Abstract
Background
The sodium glucose cotransporter‐2 inhibitors are guideline‐recommended to treat heart failure across the spectrum of left ventricular ejection fraction; however, economic evaluations of adding sodium glucose cotransporter‐2 inhibitors to standard of care in chronic heart failure across a broad left ventricular ejection fraction range are lacking.
Methods and Results
We conducted a US‐based cost‐effectiveness analysis of dapagliflozin added to standard of care in a chronic heart failure population using pooled, participant data from the DAPA‐HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) and DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure) trials. The 3‐state Markov model used estimates of transitional probabilities, effectiveness of dapagliflozin, and utilities from the pooled trials. Costs estimates were obtained from published sources, including published rebates in dapagliflozin cost. Adding dapagliflozin to standard of care was estimated to produce an additional 0.53 quality‐adjusted life years (QALYs) compared with standard of care alone. Incremental cost effectiveness ratios were $85 554/QALY when using the publicly reported full (undiscounted) Medicare cost ($515/month) and $40 081/QALY, at a published nearly 50% rebate ($263/month). The addition of dapagliflozin to standard of care would be of at least intermediate value (<$150 000/QALY) at a cost of <$872.58/month, of high value (<$50 000/QALY) at <$317.66/month, and cost saving at <$40.25/month. Dapagliflozin was of at least intermediate value in 92% of simulations when using the full (undiscounted) Medicare list cost in probabilistic sensitivity analyses. Cost effectiveness was most sensitive to the dapagliflozin cost and the effect on cardiovascular death.
Conclusions
The addition of dapagliflozin to standard of care in patients with heart failure across the spectrum of ejection fraction was at least of intermediate value at the undiscounted Medicare cost and may be potentially of higher value on the basis of the level of discount, rebates, and price negotiations offered.
Registration
URL: https://www.clinicaltrials.gov; Unique identifiers: NCT01035255 & NCT01920711.
Keywords: cost effectiveness, heart failure, SGLT2 inhibitors, value
Subject Categories: Heart Failure
Nonstandard Abbreviations and Acronyms
- DAPA‐HF
Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure
- DELIVER
Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure
- EMPEROR‐Preserved
Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction
- ICER
incremental cost effectiveness ratio
Clinical Perspective.
What Is New?
This analysis examines the cost effectiveness and economic value of dapagliflozin for the treatment of heart failure across the full spectrum of left ventricular ejection fraction.
The findings suggest that dapagliflozin is of intermediate value in heart failure at the current undiscounted cost; rebates and other discounts may improve the cost effectiveness of this therapy.
What Are the Clinical Implications?
Additional factors, including announced Medicare price negotiation for dapagliflozin as part of the Inflation Reduction Act, with effective data planned as early as 2026, may further enhance the economic value of this therapy in the near future.
The sodium glucose cotransporter‐2 inhibitors (SGLT2is) reduce cardiovascular events in patients with heart failure (HF), irrespective of chronicity of illness, care location, and left ventricular ejection fraction (LVEF). 1 Most recently, the DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure) trial, the largest trial of HF with mildly reduced or preserved ejection fraction, demonstrated an 18% reduction in cardiovascular death or worsening HF events and improvements in health‐related QoL. 2 These data add to the totality of evidence supporting the role of SGLT2is as foundational therapy in HF across the spectrum of LVEF. 1 , 3 Clinical practice guidelines now support the use of SGLT2is in patients with HF with reduced, mildly reduced, and preserved ejection fraction. 4
Despite a robust evidence base and updated guideline recommendations, implementation of SGLT2is has been slow and incomplete, which may, in part, be due to barriers to access. Previous cost effectiveness analyses based on US cost inputs have suggested that the SGLT2i dapagliflozin has intermediate economic value on the basis of conventional cost effectiveness thresholds in HF care, 5 , 6 but modeling was limited to a segment of the HF population (those with HF with reduced ejection fraction). Dedicated cost effectiveness analyses of this class in preserved ejection fraction have established low to intermediate value of this therapy based on those same thresholds but did not capture the total HF hospitalization and were limited only to those with ejection fraction (EF) that was not frankly reduced. 7 This therapy is now guideline recommended and used across the full spectrum of LVEF; however, cost effectiveness for all patients with HF is unknown. 8 , 9 , 10 We leverage pooled, individual participant data from the complementary DAPA‐HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) and DELIVER trials to examine the cost effectiveness of dapagliflozin added to standard of care in a chronic HF population across the spectrum of EF and across various available cost estimates for dapagliflozin.
METHODS
The sponsor of these trials is committed to sharing access to patient‐level data and supporting clinical documents from eligible studies with qualified external researchers. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial, in line with applicable laws and regulations. The trial data availability is according to the criteria and process described here: https://astrazenecagrouptrials.pharmacm.com/st/submission/disclosure.
Model and Patient Population
We constructed a 3‐state Markov model to estimate the cost effectiveness of dapagliflozin added to standard of care in patients with chronic HF, regardless of LVEF, using pooled, individual participant data from DAPA‐HF and DELIVER. DAPA‐HF was a double‐blind, placebo‐controlled, global trial that randomized 4744 ambulatory patients with New York Heart Association class II to IV HF and EF ≤40% enrolled from February 2017 through August 2018 to receive either dapagliflozin 10 mg daily or placebo. 11 DELIVER similarly randomized 6263 participants enrolled from August 2018 through December 2020 to dapagliflozin 10 mg daily versus placebo, but enrolled patients with HF and mildly reduced or preserved LVEF (EF >40%). 12 With the notable exception of included EF ranges, trial inclusion and exclusion criteria were similar. 11 , 12 The primary efficacy end point in both trials was a composite of the time to first worsening HF event (defined as hospitalization for HF or an urgent HF visit requiring intravenous HF therapies) or cardiovascular death. Given a recognition that inclusion of first and recurrent HF events more fully captures disease burden, 13 , 14 DAPA‐HF additionally assessed total HF hospitalizations (first and recurrent) and DELIVER assessed total HF events (first and recurrent HF hospitalization or urgent HF visit) as a component of a secondary end point. Treatment with dapagliflozin resulted in a reduction in the primary outcome compared with placebo of 26% and 18% in DAPA‐HF and DELIVER, respectively; risk reductions were also seen across these secondary end points. The trial protocols were approved by a local or central institutional review board at each participating trial center. All patients provided written informed consent. A cost effectiveness analysis of DELIVER from the US perspective was prespecified in the academic Statistical Analysis Plan, which was finalized before trial unmasking. To provide broader context, participant‐level pooled analyses of DELIVER and DAPA‐HF for efficacy and safety outcomes were additionally prespecified. 3
Our model followed a standard structure in which each month, a patient with HF had a probability of surviving, experiencing a worsening HF event, or dying. States that patients with HF were able to occupy included (1) chronic, ambulatory HF; (2) experiencing a worsening HF event; or (3) death (Figure 1).
Figure 1. Diagram of the Markov model.
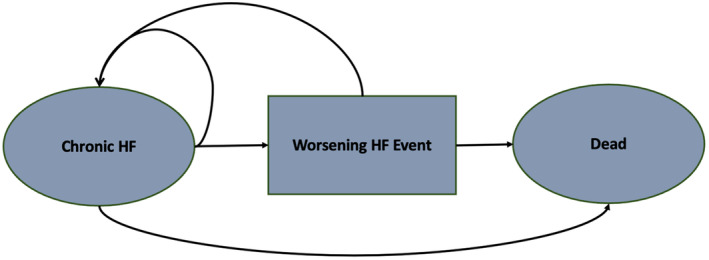
Patients occupy health states, shown in the ovals and boxes. Patients transition from different health states represented as arrows based on transition probabilities derived from participant‐level data from DAPA‐HF and DELIVER. Worsening HF events include hospitalization for HF and urgent HF visits. DAPA‐HF indicates Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; DELIVER, Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure; and HF, heart failure.
Baseline Assumptions and Modeling Inputs
Key modeling inputs were obtained from pooled, participant‐level data from DAPA‐HF and DELIVER. State transition probabilities were derived from placebo event rates for total worsening HF events and death among US participants in both trials, transformed to monthly probabilities. As DAPA‐HF evaluated total (first and recurrent) HF hospitalizations but not total (first and recurrent) urgent HF visits, we combined the placebo event rate for total HF hospitalizations from DAPA‐HF with the placebo event rate for total HF hospitalizations or urgent HF visits from DELIVER to obtain the pooled estimate. We also estimated (1) the proportion of worsening HF events attributable to HF hospitalization (versus urgent HF visit) and (2) the proportion of all‐cause death adjudicated by an independent clinical end points committee as attributable to cardiovascular causes (versus noncardiovascular causes) from US participant trial data using trial‐specific definitions. 11 , 12 Hazard ratios (dapagliflozin versus placebo) from the full trial population were applied to placebo transition probabilities for (1) worsening HF events and (2) cardiovascular death. We assumed no risk reduction with dapagliflozin versus placebo on noncardiovascular death, consistent with trial results. We also assumed dapagliflozin effectiveness was sustained over the lifetime of patients and that patients would remain adherent to therapy, without modeling discontinuation over time. As the trials reported no differences among treatment arms in prespecified safety end points, we did not model QoL penalties related to adverse drug‐related events or drug disutility. SGLT2is have consistently improved clinical outcomes in HF similarly regardless of diabetes status 15 , 16 , 17 and prior cost effectiveness analyses with dapagliflozin found similar incremental cost effectiveness ratios (ICERs) in those with and without diabetes 5 , 6 , 7 ; therefore, we used a unified model agnostic to diabetes status.
Costs
Model inputs, including costs and utilities, are reported in Table 1. 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 HF hospitalization costs were obtained from a recent US‐based systematic review 18 and were similar to cost estimates after inflation adjustment from a prior analysis using Medicare fee schedule costs. 6 In a deterministic sensitivity analysis, we used a lower‐bound HF hospitalization cost from published data 18 and an upper bound from full private payer cost estimates. 19 , 20 , 21 We estimated a cost of an urgent HF visit at $846 from an inflation‐adjusted cost from a prior cost effectiveness analysis of dapagliflozin. 6 Total cost for a worsening HF event was obtained from a weighted average of HF hospitalization and urgent HF visit costs; 93% of all worsening HF events were HF hospitalizations using pooled data from DAPA‐HF and DELIVER.
Table 1.
Model Inputs
| Value | Range | Source | |
|---|---|---|---|
| Monthly transition probabilities* | |||
| Worsening HF events | 0.021 | 0.017 to 0.027 | Trials |
| Cardiovascular death | 0.005 | 0.004 to 0.006 | Trials |
| Effectiveness of dapagliflozin vs placebo | |||
| Worsening HF events | 0.73 | 0.64 to 0.82 | Trials |
| Cardiovascular death | 0.85 | 0.75 to 0.97 | Trials |
| Proportions of events (US population) | |||
| Proportion of worsening HF events attributable to HF hospitalization | 0.93 | 0.89 to 0.95 | Trials |
| Proportion of all‐cause dortality attributable to cardiovascular death | 0.53 | 0.45 to 0.62 | Trials |
| Costs | |||
| HF costs | |||
| HF hospitalization | $13 418 | $11 125 to $29 000 | Urbich et al 18 ; private payers 19 , 20 , 21 |
| Urgent HF visit | $846 | $678 to $1016 | Isaza et al 6 |
| Chronic HF costs (annual) | $6250 | $1188 to $6779 | Gaziano et al 35 ; Dunlay et al 36 |
| Medication costs (monthly) | |||
| Dapagliflozin | $514.95 | $262.62 to $637.93 | Estimate: Medicare Part D 24 ; lower bound: 49% rebate 9 ; upper bound: 5.5% yearly increase×4 y 24 |
| Utilities | |||
| Dapagliflozin | 0.826 | 0.822 to 0.830 | Isaza et al and prior modeling 6 , 30 , 31 ; trials |
| Placebo | 0.811 | 0.806 to 0.815 | Isaza et al and prior modeling 6 , 30 , 31 ; trials |
| QoL disutility during worsening HF events | −0.0152 | −0.0066 to 0.0000 | Trials; lower bound: Isaza et al 6 |
| Discount rate, % | 3 | 0 to 5 | Hunink et al 32 ; Weinstein et al 33 ; Gold et al 34 |
DAPA‐HF indicates Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; DELIVER, Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure; HF, heart failure; and QoL, quality of life.
Transition probabilities derived from placebo event rates among US participants in both trials. Worsening HF events include hospitalization for HF and urgent HF visits. Trials refer to participant‐level data from the DAPA‐HF and DELIVER randomized clinical trials.
Determining the actual cost of dapagliflozin is challenging in the United States. In practice, pharmaceutical manufacturers have a list or wholesale acquisition cost. Publicly reported Medicare Part D drug spending reports, however, are based on the gross drug cost and do not reflect manufacturers' rebates or other price concessions. Increasingly, manufacturers may provide rebates to pharmacy benefit managers or payers (commercial or government), leading to net costs substantially lower than the list cost. 9 , 22 Further complicating matters, precise estimates of the amount of rebates are often considered trade secrets. In addition, some portion of the offered rebate is passed on to the consumer in the form of reduced medication cost at the pharmacy or reductions in overall insurance premiums, 8 , 22 , 23 making it difficult to know the true cost of medication for economic analyses. Thus, we used multiple estimates of the cost of dapagliflozin from publicly available sources and published rebate estimates.
For purposes of 1‐way deterministic and probabilistic sensitivity analyses, we used an undiscounted monthly dapagliflozin cost of $514.95 from 2020 Medicare Part D drug payments, 24 and a discounted cost of $262.62/month, reflecting a published estimate of a US‐based manufacturer discount/rebate of 49% available to payers or pharmacy benefit managers. 9 In a 1‐way sensitivity analysis, we modeled cost effectiveness around the undiscounted price from $262.62/month to $637.93/month, which represented a 5.5% yearly increase over 4 years on the basis of the Medicare gross price changes from 2018 to 2020. 24 For the discounted price, we modeled prices ranging from $125/month to $375/month.
Recognizing substantial cost variation based on payer, rebate offers, available discounts, and policies, we further explored the cost effectiveness of dapagliflozin across the various sources of dapagliflozin cost: US Wholesale Acquisition Cost ($548.83/month), 25 Federal Supply Schedule Big Four cost ($396.14/month), 26 a reduced rebate in which 20% of the overall rebate is retained by entities ($314.08/month), 9 , 22 , 27 and an inflation‐adjusted Canadian cost estimate from the Ontario Drug Benefit Formulary converted to US dollars ($68.25/month). 28 , 29 HF treatment costs were obtained from published estimates and are listed in Table 1.
Utilities
Quality‐of‐life (QoL) or utility decrements were applied on the basis of the change in Kansas City Cardiomyopathy Questionnaire overall summary scores among US participants from baseline through 32 weeks, which were prospectively collected in both DELIVER and DAPA‐HF. We used a previously reported algorithm to transform Kansas City Cardiomyopathy Questionnaire overall summary scores to EuroQol‐5D–based health‐related QoL estimates. 6 , 30 , 31 The model applied additional QoL decrements based on trial data to each month in which a patient experienced a worsening HF event.
Statistical Analysis
This cost effectiveness analysis included a lifetime horizon from a health care system perspective. Incremental costs of treatment and QALYs gained were modeled in a chronic HF population across the spectrum of EF treated with dapagliflozin 10 mg or standard of care without dapagliflozin. The primary outcome was the ICER, calculated per conventional cost effectiveness analysis guidelines. 32 Costs and QALYs were each discounted at 3% annually. 34
In the full population, we performed several 1‐way sensitivity analyses, including (1) varying the cost of dapagliflozin as previously stated, (2) varying the costs of worsening HF events, and (3) varying the efficacy of dapagliflozin at reducing worsening HF events and cardiovascular death across the 95% CIs of each pooled hazard ratio. Each sensitivity analysis was done holding all other model inputs constant. We conducted a threshold analysis to determine the monthly cost of dapagliflozin at which (1) treatment met certain predetermined willingness‐to‐pay thresholds ($50 000 and $150 000 per QALY gained) and (2) would be cost saving. As the model was most sensitive to drug costs and the effect of therapy on cardiovascular death, we performed a 2‐way sensitivity analysis based on 3 values (low, middle, high) for each variable.
A probabilistic sensitivity analysis was modeled on the basis of 100 000 iterations using input parameters for costs and effectiveness randomly drawn from the bounds of the appropriate distributions of key model inputs: β distributions for health transition probabilities bounded by 0 and 1, log‐normal distributions for hazard ratios, a triangular distribution for costs of chronic HF care, and γ distributions for all other costs. We estimated the proportion of simulations in which dapagliflozin would be cost effective at intermediate and high‐value willingness‐to‐pay thresholds. Value determinations were made on the basis of the American College of Cardiology/American Heart Association cost and value descriptions: high value, <$50 000 per QALY gained; intermediate value, $50 000 to <$150 000 per QALY gained; and low value, ≥$150 000 per QALY gained. 37
Finally, we conducted a dedicated analysis limited to patients with LVEF >40% based on individual participant‐level data from the DELIVER trial in isolation at the full undiscounted and discounted Medicare costs of dapagliflozin. Model inputs specific to participant‐level data from DELIVER are listed in Table S1; all other model assumptions are consistent with those used in Table 1.
The Markov model was created and run using DATA TreeAge Pro 2021 (Williamstown, MA) and statistical analysis using STATA version 16.1 (StataCorp, College Station, TX).
RESULTS
Model Validation
In the combined DAPA‐HF and DELIVER US populations (N=1006), median (interquartile range) age was 71 (64–77) years, 32% were women, and 18% were Black individuals. Median LVEF was 45% (30%–57%). There were 25.7 first and total HF hospitalizations or urgent visits and 10.1 deaths per 100 patient‐years in those allocated to placebo. In our model, we projected 25.7 HF hospitalizations or urgent visits and 10.4 deaths per 100 patient‐years, respectively. The modeled cohort was based on the combination of the DAPA‐HF and DELIVER populations. Median undiscounted survival without utility weighting was greater in those modeled to receive dapagliflozin in addition to standard of care (7.98 years) versus those modeled to receive standard of care alone (7.47 years). Patients experienced an average of 0.51 fewer worsening HF events over their lifetime with the addition of dapagliflozin to standard of care.
Cost Effectiveness Analysis
Using the full (undiscounted) Medicare Part D cost, treatment with standard of care alone in chronic HF across the EF spectrum was projected to generate 6.04 QALYs at a lifetime cost of $109 003. In contrast, treatment with dapagliflozin in addition to standard of care was projected to generate 6.57 QALYs at a lifetime cost of $154 512. Addition of dapagliflozin to standard of care resulted in an additional 0.53 QALYs gained at an incremental lifetime cost of $45 509 and an ICER of $85 554 per QALY gained. Using a discounted cost of $262.62/month reflecting a published estimate of a US‐based manufacturer discount/rebate of 49%, the incremental lifetime cost was $21 321, with a resultant ICER of $40 081 per QALY gained. Incremental costs and associated ICERs varied significantly across available cost estimates for dapagliflozin (Table S2). Stratification of the analysis by age, sex, and race had small effects on the ICERs. Assuming the full Medicare Part D cost, the ICERs for those aged ≥70 years versus those aged <70 years were $87 028/QALY and $84 043/QALY, respectively. Women had a higher ICER ($91 165/QALY) compared with men ($81 383/QALY). Black patients had a slightly lower ICER ($82 310/QALY) than White patients ($86 097/QALY).
The cost effectiveness of dapagliflozin in addition to standard of care was most sensitive to the cost of dapagliflozin and the effect of therapy on cardiovascular death at both the full (undiscounted) Medicare cost (Figure 2A) and the discounted cost (Figure 2B). Varying the cost of dapagliflozin at a 49% discount ($262.62/month) and a cost increase to $637.93/month from the full Medicare Part D cost yielded ICERs that ranged from $40 081 per QALY gained to $107 715 per QALY gained. Varying the benefit of therapy on cardiovascular death based on the lower and upper bounds of the 95% CIs of the pooled hazard ratio from 0.75 to 0.97 yielded ICERs that ranged from $58 666 per QALY gained to $205 969 per QALY gained. In a 2‐way sensitivity analysis, variation in the cost of dapagliflozin and the hazard ratio on cardiovascular death across each of the ranges of these values resulted in ICERs ranging from $29 691 to $262 472 per QALY gained (Table 2).
Figure 2. Tornado plot summarizing 1‐way deterministic sensitivity analysis for model parameters.
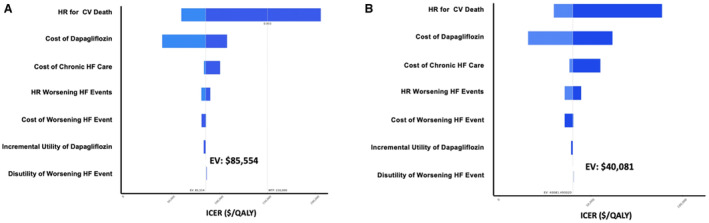
A, Model parameters were varied in this 1‐way sensitivity analysis across the ranges listed in Table 1 using the full (undiscounted) Medicare cost ($514.95/month). B, Dapagliflozin cost parameters were modeled from $125/month to $375/month using the discounted Medicare cost with 49% rebates ($262/month). All other model parameters were varied in this 1‐way sensitivity analysis across the ranges listed in Table 1. CV indicates cardiovascular; EV, expected value in base case; HF, heart failure; ICER, incremental cost effectiveness ratio; QALY, quality‐adjusted life year; and WTP, willingness‐to‐pay threshold.
Table 2.
Two‐Way Sensitivity Analysis of ICERs Varying Cost of Dapagliflozin and Hazard Ratio for Cardiovascular Death
| Hazard ratio for cardiovascular death | |||
|---|---|---|---|
| Monthly cost of dapagliflozin (US$) | 0.75 | 0.86 | 0.97 |
| $262.62 | $29 691* | $41 734* | $88 651† |
| $514.95 | $59 832† | $89 456† | $205 111‡ |
| $637.93 | $74 677† | $112 974† | $262 472‡ |
Value determinations made on the basis of the American College of Cardiology/American Heart Association cost and value descriptions: high value, <$50 000 per QALY gained; intermediate value, $50 000 to <$150 000 per QALY gained; and low‐value, ≥$150 000 per QALY gained. ICERs reported as $/QALYs gained. ICERs indicates incremental cost effectiveness ratios; and QALY, quality‐adjusted life year.
High economic value.
Intermediate economic value.
Low economic value.
Additional published estimates with and without expected rebates for the cost of dapagliflozin produced ICERs ranging from high to intermediate value (Figure 3). The addition of dapagliflozin to standard of care in the treatment of chronic HF would be of high value (ICER <$50 000 per QALY gained) at a monthly dapagliflozin cost below $317.66/month and of at least intermediate value (ICER <$150 000 per QALY gained) at a cost below $872.58/month. Treatment with dapagliflozin in chronic HF would be cost saving at a monthly cost below $40.22/month ($482.59 annually; Figure 3). The incremental cost effectiveness of dapagliflozin added to standard of care was less sensitive to other parameters, including varying hazard ratios and costs for worsening HF events at both the full (undiscounted) Medicare cost (Figure 2A) and the discounted cost (Figure 2B).
Figure 3. Incremental cost effectiveness at varying sources for monthly dapagliflozin cost.
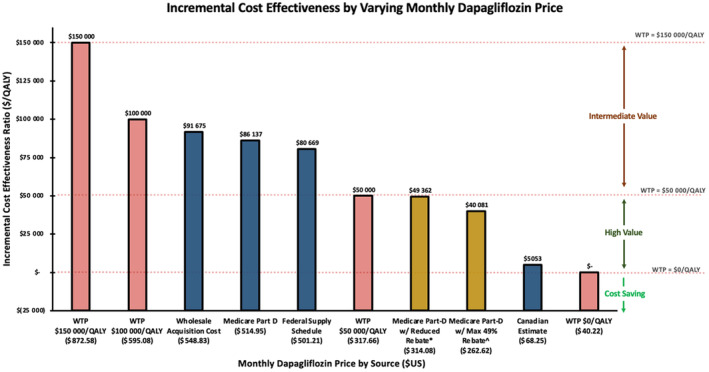
Blue bars indicate publicly available dapagliflozin costs. Yellow bars indicate potential costs with rebate amounts based on published data. Salmon bars indicate threshold costs. QALY indicates quality‐adjusted life year; and WTP, willingness‐to‐pay. *Based on published estimates of entities retaining 20% of rebate. 9 , 22 , 27 ^Based on a published estimate of 49% rebate. 9
In a probabilistic sensitivity analysis at the full (undiscounted) Medicare Part D cost, 95% of the values of the ICER for dapagliflozin in addition to standard of care occurred between $41 469 and $199 040 per QALY gained (Figure S1). Treatment with dapagliflozin was a preferred strategy at an ICER below $150 000 per QALY gained in 92% of simulations (Figure 4). Using a discounted cost of dapagliflozin ($262/month), treatment with dapagliflozin was a preferred strategy at an ICER below $150 000 per QALY gained in >99% simulations and of high value (<$50 000 per QALY gained) in 68% of simulations (Figures S2 and S3).
Figure 4. Cost‐effectiveness acceptability curve based on probabilistic sensitivity analysis.
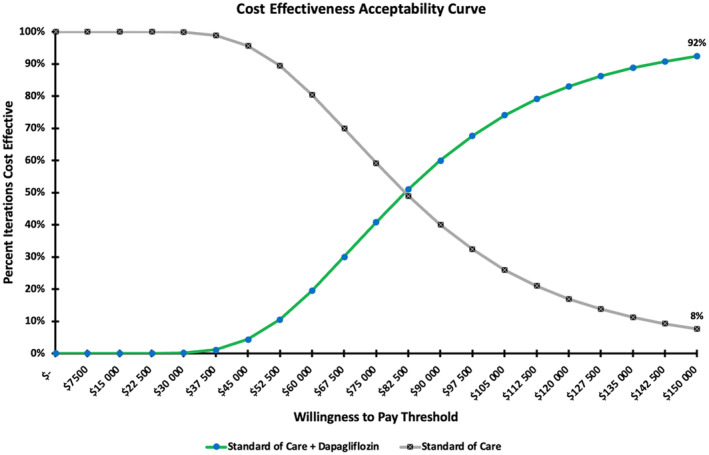
All model parameters were independently varied across their distributions in a probabilistic sensitivity analysis for 100 000 iterations using the full (undiscounted) Medicare cost. The percentage of iterations that were cost effective is plotted across various willingness‐to‐pay thresholds.
When limited to patients with mildly reduced and preserved EF modeled using participant‐level data from DELIVER (LVEF >40%), treatment with standard of care alone was projected to produce 6.17 QALYs and treatment with the addition of dapagliflozin was projected to produce 6.57 QALYs. Lifetime costs of standard of care alone were $111 561; lifetime costs with the addition of dapagliflozin were $155 622 using the undiscounted Medicare Part D cost and $131 420 using a discounted Medicare cost with 49% rebate, yielding ICERs of $108 066 per QALY gained and $48 707 per QALY gained, respectively.
DISCUSSION
In this updated cost effectiveness analysis based on model inputs from pooled individual participant‐level data from DELIVER and DAPA‐HF, we found that the addition of dapagliflozin to standard of care in patients with HF across the full spectrum of EF was of intermediate value on the basis of the (undiscounted) Medicare Part D cost and of high value when modeling a published nearly 50% rebate. Cost effectiveness was highly sensitive to drug prices. These data contextualize the expected cost effectiveness of dapagliflozin across the spectrum of EF and across various costs of therapy.
Previous cost effectiveness analyses of SGLT2is have determined the potential intermediate value of adding dapagliflozin to standard of care in HF with reduced EF. 5 , 6 Isaza et al 6 found that dapagliflozin was associated with an ICER of $68 300 per QALY gained in a simulated cohort of middle‐aged or older US adults using a Federal Supply Schedule Big Four cost, while Parizo et al 5 found an ICER of $83 650 per QALY gained using an undiscounted Medicare Part D drug payment cost. In addition, data from the EMPEROR‐Preserved (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction) trial and pooled data across preserved EF suggests that the SGLT2i empagliflozin was of low economic value. 7 A subsequent pooled trial level analysis from DELIVER and EMPEROR‐Preserved suggested SGLT2is to be of low to moderate economic value in this segment of the HF population. 38 While EMPEROR‐Preserved demonstrated the potential for attenuation in the clinical and QoL benefits of this therapy at the higher level of LVEF, 39 no such attenuation was observed with dapagliflozin. 3 , 40 In fact, a dedicated, patient‐level pooled analysis of the DAPA‐HF and DELIVER trials found consistent benefits of treatment on cardiovascular death and HF events across the full spectrum of EF; our analysis suggests that cost effectiveness of dapagliflozin treatment in patients with mildly reduced and preserved EFs is similar to that of reduced EF. In addition, these previous economic evaluations were based on published trial estimates alone, while access to participant‐level data in our cost effectiveness analysis facilitated more granular modeling of risk estimates, and pooling data from 2 large clinical trials improved precision of treatment effects, including the effect of therapy on cardiovascular death. Prior analyses evaluated the effects of therapy on reducing first HF hospitalization; the effect on the total (first and recurrent) HF hospitalizations as employed in this analysis may more appropriately reflect the disease burden. Finally, because of the consensus recommendations supporting use of this therapy given across the full spectrum of EF, evaluating cost effectiveness of this eligible population is of high clinical importance. To our knowledge, this is first report of a rigorous economic evaluation of SGLT2is inclusive of the full spectrum of LVEF.
Similar to prior analyses, we found cost effectiveness to be highly sensitive to drug costs, leading to results that are from high value to intermediate value. We used an undiscounted Medicare Part D cost and a published 49% US rebate for 1‐way deterministic and probabilistic sensitivity analyses to reflect a range of potential value of this therapy in the United States; however, true cost may vary significantly across payers and on the basis of the availability of manufacturer rebates. A recent study found that after accounting for manufacturer rebates, net costs of SGLT2is are considerably lower than list costs and have generally declined over time. 41 Additional discounts, manufacturer rebates, and cost reductions following patent expiration for dapagliflozin may result in greater value of this therapy than our model reported. Recent legislation may, over time, further reduce the cost of therapy among Medicare beneficiaries, improving the value of this therapy. Based on our modeling, a dapagliflozin cost of <$4000 annually would yield this therapy to be of high value and <$500 annually as cost saving among this broad chronic HF population irrespective of EF.
Variations in cost estimates are especially important for economic analyses in the United States; unlike in Canada and Europe, no single per‐unit cost of a drug published by a national payer exists. Given the impact the cost of dapagliflozin and other similar medications or interventions have on the results of health economic evaluations, there has been a significant demand for improved cost transparency in medications and other aspects of the health care delivery system. 27 The US system of various private pharmaceutical manufacturers; pharmacy benefit managers; commercial, self‐, and public payers; and pharmacies all involved in the competitive delivery of medications encourages rebates and other transactions that can inhibit transparency. Proponents of rebates argue that they are helpful in reducing overall costs, and opponents say they may serve to shift costs to payers such as Medicare or to patients. It is possible that both arguments may be correct; however, such a system limits the evaluation and interpretation of value results in the absence of a published true cost of medications consistent across all users. Finally, the Inflation Reduction Act recently signed into law on August 16, 2022, has implications for the cost of drugs covered by Medicare Part D; for the first time, the Centers for Medicare and Medicaid Services will be allowed to negotiate costs with manufacturers for a select number of medications, with the expectation that some costs will be reduced. In fact, dapagliflozin has been selected as 1 of the first 10 drugs covered under Medicare Part B for price negotiation under the Inflation Reduction Act, with negotiations occurring with manufacturers in 2023 and 2024, and expected negotiated price effective dates in 2026. These policies may have the potential consequences of improving cost effectiveness estimates as compared with traditionally publicly reported prices. These policy changes underscore the importance of understanding the impact of true medication costs on cost effectiveness is critical for policymakers, guideline committees, 10 and payers alike.
Therefore, in the absence of reforms that can yield more clarity on drug costs in the short term, we recommend that economic evaluations consider reporting a broad range of cost effectiveness ratios including published costs and important willingness‐to‐pay thresholds instead of relying on a single source of costs. This will allow payers and medical associations to assess whether the therapy is of high value if they know the cost they or their patients are facing. In the long term, it will be necessary for regulators of the public sector to understand the costs as they negotiate under a new legal framework.
The prevalence of HF with mildly reduced or preserved EF is poised to grow with population aging, accelerating cardiometabolic risk factor burden, and increased awareness and detection. While patients in this EF range experience lower risks of death, these patients carry a high burden of symptoms and face similar heightened longitudinal risks of HF events. This analysis, including the dedicated cost effectiveness assessment in patients with mildly reduced and preserved EF, suggests similar value of this therapy across the full range of EF, rather than the potential attenuation of benefit and economic value seen with other HF therapies. Together, these factors result in a high lifetime burden of worsening HF events and adverse health status, which have important attendant cost implications. This updated cost effectiveness analysis covers this growing patient segment and may inform coverage decisions for public payers such as Medicare that disproportionately insure an older multimorbid population.
Our analysis included the effects of dapagliflozin on total HF hospitalizations and death outcomes; however, SGTL2is may have broader effects across other important domains. Data from prior trials have demonstrated that this class of medications may reduce adverse kidney outcomes, slow kidney function decline, and prevent progression to end‐stage kidney disease. 42 , 43 , 44 In addition, among patients without diabetes, treatment with dapagliflozin may reduce the incidence of new diabetes diagnoses 45 , 46 ; patients with diabetes have been shown in prior analyses to have higher event rates and associated costs. 5 , 6 Among patients with diabetes, treatment with SGLT2is may reduce the need for insulin initiation or dose escalation and avoid the resultant costs of this therapy. 47 Additional modeling of these potential broader beneficial effects of dapagliflozin on non–HF‐related events may further improve the overall value of this therapy.
Limitations
This study has important limitations that should be acknowledged. First, the efficacy and safety of dapagliflozin were modeled from 2 large, global, randomized clinical trials; differences between the trial populations and usual care populations in the United States might affect the true cost effectiveness of this treatment in clinical practice. However, to improve the applicability of our modeling, we derived event estimates based on participants enrolled across US sites in both clinical trials. Population estimates may differ based on baseline differences and changes over time in demographics, clinical characteristics, and treatments received (eg, greater or lesser adjunctive guideline‐directed medical therapy) for patients with HF. Second, we did not directly model the impact of treatment‐related adverse events; however, several trials of SGLT2is have now demonstrated the safety of this class, with similar treatment‐related adverse events to placebo. 17 , 48 , 49 , 50 , 51 , 52 Third, our model did not consider variable adherence to treatment; suboptimal adherence in clinical practice might limit the survival and QoL benefits this treatment would otherwise offer and may reduce its value. However, we did forecast treatment estimates derived from intention‐to‐treat analyses, which accounts for drug discontinuations observed during the trials. Fourth, we were unable to model the potential benefits of dapagliflozin on preventing incident diabetes, as was done in prior cost effectiveness analysis in reduced EF, 6 as glycated hemoglobin values were not collected in follow‐up in DELIVER; therefore, our model may have underestimated the health benefits of SGLT2i therapy in patients with HF without established diabetes. Fifth, <10% of all participants across DAPA‐HF and DELIVER were treated with sacubitril/valsartan, despite guideline support for this therapy across the EF spectrum 53 ; however, there is no reported heterogeneity in the treatment benefit of dapagliflozin according to baseline sacubitril/valsartan use. 54 More prevalent background HF therapy may reduce observed event rates and associated costs but would be expected to affect dapagliflozin and standard‐of‐care groups similarly. Sixth, we applied the QoL penalty equally to an HF hospitalization or urgent HF visit as >90% of events were HF hospitalizations; smaller QoL penalties were modeled in sensitivity analyses. Seventh, we used a previously modeled approach to convert Kansas City Cardiomyopathy Questionnaire overall summary scores to health‐related QoL measures 6 , 30 , 31 ; bias in this conversion may have affected the estimates for QALYs gained but would be expected to affect both groups (dapagliflozin and standard of care) similarly. Finally, alternative modeling approaches to that of the Markov model used may provide differing cost effectiveness estimates.
CONCLUSIONS
Cost effectiveness modeling suggests that dapagliflozin, in addition to standard of care in patients with HF across the spectrum of EF, would increase QALYs at an ICER consistent with at least intermediate value at an undiscounted Medicare cost and potentially an ICER higher value based on the level of discount or price negotiation offerings available.
Sources of Funding
This study was supported by a grant from AstraZeneca to Brigham and Women's Hospital (Boston, MA).
Disclosures
Dr Bhatt has received research grant support to his institution from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute; the NIH/National Institute on Aging; the American College of Cardiology Foundation; and the Centers for Disease Control and Prevention, and has received consulting fees from Novo Nordisk and Sanofi. Dr Vaduganathan has received research grant support, served on advisory boards, or had speaker engagements with American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Chiesi, Cytokinetics, Lexicon Pharmaceuticals, Merck, Novartis, Novo Nordisk, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health, and participates on clinical trial committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics. Dr Claggett has received consulting fees from Amgen, Cardurion, Corvia, and Novartis. Dr Anand reported receiving personal fees from AstraZeneca during the conduct of the study and personal fees from Amgen, ARCA, Boston Scientific Corporation, Boehringer Ingelheim, LivaNova, and Zensun outside the submitted work. Dr Desai reports institutional grant support from Abbott, Alnylam, AstraZeneca, Bayer, Novartis and Consulting Fees from Abbott, Alnylam, AstraZeneca, Avidity, Axon Therapeutics, Bayer, Biofourmis, Boston Scientific, Cytokinetics, GlaxoSmithKline, Merck, Novartis, Parxel, Regeneron, Roche, and Verily. Dr Fang has received research grants from the NIH; has consulted with Novartis, Amgen, AstraZeneca, Boerhinger‐Ingelheim/Lilly, Abbott, Capricor, Windtree, and LabCorp; and has provided support to the American Heart Association, NIH, Heart Failure Society of America, and Heart Rhythm Society. Dr Hernandez has received research grants from American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Merck, Novartis, Somologic, and Verily; and has served as a consultant or on the Advisory Board for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cytokinetics, Eidos, Intercept, Merck, and Novartis. Dr Jhund's employer has been remunerated for his work on the DELIVER and DAPA‐HF trials by AstraZeneca; consulting and speakers' fees from Novartis, AstraZeneca, and Boheringer Ingelheim; research funding from Boehringer Ingelheim; and remuneration for clinical trial work from NovoNordisk and Bayer. Dr Kosiborod has received research grant support from AstraZeneca and Boehringer Ingelheim; has served as a consultant or on an advisory board for Alnylam, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Esperion Therapeutics, Janssen, Lexicon, Merck (Diabetes and Cardiovascular), Novo Nordisk, Sanofi, Pharmacosmos, and Vifor Pharma; has received other research support from AstraZeneca; and has received honoraria from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk. Dr Sabatine has received research grant support through Brigham and Women's Hospital from Abbott, Amgen, Anthos Therapeutics, AstraZeneca, Daiichi‐Sankyo, Eisai, Intarcia, Ionis, Medicines Company, MedImmune, Merck, Novartis, and Pfizer; and consulted for Althera, Amgen, Anthos Therapeutics, AstraZeneca, Beren Therapeutics, Bristol‐Myers Squibb, Fibrogen, Intarcia, Merck, Moderna, Novo Nordisk, and Silence Therapeutics. Additionally, Dr Sabatine is a member of the Thrombolysis in Myocardial Infarction Study Group, which has also received institutional research grant support through Brigham and Women's Hospital from ARCA Biopharma, Inc.; Janssen Research and Development, LLC; Siemens Healthcare Diagnostics, Inc.; Softcell Medical Limited; Regeneron; Roche; and Zora Biosciences. Dr Shah has received research grants from the NIH (U54 HL160273, R01 HL107577, R01 HL127028, R01 HL140731, R01 HL149423), Actelion, AstraZeneca, Corvia, Novartis, and Pfizer; and has received consulting fees from Abbott, Actelion, AstraZeneca, Amgen, Aria CV, Axon Therapies, Bayer, Boehringer‐Ingelheim, Boston Scientific, Bristol‐Myers Squibb, Cardiora, Coridea, CVRx, Cyclerion, Cytokinetics, Edwards Lifesciences, Eidos, Eisai, Imara, Impulse Dynamics, GSK, Intellia, Ionis, Ironwood, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sanofi, Sardocor, Shifamed, Tenax, Tenaya, and United Therapeutics. Dr Vardeny has received institutional research support for DELIVER from AstraZeneca and has received institutional research support from Bayer. Dr McMurray has received payments through Glasgow University for work on clinical trials, consulting, and other activities from Alnylam, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardurion, Cytokinetics, Dal‐Cor, GSK, Ionis, KBP Biosciences, Novartis, Pfizer, and Theracos; personal lecture fees from the Corpus, Abbott, Hikma, Sun Pharmaceuticals, Medscape/Heart.Org, Radcliffe Cardiology, Servier Director, and Global Clinical Trial Partners. Dr Solomon has received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Mesoblast, MyoKardia, NIH/National Heart, Lung, and Blood Institute, Neurotronik, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos, and US2.AI; and has consulted for Abbott, Action, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boeringer‐Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi‐Sankyo, GSK, Lilly, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi‐Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, Sarepta, Lexicon, Anacardio, and Akros. Dr Gaziano reported receiving research grant support from AstraZeneca during the conduct of the study; receiving personal fees from Multiple Labs and Novartis; and receiving research support from the NIH outside the submitted work.
Supporting information
Tables S1–S2
Figures S1–S3
This manuscript was sent to Sula Mazimba, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.032279
For Sources of Funding and Disclosures, see page 10.
References
- 1. Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, et al. SGLT‐2 inhibitors in patients with heart failure: a comprehensive meta‐analysis of five randomised controlled trials. Lancet. 2022;400:757–767. doi: 10.1016/S0140-6736(22)01429-5 [DOI] [PubMed] [Google Scholar]
- 2. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–1098. doi: 10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 3. Jhund PS, Kondo T, Butt JH, Docherty KF, Claggett BL, Desai AS, Vaduganathan M, Gasparyan SB, Bengtsson O, Lindholm D, et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: a patient‐level, pooled meta‐analysis of DAPA‐HF and DELIVER. Nat Med. 2022;28:1956–1964. doi: 10.1038/s41591-022-01971-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 5. Parizo JT, Goldhaber‐Fiebert JD, Salomon JA, Khush KK, Spertus JA, Heidenreich PA, Sandhu AT. Cost‐effectiveness of dapagliflozin for treatment of patients with heart failure with reduced ejection fraction. JAMA Cardiol. 2021;6:926–935. doi: 10.1001/jamacardio.2021.1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Isaza N, Calvachi P, Raber I, Liu C‐L, Bellows BK, Hernandez I, Shen C, Gavin MC, Garan AR, Kazi DS. Cost‐effectiveness of dapagliflozin for the treatment of heart failure with reduced ejection fraction. JAMA Netw Open. 2021;4:e2114501. doi: 10.1001/jamanetworkopen.2021.14501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zheng J, Parizo JT, Spertus JA, Heidenreich PA, Sandhu AT. Cost‐effectiveness of empagliflozin in patients with heart failure with preserved ejection fraction. JAMA Intern Med. 2022;182:1278–1288. doi: 10.1001/jamainternmed.2022.5010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Levy JF, Meek PD, Rosenberg MA. US‐based drug cost parameter estimation for economic evaluations. Med Decis Making. 2015;35:622–632. doi: 10.1177/0272989X14563987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ding YAO, Shi L, Lin Y, Shao HUI. 132‐OR: trends in total payments, net payments, and rebates for a 30‐day supply of glucose‐lowering drugs (GLDs) from 2010 to 2017. Diabetes. 2022;71(Suppl 1):132‐OR. doi: 10.2337/db22-132-OR [DOI] [Google Scholar]
- 10. Ostrominski JW, Hirji S, Bhatt AS, Butler J, Fiuzat M, Fonarow GC, Heidenreich PA, Januzzi JL, Lam CSP, Maddox TM, et al. Cost and value in contemporary heart failure clinical guidance documents. JACC Heart Fail. 2022;10:1–11. doi: 10.1016/j.jchf.2021.08.002 [DOI] [PubMed] [Google Scholar]
- 11. McMurray JJV, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, et al. DAPA‐HF committees and investigators. A trial to evaluate the effect of the sodium‐glucose co‐transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA‐HF). Eur J Heart Fail. 2019;21:665–675. doi: 10.1002/ejhf.1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Solomon SD, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Lindholm D, et al. Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: rationale and design of the DELIVER trial. Eur J Heart Fail. 2021;23:1217–1225. doi: 10.1002/ejhf.2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Claggett B, Pocock S, Wei LJ, Pfeffer MA, McMurray JJV, Solomon SD. Comparison of time‐to‐first event and recurrent‐event methods in randomized clinical trials. Circulation. 2018;138:570–577. doi: 10.1161/CIRCULATIONAHA.117.033065 [DOI] [PubMed] [Google Scholar]
- 14. Claggett BL, McCaw ZR, Tian L, McMurray JJV, Jhund PS, Uno H, Pfeffer MA, Solomon SD, Wei L‐J. Quantifying treatment effects in trials with multiple event‐time outcomes. NEJM Evid. 2022;1. doi: 10.1056/evidoa2200047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Belohlávek J, Böhm M, Chiang C‐E, Chopra VK, de Boer RA, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. 2020;323:1353–1368. doi: 10.1001/jama.2020.1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anker SD, Butler J, Filippatos G, Khan MS, Marx N, Lam CSP, Schnaidt S, Ofstad AP, Brueckmann M, Jamal W, et al. Effect of empagliflozin on cardiovascular and renal outcomes in patients with heart failure by baseline diabetes status: results from the EMPEROR‐reduced trial. Circulation. 2021;143:337–349. doi: 10.1161/CIRCULATIONAHA.120.051824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner‐La Rocca H‐P, Choi D‐J, Chopra V, Chuquiure‐Valenzuela E, et al; EMPEROR‐Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 18. Urbich M, Globe G, Pantiri K, Heisen M, Bennison C, Wirtz HS, Di Tanna GL. A systematic review of medical costs associated with heart failure in the USA (2014–2020). Pharmacoeconomics. 2020;38:1219–1236. doi: 10.1007/s40273-020-00952-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kilgore M, Patel HK, Kielhorn A, Maya JF, Sharma P. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy. 2017;10:63–70. doi: 10.2147/RMHP.S130341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Sullivan AK, Rubin J, Nyambose J, Kuznik A, Cohen DJ, Thompson D. Cost estimation of cardiovascular disease events in the US. Pharmacoeconomics. 2011;29:693–704. doi: 10.2165/11584620-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 21. Krawzik K, Schmidt A. 2023 DRG Expert. Volume 1 & 2. Optum; 2023. https://www.optumcoding.com/product/60739/. Accessed June 21, 2023.
- 22. Dieguez G, Alston M, Tomicki S. A primer on prescription drug rebates: insights into why rebates are a target for reducing prices. Milliman. 2018. https://www.milliman.com/en/insight/a‐primer‐on‐prescription‐drug‐rebates‐insights‐into‐why‐rebates‐are‐a‐target‐for‐reducing. Accessed June 21, 2023.
- 23. Urahn SK, Coukell A, Reynolds I, Chester A. The prescription drug landscape, explored. The PEW Charitable Trusts. 2019. https://www.pewtrusts.org/‐/media/assets/2019/03/the_prescription_drug_landscape‐explored.pdf. Accessed July 21, 2023.
- 24. Medicare Part D spending by drug . Centers for Medicare & Medicaid Services. 2022. https://data.cms.gov/summary‐statistics‐on‐use‐and‐payments/medicare‐medicaid‐spending‐by‐drug/medicare‐part‐d‐spending‐by‐drug/data. Accessed June 21, 2023.
- 25. Micromedex RED BOOK. IBM Watson Health. 2022. https://www.ibm.com/products/micromedex‐red‐book. Accessed June 21, 2023.
- 26. Pharmaceutical prices. Office of Procurement, Acquisition and Logistics (OPAL). U.S. Department of Veterans Affairs. 2022. https://www.va.gov/opal/nac/fss/pharmPrices.asp Accessed June 21, 2023.
- 27. Dusetzina SB, Conti RM, Yu NL, Bach PB. Association of prescription drug price rebates in Medicare Part D with patient out‐of‐pocket and federal spending. JAMA Intern Med. 2017;177:1185–1188. doi: 10.1001/jamainternmed.2017.1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. CADTH Canadian Drug Expert Committee Recommendation: Dapagliflozin (Forxiga—AstraZeneca Canada Inc.). Indication: in Adults, as an Adjunct to Standard of Care Therapy, for the Treatment of Heart Failure with Reduced Ejection Fraction (HFrEF) to Reduce the Risk of Cardiovascular Death, Hospitalization for Heart Failure (HF) and Urgent HF Visit. Canadian Agency for Drugs and Technologies in Health; 2021. https://www.ncbi.nlm.nih.gov/books/NBK572048/. Accessed June 21, 2023. [PubMed] [Google Scholar]
- 29. Formulary search. Ontario Drug Benefit Formulary/Comparative Drug Index. 2022. https://www.formulary.health.gov.on.ca/formulary/. Accessed June 21, 2023.
- 30. Kazi DS, Bellows BK, Baron SJ, Shen C, Cohen DJ, Spertus JA, Yeh RW, Arnold SV, Sperry BW, Maurer MS, et al. Cost‐effectiveness of tafamidis therapy for transthyretin amyloid cardiomyopathy. Circulation. 2020;141:1214–1224. doi: 10.1161/CIRCULATIONAHA.119.045093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, et al. Cardiovascular outcomes research consortium. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150:707–715. doi: 10.1016/j.ahj.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 32. Hunink MGM, Weinstein MC, Wittenberg E, Drummond MF, Pliskin JS, Wong JB, Glasziou PP. Decision Making in Health and Medicine: Integrating Evidence and Values. Cambridge University Press; 2014. doi: 10.1017/CBO9781139506779 [DOI] [Google Scholar]
- 33. Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost‐effectiveness in health and medicine. JAMA. 1996;276:1253–1258. doi: 10.1001/jama.1996.03540150055031 [DOI] [PubMed] [Google Scholar]
- 34. Gold MR, Siegel JE, Russell LB, Weinstein MC. Theoretical Foundations of Cost‐Effectiveness Analysis. Cost‐Effectiveness in Health and Medicine. Oxford, UK: Oxford University Press. 1996. [Google Scholar]
- 35. Gaziano TA, Fonarow GC, Velazquez EJ, Morrow DA, Braunwald E, Solomon SD. Cost‐effectiveness of sacubitril‐valsartan in hospitalized patients who have heart failure with reduced ejection fraction. JAMA Cardiol. 2020;5:1236–1244. doi: 10.1001/jamacardio.2020.2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dunlay SM, Shah ND, Shi Q, Morlan B, VanHouten H, Long KH, Roger VL. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes. 2011;4:68–75. doi: 10.1161/CIRCOUTCOMES.110.957225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, Halperin JL, Hlatky MA, Jacobs AK, Mark DB, et al; ACC/AHA Task Force on Performance Measures; ACC/AHA Task Force on Practice Guidelines. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129:2329–2345. doi: 10.1161/CIR.0000000000000042 [DOI] [PubMed] [Google Scholar]
- 38. Cohen LP, Isaza N, Hernandez I, Lewis GD, Ho JE, Fonarow GC, Kazi DS, Bellows BK. Cost‐effectiveness of sodium‐glucose cotransporter‐2 inhibitors for the treatment of heart failure with preserved ejection fraction. JAMA Cardiol. 2023;8:419–428. doi: 10.1001/jamacardio.2023.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Butler J, Packer M, Filippatos G, Ferreira JP, Zeller C, Schnee J, Brueckmann M, Pocock SJ, Zannad F, Anker SD. Effect of empagliflozin in patients with heart failure across the spectrum of left ventricular ejection fraction. Eur Heart J. 2022;43:416–426. doi: 10.1093/eurheartj/ehab798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bhatt AS, Kosiborod MN, Vaduganathan M, Claggett BL, Miao ZM, Kulac IJ, Lam CSP, Hernandez AF, Martinez F, Inzucchi SE, et al. Effect of dapagliflozin on health status and quality of life across the spectrum of ejection fraction: participant‐level pooled analysis from the DAPA‐HF and DELIVER trials. Eur J Heart Fail. 2023;25:981–988. doi: 10.1002/ejhf.2909 [DOI] [PubMed] [Google Scholar]
- 41. Sarpatwari A, Tessema FA, Zakarian M, Najafzadeh MN, Kesselheim AS. Diabetes drugs: list price increases were not always reflected in net price; impact of brand competition unclear. Health Aff (Millwood). 2021;40:772–778. doi: 10.1377/hlthaff.2020.01436 [DOI] [PubMed] [Google Scholar]
- 42. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, et al. CREDENCE trial investigatorsCanagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 43. The EMPA‐KIDNEY Collaborative Group ; Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, Emberson JR, Preiss D, Judge P, Mayne KJ, Ng SYA, et al. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2023;388:117–127. doi: 10.1056/NEJMoa2204233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heerspink HJL, Stefánsson BV, Correa‐Rotter R, Chertow GM, Greene T, Hou F‐F, Mann JFE, McMurray JJV, Lindberg M, Rossing P, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 45. Inzucchi SE, Docherty KF, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, Verma S, Bělohlávek J, et al. Dapagliflozin and the incidence of type 2 diabetes in patients with heart failure and reduced ejection fraction: an exploratory analysis from DAPA‐HF. Diabetes Care. 2021;44:586–594. doi: 10.2337/dc20-1675 [DOI] [PubMed] [Google Scholar]
- 46. Rossing P, Inzucchi SE, Vart P, Jongs N, Docherty KF, Jhund PS, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, et al. Dapagliflozin and new‐onset type 2 diabetes in patients with chronic kidney disease or heart failure: pooled analysis of the DAPA‐CKD and DAPA‐HF trials. Lancet Diabetes Endocrinol. 2022;10:24–34. doi: 10.1016/S2213-8587(21)00295-3 [DOI] [PubMed] [Google Scholar]
- 47. Vaduganathan M, Inzucchi SE, Sattar N, Fitchett DH, Ofstad AP, Brueckmann M, George JT, Verma S, Mattheus M, Wanner C, et al. Effects of empagliflozin on insulin initiation or intensification in patients with type 2 diabetes and cardiovascular disease: findings from the EMPA‐REG OUTCOME trial. Diabetes Obes Metab. 2021;23:2775–2784. doi: 10.1111/dom.14535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 49. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 50. Nassif ME, Windsor SL, Tang F, Khariton Y, Husain M, Inzucchi SE, McGuire DK, Pitt B, Scirica BM, Austin B, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE‐HF trial. Circulation. 2019;140:1463–1476. doi: 10.1161/CIRCULATIONAHA.119.042929 [DOI] [PubMed] [Google Scholar]
- 51. Nassif ME, Windsor SL, Borlaug BA, Kitzman DW, Shah SJ, Tang F, Khariton Y, Malik AO, Khumri T, Umpierrez G, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. 2021;27:1954–1960. doi: 10.1038/s41591-021-01536-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kosiborod MN, Angermann CE, Collins SP, Teerlink JR, Ponikowski P, Biegus J, Comin‐Colet J, Ferreira JP, Mentz RJ, Nassif ME, et al. Effects of empagliflozin on symptoms, physical limitations, and quality of life in patients hospitalized for acute heart failure: results from the EMPULSE trial. Circulation. 2022;146:279–288. doi: 10.1161/CIRCULATIONAHA.122.059725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Solomon SD, Vaduganathan M, Claggett BL, Packer M, Zile M, Swedberg K, Rouleau J, Pfeffer MA, Desai A, Lund LH, et al. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation. 2020;141:352–361. doi: 10.1161/CIRCULATIONAHA.119.044586 [DOI] [PubMed] [Google Scholar]
- 54. Solomon SD, Jhund PS, Claggett BL, Dewan P, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Inzucchi SE, et al. Effect of dapagliflozin in patients with HFrEF treated with sacubitril/valsartan: the DAPA‐HF trial. JACC Heart Fail. 2020;8:811–818. doi: 10.1016/j.jchf.2020.04.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S3


