Abstract
Background
Genetic‐guided pharmacotherapy (PGx) is not recommended in clinical guidelines for coronary artery disease (CAD). We aimed to examine the extent and quality of evidence from economic evaluations of PGx in CAD and to identify variables influential in changing conclusions on cost‐effectiveness.
Methods and Results
From systematic searches across 6 databases, 2 independent reviewers screened, included, and rated the methodological quality of economic evaluations of PGx testing to guide pharmacotherapy for patients with CAD. Of 35 economic evaluations included, most were model‐based cost‐utility analyses alone, or alongside cost‐effectiveness analyses of PGx testing to stratify patients into antiplatelets (25/35), statins (2/35), pain killers (1/35), or angiotensin‐converting enzyme inhibitors (1/35) to predict CAD risk (8/35) or to determine the coumadin doses (1/35). To stratify patients into antiplatelets (96/151 comparisons with complete findings of PGx versus non‐PGx), PGx was more effective and more costly than non‐PGx clopidogrel (28/43) but less costly than non‐PGx prasugrel (10/15) and less costly and less effective than non‐PGx ticagrelor (22/25). To predict CAD risk (51/151 comparisons), PGx using genetic risk scores was more effective and less costly than clinical risk score (13/17) but more costly than no risk score (16/19) or no treatment (9/9). The remaining comparisons were too few to observe any trend. Mortality risk was the most common variable (47/294) changing conclusions.
Conclusions
Economic evaluations to date found PGx to stratify patients with CAD into antiplatelets or to predict CAD risk to be cost‐effective, but findings varied based on the non‐PGx comparators, underscoring the importance of considering local practice in deciding whether to adopt PGx.
Keywords: cardiovascular disease, coronary artery disease, economic evaluation, genetic testing, pharmacogenetics, systematic review
Subject Categories: Precision Medicine, Cost-Effectiveness, Chronic Ischemic Heart Disease
Nonstandard Abbreviations and Acronyms
- EE
economic evaluation
- PGx
genetic‐guided pharmacotherapy
- WTP
willingness‐to‐pay
Clinical Perspective.
What Is New?
Our review found 3 main approaches of genetic‐guided pharmacotherapy (PGx) for coronary artery disease with economic evidence: (1) stratifying patients into different medications (antiplatelets, statins, pain killers, angiotensin‐converting enzyme inhibitors), (2) predicting risk of coronary artery disease followed by prescription of statins alone or with antihypertensives, (3) selecting the doses of coumadin.
We found that (1) whether a PGx is cost‐effective depends on the comparator, with most evidence available for using CYP2C19 testing to stratify patients into antiplatelets, and (2) baseline/relative risk of mortality, cost of antiplatelets, and cost of genetic testing are among variables affecting findings on cost‐effectiveness.
What Are the Clinical Implications?
In stratifying patients into antiplatelets based on CYP2C19 testing, our findings suggest that the implementation of PGx should consider the current practice: in jurisdictions where clopidogrel is the standard care, PGx based on CYP2C19 status is most likely more costly, more effective, and also cost‐effective; in jurisdictions where prasugrel is the standard care, PGx is most likely less costly and more effective (cost saving); and for ticagrelor, PGx is most likely less costly, less effective, and not cost‐effective.
Coronary artery disease (CAD) poses significant burden to health systems and incurs large economic cost. 1 , 2 , 3 To alleviate the symptoms and to prevent the adverse events, patients with CAD may undergo percutaneous coronary intervention to open the coronary arteries and receive pharmacotherapy such as dual antiplatelets (aspirin with a P2Y12‐inhibitor for up to a year) to prevent blood clots, coumadin to prevent blood clots among those with atrial fibrillation (AF), statins to lower cholesterol, angiotensin‐converting enzyme inhibitors to control blood pressure, or beta blockers to control heart rate. Those at risk of CAD may also receive pharmacotherapy to prevent CAD from developing. 4 , 5 , 6
The role of genes in predisposing to CAD and in modifying response to CAD pharmacotherapy makes genetic testing a practical tool to optimize CAD pharmacotherapy. Specifically, identifying gene variants associated with higher risk of CAD (eg, rs17114036 or rs11206510) 7 , 8 may inform primary prevention of CAD. Although the effect of each individual gene variant is small, the effects of multiple genes may be combined to generate risk scores to identify those at high risk. 9 Identifying gene variants that modify responses to CAD pharmacotherapy may also influence the choice of alternative medications or dosing regimen. For instance, CYP2C19 loss‐of‐function (LOF) gene variants (CYP2C19*2, CYP2C19*3) reduce the effects of clopidogrel (CYP2C19 encodes cytochrome P450, the enzyme responsible for metabolizing many commonly prescribed medicines, including clopidogrel), 10 , 11 a common P2Y12‐inhibitor antiplatelet (P2Y12 receptors play a central role in platelet function) prescribed alongside aspirin after percutaneous coronary intervention. Instead of clopidogrel, carriers of CYP2C19 LOF genes may receive prasugrel or ticagrelor, both P2Y12‐inhibitors that confer higher bleeding risk but are not susceptible to the effect of CYP2C19 LOF genes. These genes may be tested in combination with each other, or with a related test, such as the platelet reactivity test, which identifies patients with high on‐treatment platelet reactivity under clopidogrel who may then be prescribed an alternative P2Y12‐inhibitor. 12
Evidence from randomized controlled trials suggests that testing genes could play a part in preventing adverse outcomes due to CAD (eg, myocardial infarction) or due to the pharmacotherapy (eg, bleeding caused by antiplatelets). For instance, a meta‐analysis of randomized controlled trials 13 shows CYP2C19‐guided antiplatelet selection may reduce the risk of major adverse cardiovascular events and myocardial infarction, with similar bleeding risk as clopidogrel alone. The growing clinical evidence prompts the Clinical Pharmacogenetics Implementation Consortium to periodically updates its guidelines 14 , 15 , 16 to assist clinicians in interpreting the findings and in implementing genetic‐guided prescribing.
Although the current clinical evidence is not strong enough to recommend genetic tests as usual care, 4 , 5 , 6 better understanding of genes, decreasing cost, 17 and wider availability of genetic testing have spurred interests in economic evaluations (EEs) to examine whether genetic‐guided pharmacotherapy (PGx) could be cost‐effective for CAD. To date, the extent and quality of the evidence on cost‐effectiveness has not been investigated systematically. Although 2 recent systematic reviews of economic evaluations included 8 18 and 17 EEs, 19 respectively, on CAD, 1 18 did not use any search terms specific to CAD and neither generated insights specific to CAD, such as which variables, if change in value, may change the conclusions on cost‐effectiveness, how simulation models (hereafter models) accounted for the effect of PGx testing, or whether/how the cost of PGx has changed over time and how these factors and other study characteristics affect the conclusions. These issues, if well understood, may assist researchers and clinicians to identify settings where PGx for CAD could potentially be cost‐effective to facilitate further research or implementation.
To address these gaps, our study identifies and analyzes the economic evaluation of PGx testing in patients with CAD to examine how these findings vary according to PGx and comparators, methodological quality of included studies, and variables influential in changing the base‐case conclusions (obtained from analyses with the most likely or preferred set of assumptions and input values) on cost‐effectiveness. We further examined how model‐based EEs accounted for the effect of PGx, how the cost of PGx testing has changed over time, and how these varied based on data sources. Finally, we explored whether any study characteristics varied according to the conclusions on cost‐effectiveness.
Methods
The authors declare that all supporting data are available within the article.
This review has been prospectively registered on PROSPERO (International Prospective Register of Systematic Reviews; registration identification: CRD42019144579 20 ). It is reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement (Table S1). 21 Because this was a systematic review of published literature, neither ethics approval nor informed consent was required.
Search Strategies
We systematically searched 3 general bibliographic databases (Medline, Embase, Web of Science Core Collection) and 3 subject‐specific bibliographic databases (Econlit, National Health Service Economic Evaluation Database, Health Technology Assessment) from inception until June 27, 2022 (for National Health Service Economic Evaluation Database and Health Technology Assessment, both databases were only updated until 2015). We developed the search strategies first on Medline using a combination of Medical Subject Headings and free texts, guided by an experienced librarian and in consultation with our clinical coauthors, before adapting them to other databases. The search strategies encompass 3 concepts, economic evaluation, PGx, cardiovascular disease (CVD) (including coronary artery diseases), and their synonyms (Table S2).
The searches on bibliographic database were supplemented 22 by the searches on the websites of 4 health technology assessment agencies, the National Institute for Health and Care Excellence (United Kingdom), Haute Autorité de Santé (France), Canadian Agency for Drugs and Technologies in Health (Canada), and the Netherlands Organization for Health Research and Development (the Netherlands). In addition, we reviewed the reference lists of included articles, systematic or narrative review articles (backward searches), as well as citations of included articles on Scopus (forward searches).
Study Selection
Titles and abstracts were screened by 2 independent researchers for potential eligibility after duplicates were removed, with disagreements resolved with the third researcher through discussion. Articles were included if they reported a full EE providing both cost and outcome data and focused on genetic testing followed by pharmacotherapy for individuals with or at risk of developing CAD. Articles were excluded if they considered hypothetical genetic tests, used animals, or were review articles, study protocols, editorials, commentaries, opinions, conference abstracts, or letters. No limitations were applied on the age, sex, or ethnic background of the study populations or the setting where PGx testing took place.
Data Extraction
Using a bespoke, piloted, data extraction form in Excel, we extracted (1) authors' affiliations and study funding, (2) study design (type of economic evaluation, perspective, time horizon), (3) patient and study characteristics (country, age of the modeled or the sample population), (4) details of the interventions and the comparators, (5) analysis and findings for the base‐case conclusion (whether PGx testing was cost saving, cost‐effective, not cost‐effective, or dominated by a comparator), and sensitivity analyses. Details on the interventions and the comparators were summarized using the Template for Intervention Description and Replication checklist, 23 , 24 through which we also identified the purpose of the PGx testing. 25 Data were extracted by 1 reviewer, with duplicate extraction on key study findings (costs, effects, incremental costs, incremental effects) independently extracted by a second reviewer, and all remaining data extraction subject to open review by 1 reviewer, as counterchecks to ensure quality.
In extracting a model's variables, we indicated whether variables were tested in 1‐way deterministic sensitivity analyses and which, within the range tested, were influential in changing the base‐case conclusion. One‐way deterministic sensitivity analysis is a sensitivity analysis in economic evaluations where a point estimate of a model variable is varied while keeping the others constant, to examine whether the variable could change the base‐case conclusion (eg, from being cost‐effective to not cost‐effective).
We also extracted findings from probabilistic analyses. Probabilistic analysis is the analysis that randomly draws a set of input parameters (Monte Carlo simulations) from their respective distributions to generate outputs (cost and effectiveness). These are normally repeated between 1000 and 10 000 times 26 to give a range of cost and effectiveness values. These values are used to calculate the percentage of simulations that find an intervention cost saving (ie, less costly and more effective) or the percentage of simulations that find the intervention cost‐effective (ie, below the willingness‐to‐pay [WTP] threshold). These percentages were extracted to examine possible misclassifications of base‐case conclusions.
Quality Assessment
The methodological quality of the included EEs was assessed using the extended version of Consensus Health Economic Criteria List, 27 , 28 one of the most commonly used checklists for model‐based economic evaluations. 29 The checklist comprises 20 questions (eg, Are competing alternatives clearly described? Are the structural assumptions and the validation methods of the model properly reported?), with each answer given as yes/rather yes, no/rather no, or unclear. 27 , 28
Two reviewers assessed the methodological quality independently and reconciled their ratings via discussion or involving a third reviewer where agreement could not be reached.
Statistical Analysis
To provide an overview, we tabulated in numbers and percentages, the study characteristics (year of publication, country, funding, author's affiliation, perspective, type of economic evaluation, type of model, time horizon, age group, gene tested, purpose of PGx), and methodological quality rating. We categorized the year of publication by the median year, patients' age into ≥65 years old versus <65 years old, and time horizon into lifetime versus nonlifetime. Methodological quality is presented as the number of questions rated yes/rather yes in a bar chart.
To examine whether or how PGx testing and the comparators affect the base‐case economic evaluation conclusions, we summarized the conclusions in a 3×3 permutation matrix. 30 Where multiple outcomes were examined, the base‐case conclusions were based on quality‐adjusted life‐years, or in a minority of cases where quality‐adjusted life‐years are absent, disability‐adjusted life‐years, life‐years, or total events. Whether PGx testing was cost saving, cost‐effective, not cost‐effective, or dominated versus a comparator was determined using incremental cost‐effectiveness ratios and net monetary benefit based on the WTP thresholds reported. For EEs that did not report the WTP, we labeled the findings inconclusive.
To examine the model variables influential in changing the base‐case conclusion, we presented the number and percentages of variables tested and reported incremental cost‐effectiveness ratios in 1‐way deterministic sensitivity analyses. These variables were categorized as effectiveness/relative effectiveness, epidemiological variables, cost/resource use, or utility. In addition, we explored the approach taken by each model to account for the effect of PGx testing, which we categorized into accuracy of testing, probabilities/rates, or relative risks/odds ratios/hazard ratios. We also determined the data source for each approach, labeled based on the pyramid of evidence (meta‐analysis, interventional studies, observational studies, other EEs, or others).
To explore changes in the cost of PGx testing, we present the costs of PGx testing by the cost year. The cost of PGx testing was adjusted to 2021 US dollars by first converting the original currency to US dollars using the exchange rate at the year of costing, before inflating it to 2021 US dollars based on the gross domestic product implicit price deflator 31 , 32 (equation below). For cost data, we indicated whether the data originated from private laboratory, official document, or peer‐reviewed literature.
We present the numbers and percentages of study characteristics across PGx‐versus‐comparator pairs and base‐case conclusions. To explore the associations between study characteristics and base‐case conclusions, Fisher exact tests were performed due to all having expected counts <5.
Results
Study Inclusion
Out of 6645 articles from bibliographic databases or websites of health technology assessment agencies, 5432 unique articles were screened based on titles and abstracts, of which 427 were further screened based on full texts. Some of these full texts were later excluded because they were not empirical studies (n=123), not economic evaluations (n=94), or examined other CVDs but not CAD (n=53), among other reasons. These exclusions resulted in a total of 35 articles eligible for data extraction, including 1 found from the forward citation search (Figure S1).
Characteristics of Included EEs
The 35 included articles, each reporting 1 EE (Table S3), were published between 2011 and 2022, mostly in North America (21/35 or 60%, all from the United States), supported by public or nonprofit funders (51%) without authors affiliated to the pharmaceutical or biotechnology industry (80%). These EEs examined PGx testing for CAD mostly from the perspective of the health care system or providers (80%) using cost‐utility analyses alone (91.5%) in models (91.4%) over lifetime (40%), for patients <65 years old (69%). The EEs were mostly focused on CYP2C19 gene testing (including testing alongside other genes 33 , 34 ) to stratify patients between different antiplatelets (71%), or in genetic risk scoring to predict the risk of CAD (23%) (Table 1).
Table 1.
Study Characteristics (n=35)
| Study characteristics | n | % |
|---|---|---|
| Year of publication | ||
| 2011–2017 | 16 | 45.7 |
| 2018–2022 | 19 | 54.3 |
| Continent | ||
| North America (United States) | 21 | 60.0 |
| Europe (the Netherlands, Spain, Finland) | 6 | 17.1 |
| Asia (China, Hong Kong, Qatar, Singapore) | 6 | 17.1 |
| Australia and New Zealand | 2 | 5.7 |
| Funding | ||
| Nonprivate (public or nonprofit) | 18 | 51.4 |
| Not specified | 10 | 28.6 |
| Private | 4 | 11.4 |
| None | 3 | 8.6 |
| Any author affiliated to pharmaceutical/biotechnology industry | ||
| No | 28 | 80.0 |
| Yes | 7 | 20.0 |
| Perspective | ||
| Health care system/provider | 28 | 80.0 |
| Not stated | 4 | 11.4 |
| Societal | 2 | 5.7 |
| Private payer | 1 | 2.9 |
| Type of economic evaluations | ||
| Cost‐utility analysis only | 22 | 62.9 |
| Cost‐utility analysis and cost‐effectiveness analysis | 10 | 28.6 |
| Cost‐effectiveness analysis only | 3 | 8.6 |
| Type of study design | ||
| Model, Markov cohort ± DT | 21 | 60.0 |
| Model, DT only | 8 | 22.9 |
| Trials | 2 | 5.7 |
| Model, discrete event simulation ± DT | 1 | 2.9 |
| Model, Markov microsimulation | 1 | 2.9 |
| Model, not stated | 1 | 2.9 |
| Observational study | 1 | 2.9 |
| Time horizon | ||
| Nonlifetime | 21 | 60.0 |
| Lifetime | 14 | 40.0 |
| Age, y | ||
| <65 | 24 | 68.6 |
| Not stated | 7 | 20.0 |
| ≥65 | 4 | 11.4 |
| Gene tested* | ||
| CYP2C19 † | 25 | 71.4 |
| Not stated‡ | 7 | 20.0 |
| AT1 (rs275651, rs5182) and BK1 receptor genes (rs12050217)§ | 1 | 2.9 |
| CYP2C9/VKORC1 || | 1 | 2.9 |
| CYP2D6 ¶ | 1 | 2.9 |
| KIF6 # | 1 | 2.9 |
| LPA (rs3798220 and rs10455872)** | 1 | 2.9 |
| SLCO1B1 †† | 1 | 2.9 |
| Purpose of genetic testing* | ||
| To stratify patients between medications (stratification) | ||
| Antiplatelets, CYP2C19 | 25 | 71.4 |
| Statins, SLCO1B1, KIF6 | 2 | 5.7 |
| Pain killers, CYP2D6 | 1 | 2.9 |
| Angiotensin‐converting enzyme inhibitor, AT1 | 1 | 2.9 |
| To predict the risk of CAD (prognostic), LPA | 8 | 22.9 |
| To determine doses (differentiation) | ||
| Coumadin, CYP2C9/VKOCR1 | 1 | 2.9 |
CAD indicates coronary artery disease; and DT, decision tree.
Sums >100% because 2 studies (Hart et al, 33 and Dong et al 34 ) tested multiple genes, each for a different purpose.
Carriers of 2 nonfunctional copies/loss‐of‐function CYP2C19 variants are not able to activate antiplatelet clopidogrel.
These are studies that use a proprietary genetic test kit and/or proprietary genetic risk scores, which may specify the number of genes, but the exact genes are not stated.
AT1 and BK1 gene variants are associated with more pronounced treatment from angiotensin‐converting enzyme inhibitor (eg, perindopril).
CYP2C9/VKORC1 alleles are associated with higher risk of bleeding with coumadin (eg, warfarin).
CYP2D6 gene variants are associated with lower metabolism of tramadol (pain medication), resulting in tramadol toxicity.
KIF6 gene variants are associated with larger reduction in cardiovascular events than did noncarriers with high‐dose atorvastatin.
Both rs3798220 and rs10455872 single‐nucleotide polymorphisms are in the LPA gene and associated with higher risk of CAD; rs3798220 is also associated with lower efficacy of aspirin in women.
SLCO1B1 gene variant (rs4149056 C alleles) is associated with higher risk of myopathy with simvastatin.
Most EEs compared CYP2C19 testing followed by prescription of antiplatelet prasugrel, ticagrelor, or a mix of antiplatelets for carriers of LOF genes and clopidogrel for noncarriers versus universal use of prasugrel without CYP2C19 testing (hereafter non‐PGx prasugrel), non‐PGx ticagrelor, non‐PGx clopidogrel, or a mix of antiplatelets. These EEs assumed that CYP2C19 testing would be used alone, with its results available and used immediately to stratify patients into antiplatelets. Conversely, 2 EEs 35 , 36 examined the use of CYP2C19 testing alongside platelet reactivity test, and 1 EE 37 examined offering ticagrelor to all patients without test results for a month, after which, based on the PGx test results, noncarriers of LOF would be de‐escalated to clopidogrel (Figure 1A).
Figure 1. How (A) CYP2C19 testing and (B) genetic risk scores may be delivered and compared against a comparator in the included EEs.
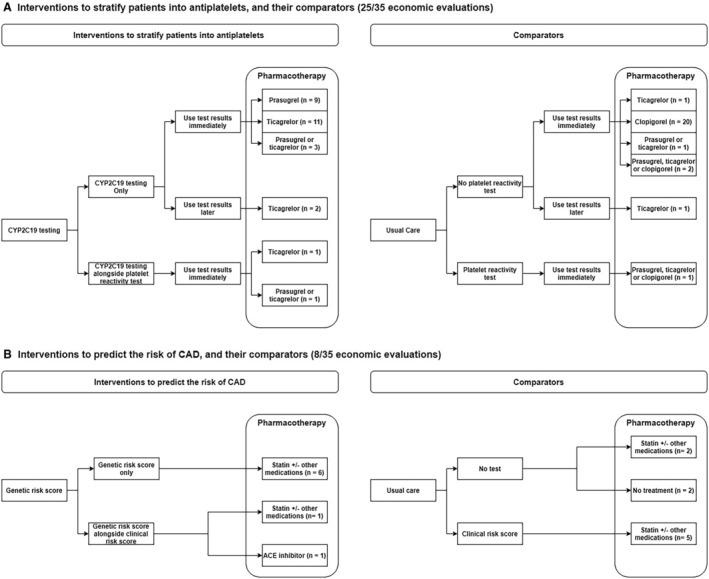
EE indicates economic evaluation.
Eight EEs examined the use of multiple gene variants to generate genetic risk scores to stratify individuals into different risk levels of CAD, such that those at certain risk levels are offered statins 38 , 39 , 40 , 41 , 42 or statin and antihypertensive medications 43 to prevent CAD. The genetic risk scores, based on up to 49 310 genetic variations (single polynucleotide polymorphisms), 40 may incorporate clinical risk factors 38 or be used alongside another clinical risk score 44 (collectively termed clinical+genetic risk score); the derivation or the validation of these scores were described or cited in the EE. The genetic risk scores may be compared with offering preventive pharmacotherapy based on clinical risk score alone or usual clinical assessment (no risk score) or no risk score and no pharmacotherapy (no treatment) (Figure 1B). Although the prescriptions triggered by the genetic test (rather than the test alone) also drive the cost‐effectiveness findings, we will only mention the genetic test in describing the findings hereafter, for brevity.
Despite the details on genes tested and the ensuing pharmacotherapy, details on the delivery of PGx testing was scant. Only 4 EEs specified the setting (hospital 45 , 46 , 47 or primary care 48 ); 3 EEs named 44 or described the test kits (the gene alleles tested 33 or buccal swab 49 ); 2 EEs 39 , 48 specified who collected the samples (eg, physicians), and 1 EE 41 accounted for genetic counseling.
Methodological Quality of Included EEs
Each EE had 6 to 18 questions (median 17 out of 20 questions) on extended version of Consensus Health Economic Criteria List checklist rated as yes/rather yes (Figure 2; Table S4).
Figure 2. Methodological quality rating based on CHEC‐Extended.
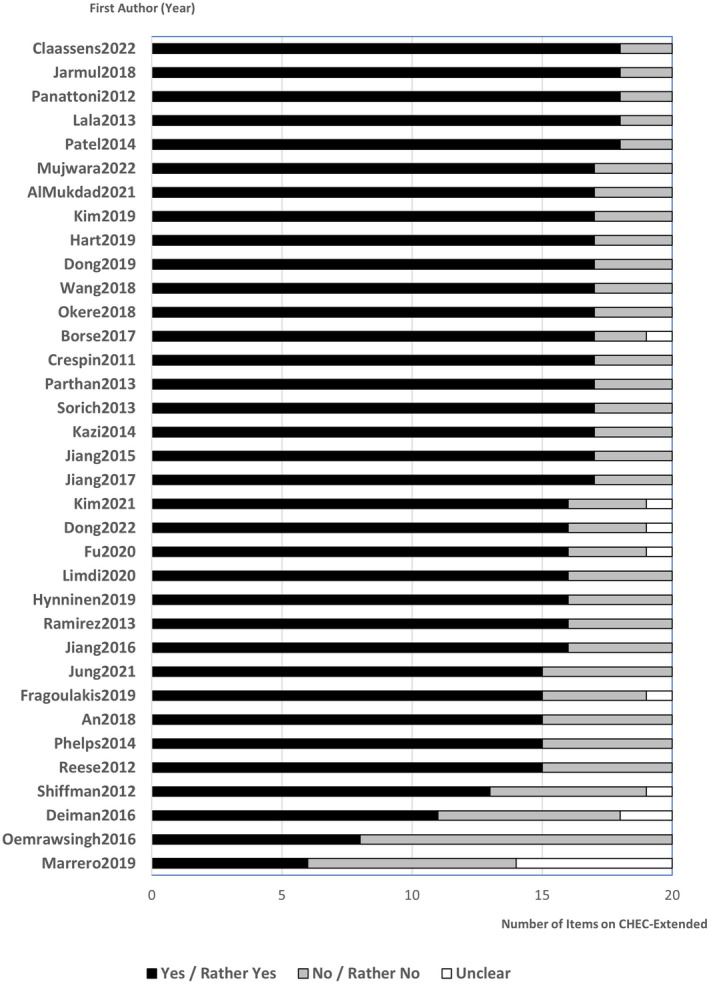
CHEC‐Extended indicates extended version of Consensus Health Economic Criteria List.
All EEs had a clear description of the study population (question 1). All but 1 EE had a clear research question (question 3), measured costs appropriately (question 9), measured outcomes appropriately (question 12), and provided conclusions that followed the data reported (question 17).
In contrast, >50% EEs did not sufficiently report structural assumptions and validation methods (question 5), discuss generalizability of findings to other settings or patient groups (question 18), or discuss ethics and distributional issues (question 20).
EE Findings Based on PGx Testing and Comparators
The 35 EEs examined 154 comparisons of PGx versus a comparator, of which 151 comparisons and their findings are summarized in Table 2. The remaining 3 comparisons, reported by a single EE, 50 could not be summarized because the articles only reported an incremental cost‐effectiveness ratio without incremental cost, incremental effectiveness, or willingness to pay separately.
Table 2.
Economic Evaluation Findings of PGx Versus Non‐PGx in a Permutation Matrix
| Relative effect | ||||
|---|---|---|---|---|
| − | 0 | + | ||
| Relative cost | + |
PGx is more costly yet less effective than comparator
To stratify patients between antiplatelets (nc=5)
To predict the risk of CAD (nc=3)
|
PGx is more costly yet similarly effective than comparator No study (nc=0) |
PGx is more costly and more effective than comparator
To stratify patients between antiplatelets (nc=35)
To predict the risk of CAD (nc=18)
To stratify patients between statins (nc=1)
To stratify patients between antiplatelets (nc=6)
To predict the risk of CAD (nc=4)
To stratify patients between antiplatelets, between statins, and to determine coumadin dosing (nc=1)
To predict the risk of CAD (nc=4)
|
| 0 |
PGx is similarly costly yet similarly effective as the comparator No study (nc=0) |
PGx is similarly costly yet similarly effective as the comparator No study (nc=0) |
PGx is similarly costly yet more effective than the comparator No study (nc=0) |
|
| − |
PGx is less costly yet less effective than the comparator
To stratify patients between antiplatelets (nc=6)
To predict the risk of CAD (nc=2)
To stratify patients between statins (nc=1)
To stratify patients between antiplatelets (nc=18)
To predict the risk of CAD (nc=3)
|
PGx is less costly yet similarly effective as the comparator
To predict the risk of CAD (nc=1) 1. Genetic + clinical risk score vs clinical risk score (nc=1) |
PGx is less costly and more effective than the comparator
To stratify patients between antiplatelets (nc=26)
To predict the risk of CAD (nc=16)
To stratify patients between antiplatelets and between pain killers (nc=1)
|
|
Interpretation: + indicates that the intervention(s) have higher cost/higher effectiveness than the comparator; 0 indicates that the intervention(s) have the same cost/same effectiveness as the comparator; − indicates that the intervention(s) have lower cost/lower effectiveness than the comparator.
Comparisons indicated with an asterisk (*) were contributed by the 2 studies (Kazi et al 56 and Phelps et al 48 ) with societal perspective.
For PGx, CYP2C19 ticagrelor/clopidogrel refers to prescribing ticagrelor for carriers of loss‐of‐function alleles in CYP2C19 testing and clopidogrel for noncarriers. Similarly, CYP2C19 prasugrel/clopidogrel refers to prasugrel for carriers of loss‐of‐function alleles and clopidogrel for noncarriers, CYP2C19 ticagrelor/prasugrel/clopidogrel refers to ticagrelor or prasugrel for carriers of loss‐of‐function alleles and clopidogrel for noncarriers.
For non‐PGx, universal clopidogrel refers to prescribing clopidogrel only to patients with CAD without CYP2C19 testing. Similarly, universal ticagrelor refers to ticagrelor only and universal prasugrel refers to prasugrel only.
All conclusions on cost‐effectiveness are for the base‐case (obtained from analyses with the most likely or preferred set of assumptions and input values), according to the willingness‐to‐pay thresholds and the time horizons deemed appropriate by the individual studies. For An et al, 54 Crespin et al, 49 Claassens et al, 69 Dong et al 34 , Mujwara et al, 42 Parthan et al, 51 who reported multiple time horizons at base case, the longest time horizon was presented. For Dong et al 34 , who used multiple WTPs at base case, the lowest WTP was presented. CAD indicates coronary artery disease; and PGx, genetic‐guided pharmacotherapy.
CYP2C19 Testing to Stratify Patients Into Antiplatelets
Most comparisons (96/151) examined stratifying patients between antiplatelets alone based on CYP2C19 status. Compared with an alternative, CYP2C19 testing was shown to be more costly and more effective (41/96), less costly and more effective (26/96), less costly and less effective (24/96), or more costly and less effective (5/96). These findings varied based on the comparators. For example, 28 out of 43 comparisons with non‐PGx clopidogrel found CYP2C19 testing more costly and more effective, the majority of which (26/28) concluded that testing was cost‐effective. Similarly, all 15 comparisons with non‐PGx prasugrel found CYP2C19 testing more effective, with 10 out of 15 finding it less costly and 5 out of 15 more costly (and cost‐effective). Conversely, 22 out of 25 comparisons with non‐PGx ticagrelor found CYP2C19 testing less costly and less effective, most (17/22) concluding that testing was not cost‐effective. The other comparisons were fewer. Comparisons with a mix of prasugrel and ticagrelor found CYP2C19 testing less costly (3/4 more effective, 1/4 less effective). Comparisons with a mix of clopidogrel, prasugrel, and ticagrelor found CYP2C19 testing more effective (4/5 more costly, 1/5 less costly). Comparisons with stratifying antiplatelets based on platelet reactivity test also found CYP2C19 testing more effective (1/2 more costly, 1/2 less costly). Comparison with ticagrelor for a month, before de‐escalation to clopidogrel, found CYP2C19 more costly (1/2 more effective, 1/2 less effective).
CAD Risk Prediction to Determine the Prescription of Statins Alone or With Antihypertensives
The next most common comparisons (51/151) examined the use of PGx to predict the risk of CAD followed by a prescription of statins alone or with antihypertensives. Compared with an alternative, these PGx testing regimens were found to be more costly and more effective (26/51), less costly and more effective (16/51), less costly and more effective (5/51), more costly and less effective (3/51), or less costly equally effective (1/51). As with CYP2C19 testing, these findings varied by choice of comparator. Notably, 17 out of 20 comparisons with a clinical risk score found a genetic risk score less costly, with 13 out of 17 comparisons being more effective, 3 out of 17 less effective (2/3 concluded cost‐effective), and 1 out of 17 equally effective. There were 16 out of 19 comparisons with no risk score that found a genetic risk score to be more costly, with 14 out of 16 being more effective (12/14 cost‐effective) and 2 out of 16 being less effective; the remaining 3 out of 19 comparisons being less costly and more effective. Compared with no treatment, a genetic risk score may also be more costly and more effective (9 comparisons, 6/9 cost‐effective, 3/9 inconclusive). Compared with myocardial perfusion imaging, a genetic risk score was found to be less costly and less effective (2 comparisons, not cost‐effective), or more costly and more effective (1 comparison, not cost‐effective).
Other Genetic Testing for Patients With CAD
The remaining comparisons examined (1) PGx to determine statin dosing (2/151), 51 of which 1 out of 2 estimates found PGx to be more costly and more effective than pravastatin, and 1 out of 2 less costly and less effective than atorvastatin, although both concluded that PGx was cost‐effective; (2) PGx to stratify patients between antiplatelets and between pain killers (1/151), 33 which found PGx to be less costly and more effective; (3) PGx to stratify patients between antiplatelets, between statins, and to determine coumadin dosing (1/151), which found PGx to be more costly and more effective. 34
Influential Model Variables
A total of 294 variables have been examined in 1‐way sensitivity analyses (Table 3) to determine whether a change in their values would change the base‐case conclusion on cost‐effectiveness. The most examined variables were baseline/relative risk of mortality (47/294), CAD (34/294), and stroke (21/294), followed by the cost of antiplatelets (21/294) and cost of events associated with CAD (20/294). Approximately 14% (40/294) of variables would change the base‐case conclusion on cost‐effectiveness. These variables were most commonly baseline/relative risk of mortality (25/40), CAD (10/40), the cost of antiplatelets (10/40), accuracy of genetic testing (2/40), baseline/relative risk of stroke (2/40), AF (2/40), and composite outcome (2/40). The other variables less frequently changed the base‐case conclusion; prevalence of CYP2C19 LOF genes (1/40), prevalence of antiplatelet response (1/40), cost of statins (1/40), utility of CAD (1/40); however, these variables were also not commonly examined.
Table 3.
Variables Tested in Deterministic Sensitivity Analyses and Shown to Change Base‐Case Conclusion
| Type of variables | Variables | No. of variables reported | % Changed conclusion | |
|---|---|---|---|---|
| ICER reported in DSA | Changed conclusion | (descending order within category) | ||
| Effectiveness/relative effectiveness | Anticholesterols (statins) | 1 | … | 0% |
| Epidemiological variables | Accuracy of genetic testing | 2 | 2 | 100% |
| Baseline/relative risk of CVD‐related events | ||||
| AF | 2 | 2 | 100% | |
| Composite outcomes | 4 | 2 | 50% | |
| CAD (including MI and CHD) | 34 | 4 | 12% | |
| Stroke | 21 | 2 | 10% | |
| Stent thrombosis | 5 | … | 0% | |
| Prevalence of antiplatelet response | 3 | 1 | 33% | |
| Prevalence CYP2C19 LOF polymorphism | 4 | 1 | 25% | |
| Baseline/relative risk of treatment‐related events | ||||
| Myalgia/myopathy | 2 | 1 | 50% | |
| Bleeding | 13 | … | 0% | |
| Adherence to test/treatment | 6 | 2 | 33% | |
| Baseline/relative risk of mortality | 47 | 10 | 21% | |
| Accuracy of platelet reactivity testing | 1 | … | 0% | |
| Patient's age at CAD | 1 | … | 0% | |
| Cost/resource use | Medications | |||
| Antiplatelets | 21 | 4 | 19% | |
| Statins | 6 | 1 | 17% | |
| Medications based on local clinical guideline | 1 | … | 0% | |
| Genetic testing | 16 | 3 | 19% | |
| Clinical risk scoring | 2 | … | 0% | |
| Laboratory tests | 2 | … | 0% | |
| CVD‐related events | ||||
| CAD (including MI and CHD) | 20 | … | 0% | |
| Stroke | 8 | … | 0% | |
| Events associated with treatment | ||||
| Bleeding | 4 | 1 | 25% | |
| Myalgia/myopathy | 1 | … | 0% | |
| Mortality | 3 | … | 0% | |
| No event | 1 | … | 0% | |
| Physician visit | 1 | … | 0% | |
| Waiting time/travel time | 6 | … | 0% | |
| Utility | CVD‐related events | |||
| Thromboembolic event | 1 | 1 | 100% | |
| CAD (including MI and CHD) | 13 | 1 | 8% | |
| Stent thrombosis | 4 | … | 0% | |
| Stroke | 8 | … | 0% | |
| Dyspnea | 1 | … | 0% | |
| Composite event | 1 | … | 0% | |
| Events associated with treatment | ||||
| Myalgia/myopathy | 6 | 1 | 17% | |
| Bleeding | 5 | … | 0% | |
| No event | 10 | 1 | 10% | |
| Mortality | 1 | … | 0% | |
| Others | Discount rate | 6 | … | 0% |
| 294 | 40 | 14% | ||
AF indicates atrial fibrillation; CAD, coronary artery disease, CHD, coronary heart disease; CVD, cardiovascular disease; DSA, deterministic sensitivity analysis; ICER, incremental cost‐effectiveness ratio; LOF, loss‐of‐function, and MI, myocardial infarction.
Variation in How Models Accounted for the Effect of PGx Testing
The 32 model‐based EEs accounted for the effect of PGx testing in at least 1 of the 3 approaches (Table 4): (1) using relative risks/odds ratios/hazard ratios of events among carrier versus noncarrier of higher‐risk genes (17/32), (2) using different probabilities for carriers versus noncarriers (15/32), or (3) accuracy of PGx testing (5/32). Across the 3 approaches, data were mostly derived from meta‐analyses or randomized controlled trials. Only a minority of case variables were based on observational studies (cohort or cross‐sectional studies), data used in other EEs, or other sources (eg, narrative reviews). Events were not equally accounted for. For instance, models that used relative risks most frequently accounted for CVD‐related events (eg, myocardial infarction, stroke, angina) (16/17), followed by treatment‐related events (eg, bleeding) (9/17), CVD mortality (5/17), and all‐cause mortality (3/17).
Table 4.
How Model‐Based EEs (n=32) Accounted for the Effect of Genetic‐Guided Pharmacotherapy on Coronary Artery Disease in their Economic Models, by Data Sources
| MA | RCTs | Observational studies | Other EEs | Others† | Total | |
|---|---|---|---|---|---|---|
| Accuracy of genetic testing‡ | … | 1 | 3 | … | 1* | 5 |
| Using different probabilities/rates data for carrier vs noncarrier of high‐risk genes | ||||||
| CVD‐related events§ | 9 | 10 | 3 | 1 | 1 | 15 |
| Treatment‐related events|| | 7 | 9 | 1 | 1 | … | 11 |
| Mortality (all‐cause) | … | 1 | 1 | 1 | … | 4 |
| Mortality (CVD) | 5 | 6 | 1 | … | … | 6 |
| Mortality (non‐CVD) | 1 | 2 | … | … | … | 2 |
| 9 | 11 | 3 | 1 | 1 | 15 | |
| Relative risks/odds ratios/hazard ratios of events for carrier vs noncarrier of high‐risk genes | ||||||
| CVD‐related events‡ | 5 | 9 | 5 | 1 | 1 | 16 |
| Treatment‐related events§ | 2 | 5 | 1 | 2 | 1 | 9 |
| Mortality (all‐cause) | 2 | 3 | … | 1 | … | 4 |
| Mortality (CVD) | 3 | 2 | 2 | … | … | 6 |
| Mortality (non‐CVD) | … | … | … | … | … | … |
| 5 | 9 | 5 | 2 | 2 | 17 | |
CVD indicates cardiovascular diseases; MA, meta‐analysis; Other EEs, other economic evaluations; Others, other sources; and RCTs, randomized controlled trials.
This study (Borse et al 55 ) did not cite the source of the data on accuracy of CYP2C19 testing.
Others includes personal communication, expert opinion, or not stated.
This refers to sensitivity and specificity of genetic testing.
CVD‐related events include myocardial infarction, stroke, stent thrombosis, angina, or major adverse cardiovascular events.
Non‐CVD events include major bleeding or bleeding unspecified.
Variation in the Cost of PGx Testing
The 35 EEs reported 37 costs of PGx testing (Figure 3), and 2 EEs 33 , 34 examined 2 types of PGx testing, CYP2C19 alone, and CYP2C19 alongside other genes. Most PGx cost data were cited from private laboratories (11/37), followed by peer‐reviewed literature (9/37), official documents (eg, formulary or reimbursement schedule) (9/37), and others such as expert opinion (5/37) or not stated (3/37).
Figure 3. The cost of genetic testing over time (2009–2020) in 2021 US dollars.
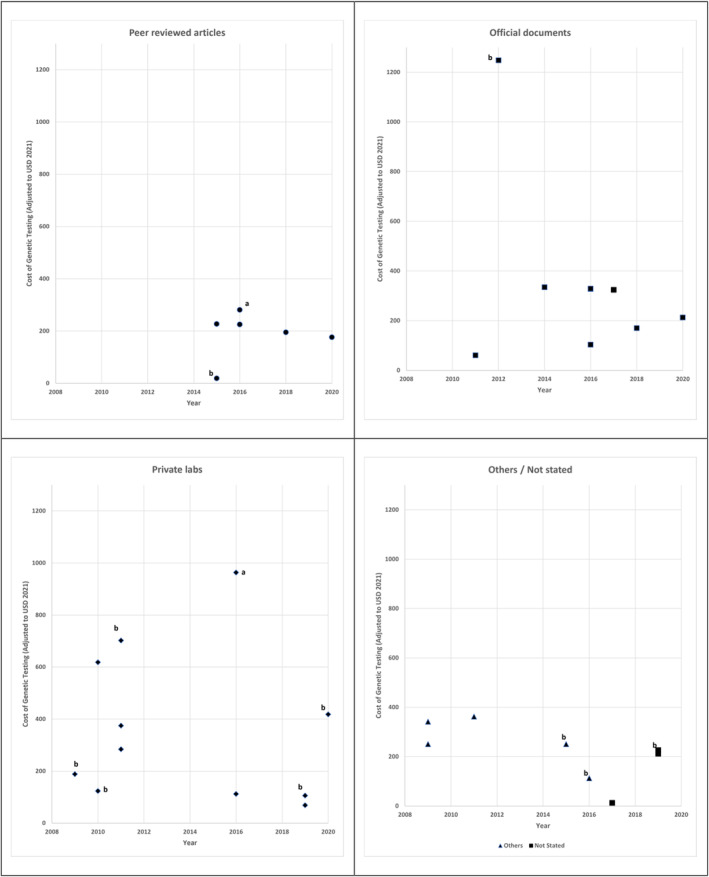
All tests are for CYP2C19 single gene (n=23), except those labeled “a” for testing CYP2C19 alongside other genes (n=2) and “b” for non‐CYP2C19 genes (n=10).
The cost of PGx testing varied between USD$13 and USD$1248 (median USD$224). Nevertheless, almost all were <USD$400, except those that came from private laboratories.
Although all EEs specifying that types of test performed and the cost data sources, only 5 EEs specified what the costs of PGx testing included, albeit in varying depth. One 49 included complete cost to the payer for the laboratory service, 1 39 included the cost of the test and a telemedicine visit, 1 42 included the cost of the assay and the bioinformatics analysis, 1 44 included the cost of the assay, the equipment, and quality control (duplicate testing for randomly selected samples), and 1 52 included the costs for obtaining samples and the associated laboratory services.
Variation in EE Conclusions Based on Study Characteristics
We tested the differences in base‐case conclusions of 147 comparisons (excluding 7 inconclusive findings) with 15 study characteristics set out in Table 1, of which 7 had statistically significant associations (P<0.05; Table S5). Specifically, the percentages of EEs concluding unfavorably of PGx (not cost‐effective or dominated by the comparator) in base‐case compared with the percentages of the same characteristics concluding favorably of PGx (cost saving or cost‐effective) were higher in studies; from recent years (2018–2022), North America, adopting a health care perspective, using a lifetime horizon, with patients ≥65 years old, accounting for the effect of PGx using probabilities or rates only, and using cost data from the peer‐reviewed literature or official documents.
Findings from probabilistic analyses (Table S6) suggest misclassification of base‐case conclusions is possible, but the possibility is low, especially for comparisons that found PGx dominated or not cost‐effective.
Discussion
In the absence of a synthesis of evidence to advise clinical practice, our review systematically examined EEs of PGx testing for individuals with or at risk of CAD. We found 35 EEs, mostly model‐based cost‐utility analyses examining CYP2C19 testing or genetic risk scores, either used alone or alongside other tests.
Our review found the most evidence for CYP2C19 testing (96/151 comparisons) in stratifying patients into antiplatelets. Whether PGx based on CYP2C19 status is cost‐effective depends on the non‐PGx comparators. Compared with non‐PGx clopidogrel, PGx based on CYP2C19 status was most likely (26/43 comparisons 36 , 37 , 46 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 ) more costly, more effective, and also cost‐effective. However, compared with non‐PGx prasugrel, PGx was most likely (10/15 comparisons 46 , 53 , 55 , 56 , 59 , 61 , 62 ) less costly and more effective (cost saving), and compared with non‐PGx ticagrelor, PGx was most likely (17/25 comparisons 46 , 49 , 54 , 56 , 57 , 58 , 60 ) less costly, less effective, and not cost‐effective. These suggest that the implementation of PGx should depend on the current practice. For example, in Singapore, where ticagrelor is the only P2Y12‐inhibitor in the local formulary, 36 CYP2C19 testing may not be advisable, because it is expected to either dominate or not be cost‐effective compared with non‐PGx ticagrelor. However, if clopidogrel or prasugrel is the most prescribed antiplatelet, CYP2C19 testing is more likely to be cost saving or cost‐effective, with local WTP threshold becoming influential in deciding whether to implement testing. Meanwhile, in jurisdictions where multiple antiplatelets are available in the market or where the use of a platelet reactivity test to choose antiplatelet is the standard practice, local research on cost‐effectiveness would be required, because our review could not find sufficient evidence in the literature.
In contrast, using genetic risk scores to predict CAD risk gave mostly favorable conclusions on cost‐effectiveness. Most comparisons found it less costly and more effective than clinical risk score (13/20 comparisons 39 , 42 ), or more costly, more effective, and also cost‐effective than no risk score (12/19 comparisons 40 , 43 , 48 ) or no treatment (6/9 comparisons 40 ). Despite the favorable findings, we would recommend validating the genetic risk scores by comparing their predictive accuracy versus non‐PGx risk scores (eg, American College of Cardiology/American Heart Association pooled cohort equation 63 ) in the local population, 64 before any decision on implementation, because the composition genes in the risk scores may vary across the population. Other purposes of PGx testing (eg, to determine coumadin doses) have also been examined, but too few studies are available to observe trends.
Our study generated a list of model variables that influence base‐case conclusions on the cost‐effectiveness of PGx for CAD. This list (eg, baseline/relative risk of mortality [see Table 3]) can inform (1) future data collection to increase the precision of variables more frequently found to change base‐case conclusions, and (2) the design of future models of PGx for CAD, which may differ from PGx for other health conditions such as AF. In our previous review of PGx for AF, 17 baseline/relative risk of bleeding was found to influence the conclusion about cost‐effectiveness, but not in our present review for CAD. This may be due to a higher risk of bleeding with coumadin used in AF than antiplatelets used in CAD. The cost of genetic testing was also found influential for AF, but not for CAD, possibly due to the decreasing cost of PGx in AF 17 but relatively stable cost of PGx in CAD over the years. These differences in influential variables between different PGx suggest caution in generalizing the findings from one health condition to another.
We also identified 3 main approaches used by simulation models to account for the effects of PGx, relative risk, probability, and accuracy. These approaches may inform future model design. For example, existing models mostly accounted for the relative risk or probability of CVD‐related events or treatment‐related events. However, accounting for these 2 events alone 55 , 57 assumes PGx has no direct impact on mortality and that any differences in mortality would indirectly result from differences in CVD‐related or treatment‐related events between PGx and non‐PGx arms. Given that risk of mortality is one of most influential variables, future models should report its assumptions on the effects of PGx and test these assumptions in sensitivity analyses.
Our exploratory analyses indicate that base‐case conclusions of EEs differ based on the publication years, geographical location, study design (perspective, time horizon, how models accounted for the effect of PGx), sample characteristics (age) and data sources (cost of genetic testing), unlike our previous exploratory analyses for PGx in AF 17 that found no such associations. These differences may be partially driven by a larger number of comparisons in the current review (147 versus 46). Most findings from the United States suggested PGx was dominant or cost‐effective compared with non‐PGx (58/86 comparisons); this may be due to study designs, because they were also mostly for patients <65 years old (53/86) from the health care perspective (60/86), characteristics that have also been associated with favorable findings (Table S5), or higher WTP thresholds (10/21 economics evaluations from the United States adopted WTP thresholds ≥USD$100,000). An issue with these exploratory analyses is the inconsistency in reporting. For example, although 2 studies 48 , 56 reported societal perspectives, neither presented societal costs (eg, productivity loss, informal care). Besides inconsistencies in reporting, gaps in reporting and poor methodological quality are also evident. For instance, few EEs discussed the structural assumptions and validation methods, generalizability to other settings or patient groups, or ethical implications of their findings. Future EEs need to be better designed to inform the implementation of PGx in practice.
Our findings have several limitations. First, the EEs included were mostly performed in the United States or Europe and did not differentiate individuals at different risk levels such as men versus women, hence the findings on cost‐effectiveness (Table 2) would be generalizable to average risk individuals in United States or Europe, but not those from other regions. Few EEs reported details on the how the PGx was delivered. Thus, findings to date are less able to inform the optimal strategies to implement PGx (eg, whether to offer PGx at primary or secondary care, which risk group to offer). 65 In addition, the EE findings (whether testing was cost saving, cost‐effective, not cost‐effective, or dominated by a comparator) in Table 2 and Table S5 rely on base‐case conclusions. Hence, they should be interpreted as representing average findings using the best possible set of point estimates that respective researchers deemed appropriate for the study populations, without having accounted for any statistical uncertainty. Findings from probabilistic analyses (Table S6) suggest that misclassification of base‐case conclusions was possible, although low. Next, our list of influential variables may not be exhaustive and may be susceptible to reporting bias, because not all EEs reported all input variables and their findings from 1‐way sensitivity analyses. Some EEs 33 , 37 used different labels in listing input variables and in presenting findings, which required some degrees of interpretation during data extraction. However, the list reflects the breadth of variables required for modeling the cost‐effectiveness of PGx for CAD that can be used as a useful guide in selecting variables for modeling and for testing in sensitivity analyses in any country. In examining the cost input variables across EEs, we focused on the cost of genetic testing, which is reported and used consistently across all EEs, but not the other variables (eg, cost of treatment), which are heterogeneous (eg, different types of treatment used at different time in the process of care) and would render any comparisons meaningless.
Despite the limitations, our study has strengths as well as implications for practice and research. As the first systematic review of EE of PGx for CAD, we identified more EEs on CAD than earlier broader reviews (35 versus 8 18 or 17 19 ). For practice, our findings suggest PGx to stratify patients into antiplatelets or to predict CAD risk may be cost‐effective against certain non‐PGx current practice. For research, our findings indicate evidence is lacking from outside of the United States or Europe and on combined testing of multiple genes. While addressing these research gaps, future trial‐based EEs may prioritize collecting more precise estimates of variables than our study found, which may change the base‐case conclusions, whereas future model‐based EEs should examine whether accounting for different events (eg, CVD related, treatment related, mortality) may affect the findings. These future EEs should strive to address the gaps and inconsistencies in reporting than what we found, such as the details on interventions, structural assumptions, model validation, and ethical issues of PGx testing.
Conclusions
Our review found 35 EEs, mostly simulation models, of PGx for individuals at risk or with CAD. Our findings suggest PGx to stratify patients for antiplatelet therapy are unlikely to be cost‐effective in places that prescribe ticagrelor as standard care but may be cost saving or cost‐effective in places that prescribe prasugrel or clopidogrel. Our findings also indicate that genetic risk scores to predict CAD risk followed by prescription of preventive pharmacotherapy is cost‐effective in places where preventive pharmacotherapy is prescribed based on clinical risk score or usual clinical assessment, or where no preventive pharmacotherapy is prescribed. However, those considering genetic risk scores should compare their predictive accuracy versus non‐PGx risk scores in their local population before deciding on implementation. For other purposes of testing (eg, to stratify patients into statins), evidence is scant, and further studies are required. We also identified 40 variables that influence conclusions on cost‐effectiveness (eg, baseline/relative risk of mortality) and 3 approaches on how simulation models accounted for the effects of PGx, both of which can inform the design and reporting of future trial‐ or model‐based EEs. Nevertheless, certain gaps in reporting and methodological quality are evident (eg, providing details on the PGx interventions and structural assumptions of models). Addressing these gaps will inform whether and when, and which, PGx to adopt for CAD.
Sources of Funding
Salary funding was received from the UK National Institute for Health Research‐Biomedical Research Centre based at Guy's and St Thomas' National Health Service Foundation Trust and King's College London (K.K.L., J.F.R., and C.D.A.W.), the British Heart Foundation (P.C.) and the UK National Institute for Health and Care Research Programme Grant for Applied Research NIHR202339 (J.F.R. and C.D.A.W.). The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health and Care Research, the British Heart Foundation, or the Department of Health and Social Care.
Disclosures
None.
Supporting information
Tables S1–S6
Figure S1
References 66–70
Acknowledgments
The authors thank King's College London librarians for help tracing full‐text articles. J.F.‐R. and R.K‐K. conceptualized and designed the study. R.K.K., C.D.A.W., P.C., and J.F.‐R. prepared the search strategies. K.K.L., R.K.K., J.F.R., and A.A.K. undertook the searches and screening of titles, abstracts, and full texts. K.K.L., R.K.K., and J.F.R. prepared and piloted the data extraction tables. K.K.L., H.F.K., and A.A.K. performed double data extractions on study findings. K.K.L. and H.F.K. extracted the remaining data. K.K.L., R.K.K., and H.F.K. double rated the study quality and discussed any ratings that could not be agreed. K.K.L. cleaned the data, performed the analyses, and prepared the first draft of the article. All authors contributed to data interpretation, revised the draft critically for important intellectual content, and agreed to the final submission.
This article was sent to Katherine C. Wu, MD, Senior Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.030058
For Sources of Funding and Disclosures, see page 16.
References
- 1. Dai H, Much AA, Maor E, Asher E, Younis A, Xu Y, Lu Y, Liu X, Shu J, Bragazzi NL. Global, regional, and national burden of ischaemic heart disease and its attributable risk factors, 1990–2017: results from the global burden of disease study 2017. Eur Heart J Qual Care Clin Outcomes. 2022;8:50–60. doi: 10.1093/ehjqcco/qcaa076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bauersachs R, Zeymer U, Brière J‐B, Marre C, Bowrin K, Huelsebeck M. Burden of coronary artery disease and peripheral artery disease: a literature review. Cardiovasc Ther. 2019;2019:8295054. doi: 10.1155/2019/8295054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bishu KG, Lekoubou A, Kirkland E, Schumann SO, Schreiner A, Heincelman M, Moran WP, Mauldin PD. Estimating the economic burden of acute myocardial infarction in the us: 12 year national data. Am J Med Sci. 2020;359:257–265. doi: 10.1016/j.amjms.2020.02.004 [DOI] [PubMed] [Google Scholar]
- 4. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck‐Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes: the task force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- 5. Lawton JS, Tamis‐Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM, Don CW, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2022;145:e18–e114. doi: 10.1161/CIR.0000000000001038 [DOI] [PubMed] [Google Scholar]
- 6. National Institute for Health & Care Excellence . Cg181: Cardiovascular disease: Risk assessment and reduction, including lipid modification 2016.
- 7. McPherson R, Tybjaerg‐Hansen A. Genetics of coronary artery disease. Circ Res. 2016;118:564–578. doi: 10.1161/CIRCRESAHA.115.306566 [DOI] [PubMed] [Google Scholar]
- 8. Roberts R, Fair J. Genetics, its role in preventing the pandemic of coronary artery disease. Clin Cardiol. 2021;44:771–779. doi: 10.1002/clc.23627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pechlivanis S, Lehmann N, Hoffmann P, Nöthen MM, Jöckel K‐H, Erbel R, Moebus S. Risk prediction for coronary heart disease by a genetic risk score–results from the heinz nixdorf recall study. BMC Med Genet. 2020;21:178. doi: 10.1186/s12881-020-01113-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klein MD, Williams AK, Lee CR, Stouffer GA. Clinical utility of CYP2C19 genotyping to guide antiplatelet therapy in patients with an acute coronary syndrome or undergoing percutaneous coronary intervention. Arterioscler Thromb Vasc Biol. 2019;39:647–652. doi: 10.1161/ATVBAHA.118.311963 [DOI] [PubMed] [Google Scholar]
- 11. Lima JJ, Thomas CD, Barbarino J, Desta Z, Van Driest SL, El Rouby N, Johnson JA, Cavallari LH, Shakhnovich V, Thacker DL, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2C19 and proton pump inhibitor dosing. Clin Pharmacol Ther. 2021;109:1417–1423. doi: 10.1002/cpt.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aradi D, Komócsi A, Price MJ, Cuisset T, Ari H, Hazarbasanov D, Trenk D, Sibbing D, Valgimigli M, Bonello L. Efficacy and safety of intensified antiplatelet therapy on the basis of platelet reactivity testing in patients after percutaneous coronary intervention: systematic review and meta‐analysis. Int J Cardiol. 2013;167:2140–2148. doi: 10.1016/j.ijcard.2012.05.100 [DOI] [PubMed] [Google Scholar]
- 13. Lyu S‐Q, Yang Y‐M, Zhu J, Wang J, Wu S, Zhang H, Shao X‐H, Ren J‐M. The efficacy and safety of CYP2C19 genotype‐guided antiplatelet therapy compared with conventional antiplatelet therapy in patients with acute coronary syndrome or undergoing percutaneous coronary intervention: a meta‐analysis of randomized controlled trials. Platelets. 2020;31:971–980. doi: 10.1080/09537104.2020.1780205 [DOI] [PubMed] [Google Scholar]
- 14. Lee CR, Luzum JA, Sangkuhl K, Gammal RS, Sabatine MS, Stein Charles M, Kisor DF, Limdi NA, Lee YM, Scott SA, et al. Clinical pharmacogenetics implementation consortium guideline for CYP2C19 genotype and clopidogrel therapy: 2022 update. Clin Pharmacol Ther. 2022;112:959–967. doi: 10.1002/cpt.2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson JA, Caudle KE, Gong L, Whirl‐Carrillo M, Stein CM, Scott SA, Lee MT, Gage BF, Kimmel SE, Perera MA, et al. Clinical pharmacogenetics implementation consortium (CPIC) guideline for pharmacogenetics‐guided warfarin dosing: 2017 update. Clin Pharmacol Ther. 2017;102:397–404. doi: 10.1002/cpt.668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilke RA, Ramsey LB, Johnson SG, Maxwell WD, McLeod HL, Voora D, Krauss RM, Roden DM, Feng Q, Cooper‐DeHoff RM, et al. The clinical pharmacogenomics implementation consortium: CPIC guideline for slco1b1 and simvastatin‐induced myopathy. Clin Pharmacol Ther. 2012;92:112–117. doi: 10.1038/clpt.2012.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamil AA, Lim KK, Koleva‐Kolarova R, Chowienczyk P, Wolfe CDA, Fox‐Rushby J. Genetic‐guided pharmacotherapy for atrial fibrillation: a systematic and critical review of economic evaluations. Value Health. 2021;25:461–472. doi: 10.1016/j.jval.2021.09.013 [DOI] [PubMed] [Google Scholar]
- 18. Berm EJJ, Looff M, Wilffert B, Boersma C, Annemans L, Vegter S, Boven JFMV, Postma MJ. Economic evaluations of pharmacogenetic and pharmacogenomic screening tests: a systematic review. Second update of the literature. PLoS One. 2016;11:e0146262. doi: 10.1371/journal.pone.0146262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu Y, Swanson KM, Rojas RL, Wang Z, St. Sauver JL, Visscher SL, Prokop LJ, Bielinski SJ, Wang L, Weinshilboum R, et al. Systematic review of the evidence on the cost‐effectiveness of pharmacogenomics‐guided treatment for cardiovascular diseases. Genet Med. 2020;22:475–486. doi: 10.1038/s41436-019-0667-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fox‐Rushby J, Koleva‐Kolarova R, Chowienczyk P, Wolfe C. Systematic Review of Economic Evaluations of Stratified Medicines for Cardiovascular Diseases: Study Protocol. 2019.
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the prisma statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ. 2005;331:1064–1065. doi: 10.1136/bmj.38636.593461.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi: 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 24. Lim KK, Koleva‐Kolarova R, Chowienczyk P, Wolfe CDA, Fox‐Rushby J. Genetic‐guided pharmacotherapy for venous thromboembolism: a systematic and critical review of economic evaluations. Pharmacogenomics. 2021;21:625–637. doi: 10.1038/s41397-021-00243-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Drucker E, Krapfenbauer K. Pitfalls and limitations in translation from biomarker discovery to clinical utility in predictive and personalised medicine. EPMA J. 2013;4:7. doi: 10.1186/1878-5085-4-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hatswell AJ, Bullement A, Briggs A, Paulden M, Stevenson MD. Probabilistic sensitivity analysis in cost‐effectiveness models: determining model convergence in cohort models. PharmacoEconomics. 2018;36:1421–1426. doi: 10.1007/s40273-018-0697-3 [DOI] [PubMed] [Google Scholar]
- 27. Evers SM, Goossens M, De Vet H, Van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. Int J Technol Assess Health Care. 2005;21:240–245. doi: 10.1017/S0266462305050324 [DOI] [PubMed] [Google Scholar]
- 28. Odnoletkova I, Goderis G, Pil L, Nobels F, Aertgeerts B, Annemans L, Ramaekers D. Cost‐effectiveness of therapeutic education to prevent the development and progression of type 2 diabetes: systematic review. J Diabetes Metab. 2014;5:1–7. doi: 10.4172/2155-6156.1000438 [DOI] [Google Scholar]
- 29. Wijnen BFM, Van Mastrigt G, Redekop WK, Majoie HJM, De Kinderen RJA, Evers S. How to prepare a systematic review of economic evaluations for informing evidence‐based healthcare decisions: data extraction, risk of bias, and transferability (part 3/3). Expert Rev Pharmacoecon Outcomes Res. 2016;16:723–732. doi: 10.1080/14737167.2016.1246961 [DOI] [PubMed] [Google Scholar]
- 30. Nixon J, Khan KS, Kleijnen J. Summarising economic evaluations in systematic reviews: a new approach. BMJ. 2001;322:1596–1598. doi: 10.1136/bmj.322.7302.1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Turner HC, Lauer JA, Tran BX, Teerawattananon Y, Jit M. Adjusting for inflation and currency changes within health economic studies. Value Health. 2019;22:1026–1032. [DOI] [PubMed] [Google Scholar]
- 32. Economic Research . U.S. Bureau of economic analysis, gross domestic product: Implicit price deflator [gdpdef]. 2021.
- 33. Hart MR, Garrison LP Jr, Doyle DL, Jarvik GP, Watkins J, Devine B. Projected cost‐effectiveness for 2 gene‐drug pairs using a multigene panel for patients undergoing percutaneous coronary intervention. Value Health. 2019;22:1231–1239. doi: 10.1016/j.jval.2019.05.015 [DOI] [PubMed] [Google Scholar]
- 34. Dong OM, Wheeler SB, Cruden G, Lee CR, Voora D, Dusetzina SB, Wiltshire T. Cost‐effectiveness of multigene pharmacogenetic testing in patients with acute coronary syndrome after percutaneous coronary intervention. Value Health. 2020;23:61–73. doi: 10.1016/j.jval.2019.08.002 [DOI] [PubMed] [Google Scholar]
- 35. Jiang M, You JHS. CYP2C19 genotype plus platelet reactivity‐guided antiplatelet therapy in acute coronary syndrome patients: a decision analysis. Pharmacogenet Genomics. 2015;25:609–617. doi: 10.1097/FPC.0000000000000177 [DOI] [PubMed] [Google Scholar]
- 36. Kim JH, Tan DS‐Y, Chan MYY. Cost‐effectiveness of CYP2C19‐guided antiplatelet therapy for acute coronary syndromes in Singapore. Pharmacogenomics J. 2021;21:243–250. doi: 10.1038/s41397-020-00204-6 [DOI] [PubMed] [Google Scholar]
- 37. Limdi NA, Cavallari LH, Lee CR, Hillegass WB, Holmes AM, Skaar TC, Pisu M, Dillon C, Beitelshees AL, Empey PE, et al. Cost‐effectiveness of CYP2C19‐guided antiplatelet therapy in patients with acute coronary syndrome and percutaneous coronary intervention informed by real‐world data. Pharmacogenomics J. 2020;20:724–735. doi: 10.1038/s41397-020-0162-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marrero WJ, Lavieri MS, Sussman JB. A simulation model to evaluate the implications of genetic testing in cholesterol treatment plans. 2019 Winter Simulation Conference (WSC) 2019:1020–2031.
- 39. Jung Y, Frisvold D, Dogan T, Dogan M, Philibert R. Cost–utility analysis of an integrated genetic/epigenetic test for assessing risk for coronary heart disease. Epigenomics. 2021;13:531–547. doi: 10.2217/epi-2021-0021 [DOI] [PubMed] [Google Scholar]
- 40. Hynninen Y, Linna M, Vilkkumaa E. Value of genetic testing in the prevention of coronary heart disease events. PLoS One. 2019;14:e0210010. doi: 10.1371/journal.pone.0210010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jarmul J, Pletcher MJ, Hassmiller Lich K, Wheeler SB, Weinberger M, Avery CL, Jonas DE, Earnshaw S, Pignone M. Cardiovascular genetic risk testing for targeting statin therapy in the primary prevention of atherosclerotic cardiovascular disease. Circ Cardiovasc Qual Outcomes. 2018;11:e004171. doi: 10.1161/CIRCOUTCOMES.117.004171 [DOI] [PubMed] [Google Scholar]
- 42. Mujwara D, Henno G, Vernon ST, Peng S, Di Domenico P, Schroeder B, Busby GB, Figtree GA, Bottà G. Integrating a polygenic risk score for coronary artery disease as a risk‐enhancing factor in the pooled cohort equation: a cost‐effectiveness analysis study. J Am Heart Assoc. 2022;11:e025236. doi: 10.1161/JAHA.121.025236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ramírez de Arellano A, Coca A, de la Figuera M, Rubio‐Terrés C, Rubio‐Rodríguez D, Gracia A, Boldeanu A, Puig‐Gilberte J, Salas E. Economic evaluation of cardio incode®, a clinical‐genetic function for coronary heart disease risk assessment. Appl Health Econ Health Policy. 2013;11:531–542. doi: 10.1007/s40258-013-0053-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oemrawsingh RM, Akkerhuis KM, Van Vark LC, Redekop WK, Rudez G, Remme WJ, Bertrand ME, Fox KM, Ferrari R, Danser AH, et al. Individualized angiotensin‐converting enzyme (ACE)‐inhibitor therapy in stable coronary artery disease based on clinical and pharmacogenetic determinants: the perindopril genetic (PERGENE) risk model. J Am Heart Assoc. 2016;5:e002688. doi: 10.1161/JAHA.115.002688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. AlMukdad S, Elewa H, Al‐Badriyeh D. Economic evaluations of CYP2C19 genotype‐guided antiplatelet therapy compared to the universal use of antiplatelets in patients with acute coronary syndrome: a systematic review. J Cardiovasc Pharmacol Ther. 2020;25:201–211. doi: 10.1177/1074248420902298 [DOI] [PubMed] [Google Scholar]
- 46. Deiman BA, Tonino PA, Kouhestani K, Schrover CE, Scharnhorst V, Dekker LR, Pijls NH. Reduced number of cardiovascular events and increased cost‐effectiveness by genotype‐guided antiplatelet therapy in patients undergoing percutaneous coronary interventions in The Netherlands. Neth Heart J. 2016;24:589–599. doi: 10.1007/s12471-016-0873-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fragoulakis V, Bartsakoulia M, Díaz‐Villamarín X, Chalikiopoulou K, Kehagia K, Ramos JGS, Martínez‐González LJ, Gkotsi M, Katrali E, Skoufas E, et al. Cost‐effectiveness analysis of pharmacogenomics‐guided clopidogrel treatment in spanish patients undergoing percutaneous coronary intervention. Pharmacogenomics J. 2019;19:438–445. doi: 10.1038/s41397-019-0069-1 [DOI] [PubMed] [Google Scholar]
- 48. Phelps CE, O'Sullivan AK, Ladapo JA, Weinstein MC, Leahy K, Douglas PS. Cost effectiveness of a gene expression score and myocardial perfusion imaging for diagnosis of coronary artery disease. Am Heart J. 2014;167:697–706.e692. doi: 10.1016/j.ahj.2014.02.005 [DOI] [PubMed] [Google Scholar]
- 49. Crespin DJ, Federspiel JJ, Biddle AK, Jonas DE, Rossi JS. Ticagrelor versus genotype‐driven antiplatelet therapy for secondary prevention after acute coronary syndrome: a cost‐effectiveness analysis. Value Health. 2011;14:483–491. doi: 10.1016/j.jval.2010.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shiffman D, Slawsky K, Fusfeld L, Devlin JJ, Goss TF. Cost‐effectiveness model of use of genetic testing as an aid in assessing the likely benefit of aspirin therapy for primary prevention of cardiovascular disease. Clin Ther. 2012;34:1387–1394. doi: 10.1016/j.clinthera.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 51. Parthan A, Leahy KJ, O'Sullivan AK, Iakoubova OA, Bare LA, Devlin JJ, Weinstein MC. Cost effectiveness of targeted high‐dose atorvastatin therapy following genotype testing in patients with acute coronary syndrome. PharmacoEconomics. 2013;31:519–531. doi: 10.1007/s40273-013-0054-5 [DOI] [PubMed] [Google Scholar]
- 52. Wang Y, Yan BP, Liew D, Lee VWY. Cost‐effectiveness of cytochrome p450 2c19 *2 genotype‐guided selection of clopidogrel or ticagrelor in chinese patients with acute coronary syndrome. Pharmacogenomics J. 2018;18:113–120. doi: 10.1038/tpj.2016.94 [DOI] [PubMed] [Google Scholar]
- 53. Patel V, Lin FJ, Ojo O, Rao S, Yu S, Zhan L, Touchette DR. Cost‐utility analysis of genotype‐guided antiplatelet therapy in patients with moderate‐to‐high risk acute coronary syndrome and planned percutaneous coronary intervention. Pharm Pract (Granada). 2014;12:438. doi: 10.4321/S1886-36552014000300007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. An XF, Zhai YJ, An HP, Wang XL, Li XY, Zeng XT, Lü J. Cost‐effectiveness analysis of CYP2C19 genetic test in guiding antiplatelet therapy. J Xi'an Jiaotong Univ (Med Sci). 2018;39:853–859. [Google Scholar]
- 55. Borse MS, Dong OM, Polasek MJ, Farley JF, Stouffer GA, Lee CR. CYP2C19‐guided antiplatelet therapy: a cost–effectiveness analysis of 30‐day and 1‐year outcomes following percutaneous coronary intervention. Pharmacogenomics. 2017;18:1155–1166. doi: 10.2217/pgs-2017-0075 [DOI] [PubMed] [Google Scholar]
- 56. Kazi DS, Garber AM, Shah RU, Dudley RA, Mell MW, Rhee C, Moshkevich S, Boothroyd DB, Owens DK, Hlatky MA. Cost‐effectiveness of genotype‐guided and dual antiplatelet therapies in acute coronary syndrome. Ann Intern Med. 2014;160:221–232. doi: 10.7326/M13-1999 [DOI] [PubMed] [Google Scholar]
- 57. Kim K, Touchette DR, Cavallari LH, Ardati AK, DiDomenico RJ. Cost‐effectiveness of strategies to personalize the selection of P2Y12 inhibitors in patients with acute coronary syndrome. Cardiovasc Drugs Ther. 2019;33:533–546. doi: 10.1007/s10557-019-06896-8 [DOI] [PubMed] [Google Scholar]
- 58. Okere AN, Ezendu K, Berthe A, Diaby V. An evaluation of the cost‐effectiveness of comprehensive mtm integrated with point‐of‐care phenotypic and genetic testing for U.S. elderly patients after percutaneous coronary intervention. J Manag Care Spec Pharm. 2018;24:142–152. doi: 10.18553/jmcp.2018.24.2.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Panattoni L, Brown PM, Te Ao B, Webster M, Gladding P. The cost effectiveness of genetic testing for CYP2C19 variants to guide thienopyridine treatment in patients with acute coronary syndromes. PharmacoEconomics. 2012;30:1067–1084. doi: 10.2165/11595080-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 60. Sorich MJ, Horowitz JD, Sorich W, Wiese MD, Pekarsky B, Karnon JD. Cost–effectiveness of using CYP2C19 genotype to guide selection of clopidogrel or ticagrelor in Australia. Pharmacogenomics. 2013;14:2013–2021. doi: 10.2217/pgs.13.164 [DOI] [PubMed] [Google Scholar]
- 61. Lala A, Berger JS, Sharma G, Hochman JS, Scott Braithwaite R, Ladapo JA. Genetic testing in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a cost‐effectiveness analysis. J Thromb Haemost. 2013;11:81–91. doi: 10.1111/jth.12059 [DOI] [PubMed] [Google Scholar]
- 62. Reese ES, Daniel Mullins C, Beitelshees AL, Onukwugha E. Cost‐effectiveness of cytochrome P450 2C19 genotype screening for selection of antiplatelet therapy with clopidogrel or prasugrel. Pharmacotherapy. 2012;32:323–332. doi: 10.1002/j.1875-9114.2012.01048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Goff DC, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 64. Mosley JD, Gupta DK, Tan J, Yao J, Wells QS, Shaffer CM, Kundu S, Robinson‐Cohen C, Psaty BM, Rich SS, et al. Predictive accuracy of a polygenic risk score compared with a clinical risk score for incident coronary heart disease. JAMA. 2020;323:627–635. doi: 10.1001/jama.2019.21782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jiang S, Mathias PC, Hendrix N, Shirts BH, Tarczy‐Hornoch P, Veenstra D, Malone D, Devine B. Implementation of pharmacogenomic clinical decision support for health systems: a cost‐utility analysis. Pharmacogenomics J. 2022;22:188–197. doi: 10.1038/s41397-022-00275-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jiang M, You JHS. CYP2C19 lof and gof‐guided antiplatelet therapy in patients with acute coronary syndrome: a cost‐effectiveness analysis. Cardiovasc Drugs Ther. 2017;31:39–49. doi: 10.1007/s10557-016-6705-y [DOI] [PubMed] [Google Scholar]
- 67. Jiang M, You JHS. Cost–effectiveness analysis of personalized antiplatelet therapy in patients with acute coronary syndrome. Pharmacogenomics. 2016;17:701–713. doi: 10.2217/pgs-2016-0008 [DOI] [PubMed] [Google Scholar]
- 68. Fu Y, Zhang X‐y, Qin S‐b, Nie X‐y, Shi L‐w, Shao H, Liu J. Cost–effectiveness of CYP2C19 lof‐guided antiplatelet therapy in chinese patients with acute coronary syndrome. Pharmacogenomics. 2019;21:33–42. doi: 10.2217/pgs-2019-0050 [DOI] [PubMed] [Google Scholar]
- 69. Claassens DMF, van Dorst PWM, Vos GJA, Bergmeijer TO, Hermanides RS, van't Hof AWJ, van der Harst P, Barbato E, Morisco C, Tjon Joe Gin RM, et al. Cost effectiveness of a Cyp2c19 genotype‐guided strategy in patients with acute myocardial infarction: results from the popular genetics trial. Am J Cardiovasc Drugs. 2022;22:195–206. doi: 10.1007/s40256-021-00496-4 [DOI] [PubMed] [Google Scholar]
- 70. Dong OM, Friede KA, Chanfreau‐Coffinier C, Voora D. Cost‐effectiveness of CYP2C19‐guided P2Y12 inhibitors in veterans undergoing percutaneous coronary intervention for acute coronary syndromes. Eur Heart J. 2022;9:qcac031. doi: 10.1093/ehjqcco/qcac031 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6
Figure S1
References 66–70


