Graphical abstract
Keywords: Dopamine type 2 receptor, Neuropsychiatric conditions, Positron emission tomography, Bayesian mixed effects modeling
Highlights
-
•
We compared human striatal and thalamic type 2 dopamine receptor (D2R) availability between healthy controls, and subjects with Parkinson’s disease (PD), antipsychotic-naïve schizophrenia, severe violent behavior, pathological gambling, depression, and overweight.
-
•
We present the mean brain maps of group specific D2R availabilities in NeuroVault (https://neurovault.org; https://identifiers.org/neurovault.collection:12799).
-
•
Dopamine type 2 receptor availability is lowered in PD in caudate nucleus, nucleus accumbens and thalamus.
-
•
Subjects with severe violent behavior had decreased correlation between the striatal and thalamic D2R availability.
-
•
Altered regional D2R availability in the striatum and thalamus is linked with motor disorders, while lowered interregional coupling in D2R might relate to violence.
Abstract
Purpose
Aberrant dopaminergic function is linked with motor, psychotic, and affective symptoms, but studies have typically compared a single patient group with healthy controls.
Methods
Here, we investigated the variation in striatal (caudate nucleus, nucleus accumbens, and putamen) and thalamic type 2 dopamine receptor (D2R) availability using [11C]raclopride positron emission tomography (PET) data from a large sample of 437 humans including healthy controls, and subjects with Parkinson’s disease (PD), antipsychotic-naïve schizophrenia, severe violent behavior, pathological gambling, depression, and overweight. We analyzed regional group differences in D2R availability. We also analyzed the interregional correlation in D2R availability within each group.
Results
Subjects with PD showed the clearest decline in D2R availability. Overall, the groups showed high interregional correlation in D2R availability, while this pattern was weaker in violent offenders. Subjects with schizophrenia, pathological gambling, depression, or overweight did not show clear changes in either the regional receptor availability or the interregional correlation.
Conclusion
We conclude that the dopaminergic changes in neuropsychiatric conditions might not only affect the overall receptor availability but also how coupled regions are across people. The region-specific receptor availability more profoundly links to the motor symptoms, while the between-region coupling might be disrupted in violence.
1. Introduction
Neurotransmitter dopamine is centrally involved in motor, motivational and emotional processes (Beaulieu et al., 2015). The four major dopaminergic pathways are i) nigrostriatal pathway running from substantia nigra to the dorsal striatum (i.e. caudate nucleus and putamen), ii) mesolimbic pathway from ventral tegmental area (VTA) to the ventral striatum (i.e. nucleus accumbens), iii) mesocortical pathway from VTA to prefrontal and cingulate cortex, and iv) tuberoinfundibular pathway from hypothalamus to pituitary gland (Harsing, 2008, Purves et al., 2018). The nigrostriatal pathway is critically involved in motor function and Parkinson’s disease (PD) (Meltzer & Stahl, 1976), while the mesolimbic pathway (also known as the reward pathway) contributes to motivation (Merims and Giladi, 2008, Purves et al., 2018). Particularly, the striatal type 2 dopamine receptor (D2R) availability has been linked with a variety of neuropsychiatric symptoms (De Keyser et al., 1988, Leggio et al., 2016, Usiello et al., 2000).
Lowered dopamine transmission in the nigrostriatal pathway is associated with motor symptoms (e.g. rigidity, and bradykinesia) in PD (Leggio et al., 2016), while hyperactivity of the dopamine neurons in the mesolimbic track might promote psychotic symptoms in schizophrenia (Meltzer & Stahl, 1976; but see also Stahl, 2018). Antipsychotic-naïve schizophrenia patients with first-episode psychosis show lowered availability of thalamic D2Rs (Plavén-Sigray et al., 2022), possibly also linked to elevated presynaptic dopamine release, observed at least in the closely located striatum (Howes et al., 2012). However, while increased presynaptic dopamine synthesis and release is characteristic for schizophrenia, only some patients have altered postsynaptic D2R, pointing towards subtypes of schizophrenia (Brugger et al., 2020).
Dopamine also contributes to impulsive behavior, such as aggressive outbursts and drug abuse (Schmidt et al., 2005, Schultz, 1998, Seo et al., 2008, Volkow et al., 2004). Mesolimbic dopamine pathway and upregulated striatal dopamine function is critical for aggressive behavior (Schmidt et al., 2005). Hyperactive striatal dopaminergic function is also consistently linked with acute effects of drug abuse in addicted subjects, who overall show diminished dopamine and D2Rs in the striatum (Volkow et al., 2004). Baseline downregulation of dopamine in addictions might associate with their commonly comorbid affective symptoms (Marshall & Farrell, 2007), such as anhedonia (lack of pleasure) and dysphoria (dissatisfaction), that are coherently connected to diminished mesocorticolimbic dopamine (Bressan & Crippa, 2005). Overall, affective symptoms are involved in numerous neurological and psychiatric conditions, including PD, schizophrenia and depression (Bressan and Crippa, 2005, Knable et al., 1997).
Finally, drugs that enhance striatal dopaminergic neurotransmission effectively treat motor symptoms (Poewe & Mahlknecht, 2020) but can also induce psychotic symptoms, impulsivity, and addictive behavior (e.g. gambling and overeating) (Meltzer and Stahl, 1976, Merims and Giladi, 2008, Witjas et al., 2005). Conversely, neuroleptics, pharmacological treatments that inhibit the striatal dopaminergic function by blocking the D2Rs, reduce psychotic symptoms (Bressan and Crippa, 2005, Kapur and Mamo, 2003) and aggressive behavior (Schmidt et al., 2005), but induce Parkinsonian-like side effects, such as rigidity and bradykinesia (Harsing, 2008, Jackson and Westlind-Danielsson, 1994, Shiraiwa et al., 2018, Stahl, 2018), and anhedonia (Bressan & Crippa, 2005). Particularly the antipsychotics that affect the nigrostriatal track in addition to the mesolimbic track might be the ones with motor side-effects (Jackson & Westlind-Danielsson, 1994), further supporting the centrality of the nigrostriatal track in motor functions.
Taken together, existing data suggest that elevated striatal dopamine activity contributes to psychotic symptoms, impulsivity, aggressive outbursts, and acute effects of reward (namely intense hedonia / pleasure), while lowered dopamine activity relates to disturbed motor functions, and blunted affect (e.g., anhedonia, diminished reward experiences). Because several neuropsychiatric conditions share symptoms (Knable et al., 1997, Seo et al., 2008), perturbations in the dopamine system might link to specific neuropsychiatric symptoms regardless of the diagnosis. To resolve the links between the pathologies with shared dopamine-regulated symptoms of voluntary movement, motivation, and affect, it is crucial to study them together. Only this allows unravelling whether differential patterns of dopaminergic dysfunctions underlie different neuropsychiatric conditions. Here, by neuropsychiatric we refer to a combined set of pathologies with an organic (neurological) and/or psychological basis.
We investigated the differences in the striatal (caudate nucleus, nucleus accumbens, and putamen), and thalamic D2R availability in a large (n = 437) sample of subjects using positron emission tomography (PET) data with radioligand [11C]raclopride acquired in resting baseline state. The dopaminergic function includes tonic and phasic dopamine firing (Grace, 2016). Because our data pertain to baseline, resting state scans, these data reflect predominantly tonic rather than phasic dopaminergic function. Our sample included healthy controls, and subjects with PD, antipsychotic-naïve schizophrenia, severe violent behavior (violence), pathological gambling (gambling), depression, and overweight. We also analyzed between-region correlation of the D2R availability in each group and assessed the effects of age and sex on D2R availability in i) the healthy subjects and ii) the other six groups (i.e. groups of interest) together.
2. Material and methods
2.1. Data
Basic subject information is shown in Table 1. This retrospective register-based study includes 437 subjects (294 males, 143 females), including healthy controls (n = 239), and subjects with PD (n = 60), schizophrenia (n = 7), severe violent behavior, i.e. prison sentence for violence (n = 10), pathological gambling (n = 12), depression (n = 12), and obesity/overweight (n = 97) who had undergone [11C]raclopride PET scan. The age range of the subjects was 19–82 years. The data were retrieved from the Turku PET Centre Aivo database (https://aivo.utu.fi), including data of imaging studies conducted at the site. See supplementary material for Original publications whose data are used in the current study. The images were acquired between the years 1989 and 2019 with six different PET scanners (GE Advance, HR+, HRRT, GE Discovery VCT PET/CT, GE Discovery 690 PET/CT, Ecat 931). The scanners are described in detail in our previous work (Malén et al., 2022), and the distribution of the different PET scanners across the subject groups are given in supplementary Table S1. The framing is given in the supplementary Table S2.
Table 1.
Age of the sample groups separately for males and females in the final sample (n = 437).
| Males (n = 294) |
Females (n = 143) |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Range | N | Mean | SD | Range | |
| Healthy controls | 174 | 28 | 11 | 19–78 | 65 | 41 | 16 | 19–82 |
| Parkinson’s disease | 31 | 62 | 12 | 34–82 | 29 | 60 | 11 | 41–81 |
| Schizophrenia | 3 | 22 | 3 | 19–25 | 4 | 36 | 13 | 24–53 |
| Violence | 10 | 31 | 7 | 24–46 | 0 | – | – | – |
| Pathological gambling | 12 | 32 | 8 | 23–49 | 0 | – | – | – |
| Depression | 3 | 38 | 10 | 25–48 | 9 | 41 | 5 | 34–50 |
| Overweight | 61 | 29 | 9 | 19–56 | 36 | 43 | 13 | 23–74 |
We retrieved the following subject information at the time of each PET scan: subject age, sex, body mass index (BMI, available for 67 % of the subjects) and group, as well as the injected dose and the PET scanner used to acquire the imaging data. Although we do not have detailed medication information for all the subjects, the exclusion criteria for the original research projects where that the subjects were drawn from contained medication affecting the central nervous system. The schizophrenia group was antipsychotic-naïve, and the depression group drug-free (mainly drug-naïve subjects with moderate depressive episode). The PD sample contained both unmedicated and medicated patients and given the mean disease duration of 10 years (median = 9 years, range from 0 to 29 years, see supplementary Figure S1 under D2R availability through PD duration), most of the patients were probably medicated at the time of imaging. The scans were, however, performed after a short washout period (subjects were withdrawn from medication at least from the night before the scan).
Patient categorization was based on their enrollment criteria in the original studies, except some healthy control subjects were classified to the overweight group if their BMI was higher than 25 (Engin, 2017). The BMI mean (standard deviation) of the remaining subjects were: PD 26.8 (5.1), schizophrenia 26.9 (3.0), severe violent behavior 27.2 (2.3), pathological gambling 27.8 (3.4), and depression 26.5 (5.2) (supplementary Table S3). Thus, some subjects in these groups are overweight, although their primary diagnosis was not obesity. However, none of the healthy controls were obese. These information regard 67 % of the total sample for who we had BMI information available (supplementary Table S3). None in the obese group are expected to have psychiatric or neurological disorders, as these disorders were a general exclusion criterion in the brain studies at the site (except in projects specifically studying those conditions). More detailed symptoms, alcohol use, or smoking status were not used in the analysis, as this information was not systematically available for all the subjects.
The potential sample of 455 subjects was cut down to 437 after excluding 18 subjects due to the following reasons. Three subjects with low injected dose < 90 megabecquerel (MBq) and seven without dose information were excluded from the sample to avoid low signal to noise ratio. The excluded subjects were from healthy control, PD, schizophrenia, and overweight groups. Due to lost or noisy data from interrupted PET scanning, we discarded eight subjects (including subjects from PD, schizophrenia, and severe violent behavior groups) whose kinetic model fits were inadequate.
2.2. PET data preprocessing and kinetic modelling
PET image preprocessing and kinetic modelling were carried out in MATLAB (The Mathworks Inc., 2021) using an in-house brain image processing pipeline Magia (Karjalainen et al., 2020) (https://github.com/tkkarjal/magia). We spatially normalized the dynamic PET images into MNI152 space with an in-house [11C]raclopride PET-template created using a subset (n = 187) of healthy control subjects, because only a subset of the sample had magnetic resonance (MR) image available (n = 249) (Karjalainen et al., 2020) (see Validation of PET atlas based spatial normalization of the [11C]raclopride binding estimates and Figures S2-S3 in supplementary material). Four bilateral regions of interest (ROI) from Harvard-Oxford atlas, caudate nucleus (caudate), nucleus accumbens (accumbens), putamen, and thalamus, were applied to the normalized PET-images. [11C]raclopride is a commonly used tracer to investigate striatal and thalamic D2R (Alakurtti et al., 2015, Elsinga et al., 2006), as its binding to D2Rs in these regions is reliable, while negligible elsewhere (Hirvonen et al., 2003, Svensson et al., 2019).
Regional specific binding of [11C]raclopride was quantified as nondisplaceable binding potential (BPND) using simplified reference tissue model (SRTM) and Harvard-Oxford atlas cerebellum as a reference region (Lammertsma & Hume, 1996). Tails beyond the cerebellum distribution's full width at half maximum were removed to ensure that the reference region excludes voxels with unusually high or low radioactivity (Karjalainen et al., 2020). BPND is a measure of receptor density and radioligand affinity and refers to the ratio at equilibrium of specifically bound radioligand to that of nondisplaceable radioligand in tissue (Innis et al., 2007). In the analyses, we used the mean of left and right hemisphere BPND as the regional estimate for each subject. Data on hemispheric symmetry in BPND is presented in supplementary Tables S4-S5 and Figures S4-S5.
Parametric BPND images were also calculated for illustrative purposes with basis function implementation of SRTM (bfSRTM) with 300 basis functions. Lower and upper bounds for theta parameter were set to 0.082 1/min and 0.6 1/min. Before the parametric image calculation, the dynamic PET images were smoothed using Gaussian kernel with 4 mm full width at half maximum to reduce the effect of noise in the voxel-level bfSRTM fit.
2.3. Statistical analysis
Statistical analysis was carried out in RStudio (Posit team, 2023) using R (R Core Team, 2023). We assessed the normality of the data with Shapiro-Wilk test with package stats (R Core Team, 2023). We analyzed the regional data using Bayesian modeling tools of brms (Bürkner, 2017, Bürkner, 2018, Bürkner, 2021) utilizing rstan (Stan Development Team, 2020). We analyzed the interregional BPND correlation in each subject group using correlation matrix with corrplot (Wei, 2021), and Mantel test (Mantel, 1967) for similarity of two matrices using ape (Paradis & Schliep, 2019). We used R (R Core Team, 2023) (particularly R packages ggplot2 (Wickham, 2016), ggsci (Xiao, 2023), bayesplot (Gabry, 2022), superheat (Barter, 2017), corrplot (Wei, 2021), and GGally (Schloerke, 2021)), MATLAB (The MathWorks Inc., 2021), and MRIcroGL (https://www.nitrc.org/projects/mricrogl) to visualize the data and findings.
2.3.1. Descriptives
We created group-specific maps of the BPND i) means and ii) coefficients of variation, to describe the homogeneity of D2R distribution between and within the groups, respectively. We calculated the statistics using MATLAB (The Mathworks Inc., 2021) and illustrated them as maps using MRIcroGL (https://www.nitrc.org/projects/mricrogl).
2.3.2. Regional group differences in D2R availability
We used linear mixed effects regression to compare regional D2R availability across the seven subject groups. In the analysis, we adjusted for the scanner used in the image acquisition, and the subject age and sex that affected striatal D2R availability in healthy controls (Malén et al., 2022).
The regional log-transformed (log) binding potentials (BPND) were modeled as a dependent variable. We analyzed the group differences in log-BPND in caudate, accumbens, putamen, and thalamus, while adjusting for age, sex, and scanner. Specifically, we applied group, standardized age, and sex as fixed effects and allowed these effects to vary between ROIs (regionally varying a.k.a. random slopes). We applied random intercepts for subjects, scanners, ROIs, and scanner-ROI combinations. This means that we allowed the log-BPND intercept to vary by subject, region, scanner, and scanner within each region. For the residual variances, we similarly applied random intercepts for scanners, ROIs, and the scanner-ROI combinations, but not for subjects as we did not expect the residual variances to depend on subject. We validated the model by comparing it with three other candidate models (see Model diagnostics and comparison in supplementary material).
The age effect was calculated for the whole sample together (and not separately for each subject group by interaction of age and group), because we wanted to maximize the age range and the sample size in the estimation of the age effect, while some of the studied groups had relatively limited age range (e.g. schizophrenia 19–25 years). Group-specific age effects were also not hypothesized as e.g. prior studies suggest that neural changes in PD do not reflect ‘accelerated aging’ (Kaasinen et al., 2015). Similarly, the sex effect was calculated for the whole sample because some of the studied groups consisted solely of males. Nevertheless, of all the studied groups, the healthy controls hold the greatest number of observations altogether and separately for males and females, as well as the widest age range. Thus, the age and sex effects observed in the healthy control group were expected to have a strong representation in the regression analysis due to large proportion of healthy controls in the total sample.
Shapiro Wilk testing (R Core Team, 2023) did not clearly support the normality of region-specific BPND in the original nor the logarithmic scale. We decided to use the log-transformed instead of the original-scale BPND as a dependent variable in the regression model, because log-transformed dependent variable allows us to directly estimate the relative (e.g. 10 %) rather than absolute (e.g. 1 unit) change in BPND between predictor levels, particularly the group specific intercepts. Relative change is more intuitive, while BPND is a ratio that describes receptor availability, and is not a direct measure of absolute receptor density (Innis et al., 2007).
We used linear regression, because based on our previous assessment (Malén et al., 2022), the relationship between age and logarithmic BPND is well approximated by a linear function in a healthy sample, that is the majority of the current sample, and as stated previously, we are not aware of group-specific age-effects. Further, modeling higher-degree associations increases the risk of overfitting, potentially resulting in poor generalization of the results (McElreath, 2020). For the remaining fixed predictors (group and sex), the estimated parameters are intercepts and not slopes.
In the absence of appropriate priors for Bayesian statistical inference from independent data, we gave a normal distribution prior (with expected value = 0 and variance = 1) for the fixed effects (age, sex, group). Such zero-centered normal prior assigns symmetrical probabilities for both positive and negative regression coefficients and does not restrict the parameter to certain values (i.e., no regression coefficient is given zero probability), while it still regularizes the model’s fit. For the standard deviations of the random effects, we set the same normal prior. As standard deviation is always positive, brms-package automatically restricts this prior to positive values, making it a half-normal distribution (Bürkner, 2017, Bürkner, 2018, Bürkner, 2021). For the rest of the modeling parameters, we used weakly informative default priors of brms (Bürkner, 2017). The model was carried out using Markov Chain Monte Carlo sampling with each four chains running 4000 iterations (including 1000 warm-up samples).
2.3.3. Validation of the age and sex effects on regional D2R availability
For validation purposes, we assessed the age and sex effects in the current sample. We tested the replicability of the age and sex effects (Malén et al., 2022) in the i) healthy control sample (almost identical sample to our earlier work (Malén et al., 2022)) and ii) groups of interest together (subjects with PD, schizophrenia, severe violent behavior, pathological gambling, depression, and overweight). These analyses are presented in Validation of the age and sex effects on regional D2R availability in supplementary material).
2.3.4. Between-region correlation of the D2R availability
We used Spearman correlation to assess how similar the BPND rank-order across subjects was between the regions of interest. This was done separately in each subject group to assess if the regional correlation patterns were consistent across groups. We computed a 4 × 4 between-region BPND correlation matrix for each subject group. These matrices were computed with the original binding estimates, because taking the logarithm of the data (group and region specific BPND) did not consistently transform it closer to normal distribution (Shapiro-Wilk), and the rank-order based Spearman correlation readily accounts for monotonic associations in the data and is unaffected by log transformations. Using Spearman and not Pearson correlation is also supported by the limited sample sizes in some of the studied groups. One-tailed testing was used as regional coupling estimates for D2R are positive rather than negative (Malén et al., 2022).
We tested for the similarity of each correlation matrix pair (e.g. correlation matrix of healthy controls compared to correlation matrix of PD patients) using one-tailed Mantel tests with default number (9 9 9) of permutations (Paradis & Schliep, 2019). These analyses allowed us to assess, whether interregional D2R coupling is altered in neuropsychopathology, and whether different neuropsychiatric conditions differ in their striatal and thalamic correlation patterns.
3. Results
3.1. Descriptives
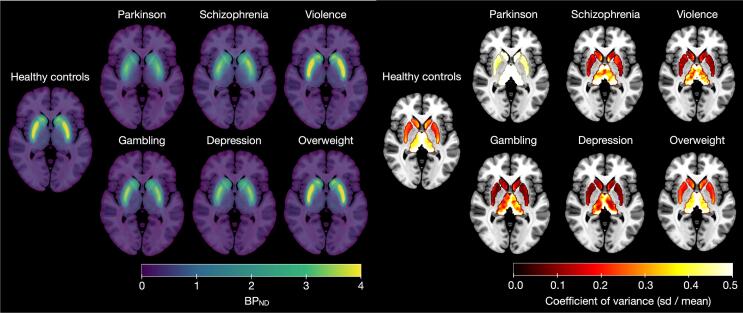
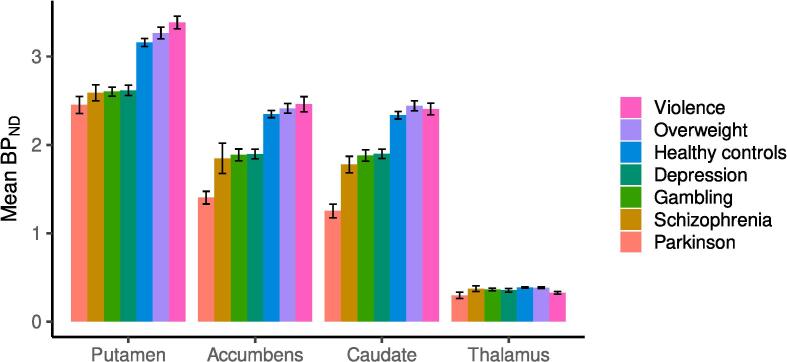
Group-specific maps for mean and coefficient of variation for D2R are shown in Fig. 1. Based on the Fig. 1, the groups show broadly similar receptor distribution in the subject groups, and the binding level shows greatest variation in the PD group. Across groups, the coefficient of variation appears greatest in thalamus. The mean D2R maps are also available in NeuroVault (https://identifiers.org/neurovault.collection:12799). Mean regional non-transformed BPND estimates for each group are shown in Fig. 2. D2R availability decreases through PD duration, please see D2R availability through PD duration and Figure S1 in the supplementary material.
Fig. 1.
Maps of the original-scale [11C]raclopride BPND estimates in the subject groups. Left: Mean BPND. Right: Coefficients of variation (standard deviation / mean) for BPND within the regions of interest (Harvard-Oxford atlas masks). The maps are presented on an MNI-template.
Fig. 2.
Barplot of the group-specific mean original-scale BPND in each ROI. The black intervals show the group-specific standard errors (sd(BPND)/sqrt(n), where sd = standard deviation, sqrt = square root, n = number of observations).
3.2. Regional group differences in D2R availability
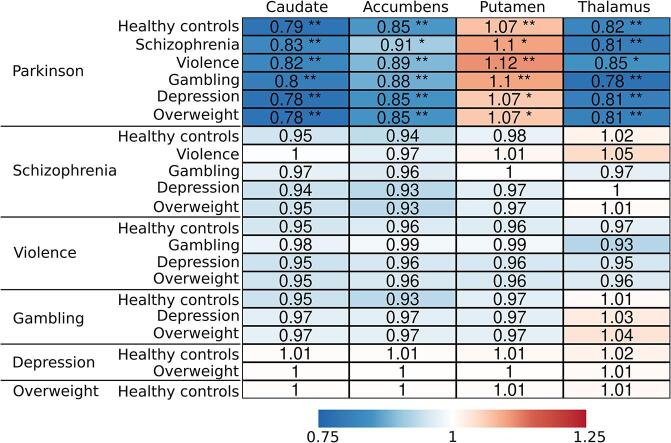
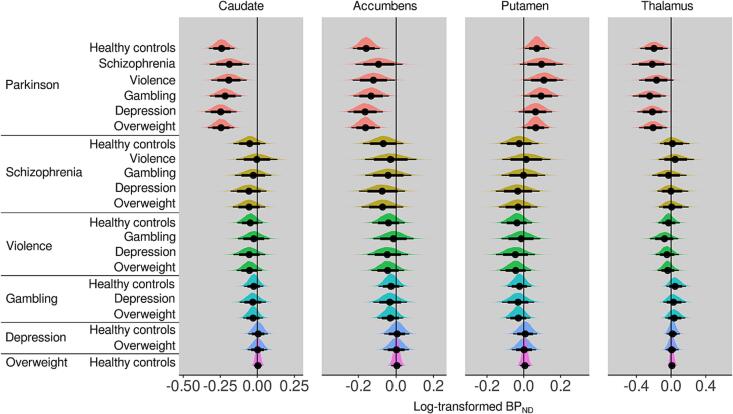
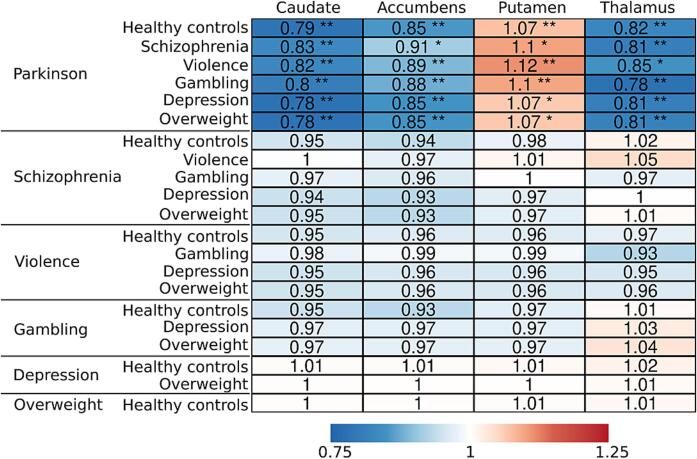
Bayesian linear mixed effects modeling (Fig. 3), adjusting for age, sex, and scanner, revealed that compared to the other groups, PD patients had approximately 10–20 % lower [11C]raclopride BPND (values transformed back to original scale) in caudate, accumbens and thalamus, while the BPND in putamen was approximately 10 % higher (Fig. 4). The other groups of interest (schizophrenia, severe violent behavior, pathological gambling, depression, and overweight) did not show clear differences in regional D2R availability when compared with healthy controls or each other. However, subjects with schizophrenia and severe violent behavior showed more support for lowered than elevated D2R availability (more probability mass below zero), although the 80 % posterior intervals (i.e. probability mass) crossed zero. Pathological gambling, depression and overweight groups showed the least support for altered D2R availability with practically non-existent difference compared to healthy controls.
Fig. 3.
Posterior probability distributions (posteriors), as well as their median (point), 80% (thick line) and 95% (thin line) posterior intervals, describing the between-group differences in log-transformed BPND. Posterior located on the negative side of the zero line suggests lower log-BPND in the reference group on the left compared with the group on the right.
Fig. 4.
Summary of the regional group comparisons. The values represent the (median) proportional BPND (values transformed back to original scale) of the reference group (on the left) compared with the group on the right. Asterisks represent comparisons were 80% (*) and 95% (**) posterior interval did not cross zero.
3.3. Validation of the age and sex effects on regional D2R availability
Similarly as in our previous analysis with healthy subjects (Malén et al., 2022), D2R availability decreased through age, both in the healthy control group that is almost identical to the sample in our previous work (Malén et al., 2022) and in the groups of interest. The lower striatal D2R availability in males versus females was only found in healthy controls. However, in thalamus, females had higher thalamic availability in both the healthy controls and the groups of interest. The results are illustrated in Figure S6. For comparison, Figure S6 also shows the age and sex effects of the main model and confirms that these effects are roughly similar between the models.
3.4. Between-region correlation of the D2R availability
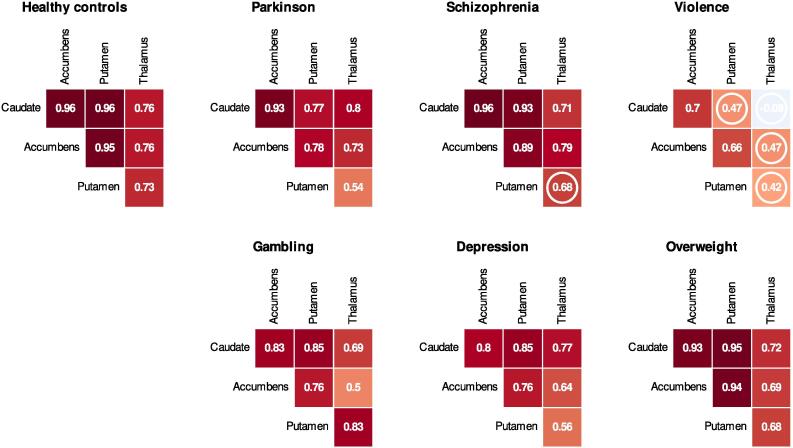
The interregional correlations of original-scale BPND are shown in Fig. 5. Additional between-region scatter, and density plots are given in supplementary Figure S7. Despite the regional BPND changes, PD patients showed high between-region BPND correlation, comparably with healthy controls. Across all groups, the weakest correlations were observed between thalamus and other regions. Subjects with severe violent behavior showed the weakest correlation between the regions.
Fig. 5.
Group-wise interregional original-scale BPND correlations (one-tailed Spearman, p-values > 0.05 marked with a circle, while the higher the p-value, the less support for non-zero correlation).
When we compared the correlation matrixes of the groups (Fig. 5) with Mantel tests, we observed similarity in the correlation patterns of the groups. Healthy controls showed some support for similarity with PD (p = 0.04), schizophrenia (p = 0.055), depression (p = 0.037), and overweight (p = 0.04). Further, similarity was suggested between PD and schizophrenia (p = 0.07), PD and depression (p = 0.038), PD and overweight (p = 0.089), and depression and overweight (p = 0.034). The remaining group comparisons, mainly including subjects with severe violent behavior and pathological gambling, did not support similarity, while the p-value was greater than 0.1 in the similarity tests.
Altogether, the groups showed strong dopaminergic correlation patterns, particularly between the striatal regions. The weakest correlations were most notably manifested with thalamus, where the estimates might be less reliable than in the striatum (Hirvonen et al., 2003). The thalamic decoupling was emphasized in the subjects with severe violent behavior, who had lowest correlation pattern also within the striatum.
4. Discussion
Striatal dopamine neurotransmission has a central role in motor functions that are disturbed particularly in PD (Kaasinen and Vahlberg, 2017, Kaasinen et al., 2021), and motivational processes that are impaired in many neuropsychiatric disorders (Bressan and Crippa, 2005, Knable et al., 1997, Schmidt et al., 2005, Schultz, 1998, Volkow et al., 2004). Our findings show that in PD, striatal (caudate and accumbens) and thalamic D2R availability is downregulated, while interregional correlation might be lowered in severe violent behavior. These results suggest that in the striatothalamic dopamine circuit, overall changes in receptor levels and specific regional decoupling patterns associate to different neuropsychiatric conditions.
4.1. Regional D2R changes in Parkinson’s disease
Striatal (caudate and accumbens) and thalamic downregulation in D2R availability was most salient in PD patients. There was limited evidence for striatal downregulation also in schizophrenia and severe violent behavior, but even the 80 % posterior intervals for these groups overlapped with zero. Our findings support a previous meta-analysis describing that the effect of the striatal D2R in schizophrenia is negligible (and possibly linked to the antipsychotic treatment) compared to the presynaptic dopamine synthesis and release (Howes et al., 2012). The other groups did not show clear alterations in striatum nor thalamus.
In the regression modeling, we used normal prior with zero mean and variance of one for the regression coefficients (beta) of the fixed effects (including group). With variance of one, the prior distribution was wide. The prior placed 50 % probability mass for beta from −0.67 to 0.67 that roughly corresponds to a range from 50 % reduction to 100 % increase in BPND in the comparison group compared to the reference group. In this sense, the prior is weakly informative and allows a wide range of regression coefficients for groups. The prior, however, places the highest probability to zero difference between groups, which could override the data from small groups and overlook some of the group differences. We, thus, believe that in our analysis of the regional group differences including small groups, there was higher probability of finding false similarity than false difference between the groups.
Compared with the other groups, PD patients exhibited both lowered (accumbens, caudate, thalamus) and elevated (putamen) striatal D2R availability when adjusting for age and sex. PD subjects had lowered D2R availability in nuclei caudate and accumbens, and thalamus. In putamen, PD patients had high D2R availability, although this effect is not discernible in the raw data where age and sex are not adjusted for.
Although age and group were both accounted for in the regression modeling, downregulation of D2R in PD might have been partly misinterpreted as an age effect, as PD patients are on average older than the other subjects (Table 1). This possible overcorrection of the age effect might have contributed to the sign switch of the observed alteration of D2R in the PD group (positive in the putamen, negative in all other ROIs). However, the age and sex effects were roughly similar in the main model (with all groups included) as in the model of only healthy controls (Figure S6). These findings suggest that the low D2R availability of PD patients did not accelerate the negative age effect in the main regression model for the group comparison. Finally, there are a few putamen observations of PD patients that exceed the ones of healthy controls (Figures S4-S5), that might have also lifted the PD intercept in putamen.
This finding requires additional validation because in PD, the D2R changes in putamen are dynamic and dependent on the disease stage. Compensatory D2R upregulation in putamen changes to downregulation around four years from PD onset (Kaasinen et al., 2021, Rinne et al., 1990). The PD duration greatly varied between the studies subjects (range: 0–29 years) and our findings cannot directly discern the effects in early and late stages, as the disease duration information was available for only half of the patients. Thus, we are unable to specify the weight of early and late stage patients in our data and analysis. For the subjects with available information, the correlation between disease duration and BPND was subtle but nevertheless negative in all tested regions (-0.17 in putamen) suggesting no prolonged upregulation with progressed PD. Consequently, our findings do not provide clear evidence against late stage D2R downregulation in putamen. Overall, the findings nonetheless highlight the region and motor symptom specificity of within-region D2R changes.
4.2. Lowered between-region correlation in severe violent behavior
Dopaminergic changes in severe violent behavior were more prominent in the regional coupling than in the overall regional receptor availability. Although in all groups we observed that thalamus showed the lowest regional correlation, this decoupling was most salient in the subjects with severe violent behavior, who also had lowest regional coupling within the striatum. Aberrant coupling in this striatal dopamine network might reflect altered communication and weakened links between the regional nodes. Alternatively, the weakened correlation of D2R availability between different regions might also be explained by selective alteration of one specific dopaminergic region, while other regions are unaffected (without interaction between regions). However, this hypothesis was not notably supported by the regional analysis where the binding in subjects with severe violent behavior did not clearly differ from healthy controls in any of the regions.
We found some support for the similarity in the regional coupling patterns (correlation matrices) between the groups, except for group comparisons including severe violent behavior and pathological gambling. However, as the matrices we compared only had six individual data points (correlation estimates of 1) caudate-accumbens, 2) caudate-putamen, 3) caudate-thalamus, 4) accumbens-putamen, 5) accumbens-thalamus, 6) putamen-thalamus), the tests might be underpowered to show significant similarity between the matrices even if similarity exists, particularly if the reliability of the six input correlations (in the matrices) are calculated from a small sample size.
Altogether, the sample size for violent offenders was small which might limit the generalizability of our findings, although some of the other groups showing stronger correlation pattern were also small. Our findings nevertheless suggest different dopaminergic correlation profiles between motor symptoms and violent aggression. The D2R (de)coupling of the regions in violence and affective disorders such as schizophrenia, pathological gambling, and depression (for which we had limited sample sizes), should be further examined in larger studies. Studies investigating the D2R correlates of specific behavioral symptoms would also complement our findings regarding D2R variation in relation to more specific clinical characterizations. Finally, we encourage future studies to address D2R alterations also in the cortex using other radioligands such as [11C]FLB457.
4.3. Unaltered baseline D2R in overweight
We observed neither regional D2R alterations nor aberrant regional coupling in schizophrenia, pathological gambling, depression, or overweight groups. While some of the effects in the first three groups might have result from limited sample sizes, overweight group included almost a hundred subjects making these findings more robust. Our results, thus, underline that dopaminergic mechanisms do not notably contribute to general weight gain (without any subgrouping).
Several recent studies have addressed the conflicting findings regarding the link between overweight and D2R (Dagher, 2012, Darcey et al., 2023, Horstmann et al., 2015). They suggest that BMI and striatal D2R availability may not be strictly linear. Mild obesity might link with low tonic and high phasic dopamine, while severe obesity would show the opposite pattern (high tonic, low phasic dopamine) (Horstmann et al., 2015). In our previous study we assessed both linear and non-linear associations between BMI (range 18–38) and D2R availability finding only subtle support for positive linear association between the measures (Malén et al., 2022). Here we instead compared the baseline intercepts of the studied groups. Our findings accords with previous empirical studies (Karlsson et al., 2015, Malén et al., 2022) as well as meta-analyses (Pak and Nummenmaa, 2023) finding no clear connections between altered D2R availability and obesity. Although overweight subjects did not differ from other studied groups in their baseline D2R availability, this does not exclude the existence of a more complex or nuanced links between weight and dopaminergic function.
Finally, our results highlight that motor symptoms, rather than affective disorders or weight gain, are most consistently linked with regional alterations in D2R availability. According to our data, PD is associated with changes in regional D2R availability, while severe violent behavior might link to disturbed correlation between the regional availabilities. Our results regarding unaltered D2R in overweight suggests that the postsynaptic type 2 dopamine receptor in the striatum and thalamus is not a central contributor of general weight gain, and possibly neither to schizophrenia, depression nor pathological gambling, although more data are needed to clarify the issue. Overall, this large multi-group dataset suggests that the dopaminergic changes observed in neuropsychiatric pathology are multifaceted, involving region-specific and between-region mechanisms of the D2R. We conclude that the changes in baseline D2R might be more profoundly linked with motor symptoms and violent aggression than with affective disorders or weight gain.
4.4. Effects of age and sex on D2R availability
We found that baseline striatal D2R availability is lowered through age both in healthy controls and in the groups of interest. Previously, we observed sex differences (female > male) in healthy controls consistently in the striatothalamic regions (Malén et al., 2022), whereas here in the groups of interest, sex differences were only found in the thalamus. However, this effect pertains to the joint analysis of the groups of interest together. Because all the groups did not include both males and females, we pooled the groups together for the analysis of age and sex, which might have overlooked group specific sex differences. Nevertheless, our data indicate that the effect of age on D2R is more consistent than the effect of sex, and generally found also in clinical populations. Accordingly, adjusting for age and sex is necessary when assessing the effects of other variables on striatothalamic D2R availability.
5. Limitations
Our data were not fully balanced. Although the dataset was large, the group size, age, sex, and scanner distribution were compromised in some of the studied groups. Combined with our statistical analysis of the regional group differences that adjusted for the age, sex, and scanner related variation in the data, some group differences may have been undetected (McElreath, 2020). In the between-region analysis, our findings mainly indicate that in the healthy control, PD, and overweight groups (where we had largest number of subjects) the between-region binding correlation is strong. Larger samples are required to validate whether the striatothalamic correlation pattern is similarly strong or decoupled in the other groups with limited sample sizes. Subjects with severe violent behavior, pathological gambling and depression were imaged with a single scanner (severe violent behavior group with GE Discovery VCT PET/ CT, pathological gambling, and depression groups with Ge Advance) (Table S1). However, the data from GE Discovery VCT PET/ CT and Ge Advance included 9 and 69 healthy control images, respectively. Additional non-healthy subjects were also imaged with these two scanners. This means that none of the scanners represented exclusively one subject group, theoretically making it possible for the model to differentiate the effects of scanner and group. In a single PET scan, it is not possible to demonstrate the exact molecule-level mechanism for altered receptor availability. Not all subjects were drug naïve. Due to the retrospective nature of the data derived from multiple projects, the collection of clinical characteristics of patient populations were not standardized. Patient categorization was based on their enrollment criteria in the original studies. Finally, it must be noted that unaltered baseline D2R level does not mean the dopaminergic responses in different environmental contexts would be similar across all studied groups. It is possible that differential patterns of dopamine firing in motivationally triggering environments exist for example, towards gambling in pathological gamblers and towards feeding in obese individuals (Grace, 2016). Investigating such effects however requires standardized challenge or activation paradigms, which could not be incorporated in the current analysis.
6. Conclusions
Dopaminergic function is altered across multiple psychiatric and neurological conditions. The regional receptor availability patterns, as well as interregional coupling of D2R levels, might distinguish between the specific conditions. PD was associated with region-specific changes in D2R availability. The role of D2R in violent behavior might rather relate to the interregional connections, as in this subject group, the between-region receptor availabilities were less correlated. In pathological gambling and overweight groups, no clear changes were observed in the striatothalamic D2R level nor the correlation structure. Our results suggest that in striatum and thalamus, motor symptoms are more profoundly linked to the region-specific D2R availability, while violence might be more associated with lowered correlation between the regions.
Ethics approval
Finnish legislation does not require ethical approval for register-based studies.
CRediT authorship contribution statement
Tuulia Malén: Writing – review & editing, Writing – original draft, Visualization, Validation, Project administration, Methodology, Formal analysis, Data curation, Conceptualization. Severi Santavirta: Writing – review & editing, Visualization, Validation, Methodology, Conceptualization. Sven De Maeyer: Writing – review & editing, Visualization, Software, Methodology. Jouni Tuisku: Writing – review & editing, Visualization, Validation, Software, Methodology, Formal analysis, Data curation. Valtteri Kaasinen: Writing – review & editing, Resources, Investigation, Data curation, Conceptualization. Tuomas Kankare: Writing – review & editing, Resources, Investigation, Data curation. Janne Isojärvi: Writing – review & editing, Software, Data curation. Juha Rinne: Writing – review & editing, Resources, Investigation. Jarmo Hietala: Writing – review & editing, Resources, Investigation. Pirjo Nuutila: Writing – review & editing, Resources, Investigation. Lauri Nummenmaa: Writing – review & editing, Visualization, Supervision, Resources, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The work was supported by the Päivikki and Sakari Sohlberg Foundation (grants to T.M. and V.K.), Finnish Governmental Research Funding (VTR) for Turku University Hospital (grants to T.M., J.T., and J.R.) and for the Western Finland collaborative area (grant to T.M.), the Finnish Brain Foundation (grant to T.M.), the Sigrid Juselius Foundation (grants to L.N. and J.R.), Academy of Finland (grant numbers 294897 and 332225 to L.N., and grant number 310962 to J.R.), and the Finnish Cultural Foundation (grants to T.M. and V.K.). We acknowledge Abbvie, Nordic Infucare; Advisory Board: Abbvie, Nordic Infucare, Adamant Health Ltd (Honoraria for Lecturing to V.K.). We thank Jose Manuel Rivera Espejo and Joni Virta for sharing their expertise on statistical modeling regarding this study. We also acknowledge Tomi Karjalainen for his work in image processing and statistical modeling, Tuomas Knuuti for quality control, and Veera Korhonen for literature searches.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2024.103578.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
We present the mean brain maps of group specific D2R availabilities in NeuroVault (https://neurovault.org; https://identifiers.org/neurovault.collection:12799).
References
- Alakurtti K., Johansson J.J., Joutsa J., Laine M., Bäckman L., Nyberg L., Rinne J.O. Long-term test–retest reliability of striatal and extrastriatal dopamine D2/3 receptor binding: study with [11C] raclopride and high-resolution PET. J. Cereb. Blood Flow Metab. 2015;35(7):1199–1205. doi: 10.1038/jcbfm.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barter R., Y. B., 2017._superheat: A Graphical Tool for Exploring Complex Datasets Using Heatmaps_. In (Version R package version 0.1.0) .
- Beaulieu J.M., Espinoza S., Gainetdinov R.R. Dopamine receptors–IUPHAR review 13. Br. J. Pharmacol. 2015;172(1):1–23. doi: 10.1111/bph.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan R.A., Crippa J.A. The role of dopamine in reward and pleasure behaviour–review of data from preclinical research. Acta Psychiatr. Scand. 2005;111:14–21. doi: 10.1111/j.1600-0447.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- Brugger S.P., Angelescu I., Abi-Dargham A., Mizrahi R., Shahrezaei V., Howes O.D. Heterogeneity of striatal dopamine function in schizophrenia: meta-analysis of variance. Biol. Psychiatry. 2020;87(3):215–224. doi: 10.1016/j.biopsych.2019.07.008. [DOI] [PubMed] [Google Scholar]
- Bürkner P.-C. brms: an R package for bayesian multilevel models using stan. J. Stat. Softw. 2017;80(1):1–28. doi: 10.18637/jss.v080.i01. [DOI] [Google Scholar]
- Bürkner P.-C. Advanced bayesian multilevel modeling with the R package brms. R Journal. 2018;10(1) doi: 10.32614/RJ-2018-017. [DOI] [Google Scholar]
- Bürkner, P.-C., 2021, 11/30. Bayesian Item Response Modeling in R with brms and Stan. J. Stat. Softw. 100 (5), 1–54. 10.18637/jss.v100.i05. [DOI]
- Dagher A. Functional brain imaging of appetite. Trends Endocrinol. Metab. 2012;23(5):250–260. doi: 10.1016/j.tem.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Darcey, V.L., Guo, J., Chi, M., Chung, S.T., Courville, A.B., Gallagher, I., Herscovitch, P., Howard, R., La Noire, M., Milley, L., 2023. Striatal dopamine tone is positively associated with body mass index in humans as determined by PET using dual dopamine type-2 receptor antagonist tracers. medRxiv, 2023.2009. 2027.23296169.
- De Keyser J., Claeys A., De Backer J.-P., Ebinger G., Roels F., Vauquelin G. Autoradiographic localization of D1 and D2 dopamine receptors in the human brain. Neurosci. Lett. 1988;91(2):142–147. doi: 10.1016/0304-3940(88)90758-6. [DOI] [PubMed] [Google Scholar]
- Elsinga P.H., Hatano K., Ishiwata K. PET tracers for imaging of the dopaminergic system. Curr. Med. Chem. 2006;13(18):2139–2153. doi: 10.2174/092986706777935258. [DOI] [PubMed] [Google Scholar]
- Engin A. The definition and prevalence of obesity and metabolic syndrome. Obesity and Lipotoxicity. 2017:1–17. doi: 10.1007/978-3-319-48382-5_1. [DOI] [PubMed] [Google Scholar]
- Gabry, J. bayesplot: Plotting for Bayesian Models.”. In (Version R package version 1.10.0). https://mc-stan.org/bayesplot/.
- Grace A.A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 2016;17(8):524–532. doi: 10.1038/nrn.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsing L., Jr . Neurotransmitter Systems; Handbook of Neurochemistry and Molecular Neurobiology: 2008. 7 Dopamine and the Dopaminergic Systems of the Brain; p. 155. [Google Scholar]
- Hirvonen J., Aalto S., Lumme V., Någren K., Kajander J., Vilkman H., Hagelberg N., Oikonen V., Hietala J. Measurement of striatal and thalamic dopamine D2 receptor binding with 11C-raclopride. Nucl. Med. Commun. 2003;24(12):1207–1214. doi: 10.1097/00006231-200312000-00002. [DOI] [PubMed] [Google Scholar]
- Horstmann A., Fenske W.K., Hankir M.K. Argument for a non-linear relationship between severity of human obesity and dopaminergic tone. Obes. Rev. 2015;16(10):821–830. doi: 10.1111/obr.12303. [DOI] [PubMed] [Google Scholar]
- Howes O.D., Kambeitz J., Kim E., Stahl D., Slifstein M., Abi-Dargham A., Kapur S. The nature of dopamine dysfunction in schizophrenia and what this means for treatment: meta-analysis of imaging studies. Arch. Gen. Psychiatry. 2012;69(8):776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis R.B., Cunningham V.J., Delforge J., Fujita M., Gjedde A., Gunn R.N., Holden J., Houle S., Huang S.-C., Ichise M. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J. Cereb. Blood Flow Metab. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Jackson D.M., Westlind-Danielsson A. Dopamine receptors: molecular biology, biochemistry and behavioural aspects. Pharmacol. Ther. 1994;64(2):291–370. doi: 10.1016/0163-7258(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Kaasinen V., Joutsa J., Noponen T., Johansson J., Seppänen M. Effects of aging and gender on striatal and extrastriatal [123I] FP-CIT binding in Parkinson's disease. Neurobiol. Aging. 2015;36(4):1757–1763. doi: 10.1016/j.neurobiolaging.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Kaasinen V., Vahlberg T. Dec). Striatal dopamine in Parkinson disease: a meta-analysis of imaging studies. Ann. Neurol. 2017;82(6):873–882. doi: 10.1002/ana.25103. [DOI] [PubMed] [Google Scholar]
- Kaasinen V., Vahlberg T., Stoessl A.J., Strafella A.P., Antonini A. Dopamine receptors in parkinson's disease: a meta-analysis of imaging studies. Mov. Disord. 2021 doi: 10.1002/mds.28632. [DOI] [PubMed] [Google Scholar]
- Kapur S., Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27(7):1081–1090. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Karjalainen T., Tuisku J., Santavirta S., Kantonen T., Bucci M., Tuominen L., Hirvonen J., Hietala J., Rinne J.O., Nummenmaa L. Magia: robust automated image processing and kinetic modeling toolbox for PET neuroinformatics. Front. Neuroinf. 2020;14:3. doi: 10.3389/fninf.2020.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson H.K., Tuominen L., Tuulari J.J., Hirvonen J., Parkkola R., Helin S., Salminen P., Nuutila P., Nummenmaa L. Obesity Is associated with decreased mu-opioid but unaltered dopamine D-2 receptor availability in the brain [Article] J. Neurosci. 2015;35(9):3959–3965. doi: 10.1523/jneurosci.4744-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knable M.B., Egan M.F., Heinz A., Gorey J., Lee K.S., Coppola R., Weinberger D.R. Altered dopaminergic function and negative symptoms in drug-free patients with schizophrenia:[123l]-iodobenzamide SPECT study. Br. J. Psychiatry. 1997;171(6):574–577. doi: 10.1192/bjp.171.6.574. [DOI] [PubMed] [Google Scholar]
- Lammertsma A.A., Hume S.P. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4(3):153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Leggio G.M., Bucolo C., Platania C.B.M., Salomone S., Drago F. Current drug treatments targeting dopamine D3 receptor. Pharmacol. Ther. 2016;165:164–177. doi: 10.1016/j.pharmthera.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Malén T., Karjalainen T., Isojärvi J., Vehtari A., Bürkner P.-C., Putkinen V., Kaasinen V., Hietala J., Nuutila P., Rinne J. Atlas of type 2 dopamine receptors in the human brain: age and sex dependent variability in a large PET cohort. Neuroimage. 2022;255 doi: 10.1016/j.neuroimage.2022.119149. [DOI] [PubMed] [Google Scholar]
- Mantel N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967;27(2_Part_1):209–220. [PubMed] [Google Scholar]
- Marshall, E.J., Farrell, M., 2007, 2007/04/01/. Substance use and psychiatric comorbidity. Medicine 35 (4), 246–249. 10.1016/j.mpmed.2007.02.010. [DOI]
- McElreath R. Chapman and Hall/CRC; 2020. Statistical rethinking: A Bayesian course with examples in R and Stan. [Google Scholar]
- Meltzer H.Y., Stahl S.M. The dopamine hypothesis of schizophrenia: a review*. Schizophr. Bull. 1976;2(1):19–76. doi: 10.1093/schbul/2.1.19. [DOI] [PubMed] [Google Scholar]
- Merims D., Giladi N. Dopamine dysregulation syndrome, addiction and behavioral changes in Parkinson's disease. Parkinsonism Relat. Disord. 2008;14(4):273–280. doi: 10.1016/j.parkreldis.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Pak, K., Nummenmaa, L., 2023. Brain dopamine receptor system is not altered in obesity: Bayesian and frequentist meta-analyses. Human brain mapping. [DOI] [PMC free article] [PubMed]
- Paradis E., Schliep K. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics. 2019;35(3):526–528. doi: 10.1093/bioinformatics/bty633. [DOI] [PubMed] [Google Scholar]
- Plavén-Sigray P., Ikonen Victorsson P., Santillo A., Matheson G.J., Lee M., Collste K., Fatouros-Bergman H., Sellgren C.M., Erhardt S., Agartz I. Thalamic dopamine D2-receptor availability in schizophrenia: a study on antipsychotic-naive patients with first-episode psychosis and a meta-analysis. Mol. Psychiatry. 2022;27(2):1233–1240. doi: 10.1038/s41380-021-01349-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poewe W., Mahlknecht P. Pharmacologic treatment of motor symptoms associated with parkinson disease. Neurol. Clin. 2020;38(2):255–267. doi: 10.1016/j.ncl.2019.12.002. [DOI] [PubMed] [Google Scholar]
- Posit team, 2023. RStudio: Integrated Development Environment for R. In Posit Software, PBC, Boston, MA. http://www.posit.co/.
- Purves, D., Augustine, G.J., Fitzpatrick, D., Hall, W.C., LaMantia, A.-S., Mooney, R.D., Platt, M.L., White, L., E., 2018. Neuroscience. Oxford University Press.
- R Core Team, 2023. R: A Language and Environment for Statistical Computing. In R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
- Rinne U., Laihinen A., Rinne J., Naågren K., Bergman J., Ruotsalainen U. Positron emission tomography demonstrates dopamine D2 receptor supersensitivity in the striatum of patients with early Parkinson's disease. Movement Disorders: Official Journal of the Movement Disorder Society. 1990;5(1):55–59. doi: 10.1002/mds.870050114. [DOI] [PubMed] [Google Scholar]
- Schloerke, B., C. D., Larmarange, J., Briatte, F., Marbach, M., Thoen, E., Elberg, A., Crowley, J., 2021. GGally: Extension to 'ggplot2'. In (Version R package version 2.1.2). https://CRAN.R-project.org/package=GGally.
- Schmidt W.J., Schmidt W., Reith M.E. Springer; 2005. Dopamine and glutamate in psychiatric disorders. [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 1998;80(1):1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Seo D., Patrick C.J., Kennealy P.J. Role of serotonin and dopamine system interactions in the neurobiology of impulsive aggression and its comorbidity with other clinical disorders. Aggress. Violent Behav. 2008;13(5):383–395. doi: 10.1016/j.avb.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraiwa N., Tamaoka A., Ohkoshi N. Clinical features of drug-induced Parkinsonism. Neurol. Int. 2018;10(4):7877. doi: 10.4081/ni.2018.7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl S.M. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. CNS Spectr. 2018;23(3):187–191. doi: 10.1017/S1092852918001013. [DOI] [PubMed] [Google Scholar]
- Stan Development Team RStan: the R interface to Stan. R Package Version. 2020;2(21):2. http://mc-stan.org/ [Google Scholar]
- Svensson J.E., Schain M., Plavén-Sigray P., Cervenka S., Tiger M., Nord M., Halldin C., Farde L., Lundberg J. Validity and reliability of extrastriatal [11C] raclopride binding quantification in the living human brain. Neuroimage. 2019;202 doi: 10.1016/j.neuroimage.2019.116143. [DOI] [PubMed] [Google Scholar]
- The Mathworks Inc., 2021. MATLAB version 9.11.0.1769968 (R2021b). In https://www.mathworks.com.
- Usiello A., Baik J.-H., Rougé-Pont F., Picetti R., Dierich A., LeMeur M., Piazza P.V., Borrelli E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408(6809):199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Fowler J.S., Wang G.-J., Swanson J.M. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol. Psychiatry. 2004;9(6):557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Wei, T.S., Viliam., 2021. R package 'corrplot': Visualization of a Correlation Matrix (Version 0.92). In (Version 0.92). https://github.com/taiyun/corrplot.
- Wickham H. In Springer-Verlag; 2016. ggplot2: Elegant Graphics for Data Analysis. [Google Scholar]
- Witjas T., Baunez C., Henry J.M., Delfini M., Regis J., Cherif A.A., Peragut J.C., Azulay J.P. Addiction in Parkinson's disease: impact of subthalamic nucleus deep brain stimulation. Movement Disorders: Official Journal of the Movement Disorder Society. 2005;20(8):1052–1055. doi: 10.1002/mds.20501. [DOI] [PubMed] [Google Scholar]
- Xiao N. In (version R Package Version 3.0.0) 2023. ggsci: Scientific Journal and Sci-Fi Themed Color Palettes for 'ggplot2'. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We present the mean brain maps of group specific D2R availabilities in NeuroVault (https://neurovault.org; https://identifiers.org/neurovault.collection:12799).