Summary
Background
Multidrug-resistant (MDR) Salmonella Infantis has disseminated worldwide, mainly linked to the consumption of poultry products. Evidence shows dissemination of this pathogen in Chile; however, studies are primarily limited to phenotypic data or involve few isolates. As human cases of Salmonella Infantis infections have substantially increased in recent years, this study aimed to characterise the genomic epidemiology and antimicrobial-resistance profiles of isolates obtained from different sources, aiming to inform effective surveillance and control measures.
Methods
We sequenced 396 Salmonella Infantis genomes and analysed them with all publicly available genomes of this pathogen from Chile (440 genomes in total), representing isolates from environmental, food, animal, and human sources obtained from 2009 to 2022. Based on bioinformatic and phenotypic methods, we assessed the population structure, dissemination among different niches, and antimicrobial resistance (AMR) profiles of Salmonella Infantis in the country.
Findings
The genomic and phylogenetic analyses showed that Salmonella Infantis from Chile comprised several clusters of highly related isolates dominated by sequence type 32. The HC20_343 cluster grouped an important proportion of all isolates. This was the only cluster associated with pESI-like megaplasmids, and up to 12 acquired AMR genes/mutations predicted to result in an MDR phenotype. Accordingly, antimicrobial-susceptibility testing revealed a strong concordance between the AMR genetic determinants and their matching phenotypic expression, indicating that a significant proportion of HC20_343 isolates produce extended-spectrum β-lactamases and have intermediate fluoroquinolone resistance. HC20_343 Salmonella Infantis were spread among environmental, animal, food, and human niches, showing a close relationship between isolates from different years and sources, and a low intra-source genomic diversity.
Interpretation
Our findings show a widespread dissemination of MDR Salmonella Infantis from the HC20_343 cluster in Chile. The high proportion of isolates with resistance to first-line antibiotics and the evidence of active transmission between the environment, animals, food, and humans highlight the urgency of improved surveillance and control measures in the country. As HC20_343 isolates predominate in the Americas, our results suggest a high prevalence of ESBL-producing Salmonella Infantis with intermediate fluoroquinolone resistance in the continent.
Funding
Partially supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services as part of an award, FDU001818, with 30% percent funded by FDA/HHS; and by Agencia de Investigación y Desarrollo de Chile (ANID) through FONDECYT de Postdoctorado Folio 3230796 and Folio 3210317, FONDECYT Regular Folio 1231082, and ANID—Millennium Science Initiative Program—ICN2021_044.
Keywords: Salmonella Infantis, Americas, Antibiotic resistance, pESI, Megaplasmid, CTX-M-65, Fluoroquinolones, Chile
Research in context.
Evidence before this study
In the last decade, emergent multidrug-resistant Salmonella Infantis has spread worldwide, primarily linked to poultry product consumption. However, in most countries from the Americas Region, such as Chile, the extent of the dissemination of emergent Salmonella Infantis and its molecular epidemiology remains unknown. In May and September 2023, an online search was conducted using the Google engine and the PMC database with the terms “Salmonella,” “Infantis,” and “Chile,” with no language restrictions. We assessed the results to select those presenting antimicrobial resistance, epidemiologic, or genomic data directly associated with isolates from Chile (13 studies). The selected studies showed that the prevalence of Salmonella Infantis in poultry-meat production systems, its resistance to different antibiotics, and the number of human cases of infection caused by this serovar have increased since 2014–2016. However, these reports were limited to phenotypic data or involved the genomic analysis of a few isolates (<50) obtained from the same source. No study has assessed the genomic epidemiology of the Salmonella Infantis population at the country level.
Added value of this study
Here, we present the first large-scale genomic epidemiology analysis of Salmonella Infantis in Chile, including isolates from environmental, food, animal, and human sources obtained from 2009 to 2022. We found that Salmonella Infantis in Chile is divided into several clusters of highly related isolates and that only a single cluster, the HC20_343, was associated with multiple antimicrobial-resistance determinants and pESI-like megaplasmids. We also report that isolates from this cluster are widespread among most sources, including irrigation water, poultry, food, and human cases. Detection of AMR determinants coupled with antimicrobial-susceptibility testing indicated that most HC20_343 isolates are ESBL-producers and have intermediate resistance to ciprofloxacin. Population structure analysis of this foodborne pathogen evidenced an active transmission of MDR Salmonella Infantis between different niches. This study reveals the widespread dissemination of MDR Salmonella Infantis in Chile.
Implications of all the available evidence
The evidence indicates that emerging Salmonella Infantis from the HC20_343 cluster is spreading among various niches, including irrigation water, poultry, and food, causing human infections in Chile. Its resistance to first-line antibiotics used for treating salmonellosis in individuals with a higher risk of severe or invasive infections is concerning. Currently, most surveillance and control efforts to reduce salmonellosis in Chile are focused on the poultry industry, and the study of outbreaks does not include whole-genome sequence analyses. Our findings highlight the urgent necessity to improve the surveillance and control measures to include agricultural waters to prevent contamination of produce and the further dissemination of resistance genes in the environment. As the HC20_343 cluster is highly prevalent in the Americas, further research involving large-scale genomic population analyses would shed light on the extent of the dissemination and transmission routes of emergent Salmonella Infantis in the continent and may contribute to informing surveillance and control policies.
Introduction
Non-typhoidal Salmonella (NTS) are one of the leading causes of foodborne disease globally, mainly affecting children under five and the elderly. In 2019, NTS caused 215,000 deaths and the loss of approximately 15 million years of healthy life worldwide, according to the estimates of the Global Burden of Disease Study.1 The burden posed by NTS is in part fuelled by the emergence and spread of antimicrobial resistance, especially to critically-relevant antimicrobials such as third-generation cephalosporins (3 GC) and fluoroquinolones, which are the first-line treatment option for severe NTS infections.2,3
In the past decade, multidrug-resistant (MDR) and extended-spectrum β-lactamase (ESBL)-producing Salmonella enterica serovar Infantis have emerged in different continents as a zoonotic pathogen causing outbreaks of foodborne illness associated with poultry products consumption.4, 5, 6 In some countries, this pathogen has displaced the historically most prevalent Salmonella serovars, such as Typhimurium and Enteritidis.7, 8, 9, 10 The success of emerging MDR Salmonella Infantis is linked to the acquisition of a ≈300 Kbp pESI-like megaplasmid, which encodes virulence, fitness-enhancing, and antibiotic-resistance factors that favour its capacity of biofilm production, adhesion, invasion, and resistance to 3 GC.4, 5, 6 Moreover, isolates of this emerging pathogen are also associated with the chromosomal gyrA (D87Y) mutation, involved in fluoroquinolone resistance.5,11 The enhanced virulence and antimicrobial resistance traits of emergent Salmonella Infantis make this pathogen a global threat to public health.
Chile is a South American country organized into 16 administrative Regions, that concentrates its population and most agricultural activities in the central area.12 Ranking third after Brazil and the United States, Chile is one of the leading exporters of poultry meat in the region.13 Recent research, primarily phenotypic or involving few isolates, has shown the spread of MDR Salmonella Infantis in the country, mainly linked to poultry. Analysis of the poultry and pig production systems indicated increased Salmonella Infantis prevalence and resistance to multiple antibiotics such as β-lactams, aminoglycosides, and tetracyclines.14,15 This was concurrent with a high proportion of reported ESBL-producing isolates in poultry products sold at Santiago de Chile's supermarkets.16, 17, 18 In 2019, the Instituto de Salud Pública of Chile (ISP; Public Health Institute of Chile) reported a 431% increase in intestinal and invasive human infections caused by Salmonella Infantis in 2018, compared to 2014,19 clearly documenting the farm-to-fork transmission of this pathogen. Furthermore, MDR strains of this pathogen were also isolated from irrigation water and a Magellanic Horned-Owl,20,21 suggesting its dissemination in the environment outside poultry sources. These data document the emergence and spread of this foodborne pathogen in Chile in recent years; however, its magnitude and molecular epidemiology remain unknown.
In this study, we conducted the first large-scale genomic analysis of the Salmonella Infantis population in the country, aiming to assess its population structure, antimicrobial resistance, and dissemination among different sources, in order to produce valuable information for the surveillance and control of this emergent pathogen and the spread of antibiotic resistance genes in food and the environment.
Methods
Study design, sample collection and isolation of Salmonella Infantis
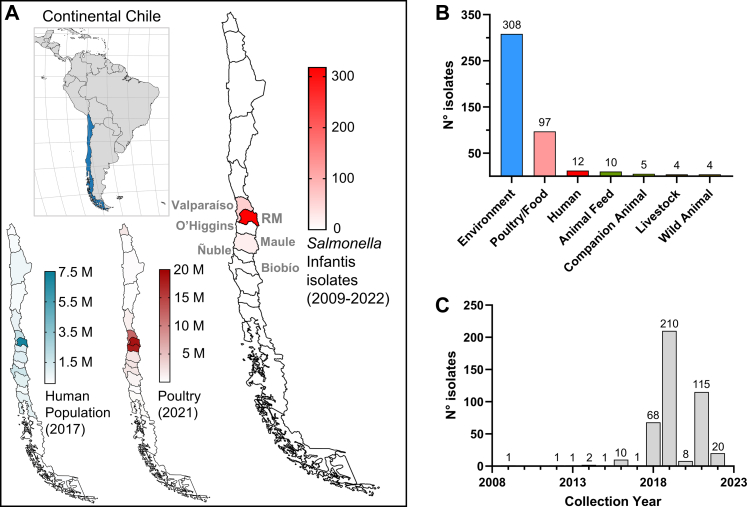
A collaborative effort between different institutions studying Salmonella (see author list and affiliations) was assembled to rapidly gather isolates of Salmonella Infantis from different sources and collection years, and to obtain and access whole-genome sequencing (WGS) data. 396 isolates (from 2014 to 2022) were obtained from environmental (surface water: 264; poultry-farm environment: 41), poultry (28), food (chicken meat: 37), animal feed (10), animal (dog feces: 5), and clinical (human: 11) sources from three regions of central Chile: Región de Valparaíso, Región Metropolitana, and Región del Maule (Fig. 1A and Supplementary Table S1).
Fig. 1.
Region of origin, isolation source, and collection year of the Salmonella Infantis isolates analysed in this study. A) Location of continental Chile in South America and distribution of its human population, poultry production, and collected Salmonella Infantis isolates among the 16 Regions (first-level administrative divisions). Note the concentration of these three variables in central Chile, especially in the Metropolitan Region. Population and poultry data were obtained from the corresponding last population and agricultural censuses carried out in 2017 and 2021, respectively (available at http://resultados.censo2017.cl/ and https://www.ine.gob.cl/censoagropecuario/resultados-finales/graficas-regionales. The administrative Regions from which the Salmonella Infantis isolates were collected are indicated. RM: Región Metropolitana. B) Salmonella Infantis isolates distribution per isolation source and per C) collection year.
Surface water samples (10 L) were collected at various points from five watersheds in central Chile (the Maipo, Mapocho, Claro, Lontué, and Mataquito rivers) using modified Moore swabs.22 Poultry-associated samples (boot swabs, chicken crops, and cecal content) were collected from poultry farms and chicken-meat production systems located in Región Metropolitana. These samples were processed according to a modified FDA-BAM protocol to isolate Salmonella as previously described.12 Isolation of Salmonella was confirmed by PCR-amplification of the invA gene with primers invAF (5′-GAATCCTCAGTTTTTCAACGTTTC-3′) and invAR (5′-TAGCCGTAACAACCAATACAAATG-3′).23 Raw meat-based dog diets and faecal samples (rectal swabs; Copan® Transystem™ 132C) from raw-fed dogs were processed according to the FDA-BAM protocol with modifications; raw food (25 g) was enriched in 225 mL lactose broth (BD Difco), and faecal samples were enriched in 10 mL buffered peptone water. Isolation of Salmonella was confirmed by PCR as described for the water isolates. Dog-samples collection was approved by the Institutional Committee for the Care and Use of Animals from the University of Chile under the code 20411-VET-UCH.
Raw and ready-to-eat poultry products were collected from supermarkets, restaurants, and meat-producer facilities by the Subsecretaría de Salud Pública from Valparaíso and transported at 0–4 °C to the Laboratorio de Salud Pública, Ambiental y Laboral (SEREMI Salud—Valparaíso). Salmonella isolation from these samples was performed according to ISO 6579-2017, and serotyping was performed at the ISP.
Human clinical samples (e.g., stool, blood, urine) representing human salmonellosis cases from different Región Metropolitana areas were received at the Laboratory of Microbiology of the UC-Christus Health network and processed for Salmonella isolation. Samples were inoculated in Hektoen Enteric agar and incubated at 35 ± 2 °C for 24–48 h in aerobiosis; then, Salmonella confirmation was performed using MALDI-TOF mass spectrometry, and serotyping was performed at the ISP. Permission to use the human clinical isolates was granted by the Comité Ético Científico de Ciencias de la Salud UC (Protocol ID 220614005).
Salmonella Infantis available from the above-described sources were all selected. All isolates were stored in 20% glycerol stocks and maintained at −80 °C.
Genome sequencing and construction of the genome dataset
Isolates from surface water were sequenced at the United States Food and Drug Administration Center for Food Safety and Applied Nutrition. Isolates from other sources were sequenced at the GenomeTrakr New York State Department of Health laboratory. Whole genome sequencing was performed on 385 isolates, and the reads were deposited in the Sequence Read Archive, NCBI. Additionally, the Salmonella Infantis strains from human cases were sequenced at SeqCenter, Pittsburgh, PA, and the reads were directly uploaded to Enterobase.24 All sequencing was conducted using Illumina platforms.
On April 20th, 2023, the Enterobase database for Salmonella was queried for genomes from Chile and the serovar Infantis as predicted by SISTR125 or SeqSero2.26 A total of 440 Salmonella Infantis records were retrieved, including the 396 sequenced by us, along with their associated metadata (Table 1; Supplementary Table S1). Only two genomes lacked the information regarding the collection year and were not included for the analysis of the isolate distribution per collection year. To avoid/reduce methodological discrepancies in genome assemblies, all genomes were downloaded from Enterobase, where they were assembled from raw reads and annotated, with the use of a standardized bioinformatic pipeline.24
Table 1.
Features of the publicly available Salmonella Infantis genomes from Chile (April 20th, 2023).
| Feature | # genomes (Total = 440) |
|---|---|
| 7-gene MLST | |
| ST32 (thrA19) | 435 (98.9%) |
| ST9835 (thrA1541) | 3 (0.7%) |
| ST9853 (thrA1552) | 2 (0.5%) |
| cgMLST + HierCC | |
| HC20_343 | 322 (73.2%) |
| HC20_775 | 83 (18.9%) |
| HC20_2398 | 8 (1.8%) |
| Other HC20 clusters | 27 (6.1%) |
| Isolation source | |
| Environmenta | 308 (70.0%) |
| Food/Poultry | 97 (22.0%) |
| Human | 12 (2.7%) |
| Other | 23 (5.2%) |
| Isolation year | |
| 2022 | 20 (4.5%) |
| 2021 | 115 (26.1%) |
| 2020 | 8 (1.8%) |
| 2019 | 210 (47.7%) |
| 2018 | 68 (15.5%) |
| 2009–2017 | 17 (3.9%) |
| Unknown | 2 (0.4%) |
Environmental isolates were obtained from surface water (n = 265) and soil/dust (n = 43).
Population structure and phylogenetic analyses
The 7-gene MLST, core genome MLST (cgMLST), hierarchical clustering based on cgMLST profiles, core SNP-based phylogeny, and minimum spanning trees were all carried out directly in Enterobase.24 Annotated genomes were used for allele calling to classify the Salmonella Infantis isolates in sequence types (STs) by MLST (based on genes aroC, dnaN, hemD, hisD, purE, sucA and thrA) or core genome STs (cgSTs) by cgMLST (based on a 3002 allele scheme, cgMLST V2; https://pubmlst.org/bigsdb?db=pubmlst_salmonella_seqdef&page=schemeInfo&scheme_id=4). HierCC V127 was used to cluster isolates based on the cgMLST profiles. Clusters grouping isolates with links no more than 20 alleles apart (HC20) were used to describe the Salmonella Infantis population. Based on cgMLST profiles, a minimum spanning tree (MST) was constructed with MSTree V2. A maximum likelihood phylogenetic tree based on core-SNPs was built with the Salmonella Infantis N55391 genome (Enterobase Barcode SAL_EA1888AA; GenBank accession NZ_CP016410.1) as a reference. The phylogenetic tree was constructed with RAxML V8 based on 2008 variant sites called in ≥95% of the genomes.
Identification of antibiotic-resistance genes/mutations and presence of pESI-like megaplasmids
All 440 genome assemblies were downloaded from Enterobase and stored locally. Antibiotic resistance genes and point mutations involved in antimicrobial resistance in Salmonella were identified using AMRFinderPlus v3.11.4.28 Since the mdsA and mdsB genes were found in all isolates and their presence did not result or explain any phenotypic resistance, these genes were not included in the analyses. The presence of pESI-like megaplasmids was assessed with ABRicate v1.0.1 (https://github.com/tseemann/abricate) and a custom database that included all 315 genes from the pESI-like megaplasmid pN55391 (GenBank accession NZ_CP016411.1).
Intra-source genomic diversity analysis
The cgMLST allelic profiles for each Salmonella Infantis isolate were downloaded from Enterobase. Pairwise allelic differences (PAD) were calculated for any pair of isolates within each source to assess the genomic diversity within each source. Only isolates representing unique cgSTs within a given source were included in the analysis. When more than one isolate per cgST was detected, only one was randomly selected and included in the analysis.
Antibiotic-susceptibility testing
A sub-sample of 23 Salmonella Infantis isolates representing the diversity of the HC20_343 cluster was selected for antimicrobial susceptibility testing. These isolates were chosen because they represented different isolation sources and collection years and had the highest intra-source genomic diversity based on their PADs. Susceptibility testing was carried out in cation-adjusted Mueller-Hinton agar by the agar-dilution method following the recommendations of the Clinical and Laboratory Standards Institute.29 The minimum inhibitory concentration (MIC) was interpreted according to the CLSI breakpoints available in the M100Ed33 document.30 The following antibiotics, or antibiotic-inhibitor, were tested: amikacin (AMK), ampicillin (AMP), ampicillin-sulbactam (SAM), cefazoline (CFZ), cefepime (FEP), cefotaxime (CTX), cefotaxime/clavulanate (CTX/CLA), ceftazidime (CAZ), ceftazidime/clavulanate (CAZ/CLA), ciprofloxacin (CIP), fosfomycin (FOS), gentamycin (GEN), imipenem (IPM), meropenem (MEM), piperacillin-tazobactam (TZP), and trimethoprim/sulfamethoxazole (SXT). ESBL production was detected when the MIC of CTX and CAZ showed ≥3 2-fold reduction in the presence of clavulanate, an ESBL-inhibitor. A multidrug-resistant phenotype was assigned to isolates that displayed resistance to one or more antibiotics from at least three different classes.
These antibiotics were selected as they are routinely tested by the United States National Antimicrobial Resistance Monitoring System for Enteric Bacteria–NARMS31 (GEN, STX, MEM, AMP, CIP), allows the detection of ESBL production (CTX, CTX/CLA, CAZ, CAZ/CLA) or represent different members of included antibiotic classes (SAM, CFZ, FEP, IPM, TZP, AMK). FOS was tested as it is a first-line antibiotic used for uncomplicated urinary tract infections (UTIs) and there are reports of UTI caused by MDR Salmonella Infantis.32
Statistical analyses and graphs
Independence between the presence of AMR genes/mutations and the results of antibiotic-susceptibility was tested with the Fisher's exact test in GraphPad Prism v10.1.0, with α = 0.05. Multiple correspondence analysis was carried out in DisplayR (https://www.displayr.com/) on metadata for all 440 genomes regarding 35 categories within 5 variables: Isolation source (8 categories), collection year (13 categories), 7-gene MLST (3 categories), HC20 cluster (9 categories), and megaplasmid presence (two categories).
Bar charts and violin plots were created in GraphPad Prism. MSTs were made with GrapeTree v1.5.1.33 The iTOL v6 online tool was used to display and annotate the phylogenetic trees (https://itol.embl.de/). The multiple correspondence analysis plot was made in DisplayR.
Reporting guidelines
This work was written following the STrengthening the REporting of Genetic Association Studies (STREGA) reporting guidelines.34
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Region of origin, isolation source, and collection year of the isolates
To study the structure of the Salmonella Infantis population from Chile, we sequenced 396 isolates of this pathogen. We analysed their genomes with all public Salmonella Infantis genomes from Chile available in Enterobase on April 20th, 2023. Our dataset was mainly composed of genomes from isolates coming from central Chile, specifically from Región de Valparaíso (n = 60), Región Metropolitana (n = 318), Región del Libertador General Bernardo O'Higgins (n = 1), Región del Maule (n = 21), Región Ñuble (n = 1), and Región del Biobío (n = 2). Most isolates (85.9%; 378/440) came from Región Metropolitana and Región de Valparaíso (Fig. 1A), and the data about the Region of origin for 37 isolates was not available. Genomes from environmental (river/creek/lagoon/irrigation water; boot swabs of soil/dust) and poultry/food (chicken carcass/crop/caecal content/faeces; chicken meat) origin made 92.0% (405/440) of total genomes, followed by human clinical cases which accounted for 2.7% (12/440 genomes) (Fig. 1B). While the collection year ranged from 2009 to 2022, most isolates (95.7%; 421/440) were distributed from 2018 to 2022 (Fig. 1C).
The Salmonella Infantis population in Chile belongs to ST32 and comprises different cgMLST clusters of highly related isolates
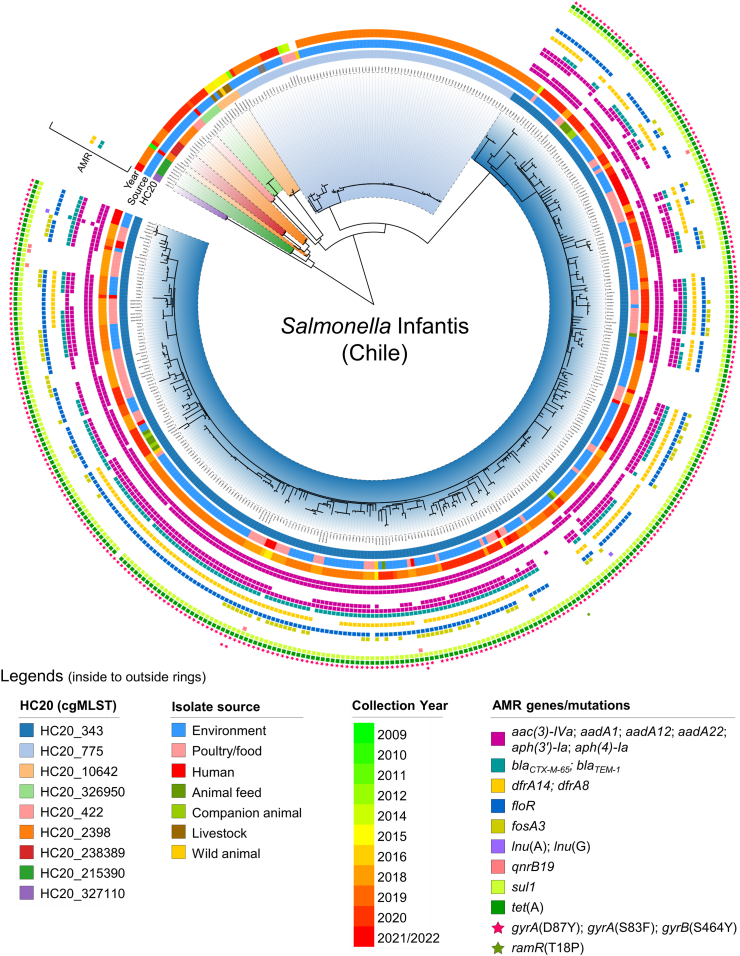
Among all isolates, 435/440 (98.9%) were ST32 (7-gene MLST), while the other five (three ST9835 and two ST9853) were single locus variants of ST32 that differed in the thrA allele (Table 1). The core SNP-based maximum likelihood phylogeny showed the presence of several clades comprising highly related genomes (Fig. 2). The hierarchical clustering of genomes linked by no more than 20 cgMLST-allele differences (HC20) revealed that the clades shown by the phylogeny corresponded with different HC20 groups, dominated by clusters HC20_343 and HC20_775 (405/440; 92.0% of genomes) (Table 1, Fig. 2). Isolates belonging to cluster HC20_343 came from the widest variety of isolation sources and some clusters (e.g., 2398, 422, 215390, 327110) seemed to group isolates only from environmental sources (Fig. 2). However, this is most likely an effect of a sample-size bias, with the most populated cluster being more likely to harbour genomes from different sources. Notably, 10 out of 12 Salmonella Infantis isolates from human infections belonged to the HC20_343 cluster while the remaining two were found in clusters HC20_215390 and HC20_2398. These HC20_343 isolates were closely related to, and shared temporal proximity with, isolates from different sources, forming subclades in the phylogeny that included poultry (chicken), food (chicken meat), and environmental (surface water and soil/dust from boot swabs) isolates (Fig. 2).
Fig. 2.
Phylogenetic analysis of Chilean Salmonella Infantis genomes. A core SNP-based maximum likelihood phylogeny (2008 variant sites; 95% presence) was constructed with 440 Chilean Salmonella Infantis genomes using Salmonella Infantis N55391 (Enterobase barcode SAL_EA1888AA), a USA strain isolated from poultry in 2014, as the reference. Additionally, metadata regarding the HC20 clusters (clade colours and first ring), isolation source (second ring), isolation year (third ring), and presence of antibiotic-resistance genes/mutations as determined by AMRFinderPlus (coloured squares/stars) were incorporated into the phylogenetic tree.
Salmonella Infantis isolates from the HC20_343 cluster include MDR ESBL-producing strains and carry pESI-like megaplasmids
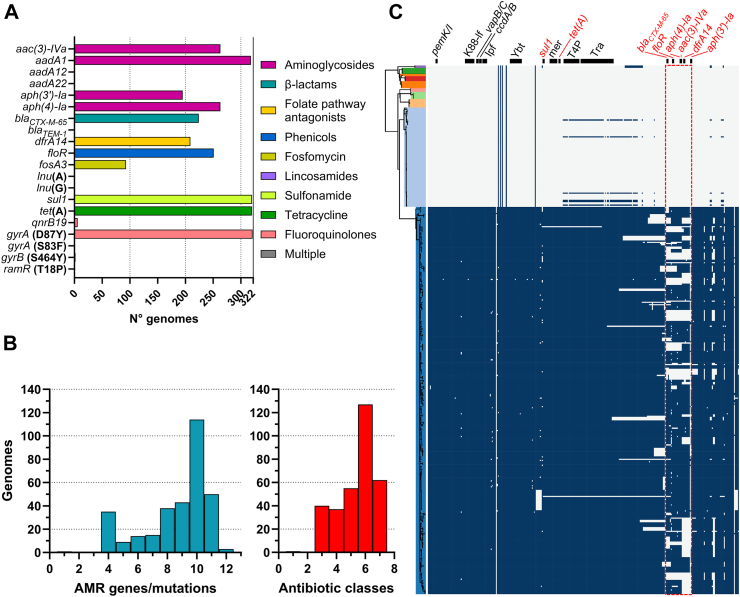
The presence of AMR genes/mutations among the 440 genomes from Chile was assessed (Fig. 2; Supplementary Tables S2 and S3). Interestingly, the HC20_343 cluster (322 genomes) was the only one associated with up to 12 acquired antimicrobial resistance genes or mutations predicted to result in resistance to aminoglycosides, cephems, folate pathway antagonists, chloramphenicol, fosfomycin, lincosamides, fluoroquinolones and tetracycline (Fig. 3A). The most frequent resistance genes among HC20_343 isolates were tet(A) (99.4%; 320/322), sul1 (99.4%; 320/322), and aadA1 (98.8%; 318/322), encoding predicted resistance to tetracycline, sulfonamide, and aminoglycosides, respectively. Genes aph(3’)-Ia, aph(4)-Ia, aac(3)-IVa (aminoglycoside resistance), blaCTX-M-65 (3 GC resistance), dfrA14 (trimethoprim resistance), and floR (phenicol resistance) were found in 60.6%–81.7% (195/322-263/322) of the isolates. Other identified resistance genes [aadA12, aadA22, blaTEM-1, lnu(A), lnu(G), and qnrB19] were present in 6/322 or less isolates only, except by fosA3 (fosfomycin resistance) that was carried by 28.9% (93/322) of isolates. Noteworthy, all but one HC20_343 isolate (99.7%; 321/322) carried the gyrA (D87Y) mutation involved in fluoroquinolone resistance.
Fig. 3.
Antibiotic-resistance determinants and pESI-like megaplasmid presence among Salmonella Infantis from the HC20_343 cluster. A) Frequency of individual antibiotic-resistant genes/mutations in the HC20_343 coloured by antibiotic class. B) Frequency of overall antibiotic-resistance genes/mutations per genome (blue bars) and antibiotic classes targeted per genome (red bars). C) Presence of pESI-like megaplasmids among the Chilean Salmonella Infantis genomes. A vertical representation of the same phylogenetic tree of Fig. 2 is coloured according to the HC20 clusters. The presence of each of the 315 genes from the pESI-like megaplasmid pN55391 was assessed by ABRicate using a custom database (blue squares). Black horizontal bars above the presence/absence matrix indicate the backbone regions of the megaplasmid (genes in black font) or the antibiotic-resistance regions (genes in red font). A red dashed line rectangle delimits the most variable region among the Chilean pESI-like megaplasmids relative to pN55391. K88-lf (K88-like fimbria), Ipf (Infantis plasmid-encoded fimbria, Ybt (Yersiniabactin synthesis cluster), mer (mercury-resistance cluster), T4P (type-IV pili encoding cluster), Tra (transfer region).
Within the HC20_343 cluster, 210/322 genomes (65.2%) harboured nine or more AMR genes/mutations, and 321/322 genomes (99.7%) carried genes/mutations predicted to encode resistance to at least one antibiotic from three or more antibiotic classes, potentially resulting in an MDR phenotype (Fig. 3B). We found association (Fisher's exact test) between the content of genetic AMR determinants and the phenotypic resistance in all the isolates for which the MIC was assessed (Supplementary Table S4). Accordingly, all blaCTX-M-65-positive isolates were resistant to the cephalosporins CFZ and CTX and displayed an ESBL-phenotype (p < 0.0001). Isolates harbouring aadA1, aac(3)-Iva, and aph(4)-Ia were resistant to GEN (p < 0.0001). Some isolates (9/23) were susceptible dose-dependent (SDD) to the fourth-generation cephalosporin FEP. However, this phenotype did not correlated with any of the identified AMR genes. The presence of aadA1 alone was not sufficient to confer GEN resistance, and aph(3′)-Ia carriage did not show agreement with the resistance profile (pAMK > 0.9999; pGEN = 0.115). All tested isolates were susceptible to AMK. SXT-resistance was found in isolates carrying sul1 plus dfrA14 (p < 0.0001), and lack of dfrA14 resulted in SXT susceptibility. All gyrA (D87Y)-positive isolates displayed intermediate resistance to CIP (p < 0.048). Notably, the presence of qnrB19 plus gyrA (D87Y) resulted in CIP-resistance (p < 0.046). Conversely, all isolates lacking at least one of the genes/mutations mentioned above were susceptible to the corresponding antibiotics.
Since many of the identified antibiotic-resistance genes have been reported to be carried by the pESI-like megaplasmid associated with emerging MDR Salmonella Infantis, we screened the entire genome dataset for the presence of the pN55391 pESI-like megaplasmid genes (Fig. 3C; Supplementary Table S5). We found that only the HC20_343 cluster harboured most of the pESI-like megaplasmid genes (from 164 to 310 out of 315 genes), including those encoding the three toxin-antitoxin systems (pemK/I, vapB/C and ccdA/B), the K88-like and Ipf fimbria, the yersiniabactin synthesis cluster, the mercury resistance cluster, and the conjugative transfer region (tra and type-IV pili-encoding genes), which are part of the pESI-like megaplasmids backbone.35 Most differences between the megaplasmids harboured by the Chilean strains and the pN55391 megaplasmid were located in the antibiotic resistance region, previously reported as a variable region.5 Nevertheless, the resistance genes contained in this region were present in most of the megaplasmid-harbouring genomes (blaCTX-M-65: 69.6%, 224/322; floR: 78.0%, 251/322; aph(4)-Ia: 81.7%, 263/322; aac(3)-IVa: 81.7%, 263/322; dfrA14: 64.9%, 209/322; and aph(3′)-Ia: 60.6%, 195/322; Supplementary Table S5). Our analyses revealed that the HC20_343 Salmonella Infantis isolates acquired multiple antimicrobial-resistance genes/mutations, partly associated with the presence of pESI-like megaplasmids.
HC20_343 Salmonella Infantis isolates are disseminated among different niches and show a low intra-source genomic diversity
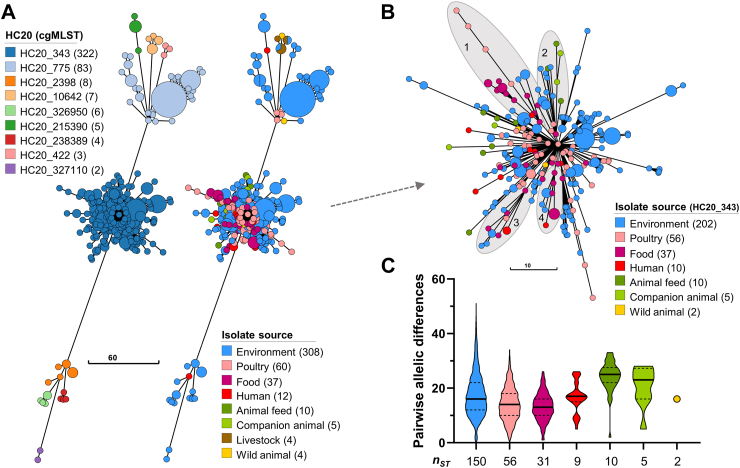
An MST was constructed to visualize the genomic structure of the Salmonella Infantis population based on the cgMLST profiles (Fig. 4A). In agreement with the core SNP-phylogeny, the MST shows the bacterial population grouped in nine HC20 clusters of highly related isolates linked by no more than 20 allele-differences. All isolates from food, animal feed, and companion animals (dogs) belonged to cluster HC20_343. Accordingly, multiple correspondence analysis (MCA), carried out with metadata of all 440 genomes (variables: isolation source, collection year, 7-gene MLST, megaplasmid presence, and HC20 cluster), also evidenced the occurrence of HC20_343 isolates with majority of sources in recent years (Supplementary File, Fig S1). MCA clustered the HC20_343 category together with megaplasmid presence, environmental, companion animal, food, and human sources, and years from 2017 to 2022. Conversely, the remaining HC20 clusters grouped with megaplasmid absence, and years 2009–2015. Together, these findings highlight the foodborne, zoonotic, and human-pathogenic potential of HC20_343 Salmonella Infantis.
Fig. 4.
Population structure of Chilean Salmonella Infantis and intra-source genomic diversity within cluster HC20_343. A) Minimum spanning tree depicting the population structure of Salmonella Infantis in Chile based on the cgMLST profiles of 3002 alleles. Nodes are coloured according to the HC20 clusters determined by HierCC or the isolation source, and their size is proportional to the number of isolates included in each node. The legends indicate the different HC20 clusters present, the isolation sources, and the number of isolates. B) Zoom-in view of the HC20_343 cluster structure with nodes coloured according to the isolation source. The legend also indicates the number of isolates per source within HC20_343. The shaded area and numbers indicate subclusters evidencing transmission of Salmonella Infantis between different sources. C) PAD between unique cgSTs from cluster HC20_343 per isolation source. Each violin plot, truncated at the highest and smallest values, represents the frequency distribution of PADs. The black unbroken and dashed lines represent the median PAD, and the 25th and 75th percentiles, respectively. The nST value indicates the number of isolates with unique cgSTs within each isolation source. Only isolates representing unique cgSTs were included in the analysis. Note: In panel 4B, the links and node sizes are the same as in panel 4A. However, panel 4B is presented at a different scale, and some branches were adjusted to prevent overlapping and improve subcluster visualization.
We carried out a more detailed analysis of cluster HC20_343 isolates. The MST evidenced putative events of transmission between different sources, as exemplified by subclusters 1 to 4 in Fig. 4B. Subclusters 1, 3, and 4 included isolates from environmental, food, and human sources, while subcluster 2 included isolates from environmental, animal feed, and companion animals. Moreover, subclusters 1, 2, and 3 also included Salmonella Infantis from poultry. Importantly, isolates from all sources within these clusters were linked to isolates obtained from food (poultry products), and, ultimately, all sources within cluster HC20_343 had links with poultry. We assessed the intra-source genomic diversity of the HC20_343 isolates regarding the PAD between any pair of isolates representing unique cgSTs (Fig. 4C; Supplementary Table S6). The environmental isolates displayed the highest diversity, with PAD values ranging from 0 to 51. All other sources harboured isolates with PADs ≤34. The median PAD per source ranged from 13 in food isolates to 25 in animal feed. Environmental, poultry, food, and human isolates had the lowest median PADs (from 13 to 17), while the isolates from animal feed and companion animals had the highest (25 and 23, respectively). Overall, a low genomic diversity was found within the different isolation sources.
Discussion
The expansion of MDR Salmonella Infantis has been reported worldwide, with the highest proportion of isolates coming from the Americas region, followed by Europe.36 The dissemination of this foodborne pathogen is mainly linked to poultry and poultry products, and different countries have reported a rise in human infections.8,15,36, 37, 38 Nevertheless, little is known about the extent of the Salmonella Infantis dissemination in different niches and its molecular epidemiology.
Here, we report the first large-scale genomic analysis of this foodborne pathogen population in Chile, finding evidence of the transmission of Salmonella Infantis carrying pESI-like megaplasmids and multiple AMR determinants (cluster HC20_343) between environmental, poultry, food, animals, and human niches. Highly related isolates from different years were found in diverse sources, indicating a constant inter-source transmission. Currently, Chile is working to enhance the surveillance and control of Salmonella (including antimicrobial resistance) (SAG, Exempt Resolution 3687; https://bcn.cl/2pap1).16 However, these efforts mainly focus on poultry and its derived products as they are known sources of Salmonella. Importantly, we report that irrigation waters are a source of Salmonella Infantis with MDR phenotypes. The presence of this pathogen in surface watersheds that supply the country's main agricultural region12 underscores the urgent necessity to improve the current monitoring of irrigation waters and establish effective control measures to prevent the contamination of produce and the dissemination of antibiotic-resistance genes in the environment.
Human infection with non-typhoidal Salmonella usually results in self-limited acute gastroenteritis. However, children under five, adults over 65, and immunocompromised people are at higher risk of developing a severe life-threatening infection.3 Antibiotics, such as 3 GC and fluoroquinolones, are recommended to prevent or treat severe diseases.3 In 2017, the World Health Organization presented a list of 12 antibiotic-resistant bacterial pathogens, categorised into critical, high, and medium priority tiers, urgently requiring research and development of novel antibiotics since available treatments are becoming limited.39 This list placed 3 GC-resistant Enterobacteriaceae and fluoroquinolone-resistant Salmonella spp. as critical and high-priority pathogens. A significant proportion of Salmonella Infantis from the HC20_343 cluster (69.6%, 224/322) harboured the ESBL-encoding gene blaCTX-M-65 in a pESI-like megaplasmid. Moreover, almost all HC20_343 isolates (321/322) harboured the chromosomal gyrA (D87Y) mutation involved in fluoroquinolone resistance. Although the resistance profiles of emergent Salmonella Infantis reported in different countries are variable,8,37,40,41 partially as a result of the diversity within the megaplasmid AMR region (see Fig. 3C and ref.5), our findings in Chile are in agreement with a recent global survey of reported AMR determinants in Salmonella Infantis.36 Importantly, we found that the aminoglycoside AMK and the carbapenems IPM and MEM were consistently active against all tested isolates from Chile. Our findings imply that the available options for preventing or treating severe infections in susceptible individuals are limited. The recently reported burden of AMR in the Americas for the year 201942 showed that the resistance to fluoroquinolones and 3 GC accounted for most of the associated deaths to AMR. However, the number of deaths caused by non-typhoidal Salmonella was among the lowest. As the burden report did not included Salmonella among the pathogens causing urinary tract infections, and the non-typhoidal Salmonella-3GC combination was also not considered, the real burden caused by this foodborne pathogen in the region could be higher, especially in the light of the widespread dissemination of MDR Salmonella Infantis in the continent (see below).
An unexpected finding was the association of the HC20_343 isolates with the presence of the pESI-like megaplasmids. Alba et al.6 found pESI-like megaplasmids carrying the blaCTX-M-1 gene in European Salmonella Infantis isolates from a higher diversity of HC20 clusters, and mainly from the HC20_7828 cluster. They also reported that megaplasmid-positive isolates from North America, or associated with traveling to South America, harboured the blaCTX-M-65 gene on the megaplasmid and belonged to the HC20_343 cluster. Similar to our study, they found that strains from the HC20_775 cluster lacked the megaplasmid. In addition to the known association of blaCTX-M-65 with American and blaCTX-M-1 with European megaplasmid-carrying Salmonella Infantis,6,8 our findings suggest that the American megaplasmid-positive Salmonella Infantis strains might be associated with the HC20_343 cluster. Testing this hypothesis might help to better understand the global dissemination of emerging Salmonella Infantis. Importantly, we found that, out of 14706 Salmonella Infantis genomes from the Americas, 60.2% (8852/14706) belonged to the HC20_343 cluster (Supplementary File, Fig. S2), suggesting a high prevalence of megaplasmid-positive ESBL-producing isolates with intermediate fluoroquinolone resistance in the continent.
The approximate time for the arrival of the emerging Salmonella Infantis into Chilean territory remains unknown. The oldest pESI-like positive genomes in our dataset date from 2016 (4 genomes) and 2017 (1 genome) (Supplementary Table S1). These genomes represent four clinical isolates and one isolate obtained from a Dominican gull (Larus dominicanus), indicating that Salmonella Infantis carrying blaCTX-M-65-positive pESI-like megaplasmids were circulating in Chile before 2016. A minimum spanning tree constructed with the cgMLST profiles from all Salmonella Infantis isolates from the Americas (available in Enterobase on July 7th, 2023) revealed that the HC20_343 isolates from the United States and Chile cluster together (Supplementary File, Fig.S2, also seen at NCBI Pathogen Detection). This finding suggests two possible scenarios in which emerging Salmonella Infantis from Chile came from the United States, or isolates from both countries share a common origin.43
Our study is not free of limitations. Our genome dataset includes isolates from 6 out of 16 Regions in the country and is primarily concentrated in two Regions. Nevertheless, it is relevant to note that these Regions harbour most of the human population and poultry production in Chile (Fig. 1A). Therefore, the dataset analysed in this study offers a substantial representation of the bacterial population potentially encountered by most individuals and poultry raising/production systems in the country. We acknowledge, however, that a complete picture of the Salmonella Infantis population in Chile would require the analysis of isolates spanning the entire territory. Another limitation is that the genome dataset does not evenly represent different isolation sources and years. Isolates from companion animals, livestock, and wildlife, as well as isolates from samples collected from 2009 to 2017 are underrepresented. This limits our capacity to draw strong conclusions about the diversity of Salmonella Infantis within those sources. As our results show a widespread dissemination of this bacterium in irrigation waters, it would be of great relevance to obtain genomic data from isolates obtained from possible sources of water contamination, as well as from produce irrigated with those waters. Importantly, the rise in human cases of MDR Salmonella Infantis reported by the ISP suggests the emergence of this pathogen in Chile around 2014–2016.19 However, the low number of genomes spanning the period before 2018 was a constraint in using the genomic data to answer questions about the time of emergence of the HC20_343 in the country. Future studies addressing questions about the emergence dynamics of MDR Salmonella Infantis in Chile should obtain and analyse genomic data from numerous isolates spanning the 2012–2017 period.
We analysed a dataset of 440 genomes representing the population structure of Salmonella Infantis in Chile. This population comprises strains lacking genetic determinants of antibiotic resistance and antibiotic-resistant strains that harbor pESI-like megaplasmids, both from the globally spread ST32. The megaplasmid-carrying strains belonged to the HC20_343 cluster, circulating among environmental, food, diverse animals, and human niches. Our results indicate that a significant proportion of the HC20_343 isolates encode ESBLs and display an intermediate resistance to fluoroquinolones, which limits the available treatments for individuals at a higher risk. Our findings and the reported increase in human cases highlight the urgent need to study the dissemination dynamics of this pathogen to devise effective surveillance and control measures.
Contributors
Conceptualisation–API, AIMS.
Data curation–API, FPA, RBM, DMAE, PG, DS, JOP, ARJ, AIMS.
Formal analysis–API, CDG, FPA, RBM, DMAE.
Funding acquisition–API, DMAE, ARJ, AIMS.
Investigation–API, CDG, FPA, RBM, DMAE, PG, DS, RCA, MT, JOP, ARJ, JM, RLB, AIMS.
Methodology–API, CDG, FPA, RBM, DMAE, PG, DS, RCA, MT, JOP, ARJ, JM, RLB, AIMS.
Software–API, CDG.
Supervision–AIMS.
Visualization–API.
Writing original draft–API, AIMS.
Writing review and editing–API, CDG, FPA, RBM, DMAE, PG, DS, RCA, MT, JOP, ARJ, JM, RLB, AIMS.
All authors read and approved the submitted version of the manuscript and had access to the raw data. API and AIMS verified the data and had the final responsibility for the decision to submit the manuscript.
Data sharing statement
All genome metadata, identified AMR genes/mutations, antimicrobial susceptibility testing results, and identified megaplasmid genes are available in the Supplementary Material. Genome assemblies are publicly available in Enterobase (https://enterobase.warwick.ac.uk/species/index/senterica) and GenBank (https://www.ncbi.nlm.nih.gov/genbank/) using the corresponding accession numbers found in the Supplementary Table S1.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
All authors declare no competing interests.
Acknowledgements
This study was partially supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) as part of an award, FDU001818, with 30% percent funded by FDA/HHS; and also by Agencia de Investigación y Desarrollo de Chile (ANID) through FONDECYT de Postdoctorado Folio 3230796 and Folio 3210317, FONDECYT Regular Folio 1231082, and ANID—Millennium Science Initiative Program—ICN2021_044.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2024.100711.
Appendix A. Supplementary data
References
- 1.GBD 2019 Antimicrobial Resistance Collaborators Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2022;400:2221–2248. doi: 10.1016/S0140-6736(22)02185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antimicrobial Resistance Collaborators Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC . 2023. Salmonella–information for Healthcare professionals and laboratories.https://www.cdc.gov/salmonella/general/technical.html Accessed Aug 23, 2023. [Google Scholar]
- 4.Aviv G., Tsyba K., Steck N., et al. A unique megaplasmid contributes to stress tolerance and pathogenicity of an emergent Salmonella enterica serovar Infantis strain. Environ Microbiol. 2014;16:977–994. doi: 10.1111/1462-2920.12351. [DOI] [PubMed] [Google Scholar]
- 5.Tate H., Folster J.P., Hsu C.-H., et al. Comparative analysis of extended-spectrum-β-lactamase CTX-M-65-producing Salmonella enterica serovar infantis isolates from humans, food animals, and retail chickens in the United States. Antimicrob Agents Chemother. 2017;61:004888–004917. doi: 10.1128/AAC.00488-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alba P., Leekitcharoenphon P., Carfora V., et al. Molecular epidemiology of Salmonella Infantis in Europe: insights into the success of the bacterial host and its parasitic pESI-like megaplasmid. Microb Genom. 2020;6 doi: 10.1099/mgen.0.000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gal-Mor O., Valinsky L., Weinberger M., et al. Multidrug resistant Salmonella enterica serovar Infantis, Israel. Emerg Infect Dis. 2010;16:1754–1757. doi: 10.3201/eid1611.100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mejía L., Medina J.L., Bayas R., et al. Genomic epidemiology of Salmonella Infantis in Ecuador: from poultry farms to human infections. Front Vet Sci. 2020;7 doi: 10.3389/fvets.2020.547891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz D., Hernandez-Carreño P.E., Velazquez D.Z., et al. Prevalence, main serovars and anti-microbial resistance profiles of non-typhoidal Salmonella in poultry samples from the Americas: a systematic review and meta-analysis. Transbound Emerg Dis. 2022;69:2544–2558. doi: 10.1111/tbed.14362. [DOI] [PubMed] [Google Scholar]
- 10.USDA FSIS . 2023. Quarterly sampling reports on Salmonella and Campylobacter.https://www.fsis.usda.gov/science-data/data-sets-visualizations/microbiology/microbiological-testing-program-rte-meat-and-7 [Google Scholar]
- 11.Bogomazova A.N., Gordeeva V.D., Krylova E.V., et al. Mega-plasmid found worldwide confers multiple antimicrobial resistance in Salmonella Infantis of broiler origin in Russia. Int J Food Microbiol. 2020;319 doi: 10.1016/j.ijfoodmicro.2019.108497. [DOI] [PubMed] [Google Scholar]
- 12.Toro M., Weller D., Ramos R., et al. Environmental and anthropogenic factors associated with the likelihood of detecting Salmonella in agricultural watersheds. Environ Pollut. 2022;306 doi: 10.1016/j.envpol.2022.119298. [DOI] [PubMed] [Google Scholar]
- 13.The observatory of economy complexity. Poultry Meat. https://oec.world/en/profile/hs/poultry-meat Accessed Sept 12, 2023.
- 14.Alegria-Moran R., Rivera D., Toledo V., Moreno-Switt A.I., Hamilton-West C. First detection and characterization of Salmonella spp. in poultry and swine raised in backyard production systems in central Chile. Epidemiol Infect. 2017;145:3180–3190. doi: 10.1017/S0950268817002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lapierre L., Cornejo J., Zavala S., et al. Phenotypic and genotypic characterization of virulence factors and susceptibility to antibiotics in Salmonella Infantis strains isolated from chicken meat: first findings in Chile. Animals. 2020;10:1049. doi: 10.3390/ani10061049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paredes-Osses E.A., Fernandez Ricci A., Duarte Boke S., et al. Estudio Piloto de Vigilancia Integrada de susceptibilidad fenotípica y presencia de genes de resistencia a antimicrobianos β-lactámicos en cepas de Salmonella enterica subsp. enterica serovar Infantis aisladas desde alimentos en Chile. Rev del Inst Salud Pública Chile. 2020;4:42–51. [Google Scholar]
- 17.Retamal P., Gaspar J., Benavides M.B., et al. Virulence and antimicrobial resistance factors in Salmonella enterica serotypes isolated from pigs and chickens in central Chile. Front Vet Sci. 2022;9 doi: 10.3389/fvets.2022.971246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krüger G.I., Pardo-Esté C., Zepeda P., et al. Mobile genetic elements drive the multidrug resistance and spread of Salmonella serotypes along a poultry meat production line. Front Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1072793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ISP . 2019. Boletin de Vigilancia de Salmonella spp. 2014-2018.http://www.ispch.cl/sites/default/files/BoletínSalmonella-12052020A.pdf [Google Scholar]
- 20.Martínez M.C., Retamal P., Rojas-Aedo J.F., Fernández J., Fernández A., Lapierre L. Multidrug-resistant outbreak-associated Salmonella strains in irrigation water from the metropolitan region, Chile. Zoonoses Public Health. 2017;64:299–304. doi: 10.1111/zph.12311. [DOI] [PubMed] [Google Scholar]
- 21.Fuentes-Castillo D., Farfán-López M., Esposito F., et al. Wild owls colonized by international clones of extended-spectrum β-lactamase (CTX-M)-producing Escherichia coli and Salmonella Infantis in the Southern Cone of America. Sci Total Environ. 2019;674:554–562. doi: 10.1016/j.scitotenv.2019.04.149. [DOI] [PubMed] [Google Scholar]
- 22.Sbodio A., Maeda S., Lopez-Velasco G., Suslow T.V. Modified Moore swab optimization and validation in capturing E. coli O157:H7 and Salmonella enterica in large volume field samples of irrigation water. Food Res Int. 2013;51:654–662. [Google Scholar]
- 23.Jeong S.K., Gang G.L., Jong S.P., et al. A novel multiplex PCR assay for rapid and simultaneous detection of five pathogenic bacteria: Escherichia coli O157:H7, Salmonella, Staphylococcus aureus, Listeria monocytogenes, and Vibrio parahaemolyticus. J Food Prot. 2007;70:1656–1662. doi: 10.4315/0362-028x-70.7.1656. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Z., Alikhan N.F., Mohamed K., Fan Y., Agama Study Group T., Achtman M. The EnteroBase user’s guide, with case studies on Salmonella transmissions, Yersinia pestis phylogeny, and Escherichia core genomic diversity. Genome Res. 2020;30:138–152. doi: 10.1101/gr.251678.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson J., Yoshida C., Kruczkiewicz P., et al. Comprehensive assessment of the quality of Salmonella whole genome sequence data available in public sequence databases using the Salmonella in silico Typing Resource (SISTR) Microb Genom. 2018;4 doi: 10.1099/mgen.0.000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang S., den Bakker H.C., Li S., et al. SeqSero2: rapid and improved Salmonella serotype determination using whole-genome sequencing data. Appl Environ Microbiol. 2019;85:017466–017519. doi: 10.1128/AEM.01746-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Z., Charlesworth J., Achtman M. HierCC: a multi-level clustering scheme for population assignments based on core genome MLST. Bioinformatics. 2021;37:3645–3646. doi: 10.1093/bioinformatics/btab234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldgarden M., Brover V., Gonzalez-Escalona N., et al. AMRFinderPlus and the Reference Gene Catalog facilitate examination of the genomic links among antimicrobial resistance, stress response, and virulence. Sci Rep. 2021;11:1–9. doi: 10.1038/s41598-021-91456-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.CLSI . 11a edn. CLSI standard M07; 2018. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 11th ed. [Google Scholar]
- 30.CLSI M100-ED33 . 33rd ed. 2023. Performance Standards for antimicrobial susceptibility testing; p. 2023.http://em100.edaptivedocs.net/GetDoc.aspx?doc=CLSIM100ED33:2023&scope=user [Google Scholar]
- 31.CDC . 2019. Antibiotics tested by NARMS.https://www.cdc.gov/narms/antibiotics-tested.html Accessed Nov 16, 2023. [Google Scholar]
- 32.CDC . 2019. Outbreak of multidrug-resistant Salmonella infections linked to raw chicken products.https://www.cdc.gov/salmonella/infantis-10-18/index.html [Google Scholar]
- 33.Zhou Z., Alikhan N.F., Sergeant M.J., et al. Grapetree: visualization of core genomic relationships among 100,000 bacterial pathogens. Genome Res. 2018;28:1395–1404. doi: 10.1101/gr.232397.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Little J., Higgins J.P.T., Ioannidis J.P.A., et al. STrengthening the REporting of genetic association studies (STREGA) — an extension of the STROBE statement. Genet Epidemiol. 2009;33:581–598. doi: 10.1002/gepi.20410. [DOI] [PubMed] [Google Scholar]
- 35.dos Santos A.M.P., Panzenhagen P., Ferrari R.G., Conte-Junior C.A. Large-scale genomic analysis reveals the pESI-like megaplasmid presence in Salmonella Agona, Muenchen, Schwarzengrund, and Senftenberg. Food Microbiol. 2022;108 doi: 10.1016/j.fm.2022.104112. [DOI] [PubMed] [Google Scholar]
- 36.Alvarez D.M., Barrón-Montenegro R., Conejeros J., Rivera D., Undurraga E.A., Moreno-Switt A.I. A review of the global emergence of multidrug-resistant Salmonella enterica subsp. enterica serovar Infantis. Int J Food Microbiol. 2023;403 doi: 10.1016/j.ijfoodmicro.2023.110297. [DOI] [PubMed] [Google Scholar]
- 37.Quino Sifuentes W., Hurtado C.V., Escalante-Maldonado O., et al. Multidrug resistance of Salmonella Infantis in Peru: a study through next generation sequencing. Rev Peru Med Exp Salud Pública. 2019;36:37–45. doi: 10.17843/rpmesp.2019.361.3934. [DOI] [PubMed] [Google Scholar]
- 38.Tyson G.H., Li C., Harrison L.B., et al. A multidrug-resistant Salmonella Infantis clone is spreading and recombining in the United States. Microb Drug Resist. 2021;27:792–799. doi: 10.1089/mdr.2020.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO . 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics.http://remed.org/wp-content/uploads/2017/03/lobal-priority-list-of-antibiotic-resistant-bacteria-2017.pdf [Google Scholar]
- 40.Brown A., Chen J., Watkins L., et al. CTX-M-65 extended-spectrum β-lactamase–producing Salmonella enterica serotype Infantis, United States. Emerg Infect Dis. 2018;24:2284–2291. doi: 10.3201/eid2412.180500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franco A., Leekitcharoenphon P., Feltrin F., et al. Emergence of a clonal lineage of multidrug-resistant ESBL-producing Salmonella Infantis transmitted from broilers and broiler meat to humans in Italy between 2011 and 2014. PLoS One. 2015;10 doi: 10.1371/journal.pone.0144802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antimicrobial Resistance Collaborators The burden of antimicrobial resistance in the Americas in 2019: a cross-country systematic analysis. Lancet Reg Health Am. 2023;25 doi: 10.1016/j.lana.2023.100561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li S., He Y., Mann D.A., Deng X. Global spread of Salmonella Enteritidis via centralized sourcing and international trade of poultry breeding stocks. Nat Commun. 2021;12:5109. doi: 10.1038/s41467-021-25319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.