Abstract
Glycosylation is a crucial post-translational modification process where sugar molecules (glycans) are covalently linked to proteins, lipids, or other biomolecules. In this highly regulated and complex process, a series of enzymes are involved in adding, modifying, or removing sugar residues. This process plays a pivotal role in various biological functions, influencing the structure, stability, and functionality of the modified molecules. Glycosylation is essential in numerous biological processes, including cell adhesion, signal transduction, immune response, and biomolecular recognition. Dysregulation of glycosylation is associated with various diseases. Glycation, a post-translational modification characterized by the non-enzymatic attachment of sugar molecules to proteins, has also emerged as a crucial factor in various diseases. This review comprehensively explores the multifaceted role of glycation in disease pathogenesis, with a specific focus on its implications in osteoarthritis (OA). Glycosylation and glycation alterations wield a profound influence on OA pathogenesis, intertwining with disease onset and progression. Diverse studies underscore the multifaceted role of aberrant glycosylation in OA, particularly emphasizing its intricate relationship with joint tissue degradation and inflammatory cascades. Distinct glycosylation patterns, including N-glycans and O-glycans, showcase correlations with inflammatory cytokines, matrix metalloproteinases, and cellular senescence pathways, amplifying the degenerative processes within cartilage. Furthermore, the impact of advanced glycation end-products (AGEs) formation in OA pathophysiology unveils critical insights into glycosylation-driven chondrocyte behavior and extracellular matrix remodeling. These findings illuminate potential therapeutic targets and diagnostic markers, signaling a promising avenue for targeted interventions in OA management. In this comprehensive review, we aim to thoroughly examine the significant impact of glycosylation or AGEs in OA and explore its varied effects on other related conditions, such as liver-related diseases, immune system disorders, and cancers, among others. By emphasizing glycosylation's role beyond OA and its implications in other diseases, we uncover insights that extend beyond the immediate focus on OA, potentially revealing novel perspectives for diagnosing and treating OA.
Keywords: Glycosylation, Glycation, Osteoarthritis, Protein modification, Disease pathogenesis
1. Osteoarthritis (OA)
Osteoarthritis (OA) is the most common age-related degenerative and progressive joint disease [1], primarily affecting the hip and knee joints [2]. It is characterized by abnormalities in subchondral bone perfusion, extracellular matrix (ECM) protein loss, sclerosis, neovascularization invading the synovium and articular cartilage, synovial inflammation, subchondral bone remodeling, osteophyte formation, degenerative changes in articular cartilage, and progressive degradation of the entire synovial joint structure [[3], [4], [5], [6], [7]]. The destruction and remodeling of cartilage in OA are gradual processes, accompanied by concurrent synovial inflammation affecting all areas of the synovial joint [8]. OA results from the damage to articular cartilage, which subsequently affects the structure of bone, synovium, and joint capsule, giving rise to characteristic clinical manifestations such as joint pain, effusion, swelling, stiffness, deformity, and limited range of motion [[9], [10], [11]]. These symptoms have varying degrees of impact on patients' daily activities and quality of life [12]. Among them, cartilage destruction, subchondral bone remodeling, and loss of matrix molecules are key features of OA's degenerative changes [[13], [14], [15]]. Chondrocytes are the main cell type in cartilage, producing glycoproteins and proteoglycans, and contributing to the maintenance of the unique ECM of cartilage with its elasticity and flexibility. The pathological process of OA involves joint deterioration with extensive chondrocyte apoptosis and cartilage breakdown [[13], [14], [15], [16]]. Unfortunately, in mature cartilage, the differentiation of relevant chondrocyte populations is halted before final maturation, rendering these cells incapable of regeneration and repair [17]. Additionally, cartilage lesions in current treatments are considered irreversible [18].
OA has traditionally been viewed as a "wear and tear" disease; It is commonly regarded as a "degenerative" disease due to its association with the gradual degradation and depletion of joint cartilage. Joint cartilage plays a crucial role in safeguarding bone surfaces and facilitating smooth joint movements. However, over time or due to injuries, this cartilage can deteriorate progressively, leading to direct bone contact, resulting in pain, swelling, and impaired joint functionality [19]. However, scientists now increasingly recognize it as a low-grade inflammatory disorder driven by metabolic dysregulation. Both preclinical animal models and studies on OA patients have reported age, obesity, and metabolic syndrome as major risk factors for OA. Furthermore, the occurrence and progression of OA are influenced and modulated by various factors, including joint injuries, inflammation, gender, genetics, and cellular senescence [20,21]. With advancing age, cellular senescence, an irreversible cell cycle arrest, becomes more prominent, often leading to chronic pain and functional disabilities in the elderly [13,14].
As the global population ages, the economic burden and prevalence of OA are steadily rising worldwide, making it an increasingly serious public health issue [15,16]. Moreover, research data support a strong association between OA and diabetes mellitus (DM), which is one of the main components of metabolic syndrome. Epidemiological studies have identified DM as an independent risk factor for knee OA, capable of triggering and exacerbating its progression [4,5]. In animal experiments, abnormal chondrocyte insulin-like growth factor 1 resistance has been observed in streptozotocin-induced DM rats [6]. Glycosylation anomalies play a crucial role in the pathogenesis of OA [1,7] Currently, joint replacement surgery remains a primary treatment for OA pain relief [8].
A hallmark of OA is defective cartilage protein production, with these cartilage proteins being secreted via the classical Golgi apparatus glycosylation mechanism. Clinical research involving OA patients has revealed impaired glycosylation mechanisms in OA, leading to shortened glycosaminoglycan chains [22], altered sulfated proteoglycan/chondroitin sulfate ratios [23]. The previous reports suggested that Glycosaminoglycans (GAGs) serve as lubricants in joints, primarily supporting connective tissues like cartilage and tendons. Moreover, GAGs have been indicated to aid in the treatment of OA [24]. The monoclonal antibody 3-B-3 corresponds to epitopes of chondroitin sulfate (CS), while the monoclonal antibody 5-D-4 corresponds to epitopes of keratan sulfate (KS). Belcher et al. reported that in comparison to the normal group, the concentration of SF-3-B-3 and the 3-B-3/GAG ratio increased in the OA patient group, despite GAG concentrations in OA patients being similar to those in normal individuals, albeit relatively lower. Furthermore, SF-5-D-4 was lower in the OA patient group compared to the control group. Changes in other glycosylated compounds, such as O-linked and N-linked glycans and glycolipids, have also been reported in OA [25]. Undoubtedly, as an inflammatory disease, OA is associated with elevated levels of inflammatory markers such as cytokines and chemokines within affected joints [26]. Associations between OA and mechanical injury have also been reported [27]. Previous research has demonstrated that under inflammatory conditions, the activity of glycosyltransferases responsible for synthesizing glycoprotein proteoglycan chains can be altered, subsequently impacting glycoprotein function. This suggests that joint destruction in arthritis is often linked to elevated levels of inflammatory cytokines and may contribute to the ongoing deterioration of arthritis [28].
2. Glycosylation
Glycosylation is a common post-translational modification for most proteins and lipids, leading to the formation of glycoproteins and glycolipids through various glycosidic bonds [[29], [30], [31]].Polysaccharides are the fundamental components of all cells in living organisms. They can interact with various types of molecules, such as proteins and lipids. By modifying the structure and function of proteins, polysaccharides influence their biological activity. The majority of glycans (glycoproteins) attached to proteins can be classified into two types: one is N-glycans, which are polysaccharides attached to asparagine (Asn) residues via nitrogen atoms, and the other is O-glycans, which are polysaccharides attached to serine or threonine residues via oxygen atoms [32]. Furthermore, specific alterations in glycosylation of glycoproteins and serum glycoproteins are associated with the pathogenesis of diseases such as cancer, infections, and autoimmune disorders. In certain diseases, protein glycosylation can serve as diagnostic biomarkers [31].
3. Characteristics and functions of glycosylation
Glycosylation has several types, for example, the well-known O-glycosylation (O-Glycans), N-glycosylation, and glycosylphosphatidylinositol (GPI) anchoring on proteins. Among them, the characteristic features of protein glycosylation mainly include O-glycosylation, N-glycosylation, O-glucosylation, and glycosaminoglycan chain modification. Protein glycosylation primarily occurs in the endoplasmic reticulum and Golgi apparatus of the secretory pathway. For instance, N-acetylgalactosamine (GalNAc) O-glycosylation takes place in the Golgi apparatus, while N-glycosylation initiates in the endoplasmic reticulum [33], undergoes further processing, and terminates in the Golgi apparatus [34]. In addition, some specific types of glycosylation are named based on the last sugar of the polysaccharide chain. These include: galactosylation (Gal), sialylation (SA), fucosylation (Fuc) (core and terminal), multibranching of glycans (bi-, tri-, tetra-, penta-antennary), and increased bisection N-acetylglucosamine (GlcNAc) [35,36]. Moreover, in higher eukaryotes, including humans, C-mannosylation (the attachment of mannose to the indole ring of tryptophan) has also been identified [37]. The biosynthesis of polysaccharides relies on the action of multiple genes, which encode enzymes like glycosyltransferases, molecular chaperones, and cell transport proteins. These enzymes collectively constitute the cellular glycosylation machinery. Polysaccharides undergo further modifications by glycosidases and undergo additional biosynthetic modifications through other enzymes [[38], [39], [40]]. These modifications are responsible for changes in enzyme activity related to glycoprotein metabolism: glycosyltransferases, which are involved in sugar transfer, and glycosidases, which are responsible for the hydrolysis of glycosidic bonds. Glycosyltransferases, being a large enzyme family, play a significant role in the formation of glycoprotein bonds [41]. The maintenance of internal balance relies on the efficient and highly controlled biosynthesis of polysaccharides, leading to proper protein glycosylation. Therefore, this is crucial for their functionality in several biological activities such as normal development, growth, and differentiation in cells and tissues [[38], [39], [40], [41], [42], [43], [44]]. However, when there is a dysregulation in the biosynthesis pathway of polysaccharides, it can lead to significant alterations in cellular glycosylation and critical changes in biological processes, ultimately triggering and modulating the pathological progression of various diseases [41]. The binding and cleavage of sugar-amino acid chains play a pivotal role in the process of glycoprotein's biological activity, which is enzyme-controlled. As a result, variations in disease-specific glycosylation can be observed in many diseases [35,45,46].
3.1. O-glycosylation
O-GlcNAcylation, also known as O-linked N-acetylglucosaminylation or O-linked glycosylation, is the attachment of O-GlcNAc modification onto serine (Ser) or threonine (Thr) residues within peptides. This post-translational modification occurs exclusively within the Golgi apparatus, where O-linked polysaccharides are attached to the free hydroxyl groups of these amino acids. It is a dynamic and reversible posttranslational modification (PTM) that plays a regulatory role in a broad spectrum of cellular events. O-GlcNAcylation regulates various processes, including but not limited to epigenetics, transcription, signaling, and rhythm within cytoplasmic, nuclear, and mitochondrial proteins [[47], [48], [49]]. Aberrant cell O-glycosylation triggers or regulates various diseases, including genetic disorders, infectious diseases, and cancers.
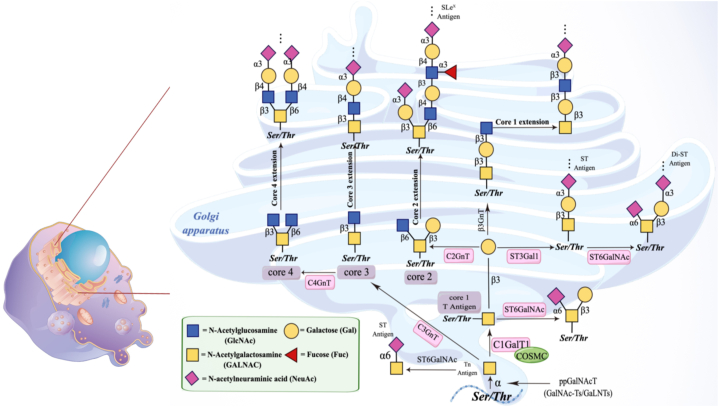
During this process, the glycosyltransferase OGT utilizes GlcNAc from nucleotide sugar UDP-GlcNAc to form O-GlcNAc modification on the hydroxyl groups of serine or threonine residues in proteins. O-GalNAc glycosylation can occur in any protein that follows the secretory pathway [50], accounting for over 80% of secreted and cell surface proteins. This type of glycosylation is highly abundant in mucins, and thus, it is commonly referred to as mucin-type O-glycosylation. Mucins, as a class of glycoproteins, contain numerous repetitive structural domains rich in Ser and Thr residues, which serve as targets for O-glycosylation [51]. All O-GalNAc glycosylation share a common structural feature, which involves the monosaccharide N-acetylgalactosamine (GalNAc) being α-linked to the hydroxyl group of Ser or Thr residues in proteins [52]. Glycoproteins exhibit a series of core structures capable of forming extended polysaccharide backbones with either linear or branched configurations. Among these, the linear structures include Galb1-3Gal- NAc- (core 1) and GlcNAcb1-3GalNAc- (core 3), while the branched structures encompass Galb1-3(GlcNAcb1-6)GalNAc- (core 2) and GlcNAcb1-3(GlcNAcb1-6)GalNAc- (core 4). Subsequently, the backbone repetitive units of N-acetyllactosamine types 1 and 2 (corresponding to Galb1-3GlcNAc- and Galb1-4GlcNAc-, respectively) are added, either as side chains I and linear I antigens (Galb1-4GlcNAcb1-3Galb1-4-), thereby forming extensions. Finally, the resulting polysaccharides undergo sialylation, fucosylation, and sulfation [53]. The mucin type O-glycans are synthesized through the aforementioned complex processes. The biological synthesis pathway of the mucin type O-glycans are depicted in [Fig. 1], as shown.
Fig. 1.
Synthesis of O-glycans.
Within the GalNAc-O-Ser/Thr structure (Tn antigen), modification is carried out by the Gal-transferase enzyme (C1GalT1). Among these, C1GalT1 is an enzyme, and its activity expression relies on its molecular chaperone (Cosmc) [54]. GalNAc glycosylation (mucin-type O-glycosylation) is a major component of mammalian glycocalyx, participating in various biological processes through glycan-protein interactions. It plays a crucial role in numerous biological and pathological processes, including tumor growth and progression.
3.2. N-glycosylation
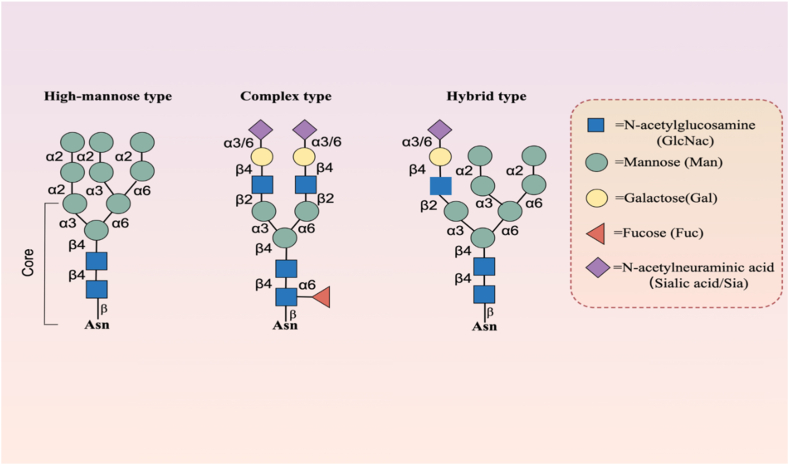
As it is widely recognized, unlike the O-linked polysaccharides with O-linked glycosylation, the core structure of N-linked polysaccharides consists of a complex "bifurcated" or "double-antennae" pentasaccharide, commonly referred to as the "biantennary" structure, composed of 3 mannose (Man) and 2 GlcNAc molecules. Within its branched structure, various types of sugars can incorporate differing numbers of additional molecules such as fucose, mannose, GlcNAc, galactose (Gal), bisecting N- GlcNAc, and N-acetylneuraminic acid (sialic acid, Sia). The complex and variable structure of N-glycans depends on the length of sugar chains, the way of branching or the arrangement order of monosaccharides. Among these, the core structure of N-linked polysaccharides can be further diversified through the action of various enzymes. However, summarily, these structures can be broadly categorized into three types: high-mannose type, hybrid type, and complex type. Both hybrid and complex types typically involve core fucosylation in most instances [Fig. 2]. As one of the most common, significant, and complex PTM occurring after protein translation [55], N-glycosylation plays a crucial role in protein biogenesis and function. Besides its potential involvement in regulating brain development and function [56], N-glycosylation exerts its important effects by influencing protein folding, cellular localization, stability, and solubility.
Fig. 2.
Major subtypes of N-glycosylation.
Typically, N-glycosylation can impact enzyme activity through the following ways: (1) promoting peptide chain folding (N-glycans are co-translationally added and can stabilize proper protein folding), (2) ensuring correct subcellular localization of proteins (preventing their accumulation in the endoplasmic reticulum and subsequent degradation), and (3) preventing protein aggregation [57]. Furthermore, within the sequence of N-glycosylation, amino acid substitutions (site-directed mutagenesis approaches) can lead to unfavorable changes in protein tertiary structure. Inhibitors of N-glycosylation may also cause side effects, such as inducing endoplasmic reticulum stress (ERS) due to the accumulation of misfolded proteins, ultimately leading to cell apoptosis [58].
N- and O- glycans are the most prevalent types of glycosylation. Due to their highly diverse and relatively hydrophilic nature, as well as the spatial flexibility of their short oligosaccharide chains formed by numerous sugar residues and the ability to transfer charges through certain sugar moieties [42,59], N-glycans are considered to be universally present in most living organisms. Additionally, they are found on the envelopes of viruses, carrying oligosaccharide chains derived from infected host cells [55,[60], [61], [62], [63], [64]]. N-glycans are typically attached to Asn residues within a consensus sequence known as Asn-X-Ser/Thr, where X can be any amino acid except proline. Furthermore, N-glycans can also attach to N-X-C, N-Q-C, N–S-G, or Q-G-T sequences [65]. At the molecular and cellular levels, N-glycans may play diverse roles, including involvement in protein folding [66,67], secretion [68], maturation [69], and intracellular transport [70]; communication and cell-cell interactions [70]; immune responses [71]; as well as mediating diseases, including cancer [72], and facilitating viral entry into host cells (e.g., SARS-CoV-2) [73]. N-glycans are synthesized by glycosyltransferases, and their impact on protein biogenesis stems from their influence on protein secondary structures and physicochemical properties [74]. Moreover, N-glycans can alter the thermodynamic stability of proteins by inducing conformational changes, as observed in the case of artificially modified Gc-MAF serum factor protein, MM1 [75], or the WW domains of human Pin 1 protein [76]. Glycosyltransferases are a complex group of enzymes widespread in most living organisms. N-glycans can influence the secretion, stability, and substrate/receptor affinity of glycosyltransferases, which may significantly impact their enzymatic activities. However, some glycosyltransferases require glycosylation to initiate their activity. Both N-glycans and glycosyltransferases play crucial roles in the pathogenesis of many diseases, including cancer.
As widely recognized, Immunoglobulin G (IgG)-type monoclonal antibodies typically feature a conserved N-glycosylation site at asparagine-297(Asn-297) within the Fc region. Additionally, about 20% of IgGs harbor an N-linked glycosylation site within the Fab region, situated on the heavy chain. The asymmetry in glycosylation levels between the two heavy chains of monoclonal antibodies further augments the diversity of glycosylated antibodies. At the forefront of immunology and glycobiology, IgG glycosylation, specifically the conserved N-linked glycan at Asn-297 in the second immunoglobulin (Ig) domain of the heavy chain (CH2) within the Fc region that helps form IgG, plays a crucial role in the structure and function of IgG. The conserved N-linked glycans on Fc portion, including the one found at Asn-297, have a special role in controlling the pro-inflammatory and anti-inflammatory functions of glycoproteins [77,78]. The effector functions of IgG vary between pro-inflammatory and anti-inflammatory states depending on its glycosylation. As the most abundant glycoprotein in circulation in the blood, IgG is composed of two identical heavy chains and two identical light chains forming its peptide backbone. Among all its structures, the most variable component is the hinge region, which connects the antigen-binding region (Fab) and the crystallizable region (Fc) of IgG. In the Fab domain, approximately 15%–25% of IgG molecules contain N-linked glycans with diverse functions [79]. Although the complex interactions required to form the final functional glycan structures cannot be solely encoded by individual genes or gene combinations [34], several genes encoding glycosyltransferases are associated with different IgG glycosylation patterns [80]. Factors driving the diverse functions of IgG include changes in galactosylation, fucosylation, and sialylation, ranging from inhibitory/anti-inflammatory effects to complement activation and promotion of antibody-dependent cellular cytotoxicity. However, inflammation is largely associated with reduced galactosylation, increased fucosylation, and increased GlcNAc splitting. Generally, alterations in Fc glycosylation subsequently influence the structural integrity and conformation of the Fc domain, as well as its affinity for Fcγ receptors (FcγRs). IgG sialylation is a key aspect of IgG's inhibitory effects.
Going back to a study conducted in 2006 by Jeffrey Ravetch's laboratory, they reported that high-dose intravenous administration of immunoglobulin IgG can treat the anti-inflammatory activity in rheumatoid arthritis (RA) due to terminal α2,6-sialylation of IgG N-glycans [77]. Consistent with this report, the team led by Thomas Rademacher found changes in IgG glycosylation in both RA and OA [81]. In a report from 1987 using lectin analysis of IgG from RA patients, it was observed that IgG present in the RA patient samples exhibited higher binding to ConA compared to the healthy control group. This finding suggests a higher prevalence of hybrid and highly fucosylated N-linked glycans associated with inflammatory diseases in RA patients [82]. IgG glycosylation reduces galactosylation [83]. Changes in galactosylation have also been reported in early-onset RA. Subsequently, this result was replicated in an experiment using RA-prone MRL-lpr/lpr mice, showing a decrease in the galactosyltransferase B4GalT1 with the onset of RA [84]. These results have stood the test of time. Over time, these results have stood the test of time.
3.3. Advanced glycation end products (AGEs)
PTMs of proteins play a pivotal role in regulating various cellular processes and are increasingly recognized as key contributors to the development and progression of numerous diseases. Among these PTMs, glycation, a non-enzymatic process involving the covalent attachment of sugar moieties to proteins, has emerged as a significant factor in disease pathogenesis. The intricate interplay between glycation and disease has sparked growing interest among researchers aiming to decipher its implications and mechanisms in different pathological contexts. In recent years, one particular area of focus within the realm of glycation-associated diseases has been OA. Osteoarthritis, characterized by the progressive degeneration of joint tissues, represents a major health burden globally. While mechanical factors have long been implicated in OA pathogenesis, emerging evidence underscores the role of biochemical alterations, including glycation, in driving disease progression. Glycation-induced modifications have been shown to affect the structural and functional integrity of joint components, exacerbating tissue damage and inflammation.
Advanced glycation end products (AGEs) are a class of compounds formed through a series of non-enzymatic reactions between reducing sugars (such as glucose) and biologically significant molecules like proteins, lipids, and nucleic acids. These reactions are commonly referred to as glycation reactions or non-enzymatic glycosylation reactions, collectively known as the Maillard reaction. This process results in the irreversible modification of biomolecules and gives rise to the formation of heterogeneous and structurally diverse AGEs. In recent years, AGEs have emerged as pivotal entities in the pathogenesis of various chronic diseases, highlighting their significance as potential therapeutic targets and biomarkers [85]. AGEs have emerged as significant contributors to the aging process and the development of various chronic diseases, impacting a wide range of physiological functions. The formation of AGEs occurs through a series of complex and interconnected pathways, ultimately resulting in the irreversible modification of biomolecules. The accumulation of AGEs has been associated with tissue dysfunction, inflammation, oxidative stress, and the pathogenesis of age-related disorders, such as diabetes, cardiovascular diseases, and neurodegenerative conditions. As proteins constituting the ECM and vascular basement membranes (BM), they are among the longest-lived proteins in the body and particularly sensitive to AGEs modifications. The presence of AGEs on the vascular BM, especially in diabetic patients, leads to an accelerated accumulation of AGEs due to the specific environment influenced by the diabetic condition, resulting in severe pathological consequences. These effects are mediated in part through their interaction with specific receptors, including the receptor for AGEs (RAGE), which amplifies cellular stress responses and inflammatory pathways [86]. AGEs were initially recognized for their role in the aging process and have since been implicated in the etiology of various age-related diseases, including diabetes, cardiovascular diseases, neurodegenerative disorders, and chronic kidney disease. The accumulation of AGEs is closely linked to oxidative stress, chronic inflammation, and the activation of various cellular pathways, which contribute to tissue damage and dysfunction. Furthermore, the interaction of AGEs with their receptor, the receptor for RAGE, triggers an array of intracellular signaling cascades that perpetuate cellular dysfunction and inflammation [87].
The formation of AGEs involves a complex process, encompassing chemical reactions between sugar molecules and amino acid residues within biologically significant molecules, often involving residues such as lysine in protein molecules. These reactions may be particularly active in high-sugar environments, such as within the bodies of diabetic patients. Once formed, AGEs can accumulate within tissues, exerting detrimental effects on the structure and function of biomolecules. Understanding the formation, metabolism, and biological effects of AGEs is crucial for unraveling their intricate involvement in disease pathogenesis. Recent advances in analytical techniques have enabled the identification and quantification of specific AGEs, facilitating the exploration of their roles in disease progression and aging. Moreover, efforts to understand the underlying mechanisms of AGEs formation and their impact on cellular function have spurred the development of therapeutic strategies aimed at mitigating AGEs-related damage. These strategies range from dietary interventions to pharmacological agents designed to interfere with AGEs accumulation and mitigate their downstream effects [88,89].
This review provides a comprehensive overview of the intricate relationship between glycation, glycosylation, and diseases, with a particular emphasis on their role in OA. By elucidating the molecular mechanisms through which they contribute to joint tissue damage, exploring relevant research findings, and integrating various perspectives, this review unravels the 'sweet mystery'. Understanding its impact on disease progression paves the way for targeted interventions and improvements in patient care.
4. Glycosylation in disease context
4.1. Glycosylation in non-neoplastic diseases
The role of glycosylation in various health conditions spans a broad spectrum, encompassing implications in liver diseases, congenital disorders, and even neurodegenerative ailments. In liver-related issues such as chronic hepatitis and liver cancer (LC), the impact of dietary long-chain fatty acids (LCFAs) on inflammation is notable. LC patients displayed rapid absorption of LCFAs through an alternative pathway involving glycosylated CD36 [[90], [91], [92]] and GLP-2 [93], potentially contributing to LC pathogenesis through altered LCFA metabolism [[94], [95], [96]]. LC patients exhibit rapid LCFA absorption, potentially contributing to the development of the disease. Moreover, in Crohn's disease (CD), abnormal glycosylation patterns in certain proteins correlate with autoimmune responses, particularly in anti-granulocyte macrophage-colony stimulating factor autoantibodies (aGMAb) [97]. These aberrations in glycosylation, when identified comprehensively, could serve as more specific biomarkers for diagnosis, especially in viral hepatitis cases with fibrosis/cirrhosis. Viral infections like Hepatitis B and C often exhibit altered glycosylation in viral envelope proteins, influencing their interaction with host cells. These glycosylated proteins play a pivotal role in the viral life cycle, particularly in cellular recognition, binding, and penetration [98,99]. Polysaccharides, as vital connecting elements with host receptors, emerge as potential targets for innovative therapeutic strategies against viral hepatitis [90,100,101]. Moreover, Congenital Disorders of Glycosylation (CDG) are metabolic disorders arising from defects in polysaccharide synthesis, impacting various organ systems and often leading to intellectual disabilities and developmental delays. In CDGs resulting from defects in protein N-glycosylation, the heterogeneity within CDGs underscores the need for considering this disorder when faced with unexplained hepatic pathologies, developmental delays, or growth failures [102,103]. In addition, N-glycosylation is closely associated with most of the pathogenic processes involved in neuroinflammation. Glycosylation emerges as a central player in neuroinflammation, affecting conditions like Alzheimer's (AD), Parkinson's (PD), and epilepsy [104]. Siew et al. pointed out that MBLs can bind to various glycan monomers, including mannose, fucose, and GlcNAc, and exert pro-inflammatory effects by activating and phagocytizing microglia. Sirko et al. found that the protein galectin-3, which requires O-glycosylation, is essential for microglia proliferation through the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway, promoting the production of the pro-inflammatory cytokine IL-6 [105]. Gligorijević and colleagues reported significant glycosylation changes in patients with liver cirrhosis (LC). This included alterations in specific carbohydrates, such as increased levels of Gal β-1,4 GlcNAc and terminal α-2,3 Sia, along with reduced core α-1,6 fucosylation and cleaved GlcNAc [106]. Various glycan-binding proteins like mannose-binding lectins (MBLs) and galectin-3 influence pro-inflammatory responses, microglia proliferation, and cytokine production, highlighting the role of glycosylation in neuronal health. Signaling pathways such as NFκB, MAPK, and JAK/STAT, besides initiating signal cascades, also require one or more glycosylation components to fine-tune pathway activity and initiate the epigenetic production of cytokines and chemokines [107]. Moreover, N-glycosylation influences the unfolded protein response (UPR) critical for neuronal survival or death, emphasizing its pivotal role in neurological disorders [106]. The complexity of the glycosylation pathway involving numerous enzymes across cellular compartments presents both challenges and opportunities for therapeutic interventions in neurological disorders. While blocking glycosylation initiation could have unintended consequences, targeting specific disrupted glycan species in distinct neurological disorders holds promise for tailored interventions. Careful consideration and design of targeted approaches for glycosylation in neurological disorders offer hope for future therapeutic strategies.
Alcoholic liver disease (ALD) has drawn significant attention in recent studies examining the intricate role of O-linked β-N-acetylglucosamine (O-GlcNAc) levels. Zhang et al. on ALD patients and alcoholic liver injury in mice revealed a decrease in O-GlcNAc modification, linked to the prevention of liver cell necrosis and fibrosis. Their focus on O-glycosylation's impact on necrotic apoptosis pathways, both transcriptionally and post-translationally, underscored the pivotal role of O-GlcNAc transferase (OGT) in inhibiting liver cell necrotic apoptosis. The absence of OGT led to progressive liver issues, including fibrosis and inflammation [107]. However, this complexity in OGT's role in liver pathology was further illuminated by Xu et al.'s findings. In contrast to Zhang et al., Xu et al. reported that OGT overexpression in the liver contributes to hepatocellular carcinoma (HCC) by activating the JNK signaling pathway [108]. This contradiction calls for a comprehensive understanding of OGT across diverse models and its integration into broader signaling networks. Moreover, insights into the dynamics of O-glycosylation in the early stages of chronic liver disease were gleaned from a mouse model of ethanol diet-induced liver injury. Despite the absence of evident fibrosis, a significant decrease in liver O-glycosylation levels accompanied liver cell injury and inflammation. This highlights O-glycosylation as an early responder to liver stress, prompting consideration of O-GlcNAc as a potential biomarker for liver disease [107]. These collective findings underscore the intricate interplay of OGT and O-GlcNAc levels, shaping our understanding of liver pathophysiology. Subtle changes in glycosylation, observed in the presence of various diseases, serve as a foundation for more sensitive and precise clinical tests. Liver diseases commonly exhibit reduced sialylation and increased polyglycan branching, while cancer demonstrates increased sialylation and fucosylation. For individuals with heavy alcohol consumption, glycosylation changes indicate elevated transferrin (Tf) levels [109], reflected in increased isoelectric focusing [[110], [111], [112], [113]] due to decreased sialic acid content. Despite a positive correlation between alcohol intoxication and cirrhosis, the presence of carbohydrate-deficient transferrin is more linked to the amount of alcohol consumed than the severity of liver disease [114]. Notably, specific glycosylation alterations in liver diseases seem to differ based on various glycoproteins, potentially attributed to differences in patient cohorts used in early or late-stage studies. In liver conditions like liver fibrosis and cirrhosis, markers such as Mac-2 Binding Protein Glycosylation Isoform (M2BPGi) demonstrate a strong association with disease severity, including correlations with hepatic venous pressure gradient levels [[97], [98], [99],115,116]. These markers might not directly predict mortality rates but could serve as indicators of impending bacterial infections, shedding light on the immunosuppressive state encountered during advanced cirrhosis [116]. Research into the intricate relationship between various diseases and glycosylation patterns of IgG has revealed compelling correlations that shed light on disease characteristics and potential diagnostic markers. Notably, cardiovascular disease (CVD) and kidney disease exhibit pronounced associations with altered IgG glycosylation, marked by increased cleavage of IgG-associated glycans and higher α2,6-sialylation alongside reduced galactosylation. Interestingly, a similar glycosylation pattern emerges in type 2 diabetes, suggesting a potential link between these conditions [117]. Inflammatory diseases like Crohn's disease, pulmonary tuberculosis [118], and juvenile idiopathic arthritis also showcase reduced IgG galactosylation, emphasizing a broader pattern across inflammatory conditions. In RA, distinct IgG glycosylation patterns have been observed, including lower galactosylation, increased fucosylation, and reduced sialylation [119], notably in RF-positive patients. These glycosylation changes align with disease activity, implicating them as potential markers for RA subsets and disease progression. Moreover, the efficacy of treatments like methotrexate (MTX) in RA appears linked to increased IgG galactosylation, suggesting a potential avenue for monitoring treatment response [120]. Similarly, systemic lupus erythematosus (SLE) patients exhibit reduced galactosylation and sialylation of IgG in serum, aligning with observations in lupus mouse models [121]. These findings collectively underscore the intricate relationship between IgG glycosylation and various diseases, paving the way for potential diagnostic and therapeutic advancements in managing these conditions.
The complexity of glycan modifications in diseases is evident in the diverse alterations observed in serum proteins. In cases of inflammation, the shifts in sialylation and branching exhibit variability, often dictated by the specific disease type under investigation. Conversely, in cancer, the trends in glycan changes appear to be protein-specific, with glycan branching either increasing or decreasing depending on the particular serum protein studied. Notably, these modifications have been harnessed for diagnostic purposes, exemplified by instances such as reduced sialylated Tf in alcohol abusers and elevated Hp and AFP aggregation in cancer tissues [122]. Furthermore, the augmentation of fucosylation emerges as a notable feature in various cancers, spanning ovarian [123], breast [119], thyroid [124], and choriocarcinoma [125]. Interestingly, the increased fucosylation in cancer appears to occur across multiple sites on serum glycoproteins without specific localization. For instance, diverse linkage patterns are observed: α-(1–3) linkage in AFP and HCG, and α-(1–3) linkage to surrounding GlcNAc residues [125] in Tf [126]and haptoglobin (Hp) [125]. Additionally, research has linked increased fucosylation in cancer to heightened activity of α-(1–3)-fucosyltransferase in the blood [127]. In contrast, the scenario with sialic acid alterations showcases nuanced differences between cancer and normal serum. While an overall increase in protein-bound sialic acid is confirmed in cancer, variations in sialic acid residue counts on Tf molecules distinguish cancer serum from normal serum [128]. These findings underscore the variability in serum protein glycosylation among diseases. For instance, cancer displays augmented sialylation and fucosylation, while liver pathology exhibits reduced sialylation and increased glycan branching.
4.2. Glycosylation in cancer
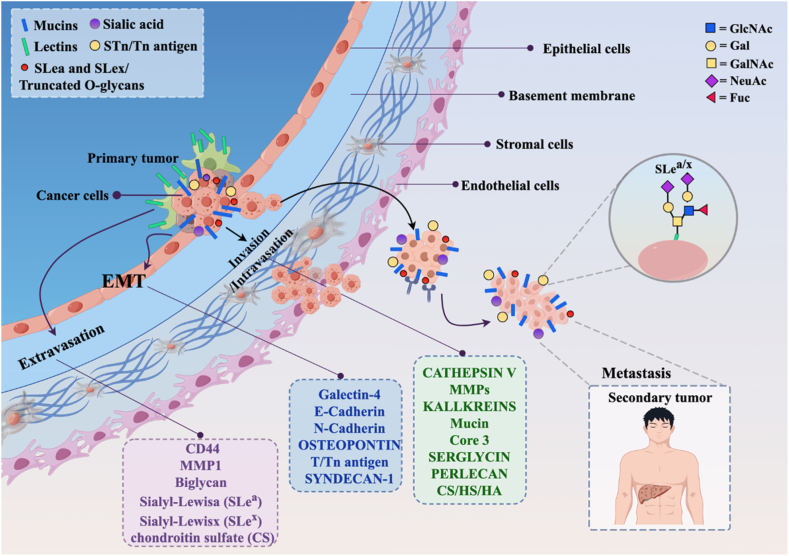
Proteins with high levels of glycosylation, such as adhesion proteins or proteases, are involved in cancer metastasis. Proteins like enzymes, transcription factors, and receptors appear repeatedly in cancer after being regulated and/or modified by O-glycosylation [129]. Compared to normal tissues, some tumor cells exhibit altered glycosylation patterns, leading to the emergence of new tumor-specific antigens. During this process, polysaccharide structures, glycosylated proteins, or glycosyltransferases participate to varying degrees, influencing various steps including epithelial-mesenchymal transition (EMT), migration, and invasion of tumor cells [Fig. 3]. Glycoproteins are mainly located on cell surfaces and extracellular matrix, playing a crucial role in regulating interactions between cells and their environment. This context underscores the key role of glycoproteins and polysaccharide structures in the metastatic process.
Fig. 3.
Abnormal glycosylation in malignant behavior of tumors.
In the realm of tumor tissues, the presence of Lewis antigens alongside T and Tn antigens stands out, facilitating the binding of tumor cells to selectins expressed by endothelial cells or blood cells. These antigens play a critical role in various cellular interactions. Among the mucin-type O-glycans carried by numerous cell surface or secreted proteins, each protein assumes distinct functions. For example, c-Myc, a nuclear phosphorylated protein, regulates cell proliferation, differentiation, and apoptosis. Cell surface proteins such as mucin 1 (MUC1), mucin 2 (MUC2), CD44, integrins, and other adhesion proteins promote cell-cell interactions, adhesion, and migration. Structural proteins like platelet hemagglutinin and β-catenin regulate cell-cell adhesion and downstream signaling pathways like Wnt/β-catenin. Meanwhile, receptors like death receptors modulate sensitivity to pro-apoptotic signals [130]. Tn antigen's prevalence in various cancers—breast, colorectal, lung, bladder, cervical, ovarian, gastric, and prostate—strongly correlates with poor prognosis and heightened metastatic potential [130]. In malignant tumors, Tn and sTn antigens often coexist, contributing significantly to tumor growth and metastasis in breast and gastric tumors. T antigen's interaction with lectin-3 further drives tumor cell adhesion to endothelial cells, furthering metastasis [131]. These antigens, carried by highly O-glycosylated proteins like MUC1, play critical roles in signaling pathways that influence growth, migration, and invasion in cancer cells [132].
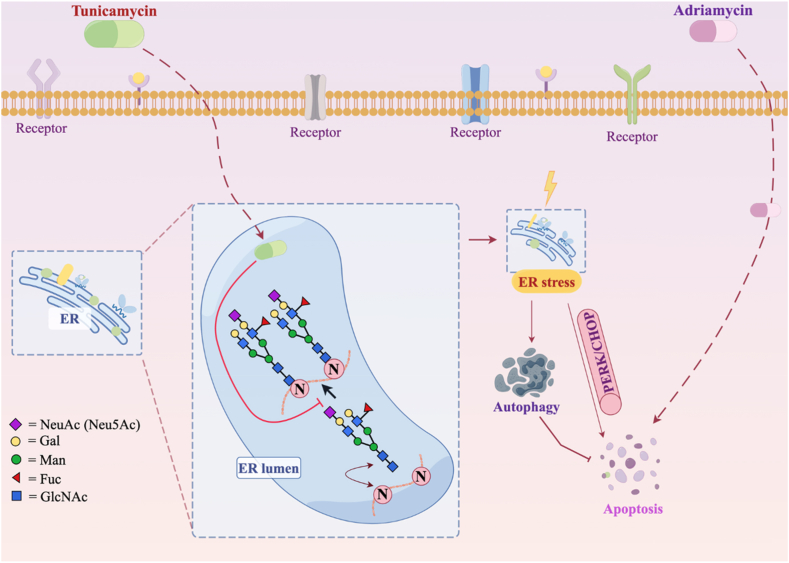
Contrarily, N-glycosylation follows a different pathway, involving the addition of polysaccharide structures to specific amino acid residues during translation, generating complex and heterogeneous structures. Over 2000 proteins exhibit motifs suitable for N-glycosylation, associated mostly with membrane-bound or secreted proteins. Variations in N-glycosylation patterns exist among different tumor types, impacting tumor stroma and tissue features. Enzymes participating in N-glycosylation, such as RPN1 (ribophorin I) and RPN2 (ribophorin II), correlate with tumor characteristics and metastasis in various cancers [[133], [134], [135], [136], [137]]. Manipulating glycosylation pathways, as seen in tunicamycin (Tu)'s inhibition of glycosylation, offers potential avenues for cancer treatment, potentially overcoming chemotherapy resistance [138][Fig. 4].
Fig. 4.
Glycosylation in drug resistance.
The understanding of glycosylation extends into tumor immunotherapy, where the glycosylation of immune checkpoint proteins like PD-1/PD-L1 plays a pivotal role in tumor immune evasion. Glycosylation's role in stabilizing PD-L1 and mediating its interaction with PD-1 inhibits T cell activation, promoting tumor-specific T cell apoptosis and rapid tumor progression [139]. Understanding these mechanisms of glycosylation, both in tumor biology and immunotherapy, holds promise for discovering diagnostic markers and therapeutic targets. By comprehending how tumors exploit glycosylation, we gain insights into disease mechanisms and potential avenues for treatment. Exploring alterations in glycosylation, particularly in proteins like PD-L1, may inform strategies to enhance the efficacy of checkpoint blockade therapies across various diseases [140]. The intricate world of protein glycosylation, as evidenced by studies on PD-L1 and other glycoproteins, underscores its profound impact on disease mechanisms and therapeutic interventions, urging further exploration into its multifaceted roles in various pathological conditions [141].
The previous discoveries have formed a crucial bridge to understanding OA. Considering the impact of glycosylation in other diseases and how this modification might play similar or related roles in the pathogenesis of different conditions — possibly by affecting similar cellular signaling, gene expression, or intercellular interactions — sheds light on the onset and progression of diseases. Revealing the common mechanisms or points of convergence of glycosylation in diverse diseases holds promise for inspiring the development of new treatment methods or intervention strategies. Moreover, the potential for a biological process or molecular mechanism to function similarly across various diseases helps expand our comprehension of the essence of these conditions. This, in turn, paves the way for understanding the pathogenesis of OA, identifying diagnostic markers, devising new intervention strategies, and advancing precision medicine, all aimed at slowing disease progression or enhancing patients' quality of life.
5. Glycosylation and glycation abnormalities in OA
In unraveling the intricate tapestry of OA pathology, a dual focus on glycation and glycosylation unveils distinct yet interconnected facets of molecular influence. The orchestrated interplay between these two glycosylative processes manifests in the joint milieu, contributing divergent yet pivotal mechanisms to the etiology of OA. Glycation, marked by non-enzymatic sugar-protein interactions, introduces structural perturbations, while glycosylation, an enzymatically guided process, intricately modifies proteins with diverse glycans. This concurrent exploration not only accentuates the nuanced nature of sugar-mediated pathways in OA but also underscores the imperative to discern and comprehend the unique contributions of glycation and glycosylation in shaping the pathophysiological landscape of joint health.
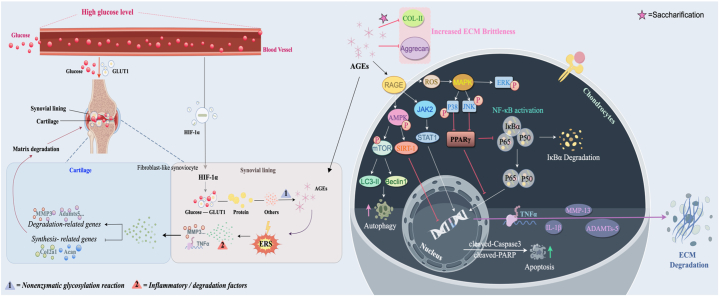
Aberrant glycosylation emerges as a crucial determinant in both OA and type 2 diabetes mellitus (T2DM), as evidenced through multifaceted investigations. Luo et al. uncovered distinct N-glycosylation patterns in OA patients with coexisting T2DM, revealing reduced N-glycosylated protein abundance and identifying specific proteins like fibronectin 1 (FN1), cartilage intermediate layer protein 1 (CILP), and collagen type VI alpha-1 chain (COL6A1) implicated in cartilage degradation [142]. Concurrently, Urita et al., Parekh et al., Bousseau et al., and Derada et al. highlighted diverse glycosylation alterations, including high-mannose type N-glycans and modifications in IgG, illuminating the wide-ranging impact of glycosylation changes in disease pathogenesis. Particularly noteworthy were observed abnormalities in chitinase 1 (CHIT1) and IgG among diabetic patients, emphasizing the diagnostic potential of N-glycosylation profiles in monitoring T2DM progression. Moreover, Li et al.'s findings underscored the profound influence of elevated blood glucose on synovium, triggering inflammatory responses and ERS, ultimately exacerbating chondrocyte degradation in OA, especially in diabetic conditions [143]. The pivotal roles of glucose transporter 1 (GLUT1) and hypoxia-inducible factor HIF-1α in mediating AGEs-induced inflammatory cascades were elucidated, highlighting potential therapeutic targets for mitigating OA progression within a high-glucose environment. RAGE, when activated by AGEs, can stimulate HIF-1α signaling across various tissues. The HIF-1α-GLUT1-AGEs–HIF–1α loop within DM-induced synovitis can exacerbate the progression of OA [143][Fig. 5]. The pivotal roles elucidated for glucose transporter 1 (GLUT1) and hypoxia-inducible factor HIF-1α in mediating inflammatory cascades induced by AGEs point towards potential therapeutic targets for mitigating OA progression within high-glucose environments. Furthermore, AGEs, in concert with the receptor for advanced glycation end-products (RAGE), actively participate in multiple pathological processes in OA, regulating gene expression, ECM degradation [144], promoting chondrocyte apoptosis [145], and inhibiting chondrocyte autophagy. These irreversible AGEs, formed through non-enzymatic glycation reactions (Maillard reaction), stand as crucial indicators of aging, implicated as a mechanistic basis for age-induced OA, highlighting their role in inducing and accelerating OA's pathological changes. AGEs' influence on chondrocyte behavior includes increased apoptosis rates, MMP13 expression, and inhibition of chondrocyte autophagy, mediated through intricate pathways involving phosphorylation of key proteins and modulation of signaling cascades [146,147].
Fig. 5.
Advanced glycation end products (AGEs) are involved in some mechanisms of osteoarthritis (OA).
The comparative study between Kashin-Beck disease (KBD) and OA sheds light on distinct pathogenic mechanisms. Lyu et al. employed proteomic and N-glycoproteomic analyses, unveiling differential protein expression in KBD and OA. Notably, various proteins like ITGB1, LRP1, ANO6, COL1A1, MXRA5, DPP4, and CSPG4, along with CRLF1 and GLG1, exhibited relevance to KBD's pathological process, influencing ECM interactions and cellular pathways [148]. While both diseases share some commonalities, KBD primarily involves chondrocyte necrosis, differing from OA's initial cartilage degeneration and inflammation [149]. ITGB1, an integrin crucial for cartilage development, displays lower expression in KBD than in OA, with altered N-glycosylation impacting its function [148]. Similarly, LRP1 variations and unique N-glycosylation sites in KBD signify distinctive pathogenic pathways compared to OA [148]. ANO6, COL1A1, and MXRA5 N-glycosylation modifications potentially influence bone and cartilage integrity, contributing to KBD's distinct pathology [148]. Han et al.'s glycoproteomic analysis further highlighted dysregulated N-glycoproteins in OA and KBD cartilage, implicating ECM interactions and cellular processes in their pathology [150]. The identified proteins like ITGB1, COL2A1, COL7A1, CHST-3, CHST-4, THBS2, BMP8A, TNC, LAMP2, and GUSB in OA and KBD cartilage underscore their involvement in ECM metabolism and cellular interactions, contributing to cartilage degeneration [150]. These findings underline the need for deeper insights into N-glycosylation roles, providing potential therapeutic targets for KBD and OA treatment.
Protein glycosylation is a fundamental player in maintaining the delicate balance within cartilage, governing both its healthy equilibrium and its distressing degenerative phases. Studies by Yoshimoto et al. spotlight the intersection of cell senescence signaling, protein glycosylation pathways, and the onset of OA in chondrocytes. Remarkably elevated expression of the N-acetylgalactosaminyltransferase (GALNT) family, particularly GALNT16, within OA chondrocytes underscores its correlation with chondrocyte senescence and the pathological landscape of OA. This enzyme family assumes a crucial role in early O-glycan biosynthesis. Beyond cartilage and chondrocytes, OA casts its impact on a spectrum of joint tissues and associated cells, fostering cellular heterogeneity that likely fuels the pathology of OA. The aggregation of aged chondrocytes within OA lesions and their release of senescence-associated secretory phenotype (SASP) factors corroborates the murine model findings, which demonstrate that removing senescent chondrocytes, either genetically or pharmacologically, ameliorates OA progression. Conversely, transplanting senescent chondrocytes precipitates cartilage degeneration, hinting at them as potential targets for OA treatment. Clinical scrutiny and animal models reveal a tight bond between abnormal glycosylation and the onset and evolution of OA. Distinct glycosylation alterations observed in human OA cartilage and degraded mouse cartilage, coupled with the regulatory role of O-linked glycosylation in chondrocyte differentiation, affirm its dual role in both normal cartilage homeostasis and the progression of degenerative cartilage diseases like OA.
The GALNT family, comprising 20 isoforms, orchestrates mucin-type O-glycosylation by initiating the transfer of N-acetylgalactosamine (GalNAc) to serine/threonine residues within the Golgi apparatus. Their distinct activities and substrate specificities intricately regulate O-glycosylation, reminiscent of their role in cancer-related glycosylation changes and congenital disorders. The increased expression of GALNTs—GALNT16 prominently—alongside GALNT1, GALNT2, GALNT7, and GALNT15 in the O-glycosylation pathway in OA chondrocytes, emphasizes their involvement [151]. Aberrations in the Golgi apparatus structure, as unearthed by Kourí et al. in an OA rat model, and the correlation between glycoprotein-bound glycans' biosynthesis and cell dynamics, as demonstrated by Yang et al. under inflammatory conditions in bovine and human chondrocytes, accentuate the intricate nexus between glycosylation and OA progression. Chondrocytes' production of glycoproteins and glycosaminoglycans—both N-linked and O-linked—plays a pivotal role in maintaining cartilage integrity. The cytokines TNFα and TGFβ emerge as pivotal players in modulating glycosylation patterns, glycoprotein expression, and glycosyltransferase activities, thereby influencing critical cell functions. TGFβ counters IL-1's inhibitory effects, aiding in cartilage recovery, while members of the TGF-β superfamily, like bone morphogenetic proteins (BMPs), govern cartilage and bone growth. The intricate connection between glycosylation and cell dynamics, regulated by TNF-α and TGFβ-1, underscores their cell type-dependent influence on glycosyltransferases and glycosylation. Notably, TNFα treatment elevates GalNAc-transferase activity in chondrocytes, suggesting its role in OA cartilage pathology. An inhibitor of GalNAc-transferase highlighted by Tian et al. unveils the pivotal role of O-glycosylation in regulating cell apoptosis, signifying its potential as a therapeutic target [28]. Huang et al.'s identification of abnormal lubricin modification and degradation in OA patients, catalyzed by cathepsin G (CG), points to a pivotal role played by synovial CG in pathological lubrication reduction through the degradation of glycosylated and non-glycosylated regions of lubricin [152].
ANGPTL4, as elucidated by Yang et al., emerges as another crucial player, enhanced in arthritis, potentially impacting conditions like RA, OA, or osteoporosis, emphasizing the multifaceted impact of glycosylated proteins in disease progression [153]. Tardio et al.'s findings on increased O-glycosylation mediating certain differentiation markers in OA shed light on the mechanism involving O-GlcNAc addition to various proteins [154]. The exploration by Riegger et al. on PUGNAc's anti-catabolic effects through the hexosamine biosynthetic pathway (HBP) inhibition underscores its potential as a cartilage protector, while Croft et al.'s investigation into CD44 splice variants suggests their association with the inflammatory state in synovial joints [155]. VN's role as a potential inhibitor of CatK, impacting collagen fibril formation, introduces a therapeutic avenue for osteoporosis and OA treatment. Notably, DeN-glycosylation of VN shows promise in diminishing CatK activity, potentially positioning VN as a therapeutic agent in OA treatment and enhancing collagen binding activity [156].
Yu et al.'s comprehensive approach integrating lectin microarray technology and qPCR revealed altered glycomic patterns associated with OA, presenting distinctive site-specific glycosylation changes and heterogeneity in OA cartilage. Notably, the heterogeneity in glycosylation at FN1_N258, ACAN_N333, and N658 emerged as novel features of OA cartilage, posing challenges in identifying site-specific glycosylation throughout OA development. The aberrant glycosylation observed in OA cartilage, including reduced high-mannose N-glycans and elevated fucose glycosylation, correlates with inflammatory conditions and MMP-13 overexpression, reflecting phenotypic changes in osteoarthritic cells [157]. The elucidation of rs11583641 within COLGALT2, a collagen post-translational modification factor, as a susceptibility target for OA, highlights the intricate impact of aberrant PTMson cartilage integrity, underscoring collagen's crucial role in OA pathology [158].
The complex interplay between glycosylation processes and OA progression is deeply intertwined, as illuminated by various studies. Yu et al. conducted extensive research, showcasing that the overexpression of FUT10 leads to escalated α-1,3 fucosylation in both OA patient cartilage and chondrocytes, rabbit OA models, and under TNF-α-induced conditions, significantly impacting chondrocyte biology. Silencing FUT10 expression curtails MMP13 and IL-1β expression, impedes α-1,3 fucosylation of TNFR1, and obstructs the NF-κB and P38/JNK-MAPK pathways, mitigating ECM degradation and chondrocyte senescence induced by TNF-α. Notably, the inflammatory state of OA chondrocytes upregulates FUT10 expression via TNF-α, driving a positive feedback loop culminating in inflammation, apoptosis, and ECM degradation. TNFR1, serving as the cell surface receptor for TNF-α, triggers intracellular events leading to NF-κB and p38/JNK MAPK pathway activation, where glycosylation, particularly N-glycosylation, plays a regulatory role. Dysregulation of pro-inflammatory cytokines, particularly TNF-α and IL-1β, spurs MMPs' expression, including ADAMTS-4, 5, and 7, contributing to cartilage degeneration.
Moreover, α-1,3/6 fucosylation alterations, Lewis antigen synthesis, and heterogeneity of glycosylation further implicate their involvement in OA onset and progression [159,160][Fig. 5]. Reboul et al. highlighted the elevation of collagenase-3 in OA chondrocytes induced by IL-1β and TNF-α, emphasizing glycosylation's role in protein expression and suggesting potential cytokine contributions to collagenase-3 elevation in OA cartilage [159,160]. Similarly, Cs-Szabó et al. emphasized the distinct glycosylation patterns in OA chondrocytes, indicative of an altered metabolic state [159]. Sun et al.'s bioinformatics analysis pinpointed key genes, including B4GALT1 and EIF4G1, downregulated in OA, implying their crucial roles in glycosylation regulation and OA pathogenesis [159]. The intricate relationship between hypermannosylated N-glycans, ECM degradation, and chondrocyte biology during OA progression is unveiled, notably impacting collagen metabolism, protein expression, and the progression of this debilitating condition [159]. Additionally, glucosamine sulfate's modulation of glycosylation pathways and its consequential influence on COX-2 expression further underscore glycosylation's pivotal role in OA mitigation [159].
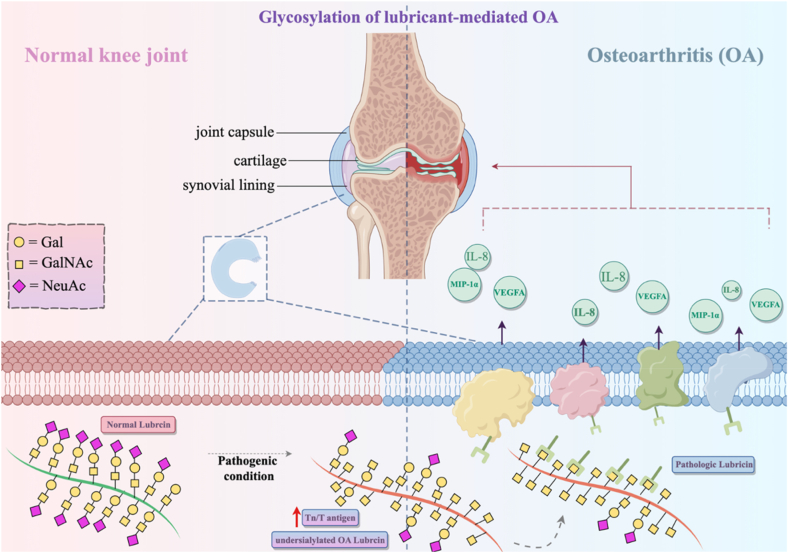
Detailed analysis using parallel reaction monitoring (PRM) revealed significant differences in N-glycosylation patterns in subchondral bone samples from OA patients, highlighting core pathogenic mechanisms. Enrichment analysis reflected associations with metabolic pathways, hinting at crucial roles in ECM interactions and protein digestion. Abnormal glycosylation in collagen proteins impacts bone integrity, closely linking to OA development. Distinct glycosylation abundance in specific proteins like COL6A3, ACAN, and MXRA5 between medial and lateral subchondral bone samples in OA patients highlights potential roles in chondrocyte regulation, suggesting therapeutic avenues for OA intervention. Studies underscore the pivotal role of specific proteins and their glycosylation patterns in OA pathogenesis, presenting avenues for diagnostic and therapeutic advancements in primary knee osteoarthritis (KOA). MXRA5's differential expression and undefined function, along with various N-glycosylation sites, hint at its possible involvement in OA progression, while LAMC1's association with multiple proteins suggests potential implications in OA and protein interactions within affected tissues [160]. Huang et al.'s identification of inflammation biomarkers unveiled distinct patterns between OA patients and controls, notably elevated plasma MCP-4 and TARC, suggesting systemic inflammation's influence in late-stage OA rather than synovial biosynthesis. Moreover, altered lubricin glycosylation in OA correlates positively with SF-IL-8, SF-MIP-1α, and SF-VEGFA, exacerbating synovial inflammation. Lubricin's highly O-glycosylated nature encoded by PRG4 gene plays pivotal roles in cartilage integrity, growth regulation, and synovial fluid lubrication, with defects in its glycosylation contributing to OA pathogenesis. The dynamics of glycosylation alterations extend beyond OA, resonating across various inflammatory conditions such as ulcerative colitis, pulmonary inflammation, and cancer, manifesting as shifts in glycan profiles and aberrant expression of specific antigens. Additionally, Toll-like receptors (TLRs) and their ligands induce a pro-inflammatory state in fibroblast-like synoviocytes (FLSs), impacting cytokine expression in OA [160][Fig. 6].
Fig. 6.
Glycosylation of lubricin in OA.
Martin et al. and Parekh et al.'s findings on IgG glycosylation underscore its potential as a distinguishing biomarker for RA and OA, revealing distinctive glycoforms associated with these conditions [160,161]. Furthermore, Legrand et al.'s discovery of glucose-derived pentosidine (GSP) as a critical biomarker in OA models highlights glycation's role in disease onset and progression, offering promise for early detection and classification algorithms [160]. Lastly, the mevalonate pathway's metabolites, including ethanol impacting protein N-glycosylation, suggest an interplay between digoxin and glycosylation in degenerative bone diseases like OA, offering insights into their metabolic connections [160]. Overall, these studies underscore the intricate and multifaceted role of glycosylation in the onset, progression, and potential treatment avenues for both OA and associated conditions like T2DM and KBD.
The intricate dance between protein glycosylation and the progression of OA unfurls a compelling narrative that intertwines the very fabric of cartilage health and disease. From the subtle alterations in glycosylation patterns within OA patients coexisting with T2DM to the distinct markers delineating KBD from OA, the revelations are profound. Glycosylation emerges as a key orchestrator, influencing chondrocyte behavior, ECM integrity, and inflammatory responses, painting a comprehensive portrait of OA's pathogenesis.
These studies, collectively, underscore the profound influence of glycosylation on various facets of OA, illuminating potential diagnostic markers and therapeutic targets. Whether unraveling the GALNT family's implications in chondrocyte senescence or spotlighting the aberrations in glycosylation pathways under inflammatory conditions, each revelation adds a stroke to the canvas of understanding OA's complexities. The glycosylation fingerprint across proteins like FN1, ACAN, or COL6A3 offers not just diagnostic possibilities but tantalizing prospects for interventions and treatments.
The intricate interplay between glycosylation and OA progression stands as a testament to the nuanced balance within cartilage, and these studies pave the way for novel interventions, diagnostic tools, and, potentially, a deeper comprehension of this degenerative joint condition. As such, these findings mark not just the culmination of diverse investigations but also herald a new chapter in the quest to decipher, intercept, and combat the throes of OA.
6. Concluding remarks
In conclusion, this review is built upon the complex interactions and paramount importance of glycosylation and glycation in the realm of diseases, with a particular focus on their significances in OA. The complex interactions between glycosylation and glycation and their involvement in the pathogenic mechanisms of diseases are recognized, particularly its multifaceted involvement in modifying proteins and impacting cellular functions. Our scrutiny of glycosylation and glycation's impact within the context of OA illuminates their significances as a contributory factor in the onset and progression of this debilitating joint disease. Understanding the nuanced mechanisms of glycosylation and glycation in various diseases, including their effect on structural proteins and inflammatory pathways, unveils promising avenues for therapeutic interventions and diagnostic advancements. The exploration of molecular mechanisms driving glycosylation and glycation-induced joint tissue damage, along with the synthesis of diverse perspectives and research findings, has deepened our understanding of the " glycosylation and glycation enigma" and their implication for disease progression. As we delve deeper into unraveling the complexities of glycosylation and glycation, their roles as a key player in disease etiology becomes more evident, offering prospects for targeted treatments and heightened disease management strategies. While seemingly contradictory research outcomes may stem from factors such as heterogeneity, it is evident that researchers possess a strong interest in unraveling the role of glycosylation and glycation in various pathological contexts.
The overall incidence of OA is on a gradual rise, posing an increasingly severe threat to patients' quality of life. It has evolved into a pressing social and public health issue, burdening both socioeconomic and healthcare systems significantly. Intervention strategies for OA currently remain confined to either conservative approaches or "complete joint replacement." However, even the methods aimed at alleviating clinical symptoms fail to decelerate or fully impede the progressive advancement of OA. In advanced stages of OA, joint replacement surgery remains the predominant viable treatment option. Nevertheless, due to the inherent "limited lifespan" of prosthetics, joint replacement is not a perpetual solution. However, several key aspects warrant further investigation.
For instance, the intricate landscape of OA unravels a compelling narrative of the decisive role played by aberrant glycosylation, an emerging determinant that transcends the boundaries of this debilitating joint disorder. Through meticulous investigations, evidence amassed by Luo et al. exposes distinctive N-glycosylation patterns in OA patients with concurrent T2DM, unveiling a marked reduction in N-glycosylated protein abundance. This revelation identifies specific proteins such as fibronectin 1 (FN1), cartilage intermediate layer protein 1 (CILP), and collagen type VI alpha-1 chain (COL6A1) as key players in the intricate orchestration of cartilage degradation [143]. Simultaneously, the scholarly endeavors of Urita et al., Parekh et al., Bousseau et al., and Derada et al. shed light on the diverse spectrum of glycosylation alterations within the context of OA and T2DM. These alterations encompass an array of modifications, from high-mannose type N-glycans to intricate changes in IgG, collectively illuminating the profound and wide-ranging impact of glycosylation dynamics on the intricate landscape of disease pathogenesis. The aberrations observed in chitinase 1 (CHIT1) and IgG among diabetic patients stand out as particularly noteworthy, underscoring the diagnostic potential inherent in discerning N-glycosylation profiles for monitoring the progression of T2DM. Furthermore, the insightful findings presented by Li et al. cast a spotlight on the overarching influence of elevated blood glucose on the synovium, triggering cascading inflammatory responses and instigating ERS. This intricate interplay culminates in the exacerbation of chondrocyte degradation in OA, particularly in the context of diabetic conditions [144]. In summation, the nexus between aberrant glycosylation and OA emerges as a critical axis, weaving together intricate molecular dynamics that influence disease onset and progression. The multifaceted revelations underscore the need for a nuanced understanding of glycosylation intricacies, positioning it as a pivotal area for further exploration and targeted therapeutic interventions in the continually evolving landscape of joint disorders.
On top of that, delving into the precise molecular pathways that regulate AGEs accumulation, examining the potential for AGEs clearance mechanisms, and elucidating the interactions between AGEs and other contributors to OA, such as inflammation and oxidative stress, are promising avenues for future research. Moreover, elucidating the spatiotemporal dynamics of AGEs deposition in joint tissues and the intricate crosstalk between various AGEs receptors and signaling pathways may offer additional layers of insight into disease development. Furthermore, as age advances, the most pronounced changes within the body involve the production and deposition of AGEs. Once formed, AGEs are challenging to eliminate upon binding to proteins, with their deposition contingent on the rate of protein degradation within the body. Presently, there are no reported mechanisms that effectively eliminate AGEs. Coupled with the sluggish turnover rate of cartilage cells, AGEs tend to accumulate within joint cartilage cells, resulting in persistent cellular damage caused by AGEs [162]. AGEs promote joint cartilage collagen glycation, curtail the synthesis of aggregate proteins within the ECM, ultimately leading to increased ECM fragility and diminished resistance to compressive and shear forces [163].
Since AGEs are one of the factors in OA pathogenesis, we can reduce the intake of exogenous AGEs and the production of endogenous AGEs to inhibit the occurrence and development of OA. Food is one of the main sources of exogenous AGEs. Research on different diet plans aims to determine the content of AGEs in different foods. In addition, high molecular weight AGEs that are bound to proteins from foreign substances can be detected. Combining the correct choice of food and cooking methods together with physical exercise can avoid the harmful effects of a high-AGEs diet on the body. Studies on endogenous AGEs have shown that substances such as aminoguanidine, pyridoxamine, and monomer amino acids (such as lysine and arginine) can effectively inhibit AGEs formation, AGEs-induced protein cross-linking, and tissue collagen accumulation and hardening. In articular cartilage, monomer amino acids can inhibit AGEs formation, thus preventing reticular collagen sclerosis. Other studies have shown that the PPARγ agonist pioglitazone and G-protein coupled receptor can reduce AGEs-induced chondrocyte inflammation, apoptosis, and ECM catabolism by inhibiting the NF-κB pathway. The specific mechanism and function of AGEs related to OA need further research and investigation to determine the function and specific action sites of AGEs and intervene there to reduce AGEs intake and production from both exogenous and endogenous sources. Additionally, chondrocytes in vitro, OA animal models, and OA patients should be investigated to further discover and verify the effects of AGEs on the pathogenesis and pathological changes of OA, which lays the foundation for OA diagnosis, prognosis, prevention, and treatment.
The specific mechanisms and functions linking AGEs to OA remain an enigma. Therefore, it might be imperative to endeavor to unravel their intricate networks, grasp the functional roles and precise target sites of AGEs, and intervene strategically in response to both exogenous and endogenous sources of AGEs. All these efforts collectively act as pivotal leverage points. Furthermore, through the integration of in vitro chondrocyte studies, OA animal models, and clinical observations of OA patients, further confirmation of the impact of AGEs on the pathogenesis and pathological changes of OA can provide a more comprehensive data foundation. This, in turn, contributes to a better understanding of OA's diagnosis, prognosis, prevention, and treatment strategies.
Incorporating data from in vitro models, animal studies, and clinical trials, along with advancements in analytical techniques, holds the potential to substantiate and refine our understanding of glycosylation and glycation's role in OA further. Ultimately, this enhanced comprehension may foster the development of innovative diagnostic approaches, prognostic indicators, preventive strategies, and targeted therapeutics tailored to individuals at risk for, or already afflicted by OA. By unraveling the complexities of glycosylation and glycation's involvement in OA and other diseases, we pave the way for transformative advancements in healthcare that could significantly improve patients' quality of life. It is important to acknowledge the limitations of the current body of research, including the variability in study designs, patient populations, and methodologies employed for assessing glycosylation and glycation-related changes in osteoarthritic tissues. Further longitudinal studies and well-controlled experiments are essential to establish causality and elucidate the precise temporal relationships between glycosylation and glycation events and disease progression. Overall, unraveling the intricate connections between glycosylation and glycation and OA holds the promise of advancing our knowledge of the disease and potentially providing novel therapeutic avenues for improving the quality of life for millions affected by this condition.
Data availability
Data will be made available on request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
CRediT authorship contribution statement
Hong-zhi Liu: Writing – original draft, Investigation, Conceptualization. Xin-qiu Song: Writing – original draft, Methodology, Investigation, Conceptualization. Hongmei Zhang: Writing – review & editing, Investigation, Conceptualization.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Acknowledgments
We thank Figdraw (http://www.figdraw.com/) for providing the drawing platform.
Contributor Information
Hong-zhi Liu, Email: doctorlhz@gmail.com.
Xin-qiu Song, Email: docxqsong@gmail.com.
Hongmei Zhang, Email: zhmwangjing@163.com.
References
- 1.Martel-Pelletier J., Barr A.J., Cicuttini F.M., Conaghan P.G., Cooper C., Goldring M.B., Goldring S.R., Jones G., Teichtahl A.J., Pelletier J.P. Osteoarthritis. Nat. Rev. Dis. Prim. 2016;2 doi: 10.1038/nrdp.2016.72. [DOI] [PubMed] [Google Scholar]
- 2.Iannone F., Lapadula G. The pathophysiology of osteoarthritis. Aging Clin. Exp. Res. 2003;15:364–372. doi: 10.1007/BF03327357. [DOI] [PubMed] [Google Scholar]
- 3.Bernabei I., So A., Busso N., Nasi S. Cartilage calcification in osteoarthritis: mechanisms and clinical relevance. Nat. Rev. Rheumatol. 2023;19:10–27. doi: 10.1038/s41584-022-00875-4. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez-Lopez E., Coras R., Torres A., Lane N.E., Guma M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022;18:258–275. doi: 10.1038/s41584-022-00749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loeser R.F., Goldring S.R., Scanzello C.R., Goldring M.B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang C.Y., Chanalaris A., Troeberg L. ADAMTS and ADAM metalloproteinases in osteoarthritis - looking beyond the 'usual suspects'. Osteoarthritis Cartilage. 2017;25:1000–1009. doi: 10.1016/j.joca.2017.02.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang H., Huang L., Welch I., Norley C., Holdsworth D.W., Beier F., Cai D. Early changes of articular cartilage and subchondral bone in the DMM mouse model of osteoarthritis. Sci. Rep. 2018;8:2855. doi: 10.1038/s41598-018-21184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glyn-Jones S., Palmer A.J., Agricola R., Price A.J., Vincent T.L., Weinans H., Carr A.J. Osteoarthritis, Lancet. 2015;386:376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 9.Sun D., Hu F., Gao H., Song Z., Xie W., Wang P., Shi L., Wang K., Li Y., Huang C., Li Z. Distribution of abnormal IgG glycosylation patterns from rheumatoid arthritis and osteoarthritis patients by MALDI-TOF-MS(n) Analyst. 2019;144:2042–2051. doi: 10.1039/c8an02014k. [DOI] [PubMed] [Google Scholar]
- 10.Sagar D.R., Ashraf S., Xu L., Burston J.J., Menhinick M.R., Poulter C.L., Bennett A.J., Walsh D.A., Chapman V. Osteoprotegerin reduces the development of pain behaviour and joint pathology in a model of osteoarthritis. Ann. Rheum. Dis. 2014;73:1558–1565. doi: 10.1136/annrheumdis-2013-203260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palazzo C., Nguyen C., Lefevre-Colau M.M., Rannou F., Poiraudeau S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59:134–138. doi: 10.1016/j.rehab.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Kulkarni K., Karssiens T., Kumar V., Pandit H. Obesity and osteoarthritis. Maturitas. 2016;89:22–28. doi: 10.1016/j.maturitas.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Kraus V.B., Burnett B., Coindreau J., Cottrell S., Eyre D., Gendreau M., Gardiner J., Garnero P., Hardin J., Henrotin Y., Heinegard D., Ko A., Lohmander L.S., Matthews G., Menetski J., Moskowitz R., Persiani S., Poole A.R., Rousseau J.C., Todman M., Group O.F.O.B.W. Application of biomarkers in the development of drugs intended for the treatment of osteoarthritis. Osteoarthritis Cartilage. 2011;19:515–542. doi: 10.1016/j.joca.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badley E.M., Wilfong J.M., Yip C., Millstone D.B., Perruccio A.V. The contribution of age and obesity to the number of painful joint sites in individuals reporting osteoarthritis: a population-based study. Rheumatology. 2020;59:3350–3357. doi: 10.1093/rheumatology/keaa138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y., Zhang Z., Li T., Xu H., Zhang H. Senescence in osteoarthritis: from mechanism to potential treatment. Arthritis Res. Ther. 2022;24:174. doi: 10.1186/s13075-022-02859-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quicke J.G., Conaghan P.G., Corp N., Peat G. Osteoarthritis year in review 2021: epidemiology & therapy. Osteoarthritis Cartilage. 2022;30:196–206. doi: 10.1016/j.joca.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis, Lancet. 2019;393:1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 18.Leifer V.P., Katz J.N., Losina E. The burden of OA-health services and economics. Osteoarthritis Cartilage. 2022;30:10–16. doi: 10.1016/j.joca.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W., Doherty M., Bardin T., Pascual E., Barskova V., Conaghan P., Gerster J., Jacobs J., Leeb B., Liote F., McCarthy G., Netter P., Nuki G., Perez-Ruiz F., Pignone A., Pimentao J., Punzi L., Roddy E., Uhlig T., Zimmermann-Gorska I., E.S.C.f.I.C.S.I. Therapeutics EULAR evidence based recommendations for gout. Part II: management. Report of a task force of the EULAR standing committee for international clinical studies including therapeutics (ESCISIT) Ann. Rheum. Dis. 2006;65:1312–1324. doi: 10.1136/ard.2006.055269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berenbaum F. Diabetes-induced osteoarthritis: from a new paradigm to a new phenotype. Ann. Rheum. Dis. 2011;70:1354–1356. doi: 10.1136/ard.2010.146399. [DOI] [PubMed] [Google Scholar]
- 21.Neumann J., Guimaraes J.B., Heilmeier U., Joseph G.B., Nevitt M.C., McCulloch C.E., Link T.M. Diabetics show accelerated progression of knee cartilage and meniscal lesions: data from the osteoarthritis initiative. Skeletal Radiol. 2019;48:919–930. doi: 10.1007/s00256-018-3088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mende M., Bednarek C., Wawryszyn M., Sauter P., Biskup M.B., Schepers U., Brase S. Chemical synthesis of glycosaminoglycans. Chem. Rev. 2016;116:8193–8255. doi: 10.1021/acs.chemrev.6b00010. [DOI] [PubMed] [Google Scholar]
- 23.Belcher C., Yaqub R., Fawthrop F., Bayliss M., Doherty M. Synovial fluid chondroitin and keratan sulphate epitopes, glycosaminoglycans, and hyaluronan in arthritic and normal knees. Ann. Rheum. Dis. 1997;56:299–307. doi: 10.1136/ard.56.5.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang Z., Zhou Z., Wang Y., Huang H., Du G., Chen J. Bio-based strategies for producing glycosaminoglycans and their oligosaccharides. Trends Biotechnol. 2018;36:806–818. doi: 10.1016/j.tibtech.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Huang S., Thomsson K.A., Jin C., Ryberg H., Das N., Struglics A., Rolfson O., Bjorkman L.I., Eisler T., Schmidt T.A., Jay G.D., Krawetz R., Karlsson N.G. Truncated lubricin glycans in osteoarthritis stimulate the synoviocyte secretion of VEGFA, IL-8, and MIP-1alpha: interplay between O-linked glycosylation and inflammatory cytokines. Front. Mol. Biosci. 2022;9 doi: 10.3389/fmolb.2022.942406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapoor M., Martel-Pelletier J., Lajeunesse D., Pelletier J.P., Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 27.Jeon O.H., Wilson D.R., Clement C.C., Rathod S., Cherry C., Powell B., Lee Z., Khalil A.M., Green J.J., Campisi J., Santambrogio L., Witwer K.W., Elisseeff J.H. Senescence cell-associated extracellular vesicles serve as osteoarthritis disease and therapeutic markers. JCI Insight. 2019;4 doi: 10.1172/jci.insight.125019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang X., Yip J., Anastassiades T., Harrison M., Brockhausen I. The action of TNFα and TGFβ include specific alterations of the glycosylation of bovine and human chondrocytes. Biochim. Biophys. Acta Mol. Cell Res. 2007;1773:264–272. doi: 10.1016/j.bbamcr.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 29.Spiro R.G. Glycoproteins. Adv. Protein Chem. 1973;27:349–467. doi: 10.1016/s0065-3233(08)60451-9. [DOI] [PubMed] [Google Scholar]
- 30.Montreuil J. Primary structure of glycoprotein glycans: basis for the molecular biology of glycoproteins. Adv. Carbohydr. Chem. Biochem. 1980;37:157–223. doi: 10.1016/s0065-2318(08)60021-9. [DOI] [PubMed] [Google Scholar]
- 31.Spiro R.G. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12:43R–56R. doi: 10.1093/glycob/12.4.43r. [DOI] [PubMed] [Google Scholar]
- 32.Kobata A. Structures and functions of the sugar chains of glycoproteins. Eur. J. Biochem. 1992;209:483–501. doi: 10.1111/j.1432-1033.1992.tb17313.x. [DOI] [PubMed] [Google Scholar]
- 33.Hang I., Lin C.W., Grant O.C., Fleurkens S., Villiger T.K., Soos M., Morbidelli M., Woods R.J., Gauss R., Aebi M. Analysis of site-specific N-glycan remodeling in the endoplasmic reticulum and the Golgi. Glycobiology. 2015;25:1335–1349. doi: 10.1093/glycob/cwv058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zoldos V., Novokmet M., Beceheli I., Lauc G. Genomics and epigenomics of the human glycome. Glycoconj. J. 2013;30:41–50. doi: 10.1007/s10719-012-9397-y. [DOI] [PubMed] [Google Scholar]
- 35.Blomme B., Van Steenkiste C., Callewaert N., Van Vlierberghe H. Alteration of protein glycosylation in liver diseases. J. Hepatol. 2009;50:592–603. doi: 10.1016/j.jhep.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 36.Gui H.L., Gao C.F., Wang H., Liu X.E., Xie Q., Dewaele S., Wang L., Zhuang H., Contreras R., Libert C., Chen C. Altered serum N-glycomics in chronic hepatitis B patients. Liver Int. 2010;30:259–267. doi: 10.1111/j.1478-3231.2009.02170.x. [DOI] [PubMed] [Google Scholar]
- 37.Krieg J., Glasner W., Vicentini A., Doucey M.A., Loffler A., Hess D., Hofsteenge J. C-Mannosylation of human RNase 2 is an intracellular process performed by a variety of cultured cells. J. Biol. Chem. 1997;272:26687–26692. doi: 10.1074/jbc.272.42.26687. [DOI] [PubMed] [Google Scholar]
- 38.Cummings R.D. The repertoire of glycan determinants in the human glycome. Mol. Biosyst. 2009;5:1087–1104. doi: 10.1039/b907931a. [DOI] [PubMed] [Google Scholar]
- 39.Yu H., Takeuchi H. Protein O-glucosylation: another essential role of glucose in biology. Curr. Opin. Struct. Biol. 2019;56:64–71. doi: 10.1016/j.sbi.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Harvey B.M., Haltiwanger R.S. Regulation of notch function by O-glycosylation. Molecular Mechanisms of Notch Signaling. 2018:59–78. doi: 10.1007/978-3-319-89512-3_4. [DOI] [PubMed] [Google Scholar]
- 41.Mereiter S., Balmaña M., Campos D., Gomes J., Reis C.A. Glycosylation in the era of cancer-targeted therapy: where are we heading? Cancer Cell. 2019;36:6–16. doi: 10.1016/j.ccell.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Moremen K.W., Tiemeyer M., Nairn A.V. Vertebrate protein glycosylation: diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012;13:448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joshi H.J., Hansen L., Narimatsu Y., Freeze H.H., Henrissat B., Bennett E., Wandall H.H., Clausen H., Schjoldager K.T. Glycosyltransferase genes that cause monogenic congenital disorders of glycosylation are distinct from glycosyltransferase genes associated with complex diseases. Glycobiology. 2018;28:284–294. doi: 10.1093/glycob/cwy015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takeuchi H., Schneider M., Williamson D.B., Ito A., Takeuchi M., Handford P.A., Haltiwanger R.S. Two novel protein O-glucosyltransferases that modify sites distinct from POGLUT1 and affect Notch trafficking and signaling. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E8395–E8402. doi: 10.1073/pnas.1804005115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arnold J.N., Saldova R., Hamid U.M., Rudd P.M. Evaluation of the serum N-linked glycome for the diagnosis of cancer and chronic inflammation. Proteomics. 2008;8:3284–3293. doi: 10.1002/pmic.200800163. [DOI] [PubMed] [Google Scholar]
- 46.Ohtsubo K., Marth J.D. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 47.Hanover J.A., Krause M.W., Love D.C. Linking metabolism to epigenetics through O-GlcNAcylation. Nat. Rev. Mol. Cell Biol. 2012;13:312–321. doi: 10.1038/nrm3334. [DOI] [PubMed] [Google Scholar]
- 48.Yang X., Qian K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2017;18:452–465. doi: 10.1038/nrm.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hart G.W., Housley M.P., Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 50.Bennett E.P., Mandel U., Clausen H., Gerken T.A., Fritz T.A., Tabak L.A. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 2012;22:736–756. doi: 10.1093/glycob/cwr182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steentoft C., Vakhrushev S.Y., Joshi H.J., Kong Y., Vester-Christensen M.B., Schjoldager K.T., Lavrsen K., Dabelsteen S., Pedersen N.B., Marcos-Silva L., Gupta R., Bennett E.P., Mandel U., Brunak S., Wandall H.H., Levery S.B., Clausen H. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013;32:1478–1488. doi: 10.1038/emboj.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Halim A., Brinkmalm G., Ruetschi U., Westman-Brinkmalm A., Portelius E., Zetterberg H., Blennow K., Larson G., Nilsson J. Site-specific characterization of threonine, serine, and tyrosine glycosylations of amyloid precursor protein/amyloid beta-peptides in human cerebrospinal fluid. Proc. Natl. Acad. Sci. U. S. A. 2011;108:11848–11853. doi: 10.1073/pnas.1102664108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corfield A.P. Mucins: a biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta. 2015;1850:236–252. doi: 10.1016/j.bbagen.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 54.Stotter B.R., Talbot B.E., Capen D.E., Artelt N., Zeng J., Matsumoto Y., Endlich N., Cummings R.D., Schlondorff J.S. Cosmc-dependent mucin-type O-linked glycosylation is essential for podocyte function. Am. J. Physiol. Ren. Physiol. 2020;318:F518–F530. doi: 10.1152/ajprenal.00399.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wacker M., Linton D., Hitchen P.G., Nita-Lazar M., Haslam S.M., North S.J., Panico M., Morris H.R., Dell A., Wren B.W., Aebi M. N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli. Science. 2002;298:1790–1793. doi: 10.1126/science.298.5599.1790. [DOI] [PubMed] [Google Scholar]
- 56.Handa-Narumi M., Yoshimura T., Konishi H., Fukata Y., Manabe Y., Tanaka K., Bao G.M., Kiyama H., Fukase K., Ikenaka K. Branched sialylated N-glycans are accumulated in brain synaptosomes and interact with siglec-H. Cell Struct. Funct. 2018;43:141–152. doi: 10.1247/csf.18009. [DOI] [PubMed] [Google Scholar]
- 57.Ryšlavá H., Doubnerová V., Kavan D., Vaněk O. Effect of posttranslational modifications on enzyme function and assembly. J. Proteonomics. 2013;92:80–109. doi: 10.1016/j.jprot.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 58.Abdullahi A., Stanojcic M., Parousis A., Patsouris D., Jeschke M.G. Modeling acute ER stress in vivo and in vitro. Shock. 2017;47:506–513. doi: 10.1097/SHK.0000000000000759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drickamer K., Taylor M.E. Evolving views of protein glycosylation. Trends Biochem. Sci. 1998;23:321–324. doi: 10.1016/s0968-0004(98)01246-8. [DOI] [PubMed] [Google Scholar]
- 60.Eichler J. Extreme sweetness: protein glycosylation in archaea. Nat. Rev. Microbiol. 2013;11:151–156. doi: 10.1038/nrmicro2957. [DOI] [PubMed] [Google Scholar]
- 61.Nothaft H., Szymanski C.M. Bacterial protein N-glycosylation: new perspectives and applications. J. Biol. Chem. 2013;288:6912–6920. doi: 10.1074/jbc.R112.417857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Samuelson J., Robbins P.W. Effects of N-glycan precursor length diversity on quality control of protein folding and on protein glycosylation. Semin. Cell Dev. Biol. 2015;41:121–128. doi: 10.1016/j.semcdb.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jarrell K.F., Ding Y., Meyer B.H., Albers S.V., Kaminski L., Eichler J. N-linked glycosylation in Archaea: a structural, functional, and genetic analysis. Microbiol. Mol. Biol. Rev. 2014;78:304–341. doi: 10.1128/MMBR.00052-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schaffer C., Messner P. Emerging facets of prokaryotic glycosylation. FEMS Microbiol. Rev. 2017;41:49–91. doi: 10.1093/femsre/fuw036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lowenthal M.S., Davis K.S., Formolo T., Kilpatrick L.E., Phinney K.W. Identification of novel N-glycosylation sites at noncanonical protein consensus motifs. J. Proteome Res. 2016;15:2087–2101. doi: 10.1021/acs.jproteome.5b00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mitra N., Sinha S., Ramya T.N., Surolia A. N-linked oligosaccharides as outfitters for glycoprotein folding, form and function. Trends Biochem. Sci. 2006;31:156–163. doi: 10.1016/j.tibs.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 67.Lee H.S., Qi Y., Im W. Effects of N-glycosylation on protein conformation and dynamics: protein Data Bank analysis and molecular dynamics simulation study. Sci. Rep. 2015;5:8926. doi: 10.1038/srep08926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fiedler K., Simons K. The role of N-glycans in the secretory pathway. Cell. 1995;81:309–312. doi: 10.1016/0092-8674(95)90380-1. [DOI] [PubMed] [Google Scholar]
- 69.Agthe M., Garbers Y., Grotzinger J., Garbers C. Two N-linked glycans differentially control maturation, trafficking and proteolysis, but not activity of the IL-11 receptor. Cell. Physiol. Biochem. 2018;45:2071–2085. doi: 10.1159/000488044. [DOI] [PubMed] [Google Scholar]
- 70.Vagin O., Kraut J.A., Sachs G. Role of N-glycosylation in trafficking of apical membrane proteins in epithelia. Am. J. Physiol. Ren. Physiol. 2009;296:F459–F469. doi: 10.1152/ajprenal.90340.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ryan S.O., Cobb B.A. Roles for major histocompatibility complex glycosylation in immune function. Semin. Immunopathol. 2012;34:425–441. doi: 10.1007/s00281-012-0309-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vajaria B.N., Patel P.S. Glycosylation: a hallmark of cancer? Glycoconj. J. 2017;34:147–156. doi: 10.1007/s10719-016-9755-2. [DOI] [PubMed] [Google Scholar]
- 73.Watanabe Y., Allen J.D., Wrapp D., McLellan J.S., Crispin M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science. 2020;369:330–333. doi: 10.1126/science.abb9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu J., Liu H., Zhang J., Wang P., Liu S., Liu G., Wu L. Effects of Asn-33 glycosylation on the thermostability of Thermomyces lanuginosus lipase. J. Appl. Microbiol. 2014;117:151–159. doi: 10.1111/jam.12519. [DOI] [PubMed] [Google Scholar]
- 75.Gavrilov Y., Shental-Bechor D., Greenblatt H.M., Levy Y. Glycosylation may reduce protein thermodynamic stability by inducing a conformational distortion. J. Phys. Chem. Lett. 2015;6:3572–3577. doi: 10.1021/acs.jpclett.5b01588. [DOI] [PubMed] [Google Scholar]
- 76.Price J.L., Shental-Bechor D., Dhar A., Turner M.J., Powers E.T., Gruebele M., Levy Y., Kelly J.W. Context-dependent effects of asparagine glycosylation on Pin WW folding kinetics and thermodynamics. J. Am. Chem. Soc. 2010;132:15359–15367. doi: 10.1021/ja106896t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaneko Y., Nimmerjahn F., Ravetch J.V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 78.Kobata A. The N-linked sugar chains of human immunoglobulin G: their unique pattern, and their functional roles. Biochim. Biophys. Acta. 2008;1780:472–478. doi: 10.1016/j.bbagen.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 79.van de Bovenkamp F.S., Hafkenscheid L., Rispens T., Rombouts Y. The emerging importance of IgG Fab glycosylation in immunity. J. Immunol. 2016;196:1435–1441. doi: 10.4049/jimmunol.1502136. [DOI] [PubMed] [Google Scholar]
- 80.Pucic M., Knezevic A., Vidic J., Adamczyk B., Novokmet M., Polasek O., Gornik O., Supraha-Goreta S., Wormald M.R., Redzic I., Campbell H., Wright A., Hastie N.D., Wilson J.F., Rudan I., Wuhrer M., Rudd P.M., Josic D., Lauc G. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol. Cell. Proteomics. 2011;10:M111. doi: 10.1074/mcp.M111.010090. 010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parekh R.B., Dwek R.A., Sutton B.J., Fernandes D.L., Leung A., Stanworth D., Rademacher T.W., Mizuochi T., Taniguchi T., Matsuta K., et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316:452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- 82.Bond A., Cooke A., Hay F.C. Glycosylation of IgG, immune complexes and IgG subclasses in the MRL-lpr/lpr mouse model of rheumatoid arthritis. Eur. J. Immunol. 1990;20:2229–2233. doi: 10.1002/eji.1830201011. [DOI] [PubMed] [Google Scholar]
- 83.Parekh R., Roitt I., Isenberg D., Dwek R., Rademacher T. Age-related galactosylation of the N-linked oligosaccharides of human serum IgG. J. Exp. Med. 1988;167:1731–1736. doi: 10.1084/jem.167.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jeddi P.A., Lund T., Bodman K.B., Sumar N., Lydyard P.M., Pouncey L., Heath L.S., Kidd V.J., Delves P.J. Reduced galactosyltransferase mRNA levels are associated with the agalactosyl IgG found in arthritis-prone MRL-lpr/lpr strain mice. Immunology. 1994;83:484–488. [PMC free article] [PubMed] [Google Scholar]
- 85.Singh V.P., Bali A., Singh N., Jaggi A.S. Advanced glycation end products and diabetic complications. KOREAN J. PHYSIOL. PHARMACOL. 2014;18:1–14. doi: 10.4196/kjpp.2014.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vlassara H., Palace M.R. Diabetes and advanced glycation endproducts. J. Intern. Med. 2002;251:87–101. doi: 10.1046/j.1365-2796.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- 87.Rabbani N., Thornalley P.J. Glyoxalase in diabetes, obesity and related disorders. Semin. Cell Dev. Biol. 2011;22:309–317. doi: 10.1016/j.semcdb.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 88.Basta G., Schmidt A.M., De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc. Res. 2004;63:582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 89.Uribarri J., Woodruff S., Goodman S., Cai W., Chen X., Pyzik R., Yong A., Striker G.E., Vlassara H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet Assoc. 2010;110:911–916 e912. doi: 10.1016/j.jada.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Drover V.A., Nguyen D.V., Bastie C.C., Darlington Y.F., Abumrad N.A., Pessin J.E., London E., Sahoo D., Phillips M.C. CD36 mediates both cellular uptake of very long chain fatty acids and their intestinal absorption in mice. J. Biol. Chem. 2008;283:13108–13115. doi: 10.1074/jbc.M708086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nassir F., Wilson B., Han X., Gross R.W., Abumrad N.A. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J. Biol. Chem. 2007;282:19493–19501. doi: 10.1074/jbc.M703330200. [DOI] [PubMed] [Google Scholar]
- 92.Nauli A.M., Nassir F., Zheng S., Yang Q., Lo C.M., Vonlehmden S.B., Lee D., Jandacek R.J., Abumrad N.A., Tso P. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology. 2006;131:1197–1207. doi: 10.1053/j.gastro.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hsieh J., Longuet C., Maida A., Bahrami J., Xu E., Baker C.L., Brubaker P.L., Drucker D.J., Adeli K. Glucagon-like peptide-2 increases intestinal lipid absorption and chylomicron production via CD36. Gastroenterology. 2009;137:997–1005. doi: 10.1053/j.gastro.2009.05.051. 1005 e1001-1004. [DOI] [PubMed] [Google Scholar]
- 94.Gligorijevic N., Minic S., Krizakova M., Katrlik J., Nedic O. Structural changes of fibrinogen as a consequence of cirrhosis. Thromb. Res. 2018;166:43–49. doi: 10.1016/j.thromres.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 95.Esmon C.T. The interactions between inflammation and coagulation. Br. J. Haematol. 2005;131:417–430. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 96.Langer B.G., Weisel J.W., Dinauer P.A., Nagaswami C., Bell W.R. Deglycosylation of fibrinogen accelerates polymerization and increases lateral aggregation of fibrin fibers. J. Biol. Chem. 1988;263:15056–15063. [PubMed] [Google Scholar]
- 97.Sethasine S., Jain D., Groszmann R.J., Garcia-Tsao G. Quantitative histological-hemodynamic correlations in cirrhosis. Hepatology. 2012;55:1146–1153. doi: 10.1002/hep.24805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rastogi A., Maiwall R., Bihari C., Ahuja A., Kumar A., Singh T., Wani Z.A., Sarin S.K. Cirrhosis histology and Laennec staging system correlate with high portal pressure. Histopathology. 2013;62:731–741. doi: 10.1111/his.12070. [DOI] [PubMed] [Google Scholar]
- 99.Kim M.Y., Cho M.Y., Baik S.K., Park H.J., Jeon H.K., Im C.K., Won C.S., Kim J.W., Kim H.S., Kwon S.O., Eom M.S., Cha S.H., Kim Y.J., Chang S.J., Lee S.S. Histological subclassification of cirrhosis using the Laennec fibrosis scoring system correlates with clinical stage and grade of portal hypertension. J. Hepatol. 2011;55:1004–1009. doi: 10.1016/j.jhep.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 100.Albillos A., Lario M., Alvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J. Hepatol. 2014;61:1385–1396. doi: 10.1016/j.jhep.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 101.Yamamoto Y., Hiasa Y., Murakami H., Ikeda Y., Yamanishi H., Abe M., Matsuura B., Onji M. Rapid alternative absorption of dietary long-chain fatty acids with upregulation of intestinal glycosylated CD36 in liver cirrhosis. Am. J. Clin. Nutr. 2012;96:90–101. doi: 10.3945/ajcn.111.033084. [DOI] [PubMed] [Google Scholar]
- 102.Wolfe L.A., Krasnewich D. Congenital disorders of glycosylation and intellectual disability. Dev Disabil Res Rev. 2013;17:211–225. doi: 10.1002/ddrr.1115. [DOI] [PubMed] [Google Scholar]
- 103.Freeze H.H. Congenital disorders of glycosylation and the pediatric liver. Semin. Liver Dis. 2001;21:501–515. doi: 10.1055/s-2001-19031. [DOI] [PubMed] [Google Scholar]
- 104.Sarlus H., Heneka M.T. Microglia in Alzheimer's disease. J. Clin. Invest. 2017;127:3240–3249. doi: 10.1172/JCI90606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sirko S., Irmler M., Gascon S., Bek S., Schneider S., Dimou L., Obermann J., De Souza Paiva D., Poirier F., Beckers J., Hauck S.M., Barde Y.A., Gotz M. Astrocyte reactivity after brain injury-: the role of galectins 1 and 3. Glia. 2015;63:2340–2361. doi: 10.1002/glia.22898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Conroy L.R., Hawkinson T.R., Young L.E.A., Gentry M.S., Sun R.C. Emerging roles of N-linked glycosylation in brain physiology and disorders. Trends Endocrinol. Metabol. 2021;32:980–993. doi: 10.1016/j.tem.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang B., Li M.D., Yin R., Liu Y., Yang Y., Mitchell-Richards K.A., Nam J.H., Li R., Wang L., Iwakiri Y., Chung D., Robert M.E., Ehrlich B.E., Bennett A.M., Yu J., Nathanson M.H., Yang X. O-GlcNAc transferase suppresses necroptosis and liver fibrosis. JCI Insight. 2019;4 doi: 10.1172/jci.insight.127709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xu W., Zhang X., Wu J.L., Fu L., Liu K., Liu D., Chen G.G., Lai P.B., Wong N., Yu J. O-GlcNAc transferase promotes fatty liver-associated liver cancer through inducing palmitic acid and activating endoplasmic reticulum stress. J. Hepatol. 2017;67:310–320. doi: 10.1016/j.jhep.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 109.Stibler H., Borg S. Evidence of a reduced sialic acid content in serum transferrin in male alcoholics. Alcohol Clin. Exp. Res. 1981;5:545–549. doi: 10.1111/j.1530-0277.1981.tb05358.x. [DOI] [PubMed] [Google Scholar]
- 110.Stibler H., Kjellin K.G. Isoelectric focusing and electrophoresis of the CSF proteins in tremor of different origins. J. Neurol. Sci. 1976;30:269–285. doi: 10.1016/0022-510x(76)90133-7. [DOI] [PubMed] [Google Scholar]
- 111.Stibler H., Sydow O., Borg S. Quantitative estimation of abnormal microheterogeneity of serum transferrin in alcoholics. Pharmacol. Biochem. Behav. 1980;13(Suppl 1):47–51. doi: 10.1016/s0091-3057(80)80008-6. [DOI] [PubMed] [Google Scholar]
- 112.van Eijk H.G., van Noort W.L., Dubelaar M.L., van der Heul C. The microheterogeneity of human transferrins in biological fluids. Clin. Chim. Acta. 1983;132:167–171. doi: 10.1016/0009-8981(83)90244-9. [DOI] [PubMed] [Google Scholar]
- 113.Vesterberg O., Petren S., Schmidt D. Increased concentrations of a transferrin variant after alcohol abuse. Clin. Chim. Acta. 1984;141:33–39. doi: 10.1016/0009-8981(84)90164-5. [DOI] [PubMed] [Google Scholar]
- 114.Stibler H., Hultcrantz R. Carbohydrate-deficient transferrin in serum in patients with liver diseases. Alcohol Clin. Exp. Res. 1987;11:468–473. doi: 10.1111/j.1530-0277.1987.tb01925.x. [DOI] [PubMed] [Google Scholar]
- 115.Narimatsu H. Development of M2BPGi: a novel fibrosis serum glyco-biomarker for chronic hepatitis/cirrhosis diagnostics. Expert Rev. Proteomics. 2015;12:683–693. doi: 10.1586/14789450.2015.1084874. [DOI] [PubMed] [Google Scholar]
- 116.Wu P.S., Hsieh Y.C., Lee K.C., Huang Y.H., Hou M.C., Lin H.C. Mac-2 binding protein glycosylation isomer is a potential biomarker to predict portal hypertension and bacterial infection in cirrhotic patients. PLoS One. 2021;16 doi: 10.1371/journal.pone.0258589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Memarian E., t Hart L.M., Slieker R.C., Lemmers R.F.L., van der Heijden A.A., Rutters F., Nijpels G., Schoep E., Lieverse A.G., Sijbrands E.J.G., Wuhrer M., van Hoek M., Dotz V. Plasma protein N-glycosylation is associated with cardiovascular disease, nephropathy, and retinopathy in type 2 diabetes. BMJ Open Diabetes Res Care. 2021;9 doi: 10.1136/bmjdrc-2021-002345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moule S.K., Peak M., Thompson S., Turner G.A. Studies of the sialylation and microheterogeneity of human serum alpha 1-acid glycoprotein in health and disease. Clin. Chim. Acta. 1987;166:177–185. doi: 10.1016/0009-8981(87)90420-7. [DOI] [PubMed] [Google Scholar]
- 119.Thompson S., Cantwell B.M., Cornell C., Turner G.A. Abnormally-fucosylated haptoglobin: a cancer marker for tumour burden but not gross liver metastasis. Br. J. Cancer. 1991;64:386–390. doi: 10.1038/bjc.1991.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Visser A., Hamza N., Kroese F.G.M., Bos N.A. Acquiring new N-glycosylation sites in variable regions of immunoglobulin genes by somatic hypermutation is a common feature of autoimmune diseases. Ann. Rheum. Dis. 2018;77 doi: 10.1136/annrheumdis-2017-212568. [DOI] [PubMed] [Google Scholar]
- 121.Huijbers M.G., Vergoossen D.L., Fillie-Grijpma Y.E., van Es I.E., Koning M.T., Slot L.M., Veelken H., Plomp J.J., van der Maarel S.M., Verschuuren J.J. MuSK myasthenia gravis monoclonal antibodies: valency dictates pathogenicity. Neurol Neuroimmunol Neuroinflamm. 2019;6:e547. doi: 10.1212/NXI.0000000000000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Turner G.A. N-glycosylation of serum proteins in disease and its investigation using lectins. Clin. Chim. Acta. 1992;208:149–171. doi: 10.1016/0009-8981(92)90073-y. [DOI] [PubMed] [Google Scholar]
- 123.Thompson S., Turner G.A. Elevated levels of abnormally-fucosylated haptoglobins in cancer sera. Br. J. Cancer. 1987;56:605–610. doi: 10.1038/bjc.1987.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yamamoto K., Tsuji T., Tarutani O., Osawa T. Structural changes of carbohydrate chains of human thyroglobulin accompanying malignant transformations of thyroid glands. Eur. J. Biochem. 1984;143:133–144. doi: 10.1111/j.1432-1033.1984.tb08352.x. [DOI] [PubMed] [Google Scholar]
- 125.Kobata A. Structures, function, and transformational changes of the sugar chains of glycohormones. J. Cell. Biochem. 1988;37:79–90. doi: 10.1002/jcb.240370108. [DOI] [PubMed] [Google Scholar]
- 126.Yamashita K., Koide N., Endo T., Iwaki Y., Kobata A. Altered glycosylation of serum transferrin of patients with hepatocellular carcinoma. J. Biol. Chem. 1989;264:2415–2423. [PubMed] [Google Scholar]
- 127.Thompson S., Cantwell B.M., Matta K.L., Turner G.A. Parallel changes in the blood levels of abnormally-fucosylated haptoglobin and alpha 1,3 fucosyltransferase in relationship to tumour burden: more evidence for a disturbance of fucose metabolism in cancer. Cancer Lett. 1992;65:115–121. doi: 10.1016/0304-3835(92)90154-n. [DOI] [PubMed] [Google Scholar]
- 128.van Eijk H.G., van Noort W.L., de Jong G., Koster J.F. Human serum sialo transferrins in diseases. Clin. Chim. Acta. 1987;165:141–145. doi: 10.1016/0009-8981(87)90157-4. [DOI] [PubMed] [Google Scholar]
- 129.Bektas M., Rubenstein D.S. The role of intracellular protein O-glycosylation in cell adhesion and disease. J Biomed Res. 2011;25:227–236. doi: 10.1016/S1674-8301(11)60031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Desai P.R. Immunoreactive T and Tn antigens in malignancy: role in carcinoma diagnosis, prognosis, and immunotherapy. Transfus. Med. Rev. 2000;14:312–325. doi: 10.1053/tmrv.2000.16229. [DOI] [PubMed] [Google Scholar]
- 131.Bian C.F., Zhang Y., Sun H., Li D.F., Wang D.C. Structural basis for distinct binding properties of the human galectins to Thomsen-Friedenreich antigen. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chou C.H., Huang M.J., Chen C.H., Shyu M.K., Huang J., Hung J.S., Huang C.S., Huang M.C. Up-regulation of C1GALT1 promotes breast cancer cell growth through MUC1-C signaling pathway. Oncotarget. 2015;6:6123–6135. doi: 10.18632/oncotarget.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wilson C.M., High S. Ribophorin I acts as a substrate-specific facilitator of N-glycosylation. J. Cell Sci. 2007;120:648–657. doi: 10.1242/jcs.000729. [DOI] [PubMed] [Google Scholar]
- 134.Milde-Langosch K., Karn T., Schmidt M., zu Eulenburg C., Oliveira-Ferrer L., Wirtz R.M., Schumacher U., Witzel I., Schutze D., Muller V. Prognostic relevance of glycosylation-associated genes in breast cancer. Breast Cancer Res. Treat. 2014;145:295–305. doi: 10.1007/s10549-014-2949-z. [DOI] [PubMed] [Google Scholar]
- 135.Ono M., Tsuda H., Kobayashi T., Takeshita F., Takahashi R.U., Tamura K., Akashi-Tanaka S., Moriya T., Yamasaki T., Kinoshita T., Yamamoto J., Fujiwara Y., Ochiya T. The expression and clinical significance of ribophorin II (RPN2) in human breast cancer. Pathol. Int. 2015;65:301–308. doi: 10.1111/pin.12297. [DOI] [PubMed] [Google Scholar]
- 136.Fujiwara T., Takahashi R.U., Kosaka N., Nezu Y., Kawai A., Ozaki T., Ochiya T. RPN2 gene confers osteosarcoma cell malignant phenotypes and determines clinical prognosis. Mol. Ther. Nucleic Acids. 2014;3:e189. doi: 10.1038/mtna.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang J., Yan B., Spath S.S., Qun H., Cornelius S., Guan D., Shao J., Hagiwara K., Van Waes C., Chen Z., Su X., Bi Y. Integrated transcriptional profiling and genomic analyses reveal RPN2 and HMGB1 as promising biomarkers in colorectal cancer. Cell Biosci. 2015;5:53. doi: 10.1186/s13578-015-0043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu J., Chen S., Liu H., Zhang Z., Ni Z., Chen J., Yang Z., Nie Y., Fan D. Tunicamycin specifically aggravates ER stress and overcomes chemoresistance in multidrug-resistant gastric cancer cells by inhibiting N-glycosylation. J. Exp. Clin. Cancer Res. 2018;37:272. doi: 10.1186/s13046-018-0935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zang X., Loke P., Kim J., Murphy K., Waitz R., Allison J.P. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10388–10392. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hsu J.-M., Li C.-W., Lai Y.-J., Hung M.-C. Posttranslational modifications of PD-L1 and their applications in cancer therapy. Cancer Res. 2018;78:6349–6353. doi: 10.1158/0008-5472.CAN-18-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li C.W., Lim S.O., Chung E.M., Kim Y.S., Park A.H., Yao J., Cha J.H., Xia W., Chan L.C., Kim T., Chang S.S., Lee H.H., Chou C.K., Liu Y.L., Yeh H.C., Perillo E.P., Dunn A.K., Kuo C.W., Khoo K.H., Hsu J.L., Wu Y., Hsu J.M., Yamaguchi H., Huang T.H., Sahin A.A., Hortobagyi G.N., Yoo S.S., Hung M.C. Eradication of triple-negative breast cancer cells by targeting glycosylated PD-L1. Cancer Cell. 2018;33:187–201 e110. doi: 10.1016/j.ccell.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Luo Y., Wu Z., Chen S., Luo H., Mo X., Wang Y., Tang J. Protein N-glycosylation aberrations and glycoproteomic network alterations in osteoarthritis and osteoarthritis with type 2 diabetes. Sci. Rep. 2022;12:6977. doi: 10.1038/s41598-022-10996-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li Q., Wen Y., Wang L., Chen B., Chen J., Wang H., Chen L. Hyperglycemia-induced accumulation of advanced glycosylation end products in fibroblast-like synoviocytes promotes knee osteoarthritis. Exp. Mol. Med. 2021;53:1735–1747. doi: 10.1038/s12276-021-00697-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen C., Ma C., Zhang Y., Zeng Y., Li Y., Wang W. Pioglitazone inhibits advanced glycation end product-induced TNF-alpha and MMP-13 expression via the antagonism of NF-kappaB activation in chondrocytes. Pharmacology. 2014;94:265–272. doi: 10.1159/000369074. [DOI] [PubMed] [Google Scholar]
- 145.Zhang H.B., Zhang Y., Chen C., Li Y.Q., Ma C., Wang Z.J. Pioglitazone inhibits advanced glycation end product-induced matrix metalloproteinases and apoptosis by suppressing the activation of MAPK and NF-kappaB. Apoptosis. 2016;21:1082–1093. doi: 10.1007/s10495-016-1280-z. [DOI] [PubMed] [Google Scholar]
- 146.Wang Z.J., Zhang H.B., Chen C., Huang H., Liang J.X. Effect of PPARG on AGEs-induced AKT/MTOR signaling-associated human chondrocytes autophagy. Cell Biol. Int. 2018;42:841–848. doi: 10.1002/cbin.10951. [DOI] [PubMed] [Google Scholar]
- 147.He C.P., Chen C., Jiang X.C., Li H., Zhu L.X., Wang P.X., Xiao T. The role of AGEs in pathogenesis of cartilage destruction in osteoarthritis. Bone Joint Res. 2022;11:292–300. doi: 10.1302/2046-3758.115.BJR-2021-0334.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lyu Y., Deng H., Qu C., Qiao L., Liu X., Xiao X., Liu J., Guo Z., Zhao Y., Han J., Lammi M.J. Identification of proteins and N-glycosylation sites of knee cartilage in Kashin-Beck disease compared with osteoarthritis. Int. J. Biol. Macromol. 2022;210:128–138. doi: 10.1016/j.ijbiomac.2022.05.014. [DOI] [PubMed] [Google Scholar]
- 149.Luo S., Shi Q., Li W., Wu W., Zha Z. ITGB1 promotes the chondrogenic differentiation of human adipose-derived mesenchymal stem cells by activating the ERK signaling. J. Mol. Histol. 2020;51:729–739. doi: 10.1007/s10735-020-09918-0. [DOI] [PubMed] [Google Scholar]
- 150.Han J., Deng H., Lyu Y., Xiao X., Zhao Y., Liu J., Guo Z., Liu X., Qiao L., Gao H., Lammi M.J. Identification of N-glycoproteins of knee cartilage from adult osteoarthritis and kashin-beck disease based on quantitative glycoproteomics, compared with normal control cartilage. Cells. 2022;11 doi: 10.3390/cells11162513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yoshimoto M., Sadamori K., Tokumura K., Tanaka Y., Fukasawa K., Hinoi E. Bioinformatic analysis reveals potential relationship between chondrocyte senescence and protein glycosylation in osteoarthritis pathogenesis. Front. Endocrinol. 2023;14 doi: 10.3389/fendo.2023.1153689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Huang S., Thomsson K.A., Jin C., Alweddi S., Struglics A., Rolfson O., Bjorkman L.I., Kalamajski S., Schmidt T.A., Jay G.D., Krawetz R., Karlsson N.G., Eisler T. Cathepsin g degrades both glycosylated and unglycosylated regions of lubricin, a synovial mucin. Sci. Rep. 2020;10:4215. doi: 10.1038/s41598-020-61161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu F.J., Xie L.Y., Li H.Z., Cao S.N., Chen Y.Z., Bin S., Wang D.D. Expression of ANGPTL4 in nucleus pulposus tissues is associated with intervertebral disc degeneration. Dis. Markers. 2021;2021 doi: 10.1155/2021/3532716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tardio L., Andres-Bergos J., Zachara N.E., Larranaga-Vera A., Rodriguez-Villar C., Herrero-Beaumont G., Largo R. O-linked N-acetylglucosamine (O-GlcNAc) protein modification is increased in the cartilage of patients with knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:259–263. doi: 10.1016/j.joca.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 155.Croft D.R., Dall P., Davies D., Jackson D.G., McIntyre P., Kramer I.M. Complex CD44 splicing combinations in synovial fibroblasts from arthritic joints. Eur. J. Immunol. 1997;27:1680–1684. doi: 10.1002/eji.1830270713. [DOI] [PubMed] [Google Scholar]
- 156.Date K., Sakagami H., Yura K. Regulatory properties of vitronectin and its glycosylation in collagen fibril formation and collagen-degrading enzyme cathepsin K activity. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-91353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yu H., Li M., Shu J., Dang L., Wu X., Wang Y., Wang X., Chang X., Bao X., Zhu B., Ren X., Chen W., Li Y. Characterization of aberrant glycosylation associated with osteoarthritis based on integrated glycomics methods. Arthritis Res. Ther. 2023;25:102. doi: 10.1186/s13075-023-03084-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kehayova Y.S., Watson E., Wilkinson J.M., Loughlin J., Rice S.J. Genetic and epigenetic interplay within a COLGALT2 enhancer associated with osteoarthritis. Arthritis Rheumatol. 2021;73:1856–1865. doi: 10.1002/art.41738. [DOI] [PubMed] [Google Scholar]
- 159.Yu H., Li M., Wen X., Yang J., Liang X., Li X., Bao X., Shu J., Ren X., Chen W., Li Z., Li Y. Elevation of alpha-1,3 fucosylation promotes the binding ability of TNFR1 to TNF-alpha and contributes to osteoarthritic cartilage destruction and apoptosis. Arthritis Res. Ther. 2022;24:93. doi: 10.1186/s13075-022-02776-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cao A., Alluqmani N., Buhari F.H.M., Wasim L., Smith L.K., Quaile A.T., Shannon M., Hakim Z., Furmli H., Owen D.M., Savchenko A., Treanor B. Galectin-9 binds IgM-BCR to regulate B cell signaling. Nat. Commun. 2018;9:3288. doi: 10.1038/s41467-018-05771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Demetriou M., Granovsky M., Quaggin S., Dennis J.W. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409:733–739. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- 162.Steenvoorden M.M., Huizinga T.W., Verzijl N., Bank R.A., Ronday H.K., Luning H.A., Lafeber F.P., Toes R.E., DeGroot J. Activation of receptor for advanced glycation end products in osteoarthritis leads to increased stimulation of chondrocytes and synoviocytes. Arthritis Rheum. 2006;54:253–263. doi: 10.1002/art.21523. [DOI] [PubMed] [Google Scholar]
- 163.Li X., Jia F., Zhu Z., Huang L. Lixisenatide attenuates advanced glycation end products (AGEs)-induced degradation of extracellular matrix in human primary chondrocytes. Artif. Cells, Nanomed. Biotechnol. 2019;47:1256–1264. doi: 10.1080/21691401.2019.1593996. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.