Abstract
Background
The International Agency for Research on Cancer (IARC) Monograph conducted a systematic review of the relationship between asbestos and ovarian cancer. However, there may have been information bias due to the undue weight given to few articles. To address this limitation, the present study performed a meta-analysis integrating studies published both before and after the 2012 IARC Monograph on Asbestos, with the aim of investigating the association between asbestos exposure and ovarian cancer.
Methods
A comprehensive search of major journal databases was conducted to identify studies examining the relationship between asbestos exposure and ovarian cancer, including those featured in the 2012 IARC Monograph on Asbestos. A meta-analysis on asbestos exposure and cancer risk was performed.
Results
The meta-analysis of studies published after the 2012 IARC Monograph on Asbestos found a summary Standardized Mortality Ratio (SMR) of 2.04 (95% CI: 1.03—4.05; p = 0.0123; 5 studies), with a significant degree of heterogeneity among the studies (I2 = 72.99%). The combined analysis of 15 studies before and after the 2012 IARC Monograph showed an overall summary SMR of 1.72 (95% CI: 1.43—2.06; p = 0.0349; 15 studies), with a moderate degree of heterogeneity (I2 = 42.99%).
Conclusion
This meta-analysis provides evidence of a significant association between asbestos exposure and ovarian cancer mortality. While the possibility of misdiagnosis in earlier studies cannot be completely ruled out, recent findings suggest a robust correlation between asbestos exposure and ovarian cancer. This highlights the importance of sustained efforts to minimize asbestos exposure and protect public health.
Keywords: Asbestos, Meta-analysis, Ovarian cancer
1. Introduction
Asbestos is a type of fibrous mineral that has been widely utilized in various industries, including construction, industry, textiles, and everyday life, due to its fire-retardant and insulating properties. However, numerous studies have shown that asbestos exposure is associated with an increased risk of asbestosis, lung cancer, and malignant mesothelioma. In 1987, the International Agency for Research on Cancer (IARC) classified all forms of asbestos as a Group 1 carcinogen [1]. In Republic of Korea, the manufacture of highly toxic types of asbestos, such as crocidolite and amosite, has been banned since 1997. Furthermore, since 2009, the domestic manufacture, importation, and use of all asbestos-containing products have been strictly prohibited in the country to prevent the harmful health effects associated with asbestos exposure [2].
As the epidemiological understanding of asbestos-related health risks has evolved, the range of diseases associated with asbestos exposure has expanded. In the 2014 update on Asbestos in Helsinki, ovarian cancer was added to the list of malignant diseases associated with asbestos exposure [3]. The Helsinki criteria were based on the studies included in the 2012 IARC Monograph on asbestos [4]. These studies served as the foundational evidence for adding ovarian cancer to the list of asbestos-related diseases. A systematic literature review conducted in 2011, which analyzed 14 cohort studies and two case-control studies, confirmed that previous exposure to asbestos was associated with an increased risk of ovarian cancer [5].
Despite these advancements, several unresolved issues persist regarding the association between asbestos exposure and ovarian cancer. For instance, while the exposure in occupational settings has been well-documented, studies suggest that women may also be exposed through reproductive, digestive, or respiratory systems [[6], [7], [8]]. Additionally, indirect exposure—such as through sexual activity or domestic cleaning when a partner has been exposed to asbestos—has been proposed as an another route for risk [6,9,10].
Furthermore, there remains a concern that cases of malignant mesothelioma in the peritoneum could be misdiagnosed as an ovarian cancer, affecting the interpretation of data. It should be noted that malignant mesothelioma in the peritoneum may be misdiagnosed as an ovarian cancer on death certificates in cases of ovarian cancer diagnosed before 1999, which could affect the interpretation of statistical significance. When the analysis was limited to pathologically confirmed cases of ovarian cancer, the risk of asbestos-related ovarian cancer became less significant [5].
Despite the publication of numerous well-designed studies on the association between asbestos exposure and ovarian cancer since the 2012 IARC Monograph on asbestos, no comprehensive meta-analysis has been conducted till date. Previous meta-analyses confirmed a positive link between asbestos exposure and ovarian cancer mortality but the potential sources of bias and the influence of newer epidemiological data have not been fully explored in the context of this association. In particular, the incorporation of advanced diagnostic technologies in recent studies may contribute to an enhanced diagnostic accuracy for asbestos-related ovarian cancer (i.e., misdiagnosed peritoneal mesothelioma). In addition to, given the latency periods of ovarian cancer and changes in asbestos use in certain industries, it may be necessary to incorporate more recent findings into the analysis.
In the present study, we aim to address these unresolved issues and to provide an updated synthesis of the evidence. A meta-analysis was conducted, integrating studies published both before and after the 2014 Helsinki update on Asbestos, to offer an advanced understanding of the link between asbestos exposure and ovarian cancer.
2. Methods
2.1. Data extraction
A systematic review of the literature available on PubMed, EMBASE, and the Cochrane Library in July 2022 was conducted to identify relevant studies. Mesh terms and queries were selected based on the advice of a librarian from the Catholic University ofKorea School of Medicine, and included key concepts such as “asbestos,” “crocidolite,” “serpentine,” “ovarian cancer,” “ovarian neoplasms,” and “ovary tumor.” Each key concept employed a wide range of synonyms, and the full terms and queries used for the search are provided in Supplements (Tables S1 and S2). In addition, we included cohort studies that were part of 2012 IARC Monograph on asbestos.
2.2. Selection criteria
We followed a two-step approach to identify eligible studies. First, we conducted a systematic search of longitudinal cohort studies published after the 2012 IARC Monograph on asbestos that investigated the association between asbestos exposure and the risk of ovarian cancer. Second, we included studies that reported outcome measures using the standardized mortality ratio (SMR), standardized incidence ratio (SIR), and 95% confidence intervals (CI). Additionally, we included the studies listed in the 2012 IARC Monograph as well as 2014 Helsinki update on asbestos. To eliminate redundancy, duplicate studies were excluded. Our search was restricted to studies published in English.
2.3. Identification of relevant studies
The Fig. 1 below illustrates the PRISMA diagram that shows the selection process of studies. Initially, 453 studies were identified through PubMed, Embase, and Cochrane databases. Studies published before the 2014 Helsinki update and duplicate studies were removed. Then, cohort studies included in the 2014 Helsinki update were added, and studies that did not meet the selection criteria were excluded. Larson TC et al. [11], and Berry et al. [12], were excluded due to their low scores (less than 4 points) in the Newcastle-Ottawa Scale (NOS) quality assessment [13]. Finally, 17 studies that met the selection criteria were included in the meta-analysis. Table 1 provides a summary of all selected literature.
Fig. 1.
PRISMA flow chart for the metaanalysis of asbestos exposure and ovarian cancer.
Table 1.
Summary of characteristics of included studies
| Author | Publication year | Country | Type of asbestos exposure | Period of follow-up | Participants | Number of women exposed | Observed case | Expected case | SMR/SIR (95% CI) | Occupational exposure | Included in 2012 IARC | Quality score∗ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dalsgaard | 2022† | Denmark | Chrysotile | 1968-2015 | All residents of a specific region | 6024 | 34 | 46.94 | SIR = 0.72(0.52 -1.01) | nonoccupation | No | 6 |
| Magnani | 2020 | Italy | not specified; likely mixed |
1970-2010 | Mixed (cement, insulation, shipbuilding, etc.) | 51801 | 43 | 31.1 | 1.38 (1.00-1.87) | occupation | No | 6 |
| Oddone E | 2017 | Italy | not specified; likely mixed |
1950-2014 | Cement | 155 | 4 | 1.1 | 3.64 (0.99-9.33) | occupation | No | 6 |
| Pira E | 2016¶ | Italy | Crocidolite, Chrysotile | 1946-2013 | Textile | 1977 | 15 | — | 3.03 (1.69-4.99) | occupation | No | 7 |
| Yang HY | 2016 | Taiwan | not specified; likely mixed |
1979-2011 | All residents of a specific region | 1216 | 6¶ | 0.68 (0.25-1.49) | occupation | No | 6 | |
| Loomis | 2009 | US | Chrysotile | 1950-2003 | Textile | 1795 | 1.23 (0.56-2.33) | occupation | Yes | 6 | ||
| Wang X | 2013 | China | Chrysotile | 1972-2008 | Textile | 279 | 1 | 0.13 | 7.69 (1.36-43.58) | occupation | No | 6 |
| Reid | 2013† | Australia | Crocidolite | 1993-2009 | All residents of a specific region | 4768 | 6 | — | SIR1: 2.63‡,§ (0.97-5.73). SIR2: 4.14‡,§ (1.51-9.01). |
nonoccupation | No | 6 |
| Ferrante D | 2007† | Italy | Crocidolite, Chrysotile | 1965-2003 | Wives of asbestos cement factory workers | 1780 | 11 | 7.7 | 1.42 (0.71—2.54) | nonoccupation | Yes | 7 |
| McDonald | 2006 | UK | Crocidolite | 1963-2003 | Gas mask assemblers | 1073 | 10 | 5.6 | 1.8 (0.9—3.3) | occupation | Yes | 6 |
| Wilczynska | 2005 | Poland | not specified; likely mixed |
1945-1999 | Asbestos product plan workers; various products | 1382 | 8 | 4.5 | 1.76 (0.76—4.08) | occupation | Yes | 6 |
| Szeszenia | 2002 | Poland | mixed | 1970-1999 | Mixed (asbestosis-mainly asbestos processing plants) | 490 | 1 | 1.27 | 0.79 (0.02—4.39) | occupation | Yes | 6 |
| Germani | 1999 | Italy | mixed | 1980-1997 | Textile, Cement | 631 | 9 | — | 4.77 (2.18—9.06) | occupation | Yes | 7 |
| Tarchi | 1994 | Italy | Chrysotile | 1965-1989 | Mining | 120 | 2 | 0.42 | 4.76 (0.58—17.2) | occupation | Yes | 7 |
| Newhouse | 1989 | UK | Chrysotile | 1941-1984 | Production of friction materials | 4346 | 11 | 10.1 | 1.08 (0.61—1.79) | occupation | Yes | 6 |
| Gardner | 1986 | UK | Chrysotile | 1941-1984 | Cement | 657 | 3 | 2.7 | 1.11 (0.23—3.25) | occupation | Yes | 7 |
| Acheson|| | 1982 | UK | Chrysotile | 1951-1980 | Gas mask assemblers (Blackburn) | 570 | 5 | 3.4 | 1.48 (0.48—3.44) | occupation | Yes | 7 |
| Acheson|| | 1982 | UK | Crocidolite | 1951-1980 | Gas mask assemblers (Leyland, Preston) | 757 | 12 | 4.4 | 2.75 (1.42—4.81) | occupation | Yes | 7 |
CI, confidence intervals; SIR, standardized incidence ratio; SMR, standardized mortality ratio.
Quality score assessed on a scale from 0 to 7, with higher scores indicating better quality.
Studies not included in the meta-analysis are marked with a dagger (†) next to the publication year.
Indicates studies that used Sstandardized incidence ratio (SIR) instead of standardized mortality ratio (SMR).
Two SIRs were reported in Reid's study: SIR1 assumes that all subjects not diagnosed with cancer were cancer-free at the end of the study and SIR2 censors subjects at their last known date to be alive.
The two entries for Acheson come from the same paper but represent separate analyses focusing on gas mask assemblers in two distinct locations: One is Blackburn and the other is Leyland and Preston.
Studies that included a pathological review of ovarian cancer cases are indicated with a section symbol (§).
2.4. Screening process
Two independent researchers (SY Kim and OH Kwon) screened the studies, and another researcher (JY Park) independently reviewed the screened studies. First, irrelevant topics were briefly removed, and then studies were excluded according to predetermined selection criteria. Disagreements between researchers were resolved through discussion. The main information extracted from the studies included the author, publication year, country, fiber types, follow-up period, participants, corrected variables, number of women exposed, observed and expected cases of ovarian cancer, and SMR and SIR with 95% CI.
2.5. Assessment of quality
The Newcastle-Ottawa Scale (NOS) was utilized to assess the quality of the included cohort studies. The NOS score ranges from 0 —9 and comprises three domains: selection of studies, comparability, and exposure [13]. Studies with a total score of less than four were deemed to be of low quality and were not included in the meta-analysis. The detailed quality assessment results of the included studies are presented in Table S3.
2.6. Statistical analyses
The metafor package (https://cran.r-project.org/web/packages/metafor/metafor.pdf) in R software version 4.2.1 (R Foundation for Statistical Computing) was used to analyze each of the SMR and SIR studies separately, and forest plots were used to display the summary effect estimates [14]. Both fixed effect and random effect models were used to estimate the weighted SMRs and 95% CIs. For the fixed effect model, the Mantel-Haenszel method was used, and for the random effects model, the DerSimonian and Laird method was employed [15,16]. Higgins' I2 was applied to estimate the percentage variation and heterogeneity in the study. A criterion of I2 ≥ 50% (∗P < 0.05) and I2 < 50% (∗P > 0.1) was adopted in the random effect and fixed effect models, respectively [16,17]. Funnel plots were used to assess publication bias [18]. Egger's regression analysis was performed to assess the asymmetry of the funnel plot when more than 10 studies were included in the meta-analysis [19].
3. Result
3.1. General characteristics of studies
The present analysis includes 17 studies published between 1982 and 2022, reporting data from 1941 to 2015 and involving more than 73,797 participants from nine countries. The cohort participants belonged to diverse occupations as well as industries of cement, textile, etc. Although most studies reported occupational asbestos exposure, some also reported cases of nonoccupational or family exposure [[20], [21], [22]].
Asbestos is categorized into two families, serpentine and amphibole, with serpentine fibers containing chrysotile, also known as chrysotile asbestos. Amphibole family minerals include amosite (brown asbestos) and crocidolite (blue asbestos), which is considered the most carcinogenic. The primary sources of exposure in most of the literature included in this study were chrysotile, crocidolite, and amosite. Among the studies analyzed, chrysotile was the most commonly reported fiber type, although crocidolite was also frequently reported in mixed form. Exposure to chrysotile was reported in 7 cohorts [20,[23], [24], [25], [26], [27], [28]]: Dalsgaard et al (2022), Loomis et al (2013), Wang X et al (2013), Tarchi et al (1994), Newhouse et al (1989), Gardner et al (1986), and Acheson et al (1982). Exposure to only crocidolite was reported in three cohorts [22,23,29]: Reid et al (2013), McDonald et al (2006), and Acheson et al (1982). Three cohorts reported exposure to both chrysotile and crocidolite [20,21,30,31]: (Pira et al (2016), Ferrante et al (2007)). In addition, two mixed types of exposure were found in (Szeszenia et al (2002) [32], Germani et al (1999) [33]), and the type of asbestos was not specified in four studies [[34], [35], [36], [37]] (Magnani et al (2020), Oddone E et al (2017), Yang HY et al (2016), Wilczynska et al. (2005)).
4. Meta-analysis
4.1. Standardized mortality ratio
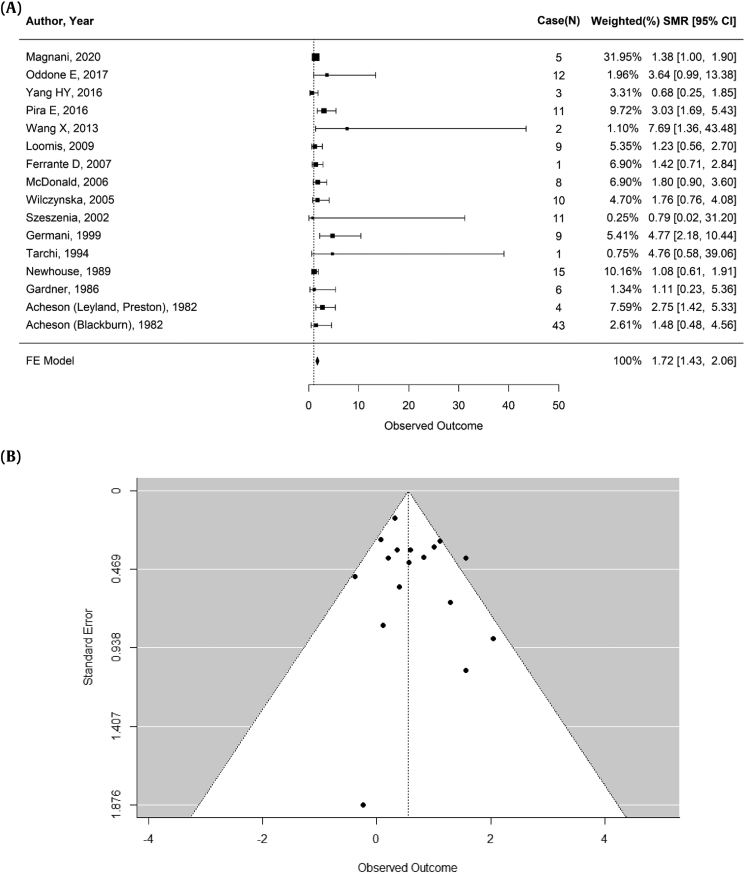
Using a fixed-effects model, an analysis of 15 studies both before and after the 2012 IARC report revealed an overall summary of SMR of 1.72 for ovarian cancer (95% CI: 1.43—2.06; p = 0.0349; 16 records) (Fig. 2A). The heterogeneity test indicated moderate heterogeneity, with a p<0.1 and an I2 of 42.99%. In Egger's test, there was a low confounding effect due to publication bias (p = 0.1830) (Fig. 2B). Excluding Ferrante D et al (2007), which included nonoccupational exposures, a summary SMR was slightly increased (summary SMR 1.74, 95% CI: 1.44—2.10), compared with a summary SMR of previous whole analysis (summary SMR 1.72, 95% CI: 1.43—2.06).
Fig. 2.
Result of pooled analysis and varification for publication analysis. (A) Meta-analysis of standardized mortality ratio for asbestos exposure and ovarian cancer. (B) Begg's funnel plots and Egger's test (p = 0.1830) for identifying publication bias in the meta-analysis of observational studies.
∗Heterogeneity: ( Q = 26.3095, df = 15, p-value = 0.0349, I2 = 42.99%).
FE Model, Fixed Effect Model, N, number of the ovarian cancer cases; SMR, Standardized Mortality Ratio.
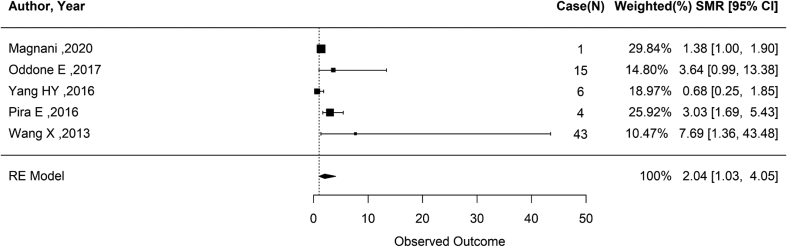
After performing an analysis using a random-effects model on studies published after the 2014 Helsinki update on Asbestos [3], based on the 2012 IARC Monograph, the summary SMR for ovarian cancer in asbestos-exposed cohorts was 2.04 (95% CI: 1.03—4.05; p = 0.0123; 5 records) (Fig. 3). The heterogeneity test showed a p< and an I2 of 72.43%, indicating high heterogeneity. Due to the small number of studies (n = 5) included, Egger's regression analysis was not performed to assess publication bias.
Fig. 3.
Meta-analysis of standardized mortality ratio for asbestos exposure and ovarian cancer (Published after 2012 IARC).
∗Heterogeneity: ( Q = 12.8050, df = 4, p-value = 0.0123, I2 = 72.99%).
N, number of the ovarian cancer cases; RE Model, Random Effect Model; SMR, Standardized Mortality Ratio.
4.2. Nonoccupational cohorts and standardized incidence ratio
Due to the majority of studies investigating the correlation between asbestos exposure and ovarian cancer being occupational cohort studies employing the SMR method, subgroup analysis on nonoccupational exposure cohorts and SIR cohorts was not feasible. However, three studies were discovered that explored the association between nonoccupational asbestos exposure and ovarian cancer [[20], [21], [22]]. Ferrante et al (2007) reported an SMR of 1.42 (95% CI: 0.71—2.54) in wives of asbestos cement factory workers [21]. Reid et al (2013) reported an SIR of 2.63 (95% CI: 0.97—7.13) in an Australian women cohort after exposure to blue asbestos [22]. However, this result excluded lost persons as 20% of the cohort was lost during follow-up. Dalsgaard et al (2022) calculated an SIR of 0.72 (95% CI: 0.52—1.00) in a women cohort attending four schools located in the neighborhood of a large asbestos cement plant in Denmark [20]. Regarding the SIR studies, the data were insufficient for a meta-analysis but showed inconsistent findings for ovarian cancer risk: one indicated a significant increase (SIR = 2.63), while the other did not (SIR = 0.72).
In the present meta-analysis of SMR cohorts, a summary SMR was 2.04 (95% CI: 1.03—4.05) for ovarian cancer among asbestos-exposed cohorts based on studies published after the 2014 Helsinki update. The heterogeneity was high, with an I2 of 72.99%. For studies conducted before and after the 2012 IARC report, the overall SMR was 1.72 (95% CI: 1.43—2.06), with moderate heterogeneity (I2 = 42.99%). Regarding the SIR cohorts, only two studies provided data: one showed a significant increase in ovarian cancer incidence (SIR = 2.63, corrected to 2.50 after adjusting for smoking), while the other showed no significant increase (SIR = 0.72). Due to the limited number of studies on SIR, we were unable to conduct a subgroup analysis for this metric.
5. Discussion
In the present study, a meta-analysis was conducted on asbestos-exposed cohort studies published both before and after the 2012 IARC Monograph. The analysis unveiled a statistically significant increase in ovarian cancer mortality. Furthermore, a subgroup analysis of studies published after the 2012 IARC Monograph showed an approximately two-fold increase in ovarian cancer mortality.
Interestingly, the estimate of effect observed in recent studies appeared higher than those from earlier studies. Wang X et al reported excessive mortality in ovarian cancer, based on one case (SMR = 7.69, 95% CI: 1.36—43.58) in a cohort of Chinese textile workers, but this result is likely biased because they overestimated the expected number of cases [28]. Pira E et al reported an SMR of 3.03 (95% CI: 1.69—4.99) in a cohort of asbestos textile workers in northern Italy, but study limitations suggest that peritoneal cancer may have been misclassified as ovarian cancer [31]. Oddone E et al reported an SMR of 3.64 (95% CI: 0.99—9.33) in a cohort of Italian cement plant workers, although the threshold of statistical significance was not reached [35]. Magnani et al (2020) reported an SMR of 1.38 (95% CI: 1.00—1.87) in a pool of 43 Italian asbestos cohorts (asbestos cement, rolling stock, shipbuilding, glasswork, harbors, insulation, and other industries) [34]. This result supports the conclusion of a previous meta-analysis that estimated a meta-analytical RR of 1.77 over 18 studies [38]. Although not included in this analysis, Luberto et al reported an SMR of 1.50 (95% CI: 0.90—2.34) for 21 asbestos cement workers cohorts in Italy, and there was a significant increase in women in the upper tertile of cumulative exposure [39].
Several potential explanations could account for this trend. Recent studies might have employed more advanced and sensitive diagnostic tools, leading to the more accurate diagnoses of ovarian cancer. Also, prolonged exposure to asbestos in occupational settings, due to historical leniencies in safety regulations, could lead to more pronounced effects in cohorts analyzed in recent studies. The delayed manifestation of the carcinogenic impact of asbestos could be more evident in the more recent cohorts, given the latency period for diseases like cancer.
The mechanism by which asbestos reaches the ovary after inhalation remains not fully understood. It is suggested that inhaled asbestos fibers might undergo phagocytosis by macrophages within the lung tissue and subsequently be transported to the abdominal cavity through the lymphatic vessels or mucous membranes [4], causing inflammation in the ovary. There is evidence to support the presence of asbestos fibers in the ovaries of women with asbestos exposure. In fact, a study found that the number of asbestos fibers detected in the ovaries of asbestos-exposed women was more than twice that in nonexposed women [6,8,40].
There is also a possibility that asbestos fibers can reach the ovary through the transvaginal route, as a recent study reported several histopathologic cases of talc (similar to asbestos fiber) found in the lymph nodes, cervix, uterine corpus, and fallopian tubes [41]. Asbestos fibers can accumulate in ovarian tissue over time and are not expelled, leading to chronic inflammation, genetic and epigenetic alterations that can promote the development of ovarian cancers [10]. In addition, recent studies have provided evidence that inflammation can contribute to the onset of ovarian cancer [42,43].
In refining our analysis by incorporating and excluding specific studies, one distinct variable that became apparent was the differentiation between occupational and nonoccupational exposures. However, when we excluded the only nonoccupational cohort of Italian wives of asbestos cement factory workers, there is just a slight increase of summary SMR. This slight increase may be due to a range of factors, such as the type, duration, and concentration of asbestos exposure. However, the overall direction of the effect estimate did not fundamentally alter our conclusions but rather increased the effect size slightly. On the other hand, exposure level was not analyzed because it was only mentioned in a few of the selected cohorts.
Additionally, due to the majority of studies investigating the correlation between asbestos exposure and ovarian cancer being occupational cohort studies employing the SMR method, a subgroup analysis on nonoccupational exposure cohorts and SIR cohorts was not feasible. However, when three cohorts with nonoccupational asbestos exposure were analyzed, there were conflicting results regarding the increased risk in the exposure group [20,21,44].
In the heterogeneity test, the meta-analysis of the studies conducted following the 2012 IARC Monograph showed high heterogeneity. However, given the limited number of studies included in the analysis, a moderation effect analysis to identify the cause of the high heterogeneity could not be conducted. Based on the literature review, it is hypothesized that differences in the type or size of asbestos fibers used in each cohort could potentially contribute to the observed heterogeneity. Specifically, Yang HY et al reported a low CI in the SMR result, possibly because they focused on a population in the nephrite production area rather than one exposed to the more common crocidolite and amosite [[45], [46], [47], [48]].
As most of the studies included in this research were occupational cohort studies using the SMR method, conducting a subgroup analysis on nonoccupational exposure cohorts and SIR cohorts was challenging. However, Dalsgaard et al demonstrated different outcomes using nonoccupational exposure cohorts and the SIR approach [20].
It is important to note that many of the studies included in this meta-analysis did not adequately account for potential confounding factors or independent risk factors for ovarian cancer. Due to the limited available information, most of these studies relied on retrospective analyses of occupational cohorts, making it difficult to comprehensively address these factors. Independent risk factors for ovarian cancer can include factors such as age at menarche or menopause, late first pregnancy, age at first delivery, and use of oral contraceptives or tubal ligation [5]. While some studies did collect data on these factors, it is generally assumed that these factors are not significantly related to asbestos exposure and therefore did not significantly impact the results of the meta-analysis [5].
As with the 2011 meta-analysis, the investigation of exposure-response relationships was limited due to the small number of ovarian cancer cases in the studies included in this analysis. Furthermore, in the pathological field, there is a possibility that serious ovarian cancer was misdiagnosed as peritoneal malignant mesothelioma in previous studies, as immunohistochemical tests to identify mesothelioma cells were developed only after 1999 [5]. This meta-analysis relies primarily on retrospective cohort studies, which inherently lack contemporaneous pathological findings. It's worth noting that only the study from the Fengtian region focusing on nephrite exposure, provided results for pathologically confirmed ovarian cancer. Given the limited number of studies meeting this criterion, performing a sensitivity analysis for pathologically confirmed cases is not feasible. Despite these limitations, the cohorts included in this meta-analysis have been followed up post-1999, and there are multiple studies indicating an elevated risk of ovarian cancer in asbestos-exposed women [28,31,34,35]. Therefore, while issues related to misdiagnosis before the advent of modern pathology techniques remain a concern, they are somewhat mitigated in the current analysis. Nevertheless, there may still be limitations to the pathological reports in studies analyzed since 2000. Therefore, if possible, the analysis in follow-up cohort studies should focus on studies monitored since 1999 to increase the accuracy of ovarian cancer diagnosis.
The significance of the current study is that it is the first meta-analysis to incorporate both the Helsinki-included studies and subsequent publications. It revealed a significant increase in SMR for ovarian cancer, even in the studies published after the 2012 IARC Monograph. To enhance future research, it is recommended to investigate women diagnosed with ovarian cancer after 1999 using the SIR method, particularly regarding environmental exposure.
Conflicts of interest
All authors declare that there is no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.shaw.2023.11.002.
Contributor Information
Ha Kyun Chang, Email: coolblue23@naver.com.
Jun-Pyo Myong, Email: dr_mjp@naver.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.IARC Overall evaluations of carcinogenicity: an updating of IARC Monographs volumes 1 to 42. IARC Monogr Eval Carcinog Risks Hum Suppl. 1987;7:1–440. [PubMed] [Google Scholar]
- 2.Kwon J. Impact of naturally occurring asbestos on asbestos ban: regulations and experience of the Republic of Korea. Int J Environ Res Public Health. 2022;19(2) doi: 10.3390/ijerph19020742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolff H., Vehmas T., Oksa P., Rantanen J., Vainio H. Asbestos, asbestosis, and cancer, the Helsinki criteria for diagnosis and attribution 2014: recommendations. Scand J Work Environ Health. 2015;41(1):5–15. doi: 10.5271/sjweh.3462. [DOI] [PubMed] [Google Scholar]
- 4.Arsenic, metals, fibres, and dusts. IARC Monogr Eval Carcinog Risks Hum. 2012;100(Pt C):11–465. [PMC free article] [PubMed] [Google Scholar]
- 5.Reid A., de Klerk N., Musk A.W. Does exposure to asbestos cause ovarian cancer? A systematic literature review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1287–1295. doi: 10.1158/1055-9965.EPI-10-1302. [DOI] [PubMed] [Google Scholar]
- 6.Heller D.S., Gordon R.E., Westhoff C., Gerber S. Asbestos exposure and ovarian fiber burden. Am J Ind Med. 1996;29(5):435–439. doi: 10.1002/(SICI)1097-0274(199605)29:5<435::AID-AJIM1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.Howe H.L., Wolfgang P.E., Burnett W.S., Nasca P.C., Youngblood L. Cancer incidence following exposure to drinking water with asbestos leachate. Public Health Rep. 1989;104(3):251. [PMC free article] [PubMed] [Google Scholar]
- 8.Steffen J.E., Tran T., Yimam M., Clancy K.M., Bird T.B., Rigler M., et al. Serous ovarian cancer caused by exposure to asbestos and fibrous talc in cosmetic talc powders-A case series. J Occup Environ Med. 2020;62(2):e65–e77. doi: 10.1097/JOM.0000000000001800. [DOI] [PubMed] [Google Scholar]
- 9.Heller D.S., Gordon R.E., Katz N. Correlation of asbestos fiber burdens in fallopian tubes and ovarian tissue. Am J Obstet Gynecol. 1999;181(2):346–347. doi: 10.1016/s0002-9378(99)70559-4. [DOI] [PubMed] [Google Scholar]
- 10.Ness R.B., Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91(17):1459–1467. doi: 10.1093/jnci/91.17.1459. [DOI] [PubMed] [Google Scholar]
- 11.Larson T.C., Williamson L., Antao V.C. Follow-up of the libby, Montana screening cohort: a 17-year mortality study. J Occup Environ Med. 2020;62(1):e1–e6. doi: 10.1097/JOM.0000000000001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berry G., Newhouse M.L., Wagner J.C. Mortality from all cancers of asbestos factory workers in east London 1933-80. Occup Environ Med. 2000;57(11):782–785. doi: 10.1136/oem.57.11.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells G.A.S.B., O'Connell D., et al. 2021. The newcastle-ottawa scale (NOS) for assessing the quality of non-randomised studies in meta-analyses.http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm [updated September 9, 2022]. Available from: [Google Scholar]
- 14.Wolfgang V. Conducting meta-analyses in R with the metafor package. J Stat Software. 2010;36(3):1–48. [Google Scholar]
- 15.VanderPluym J.H., Halker Singh R.B., Urtecho M., Morrow A.S., Nayfeh T., Torres Roldan V.D., et al. Acute treatments for episodic migraine in adults: a systematic review and meta-analysis. Jama. 2021;325(23):2357–2369. doi: 10.1001/jama.2021.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DerSimonian R., Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trial. 2007;28(2):105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Hoon C.J. Theory and practice of meta-analysis. J Rhinology. 2020;27(2):83–89. [Google Scholar]
- 18.Stuck A.E., Rubenstein L.Z., Wieland D. Bias in meta-analysis detected by a simple, graphical test. Asymmetry detected in funnel plot was probably due to true heterogeneity. Bmj. 1998;316(7129):469. author reply 70-1. [PMC free article] [PubMed] [Google Scholar]
- 19.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalsgaard S.B., Wurtz E.T., Hansen J., Roe O.D., Omland O. A cohort study on cancer incidence among women exposed to environmental asbestos in childhood with a focus on female cancers, including breast cancer. Int J Environ Res Public Health. 2022;19(4) doi: 10.3390/ijerph19042086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrante D., Bertolotti M., Todesco A., Mirabelli D., Terracini B., Magnani C. Cancer mortality and incidence of mesothelioma in a cohort of wives of asbestos workers in Casale Monferrato, Italy. Environ Health Perspect. 2007;115(10):1401–1405. doi: 10.1289/ehp.10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reid A., Franklin P., Olsen N., Sleith J., Samuel L., Aboagye-Sarfo P., et al. All-cause mortality and cancer incidence among adults exposed to blue asbestos during childhood. Am J Ind Med. 2013;56(2):133–145. doi: 10.1002/ajim.22103. [DOI] [PubMed] [Google Scholar]
- 23.Acheson E.D., Gardner M.J., Pippard E.C., Grime L.P. Mortality of two groups of women who manufactured gas masks from chrysotile and crocidolite asbestos: a 40-year follow-up. Br J Ind Med. 1982;39(4):344–348. doi: 10.1136/oem.39.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gardner M.J., Winter P.D., Pannett B., Powell C.A. Follow up study of workers manufacturing chrysotile asbestos cement products. Br J Ind Med. 1986;43(11):726–732. doi: 10.1136/oem.43.11.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loomis D., Dement J.M., Wolf S.H., Richardson D.B. Lung cancer mortality and fibre exposures among North Carolina asbestos textile workers. Occup Environ Med. 2009;66(8):535–542. doi: 10.1136/oem.2008.044362. [DOI] [PubMed] [Google Scholar]
- 26.Newhouse M.L., Sullivan K.R. A mortality study of workers manufacturing friction materials: 1941-86. Br J Ind Med. 1989;46(3):176–179. doi: 10.1136/oem.46.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarchi M., Orsi D., Comba P., De Santis M., Pirastu R., Battista G., et al. Cohort mortality study of rock salt workers in Italy. Am J Ind Med. 1994;25(2):251–256. doi: 10.1002/ajim.4700250211. [DOI] [PubMed] [Google Scholar]
- 28.Wang X., Lin S., Yu I., Qiu H., Lan Y., Yano E. Cause-specific mortality in a Chinese chrysotile textile worker cohort. Cancer Sci. 2013;104(2):245–249. doi: 10.1111/cas.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonald J.C., Harris J.M., Berry G. Sixty years on: the price of assembling military gas masks in 1940. Occup Environ Med. 2006;63(12):852–855. doi: 10.1136/oem.2006.028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magnani C., Ferrante D., Barone-Adesi F., Bertolotti M., Todesco A., Mirabelli D., et al. Cancer risk after cessation of asbestos exposure: a cohort study of Italian asbestos cement workers. Occup Environ Med. 2008;65(3):164–170. doi: 10.1136/oem.2007.032847. [DOI] [PubMed] [Google Scholar]
- 31.Pira E., Romano C., Violante F.S., Farioli A., Spatari G., La Vecchia C., et al. Updated mortality study of a cohort of asbestos textile workers. Cancer Med. 2016;5(9):2623–2628. doi: 10.1002/cam4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szeszenia-Dabrowska N., Urszula W., Szymczak W., Strzelecka A. Mortality study of workers compensated for asbestosis in Poland, 1970-1997. Int J Occup Med Environ Health. 2002;15(3):267–278. [PubMed] [Google Scholar]
- 33.Germani D., Belli S., Bruno C., Grignoli M., Nesti M., Pirastu R., et al. Cohort mortality study of women compensated for asbestosis in Italy. Am J Ind Med. 1999;36(1):129. doi: 10.1002/(sici)1097-0274(199907)36:1<129::aid-ajim18>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 34.Magnani C., Silvestri S., Angelini A., Ranucci A., Azzolina D., Cena T., et al. Italian pool of asbestos workers cohorts: asbestos related mortality by industrial sector and cumulative exposure. Ann Ist Super Sanita. 2020;56(3):292–302. doi: 10.4415/ANN_20_03_07. [DOI] [PubMed] [Google Scholar]
- 35.Oddone E., Ferrante D., Tunesi S., Magnani C. Mortality in asbestos cement workers in Pavia, Italy: a cohort study. Am J Ind Med. 2017;60(10):852–866. doi: 10.1002/ajim.22750. [DOI] [PubMed] [Google Scholar]
- 36.Wilczyńska U., Szymczak W., Szeszenia-Dabrowska N. Mortality from malignant neoplasms among workers of an asbestos processing plant in Poland: results of prolonged observation. Int J Occup Med Environ Health. 2005;18(4):313–326. [PubMed] [Google Scholar]
- 37.Yang H.Y., Huang S.H., Shie R.H., Chen P.C. Cancer mortality in a population exposed to nephrite processing. Occup Environ Med. 2016;73(8):528–536. doi: 10.1136/oemed-2016-103586. [DOI] [PubMed] [Google Scholar]
- 38.Camargo M.C., Stayner L.T., Straif K., Reina M., Al-Alem U., Demers P.A., et al. Occupational exposure to asbestos and ovarian cancer: a meta-analysis. Environ Health Perspect. 2011;119(9):1211–1217. doi: 10.1289/ehp.1003283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luberto F., Ferrante D., Silvestri S., Angelini A., Cuccaro F., Nannavecchia A.M., et al. Cumulative asbestos exposure and mortality from asbestos related diseases in a pooled analysis of 21 asbestos cement cohorts in Italy. Environ Health. 2019;18(1):71. doi: 10.1186/s12940-019-0510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vidican P., Perol O., Fevotte J., Fort E., Treilleux I., Belladame E., et al. Frequency of asbestos exposure and histological subtype of ovarian carcinoma. Int J Environ Res Public Health. 2022;19(9) doi: 10.3390/ijerph19095383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDonald S.A., Fan Y., Welch W.R., Cramer D.W., Godleski J.J. Migration of talc from the perineum to multiple pelvic organ sites. Am J Clin Pathol. 2019;152(5):590–607. doi: 10.1093/ajcp/aqz080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin H.W., Tu Y.Y., Lin S.Y., Su W.J., Lin W.L., Lin W.Z., et al. Risk of ovarian cancer in women with pelvic inflammatory disease: a population-based study. Lancet Oncol. 2011;12(9):900–904. doi: 10.1016/S1470-2045(11)70165-6. [DOI] [PubMed] [Google Scholar]
- 43.Rasmussen C.B., Faber M.T., Jensen A., Høgdall E., Høgdall C., Blaakær J., et al. Pelvic inflammatory disease and risk of invasive ovarian cancer and ovarian borderline tumors. Cancer Causes Control. 2013;24(7):1459–1464. doi: 10.1007/s10552-013-0216-y. [DOI] [PubMed] [Google Scholar]
- 44.Reid A., Segal A., Heyworth J.S., de Klerk N.H., Musk A.W. Gynecologic and breast cancers in women after exposure to blue asbestos at Wittenoom. Cancer Epidemiol Biomarkers Prev. 2009;18(1):140–147. doi: 10.1158/1055-9965.EPI-08-0746. [DOI] [PubMed] [Google Scholar]
- 45.Courtice M.N., Berman D.W., Yano E., Kohyama N., Wang X. Size- and type-specific exposure assessment of an asbestos products factory in China. J Expo Sci Environ Epidemiol. 2016;26(1):63–69. doi: 10.1038/jes.2015.46. [DOI] [PubMed] [Google Scholar]
- 46.Lippmann M. Asbestos exposure indices. Environ Res. 1988;46(1):86–106. doi: 10.1016/s0013-9351(88)80061-6. [DOI] [PubMed] [Google Scholar]
- 47.Pott F., Huth F., Friedrichs K.H. Tumorigenic effect of fibrous dusts in experimental animals. Environ Health Perspect. 1974;9:313–315. doi: 10.1289/ehp.749313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stanton M.F., Layard M., Tegeris A., Miller E., May M., Morgan E., et al. Relation of particle dimension to carcinogenicity in amphibole asbestoses and other fibrous minerals. J Natl Cancer Inst. 1981;67(5):965–975. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.