Abstract
Background
Poor neighborhood‐level access to health care, including community pharmacies, contributes to cardiovascular disparities in the United States. The authors quantified the association between pharmacy proximity, antihypertensive and statin use, and blood pressure (BP) and low‐density lipoprotein cholesterol (LDL‐C) among a large, diverse US cohort.
Methods and Results
A cross‐sectional analysis of Black and White participants in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study during 2013 to 2016 was conducted. The authors designated pharmacy proximity by census tract using road network analysis with population‐weighted centroids within a 10‐minute drive time, with 5‐ and 20‐minute sensitivity analyses. Pill bottle review measured medication use, and BP and LDL‐C were assessed using standard methods. Poisson regression was used to quantify the association between pharmacy proximity with medication use and BP control, and linear regression for LDL‐C. Among 16 150 REGARDS participants between 2013 and 2016, 8319 (51.5%) and 8569 (53.1%) had an indication for antihypertensive and statin medication, respectively, and pharmacy proximity data. The authors did not find a consistent association between living in a census tract with higher pharmacy proximity and antihypertensive medication use, BP control, or statin medication use and LDL‐C levels, regardless of whether the area was rural, suburban, or urban. Results were similar among the 5‐ and 20‐minute drive‐time analyses.
Conclusions
Living in a low pharmacy proximity census tract may be associated with antihypertensive and statin medication use, or with BP control and LDL‐C levels. Although, in this US cohort, outcomes were similar for adults living in high or low pharmacy proximity census tracts.
Keywords: antihypertensive, disparities, pharmacy proximity, rural–urban, statin
Subject Categories: Cardiovascular Disease, Risk Factors
Nonstandard Abbreviations and Acronyms
- CES‐D
Center for Epidemiologic Studies–Depression Scale
- JNC 7
Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure
- MCS
Short‐Form 12 Mental Health Component Summary
- PCS
Short‐Form 12 Physical Component Summary
- PR
prevalence ratio
- REGARDS
Reasons for Geographic and Racial Differences in Stroke
Clinical Perspective.
What Is New?
In the current analysis, US adults with hypertension who lived in census tracts with a low proximity to community pharmacies by drive time had similar antihypertensive medication use and blood pressure control as adults who lived in communities with a high proximity to community pharmacies.
Among adults with an indication for statin therapy for primary or secondary prevention, we did not find evidence that living in a low‐ versus high‐pharmacy proximity community was associated with statin medication use or low‐density lipoprotein cholesterol.
What Are the Clinical Implications?
Medication access is a significant barrier to achieving blood pressure control and appropriate low‐density lipoprotein levels, and ensuring patients have reliable pharmacy access is imperative.
Distance to the nearest pharmacy by drive time may not be a significant contributing factor to low use of antihypertensive medication or statin medication among US adults with an indication for medication use.
Hypertension and hyperlipidemia are the leading modifiable risk factors for cardiovascular disease (CVD) in the United States and disproportionally impact individuals residing in rural communities. For example, in 2017, the prevalence of hypertension was >10% higher in rural communities compared with urban communities. 1 Between 2013 and 2018, blood pressure (BP) and low‐density lipoprotein cholesterol (LDL‐C) control improved in urban communities, but such a trend was not observed in rural communities. 2 While disparities in BP and hyperlipidemia control are multifactorial, often influenced by medication adherence, clinical inertia, and complex environmental and interpersonal interactions, the degree to which differential medication access contributes to observed geographic disparities in CVD is unclear. 3 , 4 , 5
US adults living in communities with poor social and economic conditions face lower access to health care, documented through decreased health care availability and utilization. 6 , 7 , 8 Among 5096 residents in the south side of Chicago, those who had greater geographic accessibility to primary care had a lower prevalence of hypertension as well as uncontrolled hypertension. 9 Community pharmacies play a key role in health care. Access to pharmacies and pharmacy services is lower in communities that are largely minoritized populations or of low socioeconomic status, leading to “pharmacy deserts.” 10 , 11 While pharmacy delivery services (eg, “mail order”) might theoretically improve equitable access to medication, such services are predominantly used among White and high‐income households. 12 , 13 Recent estimates from the Medical Expenditure Panel Survey found that only 10.3% of US adults in 2018 received their prescriptions from mail‐order pharmacies. 12 Living in a pharmacy desert may severely limit access to antihypertensive and statin medications and impede BP control and lower LDL‐C levels.
We used data derived from a national population‐based study to quantify the association between pharmacy accessibility, as measured by pharmacy proximity, and use of antihypertensive and statin medications, separately, as well as the association with BP control and LDL‐C levels, separately. Such information might provide insights that could guide policies regarding pharmacy accessibility and strategies aimed at reducing geographic disparities in CVD within the United States.
Methods
Study Population
Briefly, the REGARDS study enrolled 30 239 adults 45 years and older throughout the United States between 2003 and 2007. 14 The goal of the REGARDS trial was to determine contributing factors to the higher stroke mortality rate among Black versus White adults in the Southeastern United States. A second in‐home examination (visit 2) was completed for 16 150 participants between 2013 and 2016, following the exclusion of 56 patients for data anomalies, 6590 who died, and 7443 lost to follow‐up or declined further participation. 15 The REGARDS study was approved by the institutional review board at the University of Alabama at Birmingham and other participating centers, and all participants provided written informed consent. Requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be submitted to the REGARDS trial executive committees at www.uab.edu/soph/regardsstudy.
We restricted our analysis to patients who participated in the second in‐home visit, as REGARDS trial coordinators collected additional patient data on perceived neighborhood characteristics at this visit. We focused on individuals receiving active treatment for hypertension or hypercholesterolemia at the time of the second in‐home visit. All participant data used in the current analysis are from the second in‐home visit.
For the analysis of antihypertensive medication use and BP control among participants with hypertension, we included individuals who: (1) had a systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg during the in‐home visit 2 or were taking at least 1 antihypertensive medication; or (2) reported “yes” to the question “Has a doctor or other health professional ever told you that you have high blood pressure?” We excluded the 21 participants who either had no valid BP measurement or who were missing information regarding antihypertensive use.
For the analysis of statin use and LDL‐C levels, we included individuals with: (1) a diagnosis of diabetes and LDL‐C ≥70 mg/dL; (2) a history of atherosclerotic CVD; (3) a predicted 10‐year atherosclerotic CVD risk of ≥7.5% as calculated by the pooled cohort equations; or (4) an LDL‐C level ≥190 mg/dL at visit 2. 16 , 17 We excluded 1281 participants who were missing variables necessary to calculate risk from the pooled cohort equations or information about antihypertensive or statin medication use at visit 2.
Data Collection
Participants provided information on age, sex, race, educational attainment, employment status, marital status, living with others, income, health insurance, and usual source of care at the time of the second in‐home visit. We additionally obtained participant‐reported information on social support defined by the score of 6 survey items of social support, perceived neighborhood characteristics defined as agreement with the following statements: “This is a close‐knit neighborhood”, “People in this neighborhood can be trusted”, and “How safe from crime do you consider your neighborhood to be?” 18 Further, car ownership, current cigarette smoking, self‐reported diabetes status, number of physical activity events per week, depressive symptoms based on the Center for Epidemiologic Studies–Depression Scale (CES‐D) score ≥4 on the 4‐item scale, physical functioning based on the Short‐Form 12 Physical Component Summary (PCS) score, mental health based on the Short‐Form 12 Mental Health Component Summary (MCS) score, Cohen's perceived stress scale score, and physical health limiting activities of daily living were obtained by computer‐assisted telephone interviews. 19 , 20
Trained health professionals measured height and weight and collected blood and urine specimens used to determine total cholesterol, high‐density lipoprotein cholesterol, and triglycerides, as well as the estimated glomerular filtration rate (calculated using the 2021 Chronic Kidney Disease Epidemiology Collaboration equation). 21
Medication use was defined by in‐home pill bottle review. 5 , 22 , 23 , 24 To determine this, participants showed study staff the containers of medications taken in the past 2 weeks. Medication use was determined for statin use, antihypertensive medication use, and total prescription medications.
We assigned rural, suburban, and urban status by population density as follows: <1000 people per square mile, 1000 to 3000 people per square mile, and >3000 people per square mile, respectively, based on the 2015 American Community Survey data from the US Census Bureau, which were used in a previous analysis focused on pharmacy access. 25
Exposure—Pharmacy Proximity
Each REGARDS trial participant was assigned a census tract based on their residence during visit 2. Only participant census tracts that were unchanged between 2010 and 2020 were used in the current analysis. We then used census blocks' population as weights to calculate the population‐weighted centroid of each census tract. The REGARDS trial coordinating center has participants' precise addresses and geolocations, but, due to privacy concerns, the center uses this information to provide the corresponding census tract for each participant. Due to the unavailability of precise home addresses, we employed a population‐weighted centroid approach, based on their residing census tract. Population‐weighted centroids account for the geographic distribution of the population within each census tract. We defined census tract–level pharmacy proximity, assessed by drive time, using the 2‐step floating catchment area method. 26 , 27 In the first step, using road network data, we determined the catchment area of a 10‐minute drive time around the geocoded location of each outpatient pharmacy from the National Council for Prescription Drug Programs data for 2015, which closely aligned with the timing of the REGARDS trial visit 2. 28 Addresses for pharmacies were geocoded using ArcGIS Pro version 3.0 (Esri). The population served by each pharmacy was then calculated by summing up the population‐weighted centroids of census tracts that fell within a 10‐minute drive time of the pharmacy. Because we did not have data on the inventory of antihypertensive and statin availability at each pharmacy, we assumed that all pharmacies offered antihypertensives and statins. Each pharmacy was then assigned a pharmacy‐to‐population ratio to represent the population served by the pharmacy. In the second step, we created a 10‐minute catchment area around the population‐weighted centroid of each census tract and summed up the pharmacy‐to‐population ratios of pharmacies within the catchment area to determine a final value of pharmacy proximity. Pharmacy proximity was categorized by quartile of proximity. In the primary analysis using a 10‐minute drive time, pharmacy proximity per 10 000 people was 0.00 to 1.34, 1.34 to 2.05, 2.05 to 3.20, and 3.20 to 21.72 for quartile 1, quartile 2, quartile 3, and quartile 4, respectively, in the hypertension cohort and 0.00 to 1.33, 1.33 to 2.03, 2.03 to 3.12, and 3.12 to 21.73 for quartile 1, quartile 2, quartile 3, and quartile 4, respectively, in the cohort with an indication for statin use. The above analysis was repeated with 5‐ and 20‐minute drive‐time catchment areas as sensitivity analysis.
Outcome
Medication use, as well as measurement of BP and LDL‐C, are defined above. BP control was defined as systolic BP <140 mm Hg and diastolic BP <90 mm Hg to be consistent with the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) at the time of the study. 29
Statistical Analysis
We calculated participant characteristics at visit 2 and determined differences in characteristics using the Jonckheere‐Terpstra test, Cochran‐Armitage test for trend, and χ2 test, as appropriate. All analyses were stratified by population density (rural, suburban, and urban), as each category has different geographic norms for expected drive‐time distances to community pharmacies. In both cohorts separately, we calculated the prevalence of antihypertensive and statin medication use by pharmacy proximity at visit 2 using a complete case analysis (missingness Tables S1 and S2). Next, we used Poisson regression with robust error variance to calculate prevalence ratios (PRs) for the medication use outcomes associated with pharmacy proximity by quartile. Although the clinical factors were assessed at the same time as exposure, it is plausible that these factors could be influenced by pharmacy proximity. 11 , 30 , 31 , 32 , 33
In order to assess the complex factors that may influence the association between pharmacy proximity with antihypertensive and statin medication use, as well as BP control and lipid levels, we employed a conceptual model based on the Aday and Anderson framework for health care utilization, the social determinants of cardiovascular disease framework proposed by Powell‐Wiley et al, and the idea proposed by Pechansky and Thomas where access is defined by “the degree of fit between the client and the system.” 34 , 35 , 36 , 37
For model 1, we adjusted for demographic information including age, sex, and race. Model 2 included all variables in model 1 plus educational attainment, employment status, and income. Model 3 included all variables in model 2 plus measures of access including health insurance, usual source of care, car ownership, level of social support (defined by the score of 6 survey items of social support), and self‐reported neighborhood characteristics (ie, agreement with “This is a close‐knit neighborhood”, “People in this neighborhood can be trusted”, “People around here are willing to help their neighbors”, and “How safe from crime do you consider your neighborhood to be?”). Model 4 was adjusted for variables in model 3 plus health status indicators including current cigarette smoking, self‐reported diabetes status, systolic BP (in the statin use and LDL‐C models only), diastolic BP (in the statin use and LDL‐C models only), body mass index, total cholesterol, physical functioning based on the PCS score, mental health based on the MCS score, aspirin use, and number of total prescription medications. Systolic and diastolic BP were not included in the BP control outcome model. Directed acyclic graphs are found in Figure S1.
We calculated variance inflation factors between the exposure variables and the covariates in the 4 main analyses to assess whether the exposure was too strongly associated with the covariates to accurately estimate the association between the exposure and outcome after covariate adjustment. 38 The variance inflation factors for the fully adjusted model in the 4 main analyses ranged from 1.12 to 1.13, indicating that covariate adjustment did not substantially reduce the precision of our estimated coefficients associated with the exposure.
All analyses were repeated for the outcome of BP control among patients taking antihypertensive medication. In addition, these analyses were repeated in the cohort with an indication for statin medication for the outcomes of statin use and LDL‐C levels. We used linear regression adjusted for the same variables as described above to determine mean differences in continuous LDL‐C associated with pharmacy proximity by quartile at visit 2. In addition, a marginal standardization method was used to estimate an average model‐based multivariable‐adjusted probability of each outcome by pharmacy proximity as a continuous variable for rural, suburban, and urban census tracts.
All analyses were repeated by the following subgroups defined at visit 2: car ownership (yes versus no), race and sex (White: man and woman versus Black: man and woman), age (≥65 years versus <65 years), and polypharmacy status (taking versus not taking ≥5 prescription medications). Interactions between each subgroup and the main exposure were tested by a product term in the regression model. All analyses were performed using R version 4.1.3 (R Core Team), and all tests were 2‐tailed with a statistical significance assigned at the 0.05 level.
Results
Baseline Characteristics
Among the 8319 included participants with hypertension, 55% (n=4599) and 45% (3720) were of White and Black race, respectively, and 57% (n=4756) were women. The median BP was 127/76 mm Hg, with 84% taking an antihypertensive. Among participants with hypertension, 35%, 30%, and 36% lived in a rural, suburban, and urban census tract, respectively (Table S3). The prevalence of antihypertensive use was 84.0%, 84.5%, and 84.7% among participants living in a rural, suburban, and urban census tract, respectively. The prevalence of BP control was 79.4%, 80.6%, and 82.3% among participants living in a rural, suburban, and urban census tract (Table 1).
Table 1.
Distribution of Medication Use, BP Control, and LDL‐C by Population Density
| Hypertension cohort | Statin cohort | |||||
|---|---|---|---|---|---|---|
| Population | No. | Prevalence of antihypertensive medication use, % | Prevalence of BP control, % | No. | Prevalence of statin medication use, % | LDL‐C, median (minimum, maximum) |
| Rural | 2875 | 84.0 | 79.4 | 3027 | 50.6 | 95.4 (11.8, 272.9) |
| Suburban | 2458 | 84.5 | 80.6 | 2541 | 50.8 | 94.3 (17.0, 278.4) |
| Urban | 2986 | 84.7 | 82.3 | 3001 | 50.0 | 94.9 (6.8, 382.8) |
BP indicates blood pressure; and LDL‐C, low‐density lipoprotein cholesterol.
Among the 8569 included participants with an indication for statin medication, 60% (5147) and 40% (3422) were of White and Black race, respectively, and 52% (n=4457) were women. The median LDL‐C was 94.9 mg/dL, with 50% of patients taking a statin medication. Among the included participants, 30% had diabetes, 34% had a history of atherosclerotic CVD, and the majority had a predicted 10‐year atherosclerotic CVD risk of ≥7.5% (97%). Among adults with an indication for a statin, 35%, 30%, and 35% lived in a rural, suburban, and urban census tract, respectively (Table S4). The prevalence of statin use was 50.6%, 50.8%, and 50.0% among participants living in a rural, suburban, and urban census tract, respectively. The median LDL‐C was 95.4 mg/dL, 94.3 mg/dL, and 94.9 mg/dL among participants living in a rural, suburban, and urban census tract, respectively (Table 1).
Association Between Pharmacy Proximity and Antihypertensive Medication Use
After complete adjustment, the PR for antihypertensive medication use was 1.02 (95% CI, 0.98–1.06) for participants in rural census tracts, 1.04 (95% CI, 0.99–1.09) for participants in suburban census tracts, and 1.01 (95% CI, 0.96–1.06) for participants in urban census tracts comparing the highest quartile of pharmacy proximity with the lowest quartile of pharmacy proximity (Table 2). Among Black male participants, living in a census tract in the highest quartile of pharmacy proximity was associated with a higher use of antihypertensives in rural census tracts only (PR, 1.17 [95% CI, 1.04–1.31]) (Table S5). Antihypertensive use was higher among adults younger than 65 years living in census tracts with the highest quartile of pharmacy proximity compared with the lowest quartile in suburban census tracts only (PR, 1.18 [95% CI, 1.00–1.40]) (Table S6). There was no association between antihypertensive medication use and pharmacy proximity by polypharmacy (Table S7) or car ownership (Table S8).
Table 2.
Association Between Antihypertensive Medication Use and BP Control With Pharmacy Proximity Among REGARDS Trial Participants During Visit 2
| PR (95% CI) | |||||
|---|---|---|---|---|---|
| Quartile, median (range) (pharmacy proximity per 10 000 persons) | Quartile 1 0.80 [0.00–1.34]) | Quartile 2 1.70 (1.34–2.05) | Quartile 3 2.53 (2.05–3.20) | Quartile 4 4.30 (3.20–21.73) | P for trend |
| Antihypertensive medication use | |||||
| Rural (n=2875) | |||||
| Cases, n | 806 | 344 | 434 | 830 | |
| Prevalence, % | 82.3 | 81.1 | 85.9 | 85.8 | |
| Unadjusted PR (95% CI) | 1.00 (Reference) | 0.99 (0.93–1.04) | 1.04 (1.00–1.09) | 1.04 (1.00–1.08) | 0.01 |
| Model 1 PR (95% CI) | 1.00 (Reference) | 0.98 (0.93–1.04) | 1.03 (0.98–1.08) | 1.03 (0.99–1.07) | 0.09 |
| Model 2 PR (95% CI) | 1.00 (Reference) | 0.98 (0.93–1.03) | 1.03 (0.99–1.08) | 1.03 (0.99–1.07) | 0.10 |
| Model 3 PR (95% CI) | 1.00 (Reference) | 0.98 (0.93–1.03) | 1.03 (0.98–1.08) | 1.02 (0.98–1.06) | 0.12 |
| Model 4 PR (95% CI) | 1.00 (Reference) | 0.98 (0.93–1.03) | 1.03 (0.98–1.07) | 1.02 (0.98–1.06) | 0.16 |
| Suburban (n=2458) | |||||
| Cases, n | 325 | 517 | 588 | 648 | |
| Prevalence, % | 82.5 | 84.8 | 84.2 | 85.7 | |
| Unadjusted PR (95% CI) | 1.00 (Reference) | 1.03 (0.97–1.09) | 1.02 (0.97–1.08) | 1.04 (0.98–1.10) | 0.22 |
| Model 1 PR (95% CI) | 1.00 (Reference) | 1.03 (0.98–1.09) | 1.04 (0.98–1.10) | 1.05 (1.00–1.11) | 0.07 |
| Model 2 PR (95% CI) | 1.00 (Reference) | 1.03 (0.97–1.09) | 1.04 (0.98–1.10) | 1.05 (0.99–1.10) | 0.11 |
| Model 3 PR (95% CI) | 1.00 (Reference) | 1.03 (0.97–1.09) | 1.04 (0.99–1.10) | 1.05 (0.99–1.10) | 0.09 |
| Model 4 PR (95% CI) | 1.00 (Reference) | 1.03 (0.97–1.08) | 1.04 (0.99–1.10) | 1.04 (0.99–1.09) | 0.13 |
| Urban (n=2986) | |||||
| Cases, n | 600 | 880 | 748 | 300 | |
| Prevalence, % | 84.9 | 84.1 | 85.3 | 84.3 | |
| Unadjusted PR (95% CI) | 1.00 (Reference) | 0.99 (0.95–1.03) | 1.01 (0.96–1.05) | 0.99 (0.94–1.05) | 0.95 |
| Model 1 PR (95% CI) | 1.00 (Reference) | 1.00 (0.96–1.05) | 1.02 (0.97–1.06) | 1.01 (0.96–1.07) | 0.53 |
| Model 2 PR (95% CI) | 1.00 (Reference) | 1.00 (0.96–1.05) | 1.01 (0.97–1.06) | 1.00 (0.95–1.06) | 0.72 |
| Model 3 PR (95% CI) | 1.00 (Reference) | 1.00 (0.96–1.04) | 1.01 (0.97–1.05) | 1.00 (0.94–1.05) | 0.87 |
| Model 4 PR (95% CI) | 1.00 (Reference) | 1.00 (0.96–1.04) | 1.01 (0.97–1.05) | 1.01 (0.96–1.06) | 0.63 |
| BP control | |||||
| Rural (n=2875) | |||||
| Cases, n | 788 | 328 | 405 | 763 | |
| Prevalence, % | 80.5 | 77.4 | 80.2 | 78.9 | |
| Unadjusted PR (95% CI) | 1.00 (Reference) | 0.96 (0.91–1.02) | 1.00 (0.94–1.05) | 0.98 (0.94–1.03) | 0.54 |
| Model 1 PR (95% CI) | 1.00 (Reference) | 0.96 (0.91–1.02) | 1.00 (0.95–1.05) | 0.98 (0.94–1.03) | 0.58 |
| Model 2 PR (95% CI) | 1.00 (Reference) | 0.96 (0.91–1.02) | 1.00 (0.95–1.05) | 0.98 (0.94–1.03) | 0.66 |
| Model 3 PR (95% CI) | 1.00 (Reference) | 0.96 (0.91–1.02) | 1.00 (0.95–1.05) | 0.99 (0.94–1.03) | 0.73 |
| Model 4 PR (95% CI) | 1.00 (Reference) | 0.96 (0.91,1.02) | 1.00 (0.95,1.05) | 0.99 (0.94,1.03) | 0.70 |
| Suburban (n=2458) | |||||
| Cases, n | 315 | 481 | 576 | 608 | |
| Prevalence, % | 79.9 | 78.9 | 82.5 | 80.4 | |
| Unadjusted PR (95% CI) | 1.00 (Reference) | 0.99 (0.92–1.05) | 1.03 (0.97–1.10) | 1.01 (0.95–1.07) | 0.50 |
| Model 1 PR (95% CI) | 1.00 (Reference) | 0.98 (0.92–1.05) | 1.02 (0.96–1.09) | 1.00 (0.94–1.06) | 0.66 |
| Model 2 PR (95% CI) | 1.00 (Reference) | 0.98 (0.92–1.05) | 1.02 (0.96–1.09) | 1.00 (0.94–1.07) | 0.60 |
| Model 3 PR (95% CI) | 1.00 (Reference) | 0.98 (0.92–1.05) | 1.02 (0.96–1.09) | 1.00 (0.94–1.07) | 0.58 |
| Model 4 PR (95% CI) | 1.00 (Reference) | 0.98 (0.92–1.05) | 1.02 (0.96–1.09) | 1.00 (0.94–1.06) | 0.66 |
| Urban (n=2986) | |||||
| Cases, n | 593 | 847 | 713 | 303 | |
| Prevalence, % | 83.9 | 81.0 | 81.3 | 85.1 | |
| Unadjusted PR (95% CI) | 1.00 (Reference) | 0.97 (0.92–1.01) | 0.97 (0.93–1.01) | 1.01 (0.96–1.07) | 0.98 |
| Model 1 PR (95% CI) | 1.00 (Reference) | 0.96 (0.92–1.00) | 0.97 (0.92–1.01) | 1.01 (0.96–1.07) | 0.90 |
| Model 2 PR (95% CI) | 1.00 (Reference) | 0.97 (0.92–1.01) | 0.97 (0.93,1–02) | 1.02 (0.96–1.08) | 0.80 |
| Model 3 PR (95% CI) | 1.00 (Reference) | 0.97 (0.92–1.01) | 0.97 (0.93–1.02) | 1.02 (0.97–1.08) | 0.78 |
| Model 4 PR (95% CI) | 1.00 (Reference) | 0.96 (0.92–1.01) | 0.97 (0.93–1.02) | 1.02 (0.97–1.08) | 0.72 |
Model 1: adjusted for age, sex, and race. Model 2: model 1 plus educational attainment, employment status, and income. Model 3: model 2 plus health insurance, usual source of care, car ownership, level of social support (defined by the score of 6 survey items of social support), and self‐reported neighborhood characteristics (ie, agreement with “This is a close‐knit neighborhood”, “People in this neighborhood can be trusted”, “People around here are willing to help their neighbors”, and “How safe from crime do you consider your neighborhood to be?”). Model 4: model 3 plus current cigarette smoking, self‐reported diabetes status, systolic blood pressure (BP; in the statin use and low‐density lipoprotein cholesterol [LDL‐C] models only), diastolic BP (in the statin use and LDL‐C models only), body mass index, total cholesterol, physical functioning based on the Short‐Form 12 Physical Component Summary score, mental health based on the Short‐Form 12 Mental Health Component Summary score, aspirin use, and number of total prescription medications. Systolic BP and diastolic BP are not included in the BP control outcome model. PR indicates prevalence ratio; and REGARDS, Reasons for Geographic and Racial Differences in Stroke.
Association Between Pharmacy Proximity and BP Control Overall and by Subgroups
The PR for the association between living in the lowest quartile compared with the highest quartile of pharmacy proximity and BP control use was 0.99 (95% CI, 0.94–1.03) for participants in rural census tracts, 1.00 (95% CI, 0.94–1.06) for residents in suburban census tracts, and 1.02 (95% CI, 0.97–1.08) for residents in urban census tracts (Table 2). In rural census tracts, Black male participants living in the highest quartile compared with the lowest quartile of pharmacy proximity had higher BP control (PR, 1.21 [95% CI, 1.01–1.45]) (Table S5). BP control was higher among adults in rural census tracts taking <5 prescription medications living in the highest quartile of pharmacy proximity (PR, 1.16 [95% CI, 1.02–1.32]) (Table S7). There was no difference in the association between pharmacy proximity and BP control for the REGARDS trial participants living in rural, suburban, and urban census tracts by age (Table S6) or car ownership (Table S8).
Association Between Pharmacy Proximity and Statin Medication Use
After complete adjustment, the PR for statin use was 0.95 (95% CI, 0.88–1.02) for participants in rural census tracts, 0.92 (95% CI, 0.83–1.03) for participants in suburban census tracts, and 0.98 (95% CI, 0.87–1.10) for participants in urban census tracts comparing the highest quartile of pharmacy proximity with the lowest quartile of pharmacy proximity (Table 3). In subgroup analysis, participants taking ≥5 prescription medications in suburban census tracts in the highest quartile of proximity to pharmacies had lower statin medication use (0.90 [95% CI, 0.81–1.00]) (Table S7). When stratified by sex (Table S5), age (Table S6), and car ownership (Table S8) among the REGARDS trial participants, there was no association between pharmacy proximity and statin use.
Table 3.
Association Between Statin Medication Use and LDL‐C Level With Pharmacy Proximity Among REGARDS Trial Participants During Visit 2
| PR (95% CI) | |||||
|---|---|---|---|---|---|
| Statin medication use | |||||
| Quartile, median (range) (pharmacy proximity per 10 000 persons) | Quartile 1 0.80 (0.00–1.33) | Quartile 2 1.69 (1.33–2.03) | Quartile 3 2.48 (2.03–3.12) | Quartile 4 4.21 (3.12–21.73) | P for trend |
| Rural (n=3027) | |||||
| Cases, n | 547 | 251 | 250 | 485 | |
| Prevalence, % | 51.8 | 54.9 | 48.4 | 48.5 | |
| Unadjusted PR (95% CI) | 1.00 (Reference) | 1.06 (0.96–1.17) | 0.93 (0.84–1.04) | 0.94 (0.86–1.02) | 0.06 |
| Model 1 PR (95% CI) | 1.00 (Reference) | 1.06 (0.95–1.17) | 0.94 (0.84–1.04) | 0.94 (0.86–1.03) | 0.08 |
| Model 2 PR (95% CI) | 1.00 (Reference) | 1.06 (0.95–1.17) | 0.94 (0.85–1.05) | 0.94 (0.87–1.03) | 0.09 |
| Model 3 PR (95% CI) | 1.00 (Reference) | 1.05 (0.95–1.16) | 0.94 (0.84–1.04) | 0.94 (0.86–1.02) | 0.07 |
| Model 4 PR (95% CI) | 1.00 (Reference) | 1.05 (0.96–1.15) | 0.93 (0.85–1.03) | 0.95 (0.88–1.02) | 0.06 |
| Suburban (n=2541) | |||||
| Cases, n | 211 | 339 | 360 | 380 | |
| Prevalence, % | 50.6 | 54.6 | 49.8 | 48.7 | |
| Unadjusted PR (95% CI) | 1.00 (Reference) | 1.08 (0.96–1.22) | 0.98 (0.87–1.11) | 0.96 (0.85–1.08) | 0.15 |
| Model 1 PR (95% CI) | 1.00 (Reference) | 1.08 (0.96–1.21) | 0.97 (0.86–1.10) | 0.98 (0.87–1.10) | 0.22 |
| Model 2 PR (95% CI) | 1.00 (Reference) | 1.07 (0.95–1.21) | 0.97 (0.86–1.10) | 0.97 (0.86–1.09) | 0.21 |
| Model 3 PR (95% CI) | 1.00 (Reference) | 1.06 (0.95–1.19) | 0.97 (0.86–1.09) | 0.96 (0.85–1.08) | 0.17 |
| Model 4 PR (95% CI) | 1.00 (Reference) | 1.03 (0.93–1.15) | 0.97 (0.87–1.08) | 0.92 (0.83–1.03) | 0.03 |
| Urban (n=3001) | |||||
| Cases, n | 335 | 534 | 457 | 176 | |
| Prevalence, % | 49.9 | 50.2 | 50.6 | 48.5 | |
| Unadjusted PR (95% CI) | 1.00 (Reference) | 1.01 (0.91–1.11) | 1.01 (0.92–1.12) | 0.97 (0.85–1.11) | 0.83 |
| Model 1 PR (95% CI) | 1.00 (Reference) | 0.99 (0.90–1.09) | 1.01 (0.91–1.12) | 0.96 (0.85–1.10) | 0.78 |
| Model 2 PR (95% CI) | 1.00 (Reference) | 0.99 (0.90–1.09) | 1.00 (0.91–1.11) | 0.95 (0.83–1.08) | 0.57 |
| Model 3 PR (95% CI) | 1.00 (Reference) | 0.99 (0.90–1.09) | 1.00 (0.90–1.10) | 0.94 (0.83–1.07) | 0.51 |
| Model 4 PR (95% CI) | 1.00 (Reference) | 1.00 (0.92–1.09) | 1.00 (0.92–1.10) | 0.98 (0.87–1.10) | 0.81 |
| LDL‐C level | |||||
| Rural (n=3027) | |||||
| Median (minimum, maximum) | 96.5 (23.7, 251) | 93.9 (31.9, 254) | 94.2 (30.0, 272.9) | 96.0 (11.8, 263.6) | |
| Unadjusted β (SE) | (Reference) | −1.81 (1.98) | −1.08 (1.86) | −0.95 (1.55) | 0.59 |
| Model 1 β (SE) | (Reference) | −1.77 (1.94) | −1.35 (1.84) | −1.34 (1.52) | 0.41 |
| Model 2 β (SE) | (Reference) | −1.73 (1.94) | −1.29 (1.84) | −1.18 (1.53) | 0.47 |
| Model 3 β (SE) | (Reference) | −1.46 (1.95) | −1.18 (1.84) | −0.89 (1.53) | 0.58 |
| Model 4 β (SE) | (Reference) | −0.3 (0.74) | 0.42 (0.66) | −0.26 (0.57) | 0.82 |
| Suburban (n=2541) | |||||
| Median (minimum, maximum) | 94.5 (22.2, 220.6) | 94.3 (17.0, 212.3) | 94.3 (26.0, 241.3) | 94.2 (23.3, 278.4) | |
| Unadjusted β (SE) | (Reference) | −1.61 (2.11) | −0.44 (2.05) | −0.19 (2.07) | 0.80 |
| Model 1 β (SE) | (Reference) | −1.62 (2.06) | −0.12 (2.01) | −1.19 (2.03) | 0.79 |
| Model 2 β (SE) | (Reference) | −1.46 (2.05) | −0.08 (2.00) | −0.86 (2.03) | 0.91 |
| Model 3 β (SE) | (Reference) | −1.32 (2.05) | −0.02 (2.00) | −0.63 (2.02) | 0.99 |
| Model 4 β (SE) | (Reference) | 0.82 (0.84) | 0.06 (0.82) | 0.79 (0.81) | 0.56 |
| Urban (n=3001) | |||||
| Median (minimum, maximum) | 95.9 (21.9, 267.2) | 93.7 (21.4, 382.8) | 95.2 (6.8, 268.2) | 94.9 (32.2, 212.8) | |
| Unadjusted β (SE) | (Reference) | −0.72 (1.73) | 0.44 (1.79) | −0.18 (2.22) | 0.82 |
| Model 1 β (SE) | (Reference) | −0.08 (1.69) | 0.55 (1.76) | 0.17 (2.18) | 0.79 |
| Model 2 β (SE) | (Reference) | 0.23 (1.70) | 1.12 (1.76) | 1.14 (2.18) | 0.46 |
| Model 3 β (SE) | (Reference) | 0.23 (1.70) | 1.27 (1.76) | 1.31 (2.18) | 0.39 |
| Model 4 β (SE) | (Reference) | 0.84 (0.68) | 1.22 (0.7) | 0.11 (0.88) | 0.46 |
Model 1: adjusted for age, sex, and race. Model 2: model 1 plus educational attainment, employment status, and income. Model 3: model 2 plus health insurance, usual source of care, car ownership, level of social support (defined by the score of 6 survey items of social support), and self‐reported neighborhood characteristics (ie, agreement with “This is a close‐knit neighborhood”, “People in this neighborhood can be trusted”, “People around here are willing to help their neighbors”, and “How safe from crime do you consider your neighborhood to be?”). Model 4: model 3 plus current cigarette smoking, self‐reported diabetes status, systolic blood pressure (BP; in the statin use and low‐density lipoprotein [LDL‐C] models only), diastolic BP (in the statin use and LDL‐C models only), body mass index, total cholesterol, physical functioning based on the Short‐Form 12 Physical Component Summary score, mental health based on the Short‐Form 12 Mental Health Component Summary score, aspirin use, and number of total prescription medications. Systolic BP and diastolic BP are not included in the BP control outcome model. PR indicates prevalence ratio; and REGARDS, Reasons for Geographic and Racial Differences in Stroke.
Association Between Pharmacy Proximity and LDL‐C Levels
The association between living in the lowest quartile compared with the highest quartile of pharmacy proximity and LDL‐C was not significant for the REGARDS trial participants in rural census tracts (beta‐estimate, −0.95 [SE, 1.55]; P=0.59), suburban census tracts (beta‐estimate, −0.19 [SE, 2.07]; P=0.80), or urban census tracts (beta‐estimate, 0.11 [SE, 0.88]; P=0.46) (Table 3). The association between living in the lowest quartile compared with the highest quartile of pharmacy proximity and LDL‐C was not significant when stratified by race and sex (Table S5). There was no significant association in LDL‐C for participants living in rural, suburban, or urban census tracts among the highest quartile of pharmacy proximity compared with the lowest quartile of pharmacy proximity by age (Table S6), polypharmacy status (Table S7), or car ownership (Table S8).
Sensitivity Analysis
In sensitivity analysis using 5‐ and 20‐minute drive time, no qualitative difference in the association of pharmacy proximity with antihypertensive use, BP control, or LDL‐C level was seen (Table S9 and S10). Using a 20‐minute drive time, statin use was lower among participants in rural and suburban census tracts with the highest pharmacy proximity (PR, 0.90 [95% CI, 0.83–0.98] and PR, 0.86 [95% CI, 0.78–0.96], respectively) (Table S10), while statin use was more prevalent among participants in urban census tracts with the highest pharmacy proximity (PR, 1.14 [95% CI, 1.04–1.26]) (Table S10).The predicted probability of antihypertensive use, BP control, statin use, or LDL‐C did not significantly differ by population density or 5‐, 10‐, or 20‐minute drive time (Figures 1 and 2).
Figure 1. Adjusted predicted probability of antihypertensive medication use and BP control with pharmacy proximity by 5‐, 10‐, and 20‐minute drive times for rural, suburban, and urban census tracts.
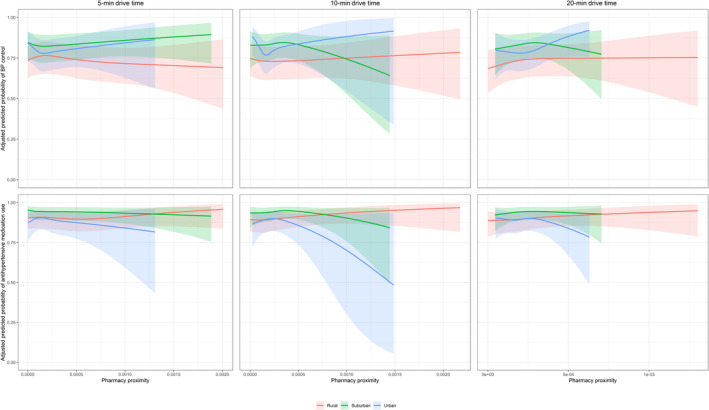
Predicted probability of antihypertensive medication use (A) and BP control (B) adjusted for age, sex, race, educational attainment, employment status, income, health insurance, usual source of care, car ownership, level of social support (defined by the score of 6 survey items of social support), self‐reported neighborhood characteristics (ie, agreement with “This is a close‐knit neighborhood”, “People in this neighborhood can be trusted”, “People around here are willing to help their neighbors”, and “How safe from crime do you consider your neighborhood to be?”), current cigarette smoking, self‐reported diabetes status, systolic BP (in the statin use and LDL‐C models only), diastolic BP (in the statin use and LDL‐C models only), body mass index, total cholesterol, physical functioning based on the Short‐Form 12 Physical Component Summary score, mental health based on the Short‐Form 12 Mental Health Component Summary score, aspirin use, and number of total prescription medications. Systolic BP and diastolic BP are not included in the BP control outcome model. BP indicates blood pressure and LDL‐C, low‐density lipoprotein cholesterol.
Figure 2. Adjusted predicted probability of statin medication use and LDL‐C with pharmacy proximity by 5‐, 10‐, and 20‐minute drive times for rural, suburban, and urban census tracts.
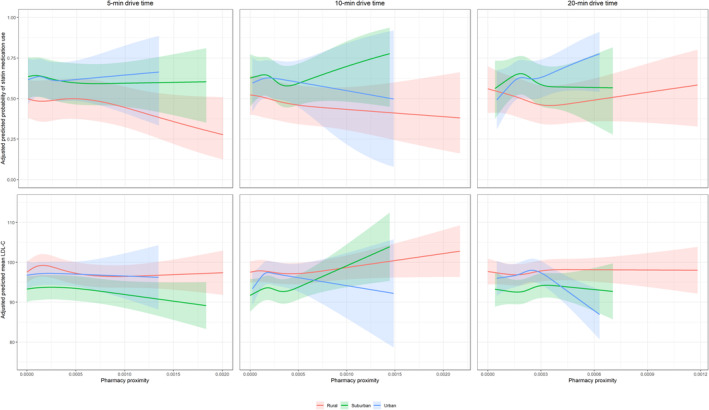
Predicted probability of antihypertensive medication use (A) and BP control (B) adjusted for age, sex, race, educational attainment, employment status, income, health insurance, usual source of care, car ownership, level of social support (defined by the score of 6 survey items of social support), self‐reported neighborhood characteristics (ie, agreement with “This is a close‐knit neighborhood”, “People in this neighborhood can be trusted”, “People around here are willing to help their neighbors”, and “How safe from crime do you consider your neighborhood to be?”), current cigarette smoking, self‐reported diabetes status, systolic BP (in the statin use and LDL‐C models only), diastolic BP (in the statin use and LDL‐C models only), body mass index, total cholesterol, physical functioning based on the Short‐Form 12 Physical Component Summary score, mental health based on the Short‐Form 12 Mental Health Component Summary score, aspirin use, and number of total prescription medications. Systolic BP and diastolic BP are not included in the BP control outcome model. BP indicates blood pressure and LDL‐C, low‐density lipoprotein cholesterol.
Discussion
Community pharmacies serve a crucial role in facilitating medication and health care access, often acting as the nearest point of contact within the health care system for patients, largely due to their neighborhood‐centric locations. 39 , 40 In the current analysis, we did not find strong evidence overall that living in a census tract with lower pharmacy proximity was associated with the use of antihypertensive medication, statin medication, BP control, or LDL‐C levels, irrespective of population density. However, we did find evidence that certain demographics, such as younger adults in suburban areas, are differentially associated with pharmacy proximity. Given the complexity of multilevel social interactions that influence health outcomes, there may be an association not captured by the current analysis. A better understanding of the impact of health care facilities and services, including pharmacies, is important in directing policies and interventions to improve equity and reduce CVD morbidity and mortality.
The REGARDS study was designed to identify factors contributing to increased stroke and CVD risk among Black adults and adults living in the Southeastern region of the United States. 14 Neighborhoods composed of predominantly Black residents face greater risk of pharmacy closure and have fewer available pharmacy services compared with White or diverse neighborhoods. 41 However, the association between living in a census tract with low pharmacy accessibility by drive time with the use of antihypertensive and statin medication has not been previously described. While in the current analysis of the REGARDS trial, we did not find a consistent association between living in a census tract with low pharmacy proximity and medication access by drive time, we did find that Black male adults living in a census tract with a higher proximity to pharmacies had a higher prevalence of antihypertensive medication use and BP control, particularly in rural census tracts. Lower neighborhood access to pharmacy and pharmacy services may lead to increased CVD risk as residents cannot access CVD risk–reducing medication as readily, particularly among residents of lower socioeconomic status and in minority predominant areas. 11
Lack of access to health care services, including pharmacies, differentially impacts both rural and urban populations. Although the age‐adjusted cardiovascular‐related mortality in the United States declined overall between 1999 and 2017, in both rural and urban settings, cardiovascular mortality remained highest among Black adults in 2017. 42 Decreased access to health care facilities in both rural and urban areas contributes to CVD‐related mortality. Since 2005, 180 hospitals have closed in rural areas of the United States, which has disproportionately impacted adults with lower socioeconomic status and education attainment. 43 In comparison, urban areas facing limited access to medical facilities, including trauma‐capable hospitals, physician offices, and pharmacies are predominantly minority and low–socioeconomic status neighborhoods. 41 , 44 , 45 In the current analysis of the REGARDS trial, we stratified participants by population density (ie, rural, suburban, and urban census tracts) due to the geographical differences that may exist, such as the drive time of a mile in an urban area versus rural area. However, living in a rural, suburban, or urban census tract did not meaningfully change the relationship between pharmacy proximity and medication use, BP control, and LDL‐C levels.
While we focused on conventional commercial pharmacies in the current analysis, there are multiple new pharmacy‐care delivery models promising to disrupt the traditional approach. For example, Amazon Pharmacy delivers throughout the entire United States using entirely mail‐order pharmacy with the promise of reduced patient cost. 46 Similarly, CostPlus has employed a standard pricing model to offer mail‐order delivery of generic medications at a reduced price. 47 Although new models of pharmacy services offer promise for increasing the availability of low‐cost, safe, and effective medication, it remains to be seen whether these new pharmacy services will actually reach underserved US populations and reduce disparities in CVD outcomes or further exacerbate disparities by simply being an additional option for well‐resourced communities.
There are several strengths and limitations to the current analysis. The REGARDS study has extensive and validated data on CVD medication availability, BP measurement, and laboratory values (ie, LDL‐C). In addition, due to the design of the REGARDS trial, in which Black adults and adults in the southeastern United States were oversampled, sampling is sufficient to detect differences in the association of living in a census tract with low pharmacy proximity between Black and White adults. 14 The composition of the REGARDS cohort, with its emphasis on residents from the southeastern United States and its focus on non‐Hispanic White and Black adults, may pose limitations to the broad generalizability of our findings. However, it is important to note that while the representativeness of the study population can influence estimates of population prevalences of disease, it generally has a lesser impact on association estimates. In our statistical analysis, we rely on a framework of health care utilization, access, and health equity for sequential model building. However, as with all statistical models, ours is prone to potential bias, including from omitted variables. As social variables have dynamic interactions it may be difficult to capture all relationships in statistical models and it is possible that there is a relationship between pharmacy proximity by drive time and antihypertensive use, statin use, BP control, and LDL‐C levels not captured by the current data and analytic approach. Also, we do not account for specific pharmacies used by individuals as this was not captured in the REGARDS trial, as well as pharmacy mail delivery services, which may attenuate the relationship between living in a census tract with low pharmacy proximity and access to medications. The decision to prescribe and take medication is multifactorial and influenced by patient and physician preferences. As such, the current analysis does not account for barriers to taking medication such as statin or antihypertensive intolerance. Medication use by pill bottle review may not accurately reflect true medication adherence and should be interpreted with caution. While we used driving time as a measure for pharmacy proximity, this metric might not accurately represent the accessibility experience of participants without car ownership; however, sensitivity analyses excluding these individuals showed consistent results, suggesting the robustness of our primary findings. Spatial autocorrelation may have been present due to the design of our analysis, although this did not appear to be strong overall for the majority of identified covariates (Figure S2). In addition, we used a population‐weighted centroid of each census tract, which minimizes bias from the modifiable areal unit problem scale effects among urban (small) and rural (large) census tracts. Our reliance on the population‐weighted centroid approach, due to the absence of specific home addresses, might introduce potential biases. This method may not account for the inherent variability within census tracts, possibly skewing accessibility perceptions for those living farther to the population‐weighted centroids. In addition, if residences are concentrated in distinct zones within a census tract, the centroids might not truly reflect the population distribution.
Conclusion
In this large cohort of US adults, we did not find evidence to suggest that living in a rural, suburban, or urban census tract of low pharmacy proximity by drive time was associated with lower use of antihypertensive and statin medication or worse BP control and LDL‐C. Limited access to health care resources remains a key barrier driving health disparities in the United States. Neighborhood‐level health care facilities are critical to ensuring that health care services are more widely available to all US residents, notably among racial/ethnic minorities and rural residents. Defining the impact of geographic restriction to pharmacies is an important step to designing and implementing interventions to improve access to medication for vulnerable populations.
Sources of Funding
REGARDS is supported by a U01 NS041588 co‐funded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIA. Representatives of the NINDS were involved in the review of the article but were not directly involved in the collection, management, analysis, or interpretation of the data.
Disclosures
F.L. Mondesir is an employee of Biogen Inc. and may hold stock in Biogen Inc. A.P. Bress is supported by K24AG080168–01, 1R01AG065805, K01HL133468, and R01HL139837. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S10
Figures S1–S2
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at: https://www.uab.edu/soph/regardsstudy/.
This article was sent to Tazeen H. Jafar, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.031717
For Sources of Funding and Disclosures, see page 12.
References
- 1. Samanic CM, Barbour KE, Liu Y, Wang Y, Fang J, Lu H, Schieb L, Greenlund KJ. Prevalence of self‐reported hypertension and antihypertensive medication use by county and rural‐urban classification–United States, 2017. MMWR Morb Mortal Wkly Rep. 2020;69:533–539. doi: 10.15585/mmwr.mm6918a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mercado CI, McKeever Bullard K, Gregg EW, Ali MK, Saydah SH, Imperatore G. Differences in U.S. rural‐urban trends in diabetes ABCS, 1999‐2018. Diabetes Care. 2021;44:1766–1773. doi: 10.2337/dc20-0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kennedy J, Wood EG. Medication costs and adherence of treatment before and after the affordable care act: 1999–2015. Am J Public Health. 2016;106:1804–1807. doi: 10.2105/AJPH.2016.303269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rolnick SJ, Pawloski PA, Hedblom BD, Asche SE, Bruzek RJ. Patient characteristics associated with medication adherence. Clin Med Res. 2013;11:54–65. doi: 10.3121/cmr.2013.1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Derington CG, King JB, Herrick JS, Schimbo D, Kronish IM, Saseen JJ, Muntner P, Moran AE, Bress AP. Trends in antihypertensive medication monotherapy and combination use among US adults, National Health and nutrition examination survey 2005–2016. Hypertension. 2020;75:973–981. doi: 10.1161/HYPERTENSIONAHA.119.14360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. LaVeist T, Pollack K, Thorpe R Jr, Fesahazion R, Gaskin D. Place, not race: disparities dissipate in southwest Baltimore when blacks and whites live under similar conditions. Health Aff. 2011;30:1880–1887. doi: 10.1377/hlthaff.2011.0640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. LaVeist TA. Racial segregation and longevity among African Americans: an individual‐level analysis. Health Serv Res. 2003;38:1719–1734. doi: 10.1111/j.1475-6773.2003.00199.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gaskin DJ, Dinwiddie GY, Chan KS, McCleary R. Residential segregation and disparities in health care services utilization. Med Care Res Rev. 2012;69:158–175. doi: 10.1177/1077558711420263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luo J, Kibriya MG, Zakin P, Craver A, Connellan L, Tasmin S, Polonsky T, Kim K, Ahsan H, Aschebrook‐Kilfoy B. Urban spatial accessibility of primary care and hypertension control and awareness on Chicago's south side: a study from the COMPASS cohort. Circ Cardiovasc Qual Outcomes. 2022;15:e008845. doi: 10.1161/CIRCOUTCOMES.121.008845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Amstislavski P, Matthews A, Sheffield S, Maroko AR, Weedon J. Medication deserts: survey of neighborhood disparities in availability of prescription medications. Int J Health Geogr. 2012;11:48. doi: 10.1186/1476-072X-11-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qato DM, Daviglus ML, Wilder J, Lee T, Qato D, Lambert B. Pharmacy deserts' are prevalent in Chicago's predominantly minority communities, raising medication access concerns. Health Aff (Millwood). 2014;33:1958–1965. doi: 10.1377/hlthaff.2013.1397 [DOI] [PubMed] [Google Scholar]
- 12. Do D, Geldsetzer P. Trends in Mail‐Order Prescription Use among U.S. Adults from 1996 to 2018: A Nationally Representative Repeated Cross‐Sectional Study. medRxiv. 2020.
- 13. Clark BE, Siracuse MV, Garis RI. A comparison of mail‐service and retail community pharmacy claims in 5 prescription benefit plans. Res Social Adm Pharm. 2009;5:133–142. doi: 10.1016/j.sapharm.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 14. Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678 [DOI] [PubMed] [Google Scholar]
- 15. Long DL, Howard G, Long DM, Judd S, Manly JJ, McClure LA, Wadley VG, Safford MM, Katz R, Glymour MM. An investigation of selection bias in estimating racial disparity in stroke risk factors. Am J Epidemiol. 2019;188:587–597. doi: 10.1093/aje/kwy253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stone NJ, Robinson JG, Lichtenstein AH, Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon Levy D, Lloyd‐Jones DM, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a [DOI] [PubMed] [Google Scholar]
- 17. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnel CJ. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 18. ENRICHD Investigators . Enhancing recovery in coronary heart disease (ENRICHD) study intervention: rationale and design. Psychosom Med. 2001;63:747–755. [PubMed] [Google Scholar]
- 19. Mondesir FL, Carson AP, Durant RW, Lewis MW, Safford MM, Levitan EB. Association of functional and structural social support with medication adherence among individuals treated for coronary heart disease risk factors: findings from the REasons for geographic and racial differences in stroke (REGARDS) study. PLoS One. 2018;13:e0198578. doi: 10.1371/journal.pone.0198578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ross CE, Mirowsky J. Disorder and decay: the concept and measurement of perceived neighborhood disorder. Urban Aff Rev. 1999;34:412–432. [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, KJW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schroff P, Gamboa CM, Durant RW, Oikeh A, Richman JS, Safford MM. Vulnerabilities to health disparities and statin use in the REGARDS (reasons for geographic and racial differences in stroke). Study J Am Heart Assoc. 2017;6:e005449. doi: 10.1161/JAHA.116.005449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Langford AT, Akinyelure OP, Moore TL Jr, Howard G, Min YI, Hillegass WB, Bress AP, Tajeu GS, Butler M, Jaeger BC. Underutilization of treatment for Black adults with apparent treatment‐resistant hypertension: JHS and the REGARDS study. Hypertension. 2020;76:1600–1607. doi: 10.1161/HYPERTENSIONAHA.120.14836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jacobs JA, Addo DK, Zheutlin AR, Derington CG, Essien UR, Navar AM, Hernandez I, Lloyd‐Jones DM, King JB, Rao S. Prevalence of statin use for primary prevention of atherosclerotic cardiovascular disease by race, ethnicity, and 10‐year disease risk in the US: National Health and nutrition examination surveys, 2013 to march 2020. JAMA Cardiol. 2023;8:443–452. doi: 10.1001/jamacardio.2023.0228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qato DM, Alexander GC, Chakraborty A, Guadamuz JS, Jackson JW. Association between pharmacy closures and adherence to cardiovascular medications among older US adults. JAMA Netw Open. 2019;2:e192606. doi: 10.1001/jamanetworkopen.2019.2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luo W, Wang F. Measures of spatial accessibility to healthcare in a GIS environment: synthesis and a case study in Chicago region. Environ Plann B Plann Des. 2003;30:865–884. doi: 10.1068/b29120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. ArcGIS . Create Drive‐Time Areas. https://doc.arcgis.com/en/arcgis‐online/analyze/create‐drive‐time‐areas.htm.
- 28. ArcGIS . ArcGIS Network Analyst Extension. https://www.esri.com/en‐us/arcgis/products/arcgis‐network‐analyst/overview.
- 29. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 30. Pednekar P, Peterson A. Mapping pharmacy deserts and determining accessibility to community pharmacy services for elderly enrolled in a state pharmaceutical assistance program. PLoS One. 2018;13:e0198173. doi: 10.1371/journal.pone.0198173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guadamuz JS, Alexander GC, Zenk SN, Kanter GP, Wilder JR, Qato DM. Access to pharmacies and pharmacy services in New York City, Los Angeles, Chicago, and Houston, 2015‐2020. J Am Pharm Assoc (2003). 2021;61:e32–e41. doi: 10.1016/j.japh.2021.07.009 [DOI] [PubMed] [Google Scholar]
- 32. Wisseh C, Hildreth K, Marshall J, Tanner A, Bazargan M, Robinson P. Social determinants of pharmacy deserts in Los Angeles County. J Racial Ethn Health Disparities. 2021;8:1424–1434. doi: 10.1007/s40615-020-00904-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hilts KE, Hudmon KS, Benson AF, Elkhadragy N. Rural‐urban disparities in tobacco use and the role of pharmacists in closing the gap. J Rural Health. 2022;38:355–359. doi: 10.1111/jrh.12607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. VanderWeele TJ. Principles of confounder selection. Eur J Epidemiol. 2019;34:211–219. doi: 10.1007/s10654-019-00494-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aday LA, Andersen R. A framework for the study of access to medical care. Health Serv Res. 1974;9:208–220. [PMC free article] [PubMed] [Google Scholar]
- 36. Powell‐Wiley TM, Baumer Y, Baah FO, Baez AS, Farmer N, Mahlobo CT, Pita MA, Potharaju KA, Tamura K, Wallen GR. Social determinants of cardiovascular disease. Circ Res. 2022;130:782–799. doi: 10.1161/CIRCRESAHA.121.319811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Penchansky R, Thomas JW. The concept of access: definition and relationship to consumer satisfaction. Med Care. 1981;19:127–140. doi: 10.1097/00005650-198102000-00001 [DOI] [PubMed] [Google Scholar]
- 38. Kock NL, Lynn G. Lateral collinearity and misleading results in variance‐based SEM: an illustration and recommendations. J Assoc Inf Syst. 2012;13:13–580. doi: 10.17705/1jais.00302 [DOI] [Google Scholar]
- 39. Berenbrok LA, Tang S, Gabriel N, Guo J, Sharareh N, Patel N, Dickson S, Hernandez I. Access to community pharmacies: a nationwide geographic information systems cross‐sectional analysis. J Am Pharm Assoc. 2022;62:1816–1822.e2. doi: 10.1016/j.japh.2022.07.003 [DOI] [PubMed] [Google Scholar]
- 40. Valliant SN, Burbage SC, Pathak S, Urick BY. Pharmacists as accessible health care providers: quantifying the opportunity. J Manag Care Spec Pharm. 2022;28:85–90. doi: 10.18553/jmcp.2022.28.1.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guadamuz JS, Wilder JR, Mouslim MC, Zenk SN, Alexander GC, Qato DM. Fewer pharmacies in Black and Hispanic/Latino neighborhoods compared with white or diverse neighborhoods, 2007‐15. Health Aff (Millwood). 2021;40:802–811. doi: 10.1377/hlthaff.2020.01699 [DOI] [PubMed] [Google Scholar]
- 42. Cross SH, Mehra MR, Bhatt DL, Nasir K, O'Donnell CJ, Califf RM, Warraich HJ. Rural‐urban differences in cardiovascular mortality in the US, 1999‐2017. JAMA. 2020;323:1852–1854. doi: 10.1001/jama.2020.2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cecil G. Sheps Center for Health Services Research. Rural Hospital Closures. Accessed June 9, 2023. https://www.shepscenter.unc.edu/programs‐projects/rural‐health/rural‐hospital‐closures/.
- 44. Tung EL, Hampton DA, Kolak M, Rogers SO, Yang JP, Peek ME. Race/ethnicity and geographic access to urban trauma care. JAMA Netw Open. 2019;2:e190138. doi: 10.1001/jamanetworkopen.2019.0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hussein M, Diez Roux AV, Field RI. Neighborhood socioeconomic status and primary health care: usual points of access and temporal trends in a major US urban area. J Urban Health. 2016;93:1027–1045. doi: 10.1007/s11524-016-0085-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Amazon . Amazon Pharmacy. Accessed July 2, 2023. https://pharmacy.amazon.com/how‐it‐works.
- 47. Company MCCD . 2023. Accessed July 2, 2023. https://costplusdrugs.com/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S10
Figures S1–S2


