Background
Cardiovascular disease remains an important comorbidity in patients with rheumatoid arthritis (RA), but traditional models do not accurately predict cardiovascular risk in patients with RA. The addition of biomarkers could improve prediction.
Methods and Results
The TARGET (Treatments Against RA and Effect on FDG PET/CT) trial assessed whether different treatment strategies in RA differentially impact cardiovascular risk as measured by the change in arterial inflammation on arterial target to background ratio on fluorodeoxyglucose positron emission tomography/computed tomography scans conducted 24 weeks apart. A group of 24 candidate biomarkers supported by prior literature was assessed at baseline and 24 weeks later. Longitudinal analyses examined the association between baseline biomarker values, measured in plasma EDTA, and the change in arterial inflammation target to background ratio. Model fit was assessed for the candidate biomarkers only, clinical variables only, and models combining both. One hundred nine patients with median (interquartile range) age 58 years (53–65 years), RA duration 1.4 years (0.5–6.6 years), and 82% women had biomarkers assessed at baseline and follow‐up. Because the main trial analyses demonstrated significant target to background ratio decreases with both treatment strategies but no difference across treatment groups, we analyzed all patients together. Baseline values of serum amyloid A, C‐reactive protein, soluble tumor necrosis factor receptor 1, adiponectin, YKL‐40, and osteoprotegerin were associated with significant change in target to background ratio. When selected candidate biomarkers were added to the clinical variables, the adjusted R 2 improved from 0.20 to 0.33 (likelihood ratio P=0.0005).
Conclusions
A candidate biomarker approach identified several promising biomarkers that associate with baseline and treatment‐associated changes in arterial inflammation in patients with RA. These will now be tested in an external validation cohort.
Keywords: biomarkers, cardiovascular disease, rheumatoid arthritis
Subject Categories: Biomarkers
Nonstandard Abbreviations and Acronyms
- JAKi
Janus kinase inhibitor
- MDS
most diseased segment
- TARGET
Treatments Against RA and Effect on FDG PET/CT
- TBR
total to background ratio
Research Perspective.
What New Question Does This Study Raise?
This is the first study to examine a broad range of candidate biomarkers as potential predictors of change in cardiovascular risk among a cohort with rheumatoid arthritis.
From this group of candidate biomarkers, several biomarkers measured at enrollment related to inflammation, bone metabolism, and angiogenesis were found associated with change in cardiovascular risk.
What Question Should Be Addressed Next?
After identifying several biomarkers in the current analyses, we will attempt to replicate them in a large clinical cohort with longer follow‐up and clinical cardiovascular end points.
Rheumatoid arthritis (RA) is the most common autoimmune inflammatory arthritis, affecting ≈2 million people in the United States and >70 million people worldwide. 1 , 2 Although pain and functional disability are the most recognized symptoms, RA is associated with a 5‐ to 6‐year decrease in life expectancy and a standardized mortality ratio of 1.5 compared with age‐ and sex‐matched controls. 3 , 4 As with the general population, cardiovascular disease (CVD) is the leading cause of death in RA, and CVD events are ≈50% more common in RA than the general population. 4 , 5
Optimal management strategies for CVD in an RA population have not been determined. There is some evidence that statins are useful in reducing CVD events in RA populations with normal low‐density lipoprotein levels. 6 As well, head‐to‐head studies of RA treatments suggest that TNF (tumor necrosis factor) inhibitors have similar effects as IL (interleukin)‐6 blockade on CVD events, 7 , 8 but JAKis (Janus kinase inhibitors) appear associated with increased CVD risk relative to TNF inhibitors in groups with known CVD risk factors. 9 However, CVD risk stratification for patients with RA lacks clarity. There is good evidence that the traditional risk factors used in the original Framingham Risk Score do not work well to predict CVD events in RA. 10 The QRISK3 prediction score for CVD includes RA diagnosis, and the American College of Cardiology/American Heart Association risk calculator (population cohort equation, PCE) includes RA as a risk enhancer. However, few patients with RA were included in either model, and no specific RA factors were considered. 11 , 12 A ×1.5 multiplier has been proposed as a calibration tool for subgroups of patients with RA, 13 but no formal derivation and validation study to judge whether this is an improvement over general population tools exists. One modification of the Framingham Risk Score considers RA factors, such as disease activity, disease duration, disability, and corticosteroid use. 14 Although this RA‐specific risk score has undergone external validation, 15 some analyses suggest that it is not any better than the PCE. 16
None of the above CVD risk stratification tools for RA have considered a broad range of biomarkers. Several biomarkers associated with RA disease activity have been tested in a multibiomarker panel and were found associated with CVD events; these biomarkers include vascular cell adhesion molecule 1 (VCAM‐1), endothelial growth factor, vascular endothelial growth factor (VEGF‐A), IL‐6, tumor necrosis factor, receptor 1 (TNF‐R1), matrix metalloproteinase 1 (MMP‐1), YKL‐40, leptin, resistin, serum amyloid A (SAA), and CRP (C‐reactive protein). 17 However, substantial evidence links a broader list of RA biomarkers to CVD (listed in Table S1). Some biomarkers are in inflammatory pathways, some angiogenesis, others are markers of thrombosis or lipid metabolism. There has not been a prior study in an RA population that has rigorously tested candidate biomarkers for their relationship with CVD and then tested for their incremental value over traditional risk scores, such as the PCE.
The current article describes a preliminary step in testing candidate biomarkers for CVD by examining the association between these biomarkers and a measure of vascular inflammation in a well‐characterized population with RA that was enrolled in the TARGET (Treatments Against RA and Effect on FDG PET/CT) trial. 18 , 19 We hypothesized that several of the candidate biomarkers measured at baseline would be significantly associated with the change in cardiovascular risk, assessed by fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT).
Methods
Study Design and Population
The TARGET trial was approved by the Mass General Brigham Human Research Committee (institutional review board protocol 2014 P002747). All subjects gave voluntary informed consent. Data from the trial and the biomarker study will be available through dbGaP (https://www.ncbi.nlm.nih.gov/gap/).
The TARGET trial was funded to assess whether different RA treatments impact CVD risk differentially. In an open‐label randomized controlled trial, we compared the effects on vascular inflammation of a tumor necrosis factor (TNF) inhibitor (TNFi, either etanercept or adalimumab) in combination with methotrexate versus triple therapy (methotrexate, sulfasalazine, and hydroxychloroquine) in patients with RA who are inadequate responders to methotrexate monotherapy. The design of the TARGET trial has been described in detail. 18 Briefly, we screened 232 patients with RA, who were inadequate responders to methotrexate, and enrolled 159 patients from 35 sites across the United States, of whom 115 had measurements of change (ie, scans at baseline and follow‐up that could be evaluated) in vascular inflammation. The primary trial outcome was change in mean of the maximum total to background ratio of the most diseased segment (meanmax total to background ratio [TBR] of the most diseased segment [MDS]).
The primary analysis of the TARGET trial showed significant reductions on TBR MDS in both arms; the difference in the reductions on TBR MDS was not different between the 2 treatment strategies. The current set of analyses examines the relationship between baseline values of candidate biomarkers and change in TBR MDS.
The study was developed with input from a multidisciplinary team supported by a public–private partnership through the Foundation for the National Institutes of Health Biomarker Consortium.
Candidate Biomarkers
Numerous commonly measured biomarkers have been associated with both conditions (see Table S1). These biomarkers derive from inflammatory pathways, including cytokines, adipokines, and regulatory molecules. Many advanced lipid markers associate with both RA and CVD. Several other proteins, many known to be associated with cardiac injury, are also found to be dysregulated in RA. Although some of these biomarkers may play causal roles in RA and CVD, it is likely that many of them reflect specific features of the systemic inflammatory activation in patients with RA. RA systemic inflammation is known to impact cytokine regulation, lipid and fat metabolism, the atherosclerotic process, and the myocardium. 20
We collected biospecimens at multiple visits across all enrolling sites. These plasma biospecimens were collected in EDTA tubes and processed locally, then sent to a central biorepository on dry ice; they were stored in a −80 oC freezer until trial completion when all samples were run concurrently. They were run in several Clinical Laboratory Improvement Amendments (CLIA)‐certified laboratories. The DiscoveryMAP and Vectra DA (RBM Myriad/Lab Quest, Austin, TX) were run at RBM laboratories using a multiplex assay previously described. 21 Several additional candidate biomarkers were run at Boston Children's Hospital using single analyte assays (NT‐proBNP [N‐terminal pro‐B‐type natriuretic peptide], high sensitivity cardiac troponin T, low‐density lipoprotein; Roche Diagnostics, Indianapolis IN).
Several biomarkers were measured in both DiscoveryMAP and Vectra (eg, leptin, SAA, MMP‐3, resistin, VCAM‐1, VEGF, and YKL‐40). As well, high‐sensitivity CRP was measured in Vectra and at Boston Children's Hospital. We used these simultaneous measurements of a single analyte to improve their precision using Deming regression. Bland‐Altman plots were evaluated for agreement and to identify outliers. We used coefficients of variation to assess quality of analytes' measurements. For all biomarkers, before analysis, we shrank outliers whose values were >2 SDs from the mean. This outlier correction guarantees that all biomarker measurements fit between ±3 SDs while their rank order is preserved. For the purposes of modeling, the biomarkers were then Z scored to standardize the units of measurement.
See Data S1 for more details on handling candidate biomarker measurements.
FDG PET/CT Outcome
The outcome of interest for these analyses was cardiovascular risk as assessed by the FDG PET/CT. In prior studies, changes in FDG PET/CT vascular signal predicted the rate of subsequent plaque expansion as measured by magnetic resonance imaging 22 or CT. 23 Studies demonstrate an association between FDG signal and cardiovascular risk factors or risk scores. 24 , 25 , 26 , 27 As well, increased arterial FDG uptake was associated with higher risk of subsequent stroke and myocardial infarction. 28 , 29 The primary measure of arterial inflammation used as the outcome of the TARGET trial was the TBR MDS). 24 , 25 This was assessed by a centralized group of readers who have substantial experience in these methods 30 , 31 ; for all vessels and time points, the intraclass correlation coefficient was >0.82, indicating good reliability of intrareader assessments of cardiovascular inflammation.
PCE Model
The PCEs were developed as a method for selecting patients who would be good candidates for lipid‐lowering treatments such as statins. 32 The PCE estimates 10‐year risk of coronary heart disease events or stroke among patients without known preexisting cardiovascular disease between the ages of 40 and 79 years. The factors used in calculating the PCE include age, sex, race (White or Black), systolic blood pressure, use of medications for hypertension, total cholesterol, high‐density lipoprotein, smoking status, and presence of diabetes. RA is considered a risk‐enhancing factor, but specific recommendations about how to use such a factor have not been described by the American College of Cardiology/American Heart Association. 32
We calculated the PCE for all subjects at baseline. A subject's PCE estimate was used as a covariate in models with TBR MDS as the outcome. We examined both the continuous values as well as categories of 10‐year risk (ie, <5% low, 5%–7.5% borderline, 7.5%–20% intermediate, and ≥20% high).
Statistical Analysis
We first examined the candidate biomarker values, calibrating ones that had been measured simultaneously using 2 different methods and trimming outliers. 33 , 34 Preprocessed values of candidate biomarkers (see Candidate Biomarker section) at baseline were compared across the 2 treatment groups (TNFi versus triple therapy) using the Welch 2‐sample t test. The baseline values of the candidate biomarkers were compared for the 2 treatment groups (TNFi versus triple therapy). No significant differences were observed (Table 1), and thus, the 2 treatment arms were combined for all further analyses.
Table 1.
Distribution of Biomarker Values Studied in the TARGET Trial Cohort by Treatment Group
| Cytokine/inflammation, units, median (IQR) | Total cohort | TNFi | Triple therapy | P value |
|---|---|---|---|---|
| n=109 | n=55 | n=54 | ||
| IL‐6, pg/mL | 11 (5–23) | 11 (5–22) | 11 (6–22) | 0.2 |
| sTNFR1, pg/mL | 1327 (1097–1712) | 1316 (1104–1718) | 1392 (1068–1699) | 0.7 |
| SAA, ng/mL | 15 478 (7674–42 875) | 14 994 (6555–51 327) | 16 371 (8560–30 635) | 0.4 |
| hsCRP, μg/mL | 4 (2–9) | 4 (1–8) | 4 (2–9) | 0.8 |
| CD‐40 ligand, ng/mL | 0.10 (0.06–0.23) | 0.11 (0.07–0.27) | 0.09 (0.05–0.17) | 0.2 |
| Adipokines | ||||
| Adiponectin, μg/mL | 7.1 (4.5–9.8) | 7.1 (4.5–11.2) | 7.2 (4.8–9.2) | 0.6 |
| Leptin, ng/mL | 22 (11–37) | 21 (12–36) | 26 (11–40) | 0.8 |
| Resistin, ng/mL | 2.84 (2.40–3.73) | 2.81 (2.44–3.67) | 2.86 (2.40–3.74) | 0.5 |
| Atherothrombosis | ||||
| Antithrombin III, μg/mL | 453 (389–522) | 453 (396–532) | 444 (381–504) | 0.6 |
| PAI‐1, ng/mL | 75 (45–117) | 70 (44–133) | 76 (48–11 209) | 0.4 |
| Lipids parameters | ||||
| Apolipoprotein C3, μg/mL | 301 (244–407) | 293 (240–398) | 328 (250–412) | 0.3 |
| Apolipoprotein A1, mg/mL | 2.10 (1.80–2.70) | 2.02 (1.85–2.60) | 2.25 (1.80–2.80) | 0.3 |
| Apolipoprotein A2, ng/mL | 329 (257–393) | 318 (244–388) | 343 (288–397) | 0.3 |
| Apolipoprotein B, μg/mL | 909 (674–1090) | 974 (714–1155) | 812 (660–1025) | 0.2 |
| Apolipoprotein C1, μg/mL | 348 (256–402) | 339 (238–433) | 354 (274–393) | 0.9 |
| Lp(a), μg/mL | 105 (48–247) | 105 (47–328) | 104 (50–236) | 0.3 |
| LDL, mg/dL | 99 (82–122) | 101 (81–126) | 98 (86–119) | 0.8 |
| Other analytes | ||||
| hsTnT, ng/L* | 60 (55%) | 32 (58%) | 28 (52%) | 0.5 |
| NT‐proBNP, pg/mL | 356 (192–737) | 350 (149–738) | 368 (258–710) | 0.9 |
| VCAM‐1, ng/mL | 608 (508–737) | 625 (507–783) | 607 (525–682) | 0.2 |
| VEGF‐A, pg/mL | 153 (99–207) | 162 (104–208) | 134 (89–200) | 0.2 |
| MMP‐1, ng/mL | 7636 (4652–11 270) | 7872 (5378–12 292) | 7545 (3955–10 558) | 0.4 |
| MMP‐3, ng/mL | 6 (4–14) | 7 (4–15) | 6 (4–13) | 0.3 |
| YKL‐40, ng/mL | 30 (21–61) | 31 (23–63) | 30 (21–61) | 0.6 |
| Cystatin‐C, ng/mL | 1140 (982–1290) | 1120 (990–1275) | 1160 (968–1380) | 0.5 |
| Osteopontin, ng/mL | 33 (25–44) | 33 (24–44) | 34 (25–44) | 0.9 |
| Osteoprotegrin, pM | 7.80 (6.50–9.70) | 7.90 (6.55–9.15) | 7.75 (6.43–10.00) | 0.3 |
CT indicates computed tomography; hsCRP, high‐sensitivity C‐reactive protein; FDG, fluorodeoxyglucose; hsTnT, high sensitivity troponin T; IL, interleukin; IQR, interquartile range; LDL, low‐density lipoprotein; Lp(a), lipoprotein(a); MMP‐1, matrix metalloproteinase 1; MMP‐3, matrix metalloproteinase 3; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PAI‐1, plasminogen activator inhibitor‐1; PET, positron emission tomography; RA, rheumatoid arthritis; SAA, serum amyloid A; sTNFR1, soluble tumor necrosis factor receptor 1; TARGET, Treatments Against RA and Effect on FDG PET/CT; TNFi, tumor necrosis factor inhibitor; VCAM‐1, vascular cell adhesion molecule 1; and VEGF‐A, vascular endothelial growth factor A.
hsTnT data represent the n (%) with detectable levels. Other subjects were below detectable range. P values were calculated from the Welch 2‐sample t test.
Next, we assessed the associations between each individual candidate biomarker Z score value (number of SDs from the mean) at baseline and change in TBR from baseline to 24 weeks in a series of adjusted linear regression models. We considered different levels of adjustment: all candidate biomarkers simultaneously, PCE alone, all biomarkers and PCE, and selected biomarkers and PCE (PCE was calculated using the R package 35 , 36 ). All biomarkers with P≤0.10 in models with all the biomarkers and PCE were advanced to the selected biomarkers models. We were interested in the association of the biomarkers with the change in TBR, as well as the model fit as measured by the R 2, the root mean square error, Akaike information criterion, and Bayesian information criterion. Further model fit was compared using the likelihood ratio test. Next, a similar set of models was run that compared baseline biomarkers with baseline TBR. Because PCE is a well‐recognized cardiovascular risk stratification tool in the general population, we also ran models with baseline biomarkers as predictor variables and baseline PCE as a secondary outcome. Sensitivity analyses were performed with models additionally adjusted for treatment assignment and glucocorticoid use.
Collinearity was assessed in all models using the variance inflation factor. All analyses were run using R (R Foundation for Statistical Computing, Vienna, Austria, version 4.2.2).
Results
TARGET Trial Population Characteristics
Of the 159 participants randomized into the TARGET trial, 138 subjects completed both the baseline and final scans. Of these, 119 participants had baseline scans that could be analyzed for TBR, and 115 had baseline and follow‐up FDG PET/CT scans that could be analyzed for TBR. Of these individuals, 109 had baseline and follow‐up biomarkers measured. All subjects were recruited and randomized between March 2016 and November 2021. The subjects included in the main analysis were well balanced across the 2 treatment groups (see Table S1) with a median age of 58.0 years, and 71% were women. The median duration of RA was 1.4 years, with a median baseline disease activity that was moderate (disease activity score in 28 joints, DAS28‐CRP 4.8). The median high‐sensitivity CRP was 3.9 mg/L, which is elevated above normal. No subjects had recent use of biologic disease modifying anti‐rheumatic drugs, and the median methotrexate dosage was 20 mg per week; oral glucocorticoids were used by 33% of subjects. Known cardiovascular disease was an exclusion. Diabetes was recorded at baseline in 1.7% of subjects, hypertension in 45.2%, hyperlipidemia in 20.0%, and current tobacco use in 12.2%. The median body mass index was 29.3 kg/m2.
The baseline characteristics of the study population were also compared across tertiles of baseline TBR MDS (see Table S1) and found to be similar. The baseline biomarkers were also compared across tertiles of baseline TBR MDS (see Table S1) and found to be similar.
Distribution of Biomarkers and Outcomes
The biomarkers were similar at baseline across the 2 treatment groups (Table 1), and the change in TBR MDS was similar across treatment groups. 19
The distribution of baseline and change in TBR MDS was examined (Figure 1A). We found that the vast majority had values >2.0; ≤2.0 is a typical measure in an adult without known coronary artery disease. 37 Approximately one‐quarter had values ≥3.0. The distribution of the change in TBR MDS values was also examined (Figure 1B). Most subjects had reductions in TBR MDS between baseline and 24‐week follow‐up; average reduction across both groups was −0.21 (7.9%). This reduction is comparable to that seen with increasing intensity of statin therapy from low to high. 30
Figure 1. Distribution of most diseased segment target to background ratio.
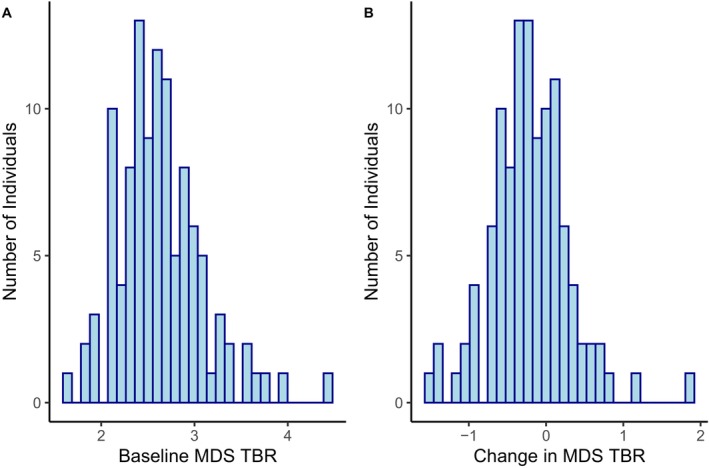
A, Baseline. B, Change in most diseased segment target to background ratio. MDS indicates most diseased segment; and TBR, target to background ratio.
Relationship Between Baseline Biomarkers and Change in Arterial Inflammation
The primary outcome was change in TBR MDS, analyzed as a linear outcome with baseline biomarker values (Z scored) as predictors (Table 2). Six biomarkers, SAA, CRP, CD‐40 ligand, adiponectin, YKL‐40, and osteoprotegerin (OPG), were advanced to the multivariable models. The model with these 6 biomarkers and PCE had moderate fit with adjusted R 2=0.32 and root mean square error=0.401. This fit was an improvement (likelihood ratio P=0.0005) over the model that contained only the clinical PCE variables (R 2=0.20 and root mean square error=0.450). A correlation matrix (see Figure 2) suggests that SAA, CRP, and CD‐40 ligand are moderately correlated, but they added to the multivariable model. In a sensitivity analysis that included RA treatments and corticosteroids, the model performed similarly, resulting in the selection of the same biomarkers and the same model fit (see Table S1).
Table 2.
Linear Regression Results Assessing Relationship Between Z Score of Candidate Biomarker at Baseline and Change in Target to Background Ratio in the TARGET Trial Cohort With Increasing Adjustment
| Biomarker | All biomarkers only, β estimate (95% CI) | Baseline PCE only, β estimate (95% CI) | All biomarkers+PCE+ baseline TBR, β estimate (95% CI) | Selected biomarkers+ PCE+baseline TBR, β estimate (95% CI) |
|---|---|---|---|---|
| Cytokine/inflammation | ||||
| IL‐6 | 0.04 (−0.09 to 0.17) | … | 0.04 (−0.09 to 0.17) | … |
| sTNFR1 | −0.10 (−0.23 to 0.02)* | … | −0.12 (−0.25 to 0.01)* , ‡ | −0.09 (−0.18 to 0.00)* , ‡ |
| SAA | −0.17 (−0.35 to 0.00)* | … | −0.17 (−0.34 to 0.01)* , ‡ | −0.19 (−0.36 to −0.03)† , ‡ |
| hsCRP | 0.17 (−0.03 to 0.36)* | … | 0.16 (−0.04 to 0.36)* , ‡ | 0.22 (0.06 to 0.39)† , ‡ |
| CD‐40 ligand | −0.07 (−0.19 to 0.05) | … | −0.05 (−0.19 to 0.09) | … |
| Adipokines | ||||
| Adiponectin | −0.12 (−0.22 to −0.01)† , ‡ | … | −0.13 (−0.24 to −0.02)* , ‡ | −0.06 (−0.15,0.02)* , ‡ |
| Leptin | −0.03 (−0.13 to 0.08) | … | −0.02 (−0.13 to 0.09) | … |
| Resistin | 0.07 (−0.03 to 0.18) | … | 0.09 (−0.02 to 0.20) | … |
| Atherothrombosis | ||||
| Antithrombin III | 0.04 (−0.07 to 0.15) | … | 0.04 (−0.08 to 0.16) | … |
| PAI‐1 | −0.05 (−0.17 to 0.07) | … | −0.05 (−0.18 to 0.08) | … |
| Lipids parameters | ||||
| Apolipoprotein A1 | 0.07 (−0.05 to 0.18) | … | 0.06 (−0.06 to 0.18) | … |
| Apolipoprotein A2 | 0.02 (−0.13 to 0.18) | … | 0.02 (−0.15 to 0.18) | … |
| Apolipoprotein B | 0.02 (−0.09 to 0.13) | … | 0.01 (−0.11 to 0.14) | … |
| Apolipoprotein C1 | 0.02 (−0.14 to 0.17) | … | 0.03 (−0.14 to 0.19) | … |
| Apolipoprotein C3 | −0.02 (−0.14 to 0.10) | … | −0.03 (−0.16 to 0.09) | … |
| Apolipoprotein H | −0.05 (−0.18 to 0.08) | … | −0.05 (−0.18 to 0.09) | … |
| Lp(a) | 0.03 (−0.06 to 0.12) | … | 0.03 (−0.07 to 0.13) | … |
| LDL | −0.04 (−0.14 to 0.07) | … | −0.04 (−0.16 to 0.07) | … |
| Other analytes | ||||
| hsTnT | −0.11 (−0.32 to 0.11) | … | −0.11 (−0.34 to 0.12) | … |
| NT‐proBNP | 0.04 (−0.06 to 0.13) | … | 0.04 (−0.08 to 0.15) | … |
| VCAM‐1 | 0.04 (−0.07 to 0.16) | … | 0.05 (−0.08 to 0.17) | … |
| VEGF‐A | −0.02 (−0.13 to 0.08) | … | −0.01 (−0.13 to 0.10) | … |
| MMP‐1 | −0.02 (−0.12 to 0.08) | … | −0.02 (−0.12 to 0.08) | … |
| MMP‐3 | 0.08 (−0.04 to 0.20) | … | 0.08 (−0.04 to 0.20) | … |
| YKL‐40 | 0.15 (0.05 to 0.25)† | … | 0.15 (0.04 to 0.26)† , ‡ | 0.11 (0.02 to 0.20)† , ‡ |
| Cystatin‐C | −0.04 (−0.14 to 0.06) | … | −0.05 (−0.17 to 0.06) | … |
| Osteopontin | −0.03 (−0.13 to 0.07) | … | −0.02 (−0.13 to 0.10) | … |
| Osteoprotegrin | −0.09 (−0.20 to 0.01)* | … | −0.09 (−0.20 to 0.02)* , ‡ | −0.12 (−0.20 to −0.03)* , ‡ |
| Pooled cohort equation | … | 0.00 (−0.02 to 0.01) | 0.00 (−0.02 to 0.02) | 0.01 (−0.01 to 0.02) |
| Model fit statistics | ||||
| Adjusted R 2 | 0.35 | 0.20 | 0.32 | 0.32 |
| RMSE | 0.349 | 0.450 | 0.352 | 0.401 |
| AIC | 141.9 | 139.7 | 143.3 | 127.3 |
| BIC | 225.3 | 150.4 | 228.5 | 153.9 |
All models include the baseline target to background ratios values from the most diseased segment (TBR MDS). PCE includes as predictor variables age, sex, race, diabetes status, cigarette use, systolic blood pressure, treatment for hypertension, total and high‐density lipoprotein cholesterol values. R 2 indicates better model fit when larger and RMSE is better when smaller. The variance inflation factors across all models were <5, which signifies low (or no) collinearity. AIC indicates Akaike information criterion; BIC, Bayesian information criterion; CT, computed tomography; FDG, fluorodeoxyglucose; hsCRP, high‐sensitivity C‐reactive protein; hsTnT, high sensitivity troponin T; IL, interleukin; LDL, low‐density lipoprotein; Lp(a), lipoprotein(a); MDS, most diseased segment; MMP‐1, matrix metalloproteinase 1; MMP‐3, matrix metalloproteinase 3; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PAI‐1, plasminogen activator inhibitor‐1; PCE, pooled cohort equation; PET, positron emission tomography; RA, rheumatoid arthritis; RMSE, root mean square error; SAA, serum amyloid A; sTNFR1, soluble tumor necrosis factor receptor 1; TARGET, Treatments Against RA and Effect on FDG PET/CT; TBR, target to background ratio; VCAM‐1, vascular cell adhesion molecule 1; and VEGF‐A, vascular endothelial growth factor A.
P<0.10.
P<0.01.
Values have P≤0.10 and were advanced to the selected biomarkers model (right column).
Figure 2. Correlation matrix for baseline biomarkers.
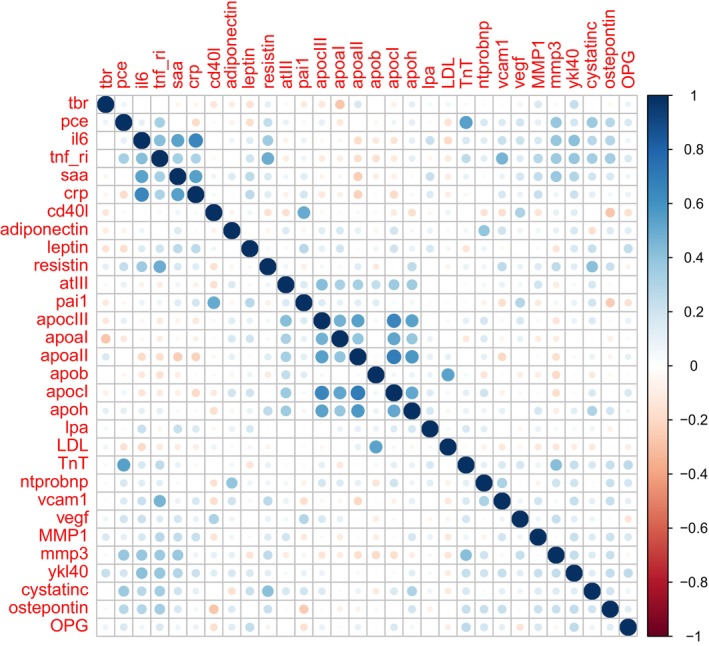
apoaI indicates apolipoprotein A1; apoaII, apolipoprotein A2; apoB, apolipoprotein B; apocI, apolipoprotein C1; apocIII, apolipoprotein C3; apoh, apolipoprotein H; atIII, antithrombin III; cd40l, CD40 ligand; crp, high‐sensitivity C‐reactive protein; cystatinc, cystatin‐C; il6, interleukin 6; LDL, low‐density lipoprotein; lpa, lipoprotein a; MMP1, matrix metalloproteinase 1; mmp3, matrix metalloproteinase 3; ntprobnp, NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide); OPG, osteoprotegerin; pai1, plasminogen activator inhibitor 1; pce, population cohort equation; saa, serum amyloid A; tbr, target to background ratio; tnf ri, tumor necrosis factor receptor 1; TnT, high‐sensitivity troponin T; vcam1, vascular cell adhesion molecule 1; vegf, vascular endothelial growth factor; and ykl40, YKL‐40.
We also examined the relationship between baseline biomarkers and baseline TBR MDS (Table 3). Although additional biomarkers (apolipoprotein A1, apolipoprotein A2, MMP‐3, and osteoprotegerin) were significantly associated with baseline TBR, a model including these biomarkers and PCE had a relatively low adjusted R 2 (0.17).
Table 3.
Linear Regression Results Assessing Relationship Between Z Score of Candidate Biomarkers at Baseline and Target to Background Ratio at Baseline in the TARGET Trial Cohort With Increasing Adjustment
| Biomarker | All biomarkers only, β estimate (95% CI) | Baseline PCE only, β estimate (95% CI) | All biomarkers+PCE, β estimate (95% CI) | Selected biomarkers+PCE, β estimate (95% CI) |
|---|---|---|---|---|
| Cytokine/inflammation | ||||
| IL‐6 | −0.05 (−0.18 to 0.08) | … | −0.06 (−0.19 to 0.08) | … |
| sTNFR1 | −0.05 (−0.17 to 0.08) | … | −0.06 (−0.19 to 0.07) | … |
| SAA | 0.03 (−0.14 to 0.21) | … | 0.04 (−0.14 to 0.22) | … |
| hsCRP | −0.01 (−0.20 to 0.19) | … | −0.01 (−0.21 to 0.20) | … |
| CD‐40 ligand | −0.10 (−0.22 to 0.03) | … | −0.09 (−0.24 to 0.05) | … |
| Adipokines | ||||
| Adiponectin | −0.06 (−0.17 to 0.04) | … | −0.07 (−0.18 to 0.04) | … |
| Leptin | −0.04 (−0.15 to 0.06) | … | −0.02 (−0.14 to 0.09) | … |
| Resistin | 0.00 (−0.10 to 0.11) | … | 0.01 (−0.10 to 0.12) | … |
| Atherothrombosis | ||||
| Antithrombin III | −0.07 (−0.18 to 0.04) | … | −0.07 (−0.19 to 0.05) | … |
| PAI‐1 | 0.00 (−0.13 to 0.12) | … | 0.00 (−0.13 to 0.13) | … |
| Lipids parameters | ||||
| Apolipoprotein A1 | −0.11 (−0.23 to 0.00)* | … | −0.11 (−0.23 to 0.01)* , ‡ | −0.19 (−0.28 to −0.10)* , ‡ |
| Apolipoprotein A2 | 0.21 (0.05 to 0.36)† | … | 0.22 (0.05 to 0.38)† , ‡ | 0.15 (0.06 to 0.24)† , ‡ |
| Apolipoprotein B | 0.01 (−0.11 to 0.12) | … | 0.01 (−0.11 to 0.13) | … |
| Apolipoprotein C1 | −0.06 (−0.22 to 0.10) | … | −0.06 (−0.22 to 0.11) | … |
| Apolipoprotein C3 | −0.10 (−0.22 to 0.02) | … | −0.10 (−0.23 to 0.03) | … |
| Apolipoprotein H | 0.05 (−0.08 to 0.19) | … | 0.05 (−0.09 to 0.19) | … |
| Lp(a) | 0.01 (−0.08 to 0.11) | … | 0.01 (−0.09 to 0.11) | … |
| LDL | 0.04 (−0.07 to 0.15) | … | 0.03 (−0.08 to 0.15) | … |
| Other analytes | ||||
| hsTnT | −0.07 (−0.28 to 0.15) | … | −0.06 (−0.29 to 0.17) | … |
| NT‐proBNP | −0.04 (−0.14 to 0.06) | … | −0.05 (−0.16 to 0.07) | … |
| VCAM‐1 | 0.06 (−0.06 to 0.18) | … | 0.07 (−0.06 to 0.20) | … |
| VEGF‐A | 0.10 (0.00 to 0.21)* | … | 0.10 (−0.01 to 0.21)* , ‡ | 0.05 (−0.04 to 0.14) |
| MMP‐1 | 0.01 (−0.09 to 0.10) | … | 0.01 (−0.09 to 0.11) | … |
| MMP‐3 | 0.13 (0.01 to 0.25)* | … | 0.13 (0.01 to 0.25)* , ‡ | 0.09 (−0.01 to 0.18)* , ‡ |
| YKL‐40 | 0.04 (−0.07 to 0.14) | … | 0.04 (−0.07 to 0.15) | … |
| Cystatin‐C | −0.03 (−0.13 to 0.07) | … | −0.05 (−0.16 to 0.07) | … |
| Osteopontin | −0.02 (−0.13 to 0.08) | … | −0.03 (−0.14 to 0.09) | … |
| Osteoprotegrin | 0.11 (0.00 to 0.21)* | … | 0.10 (−0.01 to 0.21)* , ‡ | 0.07 (−0.02 to 0.16)* , ‡ |
| Pooled cohort equation | … | 0.01 (−0.01 to 0.02) | 0.00 (−0.01 to 0.02) | 0.01 (−0.01 to 0.02) |
| Model fit statistics | ||||
| Adjusted R 2 | 0.18 | −0.004 | 0.17 | 0.17 |
| RMSE | 0.359 | 0.463 | 0.361 | 0.409 |
| AIC | 145.9 | 143.8 | 146.8 | 148.8 |
| BIC | 226.6 | 151.7 | 229.3 | 172.8 |
All models include the baseline target to background ratios values from the most diseased segment (TBR MDS). PCE includes age, sex, race, diabetes status, cigarette use, systolic blood pressure, treatment for hypertension, and high‐density lipoprotein value. R 2 indicates better model fit when larger, and RMSE is better when smaller. The variance inflation factors across all models were <5, which signifies low (or no) collinearity. AIC indicates Akaike information criterion; BIC, Bayesian information criterion; CT, computed tomography; FDG, fluorodeoxyglucose; hsCRP, high‐sensitivity C‐reactive protein; hsTnT, high sensitivity troponin T; IL, interleukin; LDL, low‐density lipoprotein; Lp(a), lipoprotein(a); MDS, most diseased segment; MMP‐1, matrix metalloproteinase 1; MMP‐3, matrix metalloproteinase 3; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PAI‐1, plasminogen activator inhibitor‐1; PCE, pooled cohort equation; PET, positron emission tomography; RA, rheumatoid arthritis; RMSE, root mean square error; SAA, serum amyloid A; sTNFR1, soluble tumor necrosis factor receptor 1; TARGET, Treatments Against RA and Effect on FDG PET/CT; TBR, target to background ratio; VCAM‐1, vascular cell adhesion molecule 1; and VEGF‐A, vascular endothelial growth factor A.
P<0.10.
P<0.01.
Values have P≤0.10 and were advanced to the selected biomarkers model (right column).
Relationship Between Baseline Biomarkers and Baseline PCE
We also examined the relationship between baseline biomarkers and baseline PCE (Table 4). We focused only on the cross‐sectional relationship, because we anticipated that most clinical variables in the PCE would not change substantially between baseline and 24 weeks. Other biomarkers, such as leptin, troponin T, NT‐proBNP, VCAM‐1, VEGF‐A, and cystatin‐C, were associated with PCE. The model fit was moderate based on R 2 and root mean square error.
Table 4.
Linear Regression Results Assessing Relationship Between Z Score of Candidate Biomarker at Baseline and Pooled Cohort Equation Score at Baseline in the TARGET Trial Cohort With Increasing Adjustment
| Biomarker | All biomarkers only, β estimate (95% CI) | Selected biomarkers only, β estimate (95% CI) |
|---|---|---|
| Cytokine/inflammation | ||
| IL‐6 | 1.2 (−0.65 to 3.0) | … |
| sTNFR1 | 1.3 (−0.53 to 3.1) | … |
| SAA | 0.03 (−2.5 to 2.5) | … |
| hsCRP | −1.7 (−4.4 to 1.1) | … |
| CD‐40 ligand | 1.1 (−0.87 to 3.0) | … |
| Adipokines | ||
| Adiponectin | −1.2 (−2.7 to 0.32) | … |
| Leptin | −2.1(−3.6 to −0.64)* | −1.6 (−2.8 to −0.36)* |
| Resistin | 0.47 (−1.1 to 2.0) | … |
| Atherothrombosis | ||
| Antithrombin III | 1.4 (−0.18 to 3.0) | … |
| PAI‐1 | −0.51 (−2.3 to 1.3) | … |
| Lipids parameters | ||
| Apolipoprotein A1 | −0.66 (−2.3 to 0.94) | … |
| Apolipoprotein A2 | −1.2 (−3.4 to 1.1) | … |
| Apolipoprotein B | −0.37 (−2.1 to 1.3) | … |
| Lp(a) | 0.64 (−0.69 to 2.0) | … |
| LDL | −0.67 (−2.2 to 0.89) | … |
| Other analytes | ||
| hsTnT | 2.6 (−0.48 to 5.7)* | 3.8 (1.4 to 6.2) |
| NT‐proBNP | 1.9 (0.38 to 3.4)* | 1.6 (0.42 to 2.9) |
| VCAM‐1 | −2.0 (−3.7 to −0.36)* | −0.66 (−1.9 to 0.54) |
| VEGF‐A | 1.1 (−0.37 to 2.6)* | 0.99 (−0.18 to 2.2) |
| MMP‐1 | 0.13 (−1.3 to 1.5) | … |
| MMP‐3 | −0.42 (−2.1 to 1.2) | … |
| YKL‐40 | 0.41 (−1.1 to 1.9) | … |
| Cystatin‐C | 1.5 (−0.03 to 3.0)* | 2.5 (1.3 to 3.7) |
| Osteopontin | 0.82 (−0.75 to 2.4) | … |
| Osteoprotegrin | 1.3 (−0.18 to 2.8) | … |
| Model fit statistics | ||
| Adjusted R 2 | 0.30 | 0.31 |
| RMSE | 4.99 | 5.63 |
| AIC | 701.5 | 683.4 |
| BIC | 781.4 | 704.8 |
All models include the baseline target to background ratios values from the most diseased segment (TBR MDS). PCE includes age, sex, race, diabetes status, cigarette use, systolic blood pressure, treatment for hypertension, total and high‐density lipoprotein value. R 2 indicates better model fit when larger, and RMSE is better when smaller. AIC indicates Akaike information criterion; BIC, Bayesian information criterion; CT, computed tomography; FDG, fluorodeoxyglucose; hsCRP, high‐sensitivity C‐reactive protein; hsTnT, high sensitivity troponin T; IL, interleukin; LDL, low‐density lipoprotein; Lp(a), lipoprotein(a); MDS, most diseased segment; MMP‐1, matrix metalloproteinase 1; MMP‐3, matrix metalloproteinase 3; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PAI‐1, plasminogen activator inhibitor‐1; PCE, pooled cohort equation; PET, positron emission tomography; RA, rheumatoid arthritis; RMSE, root mean square error; SAA, serum amyloid A; sTNFR1, soluble tumor necrosis factor receptor 1; TARGET, Treatments Against RA and Effect on FDG PET/CT; TBR, target to background ratio; VCAM‐1, vascular cell adhesion molecule 1; and VEGF‐A, vascular endothelial growth factor A.
Values have P≤0.10 and were advanced to the selected biomarkers model (right column).
Discussion
Many autoimmune conditions are associated with an increase in cardiovascular risk. 38 Among these conditions, this relationship has been best studied in patients with RA. 39 However, cardiovascular risk prediction in RA populations has been noted to be inaccurate (underestimates risk) in several studies. 10 , 40 Adding clinical characteristics of RA has been noted to improve risk prediction, 14 and a prior study suggested that using a multibiomarker panel might improve cardiovascular event prediction. 17 We took a different approach, using data from a randomized controlled trial and 24 candidate biomarkers measured at baseline and 6 months, and an imaging cardiovascular outcome with known correlation to cardiovascular events. As previously stated, we hypothesized that several of the candidate biomarkers measured at baseline would be significantly associated with the change in cardiovascular risk, assessed by FDG PET/CT. This method yielded promising results between multiple candidate biomarkers, such as SAA, high‐sensitivity CRP, sTNFR1 (soluble TNF receptor 1), adiponectin, YKL‐40, and OPG, measured at baseline, and an association with change in arterial inflammation. Thus, these selected baseline biomarkers, known to associate with both RA disease activity and CVD, may be helpful in predicting change in cardiovascular risk as measured by arterial inflammation.
It is worth reviewing the literature on the relationship with RA and with CVD for the promising biomarkers. SAA and high‐sensitivity CRP are acute‐phase reactants with strong associations with disease activity in RA. 41 , 42 SAA is a group of related small proteins that are conserved over species and consist of multiple fibrils that associate with lipid moieties. Mice prone to atherosclerosis overexpress SAA, and suppression of SAA leads to reduced atherogenesis. 43 Atherosclerotic plaques in humans have detectable SAA, 44 SAA activates the NLRP3 inflammasome in macrophages, promoting atherogenesis, 45 and the lipoprotein carrier of high‐density lipoprotein contains SAA. 46 , 47 CRP's relationship to CVD has been widely debated, 48 but it is likely that it does not play an etiopathogenic role. Rather, it has been found to be useful biomarker predicting future cardiovascular events. 49 sTNFR1 is a receptor for TNF, a cytokine known to drive disease activity in RA. Higher levels are also associated with increased cardiovascular mortality. 50
Adiponectin is secreted by adipocytes and regulates glucose and lipid metabolism; as well, it has important anti‐inflammatory effects. However, high levels are often seen in inflammatory conditions as compensation. In RA, adiponectin levels are associated with more severe radiographic progression. 51 In CVD, higher adiponectin levels predict future CVD mortality. 52 YKL‐40 is a glycoprotein produced by multiple inflammatory cells, its levels go up with age, and some consider it an acute‐phase reactant. Levels are significantly elevated in patients with active RA. 53 Plasma levels are increased after a myocardial infarction 54 ; as well, elevated levels predict cardiovascular events in patients with stable coronary artery disease. 55 OPG was first described as a cytokine receptor in the TNF receptor superfamily. It is a soluble glycoprotein, which is largely expressed by cells in the osteoblast lineage, but its expression is upregulated by IL‐1, TNF, and Wnt proteins. 56 Baseline OPG levels have been associated with higher disease activity in treated patients with RA. 57 Furthermore, OPG levels have been found across many studies to be associated with coronary artery calcium scores. 58
Although it is not entirely clear why the 3 sets of analyses suggest that different biomarkers are important correlates of CVD, there are some logical conjectures. Change in TBR MDS, the primary outcome, relates to how responsive the atherosclerotic plaques may be to interventions, such as immunomodulators that were used in the TARGET trial. 59 As well, change in TBR MDS can denote future risk of CVD. Baseline TBR MDS reflects arterial inflammation at a time when RA was active and not well controlled by methotrexate alone and may not correlate with treatment responsiveness or future cardiovascular risk. Additionally, the PCE denotes clinical risk factors that may not capture the full extent of risk in a patient with RA and systemic inflammation. This may be the reason why PCE was not a significant predictor of arterial inflammation.
This study has important strengths, such as the prospective nature of data collection in the setting of a randomized trial, the candidate biomarker approach, the consideration of known clinical risk factors for CVD, and the inclusion of longitudinal changes in arterial inflammation. However, several limitations need discussion. The sample is relatively small, and all patients had active RA at baseline; both issues limit generalizability. The outcome studied was arterial inflammation as measured by an FDG PET/CT scan. Although this outcome has moderate correlation with actual cardiovascular outcomes, the current findings wait replication in a larger cohort with cardiovascular end points. Furthermore, we tested many associations without correcting analyses for multiple comparisons. This is justified, because all biomarkers have been hypothesized correlates based on prior literature (see Table S1 for citations). It is possible that a combination of biomarkers, a composite, might be more strongly associated with CVD; this was not our hypothesis but will be pursued in future analyses. Finally, if the biomarkers replicate in external validation cohorts, the public–private partnership will facilitate dissemination of this information to the scientific community.
One of the strengths of the current study include that the data were derived from a prospective randomized controlled trial of patients with RA. The benefits of using such a population include patients who had moderately active RA disease at baseline, patients who were carefully phenotyped with respect to cardiovascular risk and RA characteristics, treatments during the trial that were carefully described, that all patients had biomarkers collected under similar conditions at consistent timepoints, and that all patients had FDG PET/CT scans at baseline and 24 weeks. A healthy control group would have been a useful comparison, but we do not believe that it would have been possible to collect a healthy control group with such a stringent protocol requiring 2 FDG PET/CT scans.
In conclusion, we found that baseline levels of several candidate biomarkers were associated with arterial inflammation, a well‐described correlate of cardiovascular events. Each of the candidate biomarkers has substantial prior literature supporting a potential role in predicting cardiovascular events in patients with RA. If validated in an external RA cohort with good information about longitudinal cardiovascular events, these biomarkers may prove useful in developing a more accurate cardiovascular risk prediction score in RA. General population cardiovascular risk scores do not work well in RA, and prior attempts to improve them have either not included biomarkers or not conducted a broad search for candidate biomarkers. We look forward to attempts to replicate this work.
Sources of Funding
This work was supported by National Institutes of Health grant RO1 HL163580 and the Foundation for the National Institutes of Health Biomarkers Consortium. O.D. is also supported by National Institutes of Health grants R21HL167173, 5K01HL135342, and 17IGMV33860009 from the American Heart Association.
Disclosures
Dr Solomon receives salary support from research contracts to Brigham and Women's Hospital from CorEvitas, Janssen, and Novartis. He also receives royalty payments from UpToDate on unrelated topics. P.M. Rist received salary support from research grants to Brigham and Women's Hospital from Bristol‐Myers‐Squibb and the American Heart Association and has received honoraria from the American Heart Association for editorial work. Dr Lau receives royalty payments from UpToDate and has consulted for UCB. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S5
References 49,50,56,60–109
Acknowledgments
Scientific and financial support for the Foundation for the National Institutes of Health Biomarkers Consortium TARGET Biomarker Study were made possible through grants, and direct and in‐kind contributions provided by Amgen Inc., Arthritis Foundation, Merck & Co., Regeneron Pharmaceuticals, Inc., Laboratory Corporation of America, Rules Based Medicine, Inc.
This article was sent to Sula Mazimba, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.032095
For Sources of Funding and Disclosures, see page 11.
References
- 1. Hunter TM, Boytsov NN, Zhang X, Schroeder K, Michaud K, Araujo AB. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004–2014. Rheumatol Int. 2017;37:1551–1557. doi: 10.1007/s00296-017-3726-1 [DOI] [PubMed] [Google Scholar]
- 2. Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, Williams B, Gabriel S, Lassere M, Johns N, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1316–1322. doi: 10.1136/annrheumdis-2013-204627 [DOI] [PubMed] [Google Scholar]
- 3. Humphreys JH, Warner A, Chipping J, Marshall T, Lunt M, Symmons DPM, Verstappen SMM. Mortality trends in patients with early rheumatoid arthritis over 20 years: results from the Norfolk Arthritis register. Arthritis Care Res. 2014;66:1296–1301. doi: 10.1002/acr.22296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Widdifield J, Paterson JM, Huang A, Bernatsky S. Causes of death in rheumatoid arthritis: how do they compare to the general population? Arthritis Care Res. 2018;70:1748–1755. doi: 10.1002/acr.23548 [DOI] [PubMed] [Google Scholar]
- 5. Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, Stampfer MJ, Curhan GC. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–1307. doi: 10.1161/01.CIR.0000054612.26458.B2 [DOI] [PubMed] [Google Scholar]
- 6. Kitas GD, Nightingale P, Armitage J, Sattar N, Belch JJF, Symmons DPM, Consortium TR. A multicenter, randomized, placebo‐controlled trial of atorvastatin for the primary prevention of cardiovascular events in patients with rheumatoid arthritis. Arthritis Rheumatol. 2019;71:1437–1449. doi: 10.1002/art.40892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giles JT, Sattar N, Gabriel S, Ridker PM, Gay S, Warne C, Musselman D, Brockwell L, Shittu E, Klearman M, et al. Cardiovascular safety of tocilizumab versus etanercept in rheumatoid arthritis: a randomized controlled trial. Arthritis Rheumatol. 2020;72:31–40. doi: 10.1002/art.41095 [DOI] [PubMed] [Google Scholar]
- 8. Kim SC, Solomon DH, Rogers JR, Gale S, Klearman M, Sarsour K, Schneeweiss S. Cardiovascular safety of tocilizumab versus tumor necrosis factor inhibitors in patients with rheumatoid arthritis: a multi‐database cohort study. Arthritis Rheumatol. 2017;69:1154–1164. doi: 10.1002/art.40084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, Germino R, Menon S, Sun Y, Wang C, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316–326. doi: 10.1056/NEJMoa2109927 [DOI] [PubMed] [Google Scholar]
- 10. Crowson CS, Matteson EL, Roger VL, Therneau TM, Gabriel SE. Usefulness of risk scores to estimate the risk of cardiovascular disease in patients with rheumatoid arthritis. Am J Cardiol. 2012;110:420–424. doi: 10.1016/j.amjcard.2012.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hippisley‐Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357:j2099. doi: 10.1136/bmj.j2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Akintoye E, Afonso L, Bengaluru Jayanna M, Bao W, Briasoulis A, Robinson J. Prognostic utility of risk enhancers and coronary artery calcium score recommended in the 2018 ACC/AHA multisociety cholesterol treatment guidelines over the pooled cohort equation: insights from 3 large prospective cohorts. J Am Heart Assoc. 2021;10:e019589. doi: 10.1161/JAHA.120.019589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peters MJ, Symmons DP, McCarey D, Dijkmans BA, Nicola P, Kvien TK, McInnes IB, Haentzschel H, Gonzalez‐Gay MA, Provan S, et al. EULAR evidence‐based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325–331. doi: 10.1136/ard.2009.113696 [DOI] [PubMed] [Google Scholar]
- 14. Solomon DH, Greenberg J, Curtis JR, Liu M, Farkouh ME, Tsao P, Kremer JM, Etzel CJ. Derivation and internal validation of an expanded cardiovascular risk prediction score for rheumatoid arthritis: a consortium of rheumatology researchers of North America registry study. Arthritis Rheumatol. 2015;67:1995–2003. doi: 10.1002/art.39195 [DOI] [PubMed] [Google Scholar]
- 15. Ljung L, Ueda P, Liao KP, Greenberg JD, Etzel CJ, Solomon DH, Askling J. Performance of the expanded cardiovascular Risk prediction score for rheumatoid arthritis in a geographically distant national register‐based cohort: an external validation. RMD Open. 2018;4:e000771. doi: 10.1136/rmdopen-2018-000771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crowson CS, Gabriel SE, Semb AG, van Riel PLCM, Karpouzas G, Dessein PH, Hitchon C, Pascual‐Ramos V, Kitas GD, Arthritis T‐ACCR. Rheumatoid arthritis‐specific cardiovascular risk scores are not superior to general risk scores: a validation analysis of patients from seven countries. Rheumatology. 2017;56:1102–1110. doi: 10.1093/rheumatology/kex038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Curtis JR, Xie F, Crowson CS, Sasso EH, Hitraya E, Chin CL, Bamford RD, Ben‐Shachar R, Gutin A, Flake DD II, et al. Derivation and internal validation of a multi‐biomarker‐based cardiovascular disease risk prediction score for rheumatoid arthritis patients. Arthritis Res Ther. 2020;22:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giles J, Rist P, Liao K, Tawakol A, Fayad Z, Venkatesh M, Ridker P, Glynn R, Lu F, Broderick R, et al. Testing the effects of disease modifying anti‐rheumatic drugs on vascular inflammation in rheumatoid arthritis: rationale and Design of the Treatments against Rheumatoid Arthritis and Effect on FDG PET‐CT (TARGET) trial. ACR Open Rheumatol. 2021;3:371–380. doi: 10.1002/acr2.11256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Solomon DH, Giles JT, Liao KP, Ridker PM, Rist PM, Glynn RJ, Broderick R, Lu F, Murray MT, Vanni K, et al. Reducing cardiovascular risk with immunomodulators: a randomised active comparator trial among patients with rheumatoid arthritis. Ann Rheum Dis. 2023;82:324–330. doi: 10.1136/ard-2022-223302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Libby P. Role of inflammation in atherosclerosis associated with rheumatoid arthritis. Am J Med. 2008;121:S21–S31. doi: 10.1016/j.amjmed.2008.06.014 [DOI] [PubMed] [Google Scholar]
- 21. Bertenshaw GP, Yip P, Seshaiah P, Zhao J, Chen T‐H, Wiggins WS, Mapes JP, Mansfield BC. Multianalyte profiling of serum antigens and autoimmune and infectious disease molecules to identify biomarkers dysregulated in epithelial ovarian cancer. Cancer Epidemiol Biomark Prev. 2008;17:2872–2881. doi: 10.1158/1055-9965.EPI-08-0464 [DOI] [PubMed] [Google Scholar]
- 22. Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, Fuster V, Ballantyne CM, Stein EA, Tardif JC, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non‐invasive multimodality imaging (dal‐PLAQUE): a randomised clinical trial. Lancet. 2011;378:1547–1559. doi: 10.1016/S0140-6736(11)61383-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abdelbaky A, Corsini E, Figueroa AL, Fontanez S, Subramanian S, Ferencik M, Brady TJ, Hoffmann U, Tawakol A. Focal arterial inflammation precedes subsequent calcification in the same location: a longitudinal FDG‐PET/CT study. Circ Cardiovasc Imaging. 2013;6:747–754. doi: 10.1161/CIRCIMAGING.113.000382 [DOI] [PubMed] [Google Scholar]
- 24. Bural GG, Torigian DA, Chamroonrat W, Houseni M, Chen W, Basu S, Kumar R, Alavi A. FDG‐PET is an effective imaging modality to detect and quantify age‐related atherosclerosis in large arteries. Eur J Nucl Med Mol Imaging. 2008;35:562–569. doi: 10.1007/s00259-007-0528-9 [DOI] [PubMed] [Google Scholar]
- 25. Joly L, Djaballah W, Koehl G, Mandry D, Dolivet G, Marie P‐Y, Benetos A. Aortic inflammation, as assessed by hybrid FDG‐PET/CT imaging, is associated with enhanced aortic stiffness in addition to concurrent calcification. Eur J Nucl Med Mol Imaging. 2009;36:979–985. doi: 10.1007/s00259-008-1047-z [DOI] [PubMed] [Google Scholar]
- 26. Rudd JH, Myers KS, Bansilal S, Machac J, Woodward M, Fuster V, Farkouh ME, Fayad ZA. Relationships among regional arterial inflammation, calcification, risk factors, and biomarkers: a prospective fluorodeoxyglucose positron‐emission tomography/computed tomography imaging study. Circ Cardiovasc Imaging. 2009;2:107–115. doi: 10.1161/CIRCIMAGING.108.811752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim TN, Kim S, Yang SJ, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM. Vascular inflammation in patients with impaired glucose tolerance and type 2 diabetes: analysis with 18F‐fluorodeoxyglucose positron emission tomography. Circ Cardiovasc Imaging. 2010;3:142–148. doi: 10.1161/CIRCIMAGING.109.888909 [DOI] [PubMed] [Google Scholar]
- 28. Rominger A, Saam T, Wolpers S, Cyran CC, Schmidt M, Foerster S, Nikolaou K, Reiser MF, Bartenstein P, Hacker M. 18F‐FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med. 2009;50:1611–1620. doi: 10.2967/jnumed.109.065151 [DOI] [PubMed] [Google Scholar]
- 29. Paulmier B, Duet M, Khayat R, Pierquet‐Ghazzar N, Laissy JP, Maunoury C, Hugonnet F, Sauvaget E, Trinquart L, Faraggi M. Arterial wall uptake of fluorodeoxyglucose on PET imaging in stable cancer disease patients indicates higher risk for cardiovascular events. J Nucl Cardiol. 2008;15:209–217. doi: 10.1016/j.nuclcard.2007.10.009 [DOI] [PubMed] [Google Scholar]
- 30. Tawakol A, Fayad ZA, Mogg R, Alon A, Klimas MT, Dansky H, Subramanian SS, Abdelbaky A, Rudd JH, Farkouh ME, et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose‐positron emission tomography/computed tomography feasibility study. J Am Coll Cardiol. 2013;62:909–917. [DOI] [PubMed] [Google Scholar]
- 31. Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, Yates D, LaMuraglia GM, Furie K, Houser S, et al. In vivo 18F‐fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818–1824. [DOI] [PubMed] [Google Scholar]
- 32. Lloyd‐Jones DM, Braun LT, Ndumele CE, Smith SC Jr, Sperling LS, Virani SS, Blumenthal RS. Use of Risk assessment tools to guide decision‐making in the primary prevention of atherosclerotic cardiovascular disease: a special report from the American Heart Association and American College of Cardiology. Circulation. 2019;139:e1162–e1177. doi: 10.1161/CIR.0000000000000638 [DOI] [PubMed] [Google Scholar]
- 33. Deming W. Statistical Adjustment of Data. Wiley; 1943. [Google Scholar]
- 34. Linnet K. Estimation of the linear relationship between the measurements of two methods with proportional errors. Stat Med. 1990;9:1463–1473. doi: 10.1002/sim.4780091210 [DOI] [PubMed] [Google Scholar]
- 35. Jaeger B. Package ‘PooledCohort’. 2022.
- 36. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van der Valk FM, Verweij SL, Zwinderman KA, Strang AC, Kaiser Y, Marquering HA, Nederveen AJ, Stroes ES, Verberne HJ, Rudd JH. Thresholds for arterial wall inflammation quantified by (18)F‐FDG PET imaging: implications for vascular interventional studies. JACC Cardiovasc Imaging. 2016;9:1198–1207. doi: 10.1016/j.jcmg.2016.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Conrad N, Verbeke G, Molenberghs G, Goetschalckx L, Callender T, Cambridge G, Mason JC, Rahimi K, McMurray JJV, Verbakel JY. Autoimmune diseases and cardiovascular risk: a population‐based study on 19 autoimmune diseases and 12 cardiovascular diseases in 22 million individuals in the UK. Lancet. 2022;400:733–743. doi: 10.1016/S0140-6736(22)01349-6 [DOI] [PubMed] [Google Scholar]
- 39. Avina‐Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta‐analysis of observational studies. Ann Rheum Dis. 2012;71:1524–1529. doi: 10.1136/annrheumdis-2011-200726 [DOI] [PubMed] [Google Scholar]
- 40. Crowson CS, Gabriel SE. Towards improving cardiovascular risk management in patients with rheumatoid arthritis: the need for accurate risk assessment. Ann Rheum Dis. 2011;70:719–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou J, Dai Y, Lin Y, Chen K. Association between serum amyloid a and rheumatoid arthritis: a systematic review and meta‐analysis. Semin Arthritis Rheum. 2022;52:151943. doi: 10.1016/j.semarthrit.2021.12.011 [DOI] [PubMed] [Google Scholar]
- 42. Vanier A, Smolen JS, Allaart CF, Van Vollenhoven R, Verschueren P, Vastesaeger N, Saevarsdottir S, Visser K, Aletaha D, Combe B, et al. An updated matrix to predict rapid radiographic progression of early rheumatoid arthritis patients: pooled analyses from several databases. Rheumatology. 2019;59:1842–1852. [DOI] [PubMed] [Google Scholar]
- 43. Vallejo A, Chami B, Dennis JM, Simone M, Ahmad G, Abdo A, Sharma A, Shihata WA, Martin N, Chin‐Dusting JP. NFkB inhibition mitigates serum amyloid A‐induced pro‐atherogenic responses in endothelial cells and leukocyte adhesion and adverse changes to endothelium function in isolated aorta. Int J Mol Sci. 2018;20:105. doi: 10.3390/ijms20010105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meek R, Urieli‐Shoval S, Benditt E. Expression of apolipoprotein serum amyloid a mRNA in human atherosclerotic lesions and cultured vascular cells: implications for serum amyloid a function. Proc Natl Acad Sci USA. 1994;91:3186–3190. doi: 10.1073/pnas.91.8.3186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Page MJ, Thomson GJ, Nunes JM, Engelbrecht A‐M, Nell TA, De Villiers WJ, De Beer MC, Engelbrecht L, Kell DB, Pretorius E. Serum amyloid a binds to fibrin (ogen), promoting fibrin amyloid formation. Sci Rep. 2019;9:3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Webb NR. High‐density lipoproteins and serum amyloid a (SAA). Curr Atheroscler Rep. 2021;23:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Van Lenten B, Hama S, De Beer F, Stafforini D, McIntyre T, Prescott S, La Du B, Fogelman A, Navab M. Anti‐inflammatory HDL becomes pro‐inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995;96:2758–2767. doi: 10.1172/JCI118345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ridker PM. Inflammation, C‐reactive protein, and cardiovascular disease: moving past the marker versus mediator debate. Circ Res. 2014;114:594–595. doi: 10.1161/CIRCRESAHA.114.303215 [DOI] [PubMed] [Google Scholar]
- 49. Collaboration ERF. C‐reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta‐analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Carlsson AC, Juhlin CC, Larsson TE, Larsson A, Ingelsson E, Sundström J, Lind L, Ärnlöv J. Soluble tumor necrosis factor receptor 1 (sTNFR1) is associated with increased total mortality due to cancer and cardiovascular causes–findings from two community based cohorts of elderly. Atherosclerosis. 2014;237:236–242. doi: 10.1016/j.atherosclerosis.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 51. Giles JT, Van Der Heijde DM, Bathon JM. Association of circulating adiponectin levels with progression of radiographic joint destruction in rheumatoid arthritis. Ann Rheum Dis. 2011;70:1562–1568. doi: 10.1136/ard.2011.150813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dekker JM, Funahashi T, Nijpels G, Pilz S, Stehouwer CD, Snijder MB, Bouter LM, Matsuzawa Y, Shimomura I, Heine RJ. Prognostic value of adiponectin for cardiovascular disease and mortality. J Clin Endocrinol Metabol. 2008;93:1489–1496. [DOI] [PubMed] [Google Scholar]
- 53. Johansen J, Jensen H, Price P. A new biochemical marker for joint injury. Analysis of YKL‐40 in serum and synovial fluid. Rheumatology. 1993;32:949–955. doi: 10.1093/rheumatology/32.11.949 [DOI] [PubMed] [Google Scholar]
- 54. Nøjgaard C, Høst NB, Christensen IJ, Poulsen SH, Egstrup K, Price PA, Johansen JS. Serum levels of YKL‐40 increases in patients with acute myocardial infarction. Coron Artery Dis. 2008;19:257–263. doi: 10.1097/MCA.0b013e3282f40dd5 [DOI] [PubMed] [Google Scholar]
- 55. Rathcke CN, Raymond I, Kistorp C, Hildebrandt P, Faber J, Vestergaard H. Low grade inflammation as measured by levels of YKL‐40: association with an increased overall and cardiovascular mortality rate in an elderly population. Int J Cardiol. 2010;143:35–42. doi: 10.1016/j.ijcard.2009.01.043 [DOI] [PubMed] [Google Scholar]
- 56. Geusens P, Landewé R, Garnero P, Chen D, Dunstan C, Lems W, Stinissen P, van der Heijde D, Van der Linden S, Boers M. The ratio of circulating osteoprotegerin to RANKL in early rheumatoid arthritis predicts later joint destruction. Arthritis Rheum. 2006;54:1772–1777. doi: 10.1002/art.21896 [DOI] [PubMed] [Google Scholar]
- 57. Raaz‐Schrauder D, Schrauder MG, Stumpf C, Lewczuk P, Kilian T, Dietel B, Garlichs CD, Schlundt C, Achenbach S, Klinghammer L. Plasma levels of sRANKL and OPG are associated with atherogenic cytokines in patients with intermediate cardiovascular risk. Heart Vessel. 2017;32:1304–1313. doi: 10.1007/s00380-017-0998-z [DOI] [PubMed] [Google Scholar]
- 58. Vazirian F, Sadeghi M, Wang D, Javidi Dashtbayaz R, Gholoobi A, Samadi S, Mohammadpour AH. Correlation between osteoprotegerin and coronary artery calcification in diabetic subjects: a systematic review of observational studies. BMC Cardiovasc Disord. 2023;23:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zupančič E, Fayad ZA, Mulder WJM. Cardiovascular immunotherapy and the role of imaging. Arterioscler Thromb Vasc Biol. 2017;37:e167–e171. doi: 10.1161/ATVBAHA.117.309227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Madhok R, Crilly A, Watson J, Capell HA. Serum interleukin 6 levels in rheumatoid arthritis: correlations with clinical and laboratory indices of disease activity. Ann Rheum Dis. 1993;52:232–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sack U, Kinne RW, Marx T, Heppt P, Bender S, Emmrich F. Interleukin‐6 in synovial fluid is closely associated with chronic synovitis in rheumatoid arthritis. Rheumatol Int. 1993;13:45–51. doi: 10.1007/BF00307733 [DOI] [PubMed] [Google Scholar]
- 62. Smolen JS, Beaulieu A, Rubbert‐Roth A, Ramos‐Remus C, Rovensky J, Alecock E, Woodworth T, Alten R, Investigators O. Effect of interleukin‐6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double‐blind, placebo‐controlled, randomised trial. Lancet. 2008;371:987–997. doi: 10.1016/S0140-6736(08)60453-5 [DOI] [PubMed] [Google Scholar]
- 63. Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin‐6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.CIR.101.15.1767 [DOI] [PubMed] [Google Scholar]
- 64. Biasucci LM, Liuzzo G, Fantuzzi G, Caligiuri G, Rebuzzi AG, Ginnetti F, Dinarello CA, Maseri A. Increasing levels of interleukin (IL)‐1Ra and IL‐6 during the first 2 days of hospitalization in unstable angina are associated with increased risk of in‐hospital coronary events. Circulation. 1999;99:2079–2084. doi: 10.1161/01.CIR.99.16.2079 [DOI] [PubMed] [Google Scholar]
- 65. Hartman J, Frishman WH. Inflammation and atherosclerosis: a review of the role of interleukin‐6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol Rev. 2014;22:147–151. doi: 10.1097/CRD.0000000000000021 [DOI] [PubMed] [Google Scholar]
- 66. Klimiuk PA, Sierakowski S, Latosiewicz R, Cylwik JP, Cylwik B, Skowronski J, Chwiecko J. Circulating tumour necrosis factor alpha and soluble tumour necrosis factor receptors in patients with different patterns of rheumatoid synovitis. Ann Rheum Dis. 2003;62:472–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, Genovese MC, Wasko MC, Moreland LW, Weaver AL, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343:1586–1593. doi: 10.1056/NEJM200011303432201 [DOI] [PubMed] [Google Scholar]
- 68. Safranow K, Dziedziejko V, Rzeuski R, Czyzycka E, Wojtarowicz A, Binczak‐Kuleta A, Jakubowska K, Olszewska M, Ciechanowicz A, Kornacewicz‐Jach Z, et al. Plasma concentrations of TNF‐alpha and its soluble receptors sTNFR1 and sTNFR2 in patients with coronary artery disease. Tissue Antigens. 2009;74:386–392. doi: 10.1111/j.1399-0039.2009.01332.x [DOI] [PubMed] [Google Scholar]
- 69. O'Hara R, Murphy EP, Whitehead AS, FitzGerald O, Bresnihan B. Acute‐phase serum amyloid a production by rheumatoid arthritis synovial tissue. Arthritis Res. 2000;2:142–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fyfe AI, Rothenberg LS, DeBeer FC, Cantor RM, Rotter JI, Lusis AJ. Association between serum amyloid a proteins and coronary artery disease: evidence from two distinct arteriosclerotic processes. Circulation. 1997;96:2914–2919. doi: 10.1161/01.CIR.96.9.2914 [DOI] [PubMed] [Google Scholar]
- 71. Dessein PH, Joffe BI, Stanwix AE. High sensitivity C‐reactive protein as a disease activity marker in rheumatoid arthritis. J Rheumatol. 2004;31:1095–1097. [PubMed] [Google Scholar]
- 72. Plant MJ, Williams AL, O'Sullivan MM, Lewis PA, Coles EC, Jessop JD. Relationship between time‐integrated C‐reactive protein levels and radiologic progression in patients with rheumatoid arthritis. Arthritis Rheum. 2000;43:1473–1477. doi: [DOI] [PubMed] [Google Scholar]
- 73. Bermudez EA, Rifai N, Buring J, Manson JE, Ridker PM. Interrelationships among circulating interleukin‐6, C‐reactive protein, and traditional cardiovascular risk factors in women. Arterioscler Thromb Vasc Biol. 2002;22:1668–1673. doi: 10.1161/01.ATV.0000029781.31325.66 [DOI] [PubMed] [Google Scholar]
- 74. Berner B, Wolf G, Hummel KM, Muller GA, Reuss‐Borst MA. Increased expression of CD40 ligand (CD154) on CD4+ T cells as a marker of disease activity in rheumatoid arthritis. Ann Rheum Dis. 2000;59:190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liu M‐F, Chao S‐C, Wang C‐R, Lei H‐Y. Expression of CD40 and CD40 ligand among cell populations within rheumatoid synovial compartment. Autoimmunity. 2001;34:107–113. [DOI] [PubMed] [Google Scholar]
- 76. Heeschen C, Dimmeler S, Hamm CW, van den Brand MJ, Boersma E, Zeiher AM, Simoons ML, Investigators CS. Soluble CD40 ligand in acute coronary syndromes. N Engl J Med. 2003;348:1104–1111. doi: 10.1056/NEJMoa022600 [DOI] [PubMed] [Google Scholar]
- 77. Andre P, Prasad KS, Denis CV, He M, Papalia JM, Hynes RO, Phillips DR, Wagner DD. CD40L stabilizes arterial thrombi by a beta3 integrin–dependent mechanism. Nat Med. 2002;8:247–252. doi: 10.1038/nm0302-247 [DOI] [PubMed] [Google Scholar]
- 78. Giles JT, Allison M, Bingham CO III, Scott WM Jr, Bathon JM. Adiponectin is a mediator of the inverse association of adiposity with radiographic damage in rheumatoid arthritis. Arthritis Rheum. 2009;61:1248–1256. doi: 10.1002/art.24789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Otero M, Lago R, Gomez R, Lago F, Dieguez C, Gomez‐Reino JJ, Gualillo O. Changes in plasma levels of fat‐derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:1198–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.ATV.0000048856.22331.50 [DOI] [PubMed] [Google Scholar]
- 81. Sierra‐Johnson J, Romero‐Corral A, Lopez‐Jimenez F, Gami AS, Sert Kuniyoshi FH, Wolk R, Somers VK. Relation of increased leptin concentrations to history of myocardial infarction and stroke in the United States population. Am J Cardiol. 2007;100:234–239. doi: 10.1016/j.amjcard.2007.02.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Muse ED, Feldman DI, Blaha MJ, Dardari ZA, Blumenthal RS, Budoff MJ, Nasir K, Criqui MH, Cushman M, McClelland RL, et al. The association of resistin with cardiovascular disease in the multi‐ethnic study of atherosclerosis. Atherosclerosis. 2015;239:101–108. doi: 10.1016/j.atherosclerosis.2014.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jones HW, Bailey R, Zhang Z, Dunne KA, Blake DR, Cox NL, Morris CJ, Winyard PG. Inactivation of antithrombin III in synovial fluid from patients with rheumatoid arthritis. Ann Rheum Dis. 1998;57:162–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Thompson SG, Fechtrup C, Squire E, Heyse U, Breithardt G, van de Loo JC, Kienast J. Antithrombin III and fibrinogen as predictors of cardiac events in patients with angina pectoris. Arterioscler Thromb Vasc Biol. 1996;16:357–362. doi: 10.1161/01.ATV.16.3.357 [DOI] [PubMed] [Google Scholar]
- 85. Saxne T, Lecander I, Geborek P. Plasminogen activators and plasminogen activator inhibitors in synovial fluid. Difference between inflammatory joint disorders and osteoarthritis. J Rheumatol. 1993;20:91–96. [PubMed] [Google Scholar]
- 86. Stoop AA, Lupu F, Pannekoek H. Colocalization of thrombin, PAI‐1, and vitronectin in the atherosclerotic vessel wall: a potential regulatory mechanism of thrombin activity by PAI‐1/vitronectin complexes. Arterioscler Thromb Vasc Biol. 2000;20:1143–1149. doi: 10.1161/01.ATV.20.4.1143 [DOI] [PubMed] [Google Scholar]
- 87. Trocme C, Marotte H, Baillet A, Pallot‐Prades B, Garin J, Grange L, Miossec P, Tebib J, Berger F, Nissen MJ, et al. Apolipoprotein A‐I and platelet factor 4 are biomarkers for infliximab response in rheumatoid arthritis. Ann Rheum Dis. 2009;68:1328–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Contois JH, McConnell JP, Sethi AA, Csako G, Devaraj S, Hoefner DM, Warnick GR; Lipoproteins a and vascular diseases division working group on best P . Apolipoprotein B and cardiovascular disease risk: position statement from the AACC lipoproteins and vascular diseases division working group on best practices. Clin Chem. 2009;55:407–419. doi: 10.1373/clinchem.2008.118356 [DOI] [PubMed] [Google Scholar]
- 89. Wang J, Hu B, Kong L, Cai H, Zhang C. Native, oxidized lipoprotein(a) and lipoprotein(a) immune complex in patients with active and inactive rheumatoid arthritis: plasma concentrations and relationship to inflammation. Clin Chim Acta. 2008;390:67–71. doi: 10.1016/j.cca.2007.12.015 [DOI] [PubMed] [Google Scholar]
- 90. Nordestgaard BG, Langsted A. Lipoprotein (a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res. 2016;57:1953–1975. doi: 10.1194/jlr.R071233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Park YJ, Cho CS, Emery P, Kim WU. LDL cholesterolemia as a novel risk factor for radiographic progression of rheumatoid arthritis: a single‐center prospective study. PLoS One. 2013;8:e68975. doi: 10.1371/journal.pone.0085221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, et al. Low‐density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European atherosclerosis society consensus panel. Eur Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bradham WS, Bian A, Oeser A, Gebretsadik T, Shintani A, Solus J, Estis J, Lu QA, Todd J, Raggi P, et al. High‐sensitivity cardiac troponin‐I is elevated in patients with rheumatoid arthritis, independent of cardiovascular risk factors and inflammation. PLoS One. 2012;7:e38930. doi: 10.1371/journal.pone.0038930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Gersh BJ, Rouleau JL, et al. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361:2538–2547. doi: 10.1056/NEJMoa0805299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. George J, Mackle G, Manoharan A, Khan F, Struthers AD. High BNP levels in rheumatoid arthritis are related to inflammation but not to left ventricular abnormalities: a prospective case‐control study. Int J Cardiol. 2014;172:e116–e118. doi: 10.1016/j.ijcard.2013.12.119 [DOI] [PubMed] [Google Scholar]
- 96. Caselli C, Prontera C, Liga R, De Graaf MA, Gaemperli O, Lorenzoni V, Ragusa R, Marinelli M, Del Ry S, Rovai D, et al. Effect of coronary atherosclerosis and myocardial ischemia on plasma levels of high‐sensitivity troponin T and NT‐proBNP in patients with stable angina. Arterioscler Thromb Vasc Biol. 2016;36:757–764. doi: 10.1161/ATVBAHA.115.306818 [DOI] [PubMed] [Google Scholar]
- 97. Luo SF, Fang RY, Hsieh HL, Chi PL, Lin CC, Hsiao LD, Wu CC, Wang JS, Yang CM. Involvement of MAPKs and NF‐kappaB in tumor necrosis factor alpha‐induced vascular cell adhesion molecule 1 expression in human rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2010;62:105–116. doi: 10.1002/art.25060 [DOI] [PubMed] [Google Scholar]
- 98. Zamani P, Schwartz GG, Olsson AG, Rifai N, Bao W, Libby P, Ganz P, Kinlay S; Myocardial ischemia reduction with aggressive cholesterol lowering study I . Inflammatory biomarkers, death, and recurrent nonfatal coronary events after an acute coronary syndrome in the MIRACL study. J Am Heart Assoc. 2013;2:e003103. doi: 10.1161/JAHA.112.003103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ozgonenel L, Cetin E, Tutun S, Tonbaklar P, Aral H, Guvenen G. The relation of serum vascular endothelial growth factor level with disease duration and activity in patients with rheumatoid arthritis. Clin Rheumatol. 2010;29:473–477. doi: 10.1007/s10067-009-1343-4 [DOI] [PubMed] [Google Scholar]
- 100. Wang HW, Lo HH, Chiu YL, Chang SJ, Huang PH, Liao KH, Tasi CF, Wu CH, Tsai TN, Cheng CC, et al. Dysregulated miR‐361‐5p/VEGF axis in the plasma and endothelial progenitor cells of patients with coronary artery disease. PLoS One. 2014;9:e98070. doi: 10.1371/journal.pone.0116382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Green MJ, Gough AK, Devlin J, Smith J, Astin P, Taylor D, Emery P. Serum MMP‐3 and MMP‐1 and progression of joint damage in early rheumatoid arthritis. Rheumatology (Oxford). 2003;42:83–88. doi: 10.1093/rheumatology/keg037 [DOI] [PubMed] [Google Scholar]
- 102. Lehrke M, Greif M, Broedl UC, Lebherz C, Laubender RP, Becker A, von Ziegler F, Tittus J, Reiser M, Becker C, et al. MMP‐1 serum levels predict coronary atherosclerosis in humans. Cardiovasc Diabetol. 2009;8:50. doi: 10.1186/1475-2840-8-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Vaananen T, Vuolteenaho K, Kautiainen H, Nieminen R, Mottonen T, Hannonen P, Korpela M, Kauppi MJ, Laiho K, Kaipiainen‐Seppanen O, et al. Glycoprotein YKL‐40: a potential biomarker of disease activity in rheumatoid arthritis during intensive treatment with csDMARDs and infliximab. Evidence from the randomised controlled NEO‐RACo trial. PLoS One. 2017;12:e0183294. doi: 10.1371/journal.pone.0183294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kjaergaard AD, Johansen JS, Bojesen SE, Nordestgaard BG. Elevated plasma YKL‐40, lipids and lipoproteins, and ischemic vascular disease in the general population. Stroke. 2015;46:329–335. doi: 10.1161/STROKEAHA.114.007657 [DOI] [PubMed] [Google Scholar]
- 105. Lertnawapan R, Bian A, Rho YH, Kawai VK, Raggi P, Oeser A, Solus JF, Gebretsadik T, Shintani A, Stein CM. Cystatin C, renal function, and atherosclerosis in rheumatoid arthritis. J Rheumatol. 2011;38:2297–2300. doi: 10.3899/jrheum.110168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lee M, Saver JL, Huang WH, Chow J, Chang KH, Ovbiagele B. Impact of elevated cystatin C level on cardiovascular disease risk in predominantly high cardiovascular risk populations: a meta‐analysis. Circ Cardiovasc Qual Outcomes. 2010;3:675–683. doi: 10.1161/CIRCOUTCOMES.110.957696 [DOI] [PubMed] [Google Scholar]
- 107. Iwadate H, Kobayashi H, Kanno T, Asano T, Saito R, Sato S, Suzuki E, Watanabe H, Ohira H. Plasma osteopontin is correlated with bone resorption markers in rheumatoid arthritis patients. Int J Rheum Dis. 2014;17:50–56. doi: 10.1111/1756-185X.12115 [DOI] [PubMed] [Google Scholar]
- 108. Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–2309. doi: 10.1161/ATVBAHA.107.144824 [DOI] [PubMed] [Google Scholar]
- 109. Lieb W, Gona P, Larson MG, Massaro JM, Lipinska I, Keaney JF Jr, Rong J, Corey D, Hoffmann U, Fox CS, et al. Biomarkers of the osteoprotegerin pathway: clinical correlates, subclinical disease, incident cardiovascular disease, and mortality. Arterioscler Thromb Vasc Biol. 2010;30:1849–1854. doi: 10.1161/ATVBAHA.109.199661 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S5
References 49,50,56,60–109


