Highlights
-
•
Neuromark analysis reveals whole-brain dysconnectivity in first-episode psychosis.
-
•
Brain regions disrupted early in psychosis are involved in key cognitive functions.
-
•
Aberrant connectivity in auditory and language networks observed early in psychosis.
-
•
Aberrant connectivity in psychosis may support a cognitive dysmetria framework.
Keywords: First-episode psychosis (FEP), Early psychosis (EP), Functional network connectivity (FNC), Schizophrenia, Resting-state fMRI, Independent component analysis (ICA)
Abstract
Psychosis (including symptoms of delusions, hallucinations, and disorganized conduct/speech) is a main feature of schizophrenia and is frequently present in other major psychiatric illnesses. Studies in individuals with first-episode (FEP) and early psychosis (EP) have the potential to interpret aberrant connectivity associated with psychosis during a period with minimal influence from medication and other confounds. The current study uses a data-driven whole-brain approach to examine patterns of aberrant functional network connectivity (FNC) in a multi-site dataset comprising resting-state functional magnetic resonance images (rs-fMRI) from 117 individuals with FEP or EP and 130 individuals without a psychiatric disorder, as controls. Accounting for age, sex, race, head motion, and multiple imaging sites, differences in FNC were identified between psychosis and control participants in cortical (namely the inferior frontal gyrus, superior medial frontal gyrus, postcentral gyrus, supplementary motor area, posterior cingulate cortex, and superior and middle temporal gyri), subcortical (the caudate, thalamus, subthalamus, and hippocampus), and cerebellar regions. The prominent pattern of reduced cerebellar connectivity in psychosis is especially noteworthy, as most studies focus on cortical and subcortical regions, neglecting the cerebellum. The dysconnectivity reported here may indicate disruptions in cortical-subcortical-cerebellar circuitry involved in rudimentary cognitive functions which may serve as reliable correlates of psychosis.
1. Introduction
1.1. Neuropathology of First-Episode and early psychosis
Psychotic symptoms are commonly experienced by individuals with schizophrenia and can be experienced by individuals with other psychiatric conditions as well, such as bipolar disorder or depression (Arciniegas, 2015). Psychosis is characterized by disruptions in a person’s thoughts (e.g., thought blocking or withdrawal, tangentiality, loose associations), irrational beliefs (e.g., delusions), aberrant perceptions (e.g., auditory hallucinations), disorganized speech, impaired cognition (reduced working memory performance), and bizarre behaviors (VandenBos, 2007). The neural underpinnings of psychosis are not fully understood, however, it has been proposed that psychotic symptoms reflect disruptions in information processing due to a breakdown in functional brain integration, or dysconnectivity (Friston, 1998, Fu et al., 2021a, Iraji et al., 2019, Iraji et al., 2022a, Iraji et al., 2022b, Khadka et al., 2013).
However, much of the existing body of psychosis research has focused on individuals with chronic symptoms, where several confounding factors such as substance use (Khokhar et al., 2018), long-term antipsychotic treatment (Ho et al., 2011, Yang et al., 2021), aging (Shahab et al., 2019), and medical conditions prevalent in patients with psychotic disorders (e.g., diabetes and hypertension; Chung and Miller, 2020, Sudarshan and Cheung, 2023, Ward and Druss, 2015) may influence the findings. Thus, further research is needed to explore whether functional abnormalities are present early in the onset of psychotic symptoms when fewer confounding factors have impacted the brain, and when potential neurobiology-guided treatments might be more effective (Hickie et al., 2019, Laurens and Cullen, 2016, Salisbury et al., 2002). To optimize the potential benefits of neurobiology-guided treatment and reduce the impact of various confounds, many studies have shifted their focus toward individuals in the earliest stages of diagnosable psychosis, particularly during first-episode (FEP) or early psychosis (EP; Breitborde et al., 2009).
Operational definitions for FEP and EP vary greatly across studies (Breitborde et al., 2009) and the terms are sometimes used interchangeably (Breitborde et al., 2009, Cattarinussi et al., 2023, Fu et al., 2021a, Jimeno et al., 2020). However, an important distinction in the literature is that FEP is more often used to describe a population that is within one (Alonso-Solís et al., 2012, Fornito et al., 2013, Lee et al., 2019) or two years (Faria et al., 2021, Ganella et al., 2018, Kim et al., 2021, Kwak et al., 2021, Lencz et al., 2022, Lesh et al., 2021, Li et al., 2021, Oh et al., 2020, Wang et al., 2019, Wang et al., 2023, Wilson et al., 2020, Yang et al., 2022) from the onset of illness, while EP samples (also referred to as early-phase or early-onset psychosis) typically include individuals within a duration of five years from the onset of illness (Deakin et al., 2018, Fu et al., 2021a, Holmes et al., 2023, Koshiyama et al., 2018, Vanes et al., 2019, Yang et al., 2022). Some studies define FEP and EP based on the first emergence of psychotic symptoms2 (Fornito et al., 2013, Fu et al., 2021a, Yang et al., 2022), while others define FEP and EP based on when treatment began, which may be considered the individual’s first contact with a clinician (Breitborde et al., 2009, Flaum et al., 1992, O’Connor et al., 2017, Reid et al., 2019), but may also account for when the individual began taking antipsychotic medication (Dempster et al., 2020, Iwashiro et al., 2016, Jauhar et al., 2018, Lencz et al., 2022, Maximo et al., 2021, Salisbury et al., 2022, Wen et al., 2023). Regardless of how FEP and EP are defined, it is important to describe the sample in a way that facilitates comparisons across relevant studies. The current study includes analyses of a sample of individuals within two years from their first clinical contact for psychosis, which we will refer to as two-year psychosis, as well as a subsample of individuals within one year from their first clinical contact for psychosis, which we will refer to as one-year psychosis.
While the primary goal of the current study is to identify patterns of aberrant brain activity in FEP and EP, doing so will enhance our understanding of the underlying neuropathology of psychosis and may aid in early detection as well. Early identification of psychosis is crucial, as early intervention is associated with improved clinical outcomes such as reduced rates of hospital admission, relapse, and suicide, as well as reduced symptom severity and treatment cost (Bird et al., 2010, Ricciardi et al., 2008). However, due to challenges such as those arising from individual differences or the sometimes gradual development of symptoms, it can be difficult to identify psychosis early on (Fusar-Poli et al., 2013, Jimeno et al., 2020, Schultze-Lutter et al., 2010). For this reason, the field would greatly benefit from tools which facilitate the detection of developing psychosis using biological criteria (Insel and Cuthbert, 2015, Keshavan and Clementz, 2023). Detecting neurophysiological patterns of dysconnectivity in individuals with FEP and EP, free from the long-term confounds of illness chronicity, will provide new leads into understanding the neural mechanisms related to psychosis. Further, such measures might contribute to the development of reliable and sensitive biomarkers for the early detection of individuals at risk for transitioning to psychosis, among whom early intervention may be most beneficial.
1.2. Aberrant Resting-State functional magnetic resonance imaging in psychosis
In recent years, resting-state functional magnetic resonance imaging (rs-fMRI) has become a popular functional imaging modality for observing disruptions in brain function at rest in individuals with psychosis (Andreou and Borgwardt, 2020, Sheffield and Barch, 2016, Suvisaari et al., 2018). Previous rs-fMRI studies in FEP have implicated aberrant brain activity across a wide range of brain regions and networks. Cattarinussi and colleagues (2023) conducted a meta-analysis of 35 rs-fMRI studies and concluded that abnormalities in the fronto-striatal circuit were characteristic of FEP. Specifically, studies utilizing measures of fractional amplitude of low-frequency fluctuations (fALFF) and regional homogeneity (ReHo) identified aberrant spontaneous brain activity associated with FEP in the bilateral striatum, superior and middle frontal gyri, the right precentral gyrus, and the right inferior frontal gyrus (Cattarinussi et al., 2023). In another meta-analysis of rs-fMRI, Gong et al. (2020) highlighted patterns of aberrant fALFF in a first-episode schizophrenia (FES) sample in the bilateral inferior parietal gyri, right precuneus, left medial prefrontal cortex, bilateral putamen, and bilateral occipital gyrus.
Lencz and colleagues (2022) observed a pattern of aberrant fALFF in bilateral orbitofrontal cortex in a notably large (N = 126) FEP sample. In a review of resting-state fMRI in FES, Mwansisya and colleagues (2017) similarly described abnormalities in prefrontal regions including dorsal lateral prefrontal cortex and orbital frontal cortex. Notably, they also identified patterns of aberrant rs-fMRI in the temporal lobe, particularly the left superior temporal gyrus (STG; Mwansisya et al., 2017).
Psychosis-related disruptions in rs-fMRI also manifest in functional connectivity (FC), which is a term that describes the temporal similarity between neuronal-related time series (Biswal et al., 1995, Satterthwaite and Baker, 2015). Abnormalities in brain functional connectivity are often described as hypoconnectivity, hyperconnectivity, or dysconnectivity, all of which have been linked to psychosis (Del Fabro et al., 2021, O’Neill et al., 2019). Similar to the findings of Mwansisya et al. (2017), Yoon et al. (2015) identified patterns of aberrant fronto-temporal FC in a FEP sample. In addition, many studies have noted disruptions in rs-fMRI associated with clinical high risk, first-episode psychosis and more chronic schizophrenia in large-scale brain networks such as the default mode network, central executive network, and salience network, as well as the cerebellum (Andreasen and Pierson, 2008, Bang et al., 2018, Del Fabro et al., 2021, Du et al., 2018a, Fu et al., 2021a, Wang et al., 2014).
The reported patterns of aberrant rs-fMRI in FEP and FES described above appear to be spread across the whole brain. Together these findings are consistent with the framework outlined by Andreasen and colleagues (1998) which postulated that the symptoms of schizophrenia are caused by a disruption in cortical-subcortical-cerebellar circuitry. However, it is difficult to draw inferences from findings across studies such as these as they vary greatly in their participant samples3 and study methodology. Furthermore, in addition to differences in the type of rs-fMRI measurement used (e.g., fALFF, ReHo, FC, etc.), the regions of interest (ROIs) involved vary greatly from study to study (Del Fabro et al., 2021, Pettersson-Yeo et al., 2011, Sheffield and Barch, 2016), and FEP and EP studies often do not account for individual differences in their selection of nodes or ROIs. Relatively few studies take a whole-brain approach which would enable us to better examine the relationships between these distributed brain regions and networks. The current study seeks to overcome these limitations by employing a data-driven whole-brain approach.
1.3. Neuromark: A Data-Driven approach
Many of the rs-fMRI studies in the literature reviews previously discussed examined FC in first-episode psychosis and schizophrenia populations using predefined ROIs and spatially-fixed nodes (e.g., Fornito et al., 2013, Oh et al., 2020, Wang et al., 2014, Zhou et al., 2007). However, these approaches have limitations as they rely on subjective ROIs that may not accurately represent functional units and fall short of considering subject specific variation (Iraji et al., 2023). In contrast, data-driven approaches extract functional sources directly from the data itself, enabling the incorporation of inter-subject spatial variability and accounting for subject-specific differences when calculating corresponding functional connectivity patterns across individuals (Iraji et al., 2020, Iraji et al., 2023, Korhonen et al., 2021). Distinct functional sources in the brain, known as intrinsic connectivity networks (ICNs), can be identified using independent component analysis (ICA; Calhoun et al., 2001, Calhoun et al., 2009), and the interactions or FC between ICNs can be described as whole-brain functional connectivity, commonly known as functional network connectivity (FNC; Jafri et al., 2008).
The current study leverages a reference-informed, data-driven approach to examine FNC in our FEP and EP sample. This approach provides notable advantages compared to other data-driven methods, including its generalizability to other studies as well as its ability to address ambiguity and uncertainty regarding the matched functional entities across individuals. Consequently, it enhances our capacity to identify consistent functional sources across individuals (Iraji et al., 2023). To address the limitations related to the discrepancy in investigated functional connectivity patterns across different studies, we utilized the Neuromark_fMRI_1.0 network template (Du et al., 2020). The Neuromark project aims to establish reliable and replicable structural and functional templates derived from large datasets, enabling findings to be comparable across studies (Du et al., 2020, Iraji et al., 2023). We employed multivariate-objective optimization ICA with reference (MOO-ICAR; Du & Fan, 2013) to estimate the corresponding ICNs for each individual in our dataset. This method has demonstrated efficacy in capturing subject-specific information and effectively removing artifacts, as evidenced by previous studies (Du et al., 2016, Du and Fan, 2013, Iraji et al., 2023). Notably, the current study examines psychosis at an earlier stage than previous studies taking a similar approach (Du et al., 2016, Du et al., 2020, Fu et al., 2021a).
2. Material and methods
2.1. Participants
The data is comprised of rs-fMRI images and diagnostic information collected from multiple sites affiliated with the University of Pittsburgh Department of Psychiatry (PITT), Pittsburgh, PA, and the Johns Hopkins School of Medicine (JH), Baltimore, MD. The data were collected with approval from the Institutional Review Board from each site. All participants received a full explanation of the study procedures, and written informed consent was obtained for all participants 18 years and older; parental consent and assent were obtained for all participants under 18. The JH participants with psychosis were recruited from local in-patient and out-patient clinics in Maryland, USA. The PITT participants with psychosis were recruited from in-patient and out-patient services of a local psychiatric hospital in Pittsburgh, Pennsylvania, USA. All participants were between 12 and 38 years old, and had no history of drug or alcohol abuse in the past three years, a traumatic brain injury, neurologic disorder, or intellectual disability. In addition, participants with an estimated premorbid intelligence quotient (IQ) below 70 were excluded. Premorbid IQ was estimated on the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 2012) for PITT participants, and the Hopkins Adult Reading Test (HART; Schretlen et al., 2009) for JH participants. The control group consisted of individuals with no personal or immediate family history of psychosis and who were not currently taking psychotropic medication. The psychosis group consisted of individuals across the psychosis continuum (see Table 1), with diagnoses based on the Structured Clinical Interview for DSM-IV (APA, 1994). Symptom severity was measured with the scale for the assessment of negative symptoms (SANS; Andreasen, 1983) and the scale for the assessment of positive symptoms (SAPS; Andreasen, 1984).
Table 1.
Demographics and clinical characteristics for the full sample are displayed in the table above. Individuals experiencing psychotic symptoms are grouped according to the following diagnostic categories: schizophrenia (SZ), major depressive disorder (MDD), bipolar disorder (BPP), schizoaffective disorder (SZAF), schizophreniform disorder (SZPH), and other psychotic disorders (Others). The following demographics are reported for all study participants by group: the number of participants, the mean and standard deviation (SD) of age in years (yrs), and the race distribution. The “Other” category for race includes participants who indicated that they belonged to a race other than those presented, indicated that they belonged to more than one race, or chose not to disclose this information. The following clinical characteristics are reported by group for the full sample of individuals experiencing psychotic symptoms: the percentage of participants with medication dosage information available, the mean chlorpromazine (CPZ) equivalent estimate in milligrams (mg), the percentage of participants with both SANS and SAPS scores, the mean SANS and SAPS global scores, the median number of months between the onset of psychotic symptoms and the magnetic resonance imaging (MRI) scan, and the median number of months between the first clinical contact and the MRI scan. IQR refers to the interquartile range.
| Control |
SZ |
MDD |
BPP |
SZAF |
SZPH |
Others |
Total |
|
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Demographics | ||||||||
| N (Male/Female) | 130 (71/59) | 60 (45/15) | 12 (7/5) | 18 (11/7) | 12 (8/4) | 4 (3/1) | 11 (7/4) | 247 (152/95) |
| Age (Range: 12–38 yrs) | 23.29 ± 4.05 | 23.27 ± 4.63 | 22.28 ± 3.41 | 24.03 ± 4.67 | 23.95 ± 3.92 | 23.64 ± 3.15 | 21.76 ± 5.17 | 23.26 ± 4.23 |
| Race (%) | ||||||||
| White | 44.62 % | 43.33 % | 25 % | 61.11 % | 58.33 % | 75 % | 45.45 % | 45.75 % |
| Black | 40 % | 38.33 % | 33.33 % | 22.22 % | 25 % | 25 % | 36.36 % | 36.84 % |
| Asian | 10 % | 3.33 % | 25 % | 5.56 % | 0 % | 0 % | 18.18 % | 8.50 % |
| Other | 5.38 % | 15 % | 16.67 % | 11.11 % | 16.67 % | 0 % | 0 % | 8.91 % |
| Medication | ||||||||
| Participants w/ CPZ (%) | --- | 88.33 % | 83.33 % | 94.44 % | 91.67 % | 75 % | 90.91 % | 88.89 % |
| CPZ equivalents (mg) | --- | 234.65 ± 244.41 | 133.06 ± 187.08 | 176.95 ± 117.88 | 246.98 ± 270.35 | 273.59 ± 106.28 | 158.30 ± 226.98 | 210.53 ± 220.87 |
| Symptom Severity & Duration | ||||||||
| Participants w/ SANS & SAPS (%) | --- | 100 % | 91.67 % | 100 % | 100 % | 100 % | 81.82 % | 97.44 % |
| SANS Global | --- | 10.58 ± 4.2 | 8.73 ± 2.28 | 5.61 ± 4.43 | 8.5 ± 4.58 | 8.5 ± 3.7 | 6.44 ± 3.17 | 9 ± 4.41 |
| SAPS Global | --- | 5.55 ± 3.82 | 3.55 ± 2.38 | 3.11 ± 4.03 | 5.67 ± 3.87 | 3 ± 1.83 | 5.67 ± 3.61 | 4.9 ± 3.76 |
| Median (IQR) months since symptom onset | --- | 15 (6.2–25.5) | 4.7 (1.1–7.4) | 6.48 (2.3–14) | 8.08 (4.7–51.9) | 2.29 (1.2–7.7) | 10.22 (3–17) | 11 (4–19.5) |
| Median (IQR) months since 1st clinical contact | --- | 3.18 (0.9–10.5) | 1.61 (0.9–4.4) | 3.78 (1–13) | 3.14 (1.6–7.5) | 2.29 (1.1–7.2) | 2 (1–10) | 2.89 (1–9.8) |
In the current study, individuals were categorized as one-year psychosis if their data was collected within one year from their first clinical contact for psychotic symptoms, and they were categorized as two-year psychosis if their data was collected within two years. These labels were used to emphasize the characteristics of the sample in the current study (see Table 1), as opposed to the widely used FEP and EP labels which can be vague and misleading due to their variable use across studies (Breitborde et al., 2009).
The final combined dataset included 247 participants (152 Male, 95 Female; mean age 23.26 years, std 4.23), including 117 individuals (81 Male, 36 Female; mean age 23.23 years, std 4.43) with psychosis and 130 individuals (71 Male, 59 Female; mean age 23.29 years, std 4.05) in the control group (see Table 1). A Fisher’s exact test (p = 0.019) indicated a significant association between sex (Male/Female) and diagnostic category (psychosis/control). We accounted for the sex differences across groups by including sex as a covariate in our analysis. We acknowledge that our inclusion of sex as a covariate was questionable (as the case/control groups differed on that variable), thus, we repeated our primary analysis without sex as a covariate to explore the effect of this decision and found that there was virtually no impact on the observed patterns of diagnostic effect on FNC (see Appendix S1). Age did not differ significantly between the psychosis and control groups (t(2 4 5) = 0.12, p = 0.907), nor did race (χ2 = 5.37, df = 3, p = 0.147).
2.2. Image acquisition and preprocessing
The imaging data were acquired from three scanners. One rs-fMRI scan (5 min, 9 sec each) was acquired for 61 participants with a 3 T Siemens TIM Trio scanner with a repetition time (TR) of 1000 ms, an echo time (TE) of 30 ms, a flip angle (FA) of 55°, and a voxel size of 2.3 × 2.3 × 2.3 mm3. In addition, a 3T Siemens Magnetom Prisma scanner was used to collect rs-fMRI scans (5 min, 46 sec each) for 64 participants. Data characteristics include a TR of 800 ms, a TE of 37 ms, a FA of 52°, and a voxel size of 2 × 2 × 2 mm3. The remaining rs-fMRI data were acquired for 122 participants (7 min, 12 sec each) using a 3T Phillips Achieva scanner with a TR of 2000 ms, a TE of 30 ms, a FA of 75°, and a voxel size of 3 × 3 × 4 mm3. Participants with absolute head motion greater than 3 mm translation or 3° rotation were excluded from the study. rs-fMRI data were preprocessed in a MATLAB 2019 environment through a pipeline utilizing functions from SPM12 (http://www.fil.ion.ucl.ac.uk/spm/) and FSL (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki). The first 10 timepoints were discarded prior to preprocessing. Preprocessing steps included distortion correction, a rigid body motion correction, slice timing correction, warping to MNI space using an echo-planar imaging (EPI) template, and resampling to 3 × 3 × 3 mm3 voxel space. Images were spatially smoothed by a Gaussian kernel with a full-width half maximum (FWHM) of 6 mm and the variance intensity of the time courses was normalized.
2.3. Analyses
2.3.1. Estimating subject-specific ICNs
We employed the Group ICA of fMRI Toolbox (GIFT) v4.0c package (http://trendscenter.org/software/gift; Iraji et al., 2021) to apply a fully automated ICA pipeline and extract ICNs. Within the pipeline, we chose the MOO-ICAR (Y. Du & Fan, 2013), a spatially constrained ICA, as our ICA algorithm and the Neuromark_fMRI_1.0 template (Du et al., 2020) as our reference to identify 53 subject-specific intrinsic connectivity networks (ICNs). The template and a comprehensive list of network labels can be accessed at http://trendscenter.org/data.
2.3.2. Calculating subject-specific FNC
Before calculating the FNC, we performed additional postprocessing on the time courses to remove the additional noise effects, including detrending, despiking, regression of head motion, and filtering with a high-frequency cutoff of 0.15 Hz (Fu et al., 2021b). We estimated whole-brain FNC by calculating Pearson correlation between the time courses of the 53 subject-specific ICNs. This resulted in a 53 × 53 symmetric FNC matrix for each individual. Prior to conducting statistical analyses, the FNC features were z-transformed. This step was performed to correct the highly skewed correlation distribution and convert it into approximately normally distributed data. By applying the z-transformation, we aimed to ensure that the data met the assumptions of normality required for subsequent statistical analyses.
2.3.3. Statistical comparisons
We conducted a statistical case/control comparison to identify differences in FNC between individuals with psychosis and controls. For each FNC, a general linear model was applied, including age, sex, race, mean framewise displacement (mean FD), and imaging site as covariates. In addition to including site as a covariate in our general linear model, we addressed the multi-site effects in our dataset with our implementation of ICA, the effectiveness of which has been demonstrated in prior studies (Chen et al., 2014, Xu et al., 2023). We adjusted for multiple comparisons using the false discovery rate (FDR) correction. We first performed this analysis with the full two-year psychosis sample and then we repeated the analysis with a one-year psychosis subset. In addition, we tested for associations between FNC features and global positive and negative symptom severity in individuals with psychosis. For each FNC showing significant differences between individuals with psychosis and controls, a general linear model was used to test for associations with SANS and SAPS global scores, respectively. Again, we included age, sex, race, mean FD, and site, as covariates and adjusted for multiple comparisons.
3. Results
3.1. Estimated ICNs
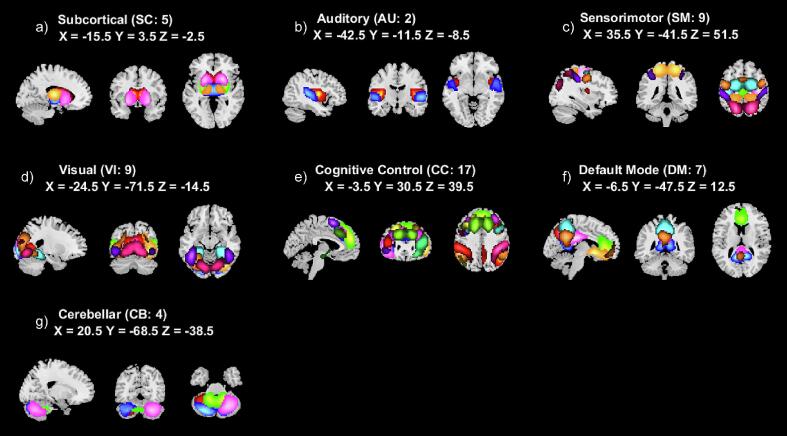
We visually inspected the estimated 53 subject-specific ICNs, which were automatically grouped into subcortical (SC; see Fig. 1a), auditory (AU; see Fig. 1b), sensorimotor (SM; see Fig. 1c), visual (VI; see Fig. 1d), cognitive control (CC; see Fig. 1e), default mode (DM; see Fig. 1f), and cerebellar (CB; see Fig. 1g) domains consistent with the labels utilized in the Neuromark_fMRI_1.0 template. We slightly modified some of the ICN labels to make them more descriptive in cases where labels were reused (e.g., the two ICNs sharing the Neuromark label “MTG” were relabeled in the current study to “anterior” and “posterior MTG”). We determined that the template ICNs were successfully identified in our dataset by calculating spatial similarity with a Pearson correlation between our estimated networks and the ICNs in the Neuromark_fMRI_1.0 template (Iraji et al., 2023). We found a mean ICN-template similarity of 0.89 (std 0.03, min 0.81, max 0.95), confirming their high correspondence (all ICNs were above a similarity threshold of 0.8; see Table 2).
Fig. 1.
A composite view of spatial maps of z-transformed average static functional network connectivity (FNC) is shown above for each of our estimated 53 intrinsic connectivity networks (ICNs) from the Neuromark_fMRI_1.0 template. The ICNs have been grouped into seven domains: (a) subcortical (SC), (b) auditory (AU), (c) sensorimotor (SM), (d) visual (VI), (e) cognitive control (CC), (f) default mode (DM), and (g) cerebellar (CB).
Table 2.
Spatial similarity measured with Pearson correlation coefficient r between the 53 estimated independent component networks (ICNs) in our dataset and the 53 ICNs from the Neuromark_fMRI_1.0 template is shown in the table above. IPL is an abbreviation for inferior parietal lobule.
| Label (ICN) | r | p | Label (ICN) | r | p |
|---|---|---|---|---|---|
| Subcortical network (SC) | Cognitive-control network (CC) | ||||
| Medial caudate (1) | 0.93 | <0.001 | Right posterior IPL (26) | 0.89 | <0.001 |
| Subthalamus/hypothalamus (2) | 0.92 | <0.001 | Insula (27) | 0.9 | <0.001 |
| Putamen (3) | 0.94 | <0.001 | Superomedial frontal gyrus (28) | 0.87 | <0.001 |
| Lateral caudate (4) | 0.93 | <0.001 | Left inferior frontal gyrus (29) | 0.87 | <0.001 |
| Thalamus (5) | 0.93 | <0.001 | Right inferior frontal gyrus (30) | 0.89 | <0.001 |
| Auditory network (AU) | Posterior middle frontal gyrus (31) | 0.87 | <0.001 | ||
| Superior temporal gyrus (6) | 0.93 | <0.001 | Bilateral inferior parietal lobule (32) | 0.9 | <0.001 |
| Anterior middle temporal gyrus (7) | 0.86 | <0.001 | Right anterior IPL (33) | 0.88 | <0.001 |
| Sensorimotor network (SM) | Supplementary motor area (34) | 0.85 | <0.001 | ||
| Anterior postcentral gyrus (8) | 0.95 | <0.001 | Superior frontal gyrus (35) | 0.85 | <0.001 |
| Left postcentral gyrus (9) | 0.91 | <0.001 | Dorsal middle frontal gyrus (36) | 0.87 | <0.001 |
| Medial paracentral lobule (10) | 0.92 | <0.001 | Anterior hippocampus (37) | 0.91 | <0.001 |
| Right postcentral gyrus (11) | 0.91 | <0.001 | Left anterior IPL (38) | 0.88 | <0.001 |
| Anterior superior parietal lobule (12) | 0.87 | <0.001 | Middle cingulate cortex (39) | 0.87 | <0.001 |
| Lateral paracentral lobule (13) | 0.88 | <0.001 | Right inferior frontal gyrus (40) | 0.86 | <0.001 |
| Precentral gyrus (14) | 0.87 | <0.001 | Anterior middle frontal gyrus (41) | 0.87 | <0.001 |
| Posterior superior parietal lobule (15) | 0.86 | <0.001 | Posterior hippocampus (42) | 0.92 | <0.001 |
| Posterior postcentral gyrus (16) | 0.88 | <0.001 | Default-mode network (DM) | ||
| Visual network (VI) | Dorsolateral precuneus (43) | 0.89 | <0.001 | ||
| Calcarine gyrus (17) | 0.89 | <0.001 | Ventral precuneus (44) | 0.89 | <0.001 |
| Middle occipital gyrus (18) | 0.91 | <0.001 | Medial anterior cingulate cortex (45) | 0.89 | <0.001 |
| Posterior middle temporal gyrus (19) | 0.89 | <0.001 | Ventral posterior cingulate cortex (46) | 0.91 | <0.001 |
| Cuneus (20) | 0.82 | <0.001 | Lateral anterior cingulate cortex (47) | 0.88 | <0.001 |
| Right middle occipital gyrus (21) | 0.83 | <0.001 | Dorsomedial precuneus (48) | 0.87 | <0.001 |
| Fusiform gyrus (22) | 0.91 | <0.001 | Dorsal posterior cingulate cortex (49) | 0.84 | <0.001 |
| Inferior occipital gyrus (23) | 0.82 | <0.001 | Cerebellar network (CB) | ||
| Lingual gyrus (24) | 0.81 | <0.001 | Left cerebellum (50) | 0.89 | <0.001 |
| Ventral middle temporal gyrus (25) | 0.88 | <0.001 | Posterior cerebellum (51) | 0.89 | <0.001 |
| Medial cerebellum (52) | 0.9 | <0.001 | |||
| Right cerebellum (53) | 0.89 | <0.001 |
3.2. Diagnostic group and FNC
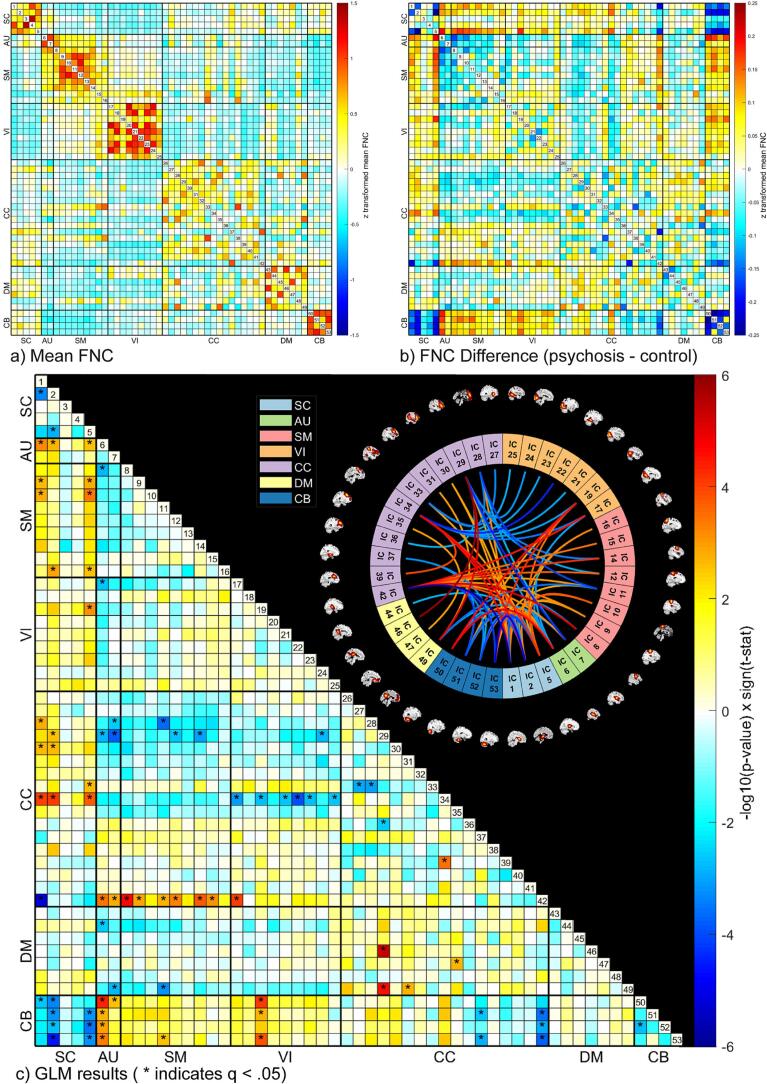
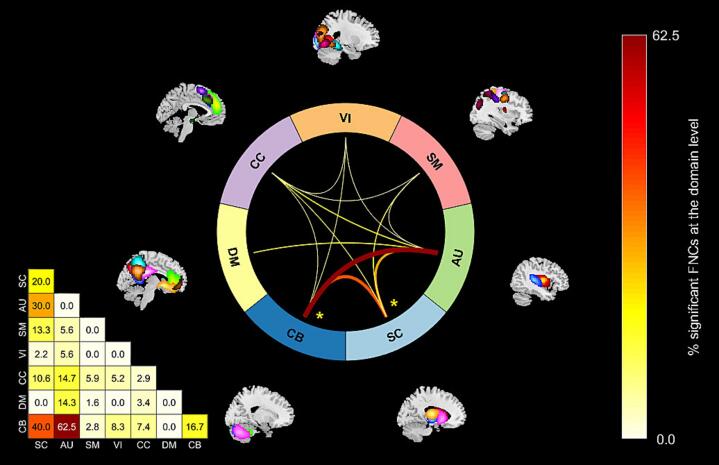
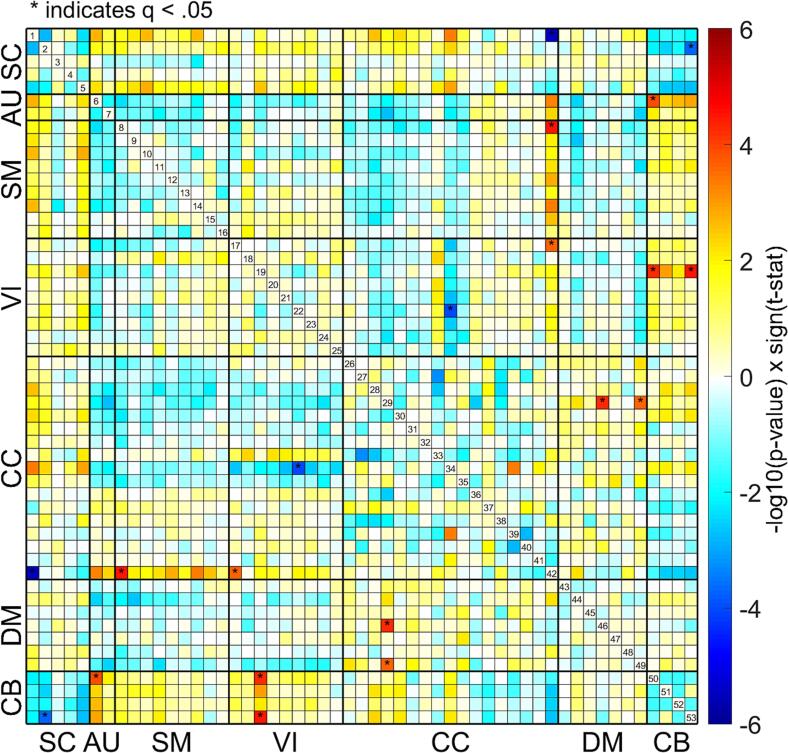
In our statistical comparisons (described in section 2.3.3) we identified differences in FNC between the two-year psychosis and control groups across the whole brain (see Fig. 2). Overall, there were greater case/control differences in FNC between domains (e.g., SC-AU) rather than within domains (e.g., SC or AU; see Fig. 2, Fig. 3). Brief summaries of the results pertaining to each domain are outlined below.
Fig. 2.
Mean functional network connectivity (FNC) for all participants is displayed in the matrix on the top left (a) with red indicating higher positive values and blue indicating lower negative values. The difference in mean FNC between diagnostic groups (psychosis - control) is displayed in the matrix on the top right (b). Results of the general linear model (GLM) for each combination of the 53 Neuromark intrinsic connectivity networks (ICNs) is displayed on the bottom (c). Significant (q < 0.05) case/control group associations with functional network connectivity (FNC) are marked with an asterisk in the matrix to the left. Sagittal slices of each ICN surround the connectogram to the right. ICNs are grouped by domain, consistent with the Neuromark_fMRI_1.0 template: subcortical (SC), auditory (AU), sensorimotor (SM), visual (VI), cognitive control (CC), default mode (DM), and cerebellar (CB). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
The percentage of significant aberrant functional network connectivity pairs (FNCs) within each domain block in the general linear model (GLM) results matrix in Fig. 2c (calculated as ) are shown above. In the connectogram on the right, between domain relationships are represented with connecting lines, while within domain relationships are represented with an asterisk. Between and within domain relationships are not shown for domain blocks where less than 5 % of the FNCs are significant (q < 0.05). Domain labels are consistent with the Neuromark_fMRI_1.0 template: subcortical (SC), auditory (AU), sensorimotor (SM), visual (VI), cognitive control (CC), default mode (DM), and cerebellar (CB).
3.2.1. SC domain
Beginning with ICNs in the SC domain, we observed hyperconnectivity (a stronger positive correlation in the psychosis group relative to the control group with q < 0.05) between ICN 1 (medial caudate; see Fig. 4a) and ICN 28 (superomedial frontal gyrus; SMFG, see Fig. 4h) in the CC domain. We also observed hyperconnectivity (a positive correlation in psychosis relative to a negative correlation in controls with q < 0.05) between ICN 2 (subthalamus/hypothalamus; see Fig. 4b) and ICN 6 (superior temporal gyrus; STG; see Fig. 4d) in the AU domain. Hypoconnectivity (2 negative correlations in psychosis relative to positive correlations in controls with q < 0.05) was observed between ICN 2 (subthalamus/hypothalamus) and ICNs 51 and 53 in the cerebellum (see Fig. 4p & r). However, the majority of our findings in the SC domain reflected dysconnectivity that manifested as weaker correlations (closer to zero in the psychosis group relative to the control group) between ICNs. This includes dysconnectivity within the SC domain (2 weaker positive correlation in psychosis relative to controls with q < 0.05) between ICN 2 (subthalamus/hypothalamus) with ICNs 1 (medial caudate) and 5 (thalamus; see Fig. 4c). Dysconnectivity was also observed between the SC and AU (2 weaker negative correlations in psychosis with q < 0.05), SM (6 weaker negative correlations in psychosis with q < 0.05), VI (1 weaker negative correlation in psychosis wtih q < 0.05), CC (7 weaker negative correlations in psychosis and 1 weaker positive correlation in psychosis with q < 0.05), and CB (6 weaker positive correlations in psychosis with q < 0.05) domains. Notably, there was a large amount of aberrant connectivity between the SC and CB domains (8 FNCs with q < 0.05). In addition, although the Neuromark template does not categorize them within the SC domain, ICNs 37 and 42 within the hippocampus (a subcortical structure; see Fig. 4k & l) displayed aberrant connectivity with a large number of other ICNs (15 FNCs with q < 0.05) across SC, AU, SM, VI, and CB domains.
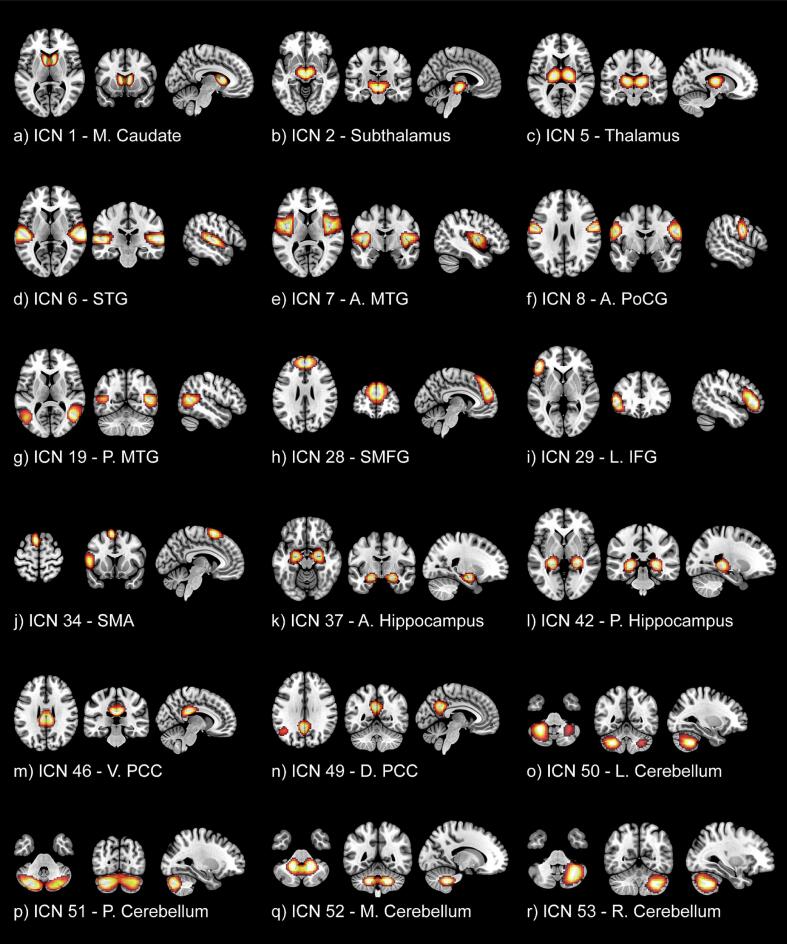
Fig. 4.
Corresponding spatial maps for the most notable intrinsic connectivity networks (ICNs) are displayed with accompanying labels derived from Du et al. (2020). From the subcortical domain (SC): (a) ICN 1 – medial caudate, (b) ICN 2 – subthalamus/hypothalamus, (c) ICN 5 – thalamus. From the auditory domain (AU): (d) ICN 6 – superior temporal gyrus (STG), (e) ICN 7 – anterior middle temporal gyrus (A. MTG). From the Sensorimotor domain (SM): (f) ICN 8 – anterior postcentral gyrus (A. PoCG). From the cognitive control domain (CC): (g) ICN 19 – posterior middle temporal gyrus (P. MTG), (h) ICN 28 – superior medial frontal gyrus (SMFG), (i) ICN 29 – left inferior frontal gyrus (L. IFG), (j) ICN 34 - supplementary motor area (SMA), (k) ICN 37 – anterior hippocampus, (l) ICN 42 – posterior hippocampus. From the Default-mode domain (DM): (m) ICN 46 – ventral posterior cingulate cortex (V. PCC), (n) ICN 49 – dorsal posterior cingulate cortex (D. PCC). And from the cerebellar domain (CB): (o) ICN 50 – left cerebellum, (p) ICN 51 – posterior cerebellum, (q) ICN 52 – medial cerebellum, (r) ICN 53 – right cerebellum.
3.2.2. AU domain
Dysconnectivity in the AU domain primarily manifested as either weaker correlations or stronger negative correlations (increased anticorrelation in psychosis). These include a weaker positive correlation in psychosis between ICN 6 (STG) in the AU domain and ICN 8 (anterior postcentral gyrus; see Fig. 4f) in the SM domain, as well as with ICN 17 (calcarine gyrus) in the VI domain. Weaker negative correlations in psychosis were observed between ICNs 6 (STG) and 7 (anterior middle temporal gyrus; A. MTG; see Fig. 4e) in the AU domain with ICN 42 (posterior hippocampus) in the CC domain. In addition, there were 5 weaker negative correlations in psychosis between AU and CB domains (q < 0.05). Increased anticorrelations in the AU domain were observed between ICN 6 (STG) and ICN 29 (left inferior frontal gyrus; L. IFG, see Fig. 4i) from the CC domain (negative correlation in psychosis relative to positive correlation in controls), as well as between ICN 7 (A. MTG) and ICNs 28 (SMFG; stronger negative correlation in psychosis relative to controls with q < 0.05) and 29 (L. IFG; negative correlations in psychosis relative to positive correlations in controls with q < 0.05). We also observed stronger anticorrelations (q < 0.05) in the AU domain between ICN 6 (STG) and ICN 44 (ventral precuneus) from the DM domain, as well as between ICN 7 (A. MTG) and ICN 49 (dorsal posterior cingulate cortex; D. PCC; see Fig. 4n). In total, the STG was associated with 17 aberrant FNCs (q < 0.05) across domains.
3.2.3. SM domain
We observed dysconnectivity (6 weaker negative correlations in psychosis with q < 0.05) between several ICNs in the SM domain (ICNs 8, 9, 11, 12, 14, & 15) and ICN 42 (hippocampus) in the CC domain. In addition, we observed a weaker negative correlation (q < 0.05) in psychosis between ICN 11 (right postcentral gyrus; R. PoCG) and ICN 53 (right cerebellum). Increased anticorrelation (a stronger negative correlation with q < 0.05) in psychosis was observed between ICN 11 (R. PoCG) and ICN 28 (SMFG), as well as between ICN 12 (anterior superior parietal lobule) and ICN 29 (L. IFG). We also observed increased anticorrelation (a stronger negative correlation with q < 0.05) in psychosis between ICN 11 (R. PoCG) and ICN 49 (dorsal posterior cingulate cortex; D. PCC) and between ICN 14 (precentral gyrus) and ICN 29 (L. IFG; negative correlation in psychosis relative to a positive correlation in controls with q < 0.05).
3.2.4. VI Domain
We also observed a trend of increased anticorrelations in psychosis between ICN 34 (supplemental motor area; SMA; see Fig. 4j) and ICNs in the VI domain (6 FNCs with q < 0.05). Hyperconnectivity (a positive correlation in psychosis relative to a negative correlation in CON, q < 0.05) was observed between ICN 17 (calcarine gyrus) and ICN 42 (P. hippocampus). And we observed dysconnectivity (q < 0.05) in the VI domain across several domains: Between VI ICN 19 (posterior MTG; see Fig. 4g) and SC ICN 5 (thalamus; weaker negative correlation in psychosis), between VI ICN 17 (calcarine gyrus) and AU ICN 6 (STG; weaker positive correlation in psychosis), and between VI ICN 24 (lingual gyrus) and CC ICN 29 (L. IFG; weaker positive correlation in psychosis). Perhaps the most notable pattern of dysconnectivity, however, is between VI ICN 19 (P. MTG) and CB ICNs 50, 51, and 53 (all weaker negative correlations in psychosis relative to controls with q < 0.05).
3.2.5. CC domain
Hyperconnectivity in the CC domain was observed in conjunction with SC (1 stronger positive correlation with q < 0.05), VI (1 positive correlation in psychosis relative to a negative correlation in controls with q < 0.05), and DM (3 stronger positive correlations with q < 0.05) domains. In addition, hyperconnectivity was observed within the CC domain (1 positive correlation in psychosis relative to a negative correlation in controls with q < 0.05). Increased anticorrelation in the CC domain was observed in conjunction with AU (2 negative correlations in psychosis relative to positive correlations in controls with q < 0.05), and VI (6 stronger negative correlations in psychosis relative to controls with q < 0.05), and domains. In addition, increased anticorrelation was observed within the CC domain (2 stronger negative correlations in psychosis with q < 0.05). Dysconnectivity in the CC domain was observed in conjunction with SC (7 weaker negative correlations in psychosis relative to controls, 1 weaker positive correlation with q < 0.05), AU (2 weaker negative correlations with q < 0.05), SM (6 weaker negative correlations with q < 0.05), VI (1 weaker positive correlation with q < 0.05), DM (1 weaker negative correlation with q < 0.05), and CB (5 weaker negative correlations with q < 0.05) domains. In addition, dysconnectivity was observed within the CC domain (1 weaker positive correlation with q < 0.05).
3.2.6. DM domain
We observed hyperconnectivity (stronger positive correlations in psychosis with q < 0.05) in the DM domain between ICN 49 (D. PCC) and ICNs 29 (L. IFG) and 31 (posterior middle frontal gyrus) in the CC domain. This was also observed between DM ICN 47 (lateral anterior cingulate cortex; L. ACC) and ICN 35 (superior frontal gyrus) in the CC domain. In contrast, we observed increased anticorrelation (stronger negative correlation in psychosis with q < 0.05) in the DM domain between ICN 49 (D. PCC) and ICNs 7 (A. MTG) and 11 (R. PoCG) from the AU and SM domains respectively. We observed this between DM ICN 44 (ventral precuneus) and AU ICN 6 (STG) as well. We observed dysconnectivity or a weaker negative correlation (q < 0.05) in psychosis between ICN 46 (Ventral PCC; see Fig. 4m) and ICN 29 (L. IFG).
3.2.7. CB domain
Many of the results pertaining to the CB domain have already been mentioned in the previous sections, however, to briefly summarize, there were 22 aberrant CB FNCs across SC, AU, SM, VI, and CC domains. Of these, 20 demonstrated hypoconnectivity or dysconnectivity either in the form of weaker positive correlations or weaker anticorrelations in psychosis relative to controls. We also observed hypoconnectivity (weaker positive correlation in psychosis with q < 0.05) within the CB between ICNs 50 (left cerebellum; see Fig. 4o) and 52 (medial cerebellum; see Fig. 4q). We observed a weaker positive correlation in psychosis (q < 0.05) between ICN 50 (L. cerebellum) and SC ICNs 1 (M. caudate) and 2 (subthalamus/hypothalamus). We also observed weaker positive correlations in psychosis (q < 0.05) between ICN 2 (subthalamus/hypothalamus) and ICN 52 (M. cerebellum) and between ICN 5 (thalamus) and CB ICNs 51, 52, and 53. We uniquely observed negative correlations in psychosis relative to positive correlations in controls (q < 0.05) between ICN 2 (subthalamus/hypothalamus) and ICNs 51 (P. cerebellum) and 53 (R. cerebellum). In the AU domain, we observed weaker negative correlations in psychosis relative to controls (q < 0.05) between ICN 6 (STG) and all cerebellar ICNS, as well as between ICN 7 (A. MTG) and ICN 50 (L. cerebellum). In the SM domain, we likewise observed a weaker negative correlation in psychosis (q < 0.05) between ICN 11 (R. PoCG) and ICN 53 (R. cerebellum). In the VI domain, again we observed weaker negative correlations in psychosis relative to controls (q < 0.05) between ICN 19 (P. MTG) and CB ICNs 50, 51, and 53. Lastly, in the CC domain we observed weaker positive correlations in psychosis (q < 0.05) between ICN 37 (A. hippocampus) and CB ICNs 51 (P. cerebellum) and 53 (R. cerebellum), as well as between ICN 42 (P. hippocampus) and CB ICNs 51, 52, and 53.
3.3. Statistical comparisons with a one-year psychosis subset
After quality control, the total sample reported in the current manuscript included 117 individuals with two-year psychosis. Our results from the diagnostic group case/control analysis highlighted 79 statistically significant aberrant FNC pairs which survived FDR correction (see Fig. 2). Acknowledging the variable definitions for FEP across studies (Breitborde et al., 2009, Faria et al., 2021, Salisbury et al., 2022, Wang et al., 2023, Yang et al., 2022), we decided to repeat our analysis including only those individuals who were within one year from their initial clinical contact for psychosis. This resulted in the exclusion of an additional 20 participants from our psychosis group (N = 97). We repeated the statistical case/control comparison to identify differences in FNC between individuals with one-year psychosis and controls. For each FNC, a linear model was used with age, sex, race, mean FD, and imaging site as covariates. All 79 of the aberrant FNC pairs reported in section 3.2 (N = 117) remained significant (p < 0.05) in the reduced sample (N = 97) prior to FDR correction. However, only 10 of these FNC pairs survived FDR correction (see Fig. 5). We compared the overall similarity of the results of our analysis with the two-year psychosis sample (N = 117) and one-year psychosis sample (N = 97) and found that the observed patterns of diagnostic effect on FNC were nearly identical (r = 0.97, p < 0.001). In other words, adjusting our psychosis sample from within two years to within one year of the first clinical contact reduced our sample size and in turn reduced our statistical power without changing the observed patterns of aberrant FNC.
Fig. 5.
Results of the general linear model (GLM) for each combination of the 53 Neuromark intrinsic connectivity networks (ICNs) in the reduced sample of psychosis within one year of their first clinical contact (N = 97) is displayed above. Significant (q < 0.05) case/control group associations with functional network connectivity (FNC) are marked with an asterisk. Note the similarity to Fig. 2c. ICNs are grouped by domain, consistent with the Neuromark_fMRI_1.0 template: subcortical (SC), auditory (AU), sensorimotor (SM), visual (VI), cognitive control (CC), default mode (DM), and cerebellar (CB).
3.4. Symptom severity and FNC
While we identified several statistically significant associations between positive (SAPS) and negative (SANS) symptom scores and FNC, none of these symptom associations survived FDR correction. We have attached a description of these findings as an appendix (see Appendix S2).
4. Discussion
4.1. Reliable and emerging patterns of aberrant connectivity
The current study utilized a data-driven approach similar to Fu et al., (2021a) and successfully identified multiple statistically significant psychosis-control group differences in FNC across all domains, many of which replicate previous findings. In addition, our results expand upon those of Fu et al., (2021a) by identifying unique patterns of aberrant connectivity in one and two-year psychosis samples which are likely more naïve (in contrast to the five-year psychosis sample utilized by Fu et al., (2021a)) to the effects of the various confounds previously mentioned. In particular, like Fu et al., (2021a), we observed pronounced altered patterns of connectivity between the cerebellum and subcortical structures (see Fig. 2, Fig. 3), including the caudate (ICN 1), thalamus (ICN 5), and subthalamic structures (ICN 2) associated with psychosis. And we expand upon the results of Fu et al., (2021a) with our observation of hypoconnectivity between the cerebellum (ICNs 51–53) and hippocampus (ICNs 37 & 42). Also like Fu et al., (2021a), we observed prominent aberrant connectivity between sensory cortex and cerebellar and subcortical structures associated with psychosis (see Fig. 3). However, unlike Fu et al., (2021a) we observed aberrant connectivity between the SMA (ICN 34) and subcortical structures as well as other sensory cortex in auditory and visual networks. Altogether, our findings appear to provide evidence of dysconnectivity in the cerebrocerebellar circuit (Buckner, 2013, McLachlan and Wilson, 2017) in individuals with one and two-year psychosis. These findings are consistent with a cognitive dysmetria theory of schizophrenia characterized by disruptions in cortical-subcortical-cerebellar circuitry (Andreasen et al., 1998). Furthermore, the patterns reported here may be characteristic of an earlier stage of psychosis, when the impact of other confounding factors is minimal.
4.2. Aberrant connectivity in auditory and language networks
Some of the most noteworthy findings in the current study pertain to auditory and language networks, many of which echo the patterns observed in individuals with psychosis in prior studies. In particular, the observed aberrant connectivity between the auditory cortex (ICNs 6, 7, & 19) and cerebellar ICNs (50–53) is consistent with results reported by Du et al. (2018) in individuals with early schizophrenia and clinical high-risk for psychosis, as well as results reported by Fu et al., (2021a) in an early-psychosis sample. In earlier work, Du et al. (2015) similarly described aberrant FNC between the cerebellum and temporal cortex associated with schizophrenia, bipolar, and schizoaffective disorders. We likewise observed these patterns (see temporal cortex ICNs 6/7/19 and the CB domain ICNs in Fig. 2, Fig. 4).
The observed dysconnectivity between a key auditory network structure (the STG, ICN 6) and subcortical networks (particularly the caudate, ICN 1; see Fig. 2) echoes the findings of Lottman et al. (2019). Also similar to our results, Zhang et al. (2021) observed STG-thalamic as well as sensorimotor-thalamic dysconnectivity. Notably, Zhang et al. (2021) emphasized their cross-sectional finding of increased sensorimotor-thalamic dysconnectivity in early-onset schizophrenia, and related it to similar findings in longitudinal studies (Anticevic et al., 2015, Cao et al., 2018), postulating that this pattern emerges early in the onset of psychosis and may contribute to the development of schizophrenia. In addition, Cao et al. (2018) posited that altered connectivity between the thalamus and sensorimotor regions might be indicative of a gating deficit responsible for aberrant subcortical sensory input to the cortex. Consistent with this, we observed statistically significant dysconnectivity in one and two-year psychosis between sensorimotor ICNs 9 and 10 (both in the postcentral gyri), and the thalamic ICN 5 (see Fig. 2, Fig. 4). Chang et al. (2017) reported aberrant connectivity between the postcentral gyrus and the language network, noting that the postcentral gyrus contains the primary somatosensory cortex, which is believed to integrate information about mouth movements into the process of speech perception (Skipper et al., 2005). In addition, we identified aberrant connectivity between the supplementary motor area (SMA; ICN 34) and various subcortical structures (ICNs 1, 2, & 5; see Fig. 2). The thalamocortical relationship reported here between ICNs 34 and 5 is comparable to findings reported in Zhang et al. (2021). Like the somatosensory cortex in the postcentral gyrus, the SMA similarly has an important role in speech and language processing through its integration of subcortical structures and functions (Hertrich et al., 2016). In general, disruptions in language-related regions and networks in psychotic disorders is a finding well-established by prior studies (Chang et al., 2017, Cui et al., 2016, DeLisi, 2001, Du et al., 2021, Lv et al., 2016, McGuire et al., 1993, Oertel-Knöchel et al., 2013, Salisbury et al., 2022).
The posterior cingulate cortex (PCC; ICNs 46 & 49; see Fig. 4m & n) is a key structure in the default mode network (DMN; Raichle et al., 2001) which is a large-scale brain network that has been widely implicated in psychotic disorder research (Buckner et al., 2008, Nair et al., 2020). In the current study, we observed hyperconnectivity between ICN 49 (D. PCC) and ICN 29 (L. IFG; see Fig. 2, Fig. 4i) which contains Broca’s area, a brain region well-known for its role in speech production and comprehension among other linguistic processes (Amunts and Zilles, 2012, Friederici, 2002, Hagoort, 2014). Interestingly, Iwashiro et al. (2016) and Salisbury et al. (2022), reported significantly decreased brain activity within the IFG in individuals with FES and FEP, respectively. The findings of Jung et al. (2012) are relevant here as well, which suggested that alterations in rs-fMRI in Broca’s area precede the onset of psychosis and that aberrant FC is associated with symptom severity. Our results appear to replicate theirs, lending further support to their conclusion; we observed multiple significant (p < 0.05) associations between FNC in the IFG and symptom severity (see Appendix S2). Also consistent with the findings of Jung et al. (2012), we observed dysconnectivity between the DMN and structures involved in language-related networks, specifically we observed increased anticorrelations between the PCC (ICN 49) and the right PoCG (ICN 11) as well as the middle temporal gyrus (MTG; ICN 7; see Fig. 4e). In contrast to these findings are those of Woodward et al., (2011) who observed hyperconnectivity between the PCC and MTG in chronic schizophrenia patients. O’Neill et al. (2019) postulated that hypoconnectivity between the DMN and regions in the language-network may underlie symptoms of psychosis. However, these mixed results across studies reporting both hyper- and hypoconnectivity between the DMN and regions in the language-network may suggest that we adopt a more general conclusion: psychosis is linked to aberrant connectivity between the DMN and regions in the language-network.
It is also interesting to note the cortico-cortical relationship with diagnostic group observed in FNC between the STG (ICN 6) and the IFG (ICN 29). Both of these regions are involved in the language network, and as a result, many studies have investigated and identified associations between the IFG, STG, and auditory verbal hallucinations (Jardri et al., 2011, Kompus et al., 2011, Kuhn and Gallinat, 2012, Zhang et al., 2015). While we did not observe associations between these regions and positive symptoms such as AVH, we did observe associations with negative symptoms (see Appendix S2). Dysfunction of Broca’s area within the IFG has previously been suggested as a contributor to the negative symptoms of schizophrenia, such as alogia (Du et al., 2021). In a FES population, Du et al. (2021) identified associations between aberrant connectivity in these language-related areas (STG and IFG) and negative symptoms, and similarly highlighted the prognostic value of these findings.
4.3. Cortico-cortical and cortical-subcortical dysconnectivity
Patterns of aberrant FNC in other sensory cortex in our results should be noted as well. Specifically, we observed widespread hypoconnectivity between the SMA (ICN 34) and ICNs in the visual network (ICNs 17, 19, 21–23, 25; see Fig. 2). As previously mentioned, the SMA has a key role in movement planning (Shima and Tanji, 1998, Tanji and Shima, 1994), which is a process that also incorporates information from the visual network. In addition, the SMA is involved in mental imagery (Zvyagintsev et al., 2013) and estimating time duration (Harvey et al., 2020, Protopapa et al., 2019). Notably, these functions are often impaired in individuals with schizophrenia (Dutschke et al., 2018, Mazhari et al., 2015, Ueda et al., 2018), which lends further support to the cognitive dysmetria framework for schizophrenia which suggests that cognitive deficits can account for the symptoms of schizophrenia (Andreasen et al., 1998). Our results are also consistent with those of Damaraju et al. (2014) and Iraji et al. (2019) who similarly observed hypoconnectivity in FNC between auditory, visual, and sensorimotor domain ICNs (the SMA corresponds with Brodmann area 6 or BA 6, which Damaraju et al., 2014 included in the sensorimotor domain rather than cognitive control as we did in the current study). While the auditory network has already been discussed at length, it should be noted that disruptions in visual processes have also been documented in psychotic disorders (Adámek et al., 2022, Türközer et al., 2019), and reduced cortical volumes have been reported in the visual areas of individuals with psychosis (Adhan et al., 2020, Reig et al., 2009). Interestingly, the aberrant FNC we observed appears to be associated with ICNs overlapping with higher-order visual areas such as visual association cortex (e.g., ICN 17) and those associated with the dorsal (DAN; e.g., ICN 34) and ventral attention networks (VAN; e.g., ICN 19) more than ICNs centered on primary visual cortex (V1; e.g., ICNs 18 & 20). Although we did not specifically test for associations between visual hallucinations and FNC, our finding that aberrant FNC in these areas was associated with psychosis appears to be consistent with van Ommen et al. (2023), who described both the absence of V1 activity during visual hallucinations and activation of higher-order visual areas. van Ommen et al. (2023) proposed that visual hallucinations may result from a dissociation of higher-order visual processing areas (e.g., DAN and VAN) from V1. The consistency between our results and this framework may serve as another example of possible disruptions in cognitive processes which may account for symptoms of psychosis.
In a meta-analysis of spontaneous brain activity (rs-fMRI) in FEP, Cattarinussi et al. (2023) highlighted a pattern of increased spontaneous neural activity in the striatum across various studies. The widespread dysconnectivity we observed between the caudate (ICN 1) and several other brain regions (see Fig. 2) appears to be consistent with this observation. In particular, our observed hyperconnectivity between the caudate (ICN 1) and the superior medial frontal gyrus (ICN 28) is also reported by Cattarinussi et al. (2023). Similarly, our observed pattern of dysconnectivity between the caudate (ICN 1) and the SMA (ICN 34; see Fig. 2) is also consistent with Cattarinussi et al. (2023). Oh et al. (2020) similarly observed hyperconnectivity between the striatum and frontal cortex in FEP. Overall, our findings further support the notion that disruptions in the fronto-striatal circuit represent key pathophysiological alterations which may serve as reliable indicators of psychosis.
4.4. Aberrant connectivity in subcortical and cerebellar regions
Another key component of Andreasen and colleagues' (1998) theory of cognitive dysmetria is the dysfunctional relationship between subcortical structures and the cerebellum. In the current study, we observed evidence of this through patterns of cerebellar-thalamic (ICNs 51–53 and ICN 5) hypoconnectivity (see Fig. 2, Fig. 4). Patterns of aberrant cerebellar-thalamic connectivity have also been reported by studies in individuals with clinical high risk for psychosis (hyperconnectivity; Cao et al., 2018), early onset-schizophrenia (hypoconnectivity; Zhang et al., 2021), early psychosis (Fu et al., 2021a), and chronic schizophrenia (hypoconnectivity; Chen et al., 2013, Wang et al., 2014). We also observed hypoconnectivity between the hippocampus (ICNs 37/42) and cerebellum (ICNs 51–53). Du et al. (2015) likewise observed disruptions in FNC between the hippocampus and cerebellum in a transdiagnostic sample including individuals with schizophrenia, bipolar, and schizoaffective disorders. Notably, Du et al., 2015, Du et al., 2018a, and Clark et al. (2020) placed a strong emphasis on cerebellum-related aberrant FC as a potential biomarker for psychotic disorders; this point is recapitulated and further supported in light of the present study which demonstrated widespread dysconnectivity (22 aberrant CB FNCs; see Fig. 2, Fig. 3) between the cerebellum and SC, AU, SM, VI, and CC domains, as well as within the cerebellum (ICNs 50 & 52; see Fig. 4). Specifically, nearly all of these FNCs were reduced (either weaker positive correlations or weaker anticorrelations) in psychosis, which is consistent with Clark et al. (2020), who exclusively observed weaker cerebellocortical FC in a chronic schizophrenia sample. It has been suggested that one of the roles of the cerebellum is to regulate attributes of cognitive processes such as speed, capacity, and appropriateness (Buckner, 2013). If the observed patterns of aberrant FNC are interpreted as disruptions in the regulation of cognitive processes, as suggested by the cognitive dysmetria framework (Andreasen et al., 1998), then weaker cerebellocortical FNC might serve as a key indicator of under-regulated cognitive processes which may manifest as symptoms of psychosis.
4.5. Limitations and future directions
While we identified several significant associations between symptoms of psychosis and FNC, these did not survive FDR correction. However, other studies have likewise failed to identify statistically significant associations with various symptom measures (Guo et al., 2018, Zhang et al., 2021, Zhang et al., 2015) and notably those reported by Fu et al., (2021a) also did not survive FDR correction. One explanation for this is that the participants in the psychosis sample included in our study are being evaluated during a recovery stage (within months to years following their first clinical contact; see Table 1) when symptoms are milder. As a result, the modest effect size does not enable these results to survive a relatively conservative statistical correction. As suggested by Fu et al., (2021a), the effect may be weakened by the heterogenous nature of psychosis, or by our statistical correction for the multiple sites where data was collected. However, even though the effects did not survive FDR correction, it is worth noting that the associated patterns we found between symptom severity and FNC were generally consistent with our findings from our analysis of the psychosis diagnostic group and FNC (see Appendix S2).
Future studies seeking to increase statistical power of relatively small symptom effects might consider strategies to increase their sample size. Many of the previous studies discussed (Bang et al., 2018, Chen et al., 2013, Du et al., 2018b, Guo et al., 2018, Iwashiro et al., 2016, Lottman et al., 2019, Salisbury et al., 2022) utilized samples less than half the size (N < 59 patients) of the current study (N = 117 patients). While our study no doubt benefitted from the relatively large sample size, future studies will likely benefit from utilizing an even larger sample as well as a more universal network template (Iraji et al., 2023). Recent work by Iraji et al., (2022a) also demonstrates how additional insight can be gained through utilizing multiple spatial scales and sex-specific alterations. Future studies might leverage features such as brain dynamics (e.g., both spatial and temporal; Damaraju et al., 2014, Iraji et al., 2019) to identify unique biomarkers of FEP through multiscale ICA (Iraji et al., 2022b).
Another important consideration are the effects of medication. While we anticipate that the effects of antipsychotic use were reduced in our sample due to the relatively short duration of illness in our participants with psychosis, we acknowledge the potential impact this confound may have had on our results. While it is difficult to determine exactly how our results may have been impacted, previous studies have linked antipsychotic use to structural and functional alterations in several brain regions in individuals with FES and FEP (Chopra et al., 2021, Ebdrup et al., 2011, Li et al., 2012, Li et al., 2018, Yang et al., 2021). Future studies might consider other strategies (e.g., recruiting drug-naïve groups or accounting for antipsychotic use in statistical analyses) for disentangling some of the effects of antipsychotic use in their analyses.
5. Conclusions
The findings in the current study have the advantage of being more informative and comprehensive due to the nature of the data-driven whole-brain approach utilized. This is evident in the wide range of FNCs associated with the psychosis group (see Fig. 2). Indeed, these results illustrate patterns of dysconnectivity in brain-wide cortical-subcortical-cerebellar networks, offering further support to existing theory (Andreasen et al., 1998, Friston, 1998). Overall, the results of the current study help to highlight key patterns of aberrant FNC associated with one and two-year psychosis. Many of these patterns of aberrant connectivity have been observed in previous studies in populations experiencing early psychosis and schizophrenia, although there are some differences in directionality (i.e., hyper- and hypoconnectivity). Our observations of these patterns within one and two years of the first clinical contact for psychosis is especially useful for the purpose of establishing stable biomarkers for psychosis at a critical period, early after the onset of symptoms. These findings will hopefully inform future research efforts as we strive to identify and better understand the biological mechanisms associated with psychosis.
CRediT authorship contribution statement
Kyle M. Jensen: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Visualization, Writing – original draft, Writing – review & editing. Vince D. Calhoun: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Software, Supervision, Writing – review & editing. Zening Fu: Data curation, Methodology, Software, Writing – review & editing. Kun Yang: Data curation, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – review & editing. Andreia V. Faria: Data curation. Koko Ishizuka: Data curation. Akira Sawa: Data curation, Funding acquisition. Pablo Andrés-Camazón: Writing – review & editing. Brian A. Coffman: Data curation. Dylan Seebold: Data curation. Jessica A. Turner: Conceptualization, Data curation, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. Dean F. Salisbury: Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – review & editing. Armin Iraji: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding Information: This work was supported by the National Institutes of Health [grant numbers MH092443 (to AS), MH094268 (to AS), MH105660 (to AS), MH107730 (to AS), MH119251 (to AI), MH118695 (to VDC), MH103204 (to DFS), MH108568 (to DFS), MH113533 (to DFS)], the Stanley Foundation (to AS), the RUSK Foundation (to AS), and the S-R Foundation (to AS). KMJ received support from the Georgia State University Second Century Initiative (2CI) Doctoral Fellowship. PAC received grant support from Programa Intramural de Impulso a la I+D+i (Instituto de Investigación Sanitaria Gregorio Marañón).
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2024.103584.
An advantage of basing the definition for FEP or EP on the emergence of symptoms is that one can examine individuals in the earliest stages of illness by accounting for untreated psychosis. Individuals often will experience psychosis for one to two years before receiving treatment from a clinician (Kane et al., 2016; Marshall et al., 2005; Maximo et al., 2020; Srihari et al., 2022). However, choosing to define FEP and EP based on the duration of treatment may be more reliable and less subjective as it is well documented by a trained clinician and does not rely on a retrospective self-reported measure.
Small sample sizes may result in underpowered studies (Pettersson-Yeo et al., 2011). Thus, further research is needed utilizing larger samples in order to elucidate and establish more stable and reliable patterns of aberrant brain connectivity in FEP and EP populations (Satterthwaite & Baker, 2015). Toward this goal, the current study combines data from multiple sites to increase the sample size and achieve more optimal power.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Adámek P., Langová V., Horáček J. Early-stage visual perception impairment in schizophrenia, bottom-up and back again. Schizophrenia. 2022;8(1):Article 1. doi: 10.1038/s41537-022-00237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhan I., Lizano P., Bannai D., Lutz O., Dhaliwal K., Zeng V., Miewald J., Montrose D., Keshavan M. Visual cortical alterations and their association with negative symptoms in antipsychotic-naïve first episode psychosis. Psychiatry Res. 2020;288 doi: 10.1016/j.psychres.2020.112957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Solís A., Corripio I., de Castro-Manglano P., Duran-Sindreu S., Garcia-Garcia M., Proal E., Nuñez-Marín F., Soutullo C., Alvarez E., Gómez-Ansón B., Kelly C., Castellanos F.X. Altered default network resting state functional connectivity in patients with a first episode of psychosis. Schizophr. Res. 2012;139(1–3):13–18. doi: 10.1016/j.schres.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K., Zilles K. Architecture and organizational principles of Broca’s region. Trends Cogn. Sci. 2012;16(8):418–426. doi: 10.1016/j.tics.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Andreasen N.C. The University of Iowa; Iowa City, Iowa: 1983. The Scale for the Assessment of Negative Symptoms (SANS) [Google Scholar]
- Andreasen N.C. The University of Iowa; Iowa City, Iowa: 1984. The Scale for the Assessment of Positive Symptoms (SAPS) [Google Scholar]
- Andreasen N.C., Paradiso S., O’Leary D.S. “Cognitive dysmetria” as an integrative theory of schizophrenia: A dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr. Bull. 1998;24(2):203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Andreasen N.C., Pierson R. The role of the cerebellum in Schizophrenia. Biol. Psychiatry. 2008;64(2):81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou C., Borgwardt S. Structural and functional imaging markers for susceptibility to psychosis. Mol. Psychiatry. 2020;25(11):2773–2785. doi: 10.1038/s41380-020-0679-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A., Haut K., Murray J.D., Repovs G., Yang G.J., Diehl C., McEwen S.C., Bearden C.E., Addington J., Goodyear B., Cadenhead K.S., Mirzakhanian H., Cornblatt B.A., Olvet D., Mathalon D.H., McGlashan T.H., Perkins D.O., Belger A., Seidman L.J., Cannon T.D. Association of Thalamic Dysconnectivity and Conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiat. 2015;72(9):882–891. doi: 10.1001/jamapsychiatry.2015.0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagnostic and statistical manual of mental disorders: DSM-IV. (1994). Washington, DC: American Psychiatric Association. (n.d.).
- Arciniegas, D.B. (2015). Psychosis. Continuum : Lifelong Learning in Neurology, 21(3 Behavioral Neurology and Neuropsychiatry), 715–736. https://doi.org/10.1212/01.CON.0000466662.89908.e7. [DOI] [PMC free article] [PubMed]
- Bang M., Park H.-J., Pae C., Park K., Lee E., Lee S.-K., An S.K. Aberrant cerebro-cerebellar functional connectivity and minimal self-disturbance in individuals at ultra-high risk for psychosis and with first-episode schizophrenia. Schizophr. Res. 2018;202:138–140. doi: 10.1016/j.schres.2018.06.031. [DOI] [PubMed] [Google Scholar]
- Bird V., Premkumar P., Kendall T., Whittington C., Mitchell J., Kuipers E. Early intervention services, cognitive–behavioural therapy and family intervention in early psychosis: Systematic review. Br. J. Psychiatry. 2010;197(5):350–356. doi: 10.1192/bjp.bp.109.074526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Zerrin Yetkin F., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn. Reson. Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Breitborde N.J.K., Srihari V.H., Woods S.W. Review of the operational definition for first-episode psychosis. Early Interv. Psychiatry. 2009;3(4):259–265. doi: 10.1111/j.1751-7893.2009.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80(3):807–815. doi: 10.1016/j.neuron.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124(1):1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Adali T., Pearlson G.D., Pekar J.J. A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp. 2001;14(3):140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V.D., Liu J., Adalı T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage. 2009;45(1):S163–S172. doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Chén O.Y., Chung Y., Forsyth J.K., McEwen S.C., Gee D.G., Bearden C.E., Addington J., Goodyear B., Cadenhead K.S., Mirzakhanian H., Cornblatt B.A., Carrión R.E., Mathalon D.H., McGlashan T.H., Perkins D.O., Belger A., Seidman L.J., Thermenos H., Cannon T.D. Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nat. Commun. 2018;9(1):3836. doi: 10.1038/s41467-018-06350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattarinussi, G., Grimaldi, D.A., & Sambataro, F. (2023). Spontaneous Brain Activity Alterations in First-Episode Psychosis: A Meta-analysis of Functional Magnetic Resonance Imaging Studies. https://doi.org/10.1093/schbul/sbad044. [DOI] [PMC free article] [PubMed]
- Chang X., Collin G., Xi Y., Cui L., Scholtens L.H., Sommer I.E., Wang H., Yin H., Kahn R.S., van den Heuvel M.P. Resting-state functional connectivity in medication-naïve schizophrenia patients with and without auditory verbal hallucinations: A preliminary report. Schizophr. Res. 2017;188:75–81. doi: 10.1016/j.schres.2017.01.024. [DOI] [PubMed] [Google Scholar]
- Chen J., Liu J., Calhoun V.D., Arias-Vasquez A., Zwiers M.P., Gupta C.N., Franke B., Turner J.A. Exploration of scanning effects in multi-site structural MRI studies. J. Neurosci. Methods. 2014;230:37–50. doi: 10.1016/j.jneumeth.2014.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.-L., Tu P.-C., Lee Y.-C., Chen Y.-S., Li C.-T., Su T.-P. Resting-state fMRI mapping of cerebellar functional dysconnections involving multiple large-scale networks in patients with schizophrenia. Schizophr. Res. 2013;149(1–3):26–34. doi: 10.1016/j.schres.2013.05.029. [DOI] [PubMed] [Google Scholar]
- Chopra S., Fornito A., Francey S.M., O’Donoghue B., Cropley V., Nelson B., Graham J., Baldwin L., Tahtalian S., Yuen H.P., Allott K., Alvarez-Jimenez M., Harrigan S., Sabaroedin K., Pantelis C., Wood S.J., McGorry P. Differentiating the effect of antipsychotic medication and illness on brain volume reductions in first-episode psychosis: A longitudinal, randomised, triple-blind, placebo-controlled MRI study. Neuropsychopharmacology. 2021;46(8):Article 8. doi: 10.1038/s41386-021-00980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J., Miller B.J. Meta-analysis of comorbid diabetes and family history of diabetes in non-affective psychosis. Schizophr. Res. 2020;216:41–47. doi: 10.1016/j.schres.2019.10.062. [DOI] [PubMed] [Google Scholar]
- Clark S.V., Tannahill A., Calhoun V.D., Bernard J.A., Bustillo J., Turner J.A. Weaker cerebellocortical connectivity within sensorimotor and executive networks in schizophrenia compared to healthy controls: Relationships with processing speed. Brain Connect. 2020;10(9):490–503. doi: 10.1089/brain.2020.0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L.-B., Liu K., Li C., Wang L.-X., Guo F., Tian P., Wu Y.-J., Guo L., Liu W.-M., Xi Y.-B., Wang H.-N., Yin H. Putamen-related regional and network functional deficits in first-episode schizophrenia with auditory verbal hallucinations. Schizophr. Res. 2016;173(1–2):13–22. doi: 10.1016/j.schres.2016.02.039. [DOI] [PubMed] [Google Scholar]
- Damaraju E., Allen E.A., Belger A. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. NeuroImage: Clinical. 2014;5:298–308. doi: 10.1016/j.nicl.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin B., Suckling J., Barnes T.R.E., Byrne K., Chaudhry I.B., Dazzan P., Drake R.J., Giordano A., Husain N., Jones P.B., Joyce E., Knox E., Krynicki C., Lawrie S.M., Lewis S., Lisiecka-Ford D.M., Nikkheslat N., Pariante C.M., Smallman R., Dunn G. The benefit of minocycline on negative symptoms of schizophrenia in patients with recent-onset psychosis (BeneMin): A randomised, double-blind, placebo-controlled trial. Lancet Psychiatry. 2018;5(11):885–894. doi: 10.1016/S2215-0366(18)30345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Fabro L., Schmidt A., Fortea L., Delvecchio G., D’Agostino A., Radua J., Borgwardt S., Brambilla P. Functional brain network dysfunctions in subjects at high-risk for psychosis: A meta-analysis of resting-state functional connectivity. Neurosci. Biobehav. Rev. 2021;128:90–101. doi: 10.1016/j.neubiorev.2021.06.020. [DOI] [PubMed] [Google Scholar]
- DeLisi L.E. Speech disorder in schizophrenia: Review of the literature and exploration of its relation to the uniquely human capacity for language. Schizophr. Bull. 2001;27(3):481–496. doi: 10.1093/oxfordjournals.schbul.a006889. [DOI] [PubMed] [Google Scholar]
- Dempster K., Jeon P., MacKinley M., Williamson P., Théberge J., Palaniyappan L. Early treatment response in first episode psychosis: A 7-T magnetic resonance spectroscopic study of glutathione and glutamate. Mol. Psychiatry. 2020;25(8):Article 8. doi: 10.1038/s41380-020-0704-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Fan Y. Group information guided ICA for fMRI data analysis. Neuroimage. 2013;69:157–197. doi: 10.1016/j.neuroimage.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Du Y., Pearlson G.D., He H., Wu L., Chen J., Calhoun V.D. 2015 IEEE 12th International Symposium on Biomedical Imaging (ISBI) 2015. Identifying brain dynamic network states via GIG-ICA: application to schizophrenia, bipolar and schizoaffective disorders; pp. 478–481. [DOI] [Google Scholar]
- Du Y., Pearlson G.D., Yu Q., He H., Lin D., Sui J., Wu L., Calhoun V.D. Interaction among subsystems within default mode network diminished in schizophrenia patients: A dynamic connectivity approach. Schizophr. Res. 2016;170(1):55–65. doi: 10.1016/j.schres.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Fryer S.L., Fu Z., Lin D., Sui J., Chen J., Damaraju E., Mennigen E., Stuart B., Loewy R.L., Mathalon D.H., Calhoun V.D. Dynamic functional connectivity impairments in early schizophrenia and clinical high-risk for psychosis. Neuroimage. 2018;180:632–645. doi: 10.1016/j.neuroimage.2017.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Fryer S.L., Lin D., Sui J., Yu Q., Chen J., Stuart B., Loewy R.L., Calhoun V.D., Mathalon D.H. Identifying functional network changing patterns in individuals at clinical high-risk for psychosis and patients with early illness schizophrenia: A group ICA study. NeuroImage: Clinical. 2018;17:335–346. doi: 10.1016/j.nicl.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Fu Z., Sui J., Gao S., Xing Y., Lin D., Salman M., Abrol A., Rahaman M.A., Chen J., Hong L.E., Kochunov P., Osuch E.A., Calhoun V.D. NeuroMark: An automated and adaptive ICA based pipeline to identify reproducible fMRI markers of brain disorders. NeuroImage: Clinical. 2020;28 doi: 10.1016/j.nicl.2020.102375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Palaniyappan L., Liu Z., Cheng W., Gong W., Zhu M., Wang J., Zhang J., Feng J. The genetic determinants of language network dysconnectivity in drug-naïve early stage schizophrenia. NPJ Schizophr. 2021;7(1):18. doi: 10.1038/s41537-021-00141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschke L.L., Stegmayer K., Ramseyer F., Bohlhalter S., Vanbellingen T., Strik W., Walther S. Gesture impairments in schizophrenia are linked to increased movement and prolonged motor planning and execution. Schizophr. Res. 2018;200:42–49. doi: 10.1016/j.schres.2017.07.012. [DOI] [PubMed] [Google Scholar]
- Ebdrup B.H., Skimminge A., Rasmussen H., Aggernaes B., Oranje B., Lublin H., Baaré W., Glenthøj B. Progressive striatal and hippocampal volume loss in initially antipsychotic-naive, first-episode schizophrenia patients treated with quetiapine: Relationship to dose and symptoms. Int. J. Neuropsychopharmacol. 2011;14(1):69–82. doi: 10.1017/S1461145710000817. [DOI] [PubMed] [Google Scholar]
- Faria A.V., Zhao Y., Ye C., Hsu J., Yang K., Cifuentes E., Wang L., Mori S., Miller M., Caffo B., Sawa A. Multimodal MRI assessment for first episode psychosis: A major change in the thalamus and an efficient stratification of a subgroup. Hum. Brain Mapp. 2021;42(4):1034–1053. doi: 10.1002/hbm.25276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaum M.A., Andreasen N.C., Arndt S. The Iowa prospective longitudinal study of recent-onset psychoses. Schizophr. Bull. 1992;18(3):481–490. doi: 10.1093/schbul/18.3.481. [DOI] [PubMed] [Google Scholar]
- Fornito A., Harrison B.J., Goodby E., Dean A., Ooi C., Nathan P.J., Lennox B.R., Jones P.B., Suckling J., Bullmore E.T. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiat. 2013;70(11):1143. doi: 10.1001/jamapsychiatry.2013.1976. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. Towards a neural basis of auditory sentence processing. Trends Cogn. Sci. 2002;6(2):78–84. doi: 10.1016/S1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- Friston K.J. The disconnection hypothesis. Schizophr. Res. 1998;30(2):115–125. doi: 10.1016/S0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- Fu Z., Iraji A., Sui J., Calhoun V.D. Whole-brain functional network connectivity abnormalities in affective and non-affective early phase psychosis. Front. Neurosci. 2021;15 doi: 10.3389/fnins.2021.682110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z., Iraji A., Turner J.A., Sui J., Miller R., Pearlson G.D., Calhoun V.D. Dynamic state with covarying brain activity-connectivity: On the pathophysiology of schizophrenia. Neuroimage. 2021;224 doi: 10.1016/j.neuroimage.2020.117385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Bechdolf A., Taylor M.J., Bonoldi I., Carpenter W.T., Yung A.R., McGuire P. At risk for schizophrenic or affective psychoses? A meta-analysis of DSM/ICD diagnostic outcomes in individuals at high clinical risk. Schizophr. Bull. 2013;39(4):923–932. doi: 10.1093/schbul/sbs060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganella E.P., Seguin C., Pantelis C., Whittle S., Baune B.T., Olver J., Amminger G.P., McGorry P.D., Cropley V., Zalesky A., Bartholomeusz C.F. Resting-state functional brain networks in first-episode psychosis: A 12-month follow-up study. Aust. N. Z. J. Psychiatry. 2018;52(9):864–875. doi: 10.1177/0004867418775833. [DOI] [PubMed] [Google Scholar]
- Gong J., Wang J., Luo X., Chen G., Huang H., Huang R., Huang L., Wang Y. Abnormalities of intrinsic regional brain activity in first-episode and chronic schizophrenia: A meta-analysis of resting-state functional MRI. J. Psychiatry Neurosci. 2020;45(1):55–68. doi: 10.1503/jpn.180245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Zhang F., Liu F., Chen J., Wu R., Chen D.Q., Zhang Z., Zhai J., Zhao J. Cerebellar abnormalities in first-episode, drug-naive schizophrenia at rest. Psychiatry Res. Neuroimaging. 2018;276:73–79. doi: 10.1016/j.pscychresns.2018.03.010. [DOI] [PubMed] [Google Scholar]
- Hagoort P. Nodes and networks in the neural architecture for language: Broca’s region and beyond. Curr. Opin. Neurobiol. 2014;28:136–141. doi: 10.1016/j.conb.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Harvey B.M., Dumoulin S.O., Fracasso A., Paul J.M. A network of topographic maps in human association cortex hierarchically transforms visual timing-selective responses. Curr. Biol. 2020;30(8):1424–1434.e6. doi: 10.1016/j.cub.2020.01.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertrich I., Dietrich S., Ackermann H. The role of the supplementary motor area for speech and language processing. Neurosci. Biobehav. Rev. 2016;68:602–610. doi: 10.1016/j.neubiorev.2016.06.030. [DOI] [PubMed] [Google Scholar]
- Hickie I.B., Scott E.M., Cross S.P., Iorfino F., Davenport T.A., Guastella A.J., Naismith S.L., Carpenter J.S., Rohleder C., Crouse J.J., Hermens D.F., Koethe D., Markus Leweke F., Tickell A.M., Sawrikar V., Scott J. Right care, first time: A highly personalised and measurement-based care model to manage youth mental health. Med. J. Aust. 2019;211(Suppl 9):S3–S46. doi: 10.5694/mja2.50383. [DOI] [PubMed] [Google Scholar]
- Ho B.-C., Andreasen N.C., Ziebell S., Pierson R., Magnotta V. Long-term antipsychotic treatment and brain volumes: A longitudinal study of first-episode schizophrenia. Arch. Gen. Psychiatry. 2011;68(2):128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A., Levi P.T., Chen Y.-C., Chopra S., Aquino K.M., Pang J.C., Fornito A. Disruptions of hierarchical cortical organization in early psychosis and schizophrenia. Biol. Psychiatr.: Cogn. Neurosci. Neuroimaging. 2023 doi: 10.1016/j.bpsc.2023.08.008. [DOI] [PubMed] [Google Scholar]
- Insel T.R., Cuthbert B.N. Brain disorders? Precisely. Science. 2015;348(6234):499–500. doi: 10.1126/science.aab2358. [DOI] [PubMed] [Google Scholar]
- Iraji A., Deramus T.P., Lewis N., Yaesoubi M., Stephen J.M., Erhardt E., Belger A., Ford J.M., McEwen S., Mathalon D.H., Mueller B.A., Pearlson G.D., Potkin S.G., Preda A., Turner J.A., Vaidya J.G., van Erp T.G.M., Calhoun V.D. The spatial chronnectome reveals a dynamic interplay between functional segregation and integration. Hum. Brain Mapp. 2019;40(10):3058–3077. doi: 10.1002/hbm.24580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraji A., Miller R., Adali T., Calhoun V.D. Space: A missing piece of the dynamic puzzle. Trends Cogn. Sci. 2020;24(2):135–149. doi: 10.1016/j.tics.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraji A., Faghiri A., Lewis N., Fu Z., Rachakonda S., Calhoun V.D. Tools of the trade: Estimating time-varying connectivity patterns from fMRI data. Soc. Cogn. Affect. Neurosci. 2021;16(8):849–874. doi: 10.1093/scan/nsaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraji A., Faghiri A., Fu Z., Kochunov P., Adhikari B.M., Belger A., Ford J.M., McEwen S., Mathalon D.H., Pearlson G.D., Potkin S.G., Preda A., Turner J.A., Van Erp T.G.M., Chang C., Calhoun V.D. Moving beyond the ‘CAP’ of the Iceberg: Intrinsic connectivity networks in fMRI are continuously engaging and overlapping. Neuroimage. 2022;251 doi: 10.1016/j.neuroimage.2022.119013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraji A., Faghiri A., Fu Z., Rachakonda S., Kochunov P., Belger A., Ford J.M., McEwen S., Mathalon D.H., Mueller B.A., Pearlson G.D., Potkin S.G., Preda A., Turner J.A., van Erp T.G.M., Calhoun V.D. Multi-spatial-scale dynamic interactions between functional sources reveal sex-specific changes in schizophrenia. Network Neurosci. 2022;6(2):357–381. doi: 10.1162/netn_a_00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraji A., Fu Z., Faghiri A., Duda M., Chen J., Rachakonda S., DeRamus T., Kochunov P., Adhikari B.M., Belger A., Ford J.M., Mathalon D.H., Pearlson G.D., Potkin S.G., Preda A., Turner J.A., van Erp T.G.M., Bustillo J.R., Yang K., Ishizuka K., Faria A., Sawa A., Hutchison K., Osuch E.A., Theberge J., Abbott C., Mueller B.A., Zhi D., Zhuo C., Liu S., Xu Y., Salman M., Liu J., Du Y., Sui J., Adali T., Calhoun V.D. Identifying canonical and replicable multi-scale intrinsic connectivity networks in 100k+ resting-state fMRI datasets. Hum. Brain Mapp. 2023;44(17):5729–5748. doi: 10.1002/hbm.26472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashiro N., Koike S., Satomura Y., Suga M., Nagai T., Natsubori T., Tada M., Gonoi W., Takizawa R., Kunimatsu A., Yamasue H., Kasai K. Association between impaired brain activity and volume at the sub-region of Broca’s area in ultra-high risk and first-episode schizophrenia: A multi-modal neuroimaging study. Schizophr. Res. 2016;172(1):9–15. doi: 10.1016/j.schres.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Jafri M.J., Pearlson G.D., Stevens M., Calhoun V.D. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39(4):1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardri R., Pouchet A., Pins D., Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: A coordinate-based meta-analysis. Am. J. Psychiatry. 2011;168(1):73–81. doi: 10.1176/appi.ajp.2010.09101522. [DOI] [PubMed] [Google Scholar]
- Jauhar S., McCutcheon R., Borgan F., Veronese M., Nour M., Pepper F., Rogdaki M., Stone J., Egerton A., Turkheimer F., McGuire P., Howes O.D. The relationship between cortical glutamate and striatal dopamine in first-episode psychosis: A cross-sectional multimodal PET and magnetic resonance spectroscopy imaging study. Lancet Psychiatry. 2018;5(10):816–823. doi: 10.1016/S2215-0366(18)30268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno N., Gomez-Pilar J., Poza J., Hornero R., Vogeley K., Meisenzahl E., Haidl T., Rosen M., Klosterkötter J., Schultze-Lutter F. Main symptomatic treatment targets in suspected and early psychosis: new insights from network analysis. Schizophr. Bull. 2020;46(4):884–895. doi: 10.1093/schbul/sbz140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung W.H., Jang J.H., Shin N.Y., Kim S.N., Choi C.-H., An S.K., Kwon J.S. Regional brain atrophy and functional disconnection in Broca’s area in individuals at ultra-high risk for psychosis and schizophrenia. PLoS One. 2012;7(12):e51975. doi: 10.1371/journal.pone.0051975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J.M., Robinson D.G., Schooler N.R., Mueser K.T., Penn D.L., Rosenheck R.A., Addington J., Brunette M.F., Correll C.U., Estroff S.E., Marcy P., Robinson J., Meyer-Kalos P.S., Gottlieb J.D., Glynn S.M., Lynde D.W., Pipes R., Kurian B.T., Miller A.L., Heinssen R.K. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program. Am. J. Psychiatry. 2016;173(4):362–372. doi: 10.1176/appi.ajp.2015.15050632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan M.S., Clementz B.A. Precision medicine for psychosis: A revolution at the interface of psychiatry and neurology. Nat. Rev. Neurol. 2023 doi: 10.1038/s41582-023-00788-0. [DOI] [PubMed] [Google Scholar]
- Khadka S., Meda S.A., Stevens M.C., Glahn D.C., Calhoun V.D., Sweeney J.A., Tamminga C.A., Keshavan M.S., O’Neil K., Schretlen D., Pearlson G.D. Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biol. Psychiatry. 2013;74(6):458–466. doi: 10.1016/j.biopsych.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhar J.Y., Dwiel L., Henricks A., Doucette W.T., Green A.I. The link between schizophrenia and substance use disorder: A unifying hypothesis. Schizophr. Res. 2018;194:78–85. doi: 10.1016/j.schres.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Shin S.H., Santangelo B., Veronese M., Kang S.K., Lee J.S., Cheon G.J., Lee W., Kwon J.S., Howes O.D., Kim E. Dopamine dysregulation in psychotic relapse after antipsychotic discontinuation: An [18F]DOPA and [11C]raclopride PET study in first-episode psychosis. Mol. Psychiatry. 2021;26(7):Article 7. doi: 10.1038/s41380-020-00879-0. [DOI] [PubMed] [Google Scholar]
- Kompus K., Westerhausen R., Hugdahl K. The “paradoxical” engagement of the primary auditory cortex in patients with auditory verbal hallucinations: A meta-analysis of functional neuroimaging studies. Neuropsychologia. 2011;49(12):3361–3369. doi: 10.1016/j.neuropsychologia.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Korhonen O., Zanin M., Papo D. Principles and open questions in functional brain network reconstruction. Hum. Brain Mapp. 2021;42(11):3680–3711. doi: 10.1002/hbm.25462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiyama D., Kirihara K., Tada M., Nagai T., Fujioka M., Ichikawa E., Ohta K., Tani M., Tsuchiya M., Kanehara A., Morita K., Sawada K., Matsuoka J., Satomura Y., Koike S., Suga M., Araki T., Kasai K. Electrophysiological evidence for abnormal glutamate-GABA association following psychosis onset. Transl. Psychiatry. 2018;8(1):Article 1. doi: 10.1038/s41398-018-0261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S., Gallinat J. Quantitative meta-analysis on state and trait aspects of auditory verbal hallucinations in schizophrenia. Schizophr. Bull. 2012;38(4):779–786. doi: 10.1093/schbul/sbq152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak Y.B., Cho K.I.K., Hwang W.J., Kim A., Ha M., Park H., Lee J., Lee T.Y., Kim M., Kwon J.S. Mapping thalamocortical functional connectivity with large-scale brain networks in patients with first-episode psychosis. Sci. Rep. 2021;11(1):Article 1. doi: 10.1038/s41598-021-99170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurens K.R., Cullen A.E. Toward earlier identification and preventative intervention in schizophrenia: Evidence from the London child health and development study. Soc. Psychiatry Psychiatr. Epidemiol. 2016;51(4):475–491. doi: 10.1007/s00127-015-1151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.-H., Oh H., Suh J.S., Cho K.I.K., Yoon Y.B., Shin W.-G., Lee T.Y., Kwon J.S. Functional and structural connectivity of the cerebellar nuclei with the striatum and cerebral cortex in first-episode psychosis. J. Neuropsychiatr. Clin. Neurosci. 2019;31(2):143–151. doi: 10.1176/appi.neuropsych.17110276. [DOI] [PubMed] [Google Scholar]
- Lencz T., Moyett A., Argyelan M., Barber A.D., Cholewa J., Birnbaum M.L., Gallego J.A., John M., Szeszko P.R., Robinson D.G., Malhotra A.K. Frontal lobe fALFF measured from resting-state fMRI as a prognostic biomarker in first-episode psychosis. Neuropsychopharmacology. 2022;47(13):2245–2251. doi: 10.1038/s41386-022-01470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesh T.A., Maddock R.J., Howell A., Wang H., Tanase C., Daniel Ragland J., Niendam T.A., Carter C.S. Extracellular free water and glutathione in first-episode psychosis—A multimodal investigation of an inflammatory model for psychosis. Mol. Psychiatry. 2021;26(3):Article 3. doi: 10.1038/s41380-019-0428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Chen Z., Deng W., He Z., Wang Q., Jiang L., Ma X., Wang Y., Chua S.E., Cheung C., McAlonan G.M., Sham P.C., Collier D.A., Gong Q., Li T. Volume increases in putamen associated with positive symptom reduction in previously drug-naive schizophrenia after 6 weeks antipsychotic treatment. Psychol. Med. 2012;42(7):1475–1483. doi: 10.1017/S0033291711002157. [DOI] [PubMed] [Google Scholar]
- Li W., Li K., Guan P., Chen Y., Xiao Y., Lui S., Sweeney J.A., Gong Q. Volume alteration of hippocampal subfields in first-episode antipsychotic-naïve schizophrenia patients before and after acute antipsychotic treatment. NeuroImage: Clinical. 2018;20:169–176. doi: 10.1016/j.nicl.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Li W., Wei Y., Gui G., Zhang R., Liu H., Chen Y., Jiang Y. Deep learning based automatic diagnosis of first-episode psychosis, bipolar disorder and healthy controls. Comput. Med. Imaging Graph. 2021;89 doi: 10.1016/j.compmedimag.2021.101882. [DOI] [PubMed] [Google Scholar]
- Lottman K.K., Gawne T.J., Kraguljac N.V., Killen J.F., Reid M.A., Lahti A.C. Examining resting-state functional connectivity in first-episode schizophrenia with 7T fMRI and MEG. NeuroImage: Clinical. 2019;24 doi: 10.1016/j.nicl.2019.101959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv D., Lin W., Xue Z., Pu W., Yang Q., Huang X., Zhou L., Yang L., Liu Z. Decreased functional connectivity in the language regions in bipolar patients during depressive episodes but not remission. J. Affect. Disord. 2016;197:116–124. doi: 10.1016/j.jad.2016.03.026. [DOI] [PubMed] [Google Scholar]
- Marshall M., Lewis S., Lockwood A., Drake R., Jones P., Croudace T. Association between duration of untreated psychosis and outcome in cohorts of first-episode patients: A systematic review. Arch. Gen. Psychiatry. 2005;62(9):975–983. doi: 10.1001/archpsyc.62.9.975. [DOI] [PubMed] [Google Scholar]
- Maximo J.O., Nelson E.A., Armstrong W.P., Kraguljac N.V., Lahti A.C. Duration of untreated psychosis correlates with brain connectivity and morphology in medication-naïve patients with first-episode psychosis. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging. 2020;5(2):231–238. doi: 10.1016/j.bpsc.2019.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximo J.O., Kraguljac N.V., Rountree B.G., Lahti A.C. Structural and functional default mode network connectivity and antipsychotic treatment response in medication-naïve first episode psychosis patients. Schizophrenia Bull. Open. 2021;2(1):sgab032. doi: 10.1093/schizbullopen/sgab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazhari S., Tabrizi Y.M., Nejad A.G. Neural evidence for compromised mental imagery in individuals with chronic schizophrenia. J. Neuropsychiatry Clin. Neurosci. 2015;27(2):127–132. doi: 10.1176/appi.neuropsych.13120392. [DOI] [PubMed] [Google Scholar]
- McGuire P.K., Murray R.M., Shah G.M.S. Increased blood flow in Broca’s area during auditory hallucinations in schizophrenia. Lancet. 1993;342(8873):703–706. doi: 10.1016/0140-6736(93)91707-S. [DOI] [PubMed] [Google Scholar]
- McLachlan N.M., Wilson S.J. The contribution of brainstem and cerebellar pathways to auditory recognition. Front. Psychol. 2017;08 doi: 10.3389/fpsyg.2017.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwansisya T.E., Hu A., Li Y., Chen X., Wu G., Huang X., Lv D., Li Z., Liu C., Xue Z., Feng J., Liu Z. Task and resting-state fMRI studies in first-episode schizophrenia: A systematic review. Schizophr. Res. 2017;189:9–18. doi: 10.1016/j.schres.2017.02.026. [DOI] [PubMed] [Google Scholar]
- Nair A., Jolliffe M., Lograsso Y.S.S., Bearden C.E. A review of default mode network connectivity and its association with social cognition in adolescents with autism Spectrum disorder and early-onset psychosis. Front. Psychiatry. 2020;11 doi: 10.3389/fpsyt.2020.00614. https://www.frontiersin.org/articles/10.3389/fpsyt.2020.00614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor J.A., Ellett L., Ajnakina O., Schoeler T., Kollliakou A., Trotta A., Wiffen B.D., Falcone A.M., Di Forti M., Murray R.M., Bhattacharyya S., David A.S. Can cognitive insight predict symptom remission in a first episode psychosis cohort? BMC Psychiatry. 2017;17:54. doi: 10.1186/s12888-017-1210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill A., Mechelli A., Bhattacharyya S. Dysconnectivity of large-scale functional networks in early psychosis: A meta-analysis. Schizophr. Bull. 2019;45(3):579–590. doi: 10.1093/schbul/sby094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel-Knöchel V., Knöchel C., Matura S., Prvulovic D., Linden D.E.J., van de Ven V. Reduced functional connectivity and asymmetry of the planum temporale in patients with schizophrenia and first-degree relatives. Schizophr. Res. 2013;147(2–3):331–338. doi: 10.1016/j.schres.2013.04.024. [DOI] [PubMed] [Google Scholar]
- Oh S., Kim M., Kim T., Lee T.Y., Kwon J.S. Resting-state functional connectivity of the striatum predicts improvement in negative symptoms and general functioning in patients with first-episode psychosis: A 1-year naturalistic follow-up study. Aust. N. Z. J. Psychiatry. 2020;54(5):509–518. doi: 10.1177/00048674198854. [DOI] [PubMed] [Google Scholar]
- Pettersson-Yeo W., Allen P., Benetti S., McGuire P., Mechelli A. Dysconnectivity in schizophrenia: Where are we now? Neurosci. Biobehav. Rev. 2011;35(5):1110–1124. doi: 10.1016/j.neubiorev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Protopapa F., Hayashi M.J., Kulashekhar S., Van Der Zwaag W., Battistella G., Murray M.M., Kanai R., Bueti D. Chronotopic maps in human supplementary motor area. PLoS Biol. 2019;17(3):e3000026. doi: 10.1371/journal.pbio.3000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid M.A., Salibi N., White D.M., Gawne T.J., Denney T.S., Lahti A.C. 7T proton magnetic resonance spectroscopy of the anterior cingulate cortex in first-episode schizophrenia. Schizophr. Bull. 2019;45(1):180–189. doi: 10.1093/schbul/sbx190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reig S., Moreno C., Moreno D., Burdalo M., Janssen J., Parellada M., Zabala A., Desco M., Arango C. Progression of brain volume changes in adolescent-onset psychosis. Schizophr. Bull. 2009;35(1):233–243. doi: 10.1093/schbul/sbm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi A., McAllister V., Dazzan P. Is early intervention in psychosis effective? Epidemiol. Psichiatr. Soc. 2008;17(3):227–235. doi: 10.1017/S1121189X00001329. [DOI] [PubMed] [Google Scholar]
- Salisbury D.F., Shenton M.E., Griggs C.B., Bonner-Jackson A., McCarley R.W. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch. Gen. Psychiatry. 2002;59(8):686–694. doi: 10.1001/archpsyc.59.8.686. [DOI] [PubMed] [Google Scholar]
- Salisbury D.F., Curtis M., Longenecker J., Yeh F.-C., Kim T., Coffman B.A. Pathological resting-state executive and language system perfusion in first-episode psychosis. NeuroImage: Clinical. 2022;36 doi: 10.1016/j.nicl.2022.103261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Baker J.T. How can studies of resting-state functional connectivity help us understand psychosis as a disorder of brain development? Curr. Opin. Neurobiol. 2015;30:85–91. doi: 10.1016/j.conb.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schretlen D.J., Winicki J.M., Meyer S.M., Testa S.M., Pearlson G.D., Gordon B. Development, psychometric properties, and validity of the Hopkins adult Reading test (HART) Clin. Neuropsychol. 2009;23(6):926–943. doi: 10.1080/13854040802603684. [DOI] [PubMed] [Google Scholar]
- Schultze-Lutter F., Ruhrmann S., Berning J., Maier W., Klosterkotter J. Basic symptoms and ultrahigh risk criteria: Symptom development in the initial prodromal state. Schizophr. Bull. 2010;36(1):182–191. doi: 10.1093/schbul/sbn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahab S., Mulsant B.H., Levesque M.L., Calarco N., Nazeri A., Wheeler A.L., Foussias G., Rajji T.K., Voineskos A.N. Brain structure, cognition, and brain age in schizophrenia, bipolar disorder, and healthy controls. Neuropsychopharmacology. 2019;44(5):898–906. doi: 10.1038/s41386-018-0298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield J.M., Barch D.M. Cognition and resting-state functional connectivity in schizophrenia. Neurosci. Biobehav. Rev. 2016;61:108–120. doi: 10.1016/j.neubiorev.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K., Tanji J. Both supplementary and presupplementary motor areas are crucial for the temporal organization of multiple movements. J. Neurophysiol. 1998;80(6):3247–3260. doi: 10.1152/jn.1998.80.6.3247. [DOI] [PubMed] [Google Scholar]
- Skipper J.I., Nusbaum H.C., Small S.L. Listening to talking faces: Motor cortical activation during speech perception. Neuroimage. 2005;25(1):76–89. doi: 10.1016/j.neuroimage.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Srihari V.H., Ferrara M., Li F., Kline E., Gülöksüz S., Pollard J.M., Cahill J.D., Mathis W.S., Yoviene Sykes L., Walsh B.C., McDermott G., Seidman L.J., Gueorguieva R., Woods S.W., Tek C., Keshavan M.S. Reducing the duration of untreated psychosis (DUP) in a US community: A quasi-experimental trial. Schizophrenia Bull. Open. 2022;3(1):sgab057. doi: 10.1093/schizbullopen/sgab057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarshan Y., Cheung B.M.Y. Hypertension and psychosis. Postgrad. Med. J. 2023;99(1171):411–415. doi: 10.1136/postgradmedj-2021-141386. [DOI] [PubMed] [Google Scholar]
- Suvisaari J., Mantere O., Keinänen J., Mäntylä T., Rikandi E., Lindgren M., Kieseppä T., Raij T.T. Is it possible to predict the future in first-episode psychosis? Front. Psych. 2018;9:580. doi: 10.3389/fpsyt.2018.00580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J., Shima K. Role for supplementary motor area cells in planning several movements ahead. Nature. 1994;371(6496) doi: 10.1038/371413a0. [DOI] [PubMed] [Google Scholar]
- Türközer H.B., Hasoğlu T., Chen Y., Norris L.A., Brown M., Delaney-Busch N., Kale E.H., Pamir Z., Boyacı H., Kuperberg G., Lewandowski K.E., Topçuoğlu V., Öngür D. Integrated assessment of visual perception abnormalities in psychotic disorders and relationship with clinical characteristics. Psychol. Med. 2019;49(10):1740–1748. doi: 10.1017/S0033291718002477. [DOI] [PubMed] [Google Scholar]
- Ueda N., Maruo K., Sumiyoshi T. Positive symptoms and time perception in schizophrenia: A meta-analysis. Schizophrenia Res. Cogn. 2018;13:3–6. doi: 10.1016/j.scog.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ommen M.M., van Laar T., Renken R., Cornelissen F.W., Bruggeman R. Visual hallucinations in psychosis: The curious absence of the primary visual cortex. Schizophr. Bull. 2023;49(Supplement_1):S68–S81. doi: 10.1093/schbul/sbac140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandenBos, G.R. (2007). APA Dictionary of Psychology. American Psychological Association. https://dictionary.apa.org/.
- Vanes L.D., Mouchlianitis E., Patel K., Barry E., Wong K., Thomas M., Szentgyorgyi T., Joyce D., Shergill S. Neural correlates of positive and negative symptoms through the illness course: An fMRI study in early psychosis and chronic schizophrenia. Sci. Rep. 2019;9(1):Article 1. doi: 10.1038/s41598-019-51023-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Barker P.B., Cascella N.G., Coughlin J.M., Nestadt G., Nucifora F.C., Sedlak T.W., Kelly A., Younes L., Geman D., Palaniyappan L., Sawa A., Yang K. Longitudinal changes in brain metabolites in healthy controls and patients with first episode psychosis: A 7-Tesla MRS study. Mol. Psychiatry. 2023 doi: 10.1038/s41380-023-01969-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Zhuo K., Zhu Y., Liu D., Li Y. 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 2019. Abnormal interhemispheric functional interactions in drug-naïve adult-onset first episode psychosis patients; pp. 4346–4349. [DOI] [PubMed] [Google Scholar]
- Wang L., Zou F., Shao Y., Ye E., Jin X., Tan S., Hu D., Yang Z. Disruptive changes of cerebellar functional connectivity with the default mode network in schizophrenia. Schizophr. Res. 2014;160(1–3):67–72. doi: 10.1016/j.schres.2014.09.034. [DOI] [PubMed] [Google Scholar]
- Ward M., Druss B. The epidemiology of diabetes in psychotic disorders. Lancet Psychiatry. 2015;2(5):431–451. doi: 10.1016/S2215-0366(15)00007-3. [DOI] [PubMed] [Google Scholar]
- Wechsler, D. (2012). Wechsler Abbreviated Scale of Intelligence [dataset]. https://doi.org/10.1037/t15170-000.
- Wen Y., Zhou C., Chen L., Deng Y., Cleusix M., Jenni R., Conus P., Do K.Q., Xin L. Bridging structural MRI with cognitive function for individual level classification of early psychosis via deep learning. Front. Psychiatr. 2023;13 doi: 10.3389/fpsyt.2022.1075564. https://www.frontiersin.org/articles/10.3389/fpsyt.2022.1075564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R.S., Yung A.R., Morrison A.P. Comorbidity rates of depression and anxiety in first episode psychosis: A systematic review and meta-analysis. Schizophr. Res. 2020;216:322–329. doi: 10.1016/j.schres.2019.11.035. [DOI] [PubMed] [Google Scholar]
- Woodward N.D., Rogers B., Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr. Res. 2011;130(1–3):86–93. doi: 10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Hao Y., Zhang Y., Zhou D., Kärkkäinen T., Nickerson L.D., Li H., Cong F. Harmonization of multi-site functional MRI data with dual-projection based ICA model. Front. Neurosci. 2023;17 doi: 10.3389/fnins.2023.1225606. https://www.frontiersin.org/articles/10.3389/fnins.2023.1225606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K., Longo L., Narita Z., Cascella N., Nucifora F.C., Coughlin J.M., Nestadt G., Sedlak T.W., Mihaljevic M., Wang M., Kenkare A., Nagpal A., Sethi M., Kelly A., Di Carlo P., Kamath V., Faria A., Barker P., Sawa A. A multimodal study of a first episode psychosis cohort: Potential markers of antipsychotic treatment resistance. Mol. Psychiatry. 2022;27(2):1184–1191. doi: 10.1038/s41380-021-01331-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Tang J., Liu N., Yao L., Xu M., Sun H., Tao B., Gong Q., Cao H., Zhang W., Lui S. The effects of antipsychotic treatment on the brain of patients with first-episode schizophrenia: A selective review of longitudinal MRI studies. Front. Psych. 2021;12 doi: 10.3389/fpsyt.2021.593703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y.B., Yun J.-Y., Jung W.H., Cho K.I.K., Kim S.N., Lee T.Y., Park H.Y., Kwon J.S. Altered fronto-temporal functional connectivity in individuals at ultra-high-risk of developing psychosis. PLoS One. 2015;10(8):e0135347. doi: 10.1371/journal.pone.0135347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Palaniyappan L., Deng M., Zhang W., Pan Y., Fan Z., Tan W., Wu G., Liu Z., Pu W. Abnormal thalamocortical circuit in adolescents with early-onset schizophrenia. J. Am. Acad. Child Adolesc. Psychiatry. 2021;60(4):479–489. doi: 10.1016/j.jaac.2020.07.903. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zheng J., Fan X., Guo X., Guo W., Yang G., Chen H., Zhao J., Lv L. Dysfunctional resting-state connectivities of brain regions with structural deficits in drug-naive first-episode schizophrenia adolescents. Schizophr. Res. 2015;168(1–2):353–359. doi: 10.1016/j.schres.2015.07.031. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Liang M., Jiang T., Tian L., Liu Y., Liu Z., Liu H., Kuang F. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci. Lett. 2007;417(3):297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]
- Zvyagintsev M., Clemens B., Chechko N., Mathiak K.A., Sack A.T., Mathiak K. Brain networks underlying mental imagery of auditory and visual information. Eur. J. Neurosci. 2013;37(9):1421–1434. doi: 10.1111/ejn.12140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.