Abstract
Background
Prior studies investigating the impact of residual mitral regurgitation (MR), tricuspid regurgitation (TR), and elevated predischarge transmitral mean pressure gradient (TMPG) on outcomes after mitral transcatheter edge‐to‐edge repair (TEER) have assessed each parameter in isolation. We sought to examine the prognostic value of combining predischarge MR, TR, and TMPG to study long‐term outcomes after TEER.
Methods and Results
We reviewed the records of 291 patients who underwent successful mitral TEER at our institution between March 2014 and June 2022. Using well‐established outcomes‐related cutoffs for predischarge MR (≥moderate), TR (≥moderate), and TMPG (≥5 mm Hg), 3 echo profiles were developed based on the number of risk factors present (optimal: 0 risk factors, mixed: 1 risk factor, poor: ≥2 risk factors). Discrimination of the profiles for predicting the primary composite end point of all‐cause mortality and heart failure hospitalization at 2 years was examined using Cox regression. Overall, mean age was 76.7±10.6 years, 43.3% were women, and 53% had primary MR. Two‐year event‐free survival was 61%. Predischarge TR≥moderate, MR≥moderate, and TMPG≥5 mm Hg were risk factors associated with the primary end point. Compared with the optimal profile, there was an incremental risk in 2‐year event‐rate with each worsening profile (optimal as reference; mixed profile: hazard ratio (HR), 2.87 [95% CI, 1.71–5.17], P<0.001; poor profile: HR, 3.76 [95% CI, 1.84–6.53], P<0.001). Echocardiographic profile was statistically associated with the 2‐year mortality end point (optimal as reference; mixed profile: HR, 3.55 [95% CI, 1.81–5.96], P<0.001; poor profile: HR, 3.39 [95% CI, 2.56–7.33], P=0.02).
Conclusions
The echocardiographic profile integrating predischarge TR, MR, and TMPG presents a novel prognostic stratification tool for patients undergoing mitral TEER.
Keywords: echocardiographic profile, transcatheter edge‐to‐edge repair, transmitral pressure gradient, tricuspid regurgitation
Subject Categories: Valvular Heart Disease
Nonstandard Abbreviations and Acronyms
- MR
mitral regurgitation
- TEE
transesophageal echocardiography
- TEER
transcatheter edge‐to‐edge repair
- TR
tricuspid regurgitation
Clinical Perspective.
What Is New?
This is the first study to report the combined impact of noninvasive echocardiographic parameters assessed at a standardized time point consistently applied to all patients.
Echocardiographic profiling based on predischarge tricuspid regurgitation severity, mitral regurgitation, and transmitral mean pressure gradient provides an important and easily attainable prognostication tool that predicts mortality and the composite of mortality and heart failure hospitalization after mitral transcatheter edge‐to‐edge repair.
What Are the Clinical Implications?
This is a new prognostication method for assessing patients undergoing mitral transcatheter edge‐to‐edge repair using easily measured echocardiographic parameters.
Validation of our findings in a larger independent cohort along with longer‐term follow‐up, specifically in both patients with primary and secondarymitral regurgitation, is warranted.
Mitral regurgitation (MR) is the most prevalent valvular heart disease in the United States and the third most common worldwide. 1 Mitral transcatheter edge‐to‐edge repair (TEER) has emerged as a safe and effective treatment for patients with significant primary MR who are at high surgical risk and those with secondary MR who have persistent symptoms and significant MR despite optimization of guideline‐directed medical therapy. 2 , 3 , 4 As newer generations of mitral TEER devices become available, ongoing research has focused on identifying the patient population that derives the greatest benefit from this intervention. Studies have examined the impact of residual MR, tricuspid regurgitation (TR), and elevated transmitral mean pressure gradient (TMPG) on long‐term outcomes after mitral TEER. 5 , 6 , 7 , 8 , 9 , 10 Optimal MR reduction after mitral TEER has been associated with improved overall survival and reduced rates of heart failure hospitalizations (HFH). 5 , 6 Notably, in light of increasing evidence regarding the effect of significant TR on long‐term outcomes after transcatheter procedures, it has been found that baseline moderate or severe TR is associated with higher risk of mortality and HFH in patients undergoing mitral TEER. 7 , 8 Conversely, the effect of elevated TMPG after mitral TEER remains a subject of debate. 9 , 10
Considering the temporal discrepancies in assessing baseline TR between patients, as well as inconsistencies in reporting outcomes between intraoperative residual and predischarge MR severity and TMPG, our study aimed to assess the clinical significance of these noninvasive Doppler parameters at a uniform time point, consistently applied to all patients. We therefore sought to investigate the combined effect of these predischarge echocardiographic factors by creating profiles that best predicted outcomes after mitral TEER.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Data Source
We reviewed the records of 298 consecutive patients with moderate–severe or severe MR who underwent mitral TEER with MitraClip (Abbott Vascular, Santa Clara, CA) at Houston Methodist Hospital (Houston, TX) from March 2014 to September 2022. As determined by a multidisciplinary heart team based on current guidelines, patients with symptomatic primary MR at high surgical risk and those with secondary MR on optimized guideline‐directed medical therapy underwent the procedure. Among 298 patients who had successful MitraClip implantation, 291 patients had satisfactory color Doppler MR evaluation on echocardiography before, during, and after the procedure and comprised the primary study cohort. All study procedures were conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all patients before the procedure. This observational study was approved by the Houston Methodist Institutional Review Board.
Invasive Hemodynamics
The procedure was performed under general anesthesia and guided by transesophageal echocardiography (TEE) and fluoroscopy. Following a transseptal puncture, a 24‐F transseptal sheath was used to measure the mean left atrial pressure (LAP) and v‐wave pressure before inserting the clip delivery system. Throughout the procedure, LAP and v‐wave were continuously monitored. Once the final clip was deployed, the sheath was withdrawn from the left atrium to the right atrium, and direct LAP and v‐wave measurements were obtained.
Echocardiographic Analysis
All patients had preprocedural transthoracic echocardiography and TEE using a standard echocardiography system (i33 instruments; Philips Technology, Amsterdam, the Netherlands). At discharge, the mean gradient across the mitral valve was measured by transthoracic echocardiography using continuous wave Doppler of the inflow tracing. The severity of MR and TR was assessed based on the American Society of Echocardiography guidelines, categorizing them as mild (1+), moderate (2+), moderate to severe (3+), or severe (4+). The mechanism of MR was classified as primary/degenerative, secondary, or mixed based on guidelines. 11
Statistical Analysis
Clinical, procedural, and echocardiographic characteristics were collected from patients' records before and after undergoing the procedure. For continuous variables, means with SD or medians with interquartile range were reported depending on the data distribution, and categorical variables were presented as frequencies and proportions. The normality of continuous data was assessed using the Kolmogorov–Smirnov test. The primary end point of the study was a composite of all‐cause mortality and HFH at 2 years and the secondary end point was all‐cause mortality at 2 years. Univariable Cox regression analysis was conducted to identify variables associated with 2‐year mortality/HFH. Variables with a P value <0.10 from the univariable analysis, along with the most predictive variables associated the outcomes of interest, were considered for the multivariable Cox regression analysis. Only variables with a P value <0.05 were included in the final model.
Patients were stratified into different echocardiographic profiles based on the number of risk factors present. These profiles were defined as optimal (no risk factors), mixed (1 risk factor), and poor (≥2 risk factors). Differences among the 3 profiles were assessed using analysis of variance for continuous variables and chi‐square or Fisher's exact test for categorical variables. Kaplan–Meier analysis was used to estimate survival rates for the primary end point in the overall population, and differences in survival among the profiles were compared using the log‐rank test. The area under the curve for optimal versus poor profile was 0.73 (95% CI, 0.65–0.81) and the area under the curve of optimal profile versus mixed was 0.66 (95% CI, 0.60–0.73) in predicting the composite end point. The impact of the echocardiographic profile on the end points was determined using Cox regression analysis. In assessing the proportionality assumption, we used the “Cox.zph” function in R, which allowed us to examine the risk factor by log(time) interactions. Specifically, we conducted log‐rank tests for each covariate to assess whether the hazard ratios varied over time. The results of these tests were consistently nonsignificant with P values >0.05 across all cases, providing evidence in support of the proportionality assumption.
A 2‐sided P value <0.05 was considered statistically significant, and all statistical analyses were performed using SPSS version 28.0 (IBM, Armonk, NY) and R (version 4.3.0).
Results
Baseline Clinical, Echocardiographic, and Procedural Characteristics
Of the 291 patients (mean age 76.7±10.6 years, 43.3% women) included in our final analysis, 156 (53.6%) patients had primary MR, 108 (37.2%) patients had secondary MR, and 27 (9.3%) patients had MR of mixed cause. All patients had MR grade ≥3+ with the vast majority having 4+ (79.7%) MR. New York Heart Association Class III/IV was present in 225 (78.9%) patients. Baseline clinical and echocardiographic characteristics are listed in Table 1.
Table 1.
Baseline Clinical and Echocardiographic Characteristics
| Clinical characteristics | Overall N=291 | Optimal N=103 | Mixed N=125 | Poor N=63 | P value |
|---|---|---|---|---|---|
| Age, y | 76.7 [10.6] | 77.7 [10.2] | 77.4 [9.0] | 73.8 [13.6] | 0.04* |
| Female sex | 126 (43.3) | 42 (40.8) | 52 (41.6) | 32 (50.8) | 0.39 |
| Hypertension | 207 (71.1) | 77 (74.8) | 89 (71.2) | 41 (65.1) | 0.41 |
| Atrial fibrillation | 174 (60.2) | 58 (56.9) | 74 (59.7) | 42 (66.7) | 0.45 |
| Smoking | 43 (14.8) | 12 (11.7) | 22 (17.6) | 9 (14.3) | 0.44 |
| Coronary artery disease | 106 (36.4) | 37 (35.9) | 43 (34.4) | 26 (41.3) | 0.64 |
| Frailty | 201 (69.1) | 67 (65.0) | 94 (75.2) | 40 (63.5) | 0.14 |
| Diabetes | 80 (27.5) | 28 (27.2) | 31 (24.8) | 21 (33.3) | 0.46 |
| Prior stroke | 36 (12.4) | 11 (10.7) | 14 (11.2) | 11 (17.5) | 0.38 |
| Dialysis | 19 (6.5) | 3 (2.8) | 10 (8.3) | 6 (9.7) | 0.12 |
| Prior myocardial infarction | 50 (17.2) | 15 (14.6) | 21 (16.9) | 14 (22.2) | 0.44 |
| Prior pacemaker | 46 (15.8) | 9 (8.7) | 26 (20.8) | 11 (17.5) | 0.04* |
| Prior implantable cardioverter‐defibrillator | 45 (15.5) | 12 (11.7) | 20 (16.0) | 13 (20.6) | 0.29 |
| Prior coronary artery bypass graft | 68 (23.4) | 26 (25.2) | 27 (21.6) | 15 (23.8) | 0.80 |
| Prior percutaneous coronary intervention | 64 (22.0) | 22 (21.4) | 26 (20.8) | 16 (25.4) | 0.75 |
| Body mass index, kg/m2 | 26.1 [6.0] | 25.5 [5.5] | 26.0 [5.6] | 27.5 [7.4] | 0.10 |
| New York Heart Association class | |||||
| III | 185 (64.9) | 72 (71.3) | 78 (63.9) | 35 (56.5) | 0.43 |
| IV | 40 (14.0) | 11 (10.9) | 16 (13.1) | 13 (21.0) | |
| STS risk MV replacement (%) | 7.3 [6.5] | 6.9 [5.4] | 7.1 [5.2] | 8.4 [10.1] | 0.41 |
| STS risk MV repair (%) | 5.3 [5.6] | 5.0 [4.7] | 5.1 [4.7] | 6.0 [8.2] | 0.52 |
| Hemoglobin, g/dL | 11.5 [2.1] | 11.9 [1.7] | 11.5 [2.0] | 10.7 [1.8] | <0.001* |
| Creatinine, mg/dL | 1.6 [1.1] | 1.5 [1.1] | 1.7 [1.0] | 1.7 [1.2] | 0.17 |
| Echocardiographic characteristics | |||||
| MR cause | |||||
| Primary | 156 (53.6) | 69 (67.0) | 61 (48.8) | 26 (41.3) | 0.001* |
| Secondary | 108 (37.2) | 29 (28.2) | 49 (39.2) | 30 (47.6) | 0.03* |
| Mixed | 27 (9.3) | 5 (4.9) | 15 (12.0) | 7 (11.1) | 0.15 |
| MR severity | |||||
| Moderate–severe | 59 (20.3) | 23 (22.3) | 25 (20.0) | 11 (17.5) | 0.43 |
| Severe | 232 (79.7) | 80 (77.7) | 100 (80.0) | 52 (82.5) | |
| Mitral valve area, cm2 | 5.2 [1.7] | 5.2 [1.7] | 5.3 [1.6] | 5.2 [1.8] | 0.81 |
| Tricuspid regurgitation≥moderate | 109 (37.5) | 15 (14.6) | 47 (37.6) | 47 (74.6) | <0.001* |
| Pulmonary artery systolic pressure, mm Hg | 52.8 [17.6] | 47.7 [17.3] | 55.0 [16.9] | 56.6 [17.7] | 0.004* |
| Mitral annular calcification | 92 (31.6) | 30 (29.1) | 43 (34.4) | 19 (30.2) | 0.66 |
| Right atrial pressure, mm Hg | 11.6 [5.8] | 10.0 [5.2] | 12.0 [5.9] | 13.4 [5.8] | 0.002* |
| Left ventricle ejection fraction (%) | 51.3 [14.6] | 53.5 [13.6] | 50.5 [15.4] | 49.1 [14.5] | 0.12 |
| LVIDs, cm | 3.7 [1.1] | 3.7 [1.0] | 3.7 [1.2] | 3.8 [1.2] | 0.68 |
| LVIDd, cm | 5.3 [0.9] | 5.3 [0.8] | 5.3 [0.9] | 5.2 [1.2] | 0.95 |
| LA volume, mL | 118.5 [52.2] | 108.9 [46.5] | 119.4 [51.5] | 132.3 [59.4] | 0.02* |
| LA volume index, mL/m2 | 62.9 [25.5] | 58.6 [22.7] | 62.8 [24.5] | 70.0 [30.3] | 0.02* |
Values are expressed as mean [SD] or N (%). LA indicates left atrium; LVIDs/d, left ventricle internal diameter (systole/diastole); MR, mitral regurgitation; and STS, Society for Thoracic Surgeons.
P value is significant if <0.05.
Procedural Characteristics
Procedural details are summarized in Table 2. Total number of clips deployed averaged 1.5±0.6 per patient, with 53.6% receiving 1 MitraClip and 46.4% patients receiving >1 device. MR reduction to ≤1+ MR was achieved in 83.4% at the end of the procedure, with a mean reduction of 2.7±0.8 grades. TEE‐derived postprocedural TMPG averaged 3.4±1.4 mm Hg. Mitral TEER was associated with a significant reduction in invasive mean LAP (from 20.0±8.1 mm Hg to 15.1±5.9 mm Hg, P<0.001) and v‐wave (from 35.0±17.0 mm Hg to 22.0±9.4 mm Hg, P<0.001).
Table 2.
Procedural Characteristics
| Procedural characteristics | Overall N=291 | Optimal N=103 | Mixed N=125 | Poor N=63 | P value |
|---|---|---|---|---|---|
| Number of clips | |||||
| Total | 1.5 [0.6] | 1.4 [0.5] | 1.5 [0.6] | 1.7 [0.7] | 0.001* |
| 1 clip | 156 (53.6) | 66 (64.1) | 63 (50.4) | 27 (42.9) | 0.01* |
| >1 clip | 135 (46.4) | 37 (35.9) | 62 (49.6) | 36 (57.1) | |
| Type of MitraClip | |||||
| Old generation | 115 (39.5) | 37 (35.9) | 51 (40.8) | 27 (42.9) | 0.52 |
| NT classic | 15 (5.1) | 4 (3.9) | 6 (4.8) | 5 (7.9) | 0.55 |
| NTR | 31 (10.7) | 11 (10.7) | 13 (10.4) | 7 (11.1) | 0.81 |
| XT | 13 (4.4) | 4 (3.9) | 7 (5.6) | 2 (3.2) | 0.80 |
| NTW | 40 (13.7) | 9 (8.7) | 20 (16.0) | 11 (17.5) | 0.38 |
| XTW | 65 (22.3) | 30 (29.1) | 21 (16.8) | 14 (22.2) | 0.07 |
| XTR | 55 (18.9) | 20 (19.4) | 25 (20.0) | 10 (15.8) | 0.88 |
| Fluoroscopy time, min | 23.0 [17.2] | 20.4 [11.1] | 22.4 [13.5] | 28.3 [27.7] | 0.01* |
| LAP, mm Hg | 20.0 [8.1] | 17.5 [7.4] | 21.3 [8.7] | 22.7 [7.3] | <0.001* |
| V‐wave, mm Hg | 35.0 [17.0] | 31.1 [16.6] | 35.8 [17.0] | 39.5 [17.3] | 0.008* |
| Postclip | |||||
| MR severity ≥moderate | 48 (16.6) | 0 (0) | 20 (16.0) | 28 (44.4) | <0.001* |
| LAP, mm Hg | 15.1 [5.9] | 12.5 [4.9] | 15.8 [5.6] | 18.3 [6.2] | <0.001* |
| V‐wave, mm Hg | 22.0 [9.4] | 17.7 [7.6] | 22.7 [9.0] | 27.9 [9.8] | <0.001* |
| Transesophageal echocardiography‐derived transmitral mean pressure gradient | 3.4 [1.4] | 2.7 [1.1] | 3.5 [1.4] | 4.3 [1.5] | <0.001* |
| Difference pre‐ and post | |||||
| MR reduction (grade) | 2.7 [0.8] | 2.9 [0.6] | 2.7 [0.8] | 2.2 [1.0] | <0.001* |
| LAP reduction, mm Hg | 5.1 [6.9] | 5.2 [6.3] | 5.7 [7.5] | 3.9 [6.7] | 0.26 |
| V‐wave reduction, mm Hg | 13.5 [15.0] | 14.0 [15.4] | 13.5 [14.2] | 12.8 [16.2] | 0.90 |
Values are expressed as mean [SD] or N (%). LAP indicates left atrial pressure; and MR, mitral regurgitation.
P value is significant if <0.05.
Outcomes
Outcomes after Mitral TEER are summarized in Table 3. The median length of stay was 2 days (interquartile range, 1–3). There was 1 in‐hospital death. At 2 years, the primary composite end point of mortality and HFH occurred in 113 (38.8%) patients overall, with mortality and HFH rate of 28.9% and 15.8%, respectively.
Table 3.
Outcomes
| Outcomes | Overall N=291 | Optimal N=103 | Mixed N=125 | Poor N=63 | P value |
|---|---|---|---|---|---|
| In‐hospital outcomes | |||||
| Length of stay, d | 2 (1–3) | 1 (1–2) | 2 (1–4) | 2 (1–5) | 0.08 |
| In‐hospital mortality | 1 (0.3) | 1 (0.9) | 0 (0) | 0 (0) | … |
| TMPG, mm Hg | 4.2 [1.9] | 3.0 [0.9] | 4.5 [2.0] | 5.7 [2.0] | <0.001* |
| TMPG≥5, mm Hg | 99 (34.0) | 0 (0) | 54 (43.2) | 45 (71.4) | <0.001* |
| MR≥ moderate | 67 (23.1) | 0 (0) | 27 (21.6) | 40 (63.5) | <0.001* |
| TR≥ moderate | 95 (32.6) | 0 (0) | 44 (35.2) | 51 (81.0) | <0.001* |
| 30‐d outcomes | |||||
| Mortality | 10 (3.4) | 2 (1.9) | 4 (3.2) | 4 (3.2) | 0.31 |
| HFH | 6 (2.0) | 0 (0) | 4 (3.2) | 2 (3.2) | 0.18 |
| New York Heart Association functional class III or IV | 32 (15.2) | 6 (8.1) | 15 (15.6) | 11 (27.5) | 0.17 |
| LA volume, mL | 121.8 [49.6] | 116.8 [41.0] | 123.1 [55.4] | 127.6 [50.0] | 0.47 |
| LAVI, mL/m2 | 68.1 [25.7] | 64.2 [21.5] | 68.4 [28.1] | 73.9 [26.3] | 0.14 |
| Pulmonary artery systolic pressure, mm Hg | 47.2 [14.5] | 44.1 [13.8] | 46.5 [14.3] | 53.8 [14.4] | 0.002* |
| Left ventricle ejection fraction (%) | 48.1 [14.7] | 49.6 [13.0] | 46.4 [15.7] | 49.2 [15.2] | 0.25 |
| LVIDs, cm | 3.7 [1.1] | 3.5 [0.9] | 3.8 [1.2] | 3.8 [1.1] | 0.22 |
| LVIDd, cm | 5.1 [0.9] | 5.0 [0.8] | 5.2 [0.9] | 5.1 [0.9] | 0.26 |
| MR≥moderate | 89 (34.9) | 16 (17.6) | 45 (42.4) | 28 (53.8) | 0.005* |
| TMPG, mm Hg | 4.4 [2.2] | 3.4 [1.4] | 4.6 [2.3] | 5.9 [2.4] | 0.001* |
| TR severity ≥moderate | 96 (37.6) | 9 (10.1) | 42 (37.5) | 35 (64.9) | <0.001* |
| 1‐y outcomes | |||||
| Mortality | 62 (21.3) | 7 (6.8) | 34 (27.2) | 21 (33.3) | <0.001* |
| HFH | 34 (11.7) | 3 (2.9) | 18 (14.4) | 13 (20.6) | 0.001* |
| Composite | 85 (29.2) | 10 (9.7) | 45 (36.0) | 30 (47.6) | <0.001* |
| LA volume, mL | 119.0 [44.5] | 113.7 [39.2] | 123.6 [49.7] | 128.2 [44.0] | 0.30 |
| LAVI, mL/m2 | 64.6 [24.9] | 61.6 [19.9] | 67.3 [29.5] | 69.2 [25.1] | 0.32 |
| LVIDs, cm | 3.6 [1.1] | 3.4 [1.0] | 3.8 [1.2] | 3.7 [1.0] | 0.07 |
| LVIDd, cm | 5.1 [0.9] | 5.0 [0.8] | 5.2 [1.0] | 5.1 [0.7] | 0.43 |
| MR severity ≥moderate | 65 (35.1) | 16 (21.3) | 33 (42.3) | 16 (50.0) | 0.004* |
| TMPG, mm Hg | 4.2 [2.2] | 3.6 [1.8] | 4.5 [2.3] | 5.3 [2.3] | 0.002* |
| TR severity ≥moderate | 43 (26.3) | 11 (15.8) | 20 (27.0) | 13 (50.0) | <0.001* |
| 2‐y outcomes | |||||
| Mortality | 84 (28.9) | 11 (10.7) | 46 (36.8) | 27 (42.9) | <0.001* |
| HFH | 46 (15.8) | 6 (5.8) | 23 (18.4) | 17 (27.0) | <0.001* |
| Composite | 113 (38.8) | 17 (16.5) | 58 (46.4) | 38 (60.3) | <0.001* |
| Last follow‐up | |||||
| Mortality | 140 (48.1) | 34 (33.0) | 66 (52.8) | 40 (63.5) | <0.001* |
| HFH | 59 (20.3) | 10 (9.7) | 29 (23.2) | 20 (31.7) | 0.002* |
| Composite | 169 (58.1) | 40 (38.8) | 80 (64.0) | 49 (77.8) | <0.001* |
| Mitral valve reintervention | 9 (3.3) | 2 (2.1) | 4 (3.3) | 3 (5.0) | 0.60 |
Values are expressed as mean [SD] or N (%). HFH indicates heart failure hospitalization; LA, left atrium; LAVI, left atrium volume index; LVIDs/d, left ventricle internal diameter (systole/diastole); MR, mitral regurgitation; TMPG, transmitral mean pressure gradient; and TR, tricuspid regurgitation.
P value is significant if <0.05.
Echocardiographic Profiling
Based on well‐validated literature cutoffs, we identified residual MR≥moderate, predischarge TR≥moderate, and TMPG ≥5 mm Hg as the most predictive thresholds that were associated with the composite of 2‐year mortality/HFH. In our study population, 23.1% had residual MR≥moderate, 32.6% had predischarge TR≥moderate, and 34.0% had residual predischarge TMPG ≥5 mm Hg. Based on the number of these risk factors present, we created 3 echocardiographic profiles defined as optimal (0 risk factors), mixed (1 risk factor), and poor (≥2 risk factors), which were observed in 35.4%, 42.9%, and 21.7% of cases, respectively (Figure 1).
Figure 1. Echocardiographic profile.
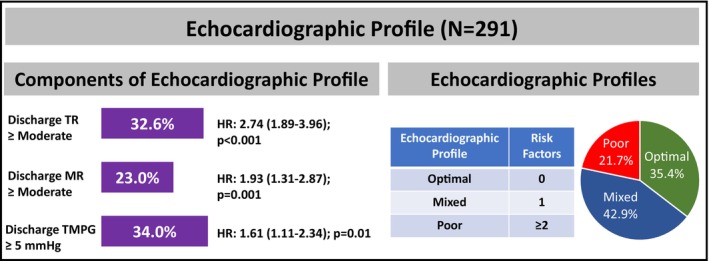
Predischarge TR≥moderate, MR≥moderate, and predischarge TMPG ≥5 mm Hg were the 3 components of the echocardiographic profile. Echocardiographic profiles were labeled as optimal, mixed, or poor based on the presence of 0, 1, or ≥2 of these components, respectively. HRs represents the univariable hazard ratio associated with the 2‐year composite end point of mortality and heart failure hospitalization. HR indicates hazard ratio; MR, mitral regurgitation; TMPG, transmitral mean pressure gradient; and TR, tricuspid regurgitation.
Clinical Characteristics According to Echocardiographic Profile
Baseline Characteristics
Compared with the mixed/poor profile, patients in the optimal profile were older (77.7 versus 77.4 versus 73.8 years, P=0.04) and had a lower prevalence of prior pacemaker (8.7% versus 20.8% versus 17.5%, P=0.04). Patients with an optimal profile had lower baseline pulmonary artery systolic pressure (47.7±17.3 versus 55.0±16.9 versus 56.6±17.7 mm Hg, P=0.004) and smaller left atrial volume index (58.6±22.7 versus 62.8±24.5 versus 70.0±30.3 mL/m2, P=0.02). Patients with the optimal profile had more frequent primary MR (67.0% versus 48.8% versus 41.3%, P=0.001) and less secondary MR (28.2% vs 39.2% versus 47.6%, P=0.03) There were no significant differences in other baseline clinical and echocardiographic characteristics between groups (Table 1).
Procedural Characteristics
Compared with the mixed/poor profile, patients in the optimal profile averaged fewer devices (1.4±0.5 versus 1.5±0.6 versus 1.7±0.7, P=0.001), had lower baseline mean LAP (17.5±7.4 versus 21.3±8.7 versus 22.7±7.3 mm Hg, P<0.001), and had lower left atrial v‐wave pressure (31.1±16.6 versus 35.8±17.0 versus 39.5±17.3 mm Hg, P=0.008). No other differences were observed in terms of baseline procedural characteristics (Table 3).
MitraClip implantation was associated with a significant reduction in MR severity along with a significant change in invasive hemodynamics within each group (all P<0.05). Compared with the mixed/poor profile group, the degree of MR reduction was statistically higher in the optimal group (2.9±0.6 versus 2.7±0.8 versus 2.2±1.0 grades, P<0.001) (Table 3 and Figure 2).
Figure 2. Mitral regurgitation severity before and after MitraClip.
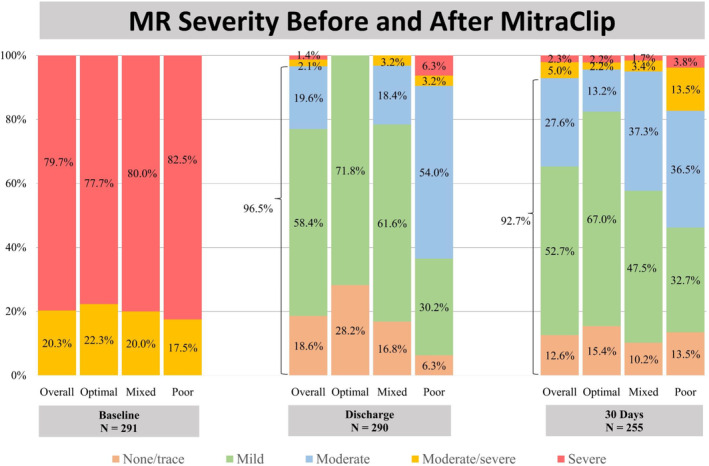
There was a significant reduction in MR severity after MitraClip implantation with ≤moderate MR in 96.5% at discharge. MR indicates mitral regurgitation.
At the end of the procedure, <moderate MR was achieved in 83.6% of patients, with a significantly greater proportion in the optimal group (100% versus 84.0% versus 55.6%, P<0.001). The optimal profile had a lower invasive mean LAP (12.5±4.9 versus 15.8±5.6 versus 18.3±6.2 mm Hg, P<0.001), left atrial v‐wave pressure (17.7±7.6 versus 22.7±9.0 versus 27.9±9.8 mm Hg, P<0.001), and TEE‐derived TMPG (2.7±1.1 versus 3.5±1.4 versus 4.3±1.5 mm Hg, P<0.001).
Outcomes According to Echocardiographic Profile
At discharge, the optimal profile group had lower proportions of patients with MR≥moderate, TR≥moderate, and TMPG≥5 mm Hg (all P<0.001). There was 1 in‐hospital mortality. At 30 days, 10 patients died and 6 had HFH, with no statistically significant differences between the groups. At 1 year, patients with the poor profile exhibited higher mortality, HFH, and the composite of mortality and HFH (all P<0.05). The proportion of MR≥moderate, TR≥moderate, and TMPG≥5 mm Hg remained statistically lower in patients with the optimal profile at 1 month and 1 year (all P<0.05).
At a median follow‐up of 23 months (interquartile range, 12–40) after mitral TEER, cumulative event‐free survival was 70.8% at 1 year and 61.2% at 2 years. Each component of the echocardiographic profile was associated with worse outcomes. When stratified by individual components, patients with residual MR≥moderate had a lower 2‐year event‐free survival (43.7% versus 65.0%; hazard ratio [HR], 1.93 [95% CI, 1.31–2.87], P=0.001), as well as those with TR≥moderate (38.4% versus 69.5%, HR, 2.74 [95% CI, 1.89–3.96], P<0.001), and those with TMPG ≥5 mm Hg (49.2% versus 65.5%, HR, 1.61 [95% CI, 1.11–2.34], P=0.01). Echocardiographic profile was associated with the primary end point with a higher 2‐year event‐free survival in the optimal profile group (84.1% versus 51.7% mixed versus 37.1% poor, P<0.001), and an overall incremental risk of mortality/HFH per profile (HR, 2.12 [95% CI, 1.65–2.73], P<0.001) and within primary (HR, 1.98/profile [95% CI, 1.37–2.85], P<0.001) and secondary MR (HR, 2.05/profile [95% CI, 1.39–3.03], P<0.001) cohorts (Figure 3). Moreover, the echocardiographic profile was associated with overall survival alone, with a higher 2‐year cumulative survival in the optimal profile (89.6% versus 60.6% mixed versus 52.3% poor, P<0.004), and an overall incremental risk of mortality per profile (HR, 2.05 [95% CI, 1.53–2.73], P<0.001) (Figure 4).
Figure 3. Event‐free survival based on individual components and echocardiographic profile.
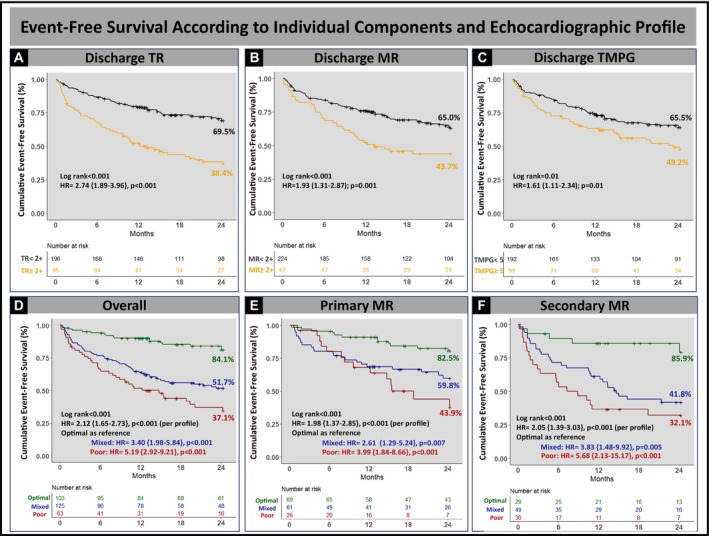
Cumulative event‐free survival was associated with each component of the hemodynamic profile, with lower 2‐year cumulative event‐free survival in patients with predischarge TR≥moderate (A), MR≥moderate (B), and predischarge TMPG ≥5 mm Hg (C); and the intraprocedural hemodynamic profile with lower 2‐year cumulative event‐free survival overall. Compared with patients with poor profile, optimal profile had superior event survival overall (D) and in patients with primary (E) and secondary (F) MR. HR indicates hazard ratio; MR, mitral regurgitation; TMPG, transmitral mean pressure gradient; and TR, tricuspid regurgitation.
Figure 4. Cumulative survival based on echocardiographic profile.
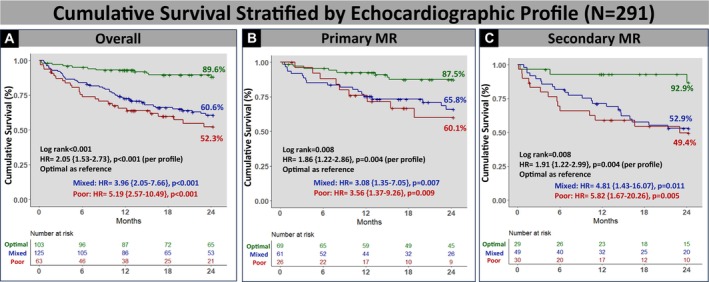
Compared with patients with poor profile, optimal profile had superior overall (A) survival and in patients with primary (B) and secondary (C) MR. HR indicates hazard ratio; MR, mitral regurgitation; TMPG, transmitral mean pressure gradient; and TR, tricuspid regurgitation.
Predictors Associated With Outcomes After Mitral TEER
The univariable analysis showed that the primary end point was associated with secondary MR and the individual components of the profile. Multivariable Cox regression showed that the individual components of the profile and the profile overall were predictors statistically associated with the primary end point. The echocardiographic profile was also a predictor associated with 2‐year mortality after mitral TEER (optimal as reference; mixed profile: HR, 3.55 [95% CI, 1.81–5.96], P<0.001; poor profile: HR, 3.39 [95% CI, 2.56–7.33], P=0.02) (Table 4).
Table 4.
Predictors Associated With the Primary End Point of 2‐Year Mortality/HFH and 2‐Year Mortality Alone
| Univariable | HR | CI lower | CI upper | P value | Multivariable | HR | CI lower | CI upper | P value |
|---|---|---|---|---|---|---|---|---|---|
| Forest plot of predictors of 2‐year mortality + HFH | |||||||||
| Age | 1.01 | 0.98 | 1.02 | 0.441 | 1.02 | 1.01 | 1.03 | 0.03† | |
| MR cause | 1.35 | 1.04 | 1.75 | 0.02† | 0.79 | 0.56 | 1.12 | 0.19 | |
| Residual MR* | 1.93 | 1.31 | 2.87 | 0.001† | 1.76 | 1.14 | 2.69 | 0.009† | |
| Residual tricuspid regurgitation* | 2.74 | 1.89 | 3.96 | <0.001† | 1.9 | 1.25 | 2.88 | 0.002† | |
| Residual transmitral pressure gradient* | 1.61 | 1.11 | 2.34 | 0.01† | 1.51 | 1.01 | 2.24 | 0.042† | |
| Poor profile (Optimal as reference) | 5.19 | 2.92 | 9.21 | <0.001† | 3.47 | 1.84 | 6.53 | <0.001† | |
| Mixed profile (Optimal as reference) | 3.40 | 1.98 | 5.84 | <0.001† | 2.97 | 1.71 | 5.17 | <0.001† | |
| Atrial fibrillation | 1.41 | 0.89 | 2.21 | 0.136 | |||||
| MAC | 1.32 | 0.84 | 2.05 | 0.219 | |||||
| Prior stroke | 1.49 | 0.83 | 2.64 | 0.173 | |||||
| Post LAP | 1.07 | 1.05 | 1.11 | <0.001† | 1.04 | 1.01 | 1.07 | 0.01† | |
| Baseline creatinine | 1.08 | 0.98 | 1.18 | 0.09 | |||||
| PASP | 1.00 | 0.98 | 1.01 | 0.735 | |||||
| Baseline Hgb | 0.89 | 0.8 | 0.98 | 0.03† | 0.89 | 0.81 | 0.98 | 0.02† | |
| LVEF | 0.97 | 0.95 | 0.98 | <0.001† | 0.97 | 0.96 | 0.99 | 0.002† | |
| Forest plot of predictors of 2‐year mortality | |||||||||
| Age | 1.02 | 0.99 | 1.03 | 0.154 | |||||
| MR cause | 1.35 | 1.01 | 1.83 | 0.048† | 0.86 | 0.58 | 1.27 | 0.45 | |
| Atrial fibrillation | 1.48 | 0.87 | 2.53 | 0.145 | |||||
| Poor profile (Optimal as reference) | 5.19 | 2.57 | 10.49 | <0.001† | 3.39 | 2.56 | 7.33 | 0.02† | |
| Mixed profile (Optimal as reference) | 3.96 | 2.05 | 7.66 | <0.001† | 3.55 | 1.81 | 5.96 | <0.001† | |
| MAC | 1.44 | 0.36 | 2.41 | 0.159 | |||||
| Prior stroke | 1.82 | 0.97 | 3.43 | 0.061 | |||||
| Post LAP | 1.06 | 1.03 | 1.1 | <0.001† | 1.04 | 0.99 | 1.07 | 0.08 | |
| Baseline creatinine | 1.08 | 0.97 | 1.2 | 0.156 | |||||
| PASP | 1.00 | 0.98 | 1.01 | 0.71 | |||||
| Baseline Hgb | 0.86 | 0.76 | 0.97 | 0.014† | 0.89 | 0.79 | 0.99 | 0.04† | |
| LVEF | 0.97 | 0.96 | 0.99 | 0.004† | 0.98 | 0.97 | 0.99 | 0.04† | |
Hgb indicates hemoglobin; HFH, heart failure hospitalization; HR, hazard ratio; LAP, left atrial pressure; LVEF, left ventricular ejection fraction; MAC, mitral annular calcification, MR, mitral regurgitation; PASP, pulmonary artery systolic pressure.
These variables were excluded from the model that incorporates the profile, as they serve as integral components directly associated with the profile.
P value is significant if <0.05.
Predictors Associated With the Poor Profile
In the univariable analysis, factors such as age, postprocedural LAP, baseline hemoglobin, and body mass index demonstrated associations with the composite end point. However, in the multivariable Cox regression analysis, it was revealed that postprocedural LAP (HR, 1.03 [95% CI, 1.01–1.04], P<0.001) and baseline hemoglobin (HR, 0.93 [95% CI, 0.89–0.97], P=0.001) were predictors statistically associated with the primary composite end point (Table S1).
Discussion
In this retrospective single‐center study consisting of 291 patients undergoing mitral TEER, the prognostic utility of integrating predischarge noninvasive Doppler parameters into an echocardiographic profile was examined to study outcomes after MitraClip. Our key findings are as follows (Figure 5): First, predischarge TR≥moderate, MR≥moderate, and predischarge TMPG ≥5 mm Hg were predictors statistically associated with the primary composite end point of 2‐year mortality and HFH. Second, echocardiographic profiles generated based on the number of risk factors present showed that optimal (0 risk factor), mixed (1 risk factor), and poor (≥2 risk factors) profiles were observed in 35.4%, 42.9%, and 21.7% of patients, respectively. Third, patients with the optimal profile had superior outcomes overall. There was an incremental risk of both mortality and mortality/HFH with each worsening profile. Finally, the echocardiographic profile was a predictor statistically associated with the 2‐year mortality and composite end point after mitral TEER.
Figure 5. TEER outcomes according to echocardiographic profile.
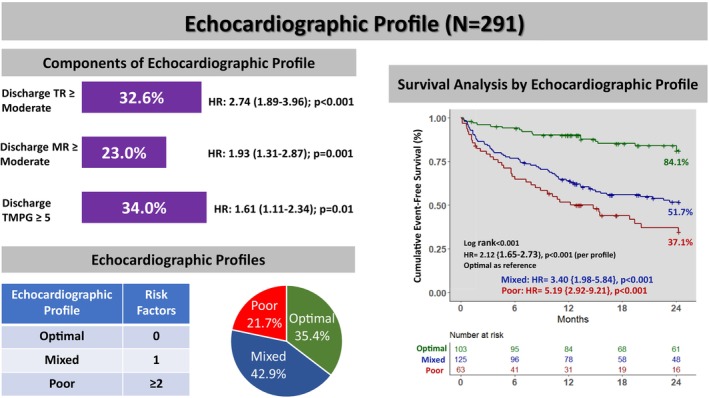
Using well‐studied thresholds for predischarge TR (≥ moderate), MR (≥ moderate), and predischarge TMPG (≥5 mm Hg), 3 echocardiographic profiles were developed: optimal (0 risk factors), mixed (1 risk factor), and poor (≥2 risk factors). Compared with mixed/poor groups, optimal profile was associated with higher 2‐year cumulative event‐free survival (log‐rank P<0.001), with an incremental risk of mortality and heart failure hospitalization (HR, 2.12 [95% CI, 1.65–2.73], P<0.001) with each profile. HR indicates hazard ratio; MR, mitral regurgitation; TMPG, transmitral mean pressure gradient; and TR, tricuspid regurgitation.
The impact of the individual risk factors in our study have been studied in isolation. Previous studies have investigated baseline TR for predicting outcomes after mitral TEER. 7 , 8 For all patients, preprocedural transthoracic echocardiography and TEE was done at different times, rendering the proper assessment of baseline TR. Inconsistencies in reporting outcomes between intraoperative and predischarge MR and TMPG have also been described. 3 , 4 , 5 , 6 , 9 , 10 This is the first study to investigate the outcomes and impact of all 3 noninvasive echocardiographic parameters at a standardized time point consistently applied to all patients. The study uses these parameters to create profiles that offer valuable prognostic insights for patients undergoing mitral TEER.
Prognostic Value of TR in Mitral TEER
TR is often present in patients with severe MR and may be due to secondary pulmonary hypertension, with or without right ventricular dysfunction/dilatation. In many single‐center and multicenter studies, greater severity of TR was associated with adverse cardiovascular outcomes. 7 , 12 , 13 Chronic pulmonary hypertension secondary to left heart disease may result in right ventricular dilatation and dysfunction, leading to tricuspid valve tethering and progressive functional TR. 14 This mechanism is further supported by our observation that patients with an optimal profile and less than moderate TR at discharge had lower pulmonary artery systolic pressure when compared with patients in the mixed and poor profile groups and lower baseline LAP. Previous studies have examined the impact of TR in secondary and primary MR populations. 7 , 8 , 12 , 13 In our cohort (≈53% primary MR), predischarge TR≥moderate was a predictor statistically associated with the 2‐year mortality and HFH endpoint after TEER (HR, 1.90 [95% CI, 1.25–2.88], P=0.002). Evidence on concomitant transcatheter or surgical tricuspid intervention during MitraClip or mitral valve surgery was associated with a lower incidence of the primary endpoint. 15 , 16 In our study, TR severity did not significantly improve after mitral TEER (Figure S1); the question of whether TR intervention should be performed concurrently or sequentially with mitral TEER remains unanswered, especially considering studies showing improved TR severity after mitral TEER. 12 , 17
Prognostic Value of Predischarge TMPG in Mitral TEER
The goal of mitral TEER is to reduce MR while avoiding significant mitral stenosis. According to most studies, there was a substantial correlation between ≥moderate residual MR and 1‐year mortality. 4 , 5 , 6 On the other hand, the significance of TMPG is debatable. Two studies showed that postprocedural TMPG was associated with worse outcomes 18 , 19 in contrary to predischarge TMPG where 1 out of 3 studies showed association between the primary end point and elevated TMPG. 9 , 10 , 20 It is important to note that in our population, postprocedural TMPG was not statistically associated with the primary end point, whereas predischarge TMPG ≥5 mm Hg was associated with worse outcomes (HR, 1.51 [95% CI, 1.01–2.24], P=0.042). Our findings underscore the importance of integrating predischarge MR, TR, and TMPG, which could help prognosticate patients undergoing mitral TEER.
Echocardiographic Profiling in Mitral TEER
Echocardiographic studies exploring outcomes in mitral TEER have been performed in isolation, and no prior studies have integrated multiple echocardiographic parameters to study long‐term outcomes after MitraClip. Echocardiographic profiling by integrating noninvasive Doppler parameters may further prognosticate patients undergoing mitral TEER to predict long‐term clinical outcomes. We used well‐described thresholds for each risk factor to develop 3 echocardiographic profiles based on the number of risk factors present: optimal (0 risk factor), mixed (1 risk factor), and poor (≥2 risk factors). We found that there was an incremental risk of both mortality and mortality/HFH with each profile. Additionally, echocardiographic profile was statistically associated with the 2‐year mortality end point after TEER.
Despite being older, patients in the optimal profile in our study had superior outcomes compared with the mixed/poor groups. At baseline, all 3 groups had comparable left ventricle dimensions, ejection fraction, and MR severity. Patients in the optimal profile had significantly lower baseline mean LAP when compared with patients in the mixed and poor profile (P=0.04). This group likely represents patients with the least remodeling and those who derive the most benefit from MitraClip. This assumption is further reinforced by the smaller baseline left atrial volume and lower baseline pulmonary artery systolic pressure with lower invasive mean LAP and v‐wave after MitraClip.
Patients in the optimal profile had more primary MR and less secondary MR when compared with patients in the mixed and poor profiles. However, patients with optimal profile had superior outcomes within each cause when compared with patients with mixed/poor profiles, which further strengthens the discrimination of the profile. Multivariable analysis showed that the echocardiographic profile was statistically associated with the primary end point after adjusting for MR cause, age, sex, and baseline clinical characteristics (Table 4).
Postprocedural LAP serves as a hemodynamic parameter that reflects changes in left ventricular and atrial compliance, along with pre‐ and postprocedural mitral stenosis. Additionally, it indicates the presence of residual MR postprocedurally. Moreover, it is acknowledged as a component influencing pulsatile right ventricular afterload, a factor linked to right ventricular dysfunction and TR. Considering the aforementioned factors, an association between higher postprocedural LAP and patients with the poor profile is not surprising, leading to worse clinical outcomes after mitral TEER (Table S1).
These observations are significant considering recent research that emphasizes the importance of identifying individuals who respond positively to mitral TEER therapy. 21 , 22 Therefore, the ability to stratify these patients based on readily available and easily obtainable echocardiographic parameters may prove beneficial for further prognostication.
Study Limitations
First, this study includes a relatively small number of patients undergoing TEER at a single institution. Second, the retrospective nature of this study at a single institution has inherent limitations and biases, including time bias as different TEER device generations were included. Second, there was no independent echocardiographic core laboratory to assess the echocardiographic parameters before and after the procedure. However, a single experienced echocardiographer (P.W.) performed all echocardiographic evaluation. Third, the limited number of patients might have affected the overall follow‐up and carrying out of meaningful analysis.
Conclusions
Echocardiographic profile integrating noninvasive Doppler parameters presents a novel prognostication tool for patients undergoing mitral TEER. The profile integrating predischarge TR, MR, and TMPG presents a prognostic tool to further stratify these patients. However, it is important to note that further validation of these findings in a larger independent cohort is necessary, and additional longer‐term studies are warranted.
Sources of Funding
None.
Disclosures
Dr Reardon is a consultant for Medtronic, Boston Scientific, Abbott, and W L Gore & Associates. Dr Atkins is a consultant for W L Gore & Associates. Dr Kleiman is a local principal investigator in trials sponsored by Boston Scientific, Medtronic, Abbott, and Edwards Lifesciences. Dr Goel is a Consultant for Medtronic, W L Gore & Associates, and on the speakers bureau for Abbott Structural Heart. The remaining authors have no disclosures to report.
Supporting information
Table S1
Figure S1
This study was presented as a Moderated Abstract at the Transcatheter Cardiovascular Therapeutics (TCT) Conference, October 23–26, 2023, in San Francisco, CA.
This article was sent to Amgad Mentias, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.032784
For Sources of Funding and Disclosures, see page 12.
References
- 1. Enriquez‐Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet. 2009;373:1382–1394. doi: 10.1016/S0140-6736(09)60692-9 [DOI] [PubMed] [Google Scholar]
- 2. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP III, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2021;143:e72–e227. doi: 10.1161/CIR.0000000000000923 [DOI] [PubMed] [Google Scholar]
- 3. Feldman T, Kar S, Elmariah S, Smart SC, Trento A, Siegel RJ, Apruzzese P, Fail P, Rinaldi MJ, Smalling RW, et al. Randomized comparison of percutaneous repair and surgery for mitral regurgitation: 5‐year results of EVEREST II. J Am Coll Cardiol. 2015;66:2844–2854. doi: 10.1016/j.jacc.2015.10.018 [DOI] [PubMed] [Google Scholar]
- 4. Stone GW, Abraham WT, Lindenfeld J, Kar S, Grayburn PA, Lim DS, Mishell JM, Whisenant B, Rinaldi M, Kapadia SR, et al. Five‐year follow‐up after transcatheter repair of secondary mitral regurgitation. N Engl J Med. 2023;388:2037–2048. doi: 10.1056/NEJMoa2300213 [DOI] [PubMed] [Google Scholar]
- 5. Higuchi S, Orban M, Stolz L, Karam N, Praz F, Kalbacher D, Ludwig S, Braun D, Näbauer M, Wild MG, et al. Impact of residual mitral regurgitation on survival after transcatheter edge‐to‐edge repair for secondary mitral regurgitation. JACC Cardiovasc Interv. 2021;14:1243–1253. doi: 10.1016/j.jcin.2021.03.050 [DOI] [PubMed] [Google Scholar]
- 6. Boekstegers P, Hausleiter J, Schmitz T, Bufe A, Comberg T, Seyfarth M, Frerker C, Beucher H, Rottländer D, Higuchi S, et al. Intraprocedural residual mitral regurgitation and survival after transcatheter edge‐to‐edge repair: prospective German multicenter registry (MITRA‐PRO). JACC Cardiovasc Interv. 2023;16:574–585. doi: 10.1016/j.jcin.2022.12.015 [DOI] [PubMed] [Google Scholar]
- 7. Hahn RT, Asch F, Weissman NJ, Grayburn P, Kar S, Lim S, Ben‐Yehuda O, Shahim B, Chen S, Liu M, et al. Impact of tricuspid regurgitation on clinical outcomes: the COAPT trial. J Am Coll Cardiol. 2020;76:1305–1314. doi: 10.1016/j.jacc.2020.07.035 [DOI] [PubMed] [Google Scholar]
- 8. Chitturi KR, Bhardwaj B, Murtaza G, Karuparthi PR, Faza NN, Goel SS, Reardon MJ, Kleiman NS, Aggarwal K. Clinical impact of tricuspid regurgitation on transcatheter edge‐to‐edge mitral valve repair for mitral regurgitation. Cardiovasc Revasc Med. 2022;41:1–9. doi: 10.1016/j.carrev.2022.01.027 [DOI] [PubMed] [Google Scholar]
- 9. Koell B, Ludwig S, Weimann J, Waldschmidt L, Hildebrandt A, Schofer N, Schirmer J, Westermann D, Reichenspurner H, Blankenberg S, et al. Long‐term outcomes of patients with elevated mitral valve pressure gradient after mitral valve edge‐to‐edge repair. JACC Cardiovasc Interv. 2022;15:922–934. doi: 10.1016/j.jcin.2021.12.007 [DOI] [PubMed] [Google Scholar]
- 10. Halaby R, Herrmann HC, Gertz ZM, Lim S, Kar S, Lindenfeld J, Abraham WT, Grayburn PA, Naidu S, Asch FM, et al. Effect of mitral valve gradient after MitraClip on outcomes in secondary mitral regurgitation: results from the COAPT trial. JACC Cardiovasc Interv. 2021;14:879–889. doi: 10.1016/j.jcin.2021.01.049 [DOI] [PubMed] [Google Scholar]
- 11. Zoghbi WA, Enriquez‐Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3 [DOI] [PubMed] [Google Scholar]
- 12. Ohno Y, Attizzani GF, Capodanno D, Cannata S, Dipasqua F, Immé S, Barbanti M, Ministeri M, Caggegi A, Pistritto AM, et al. Association of tricuspid regurgitation with clinical and echocardiographic outcomes after percutaneous mitral valve repair with the MitraClip system: 30‐day and 12‐month follow‐up from the GRASP registry. Eur Heart J Cardiovasc Imaging. 2014;15:1246–1255. doi: 10.1093/ehjci/jeu114 [DOI] [PubMed] [Google Scholar]
- 13. Sorajja P, Vemulapalli S, Feldman T, Mack M, Holmes DR Jr, Stebbins A, Kar S, Thourani V, Ailawadi G, et al. Outcomes with transcatheter mitral valve repair in the United States: an STS/ACC TVT registry report. J Am Coll Cardiol. 2017;70:2315–2327. doi: 10.1016/j.jacc.2017.09.015 [DOI] [PubMed] [Google Scholar]
- 14. Hahn RT, Waxman AB, Denti P, Delhaas T. Anatomic relationship of the complex tricuspid valve, right ventricle, and pulmonary vasculature: a review. JAMA Cardiol. 2019;4:478–487. doi: 10.1001/jamacardio.2019.0535 [DOI] [PubMed] [Google Scholar]
- 15. Mehr M, Karam N, Taramasso M, Ouarrak T, Schneider S, Lurz P, von Bardeleben RS, Fam N, Pozzoli A, Lubos E. Combined tricuspid and mitral versus isolated mitral valve repair for severe MR and TR: an analysis from the TriValve and TRAMI registries. JACC Cardiovasc Interv. 2020;13:543–550. doi: 10.1016/j.jcin.2019.10.023 [DOI] [PubMed] [Google Scholar]
- 16. Gammie JS, Chu MWA, Falk V, Overbey JR, Moskowitz AJ, Gillinov M, Mack MJ, Voisine P, Krane M, Yerokun B. Concomitant tricuspid repair in patients with degenerative mitral regurgitation. N Engl J Med. 2022;386:327–339. doi: 10.1056/NEJMoa2115961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frangieh AH, Gruner C, Mikulicic F, Attinger‐Toller A, Tanner FC, Taramasso M, Corti R, Grünenfelder J, Lüsche TF, Ruschitzka F, et al. Impact of percutaneous mitral valve repair using the MitraClip system on tricuspid regurgitation. EuroIntervention. 2016;11:E1680–E1686. doi: 10.4244/EIJV11I14A320 [DOI] [PubMed] [Google Scholar]
- 18. Neuss M, Schau T, Isotani A, Pilz M, Schöpp M, Butter C. Elevated mitral valve pressure gradient after MitraClip implantation deteriorates long‐term outcome in patients with severe mitral regurgitation and severe heart failure. J Am Coll Cardiol Intv. 2017;10:931–939. doi: 10.1016/j.jcin.2016.12.280 [DOI] [PubMed] [Google Scholar]
- 19. Patzelt J, Zhang W, Sauter R, Mezger M, Nording H, Ulrich M, Becker A, Patzelt T, Rudolph V, Eitel I, et al. Elevated mitral valve pressure gradient is predictive of long‐term outcome after percutaneous edge‐to‐edge mitral valve repair in patients with degenerative mitral regurgitation (MR), but not in functional MR. J Am Heart Assoc. 2019;8:e011366. doi: 10.1161/JAHA.118.011366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoon SH, Makar M, Kar S, Chakravarty T, Oakley L, Sekhon N, Koseki K, Enta Y, Nakamura M, Hamilton M, et al. Prognostic value of increased mitral valve gradient after transcatheter edge‐to‐edge repair for primary mitral regurgitation. JACC Cardiovasc Interv. 2022;15:935–945. doi: 10.1016/j.jcin.2022.01.281 [DOI] [PubMed] [Google Scholar]
- 21. Hatab T, Bou Chaaya RG, Zaid S, Wessly P, Satish P, Villanueva V, Faza N, Little SH, Atkins MD, Reardon MJ, et al. Feasibility and outcomes of mitral transcatheter edge‐to‐edge repair in patients with variable degrees of mitral annular calcification. J Am Heart Assoc. 2023;12:e031118. doi: 10.1161/JAHA.123.031118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zaid S, Wessly P, Hatab T, Faza N, Little SH, Atkins MD, Reardon MJ, Kleiman NS, Zoghbi WA, Goel SS. Intraprocedural doppler and invasive hemodynamic profiling predict clinical outcomes after mitral TEER. J Am Coll Cardiol Img. 2023;S1936‐878X(23)00476‐X. Online ahead of print. doi: 10.1016/j.jcmg.2023.10.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figure S1


