malperfusion in SV‐AO. Fetal vascular malperfusion is a consequence of partial or complete obstructions in fetoplacental blood flow leading to impaired fetal perfusion downstream in the placenta. Fetal vascular thrombi form as a result of hemodynamic changes, hypercoagulability, or endothelial injury, collectively referred to as Virchow's triad. 38 Although several factors can trigger fetal vascular malperfusion, fetal cardiac dysfunction is recognized as a significant contributing factor. 38 Notably, SV‐AO is a condition characterized by ineffective functioning of the left ventricle, leading to diminished blood flow to the systemic vascular bed and placenta. 5 As a result, there is a reduction in perfusion through the umbilical arteries, which may be the underlying factor of the higher incidence of fetal vascular malperfusion in SV‐AO. However, this finding should be interpreted with caution, because fetal thrombosis occurred in a relatively small sample of only 10 neonates. To summarize, the placental abnormalities identified in this cohort suggest that the cause of placental pathology in CHD is likely multifactorial, including lesions that are developmental in origin as well as lesions that arise due to changes in maternal and fetal hemodynamics.
As the placenta plays an essential role in fetal organogenesis and overall fetal growth, placental pathology may have significant adverse effects on these processes. 12 This is the first study investigating the association between placental pathology and postpartum, preoperative brain volumes in neonates with isolated CHD. Higher placental pathology severity scores were associated with smaller postnatal volumes, particularly in the cerebellum and cortical gray matter. Fetal cerebral growth in the third trimester is most pronounced in the cerebellum and cortex, both contributing substantially to the overall increase in total brain volume. Although white matter volume also accounts for a significant proportion of total brain volume, its growth peaks early in the third trimester. 39 Our results, therefore, suggest that placental pathology predominantly is associated with brain regions undergoing the most rapid growth throughout the third trimester. This period is marked by increasing cerebral metabolic demand, and as a result, placental dysfunction may have a more pronounced effect in late gestation. 7 Smaller neonatal volumes of the cerebellum, cortex, and total brain have been described to associate with poorer cognitive, language, and motor outcomes in CHD, 40 , 41 , 42 which might indicate that placental pathology is an early biomarker for adverse neurodevelopment. 21
Multiple biological mechanisms may be involved in the suggested impact of placental pathology on brain development in CHD. During the third trimester, the fetal brain experiences a period of rapid volumetric growth and undergoes crucial developmental processes, such as synaptogenesis, neuronal migration, and myelination, requiring continuing supply of oxygen and nutrients from the placenta. In addition, the placenta regulates fetal brain development by synthesis of neurotransmitters and hormones. 43 Thus, placental abnormalities might compromise brain development by rendering inadequate metabolic and endocrine support. Moreover, acute or chronic inflammatory placental lesions may trigger a fetal inflammatory response that disrupts fetal brain development through the activation of microglia and astrocytes, which release inflammatory factors such as cytokines, chemokines, and reactive oxygen species. 44 , 45 Another potential mechanism by which placental pathology could affect fetal brain development is through increased exposure to maternal stress. The placenta regulates maternal cortisol levels by expressing the enzyme 11‐β‐hydrosteroid dehydrogenase type 2, which converts cortisol to the inactive metabolite cortisone, thereby minimizing fetal exposure to maternal cortisol. 46 Placental lesions have been linked to reduced levels of this enzyme. 47 Markedly, in pregnancies complicated by fetal CHD, maternal psychological distress is prevalent and has been associated with smaller cerebellar and hippocampal volumes. 48
In our study, the level of placental pathology was not associated with preoperative hemorrhagic or ischemic brain injury, implying that placental lesions primarily affect fetal growth rather than directly leading to postnatal brain injury. Presurgical brain injury is more likely to result from postnatal events that lead to acute hemodynamic instability, such as the delivery process or urgent cardiac interventions. 3 Comparable to our results, Schlatterer et al did not find significant correlations between abnormal placentation and brain injury, but did observe a statistical trend toward more severe brain lesions in cases with placental abnormalities. 19 Although placental pathology may not directly cause postnatal brain injury, it could have an indirect effect, since reduced volumetric growth has been associated with increased susceptibility to postnatal brain injury. 6 Previous studies in other neonatal populations at risk for brain injury have reported associations between placental abnormalities and brain damage. In infants with hypoxic–ischemic encephalopathy, chorioamnionitis and chronic villitis have been linked to both the severity and location of the encephalopathy. 27 , 49 In infants born preterm, chorioamnionitis has been identified as a risk factor for periventricular leukomalacia, intraventricular hemorrhages, and ventriculomegaly. 50 , 51
The strengths of this study include its prospective study design, robust sample size, high‐quality imaging data, and detailed standardized review of the placenta. 26 However, there are certain limitations to this study. First, the scoring system used to measure the extent and severity of pathology in each placenta was based on a score described in the study by Harteman et al, in which the effect of placental lesions on cerebral injury in infants with hypoxic–ischemic encephalopathy was examined. 27 This pathology score has not been validated nor has it been specified for use in neonates with CHD. Second, although the pathologists were blinded to CHD subtypes and clinical outcomes, they were aware that the neonates had CHD, which may have introduced bias. Third, although the study included an exploratory subgroup analysis, our sample size was underpowered to draw definitive conclusions about the variation in placental pathology between CHD subtypes. Fourth, it is worth noting that despite our exclusion of cases with genetic or chromosomal disorders, not all included neonates underwent pre‐ or postnatal genetic testing. As a result, the presence of undiagnosed genetic conditions among the untested cases remains a possibility. Finally, the absence of a control group of neonates with comparable placental lesions makes it challenging to assess the causal relationship and interplay between placental pathology and brain development in CHD. Future research endeavors should consider including preterm‐born children with CHD as well, given that the prevalence of placental pathology may be even higher in this particular group. Additionally, conducting longitudinal studies investigating the relationship between structural and functional placental abnormalities, fetal hemodynamics, and fetal growth throughout gestation holds the potential to determine the precise timing of placental dysfunction and disruptions in the growth trajectory. This could be achieved by combining histological reports with fetal biometry and advanced imaging techniques, such as functional placenta MRI and Doppler ultrasound.
Conclusions
This study shows that placental pathology is associated with smaller total and regional brain volumes in neonates with CHD. Placental abnormalities were highly prevalent and included lesions indicating disruptions in the uteroplacental circulation, as well as lesions suggesting compromised fetoplacental circulation. A higher placental pathology severity score was associated with reductions in postnatal cortical gray matter, cerebellar, and total brain volumes, which suggests that placental abnormalities may predominantly affect brain structures undergoing rapid growth in the third trimester of pregnancy. Our findings underline the importance of monitoring placental health and evaluation of the placenta by an experienced perinatal pathologist in pregnancies with fetal CHD. To unravel the underlying causes of placental abnormalities in CHD and their impact on placental functioning, fetal hemodynamics, and neurodevelopmental outcomes, further investigations into the placenta‐heart‐brain axis are warranted. Ultimately, targeting placental pathology may hold promise as a prenatal intervention for improving neurodevelopmental outcomes in CHD.
Appendix
Congenital Heart Disease LifeSpan Study Group
Department of Pediatric Intensive Care, Wilhelmina Children's Hospital, University Medical Center Utrecht, Utrecht, the Netherlands: Joppe Nijman, Thomas Alderliesten, Rian Bosch, and Roelie M. Wösten‐van Asperen. Department of Pediatric Cardiology, Wilhelmina Children's Hospital, University Medical Center Utrecht, Utrecht, the Netherlands: Martijn G. Slieker, Gabrielle G. van Iperen, and Heynric B. Grotenhuis. Department of Anesthesiology, Wilhelmina Children's Hospital, University Medical Center Utrecht, Utrecht, the Netherlands: Kim van Loon and Erik Koomen. Department of Congenital Cardiothoracic Surgery, Wilhelmina Children's Hospital, University Medical Center Utrecht, Utrecht, the Netherlands: Bram van Wijk, Paul H. Schoof, and Hanna Talacua. Department of Pediatric Psychology, Sector of Neuropsychology, Wilhelmina Children's Hospital, University Medical Center Utrecht, the Netherlands: Monique M. J. van Schooneveld. Center of Child Development, Exercise and Physical Literacy, Wilhelmina Children's Hospital, University Medical Center Utrecht, Utrecht, the Netherlands: Maaike C. A. Sprong. Department of Obstetrics and Fetal Medicine, Leiden University Medical Center, Leiden, the Netherlands: Monique C. Haak and Maartje C. Snoep.
Sources of Funding
The PhD position of Maaike Nijman was supported by the Dutch Organization for Health Research and Development (ZonMw); project number 848042002, CeRebrUm and CardIac Protection with ALlopurinol in Neonates with Critical Congenital Heart Disease Requiring Cardiac Surgery with Cardiopulmonary Bypass (CRUCIAL trial). The subsidizing party did not play a role in data collection, data analysis, or writing of the article.
Disclosures
None.
Acknowledgments
The authors thank the Congenital Heart Disease LifeSpan Study group, which includes staff from the Departments of Obstetrics, Neonatology, Pediatric Cardiology, Pediatric Intensive Care, Congenital Cardiothoracic Surgery, Anesthesiology, Radiology, Child Development and Exercise Center, Medical Psychology, and Social Work in Wilhelmina Children's Hospital, University Medical Center Utrecht.
This article was sent to John L. Jefferies, MD, MPH, Guest Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 10.
Contributor Information
Manon J. N. L. Benders, Email: m.benders@umcutrecht.nl.
CHD LifeSpan Study Group:
Joppe Nijman, Thomas Alderliesten, Rian Bosch, Roelie M. Wösten‐van Asperen, Martijn G. Slieker, Gabrielle G. van Iperen, Heynric B. Grotenhuis, Kim van Loon, Erik Koomen, Bram van Wijk, Paul H. Schoof, Hanna Talacua, Monique M. J. van Schooneveld, Maaike C. A. Sprong, Monique C. Haak, and Maartje C. Snoep
References
- 1. Oster ME, Lee KA, Honein MA, Riehle‐Colarusso T, Shin M, Correa A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics. 2013;131:e1502–e1508. doi: 10.1542/peds.2012-3435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liamlahi R, Latal B. Neurodevelopmental outcome of children with congenital heart disease. Handb Clin Neurol. 2019;162:329–345. doi: 10.1016/B978-0-444-64029-1.00016-3 [DOI] [PubMed] [Google Scholar]
- 3. Bonthrone AF, Stegeman R, Feldmann M, Claessens NHP, Nijman M, Jansen NJG, Nijman J, Groenendaal F, de Vries LS, Benders MJNL, et al. Risk factors for perioperative brain lesions in infants with congenital heart disease: a European collaboration. Stroke. 2022;53:3652–3661. doi: 10.1161/STROKEAHA.122.039492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu S, Sai X, Lin J, Deng G, Zhao M, Nasser MI, Zhu P. Mechanisms of perioperative brain damage in children with congenital heart disease. Biomed Pharmacother. 2020;132:110957. doi: 10.1016/j.biopha.2020.110957 [DOI] [PubMed] [Google Scholar]
- 5. Sun L, Macgowan CK, Sled JG, Yoo SJ, Manlhiot C, Porayette P, Grosse‐Wortmann L, Jaeggi E, McCrindle BW, Kingdom J, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation. 2015;131:1313–1323. doi: 10.1161/CIRCULATIONAHA.114.013051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Claessens NHP, Khalili N, Isgum I, Ter Heide H, Steenhuis TJ, Turk E, Jansen NJG, de Vries LS, Breur JMPJ, de Heus R, et al. Brain and CSF volumes in fetuses and neonates with antenatal diagnosis of critical congenital heart disease: a longitudinal MRI study. AJNR Am J Neuroradiol. 2019;40:885–891. doi: 10.3174/ajnr.A6021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee FT, Seed M, Sun L, Marini D. Fetal brain issues in congenital heart disease. Transl Pediatr. 2021;10:2182–2196. doi: 10.21037/tp-20-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL Jr, Guizard N, McGrath E, Geva J, Annese D, et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. doi: 10.1161/CIRCULATIONAHA.109.865568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sadhwani A, Wypij D, Rofeberg V, Gholipour A, Mittleman M, Rohde J, Velasco‐Annis C, Calderon J, Friedman KG, Tworetzky W, et al. Fetal brain volume predicts neurodevelopment in congenital heart disease. Circulation. 2022;145:1108–1119. doi: 10.1161/CIRCULATIONAHA.121.056305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zeng S, Zhou J, Peng Q, Tian L, Xu G, Zhao Y, Wang T, Zhou Q. Assessment by three‐dimensional power doppler ultrasound of cerebral blood flow perfusion in fetuses with congenital heart disease. Ultrasound Obstet Gynecol. 2015;45:649–656. doi: 10.1002/uog.14798 [DOI] [PubMed] [Google Scholar]
- 11. Licht DJ, Jacobwitz M, Lynch JM, Ko T, Boorady T, Devarajan M, Heye KN, Mensah‐Brown K, Newland JJ, Schmidt A, et al. Impaired maternal‐fetal environment and risk for preoperative focal white matter injury in neonates with complex congenital heart disease. J Am Heart Assoc. 2023;12:e025516. doi: 10.1161/JAHA.122.025516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gude NM, Roberts CT, Kalionis B, King RG. Growth and function of the normal human placenta. Thromb Res. 2004;114:397–407. doi: 10.1016/j.thromres.2004.06.038 [DOI] [PubMed] [Google Scholar]
- 13. Courtney JA, Cnota JF, Jones HN. The role of abnormal placentation in congenital heart disease; cause, correlate, or consequence? Front Physiol. 2018;9:1045. doi: 10.3389/fphys.2018.01045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ernst LM. Maternal vascular malperfusion of the placental bed. APMIS. 2018;126:551–560. doi: 10.1111/apm.12833 [DOI] [PubMed] [Google Scholar]
- 15. Auger N, Fraser WD, Healy‐Profitos J, Arbour L. Association between preeclampsia and congenital heart defects. JAMA. 2015;314:1588–1598. doi: 10.1001/jama.2015.12505 [DOI] [PubMed] [Google Scholar]
- 16. Jones HN, Olbrych SK, Smith KL, Cnota JF, Habli M, Ramos‐Gonzales O, Owens KJ, Hinton AC, Polzin WJ, Muglia LJ, et al. Hypoplastic left heart syndrome is associated with structural and vascular placental abnormalities and leptin dysregulation. Placenta. 2015;36:1078–1086. doi: 10.1016/j.placenta.2015.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matthiesen NB, Henriksen TB, Agergaard P, Gaynor JW, Bach CC, Hjortdal VE, Østergaard JR. Congenital heart defects and indices of placental and fetal growth in a nationwide study of 924 422 liveborn infants. Circulation. 2016;134:1546–1556. doi: 10.1161/CIRCULATIONAHA.116.021793 [DOI] [PubMed] [Google Scholar]
- 18. Rychik J, Goff D, McKay E, Mott A, Tian Z, Licht DJ, Gaynor JW. Characterization of the placenta in the newborn with congenital heart disease: distinctions based on type of cardiac malformation. Pediatr Cardiol. 2018;39:1165–1171. doi: 10.1007/s00246-018-1876-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schlatterer SD, Murnick J, Jacobs M, White L, Donofrio MT, Limperopoulos C. Placental pathology and neuroimaging correlates in neonates with congenital heart disease. Sci Rep. 2019;9:4137. doi: 10.1038/s41598-019-40894-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leon RL, Sharma K, Mir IN, Herrera CL, Brown SL, Spong CY, Chalak LF. Placental vascular malperfusion lesions in fetal congenital heart disease. Am J Obstet Gynecol. 2022;227:620.e1–620.e8. doi: 10.1016/j.ajog.2022.05.038 [DOI] [PubMed] [Google Scholar]
- 21. Segar DE, Zhang J, Yan K, Reid A, Frommelt M, Cohen S. The relationship between placental pathology and neurodevelopmental outcomes in complex congenital heart disease. Pediatr Cardiol. 2023;44:1143–1149. doi: 10.1007/s00246-022-03018-4 [DOI] [PubMed] [Google Scholar]
- 22. O'Hare CB, Mangin‐Heimos KS, Gu H, Edmunds M, Bebbington M, Lee CK, He M, Ortinau CM. Placental delayed villous maturation is associated with fetal congenital heart disease. Am J Obstet Gynecol. 2023;228:231.e1–231.e11. doi: 10.1016/j.ajog.2022.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leon RL, Mir IN, Herrera CL, Sharma K, Spong CY, Twickler DM, Chalak LF. Neuroplacentology in congenital heart disease: placental connections to neurodevelopmental outcomes. Pediatr Res. 2022;91:787–794. doi: 10.1038/s41390-021-01521-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/S0735-1097(02)01886-7 [DOI] [PubMed] [Google Scholar]
- 25. Pinar H, Sung CJ, Oyer CE, Singer DB. Reference values for singleton and twin placental weights. Pediatr Pathol Lab Med. 1996;16:901–907. doi: 10.1080/15513819609168713 [DOI] [PubMed] [Google Scholar]
- 26. Khong TY, Mooney EE, Ariel I, Balmus NC, Boyd TK, Brundler MA, Derricott H, Evans MJ, Faye‐Petersen OM, Gillan JE, et al. Sampling and definitions of placental lesions: Amsterdam Placental Workshop Group consensus statement. Arch Pathol Lab Med. 2016;140:698–713. doi: 10.5858/arpa.2015-0225-CC [DOI] [PubMed] [Google Scholar]
- 27. Harteman JC, Nikkels PG, Benders MJNL, Kwee A, Groenendaal F, de Vries LS. Placental pathology in full‐term infants with hypoxic‐ischemic neonatal encephalopathy and association with magnetic resonance imaging pattern of brain injury. J Pediatr. 2013;163:968–995.e2. doi: 10.1016/j.jpeds.2013.06.010 [DOI] [PubMed] [Google Scholar]
- 28. Stegeman R, Nijman M, Breur JMPJ, Groenendaal F, Haas F, Derks JB, Nijman J, van Beynum IM, Taverne YJHJ, Bogers AJJC, et al; CRUCIAL trial consortium . CeRebrUm and CardIac protection with ALlopurinol in neonates with critical congenital heart disease requiring cardiac surgery with cardiopulmonary bypass (CRUCIAL): study protocol of a phase III, randomized, quadruple‐blinded, placebo‐controlled, Dutch multicenter trial. Trials. 2022;23:174. doi: 10.1186/s13063-022-06098-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stegeman R, Feldmann M, Claessens NHP, Jansen NJG, Breur JMPJ, de Vries LS, Logeswaran T, Reich B, Knirsch W, Kottke R, et al. A uniform description of perioperative brain MRI findings in infants with severe congenital heart disease: results of a European collaboration. AJNR Am J Neuroradiol. 2021;42:2034–2039. doi: 10.3174/ajnr.A7328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Makropoulos A, Robinson EC, Schuh A, Wright R, Fitzgibbon S, Bozek J, Counsell SJ, Steinweg J, Vecchiato K, Passerat‐Palmbach J, et al. The developing human connectome project: a minimal processing pipeline for neonatal cortical surface reconstruction. Neuroimage. 2018;173:88–112. doi: 10.1016/j.neuroimage.2018.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Makropoulos A, Aljabar P, Wright R, Huning B, Merchant N, Arichi T, Tusor N, Hajnal JV, Edwards AD, Counsell SJ, et al. Regional growth and atlasing of the developing human brain. Neuroimage. 2016;125:456–478. doi: 10.1016/j.neuroimage.2015.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User‐guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015 [DOI] [PubMed] [Google Scholar]
- 33. Bozek J, Makropoulos A, Schuh A, Fitzgibbon S, Wright R, Glasser MF, Coalson TS, O'Muircheartaigh J, Hutter J, Price AN, et al. Construction of a neonatal cortical surface atlas using multimodal surface matching in the developing human connectome project. Neuroimage. 2018;179:11–29. doi: 10.1016/j.neuroimage.2018.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hermansen MC. Nucleated red blood cells in the fetus and newborn. Arch Dis Child Fetal Neonatal Ed. 2001;84:F211–F215. doi: 10.1136/fn.84.3.F211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Redline RW, Bagby C, Ravishankar S. Hypervascularity. In: Khong TY, Mooney EE, Nikkels PGJ, Morgan TK, Gordijn SJ, eds. Pathology of the Placenta: A Practical Guide. Cham: Springer International Publishing; 2019:163–172. doi: 10.1007/978-3-319-97214-5_24 [DOI] [Google Scholar]
- 36. Huynh J, Dawson D, Roberts D, Bentley‐Lewis R. A systematic review of placental pathology in maternal diabetes mellitus. Placenta. 2015;36:101–114. doi: 10.1016/j.placenta.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. de Laat MW, van der Meij JJ, Visser GH, Franx A, Nikkels PG. Hypercoiling of the umbilical cord and placental maturation defect: associated pathology? Pediatr Dev Pathol. 2007;10:293–299. doi: 10.2350/06-01-0015.1 [DOI] [PubMed] [Google Scholar]
- 38. Redline RW, Ravishankar S. Fetal vascular malperfusion, an update. APMIS. 2018;126:561–569. doi: 10.1111/apm.12849 [DOI] [PubMed] [Google Scholar]
- 39. Andescavage NN, du Plessis A, McCarter R, Serag A, Evangelou I, Vezina G, Robertson R, Limperopoulos C. Complex trajectories of brain development in the healthy human fetus. Cereb Cortex. 2017;27:5274–5283. doi: 10.1093/cercor/bhw306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stegeman R, Sprong MCA, Breur JMPJ, Groenendaal F, de Vries LS, Haas F, van der Net J, Jansen NJG, Benders MJNL, Claessens NHP. Early motor outcomes in infants with critical congenital heart disease are related to neonatal brain development and brain injury. Dev Med Child Neurol. 2022;64:192–199. doi: 10.1111/dmcn.15024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bonthrone AF, Dimitrova R, Chew A, Kelly CJ, Cordero‐Grande L, Carney O, Egloff A, Hughes E, Vecchiato K, Simpson J, et al. Individualized brain development and cognitive outcome in infants with congenital heart disease. Brain Commun. 2021;3:fcab046. doi: 10.1093/braincomms/fcab046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meuwly E, Feldmann M, Knirsch W, von Rhein M, Payette K, Dave H, O'Gorman Tuura R, Kottke R, Hagmann C, Latal B, et al. Postoperative brain volumes are associated with one‐year neurodevelopmental outcome in children with severe congenital heart disease. Sci Rep. 2019;9:10885. doi: 10.1038/s41598-019-47328-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rosenfeld CS. The placenta‐brain‐axis. J Neurosci Res. 2021;99:271–283. doi: 10.1002/jnr.24603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goldstein JA, Gallagher K, Beck C, Kumar R, Gernand AD. Maternal‐fetal inflammation in the placenta and the developmental origins of health and disease. Front Immunol. 2020;11:531543. doi: 10.3389/fimmu.2020.531543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Volpe JJ. Brain injury in the premature infant. Neuropathology, clinical aspects, pathogenesis, and prevention. Clin Perinatol. 1997;24:567–587. doi: 10.1016/S0095-5108(18)30159-3 [DOI] [PubMed] [Google Scholar]
- 46. Chapman KE, Coutinho AE, Zhang Z, Kipari T, Savill JS, Seckl JR. Changing glucocorticoid action: 11β‐hydroxysteroid dehydrogenase type 1 in acute and chronic inflammation. J Steroid Biochem Mol Biol. 2013;137:82–92. doi: 10.1016/j.jsbmb.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Johnstone JF, Bocking AD, Unlugedik E, Challis JR. The effects of chorioamnionitis and betamethasone on 11beta hydroxysteroid dehydrogenase types 1 and 2 and the glucocorticoid receptor in preterm human placenta. J Soc Gynecol Investig. 2005;12:238–245. doi: 10.1016/j.jsgi.2005.01.029 [DOI] [PubMed] [Google Scholar]
- 48. Wu Y, Kapse K, Jacobs M, Niforatos‐Andescavage N, Donofrio MT, Krishnan A, Vezina G, Wessel D, du Plessis A, Limperopoulos C. Association of maternal psychological distress with in utero brain development in fetuses with congenital heart disease. JAMA Pediatr. 2020;174:e195316. doi: 10.1001/jamapediatrics.2019.5316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mir IN, Johnson‐Welch SF, Nelson DB, Brown LS, Rosenfeld CR, Chalak LF. Placental pathology is associated with severity of neonatal encephalopathy and adverse developmental outcomes following hypothermia. Am J Obstet Gynecol. 2015;213: 849.e1–849.e7. doi: 10.1016/j.ajog.2015.09.072 [DOI] [PubMed] [Google Scholar]
- 50. Lu HY, Zhang Q, Wang QX, Lu JY. Contribution of histologic chorioamnionitis and fetal inflammatory response syndrome to increased risk of brain injury in infants with preterm premature rupture of membranes. Pediatr Neurol. 2016;61:94–98.e1. doi: 10.1016/j.pediatrneurol.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 51. De Felice C, Toti P, Laurini RN, Stumpo M, Picciolini E, Todros T, Tanganelli P, Buonocore G, Bracci R. Early neonatal brain injury in histologic chorioamnionitis. J Pediatr. 2001;138:101–104. doi: 10.1067/mpd.2001.109605 [DOI] [PubMed] [Google Scholar]


